Abstract
Background and aims
Numerous studies have found an association between vitamin deficiency and thyroid disorders (TD). The presence of anti-parietal cell antibodies is indicative of reduced ability to absorb vitamin B12. Thus, this study reviewed the existing studies with the objective of assessing differences in the serum levels of vitamin B12 among patients with and without TD, the frequency of vitamin B12 deficiency in patients with TD, and the presence of anti-parietal cell antibodies in patients with TD.
Methods
A meta-analysis of random-effects model was conducted to calculate pooled frequencies, mean differences (MD), and their respective 95% confidence intervals (CI). We identified 64 studies that met our inclusion criteria (n = 28597).
Results
We found that patients with hypothyroidism had lower vitamin B12 levels than healthy participants (MD: −60.67 pg/mL; 95% CI: −107.31 to −14.03 pg/mL; p = 0.01). No significant differences in vitamin B12 levels were observed between healthy participants and patients with hyperthyroidism (p = 0.78), autoimmune thyroid disease (AITD) (p = 0.22), or subclinical hypothyroidism (SH) (p = 0.79). The frequencies of vitamin B12 deficiency among patients with hypothyroidism, hyperthyroidism, SH, and AITD were 27%, 6%, 27%, and 18%, respectively.
Conclusions
Patients with hypothyroidism had lower levels of vitamin B12 than healthy participants. No significant differences were observed between vitamin B12 levels and hyperthyroidism, AITD, or SH.
Systematic Review Registration
https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=324422, identifier (CRD42022324422).
Keywords: thyroid, vitamin B12, autoimmune thyroid disease, hypothyroidism, hyperthyroidism, subclinical hypothyroidism
1. Introduction
Thyroid disorders (TD) are a heterogeneous group of diseases that affect the thyroid’s anatomy or function (1), including hypothyroidism, hyperthyroidism, subclinical hypothyroidism (SH), subclinical hyperthyroidism, structural abnormalities, and cancer (1, 2). The increasing life expectancy of the global population has significantly increased the incidence of TD and its global burden, especially among older adults (3). The frequency and incidence of TD differ among regions. However, it has been estimated that some TD, such as hypothyroidism, affect 5% of the global population (4), whereas hyperthyroidism affects 0.8% and 1.3% of the population in Europe and the USA, respectively (2, 5). Also, the global age-standardized thyroid cancer (TC) rates are 10.1 per 100 000 women and 3.1 per 100 000 men (6).
Thyroid function is regulated by various nutrients, primarily iodine and selenium. Iodine is an essential micronutrient required for thyroid hormone synthesis, whereas selenium is a cofactor of thyroid enzymes (7, 8). Certain vitamins also play moderating roles in thyroid function, such as vitamins A, E, D and B. Previous studies have reported vitamin deficiencies in patients with TD (7, 9). Regarding the B complex vitamins, B12 is one of the most important as it is indispensable to several biochemical processes. In fact, Vitamin B12, or cobalamin, plays a central role in hematopoiesis and is a component of enzymes, such as methylmalonyl-coenzyme. Although the causes of vitamin B12 deficiency in patients with TD may be multifactorial, they would be predominantly related to the comorbidity of other autoimmune disorders and dietary habits (10–12).
The intrinsic factor of Castle is a mucoprotein essential for the absorption of vitamin B12 at the distal ileum that is synthesized and secreted by the parietal cells of the stomach (13). Therefore, these cells play a key role in pathologies associated with vitamin B12 deficiency, such as pernicious anemia (PA) and autoimmune atrophic gastritis (AAG). Thus, the detection of anti-parietal cell antibodies (APCA) has emerged as a means for screening these pathologies (14).
As aforementioned, the frequency of TD has been increasing in the last decades. Thus, an in-depth assessment of the vitamin B12 serum levels among patients with and without TD, frequency of vitamin B12 deficiency, and presence of APCA in patients with TD has great clinical relevance and impact. Even though a narrative review described the association between vitamin B12 levels and TD (15), there was not an adequate data search and selection strategy, which are necessary to systematize the available evidence on this association. Therefore, the main objective of this systematic review was to evaluate the differences in the serum levels of vitamin B12 among patients with and without TD. The secondary objectives were to evaluate the frequency of vitamin B12 deficiency in patients with TD and the frequency of APCA in patients with autoimmune thyroid diseases (AITD).
2. Methods
2.1. Registration and search strategy
This systematic review was conducted in accordance with the tenets of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (16) and the Cochrane Handbook for Systematic Reviews. In addition, a summary of the protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) [CRD42022324422].
A systematic search was performed in four databases (PubMed, Scopus, Web of Science, and Embase) on April 3, 2022, with no restrictions regarding language or year of publication. The search included the following keywords: “thyroid diseases” and “vitamin B12”. We also conducted a manual search on preprint platforms (medRxiv and Research Square) and other databases (CINAHL, China National Knowledge Internet databases, Wanfang Database, and Scielo).
2.2. Eligibility criteria
We included studies on adult participants (≥18 years) that met the following criteria: (1) studies assessing the frequency of B12 deficiency in patients with TD, (2) studies evaluating differences in the B12 levels between patients with TD and healthy participants, and (3) studies evaluating the frequency of APCA in patients with TD. We excluded: (1) case reports, (2) editorials, and (3) any type of review.
2.3. Study selection
The articles obtained from the electronic search were uploaded to the data management software Rayyan QCRI (Rayyan Systems Inc. ©, Cambridge, MA, USA). Four of the authors (VAB-Z, JRU-B, EA-B, and EAH-B) independently screened the titles and abstracts of each article to identify potentially eligible studies. Then, they read the full text of the articles identified in the previous stage to find those that met our selection criteria. All studies that did not fully meet the selection criteria were excluded from our review. Any disagreements were resolved by discussion until reaching a consensus among all authors.
2.4. Data extraction
A standardized data collection sheet was created in Microsoft Excel. Two authors (AA-C and PH-A) independently extracted the following information from each article: title, author, year, country, number of participants, age, sex, vitamin B12 assay method, vitamin B12 levels (pg/mL) of healthy participants, vitamin B12 levels of patients with TD, frequency of vitamin B12 deficiency, and frequency of APCA (+). In cases of missing information, the corresponding author was contacted via email to request the missing data.
2.5. Quality assessment
The quality of each study was independently assessed by four reviewers (VAB-Z, JRUB, AA-C, and EAH-B) using the Newcastle–Ottawa Scale (NOS) for the cohort and case–control studies, and an adaptation of the NOS for cross-sectional studies (NOS-CS) (17, 18). Both scales consist of a checklist covering three domains: selection, comparability, and outcome/exposure. For this study, articles with a score of seven or more on these scales were deemed to have a low risk of bias, whereas those with a score of less than seven were deemed to have a high risk of bias. This is the rating system recommended for the NOS and NOS-CS. In case of disagreements over the rating of a study, all authors examined the article and reached a consensus.
2.6. Statistical analysis
The information obtained from the included articles was combined using the Review Manager v.5.4 (The Cochrane Collaboration, Copenhagen, Denmark) and STATA v.17.0 software (College Station, TX: StataCorp LLC). All the meta-analyses were conducted using a random-effects model. The DerSimonian and Laird method was employed to estimate the between-study variance. For the pooled analysis of mean differences (MD), the data from those studies that used medians and interquartile ranges (IQR) were converted to means and standard deviations (SD) using Hozo’s method (19). For variables with the standard errors (SE) reported, SD was determined using the following equation: SE × √ (sample size). For the pooled analysis of proportions, we employed the Clopper–Pearson method to calculate the 95% confidence intervals (CI) and the Freeman–Tukey double arcsine transformation as the variance-stabilizing transformation. The between-study heterogeneity was evaluated using a chi-squared test and the I2 statistic. For the chi-squared test, P-values < 0.1 were considered indicative of heterogeneity. For the I2 statistic, heterogeneity was classified as low if I2 < 30%, moderate if I2 = 30%–60%, and high if I2 > 60%. We conducted subgroup analyses based on the continents where the studies were carried out. Also, sensitivity analysis was also conducted eliminating studies with a high risk of bias. Finally, publication bias was assessed through funnel plots and Egger’s test.
3. Results
3.1. Search results
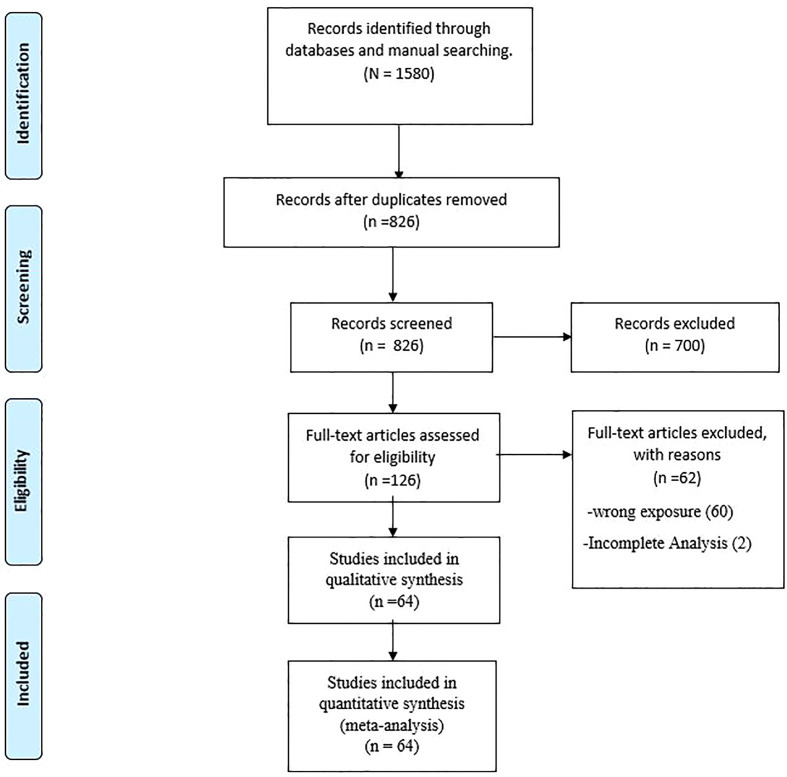
Our electronic search identified 1580 articles, from which 754 duplicates were excluded. The screening of the titles and abstracts led to the exclusion of a further 700 studies. The whole manuscript assessments resulted in the exclusion of 62 studies. Finally, a total of 64 studies were included in our systematic review and meta-analysis (12, 20–82). A flowchart of the selection process is presented in Figure 1 .
Figure 1.
PRISMA Flow Diagram.
3.2. Study characteristics
The characteristics of the included studies are summarized in Table 1 . We included 64 studies published between 1967 and 2022 in 20 countries. A total of 28597 participants (14915 male and 13682 female) were evaluated; however, 12 studies did not report the number of participants by gender. The participants were aged 19–94 years; yet, 15 studies did not provide this information. As well, the cut-off points for defining vitamin B12 deficiency were reported in 31 studies and ranged from 130 to 400 pg/mL.
Table 1.
Characteristics of the studies included in this review.
| Author | Year | Country | Participants (female/male) |
Mean/median age (SD/IQR) | Threshold for vitamin B12 deficiency (pg/mL) | TD evaluated |
Vitamin B12 levels in healthy participants Mean (SD) |
Vitamin B12 levels in patients with TD Mean (SD) |
Patients with TD and vitamin B12 deficiency (frequency %) | Patients with TD and normal/high levels of vitamin B12 (frequency %) | Vitamin B12 assay method | Patients with AITD and APCA (+) (frequency %) | Patients with AITD and APCA(-) (frequency %) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Das et al. | 2012 | India | 60(42/18) | 36(19 - 67) | NR | Hypothyroidism | NR | NR | 9(15) | 51(85) | NR | NR | NR |
| Wang et al. | 2012 | Taiwan | 380(346/34) | 62 (12) | <200 | AITD | 701.9(181.4) | 665.5(286) | 12(6.32) | 178(93.68) | NR | 48(12.7) | 332(87.3) |
| Velarde-Mayol et al. | 2014 | Spain | 409(342/34) | 78(8) | <199 | AITD | NR | NR | 76(18.58) | 333(81.42) | NR | NR | NR |
| Venerito et al. | 2015 | Germany | 34(NR/NR) | 55 (13) | NR | AITD | NR | NR | 1(2.94) | 33(97.06) | NR | 11(32.35) | 23(64.65) |
| Bhuta et al. | 2019 | India | 60(48/12) | NR(NR) | <210 | Hypothyroidism | NR | NR | 17(28.3) | 43(71.7) | FEI | NR | NR |
| Sattar-Lakho et al. | 2018 | Pakistan | 145(48/97) | 42(8.9) | <150 | Hypothyroidism | NR | NR | 105(72.41) | 40(27.6) | NR | NR | NR |
| Kumari et al. | 2015 | India | 350(250/100) | 32 (11) | <200 | AITD | NR | NR | 194(55.43) | 156 (44.57) | NR | NR | NR |
| Carrol et al. | 2015 | United States of America | 80(61/19) | 55(17) | <200 | AITD | 655.8 (342.7) | 606.8 (281.8) | 0. 0(0) | 36 (100) | ECLIA | 4(11.11) | 32(88.89) |
| Siddique et al. | 2017 | Pakistan | 225(122/103) | 47(7) | NR | Hypothyroidism | NR | NR | 54(24) | 171(76) | CLIA | NR | NR |
| Jabbar et al. | 2008 | Pakistan | 116(95/21) | 44 (13) | <200 | Hypothyroidism | NR | NR | 47(41.52) | 69(59.48) | RIA | NR | NR |
| Jabeen et al. | 2016 | Pakistan | 204(197/7) | 37 (11) | <200 | Hypothyroidism | NR | NR | 112(54.9) | 92(45.1) | RIA | NR | NR |
| Adnan et al. | 2019 | Iraq | 70(NR/NR) | NR(NR) | <400 | Hypothyroidism | 788.62(138.21) | 462.06(224.93) | NR | NR | Spectrophotometry | NR | NR |
| Şanver et al. | 2022 | Turkey | 261(261/0) | 46(15) | NR | AITD | 377(287) | 351(188) | NR | NR | CLIA | NR | NR |
| Twito et al. | 2015 | Israel | 120(108/12) | 50(16) | NR | AITD | NR | NR | NR | NR | NR | 34(28.3) | 86(71.3) |
| Utiyama et al. | 2017 | Brazil | 243(213/30) | 45 (13) | NR | AITD | NR | NR | NR | NR | NR | 49(20.16) | 194(75.84) |
| Howel et al. | 1967 | England | 74(65/9) | NR(NR) | NR | AITD | NR | NR | NR | NR | NR | 5(6.76) | 69(93.24) |
| Tozzoli et al | 2010 | Italy | 208(187/21) | 43(29) | NR | AITD | NR | NR | NR | NR | NR | 51(24.5) | 157(75.48) |
| Gerenova et al. | 2013 | Bulgaria | 151(142/9) | 49(1.2) | NR | AITD | NR | NR | NR | NR | NR | 51(33.77) | 100(66.23) |
| Yadav et al. | 2019 | India | 100(88/12) | 33(7) | <211 | Hypothyroidism | NR | NR | 12(12) | 88(88) | NR | NR | NR |
| Checchi et al. | 2008 | Italy | 391(351/40) | 55.3(15) | NR | AITD | NR | NR | NR | NR | NR | 155(39.6) | 236(60.4) |
| Lahner et al. | 2008 | Italy | 128(107/21) | 54 (20–76) | NR | AITD | NR | NR | NR | NR | NR | 110(86.7) | 18(13.3) |
| Chan et al. | 2009 | China | 56(40/16) | 75(46) | NR | AITD | NR | NR | NR | NR | NR | 37(66.07) | 19(33.93) |
| Morawiec-Szymonik et al. | 2019 | Poland | 51(35/16) | NR(NR) | NR | AITD | NR | NR | NR | NR | NR | 29(56.86) | 22(43.14) |
| Khan et al. | 2019 | India | 75(45/30) | NR(NR) | <211 | Hypothyroidism | NR | NR | 45(60) | 30(40) | NR | NR | NR |
| Colleran et al. | 2003 | United States of America | 31(NR/NR) | NR(NR) | <200 | Hyperthyroidism | 418 (219) | 514 (210) | 1(4.76) | 20(95.24) | CLIA | NR | NR |
| Alperin et al. | 1970 | United States of America | 88(NR/NR) | NR(NR) | <200 | Hyperthyroidism | 572(183) | 347(144) | 2(5.9) | 32(94.1) | NR | NR | NR |
| Nitu et al. | 2016 | India | 100(100/0) | NR(NR) | NR | Hyperthyroidism | 276.08 (120.01) | 341.5(25.58 ) |
NR | NR | HPLC | NR | NR |
| Kumar et al. | 2019 | India | 400(NR/NR) | NR(NR) | NR | Hypothyroidism | 456.43(243.54) | 211.34(121.45) | NR | NR | CLIA | NR | NR |
| Tripathi et al. | 2019 | India | 350(214/136) | 33.78(13.9) | NR | Hypothyroidism | 483.93(264.7 4) | 210.45(129.3) | NR | NR | NR | NR | NR |
| Berker et al. | 2009 | Turkey | 42(42/0) | 24 (2.8) | NR | Hyperthyroidism | 235 (86.9) | 209.5 (36.3) | 0(0) | 42(100) | CLIA | NR | NR |
| Khubchandani et al. | 2015 | India | 100(72/28) | 39.48(14.19) | <200 | Hypothyroidism | 365.17(45.82) | 187.38(35.89) | 32(64) | 18(36) | CLIA | NR | NR |
| Choudhary et al. | 2021 | India | 150(86/64) | NR(NR) | <200 | Hypothyroidism | NR | NR | 38(25.3) | 112(74.7) | NR | NR | NR |
| Garcia-Garcia | 2010 | Spain | 148(137/11) | 45(15) | NR | AITD | NR | NR | NR | NR | NR | 30(20.27) | 118(79.73) |
| Castoro et al. | 2016 | Italy | 242(207/35) | 41 (12–78) | NR | AITD | NR | NR | NR | NR | NR | 57(23.55) | 185(76.45) |
| Alexandraki et al. | 2014 | Greece | 120(98/22) | 51 (13) | NR | AITD | NR | NR | NR | NR | NR | 38(31.67) | 82(68.33) |
| Souka et al. | 2018 | United Arab Emirates | 60(60/0) | 40.8(9.5) | NR | Hypothyroidism | 371.75(201.48) | 279.25(362.9) | NR | NR | ECLIA | NR | NR |
| Ozmen et al. | 2006 | Turkey | 47(35/12) | 51(35–66) | NR | Hypothyroidism | 474.93(327.21) | 475.61(172.63) | NR | NR | CLIA | NR | NR |
| Min-Yu et al. | 2014 | South Korea | 17541(6209/11332) | 41.8 (10) | NR | Hypothyroidism | 421(95.3) | 423 (91.3) | NR | NR | CLIA | NR | NR |
| SH | 421(95.3) | 423 (91.3) | NR | NR | |||||||||
| Srikrishna et al. | 2015 | India | 440(377/63) | 48.41(11.65) | <211 | Hypothyroidism | 412(93) | 327(90) | NR | NR | ECLIA | NR | NR |
| SH | 412(93) | 393(99) | NR | NR | |||||||||
| Sengul et al. | 2004 | Turkey | 58(58/0) | 42.3 (10.85) | NR | Hypothyroidism | 241.4 (33.47) | 250.69 (110.13) | NR | NR | CLIA | NR | NR |
| SH | 241.4 (33.47) | 250.69 (110.13) | NR | NR | |||||||||
| Luboshitzky et al. | 2002 | Israel | 91(91/0) | 48(13) | <192 | Hypothyroidism | 298(135) | 317(140) | NR | NR | CLIA | NR | NR |
| SH | 298(135) | 317(140) | NR | NR | |||||||||
| Çakal et al. | 2007 | Turkey | 46(NR/NR) | 41.4(14.1) | <145 | Hypothyroidism | 244.2(47.2) | 236.4(102) | NR | NR | CLIA | NR | NR |
| SH | 244.2(47.2) | 245.1(88.8) | NR | NR | |||||||||
| Nedrebo et al. | 1998 | Norway | 438(250/188) | 48(19–89) | NR | Hypothyroidism | 508.13(39.02) | 724.93(122.45) | NR | NR | CLIA | NR | NR |
| Hyperthyroidism | 508.13(39.02) | 577.23(104.37) | NR | NR | |||||||||
| Diekman et al. | 2001 | Netherlands | 96(75/21) | 38 (22–79) | NR | Hypothyroidism | 410.56(161.24) | 453.92(253.38) | NR | NR | RIA | NR | NR |
| Hyperthyroidism | 471.54(197.83) | 462.05(188.34) | NR | NR | |||||||||
| Calcaterra et al. | 2019 | Italy | 220(184/36) | NR(NR) | NR | AITD | NR | NR | 4(40) | 6(60) | CLIA | 10(4.55) | 210(95.45) |
| Hypothyroidism | NR | NR | 3(37.5) | 5(62.5) | |||||||||
| Hyperthyroidism | NR | NR | 1(33.3) | 2(66.7) | |||||||||
| Dagdelen et al. | 2012 | Turkey | 327(NR/NR) | NR(NR) | <200 | AITD | NR | NR | 51(15.6) | 276(84.4) | NR | NR | NR |
| Hypothyroidism | NR | NR | 50(18.25) | 224(81.75) | |||||||||
| Hyperthyroidism | NR | NR | 1(1.9) | 52(98.1) | |||||||||
| Leineweber et al. | 2016 | United States of America | 494(NR/NR) | NR(NR) | <200 | AITD | NR | NR | 19(4.8) | 379(95.2) | NR | 88(22.1) | 310(77.9) |
| Hypothyroidism | NR | NR | 18(4) | 439(96) | |||||||||
| Hyperthyroidism | NR | NR | 4(10.8) | 33(89.2) | |||||||||
| Meling et al. | 2022 | Norway | 458(331/127) | NR(NR) | <200 | AITD | NR | NR | 48(10.5) | 410(89.5) | NR | NR | NR |
| Hypothyroidism | NR | NR | 36(9.5) | 344(90.5) | |||||||||
| Hyperthyroidism | NR | NR | 12(15.4) | 66(84.6) | |||||||||
| Wiebolt et al. | 2011 | Netherlands | 882(751/132) | 50(14) | <130 | AITD | NR | NR | 66(8.85) | 680(91.15) | NR | 73(12.2) | 525(87.8) |
| Hypothyroidism | NR | NR | 30(8.8) | 310(91.2) | |||||||||
| Hyperthyroidism | NR | NR | 36(7.6) | 436(92.4) | |||||||||
| Raju et al. | 2021 | India | 50(27/23) | 45(12.8) | <200 | AITD | NR | NR | 17(70) | 7(30) | RIA | NR | NR |
| Hypothyroidism | NR | NR | 26(52) | 24(48) | |||||||||
| SH | NR | NR | 16(57.14) | 12(42.86) | |||||||||
| Ness-Abramof et al. | 2006 | Israel | 115(108/7) | 47 (15) | <133 | AITD | NR | NR | 32(27.8) | 83(72.2) | CLIA | NR | NR |
| Hypothyroidism | NR | NR | 27(28.7) | 67(71.3) | |||||||||
| Hyperthyroidism | NR | NR | 3(17.6) | 14(82.4) | |||||||||
| Morel et al. | 2009 | France | 226(NR/NR) | 56 (20–94) | <180 | AITD | 435(574.8) | 588.75(972.59) | NR | NR | CLIA | NR NR | |
| Hypothyroidism | 435(574.8) | 557(1001.4) | NR | NR | |||||||||
| Mehmet et al. | 2012 | Turkey | 200(173/27) | 44.9(14.2) | <189 | Hypothyroidism | 299.1 (205.5) |
400.2 (314.5) |
18(18) | 82(82) | NR | NR | NR |
| SH | 299.1 (205.5) |
348.5(211.8) | 25(25) | 75(75) | |||||||||
| Caplan et al. | 1975 | United States of America | 103(NR/NR) | 56.8 (2.1) | <200 | Hypothyroidism | 412 (213.12) | 450 (231.98) | NR | NR | Microbiological assay | NR | NR |
| Hyperthyroidism | 412 (213.12) | 499 (253.65) | NR | NR | |||||||||
| Ranjan et al. | 2020 | India | 150(NR/NR) | NR(NR) | NR | Hypothyroidism | 314.85 (41.1) | 277.2 (37.89) | NR | NR | CLIA | NR | NR |
| SH | 314.85 (41.1) | 277.2 (37.89) | NR | NR | |||||||||
| Onat et al. | 2003 | Turkey | 85(74/11) | 59.93 (19.84) | NR | Hypothyroidism | 521.92 (121.45) | 225.6 (97.16) | NR | NR | CLIA | NR | NR |
| Hyperthyroidism | 521.92 (121.45) | 359.36 (323.15) | NR | NR | |||||||||
| Photam et al. | 2014 | India | 47(NR/NR) | NR(NR) | NR | Hyperthyroidism | 205(111.85) | 249(165.18) | NR | NR | NR | NR | NR |
| AITD | 205(111.85) | 249(165.18) | NR | NR | |||||||||
| Miskiewicz et al. | 2015 | Poland | 8(5/3) | 33 (22–68) | NR | AITD | NR | NR | 2(25) | 6(75) | NR | 1(12.5) | 7(87.5) |
| Hyperthyroidism | NR | NR | 2(25) | 6(75) | |||||||||
| Orzechowska-Pawilojc et al. | 2007 | Poland | 61(61/0) | 37.9(10.3) | <179 | AITD | 420.83(142.07) | 329.69(154.37) | 3(9.7) | 28(90.3) | CLIA | NR | NR |
| Hypothyroidism | 420.83(142.07) | 329.69(154.37) | 3(9.7) | 28(90.3) | |||||||||
| Nicolaou et al. | 2014 | Greece | 115(99/16) | 47.7(12.9) | NR | AITD | NR | NR | 19(16.5) | 96(83.5) | NR | 33(28.7) | 82(71.3) |
| Hypothyroidism | NR | NR | 19(16.5) | 96(83.5) | |||||||||
| Aon et al. | 2022 | Kuwait | 93(80/13) | 34(14) | <133 | Hypothyroidism | NR | NR | 15(33.3) | 30(66.7) | CLIA | NR | NR |
| SH | NR | NR | 23(47.9) | 25(52.1) | |||||||||
| Aktaş et al. | 2020 | Turkey | 130(115/15) | 41.4(1.9) | <200 | AITD | NR | NR | 60(46.15) | 70(53.85) | CLIA | NR | NR |
| Hypothyroidism | NR | NR | 60(46.15) | 70(53.85) | |||||||||
| Nalbant et al. | 2016 | Turkey | 211(194/17) | 39.31(11.44) | NR | AITD | 261.5(109.3) | 259(105.1) | 51(24.17) | 160(75.83) | CLIA | NR | NR |
| Hyperthyroidism | 261.5(109.3) | 239(68.7) | NR | NR | |||||||||
| Hypothyroidism | 261.5(109.3) | 249.3(85.9) | 51(24.17) | 160(75.83) | |||||||||
| Erdal et al. | 2008 | Turkey | 43(39/4) | 48.5(4.7) | <193 | AITD | NR | NR | 0(0) | 43(100) | CLIA | NR | NR |
| Hypothyroidism | NR | NR | 0(0) | 43(100) | |||||||||
| SH | NR | NR | 0(0) | 43(100) | |||||||||
AITD, autoimmune thyroid disease; APCA, anti-parietal cell antibodies; CLIA, chemiluminescence immunoassay; ECLIA, electrochemiluminescence immunoassay; FEI, fluorescence enzyme immunoassay; HPLC, high-performance liquid chromatography; IQR, interquartile range; NR, not reported; RIA, radio immunoassay; SH, subclinical hypothyroidism; SD, standard deviation; TD, thyroid disorder; 95% CI, 95% confidence interval.
We sent emails requesting the missing information to the authors, but received no reply. A total of 37 studies were classified as having a low risk of bias, and 27 as having a high risk of bias ( Supplemental Table S1 ).
Of the studies included, 40 (n = 24835) evaluated vitamin B12 abnormalities in patients with hypothyroidism. Of these, 20 determined the frequency of vitamin B12 deficiency in patients with hypothyroidism, 16 determined the MD between the vitamin B12 levels of healthy participants and those with hypothyroidism, and 4 evaluated both the MD and the frequencies.
A total of 17 studies (n = 3795) evaluated the B12 levels in patients with hyperthyroidism. Of these, 7 evaluated the frequency of B12 deficiency in patients with hyperthyroidism. As well, 7 evaluated the MD in the B12 levels between healthy participants and patients with hyperthyroidism. The remaining 3 studies evaluated both the MD and the frequencies.
We found 21 studies (n = 4901) that evaluated B12 deficiencies in patients with AITD. Of these, 14 evaluated the frequency of B12 deficiency in AITD, 3 evaluated the MDs in the vitamin B12 levels between healthy participants and AITD patients, and 4 evaluated both the MDs and frequencies.
A total of 10 studies (n = 18712) evaluated B12 deficiencies in patients with SH. Of these, 3 assessed the frequency of B12 deficiency in SH, 6 evaluated the MDs in vitamin B12 levels between healthy participants and patients with SH, and only 1 evaluated both the MD and frequency.
3.3. Differences in the serum levels of vitamin B12 among patients with and without TD
3.3.1. Differences between the vitamin B12 levels of patients with hypothyroidism and healthy participants
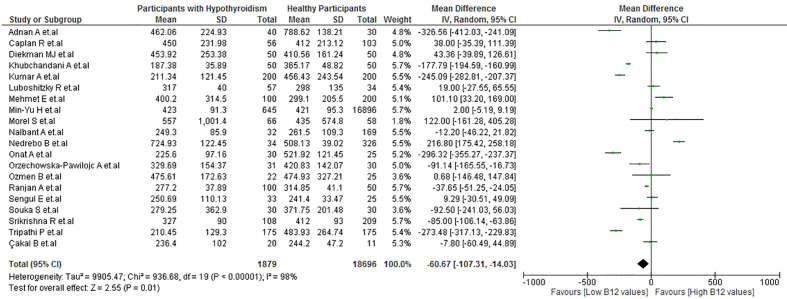
Patients with hypothyroidism had lower B12 levels than healthy participants (MD: −60.67 pg/mL; 95% CI: −107.31 to −14.03 pg/mL; p = 0.01, I2 = 98%) ( Figure 2 ). A subgroup analysis by continent ( Supplemental Figure S1 ) revealed that, in Asian countries, the statistical significance of this difference remained with high heterogeneity (MD: −133.04 pg/mL; 95% CI: −197.84 to −68.23 pg/mL; P < 0.001, I2 = 99%); meanwhile, no statistical significance was observed in European countries (MD: 3.11 pg/mL; 95% CI: −88.39 to 94.62 pg/mL; p = 0.95). In the sensitivity analysis, after removing the studies with a high risk of bias, there was a decrease in heterogeneity (I2 = 53%) ( Supplemental Figure S2 ).
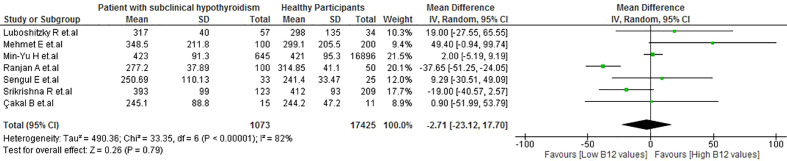
Figure 2.
Vitamin B12 values in patients with hypothyroidism vs healthy patients.
3.3.2. Differences between vitamin B12 levels of patients with hyperthyroidism and healthy participants
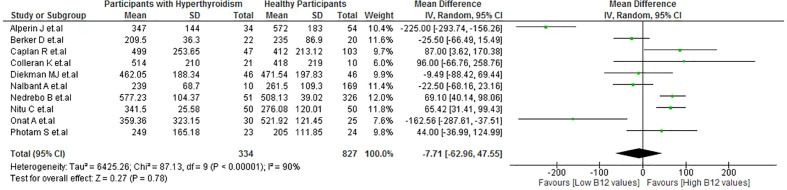
No significant difference was observed in the B12 levels between patients with hyperthyroidism and healthy participants (MD: −7.71 pg/mL; 95% CI: −62.96 to 47.55 pg/mL; p = 0.78, I2 = 90%) ( Figure 3 ). There were also no significant differences in the subgroups analysis ( Supplemental Figure S3 ). In the sensitivity analysis, after removing the studies with a high risk of bias, there was a decrease in heterogeneity (I2 = 0%) ( Supplemental Figure S4 ).
Figure 3.
Vitamin B12 values in patients with hyperthyroidism vs healthy patients.
3.3.3. Differences between the vitamin B12 levels of patients with autoimmune thyroid disease and healthy participants
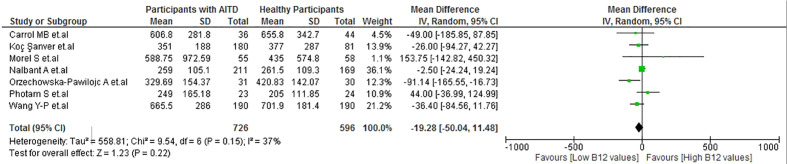
No significant difference was observed in the B12 levels between patients with AITD and healthy participants (MD: −19.28 pg/mL; 95% CI: −50.04 to 11.48 pg/mL; p = 0.22, I2 = 37%) ( Figure 4 ). As well, there were no significant differences in the subgroup analysis ( Supplemental Figure S5 ). In the sensitivity analysis, after removing studies with a high risk of bias, low heterogeneity remained (I2 = 33%) ( Supplemental Figure S6 ).
Figure 4.
Vitamin B12 values in patients with AITD vs healthy participants.
3.3.4. Differences between the vitamin B12 levels of patients with subclinical hypothyroidism and healthy participants
No significant differences in the levels of vitamin B12 were observed between healthy participants and patients with SH (MD: −2.71 pg/mL; 95% CI: −23.12 to 17.7 pg/mL; p = 0.79, I2 = 82%) ( Figure 5 ). Also, there were no significant differences in the subgroup analysis ( Supplemental Figure S7 ). Regarding the sensitivity analysis, after removing the studies with a high risk of bias, high heterogeneity remained (I2 = 70%) ( Supplemental Figure S8 ).
Figure 5.
Vitamin B12 values in patients with SH vs healthy participants.
3.4. Frequency of vitamin B12 deficiency in patients with TD
3.4.1. Evaluation of the frequency of vitamin B12 deficiency in hypothyroidism
The frequency of vitamin B12 deficiency in patients with hypothyroidism was 27.0% (95% CI: 19.0% to 36.0%), with high heterogeneity among studies (I2 = 97%) ( Supplemental Figure S9 ). In the sensitivity analysis, after removing the studies with a high risk of bias, high heterogeneity remained (I2 = 96.5%) ( Supplemental Figure S10 ).
3.4.2. Evaluation of the frequency of vitamin B12 deficiency in hyperthyroidism
The frequency of B12 deficiency in patients with hyperthyroidism was 6.0% (95% CI: 2.0% to 11.0%) with moderate heterogeneity among studies (I2 = 60%) ( Supplemental Figure S11 ). In the sensitivity analysis, after removing the studies with a high risk of bias, there was a decrease in heterogeneity (I2 = 28.03%) ( Supplemental Figure S12 ).
3.4.3. Evaluation of the frequency of vitamin B12 deficiency in autoimmune thyroid disease
The frequency of vitamin B12 deficiency in patients with hypothyroidism was 18.0% (95% CI: 11.0% to 27.0%), with high heterogeneity among studies (I2 = 97%) ( Supplemental Figure S13 ). In the sensitivity analysis, after removing the studies with a high risk of bias, high heterogeneity remained (I2 = 97%) ( Supplemental Figure S14 ).
3.4.4. Evaluation of the frequency of vitamin B12 deficiency in subclinical hypothyroidism
The frequency of vitamin B12 deficiency in patients with SH was 27.0% (95% CI: 5.0% to 57.0%), with high heterogeneity among studies (I2 = 95%) ( Supplemental Figure S15 ).
3.5. Anti-parietal cell antibodies and autoimmune thyroid disease
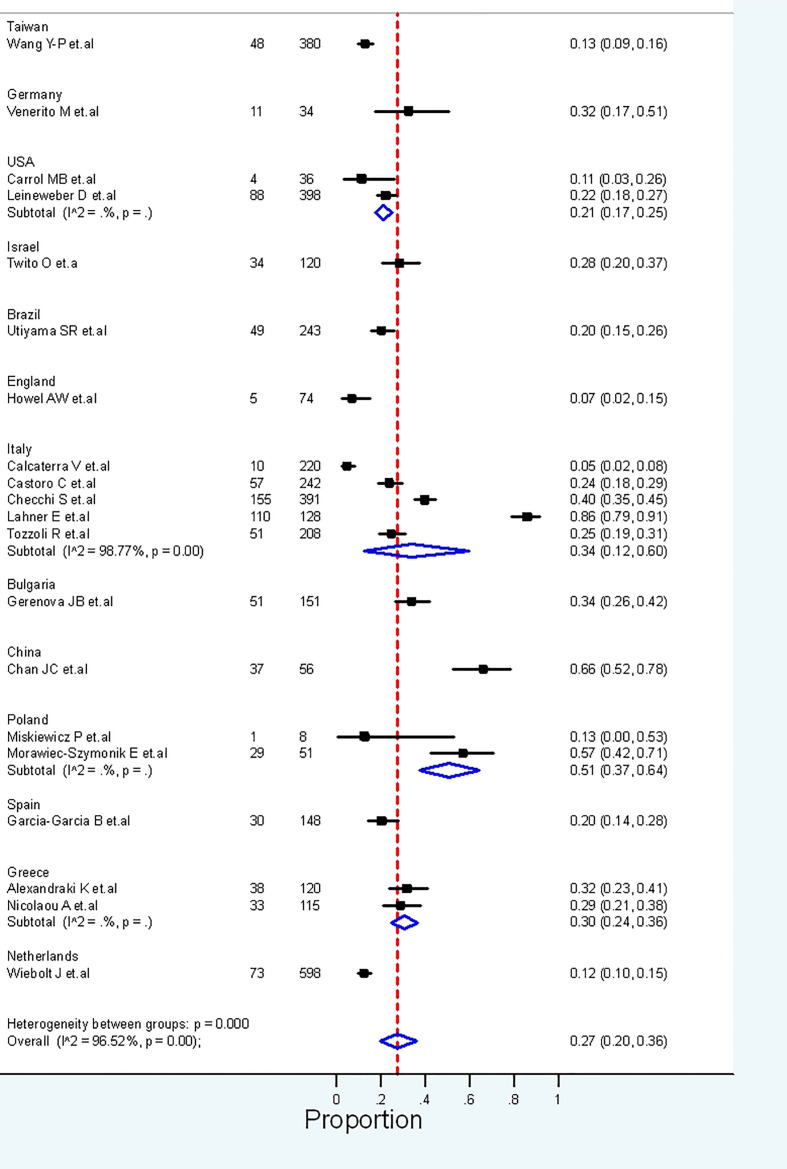
The frequency of APCA in patients with AITD was evaluated in 20 studies (n = 3721). Overall, these studies found APCA to be present in 27.0% (95% CI: 20.0% to 36.0%) of patients with AITD, with high heterogeneity between studies (I2 = 96%) ( Figure 6 ). Regarding the sensitivity analysis, after removing the studies with a high risk of bias, high heterogeneity remained (I2 = 98.19%) ( Supplemental Figure S16 ).
Figure 6.
Frequency of the presence of APCA in AITD.
3.6. Publication bias
When using the Egger test, no publication bias was found in the evaluations of vitamin B12 levels in hypothyroidism (p = 0.495), hyperthyroidism (p = 0.632), AITD (p = 0.687), and SH (p = 0.159). A funnel plot showed no asymmetry in any of the scenarios ( Supplemental Figures S17 - S20 ).
4. Discussion
The main finding of our study was the significant difference in the vitamin B12 levels between patients with hypothyroidism and healthy participants. However, no significant difference was observed in the vitamin B12 levels between patients with hyperthyroidism/AITD/SH and healthy participants. The frequencies of vitamin B12 deficiency in patients with SH, hypothyroidism, hyperthyroidism, and AITD were 27%, 27%, 6%, and 18%, respectively, and the frequency of APCA in AITD was 27%. Our results should serve as a basis for the modification of clinical practice guidelines on TD.
Vitamin B12 is synthesized by intestinal anaerobic microorganisms, albeit its impact is still unexplored; while oral dietary intake provides 50% of B12 requirements (83, 84). Vitamin B12 is naturally found in foods of animal origin and in crystalline form in supplements and fortified foods. It comes in the form of cyanocobalamin or hydroxocobalamin, which can be converted to either of the two forms of vitamin B12 cofactors, whose transformation reactions are essential for the synthesis of nucleic acids, myelination of the nerves and axons of the central nervous system, and efficient bone marrow erythropoiesis (15). Thus, an adequate supply of this vitamin is required to maintain these biological processes.
Inadequate dietary intake and malabsorption are the major causes of vitamin B12 deficiency (83), though the first rarely occurs in high-income countries, as foods of animal origin are an important component of the diet. Nevertheless, it may occur in strict vegetarians and malnourished older adults (85). On the other hand, B12 malabsorption is found in some medical conditions such as AAG, Helicobacter pylori infection, PA, and long-term antacid treatment (15). It can also be a consequence of surgeries such as partial gastrectomy and gastric bypass (15).
Previously, a narrative review found a frequency of B12 deficiency ranging from 10% to 40.5% in patients with hypothyroidism and from 6.3% to 55.5% in patients with AITD (15). However, it was not as comprehensive as our systematic review, in which we conducted a meta-analysis. Our results were based on a larger number of studies, thus decreasing variations and generating a more accurate frequency. Nonetheless, the prevalence of B12 deficiency varies depending on the cut-off used to define it. The B12 level that constitutes deficiency depends on both the population and the method employed to measure the B12 levels. For instance, serum vitamin B12 levels < 148 pmol/L are generally considered deficient in high-income countries. Using this parameter, vitamin B12 deficiency has been shown to increase with age, from 3% in young adults to 10% in older adults (82). The incidence of subclinical B12 deficiency, defined as serum vitamin B12 levels of 148–221 pmol/L, affects about 20% of older adults (82). Contrarily, the incidence is higher in low-income countries, where low and borderline B12 levels are detected in around 70% of adults (82). It is important to note that serum B12 is the primary test used in clinical practice, in spite of its poor sensitivity and specificity for identifying B12 deficiency (82). Although more sensitive tests have been developed, including plasma methylmalonic acid (MMA), homocysteine, and serum holotranscobalamin, they are expensive and not routinely available. Moreover, they do not have defined cut-off points to denote deficiency. Accordingly, their role in clinical practice is still unclear (86).
The association between vitamin B12 deficiency and TD has been studied with a particular focus on patients with AITD (15). AITD encompasses a group of disorders characterized by the production of antibodies against the thyroid gland, with Graves’ disease and Hashimoto’s thyroiditis being the most common (15). Even though the causes of vitamin B12 deficiency among these patients are likely to be multifactorial, they would be predominantly related to the comorbidity of other autoimmune disorders, such as AAG, PA (10), and celiac disease (11). Indeed, the frequency of AAG among patients with AITD ranges from 35% to 40%; meanwhile, the frequency of PA in the same group of patients reaches 16% (53, 87). As well, 26% of patients with celiac disease also have AITD (11).
In the absence of AITD, the causes of vitamin B12 deficiency in those with hypothyroidism have been studied in less detail; however, they might be related to dietary habits (12). Alterations in the composition of the microbiota, bacterial overgrowth, and slow intestinal motility have also been proposed as potential causes in these patients (8, 12). Given the increased frequency of hypothyroidism and vitamin B12 deficiency with ageing, age should also be considered as a contributing factor (5, 88). This convergence of factors would explain not only the vitamin B12 deficiency but also the deficiencies in trace elements, which are also frequently evidenced in patients with hypothyroidism and SH (89). In a meta-analysis carried out in India, the frequency of B12 deficiency in healthy adults was 48% (90), being higher than the frequencies of all the TDs we obtained.
While tests for the presence of APCA are considered the most sensitive form of AAG diagnosis (91), some studies have suggested that these antibodies can also be found in 7.8% of healthy individuals and 19.5% of patients infected with H. pylori (14, 91). Their association with other autoimmune diseases, such as type 1 diabetes mellitus, vitiligo, and celiac disease, is also well known (26, 92). Several studies have demonstrated that APCA-positive patients have a higher incidence of anemia as the involved antibodies can induce the destruction of gastric parietal cells, preventing the production of intrinsic factor, and leading to insufficient vitamin B12 absorption and PA (47, 48, 93). Variations in the frequency of APCA would differ due to the heterogeneity of the studies, but some researchers have suggested that this variation may also be attributable to the AITD type. Utiyama et al. found that 20.16% of patients with AITD tested positive for APCA, with a frequency of 21.3% among those with Graves’ disease and 18.6% among those with Hashimoto’s thyroiditis (48).
Our findings are particularly useful in clinical settings as they emphasize the necessity of in-depth evaluations of vitamin B12 levels in patients with TD. Nearly one in four patients with either SH or hypothyroidism suffers from B12 deficiency. Although it is tempting to suggest routine vitamin B12 assessment in patients with TD, more studies are needed to support this practice. There is still scarce evidence suggesting that the administration of vitamins with antioxidant properties in patients with TD, such as hyperthyroidism, can decrease the severity of clinical symptoms (94). Likewise, some studies suggest vitamin D supplementation can have a beneficial effect on bone system among these patients (94). Nevertheless, the role of vitamin D is controversial. A systematic review revealed that although there are various health benefits of dietary supplements in the prevention and treatment of several TD, there are also many risks associated with the use of these supplements (95). In this regard, clinical practice guidelines should include nutritional assessments as part of the management of TD patients. We found that many of the current guidelines on TD do not require a comprehensive nutritional evaluation as part of their management plan, nor do they recommend assessing and addressing B12 deficiencies (96–98).
4.1. Limitations
This study has some limitations that need to be considered. Firstly, most of the studies included were conducted on the Asian continent, with few were from other continents. Differences in B12 levels among people of different ethnicities should be measured and compared to determine whether the results of this review can be ethnically generalised. Secondly, there was high statistical heterogeneity caused by clinical and methodological differences, and our sensitivity analysis was only able to reduce it in some cases. Thirdly, there were few studies assessing the association between B12 vitamin and the levels of TPO-Ab and Tg-Ab. We therefore encourage further evaluation of this association in future studies. Finally, vitamin B12 levels were not adjusted for sociodemographic variables or comorbidities. Such adjustment could allow a cut-off consensus to be obtained according to the conditions of each population and should be considered in future studies.
5. Conclusion
Patients with hypothyroidism had lower levels of vitamin B12 than healthy participants. No significant differences were found between vitamin B12 levels and hyperthyroidism, AITD, or SH. The co-occurrence of APCA and vitamin B12 deficiency in TD patients did not exceed 30% in any of the reviewed studies.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Author contributions
Conceptualization, VB-Z, JU-B, EA-B, EH-B, AA-C, PH-A and FI-C. Data curation, VB-Z, JU-B, EA-B and EH-B. Formal analysis, JU-B, EA-B and VB-Z. Methodology, VB-Z, JU-B, EA-B, and EH-B. Writing original draft, VB-Z, JU-B, EA-B, EH-B, AA-C, PH-A and FI-C. Review and editing, JU-B, AA-C, FI-C, PH-A and VB-Z. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1070592/full#supplementary-material
References
- 1. Alarcon G, Figueredo V, Tarkoff J. Thyroid disorders. Pediatr Rev (2021) 42:604–18. doi: 10.1542/pir.2020-001420 [DOI] [PubMed] [Google Scholar]
- 2. Hollowell JG, Staehling NW, Dana Flanders W, Harry Hannon W, Gunter EW, Spencer CA, et al. Serum TSH, T(4), and thyroid antibodies in the united states population (1988 to 1994): National health and nutrition examination survey (NHANES III). J Clin Endocrinol Metab (2002) 87:489–99. doi: 10.1210/jcem.87.2.8182 [DOI] [PubMed] [Google Scholar]
- 3. Mariotti S, Franceschi C, Cossarizza A, Pinchera A. The aging thyroid. Endocr Rev (1995) 16:686–715. doi: 10.1210/edrv-16-6-686 [DOI] [PubMed] [Google Scholar]
- 4. Chiovato L, Magri F, Carlé A. Hypothyroidism in context: Where we’ve been and where we’re going. Adv Ther (2019) 36:47–58. doi: 10.1007/s12325-019-01080-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garmendia Madariaga A, Santos Palacios S, Guillén-Grima F, Galofré JC. The incidence and prevalence of thyroid dysfunction in Europe: A meta-analysis. J Clin Endocrinol Metab (2014) 99:923–31. doi: 10.1210/jc.2013-2409 [DOI] [PubMed] [Google Scholar]
- 6. Pizzato M, Li M, Vignat J, Laversanne M, Singh D, La Vecchia C, et al. The epidemiological landscape of thyroid cancer worldwide: GLOBOCAN estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol (2022) 10:264–72. doi: 10.1016/S2213-8587(22)00035-3 [DOI] [PubMed] [Google Scholar]
- 7. Triggiani V, Tafaro E, Giagulli V, Sabba C, Resta F, Licchelli B, et al. Role of iodine, selenium and other micronutrients in thyroid function and disorders. Endocr Metab Immune Disord Drug Targets (2009) 9:277–94. doi: 10.2174/187153009789044392 [DOI] [PubMed] [Google Scholar]
- 8. Knezevic J, Starchl C, Tmava Berisha A, Amrein K. Thyroid-Gut-Axis: How does the microbiota influence thyroid function? Nutrients (2020) 12(6):1769. doi: 10.3390/nu12061769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taheriniya S, Arab A, Hadi A, Fadel A, Askari G. Vitamin d and thyroid disorders: A systematic review and meta-analysis of observational studies. BMC Endocr Disord (2021) 21(1):171. doi: 10.1186/s12902-021-00831-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iddah MA, Macharia BN. Autoimmune thyroid disorders. Int Sch Res Notices (2013) 11(1):e0147601. doi: 10.1371/journal.pone.0147601 [DOI] [Google Scholar]
- 11. Lauret E, Rodrigo L. Celiac disease and autoimmune-associated conditions. BioMed Res Int (2013) 22(10):985–96. doi: 10.2174/1871530322666220419125131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jabbar A, Yawar A, Waseem S, Islam N, Haque NU, Zuberi L, et al. Vitamin B12 deficiency common in primary hypothyroidism. J Pak Med Assoc (2008) 58:258–61. Available at: https://jpma.org.pk/article-details/1394?article_id=1394. [PubMed] [Google Scholar]
- 13. Green R, Allen LH, Bjørke-Monsen AL, Brito A, Guéant JL, Miller JW, et al. Vitamin B12 deficiency. Nat Rev Dis Primers (2017) 3:17040. doi: 10.1038/nrdp.2017.40 [DOI] [PubMed] [Google Scholar]
- 14. Rusak E, Chobot A, Krzywicka A, Wenzlau J. Anti-parietal cell antibodies – diagnostic significance. Adv Med Sci (2016) 61:175–9. doi: 10.1016/j.advms.2015.12.004 [DOI] [PubMed] [Google Scholar]
- 15. Collins AB, Pawlak R. Prevalence of vitamin b-12 deficiency among patients with thyroid dysfunction. Asia Pac J Clin Nutr (2016) 25:221–6. doi: 10.6133/apjcn.2016.25.2.22 [DOI] [PubMed] [Google Scholar]
- 16. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (2009) 339:b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alarcón-Braga EA, Hernandez-Bustamante EA, Salazar-Valdivia FE, Valdez-Cornejo VA, Mosquera-Rojas MD, Ulloque-Badaracco JR, et al. Acceptance towards COVID-19 vaccination in Latin America and the Caribbean: A systematic review and meta-analysis. Travel Med Infect Dis (2022) 49:102369. doi: 10.1016/j.tmaid.2022.102369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Modesti PA, Reboldi G, Cappuccio FP, Agyemang C, Remuzzi G, Rapi S, et al. Panethnic differences in blood pressure in Europe: A systematic review and meta-analysis. PloS One (2016) 11. doi: 10.1371/journal.pone.0147601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Method (2005) 5:1–10. doi: 10.1186/1471-2288-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Das C, Sahana P, Sengupta N, Giri D, Roy M, Mukhopadhyay P. Etiology of anemia in primary hypothyroid subjects in a tertiary care center in Eastern India. Indian J Endocrinol Metab (2012) 16:S361–363. doi: 10.4103/2230-8210.104093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bhuta P, Shah A, Muley AA. Study of anemia in hypothyroidism with reference to vitamin B12 deficiency. Int J Adv Med (2019) 6:1667–71. doi: 10.18203/2349-3933.ijam20194240 [DOI] [Google Scholar]
- 22. Velarde-Mayol C, de la Hoz-García B, del Cañizo-Fernández-Roldán C, Hernández-López AM, Loza-Candia I, Cardona-Hernández A. Anemia perniciosa y enfermedades tiroideas autoinmunes en una población mayor de 65 años. REGG (2015) 50:126–8. doi: 10.1016/j.regg.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 23. Calcaterra V, Montalbano C, Miceli E, Luinetti O, Albertini R, Vinci F, et al. Anti-gastric parietal cell antibodies for autoimmune gastritis screening in juvenile autoimmune thyroid disease. J Endocrinol Invest (2020) 43:81–6. doi: 10.1007/s40618-019-01081-y [DOI] [PubMed] [Google Scholar]
- 24. Duran İ.D., Ağbaht K, Soykan İ., Güllü S. Coexistence of autoimmune and allergic diseases with autoimmune thyroid diseases. Turk J Endocrinol Metab (2017) 21:120–6. doi: 10.25179/tjem.2017-58031 [DOI] [Google Scholar]
- 25. Morel S, Georges A, Bordenave L, Corcuff JB. Thyroid and gastric autoimmune diseasesMaladies auto-immunes thyroïdiennes et gastriques. Ann Endocrinol (2009) 70:55–8. doi: 10.1016/j.ando.2008.11.003 [DOI] [PubMed] [Google Scholar]
- 26. Venerito M, Radünz M, Reschke K, Reinhold D, Frauenschläger K, Jechorek D, et al. Autoimmune gastritis in autoimmune thyroid disease. Aliment Pharmacol Ther (2015) 41:686–93. doi: 10.1111/apt.13097 [DOI] [PubMed] [Google Scholar]
- 27. Leineweber D, Galling B, Schurmeyer TH. Autoimmune thyroid diseases (AITD) and mental illness: An evaluation of parietal cell antibodies, intrinsic factor antibodies, vitamin B12 and psychiatric symptoms in patients with AITD and non-AITD controls. Endocr abstr (2016) 41:EP100. doi: 10.1530/endoabs.41.EP1000 [DOI] [Google Scholar]
- 28. Meling Stokland A-E, Ueland G, Lima K, Grønning K, Finnes TE, Svendsen M, et al. Autoimmune thyroid disorders in autoimmune Addison disease. J Clin Endocr Metab (2022) 107:e2331–8. doi: 10.1210/clinem/dgac089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang YP, Lin HP, Chen HM, Kuo YS, Lang MJ, Sun A. Hemoglobin, iron, and vitamin B12 deficiencies and high blood homocysteine levels in patients with anti-thyroid autoantibodies. J Formos Med Assoc (2014) 113:155–60. doi: 10.1016/j.jfma.2012.04.003 [DOI] [PubMed] [Google Scholar]
- 30. Mehmet E, Aybike K, Ganidagli S, Mustafa K. Characteristics of anemia in subclinical and overt hypothyroid patients. Endocr J (2012) 59:213–20. doi: 10.1507/endocrj.ej11-0096 [DOI] [PubMed] [Google Scholar]
- 31. Wiebolt J, Achterbergh R, Den Boer A, van der Leij S, Marsch E, Suelmann B, et al. Clustering of additional autoimmunity behaves differently in hashimoto's patients compared with graves' patients. Eur J Endocrinol (2011) 164:789–94. doi: 10.1530/eje-10-1172 [DOI] [PubMed] [Google Scholar]
- 32. Lakho AS, Channa AA, Dars AG, Shah SZA, Iqbal M. Vitamin B12 deficiency; vitamin B12 deficiency in patients with hypothyroidism. Prof Med J (2018) 25:753–8. doi: 10.29309/TPMJ/2018.25.05.321 [DOI] [Google Scholar]
- 33. Jaya Kumari S, Bantwal G, Devanath A, Aiyyar V, Patil M. Evaluation of serum vitamin B12 levels and its correlation with anti-thyroperoxidase antibody in patients with autoimmune thyroid disorders. Indian J Clin Biochem (2015) 30:217–20. doi: 10.1007/s12291-014-0418-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miskiewicz P, Gos-Zajac A, Kurylowicz A, Plazinska TM, Franaszczyk M, Bartoszewicz Z, et al. HLA DQ2 haplotype, early onset of graves disease, and positive family history of autoimmune disorders are risk factors for developing celiac disease in patients with graves disease. Endocr Pract (2015) 21:993–1000. doi: 10.4158/EP15700.OR [DOI] [PubMed] [Google Scholar]
- 35. Orzechowska-Pawilojc A, Sworczak K, Lewczuk A, Babinska A. Homocysteine, folate and cobalamin levels in hypothyroid women before and after treatment. Endocr J (2007) 54:471–6. doi: 10.1507/endocrj.k06-112 [DOI] [PubMed] [Google Scholar]
- 36. Raju P, Kumar S. Incidence of vitamin B12 deficiency in patients with hypothyroidism. J Evid Based Med Healthc (2021) 8:415–9. doi: 10.18410/jebmh/2021/81 [DOI] [Google Scholar]
- 37. Nicolaou A, Thomas D, Alexandraki KI, Sougioultzis S, Tsolakis AV, Kaltsas G. Predictive value of gastrin levels for the diagnosis of gastric enterochromaffin-like cell hyperplasia in patients with hashimoto's thyroiditis. Neuroendocrinology (2014) 99:118–22. doi: 10.1159/000362879 [DOI] [PubMed] [Google Scholar]
- 38. Ness-Abramof R, Nabriski DA, Braverman LE, Shilo L, Weiss E, Reshef T, et al. Prevalence and evaluation of B12 deficiency in patients with autoimmune thyroid disease. Am J Med Sci (2006) 332:119–22. doi: 10.1097/00000441-200609000-00004 [DOI] [PubMed] [Google Scholar]
- 39. Carroll MB, Tessier CM. Prevalence of anti-gastric parietal cell antibodies and vitamin B12 deficiency in rheumatoid arthritis. J Musculoskelet Disord Treat (2015) 1:003. Available at: https://clinmedjournals.org/articles/jmdt/journal-of-musculoskeletal-disorders-and-treatment-jmdt-1-003.pdf. [Google Scholar]
- 40. Siddique M, Akhtar Parvez M, Zafar H, Mustafa A, Shafi A. Prevalence of vitamin B12 deficiency in hypothyroid patients. APMC (2017) 11:252–6. doi: 10.29054/apmc/2017.206 [DOI] [Google Scholar]
- 41. Aktas SH. Vitamin B12 and vitamin d levels in patients with autoimmune hypothyroidism and their correlation with anti-thyroid peroxidase antibodies. Med Princ Pract (2020) 29:364–70. doi: 10.1159/000505094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jabeen A, Mushtaq S, Raza H. Vitamin B12 deficiency: prevalence and evaluation of a reversible co-morbidity in hypothyroid patients. Pakistan J Nucl Med (2016) 6:23–9. Available at: https://pjnmed.com/article?mno=284653. [Google Scholar]
- 43. Adnan Otla A, Ahmed Saleh N. Estimation of the level of homocysteine and vitamin B12 in serum of patients with hypothyroidism. Tikrit J Pure Sci (2019) 24:70–3. doi: 10.25130/tjps.24.2019.011 [DOI] [Google Scholar]
- 44. Koc S, Güngör K, Dokuzeylül Güngör N, Uzunlulu M. Iron deficiency in women with thyroid-specific autoantibodies: A case control study. J Exp Clin Med (2022) 39:194–8. doi: 10.52142/omujecm.39.1.38 [DOI] [Google Scholar]
- 45. Makalesi A, Üniversitesi Tıp Fakültesi S, Nalbant A, Aydin A, Ti̇lla H, Ci̇nemre H. HaşhimotoTiroiditli olguların klinik ve laboratuvar bulgularının değerlendirilmesi. Cilt 1 Sayı (2016) 1(3):8–20. Available at: https://dergipark.org.tr/tr/pub/otjhs/issue/24684/261028#article_cite. [Google Scholar]
- 46. Erdal M, Sahin M, Hasimi A, Uckaya G, Kutlu M, Saglam K. Trace element levels in hashimoto thyroiditis patients with subclinical hypothyroidism. Biol Trace Elem Res (2008) 123:1–7. doi: 10.1007/s12011-008-8117-8 [DOI] [PubMed] [Google Scholar]
- 47. Twito O, Shapiro Y, Golan-Cohen A, Dickstein Y, Ness-Abramof R, Shapiro M. Anti-thyroid antibodies, parietal cell antibodies and tissue transglutaminase antibodies in patients with autoimmune thyroid disease. Arch Med Sci (2018) 14:516–20. doi: 10.5114/aoms.2016.58743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Utiyama SRR, De Bem RS, Skare TL, De Carvalho GA, Teixeira LM, Bertolazo M, et al. Anti-parietal cell antibodies in patients with autoimmune thyroid diseases. J Endocrinol Invest (2018) 41:523–9. doi: 10.1007/s40618-017-0755-2 [DOI] [PubMed] [Google Scholar]
- 49. Evans AW, Woodrow JC, McDougall CD, Chew AR, Evans RW. Antibodies in the families of thyrotoxic patients. Lancet (1967) 289:636–41. doi: 10.1016/S0140-6736(67)92539-1 [DOI] [PubMed] [Google Scholar]
- 50. Tozzoli R, Kodermaz G, Perosa AR, Tampoia M, Zucano A, Antico A, et al. Autoantibodies to parietal cells as predictors of atrophic body gastritis: a five-year prospective study in patients with autoimmune thyroid diseases. Autoimmun Rev (2010) 10:80–3. doi: 10.1016/j.autrev.2010.08.006 [DOI] [PubMed] [Google Scholar]
- 51. Gerenova JB, Manolova IM, Tzoneva VI. Clinical significance of autoantibodies to parietal cells in patients with autoimmune thyroid diseases. Folia Med (2013) 55:26–32. doi: 10.2478/folmed-2013-0014 [DOI] [PubMed] [Google Scholar]
- 52. Checchi S, Montanaro A, Pasqui L, Ciuoli C, De Palo V, Chiappetta MC, et al. L-thyroxine requirement in patients with autoimmune hypothyroidism and parietal cell antibodies. J Clin Endocrinol Metab (2008) 93:465–9. doi: 10.1210/jc.2007-1544 [DOI] [PubMed] [Google Scholar]
- 53. Lahner E, Centanni M, Agnello G, Gargano L, Vannella L, Iannoni C, et al. Occurrence and risk factors for autoimmune thyroid disease in patients with atrophic body gastritis. Am J Med (2008) 121:136–41. doi: 10.1016/j.amjmed.2007.09.025 [DOI] [PubMed] [Google Scholar]
- 54. Chan JCW, Liu HSY, Kho BCS, Lau TKH, Li VL, Chan FHY, et al. Pattern of thyroid autoimmunity in chinese patients with pernicious anemia. Am J Med Sci (2009) 337:432–7. doi: 10.1097/maj.0b013e31819c0ecf [DOI] [PubMed] [Google Scholar]
- 55. Morawiec-Szymonik E, Foltyn W, Marek B, Kos-Kudła B, Kajdaniuk D. Pernicious anaemia and endocrine glands antibodies. Endokryn Pol (2019) 70:143–50. doi: 10.5603/ep.a2018.0086 [DOI] [PubMed] [Google Scholar]
- 56. García García B, Gimeno Orna JA, Aguillo Gutiérrez E, Altemir Trallero J, Cabrejas Gómez C, Ilundaín González A, et al. [Prevalence and predictive factors of parietal cell antibody positivity in autoimmune thyroid disease]. Endocrinol Nutr (2010) 57:49–53. doi: 10.1016/j.endonu.2009.11.001 [DOI] [PubMed] [Google Scholar]
- 57. Castoro C, Le Moli R, Arpi ML, Tavarelli M, Sapuppo G, Frittitta L, et al. Association of autoimmune thyroid diseases, chronic atrophic gastritis and gastric carcinoid: experience from a single institution. J Endocrinol Invest (2016) 39:779–84. doi: 10.1007/s40618-016-0445-5 [DOI] [PubMed] [Google Scholar]
- 58. Alexandraki KI, Nikolaou A, Thomas D, Syriou V, Korkolopoulou P, Sougioultzis S, et al. Are patients with autoimmune thyroid disease and autoimmune gastritis at risk of gastric neuroendocrine neoplasms type 1? Clin Endocrinol (2014) 80:685–90. doi: 10.1111/cen.12346 [DOI] [PubMed] [Google Scholar]
- 59. Yadav RK, Ahmad M, Mathur MK, Dhingra V. Study of prevalence and types of anemia in primary hypothyroidism. Int JAdv Med (2019) 6:1574–9. doi: 10.18203/2349-3933.ijam20194221 [DOI] [Google Scholar]
- 60. Caplan RH, Davis K, Bengston B, Smith MJ. Serum folate and vitamin B12 levels in hypothyroid and hyperthyroid patients. Arch Intern Med (1975) 135:701–4. doi: 10.1001/archinte.1975.00330050075013 [DOI] [PubMed] [Google Scholar]
- 61. Khan A, Shah N, Thakkar M. Study of clinical profile of hypothyroidism with emphasis on vitamin B12 levels. IOSR JDMS (2019) 18:31–4. doi: 10.9790/0853-1811043134 [DOI] [Google Scholar]
- 62. Colleran KM, Ratliff DM, Burge MR. Potential association of thyrotoxicosis with vitamin b and folate deficiencies, resulting in risk for hyperhomocysteinemia and subsequent thromboembolic events. Endocr Pract (2003) 9:290–5. doi: 10.4158/EP.9.4.290 [DOI] [PubMed] [Google Scholar]
- 63. Alperin JB, Haggard ME, Haynie TPA. Study of vitamin B12 requirements in a patient with pernicious anemia and thyrotoxicosis: Evidence of an increased need for vitamin B12 in the presence of hyperthyroidism. Blood (1970) 36:632–41. doi: 10.1182/blood.V36.5.632.632 [DOI] [PubMed] [Google Scholar]
- 64. Ranjan A, Sudha K, Nandini M, Durgarao Y. Novel evidence of relationship between serum vitamin B12 and folate with total vitamin d in subclinical hypothyroidism. Biomedicine (2020) 40:278–80. doi: 10.51248/.v40i3.7 [DOI] [Google Scholar]
- 65. Nitu C, Rohitash K, Author C, Kumar R. The comparative study of homocysteine, folate and cobalamine level in hyperthyroid women and normal healthy women. JMSCR (2016) 4:9717–21. doi: 10.18535/jmscr/v4i3.23 [DOI] [Google Scholar]
- 66. Kumar A, Kapoor S, Bhattacharya I, Professor A. To compare the levels of vitamin B12, folate and ferritin with thyroid hormones in hypothyroidism patients: An institutional based study. Int J Med Res Prof (2019) 5:301–3. doi: 10.21276/ijmrp.2019.5.4.075 [DOI] [Google Scholar]
- 67. Tripathi P, Saxena N, Verma MK, Singh AN. Association of vitamin B12, folate and ferritin with thyroid hormones in hypothyroidism. Ann Int Med Den Res (2019) 5:BC01–6. Available at: https://aimdrjournal.com/wp-content/uploads/2021/06/BC1_OA_Manish-1-edit.pdf. [Google Scholar]
- 68. Onat A, Gönenç A, Gürcan S, Torun M. İron metabolism in patients with impaired thyroid function tiroid fonksiyon bozukluğu olan hastalarda demir metabolizmasi. J Fac Pharm Ankara (2003) 32:221–30. doi: 10.1501/Eczfak_0000000391 [DOI] [Google Scholar]
- 69. Berker D, Isik S, Aydin Y, Ozuguz U, Tutuncu Y, Erden G, et al. Evaluation of serum homocysteine levels in pregnant patients with graves' disease. Turkiye Klinikleri J Gynecol Obst (2009) 19:155–9. Available at: https://www.jcog.com.tr/article/en-evaluation-of-serum-homocysteine-levels-in-pregnant-patients-with-graves-disease-54410.html. [Google Scholar]
- 70. Potham SK, Vaikkakara S, Sachan A, Rao SP, Kalawat TC, Ravi P, et al. Vitamin B12 levels are not affected by radioiodine ablation of the thyroid. Endocr Regul (2014) 48:77–85. doi: 10.4149/endo_2014_02_77 [DOI] [PubMed] [Google Scholar]
- 71. Khubchandani A, Parmar V, Shah P, Malek R. Vitamin B12 deficiency common in primary hypothyroidism. Int J Res Med (2015) 4:158–60. Available at: https://ijorim.com/abstract.php?art_id=693&year=2015&issue=VOLUME%204,%20ISSUE%204. [Google Scholar]
- 72. Choudhary O, Kalia A, Jat R, Gupta D, Narayan Saxena G. To study the prevalence of vitamin b 12 deficiency among patients with hypothyroidism. J Mahatma Gandhi Univ Med Sci Tech (2021) 6:3–4. doi: 10.5005/jp-journals-10057-0154 [DOI] [Google Scholar]
- 73. Cakal B, Cakal E, Demirbaş B, Ozkaya M, Karaahmetoğlu S, Serter R, et al. Homocysteine and fibrinogen changes with l-thyroxine in subclinical hypothyroid patients. J Korean Med Sci (2007) 22:431–5. doi: 10.3346/jkms.2007.22.3.431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nedrebø BG, Ericsson UB, Nygård O, Refsum H, Ueland PM, Aakvaag A, et al. Plasma total homocysteine levels in hyperthyroid and hypothyroid patients. Metab Clin Exp (1998) 47:89–93. doi: 10.1016/S0026-0495(98)90198-6 [DOI] [PubMed] [Google Scholar]
- 75. Diekman MJM, van der Put NM, Blom HJ, Tijssen JGP, Wiersinga WM. Determinants of changes in plasma homocysteine in hyperthyroidism and hypothyroidism. Clin Endocrinol (2001) 54:197–204. doi: 10.1046/j.1365-2265.2001.01170.x [DOI] [PubMed] [Google Scholar]
- 76. Luboshitzky R, Aviv A, Herer P, Lavie L. Risk factors for cardiovascular disease in women with subclinical hypothyroidism. Thyroid (2002) 12:421–5. doi: 10.1089/105072502760043512 [DOI] [PubMed] [Google Scholar]
- 77. Sengul E, Cetinarslan B, Tarkun I, Cantürk Z, Türemen E. Homocysteine concentrations in subclinical hypothyroidism. Endocr Res (2004) 30:351–9. doi: 10.1081/erc-200033558 [DOI] [PubMed] [Google Scholar]
- 78. Srikrishna R, Girishbabu R, Ramesh ST. Study of association of anemia in Sub-clinical and overt hypothyroid patients. RJPBCS (2015) 6:368–73. Available at: https://www.rjpbcs.com/pdf/2015_6(3)/[49].pdf. [Google Scholar]
- 79. Özmen B, Özmen D, Parildar Z, Mutaf I, Turgan N, Bayindir O. Impact of renal function or folate status on altered plasma homocysteine levels in hypothyroidism. Endocr J (2006) 53:119–24. doi: 10.1507/endocrj.53.119 [DOI] [PubMed] [Google Scholar]
- 80. Min Yu H, Seo Park K, Min Lee J. The value of red blood cell distribution width in subclinical hypothyroidism. Arq Bras Endocrinol Metab (2014) 58:30–6. doi: 10.1590/0004-2730000002836 [DOI] [PubMed] [Google Scholar]
- 81. Souka S, Kandil H, Korraa S, Hameed AAA, Hassan M. Homocysteinemia in relation to anemia in hypothyroid patients. Sci J Al-Azhar Med Fac Girls (2018) 2:171–80. doi: 10.4103/sjamf.sjamf_31_18 [DOI] [Google Scholar]
- 82. Aon M, Taha S, Mahfouz K, Ibrahim MM, Aoun AH. Vitamin B12 (Cobalamin) deficiency in overt and subclinical primary hypothyroidism. Clin Med Insights Endocrinol Diabetes (2022) 15:1–8. doi: 10.1177/11795514221086634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Watanabe F, Bito T. Vitamin B12 sources and microbial interaction. Exp Biol Med (2018) 243:148–58. doi: 10.1177/1535370217746612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Guetterman HM, Huey SL, Knight R, Fox AM, Mehta S, Finkelstein JL. Vitamin b-12 and the gastrointestinal microbiome: A systematic review. Adv Nutr (2022) 13(2):530–58. doi: 10.1093/advances/nmab123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Allen LH. Causes of vitamin B12 and folate deficiency. Food Nutr Bull (2008) 29:S20–34. doi: 10.1177/15648265080292s105 [DOI] [PubMed] [Google Scholar]
- 86. Devalia V, Hamilton MS, Molloy AM. British Committee for standards in haematology. guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol (2014) 166:496–513. doi: 10.1111/bjh.12959 [DOI] [PubMed] [Google Scholar]
- 87. Centanni M, Marignani M, Gargano L, Corleto VD, Casini A, Delle Fave G, et al. Atrophic body gastritis in patients with autoimmune thyroid disease: an underdiagnosed association. Arch Intern Med (1999) 159:1726–30. doi: 10.1001/archinte.159.15.1726 [DOI] [PubMed] [Google Scholar]
- 88. Allen LH. How common is vitamin b-12 deficiency? Am J Clin Nutr (2009) 89:693S–6S. doi: 10.3945/ajcn.2008.26947a [DOI] [PubMed] [Google Scholar]
- 89. Talebi S, Ghaedi E, Sadeghi E, Mohammadi H, Hadi A, Clark CCT, et al. Trace element status and hypothyroidism: A systematic review and meta-analysis. Biol Trace Elem Res (2020) 197:1–14. doi: 10.1007/s12011-019-01963-5 [DOI] [PubMed] [Google Scholar]
- 90. Venkatesh U, Sharma A, Ananthan VA, Subbiah P, Durga R. Micronutrient's deficiency in India: a systematic review and meta-analysis. J Nutr Sci (2021) 10:e110. doi: 10.1017/jns.2021.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Smyk DS. Helicobacter pylori and autoimmune disease: Cause or bystander. World J Gastroenterol (2014) 20:613–29. doi: 10.3748/wjg.v20.i3.613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cellini M, Santaguida MG, Virili C, Capriello S, Brusca N, Gargano L, et al. Hashimoto’s thyroiditis and autoimmune gastritis. Front Endocrinol (2017) 8:92. doi: 10.3389/fendo.2017.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Boutzios G, Koukoulioti E, Goules AV, Kalliakmanis I, Giovannopoulos I, Vlachoyiannopoulos P, et al. Hashimoto thyroiditis, anti-parietal cell antibodies: Associations with autoimmune diseases and malignancies. Front Endocrino (2022) 13:860880. doi: 10.3389/fendo.2022.860880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sworczak K, Wiśniewski P. The role of vitamins in the prevention and treatment of thyroid disorders. Endokryn Pol (2011) 62:340–4. Available at: https://journals.viamedica.pl/endokrynologia_polska/article/view/2526. [PubMed] [Google Scholar]
- 95. Dahiya V, Vasudeva N, Sharma S, Kumar A. Role of dietary supplements in thyroid diseases. Endocr Metab Immune Disord Drug Targets (2022) 22. doi: 10.2174/1871530322666220419125131 [DOI] [PubMed] [Google Scholar]
- 96. Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, et al. American Association of clinical endocrinologists and American thyroid association taskforce on hypothyroidism in adults. clinical practice guidelines for hypothyroidism in adults: cosponsored by the American association of clinical endocrinologists and the American thyroid association. Endocr Pract (2012) 18:988–1028. doi: 10.4158/ep12280.gl [DOI] [PubMed] [Google Scholar]
- 97. Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, et al. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid (2014) 24:1670–751. doi: 10.1089/thy.2014.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kahaly J, George, Bartalena L, Hegedüs L, Leenhardt L, Poppe K, et al. 2018 European thyroid association guideline for the management of graves’ hyperthyroidism. Eur Thyroid J (2018) 7:167–86. doi: 10.1159/000490384 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.