Abstract
The splicing factor U2AF is required for the recruitment of U2 small nuclear RNP to pre-mRNAs in higher eukaryotes. The 65-kDa subunit of U2AF (U2AF65) binds to the polypyrimidine (Py) tract preceding the 3′ splice site, while the 35-kDa subunit (U2AF35) contacts the conserved AG dinucleotide at the 3′ end of the intron. It has been shown that the interaction between U2AF35 and the 3′ splice site AG can stabilize U2AF65 binding to weak Py tracts characteristic of so-called AG-dependent pre-mRNAs. U2AF35 has also been implicated in arginine-serine (RS) domain-mediated bridging interactions with splicing factors of the SR protein family bound to exonic splicing enhancers (ESE), and these interactions can also stabilize U2AF65 binding. Complementation of the splicing activity of nuclear extracts depleted of U2AF by chromatography in oligo(dT)-cellulose requires, for some pre-mRNAs, only the presence of U2AF65. In contrast, splicing of a mouse immunoglobulin M (IgM) M1-M2 pre-mRNA requires both U2AF subunits. In this report we have investigated the sequence elements (e.g., Py tract strength, 3′ splice site AG, ESE) responsible for the U2AF35 dependence of IgM. The results indicate that (i) the IgM substrate is an AG-dependent pre-mRNA, (ii) U2AF35 dependence correlates with AG dependence, and (iii) the identity of the first nucleotide of exon 2 is important for U2AF35 function. In contrast, RS domain-mediated interactions with SR proteins bound to the ESE appear to be dispensable, because the purine-rich ESE present in exon M2 is not essential for U2AF35 activity and because a truncation mutant of U2AF35 consisting only of the pseudo-RNA recognition motif domain and lacking the RS domain is active in our complementation assays. While some of the effects of U2AF35 can be explained in terms of enhanced U2AF65 binding, other activities of U2AF35 do not correlate with increased cross-linking of U2AF65 to the Py tract. Collectively, the results argue that interaction of U2AF35 with a consensus 3′ splice site triggers events in spliceosome assembly in addition to stabilizing U2AF65 binding, thus revealing a dual function for U2AF35 in pre-mRNA splicing.
Intron removal from mRNA precursors (pre-mRNA splicing) is an essential step of gene expression in eukaryotes. The precise recognition of the intron boundaries, the 5′ and 3′ splice sites, is achieved by small nuclear RNPs (snRNPs) and non-snRNP proteins. The 5′ splice site is initially recognized by U1 snRNP, and the 3′ splice site region is recognized by U2 snRNP. Subsequent addition of the U4/U6/U5 tri-snRNP forms the spliceosome, the macromolecular complex within which splicing catalysis takes place (reviewed in references 6 and 23).
Several sequence elements help to define the 3′ splice site region in higher eukaryotes (reviewed in reference 35) : the branchpoint (BP) sequence, usually followed by a pyrimidine-rich sequence (the polypyrimidine tract or Py tract), and a conserved AG dinucleotide at the 3′ end of the intron. The BP contains an adenosine residue that forms a 2′ to 5′ phosphodiester bond with the 5′ end of the intron during the first catalytic step of the splicing reaction (39). U2 snRNP binds to the BP through base pairing interactions between this sequence and U2 snRNA (31, 33, 50, 56). U2 snRNP binding requires auxiliary factors, including SF1/mBBP and U2AF (22, 24, 40). SF1/mBBP has been shown to specifically recognize the BP (2, 34) and play a kinetic role in spliceosome assembly (17, 41). U2AF is a heterodimer of 65 and 35-kDa subunits (52). U2AF65 binds specifically to the Py tract via its RNA recognition motifs (RRMs) (53) and contacts the BP via its RS domain (11, 44), whereas U2AF35 contacts the AG dinucleotide at the 3′ splice site (30, 51, 58).
The 3′ splice site AG marks the 3′ intron boundary and is involved in exon ligation, the second catalytic step of the splicing reaction. For some AG-dependent substrates, however, this dinucleotide is already required for early steps of spliceosome assembly prior to catalysis (36). AG-dependent substrates typically contain weak Py tracts, and substrates with strong Py tracts generally do not require the presence of the 3′ splice site AG before the second catalytic step and are considered AG independent. Interaction between U2AF35 and the 3′ splice site AG dinucleotide was shown to stabilize U2AF65 binding to a weak Py tract and to be essential for splicing of AG-dependent substrates (51).
An alternative set of interactions has been proposed for U2AF35. The arginine-serine (RS) region of U2AF35 has been shown to establish protein-protein interactions with splicing factors of the SR family (49) (reviewed in references 10, 13, 29, and 45). One type of sequences bound by SR proteins are purine-rich exonic splicing enhancers (ESE), which stimulate splicing of pre-mRNAs containing weak 3′ splice sites (reviewed in references 7 and 42). Based upon experiments using purified components, Zuo and Maniatis (60) proposed that SR proteins bound to ESEs facilitate recruitment of U2AF65 to the Py tract via bridging interactions mediated by U2AF35 (4, 13, 14, 37). Other results, however, argued that U2AF65 recruitment was not the rate-limiting step in ESE-dependent splicing (20, 26).
We have previously shown that splicing of a mouse immunoglobulin M (IgM) M1-M2 pre-mRNA substrate requires both U2AF65 and U2AF35 (16). Exon M2 contains the founding member of the purine-rich class of ESEs (48). Because of the different sets of proposed U2AF35-mediated interactions mentioned above, we set out to investigate whether the dependence on U2AF35 for IgM splicing correlated with the presence of the purine-rich ESE or with sequences at the 3′ splice site. Our results indicate that IgM M1-M2 is an AG-dependent pre-mRNA and that U2AF35 dependence correlates with AG dependence but not with the presence of the purine-rich ESE.
One common feature of the two models for U2AF35 function is that direct or indirect interactions of U2AF35 with nearby sequences stabilize U2AF65 binding to the Py tract. Here we show that although interaction of U2AF35 with the 3′ splice site AG can stabilize U2AF65 binding, not all the activities of U2AF35 correlate with increased cross-linking of U2AF65 to the Py tract. The additional function is strongly dependent on the identity of the first nucleotide of the 3′ exon and requires only the pseudo-RRM (ΨRRM) motif present in U2AF35. Taken together, the data indicate that U2AF35 has a dual function in the splicing of AG-dependent pre-mRNAs.
MATERIALS AND METHODS
Plasmids.
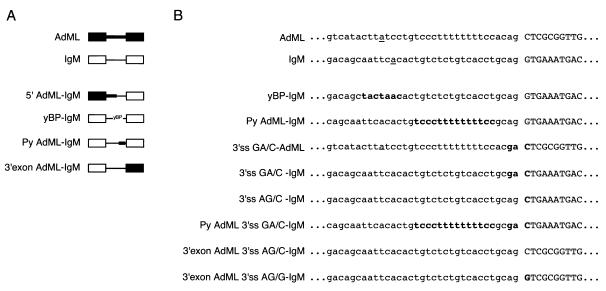
pAdML and pμM (IgM M1-M2) were described previously (48, 57). 5′ AdML (for adenovirus major late transcripts)-IgM was prepared by replacing the 3′ half of the intron and exon 2 of AdML with the corresponding part of the IgM substrate. In 3′ exon AdML-IgM, the enhancer containing exon M2 of IgM was replaced by exon 2 of AdML. In both cases the insert (3′ half of IgM and 3′ exon AdML) and the corresponding part of the vector (pAdML and pμM) were amplified by PCR and joined by blunt-end ligation. All other mutants were prepared via PCR-based site-directed mutagenesis of the plasmids mentioned above, as described elsewhere (19), using TaqPlus Precision DNA polymerase (Stratagene). All mutant clones were confirmed by sequencing.
Preparation of HeLa nuclear extract.
HeLa nuclear extract was prepared as described by Dignam et al. (8).
Depletion of U2AF by oligo(dT)-cellulose chromatography.
HeLa nuclear extract was depleted of U2AF exactly as described previously (46) by passing the extract over an oligo(dT)-cellulose column at 1 M KCl. The column flow-through of this procedure yields the depleted nuclear extract (odTΔNE).
Expression and purification of recombinant proteins.
U2AF65 was expressed as a glutathione S-transferase (GST) fusion protein in Escherichia coli as described previously (27). The plasmid used for expression was described previously (53). The purified protein was dialyzed against buffer D (20 mM HEPES [pH 8.0], 0.5 mM EDTA, 20% glycerol, 1 mM dithiothreitol [DTT], 0.05% NP-40) with 100 mM KCl.
U2AF35 was expressed with an amino-terminal six-His tag and was purified from baculovirus-infected insect cells under standard denaturing conditions using Ni-NTA agarose beads (Qiagen) (60). After purification the protein was renatured by dialysis against a solution containing 20 mM Tris-HCl (pH 8.0), 850 mM KCl, 20% glycerol. Prior to use the protein was diluted in buffer D without salt to yield a final KCl concentration of 100 mM.
His-U2AF35 ΨRRM was generated by cloning a fragment of U2AF35 cDNA encoding amino acids 38 to 153 in frame with a histidine tag in plasmid pET9 (gift from G. Stier, EMBL Heidelberg). The protein was expressed in BL21(DE3) E. coli cells induced with 1 mM isopropyl-d-thiogalactopyranoside for 5 h at 25°C. Cells were harvested and resuspended in a solution containing 10% glycerol, 0.1% Triton X-100, 50 mM Tris-HCl (pH 7.5), 250 mM NaCl, 5 mM 2-mercaptoethanol and were disrupted by sonication. The cell debris was sedimented by centrifugation. Recombinant His-U2AF35 ψRRM was purified by affinity chromatography on Ni-NTA columns (Qiagen) equilibrated with buffer A (50mM Tris-HCl [pH 7.5], 200 mM NaCl, 20 mM imidazole, 5 mM 2-mercaptoethanol). The Ni-NTA resin was subsequently incubated with the clear lysate of cells expressing U2AF65, the column was extensively washed with buffer A, and bound proteins were eluted with 300 mM imidazole. U2AF65/U2AF35 ψRRM complexes were separated from free U2AF35 ψRRM by gel filtration on Superdex 75 (Amersham Pharmacia Biotech) in a solution of 20 mM sodium phosphate [pH 6.3], 100 mM NaCl, 2 mM DTT.
In vitro transcription of splicing substrates.
Transcription templates were generated by PCR using plasmids harboring the sequences of AdML, IgM M1-M2 (pμM), or mutant derivatives of both substrates (Fig. 1) preceded by an SP6 promoter. SP6 primer and a reverse primer annealing to the IgM exon enhancer sequence or AdML exon 2 were used to generate templates for full-length transcription templates. The same reverse primers were used for the 3′-half substrates in combination with a forward primer containing the T7 promoter followed by a sequence annealing approximately 20 nucleotides upstream of the BP.
FIG. 1.
Pre-mRNA splicing substrates. (A) Schematic representation of AdML, IgM, and mutant splicing substrates. Black boxes and thick lines represent the AdML exons and intron, respectively; white boxes and thin lines depict the IgM M1-M2 exons and intron. yBP indicates the yeast consensus BP sequence, TACTAAC. (B) Sequence of splicing substrates at the 3′ splice site, including BP, Py tract, and part of the 3′ exon (in capitals). The BP in the wild-type substrates is underlined; sequences in bold indicate mutated nucleotides.
Full-length substrates were transcribed in the presence of a CAP analog [m7G (5′) ppp (5′) G] (New England Biolabs) and [α-32P]UTP (Amersham) as described previously (16). For transcription of 3′-half RNAs the CAP analog was omitted from the reaction mix and the GTP concentration was raised accordingly. After a 2-h incubation at 37°C, the transcripts were gel purified, ethanol precipitated, and resuspended in water.
In vitro splicing assays and spliceosome assembly reactions.
Splicing reactions, splicing complementation assays, and spliceosome assembly reactions were performed as described previously (16). Spliced products were resolved on 13% denaturing polyacrylamide gels in Tris-Borate-EDTA buffer and spliceosomal complexes were resolved on native 4% acrylamide:bisacrylamide (80:1)–0.5% agarose gels in 50 mM Tris base–50 mM glycine buffer. Gels were exposed to PhosphorImager screens (Fuji BAS-MP).
UV cross-linking and immunoprecipitation.
The UV cross-linking and immunoprecipitation experiments were performed exactly as described elsewhere (16). Gels were exposed to PhosphorImager screens, and the intensity of the bands was quantified.
RESULTS
Mapping sequences that make IgM M1-M2 pre-mRNA U2AF35 dependent.
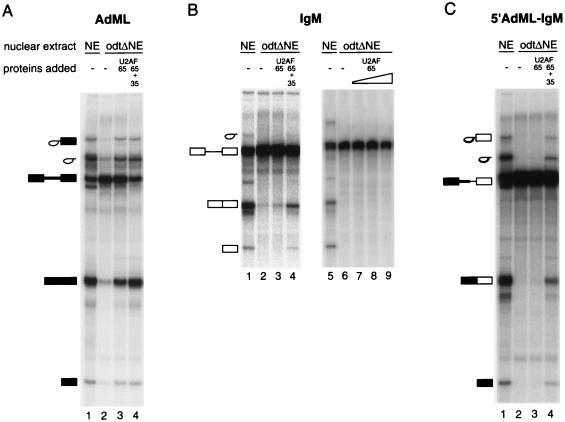
We have used oligo(dT) chromatography at 1 M KCl to deplete U2AF from HeLa nuclear extracts (46). The flow-through of the column represents an extract unable to support in vitro splicing assays unless complemented with U2AF activity. For some splicing substrates that contain strong 3′ splice site signals, e.g., AdML or β-globin, complementation can be achieved with U2AF65 alone (16, 44, 46, 53) (Fig. 2A). For other pre-mRNAs, e.g., IgM M1-M2 pre-mRNA (referred to as IgM), which contains relatively weak Py tract and BP sequences, significant levels of complementation require the presence of both U2AF subunits (16) (Fig. 2B). As a first step to determine sequence elements responsible for the U2AF35 dependence exhibited by IgM pre-mRNA, chimeric RNAs comprising parts of AdML and IgM as well as additional IgM RNAs containing mutations within the 3′ splice site signals were generated. Figure 1 shows the mutants used in this study.
FIG. 2.
In vitro splicing reconstitution assay. Radioactively labeled pre-mRNAs were incubated under splicing conditions, and RNAs were isolated and fractionated by electrophoresis on denaturing 13% polyacrylamide gels. Pre-mRNAs were incubated in HeLa nuclear extract (NE) or odtΔNE in the absence or presence of recombinant GST-U2AF65 and His-U2AF35 (for protein concentrations, see below). Splicing substrates and products are indicated schematically on the left of each panel, represented as in Fig. 1A. (A) AdML pre-mRNA, with 90 nM GST-U2AF65 in lanes 3 and 4 and 210 nM His-U2AF35 in lane 4; (B) mouse IgM M1-M2 minigene, with 90nM GST-U2AF65 in lanes 3 and 4 and 210 nM His-U2AF35 in lane 4 and 90, 180, and 270 nM GST-U2AF65 in lanes 7, 8, and 9, respectively; (C) 5′ AdML-IgM, a chimeric RNA comprising the 5′ half of the AdML and the 3′ half of the IgM substrate, with 90nM GST-U2AF65 in lanes 3 and 4 and 210 nM His-U2AF35 in lane 4.
When an RNA containing the 5′ exon and the first half of the intron of the AdML substrate fused to the 3′ half of the intron and the 3′ exon of IgM (designated 5′ AdML-IgM; Fig. 1A) was tested, addition of both U2AF65 and U2AF35 were necessary to restore the splicing activity of the depleted extracts (Fig. 2C). These results indicate that the sequences responsible for U2AF35 dependence do not lie within the 5′ half of the IgM pre-mRNA. Therefore, the 5′ splice site of IgM does not play an essential role in making this pre-mRNA U2AF35 dependent.
Next the role of sequence elements within the 3′ half of the RNA (BP, Py tract, 3′ splice site, and ESE) was tested. The BP sequence of IgM AAUUCAC (the underlined residue indicates the experimentally determined BP adenosine; data not shown) diverges significantly from the consensus sequence YNCURAY (Y is C/U, R is A/G, and N is any nucleotide). When the IgM BP was replaced by the yeast consensus BP UACUAAC (yBP-IgM; Fig. 1A and B), which is also the preferred site in the metazoan system (55), the requirement for U2AF35 was maintained (Fig. 3A). In contrast, when the weak 12-nucleotide Py tract of IgM was replaced by the U-rich 14-nucleotide Py tract of AdML (Py AdML-IgM; Fig. 1A and B), splicing was partially restored by addition of only U2AF65 to the depleted extracts (Fig. 3B), as was the case for the AdML substrate (Fig. 2A). Therefore, a U-rich Py tract, which represents a high affinity binding site for U2AF65, relieved the absolute requirement for U2AF35. Although these and other related results (14) are compatible with a role of U2AF35 in promoting U2AF65 binding, experiments described below indicate that U2AF35 has an additional function in spliceosome assembly.
FIG. 3.
A U-rich Py tract, but not a consensus BP, renders IgM U2AF35 independent. Radioactively labeled yBP-IgM (A) or Py AdML-IgM RNA (B) was incubated in HeLa nuclear extract (NE) or odtΔNE in the absence or presence of 90 nM GST-U2AF65 and 210 nM U2AF35 and were analyzed as described for Fig. 2. Splicing products and intermediates are indicated on the left of each panel and are represented as in Fig. 1A.
IgM is an AG-dependent substrate.
Substrates with weak Py tracts are usually AG dependent, i.e., the AG dinucleotide at the 3′ splice site is already required for spliceosome assembly and the first catalytic step. In at least one β-globin pre-mRNA derivative, such AG dependence correlated with a requirement for U2AF35 (51). To investigate whether the requirement for U2AF35 in IgM splicing also correlated with the AG dependence of this substrate, the sequence at the 3′ splice site of IgM was changed from AG/G into GA/C (designated 3′ss GA/C-IgM; Fig. 1B). This pre-mRNA remained completely unspliced (Fig. 4A, lane 4) under conditions that allowed accumulation of substantial amounts of spliced products from the wild-type RNA (Fig. 4A, lane 2). Concurrently, formation of splicing complexes was significantly reduced in the 3′ splice site GA/C mutant (Fig. 4B, compare lanes 2 and 4). This is in contrast with results obtained with AdML, where the same mutation (designated 3′ss GA/C-AdML; Fig. 1B) still allowed the first catalytic step of splicing to occur, leading to an accumulation of splicing intermediates (Fig. 4A, lane 15). Consistent with our predictions and previous observations (36), combination of the GA/C substitution with replacement of the IgM Py tract by that of AdML (designated Py AdML 3′ss GA/C-IgM; Fig. 1B) resulted in a pre-mRNA which could undergo the first step of splicing, leading to accumulation of splicing intermediates (Fig. 4A, lanes 7 and 8). For this substrate the first step of splicing could be partially restored by addition of U2AF65 alone (Fig. 4A, lane 10), as was the case for AdML. As the AdML Py tract rendered IgM pre-mRNA splicing both AG independent (Fig. 4A) and U2AF35 independent (Fig. 3B), these results are consistent with the idea that interaction between U2AF35 and the 3′ splice site AG is important for splicing of the U2AF35-dependent IgM pre-mRNA.
FIG. 4.
IgM is an AG-dependent substrate. (A) Splicing assays. The splicing substrates indicated at the top of each panel were incubated in HeLa nuclear extract (NE) in the presence or absence of ATP or in odtΔNE in the presence of recombinant U2AF subunits (90 nM U2AF65, 210 nM U2AF35) as indicated. Products were analyzed as described for Fig. 2. IgM splicing products are indicated on the left, and AdML splicing products are indicated on the right. (B) Spliceosome assembly assay. IgM and 3′ss GA/C-IgM RNAs were incubated in HeLa nuclear extract (NE) in the absence or presence of ATP. IgM was also incubated in odtΔNE (right panel) in the presence of recombinant U2AF subunits or the U2AF-containing column eluate (GUA). After incubation for 20 min the mixtures were loaded onto native polyacrylamide composite gels to separate ATP-independent hnRNP complexes (complex H) from ATP-dependent prespliceosomes (complex A) and two conformations of the spliceosome (complex B/C). wt, wild type.
The results presented in the right panel of Fig. 4B indicate that U2AF35 promotes U2 snRNP recruitment. While no detectable spliceosomal A or B/C complexes were observed in the presence of U2AF65 alone (lane 8), addition of U2AF65 and U2AF35 restored complex A formation (lane 9), correlating with splicing activation (Fig. 2B).
Dual function of U2AF35.
Wu et al. (51) demonstrated that in vitro splicing of an AG-dependent derivative of the β-globin pre-mRNA was strongly stimulated by U2AF35 and that U2AF35 increased binding of U2AF65 to the Py tract of this substrate. To test whether this was also the case for IgM under our experimental conditions, we carried out UV cross-linking and immunoprecipitation of U2AF65 from reactions set up under splicing conditions. The 3′-half RNAs corresponding to wild-type IgM, 3′ss AG/C-IgM, and 3′ss GA/C-IgM, comprising approximately 20 nucleotides upstream from the BP, Py tract, and the downstream exon including the purine-rich ESE, were utilized. U2AF65 cross-linking to these RNAs was specific to the Py tract and could no longer be detected when the Py tract was mutated or deleted (16 and data not shown).
When recombinant U2AF65 was added to U2AF-depleted extracts the cross-linking signal to IgM increased with increasing protein concentrations (Fig. 5, lanes 3 to 5 and 11 to 13), arguing that the assay can detect increases in U2AF65 binding. Although occupancy by U2AF65 increased with the concentration of the protein, splicing was not detectable above background even at the highest concentration of U2AF65 (Fig. 2B, lanes 7 to 9) (16). Addition of U2AF35 to the reaction mixtures increased the U2AF65 cross-linking signal (Fig. 5, compare lanes 3 to 5 with 6 to 8 and lane 11 with 14), coincident with splicing activation (Fig. 2B, lane 4). Mutation of the 3′ splice site AG/G to AG/C or GA/C resulted both in the loss of the stimulatory effect of U2AF35 on U2AF65 cross-linking (compare lanes 17 and 18 and 21 and 22) and in the absence of splicing in complementation reactions (Fig. 6C and 4A, lanes 3 to 5). Collectively these results are consistent with the idea that U2AF35 enhances U2AF65 binding to promote IgM splicing. However, the levels of U2AF65 cross-linking did not always correlate with the efficiency of splicing. Thus, although high concentrations of U2AF65 resulted in similar or higher cross-linking signals than those observed in the presence of both subunits (Fig. 5, compare lanes 13 and 14), no splicing was observed in the presence of U2AF65 alone at any concentration tested (Fig. 2B, lanes 7 to 9). These observations argue that although U2AF35 can increase U2AF65 binding, this is not sufficient to promote splicing and, therefore, occupancy of the Py tract by U2AF65 is not the rate-limiting step facilitated by U2AF35 under these experimental conditions.
FIG. 5.
Cross-linking of U2AF65 to IgM and 3′ss GA/C-IgM. The radioactively labeled 3′-half RNAs of IgM and 3′ splice site mutants (3′ ss AG/C-IgM and 3′ss GA/C-IgM) were incubated under splicing condition in HeLa nuclear extracts (NE) or odtΔNE, with or without GST-U2AF65 (concentrations [conc.] are indicated above each lane) or 210 nM His-U2AF35. The mixtures were irradiated with UV light and U2AF65 immunoprecipitated with specific anti-U2AF65 antibodies. The precipitates were fractionated on sodium dodecyl sulfate–10% polyacrylamide gels, and the dried gels were exposed to a phosphorimager screen. The positions of GST-U2AF65, endogenous U2AF65, and U2AF35 are indicated on the left. Quantification of the phosphorimager signals corresponding to GST-U2AF65 cross-linking is shown in the lower panel. The signal obtained in odtΔNE without added protein was used as background, and the value was deducted from values obtained for GST-U2AF65 cross-linking. The value for 90 nM GST-U2AF65 was set to 300 arbitrary scan units, and the remaining values were scaled accordingly to be able to directly compare them. wt, wild type.
FIG. 6.
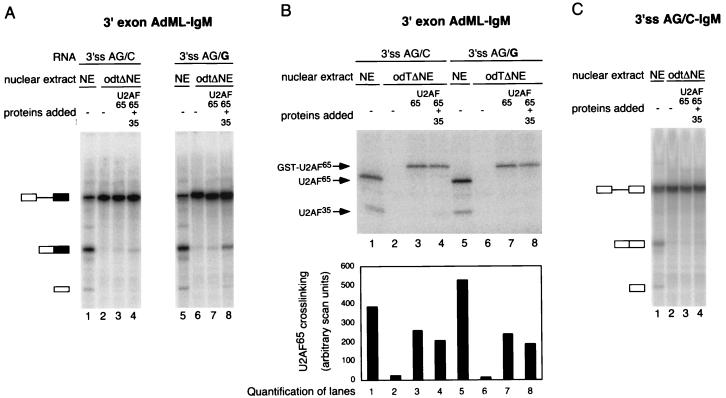
A consensus 3′ splice site, but not the ESE, is required for optimal U2AF35 function on IgM. (A) Splicing complementation assay using 3′ exon AdML 3′ss AG/C-IgM and 3′ exon AdML 3′ss AG/G-IgM pre-mRNA substrates in HeLa nuclear extract (NE) or odtΔNE supplemented with 90 nM GST-U2AF65 and 210 nM His-U2AF35 as indicated above each lane. After incubation the RNA was isolated and splicing products were separated on denaturing 13% polyacrylamide gels. (B) Cross-linking of U2AF65 in the reactions shown in panel A. The 3′-half RNAs corresponding to 3′ exon AdML 3′ss AG/C-IgM and 3′ exon AdML 3′ss AG/G-IgM were incubated under the same conditions as those described for panel A. The mixtures were then irradiated with UV light, and U2AF65 was immunoprecipitated with specific anti-U2AF65 antibodies. Precipitated proteins were separated on sodium dodecyl sulfate–10% polyacrylamide gels and exposed to a phosphorimager screen. The positions of GST-U2AF65, U2AF65, and U2AF35 are indicated on the left. The lower panel shows a quantification of the signals corresponding to U2AF65. (C) Splicing complementation assay using the 3′ss AG/C-IgM pre-mRNA performed as described for panel A.
The additional function of U2AF35 is dependent upon the presence of a consensus 3′ splice site.
To investigate whether the purine-rich ESE present in IgM exon M2 plays a role in U2AF35 dependence, this sequence was deleted. Accumulation of spliced products from this substrate was significantly reduced under our experimental conditions (data not shown), thus precluding further analysis in depleted extracts. As a second attempt to study the role of the M2 ESE, a mutant was generated in which exon M2 was replaced by exon 2 of AdML pre-mRNA, which does not contain an identifiable purine-rich ESE. This chimeric RNA (designated 3′ exon AdML 3′ss AG/C-IgM; Fig. 1A and B) could be spliced in nuclear extracts, arguing that the purine-rich ESE present in exon M2 is not essential for splicing of the IgM intron. Surprisingly, however, addition of U2AF65 and U2AF35 failed to restore significant levels of splicing in U2AF-depleted extracts (Fig. 6A, lanes 1 to 4). One possible explanation for this observation was that although it is dispensable for splicing in complete extracts, the M2 ESE was required for complementation by the U2AF heterodimer in oligo(dT)-depleted extracts. An alternative possibility was that other sequences within AdML exon 2 were suboptimal for the function of the heterodimer in the complementation assay. One difference between wild-type IgM and 3′exon AdML 3′ss AG/C-IgM is that the first nucleotide of the AdML exon 2 is a cytidine, and therefore the chimeric 3′ splice site diverges from the consensus AG/G, which is present in IgM. To test whether the presence of a consensus 3′ splice site was important for complementation, the first nucleotide of AdML exon 2 was changed from C to G to re-create a consensus 3′ splice site (designated 3′exon AdML-3′ss AG/G-IgM; Fig. 1B). This RNA was spliced in nuclear extracts, and addition of U2AF65 and U2AF35 could restore splicing in depleted extracts (Fig. 6A, lanes 5 to 8). Phosphorimager-based quantification of the signals revealed that the levels of activation [ratio between products of splicing and unspliced pre-mRNA compared to the background in oligo(dT)-depleted extracts] obtained by addition of U2AF65 and U2AF35 to depleted extracts were comparable for IgM and 3′exon AdML-3′ss AG/G-IgM substrates (between 3- and 3.5-fold in three independent experiments), whereas U2AF65 alone failed to activate splicing (Fig. 2B and 6A). We conclude that the presence of a consensus 3′ splice site is important for the activity of U2AF35 in the complementation assay.
As the presence of a consensus 3′ splice site was required for U2AF35 activity, we asked whether the presence of a consensus site had any effect on U2AF65 cross-linking to the Py tract. UV cross-linking and immunoprecipitation of U2AF65 showed that cross-linking of recombinant U2AF65 to either substrate was similar and was not enhanced by the presence of recombinant U2AF35 (Fig. 6B). As splicing occurred with the 3′ splice site AG/G substrate but not with the 3′ splice site AG/C substrate, these observations further support the idea that splicing activation by U2AF35 can be uncoupled from its effects on U2AF65 binding.
To further confirm the role of the guanidine nucleotide at position +1 of IgM exon M2, this position was changed to cytidine in the original IgM pre-mRNA (3′ss AG/C-IgM; Fig. 1B). Figure 6C shows that this substrate could be spliced in nuclear extracts (lane 1), albeit less efficiently than wild-type IgM (IgM ∼50%, 3′ss AG/C-IgM ∼20%). Addition of U2AF65 and U2AF35 to depleted extracts, however, failed to activate splicing of 3′ss AG/C-IgM in the depleted extracts. This result confirms the importance of a consensus 3′ splice site for U2AF35 activity.
In summary, we conclude that splicing activation by U2AF35 in oligo(dT)-depleted extracts depends on a consensus 3′ splice site and does not fully correlate with increases in U2AF65 cross-linking to the Py tract. The results also argue that the presence of the purine-rich ESE found in exon M2 is not essential for the activity of U2AF35 in these assays.
U2AF35 ψRRM is sufficient to activate IgM splicing.
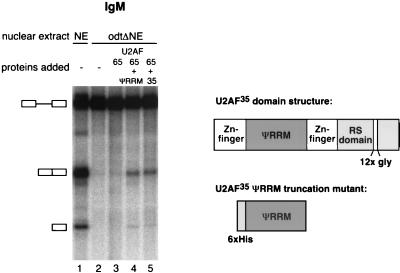
To further investigate a possible role for ESEs in U2AF35-dependent splicing, an experiment was designed to test a critical tenet of the recruitment model for exon enhancer function: SR proteins bound to the enhancer sequence establish protein-protein interactions with U2AF35 that increase the local concentration of the U2AF heterodimer and facilitate U2AF65 binding to the Py tract (13). Evidence has accumulated that these protein-protein interactions involve the RS domains present in both SR protein family members and U2AF35 (1, 15, 21, 25, 49). Recently, Zhu and Krainer have shown that RS domains are particularly critical for the activity of enhancer-bound SR proteins when the substrate analyzed has a weak Py tract and is U2AF35 dependent, as is the case for IgM (54). U2AF35 consists of a carboxy-terminal RS domain, two zinc fingers, and a region that, although significantly homologous to RRMs, shows some unusual sequence features and has therefore been dubbed ΨRRM (3). The result presented in Fig. 7 indicates that a U2AF heterodimer consisting of U2AF65 and only the ΨRRM of U2AF35 can restore splicing of IgM in oligo(dT)-depleted extracts (Fig. 7, lane 4). This result indicates that the RS domain of U2AF35 is not essential for the biochemical activity tested in our assays and therefore that RS-mediated protein-protein interactions with SR proteins bound to the IgM ESE are not indispensable for the function of U2AF35 in our experimental system. Consistent with the proposal that U2AF35 needs to recognize the 3′ splice site to restore activity to oligo(dT)-depleted extracts, preliminary results indicate that the ΨRRM domain can be cross-linked to a pre-mRNA site-specifically labeled at the 3′ splice site (S. Guth and E. Kellenberger, unpublished data).
FIG. 7.
The U2AF35 ψRRM is sufficient to provide U2AF35 activity for splicing of IgM in oligo(dT)-depleted nuclear extracts. Radioactively labeled IgM RNA was incubated in NE or odtΔNE supplemented with either U2AF65 alone, with additional U2AF35 ΨRRM, or with full-length U2AF35 and was analyzed as described for Fig. 2. A schematic representation of the U2AF35 domain structure and the truncation mutant are shown on the right.
Taken together, our analyses of pre-mRNA sequences and protein domains important for U2AF35 function argue that RS domain-mediated bridging interactions between enhancer-bound SR proteins and U2AF35 are not critical for splicing of IgM in oligo(dT)-depleted extracts. The results are more compatible with a model in which U2AF35 recognizes the 3′ splice site AG/G sequence to stimulate spliceosome assembly at a step subsequent to U2AF65 binding to the Py tract.
DISCUSSION
The tight association of U2AF35 with the essential splicing factor U2AF65, the significant phylogenetic conservation of U2AF35 across metazoa, and the essential nature of the gene in Drosophila melanogaster and Caenorhabditis elegans argue that U2AF35 plays an important function in the splicing process (38, 59). Early biochemical results, however, failed to show an absolute requirement for this factor in the splicing of model substrates in vitro (16, 44, 46, 53) (Fig. 2). More recent work indicated that, although not essential to complement U2AF-depleted nuclear extracts, U2AF35 did enhance splicing (14, 60). This enhancement was found to be particularly critical for achieving significant levels of splicing of pre-mRNAs like mouse IgM, suggesting substrate-specific differences in the requirement of U2AF35 (16). It is also important to point out that the apparent degree of U2AF35 dependence is affected by the precise conditions of the biochemical assays. Thus, U2AF35 requirement is more stringent when extracts are depleted of U2AF by chromatography in oligo(dT)-cellulose than when they are immunodepleted by using antibodies against U2AF65 (16, 20). It is possible that chromatographic depletion of factors in addition to U2AF [e.g., PUF60 (32)] makes U2AF35 function more rate limiting in oligo(dT)-depleted extracts. Therefore it seems likely that discrepancies in U2AF35 requirement observed in different laboratories (14, 16, 20, 60) result from differences in the sources of depleted extracts and recombinant proteins as well as on the particular pre-mRNA tested. Codepletion of other factors could also explain why some of the substrates used in this manuscript (e.g., 3′ exon AdML-IgM and 3′ss AG/C-IgM) failed to be spliced in depleted extracts complemented by U2AF65 and U2AF35 but were processed in complete extracts. Differences in assays and specific RNA constructs could also be at the basis of discrepancies on whether or not ESE sequences are required for IgM splicing, depending on the presence or absence of other exonic sequences, including a downstream inhibitory sequence element (14, 20).
The data presented in this report indicate that U2AF35 dependence correlates with AG dependence (Fig. 2, 4A, and 5). In addition, the nature of the first nucleotide in exon 2 has a strong influence on U2AF35 function, with guanosine being significantly more efficient than cytidine (Fig. 6). This result is consistent with in vitro selection studies by Wu et al. showing that while only uridine-rich sequences were selected by U2AF65, an adjacent AG/G sequence was selected when the process was carried out using the U2AF65/35 heterodimer (51). Interaction between U2AF35 and the 3′ splice site AG/G stabilizes U2AF65 binding (30, 51, 58), and the cross-linking data in Fig. 5 are certainly consistent with this. The question, however, is whether U2AF65 binding is rate limiting for splicing under the conditions of a specific substrate and assay. The combined results of Fig. 2B and 5, together with those of Fig. 6, suggest that U2AF65 binding is not rate limiting for splicing under the conditions used in our assays. While similar levels of cross-linking were observed in the presence of U2AF65 (90 nM) and U2AF35 or in the presence of higher concentrations (270 nM) of U2AF65 alone (Fig. 5), significant levels of splicing were observed only in the presence of U2AF35 (Fig. 2B). This argues for a function of U2AF35 in addition to assisting U2AF65 binding. The fact that providing a Py tract with higher affinity for U2AF65 results in U2AF35 independence (Fig. 3C) or ESE independence (14, 28, 43) does not necessarily mean that the exclusive role of U2AF35 is to improve U2AF35 binding. Given the highly cooperative nature of spliceosome assembly, the additional role provided by U2AF35 in our experimental conditions could promote a set of interactions whose final effect can also be achieved by U2AF65-Py tract interactions of higher affinity. It is worth pointing out in this context that the stimulation of U2AF65 cross-linking by U2AF35 reported here using the 3′-half RNAs is stronger than that observed using a full-length IgM RNA specifically labeled at the Py tract region (16). A possible explanation for these observations is that factors bound to the 5′ splice site stabilize U2AF65 binding in a U2AF35-independent manner, thus attenuating the apparent contribution of U2AF35 to U2AF65 binding.
Graveley et al. have recently reported that splicing of an IgM-derived construct, in which an ESE was replaced by a binding site for the bacteriophage RNA binding protein MS2, was strictly dependent upon addition of a fusion protein between MS2 and an SR protein RS domain (14). Efficient splicing reconstitution with this substrate in oligo(dT)-depleted extracts required, in addition to MS2-RS, both U2AF65 and U2AF35, which is consistent with our observations using ESE-containing IgM. These observations supported the notion that RS-mediated interactions are important for splicing activation and required U2AF35. As MS2-RS also increased cross-linking of U2AF65 and U2AF35 to the pre-mRNA under conditions of limited amounts of nuclear extract, these observations were in agreement with the idea that RS domain-mediated interactions facilitate U2AF recruitment (14), possibly through interactions with the RS domain of U2AF35 (49, 60). The levels of U2AF65 cross-linking in the reconstituted reaction mixtures, however, were not analyzed in these experiments. As a direct correlation between splicing and U2AF65 cross-linking in parallel reconstitution reactions was not established, it is possible that recruitment of U2AF65 is not the rate-limiting step in oligo(dT)-depleted extracts supplemented with MS2-RS, U2AF65, and U2AF35 using the IgM-MS2 substrate, as was the case in our experiments using wild-type IgM. These arguments do not detract from the fact that the experiments of Graveley et al. persuasively argue that SR proteins bound to the IgM ESE play an important role in IgM splicing. This function could be related to antagonizing silencing effects of other sequences (20) and/or facilitating other steps in spliceosome assembly, including U1 snRNP binding to the 5′ splice site through interactions with the splicing coactivator SRm160/300 (4, 9, 13, 26). Indeed, recent results strongly argue that ESEs can act through different mechanisms depending on the specific sequence configuration of the 3′ splice site (54). This is consistent with the widespread role that ESEs play in both constitutive and regulated splicing, as highlighted by the high incidence of ESE mutations in disease (reviewed in reference 18).
Alternatively (or additionally), an important role of ESEs in normal extracts could indeed be to facilitate U2AF recruitment, and results from a number of laboratories and different pre-mRNA substrates are certainly consistent with this (5, 14, 37, 47). Also consistent are the results presented in Fig. 4 and 5, showing that replacement of the 3′ splice site AG/G by GA/C, a mutation that abolishes U2AF35 binding (30, 51, 58), decreased endogenous U2AF65 cross-linking (Fig. 5, lanes 9 and 19) concomitantly with decreased spliceosome assembly (Fig. 4B) and failure to undergo splicing (Fig. 4A).
Reconstitution of depleted extracts requires amounts of recombinant subunits apparently in excess of the levels of the endogenous factors present in undepleted nuclear extracts (data not shown). It is conceivable that this excess, combined with other effects derived from the chromatographic depletion procedure, can change the rate-limiting step for splicing. Although this could, in principle, question the significance of results obtained by using depleted nuclear extracts, we believe that the experimental conditions described here have been useful to unravel a function for U2AF35 in splicesome assembly in addition to its role in promoting U2AF65 binding to the Py tract. This function is influenced by the nature of the first nucleotide after the 3′ splice site. Proper interaction of U2AF35 with this residue could result in a particular conformation of U2AF35 (or even U2AF65) that exposes domains required, directly or indirectly, for U2 snRNP recruitment (e.g., interactions with the 17S U2 snRNP component SAP155 [12]). Another possible scenario is that U2AF35 facilitates binding of SR proteins to the downstream exon enhancer, which in turn could lead to the recruitment of other factors and complexes that activate splicing—including SRm160/300 (4)—or that antagonize inhibitory signals (20). Intriguingly, the result with the U2AF35 deletion mutant of Fig. 7 suggests that whatever the nature of this function, it can be provided by the ψRRM alone.
In summary, we propose that U2AF35 plays a dual function in splicing, separable under different experimental conditions, and that the precise sequence context of the 3′ splice site strongly influences the effects of U2AF35. The two subunits of U2AF work in concert to recognize the 3′ splice site region and promote U2 snRNP binding. Depending on the given sequence context of different pre-mRNAs, the role of U2AF35 may be critical for either one or both of its functions. The multiple interactions and their different relative contributions can set the stage for versatile substrate-specific regulation of splice site recognition at the earliest steps of spliceosome assembly.
ACKNOWLEDGMENTS
We thank Tom Maniatis for the gift of U2AF35-baculovirus stocks and Michael Sattler, Gunther Stier, and members of the EMBL Gene Expression Programme for advice, discussions, and critical reading of the manuscript.
T.O/.T. was supported by an EMBO short-term fellowship. E. K. was supported by the Alexander von Humboldt Stiftung.
REFERENCES
- 1.Amrein H, Hedley M, Maniatis T. The role of specific protein-RNA and protein-protein interactions in positive and negative control of pre-mRNA splicing by Transformer-2. Cell. 1994;76:735–746. doi: 10.1016/0092-8674(94)90512-6. [DOI] [PubMed] [Google Scholar]
- 2.Berglund J, Chua K, Abovich N, Reed R, Rosbash M. The splicing factor BBP interacts specifically with the pre-mRNA branchpoint sequence UACUAAC. Cell. 1997;89:781–787. doi: 10.1016/s0092-8674(00)80261-5. [DOI] [PubMed] [Google Scholar]
- 3.Birney E, Kumar S, Krainer A. Analysis of the RNA-recognition motif and RS and RGG domains: conservation in metazoan pre-mRNA splicing factors. Nucleic Acids Res. 1993;21:5803–5816. doi: 10.1093/nar/21.25.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blencowe B. Exonic splicing enhancers: mechanism of action, diversity and role in human genetic diseases. Trends Biochem Sci. 2000;25:106–110. doi: 10.1016/s0968-0004(00)01549-8. [DOI] [PubMed] [Google Scholar]
- 5.Bouck J, Fu X, Skalka A, Katz R. Role of the constitutive splicing factors U2AF65 and SAP49 in suboptimal RNA splicing of novel retroviral mutants. J Biol Chem. 1998;273:15169–15176. doi: 10.1074/jbc.273.24.15169. [DOI] [PubMed] [Google Scholar]
- 6.Burge C, Tuschl T, Sharp P. Splicing of precursors to mRNAs by the spliceosome. In: Gesteland R, Cech T, Atkins J, editors. The RNA world. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1999. pp. 525–60. [Google Scholar]
- 7.Cooper T. Defining pre-mRNA cis elements that regulate cell-specific splicing. Methods Mol Biol. 1999;118:391–403. doi: 10.1385/1-59259-676-2:391. [DOI] [PubMed] [Google Scholar]
- 8.Dignam J, Lebovitz R, Roeder R. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eldridge A, Li Y, Sharp P, Blencowe B. The SRm160/300 splicing coactivator is required for exon-enhancer function. Proc Natl Acad Sci USA. 1999;96:6125–6130. doi: 10.1073/pnas.96.11.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu X-D. The superfamily of arginine/serine-rich splicing factors. RNA. 1995;1:663–680. [PMC free article] [PubMed] [Google Scholar]
- 11.Gaur R, Valcárcel J, Green M. Sequential recognition of the pre-mRNA branch point by U2AF65 and a novel spliceosome-associated 28 kDa polypeptide. RNA. 1995;1:407–417. [PMC free article] [PubMed] [Google Scholar]
- 12.Gozani O, Potashkin J, Reed R. A potential role for U2AF-SAP155 interaction in recruiting U2 snRNP to the branch site. Mol Cell Biol. 1998;18:4752–4760. doi: 10.1128/mcb.18.8.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graveley B. Sorting out the complexity of SR protein functions. RNA. 2000;6:1197–1211. doi: 10.1017/s1355838200000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graveley B, Hertel K, Maniatis T. The role of U2AF35 and U2AF65 in enhancer-dependent splicing. RNA. 2001;7:806–818. doi: 10.1017/s1355838201010317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graveley B, Maniatis T. Arginine/serine-rich domains of SR proteins can function as activators of pre-mRNA splicing. Mol Cell. 1998;1:765–771. doi: 10.1016/s1097-2765(00)80076-3. [DOI] [PubMed] [Google Scholar]
- 16.Guth S, Martinez C, Gaur R, Valcarcel J. Evidence for substrate-specific requirement of the splicing factor U2AF(35) and for its function after polypyrimidine tract recognition by U2AF(65) Mol Cell Biol. 1999;19:8263–8271. doi: 10.1128/mcb.19.12.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guth S, Valcárcel J. Kinetic role for mammalian SF1/mBBP in spliceosome assembly and function after polypyrimidine tract recognition by U2AF65. J Biol Chem. 2000;275:38059–38066. doi: 10.1074/jbc.M001483200. [DOI] [PubMed] [Google Scholar]
- 18.Hastings M, Krainer A. Pre-mRNA splicing in the new millennium. Curr Opin Cell Biol. 2001;13:302–309. doi: 10.1016/s0955-0674(00)00212-x. [DOI] [PubMed] [Google Scholar]
- 19.Hemsley A, Arnheim N, Toney M, Cortopassi G, Galas D. A simple method for site-directed mutagenesis using the polymerase chain reaction. Nucleic Acids Res. 1989;17:6545–6551. doi: 10.1093/nar/17.16.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kan J, Green M. Pre-mRNA splicing of IgM exons M1 and M2 is directed by a juxtaposed splicing enhancer and inhibitor. Genes Dev. 1999;13:462–471. doi: 10.1101/gad.13.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohtz J, Jamison S, Will C, Zuo P, Lührmann R, Garcia-Blanco M, Manley J. Protein-protein interactions and 5′-splice-site recognition in mammalian mRNA precursors. Nature. 1994;368:119–124. doi: 10.1038/368119a0. [DOI] [PubMed] [Google Scholar]
- 22.Krämer A. Presplicing complex formation requires two proteins and U2 snRNP. Genes Dev. 1988;2:1155–1167. doi: 10.1101/gad.2.9.1155. [DOI] [PubMed] [Google Scholar]
- 23.Krämer A. The structure and function of proteins involved in mammalian pre-mRNA splicing. Annu Rev Biochem. 1996;65:367–409. doi: 10.1146/annurev.bi.65.070196.002055. [DOI] [PubMed] [Google Scholar]
- 24.Krämer A, Utans U. Three protein factors (SF1, SF3 and U2AF) function in pre-splicing complex formation in addition to snRNPs. EMBO J. 1991;10:1503–1509. doi: 10.1002/j.1460-2075.1991.tb07670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Labourier E, Bourbon H, Gallouzi I E, Fostier M, Allemand E, Tazi J. Antagonism between RSF1 and SR proteins for both splice-site recognition in vitro and Drosophila development. Genes Dev. 1999;13:740–753. doi: 10.1101/gad.13.6.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Blencowe B. Distinct factor requirements for exonic splicing enhancer function and binding of U2AF to the polypyrimidine tract. J Biol Chem. 1999;274:35074–35079. doi: 10.1074/jbc.274.49.35074. [DOI] [PubMed] [Google Scholar]
- 27.Lin Y, Green M. Mechanism of action of an acidic transcriptional activator in vitro. Cell. 1991;64:971–981. doi: 10.1016/0092-8674(91)90321-o. [DOI] [PubMed] [Google Scholar]
- 28.Lorson C, Androphy E. An exonic enhancer is required for inclusion of an essential exon in the SMA-determining gene SMN. Hum Mol Genet. 2000;9:259–265. doi: 10.1093/hmg/9.2.259. [DOI] [PubMed] [Google Scholar]
- 29.Manley J, Tacke R. SR proteins and splicing control. Genes Dev. 1996;10:1569–1579. doi: 10.1101/gad.10.13.1569. [DOI] [PubMed] [Google Scholar]
- 30.Merendino L, Guth S, Bilbao D, Martinez C, Valcarcel J. Inhibition of ms1–2 splicing by Sex-lethal reveals interaction between U2AF35 and the 3′ splice site AG. Nature. 1999;402:838–841. doi: 10.1038/45602. [DOI] [PubMed] [Google Scholar]
- 31.Nelson K, Green M. Mammalian U2 snRNP has a sequence-specific RNA-binding activity. Genes Dev. 1989;3:1562–1571. doi: 10.1101/gad.3.10.1562. [DOI] [PubMed] [Google Scholar]
- 32.Page-McCaw P, Amonlirdviman K, Sharp P. PUF60: a novel U2AF65-related splicing activity. RNA. 1999;5:1548–1560. doi: 10.1017/s1355838299991938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parker R, Siciliano P, Guthrie C. Recognition of the TACTAAC box during mRNA splicing in yeast involves base pairing to the U2-like snRNA. Cell. 1987;49:229–239. doi: 10.1016/0092-8674(87)90564-2. [DOI] [PubMed] [Google Scholar]
- 34.Rain J, Rafi Z, Rhani Z, Legrain P, Krämer A. Conservation of functional domains involved in RNA binding and protein-protein interactions in human and Saccharomyces cervisiae pre-mRNA splicing factor SF1. RNA. 1998;4:551–565. doi: 10.1017/s1355838298980335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reed R. Mechanisms of fidelity in pre-mRNA splicing. Curr Opin Cell Biol. 2000;12:340–345. doi: 10.1016/s0955-0674(00)00097-1. [DOI] [PubMed] [Google Scholar]
- 36.Reed R. The organization of 3′ splice-site sequences in mammalian introns. Genes Dev. 1989;3:2113–2123. doi: 10.1101/gad.3.12b.2113. [DOI] [PubMed] [Google Scholar]
- 37.Romfo C, Maroney P, Wu S, Nilsen T. 3′ splice site recognition in nematode trans-splicing involves enhancer-dependent recruitment of U2 snRNP. RNA. 2001;7:785–792. doi: 10.1017/s1355838201010263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rudner D, Kanaar R, Breger K, Rio D. Mutations in the small subunit of the Drosophila U2AF splicing factor cause lethality and developmental defects. Proc Natl Acad Sci USA. 1996;93:10333–10337. doi: 10.1073/pnas.93.19.10333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruskin B, Krainer A, Maniatis T, Green M. Excision of an intact intron as a novel lariat structure during pre-mRNA splicing in vitro. Cell. 1984;38:317–331. doi: 10.1016/0092-8674(84)90553-1. [DOI] [PubMed] [Google Scholar]
- 40.Ruskin B, Zamore P, Green M. A factor, U2AF, is required for U2 snRNP binding and splicing complex assembly. Cell. 1988;52:207–219. doi: 10.1016/0092-8674(88)90509-0. [DOI] [PubMed] [Google Scholar]
- 41.Rutz B, Seraphin B. Transient interaction of BBP/ScSF1 and Mud2 with the splicing machinery affects the kinetics of spliceosome assembly. RNA. 1999;5:819–831. doi: 10.1017/s1355838299982286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tacke R, Manley J. Determinants of SR protein specificity. Curr Opin Cell Biol. 1999;11:358–362. doi: 10.1016/S0955-0674(99)80050-7. [DOI] [PubMed] [Google Scholar]
- 43.Tian M, Maniatis T. A splicing enhancer exhibits both constitutive and regulated activities. Genes Dev. 1994;8:1703–1712. doi: 10.1101/gad.8.14.1703. [DOI] [PubMed] [Google Scholar]
- 44.Valcárcel J, Gaur R, Singh R, Green M. Interaction of U2AF65 RS region with pre-mRNA branch point and promotion of base pairing with U2 snRNA. Science. 1996;273:1706–1709. doi: 10.1126/science.273.5282.1706. [DOI] [PubMed] [Google Scholar]
- 45.Valcárcel J, Green M. The SR protein family: pleiotropic effects in pre-mRNA splicing. Trends Biochem Sci. 1996;21:296–301. [PubMed] [Google Scholar]
- 46.Valcárcel J, Martínez C, Green M. Functional analysis of splicing factors and regulators. In: Richter J D, editor. mRNA formation and function. New York, N.Y: Academic Press; 1997. pp. 31–53. [Google Scholar]
- 47.Wang Z, Hoffmann H, Grabowski P. Intrinsic U2AF binding is modulated by exon enhancer signals in parallel with changes in splicing activity. RNA. 1995;1:21–35. [PMC free article] [PubMed] [Google Scholar]
- 48.Watakabe A, Tanaka K, Shimura Y. The role of exon sequences in splice site selection. Genes Dev. 1993;7:407–418. doi: 10.1101/gad.7.3.407. [DOI] [PubMed] [Google Scholar]
- 49.Wu J, Maniatis T. Specific interactions between proteins implicated in splice site selection and regulated alternative splicing. Cell. 1993;75:1061–1070. doi: 10.1016/0092-8674(93)90316-i. [DOI] [PubMed] [Google Scholar]
- 50.Wu J, Manley J. Mammalian pre-mRNA branch site selection by U2 snRNP involves base-pairing. Genes Dev. 1989;3:1553–1561. doi: 10.1101/gad.3.10.1553. [DOI] [PubMed] [Google Scholar]
- 51.Wu S, Romfo C, Nilsen T, Green M. Functional recognition of the 3′ splice site AG by the splicing factor U2AF35. Nature. 1999;402:832–835. doi: 10.1038/45590. [DOI] [PubMed] [Google Scholar]
- 52.Zamore P, Green M. Identification, purification and biochemical characterization of U2 small nuclear ribonucleoprotein auxiliary factor. Proc Natl Acad Sci USA. 1989;86:9243–9247. doi: 10.1073/pnas.86.23.9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zamore P, Patton J, Green M. Cloning and domain structure of the mammalian splicing factor U2AF. Nature. 1992;355:609–614. doi: 10.1038/355609a0. [DOI] [PubMed] [Google Scholar]
- 54.Zhu J, Krainer A. Pre-mRNA splicing in the absence of an SR protein RS domain. Genes Dev. 2000;14:3166–3178. doi: 10.1101/gad.189500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhuang Y, Goldstein A, Weiner A. UACUAAC is the preferred branch site for mammalian pre-mRNA splicing. Proc Natl Acad Sci USA. 1989;86:2752–2756. doi: 10.1073/pnas.86.8.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhuang Y, Weiner A. A compensatory base change in human U2 snRNA can suppress a branch site mutation. Genes Dev. 1989;3:1545–1552. doi: 10.1101/gad.3.10.1545. [DOI] [PubMed] [Google Scholar]
- 57.Zillmann M, Zapp M, Berget S. Gel electrophoretic isolation of splicing complexes containing U1 small nuclear ribonucleoprotein particles. Mol Cell Biol. 1988;8:814–821. doi: 10.1128/mcb.8.2.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zorio D, Blumenthal T. Both subunits of U2AF recognize the 3′ splice site in Caenorhabditis elegans. Nature. 1999;402:835–838. doi: 10.1038/45597. [DOI] [PubMed] [Google Scholar]
- 59.Zorio D, Blumenthal T. U2AF35 is encoded by an essential gene clustered in an operon with RRM/cyclophilin in Caenorhabditis elegans. RNA. 1999;5:487–494. doi: 10.1017/s1355838299982225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zuo P, Maniatis T. The splicing factor U2AF35 mediates critical protein-protein interactions in constitutive and enhancer-dependent splicing. Genes Dev. 1996;10:1356–1368. doi: 10.1101/gad.10.11.1356. [DOI] [PubMed] [Google Scholar]