Abstract
BACKGROUND:
Noninvasive ventilation (NIV) has become more studied in immunocompromised patients. However, it has not been studied in hematopoietic cell transplantation (HCT) recipients, who have higher mortality and higher pulmonary complication rates than other immunocompromised patients. This population may be prone to negative effects from this treatment modality. The aim of this study was to determine whether NIV use is associated with worse outcomes in this vulnerable patient population.
METHODS:
A secondary analysis of a retrospective multi-center database was performed. Twelve pediatric ICUs across the United States enrolled HCT subjects from 2009–2014 that were admitted to the pediatric ICU (PICU) with the diagnosis of acute respiratory failure. Subjects exposed to NIV prior to intubation were compared against those not exposed to NIV. Our primary outcome was all-cause mortality at 90 d; secondary outcomes included ventilator-free days (VFD) at 28 d and development of pediatric ARDS. Multivariable logistic and linear regression models were constructed using variables significant on univariable analysis.
RESULTS:
Two-hundred eleven subjects were included. Of these, 82 (39%) received NIV prior to intubation. Those that received NIV prior to intubation were older (13 vs 6 y, P < .001) and more commonly diagnosed with respiratory distress (90% vs 74%, P = .004). On multivariable analysis, NIV use prior to intubation was associated with a higher PICU mortality (hazard ratio 1.51 [95% CI 1.18–2.28], P = .02) and fewer VFD at 28 d (β −3.50 [95% CI −6.09 to 0.91], P = .008). Those with NIV exposure prior to intubation also had higher rates of development of pediatric ARDS (95% vs 78%, P = .001).
CONCLUSIONS:
In this cohort of children post-HCT, NIV use prior to intubation was associated with worse outcomes. The benefits and risks of NIV in this patient population should be carefully evaluated prior to its use, and careful patient selection is crucial for its optimal utilization.
Keywords: hematopoietic stem cell transplantation, noninvasive ventilation, artificial respiration, mortality, critical care, pediatrics
Introduction
Hematopoietic cell transplantation (HCT) is a potentially curative therapy for a diverse array of diagnoses. Children post-HCT, however, frequently have high rates of complications and toxicities that can limit treatment effects. In the pediatric HCT population, pulmonary complications range from 12–74% and are associated with high mortality.1-4 Multiple factors likely contribute to the high pulmonary complication rate including conditioning regimens, disordered immune regulation, infectious etiologies, and systemic inflammation peri-engraftment. At various stages post-HCT, there are differing mechanisms of injury, from the initial conditioning regimens early to the later chronic changes from fibrinolytic damage and idiopathic pneumonia syndrome.5 The lung post-HCT is a vulnerable organ. Careful consideration of supportive care is needed to mitigate further ongoing damage and improve survival. A large, multi-center study examining respiratory parameters in children post-HCT identified certain risk factors associated with higher mortality rates.6 One such risk factor identified was the utilization of noninvasive ventilation (NIV) in HCT patients prior to invasive mechanical ventilation.
Noninvasive respiratory support, including bi-level positive airway pressure (BPAP) and CPAP, has been increasingly utilized in both pediatric and adult critical care.7-9 Whereas studies have demonstrated multiple beneficial effects including decreased intubation rates and shorter length of stays, its role in ARDS is less clear.10,11 In adults with ARDS, there is a high rate of NIV failure, and NIV failure is associated with worse outcomes in this population.12 Due to this, the current European Respiratory Society and American Thoracic Society (ERS/ATS) guidelines were not able to make a recommendation for or against its use in de novo acute respiratory failure.13 The pediatric literature is sparser, with multiple single-center studies describing its utilization and demonstrating its safety.14,15 However, a multi-center randomized controlled trial examining early NIV in immunocompromised subjects demonstrated a higher mortality in the NIV cohort.16 Whereas this study enrolled immunocompromised subjects, it was, unfortunately, underpowered for the primary outcome, and larger study of this at-risk group is warranted. Also, a secondary analysis of the RESTORE trial demonstrated that pre-intubation NIV was associated with worse outcomes when compared to no pre-intubation NIV.17 Both of these studies were undertaken in immunocompromised subjects and likely have significant differences from the HCT patient population, which is predisposed to many deleterious lung phenomena. Given the sparse pediatric data regarding safety and timing of using NIV in the setting of pediatric acute respiratory failure, we sought to investigate NIV in a unique critically ill patient population with high mortality rates. The objective of this study was to determine whether NIV use prior to intubation is associated with worse outcomes in this particularly susceptible patient population after controlling for other important risk factors in order to add to the growing literature about the use of NIV in immunocompromised children.
QUICK LOOK.
Current Knowledge
Noninvasive ventilation (NIV) is frequently utilized for acute respiratory failure in both adult and pediatric patients. In adults with ARDS, its use is associated with a high rate of NIV failure, and NIV failure is associated with worse outcomes. In pediatrics, the data are limited; and in certain high-risk patient populations, such as the hematopoietic cell transplantation (HCT) population, the role of NIV has not been well established.
What This Paper Contributes to Our Knowledge
In a multi-center retrospective analysis of HCT recipients with acute respiratory failure, the use of NIV prior to intubation was associated with worse outcomes. Specifically, NIV use prior to intubation was associated with higher mortality, fewer ventilator-free days, and higher incidence and severity of pediatric ARDS.
Methods
This was a secondary analysis of a multi-center retrospective cohort study of children and young adults that experienced respiratory failure post-allogeneic HCT.6 Between 2009–2014, 12 centers contributed their 25 most recent subjects. Regulatory approval was obtained at each individual center prior to the start of data collection. Subjects were included if they were age 1 month–21 y, had received an allogeneic HCT for any indication, and experienced respiratory failure defined as the need for invasive mechanical ventilation for critical illness. Patients intubated solely for procedures and patients only requiring CPAP therapy were excluded from study.
Demographic and transplant characteristic data were collected. Diagnosis of respiratory distress, hemodynamic instability, altered mental status, and fluid overload/renal failure were documented if present on pediatric ICU (PICU) admission. Granular data on vital signs and respiratory parameters were collected starting on the day prior to intubation. NIV was defined as use of BPAP. Duration of NIV was determined and reported in days. Subjects were categorized into those who received NIV prior to intubation and compared to those who did not receive NIV prior to intubation. NIV use following extubation was not considered. The primary outcome was all-cause mortality at 90 d. Secondary outcomes included ventilator-free days (VFD) at 28 d and development of pediatric ARDS. Both development of pediatric ARDS and severity of pediatric ARDS were defined using the Pediatric Acute Lung Injury Consensus Conference (PALICC) criteria.18
Statistical Analysis
Data were summarized and distributions examined. Nonparametric continuous variables were presented as medians with interquartile ranges and compared with a Mann-Whitney U test. Categorical variables were presented as counts and frequencies. Comparisons of categorical variables were done using chi-square and logistic regression. Multivariable logistic and linear regression models were constructed using variables significant on univariable analysis (P < .05). Backward stepwise regression was utilized for construction of multivariable models. Unadjusted and multivariable adjusted time-to-event analyses were performed using Kaplan-Meier and Cox proportional hazards models, respectively. Kaplan-Meier survival analysis was used comparing those who received NIV prior to intubation to those who did not. Center effects were explored for the primary outcome by adding center to multivariable models. A 2-tailed P value < .05 was considered statistically significant.
Results
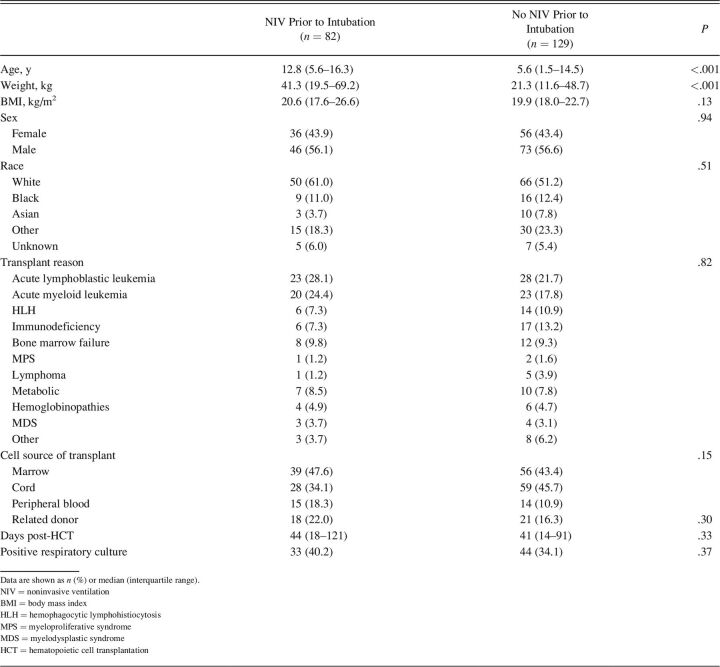
A total of 211 subjects, with a median age of 8.4 y (interquartile range [IQR] 2.0–15.5) were included in the study. See Figure S1 (see related supplementary materials at http://www.rcjournal.com). Of these, 39% (n = 82) received NIV prior to intubation. There was no difference in sex, race, underlying malignant diagnosis, or transplant characteristics between those that received NIV prior to intubation compared to those that did not; see Table 1. Those that received NIV prior, however, were older (12.8 y vs 5.6 y, P < .001) and weighed more (41.3 kg vs 21.3 kg, P < .001). Respiratory distress was the most common diagnosis present on PICU admission, affecting 80% of the entire cohort and was more common in subjects that received NIV prior to intubation (90% vs 74%, P = .004). Other diagnoses at PICU admission were not different (Table S1, see related supplementary materials at http://www.rcjournal.com). Vasoactive/inotropic agents were utilized in more subjects that received NIV prior to intubation (83% vs 68%, P = .02).
Table 1.
Subject Characteristics
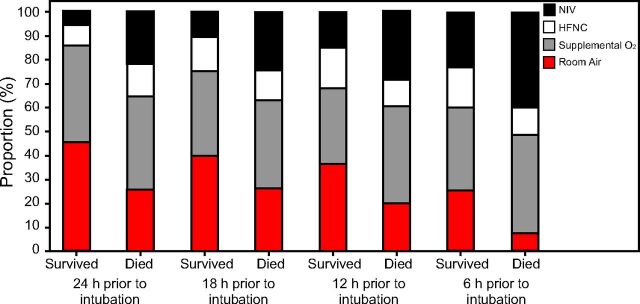
Duration of NIV was variable, with a median of 1 d (IQR 0–5). Examining the 24 h immediately preceding intubation, there was an increase in the percentage of subjects that were exposed to NIV as the time got closer to intubation (Fig. 1) (Table S2, see related supplementary materials at http://www.rcjournal.com).
Fig. 1.
Graphical representation of different respiratory support used in the preceding 24 hours prior to intubation. Higher rates of noninvasive ventilation (NIV) are seen in the cohort who died at each time period documented. HFNC = high-flow nasal cannula.
NIV Association With PICU Mortality
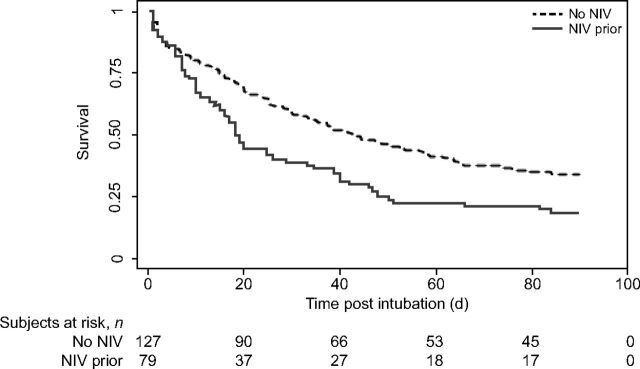
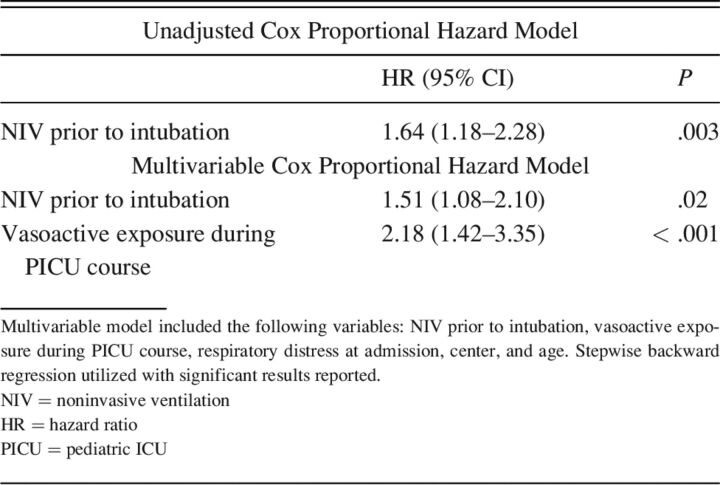
PICU mortality was higher in those treated with NIV prior to intubation (n = 67, 81%) compared with those not treated with NIV prior to intubation (n = 85, 65%); P = .01. Figure 2 illustrates the survival difference over time for those that were exposed to NIV prior to intubation compared to those who were not. On univariable analysis, subjects with NIV exposure prior to intubation had increased odds of PICU mortality (P = .003). NIV exposure continued to be associated with PICU mortality on multivariable analysis adjusting for age, respiratory distress at admission, center, and vasoactive exposure (P = .02) (Table 2).
Fig. 2.
Kaplan-Meier survival estimates assessed by exposure or no exposure to noninvasive ventilation (NIV) prior to intubation. The dotted line denotes subjects who were not exposed to NIV prior to intubation. The solid line shows subjects who were exposed to NIV prior to intubation. Subjects exposed to NIV prior to intubation had higher odds of death as evidenced by log-rank P value .003.
Table 2.
Increased Risk of Pediatric ICU Mortality for Those Exposed to NIV Prior to Intubation
Time to Intubation Association With NIV and Mortality
NIV use prior to invasive mechanical ventilation was associated with a longer time between PICU admission and intubation. Those exposed to NIV prior to intubation had a median of 1 d (IQR 0–4) prior to intubation versus 0 d (IQR 0–1) prior to intubation in those not exposed to NIV. Longer time to intubation was also associated with increased odds of mortality (P = .007).
NIV Association With Duration of Mechanical Ventilation
Those exposed to NIV prior to intubation had fewer VFD; median (IQR) of those with NIV prior to intubation, 0 (IQR 0), versus those without NIV exposure prior to intubation, 0 (IQR 0–19); P = .001. As the median is 0 in both groups, we also report the mean (SD) to fully display the data: those with NIV prior to intubation, 3.8 ± 7.8 d, compared to those without NIV exposure prior to intubation, 8.4 ± 10.7 d. On univariable analysis, exposure to NIV prior to intubation was associated with fewer VFD at 28 d (β −4.5 [95% CI −7.2 to −1.8], P = .001). Upon multivariable analysis after adjusting for age, center, respiratory distress at admission, and vasoactive exposure, exposure to NIV prior to intubation remained associated with fewer VFD at 28 d (β −3.5 [95% CI −6.1 to −0.9], P = .008). Therefore, NIV use prior to intubation was associated with 4.5 and 3.5 fewer VFD at 28 d on univariable and multivariable analyses, respectively.
NIV Association With the Development of Pediatric ARDS
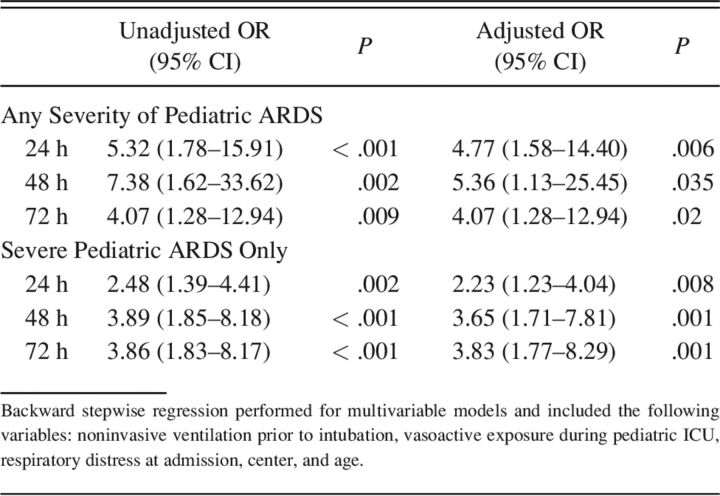
At time of intubation, subjects that were exposed to NIV prior to intubation had a lower median peripheral oxygen saturation (95 [IQR 92–99] %) compared to those without NIV exposure (99 [IQR 96–100] %, P < .001), although this is likely not clinically important. Those exposed to NIV prior to intubation had higher initial FIO2 (0.7 vs 0.6); P = .046. There was a trend toward lower SpO2/FIO2 (110 vs 124) in the NIV exposed group, but this did not reach statistical significance; P = .08. In the 6 h prior to invasive ventilation, there was a significant decrease in SpO2/FIO2 scores in the cohort exposed to NIV prior (117.5 [IQR 94.0–162.0] vs 133.0 [IQR 96.0–323.0]); P = .02; however, given the high number of saturations > 97%, there were many SpO2/FIO2 that were unable to be measured (total n = 108). During the PICU course, those exposed to NIV prior to intubation had a higher rate of high-frequency oscillatory ventilation use (55% [n = 45] vs 29% [n = 38], P < .001) and inhaled nitric oxide use (38% [n = 31] vs 18% [n = 23], P = .001). All these parameters together suggested worse oxygenation. Therefore, the occurrence of pediatric ARDS within the first 72 h of invasive mechanical ventilation was analyzed. Compared to those who did not receive NIV, more subjects exposed to NIV prior to intubation had presence of pediatric ARDS at 24 h of invasive ventilation (95% vs 78%, P = .001), 48 h of invasive ventilation (97% vs 81%, P = .004), and 72 h of invasive ventilation (93% vs 78%, P = .01), (Table S3, see related supplementary materials at http://www.rcjournal.com). Not only was there an increase in the presence of pediatric ARDS, but there was also more severe pediatric ARDS in the NIV group at all 3 time points (Table S4, see related supplementary materials at http://www.rcjournal.com). There was an increase in the risk of pediatric ARDS development for subjects that were exposed to NIV prior, particularly when isolating for severe pediatric ARDS (Table 3) (Figure S2, see related supplementary materials at http://www.rcjournal.com). Upon multivariable analysis after adjusting for age, center, respiratory distress on admission, and vasoactive exposure, NIV prior to intubation was still associated with increased presence of pediatric ARDS at all time points (Table 3).
Table 3.
Odds of Pediatric ARDS and Severity at 24, 48, and 72 Hours of Intubation With the Use of Noninvasive Ventilation Prior to Intubation
Discussion
This is a secondary analysis of a large multi-center cohort of children post-HCT that describes clinically relevant outcomes related to pre-intubation NIV use. In this cohort, the use of NIV pre-intubation was associated with higher mortality, fewer VFD at 28 d, and the development and severity of pediatric ARDS. Thoughtful consideration with a focus on not delaying more definitive care should be prioritized when considering use of NIV in this particularly vulnerable patient population.
This analysis demonstrates an increased risk of mortality in subjects who received NIV prior to intubation. In the adult literature, the main risk of NIV for the indication of de novo acute respiratory failure is the delay in a needed intubation.19 Our results further support these findings given the longer time to intubation in the cohort exposed to NIV, implying a delay in needed intubation. Importantly in this cohort, the time to intubation was also independently associated with mortality. Also, predictors of NIV failure in adults are severity of illness and diagnosis of ARDS.20 This framework may explain the increased risk of mortality in this patient population. Previous work by Rowan et al21 described a high incidence of pediatric ARDS diagnosis in this cohort, with 55% of subjects meeting severe pediatric ARDS criteria per PALICC definitions. This cohort likely has much higher severity of illness, higher rates of pediatric ARDS, and is much more likely to ultimately need intubation and invasive mechanical ventilation support. The single-center SCARF trial in which immunocompromised pediatric subjects were randomized to early CPAP or standard care demonstrated higher mortality in those receiving NIV.16 Our larger multi-center study also shows an association with higher mortality in subjects who received NIV. This study adds to the existing literature about the risk of NIV when utilized in patients with higher severity of illness and pediatric ARDS.
The high preponderance of preexisting lung damage in the HCT patient population may make this cohort especially sensitive to ventilator-induced lung injury. Animal models have demonstrated that lungs with preexisting injury are more susceptible to ventilation-induced lung injury.22,23 This is coined the multiple-hit hypothesis and is thought to be an important factor in ventilator-induced lung injury.24 In adults, NIV can cause unregulated changes in tidal volume and transpulmonary pressures that could be lung injurious itself.25 It has also been demonstrated that adults with ARDS who are managed with NIV have higher tidal volumes per kilogram and lower PEEP when compared to invasively ventilated counterparts.25,26 Therefore, those managed with NIV may be more likely to be exposed to ventilation strategies not in line with lung-protective settings. Whereas this study did not evaluate tidal volumes or PEEP on NIV for this cohort, these same factors could be present. These deleterious effects are likely more problematic in the HCT patient with an already vulnerable lung and perhaps account for some of the increased mortality, length of mechanical ventilation, and severity of illness.
In this cohort, NIV use pre-intubation was associated with fewer VFD at 28 days and was associated with increased development and severity of pediatric ARDS. The fewer VFD seen in the NIV cohort could be attributed to the deleterious effects as described above. Another potentially important factor is the appropriate selection of patients who will be good candidates for NIV. The current PALICC guidelines recommend NIV only for mild ARDS and recommend against its use in moderate-to-severe ARDS.18 In previous work by Rowan et al,6 only 19% of the subjects from this cohort would have met the PALICC guidelines recommendations for trial of NIV, whereas 41% of the cohort received NIV. As the PALICC guidelines were released after this study period, in the future careful patient selection may lead to less of a difference between the cohorts.
There are some limitations of this study. In addition to the typical limitations of a retrospective study, one of the main limitations is that relevant information regarding ongoing goals of care and possible end-of-life discussions was not captured in this data set. The reason NIV was used or not used is also unable to be determined. There was a significant difference in age, and that could be in part due to difficulty obtaining appropriately sized masks for the younger subjects. Due to these important topics, there may be selection bias in subjects who were put on NIV versus those who were not. As this is a retrospective study, the precise indication for intubation is challenging to determine. Therefore, there may be relevant differences in the need for intubation between subjects that were treated with NIV and those who were not. Another limitation of this study is that this cohort did not include patients who did not require invasive mechanical ventilation; there is likely a subset of HCT patients with acute respiratory failure who may benefit from use of NIV that is not captured in this data set. Recent work by Rowan et al27 have examined NIV failure and proposed a model to inform the use of NIV in this unique cohort. Another limitation of this study is that the parent study did not collect severity of illness scores, and therefore, we were not able to compare baseline severity of illness in the cohorts. Due to this, we adjusted for exposure to vasoactive medications as a surrogate for severity of illness scores to attempt to adjust for patient acuity. Future prospective study that accounts for the indication of why or why not NIV was utilized will be extremely important.
Conclusions
As the incidence of pediatric HCT continues to rise, and outcomes improve, we will continue to have to investigate best practices in the management of these medically complex patients. In this multi-center, retrospective analysis of pediatric subjects who underwent HCT, the use of NIV was associated with higher mortality, fewer VFD, and higher incidence and severity of pediatric ARDS. Thoughtful and judicious use of this respiratory modality in this patient population is warranted until more prospective studies can be done to examine the clinical utility of NIV in the HCT patient population. The benefits and risks of NIV along with appropriate patient selection, timing of initiation, and frequent assessments for NIV failure should be carefully considered in this at-risk patient population.
Supplementary Material
Footnotes
Dr Rowan is funded by the NIH (1K23HL150244-01A21) The remaining authors have disclosed no conflicts of interest.
Supplementary material related to this paper is available at http://www.rcjournal.com.
References
- 1.Eikenberry M, Bartakova H, Defor T, Haddad IY, Ramsay NK, Blazar BR, et al. Natural history of pulmonary complications in children after bone marrow transplantation. Biol Blood Marrow Transplant 2005;11(1):56-64. [DOI] [PubMed] [Google Scholar]
- 2.Ciki K, Dogru D, Kuskonmaz B, Emiralioglu N, Yalcin E, Ozcelik U, et al. Pulmonary complications following hematopoietic stem cell transplantation in children. Turk J Pediatr 2019;61(1):59-60. [DOI] [PubMed] [Google Scholar]
- 3.Broglie L, Fretham C, Al-Seraihy A, George B, Kurtzberg J, Loren A, et al. Pulmonary complications in pediatric and adolescent patients following allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2019;25(10):2024-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaya Z, Weiner DJ, Yilmaz D, Rowan J, Goyal RK. Lung function, pulmonary complications, and mortality after allogeneic blood and marrow transplantation in children. Biol Blood Marrow Transplant 2009;15(7):817-826. [DOI] [PubMed] [Google Scholar]
- 5.Haider S, Durairajan N, Soubani AO. Noninfectious pulmonary complications of hematopoietic stem cell transplantation. Eur Respir Rev 2020;29(156):190119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rowan CM, Gertz SJ, McArthur J, Fitzgerald JC, Nitu ME, Loomis A, et al. ; Investigators of the Pediatric Acute Lung Injury and Sepsis Network. Invasive mechanical ventilation and mortality in pediatric hematopoietic stem cell transplantation: a multi-center study. Pediatr Crit Care Med 2016;17(4):294-302. [DOI] [PubMed] [Google Scholar]
- 7.Davies JD. 2018 year in review: noninvasive respiratory support. Respir Care 2019;64(9):1139-1145. [DOI] [PubMed] [Google Scholar]
- 8.Santschi M, Jouvet P, Leclerc F, Gauvin F, Newth CJ, Carroll CL, et al. ; European Society of Pediatric and Neonatal Intensive Care (ESPNIC). Acute lung injury in children: therapeutic practice and feasibility of international clinical trials. Pediatr Crit Care Med 2010;11(6):681-689. [DOI] [PubMed] [Google Scholar]
- 9.Essouri S, Chevret L, Durand P, Haas V, Fauroux B, Devictor D. Noninvasive positive-pressure ventilation: five years of experience in a pediatric intensive care unit. Pediatr Crit Care Med 2006;7(4):329-334. [DOI] [PubMed] [Google Scholar]
- 10.He H, Sun B, Liang L, Li Y, Wang H, Wei L, et al. ; ENIVA Study Group. A multi-center RCT of noninvasive ventilation in pneumonia-induced early mild acute respiratory distress syndrome. Crit Care 2019;23(1):300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taha A, Larumbe-Zabala E, Abugroun A, Mohammedzein A, Naguib MT, Patel M. Outcomes of noninvasive positive-pressure ventilation in acute respiratory distress syndrome and their predictors: a national cohort. Crit Care Res Pract 2019;2019:8106145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demoule A, Girou E, Richard JC, Taille S, Brochard L. Benefits and risks of success or failure of noninvasive ventilation. Intensive Care Med 2006;32(11):1756-1765. [DOI] [PubMed] [Google Scholar]
- 13.Rochwerg B, Brochard L, Elliott MW, Hess D, Hill NS, Nava S, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J 2017;50(2):1602426. [DOI] [PubMed] [Google Scholar]
- 14.Bernet V, Hug MI, Frey B. Predictive factors for the success of noninvasive mask ventilation in infants and children with acute respiratory failure. Pediatr Crit Care Med 2005;6(6):660-664. [DOI] [PubMed] [Google Scholar]
- 15.Dohna-Schwake C, Stehling F, Tschiedel E, Wallot M, Mellies U. Noninvasive ventilation on a pediatric intensive care unit: feasibility, efficacy, and predictors of success. Pediatr Pulmonol 2011;46(11):1114-1120. [DOI] [PubMed] [Google Scholar]
- 16.Peters MJ, Agbeko R, Davis P, Klein N, Zenasni Z, Jones A, et al. Randomized study of early continuous positive airways pressure in acute respiratory failure in children with impaired immunity (SCARF) ISRCTN82853500. Pediatr Crit Care Med 2018;19(10):939-948. [DOI] [PubMed] [Google Scholar]
- 17.Kopp W, Gedeit RG, Asaro LA, McLaughlin GE, Wypij D, Curley MAQ. The impact of Pre-intubation noninvasive ventilation on outcomes in pediatric acute respiratory distress syndrome. Crit Care Med 2021;49(5):816-827. [DOI] [PubMed] [Google Scholar]
- 18.Pediatric Acute Lung Injury Consensus Conference Group . Pediatric acute respiratory distress syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 2015;16(5):428-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brochard L, Lefebvre JC, Cordioli RL, Akoumianaki E, Richard JC. Noninvasive ventilation for patients with hypoxemic acute respiratory failure. Semin Respir Crit Care Med 2014;35(4):492-500. [DOI] [PubMed] [Google Scholar]
- 20.Antonelli M, Conti G, Moro ML, Esquinas A, Gonzalez-Diaz G, Confalonieri M, et al. Predictors of failure of noninvasive positive pressure ventilation in patients with acute hypoxemic respiratory failure: a multi-center study. Intensive Care Med 2001;27(11):1718-1728. [DOI] [PubMed] [Google Scholar]
- 21.Rowan CM, Smith LS, Loomis A, McArthur J, Gertz SJ, Fitzgerald JC, et al. Pediatric acute respiratory distress syndrome in pediatric allogeneic hematopoietic stem cell transplants: a multi-center study. Pediatr Crit Care Med 2017;18(4):304-309. [DOI] [PubMed] [Google Scholar]
- 22.Bouadma L, Dreyfuss D, Ricard JD, Martet G, Saumon G. Mechanical ventilation and hemorrhagic shock–resuscitation interact to incre-ase inflammatory cytokine release in rats. Crit Care Med 2007;35(11):2601-2606. [DOI] [PubMed] [Google Scholar]
- 23.Bouadma L, Schortgen F, Ricard JD, Martet G, Dreyfuss D, Saumon G. Ventilation strategy affects cytokine release after mesenteric ischemia-reperfusion in rats. Crit Care Med 2004;32(7):1563-1569. [DOI] [PubMed] [Google Scholar]
- 24.Litell JM, Gong MN, Talmor D, Gajic O. Acute lung injury: prevention may be the best medicine. Respir Care 2011;56(10):1546-1554. [DOI] [PubMed] [Google Scholar]
- 25.Bellani G, Laffey JG, Pham T, Madotto F, Fan E, Brochard L, et al. ; ESICM Trials Group. Noninvasive ventilation of patients with acute respiratory distress syndrome. Insights from the LUNG SAFE study. Am J Respir Crit Care Med 2017;195(1):67-77. [DOI] [PubMed] [Google Scholar]
- 26.Carteaux G, Millan-Guilarte T, De Prost N, Razazi K, Abid S, Thille AW, et al. Failure of noninvasive ventilation for de novo acute hypoxemic respiratory failure: role of tidal volume. Crit Care Med 2016;44(2):282-290. [DOI] [PubMed] [Google Scholar]
- 27.Rowan CM, Fitzgerald JC, Agulnik A, Zinter MS, Sharron MP, Slaven JE, et al. Risk factors for noninvasive ventilation failure in children post-hematopoietic cell transplant. Front Oncol 2021;11(653607). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.