Abstract
Introduction
World Trade Center (WTC) responders are experiencing a high risk of mild cognitive impairment (MCI) and dementia, though the etiology remains inadequately characterized. This study investigated whether WTC exposures and chronic post‐traumatic stress disorder (PTSD) were correlated with plasma biomarkers characteristic of Alzheimer's disease (AD) neuropathology.
Methods
Eligible participants included WTC‐exposed individuals with a baseline cognitive assessment and available plasma sample. We examined levels of the amyloid beta (Aβ)40/42 ratio, phosphorylated tau 181 (p‐tau181), and neurofilament light chain (NfL) and associations with a WTC exposures (duration on site ≥15 weeks, dust cloud), the PTSD Symptom Checklist for Diagnostic and Statistical Manual of Mental Disorders, 4th edition PTSD, and classification of amyloid/tau/neurodegeneration (AT[N]) profiles. Multinomial logistic regressions assessed whether biomarkers predicted increased risk of MCI or dementia.
Results
Of 1179 eligible responders, 93.0% were male, mean (standard deviation) age 56.6 years (7.8). Aβ40/42, p‐tau181, and NfL intercorrelated and increased with age. In subgroup analyses of responders with available neuroimaging data (n = 75), Aβ40/42 and p‐tau181 were further associated with decreased hippocampal volume (Spearman's ρ = −0.3). Overall, 58.08% of responders with dementia had ≥1 elevated biomarker, and 3.45% had elevations across all biomarkers. In total, 248 (21.05%) had MCI and 70 (5.94%) had dementia. Increased risk of dementia was associated with plasma AT(N) profile T+ or A+N+. Exposure on site ≥15 weeks was independently associated with T+ (adjusted risk ratio [aRR] = 1.03 [1.01−1.05], P = 0.009), and T+N+ profile (aRR = 2.34 [1.12−4.87]). The presence of PTSD was independently associated with risk of A+ (aRR = 1.77 [1.11−2.82]).
Discussion
WTC exposures and chronic PTSD are associated with plasma biomarkers consistent with neurodegenerative disease.
1. INTRODUCTION
The events of September 11, 2001 at the World Trade Center (WTC) in New York, New York, USA, have been widely documented and several groups have monitored the long‐term psychiatric and disease outcomes among distinct WTC‐affected populations. 1 , 2 , 3 Research reports high rates of chronic post‐traumatic stress disorder (PTSD) 22 , 23 , 24 , 25 among WTC responders. WTC responders were men and women whose employment required their service or who volunteered at the site. While there, they inhaled airborne toxins, and participated in a range of high‐risk activities including, for example, digging through the rubble while looking for survivors, sifting through the debris looking for remains, or working on bucket brigades. 4 , 5
Over the past decade, studies have reported a higher than expected incidence of mild cognitive impairment (MCI), 6 more rapid cognitive decline, 7 and greater than age‐expected impairments in physical functioning in WTC responders. 8 Recent neuroimaging work examining the reasons for these changes in WTC responders with chronic PTSD have identified reduced cortical complexity, 9 and evidence of cortical and hippocampal atrophy, 10 , 11 as well as widespread changes to the brain's connectome. 12 , 13 These changes raised concerns that WTC exposures and related PTSD may have accelerated brain atrophy, resulting in a neurodegenerative disease that is emerging at mid‐life, 20 years after response efforts ended. 12 The evidence suggesting the presence of earlier than age‐expected cognitive impairment (CI) and decline requires a better understanding of the nature of aging and the onset of neuropathology in this cohort. WTC responders are now at mid‐life; it is therefore important to understand how these exposures might change expectations for aging in this population.
Alzheimer's disease (AD) is the most common cause of dementia worldwide and sixth leading cause of death in the United States. 14 , 15 The neuropathological course of disease is heterogeneous and insidious with the preclinical phase of AD evident nearly three decades prior to the emergence of clinical symptoms. 15 With the implementation of the National Institute on Aging and Alzheimer's Association (NIA‐AA) Research Framework, using a biological definition of AD to map the presence of amyloid beta (Aβ)‐induced amyloidosis (Α), hyperphosphorylated tau‐induced neurofibrillary tangles and tauopathy (Τ), and neurodegeneration (Ν; i.e., the AT[N] biomarker profile) has become a public health priority. 16 , 17 Growing evidence supports the use of plasma biomarkers, which are cost effective and minimally invasive. However, profiles of preclinical plasma AD and neuronal injury biomarkers have not been fully characterized. For example, recent investigations have reported that plasma AT(N) biomarker classification may outperform a single biomarker in predicting conversion to AD in cognitively unimpaired older adults. 18 There is relatively limited information about the clinical performance of these metrics among individuals whose cognition may be diminished due to chronic stress or environmental exposure. 18 , 19 , 20 , 21 , 22 , 23 , 24
Plasma biomarkers have increasingly been examined in relation to cognition and AD‐related neuropathologic changes in the brain. 25 For example, prior studies have reported that reduced concentrations of plasma Aβ42 are associated with greater rates of cognitive decline in healthy older adults, 26 and conversion to all‐cause dementia and AD. 27 , 28 Several studies report that higher concentrations of plasma phosphorylated tau 181 (p‐tau181) correlate with neuroimaging biomarkers of AD pathology and can reliably differentiate AD neuropathologic changes from non‐AD tau pathology. 29 , 30 Finally, considerable evidence supports the use of plasma neurofilament light chain (NfL) as a marker of neuronal and axonal injury to monitor the course of disease in preclinical AD. Plasma NfL correlates with cerebrospinal fluid (CSF) NfL, 31 brain atrophy, 21 and changes in attentional control. 22 Some evidence suggests that plasma NfL has a high level of accuracy in the identification of neurodegeneration in younger versus older individuals, potentially suggesting that plasma NfL may be particularly informative in the detection of individuals at early stages of risk and across diseases emerging from different neurodegenerative etiologies. 23
Although work to date confirms associations of CI with PTSD, 32 lengthier exposures to the WTC work sites, 33 , 34 and brain morphological changes, 35 , 36 , 37 existing work has not been able to clarify whether these findings are consistent with early‐onset AD, a related phenotype (i.e., parietal‐dominant or another AD‐related dementia), or some other unspecified WTC‐related neurological disorder. The goals of the present report were to test the hypothesis that plasma AT(N) pathology might help to explain the high incidence of MCI seen among high‐exposure WTC responders who continue to report symptoms of WTC‐related PTSD. First, owing to the limited data available on the characterization of plasma biomarkers among younger populations, we characterized plasma biomarker AT(N) profiles using the NIA‐AA Research Framework and determined whether they were associated with MCI and dementia as well as neuroimaging data in the WTC cohort. Second, we examined the extent to which WTC‐related stress and exposures were associated with plasma biomarker levels. Third, in subgroup analyses of responders with concurrent neuroimaging data (n = 75), we examined associations between plasma biomarkers and AT(N) classification with region‐specific brain atrophy.
2. METHODS
2.1. Study design and participants
In this population‐based cohort, we analyzed data from 1179 adults at mid‐life enrolled in the WTC Aging Study (WTC‐AS) from 2014 through 2019. The WTC‐AS is a longitudinal epidemiological study, with the primary purpose of determining the incidence of MCI among WTC‐exposed individuals. 31 Additional inclusion criteria were that participants had a baseline cognitive assessment and an available plasma sample collected in 2019. 32 , 33 Of those in the WTC‐AS whose blood was analyzed, 15 declined all cognitive assessments. Individuals who declined cognitive assessments were like study participants included in the present investigation (P = 0.168).
RESEARCH IN CONTEXT
Systematic Review: On September 11, 2001, terrorists attacked the World Trade Center (WTC) in New York, New York, USA. Since then, the individuals who helped in the search, rescue, and recovery operations have been monitored for the emergence of novel conditions. A recent review reported that lengthier WTC exposures are associated with an increased risk of cognitive dysfunction and impairment. Seeking an understanding of these causes, follow‐up research has noted that WTC responders with cognitive impairment have evidence of cortical atrophy in the cerebral and cerebellar cortices. Recent advances in biomarker detection have allowed researchers to examine the presence of amyloid beta 1‐42 alongside phosphorylated tau 181 and neurofilament light chain to indicate potential dysregulation in Alzheimer's disease–related biomarkers. While studies are clear that exposures and subsequent medical sequelae may implicate changes in cognitive functioning, the mechanisms tying exposure to neurological changes are unclear.
Interpretation: This cross‐sectional study allowed us to follow the National Institute on Aging and Alzheimer's Association Research Framework to examine the potential role of WTC exposures in predicting changes in amyloid, tau, and neurodegeneration (AT[N]) as measured using serology. In the first study to examine AT(N) biomarkers in a large group of WTC responders, we found that serological AT(N) biomarkers were associated with both cognitive outcomes and with WTC exposures (e.g., lengthy exposures, actively laboring in the dust on site, and inhaling particulate matter from the dust cloud).
Future Directions: WTC responders may be at high risk of experiencing a WTC‐related neuropathological condition, and screening for and monitoring the progression of neurodegenerative disease may be supported. The long‐term prognosis of biomarkers is not yet established; however, a follow‐up study is ongoing.
2.2. Ethics
This study was approved by the Stony Brook University Institutional Review Board (IRB #604113). All participants provided written informed consent.
2.3. Outcome: plasma biomarkers
Our outcome in this study was levels of ratio Aβ40/42, p‐tau181, and NfL measured in plasma. Non‐fasting plasma samples were collected through venipuncture in 2019 as previously described. 38 Briefly, we used the Simoa Human Neurology four‐plex assay to measure Aβ1−42 (lower limit of quantitation [LLOQ] = 0.38 pg/mL), Aβ1−40 (LLOQ = 1.02 pg/mL), and NfL (LLOQ = 0.40 pg/mL) accompanied by Phosphorylated Tau single‐assay kits (LLOQ = 0.09 pg/mL). All assays passed quality control procedures, and mean coefficients of variation (CV) were CV = 2.35% (Aβ1−42), 2.24% (Aβ1−40), 4.40% (p‐tau181), and 3.54% (NfL), making them less than 5% and therefore within acceptable ranges. A few samples (five p‐tau181, one NfL, and one Aβ1−42) had analytes below the LLOQ. Due to instrumentation error, five participants (0.38%) were missing information on Aβ1−42, Aβ1‐40, and NfL, and 12 (0.92%) were missing information on p‐tau181. We examined the impact of imputing these using the LLOQ as their values, and results were similar with or without these values so imputed values were retained. Participants with missing plasma samples did not differ in terms of demographics, cognitive status, WTC exposures, or across biomarker concentration levels compared to those whose data was successfully assayed.
2.4. Classification of plasma AT(N) biomarker profiles
High levels of plasma Aβ40/42 ratios (A), phosphorylated tau‐181 (T), and neurofilament light (N) are reported in the early stages of sporadic AD. 39 However, given limited available data for cut‐off values across preclinical AD plasma and neuronal injury biomarkers to differentiate at risk from cognitively healthy individuals in epidemiological studies of healthy individuals at mid‐life and based on earlier work from our group, 38 we defined cutoffs based on distribution of our sample as sex‐stratified top quantiles for A+, T+, and N+ status (see Table S1 in supporting information for cutoff values).
2.5. Classification of cognitive status
CI was operationalized algorithmically following standard criteria for MCI, 40 and dementia, 41 using the Montreal Cognitive Assessment (MoCA), an instrument developed to reliably identify age‐related cognitive impairment. 42 To avoid test–retest biases resulting in increased scores on cognitive assessments, alternate test versions were used at each screening visit. Eligibility criterion used to identify MCI and dementia relies on conservative cutoff scores of 21 to 23 and ≤20, 13 respectively, consistent with current guidance in population‐based cognitive monitoring studies. 13 , 19
2.6. Subgroup analysis of plasma and neuroimaging biomarkers
Data for a subsample of responders (n = 75) with available plasma biomarkers and neuroimaging data were examined to further characterize associations between the burden of plasma AT(N) biomarker profiles with region‐specific brain changes. Structural neuroimaging data were all available from previously published papers. 10 , 11 Briefly, scans were completed as part of a separate study using a Siemen's 3T multimodal magnetic resonance imaging machine using a T1‐MPRAGE protocol. Quantification was completed using standard imaging methods with reported mean cortical thickness, mean gray matter volume (% of intracranial volume [%ICV]), mean hippocampal volume (%ICV), white matter hypointensity burden, and cortical complexity as measured using the fractal dimension were reported. 11 , 36 , 43
2.7. WTC exposures and PTSD
Participants were stratified by occupational role (supervisor, non‐supervisor) reported while on site at the WTC. Duration on site has been identified as a primary risk factor for CI in this cohort and was included here as ≥15 weeks (the top quintile of exposure durations). Exposure to the dust cloud on the morning of 9/11 has been identified as the most intense and caustic period of particle and gas exposure and has been implicated in the severity of long‐term health outcomes among WTC‐affected populations so we included dust cloud exposure. 44 , 45
Probable PTSD was assessed using the 17‐item PTSD Checklist (PCL‐C) for Diagnostic and Statistical Manual of Mental Disorders, 4th edition. 46 The index was created by summing symptoms (Cronbach's α = 0.95), and a cutoff of PCL ≥44 was used to determine WTC‐related probable PTSD (hereafter, PTSD).
2.8. Demographic and medical covariates
We adjusted for potential confounders (retrieved from monitoring visit questionnaires and medical records) in the relationship with plasma biomarkers and cognitive status, including sociodemographic characteristics (age, sex, race/ethnicity), and educational attainment. We further adjusted for medical conditions associated with increased risk of AD including history of hypertension and diabetes, as well as those that are common in this cohort (i.e., obstructive airway disease, cancer) and have been linked to cognitive decline.
2.9. Statistical analyses
Our analyses had four main steps. First, we describe the study cohort using categorical variables expressed by percentages and continuous variables expressed by means (standard deviation). Second, we examined unadjusted correlations between plasma biomarkers with continuous predictors using Spearma'sn rho (ρ). Third, we examined correlates of plasma biomarkers with clinical profiles. Specifically, we first used ρ to examine associations between plasma AT(N) biomarkers with structural indicators of neurodegeneration in the imaging subgroup (n = 75). We examined the prevalence of different plasma AT(N) profiles by calculating the observed/expected ratio based on assumptions of independence and reported P‐values derived from tests of proportions.
Next, we used multinomial logistic regressions adjusted for covariates to estimate multivariable‐adjusted risk ratios for associations between plasma AT(N) biomarker profiles and the presence of MCI or dementia. Fourth, we examined WTC exposure‐related predictors including PTSD with AT(N) biomarker profiles by first relying on generalized linear modeling with Poisson regression using a robust standard error to estimate the relative risk of having plasma biomarkers in the top quintile. Poisson regression is preferable to logistic in situations in which outcomes are common. 47 Next, we supplemented these analyses using multinomial logistic regression to examine combinations between overlapping plasma biomarker profiles to characterize a preclinical AD plasma biomarker model consistent with the AT(N) research framework. 3 Multivariable‐adjusted relative risks (aRR) and accompanying 95% confidence intervals were reported alongside P‐values. Analyses were completed using Stata 17/MP (StataCorp).
2.10. Role of the funding source
The funding agency played no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all data in the study. SAPC had final responsibility for the decision to submit for publication.
3. RESULTS
Among 1179 participants (Table 1), 1104 (93.0%) were male, with a mean age of 56.55 (7.81) at the time of blood collection. Overall, 346 (29.12%) were volunteer and construction workers, while the remainder worked as police or other security officers, and 166 (13.97%) reported working as supervisors on site at the WTC.
TABLE 1.
World Trade Center responder sample characteristics.
| Participant characteristic | N (%) or Mean (SD) |
|---|---|
| Demographics | |
| Age, years | 57.48 (7.76) |
| Female sex | 86 (7.29) |
| Educational attainment | |
| High school or less | 312 (26.46) |
| Some college | 545 (46.23) |
| University degree | 322 (27.31) |
| Biomarkers | |
| Amyloid beta 40/42 ratio, pg/mL | 15.87 (3.99) |
| Phosphorylated tau 181, pg/mL | 1.70 (1.09) |
| Neurofilament light chain, pg/mL | 10.94 (6.93) |
| Cognitive status | |
| Unimpaired | 913 (79.39) |
| Mild cognitive impairment | 188 (16.35) |
| Possible dementia | 49 (4.26) |
| WTC exposure | |
| Untrained responder | 337 (28.58) |
| Supervisor | 163 (13.83) |
| Probable post‐traumatic stress disorder at enrollment | 129 (11.22) |
| ≥ 15 weeks on site | 253 (21.46) |
| Dust cloud exposure | 552 (47.42) |
| Medical comorbidities | |
| Hypertension | 325 (27.85) |
| Diabetes | 65 (5.58) |
| Obstructive airway disease | 55 (4.66) |
| Cancer | 179 (15.18) |
Note: Data are N (%), mean (SD) for the enrolled study cohort. Non‐supervisors included volunteers, construction workers, police, and security officers; Probable post‐traumatic stress disorder based on cut‐off score of 44 on PCL‐C.
Abbreviations: PCL‐C, PTSD Checklist; pg/mL, picogram per millimeter; SD, standard deviation; WTC, World Trade Center.
All four biomarkers were positively intercorrelated and increased with age (Table 2). Unadjusted associations with overall cognition were weak to moderate in size; however, we identified associations between NfL with overall cognition and processing speed with Aβ40/42 and NfL, and abstraction with Aβ40/42.
TABLE 2.
Associations of amyloid beta 40/42 ratio, phosphorylated tau 181, and neurofilament light chain biomarkers with age and cognition at baseline. Spearman's rho examining correlations between plasma biomarkers, age, and cognition at baseline in the World Trade Center responder sample. Bold figures are statistically significant (P < 0.05), red shading shows cells with stronger associations
| Characteristics |
1 Age |
2 Aβ40/42 |
3 p‐tau181 |
4 NfL |
|
|---|---|---|---|---|---|
| 1 | Age, years | 1.000 | |||
| 2 | Amyloid beta 40/42 ratio | 0.191 | 1.000 | ||
| 4 | Phosphorylated tau 181 | 0.276 | 0.138 | 1.000 | |
| 5 | Neurofilament light chain | 0.553 | 0.186 | 0.353 | 1.000 |
| 6 | Montreal Cognitive Assessment score | 0.096 | 0.013 | 0.014 | 0.051 |
| 7 | Attention | 0.082 | 0.067 | −0.004 | 0.041 |
| 8 | Abstraction | 0.053 | 0.008 | 0.014 | 0.023 |
| 9 | Episodic memory | 0.072 | 0.001 | 0.002 | 0.044 |
| 10 | Processing speed | 0.152 | −0.068 | −0.019 | 0.057 |
| 11 | Calculation | 0.097 | 0.033 | 0.026 | 0.013 |
| 12 | Language | 0.041 | ‐0.020 | 0.039 | 0.005 |
| 13 | Executive function | −0.005 | 0.024 | 0.027 | 0.031 |
| 14 | Orientation | 0.092 | 0.034 | 0.006 | 0.038 |
| 15 | Visuospatial function | 0.062 | 0.010 | 0.030 | 0.023 |
Abbreviations: Aβ40/42, ratio of levels of amyloid beta 1‐40 to amyloid beta 1‐42; NfL, neurofilament light chain; pg/mL, picogram per milliliter; p‐tau181, levels of phosphorylated tau 181.
Analyses in a subset of responders (n = 75) with available neuroimaging data (average age = 56.1 [4.8], 17.3% female) revealed some significant associations between plasma biomarkers with region‐specific brain atrophy (Figure 1). After adjusting for ICV, age, and participant sex we found that p‐tau181 and Aβ40/42 were correlated with hippocampal volume.
FIGURE 1.

Associations of amyloid beta (Aβ)40/42 ratio, phosphorylated tau 181, and neurofilament light chain biomarkers with neuroimaging regions of interest. Spearman's rho showing associations between the distribution of plasma ratio Aβ1‐40/42 (A), phosphorylated tau 181 (T), and neurofilament light chain (N) biomarker levels with region‐specific brain atrophy among a subset of participants (n = 75) with available neuroimaging data. pg/mL, picogram per millimeter. Black typeface shows trends (P < 0.10); bold typeface shows nominally statistically significant (P < 0.05); italicized numbers are those determined to be statistically significant after accounting for the false discovery rate. Blue versus red coloring shows direction of relationship, while hue shows strength of the relationship. Associations have all been adjusted for age, sex, and intracranial volume (ICV).
To understand the distribution of biomarker profiles in WTC responders, we compared the prevalence of overlapping AT(N) categories to the expected prevalence in the case that biomarkers were uncorrelated (Table 3). This analysis showed that the prevalence of the overlapping categories of A+T+N+, and T+N+(A−) were relatively more common than expected while A+T+(N−) and N+(A−T−) were relatively less common than expected from chance alone.
TABLE 3.
Prevalence of amyloid beta 40/42 ratio, phosphorylated tau 181, and neurofilament light chain in sample.
| Neurofilament status | ||||
|---|---|---|---|---|
| Amyloid status | Tau status | N+ | N− | |
| A+ | T+ | % | 3.45% | 1.87% |
| (2.6–4.7) | (1.29–2.85) | |||
| O/E, P | 4.32, P <0.001 | 0.58, P = 0.013 | ||
| T− | % | 3.55% | 11.67% | |
| (2.68–4.8) | (10–13.67) | |||
| O/E, P | 1.11, P = 0.518 | 0.91, P = 0.269 | ||
| A | T+ | % | 5.32% | 9.52% |
| (4.22–6.8) | (8.02–11.38) | |||
| O/E, P | 1.66, P < 0.001 | 0.74, P = 0.001 | ||
| T− | % | 7.66% | 56.96% | |
| (6.32–9.36) | (54.15–59.81) | |||
| O/E, P | 0.60, P < 0.001 | 1.11, P = 0.998 | ||
Notes: Bold typeface shows statistically significant results. P‐values were derived from tests of proportions examining the hypothesis that the cell proportion was different from expected depending on random chance.
Abbreviations: A+, amyloid beta 40/42 ratios in the top sex‐specific quintile; N+, neurofilament light chain results in the top sex‐specific quintile; T+, phosphorylated tau 181 results in the top sex‐specific quintiles. A‐, T‐, and N‐ indicate results that are not in the positive category.
Next, we examined risk ratios of associations between plasma AT(N) profiles and cognitive status (Figure 2). Risk of dementia was associated with the presence of plasma A+N+ (aRR = 2.70 [1.04−6.99], P = 0.040) and T+ (aRR = 4.60 [1.15−18.46], P = 0.031) profiles. Overall, 58.08% (50.88–65.04) of participants with dementia had abnormal plasma AT(N) biomarker levels.
FIGURE 2.
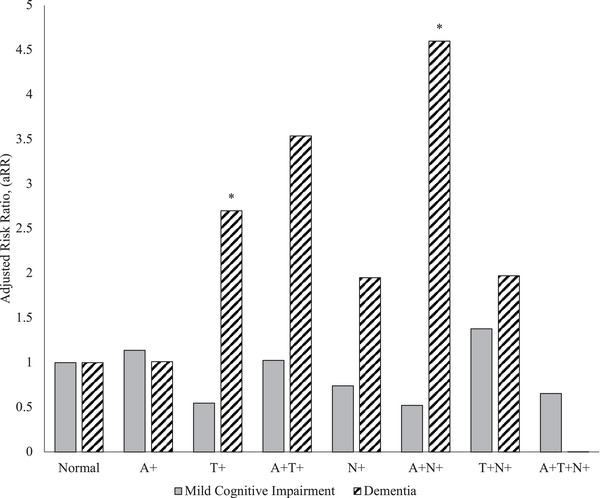
Associations of plasma AT(N) biomarker status amyloid beta (Aβ)40/42 ratio, phosphorylated tau 181, and neurofilament light chain biomarkers with cognitive status. Degree of association between the plasma Aβ40/42 (A), phosphorylated tau 181 (T), and/or neurofilament light chain (N) profiles and cognitive status of mild cognitive impairment or dementia among participants compared to the lowest risk group. * P < 0.05. A+ indicates that Aβ40/42 ratios were in the top sex‐specific quintile. T+ indicates that plasma phosphorylated tau 181 results were in the top sex‐specific quintile. N+ indicates that plasma neurofilament light results were in the top sex‐specific quintile. Model reports relative risks derived from multinomial logistic regression models estimating the risk of mild cognitive impairment and dementia adjusting for age, sex, race/ethnicity, and education.
Multivariable adjusted modeling was used to examine WTC‐related exposures and PTSD as predictors associated independently with plasma biomarker levels (Table 4). Older age was associated with increased risk of higher levels of plasma p‐tau181 and NfL, but not with Aβ40/42. Analyses further revealed that greater time on site and occupational role (e.g., supervisor vs. non‐supervisor) were associated with increased risks of higher p‐tau181.
TABLE 4.
Multivariable‐adjusted risk ratios showing degree of association between World Trade Center related PTSD and exposures with plasma AT(N), Aβ40/42 ratio (A), phosphorylated tau 181 (T), and neurofilament‐light (N) status.
|
Model 1 Amyloid beta 40/42 (A+), ratio |
Model 2 Phosphorylated tau 181 positive (T+), pg/mL |
Model 3 Neurofilament light positive (N+), pg/mL |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | aRR | 95% CI | P | aRR | 95% CI | P | aRR | 95% CI | P |
| Age, years | 1.044 | 1.03–1.06 | <0.001 | 1.059 | 1.04–1.08 | <0.001 | 1.114 | 1.1–1.13 | <0.001 |
| ≥15 weeks on‐site | 1.000 | 0.97–1.03 | 0.982 | 1.031 | 1.01–1.05 | 0.009 | 1.001 | 0.98–1.03 | 0.936 |
| PTSD | 1.028 | 1.00–1.06 | 0.089 | 1.012 | 0.98–1.05 | 0.504 | 1.018 | 0.98–1.05 | 0.323 |
| Non‐supervisorial role | 1.049 | 0.73–1.51 | 0.795 | 1.733 | 1.09–2.75 | 0.020 | 1.027 | 0.73–1.44 | 0.878 |
| Dust cloud | 1.226 | 0.94–1.59 | 0.127 | 0.815 | 0.61–1.08 | 0.157 | 1.010 | 0.77–1.32 | 0.944 |
Note: Bold typeface shows statistically significant results.
Abbreviations: 95% CI, 95% confidence interval; A+, those with elevated Aβ40/42 ratio; Aβ, amyloid beta; aRR, adjusted risk ratio, models adjusted for demographics including age, race/ethnicity, sex, and education; N+, those with elevated neurofilament light chain; PTSD, post‐traumatic stress disorder; Occupation role, responders self‐reported classification as supervisor/non‐supervisor while on site at the World Trade Center; T+, those with elevated phosphorylated tau 181.
Multivariable‐adjusted multinomial logistic regression modeling was used to assess increased risks of single versus combined plasma biomarker levels with WTC‐related PTSD and exposures (Table 5; different combinations of biomarkers shown in models 1–3). Overall, age was associated with increased pathology across nearly all plasma biomarker profiles. Additionally, PTSD was associated with increased risk of plasma A+ biomarker status. WTC exposure on site, reported as ≥15 weeks at the WTC, was associated with increased risk of plasma T+N+ status. Although not reported, while most medical comorbidities were not significantly associated with plasma biomarker levels or plasma AT(N) biomarker profiles, a history of diabetes was associated with both the presence of N+ (aRR = 2.85 [1.30−6.26], P = 0.009) and T+N+ (aRR = 3.01 [1.30−7.01], P = 0.010) status.
TABLE 5.
Multivariable‐adjusted risk ratios between World Trade Center exposures with plasma ΑΤ(Ν) biomarker classification based on combinations of ΑΤ(Ν).
| Age, years | ≥15 weeks on site | Post‐traumatic stress disorder | Non‐supervisorial role | Dust cloud | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AT(N) status | aRR | 95% CI | aRR | 95% CI | aRR | 95% CI | aRR | 95% CI | aRR | 95% CI |
| A+ | 0.99 | 0.96–1.01 | 1.50 | 0.84–2.67 | 1.77 | 1.11–2.82 | 1.13 | 0.67–1.89 | 1.16 | 0.83–1.61 |
| T+ | 1.03 | 1.00–1.07 | 1.63 | 0.79–3.32 | 1.03 | 0.50–2.10 | 1.76 | 0.86–3.61 | 0.68 | 0.44–1.06 |
| A+T+ | 1.05 | 0.99–1.12 | 2.65 | 0.84–8.34 | – | – | 5.66 | 0.73–43.84 | 1.48 | 0.67–3.25 |
| N+ | 1.16 | 1.12–1.20 | 1.81 | 0.93–3.50 | 0.68 | 0.30–1.52 | 1.24 | 0.69–2.24 | 0.83 | 0.54–1.29 |
| A+N+ | 1.18 | 1.11–1.26 | 0.41 | 0.05–3.26 | 2.14 | 0.74–6.18 | 0.71 | 0.24–2.08 | 1.16 | 0.50–2.73 |
| T+N+ | 1.22 | 1.18–1.27 | 2.34 | 1.12–4.87 | 1.22 | 0.55–2.72 | 1.86 | 0.90–3.86 | 0.85 | 0.50–1.42 |
| A+T+N+ | 1.30 | 1.14–1.49 | 5.49 | 0.71–42.42 | 3.30 | 0.26–42.06 | 0.68 | 0.11–4.22 | 1.85 | 0.34–10.02 |
Note: Bold typeface shows statistically significant results. Models additionally adjusted for sex, educational attainment, race/ethnicity, diabetes, hypertension, obstructive airway disease, and cancer. Results labeled with a dash did not converge.
Abbreviations: 95% CI, 95% confidence interval; A+, amyloid beta 40/42 ratios in the highest sex‐specific quintile; aRR, multivariable‐adjusted risk ratio derived from multinomial logistic regression; N+, neurofilament light levels in the highest sex‐specific quintile; T+, phosphorylated tau 181 levels in the highest sex‐specific quintile.
4. DISCUSSION
There is an increasing interest in understanding the utility of plasma‐based biomarkers in different populations and in the monitoring of AD and AD‐related dementias (ADRD). To our knowledge, this is the first and largest population‐based study to characterize preclinical AD and neuronal injury plasma biomarkers with cognitive status at mid‐life among a well‐characterized cohort at high risk of aging‐related diseases. We used the NIA‐AA Research Framework to create plasma biomarker AT(N) classification and found that plasma T+ and A+N+ profiles were associated with increased risk of dementia in WTC responders. Next, we identified distinct plasma AT(N) biomarker profiles consistent with neurodegenerative disease that were significantly associated with WTC‐related exposures and PTSD.
Among individuals who more than 20 years since 9/11 met screening criteria for WTC‐related probable PTSD, we found that PTSD was further associated with distinct plasma AD biomarker profiles. Animal models have reported that stress accelerates Aβ production 48 and population‐based studies have indicated that PTSD increases vulnerability to oxidative stress, in turn increasing risk of dementia. 49 , 50 The association we report here is consistent with this research. Although we did not find evidence of associations for PTSD with other plasma A+ profiles including A+T+, there was a trend toward potential classification of plasma A+N+ status. Additional longitudinal studies are needed to determine whether plasma A+ profiles among WTC responders with PTSD are pathogenic.
In the present study, we extend previous work in which we identified that decreases in plasma Aβ42 were associated with increased risk of CI. 51 Using the NIA‐AA Research Framework, we now demonstrate that profiles of plasma biomarkers consistent with the AT(N) classification system are associated with CI and WTC exposures. Aβ40/42 has been previously associated with conversion to all‐cause dementia and AD. 52 Similar to CSF, plasma p‐tau181 has a high degree of sensitivity and specificity in discriminating AD dementia, 53 with several studies reporting higher p‐tau181 in AD versus healthy controls. 54 Together, our results suggest that Aβ40/42, p‐tau181, and NfL may be associated with an early‐onset dementia among younger populations, with similar molecular mechanisms to AD. Preclinical phases of possible AD pathology are suspected to begin around 40 years of age; however, profiles of preclinical AD plasma biomarkers in younger populations have not been well characterized. Thus, although some evidence indicates that early‐onset AD may be exacerbated by tauopathy, the temporal course of tau and amyloid in this population remains largely unknown.
Extending prior plasma AT(N) research with WTC responders, we here found that the duration of time on site at the WTC was associated with increased risk of plasma AT(N) biomarker profiles and with T+N+ profiles in particular. 16 Previous studies have reported that plasma NfL strongly correlates with CSF NfL, 31 is associated with decline in hippocampal volume and cortical thickness, 29 and several reports support the utility of plasma NfL as a reliable staging biomarker in pre‐symptomatic AD. 21 Related to the present investigation, emerging studies further support the utility of plasma NfL as a promising biomarker in monitoring AD progression among younger at‐risk individuals. 23 Specifically, among individuals aged 65 years and younger, plasma concentrations of NfL were helpful in differentiating neurodegeneration among younger versus older controls. 23
4.1. Limitations
Our study has several limitations. First, apolipoprotein E genotyping to identify carriers and non‐carriers of the ε4 allele, the most significant risk factor associated with AD, was not available at the time of this study. Second, the plasma biomarkers in the present report have only been validated among older populations; thus, determining a threshold for cut points was challenging among younger individuals at mid‐life. Third, WTC‐related PTSD symptoms were assessed with a self‐reported rating scale. Although the PCL is a well‐validated measure of PTSD extensively used for screening of individuals with probable PTSD, it does not confirm diagnosis. Importantly given the complex and synergistic effects of occupational, neurotoxic, and psychological exposures that WTC‐exposed individuals endured, plasma biomarkers levels of amyloid and neuronal injury may not be applicable to same‐aged non–WTC‐exposed populations. We did not characterize these plasma biomarkers in a same‐age, sex‐matched non–WTC‐exposed control group. Furthermore, although we found that the plasma A+T+N+ profile was more common than expected and was associated with decreased hippocampal volume among responders with neuroimaging data (n = 75), we may be underpowered to further characterize these results owing to sample size limitations. Nonetheless, given the large numbers of WTC‐exposed survivors who did not participate in rescue and recovery efforts, we believe that it is in WTC monitoring programs and similar research settings that these plasma biomarkers have the greatest clinical significance.
5. CONCLUSION
In the two decades since 9/11, PTSD, an identified correlate of aging‐related diseases including dementia, 49 remains the most frequently endorsed psychiatric condition among WTC‐exposed populations. Emerging research further indicates that responders are experiencing a higher risk of MCI earlier in life than expected. 32 Ongoing efforts within our group are attempting to characterize the neurobiological etiology for these observations, as identifying the underlying pathogenesis would enable the development of targeted clinical interventions to slow the progression of neurodegeneration. In this study, we applied the NIA‐AA Research Framework to the study of WTC responders with available plasma biomarkers characteristic of AD neuropathology and found that a variety of diagnostic phenotypes were relatively more common in this cohort than expected, including the plasma A+T+N+, and T+N+ phenotypes. Additionally, we found that the duration on site at the WTC was associated with increased risk of T+ status and with T+N+(A−) in particular. Additionally, we found that probable PTSD was associated with plasma A+(T−N−) status. These results may suggest the presence of a non‐amyloid exposure‐related tauopathy (T+N+[A−]) among high exposure responders and highlight the potential for an AD‐like condition (A+[T−N−]) among responders with chronic PTSD. Our findings using novel plasma biomarkers provide additional evidence in support of an ongoing neuropathological process that does not necessarily fit the NIA‐AA, AD research framework of AT(N) biomarkers, yet mirrors the complex neurotoxic exposures and WTC‐related stressors endured by responders.
CONFLICTS OF INTEREST STATEMENT
The authors declare no conflicts of interest. Author disclosures are available in the supporting information.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (NIH/NIA R01 AG049953, P30 AG066514) and the Centers for Disease Control and Prevention (CDC/NIOSH 200‐2011‐39361).
Kritikos M, Diminich ED, Meliker J, et al. Plasma amyloid beta 40/42, phosphorylated tau 181, and neurofilament light are associated with cognitive impairment and neuropathological changes among World Trade Center responders: A prospective cohort study of exposures and cognitive aging at midlife. Alzheimer's Dement. 2023;15:e12409. 10.1002/dad2.12409
Minos Kritikos and Erica D. Diminich contributed equally to this study.
DATA AVAILABILITY STATEMENT
All data in this study are considered private health information. A limited dataset for this study can be made available to any institutionally affiliated researcher after execution of a data usage agreement. Interested parties should contact Dr. Clouston (sean.clouston@stonybrookmedicine.edu) for details about this process.
REFERENCES
- 1. Neria Y, DiGrande L, Adams BG. Posttraumatic stress disorder following the September 11, 2001, terrorist attacks a review of the literature among highly exposed populations. Am Psychol. 2011;66(6):429‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clouston SAP, Hall CB, Kritikos M, et al. Cognitive impairment and World Trade Centre‐related exposures. Nat Rev Neurol. 2022;18(2):103‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cleven KL, Rosenzvit C, Nolan A, et al. Twenty‐year reflection on the impact of World Trade Center exposure on pulmonary outcomes in Fire Department of the City of New York (FDNY) rescue and recovery workers. Lung. 2021;199(6):569‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clouston SAP, Hall CB, Kritikos M, et al. Cognitive impairment and World Trade Centre‐related exposures: considerations when monitoring World Trade Center affected individuals. Nat Rev Neurol. 2022;18(2):103‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kritikos M, Gandy SE, Meliker JR, Luft BJ, Clouston SAP. Acute versus chronic exposures to inhaled particulate matter and neurocognitive dysfunction: pathways to Alzheimer's disease or a related dementia. J Alzheimers Dis. 2020;78(3):871‐886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clouston SA, Kotov R, Pietrzak RH, et al. Cognitive impairment among World Trade Center responders: long‐term implications of re‐experiencing the 9/11 terrorist attacks. Alzheimers Dement (Amst). 2016;4:67‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clouston SA, Diminich ED, Kotov R, et al. Incidence of mild cognitive impairment in World Trade Center responders: long‐term consequences of re‐experiencing the events on 9/11/2001. Alzheimers Dement (Amst). 2019;11(1):628‐636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Diminich ED, Clouston SAP, Kranidis A, et al. Chronic posttraumatic stress disorder and comorbid cognitive and physical impairments in World Trade Center Responders. J Trauma Stress. 2021;34(3):616‐627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kritikos M, Clouston SA, Huang C, et al. Cortical complexity in world trade center responders with chronic posttraumatic stress disorder. Translational Psychiatry. 2021;11(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clouston SAP, Deri Y, Horton M, et al. Reduced cortical thickness in World Trade Center responders with cognitive impairment. Alzheimers Dement (Amst). 2020;12(1):e12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deri Y, Clouston SA, DeLorenzo C, et al. Selective hippocampal subfield volume reductions in World Trade Center responders with cognitive impairment. Alzheimers Dement (Amst). 2021;13(1):e12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang C, Kritikos M, Clouston SA, et al. White matter connectivity in incident mild cognitive impairment: a Diffusion Spectrum Imaging Study of World Trade Center Responders at midlife. J Alzheimers Dis. 2021;80(3):1209‐1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang C, Kritikos M, Sosa MS, et al. World Trade Center site exposure duration is associated with hippocampal and cerebral white matter neuroinflammation. Mol Neurobiol. 2023;60(1):160‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alzheimer's Association . 2018 Alzheimer's disease facts and figures. Alzheimers Dementia. 2018;14(3):367‐429. [Google Scholar]
- 15. Gordon BA, Blazey TM, Su Y, et al. Spatial patterns of neuroimaging biomarker change in individuals from families with autosomal dominant Alzheimer's disease: a longitudinal study. Lancet Neurol. 2018;17(3):241‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jack CR, Jr. , Bennett DA, Blennow K, et al. NIA‐AA Research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grossberg GT, Tong G, Burke AD, Tariot PN. Present algorithms and future treatments for Alzheimer's disease. J Alzheimers Disease. 2019;67(4):1157‐1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cullen NC, Leuzy A, Janelidze S, et al. Plasma biomarkers of Alzheimer's disease improve prediction of cognitive decline in cognitively unimpaired elderly populations. Nat Commun. 2021;12(1):3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cacciottolo M, Morgan TE, Saffari AA, et al. Traffic‐related air pollutants (TRAP‐PM) promote neuronal amyloidogenesis through oxidative damage to lipid rafts. Free Radical Biol Med. 2020;147:242‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Forman HJ, Finch CE. A critical review of assays for hazardous components of air pollution. Free Radical Biol Med. 2018;117:202‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Preische O, Schultz SA, Apel A, et al. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer's disease. Nat Med. 2019;25(2):277‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aschenbrenner AJ, Gordon BA, Fagan AM, et al. Neurofilament light predicts decline in attention but not episodic memory in preclinical Alzheimer's disease. J Alzheimers Disease. 2020;74(4):1119‐1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ashton NJ, Janelidze S, Al Khleifat A, et al. A multicentre validation study of the diagnostic value of plasma neurofilament light. Nat Commun. 2021;12(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gauthier S, Rosa‐Neto P, Morais J, Webster C; Alzheimer's Disease International . World Alzheimer Report 2021: Journey Through the Diagnosis of Dementia. Alzheimer's Disease International; 2021. [Google Scholar]
- 25. Palmqvist S, Tideman P, Cullen N, et al. Prediction of future Alzheimer's disease dementia using plasma phospho‐tau combined with other accessible measures. Nat Med. 2021;27(6):1034‐1042. [DOI] [PubMed] [Google Scholar]
- 26. Stockmann J, Verberk IM, Timmesfeld N, et al. Amyloid‐β misfolding as a plasma biomarker indicates risk for future clinical Alzheimer's disease in individuals with subjective cognitive decline. Alzheimers Res Ther. 2020;12(1):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mattsson N, Cullen NC, Andreasson U, Zetterberg H, Blennow K. Association between longitudinal plasma neurofilament light and neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2019;76(7):791‐799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schindler SE, Bollinger JG, Ovod V, et al. High‐precision plasma beta‐amyloid 42/40 predicts current and future brain amyloidosis. Neurology. 2019;93(17):e1647‐e1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mielke MM, Syrjanen JA, Blennow K, et al. Plasma and CSF neurofilament light: relation to longitudinal neuroimaging and cognitive measures. Neurology. 2019;93(3):e252‐e260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Janelidze S, Mattsson N, Palmqvist S, et al. Plasma P‐tau181 in Alzheimer's disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer's dementia. Nat Med. 2020;26(3):379‐386. [DOI] [PubMed] [Google Scholar]
- 31. Milà‐Alomà M, Suárez‐Calvet M, Molinuevo JL. Latest advances in cerebrospinal fluid and blood biomarkers of Alzheimer's disease. Ther Adv Neurol Disord. 2019;12:1756286419888819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Clouston S, Diminich ED, Kotov R, et al. Incidence of mild cognitive impairment in World Trade Center responders: long‐term consequences of re‐experiencing the events on 9/11/2001. Alzheimers Dement (Amst). 2019;11:628‐636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Clouston S, Quesnel‐Vallée A. The role of defamilialization in the relationship between partnership and self‐rated health: a cross‐national comparison of Canada and the United States. Soc Sci Med. 2012;75(8):1342‐1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen APF, Clouston SAP, Kritikos M, et al. A deep learning approach for monitoring parietal‐dominant Alzheimer's disease in World Trade Center responders at midlife. Brain Commun. 2021;3(3):fcab145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Deri Y, Clouston S, DeLorenzo C, et al. Selective hippocampal subfield volume reductions in World Trade Center responders with cognitive impairment. Alzheimers Dement (Amst). 2021;13(1):e12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Clouston S, Deri Y, Horton M, et al. Reduced cortical thickness in World Trade Center responders with cognitive impairment: neuroimaging/differential diagnosis. Alzheimers Dementia. 2020;16:e039996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huang C, Kritikos M, Clouston SAP, et al. White matter connectivity in incident mild cognitive impairment: a Diffusion Spectrum Imaging Study of World Trade Center Responders at midlife. J Alzheimers Disease. 2021;80:1209‐1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kritikos M, Clouston SAP, Diminich ED, et al. Pathway analysis for plasma β‐Amyloid, Tau and Neurofilament Light (ATN) in World Trade Center Responders at midlife. Neurol Ther. 2020;9(1):159‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hansson O, Cullen N, Zetterberg H, Blennow K, Mattsson‐Carlgren N; Alzheimer's Disease Neuroimaging Initiative . Plasma phosphorylated tau181 and neurodegeneration in Alzheimer's disease. Ann Clin Transl Neurol. 2021;8(1):259‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers dementia. 2011;7(3):263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695‐699. [DOI] [PubMed] [Google Scholar]
- 43. Kritikos M, Clouston SAP, Huang C, et al. Cortical complexity in world trade center responders with chronic posttraumatic stress disorder. Transl Psychiatry. 2021;11(1):597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lioy PJ, Georgopoulos P. The anatomy of the exposures that occurred around the World Trade Center site: 9/11 and beyond. Ann N Y Acad Sci. 2006;1076:54‐79. [DOI] [PubMed] [Google Scholar]
- 45. Lippmann M, Cohen MD, Chen LC. Health effects of World Trade Center (WTC) Dust: an unprecedented disaster's inadequate risk management. Crit Rev Toxicol. 2015;45(6):492‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Weathers FW, Litz BT, Herman DS, Huska JA, Keane TM. The PTSD Checklist (PCL): Reliability, validity, and diagnostic utility. annual meeting of the international society for traumatic stress studies. Paper Presented at: The Annual Convention of the International Society for Traumatic Stress Studies; 1993; San Antonio, TX. [Google Scholar]
- 47. Chen W, Qian L, Shi J, Franklin M. Comparing performance between log‐binomial and robust Poisson regression models for estimating risk ratios under model misspecification. BMC Med Res Methodol. 2018;18(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Baglietto‐Vargas D, Chen Y, Suh D, et al. Short‐term modern life‐like stress exacerbates Aβ‐pathology and synapse loss in 3xTg‐AD mice. J Neurochem. 2015;134(5):915‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Greenberg MS, Tanev K, Marin MF, Pitman RK. Stress, PTSD, and dementia. Alzheimers Dementia. 2014;10:S155‐S165. [DOI] [PubMed] [Google Scholar]
- 50. Flatt JD, Gilsanz P, Quesenberry CP, Jr., Albers KB, Whitmer RA. Post‐traumatic stress disorder and risk of dementia among members of a health care delivery system. Alzheimers Dementia. 2018;14(1):28‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kritikos M, Clouston S, Diminich ED, et al. Pathway analysis for Plasma β‐Amyloid, Tau and Neurofilament Light (ATN) in World Trade Center Responders at midlife. Neurol Ther. 2020;9(1):159‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. de Wolf F, Ghanbari M, Licher S, et al. Plasma tau, neurofilament light chain and amyloid‐β levels and risk of dementia; a population‐based cohort study. Brain. 2020;143(4):1220‐1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Karikari TK, Pascoal TA, Ashton NJ, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer's disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020;19(5):422‐433. [DOI] [PubMed] [Google Scholar]
- 54. Clark AL, Weigand AJ, Bangen KJ, et al. Higher cerebrospinal fluid tau is associated with history of traumatic brain injury and reduced processing speed in Vietnam‐era veterans: a Department of Defense Alzheimer's Disease Neuroimaging Initiative (DOD‐ADNI) study. Alzheimers Dement (Amst). 2021;13(1):e12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Data Availability Statement
All data in this study are considered private health information. A limited dataset for this study can be made available to any institutionally affiliated researcher after execution of a data usage agreement. Interested parties should contact Dr. Clouston (sean.clouston@stonybrookmedicine.edu) for details about this process.


