Abstract
Background:
Attention-deficit/hyperactivity disorder (ADHD) and obesity have been independently associated with deficient cognitive control and heightened preference for immediate reward.
Objectives:
We aimed to identify specific shared and distinct neurobehavioral phenotypes of child obesity and ADHD by simultaneously measuring cognitive control and preference for immediate reward in children with and without ADHD who varied in body weight
Methods:
This case-control study included 323 8–12 year olds (ADHD n=215, typically developing screened for ADHD symptoms [TD] controls n=108) varying in body weight. Children completed a go/no-go task (assessing cognitive control), as well as a classical money delay discounting (DD) task and novel experiential game time DD task (assessing preference for immediate over delayed rewards).
Results:
Game time DD showed a BMIz*ADHD interaction, such that TD children with overweight/obesity showed game time DD levels that were greater than those of TD children without overweight/obesity and similar to those of children with ADHD. Only children with ADHD showed poorer cognitive control compared to TD children, with no effects of body weight.
Conclusions:
Heightened game time DD with delays and rewards experienced in real-time may represent a neurobehavioral phenotype that is shared between ADHD and overweight/obesity in childhood, whereas deficient cognitive control may be specific to children with ADHD.
Keywords: BMI, childhood obesity, cognitive function
Introduction
Obesity and ADHD often co-occur in children and even more so in adults, with two recent meta-analyses revealing increased odds ratios (OR) as high as 1.55 (1,2). ADHD and obesity may combine to worsen outcomes such that their co-occurrence not only presents a dual mental and physical health burden but also complicates intervention, with some findings suggesting that comorbid ADHD negatively impacts the treatment outcome of obesity (3). Empirical evidence guiding treatment of patients with comorbid ADHD and obesity is limited, as is our understanding of the neurobehavioral risk mechanisms for their co-occurrence, which could have important implications for prevention and intervention.
ADHD and obesity have each been associated with the neurobehavioral risk factors of impaired cognitive control and atypical response to reward, as well as anomalous structure and function of related neural circuits (4).The underlying mechanisms of the association between ADHD and obesity remain unclear, but theoretical models suggest a shared neurocognitive phenotype thought to be determined by genetic and epigenetic pathways disrupting the development of self regulation circuits (5). In support of this theory, findings from the separate obesity and ADHD literatures suggest that diminished cognitive control and heightened delay discounting may serve as a shared neurobehavioral phenotype of these disorders (6). However, there is a lack of research examining these features in both ADHD and obesity relative to TD children without obesity. A few studies have examined associations between BMIz and executive dysfunction among children with ADHD (6–10), finding either a positive relationship (7,8) or no relationship (9,10). Notably, few studies have included a non-ADHD control group with mixed findings among those that have, including a positive association between BMI and executive dysfunction in the ADHD group but a negative association in the control group (11) or no association (12). Identification of the shared and distinct phenotypes underlying obesity and ADHD has clinical value, as this could facilitate a precision medicine approach to prevention and treatment of independent or comorbid obesity and ADHD(13). For example, a recent study found that cognitive training improved weight loss at the group level among 8–14 year-olds in a weight loss program (14), although there was individual variability in treatment response. Such treatments may be most effective for children with obesity and ADHD diagnosis/symptoms, who may be more likely to have deficient cognitive control (CC).
Cognitive control deficits are ubiquitous among studies of children with ADHD (15) and thought to be a core feature of the disorder contributing to the defining symptoms of age-inappropriate attention dysregulation, hyperactivity, and impulsivity. Supporting this, findings in ADHD include failures to withhold a prepotent tendency to act or response inhibition (e.g., increased commission errors in a go/no-go [GNG] task) and failures to efficiently direct attention (e.g. increased reaction time [RT] variability on a GNG task, particularly very prolonged RTs reflecting attention lapses) (16). Obesity has also been associated with poorer CC task performance (e.g., poorer response inhibition, higher response variability) in a number of studies(17), although several have suggested that effects that are specific to food-adapted (vs. standard) versions of tasks including the GNG (18) and stop signal (19). Studies investigating whether body weight is related to CC among children with ADHD have produced mixed results (7,10,12). However a single study of GNG performance found that greater standardized body weight (body mass index [BMI]) was associated with more GNG commission errors and greater RT variability in 8–17 year-olds with ADHD, suggesting that ADHD and obesity could have additive effects on CC (11).
Atypical response to reward is also implicated in obesity and ADHD, including a stronger preference for smaller, immediate rewards vs. larger, delayed rewards, assessed with delay of gratification (DoG) (20) and delay discounting (DD) tasks (21). DoG refers to the ability to delay an immediate reward to get a bigger reward in the future, whereas DD refers to the phenomenon that the present value of delayed rewards declines as a function of the delay interval. There is general support for poorer DoG and greater DD in children with ADHD (22) but mounting evidence suggests effects may depend on whether tasks use concrete (vs. abstract) rewards and delivered in real-time (vs. hypothetical) delays (23,24). Studies of obesity have produced more mixed results, with substantial variation in findings by developmental stage, sample characteristics (e.g., ADHD symptoms, pubertal stage, IQ), and task type (e.g., real vs. hypothetical) (17,25). Pubertal status may be a particularly important confounder to consider given the impact of puberty on body weight (26), eating behavior(27), and brain development(28).
As far as we are aware, no published studies have examined whether experiential DD using a salient, concrete non-food reward varies by body weight in either typically developing (TD) children or children with ADHD. In an “experiential task” participants directly experience the delays and rewards associated with their choices in real-time, thereby experiencing the consequence of their choices during the task. In this study, we examined whether the behavioral phenotype captured by a novel experiential “game time” DD task we developed may represent a shared phenotype for childhood obesity and ADHD that could potentially be used to target interventions for obesity, ADHD, and their co-occurrence, and to predict weight and ADHD symptom trajectories and treatment outcomes. We have previously shown this game time DD task to be more sensitive to ADHD symptomatology than classic monetary delay discounting tasks (23), supporting the validity of this task for assessing DD in this age range.
Our goal was to identify shared and independent neurobehavioral phenotypes associated with ADHD and excess weight in childhood. We therefore administered a GNG task to assess CC, and a traditional money DD task and a novel experiential game time DD task to assess preference for immediate over delayed rewards, in 323 8–12 year-old children varying by ADHD diagnosis (ADHD vs. TD), and body weight. Our rationale for using the game time DD task was that a concrete, experiential task (thereby limiting demands on abstract reasoning abilities) with a preferable activity (e.g., video games) as an immediate reward may be maximally sensitive to neurobehavioral differences in both ADHD and obesity in children as it better approximates “real-world” ability to resist temptation than the money DD task. Critically, TD children had ADHD symptom scores that were substantially below clinical cutoffs on all parent/teacher rating scales, allowing us to identify dimensions of CC and DD specific to obesity in the absence of other co-morbidities, and supplementary analyses controlled for stimulant use, which could obscure relationships with weight (29) due to appetite suppression and potentially via improved self-control. The sample was predominantly pre-pubertal, important both to avoid confounding from hormonal effects on body weight, eating behavior and brain development (26–28), and because characterizing prepubertal neurobehavioral risk factors for later obesity or ADHD (30–32) could inform early life prevention and intervention approaches.
We hypothesized that ADHD and higher BMI-z scores (BMIz) would both be associated with greater DD (reflecting a shared phenotype), with greatest DD in children with both higher BMIz and ADHD, and greater effects for the game time DD than the money DD task. We further hypothesized that ADHD would be more strongly associated with CC (response inhibition and variability) than would BMIz (supporting partially distinct phenotypes).
Methods
Participants
In this case-control study, data from 323 8–12 year-old children were examined, including 215 children with ADHD (55 girls) and 108 TD children (41 girls). Participants were recruited through local schools, community-wide advertisement, volunteer organizations, medical institutions, pediatrician’s offices, and word of mouth. The analysis sample is a subset of a larger sample of 901 participants enrolled in various studies of children with ADHD (n=440) and TD controls (n=470) at our research center. In the past several years, these studies have extended beyond the 8–12 year-old age range restricted to prepubertal children (i.e., Tanners Stage 1–2) to include adolescents between the ages of 12–17 with no restrictions on pubertal status. After providing a complete study description to the participants, oral informed consent was obtained from a parent/guardian prior to the initial phone screening conducted with the parent/guardian. Children not meeting criteria for either the TD or ADHD group (as defined below) or with a history of intellectual disability, learning disability, seizures, traumatic brain injury, incidental finding on a brain MRI, or other neurological illnesses or neurodevelopmental disorders (including Autism Spectrum Disorder and tic disorders) were excluded (n=55), with n=855 participants remaining (TD n=446, ADHD n=227). Next, analyses were restricted to those participants that were between the ages of 8–12 for whom we obtained measures of height and weight and behavioral task data for the GNG and DD tasks (n=341; ADHD n=227; TD n=114). Of these participants, 18 sibling pairs were enrolled across the various studies. The sibling with the most complete data for our analyses were included in the final analysis sample of n=323 with data collected between December of 2013 and August of 2021. Given that there were missing data across the GNG and DD tasks due to tasks being introduced at different times across these studies (Money DD n=309, Game DD n=245, GNG n=317), we also conducted secondary analyses including only participants that performed all 4 tasks (n=237). This study was approved by the Johns Hopkins University School of Medicine Institutional Review Board.
Eligible participants and their parents attended two laboratory sessions. At the initial laboratory visit, written informed consent and assent were obtained from the parent/guardian and the child and intellectual ability was assessed using the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV, n=104) or Fifth Edition (WISC-V, n=211), or the Wechsler Abbreviated Scale for Intelligence, Second Edition (WASI-II, n=3). Participants with General Ability Index (GAI) score derived from WISC-IV or -V (a composite score based on 3 Verbal Comprehension and 3 Perceptual Reasoning subsets that does not factor in working memory and processing speed scores), where children with scores below 80 were excluded. To screen for reading disorders, children were administered the Word Reading subtest from the Wechsler Individual Achievement Test, Second Edition (WIAT-II, n=111) or Third Edition (WIAT-III, n=205) and were excluded for standard scores below 85. Diagnostic status was established through administration of either the Diagnostic Interview for Children and Adolescents, Fourth Edition (DICA-IV; n=94) or the Kiddie Schedule for Affective Disorders and Schizophrenia for School Aged Children Present Lifetime version (KSADS-PL; n=229)(33). Parents and teachers (when available) also completed the Conners Parent and Teacher Rating Scales-Revised Long Version or the Conners-3 (CPRS and CTRS)(34–36) and the ADHD Rating Scale-IV (ADHD-RS), home and school versions(37). A diagnosis of ADHD was confirmed by a child neurologist and/or psychologist based on the diagnostic interview, which considered information provided by the parent about functioning at school, in addition to onset, course, duration, and frequency of symptoms, and parent/teacher rating scales (i.e., T-scores ≥ 65 or ≥ 6 symptoms in either symptom domain endorsed on at least one rating scale). Inclusion in the TD group required scores below clinical cutoffs (i.e., T-scores ≤ 60 and ≤ 4 symptoms endorsed) on all parent/teacher rating scales.
BMI z-scores based on Center for Disease Control (CDC) reference data from 2000 were calculated based on height and weight assessed by study personnel. Relevant demographic information is provided in Table 1.
Table 1.
Demographic and clinical characteristics for children with ADHD and typically developing (TD) control groups.
| ADHD (n=215) | TD (n=108) | Group comparison p-value | |
|---|---|---|---|
|
| |||
| BMIz | 0.29 (1.05) | 0.43 (1.11) | .260 |
| Weight Group (n, % OV/OB, ≥85th percentile) | 51 (24%) | 35 (32%) | .110 |
| Sex (% male) | 160 (74%) | 67 (62%) | .022 |
| Age in years | 10.2 (1.4) | 10.2 (1.2) | .971 |
| Pubertal status (% pre-pubertal) | 200 (92%) | 97 (90%) | .422 |
| Race (% Caucasian) | 138 (64%) | 65 (60%) | .483 |
| SES | 52.6 (9.9) | 55.9 (9.08) | .005 |
| WISC GAI | 107.8 (12.4) | 115.4 (12.5) | <.001 |
| DuPaul IA Raw score | 19.0 (4.7) | 3.3 (2.7) | <.001 |
| Conners IA T score | 76.4 (10.3) | 46.2 (7.0) | <.001 |
| DuPaul HI Raw score | 15.0 (6.3) | 2.3 (2.5) | <.001 |
| Conners HI T score | 76.0 (13.3) | 46.2 (6.7) | <.001 |
| ODD (n, %) | 62 (29%) | 0 | n/a |
| Anxiety (n,%) | 49 (23%) | 0 | n/a |
| Depression (n, %) | 10 (5%) | 0 | n/a |
| Stimulant Medication | 115 (54%) | 0 | n/a |
| Non-Stimulant Medication* | 21 (10%) | 0 | n/a |
Mean (SD) reported unless otherwise noted.
The vast majority of participants were prepubertal (92% with Tanners Stage 1 or 2) based on initial requirements for study inclusion that were later expanded, with no significant differences in the percentage of prepubertal participants across diagnostic groups. Analyses were also conducted excluding participants beyond Tanners Stage 2 (n=25) and results did not change. Therefore, we chose to include all available data to preserve statistical power. Intellectual reasoning ability as measured by the GAI (38,39), was at least 80 for inclusion in both groups. Children meeting criteria for a current diagnosis of oppositional defiant disorder (ODD, n=62), major depressive disorder (n=3), dysthymia (n=1), depression NOS (n=6), adjustment disorder (n=1), panic disorder (n=1), separation anxiety (n=1), social anxiety (n=6), specific phobia (n=27), generalized anxiety disorder (n=38), anxiety NOS (n=8), or chronic tic disorder (n=1) were included in the study. Children taking psychotropic medications other than stimulants (n=4 taking a non-stimulant medication for ADHD and n=17 taking an SSRI or other psychotropic medication for anxiety or depression) were included and did not discontinue their medication to participate in the study whereas children taking stimulant medication were asked to withhold medication the day prior to and day of testing.
Behavioral Tasks
Game Time Delay Discounting.
As previously described (23), participants make nine choices between getting to play a preferred game for a shorter amount of time (either 15, 30, or 45 s) immediately or for a fixed longer amount of time (60 s) after waiting (either 25, 50, or 100 s). Participants could bring their own game and were offered several game options (handheld video game, coloring, Lego, etc.) to maximize the rewarding value for each individual. Their preferred game was placed in a clear box in front of them when they made their choices and while waiting to play. The majority of participants selected to play a video game (82%) with the remaining participants choosing either to draw/color or play with magnetic tiles or Lego. Once the participant makes a choice, they experience the delays and rewards associated with that choice in real-time before making their next choice. Area over the curve (AOC) was examined as in prior studies such that higher values indicate greater DD (23,40,41).
Money Delay Discounting.
Participants completed a 10–15 min computer-based DD task involving 91 choices between a varying amount of money now ($0–$10.50 in $0.50 increments) or $10.00 after a varying delay (1, 7, 30, or 90 days) (41). Participants were told that some of the choices were real and they would actually receive the amount of money at the specified delay that they chose for some of the items in the form of gift cards or prizes (two choices semi-randomly selected). AOC was examined as described above. This task has been validated for use among children as young as 7 years old (41).
Go/No-Go Task.
Participants completed a Go/No-Go (GNG) task described previously(15). Participants were seated in front of a computer monitor with a keyboard. The task stimuli consisted of green spaceships for “Go” trials (80%) and red spaceships for “No-Go” trials (20%), presented one at a time. Participants were instructed to push the spacebar as quickly as possible in response to green spaceships and to withhold their response for red spaceships. There were 240 experimental trials with go and no-go trials appearing in pseudorandom order. We examined commission error rate ComRate (response inhibition errors) and tau (ex-Gaussian indicator of response variability thought to reflect attention dysregulation) (42). We also examined ex-Gaussian parameters mu and sigma (measure of response speed and variability, respectively, in the Gaussian portion of the reaction time distribution), but there were no associations with ADHD or BMI/weight group. Those results are not reported here to maintain the focus on cognitive deficits hypothesized to be most relevant for ADHD and obesity.
Statistical Analyses
Analyses were performed using SPSS statistics. Variables were tested for normality using a Shapiro-Wilk test. Correlations between variables were evaluated using Spearman’s rho for non-parametric data and Pearson’s correlation for parametric data. Bivariate correlations, ANOVA, and chi-square tests were examined to identify demographic (age, sex, GAI, socioeconomic status (SES), race), diagnostic (comorbid anxiety/depression), and medication variables (current stimulant and non-stimulant psychotropic medications) that varied with BMIz-scores and diagnostic group (ADHD vs. TD) or dimensional ADHD symptoms (DuPaul Rating Scale Total Raw Score). BMIz and/or diagnostic group were associated with (GAI), SES, race (white vs. non-white), stimulant and non-stimulant medication, and comorbid anxiety/depression (see Supplementary Table S1). Analyses reported below were therefore conducted with and without inclusion of these covariates, with the exception of SES as this was missing for 10 participants, although results did not change with SES as a covariate. Since findings were similar in each set of analyses, we present unadjusted analyses as primary results, and sensitivity analyses with relevant covariates in Supplementary Materials.
As a first step, correlation analyses were performed using Spearman’s rho (rs) to test for intercorrelations between the DD and GNG task measures, and to investigate unadjusted associations between task performance, BMIz, and ADHD diagnosis/symptoms across the whole sample. Regressions were conducted using the Hayes PROCESS macro (v3.5) in SPSS (v27) to test whether the relationship between BMIz and behavioral task performance was moderated by ADHD diagnosis, controlling for age, sex, race, GAI, stim med, non-stim med, anxiety and depression. All variables were standardized to avoid multi-collinearity, moderation analysis was performed using bootstrapping confidence intervals and a heteroscedasticity consistent standard error, which does not assume normality and homoscedasticity. Further, in order to 1) test whether children with both ADHD and overweight/obesity differed from children with either ADHD or overweight/obesity, and 2) test whether the effect of overweight/obesity among TD children was comparable to the effect of ADHD diagnosis vs. TD, we categorized participants into weight groups (OV/OB: ≥85th percentile n=86 (n=37 overweight i.e. ≥85th<95th, n=49 obesity i.e. ≥95th), vs. Non-OV/OB: <85th percentile, n=237) and compared performance across diagnostic (ADHD vs. TD) and weight groups using ANOVA.
Finally, to explore potential stimulant effects we compared associations among BMIz and DD and GNG task performance among children with ADHD currently prescribed a stimulant medication (n=115) and those that were not (n=100). Since there was no evidence that stimulant medication effects moderated relationships between BMIz and DD/GNG task performance, these results are presented in the Supplementary Materials (Table S3, Figure S1a–b).
Results
Correlation and regression analyses
Correlation analyses conducted between the GNG and DD tasks across the whole sample revealed that money and game DD were correlated (rs =.194, p=.002) as were GNG ComRate and tau (rs =.353, p<.001), suggesting that these tasks are assessing partly different aspects of DD and CC among children in this age range. DD and GNG Comrate were not correlated (ps>.40) although tau was significantly correlated with money DD (rs =.146, p=.011) with a similar trend for game DD (rs =.112, p=.084). BMIz was uncorrelated with ADHD diagnosis and symptoms and performance on DD and GNG tasks (see Supplementary Table S2).
Regression results are presented in Table 2 and Figure 1. For game DD, higher BMIz was associated with greater DD (p=.014), with a strong trend for greater DD in children with ADHD vs. TD children (p=.054). There was also a BMIz*ADHD interaction (p=.017), such that there was a positive relationship between BMIz and DD within the TD group (rs =.26, p=.015) but not within the ADHD group (rs =−.045, p=.574). For money DD, there was no relationship between DD and BMIz (p=.377), ADHD Diagnosis (p=.354) or BMIz*ADHD (p=.933). Analysis of GNG performance revealed effects of ADHD Diagnosis only, with higher ComRate (p=.003) and tau (p<.001) in the ADHD vs. TD group, but no associations with BMIz. These relationships were similar with all covariates included.
Table 2.
Regression analyses testing moderation effects of ADHD diagnosis, BMIz, and their interaction (BMIz*ADHD) with performance on the delay discounting and go/no-go tasks.
| DV | n | IV | β | SE | p | p covariates |
|---|---|---|---|---|---|---|
|
| ||||||
| Game DD | 245 | BMIz | .036 | .015 | .014 | .026 |
| ADHD | .043 | .022 | .054 | .043 | ||
| BMIz*ADHD | −.046 | .019 | .017 | .022 | ||
| Money DD | 309 | BMIz | .023 | .026 | .377 | .554 |
| ADHD | .035 | .038 | .354 | .484 | ||
| BMIz*ADHD | −.003 | .032 | .933 | .810 | ||
| GNG Com | 315 | BMIz | −.014 | .016 | .403 | .654 |
| ADHD | .071 | .023 | .003 | .083 | ||
| BMIz*ADHD | .028 | .020 | .169 | .259 | ||
| GNG Tau | 315 | BMIz | −1.082 | 5.39 | .840 | .999 |
| ADHD | 36.82 | 7.81 | <.001 | .015 | ||
| BMIz*ADHD | 5.35 | 6.76 | .430 | .660 | ||
Statistically significant predictors appear in bold. DD=delay discounting, Com=commission error rate
Figure 1:
Moderation effects of diagnostic groups on the relationship between BMIz scores and delay discounting and go/no-go task performance in ADHD and TD groups. Footnote: restricting analyses to subjects that performed all 4 tasks i.e. (n=227) did not change our findings.
Group Comparisons
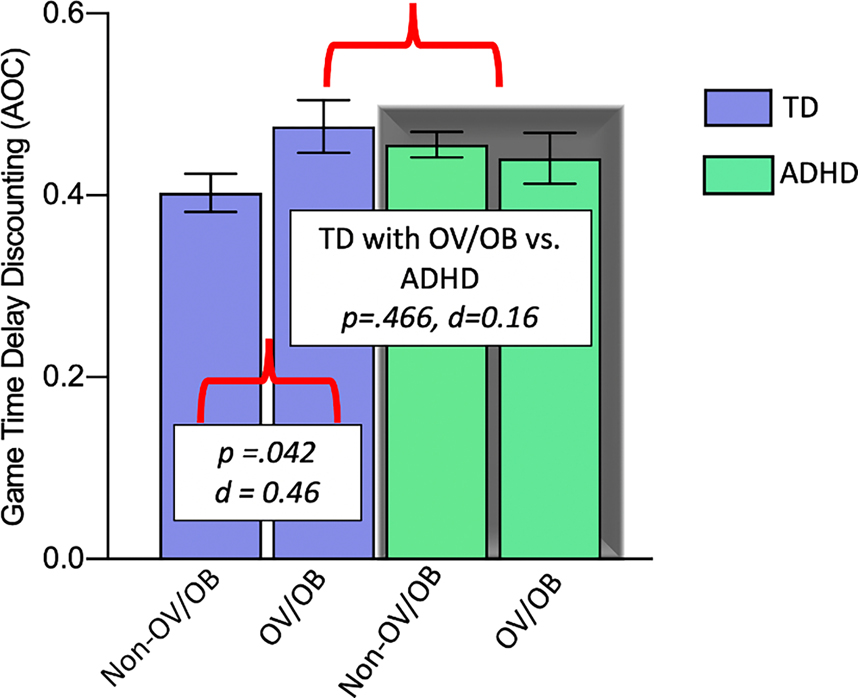
Consistent with regression results revealing a BMIz*ADHD interaction for game DD, clinical group comparisons of children with OV/OB (vs. children without OV/OB; non-OV/OB) and ADHD (vs. TD) revealed that TD children with OV/OB showed similar game DD as children with ADHD (regardless of weight group) (p=.466, d=0.16), and greater game DD compared to TD children without OV/OB (non-OV/OB) (p=.042, d=0.46), suggesting that TD children with excess weight demonstrate a profile of raised DD that is similar to that of children with ADHD. Children with both OV/OB and ADHD did not differ from children with ADHD without OV/OB (non-OV/OB) (p=.625, d=0.10) or from TD children with OV/OB (p=.34, d=.25), supporting an absence of an effect of excess weight within the ADHD group (Figure 2).
Figure 2:
Group comparisons of delay discounting among diagnostic (ADHD vs. TD) and weight groups (children with OV/OB vs. children without OV/OB; non-OV/OB). Error bars represent standard error of mean (SEM) for results without any covariates.
Discussion
To identify shared and distinct neurobehavioral phenotypes underlying ADHD and obesity in childhood, we examined performance on task-based measures of cognitive control and delay discounting among 8 to 12 year-olds with ADHD relative to TD children screened for ADHD symptoms. The relationship between BMIz and DD was moderated by diagnosis, such that TD children with OV/OB showed discounting levels that were greater than those of TD children without OV/OB and similar to those of children with ADHD, regardless of body weight. Interestingly, this was specific to an experiential DD task for a preferred sedentary activity (game time). This finding suggests that heightened game DD in TD children with OV/OB resembles the response style commonly seen in children with ADHD, consistent with DD being a shared phenotype of overweight and ADHD. In contrast, analyses of GNG task performance revealed only a diagnosis effect (with no effect of body weight) such that children with ADHD showed poorer response inhibition (ComRate) and greater response variability (tau) compared to TD children, suggestive of a phenotype unique to ADHD. This pattern of results was unchanged when controlling for stimulant use. Hence, game DD may represent a phenotype associated with obesity in some children that do not display impairments on cognitive control (e.g., poor response inhibition and increased response variability).
The finding of heightened DD in relation to greater BMIz and to ADHD was specific to the game DD task. In addition, we did not observe any association between money DD and BMI-z score in TD or ADHD children, which is in line with our previous work (23), but is in contrast with a previous meta-analysis (25) that showed a positive association between money DD and obesity. We believe the discrepancy in findings may be attributed to methodological and sample heterogeneity as well as varying screening criteria across obesity studies, with only a couple of these studies excluding children with ADHD symptoms. The experiential nature of the game DD task includes several features that make the task developmentally appropriate for this age range while also enhancing the salience of the reward and potential for negative affect associated with waiting, as well as reducing demand for abstract conceptual reasoning. In this task, choices involve short delays for primary (rather than secondary) rewards, the chosen reward (i.e., their preferred game) is displayed while making choices, children experience waiting their chosen amount of time before receiving the reward, and they actually receive/consume the reward before making their next choice. In contrast, the money DD task involves choices at much longer delays (up to 90 days) for secondary rewards (money, that they rely on an adult to spend), that are not experienced as the choices are being made. Thus, the money DD task may rely more on attention, EF, and intellectual reasoning ability than the game DD, consistent with prior work showing the heightened money DD in ADHD on a nearly identical task is not independent of IQ (41). Future research should include more comprehensive measures of EF and DD to elucidate their relationship.
Cognitive control assessed with the GNG task was highly sensitive to ADHD, as evidenced by significantly greater commission rate errors and RT variability among children with ADHD relative to TD children regardless of body weight. Greater commission error rate and RT variability in children with ADHD have been extensively described (15,43). Although there is heterogeneity within the ADHD group (44,45), our findings suggest that this is not related to weight status. In addition, the absence of BMIz associations and weight group differences in the TD group for GNG task performance is inconsistent with some previous research (17) although other studies have similarly shown that individuals with obesity perform as well as controls on EF measures assessing inhibitory control and working memory (46). This may be related to our exclusion of children with elevated symptoms of ADHD from the TD group and below average IQ across both groups, which is often not applied in obesity studies. The presence of ADHD symptoms or low IQ may therefore have contributed to prior findings of deficient cognitive control in relation to overweight/obesity. Although our overweight/obesity sample may not be representative of the broader population due to these exclusions, an important implication of our results is that experiential DD may contribute to obesity in the absence of deficient cognitive control. Future studies examining response inhibition, or other cognitive functions, in overweight/obesity may benefit from screening for, or at least accounting for symptoms of ADHD, in order to better understand shared and distinct underlying neurobehavioral phenotypes.
Moreover, cognitive control (e.g., commission errors) and DD were uncorrelated, suggesting that these tasks may assess distinct aspects of EF that are differentially impacted in children with ADHD and overweight children and may have a separable biological basis (47). This is consistent with the classification of EFs as “cool” versus “hot” describing the extent to which they occur in an emotional or motivational context and the underlying neural circuitry(48). Cool EFs, broadly involving cognitive control and related dorso- and ventrolateral prefrontal cortex, are thought to be captured by clinic- or laboratory-based neuropsychological tasks assessing inhibition, attentional control, working memory, and cognitive flexibility. Hot EFs refer to self-control in the context of emotionally arousing or rewarding stimuli often encountered in the “real-world”, including resisting temptation, with greater engagement of the ventral medial prefrontal cortex in concert with subcortical regions governing response to reward (49,50). Within the ADHD literature, a dual pathway model has been proposed postulating multiple neuropsychological pathways to ADHD involving separate, but related, cognitive (“cool” EF) and motivational (“hot” EF) deficits (51). This model has also been applied to examine the underlying mechanisms of the association between ADHD and obesity in adults (46), but further research is needed to understand the role of “cool” vs. “hot” EFs in relation to obesity and ADHD.
Our results suggest game time DD (involving “hot” EF) may be a sensitive index of difficulty resisting temptation or devaluing delayed rewards, among school-age children and perhaps a shared neurobehavioral marker of ADHD and obesity. Further research is needed to understand the mechanistic pathway from heightened delay discounting to obesity. There is some evidence that obesity with binge-eating rather than obesity alone was associated with greater delay discounting, driven by inattention (46), although it has also been suggested that EF deficits may increase the risk of obesity via disordered eating patterns (such as binge eating) that are aggravated by disrupted sleep patterns, resulting in increased food intake (5). Thus, it may be that stronger preference for immediate reward leads to binge-eating behavior or eating more than is intended without consideration of the potential negative consequences due to greater valuation of immediate over delayed rewards. In the present study, we did not assess disordered eating behavior or delay discounting for food rewards, but this is warranted for future studies aiming to understand these relationships and their contribution to obesity. It is also notable that increased computer game use is somewhat associated with both obesity (52) and ADHD symptomatology (53). The reward used in the game DD task, which was most frequently selected to be a video game, may therefore be of particular relevance to both conditions, with game time DD being a uniquely promising intervention target.
Given that stimulants such as methylphenidate and amphetamine are often prescribed to children with ADHD and obesity to reduce ADHD symptoms, and some (54,55) but not all (56) studies have supported stimulant-associated reductions in appetite and weight, supplementary preliminary analyses explored the impact of stimulant medication on our results. Our main findings did not change as a function of current stimulant medication use. However, the number of participants with ADHD taking stimulant medication in the overweight group was small (n=22) and we did not collect information about duration of stimulant use or associated weight loss. Another potential limitation is that the vast majority of our sample was prepubertal (92% below Tanners Stage 3) which may have led to decreased representation of early-maturing children including heavier children who reach puberty earlier (57). However this feature also strengthens our analyses by removing the confounding effects of puberty on neurobehavioral outcomes(58).
Finally, as with all studies of children with ADHD in which comorbid psychiatric and learning disorders are common(59), the balance of generalizability with sample homogeneity must be considered. We excluded children with a history of reading disorders and/or below average performance on a measure of single word reading and only allowed certain psychiatric comorbidities (e.g., ODD, anxiety, and depressive disorders). This was necessary as we also obtained neuroimaging data for these participants and altered brain structure and function is associated with these conditions. Our chosen exclusion criteria improve our ability to draw conclusions about effects that may be specific to ADHD. However they may also limit the generalizability of our findings, supporting future research in larger samples to investigate the impact of these comorbidities.
To conclude, our results suggest that experiential game time DD may represent a shared neurobehavioral phenotype of heightened preference for immediate reward common to both ADHD and obesity in childhood. In light of new research showing shared genetic risk between ADHD and BMI and shared neural correlates of impairments in inhibitory control (60), ability to tolerate delay for a salient preferred game reward with real-time delays may be a promising neurobehavioral intervention target for children with ADHD or obesity alone, and a particularly parsimonious target for children with comorbid ADHD and obesity. Furthermore, reward processing and cognitive control may be somewhat independent contributors to population variation in body weight, with a separable neural basis, and ensuing implications for individualized pharmacological and behavioral treatment approaches. Our results also suggest that ADHD symptoms among typically developing samples, and stimulant use among ADHD samples, should be evaluated, reported, and accounted for in future studies of neurobehavioral correlates of ADHD and overweight/obesity.
Supplementary Material
ACKNOWLEGDEMENTS :
Drs Rosch, Carnell and Mostofsky conceptualized and designed the study. Drs Rosch and Mostofsky coordinated and supervised data collection. Drs Thapaliya and Rosch carried out the analyses, drafted the initial manuscript and revised the manuscript. Dr Carnell reviewed and revised the manuscript, and Dr Mostofsky critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work. All phases of this study were supported by: NIH grant: R01DK113286 [SC, GT], NIH grant: K23MH101322 [KSR], NIH grant: R01MH078160 [SHM, KSR], NIH grant: R01MH085328 [SHM, KSR], the Intellectual and Developmental Disabilities Research Center at Kennedy Krieger Institute and The Johns Hopkins University NIH grant: P50HD103538 [to SHM], and Kirby Center for Functional Brain Imaging: P41 EB015909–19.
Footnotes
DISCLOSURE: The author(s) have no potential conflicts of interest to disclose
References
- 1.Nigg JT, Johnstone JM, Musser ED, Long HG, Willoughby M, Shannon J. Attention-deficit/hyperactivity disorder (ADHD) and being overweight/obesity: New data and meta-analysis. Clin Psychol Rev [Internet]. 2016;43:67–79. Available from: 10.1016/j.cpr.2015.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cortese S, Moreira-Maia CR, St Fleur D, Morcillo-Peñalver C, Rohde LA, Faraone SV. Association between ADHD and obesity: A systematic review and meta-analysis. Am J Psychiatry. 2016;173(1):34–43. [DOI] [PubMed] [Google Scholar]
- 3.Cortese S, Castellanos FX. The relationship between ADHD and obesity: implications for therapy. Expert Rev Neurother. 2014. May;14(5):473–9. [DOI] [PubMed] [Google Scholar]
- 4.Rubia K Cognitive Neuroscience of Attention Deficit Hyperactivity Disorder (ADHD) and Its Clinical Translation. Front Hum Neurosci [Internet]. 2018;12:100. Available from: https://www.frontiersin.org/article/10.3389/fnhum.2018.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanć T, Cortese S. Attention deficit/hyperactivity-disorder and obesity: A review and model of current hypotheses explaining their comorbidity. Neurosci Biobehav Rev. 2018. Sep;92:16–28. [DOI] [PubMed] [Google Scholar]
- 6.Seymour KE, Reinblatt SP, Benson L, Carnell S. Overlapping neurobehavioral circuits in ADHD, obesity, and binge eating: evidence from neuroimaging research. CNS Spectr. 2015;20(04):401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graziano PA, Bagner DM, Waxmonsky JG, Reid A, McNamara JP, Geffken GR. Co-occurring weight problems among children with attention deficit/hyperactivity disorder: The role of executive functioning. Int J Obes [Internet]. 2012;36(4):567–72. Available from: 10.1038/ijo.2011.245 [DOI] [PubMed] [Google Scholar]
- 8.Reinblatt SP, Mahone EM, Tanofsky-Kraff M, Lee-Winn AE, Yenokyan G, Leoutsakos JMS, et al. Pediatric loss of control eating syndrome: Association with attention-deficit/hyperactivity disorder and impulsivity. Int J Eat Disord. 2015;48(6):580–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choudhry Z, Sengupta SM, Grizenko N, Harvey WJ, Fortier M-È, Schmitz N, et al. Body weight and ADHD: examining the role of self-regulation. PLoS One. 2013;8(1):e55351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanć T, Dmitrzak-Węglarz M, Borkowska A, Wolańczyk T, Pytlińska N, Rybakowski F, et al. Overweight in Boys With ADHD Is Related to Candidate Genes and Not to Deficits in Cognitive Functions. J Atten Disord. 2018;22(12):1158–72. [DOI] [PubMed] [Google Scholar]
- 11.Racicka-Pawlukiewicz E, Kuć K, Bielecki M, Hanć T, Cybulska-Klosowicz A, Bryńska A. The association between executive functions and body weight/BMI in children and adolescents with ADHD. Brain Sci. 2021;11(2):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fliers EA, Buitelaar JK, Maras A, Bul K, Höhle E, Faraone SV., et al. ADHD is a risk factor for overweight and obesity in children. J Dev Behav Pediatr. 2013;34(8):566–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satyal MK, Basso JC, Tegge AN, Metpally AR, Bickel WK. A novel model of obesity prediction: Neurobehaviors as targets for treatment. Behav Neurosci. 2021. Jun;135(3):426–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verbeken S, Braet C, Goossens L, van der Oord S. Executive function training with game elements for obese children: a novel treatment to enhance self-regulatory abilities for weight-control. Behav Res Ther. 2013. Jun;51(6):290–9. [DOI] [PubMed] [Google Scholar]
- 15.Seymour KE, Mostofsky SH, Rosch KS. Cognitive Load Differentially Impacts Response Control in Girls and Boys with ADHD. J Abnorm Child Psychol [Internet]. 2016. Jan;44(1):141–54. Available from: https://pubmed.ncbi.nlm.nih.gov/25624066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol. 2013. Sep;108:44–79. [DOI] [PubMed] [Google Scholar]
- 17.Pearce AL, Leonhardt CA, Vaidya CJ. Executive and Reward-Related Function in Pediatric Obesity: A Meta-Analysis. Child Obes. 2018;14(5):265–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.da Costa KG, Price M, Bortolotti H, de Medeiros Rêgo ML, Cabral DAR, Langer RD, et al. Fat mass predicts food-specific inhibitory control in children. Physiol Behav. 2019;204(August 2018):155–61. [DOI] [PubMed] [Google Scholar]
- 19.Nederkoorn C, Coelho JS, Guerrieri R, Houben K, Jansen A. Specificity of the failure to inhibit responses in overweight children. Appetite. 2012. Oct;59(2):409–13. [DOI] [PubMed] [Google Scholar]
- 20.Johnson WG, Parry W, Drabman RS. The performance of obese and normal size children on a delay of gratification task. Addict Behav [Internet]. 1978;3(3):205–8. Available from: https://www.sciencedirect.com/science/article/pii/0306460378900205 [DOI] [PubMed] [Google Scholar]
- 21.Myerson J, Green L. Discounting of delayed rewards: Models of individual choice. J Exp Anal Behav. 1995. Nov;64(3):263–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patros CHG, Alderson RM, Kasper LJ, Tarle SJ, Lea SE, Hudec KL. Choice-impulsivity in children and adolescents with attention-deficit/hyperactivity disorder (ADHD): A meta-analytic review. Clin Psychol Rev. 2016. Feb;43:162–74. [DOI] [PubMed] [Google Scholar]
- 23.Rosch KS, Mostofsky SH. Increased Delay Discounting on a Novel Real-Time Task among Girls, but not Boys, with ADHD. J Int Neuropsychol Soc [Internet]. 2015/11/09. 2016. Jan;22(1):12–23. Available from: https://pubmed.ncbi.nlm.nih.gov/26549118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marx I, Hacker T, Yu X, Cortese S, Sonuga-Barke E. ADHD and the Choice of Small Immediate Over Larger Delayed Rewards: A Comparative Meta-Analysis of Performance on Simple Choice-Delay and Temporal Discounting Paradigms. J Atten Disord. 2021;25(2):171–87. [DOI] [PubMed] [Google Scholar]
- 25.Tang J, Chrzanowski-Smith OJ, Hutchinson G, Kee F, Hunter RF. Relationship between monetary delay discounting and obesity: a systematic review and meta-regression. Int J Obes [Internet]. 2019;43(6):1135–46. Available from: 10.1038/s41366-018-0265-0 [DOI] [PubMed] [Google Scholar]
- 26.Zhou X, Hu Y, Yang Z, Gong Z, Zhang S, Liu X, et al. Overweight/Obesity in Childhood and the Risk of Early Puberty: A Systematic Review and Meta-Analysis. Vol. 10, Frontiers in pediatrics. Switzerland; 2022. p. 795596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mikhail ME, Anaya C, Culbert KM, Sisk CL, Johnson A, Klump KL. Gonadal Hormone Influences on Sex Differences in Binge Eating Across Development. Curr Psychiatry Rep. 2021. Oct;23(11):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goddings A-L, Beltz A, Peper JS, Crone EA, Braams BR. Understanding the Role of Puberty in Structural and Functional Development of the Adolescent Brain. J Res Adolesc Off J Soc Res Adolesc. 2019. Mar;29(1):32–53. [DOI] [PubMed] [Google Scholar]
- 29.Cortese S, Tessari L. Attention-Deficit/Hyperactivity Disorder (ADHD) and Obesity: Update 2016. Curr Psychiatry Rep. 2017;19(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leventakou V, Herle M, Kampouri M, Margetaki K, Vafeiadi M, Kogevinas M, et al. The longitudinal association of eating behaviour and ADHD symptoms in school age children: a follow-up study in the RHEA cohort. Eur Child Adolesc Psychiatry. 2022. Mar;31(3):511–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bowling AB, Tiemeier HW, Jaddoe VW V, Barker ED, Jansen PW. ADHD symptoms and body composition changes in childhood: a longitudinal study evaluating directionality of associations. Pediatr Obes. 2018. Sep;13(9):567–75. [DOI] [PubMed] [Google Scholar]
- 32.Sonneville KR, Calzo JP, Horton NJ, Field AE, Crosby RD, Solmi F, et al. Childhood hyperactivity/inattention and eating disturbances predict binge eating in adolescence. Psychol Med. 2015;45(12):2511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997. Jul;36(7):980–8. [DOI] [PubMed] [Google Scholar]
- 34.Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998. Aug;26(4):257–68. [DOI] [PubMed] [Google Scholar]
- 35.Conners CK, Sitarenios G, Parker JD, Epstein JN. Revision and restandardization of the Conners Teacher Rating Scale (CTRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998. Aug;26(4):279–91. [DOI] [PubMed] [Google Scholar]
- 36.Conners CK, Pitkanen J, Rzepa SR. Conners 3rd Edition (Conners 3; Conners 2008). In: Kreutzer JS, DeLuca J, Caplan B, editors. Encyclopedia of Clinical Neuropsychology [Internet]. New York, NY: Springer New York; 2011. p. 675–8. Available from: 10.1007/978-0-387-79948-3_1534 [DOI] [Google Scholar]
- 37.DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale—IV: Checklists, norms, and clinical interpretation. ADHD Rating Scale—IV: Checklists, norms, and clinical interpretation. New York, NY, US: Guilford Press; 1998. viii, 79–viii, 79. [Google Scholar]
- 38.Wechsler D L Wechsler Intelligence Scale for Children, Fourth Edition. In: Goldstein S, Naglieri JA, editors. The Psychological Corporation [Internet]. Boston, MA: Springer US; 2003. Available from: 10.1007/978-0-387-79061-9_3066 [DOI] [Google Scholar]
- 39.Weschler D L Wechsler Intelligence Scale for Children, Fifth Edition (WISC-V). In: Goldstein, Naglieri, editors. Boston, MA: Springer US; 2014. Available from: 10.1007/978-0-387-79061-9_3066 [DOI] [Google Scholar]
- 40.Patros CHG L Sweeney K, Mahone EM, Mostofsky SH, Rosch KS. Greater delay discounting among girls, but not boys, with ADHD correlates with cognitive control. Child Neuropsychol a J Norm Abnorm Dev Child Adolesc 2018. Nov;24(8):1026–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson VB, Mitchell SH, Musser ED, Schmitt CF, Nigg JT. Delay discounting of reward in ADHD: application in young children. J Child Psychol Psychiatry. 2011. Mar;52(3):256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leth-Steensen C, Elbaz ZK, Douglas VI. Mean response times, variability, and skew in the responding of ADHD children: a response time distributional approach. Acta Psychol (Amst). 2000. May;104(2):167–90. [DOI] [PubMed] [Google Scholar]
- 43.Metin B, Roeyers H, Wiersema JR, Van Der Meere J, Sonuga-Barke E. A meta-analytic study of event rate effects on Go/No-Go performance in attention-deficit/hyperactivity disorder. Biol Psychiatry [Internet]. 2012;72(12):990–6. Available from: 10.1016/j.biopsych.2012.08.023 [DOI] [PubMed] [Google Scholar]
- 44.Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005. Jun;57(11):1336–46. [DOI] [PubMed] [Google Scholar]
- 45.Nigg JT, Willcutt EG, Doyle AE, Sonuga-Barke EJS. Causal heterogeneity in attention-deficit/hyperactivity disorder: do we need neuropsychologically impaired subtypes? Biol Psychiatry. 2005. Jun;57(11):1224–30. [DOI] [PubMed] [Google Scholar]
- 46.Van der Oord S, Braet C, Cortese S, Claes L. Testing the dual pathway model of ADHD in obesity: a pilot study. Eat Weight Disord. 2018;23(4):507–12. [DOI] [PubMed] [Google Scholar]
- 47.Wang Q, Chen C, Cai Y, Li S, Zhao X, Zheng L, et al. Dissociated neural substrates underlying impulsive choice and impulsive action. Neuroimage. 2016. Jul;134:540–9. [DOI] [PubMed] [Google Scholar]
- 48.Salehinejad MA, Ghanavati E, Rashid MHA, Nitsche MA. Hot and cold executive functions in the brain: A prefrontal-cingular network. Brain Neurosci Adv. 2021;5:23982128211007770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diamond A Executive functions. Annu Rev Psychol. 2013;64:135–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hongwanishkul D, Happaney KR, Lee WSC, Zelazo PD. Assessment of hot and cool executive function in young children: age-related changes and individual differences. Dev Neuropsychol. 2005;28(2):617–44. [DOI] [PubMed] [Google Scholar]
- 51.Sonuga-Barke E, Bitsakou P, Thompson M. Beyond the dual pathway model: evidence for the dissociation of timing, inhibitory, and delay-related impairments in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010. Apr;49(4):345–55. [DOI] [PubMed] [Google Scholar]
- 52.Falbe J, Rosner B, Willett WC, Sonneville KR, Hu FB, Field AE. Adiposity and different types of screen time. Pediatrics [Internet]. 2013/11/25. 2013. Dec;132(6):e1497–505. Available from: https://pubmed.ncbi.nlm.nih.gov/24276840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nikkelen SWC, Valkenburg PM, Huizinga M, Bushman BJ. Media use and ADHD-related behaviors in children and adolescents: A meta-analysis. Dev Psychol. 2014. Sep;50(9):2228–41. [DOI] [PubMed] [Google Scholar]
- 54.Faraone SV, Biederman J, Morley CP, Spencer TJ. Effect of stimulants on height and weight: a review of the literature. J Am Acad Child Adolesc Psychiatry. 2008. Sep;47(9):994–1009. [DOI] [PubMed] [Google Scholar]
- 55.Mellström E, Forsman C, Engh L, Hallerbäck MU, Wikström S. Methylphenidate and Reduced Overweight in Children With ADHD. J Atten Disord [Internet]. 2020;24(2):246–54. Available from: 10.1177/1087054718808045 [DOI] [PubMed] [Google Scholar]
- 56.Aguirre Castaneda RL, Kumar S, Voigt RG, Leibson CL, Barbaresi WJ, Weaver AL, et al. Childhood Attention-Deficit/Hyperactivity Disorder, Sex, and Obesity: A Longitudinal Population-Based Study. Mayo Clin Proc [Internet]. 2016/02/04. 2016. Mar;91(3):352–61. Available from: https://pubmed.ncbi.nlm.nih.gov/26853710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brix N, Ernst A, Lauridsen LLB, Parner ET, Arah OA, Olsen J, et al. Childhood overweight and obesity and timing of puberty in boys and girls: cohort and sibling-matched analyses. Int J Epidemiol [Internet]. 2020. Jun 1;49(3):834–44. Available from: 10.1093/ije/dyaa056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poon K Hot and Cool Executive Functions in Adolescence: Development and Contributions to Important Developmental Outcomes. Front Psychol. 2017;8:2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reale L, Bartoli B, Cartabia M, Zanetti M, Costantino MA, Canevini MP, et al. Comorbidity prevalence and treatment outcome in children and adolescents with ADHD. Eur Child Adolesc Psychiatry. 2017. Dec;26(12):1443–57. [DOI] [PubMed] [Google Scholar]
- 60.Barker ED, Ing A, Biondo F, Jia T, Pingault JB, Du Rietz E, et al. Do ADHD-impulsivity and BMI have shared polygenic and neural correlates? Mol Psychiatry [Internet]. 2021;26(3):1019–28. Available from: 10.1038/s41380-019-0444-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.