Abstract
Purpose.
To investigate the effects of smoking on optic nerve head capillary density measured by optical coherence tomography angiography (OCTA) in patients with open angle glaucoma (OAG).
Methods.
In this retrospective cross-sectional study, perimetric and preperimetric glaucoma patients enrolled in the Diagnostic Innovations in Glaucoma Study (DIGS) with OCTA follow up were included. Univariable and multivariable linear mixed analysis were performed to determine the effects of different variables on the superficial whole image capillary density (wiCD).
Results.
A total of 432 eyes of 271 glaucoma patients comprising 63 preperimetric (106 eyes) and 208 perimetric OAG (326 eyes) were included. A history of tobacco consumption was reported in 105 (38.8%). Among smokers, mean (95% CI) smoking intensity was 12.8 (10.2, 15.5) pack-years. After adjusting for age, glaucoma severity and other confounders, each 10 pack-year increase in smoking intensity (95% CI) was associated with −0.54 (−1.06, −0.02) % lower wiCD (P=0.041).
Conclusions.
Smoking intensity is associated with reduced optic nerve vessel density in glaucoma.
Keywords: glaucoma, optical coherence tomography, smoking
Introduction
Cigarette smoking has been regarded as a risk factor for the incidence of a wide variety of chronic illness, including cardiovascular disease,1 respiratory disorders,2 and different types of carcinomas.3 As a highly modifiable environmental factor, smoking has been associated with a broad range of ocular disorders, including age-related macular degeneration (AMD), ischemic optic neuropathy, retinal vein occlusion. The relationship between smoking and glaucoma has been conflicting. It is not as well characterized as that between smoking and other ocular diseases such as cataract and AMD.4–9
Glaucoma is characterized by loss of retinal ganglion cells (RGC) which results in progressive functional loss with loss of visual field (VF). The onset and progression of glaucoma has been attributed to both vascular and mechanical factors.10,11 Identifying evidence-based risk factors can influence the estimation of risk of developing glaucoma, as well as its possible progression.12,13 In this regard, the association between social history (i.e., smoking, alcohol, and body mass index (BMI)) and glaucoma has been widely investigated.4,6–9,14–17 Decreased ocular perfusion pressure has been associated with an increase in glaucoma prevalence, incidence, and worsening.18–21 This suggests that microvascular abnormalities may contribute to the pathophysiology of glaucoma.18–21 As the relationship between smoking and the risk of peripheral vascular disease has also been documented, it is possible that reduced optic disc blood flow in smokers contributes to further retinal nerve fiber injury in glaucoma eyes and susceptibility to progression.
In addition to reduced optic disc and macular blood flow, high oxidative stress with the production of free radicals also may be associated with glaucoma, and smoking has been reported to increase free radical generation.22,23 Free radicals have been reported to damage both trabecular meshwork cells (TMC) and RGCs damage.22 Smoking has been suggested to influence the microcirculation with endothelial-dependent vasorelaxation by abnormal nitric oxide activity, platelet aggregation, and endothelial cell dysfunction. These changes can reduce vascular blood flow in vivo and vitro24 and therefore, may worsen glaucoma. Consequently, smoking may have a role in developing open angle glaucoma (OAG) and its progression.
Optical coherence tomography angiography (OCTA) is a non-invasive technique of imaging optic nerve head (ONH) and retinal microvasculature.25,26 In an earlier study, a decrease in choriocapillaris blood flow was shown after use of cigarettes in healthy controls.27 The effect of smoking on optic nerve head vasculature in glaucoma is still unknown. In the present study, we evaluated the effect of smoking on ONH microvasculature using OCTA in patients with OAG.
Methods
Participants
This is a cross-sectional study of perimetric and preperimetric OAG patients enrolled in the Diagnostic Innovations in Glaucoma Study (DIGS)28 who underwent OCTA (Angiovue; Optovue Inc., Fremont, CA) imaging. This prospectively designed study received the Human Subject Committee approval of the University of California, San Diego (NCT00221897) and the methodology adhered to the tenets of the Declaration of Helsinki for research involving human subjects and to the Health Insurance Portability and Accountability Act. Details of the DIGS protocol and eligibility criteria have been described previously.28,29 Briefly, all participants underwent an annual comprehensive ophthalmologic examination and the semi-annual examination included intraocular pressure (IOP), spectral-domain OCT imaging, and VF testing by standard automated perimetry (Humphrey Field Analyzer; Carl Zeiss Meditec, Dublin, CA), and OCTA.
Self-reported history of alcohol and tobacco consumption and BMI were also collected. participants were asked if they have ever smoked. If positive, the smoking intensity was calculated as the pack-year index. Current smokers were not included in this study. The most recent good quality OCTA image was selected for each patient. Preperimetric glaucoma included eyes with elevated IOP (≥22mmHg) or glaucomatous-appearing optic discs (glaucomatous optic neuropathy) without the presence of repeatable glaucomatous VF damage. Eyes were classified as perimetric glaucoma if they had repeatable (at least 2 consecutive) abnormal VF test results with evidence of glaucomatous optic neuropathy. Glaucomatous optic neuropathy was defined as excavation, the presence of focal thinning, notching of neuroretinal rim, or localized or diffuse atrophy of the RNFL based on masked grading of optic disc photographs by 2 graders or clinical examination by a glaucoma specialist. An abnormal VF test was defined as a pattern standard deviation outside of the 95% normal confidence limits or a Glaucoma Hemifield Test result outside normal limits. Glaucoma disease severity was classified as early (24–2 VF mean deviation (MD) >−6 dB), and moderate to advanced (24–2 VF MD ≤−6 dB).
Inclusion criteria included (1) older than 18 years of age, (2) open angles on gonioscopy, and (3) best-corrected visual acuity of 20/40 or better at baseline visit. Exclusion criteria included (1) history of trauma or intraocular surgery (except for uncomplicated cataract surgery or glaucoma surgery), (2) coexisting retinal disease, (3) uveitis, (4) non-glaucomatous optic neuropathy, or (5) axial length of 27 mm or greater. Participants with the diagnosis of systemic diseases such as Parkinson’s disease, Alzheimer’s disease, dementia, or a history of stroke were excluded.
Optical Coherence Tomography Angiography
ONH microvasculature was evaluated using the AngioVue OCT system (software version 2018.1.1.63). This system has been described previously.30 Vessel density was automatically calculated as the proportion of the measured area occupied by flowing blood, which is defined as pixels having decorrelation values – acquired by the split-spectrum amplitude-decorrelation angiography algorithm – above the threshold level. Whole image capillary density (wiCD) from 4.5 × 4.5-mm2 scans (304 B-scans x 304 A-scans per B-scan) centered on the ONH were obtained with this system. Image quality review was completed on all scans according to the University of California, San Diego, Imaging Data Evaluation and Analysis Reading Center standard protocol. Trained graders reviewed scans and excluded poor-quality images, defined as images with (1) a scan quality less than 4, (2) poor clarity, (3) residual motion artifacts visible as irregular vessel pattern or disc boundary on the en face angiogram, (4) local weak signal, or (5) severe segmentation failures. Modifiable segmentation failure was adjusted manually as needed.
Statistical analysis
Patient and eye characteristics data were presented as mean (95% confidence interval (CI)) for continuous variables and count (%) for categorical variables. Categorical variables were compared using the chi-square test. Linear Mixed-effects modeling was used to compare ocular parameters among groups. Measurements of bilateral eyes were nested within participant to take account for within- participant associations. Univariable and multivariable linear mixed analysis were performed to determine the factors associated with wiCD. In addition to each social history parameters, the effect of potential predictors – age, IOP, and any other variable in which the P value was <0.10 in univariable analysis – on wiCD were also introduced in the multivariable model. Statistical analyses were performed using Stata version 16.0 (StataCorp, College Station, TX). P values of less than 0.05 were considered statistically significant for all analyses.
Results
A total of 432 eyes of 271 glaucoma patients, including 63 preperimetric (106 eyes), and 208 perimetric OAG (326 eyes) patients were included in the analysis. Mean age (95% CI) was 73.0 (71.7, 74.3) years. Mean 24–2 VF MD (95% CI) was −4.9 (−5.4, −4.4) dB, while mean wiCD (95% CI) was 40.5 (40.0, 41.0) %. One hundred five (38.8%) patients had reported prior tobacco consumption. Among smokers, mean (95% CI) smoking intensity was 12.8 (10.2, 15.5) pack-years. Current alcohol consumption was reported in 132 (48.7%), and mean (95% CI) BMI was 26.9 (26.2, 27.6) kg/m2. Demographics and baseline clinical characteristics of the subjects are presented in Table 1.
Table 1.
Demographics and Baseline Clinical Characteristics of the Subjects
| Characteristic | n=432 eyes of 271 patients |
|---|---|
| Age (years) | 73.0 (71.7, 74.3) |
| Sex (Female/ Male) | 139/132 |
| Race (African American/ Non-African American) |
59/212 |
| Ever reported tobacco consumption, n (%) | 105 (38.8%) |
| >0–10 pack-years | 59 (21.8%) |
| >10 −20 pack-years | 19 (7.0 %) |
| 20+ pack-years | 27 (10.0%) |
| Smoking intensity among ever smokers, pack-year | 12.8 (10.2, 15.5) |
| Current alcohol consumption, n (%) | 132 (48.7%) |
| BMI (kg/m2) | 26.9 (26.2, 27.6) |
| Time between OCTA and smoking history (years) | 3.1 (2.9, 3.3) |
| Self-reported hypertension, n (%) | 162 (59.8%) |
| Self-reported diabetes, n (%) | 49 (18.1%) |
| Diagnosis Perimetric Glaucoma/ Preperimetric Eyes (n) |
326/106 |
| Disease Severity by baseline VF MD | |
| Early glaucoma, Eye No. (%) | 203 (62.3%) |
| Moderate and advanced glaucoma, Eye No. (%) | 123 (37.7%) |
| Axial length (mm) | 24.3 (24.2, 24.5) |
| CCT (μm) | 538.5 (534.5, 542.6) |
| IOP (mmHg) | 14.5 (14.1, 15.0) |
| 24–2 VF MD (dB) | −4.9 (−5.4, −4.4) |
| Average wiCD (%) | 40.5 (40.0, 41.0) |
| Average SSI | 60.4 (59.5, 61.2) |
BMI = body mass index; CCT = central corneal thickness; IOP = intraocular pressure; MD = mean deviation; SSI = signal strength index; VF = visual field; wiCD = whole image capillary density. Values are shown in mean (95% confidence interval), unless otherwise indicated.
Table 2 summarizes the characteristics of eyes categorized by smoking history. The smoking group was characterized by older age, and a higher proportion of self-reported hypertension (P<0.001 and P=0.019, respectively). No significant differences were seen in sex, race, presence of diabetes mellitus, axial length, central corneal thickness (CCT), IOP, diagnosis, distribution of disease severity based on 24–2 VF MD, average wiCD, and signal strength index (SSI) (P>0.05 for all).
Table 2.
Characteristics of Eyes Categorized by Smoking History
| Smoking history (+) | Smoking history (−) | P value | |
|---|---|---|---|
|
| |||
| Characteristic | n=162 eyes of 105 patients | n=270 eyes of 166 patients | |
| Age (years) | 75.9 (74.2, 77.5) | 71.2 (69.4, 73.0) | <0.001 |
| Sex (Female/ Male) | 48/57 | 91/75 | 0.144 |
| Race (African American/ Non-African American) |
21/84 | 38/128 | 0.574 |
| Self-reported hypertension, n (%) | 72 (68.6%) | 90 (54.2%) | 0.019 |
| Self-reported diabetes, n (%) | 21 (20.0%) | 28 (16.9%) | 0.514 |
| Diagnosis Perimetric Glaucoma/ Preperimetric Eyes (n) |
121/41 | 203/67 | 0.909 |
| Disease Severity by baseline VF MD | 0.750 | ||
| Early glaucoma, Eye No. (%) | 74 (61.2%) | 129 (62.9%) | |
| Moderate and advanced glaucoma, Eye No. (%) | 47 (38.8%) | 76 (37.1%) | |
| Axial length (mm) | 24.3 (24.1, 24.4) | 24.4 (24.2, 24.5) | 0.367 |
| CCT (μm) | 538.8 (532.3, 545.2) | 538.4 (533.1, 543.7) | 0.947 |
| IOP (mmHg) | 14.5 (13.9, 15.2) | 14.5 (13.9, 15.1) | 0.915 |
| 24–2 VF MD (dB) | −4.9 (−5.8, −3.9) | −4.9 (−5.6, −4.3) | 0.886 |
| Average wiCD (%) | 40.8 (39.9, 41.6) | 40.4 (39.8, 41.0) | 0.540 |
| Average SSI | 59.6 (58.2, 61.0) | 60.8 (59.8, 61.9) | 0.232 |
BMI = body mass index; CCT = central corneal thickness; IOP = intraocular pressure; MD = mean deviation; SSI = signal strength index; VF = visual field; wiCD = whole image capillary density. Values are shown in mean (95% confidence interval), unless otherwise indicated. Bold text indicates a statistically significant difference with a p-value less than 0.05.
Table 3 summarizes the characteristics of eyes by race. There was a higher BMI, higher proportion of self-reported hypertension and diabetes in the African descent group (P<0.05 for all), whereas the proportion of ever reported tobacco consumption, smoking intensity, and the proportion of current alcohol consumption did not show significant differences in two groups. Average wiCD was significantly higher in African descent group compared to Non-African descent group (43.1 (42.1, 44.1) vs 39.8 (39.2, 40.3), respectively P<0.001). The non-African descent group had a higher proportion of eyes with advanced glaucoma, while no significant differences were seen in overall study population 24–2 VF MD (P=0.295).
Table 3.
Characteristics of Eyes Categorized by Race
| African descent | Non-African descent | P value | |
|---|---|---|---|
|
| |||
| Characteristic | n=100 eyes of 59 patients | n=332 eyes of 212 patients | |
| Age (years) | 73.1 (70.5, 75.7) | 73.0 (71.5, 74.5) | 0.927 |
| Sex (Female/ Male) | 33/26 | 106/106 | 0.420 |
| Ever reported tobacco consumption, n (%) | 21 (35.6%) | 84 (39.6%) | 0.574 |
| Smoking intensity, pack-year | 11.7 (5.6, 17.8) | 13.1 (10.2, 16.1) | 0.663 |
| Current alcohol consumption, n (%) | 26 (44.1%) | 106 (50.0%) | 0.420 |
| BMI (kg/m2) | 30.5 (29.0, 32.0) | 25.9 (25.2, 26.6) | <0.001 |
| Self-reported hypertension, n (%) | 49 (83.1%) | 113 (53.3%) | <0.001 |
| Self-reported diabetes, n (%) | 24 (40.7%) | 25 (11.8%) | <0.001 |
| Diagnosis Perimetric Glaucoma/ Preperimetric Eyes (n) |
75/25 | 251/81 | 0.902 |
| Disease Severity by baseline VF MD | 0.012 | ||
| Early glaucoma, Eye No. (%) | 56 (74.7%) | 147 (58.6%) | |
| Moderate and advanced glaucoma, Eye No. (%) | 19 (25.3%) | 104 (41.4%) | |
| Axial length (mm) | 24.1 (23.8, 24.3) | 24.4 (24.3, 24.6) | 0.089 |
| CCT (μm) | 536.7 (529.2, 544.2) | 539.1 (534.3, 543.9) | 0.680 |
| IOP (mmHg) | 15.3 (14.4, 16.2) | 14.3 (13.8, 14.8) | 0.100 |
| 24–2 VF MD (dB) | −4.4 (−5.4, −3.4) | −5.1 (−5.7, −4.5) | 0.295 |
| Average wiCD (%) | 43.1 (42.1, 44.1) | 39.8 (39.2, 40.3) | <0.001 |
| Average SSI | 62.5 (60.8, 64.3) | 59.7 (58.7, 60.6) | 0.019 |
BMI = body mass index; CCT = central corneal thickness; IOP = intraocular pressure; MD = mean deviation; SSI = signal strength index; VF = visual field; wiCD = whole image capillary density. Values are shown in mean (95% confidence interval), unless otherwise indicated. Bold text indicates a statistically significant difference with a p-value less than 0.05.
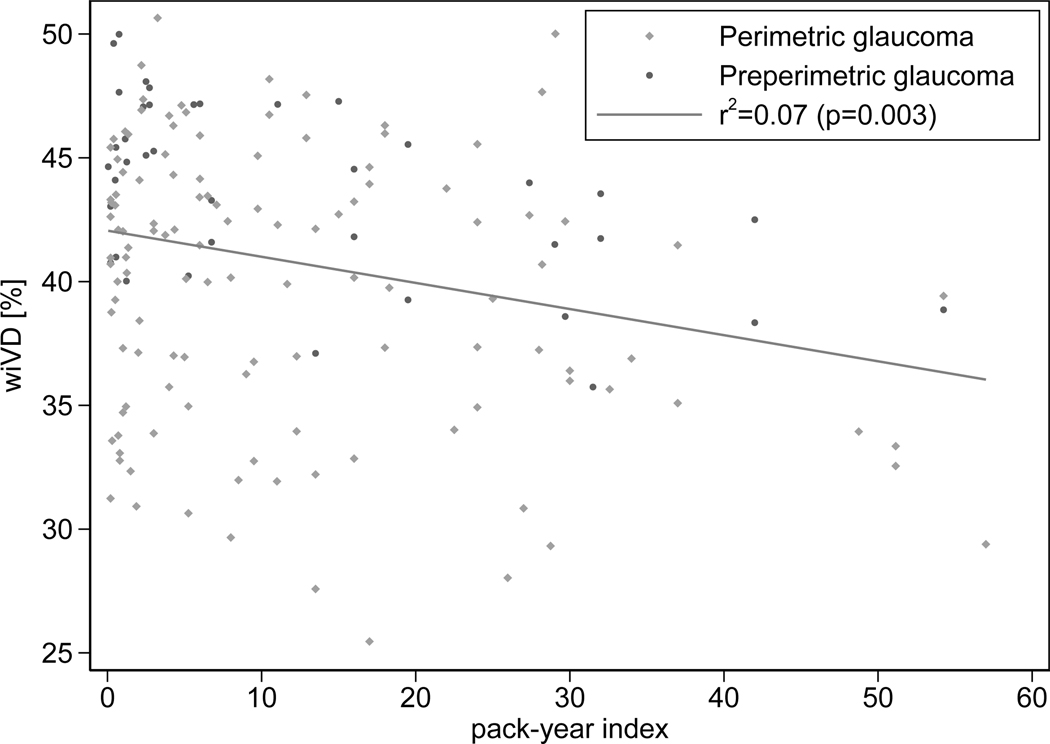
Table 4 summarize the effect of social history on wiCD in univariable and multivariable method, respectively. In univariable models, higher smoking intensity and BMI were significantly associated with wiCD (coefficient (95% CI) −1.09 (−1.81, −0.36)- % per 10 pack-year higher; P=0.003 (r2=0.07) (Figure 1), and 0.12 (0.01, 0.23) % per 1 kg/m2 higher BMI; P=0.031, respectively), while past smoking history, duration of time after quitting smoking, and current alcohol consumption were not (P>0.10 for all). In multivariable model, smoking history, current alcohol consumption, and BMI were not significantly associated with wiCD (P>0.05 for all), while smoking intensity remained significantly associated with wiCD (coefficient (95% CI) −0.54 (−1.06, −0.02) % per 10 pack-year higher; P=0.041). Factors including older age, being non-African American, worse 24–2 VF MD, and lower SSI were also found to be associated with lower wiCD (P<0.05 for all).
Table 4.
Factors Correlated with wiCD by Univariable and Multivariable Linear Mixed Analysis
| Variables | Univariable Model | Multivariable Model | ||
|---|---|---|---|---|
|
| ||||
| coefficient, 95% CI | P value | coefficient, 95% CI | P value | |
|
| ||||
| Smoking history, yes | 0.04 (−1.18, 1.27) | 0.944 | ||
| Smoking intensity, per 10 pack-year higher | −1.09 (−1.81, −0.36) | 0.003 | −0.54 (−1.06, −0.02) | 0.041 |
| Duration of time after quitting smoking, years | 0.00 (−0.03, 0.03) | 0.966 | ||
| Current alcohol consumption, yes | −0.10 (−1.46, 1.27) | 0.887 | −0.01 (−1.09, 1.08) | 0.991 |
| BMI, per 1 kg/m2 higher | 0.12 (0.01, 0.23) | 0.031 | 0.02 (−0.08, 0.13) | 0.654 |
| Age (year) per 10 years | −0.93 (−1.48, −0.39) | 0.001 | −0.85 (−1.37, −0.32) | 0.002 |
| Sex: female | 2.68 (1.53, 3.83) | <0.001 | 1.05 (−0.02, 2.12) | 0.055 |
| Race: African American | 3.33 (1.96, 4.70) | <0.001 | 2.42 (1.11, 3.73) | <0.001 |
| Self-reported hypertension | −0.15 (−1.37, 1.06) | 0.807 | ||
| Self-reported diabetes | 1.78 (0.25, 3.31) | 0.022 | 1.12 (−0.28, 2.51) | 0.117 |
| Axial length, per 1mm longer | −0.44 (−0.88, 0.00) | 0.050 | −0.40 (−0.83, 0.03) | 0.071 |
| CCT, per 100 μm thinner | −0.76 (−2.08, 0.56) | 0.261 | ||
| IOP, per 1 mmHg higher | 0.09 (−0.02, 0.20) | 0.108 | −0.06 (−0.16, 0.04) | 0.251 |
| 24–2 VF MD, per 1 dB worse | −0.56 (−0.63, −0.48) | <0.001 | −0.54 (−0.63, −0.46) | <0.001 |
| Average SSI, per 1 higher | 0.17 (0.11, 0.22) | <0.001 | 0.05 (0.00, 0.10) | 0.039 |
BMI = body mass index; CCT = central corneal thickness; IOP = intraocular pressure; MD = mean deviation; SSI = signal strength index; VF = visual field; wiCD = whole image capillary density. Values are shown in mean (95% confidence interval), unless otherwise indicated. Bold text indicates p-value with <0.05.
Figure 1.
Scatterplot illustrating the association between wiCD and smoking severity
Discussion
After adjusting for confounding factors, wiCD was significantly associated with smoking intensity rather than history of smoking. Each 10 pack-year increase in smoking intensity was associated with 0.5% lower wiCD. Our findings highlight the possible role of microvasculature changes on the pathogenesis of glaucoma and tobacco-related glaucoma progression. These results suggest that evaluation of smoking intensity adds essential information to the assessment of the risk of glaucoma progression.
In contrast to the already known risk factors for OAG, such as advanced age, a family history of glaucoma and African ethnicity, tobacco smoking is a modifiable risk factor. There is controversy regarding existing data on the association between tobacco smoking and glaucoma. 31–39 One meta-analysis and one systematic review concluded that heavy smoking may increase the risk of OAG (odds ratio [OR]=1.37, 95% CI: 1.00–1.87), while past smoking history did not appear to affect that risk (OR=1.03, 95% CI: 0.77–1.38). 40 Likewise, a systematic review concluded that the evidence for a link between current smoking and OAG appeared stronger than that of past smoking. Recent studies have suggested that heavy smoking may increase the risk of OAG. 41 However, they also have suggested that further studies are needed to investigate the possible positive association between heavy smoking and OAG by stratifying participants by pack years and age. 41 In contrast, another meta-analysis found that both current smokers and former smokers were not significantly associated with the risk of development of OAG. 42 The discrepancies among studies on the association between smoking and glaucoma may be partly due to the complexity of the relationship.
We recently showed that higher smoking intensity was associated with faster VF loss in glaucoma patients.43 It is noteworthy that most studies did not consider the number of pack-years smoked. In NHANES and Sun studies, the association between smoking and glaucoma lost significance in adjusted analysis suggesting that the associations were due to confounding factors.15,44 However, among smokers, greater pack per day of smoking history was associated with higher odds of developing glaucoma after adjustment for confounders. 15,44 Likewise, in our study, the history of smoking was not associated with wiCD changes after adjustment for confounding factors. However, the intensity of smoking remained significant after adjusting the confounding factors in multivariate analysis.
It is known that cigarette smoking contributes to vascular disease by occluding arterial lumina with atherosclerotic plaques and intimal thickening.45 Cigarette smoking directly causes alterations in choroicapillaries blood flow 42,46–49 Moreover, it may cause vasoconstriction of the episcleral veins.50 Cigarette smoking also has been associated with low blood velocity of the ophthalmic artery.51,52 In contrast, Robinson et al. 53 showed a significant increase in macular leukocyte velocity and blood flow with smoking. Similarly, increased blood velocity in visible surface vessels of the optic nerve head and choroid-retina were confirmed in habitual smokers by using laser speckle technology.54 It was also shown that smoking could also result in reduced choroidal thickness that could be associated with decreased blood flow to the choroid following smoking.55–57
Several studies evaluated the immediate effect of smoking by OCTA on the optic nerve and macular perfusion among smokers and found controversial effects.58–63 Aayhan et al.59 did not find any change in macular vessel density or FAZ area one hour after smoking. However, they showed that smoking decreases the blood flow index of the choriocapillary area due to the acute effects of nicotine and harmful substances in cigarettes on the peripheral vascular structure.59 Others found no immediate influence of smoking on vessel density parameters in healthy habitual smokers.60,64
We found that higher smoking intensity was associated with lower ONH vessel density. These effects may be related at least in part to microinfarction and capillary non-perfusion, resulting in impairment and decreased blood flow in capillaries. In addition, cigarette smoking also was associated with reduced retinal microcirculation in the macula, especially in the deep and superficial fovea and parafoveal area through decreased macular fovea thickness, decreased deep and superficial foveal and parafoveal vessel density, and elevated circumference and area of the macular foveal avascular zone (FAZ) in previous macular OCTA studies.65–70 The complex anatomical structure, the proximity to the increased metabolic need of the outer retina, and the distance from the larger arterioles may make the deep capillary plexus more prone to be damaged by the effect of smoking.71 Çiloglu et al.66 showed that FAZ area, superficial foveal vessel density (VD), and deep foveal VD are decreased in smokers with more than 10 pack-years compared to those who smoked less than 10 pack- years.66 Dogan et al.68 showed that the early impact of smoking could be seen on microvascular density even by low-pack year smoking exposure (average of 3 packs-year smoking), suggesting that smoking may effect the local ischemic microenvironment. Another study also showed that the number of daily cigarettes and pack-years of smoking were inversely related to macular thickness, while they were positively associated with the circumference and the area of FAZ.65 The chronic and acute effects of smoking on the vascular system, organs and tissue is complex. Moreover, the above-mentioned effects of smoking might be more detrimental in susceptible glaucoma patients who have other risk factors. Further studies are needed to compare the effects of smoking on healthy individuals compared with glaucoma patients.
This study has several limitations. First, there is some evidence that topical glaucoma medications may influence ocular blood flow.72,73 Therefore, we cannot exclude the possibility that the use of topical eye drops accounts for the observed vascular differences between the study groups. Similarly, it is unclear whether systemic medications had an effect on macular vascular changes.72 Second, our study did not consider the effect of passive smoking on glaucoma. OAG patients with non-smoking history might have been passive smokers. Likewise, active smokers are also more likely to be passive smokers so that we may be overestimating the coefficient on structural changes. Last, vascular density varies widely in individuals, and a lower vascular density does not necessarily indicate more advanced stage of glaucoma. However, this does not undermine the value of our findings that smoking intensity was associated with lower vessel density, and it is of interest to investigate the longitudinal vessel density change and smoking status. Further longitudinal studies are needed to clarify the relationship between smoking intensity and glaucoma progression.
In conclusion, higher intensity of smoking was significantly associated with decreased ONH microvasculature. This supports the value of obtaining an accurate history of smoking and, particularly, the intensity as it may help in screening and monitoring glaucoma to stratify the patients with OAG.
Footnotes
Précis: Decreased superficial whole image capillary density was observed in OAG patients with high smoking intensity.
References
- 1.Nance R, Delaney J, McEvoy JW, et al. Smoking intensity (pack/day) is a better measure than pack-years or smoking status for modeling cardiovascular disease outcomes. Journal of Clinical Epidemiology. Jan 2017;81:111–119. doi: 10.1016/j.jclinepi.2016.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hedley AJ, Lam TH, McGhee SM, et al. Passive smoking - Secondhand smoke does cause respiratory disease. Brit Med J. Aug 30 2003;327(7413):502–502. doi:DOI 10.1136/bmj.327.7413.502-a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joseph AM, Rothman AJ, Almirall D, et al. Lung Cancer Screening and Smoking Cessation Clinical Trials SCALE (Smoking Cessation within the Context of Lung Cancer Screening) Collaboration. American Journal of Respiratory and Critical Care Medicine. Jan 15 2018;197(2):172–182. doi: 10.1164/rccm.201705-0909CI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang JH, Pasquale LR, Rosner BA, et al. Prospective study of cigarette smoking and the risk of primary open-angle glaucoma. Archives of ophthalmology. 2003;121(12):1762–1768. [DOI] [PubMed] [Google Scholar]
- 5.Founti P, Bunce C, Khawaja AP, et al. Risk Factors for Visual Field Deterioration in the United Kingdom Glaucoma Treatment Study. Ophthalmology. 2020/12/01/ 2020;127(12):1642–1651. doi: 10.1016/j.ophtha.2020.06.009 [DOI] [PubMed] [Google Scholar]
- 6.Pérez-de-Arcelus M, Toledo E, Martínez-González MÁ, et al. Smoking and incidence of glaucoma: The SUN Cohort. Medicine (Baltimore). 2017;96(1):e5761–e5761. doi: 10.1097/MD.0000000000005761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahn HA, Milton RC. Alternative Definitions of Open-Angle Glaucoma: Effect on Prevalence and Associations in the Framingham Eye Study. Archives of Ophthalmology. 1980;98(12):2172–2177. doi: 10.1001/archopht.1980.01020041024003 [DOI] [PubMed] [Google Scholar]
- 8.Chiotoroiu SM, Pop de Popa D, Ştefăniu GI, et al. The importance of alcohol abuse and smoking in the evolution of glaucoma disease. J Med Life. 2013;6(2):226–229. [PMC free article] [PubMed] [Google Scholar]
- 9.Leske MC, Connell A, Wu S-Y, et al. Risk factors for open-angle glaucoma: the Barbados Eye Study. Archives of ophthalmology. 1995;113(7):918–924. [DOI] [PubMed] [Google Scholar]
- 10.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. May 14 2014;311(18):1901–11. doi: 10.1001/jama.2014.3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. May 22 2004;363(9422):1711–20. doi: 10.1016/S0140-6736(04)16257-0 [DOI] [PubMed] [Google Scholar]
- 12.Leske MC, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Archives of ophthalmology. 2003;121(1):48–56. [DOI] [PubMed] [Google Scholar]
- 13.Weinreb RN, Aung T, Medeiros FA. The Pathophysiology and Treatment of Glaucoma: A Review. JAMA. 2014;311(18):1901–1911. doi: 10.1001/jama.2014.3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Founti P, Bunce C, Khawaja AP, et al. Risk Factors for Visual Field Deterioration in the United Kingdom Glaucoma Treatment Study. Ophthalmology. Dec 2020;127(12):1642–1651. doi: 10.1016/j.ophtha.2020.06.009 [DOI] [PubMed] [Google Scholar]
- 15.Perez-de-Arcelus M, Toledo E, Martinez-Gonzalez MA, et al. Smoking and incidence of glaucoma: The SUN Cohort. Medicine (Baltimore). Jan 2017;96(1):e5761. doi: 10.1097/MD.0000000000005761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han YS, Kim YW, Kim YJ, et al. Alcohol consumption is associated with glaucoma severity regardless of ALDH2 polymorphism. Scientific Reports. 2020;10(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim H-A, Han K, Lee Y-A, et al. Differential association of metabolic risk factors with open angle glaucoma according to obesity in a Korean population. Scientific reports. 2016;6(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leske MC, Wu SY, Nemesure B, et al. Incident open-angle glaucoma and blood pressure. Arch Ophthalmol. Jul 2002;120(7):954–9. doi:eeb10014 [pii] [DOI] [PubMed] [Google Scholar]
- 19.Mansouri K, Leite MT, Weinreb RN. 24-hour ocular perfusion pressure in glaucoma patients. Br J Ophthalmol. Aug 2011;95(8):1175–6. doi:bjophthalmol-2011–300160 [pii] 10.1136/bjophthalmol-2011-300160 [DOI] [PubMed] [Google Scholar]
- 20.Tielsch JM, Katz J, Sommer A, et al. Hypertension, perfusion pressure, and primary open-angle glaucoma. A population-based assessment. Arch Ophthalmol. Feb 1995;113(2):216–21. [DOI] [PubMed] [Google Scholar]
- 21.Zeitz O, Galambos P, Wagenfeld L, et al. Glaucoma progression is associated with decreased blood flow velocities in the short posterior ciliary artery. Br J Ophthalmol. Oct 2006;90(10):1245–8. doi: 10.1136/bjo.2006.093633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zanon-Moreno V, Garcia-Medina JJ, Zanon-Viguer V, et al. Smoking, an additional risk factor in elder women with primary open-angle glaucoma. Mol Vis. Dec 31 2009;15:2953–9. [PMC free article] [PubMed] [Google Scholar]
- 23.Moghimi S, Zangwill LM, Penteado RC, et al. Macular and Optic Nerve Head Vessel Density and Progressive Retinal Nerve Fiber Layer Loss in Glaucoma. Ophthalmology. Nov 2018;125(11):1720–1728. doi: 10.1016/j.ophtha.2018.05.006 [DOI] [PubMed] [Google Scholar]
- 24.Barua RS, Ambrose JA, Eales-Reynolds L-J, et al. Dysfunctional endothelial nitric oxide biosynthesis in healthy smokers with impaired endothelium-dependent vasodilatation. Circulation. 2001;104(16):1905–1910. [DOI] [PubMed] [Google Scholar]
- 25.Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Optical Coherence Tomography Angiography Vessel Density in Healthy, Glaucoma Suspect, and Glaucoma Eyes. Invest Ophthalmol Vis Sci. Jul 1 2016;57(9):OCT451–9. doi: 10.1167/iovs.15-18944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishida T, Moghimi S, David RCC, et al. Rates of Circumpapillary Retinal Nerve Fiber Layer Thinning and Capillary Density Loss in Glaucomatous Eyes with Disc Hemorrhage. Am J Ophthalmol. Mar 2022;235:24–31. doi: 10.1016/j.ajo.2021.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ayhan Z, Kaya M, Ozturk T, et al. Evaluation of Macular Perfusion in Healthy Smokers by Using Optical Coherence Tomography Angiography. Ophthalmic Surg Lasers Imaging Retina. Aug 1 2017;48(8):617–622. doi: 10.3928/23258160-20170802-03 [DOI] [PubMed] [Google Scholar]
- 28.Sample PA, Girkin CA, Zangwill LM, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol. Sep 2009;127(9):1136–45. doi: 10.1001/archophthalmol.2009.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Girkin CA, Sample PA, Liebmann JM, et al. African Descent and Glaucoma Evaluation Study (ADAGES): II. Ancestry differences in optic disc, retinal nerve fiber layer, and macular structure in healthy subjects. Arch Ophthalmol. May 2010;128(5):541–50. doi: 10.1001/archophthalmol.2010.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu L, Jia Y, Takusagawa HL, et al. Optical Coherence Tomography Angiography of the Peripapillary Retina in Glaucoma. JAMA Ophthalmol. Sep 2015;133(9):1045–52. doi: 10.1001/jamaophthalmol.2015.2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doshi V, Ying-Lai M, Azen SP, et al. Sociodemographic, family history, and lifestyle risk factors for open-angle glaucoma and ocular hypertension. The Los Angeles Latino Eye Study. Ophthalmology. Apr 2008;115(4):639–647 e2. doi: 10.1016/j.ophtha.2007.05.032 [DOI] [PubMed] [Google Scholar]
- 32.Ramdas WD, Wolfs RC, Hofman A, et al. Lifestyle and risk of developing open-angle glaucoma: the Rotterdam study. Archives of ophthalmology. 2011;129(6):767–772. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X, Kahende J, Fan AZ, et al. Peer reviewed: smoking and visual impairment among older adults with age-related eye diseases. Preventing chronic disease. 2011;8(4) [PMC free article] [PubMed] [Google Scholar]
- 34.Topouzis F, Wilson MR, Harris A, et al. Risk factors for primary open-angle glaucoma and pseudoexfoliative glaucoma in the Thessaloniki eye study. American journal of ophthalmology. 2011;152(2):219–228. e1. [DOI] [PubMed] [Google Scholar]
- 35.Klein BE, Klein R, Ritter LL. Relationship of drinking alcohol and smoking to prevalence of open-angle glaucoma: the Beaver Dam Eye Study. Ophthalmology. 1993;100(11):1609–1613. [DOI] [PubMed] [Google Scholar]
- 36.Wise LA, Rosenberg L, Radin RG, et al. A prospective study of diabetes, lifestyle factors, and glaucoma among African-American women. Annals of epidemiology. 2011;21(6):430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Renard JP, Rouland JF, Bron A, et al. Nutritional, lifestyle and environmental factors in ocular hypertension and primary open‐angle glaucoma: an exploratory case–control study. Acta ophthalmologica. 2013;91(6):505–513. [DOI] [PubMed] [Google Scholar]
- 38.Pérez-de-Arcelus M, Toledo E, Martínez-González MÁ, et al. Smoking and incidence of glaucoma: The SUN Cohort. Medicine (Baltimore). 2017;96(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Law S, Lu X, Yu F, et al. Cigarette smoking and glaucoma in the United States population. Eye. 2018;32(4):716–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonovas S, Filioussi K, Tsantes A, et al. Epidemiological association between cigarette smoking and primary open-angle glaucoma: a meta-analysis. Public Health. Jun 2004;118(4):256–61. doi: 10.1016/j.puhe.2003.09.009 [DOI] [PubMed] [Google Scholar]
- 41.Jain V, Jain M, Abdull MM, et al. The association between cigarette smoking and primary open-angle glaucoma: a systematic review. Int Ophthalmol. Feb 2017;37(1):291–301. doi: 10.1007/s10792-016-0245-0 [DOI] [PubMed] [Google Scholar]
- 42.Zhou Y, Zhu W, Wang C. The effect of smoking on the risk of primary open-angle glaucoma: an updated meta-analysis of six observational studies. Public Health. Nov 2016;140:84–90. doi: 10.1016/j.puhe.2016.04.016 [DOI] [PubMed] [Google Scholar]
- 43.Mahmoudinezhad G, Nishida T, Weinreb RN, et al. Impact of Smoking on Visual Field Progression. Investigative Ophthalmology & Visual Science. 2022;63(7):2266–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Law SM, Lu X, Yu F, et al. Cigarette smoking and glaucoma in the United States population. Eye (Lond). Apr 2018;32(4):716–725. doi: 10.1038/eye.2017.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaiser HJ, Schoetzau A, Flammer J. Blood flow velocity in the extraocular vessels in chronic smokers. Br J Ophthalmol. Feb 1997;81(2):133–5. doi: 10.1136/bjo.81.2.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williamson TH, Lowe GD, Baxter GM. Influence of age, systemic blood pressure, smoking, and blood viscosity on orbital blood velocities. Br J Ophthalmol. Jan 1995;79(1):17–22. doi: 10.1136/bjo.79.1.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rojanapongpun P, Drance SM. The effects of nicotine on the blood flow of the ophthalmic artery and the finger circulation. Graefes Arch Clin Exp Ophthalmol. Jul 1993;231(7):371–4. doi: 10.1007/BF00919642 [DOI] [PubMed] [Google Scholar]
- 48.Cheng AC, Pang CP, Leung AT, et al. The association between cigarette smoking and ocular diseases. Hong Kong medical journal = Xianggang yi xue za zhi. Jun 2000;6(2):195–202. [PubMed] [Google Scholar]
- 49.Erb C, Heinke M. Oxidative stress in primary open-angle glaucoma. Front Biosci (Elite Ed). Jun 1 2011;3:1524–33. doi: 10.2741/e353 [DOI] [PubMed] [Google Scholar]
- 50.Solberg Y, Rosner M, Belkin M. The association between cigarette smoking and ocular diseases. Surv Ophthalmol. May-Jun 1998;42(6):535–47. doi: 10.1016/s0039-6257(98)00002-2 [DOI] [PubMed] [Google Scholar]
- 51.Wimpissinger B, Resch H, Berisha F, et al. Effects of isometric exercise on subfoveal choroidal blood flow in smokers and nonsmokers. Investigative ophthalmology & visual science. 2003;44(11):4859–4863. [DOI] [PubMed] [Google Scholar]
- 52.Rojanapongpun P, Drance SM. The effects of nicotine on the blood flow of the ophthalmic artery and the finger circulation. Graefe’s archive for clinical and experimental ophthalmology. 1993;231(7):371–374. [DOI] [PubMed] [Google Scholar]
- 53.Robinson F, Petrig B, Riva CE. The acute effect of cigarette smoking on macular capillary blood flow in humans. Investigative ophthalmology & visual science. 1985;26(5):609–613. [PubMed] [Google Scholar]
- 54.Tamaki Y, Araie M, Nagahara M, et al. Acute effects of cigarette smoking on tissue circulation in human optic nerve head and choroid-retina. Ophthalmology. 1999;106(3):564–569. [DOI] [PubMed] [Google Scholar]
- 55.Rose K, Flanagan JG, Patel SR, et al. Retinal Blood Flow and Vascular Reactivity in Chronic Smokers. Investigative Ophthalmology & Visual Science. 2014;55(7):4266–4276. doi: 10.1167/iovs.14-14022 [DOI] [PubMed] [Google Scholar]
- 56.Williamson TH, Lowe G, Baxter GM. Influence of age, systemic blood pressure, smoking, and blood viscosity on orbital blood velocities. British Journal of Ophthalmology. 1995;79(1):17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eriş E, Aydin E, Özçift SG. The effect of the smoking on choroidal thickness, central macular vascular and optic disc perfusion. Photodiagnosis and Photodynamic Therapy. 2019/12/01/ 2019;28:142–145. doi: 10.1016/j.pdpdt.2019.09.005 [DOI] [PubMed] [Google Scholar]
- 58.Ciesielski M, Rakowicz P, Stopa M. Immediate effects of smoking on optic nerve and macular perfusion measured by optical coherence tomography angiography. Scientific reports. 2019;9(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ayhan Z, Kaya M, Ozturk T, et al. Evaluation of macular perfusion in healthy smokers by using optical coherence tomography angiography. Ophthalmic Surgery, Lasers and Imaging Retina. 2017;48(8):617–622. [DOI] [PubMed] [Google Scholar]
- 60.Holló G.No acute effect of smoking on peripapillary and macular vessel density in healthy middle-aged smokers. Journal of glaucoma. 2019;28(5):e86–e88. [DOI] [PubMed] [Google Scholar]
- 61.Işik MU, Akay F, Akmaz B, et al. Evaluation of subclinical alterations in retinal layers and microvascular structures with OCT and OCTA in healthy young short-term smokers. Photodiagnosis and Photodynamic Therapy. 2021/12/01/ 2021;36:102482. doi: 10.1016/j.pdpdt.2021.102482 [DOI] [PubMed] [Google Scholar]
- 62.Kaymaz A, Ulaş F, Toprak G, et al. Evaluation of the acute effects of cigarette smoking on the eye of non-Smoking healthy young male subjects by optical coherence tomography angiography. Cutan Ocul Toxicol. Jun 2020;39(2):165–170. doi: 10.1080/15569527.2020.1753762 [DOI] [PubMed] [Google Scholar]
- 63.Cınar E, Yuce B, Zengin MO, et al. The Effect of Nicotine on Macular Microcirculation in Healthy Subjects. Ophthalmic Surgery, Lasers and Imaging Retina. 2019;50(11):691–700. doi:doi: 10.3928/23258160-20191031-04 [DOI] [PubMed] [Google Scholar]
- 64.Ciesielski M, Rakowicz P, Stopa M. Immediate effects of smoking on optic nerve and macular perfusion measured by optical coherence tomography angiography. Scientific Reports. 2019/07/15 2019;9(1):10161. doi: 10.1038/s41598-019-46746-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cui B, He K, Zhang X, et al. Association of cigarette smoking with retinal thickness and vascular structure in an elderly Chinese population. Photodiagnosis and Photodynamic Therapy. 2021/12/01/ 2021;36:102481. doi: 10.1016/j.pdpdt.2021.102481 [DOI] [PubMed] [Google Scholar]
- 66.Çiloğlu E, Unal F, Sukgen EA, et al. Evaluation of foveal avascular zone and capillary plexus in smokers using optical coherence tomography angiography. Journal of current ophthalmology. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aboud SA-A, Hammouda LM, Saif MYS, et al. Effect of smoking on the macula and optic nerve integrity using optical coherence tomography angiography. European Journal of Ophthalmology. 2021:1120672121992960. doi: 10.1177/1120672121992960 [DOI] [PubMed] [Google Scholar]
- 68.Dogan M, Akdogan M, Gulyesil FF, et al. Cigarette smoking reduces deep retinal vascular density. Clinical and Experimental Optometry. 2020;103(6):838–842. [DOI] [PubMed] [Google Scholar]
- 69.Steigerwalt RD Jr, Laurora G, Incandela L, et al. Ocular and orbital blood flow in cigarette smokers. Retina (Philadelphia, Pa). 2000;20(4):394–397. [DOI] [PubMed] [Google Scholar]
- 70.Lehr H-A. Microcirculatory dysfunction induced by cigarette smoking. Microcirculation. 2000;7(6):367–384. [PubMed] [Google Scholar]
- 71.Nakahara T, Hoshino M, Hoshino S-i, et al. Structural and functional changes in retinal vasculature induced by retinal ischemia-reperfusion in rats. Experimental eye research. 2015;135:134–145. [DOI] [PubMed] [Google Scholar]
- 72.Mayama C, Araie M. Effects of antiglaucoma drugs on blood flow of optic nerve heads and related structures. Jpn J Ophthalmol. Mar 2013;57(2):133–49. doi: 10.1007/s10384-012-0220-x [DOI] [PubMed] [Google Scholar]
- 73.Chihara E, Dimitrova G, Chihara T. Increase in the OCT angiographic peripapillary vessel density by ROCK inhibitor ripasudil instillation: a comparison with brimonidine. Graefes Arch Clin Exp Ophthalmol. Jul 2018;256(7):1257–1264. doi: 10.1007/s00417-018-3945-5 [DOI] [PMC free article] [PubMed] [Google Scholar]