Abstract
Renal ischemia-reperfusion injury is an important contributor to the development of delayed graft function after transplantation, which is associated with higher rejection rates and worse long-term outcomes. One of the earliest impairments during ischemia is Na+/K+-ATPase (Na/K pump) dysfunction due to insufficient ATP supply, resulting in subsequent cellular damage. Therefore, strategies that preserve ATP or maintain Na/K pump function may limit the extent of renal injury during ischemia-reperfusion. In this study, we presented a technique using a synchronization modulation electric field to activate the Na/K pumps, thereby maintaining cellular functions under ATP insufficient conditions. We tested the effectiveness of this technique in two models of ischemic renal injury: an in situ renal ischemia-reperfusion injury model (predominantly warm ischemia) and a kidney transplantation model (predominantly cold ischemia). Our results showed that application of the synchronization modulation electric field preserved Na/K pump activity, thereby alleviating kidney injury reflected by 40% lower plasma creatine (1.17±0.03 mg/dL) in the electric field treated group than untreated control group (1.89±0.06 mg/dL) in a mouse renal ischemia-reperfusion model. More importantly, in the mouse kidney transplantation model, renal graft function was improved by 54.20% with the application of the synchronization modulation electric field according to the GFR measurements (85.40±5.44 μl/min for the untreated group and 142.80±5.21 μl/min for the electric field treated group). This technique for preserving Na/K pump function may have therapeutic potential not only for ischemic kidney injury, but also for other diseases associated with Na/K pump dysfunction due to inadequate ATPs.
One Sentence Summary:
Kidney injury can be reduced by electrically fueling Na/K pumps without ATP consumption during renal ischemia.
INTRODUCTION
Kidney transplantation can prolong the lives of individuals with end-stage kidney disease. Transplanted organs experience several episodes of ischemia and ischemia-reperfusion injury (IRI) before and during procurement and transplantation. IRI is considered as one of the most important factors affecting allograft dysfunction and long-term graft survival (1–6).
Na+/K+-ATPase (Na/K pump) is an active membrane transporter expressed in almost all types of cells that extrudes three Na+ ions in exchange for two K+ ions by consuming one ATP molecule. The Na/K pump establishes and maintains high K+ and low Na+ concentrations intracellularly, which is essential in maintaining the osmotic balance and the volume of cells, as well as the excitable properties of muscles and nerves (7, 8). Ischemia reduces oxygen delivery to the cells which subsequently affects ATP synthesis and impairs Na/K pump activity. The impaired Na/K pump activity disrupts the equilibrium of Na+ and K+ concentration gradients, leading to altered cell volume, activated proteases, phospholipases and caspases, and increased cellular accumulation of hypoxanthine and reactive oxygen species (ROS) (9, 10). These changes promote the development of inflammation, apoptosis and necrosis (11–13). Therefore, maintaining Na/K pump functions may attenuate the abnormal milieu induced by ischemia and ameliorate cell injury. However, to date no strategies can maintain or stimulate the pump function in the absence of sufficient ATP supply.
Given that Na/K pumps are sensitive to membrane potential, one approach to activate them is via the application of an electric field (14, 15). The study by Tsong and Tiessie demonstrated that an oscillating electric field with megahertz and kilohertz frequencies can activate Na- and K-transports, respectively (16). Subsequently, various models were developed to study the interactions between the electric fields and Na/K pumps, including Brownian motors (17, 18), electric resonance (19–21), adiabatic process (22, 23), electronic vibration (24, 25), thermal noise effect (26), and Soliton propagation (27, 28). However, none have been able to maintain Na/K pump activities under ATP-insufficient conditions.
We chose to focus on the pumping cycle, wherein the outward Na+ and inward K+ transports result in alternating opposite pump currents. Thus, application of an oscillating electric field may be able to activate the pump functions by alternately activating two ion-transport steps (29–32). However, there are millions of pump molecules in each cell, running independently with random paces and different pump rates, making it impossible to use one electric field to control all the pump molecules. We previously addressed this limitation by developing a technique that applies an oscillating electrical field to first synchronize the pump molecules, and then modulate their pumping rates (29–35). We dubbed this technique as the synchronization modulation electric field (SMEF).
The first generation of this technique (1stgen-SMEF) (36) synchronizes the two ion-transport steps in the pumping cycle, and controls the pump rates (29–32). We subsequently improved this technique so that the Na/K pumps could be synchronized down to the individual steps of the pump cycle, resulting in the real-time semi-single pump current (2ndgen-SMEF) (37). Both the Na and K pump currents are transient with extremely short time-course comparable to the membrane capacitance currents, indicating that Na+ and K+ ions are quickly moved across the cell membrane. However, for both the 1st and 2ndgen-SMEF techniques, adequate ATP supply is required. To overcome this limitation, the 3rd generation SMEF (3rdgen-SMEF) (38) was developed. The 3rdgen-SMEF consists of 3 phases: synchronization (phase 1, dubbed 3rdgen-SEF), modulation (phase 2), and maintenance (phase 3). The electric field first synchronizes the pump molecules by utilizing ATPs to actively transport Na+ and K+ ions. Meanwhile, it applies electric energy to the pump molecules so that the pumps can synthesize one ATP at the end of each pumping cycle. Thus, ATP consumption is drastically reduced. The pumping rates are then gradually modulated (increase or decrease) to the desired values and the pump rates are sustained at the target value throughout the maintenance phase.
Here we tested whether the Na/K pump function, preserved with the 3rdgen-SMEF, could decrease the magnitude of tissue injury during ischemia. We first confirmed the efficacy of this technique in driving Na/K pump function in the face of insufficient ATP supply, and then tested whether it ameliorated renal injury in an in-situ model of renal IRI. Last, we investigated the applicability of this technique to kidney transplantation. Our results suggested that application of the 3rdgen-SMEF effectively preserved Na/K pump activities, thereby alleviating kidney injury and improving renal allograft function. These studies provide evidence that utilizing electric energy to stimulate Na/K pump activity can prevent or ameliorate IRI.
RESULTS
Na/K pump current generation by the 3rdgen-SEF in ATP-depleted muscle fibers
To test whether our 3rdgen-SMEF technique could fuel Na/K pump activity under the insufficient supplies of ATP, we performed proof-of-concept experiments in isolated muscle fibers from American bullfrogs. The right panel of Fig. 1A illustrates the waveform of the 3rdgen-SEF. It is a pulsed, symmetric oscillating electric field with a frequency of 50 Hz, comparable to the turnover rate of the native Na/K pumps (34, 35). Each half-pulse consists of two narrow overshoots with different durations and magnitudes (90 mV for 0.5 ms and 70 mV for 1.5 ms) separated by an 8 ms 20 mV plateau. The formal is activation overshoot while the latter is the energy well. The symmetric waveform of the oscillating electric field can equally affect the pump molecules on both hemispheres of the cell. A pre-pulse (Fig. 1A, left panel) was applied before the application oscillating electric field (Fig. 1A, right panel) to identify the pump currents by subtracting the pre-pulse (as a reference) from the current generated by each oscillating pulse. This process and resulting currents are represented in Fig. 1B.
Figure 1. 3rdgen-SEF drives Na/K pump activity in ATP-depleted muscle fibers.
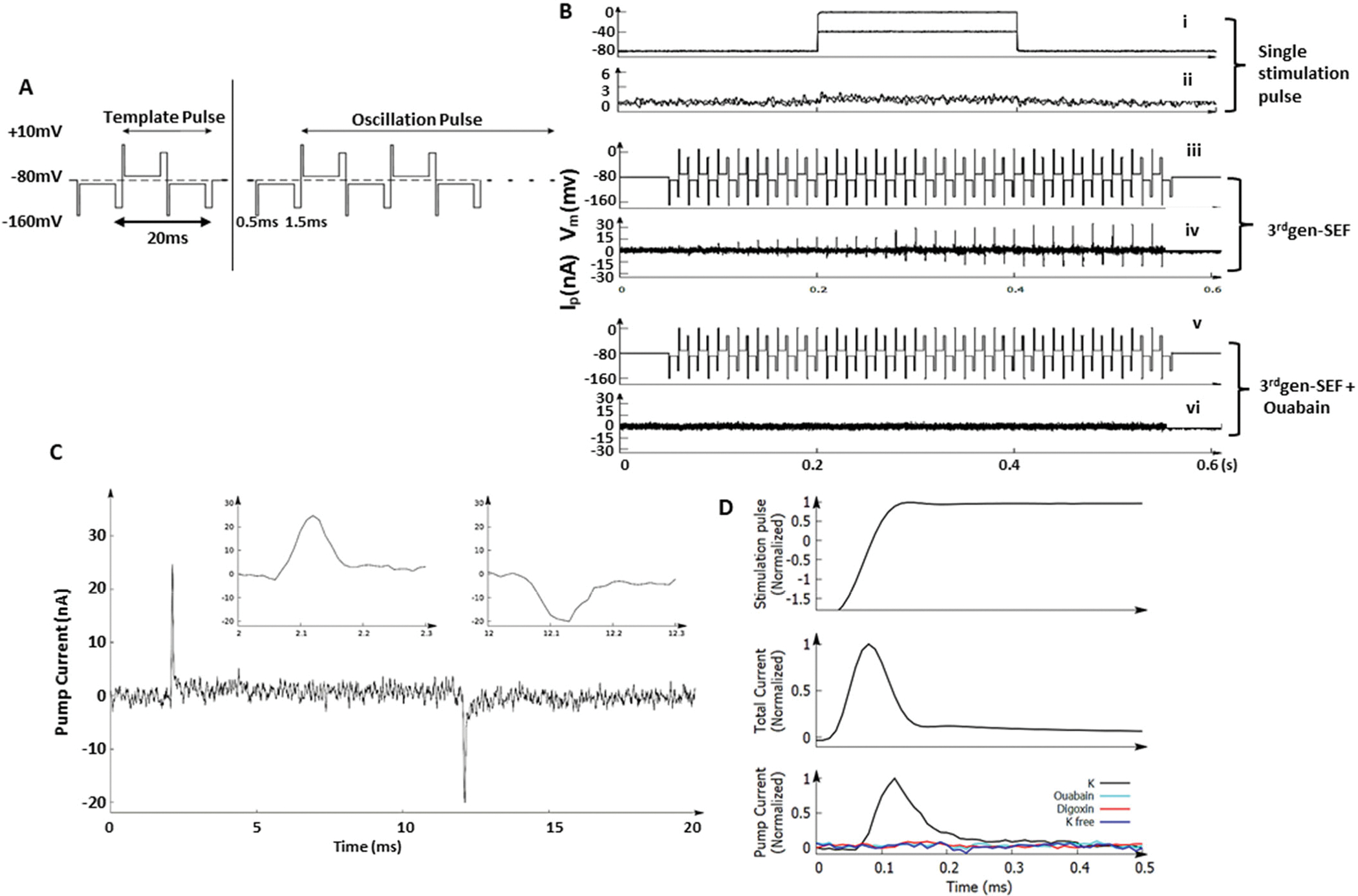
After depleting ATP from the isolated American bullfrog muscle fibers, Na/K pump activity in response to two distinct electrical fields was measured. (A). Waveforms generated by the 3rdgen-SEF. The left panel shows the pre-pulse, whereas the right panel shows the oscillating pulses. The pump current was gained by subtracting the current generated by pre-pulse (a reference) from the current generated by each oscillating pulse. The results are the pump currents responding to this specific oscillating pulse. For the oscillating pulse, each half-pulse consisted of three components: the first overshoot (0.5ms, 90mV), the speeding plateau (8ms, 20mV), and the second overshoot (1.5ms, 70mV). (B). Different electrical fields applied (the traditional single stimulation pulse and the 3rdgen-SEF) and the resultant pump currents. The 3rdgen-SEF was able to generate pump currents in this ATP-depleted milieu (traces iii and iv); these pump currents were eliminated by ouabain, a specific inhibitor of the Na/K pumps (traces v and vi). (C) Representative traces of a pair of synchronized pump currents. The inserts showed the details of Na (left) and K (right) currents with a duration of about 100 μs. (D). Currents measured after reducing the gain of the voltage-clamp. The upper, middle and lower panels depicted the rising-phase of the membrane potential of the first overshot, total transmembrane currents, and the Na/K pump currents, respectively. (In the bottom panel, the black trace was Na/K pump current measured in regular physiological solution; teal trace in solution with ouabain; red trace in solution with Digoxin and blue trace in K-free solution.) The pump and transmembrane capacitance currents have similar durations.
ATP molecules were deleted from the isolated bullfrog muscle fibers by exchanging the physiologic internal solution with an ATP-depleting solution and then the pump currents were measured. We first measured Na/K pump currents using the traditional method of applying a single stimulation pulse of 80 mV for 100 ms (Fig. 1B, trace ‘i’). No Na/K pump currents were generated, indicating that the ATP concentration was insufficient to drive the Na/K pumps (Fig. 1B, trace “ii”). We then applied the 3rdgen-SEF (Fig. 1B, trace “iii”) to the muscle fibers. The separate, transient outward and inward pump currents (Fig. 1B, trace “iv”) were generated in response to the activation phase of the first overshoot pulse in the positive and negative half-cycles, respectively. The transient pump currents were initially zero and gradually increased until saturation, suggesting that pump synchronization is a progressive process. The ability of the 3rdgen-SEF to sustain the pump currents in the ATP-depleted milieu implied that the electrical energy was used to fuel the Na/K pumps. We then confirmed that these separated transient currents were Na/K pump currents by demonstrating that they were inhibited by ouabain (Fig. 1B, trace “v” and “vi”). The details of one pair of pump currents are shown in Fig.1C. Magnitudes of the transient currents were 25 nA and −19 nA, respectively, and had a ratio of 3:2, the stoichiometric ratio of Na/K pumps, which further confirms that the transient outward and inward currents are the Na/K pump currents (39). Both the transient outward and inward pump currents had an extremely short duration of about 100 μs, comparable to the membrane capacitance currents (Fig. 1C, inserts).
To verify that the Na/K pump currents and membrane capacitance currents (generated by ion-drifting in the absence of a biological event) had similar durations, the gain of the voltage-clamp was increased or decreased to shorten or expand the duration of transmembrane currents (mainly the capacitance currents). Regardless of the changes in gain, the pump currents had similar durations to the membrane capacitance currents under the normalized membrane potential (Fig. 1D, upper panel). Reduction in gain expanded the transmembrane currents to about 400 μs (Fig. 1D, middle panel), which was similar to the increase in the pump current (Fig. 1D, lower panel). The similar duration of the capacitance current and pump currents suggested that the Na+ and K+ ions were moving freely across the cell membrane by the pump molecules.
Maintenance of the transepithelial potential difference (TEPD) of ATP-depleted kidneys by the 3rdgen-SMEF
In kidneys, Na/K pump activity in the proximal convoluted tubule (PCT) establishes the Na+ gradient and the TEPD, creating the necessary driving forces for cotransporters and antiporters (40). Because Na/K pump function is dependent on adequate ATP, ischemia will impair the pump function and reduce the TEPD. The TEPD has been considered a measure of Na/K pump function (41, 42). Therefore, we next tested whether the 3rdgen-SMEF could maintain the TEPD in ATP-deficient PCTs. The TEPDs were measured in the PCTs of the isolated non-perfused rat kidneys in the presence and absence of 3rdgen-SMEF. After the pumps being synchronized, the synchronization frequency was gradually increased from 50 Hz to 150 Hz in the modulation phase, and then sustained at this rate during the maintenance phase (Fig. 2A). As the field frequency was increased (Fig. 2B, top panel), both magnitude and frequency of the transient pump currents progressively increased (Fig. 2B, bottom panel), indicating that an increasing number of ions were being transported across the cell membrane per second.
Figure 2. The 3rdgen-SMEF maintains the TEPD of ATP-depleted kidneys.
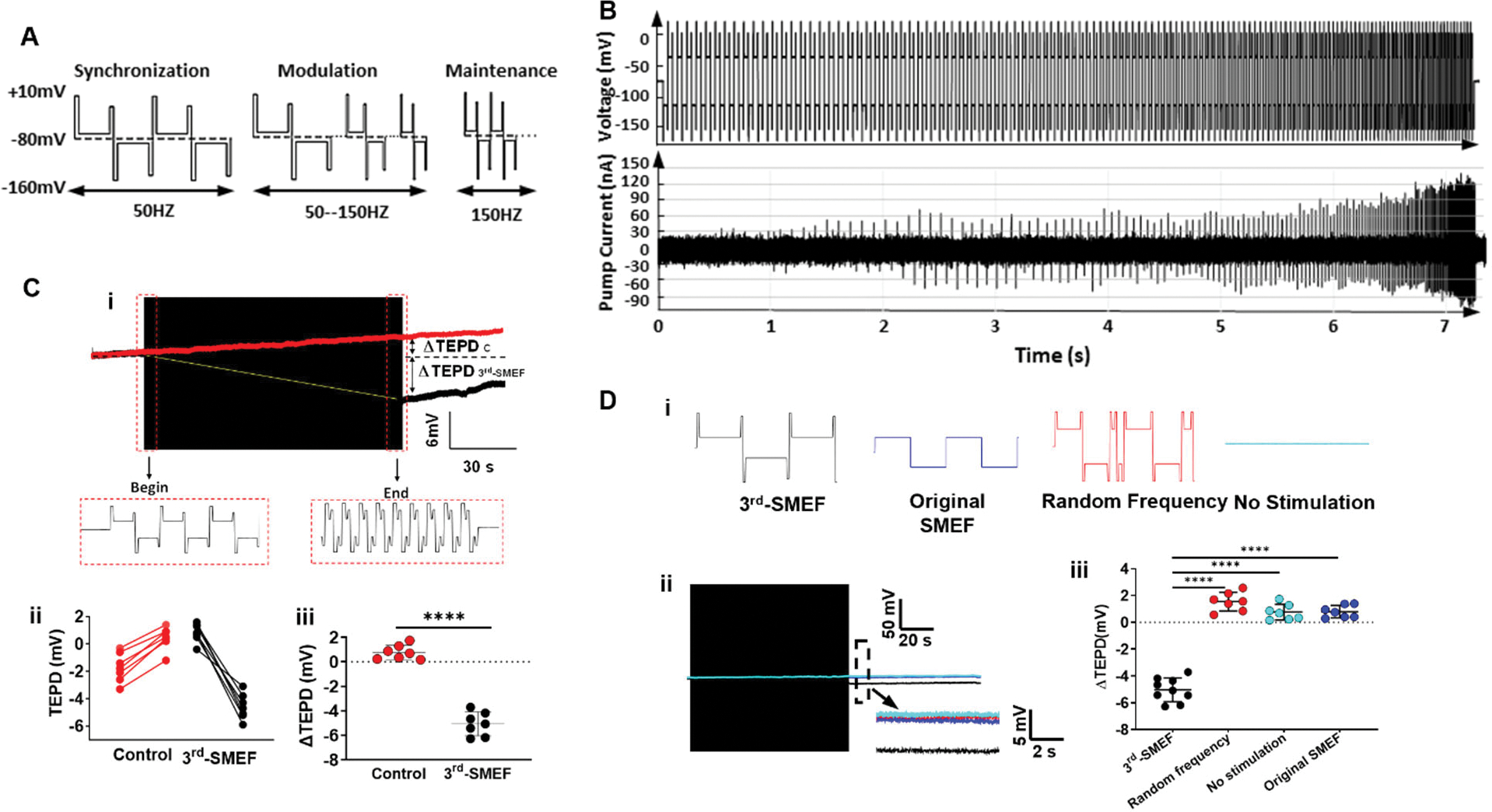
(A). Waveform of all three phases for the 3rdgen-SMEF applied: phase 1 synchronization (left), phase 2 modulation (middle), and phase 3 maintenance (right). (B). Pump currents resulting from this oscillating electrical field. The synchronization frequency was gradually increased in a stepwise pattern (change of 5% to 10% for every 10 to 20 oscillating pulses) up to the target frequency of 150 Hz. The increasing field frequency was accompanied by increases in the magnitude and density of the transient pump currents. (C). The TEPD of PCTs in the isolated Sprague Dawley rat kidneys in the presence (black trace in subpanel i) and absence (red trace in subpanel i) of 3rdgen-SMEF. TEPD was measured with a microelectrode, which was placed in the proximal tubule of the isolated kidneys. The black box was the compressed oscillating electric field of the 3rdgen-SMEF. Panels ii and iii summarized the results from the experiments performed. Significant differences were determined with student t-test. (****P<0.0001, n = 7 kidneys/group) (D). Effects of different electric fields (subpanel i, no stimulation-teal; random frequency-red; original SMEF-blue; and 3rdgen-SMEF-black) on TEPD in PCTs. Detailed TEPD changes right after the application of the different electric fields (black box) were measured in the dash-line box shown as the inserts in D-ii. The results were summarized in D-iii. Results are presented as mean ± SD. Significant differences were determined with one-way ANOVA followed by Dunnett’s multiple comparisons test. (****P<0.0001, n = 7–9 kidneys/group).
The TEPD of PCTs in the untreated isolated kidneys decreased from −1 mV to 1 mV over ~120 seconds (Fig. 2Ci, red traces). The change of the TEPD in these control PCTs was 2 mV (Fig. 2Cii–iii). This loss of polarity was consistent with Na/K pump dysfunction, as reported in previous studies (43). Then, application of the 3rdgen-SMEF to the isolated kidneys not only prevented the decrease in TEPD, but in fact increased it to −5 mV (Fig. 2.Ci, black traces). The net change in TEPD was 6 mV (Fig. 2Cii–iii). Thus, 3rdgen-SMEF can not only maintain TEPD of PCTs in the isolated kidney but can even hyperpolarize them.
We next compared the effectiveness of the 3rdgen-SMEF in hyperpolarizing TEPD in PCTs of the isolated kidneys, against the controls of no stimulation (without field application), the original SMEF (1stgen-SEF), and the 3rdgen-SEF waveform but with random frequency. Only the 3rdgen-SMEF was able to increase the TEPD to −5 mv, whereas the controls decreased it to 1mV.
Modulation of renal tubular sodium excretion by the decelerating- and accelerating-3rdgen-SMEF in vivo
Modifying kidney Na/K pump activities in vivo should alter kidney sodium reabsorption and excretion ability. Therefore, we tested whether modulating Na/K activity by applying either the decelerating-3rdgen-SMEF (to decrease the pump activity) or the accelerating-3rdgen-SMEF (to increase pump activity) altered renal sodium handling by the kidney. For this, we applied both modes of the 3rdgen-SMEF in vivo to rat kidneys and measured the urinary flow rates and renal sodium excretion under physiologic conditions and during an acute volume challenge (fig. S1).
We first determined the effects of decreasing the Na/K pump activity on sodium excretion rates under baseline conditions. Basal urine flow and sodium excretion rates of each kidney were the same during a 30-minute control period (fig. S1A). Application of the decelerating-3rdgen-SMEF (down to a frequency of 10 Hz) to the left kidneys quickly increased urinary flow rates and sodium excretion rates. The right kidneys without the electric field application were considered as the controls, where both the urinary flow and sodium excretion rates were unchanged over the time course of the experiments (fig. S1Ai–ii). These rates returned to baseline upon turning off the electrical field.
We next examined whether we could manipulate the physiologic response to acute volume expansion. Because the normal physiologic response of the kidney to a volume challenge is inhibition of sodium reabsorption, resulting in increased urine sodium excretion and volume (44, 45), we hypothesized that the accelerating-3rdgen-SMEF (from 50 to 150 Hz) would increase Na/K pump activity, thereby preventing the inhibition of sodium reabsorption and its consequent effects on urine volume. To test this, we performed unpaired experiments in which rats were subjected to an acute volume expansion (an intravenous bolus of 0.9% saline) in the absence or presence of accelerating-3rdgen-SMEF on both kidneys. Basal urine flow rates were similar in the untreated and experimental groups (3.95±0.12 vs. 3.80±0.31 μl/min/g KW, respectively, fig. S1B). After the initial control period, both groups were subjected to the saline bolus, with the experimental group also receiving the accelerating-3rd-gen-SMEF at the same time as the bolus. Volume expansion elicited a 400% increase in urine flow rate to 15.63±1.55 μl/min/g KW at 30 minutes after the bolus in the control group (fig. S1B). However, this increase in urine flow rate was limited by the application of accelerating-3rdgen-SMEF (urine flow increased to 6.81±0.63μl/min/g KW with application of the intervention). This natriuretic response waned over the ensuing hour in both groups. Ninety minutes after the fluid bolus, the accelerating-3rdgen-SMEF was stopped, and urine flow increased to the same rate as the untreated group by 120 minutes, demonstrating the reversibility of the effects of accelerating-3rdgen-SMEF on renal sodium handling.
Preserved ATP content and mitochondrial function with the application of the accelerating-3rdgen-SMEF
Regeneration of ATP molecules is compromised during ischemia, leading to decreased ATP concentrations. Mitochondria are responsible for the generation of ATP by oxidative phosphorylation. During ischemia, ATP depletion increases the osmotic gradient, resulting in mitochondrial matrix swelling (46), which subsequently inhibits mitochondrial respiration (47). Because the accelerating-3rdgen-SMEF utilizes electric energy to activate the Na/K pumps in vitro in place of ATPs, we tested the effects of accelerating-3rdgen-SMEF on ATP concentrations and mitochondrial bioenergetics during kidney ischemia.
Warm ischemia was induced by clamping both renal pedicles in mice at the body temperature of 37°C, with the accelerating-3rdgen-SMEF applied to one kidney while the other kidney was untreated as a control. ATP content in the kidney tissue was measured with a colorimetric assay. Warm ischemia resulted in a rapid decline in ATP content to almost 50% of basal value within 5 minutes of clamping and to 0 by 20 minutes in the untreated kidneys (Fig. 3A). Application of the accelerating-3rdgen-SMEF delayed the depletion of ATP during the ischemia compared with the untreated kidneys, with 78.14±4.44 and 43.48±1.81 % of basal value ATP maintained in the kidney tissue at 5 and 20 minutes of clamping, respectively. At 30 minutes of warm ischemia, 17.98% ATP still remained. We then measured the oxygen consumption rates (OCR) in the isolated kidney mitochondria (Fig. 3B) with a Seahorse XFe24 analyzer after 15 min of ischemia and compared the basal, ATP product, maximal respirations and spare respiratory capacities between the accelerating-3rdgen-SMEF treated and control groups. Although the basal respiration, ATP product, maximal respiration and spare respiration capacities in the accelerating-3rdgen-SMEF treated group dropped by 13–30% compared to the sham group, these parameters were about 20–30% higher than those in the untreated group which decreased 31–62% of sham group (Fig. 3C, i–iv). These data demonstrate that mitochondrial respiration is impaired during warm ischemia and that applying the accelerating-3rdgen-SMEF to the ischemic kidney facilitate the preservation of mitochondrial respiratory function.
Figure 3. ATP content and mitochondrial function are preserved with the application of accelerating-3rdgen-SMEF.
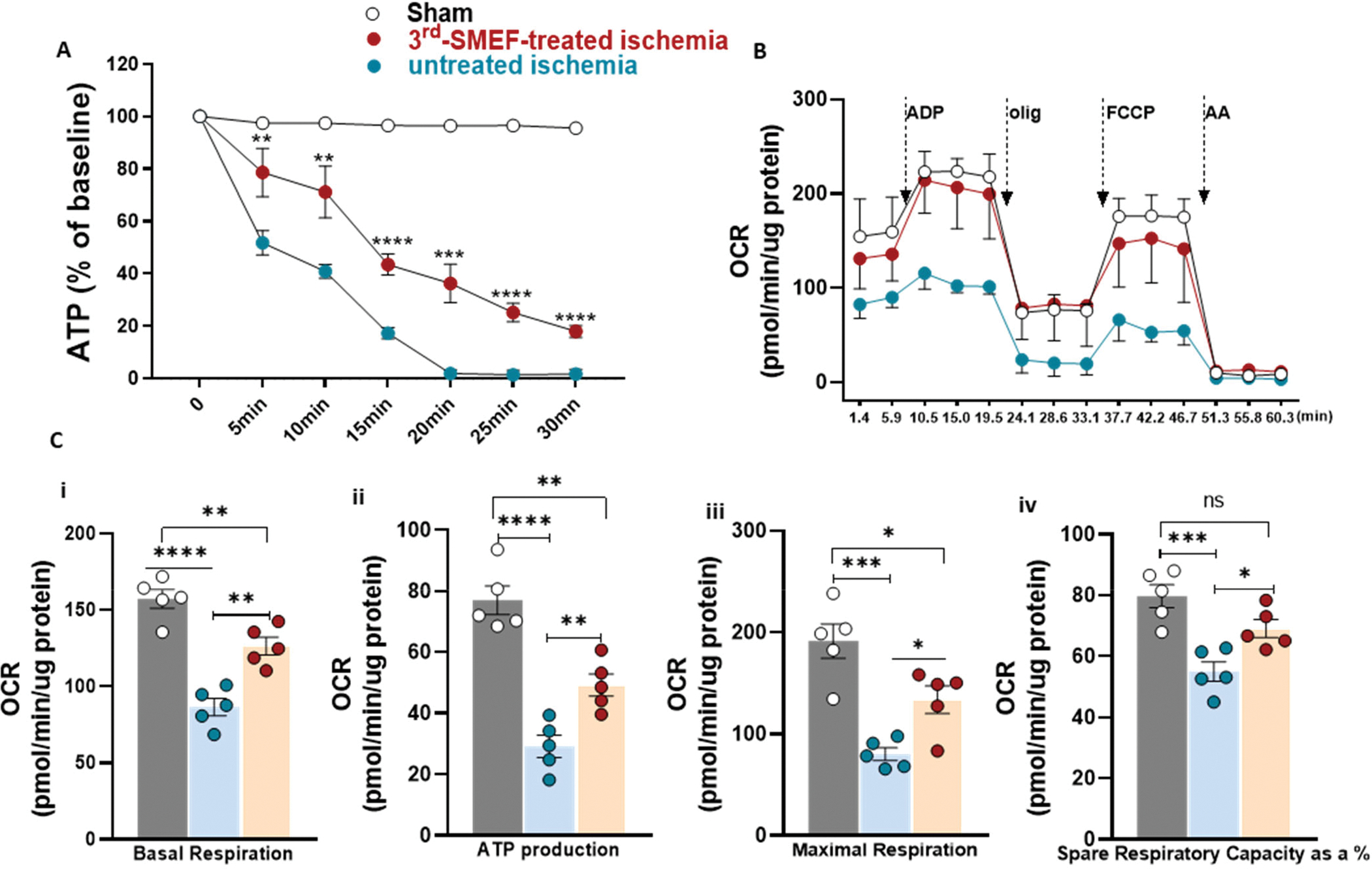
(A). ATP content in kidney tissue was measured at different time points of ischemia without reperfusion. Both renal pedicles were clamped, one as untreated control and the other one treated with accelerating-3rdgen-SMEF. Data are presented as mean ± SEM. Two-way ANOVA followed by Tukey multiple comparisons test have been performed (**p<0.01; ***p < 0.001; ****p<0.0001, n = 5 mice/group). (B) Oxygen consumption rate (OCR) traces of isolated mitochondria from C57BL/6J mouse kidneys underwent 15 min of ischemia with or without accelerating-3rdgen-SMEF treatment, expressed as picomoles of O2 per minute, under basal conditions and after the injection of ADP (1mM), oligomycin (2 μM), FCCP (4 μM), and AA+ rotenone (2 μM). Oligo, oligomycin; FCCP, carbonyl cyanide 4-[trifluoromethoxy]phenylhydrazone; AA, antimycin A. (C). Analysis of mitochondrial respiratory parameters obtained from normalized XFe24 graphs (B). Data are presented as mean ± sem. One-way ANOVA followed by Tukey multiple comparisons test have been performed (*p<0.05; **p<0.01; ***p < 0.001; ****p<0.0001, n = 5 kidneys/group).
Attenuated IRI-induced acute kidney injury (AKI) by application of the accelerating-3rdgen-SMEF
IRI is a common cause of AKI and one of the most serious hurdles for transplant graft survival (6, 48, 49). Decreased Na/K pump activities due to insufficient ATP supply during ischemia not only impairs renal tubular function, but also results in progressive cellular damage and death (13, 50). We then examined the effect of accelerating-3rdgen-SMEF on the kidney injury and function in a mouse model of IRI-induced AKI.
We induced unilateral kidney ischemia in mice with or without application of the accelerating-3rdgen-SMEF for 15min, followed by nephrectomy of the counter kidney. The untreated group suffered from severe AKI and had a mortality of 50% at one week. In contrast, all the mice treated with the accelerating-3rdgen-SMEF survived (Fig. 4A). The severity of AKI was determined on 1, 3 and 7 days after reperfusion. Basal values of plasma creatinine were similar in all groups of mice. IRI increased plasma creatinine concentrations to 1.89±0.06, 1.22±0.04 and 0.69±0.02 mg/dL at 1, 3 and 7 days after IRI in the untreated control mice. Application of the accelerating-3rdgen-SMEF blunted the increase of plasma creatinine to 1.17±0.03, 0.52±0.03 and 0.26±0.02 mg/dL at 1, 3 and 7 days after IRI, which were 37–60% less than the untreated group (Fig. 4B, p<0.0001). Plasma neutrophil gelatinase-associated lipocalin (NGAL) measurements showed a similar pattern as the plasma creatinine (Fig. 4C). Kidney function was assessed by glomerular filtration rate (GFR) measurement in conscious mice on day 7 post IRI (Fig. 4D). GFR reduced by about 55% in the untreated IRI group (85.33±14.84μl/min) and by 30% in accelerating-3rdgen-SMEF treated group (138.14±19.8μl/min) compared with the sham group (195.71±17.72μl/min). Application of accelerating-3rdgen-SMEF blunted the drop of GFR, resulting in about 38% higher GFR values than untreated mice. Histologic analysis of the kidneys at one week after injury confirmed the changes in morphologic parameters of injury (Fig. 4E and F). IRI caused severe tubular necrosis was associated with increased fibrosis (Masson’s Trichrome staining), apoptosis (TUNEL staining), and less peritubular capillary endothelial cell density (CD31 positive area). All of these parameters were less severe in the mice treated with accelerating-3rdgen-SMEF. These results suggested that the application of accelerating-3rdgen-SMEF during ischemia can ameliorate AKI induced by warm ischemia/reperfusion.
Figure 4. Ischemia reperfusion-induced AKI is attenuated by accelerating-3rdgen-SMEF.
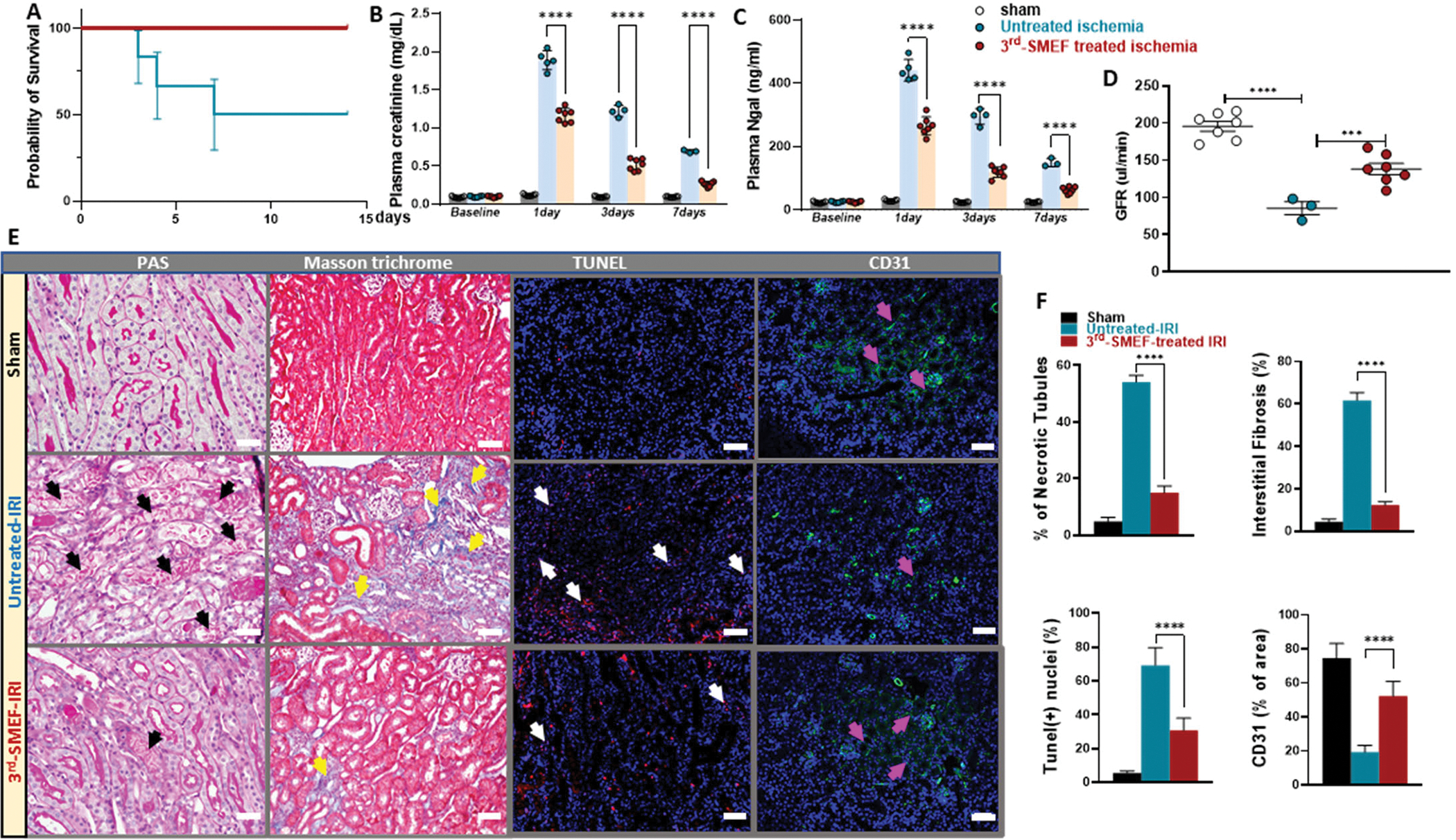
Unilateral kidney ischemia was induced by clamping left renal pedicle for 15 min in C57BL/6J mice with or without application of the accelerating-3rdgen-SMEF, followed by nephrectomy of the counter kidney. Survival rates were monitored for 14 days. Survival curves were analyzed using the log-rank (Mantel–Cox) test (A). Kidney injury was evaluated by plasma creatinine (B) and NGAL (C) measurements at 1-, 3- and 7-days following IR. Two-way ANOVA followed by Tukey multiple comparisons test have been performed (***P < 0.001; ****P<0.0001, n = 3 to 7 mice/group). Kidney function was assessed by GFR measurement in conscious mice at 7 days following IR (D). One-way ANOVA followed by Tukey multiple comparisons test was performed to determine the significance (***P < 0.001; ****P<0.0001, n = 3 to 7 mice/group). Representative morphologic evidence of kidney injury was assessed at 7 days following IR by PAS staining, Masson’s Trichrome staining, TUNEL staining, and CD31 immunofluorescent staining. (black arrows demonstrate necrotic tubules, yellow arrows denote areas of tubulointerstitial fibrosis, white arrows denote apoptosis and pink arrows show peritubular capillary endothelial cell). Scale bars were 50 μm (E). Histological data was quantified with Image-J (F) as described in Supplementary Materials and Methods. Data are presented as mean ± SEM. Student t-test was used to determine the significance (****P<0.0001, n = 3 to 7 kidneys/group).
Preserved kidney graft function by application of accelerating-3rdgen-SMEF
We next investigated the effects of accelerating-3rdgen-SMEF on donor kidneys during cold storage in a mouse kidney transplantation model. The donor kidney and associated vessels were isolated and stored in 4 °C cold saline with or without the application of the accelerating-3rdgen-SMEF for 1.5 hours. The kidney was then transplanted into a same sex recipient. Renal graft function was determined on days 5 and 8 via plasma creatinine and NGAL (Fig. 5A and B) and on day 11 via GFR measurement (Fig. 5C). Plasma creatinine increased by over 10 and 4 folds on days 5 and 8 after the transplantation in the untreated group. Applying the accelerating-3rdgen-SMEF during the cold ischemia blunted the post-transplantation increases in plasma creatinine to a less than 5-fold increase at day 5 that returned to baseline on day 8 after surgery (Fig. 5A). Plasma NGAL measurements showed similar patterns as the plasma creatinine (Fig. 5B). Application of the accelerating-3rdgen-SMEF improved GFRs by more than 40% compared with the untreated group (142.80±11.65 vs. 85.40±12.18μl/min for accelerating-3rdgen-SMEF and untreated groups, respectively) (Fig. 5C). The improved functional parameters correlated with decreased fibrosis (Masson’s Trichrome staining) and apoptosis cells (TUNEL staining), as well as higher peritubular capillary density (CD31 positive area) (Fig. 5D and E). Thus, these results suggested that accelerating-3rdgen-SMEF protected against the ischemic injury during cold storage and improved transplanted graft function.
Figure 5. The accelerating-3rdgen-SMEF blunts cold-ischemia induced injury in a murine model of kidney transplantation.
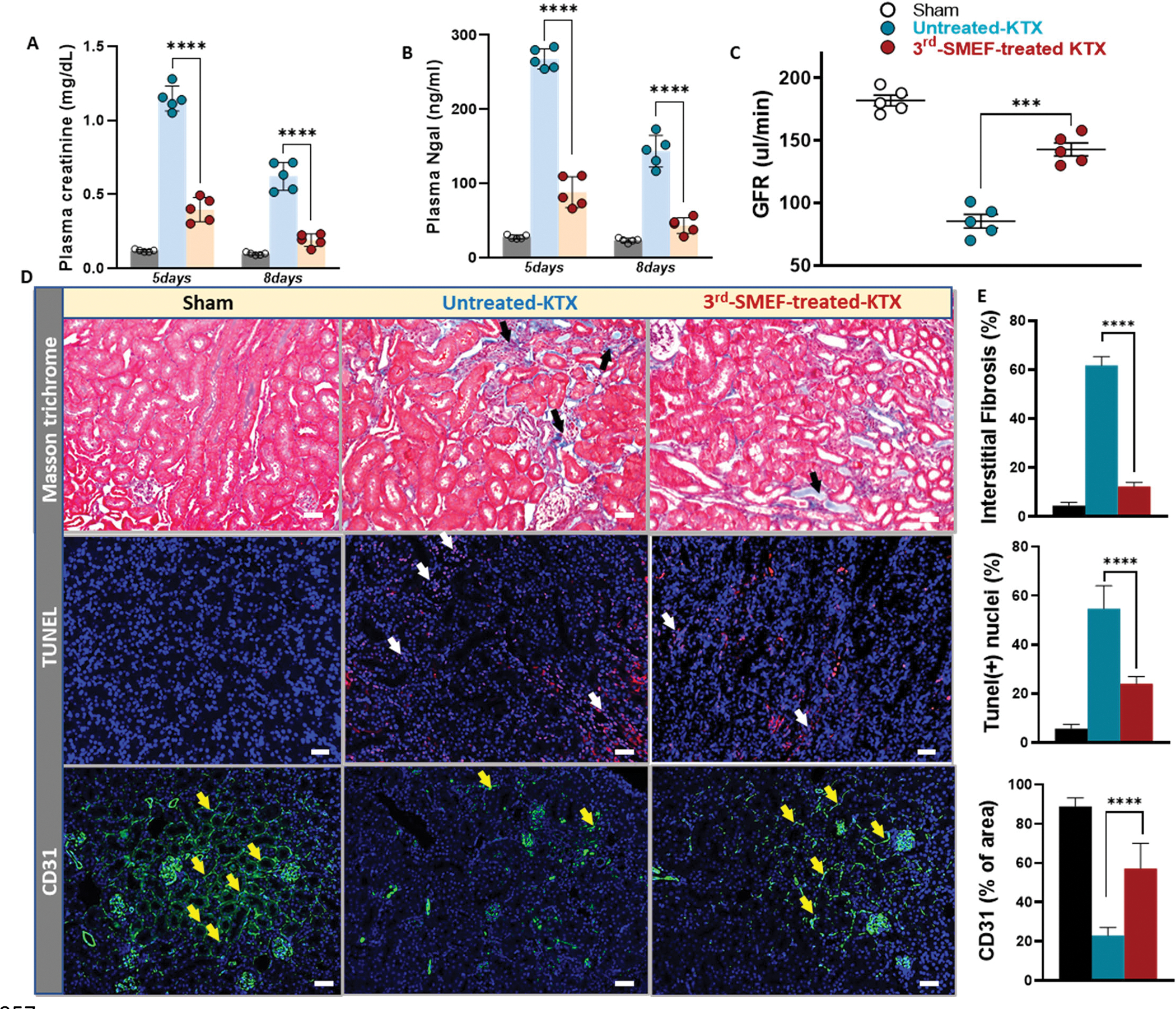
Plasma creatinine (A) and NGAL (B) measurements on day 5 and day 8 following transplant. ****P<0.0001, n = 5 mouse recipients/group, Two-way ANOVA followed by Tukey multiple comparisons test have been performed. (C) GFR measurement in the conscious recipients on day 11 following transplant. Data are presented as mean ± SD. ***P<0.001, Student’s t test comparing untreated group to accelerating-3rdgen-SMEF treated group was performed. (n=5 mouse recipients/group). (D) Representative kidney tissue sections stained with Masson’s Trichrome, TUNEL and immunofluorescent anti-CD31. (black arrows, tubulointerstitial fibrosis; white arrows, apoptosis and yellow arrows, peritubular capillary endothelial cells). (F) Quantification of tubulointerstitial fibrosis area over whole kidney sections, TUNEL-positive cells/nuclei as the percentage (%) of TUNEL and DAPI double-positive cells relative to total cells and CD31-positive area over the kidney section as described in Supplementary Materials and Methods. Data are presented as mean ± SEM. One-way ANOVA followed by Tukey multiple comparisons test have been performed (****P<0.0001, n = 5 kidneys/group).
Preliminary evidence of protection in human donor kidneys with the application of accelerating-3rdgen-SMEF
Last, we obtained five pairs of human kidneys to test whether accelerating-3rdgen-SMEF could be used to confer protection against cold storage-related injury in humans. The kidneys, which were deemed unsuitable for transplantation, were handled identically and immediately placed in University of Wisconsin (UW) cold storage solution for a 24-hour storage period. For each pair kidneys from the same donor, one kidney was taken as the untreated control and the other subjected to accelerating-3rdgen-SMEF during the storage period. The kidneys were then evaluated for morphologic evidence of injury at the end of cold storage. The PAS-stained kidney sections from the untreated control group exhibited more severe tubular injury, including loss of brush borders, cytoplasmic vacuolization and nuclear drop out, than the treated group (Fig. 6A). The accelerating-3rdgen-SMEF-treated kidneys showed more preserved histological structures, including relatively intact epithelial cells with preserved brush borders and maintenance of cytosolic integrity. Transmission electron microscopy (TEM) examination of the kidneys also demonstrated that the untreated kidneys had more ultrastructural damage, such as increased lysed mitochondria, apical cytoplasm blebbing and cytoplasmic vacuolization, than the accelerating-3rdgen-SMEF-treated kidneys (Fig. 6B). These preliminary observations raise the possibility for the translation of our mouse transplant results to humans.
Figure 6. Preliminary evidence suggests that accelerating-3rdgen-SMEF may protect human donor kidneys during cold storage.
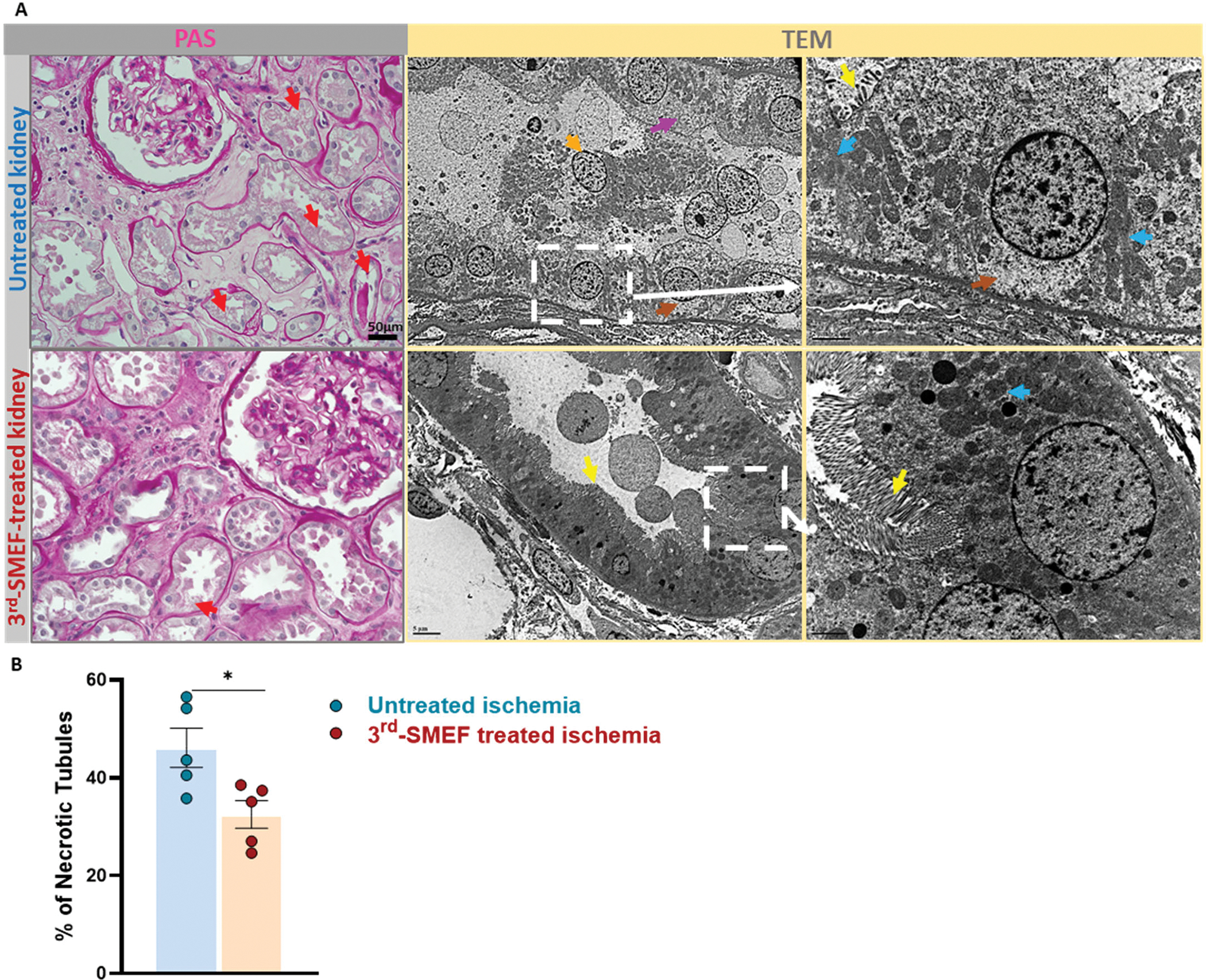
Accelerating-3rdgen-SMEF was applied to five pairs of human kidneys during a 24-hour storage period after which morphology was evaluated. (A). The left two panels are representative images of kidney slices stained by PAS. The red arrows indicate the injured tubules (loss of brush borders, cytoplasmic vacuolization and nuclear drop out). Scale bars are 50μm. The right 4 panels are TEM images (yellow arrows, brush border; orange arrows, epithelial cells sloughing into tubular lumen; blue arrows, mitochondria; brown arrows, cytoplasmic vacuolization and pink arrows, apical cytoplasm blebbing). Scale bars are 5μm. (B). The percentage of necrotic tubules based on PAS staining was quantified with Image J as described in Supplementary Materials and Methods. Paired t -test has been performed (*P < 0.05, n = 5 kidneys/group).
DISCUSSION
Renal IRI during the transplant procedure underlies the clinical entity of delayed graft function (DGF) (6). Although IRI is an underlying multifactorial pathophysiological process (6, 48, 49), the main characteristic associated with ischemia is the reduction in the availability of ATP, which impairs Na/K pump function thus resulting in cellular damage and death (13, 50). Therefore, maintaining the activity of Na/K pump molecules may be beneficial in the protection against IRI. Here, we examined the application of the 3rdgen-SMEF in maintaining the Na/K pump functions under ATP insufficient or depleted conditions. Our results suggested that application of the 3rdgen-SMEF can drive and activate Na/K pump activities, maintain tubular functions, and therefore, protect the kidney against the ischemia-induced injury.
The 3rdgen-SMEF was developed based on the combination of two modes of the Na/K pumps (physiological model actively transporting Na and K ions by consuming ATPs and non-canonical mode using ionic concentration gradient to synthesize ATPs). The key feature of the 3rdgen-SMEF is the ability to drive the Na/K pumps under the ATP insufficient conditions. Under this electric field, the pumps first extrude 3 Na+ ions by exchanging 2 K+ ions consuming one ATP, and then, utilize the electric energy to synthesize one ATP. The less or zero ATP consumption allows us to maintain and activate the functions of Na/K pumps even under the situations with insufficient ATP supply (38).
In this study, we first confirmed the efficacy of the 3rdgen-SMEF in the maintenance of the Na/K pump activities in muscle fibers where ATP was depleted. The results demonstrated that the Na/K pumping activities could be maintained and activated in an ATP-poor environment by using an oscillating electric field. These experiments set the foundation for applying the 3rdgen-SMEF to the whole kidney and thus, it may prove to be clinically applicable.
In kidneys, Na/K pumps are highly expressed on the basolateral surface in tubules. and are essential in maintenance of electrolyte, acid-base and volume homeostasis (7, 8). We then tested whether the 3rdgen-SMEF could effectively modulate TEPD in renal tubules, and subsequently tubular sodium transport in the kidney. Our isolated kidney studies showed that TEPD fell in the untreated nonperfused kidneys, but the 3rdgen-SMEF could not only maintain but also hyperpolarize the TEPD in the isolated kidney. We then extended these findings to the modulation of changes in renal sodium excretion in vivo. The results demonstrated that the decelerating-3rdgen-SMEF can increase renal sodium excretion rates under the basal conditions and the accelerating-3rdgen-SMEF could ameliorate the normal natriuretic response to volume expansion.
Ischemia induces impaired function of Na/K pumps due to lack of ATPs. IRI-induced kidney injury is unavoidable during organ transplantation and can result in DGF, which is one of the most important determinants of the longevity of transplant graft survival (1–5, 49). Our study provided pre-clinical evidence that the accelerating-3rdgen-SMEF could protect kidneys against IRI-induced AKI. Its application in a mouse model of kidney transplant further revealed protection of the renal graft function, which was associated with decreased tubular necrosis, fibrosis and apoptosis. Although we could not test this in humans at this time, our preliminary results in the five pairs of human kidneys raised the possibility that the technique can be translated to humans.
The main limitation of our study is the uncertainty about the transferability of the effects of 3rd-gen-SMEF from animals to humans. Therefore, test of 3rd-gen-SMEF in preclinical large animals is essential to demonstrate translational significance. In addition, further assessment and optimization of the application of 3rd-gen-SMEF is needed. We have not yet detailed the impact of different cold-ischemia times on the efficacy of 3rd-gen-SMEF. Furthermore, the effects of the 3rd-gen-SMEF on donor kidneys that are stored at different temperatures need to be examined. Finally, the efficiency of combination of the 3rd-gen-SMEF with machine perfusion during donor storage also needs to be determined in the future studies.
The direct application of the electrical field via 3rdgen-SMEF to the tissues does not result in any identifiable adverse effects on tissue integrity. This field strength is unlikely to change the integrity of cell membrane because it has been specifically designed to avoid damaging the cell membrane. We kept the magnitude of two overshoot pulses within the physiological range (between +100 and −100 mV), which is much lower than the thresholds necessary to induce membrane electroporation (51) or protein denaturation (52). The electric field is also unlikely to noticeably alter other membrane proteins or other ion pumps because the short duration of the overshoot pulses (a few hundred microseconds) is not long enough to open the voltage-gated ion channels including the Na-channel, which has the fastest electric response time of 1million seconds (53). Finally, the 50 Hz frequency of the electric field, needed to drive the Na/K cations in the opposite directions, is much lower than the frequency needed to drive other pumps (the Ca2+ pump requires 500 Hz, for example) (54).
In summary, our study describes a technique, named the 3rdgen-SMEF that is able to maintain Na/K pump activities and control the pumping rates (acceleration or deceleration) under ATP-insufficient or -depleted conditions. Application of the 3rdgen-SMEF protected the kidney against injury induced by warm and cold ischemia and improved transplanted graft function in murine models. Further studies are required to translate this technique to humans both to improve graft survival and to expand the donor organ pool.
MATERIALS AND METHODS
Study design
The aim of this study was to test the efficacy of a technique, 3rdgen-SMEF, on the maintenance of Na/K pump activities under ATP depleted conditions using animal models of ischemia in vitro and in vivo. Bullfrogs, rats, and mice were utilized in this study. we first confirmed the efficacy of the 3rdgen-SMEF in the maintenance of the Na/K pump activities in vitro models in American bullfrog muscle fibers and isolated rat kidneys where ATP was depleted. For expansion of the findings from in vitro to in vivo, renal sodium excretion responses to 3rdgen-SMEF upon acute volume expansion were tested. IRI-induced AKI and kidney transplantation in mice revealed protective effect of 3rdgen-SMEF on kidneys against injury and improvement of the renal graft function based on several evaluated parameters (plasma creatinine, plasma NGAL, GFR and histological analysis).
Sample sizes were determined based on variability observed in pilot experiments and on availability of primary blasts and were confirmed using the G-power v3.1.9.2 software. Eight successful experiments were needed to achieve a power of (1-β) of 0.85 with a Type I error (α) probability of 0.05. For all the studies, the animals were of the same ages and were weighed at the beginning of study. Animals were randomized to groups (5 to 10 mice per group) to ensure equal average weights between different groups. No animals were excluded from the experiments unless died in the middle of experiment due to severe kidney injury. The administration of 3rdgen-SMEF, surgical operations and sample handlings were carried out by different individuals in a blinded manner.
Animals
Adult American bullfrogs (R. catesbeiana) weighing from 150–180g were purchased from a commercial supplier (Charles D. Sullivan Co. Inc.). The frogs were housed in the Animal Care facility in large aquaria, which include both aquatic and terrestrial regions, with the temperature maintained at 20–23°C. Male C57BL/6J mice, aged 10–12 weeks (25–30g) were obtained from a vendor (Jackson Laboratories). Male Sprague Dawley (SD) rats, aged 8–10 weeks (250–300g), were ordered from Envigo. After arrival, the animals were housed in a temperature-controlled environment with 12:12h light-dark cycle and ad libitum access to water and food for 1 week before experiments. The animals were randomly assigned into control or treatment groups and donors or recipients based on the requirement of the experiments. All procedures and experiments were performed with the approval of the Institutional Animal Care and Use Committee at the University of South Florida College of Medicine and in accordance with all federal guidelines. All chemicals were purchased from Sigma-Aldrich unless otherwise indicated. Animals were euthanized according to the guidelines set forth by the American Veterinary Medicine Association.
Muscle fiber preparation and Na/K pump current measurement
Fast twitch skeletal muscles, semitendinosus and iliopsoas, were dissected from American bullfrogs. Then, a single fiber was hand dissected under microscope (Nikon SMZ 1500) in relaxing solution (see Supplementary Materials and Methods) and mounted into a custom-made chamber as described in previous publications (55, 56).
Pump currents were measured using the double Vaseline-gap voltage-clamp (Dagon T200 TEVC) with the software pClamp 10 (Molecular Devices) on skeletal twitch muscle fibers following the previous protocol (55, 56). Two electric fields, single stimulation pulse and 3rdgen-SEF, were applied in this experiment. For the experiments having extremely low ATP concentration, the internal solution was changed to the washing-out solution (without ATP) by washing the end-pool at least five times to minimize the ATP concentration in the fiber. Composition of the solutions are detailed in the Supplementary Materials and Methods.
To identify the synchronized pump currents, we introduced the template-subtraction method based on the p/4 method in the study of ion-channels. The pre- or post-pulse (Fig. 1A, left panel), having the same waveform as the oscillating pulses, was applied to the cell membrane. The generated current by the pre-pulse functioned as a reference to be subtracted from the current generated by each oscillating pulse. The resultant transient separated outward and inward currents responding to the rising phase of two half-pulses were the synchronized pump currents. All the data was collected at least 10 minutes after changing the solutions when the parameters reached the equilibrium.
Transepithelial potential difference (TEPD) measurements in ischemia-injured kidneys.
Rat kidneys were isolated and removed. A microelectrode was used to puncture into the PCT, and the reference electrode was placed on the kidney surface to measure the TEPD at room temperature. The microelectrode was first calibrated to zero mV in the experimental solution. As the microelectrode was punctured into the surface of kidney and when the potential reading suddenly changed from 0 mV to about −40 mv, it indicated that the electrode was in the epithelial cell. When the microelectrode was moved further in, the potential quickly reduced to a few mV, which showed that the electrode was in the lumen of the renal tubule.
Then, the different electric fields were applied on the surface of the kidney through two thin wire electrodes touching the kidney surface and the TEPDs were continuously measured. The experiments were performed within one hour of surgery.
Application of 3rdgen-SMEF on rat and mouse kidneys
An abdominal incision was made, and the kidneys were exposed. The 3rdgen-SMEF was applied to the kidney by two pairs of stainless-steel electrodes. The two pairs of electrodes, attached on a piece of fabric, were placed on kidneys perpendicular to each other to ensure the electric field evenly surrounding the whole kidney surface (similar as showed in fig. S2). The 3rdgen-SMEF was generated by a custom-made signal generator with a magnitude of about 1 V. There are three phases within a synchronization modulation train. Phase I (synchronization): oscillating electric field with a frequency of 50 Hz (natural turnover rate of Na+/K+-ATPase) to synchronize the Na/K pump molecules. Phase II (modulation): once the Na/K pumps were synchronized, the frequency of oscillating pulses was increased or decreased in a stepwise manner (2% change for each step) to accelerate or decelerate the pumping rates. Eventually, the turnover rate of Na/K pump was gradually modulated or entrained to the pre-determined frequencies. Phase III (maintenance): the frequency of the electric field was then kept at the targeted frequency to maintain the pumping rate till the end of experiment. The system automatically switched from phase I to phase II and phase III with no gaps. The potential difference applied between each pair of electrodes was 200 mV.
Urinary flow and Na+ excretion under physiological and acute volume challenged conditions
Sprague Dawley (SD) rats (age: 8–10 weeks, n=5–7 /group) were used. The surgical procedures were similar as described previously (57, 58). The details of the surgeries and parameters measurement were described in the Supplementary Materials and Methods.
Measurement of ATP
To test the effects of accelerating-3rdgen-SMEF on the depletion of kidney ATP during ischemia, we induced bilateral kidney ischemia by clamping both renal pedicles in C57BL/6J mice, with one kidney serving as the untreated control and the other one subjected to accelerating-3rdgen-SMEF. The kidney tissue was collected and homogenized with a Dounce homogenizer at the end of different ischemia times (5, 10, 15, 20, 25 and 30 minutes) without perfusion. The total ATP content in the kidney tissue was determined with an ATP colorimetric assay kit (K354, Biovision) according to the manufacturer’s instructions. Briefly, 20 mg of kidney tissue was lysed in ATP assay buffer followed by deproteinization and neutralization with Deproteinizing Sample Preparation Kit (K808). The absorbance of the samples was taken at 570 nm using a micro-plate reader (Synergy HTX, Biotek).
Mitochondrial isolation and bioenergetics measurement
In C57BL/6J mice, bilateral kidney ischemia was induced by clamping both renal pedicles for 15 min, with one kidney serving as the untreated control and the other one with the application of accelerating-3rdgen-SMEF. At the end of ischemia, mitochondria protein was isolated by gradient centrifugation as previously described (59–61). Briefly, kidneys were collected, and cortical tissue was homogenized in ice-cold isolation buffer (0.1 M Tris-MOPS, 5 ml 0.1 M EGTA-Tris, 100 ml 1 M sucrose, 5mM magnesium sulfate, Ph 7.4) using a Dounce homogenizer. The homogenate was then centrifuged first at 600 g, 4°C for 10 min to remove debris, then the supernatant was centrifuged at 1,400 g, 4°C for 10 min and 10,000 g, 4°C for 10 min to obtain a mitochondrial pellet which was then resuspended in a small amount of isolation buffer. The mitochondrial protein content was measured with a BCA protein assay kit (Pierce, 23227) and the purity was assessed by Western blot (fig. S2).
Mitochondrial oxygen consumption rates (OCR) were assessed with a Seahorse XFe24 Analyzer (Agilent Technologies, Inc.) as previously described (59). Two micrograms of freshly prepared mitochondria in mitochondrial assay solution (MAS) (70 mM sucrose, 220 mM mannitol, 10 mM KH2PO4, 5 mM MgCl2, 2 mM HEPES, 1 mM EGTA, and 0.2% BSA in nanopure water, pH 7.4, adjusted using KOH) were loaded onto the 24-well SeaHorse plates. Succinate (10 mM) and Rotenone (2 μM) were added to the MAS and the resulting solution was used to make 10 × stocks of the respiratory inhibitors and uncouplers. The respiratory stocks were loaded into the drug ports of a hydrated sensor cartridge in the following order: (A) ADP (1mM, final), (B) oligomycin (2 μM, final), (C) carbonyl-cyanide-4-(trifluoromethoxy) phenyhydrazone (FCCP, 4 μM final) and (D) rotenone + antimycin A (2 μM final). The tissue respiration assay protocol included a minimum of three cycles of OCR measurements for each measurement period. Each cycle consisted of a 1 min ‘mix’ period and 2 min ‘wait’ period, followed by a 3 min ‘measure’ period. Results were analyzed using the Wave software (Agilent Technologies, Inc.) export option and GraphPad Prism. The following parameters were monitored and analyzed: basal respiration (OCR before ADP injection), ATP-linked respiration (= last OCR before injection of oligomycin – minimum OCR after injection of oligomycin), maximal respiration (= maximum OCR after FCCP injection) and spare respiratory capacity (= maximal respiration - basal respiration).
Renal ischemia reperfusion-induced acute kidney injury (IRI-AKI)
Unilateral IRI was performed in C57BL/6J mice similarly as previously described with slight modifications (62). The accelerating-3rdgen-SMEF was applied to the left kidney as described above. The renal pedicle was clamped using microvascular clamps for 15 min with the body temperature at 37°C. The accelerating-3rdgen-SMEF was turned on simultaneously with clamping. Immediately following the release of the clip, the accelerating-3rdgen-SMEF was turned off and the electrodes were removed. The right kidney was then nephrectomized and the abdomen was closed.
Kidney Transplantation and accelerating-3rdgen-SMEF application in mice
Kidney Transplantation between same-sex C57BL/6J mice was completed as recently reported (58, 63, 64). The donor kidney was procured and stored in 4 °C cold saline with or without accelerating-3rdgen-SMEF application for 1.5 hours. The donor kidney was then transplanted into a recipient. The remaining contralateral native kidney of the recipient was removed. Last, the abdomen was closed. Mice with right kidney nephrectomy served as sham controls. Ten mice for each group were used in the transplantation experiment, with five mice as donors (non-survival) and five as recipients (survival).
Kidney injury and function evaluation
Plasma creatinine and NGAL concentrations were used to assess kidney injuries. GFR was measured to evaluate kidney functions in conscious mice by a single bolus intravenous injection of fluorescein isothiocyanate-sinistrin (FITC-sinistrin) (Fresenius Kabi Austria GmbH) as recently described (62, 63). The details of the measurement have been described in the Supplementary Materials and Methods.
Histology and Immunofluorescence
Periodic acid–Schiff (PAS), Masson’s Trichrome, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) and CD31 immunofluorescence staining were used to examine morphologic changes, fibrosis, apoptosis and endothelial cells at peritubular capillaries, respectively. The detailed methods are described in the Supplementary Materials and Methods.
The application of accelerating-3rdgen-SMEF to discarded human renal allografts
For human kidney donors who were not viable for transplant and discarded, we requested access to these organs through our local organ procurement organization for experimental research. In total, 5 pairs of human kidneys were used in this study. Once we received the pair of donor kidneys, one kidney was stored in UW solution as the untreated control and the other kidney from the same donor was subjected to 3rdgen-SMEF during the subsequent storage as shown in fig. S3. The total storage time was 24 hours. Kidney tissue was then collected to evaluate histological changes by PAS staining and TEM. (n= 5 pairs of kidneys from 5 kidney donors)
Transmission electron microscopy (TEM)
To perform TEM, the kidney tissues were cut into about 1 mm3 pieces and fixed in 2% glutaraldehyde in 0.1 M sodium cacodylate buffer overnight. Then the kidney tissues were treated with 1% osmium tetroxide, dehydrated with a series of graded ethanol treatments, sequentially infiltrated with 2:1, 1:1, and 1:2 mixtures of acetone and resin, and embedded in 100% resin. Thin sections (approximately 90–100 nm) were cut and examined with the transmission electron microscope (JEM-1400Plus).
Histologic examination of all kidney sections was performed by an experienced renal pathologist (L. Fu) blinded to the experimental procedures.
Statistical analysis
All values are presented as means ± SEM unless otherwise indicated in the figure legends. All statistical tests performed have been mentioned in figure legends for each dataset with statistical significance defined as p< 0.05. Comparison of the trend of survival curve was done using the log-rank (Mantel–Cox) test. Comparison of the data for two groups was used student’s t test. Comparison of three or more datasets was performed using one way analysis of variance (ANOVA) ANOVA followed by Tukey’s multiple comparison test. Comparison of three or more datasets at multiple time points was performed using two-way ANOVA followed by Tukey’s multiple comparison test. Sample sizes are provided in the figure legends. All statistics were performed using GraphPad Prism, version 9.0 (GraphPad Software), except the data analysis of the pump currents and TEPD, which was performed by using the pClamp10 (Molecular Devices) and a Java program and the statistical significance defined as p< 0.05 was determined with Student’s t test.
Supplementary Material
Acknowledgements
We thank the O’Brien Center at the University of Alabama at Birmingham for technical assistance in the measurement of plasma creatinine concentrations with HPLC. We thank Tampa General Hospital for assistance with human kidney sample collection and LifeLink for the transportation and delivery of the human kidneys for our study.
Funding:
This work was supported by grants from NIH (GM 50785 to W.C., HL137987 to R.L, HL142414 to R.L., DK122050 to L.W. and R41DK130764. to R.L. and W.C.) and National Science Foundation (NSF0515787 to W.C) as well as American Heart Association Career Development Award 18CDA34110441 to L.W.
Footnotes
Competing Interests: C.W has a patent application pending for “Method of electrogenically controlling pump molecules” (# 8,073,549) and “System and method utilizing electrical energy to fuel and activate ion transporters without consumption of adenosine triphosphate molecules” (#1372.1285 PR). C.W. and L.R. are inventors on a filed patent application (Serial #: 63/162,169): “System and Method Utilizing Electrical Energy to Fuel and Activate Active Ion Transporter without Consumption of Adenosine Triphosphate (ATP) Molecules: Clinical Ischemia Application on Kidney”. All other authors declare that they have no competing interests.
List of Supplementary Materials
Other Supplementary Material for this manuscript includes the following: Data files S1
Data and materials availability:
All data associated with this study are present in the paper or the Supplementary Materials and Methods.
References and Notes
- 1.Halloran PF, Aprile MA, Farewell V, Ludwin D, Smith EK, Tsai SY, Bear RA, Cole EH, Fenton SS, Cattran DC, Early function as the principal correlate of graft survival. A multivariate analysis of 200 cadaveric renal transplants treated with a protocol incorporating antilymphocyte globulin and cyclosporine. Transplantation 46, 223–228 (1988). [PubMed] [Google Scholar]
- 2.Womer KL, Vella JP, Sayegh MH, Chronic allograft dysfunction: mechanisms and new approaches to therapy. Semin. Nephrol 20, 126–147 (2000). [PubMed] [Google Scholar]
- 3.Nehus EJ, Devarajan P, Acute kidney injury: AKI in kidney transplant recipients--here to stay. Nat. Rev Nephrol 8, 198–199 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Daemen MA, de VB, Buurman WA, Apoptosis and inflammation in renal reperfusion injury. Transplantation 73, 1693–1700 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Cooper JE, Wiseman AC, Acute kidney injury in kidney transplantation. Curr. Opin. Nephrol. Hypertens 22, 698–703 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Perico N, Cattaneo D, Sayegh MH, Remuzzi G, Delayed graft function in kidney transplantation. Lancet 364, 1814–1827 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Juhaszova M, Blaustein MP, Distinct distribution of different Na+ pump alpha subunit isoforms in plasmalemma. Physiological implications. Ann N Y Acad Sci 834, 524–536 (1997). [DOI] [PubMed] [Google Scholar]
- 8.Kaplan JH, Biochemistry of Na,K-ATPase. Annu Rev Biochem 71, 511–535 (2002). [DOI] [PubMed] [Google Scholar]
- 9.Wu MY, Yiang GT, Liao WT, Tsai AP, Cheng YL, Cheng PW, Li CY, Li CJ, Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell Physiol Biochem 46, 1650–1667 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Sen N, Das BB, Ganguly A, Mukherjee T, Bandyopadhyay S, Majumder HK, Camptothecin-induced imbalance in intracellular cation homeostasis regulates programmed cell death in unicellular hemoflagellate Leishmania donovani. J Biol Chem 279, 52366–52375 (2004). [DOI] [PubMed] [Google Scholar]
- 11.De RS, Antonelli M, Ronco C, Hypothermia and kidney: a focus on ischaemia-reperfusion injury. Nephrol Dial. Transplant 32, 241–247 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Ponticelli CE, The impact of cold ischemia time on renal transplant outcome. Kidney Int 87, 272–275 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Devarajan P, Cellular and molecular derangements in acute tubular necrosis. Curr Opin Pediatr 17, 193–199 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Moreno C, Yano S, Bezanilla F, Latorre R, Holmgren M, Transient Electrical Currents Mediated by the Na(+)/K(+)-ATPase: A Tour from Basic Biophysics to Human Diseases. Biophys J 119, 236–242 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glitsch HG, Electrophysiology of the sodium-potassium-ATPase in cardiac cells. Physiol Rev 81, 1791–1826 (2001). [DOI] [PubMed] [Google Scholar]
- 16.Teissie J, Tsong TY, Evidence of voltage-induced channel opening in Na/K ATPase of human erythrocyte membrane. J Membr Biol 55, 133–140 (1980). [DOI] [PubMed] [Google Scholar]
- 17.Tsong TY, Na,K-ATPase as A Brownian Motor: Electric Field-InducedConformational Fluctuation Leads to Uphill Pumping of Cation inthe Absence of ATP. Journal of biological physics 28, 309–325 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Astumian RD, Thermodynamics and kinetics of a Brownian motor. Science (New York, N.Y.) 276, 917–922 (1997). [DOI] [PubMed] [Google Scholar]
- 19.Tsong TY, Astumian RD, Electroconformational coupling and membrane protein function. Prog Biophys Mol Biol 50, 1–45 (1987). [DOI] [PubMed] [Google Scholar]
- 20.Liu DS, Astumian RD, Tsong TY, Activation of Na+ and K+ pumping modes of (Na,K)-ATPase by an oscillating electric field. J Biol Chem 265, 7260–7267 (1990). [PubMed] [Google Scholar]
- 21.Xie TD, Chen Y, Marszalek P, Tsong TY, Fluctuation-driven directional flow in biochemical cycle: further study of electric activation of Na,K pumps. Biophys J 72, 2496–2502 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robertson B, Astumian RD, Tsong TY, in Charge and Field Effects in Biosystems—2, Allen MJ, Cleary SF, Hawkridge FM, Eds. (Springer US, Boston, MA, 1989), pp. 191–209. [Google Scholar]
- 23.Astumian RD, Adiabatic Pumping Mechanism for Ion Motive ATPases. Physical Review Letters 91, 118102 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Blank M, Soo L, The effects of alternating currents on Na,K-ATPase function. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry 276, 313–322 (1989). [Google Scholar]
- 25.Blank M, Soo L, Ion activation of the Na,K-ATPase in alternating currents. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry 299, 51–61 (1990). [Google Scholar]
- 26.Weaver JC, Electromagnetic field dosimetry: Issues relating to background, noise, and interaction mechanisms. 13, 115–117 (1992). [DOI] [PubMed] [Google Scholar]
- 27.Brizhik L, Eremko A, Piette B, Zakrzewski W, Solitons in alpha-helical proteins. Physical review. E, Statistical, nonlinear, and soft matter physics 70, 031914 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Lawrence AF, McDaniel JC, Chang DB, Birge RR, The nature of phonons and solitary waves in alpha-helical proteins. Biophysical journal 51, 785–793 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen W, Zhang ZS, Synchronization of Na/K pump molecules by a train of squared pulses. J Bioenerg Biomembr 38, 319–325 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Chen W, Zhang Z, Huang F, Synchronization of Na/K pump molecules by an oscillating electric field. J Bioenerg Biomembr 40, 347–357 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Chen W, Zhang Z, Huang F, Entrainment of Na/K pumps by a synchronization modulation electric field. J Bioenerg Biomembr 39, 331–339 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Fang Z, Chen W, Quick and effective hyperpolarization of the membrane potential in intact smooth muscle cells of blood vessels by synchronization modulation electric field. J Bioenerg Biomembr 44, 385–395 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Tran V, Zhang X, Cao L, Li H, Lee B, So M, Sun Y, Chen W, Zhao M, Synchronization modulation increases transepithelial potentials in MDCK monolayers through Na/K pumps. PLoS One 8, e61509 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang P, Mast J, Chen W, Synchronization Modulation of Na/K Pumps Induced Membrane Potential Hyperpolarization in Both Physiological and Hyperkalemic Conditions. J Membr Biol 252, 577–586 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Chen W, Synchronization of ion exchangers by an oscillating electric field: theory. J Phys Chem B 112, 10064–10070 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Chen W, Method of electrogenically controlling pump molecules. Patent No.8073549, (2011).
- 37.Chen W, Synchronization of the Na/K pump down to individual steps in the pumping cycle. Patent pending, (2020).
- 38.Chen W 3rd generation synchronization modulation technique can utilize electric energy to substitute ATP in fueling the Na/K pumps. Patent pending, (2021). [Google Scholar]
- 39.Horisberger JD, Kharoubi-Hess S, Guennoun S, Michielin O, The fourth transmembrane segment of the Na,K-ATPase alpha subunit: a systematic mutagenesis study. J Biol Chem 279, 29542–29550 (2004). [DOI] [PubMed] [Google Scholar]
- 40.el Mernissi G, Barlet-Bas C, Khadouri C, Marsy S, Cheval L, Doucet A, Characterization and localization of ouabain-insensitive Na-dependent ATPase activities along the rat nephron. Biochim Biophys Acta 1064, 205–211 (1991). [DOI] [PubMed] [Google Scholar]
- 41.Koeppen BM, Giebisch G, Biagi BA, Electrophysiology of mammalian renal tubules: inferences from intracellular microelectrode studies. Annu Rev Physiol 45, 497–517 (1983). [DOI] [PubMed] [Google Scholar]
- 42.Katz AI, Lindheimer MD, Relation of Na-K-ATPase to acute changes in renal tubular sodium and potassium transport. J Gen Physiol 66, 209–222 (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molitoris BA, Chan LK, Shapiro JI, Conger JD, Falk SA, Loss of epithelial polarity: a novel hypothesis for reduced proximal tubule Na+ transport following ischemic injury. J Membr Biol 107, 119–127 (1989). [DOI] [PubMed] [Google Scholar]
- 44.Buckalew VM Jr., Martinez FJ, Green WE, The effect of dialysates and ultrafiltrates of plasma of saline-loaded dogs on toad bladder sodium transport. J Clin Invest 49, 926–935 (1970). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gonick HC, Kramer HJ, Paul W, Lu E, Circulating inhibitor of sodium-potassium-activated adenosine triphosphatase after expansion of extracellular fluid volume in rats. Clin Sci Mol Med 53, 329–334 (1977). [DOI] [PubMed] [Google Scholar]
- 46.Kaasik A, Safiulina D, Zharkovsky A, Veksler V, Regulation of mitochondrial matrix volume. Am J Physiol Cell Physiol 292, C157–163 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Szeto HH, Liu S, Soong Y, Wu D, Darrah SF, Cheng FY, Zhao Z, Ganger M, Tow CY, Seshan SV, Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J Am Soc Nephrol 22, 1041–1052 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thadhani R, Pascual M, Bonventre JV, Acute renal failure. N Engl J Med 334, 1448–1460 (1996). [DOI] [PubMed] [Google Scholar]
- 49.Jassem W, Heaton ND, The role of mitochondria in ischemia/reperfusion injury in organ transplantation. Kidney Int 66, 514–517 (2004). [DOI] [PubMed] [Google Scholar]
- 50.De Rosa S, Antonelli M, Ronco C, Hypothermia and kidney: a focus on ischaemia-reperfusion injury. Nephrol Dial Transplant 32, 241–247 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Weaver JC, Smith KC, Esser AT, Son RS, Gowrishankar TR, A brief overview of electroporation pulse strength-duration space: a region where additional intracellular effects are expected. Bioelectrochemistry 87, 236–243 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang F, Fang Z, Mast J, Chen W, Comparison of membrane electroporation and protein denature in response to pulsed electric field with different durations. Bioelectromagnetics 34, 253–263 (2013). [DOI] [PubMed] [Google Scholar]
- 53.Hille B, Ion channels of exitable membranes. (Sinauer Associates, Inc, ed. Third edition, 2001), pp. 37. [Google Scholar]
- 54.Lauger P, lectrogenic Ion Pumps. (Sinauer Associates Inc, 1991). [Google Scholar]
- 55.Chen W, Lee RC, An improved double vaseline gap voltage clamp to study electroporated skeletal muscle fibers. Biophys J 66, 700–709 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen W, Hui CS, Differential blockage of charge movement components in frog cut twitch fibres by nifedipine. J Physiol 444, 579–603 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang X, Chandrashekar K, Wang L, Lai EY, Wei J, Zhang G, Wang S, Zhang J, Juncos LA, Liu R, Inhibition of Nitric Oxide Synthase 1 Induces Salt-Sensitive Hypertension in Nitric Oxide Synthase 1alpha Knockout and Wild-Type Mice. Hypertension 67, 792–799 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang L, Wang X, Qu HY, Jiang S, Zhang J, Fu L, Buggs J, Pang B, Wei J, Liu R, Role of Kidneys in Sex Differences in Angiotensin II-Induced Hypertension. Hypertension 70, 1219–1227 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McCrimmon A, Domondon M, Sultanova RF, Ilatovskaya DV, Stadler K, Comprehensive assessment of mitochondrial respiratory function in freshly isolated nephron segments. Am J Physiol Renal Physiol 318, F1237–F1245 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ruggiero C, Ehrenshaft M, Cleland E, Stadler K, High-fat diet induces an initial adaptation of mitochondrial bioenergetics in the kidney despite evident oxidative stress and mitochondrial ROS production. Am J Physiol Endocrinol Metab 300, E1047–1058 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Micakovic T, Banczyk WZ, Clark E, Kranzlin B, Peters J, Hoffmann SC, Isolation of Pure Mitochondria from Rat Kidneys and Western Blot of Mitochondrial Respiratory Chain Complexes. Bio Protoc 9, e3379 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang J, Wang X, Wei J, Wang L, Jiang S, Xu L, Qu L, Yang K, Fu L, Buggs J, Cheng F, Liu R, A two-stage bilateral ischemia-reperfusion injury-induced AKI to CKD transition model in mice. Am J Physiol Renal Physiol 319, F304–F311 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang L, Wang X, Jiang S, Wei J, Buggs J, Fu L, Zhang J, Liu R, Graft function assessment in mouse models of single- and dual-kidney transplantation. Am J Physiol Renal Physiol 315, F628–F636 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang L, Wei J, Jiang S, Li HH, Fu L, Zhang J, Liu R, Effects of different storage solutions on renal ischemia tolerance after kidney transplantation in mice. Am J Physiol Renal Physiol 314, F381–F387 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu Y, Wei J, Stec DE, Roman RJ, Ge Y, Cheng L, Liu EY, Zhang J, Hansen PB, Fan F, Juncos LA, Wang L, Pollock J, Huang PL, Fu Y, Wang S, Liu R, Macula Densa Nitric Oxide Synthase 1beta Protects against Salt-Sensitive Hypertension. J Am Soc Nephrol 27, 2346–2356 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang J, Wei J, Jiang S, Xu L, Wang L, Cheng F, Buggs J, Koepsell H, Vallon V, Liu R, Macula Densa SGLT1-NOS1-Tubuloglomerular Feedback Pathway, a New Mechanism for Glomerular Hyperfiltration during Hyperglycemia. J Am Soc Nephrol 30, 578–593 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wei J, Zhang J, Jiang S, Wang L, Persson AEG, Liu R, High-Protein Diet-Induced Glomerular Hyperfiltration Is Dependent on Neuronal Nitric Oxide Synthase beta in the Macula Densa via Tubuloglomerular Feedback Response. Hypertension 74, 864–871 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this study are present in the paper or the Supplementary Materials and Methods.


