Abstract
The ability to walk contributes considerably to physical health and overall well-being, particularly in children with motor disability, and is therefore prioritized as a rehabilitation goal. However, half of ambulatory children with cerebral palsy (CP), the most prevalent childhood movement disorder, cease to walk in adulthood. Robotic gait trainers have shown positive outcomes in initial studies, but these clinic-based systems are limited to short-term programs of insufficient length to maintain improved function in a lifelong disability such as CP. Sophisticated wearable exoskeletons are now available, but their utility in treating childhood movement disorders remains unknown. We evaluated an exoskeleton for the treatment of crouch (or flexed-knee) gait, one of the most debilitating pathologies in CP. We show that the exoskeleton reduced crouch in a cohort of ambulatory children with CP during overground walking. The exoskeleton was safe and well tolerated, and all children were able to walk independently with the device. Rather than guiding the lower limbs, the exoskeleton dynamically changed the posture by introducing bursts of knee extension assistance during discrete portions of the walking cycle, a perturbation that resulted in maintained or increased knee extensor muscle activity during exoskeleton use. Six of seven participants exhibited postural improvements equivalent to outcomes reported from invasive orthopedic surgery. We also demonstrate that improvements in crouch increased over the course of our multiweek exploratory trial. Together, these results provide evidence supporting the use of wearable exoskeletons as a treatment strategy to improve walking in children with CP.
INTRODUCTION
Injury to the central nervous system during early development can lead to neuromotor disorders, such as cerebral palsy (CP), that negatively affect quality of life (1). Motor deficits in CP produce pathological walking patterns (2). Crouch gait, characterized by excessive knee flexion (3), is a common and debilitating gait disorder in CP. No consensus exists on how much knee flexion constitutes crouch (4). In children, the mean minimum knee angle during stance is typically 4° ± 6° (5); in those with crouch, the knee angle is beyond this range and may exceed 40° in severe cases (6). Surgery (7, 8), toxin injections (9), and physical therapy (2, 10) may temporarily improve crouch, but long-term deficits frequently remain (11, 12). Although not progressive from a neurological perspective, crouch gait is more energetically costly than typical walking (13), so children with CP often decline in functional capability as they age (14). Muscle strength and endurance do not keep pace with body mass during growth, exacerbating locomotor deterioration (15, 16), ultimately leading to mobility loss in 50% of ambulatory adolescents with CP in adulthood (17).
Technology-driven gait rehabilitation has led to new treatment options. Treadmill-based body weight support locomotor training (18) and robot-assisted gait trainers (RAGTs) (19) are more efficient than traditional approaches and have shown some effectiveness in children and adults with CP. However, in controlled trials, improvements were not superior to therapies of equal intensity, indicating that these new treatments should not replace overground training (20). Further, treadmill-based approaches are constrained to clinical or research facilities, which limits treatment frequency and duration; thus, retention of improvements is unlikely after training ends (20). Alternatively, whole-body vibration training may offer some gains in muscle and bone mass (21) but does not address specific gait deficits.
A clear need exists for more effective and sustainable treatments for crouch gait. Powered exoskeletons provide an untapped resource for this population. Wearable robotic devices for gait rehabilitation have primarily focused on restoring lost function due to paralysis in adults after spinal cord injury (22) or stroke (23). Typically, these systems use impedance-based controllers to achieve desired joint trajectories (24), with the amount of robotic guidance dependent on the user’s capability (25). Fundamentally, this approach mirrors the assist-as-needed strategy of RAGT, albeit in an overground setting. Translation of these technologies to children with gait pathology is not straightforward because the primary goal is to train a different walking pattern rather than to restore lost walking capability. Further, the neurological deficits underlying gait pathology from CP, including spasticity, poor selective motor control, and muscle weakness make the effect of motorized assistance difficult to predict in this population. An alternative strategy is to deploy motorized assistance to dynamically change posture during each gait cycle without imposing a strict trajectory, instead requiring the user to adjust to the perturbation using volitional muscle activity. Repeated exposure to such perturbations can recalibrate the motor strategy, but whether this recalibrated strategy can persist and replace the user’s previous one remains an open question (26).
To our knowledge, no previous studies have assessed wearable exoskeletons to treat biomechanical gait deficits in children and adolescents. Thus, their suitability for pediatric rehabilitation remains largely unknown. Here, we present findings from a multiweek exploratory trial of a pediatric exoskeleton (27) designed to alleviate crouch gait from CP. This study is the first step toward the long-term goal of implementing a novel device-based approach to treating crouch. The primary aims were to determine whether motorized knee extension assistance safely and effectively reduced crouch during overground walking in ambulatory children with CP, evaluate its effect on voluntary muscle activity during walking, and elucidate short-term changes in gait biomechanics in response to robotic assistance. We demonstrate that wearable exoskeletons can reliably reduce crouch within a short time frame in a cohort of children with CP as young as 5 years of age and that children with CP are capable of acclimating to robotic assistance without reducing knee extensor muscle activity, suggesting that exoskeletons are viable for overground gait training in this population.
RESULTS
Timing of exoskeleton assistance affects reduction in crouch
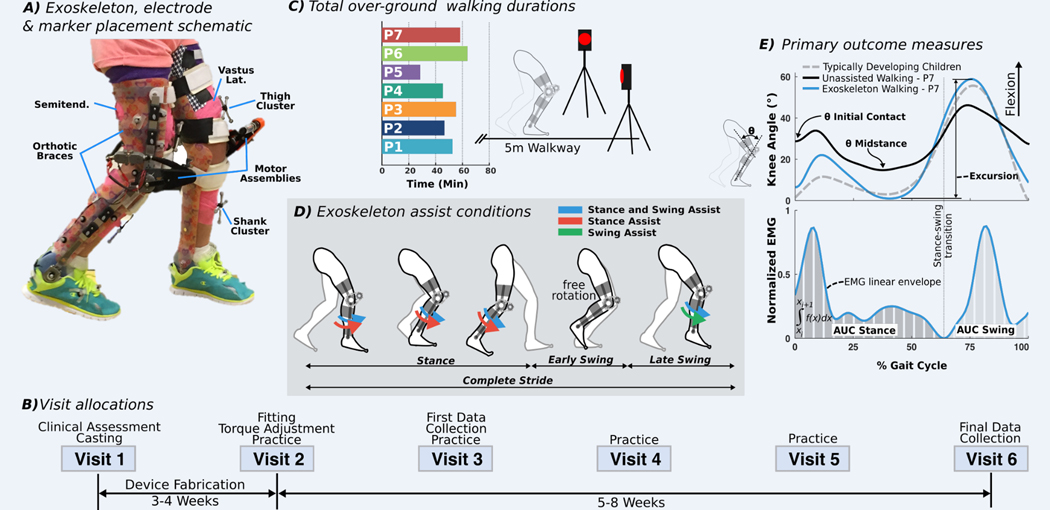
The exoskeleton provided powered knee extension assistance during the stance and late swing phases of walking in the form of a dynamic extensor torque about the knee rather than position- or impedance-controlled limb guidance (Fig. 1A). A proportional-integral-derivative (PID) motor controller compensated for friction and inertia to achieve the specified torque output at each state, including zero torque when no assistance was provided. The ankle joint was passively hinged, allowing free rotation. In-depth technical details can be found in (27). Seven participants completed the multiweek trial (Fig. 1B and Table 1). After initial fit adjustment and exoskeleton tuning, participants completed five additional visits to the National Institutes of Health Clinical Center, each lasting 2 to 3 hours. Similar to lower-extremity orthotic studies (28), participants acclimated to the exoskeleton during four laboratory-based practice sessions before the final biomechanical assessment of differences between baseline and exoskeleton-assisted gait (Fig. 1C). An initial gait assessment was also performed once the participant was able to walk independently with the exoskeleton. Total practice amount varied by individual because of fitness level, walking ability, age, and compliance with experimenter directions. Each assessment included the participant’s everyday (baseline) walking condition, walking with exoskeleton assistance, and the null exoskeleton condition (zero-torque mode) during which no assistance was provided, but the controller compensated for the inertia of the device. Three different modes of knee extension assistance were implemented: during stance phase only, swing phase only, or both stance and swing phases (Fig. 1D). For each participant, the assistive torque in each mode was held constant across the assessments. All participants achieved independent overground walking in the exoskeleton without a mobility aid or therapist assistance, with six of seven doing so within the first practice session. No falls were observed in any participant after this milestone.
Fig. 1. Lower-extremity exoskeleton study description.
(A) Image illustrating exoskeleton components on a study participant. To ensure accurate kinematic comparison between conditions, marker clusters were placed on limb locations free from interference with the exoskeleton and were not removed between conditions. EMG electrodes were placed over the vastus lateralis (vastus lat.) and semitendinosus (semitend.). (B) Timeline of the six visits, including two data collection assessments and practice visits. (C) Time spent walking over ground with the exoskeleton by each participant. (D) Schematic of gait cycle phases and the timing for the three modes of knee extension assistance: stance and swing (blue), stance only (red), and swing only (green). (E) Kinematic outcome measures included knee angle at initial contact (θ initial contact), peak knee angle during midstance (θ midstance), and total knee excursion. Knee angle data of a representative participant (P7) at baseline and with the exoskeleton and reference data from typically developing children and young adults from our clinical laboratory database are shown. EMG outcome measures included the integrated muscle activity (area under the EMG percent gait cycle curve) during stance [area under the curve (AUC) stance] and swing (AUC swing), normalized by the peak value of each muscle during baseline.
Table 1. Participant information.
GMFCS, Gross Motor Function Classification System; MAS, modified Ashworth Scale for knee flexors of the more- and less-affected limbs; AFO, ankle-foot orthosis.
| Patient (ID) | Age (years) | Gender | Height (m) | Body mass (kg) | GMFCS level | MAS (more/less affected) | Baseline condition | Exoskeleton mass (kg)* |
|---|---|---|---|---|---|---|---|---|
| P1 | 10 | Female | 1.48 | 42.5 | II | 1+/1+ | AFO | 3.7 |
| P2 | 12 | Male | 1.72 | 69.3 | I | 1/1 | Shod | 3.8 |
| P3 | 19 | Female | 1.48 | 65.1 | II† | 1/1 | Shod | 4.5 |
| P4 | 11 | Male | 1.35 | 32.0 | II | 2/1+ | Shod | 3.0 |
| P5 | 6 | Male | 1.11 | 20.0 | II | 0/0 | AFO | 3.1 |
| P6 | 11 | Female | 1.56 | 40.8 | II | 1+/1+ | Shod | 3.6 |
| P7 | 5 | Male | 1.15 | 20.6 | II | 1+/1 | AFO | 2.6 |
Exoskeleton mass is the combined total for both limbs.
P3 typically walks with crutches and was originally classified as GMFCS III; however, when prompted, she walked without assistance and was reclassified as GMFCS II.
For the stance and swing assist condition, the average knee extensor torque provided by the exoskeleton was 0.17 ± 0.06 Nm/kg (mean ± SD) during stance and 0.06 ± 0.02 Nm/kg during swing (table S1). For the stance assist condition the average torque was 0.15 ± 0.05 Nm/kg, and for the swing assist condition, the average torque was 0.07 ± 0.03 Nm/kg. For the null (zero-torque) exoskeleton condition, the average torque was 0.0013 ± 0.002 Nm/kg. The average torque in the early swing phase of the assist modes, when the setting was also zero, was 0.019 ± 0.02 Nm/kg.
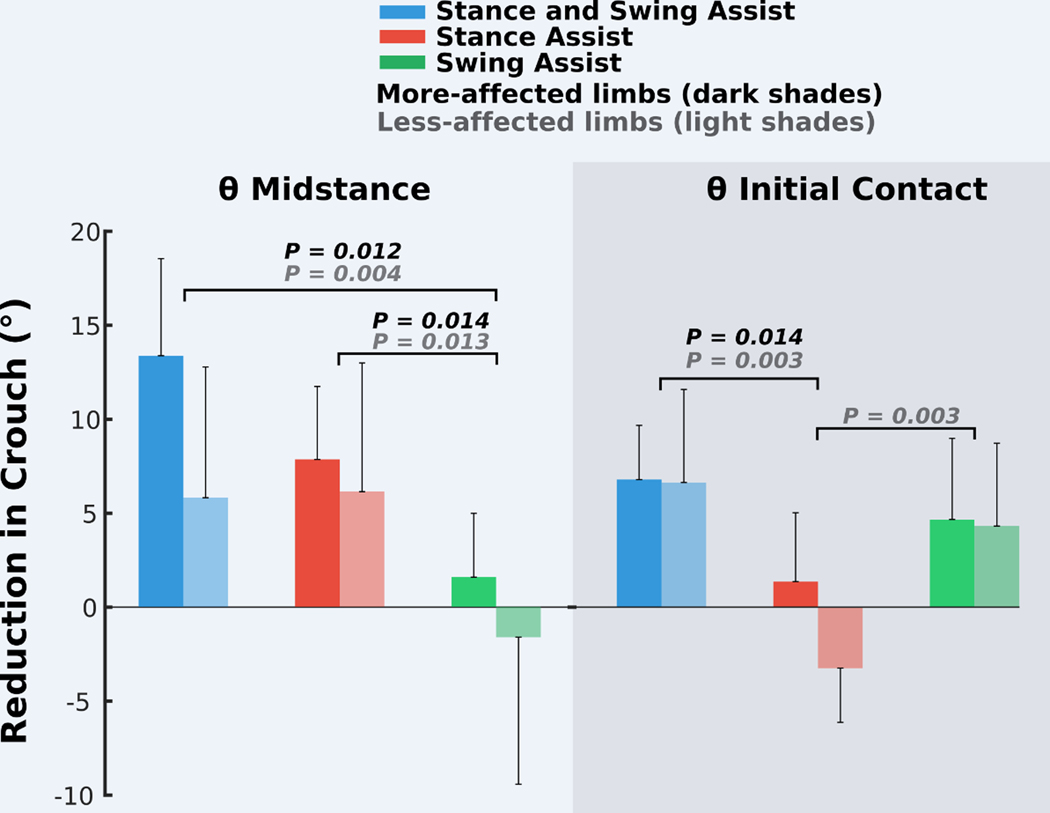
The mode of exoskeleton assistance significantly affected knee angle outcome measures [Fig. 2; θ initial contact analysis of variance (ANOVA), P = 0.002; θ midstance ANOVA, P = 0.001]. The stance and swing assist condition resulted in significantly greater mean improvements in knee extension during midstance compared to the swing assist condition [more-affected limb, 11.8° (P = 0.012); less-affected limb, 7.4° (P = 0.004)] and at initial contact compared to the stance assist condition [more-affected limb, 5.5° (P = 0.014); less-affected limb, 10° (P = 0.003)] (Fig. 2) and was therefore selected for all subsequent in-depth analyses. Stance assist resulted in more knee extension during midstance compared to swing assist [more-affected limb, 6.3° (P = 0.014); less-affected limb, 7.7° (P = 0.013)]. Swing assist elicited a 7.5° reduction in knee flexion for the less-affected limbs compared to stance assist (P = 0.003). The response to each assistive mode varied across individuals (fig. S1).
Fig. 2. Effect of exoskeleton assistance mode on crouch.
Reduction in crouch, measured as increased knee extension angle compared to baseline walking during early (θ initial contact) and midstance (θ midstance) for the three assistance modes: stance and swing, stance only, and swing only. The darker and lighter bars depict group mean (n = 7) values of the more- and less-affected limbs, respectively. Error bars denote 1/2 SD; the large error bars indicate the large interparticipant variability (fig. S1). Significant differences between conditions are indicated (post hoc paired t tests with Bonferroni correction, P < 0.05).
Exoskeleton assistance improved knee extension without reducing volitional muscle activity
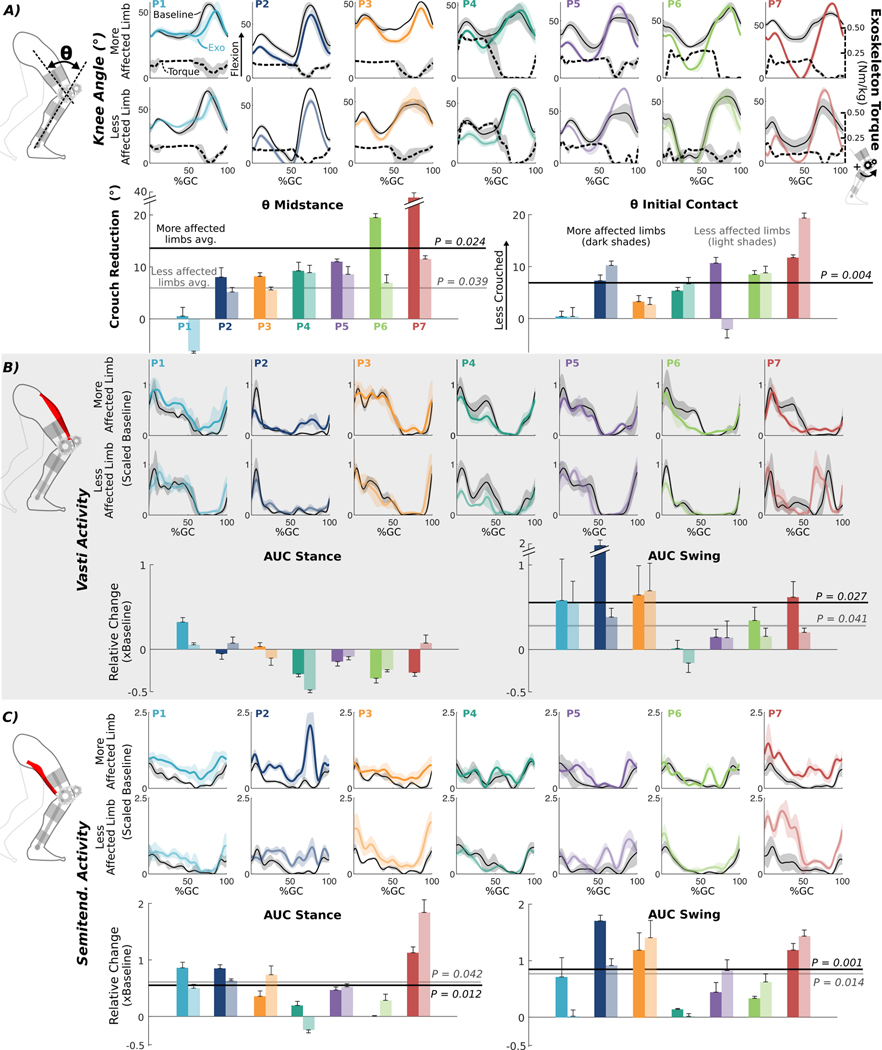
Kinematic responses to extension assistance varied across individuals and between limbs within an individual at the final assessment (Fig. 3). Significant reductions in knee flexion at initial contact and midstance were observed in six of the seven participants when walking with the exoskeleton; the largest individual improvement was a 36.7° increase in midstance knee extension. At the group level, walking with the exoskeleton in stance and swing assist mode improved mean stance-phase knee extension by 13.3° (P = 0.024) and 5.8° (P = 0.039) for the more- and less-affected limbs, respectively (Fig. 3A). Knee extension at initial contact was improved by 6.8° for the more-affected limbs (P = 0.004). Vastus lateralis activity increased during swing [more-affected limb, 56% (P = 0.027); less-affected limb, 28% (P = 0.041)] (Fig. 3B). Semitendinosus activity increased during stance [more-affected limb, 55% (P = 0.012); less-affected limb, 61% (P = 0.042)] and swing [more-affected limb, 81% (P = 0.001); less-affected limb, 75% (P = 0.014)] (Fig. 3C).
Fig. 3. Biomechanical effects of exoskeleton extension assistance.
(A) Knee angles across the gait cycle (GC) for each participant’s more- and less-affected limbs during exoskeleton walking under stance and swing assist (colored lines) and at baseline (thin black lines). Measured exoskeleton torques across the gait cycle (black dashed lines) for each limb, normalized by body mass, are plotted relative to the right axis. Bar charts below show the mean reduction in crouch during exoskeleton walking at midstance (left) and initial contact (right) for each individual. Vastus lateralis (B) and semitendinosus (C) EMG activity across the gait cycle for each participant’s more- and less-affected limbs during exoskeleton walking (colored lines) and baseline (thin black lines); EMG activity was scaled to baseline. Bars below are the mean change in EMG during exoskeleton walking relative to baseline during stance (left) and swing (right). For all bar plots, participants are presented from left to right by least-to-most improvement in crouch for their more-affected limbs. The darker and lighter bars depict the more- and less-affected limbs, respectively, and error bars denote 1/2 SD. Lines across the bar charts indicate group-level significant mean (avg.) changes from baseline for more-affected (dark shade) and less-affected (light shade) limbs (θ midstance for less-affected limbs: one-sided Wilcoxon signed-rank test, P < 0.05; all other comparisons: paired t tests, P < 0.05). All gait cycle plots are individual means ± 1 SD in shaded regions; darker and lighter lines depict more- and less-affected limbs, respectively.
Comparing baseline and null exoskeleton conditions, no group-level significant differences were seen in knee angle at midstance (more-affected limb, P = 0.81; less-affected limb, P = 0.12) or initial contact (more-affected limb, P = 0.58; less-affected limb, P = 0.17) (fig. S2). The only significant differences observed in electromyography (EMG) were increases in both semitendinosus (51%; P = 0.001) and vasti (46%; P = 0.002) muscle activity during swing for the more-affected limbs (fig. S2), an expected result due to the added mass of the exoskeleton.
Three participants used AFOs in their baseline walking condition (P1, P5, and P7). Although we did not compare the AFO to the no-AFO condition, the null exoskeleton (and the exoskeleton) had a free ankle. One individual (P1) was more crouched in the null exoskeleton condition compared to baseline with AFOs (fig. S2), which likely decreased the effectiveness of the exoskeleton in reducing her crouch. No difference was observed between the AFO baseline and the null exoskeleton for the other participant (P7) for which data were available.
Mean total knee angle excursion (more-affected limb, P = 0.06; less-affected limb, P = 0.15), step length (more-affected limb, P = 0.22; less-affected limb, P = 0.22), and cadence (more-affected limb, P = 0.07; less-affected limb, P = 0.17) were not significantly different between baseline and exoskeleton walking during the final assessment (fig. S3). Baseline spasticity (knee extensors, P = 0.77; knee flexors, P = 0.80) and body mass–normalized muscle strength (knee extensors, P = 0.41; knee flexors, P = 0.59) were not significantly associated with the changes in crouch (Fig. 4). Mean extension torque provided by the exoskeleton during stance amounted to 27 ± 13% of mean knee extensor strength (table S1).
Fig. 4. Improvement in crouch does not correlate with spasticity or strength.
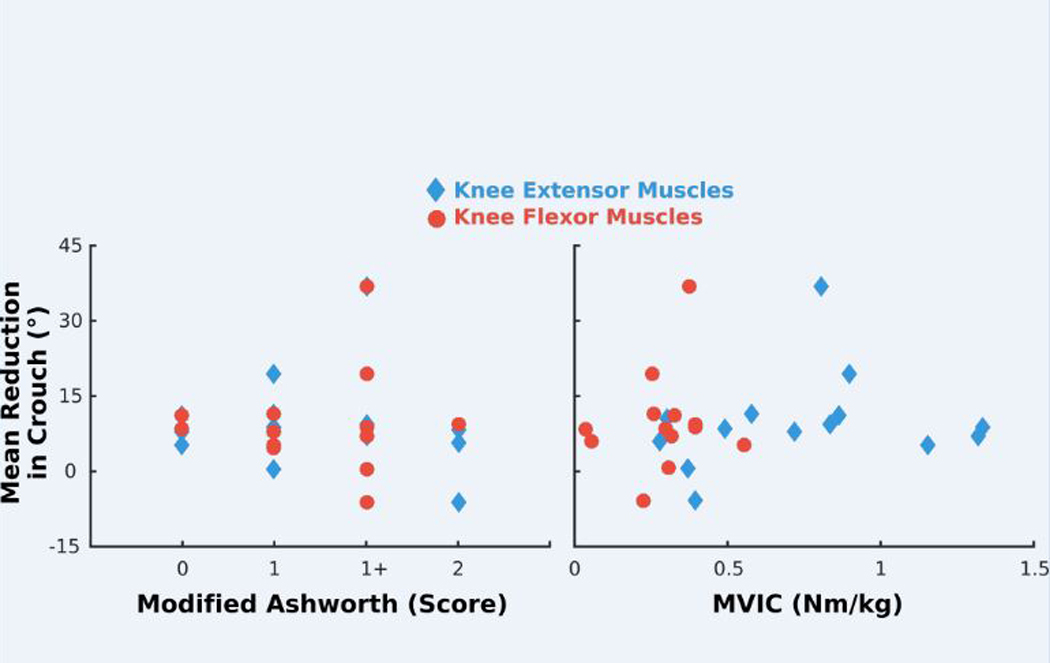
Mean reduction in crouch at midstance during exoskeleton walking plotted versus clinical measure of spasticity (modified Ashworth score; left) and body mass–normalized strength (MVIC, maximum voluntary isometric contraction; right) for the knee extensor (diamond) and knee flexor (circle) muscles. Pearson’s product-moment correlation analysis revealed no significant relationships between crouch reduction and spasticity (knee extensors, P = 0.77; knee flexors, P = 0.80) or strength (knee extensors, P = 0.41; knee flexors, P = 0.59).
Improvement in knee extension accrued with practice
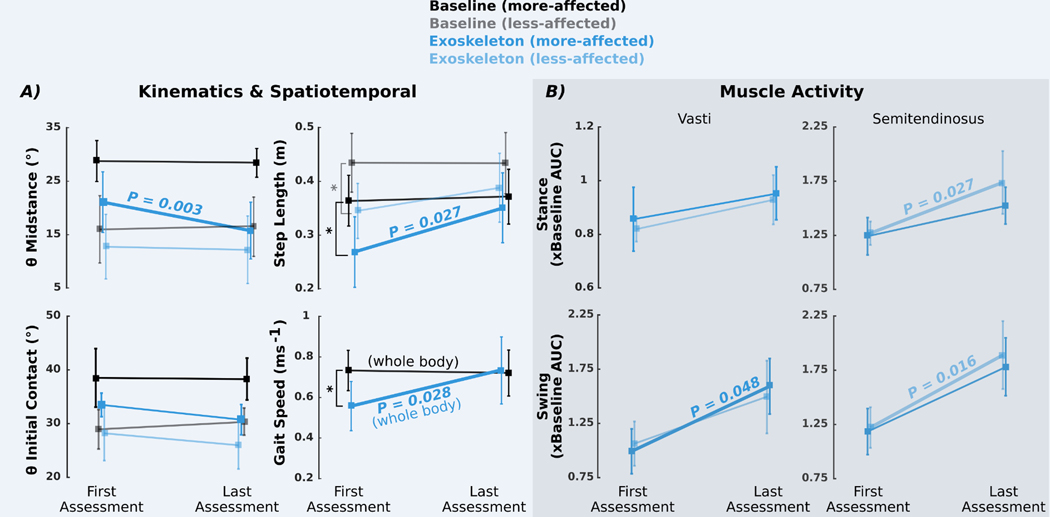
Marked changes in gait were observed between the first and final sessions with the powered exoskeleton. Stance-phase knee extension improved by 5.3° (P = 0.003) on the more-affected limbs between assessments (Fig. 5 and fig. S4), indicating a progressive reduction in crouch with continued exoskeleton use. When participants first walked with the exoskeleton, significant reductions in step length (more-affected limb, P = 0.025; less-affected limb, P = 0.039) and gait speed (P = 0.023) were seen compared to baseline walking (Fig. 5A). However, both measures returned to baseline values during the final assessment with step length increasing by 33% (0.09 m; P = 0.027) and gait speed increasing by 32% (0.18 m/s; P = 0.028) across assessments (Fig. 5A). Increased muscle activity during exoskeleton use was also observed between assessments; relative vasti activity increased by 61% (P = 0.048) during swing in the more-affected limbs, whereas relative semitendinosus activity increased during stance by 36% (P = 0.027) and swing by 55% (P = 0.016) for the less-affected limbs (Fig. 5B).
Fig. 5. Change in biomechanical measures between first and last assessments.
(A) Knee angle during midstance (θ midstance), knee angle at initial contact (θ initial contact), and step length for more-affected (dark shades) and less-affected (light shades) limbs and gait speed at the first and last assessment visits during baseline (black/ gray) and exoskeleton stance and swing assist (dark/light blue) walking. Asterisks indicate significant differences between conditions during the first assessment (paired t tests, P < 0.05) in step length (more-affected limb, P = 0.025; less-affected limb, P = 0.039) and gait speed (P = 0.023). Differences between conditions at the last assessment are shown in Fig. 3 and fig. S3. (B) Change in vastus lateralis and semitendinosus activity during exoskeleton walking relative to baseline as measured by area under the curve. EMG data during the exoskeleton trials were normalized by the EMG from baseline walking within visits. All values plotted are group means (n = 7); error bars denote ±1/2 SD. Bold lines and P values indicate significant differences between visits (paired t tests, P < 0.05).
There were no significant changes in stance-phase knee extension (more-affected limb, P = 0.99; less-affected limb, P = 0.26) or gait speed (P = 0.05) when walking in the null exoskeleton condition (zero assistive torque) between assessments (fig. S5). On the more-affected limbs, step length increased by 10% (0.04 m; P = 0.024), and knee angle at initial contact increased by 3.2° (P = 0.039). There were no significant changes in stance or swing phase knee extensor and flexor muscle activity between assessments in the null exoskeleton condition (all P > 0.05; fig. S5).
DISCUSSION
We developed a robotic exoskeleton designed to ameliorate crouch gait. Our results demonstrate that robotic assistance provided during overground walking significantly reduces crouch in children and adolescents with CP. We observed improvements in knee extension in six of seven participants with gains (8° to 37°) similar to or greater than those reported from invasive surgical interventions (7, 8). Walking with the exoskeleton was safe and well tolerated with all participants able to walk independently after an initial acclimation period. Our results suggest that powered knee exoskeletons should be investigated as an alternative to or in conjunction with existing treatments for crouch gait, including orthopedic surgery, muscle injections, and physical therapy.
Effects of knee extension assistance were specific to the gait phase during which it was provided (Fig. 2). Powered assistance during stance reduced crouch at midstance but did not improve knee angle at initial contact. Similarly, assistance in late swing improved knee extension at initial contact, but improvements did not persist throughout stance. However, the effects were additive such that combined stance and swing assist mode provided the greatest improvement in knee extension across the entire gait cycle.
The increase in gait speed across assessments, with no difference from baseline speed at the second assessment, indicates that participants acclimated favorably to the exoskeleton. Crouch, as assessed by knee extension during midstance, and step length also improved between assessments in most of the participants despite the amount of exoskeleton assistance being held constant. The lack of intermediate visit data precluded conclusions regarding the time course of crouch improvement during the acclimation period. It is likely that additional practice or increased amounts of assistive torque would have enhanced gains in crouch reduction in some participants. Long-term studies of the effects of assistance on crouch are needed to elucidate the mechanisms of improvement over time.
Many robotic gait trainers and exoskeletons use impedance-based controllers to guide the limbs along predefined trajectories (29). We implemented a different strategy of providing dynamic extension assistance at multiple points in the gait cycle, a paradigm intended to perturb the user’s baseline walking pattern. Most participants adjusted to the assistance after a short amount of practice as evidenced by the ability to walk independently on the first visit. Our results demonstrate that ambulatory children with CP did not reduce, but instead maintained or increased, knee extensor muscle activity when walking with the exoskeleton, suggesting that robotic assistance augmented, rather than replaced, volitional knee extensor muscle activity to elicit improvements in gait mechanics, despite unwanted concurrent increases in antagonist muscle activity in some individuals. Notably, knee extensor activity was maintained or improved between the first and final assessments, a result which has positive implications for retaining or potentially increasing muscle strength with long-term use of phasic wearable assistance. Previous studies of robotic assistance during walking in healthy individuals have found that users respond by reducing their voluntary effort to maintain baseline kinematics. Initial increases in ankle plantar flexion in the presence of assistance were transient and relatively quickly discarded with continued practice (30). Similarly, robotic hip flexion assistance resulted in decreased muscle activity and peak hip flexion angle (31). These results at the hip and ankle, combined with our results at the knee, suggest that the biomechanical effect of isolated exoskeleton assistance may differ between healthy individuals and those with neurological deficits.
The exoskeleton was most effective at reducing crouch during midstance, whereas improving knee extension at initial contact was more difficult. Torque transfer to the knee was more effective in stance, when the average specified torque was 0.19 ± 0.08 Nm/kg and the measured assistance was 0.17 ± 0.06 Nm/kg, as compared to swing, when specified torque was 0.18 ± 0.04 Nm/kg but measured assistance was 0.06 ± 0.02 Nm/kg. Delivery of torque during late swing is challenging because knee extension velocity approaches the motor no-load speed, and the motor latency (~50 ms) constitutes a considerable percentage of this gait phase, limiting the available time to deliver assistance (27). However, neural responses to robotic assistance may also contribute to the gap in exoskeleton-mediated crouch reduction between stance and swing. Knee extensor and flexor muscle response to the exoskeleton was greater in swing despite delivery of less-assistive torque. Part, although not all, of this increased activity was due to the inertia of the exoskeleton (fig. S2). Coactivation of antagonist muscles, either due to abnormal reciprocal inhibition (32), reciprocal excitation (33), attenuated presynaptic inhibition (34), or dystonia, may be responsible for the observed increases in both muscle groups, and spasticity may have contributed to increased flexor activation during late swing knee extension. Another possibility is that the elevated muscle activity in late swing was not a reflex but rather an attempt to resist postural changes at foot contact caused by robotic assistance because cocontraction responses improve stability for control over joint angles (35), and such control has been previously demonstrated in children with CP (36).
In stance phase, vasti activity remained at baseline values with the exoskeleton, whereas hamstring activity increased in five of seven participants in response to assistance (Fig. 3). There are several possible explanations for this increased activation. Spasticity is a potential primary contributor because six of seven children in our cohort had mild spasticity measured clinically (Table 1). More rapid knee extension after loading response may have elicited a stretch response in some individuals (P2, P3, P5, and P7), although these presumed stretch responses observed in EMG were not always consistent with clinical assessment of spasticity. This could be explained by a lack of sensitivity of the MAS, particularly in the lower extremity (37), or because knee joint velocity during MAS assessment is slower than that during assisted gait. Alternatively, the elevated hamstring EMG may be an attempt to maintain stability because reduction in crouch during midstance without a concurrent increase in hip extension would anteriorly shift the center of mass. Increased hamstring activity may also constitute a maladaptive response because of the external torque provided by the motor exceeding the internal flexor moment (38). Additional improvements in posture and muscle patterns may be achieved by refinement of robotic control algorithms to account for internal joint mechanics (38). More practice time may also enhance coordination of muscle activity with the external assistance.
In addition to alleviating crouch, the perturbation-like dynamic assistance may also provide the opportunity to train a new strategy for walking. Modifying locomotor strategies via motor adaptation has been studied extensively (39). Individuals with brain lesions from stroke (40) and CP (41) have shown improved short-term step symmetry in response to perturbations. Such changes are transient; the newly adapted movement patterns are rapidly abandoned after the perturbation is removed. The beneficial effects of short-term adaptation may be extended by repetitive training (42) and reinforcement (43), supporting the idea that long-term training in the presence of a perturbation may form new motor patterns (26). An exoskeleton that introduces postural changes as a perturbation, as in this study, may therefore provide an opportunity for long-term rehabilitation gains by reinforcing short-term adaptations through extensive home-based training, a potential advantage over traditional therapy settings. Transfer of these new patterns outside the environment within which they were learned (in this case, to walking without the exoskeleton) remains a key challenge. Gradually increasing the perturbation over time may result in more effective generalization of the new motor pattern (44). Future studies focusing on systematic follow-up after intensive training with the exoskeleton will be vital in determining the dosage of assistance required to achieve long-term biomechanical improvements.
Exoskeleton use may confer other benefits even in the absence of long-term crouch reduction without the device. A flexed posture significantly increases the muscular effort (6) and metabolic cost (45) associated with walking. Thus, the improved knee extension achieved using our device may ease the energy burden of walking and enhance physical activity in those with crouch, which may subsequently help halt or reverse the downward cycle of impaired mobility (14, 46). Detailed studies of walking in the exoskeleton over longer time periods are necessary to fully assess the effect on metabolic cost and energy expenditure.
We observed asymmetric biomechanical responses to robotic assistance with greater kinematic improvements generally observed on the more-affected limbs. The less-affected limb, by definition, had a smaller range over which kinematic improvements could take place. In some cases, the disparity in knee extension improvements between limbs concurred with muscle activity results. For example, P5 and P7 had greater increases in hamstring activity and less crouch reduction with stance phase assistance on the less-affected limb. This result is in agreement with recent studies of unilateral leg weighting in children with CP in which adaptation effects differed between dominant (less-affected) and nondominant (more-affected) legs (47) suggesting that, like healthy individuals, children with CP have separate neural circuits for controlling each leg during walking that can adapt independently (48). Although we tuned the torque setting for each leg independently, outcomes may be further improved by using distinct control strategies for each limb.
Heterogeneity in the type and timing of neurological insults leading to CP creates variability in both the degree of motor disability (49) and the response to interventions (50, 51). However, we observed significant crouch reduction in six of seven participants. These improvements were not correlated with clinical assessment of spasticity or extensor strength. The former result was not surprising because spasticity is only weakly correlated with reduced walking ability (52). Conversely, the same study found that strength was strongly correlated to gait function. Our participants had sufficient strength to ambulate independently; a stronger correlation between strength and exoskeleton response may become more evident in individuals with greater weakness and mobility challenges. Nonetheless, the exoskeleton improved upright posture in children with widely varying crouch severity (21° to 51° of knee flexion at initial contact) and thus may be a broadly applicable treatment for crouch across the CP population.
Our findings pave the way for the longer-term investigation of wearable exoskeletons for gait rehabilitation in children with neurological disorders. Even the participant (P1) who did not exhibit reduced crouch with the exoskeleton compared to baseline at the final assessment improved over the course of the study. This participant had the slowest absolute and height-normalized walking speed during baseline and exoskeleton conditions. On her first assessment with the exoskeleton, she ambulated via noncontinuous steps. However, she increased her gait speed by 140% and her more-affected limb knee extension by 20% between assessments, suggesting that additional practice with the exoskeleton may have improved her posture.
Limitations of this study were the small number of participants and that all were at Gross Motor Function Classification System (GMFCS) level I or II. To isolate the biomechanical effects of robotic assistance, only participants who could walk without external assistance were included, limiting the generalizability of our findings. Many individuals at GMFCS levels III and IV are at higher risk for losing functional mobility in and after adolescence (53, 54) and thus would likely benefit from exoskeleton assistance. Similarly, children with lower limb weakness as a result of muscular dystrophy, spina bifida, or incomplete spinal cord injury are also potential candidates for training with this type of device. Although more research is needed, the diversity in age range, anthropometrics, and crouch severity was a strength of our small cohort, which lends credence to broad applicability of exoskeletons for gait training.
In conclusion, our findings demonstrate that a powered lower extremity exoskeleton providing dynamic extension assistance produces clinically meaningful improvements in crouch during overground walking in children and adolescents with CP. The exoskeleton was well tolerated, and biomechanical improvements accrued after a small amount of time in the device. Collectively, these results provide evidence to support larger controlled intervention studies of pediatric exoskeleton efficacy for gait rehabilitation in CP and other disorders.
MATERIALS AND METHODS
Study design
The goal of this study was to assess the effects of powered knee extension assistance during overground walking in children with crouch gait from CP. Biomechanical effects were measured in a motion capture laboratory, with primary outcome measures of knee extension angle and knee extensor (vastus lateralis) and flexor (semitendinosus) EMG. This was a cohort study with two data collection time points. Primary outcome measures were evaluated at the final assessment, allowing all participants four practice sessions to acclimate to the exoskeleton. These acclimation periods are common in studies of lower extremity orthoses (28). To examine short-term biomechanical changes during acclimation, an initial data collection was performed when each participant was first able to walk independently with the exoskeleton. At each assessment, data were collected in the baseline walking condition, with the exoskeleton in three assist modes (stance only, swing only, and stance and swing) and a null exoskeleton mode in which no assistance was provided, but the controller provided inertia compensation for the exoskeleton. Within each session, the order of exoskeleton modes was randomized.
Our protocol (#13-CC-0210) was approved by the National Institutes of Health institutional review board. Individuals between the ages of 5 and 19 were recruited to participate in our study that included six visits over 8 to 12 weeks (Fig. 1B). In addition to a diagnosis of crouch gait from CP, inclusion criteria were GMFCS levels I and II, thigh-foot angle from 10° internal tibial rotation to 25° external rotation, and less than 5° and 10° knee flexion and plantar flexion contractures, respectively. Participants were excluded if they had any health condition other than CP that would affect their participation. Eight participants with spastic diplegic CP met all the criteria. Informed assent and consent were obtained from each child and their parents, respectively. The parents of one participant, a 5-year-old who had difficulty complying with study procedures, withdrew the child from the study. Participant information for the seven individuals who completed all six visits is shown in Table 1.
Exoskeleton design
A full technical description of the exoskeleton used in this study can be found in (27). The device is based on a standard knee AFO with a passive adjustable dynamic ankle joint and an actuated knee joint containing a back-drivable 24-V, 90-W brushless motor (Maxon Motor) with a custom-designed two-stage transmission. The exoskeleton’s maximum torque is 16.1 Nm, and the maximum no-load speed is 314°/s. The exoskeleton weight varied by individual ranging from 2.6 to 4.5 kg for both legs combined (Table 1). Each limb’s exoskeleton contained a quadrature encoder (Maxon Motor), a torque sensor (Transducer Techniques), and a force sensitive resistor (Interlink Electronics) to track knee angle and angular velocity, knee joint torque, and foot-ground contact, respectively. The gait cycle was split into three discrete states (stance, early swing, and late swing) in real time using sensor feedback and a rule-based finite state machine. Within each phase, a PID controller was used to achieve the desired torque output. PID gains were tuned manually during benchtop testing before each participant donned the device and remained the same for all experimental sessions. The mean latency was 53 ms for torque activation and 57 ms for deactivation (27); thus, the desired step input changes in torque set points within each gait phase were achieved as ramp responses in practice. The control system was implemented in a single-board computer (LX800, LiPPERT Embedded Computers) running Debian Linux with Xenomai real-time kernel. The exoskeleton can be powered by a DC power source or an onboard 22.2-V lithium-ion battery. The control box weighed 2.0 kg and was carried by an experimental assistant walking behind the participant.
Study protocol
On the first visit, participants completed baseline overground gait analysis and knee flexor and extensor spasticity and strength assessment (System 4, Biodex). An orthotist cast each participant’s legs for fabrication of the custom exoskeleton. On the second, fourth, and fifth visits, each lasting 2 to 3 hours, participants donned their exoskeleton and practiced walking overground and on a treadmill (Fig. 1C). Exoskeleton assistance could be provided during the stance and late swing phases to assist knee extension. Assistance was provided in the form of a step change in torque set point. Whenever assistance was not active, the control algorithm specified zero torque (free rotation) as was always the case during early swing to allow the knee to flex without resistance and to maintain toe clearance. The amount of assistance was tuned during the second visit with the goal of maximizing knee extension without compromising walking stability. The torque set point was incrementally increased, and the effect was evaluated visually and through verbal feedback with the user until the optimal setting within the limits of the device that could be comfortably accommodated by each participant was reached, an approach similar to clinical tuning of commercially available prosthetics and orthotics. Torque set points were tuned separately for each limb and gait phase (stance and swing) and remained the same during all data collection sessions to allow acclimation and to preclude confounding effects of varying the amount of assistance. We evaluated three different modes of exoskeleton assistance: (i) stance assist, (ii) swing assist, and (iii) combined stance and swing assist (Fig. 1D).
Data collection was allocated to two visits: The first assessment occurred on the third visit except for one participant (P5) who was unable to walk independently with the exoskeleton until the fourth visit, so the fifth visit was his first assessment, and the final assessment took place on the last visit. At the start of the data collection visits, which lasted 3 to 4 hours, participants completed an overground walking trial in their normal baseline condition (shoes with or without prescribed AFOs). Each walking trial comprised a minimum of four passes across the 5-m motion capture space. Next, participants donned the exoskeleton and completed each exoskeleton walking condition over ground. A safety line, attached to a torso-mounted gait belt, was in place but untensioned as long as the participant remained upright. The exoskeleton was tethered to a power supply to guarantee continuous operation during testing. Participants were provided frequent rest periods to minimize fatigue. One participant (P5) was unable to complete the null exoskeleton condition during the final assessment.
Gait assessments included motion capture and EMG. Ten infrared cameras (Vicon Motion Systems) recording at 100 Hz captured the three-dimensional positions of retroreflective markers placed according to a custom marker set (27). Wireless EMG electrodes (Trigno, Delsys) recording at 1000 Hz were placed bilaterally on the vastus lateralis and semitendinosus. Motion capture, EMG, and exoskeleton data were synchronized via an electrical pulse.
Biomechanical analysis
Joint angles were computed in Visual 3D (C-Motion). MATLAB version 2016a (MathWorks Inc.) was used to postprocess the joint angle, exoskeleton torque, and EMG data. EMG data were band-pass–filtered (15 to 380 Hz), full wave–rectified, and low-pass–filtered with a 7-Hz cutoff frequency to generate linear envelopes (55). The area under the stance and swing portions of the EMG versus percent gait cycle curve were calculated by numerical integration (Fig. 1E).
For each subject, strides were normalized to percent gait cycle and averaged across gait cycles for each walking condition. The leg with the greatest crouch during stance was designated as the more-affected limb. Only independent walking strides without external assistance in which the exoskeleton was functioning properly were analyzed.
Kinematic outcome measures included knee angle at initial contact (θ initial contact), minimum knee flexion during stance (θ midstance), and total knee excursion (Fig. 1E), with a reduction in knee flexion angle (increased knee extension) considered an improvement in posture. Spatiotemporal outcome measures included step length, cadence, and gait velocity. Neuromuscular outcome measures included integrated EMG during stance and swing. To compare between exoskeleton and baseline walking conditions within a visit, EMG data were normalized by the peak value of each muscle’s activity during the baseline trials. To compare the exoskeleton conditions between visits, EMG data for each muscle during the exoskeleton trials were normalized by the EMG data during the baseline trials for that visit.
Statistical analysis
For each condition and outcome measure, means and SDs were calculated for the more- and less-affected limbs. One-factor repeated-measures ANOVA procedures were used to determine the effects of the three exoskeleton assist conditions on knee flexion at initial contact and midstance. When indicated, post hoc comparisons were performed using paired t tests with Bonferroni multiple comparisons correction. Paired t tests were used to evaluate a small number of a priori comparisons: between baseline and exoskeleton walking conditions under optimized torque settings (and the null exoskeleton condition) on the final assessment and between the two assessments during walking with the exoskeleton operating at same torque settings. For each comparison, the Jarque-Bera two-sided goodness-of-fit test was used to check for normality. Wilcoxon signed-rank tests replaced paired t tests for distributions that failed normality. Pearson’s product-moment correlation analysis was used to relate knee extensor and flexor strength and spasticity to changes in crouch. Statistical significance was set at α < 0.05, except when adjusted for multiple comparisons. Individual subject-level data are shown in table S2.
Supplementary Material
Fig. S1. Effect of exoskeleton assistance mode on crouch by individual.
Fig. S2. Biomechanical effects of walking with the null exoskeleton.
Fig. S3. Effect of exoskeleton assistance on gait parameters.
Fig. S4. Individual differences in crouch between first and last assessments.
Fig. S5. Group-level change in biomechanical measures between first and last assessments during walking with the null exoskeleton.
Table S1. Knee torque measurement by exoskeleton condition and muscle strength.
Table S2. Individual subject-level data.
Acknowledgments:
We thank M. DeHarde and Ultraflex Systems Inc. for fabricating the orthotics for each exoskeleton. We thank H. S. Park and J. Kim for their contributions to the design of the exoskeleton and control electronics. We also thank K. Alter, A. Gravunder, C. Stanley, C. Zampieri-Gallagher, S. Galey, A. Sharma, and L. Ohlrich for their assistance with clinical assessments and data collection. Z.F.L. was with the NIH and is currently with the Department of Mechanical Engineering, Northern Arizona University, Flagstaff, AZ.
Funding: This work was supported by the intramural research program at the NIH Clinical Center (protocol #13-CC-0210).
Footnotes
Competing interests: Z.F.L., D.L.D., and T.C.B. are named inventors on a provisional patent application (U.S. patent application no. 62/368,926, “Powered Gait Assistance Systems”) covering the exoskeleton used in the study, which is held by the NIH.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from T.C.B. (thomas.bulea@nih.gov).
SUPPLEMENTARY MATERIALS
www.sciencetranslationalmedicine.org/cgi/content/full/9/404/eaam9145/DC1
REFERENCES AND NOTES
- 1.Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, Dan B, Jacobsson B, A report: The definition and classification of cerebral palsy April 2006. Dev. Med. Child Neurol. Suppl 109, 8–14 (2007). [PubMed] [Google Scholar]
- 2.Damiano DL, Alter KE, Chambers H, New clinical and research trends in lower extremity management for ambulatory children with cerebral palsy. Phys. Med. Rehabil. Clin. N. Am 20, 469–491 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wren TAL, Rethlefsen S, Kay RM, Prevalence of specific gait abnormalities in children with cerebral palsy: Influence of cerebral palsy subtype, age, and previous surgery. J. Pediatr. Orthop 25, 79–83 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Galey SA, Lerner ZF, Bulea TC, Zimbler S, Damiano DL, Effectiveness of surgical and non-surgical management of crouch gait in cerebral palsy: A systematic review. Gait Posture 54, 93–105 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ounpuu S, Gage JR, Davis RB, Three-dimensional lower extremity joint kinetics in normal pediatric gait. J. Pediatr. Orthop 11, 341–349 (1991). [PubMed] [Google Scholar]
- 6.Hicks JL, Schwartz MH, Arnold AS, Delp SL, Crouched postures reduce the capacity of muscles to extend the hip and knee during the single-limb stance phase of gait. J. Biomech 41, 960–967 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Mattos C, Do KP, Pierce R, Feng J, Aiona M, Sussman M., Comparison of hamstring transfer with hamstring lengthening in ambulatory children with cerebral palsy: Further follow-up. J. Child. Orthop 8, 513–520 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stout JL, Gage JR, Schwartz MH, Novacheck TF, Distal femoral extension osteotomy and patellar tendon advancement to treat persistent crouch gait in cerebral palsy. J. Bone Joint Surg. Am 90, 2470–2484 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Corry IS, Cosgrove AP, Duffy CM, Taylor TC, Graham HK, Botulinum toxin A in hamstring spasticity. Gait Posture 10, 206–210 (1999). [DOI] [PubMed] [Google Scholar]
- 10.Damiano DL, Arnold AS, Steele KM, Delp SL, Can strength training predictably improve gait kinematics? A pilot study on the effects of hip and knee extensor strengthening on lower-extremity alignment in cerebral palsy. Phys. Ther 90, 269–279 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dreher T, Vegvari D, Wolf SI, Geisbusch A, Gantz S, Wenz W, Braatz F, Development of knee function after hamstring lengthening as a part of multilevel surgery in children with spastic diplegia: A long-term outcome study. J. Bone Joint Surg. Am 94, 121–130 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Rethlefsen SA, Yasmeh S, Wren TAL, Kay RM, Repeat hamstring lengthening for crouch gait in children with cerebral palsy. J. Pediatr. Orthop 33, 501–504 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Rose J, Gamble JG, Burgos A, Medeiros J, Haskell WL, Energy expenditure index of walking for normal children and for children with cerebral palsy. Dev. Med. Child Neurol 32, 333–340 (1990). [DOI] [PubMed] [Google Scholar]
- 14.Bjornson KF, Belza B, Kartin D, Logsdon R, McLaughlin JF, Ambulatory physical activity performance in youth with cerebral palsy and youth who are developing typically. Phys. Ther 87, 248–257 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bell KJ, Ounpuu S, DeLuca PA, Romness MJ, Natural progression of gait in children with cerebral palsy. J. Pediatr. Orthop 22, 677–682 (2002). [PubMed] [Google Scholar]
- 16.Johnson DC, Damiano DL, Abel MF, The evolution of gait in childhood and adolescent cerebral palsy. J. Pediatr. Orthop 17, 392–396 (1997). [PubMed] [Google Scholar]
- 17.Bottos M, Gericke C, Ambulatory capacity in cerebral palsy: Prognostic criteria and consequences for intervention. Dev. Med. Child Neurol 45, 786–790 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Dodd KJ, Foley S, Partial body-weight-supported treadmill training can improve walking in children with cerebral palsy: A clinical controlled trial. Dev. Med. Child Neurol 49, 101–105 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Borggraefe I, Meyer-Heim A, Kumar A, Schaefer JS, Berweck S, Heinen F, Improved gait parameters after robotic-assisted locomotor treadmill therapy in a 6-year-old child with cerebral palsy. Mov. Disord 23, 280–283 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Dobkin BH, Duncan PW, Should body weight–supported treadmill training and robotic-assistive steppers for locomotor training trot back to the starting gate? Neurorehabil. Neural Repair 26, 308–317 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gusso S, Munns CF, Colle P, Derraik JGB, Biggs JB, Cutfield WS, Hofman PL, Effects of whole-body vibration training on physical function, bone and muscle mass in adolescents and young adults with cerebral palsy. Sci. Rep 6, 22518 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farris RJ, Quintero HA, Murray SA, Ha KH, Hartigan C, Goldfarb M, A preliminary assessment of legged mobility provided by a lower limb exoskeleton for persons with paraplegia. IEEE Trans. Neural Syst. Rehabil. Eng 22, 482–490 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bortole M, Venkatakrishnan A, Zhu F, Moreno JC, Francisco GE, Pons JL, Contreras-Vidal JL, The H2 robotic exoskeleton for gait rehabilitation after stroke: Early findings from a clinical study. J. Neuroeng. Rehabil 12, 54 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marchal-Crespo L, Reinkensmeyer DJ, Review of control strategies for robotic movement training after neurologic injury. J. Neuroeng. Rehabil 6, 20 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banala SK, Kim SH, Agrawal SK, Scholz JP, Robot assisted gait training with active leg exoskeleton (ALEX). IEEE Trans. Neural Syst. Rehabil. Eng 17, 2–8 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Bastian AJ, Understanding sensorimotor adaptation and learning for rehabilitation. Curr. Opin. Neurol 21, 628–633 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lerner ZF, Damiano DL, Park HS, Gravunder AJ, Bulea TC, A robotic exoskeleton for treatment of crouch gait in children with cerebral palsy: Design and initial application. IEEE Trans. Neural Syst. Rehabil. Eng 25, 650–659 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carlson WE, Vaughan CL, Damiano DL, Abel MF, Orthotic management of gait in spastic diplegia. Am. J. Phys. Med. Rehabil 76, 219–225 (1997). [DOI] [PubMed] [Google Scholar]
- 29.Riener R, Lunenburger L, Jezernik S, Anderschitz M, Colombo G, Dietz V, Patient-cooperative strategies for robot-aided treadmill training: First experimental results. IEEE Trans. Neural Syst. Rehabil. Eng 13, 380–394 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Gordon KE, Ferris DP, Learning to walk with a robotic ankle exoskeleton. J. Biomech 40, 2636–2644 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Lenzi T, Carrozza MC, Agrawal SK, Powered hip exoskeletons can reduce the user’s hip and ankle muscle activations during walking. IEEE Trans. Neural Syst. Rehabil. Eng 21, 938–948 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Leonard CT, Moritani T, Hirschfeld H, Forssberg H, Deficits in reciprocal inhibition of children with cerebral palsy as revealed by H reflex testing. Dev. Med. Child Neurol 32, 974–984 (1990). [DOI] [PubMed] [Google Scholar]
- 33.Myklebust BM, Gottlieb GL, Penn RD, Agarwal GC, Reciprocal excitation of antagonistic muscles as a differentiating feature in spasticity. Ann. Neurol 12, 367–374 (1982). [DOI] [PubMed] [Google Scholar]
- 34.Dietz V, Sinkjaer T, Spastic movement disorder: Impaired reflex function and altered muscle mechanics. Lancet Neurol. 6, 725–733 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Aagaard P, Simonsen EB, Andersen JL, Magnusson SP, Bojsen-Moller F, Dyhre-Poulsen P, Antagonist muscle coactivation during isokinetic knee extension. Scand. J. Med. Sci. Sports 10, 58–67 (2000). [DOI] [PubMed] [Google Scholar]
- 36.van Roon D, Steenbergen B, Meulenbroek RG , Trunk use and co-contraction in cerebral palsy as regulatory mechanisms for accuracy control. Neuropsychologia 43, 497–508 (2005). [DOI] [PubMed] [Google Scholar]
- 37.Pandyan AD, Johnson GR, Price CI, Curless RH, Barnes MP, Rodgers H, A review of the properties and limitations of the Ashworth and modified Ashworth Scales as measures of spasticity. Clin. Rehabil 13, 373–383 (1999). [DOI] [PubMed] [Google Scholar]
- 38.Lerner ZF, Damiano DL, Bulea TC, Estimating the mechanical behavior of the knee joint during crouch gait: Implications for real-time motor control of robotic knee orthoses. IEEE Trans. Neural Syst. Rehabil. Eng 24, 621–629 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torres-Oviedo G, Vasudevan E, Malone L, Bastian AJ, Locomotor adaptation. Prog. Brain Res 191, 65–74 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malone LA, Bastian AJ, Spatial and temporal asymmetries in gait predict split-belt adaptation behavior in stroke. Neurorehabil. Neural Repair 28, 230–240 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Damiano D, Stanley CJ, Bulea TC, Park HS, Motor learning abilities are similar in hemiplegic cerebral palsy compared to controls as assessed by adaptation to unilateral leg-weighting during gait: Part 1. Front. Hum. Neurosci 11, 49 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reisman DS, McLean H, Keller J, Danks KA, Bastian AJ, Repeated split-belt treadmill training improves poststroke step length asymmetry. Neurorehabil. Neural Repair 27, 460–468 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bar-Haim S, Harries N, Nammourah I, Oraibi S, Malhees W, Loeppky J, Perkins NJ, Belokopytov M, Kaplanski J, Lahat E, Effectiveness of motor learning coaching in children with cerebral palsy: A randomized controlled trial. Clin. Rehabil 24, 1009–1020 (2010). [DOI] [PubMed] [Google Scholar]
- 44.Kluzik J, Diedrichsen J, Shadmehr R, Bastian AJ, Reach adaptation: What determines whether we learn an internal model of the tool or adapt the model of our arm? J. Neurophysiol 100, 1455–1464 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waters RL, Mulroy S, The energy expenditure of normal and pathologic gait. Gait Posture 9, 207–231 (1999). [DOI] [PubMed] [Google Scholar]
- 46.Cooper RA, Quatrano LA, Axelson PW, Harlan W, Stineman M, Franklin B, Krause JS, Bach J, Chambers H, Chao EY, Alexander M, Painter P, Research on physical activity and health among people with disabilities: A consensus statement. J. Rehabil. Res. Dev 36, 142–154 (1999). [PubMed] [Google Scholar]
- 47.Bulea TC, Stanley CJ, Damiano DL, Part 2: Adaptation of gait kinematics in unilateral cerebral palsy demonstrates preserved independent neural control of each limb. Front. Hum. Neurosci 11, 50 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi JT, Bastian AJ, Adaptation reveals independent control networks for human walking. Nat. Neurosci 10, 1055–1062 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Damiano DL, Quinlivan J, Owen BF, Shaffrey M, Abel MF, Spasticity versus strength in cerebral palsy: Relationships among involuntary resistance, voluntary torque, and motor function. Eur. J. Neurol 8 (suppl. 5), 40–49 (2001). [DOI] [PubMed] [Google Scholar]
- 50.Steele KM, Damiano DL, Eek MN, Unger M, Delp SL, Characteristics associated with improved knee extension after strength training for individuals with cerebral palsy and crouch gait. J. Pediatr. Rehabil. Med 5, 99–106 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Damiano DL, Meaningfulness of mean group results for determining the optimal motor rehabilitation program for an individual child with cerebral palsy. Dev. Med. Child Neurol 56, 1141–1146 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ross SA, Engsberg JR, Relationships between spasticity, strength, gait, and the GMFM-66 in persons with spastic diplegia cerebral palsy. Arch. Phys. Med. Rehabil 88, 1114–1120 (2007). [DOI] [PubMed] [Google Scholar]
- 53.Hanna SE, Rosenbaum PL, Bartlett DJ, Palisano RJ, Walter SD, Avery L, Russell DJ, Stability and decline in gross motor function among children and youth with cerebral palsy aged 2 to 21 years. Dev. Med. Child Neurol 51, 295–302 (2009). [DOI] [PubMed] [Google Scholar]
- 54.Palisano RJ, Rosenbaum P, Bartlett D, Livingston MH, Content validity of the expanded and revised Gross Motor Function Classification System. Dev. Med. Child Neurol 50, 744–750 (2008). [DOI] [PubMed] [Google Scholar]
- 55.Lerner ZF, Board WJ, Browning RC, Pediatric obesity and walking duration increase medial tibiofemoral compartment contact forces. J. Orthop. Res 34, 97–105 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Effect of exoskeleton assistance mode on crouch by individual.
Fig. S2. Biomechanical effects of walking with the null exoskeleton.
Fig. S3. Effect of exoskeleton assistance on gait parameters.
Fig. S4. Individual differences in crouch between first and last assessments.
Fig. S5. Group-level change in biomechanical measures between first and last assessments during walking with the null exoskeleton.
Table S1. Knee torque measurement by exoskeleton condition and muscle strength.
Table S2. Individual subject-level data.