Summary
Evergreen species are widespread across the globe, representing two major plant functional forms in terrestrial models. We reviewed and analysed the responses of photosynthesis and respiration to warming in 101 evergreen species from boreal to tropical biomes. Summertime temperatures affected both latitudinal gas exchange rates and the degree of responsiveness to experimental warming. The decrease in net photosynthesis at 25°C (A net25) was larger with warming in tropical climates than cooler ones. Respiration at 25°C (R 25) was reduced by 14% in response to warming across species and biomes. Gymnosperms were more sensitive to greater amounts of warming than broadleaved evergreens, with A net25 and R 25 reduced c. 30–40% with > 10°C warming. While standardised rates of carboxylation (V cmax25) and electron transport (J max25) adjusted to warming, the magnitude of this adjustment was not related to warming amount (range 0.6–16°C). The temperature optimum of photosynthesis (T optA) increased on average 0.34°C per °C warming. The combination of more constrained acclimation of photosynthesis and increasing respiration rates with warming could possibly result in a reduced carbon sink in future warmer climates. The predictable patterns of thermal acclimation across biomes provide a strong basis to improve modelling predictions of the future terrestrial carbon sink with warming.
Keywords: acclimation, biome, nitrogen, photosynthesis, respiration, temperature, warming
1.
| Contents | ||
|---|---|---|
| Summary | 353 | |
| I. | Introduction | 354 |
| II. | Latitudinal patterns | 354 |
| III. | Acclimation of photosynthesis to warming | 355 |
| IV. | Acclimation of leaf respiration to warming | 362 |
| V. | Limits to the thermal acclimation capacity | 365 |
| VI. | Can spatial gradients and seasonal changes help to predict warming responses? | 365 |
| VII. | Including acclimation capacity of photosynthesis and respiration in land surface models | 366 |
| VIII. | Conclusions | 367 |
| Acknowledgements | 367 | |
| References | 367 | |
| Appendix A1 | 372 | |
| Appendix A2 | 373 | |
I. Introduction
Evergreen tree species are found at most latitudes and represent key dominant plant functional types (PFTs) employed in process‐based vegetation models. Boreal forests are dominated by needle‐leaved evergreen species and extensively cover the higher northern latitudes, accounting for c. 20% of the global aboveground biomass. The mid‐latitudes contain a mixture of evergreen conifers and deciduous species, with a transition towards mostly broadleaved evergreen species in subtropical and tropical latitudes. Together, boreal and tropical forests are the strongest contributors across all biomes to the global terrestrial carbon sink; c. 27% each corresponding to c. 0.30 Pg C yr−1 in each biome (Beer et al., 2010; Tagesson et al., 2020). With a projected future warming of 2.6–5°C by the end of this century (Stocker et al., 2013; Masson‐Delmotte et al., 2022), understanding how evergreen species will respond as the climate warms is key to estimating the strength of the future terrestrial carbon sink.
Although temperature has increased on average by c. 1.1°C globally, the amount of warming is not equally distributed across latitudes. Boreal regions are predicted to warm up to 10°C while tropical regions may experience warming of about 3–4°C by the end of this century (Masson‐Delmotte et al., 2022). Projecting evergreen tree responses to warming is complicated due to key uncertainties regarding the temperature responses of photosynthesis and respiration and their feedbacks to the global carbon cycle (Lombardozzi et al., 2015; Mercado et al., 2018). Importantly, small adjustments of photosynthesis and respiration in response to climate warming can have a large impact on carbon uptake and therefore the size of the future terrestrial carbon sink (Huntingford et al., 2017; Dusenge et al., 2019). There is extensive evidence that both photosynthesis and respiration physiologically adjust to changes in growth temperature over a timeframe of weeks to years, the so‐called thermal acclimation (Atkin et al., 2005a). Taking this physiological acclimation into account will result in more accurate estimates of global carbon exchange (Atkin et al., 2008; Smith & Dukes, 2013; Smith et al., 2016; Dusenge et al., 2019).
In this review, we focus on the capacity of evergreen species to physiologically acclimate to warming and investigate how warming responses vary with biome. Evergreen tree species, and especially tropical broadleaf evergreen species, generally have formed a small component in previous analyses investigating plant physiological responses to warming across a broad set of species (Saxe et al., 2001; Way & Oren, 2010; Chung et al., 2013; Liang et al., 2013; Dusenge et al., 2019). Here we aimed to bring together an understanding of how evergreen tree physiology responds to warming. We focus on the response of photosynthesis and respiration and related variables. Whole‐plant metrics or other aspects of warming responses such as growth (Saxe et al., 2001; Way & Oren, 2010), phenology (Chambers et al., 2013; Chung et al., 2013) and environmental interactions with warming (Teskey et al., 2015; Ruehr et al., 2016) are beyond the scope here and are reviewed elsewhere. However, in many cases these responses are derived from the gas exchange processes affected by warming that we consider in this review.
In addition to reviewing the literature involving how evergreen species adjust to a change in growth temperatures, we collected photosynthetic and respiratory variables from warming studies on evergreen species from both the field and controlled environment experiments, which we will refer to as the ‘Evergreen species in a warming environment’ (ESWE) dataset. The ESWE dataset contained 101 evergreen species across two plant groups with different leaf form, that is needleleaf and broadleaf, representing a total of 23 gymnosperm and 78 angiosperm evergreen species across three biomes: boreal, temperate and tropical, based on the coordinates indicated in the study (Supporting Information Table S1). Each coordinate was linked to a mean annual temperature and a mean temperature of the warmest quarter from BioCLIM (Hijmans et al., 2005), both of which corresponded well with biome (Fig. S1). The ESWE dataset represented a realistic diversity of evergreen species and leaf forms across latitudes with 13 needleleaf evergreen species in the boreal zone, a mixture of needleleaf (10 species) and broadleaf (31 species) evergreen species in the temperate zone and 51 broadleaf evergreen species in the tropical zone (Table S1). The amount of warming across the ESWE dataset spanned from 0.6°C to 16°C warming. See Appendix A1 for more details on the variables and the statistical analyses and Appendix A2 for the studies used in the analyses.
We evaluated the following questions: (1) Are there any intrinsic latitudinal patterns using ambient (i.e. control only) conditions across biomes and leaf form (Section II), (2) How do photosynthesis and respiration parameters respond to experimental warming? Is that response different across biomes and/or leaf forms? (3) How are these parameters related to the amount of warming applied? (Sections III; IV and V). Finally, we discuss whether we could use spatial gradients in the environment as a proxy for time (i.e. future projected warming) to predict temporal changes (Section VI) and consider how our review can inform land surface models (Section VII).
II. Latitudinal patterns
Examining variation across latitudinal gradients provides insight into how climate has shaped plant functioning to survive, grow and reproduce, including physiological processes (Brown et al., 1996; Willig et al., 2003). Evergreen species are an ideal ‘model’ to examine biogeographic patterns due to their wide occurrence, allowing physiological responses to be contrasted across species originating from different climates. Understanding how physiological traits are related to the current climate conditions is important to evaluate responses to warming across a large set of species (see Appendix A1 for statistical details).
Plants have been hypothesised to balance functional trade‐offs to optimally utilise their available resources (Dewar et al., 2009; Togashi et al., 2018; Harrison et al., 2021). Plants invest a lot of nutrients toward metabolism (Evans, 1989) and balance how nutrients are allocated to achieve the best resource‐use efficiency at the least cost (Togashi et al., 2018). Global patterns of leaf nitrogen (N) and phosphorus (P) have been related to latitude, which strongly co‐varies with temperature (Reich & Oleksyn, 2004). Leaf N and P declined from high‐latitudes towards the equator, suggesting a response of declining N as average temperatures increase (Reich & Oleksyn, 2004). This trend in leaf nutrients has been supported by many studies, finding higher N in cool‐grown plants and lower N observed in response to warmer growth temperatures (Strand et al., 1999; Hikosaka, 2005; Yamori et al., 2005; Way & Sage, 2008; Crous et al., 2018). In the ESWE dataset, area‐based leaf N (Na) decreased as site temperature increased both with the mean temperature of the warmest quarter, a proxy for summer growing temperature based on latitude (P < 0.0001, R 2 = 0.14; Table 1; Figs 1a, S2) and with experimental growth temperature (Fig. S3), supporting that leaf N content declines with higher growth temperatures.
Table 1.
ANCOVA results (F‐statistic and P‐value) with leaf form as categorical variable and mean temperature of the warmest quarter (meanTWQ) as the covariate using the values of the control treatments only, transformed when necessary.
| Control conditions | Total obs. |
Leaf form df = 1 |
meanTWQ df = 1 |
||
|---|---|---|---|---|---|
| Variable | F | P | F | P | |
| V cmax25 | 69 | 4.32 | 0.042 | 8.63 | 0.0046 |
| J max25 | 68 | 9.66 | 0.0028 | 26.09 | < 0.0001 |
| J : V 25 | 53 | 8.69 | 0.005 | 19.95 | < 0.0001 |
| A net25 | 106 | 0.74 | 0.39 | 7.08 | 0.0091 |
| T optA | 56 | 0.019 | 0.89 | 20.17 | < 0.0001 |
| A growth | 54 | 8.48 | 0.0056 | 0.48 | 0.49 |
| R growth | 126 | 0.068 | 0.79 | 0.84 | 0.36 |
| R d25 | 129 | 0.001 | 0.97 | 9.52 | 0.0026 |
| Na | 109 | 0.99 | 0.32 | 19.10 | < 0.0001 |
Sources of the model are the columns and variables form the rows, df is the degree of freedom for each source and the sample size for each variable is found in the second column (Total obs.). P‐values are indicated in bold when significant < 0.05.
Variables are: the maximum carboxylation rate (V cmax25); the maximum electron transport rate (J max25); the ratio of J max25 over V cmax25 (J : V 25); and net photosynthesis (A net25). All parameters were measured at a common temperature of 25°C, the temperature optimum of photosynthesis (T optA); photosynthesis and respiration were measured at their growth temperatures (A growth and R growth respectively); mitochondrial dark respiration was measured at 25°C (R d25); and area‐based leaf nitrogen content (Na).
Fig. 1.
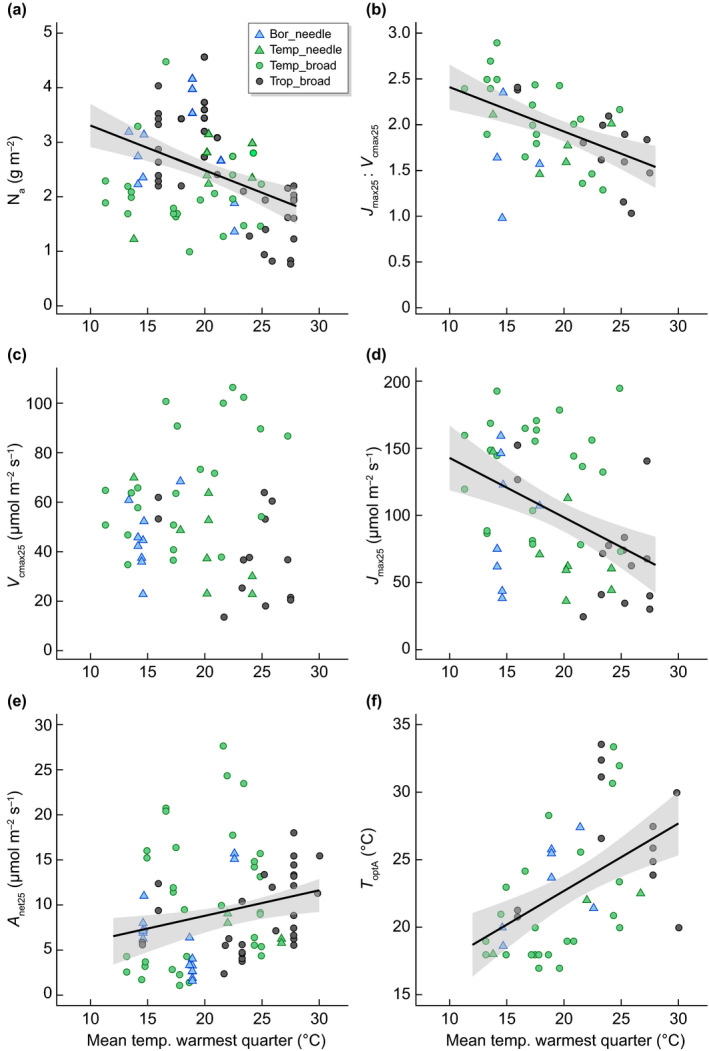
Linear regression relationships with 95% confidence intervals (grey area) between several variables against mean temperature of the warmest quarter (or average summer temperatures) to test for latitudinal patterns across biomes (Bor, boreal in skyblue; Temp, temperate in green; Trop, tropical in black) in control conditions based on latitudinal information for (a) nitrogen content (Na = −0.0822x + 4.128, R 2 = 0.16, P < 0.0001), (b) the ratio of maximum carboxylation rate at 25°C (V cmax25) to maximum electron transport rate at 25°C (J max25) (J max25 : V cmax25 = −0.048 + 2.89, R 2 = 0.25, P = 0.0001), (c) maximum carboxylation rate at 25°C (V cmax25), (d) maximum electron transport rates at 25°C (J max25 = −4.43x + 187.09, R 2 = 0.19, P = 0.0002), (e) net photosynthesis measured at a common temperature of 25°C (A net25) (A net25 = 0.28x + 3.15, R 2 = 0.06, P = 0.0088), and (f) the temperature optimum of photosynthesis (T optA) (T optA = 0.499x + 12.698, R 2 = 0.26, P < 0.0001). The partial regressions from the analyses of covariance (ANCOVA) were similar (except for V cmax25) and found in Supporting Information Fig. S2. Different symbols indicate leaf form with triangles for needleleaf and circles for broadleaf species.
Given the global patterns of declining N and P with warmer growth temperatures in combination with the large amount of N invested in the enzymes driving photosynthetic capacity, defined as the maximum carboxylation rate, V cmax25 and the maximum electron transport rate measured at 25°C (J max25), we expected lower V cmax25 and J max25 rates in response to warmer growth temperatures (Dusenge et al., 2020; Wang et al., 2020). Examining our ESWE dataset, we found that most photosynthetic variables in ambient conditions showed significant relationships with mean temperature of the warmest quarter (Table 1) and with mean annual temperature (Table S2) and differed depending on leaf form (Table 1). As Na declined with mean temperatures of the warmest quarter (Fig. 1a), the maximum rates of carboxylation and electron transport at a common temperature of 25°C as well as their ratio all declined with increasing mean temperatures of the warmest quarter (Table 1; Figs 1, S2a). Grouped per biome, V cmax25 and J max25 were 25 ± 10% and 26 ± 8% lower, respectively in the tropical biome compared to temperate/boreal biomes, but temperate and boreal biomes were not different from each other. Net photosynthesis measured at 25°C, A net25 slightly increased with higher mean temperature of the warmest quarter (Fig. 1e, R 2 = 0.06). Similar trends were observed with mean annual temperatures (Fig. S4; Table S2) and to some extent with experimental growing conditions (Fig. S3). Dark respiration rates measured at a common temperature of 25°C, R 25 rates reduced with higher mean temperatures of the warmest quarter (Fig. S2e; Table 1), similar to Atkin et al. (2015), reporting lower rates in the tropics than the Arctic measured at a common temperature.
Taken together, there were clear latitudinal patterns in photosynthetic and respiratory variables (V cmax25, J max25 and J : V 25, R 25) for which rates in the tropical biomes were lower compared with the other biomes, are likely to be linked to a corresponding decline in Na. Although standardised photosynthesis and respiration declined in warmer climates, actual rates at prevailing growth temperatures (i.e. A growth and R growth) were similar across latitudes (Table 1). This implies that negative effects on biochemical capacities at 25°C (i.e. V cmax25 and J max25) were balanced by positive direct effects of higher growth temperatures on enzyme reaction rates (up to the biochemical optimum temperatures, which are higher for V cmax25 and J max25 than those of A net), resulting in overall similar gas exchange rates across biomes.
Leaf form also influenced the rates of V cmax25 and J max25, in which needle‐leaved evergreens had lower rates compared with broadleaf evergreen species (−19% for V cmax25, −20% for J max25, −8% for J : V 25; P < 0.05; Table 1). At prevailing growth temperatures, A growth had significantly lower rates in needleleaf compared with broadleaf species (−34%, P = 0.0056; Table 1). Given that growth temperatures varied among studies, the A growth response could reflect lower growth temperatures used in experiments with needleleaf compared with broadleaf species.
III. Acclimation of photosynthesis to warming
The temperature response of net photosynthesis is usually described as an increase in a maximum net photosynthesis rate at an optimum temperature (T optA, black triangle in Fig. 2) followed by a relatively rapid decline in photosynthesis at higher temperatures (Berry & Björkman, 1980; Sage & Kubien, 2007; Yamori et al., 2014). The temperature response of net photosynthesis can be empirically modelled as:
| (Eqn 1) |
where T optA is the temperature optimum (°C) at which the highest rate of photosynthesis occurs (A opt) and b represents the broadness of the parabolic curvature.
Fig. 2.
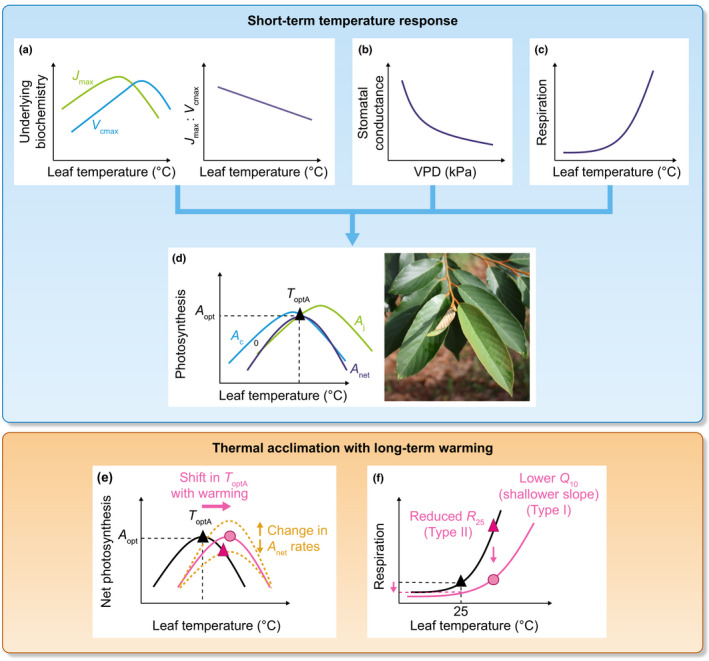
The photosynthesis–temperature (T) response is governed by underlying biochemistry (maximum carboxylation rate, V cmax and maximum electron transport rates, J max) (a) and stomatal processes by their responses to vapour pressure deficit (VPD) (b) and leaf respiration during the day (c), each of which has their own temperature response. Together these processes result in a net photosynthesis–temperature curve with a temperature optimum of photosynthesis (T optA black triangle) and a maximum photosynthesis rate at the T opt (A opt) (d), (curve differences emphasised for clarity). While the T opt of J max < T opt of V cmax, Rubisco‐limited photosynthesis (A c) usually declines faster at high temperatures than V cmax, resulting in T opt of A c < T opt of A j (RuBP‐limited photosynthesis). Changes in the temperature response curve of photosynthesis due to thermal acclimation is represented in (e) with a shift in T optA (pink arrow) and/or changes in photosynthesis rates (orange arrows, dotted lines). Changes in the temperature response curve of respiration due to thermal acclimation is represented in (f) with a lower temperature sensitivity (Q 10) and reduced respiration rates at a given temperature (pink arrow). In (e, f), black lines are representing instantaneous temperature responses under current growth temperatures, whereas the pink lines represent the adjustments to long‐term warming for photosynthesis and respiration. The pink triangles represent the short‐term response to warming without thermal acclimation (black and pink triangles, respectively). Pink circles represent the long‐term response to warming. Both lower respiration rates and similar to higher photosynthesis rates at higher temperatures due to acclimation improve the carbon gain balance compared with when these adjustments would not have occurred, compare pink triangles with pink circles in (e, f).
Stomatal conductance generally has a large control over photosynthesis and often declines in response to high vapour pressure deficit (VPD) associated with higher temperatures (Fig. 2b) (Monteith, 1995; Lloyd & Farquhar, 2008; Grossiord et al., 2020). This decline in stomatal conductance can affect the shape of the temperature response of photosynthesis. Lin et al. (2012) showed that low stomatal conductance decreased the T optA of photosynthesis. One approach to examine photosynthetic biochemistry without confounding influences from stomatal limitations is to estimate photosynthesis at a common internal CO2 concentration, C i (or at a common chloroplastic CO2 concentration, C c) using the Farquhar et al. (1980) model of photosynthesis (Vårhammar et al., 2015; Kumarathunge et al., 2019; Dusenge et al., 2021). It is currently more common to use C i than C c as data on the temperature response of mesophyll conductance are rare (Von Caemmerer & Evans, 2015) and it is hard to measure accurately in the field.
The temperature response of photosynthetic biochemistry is determined by changes in the maximum rates of carboxylation of ribulose‐1,5‐bisphophate (RuBP) (V cmax) and regeneration of RuBP (i.e. the maximum rate of electron transport, J max), and mitochondrial respiration (R d) (Farquhar et al., 1980). Biochemical processes underlying photosynthesis are mechanistically independent from stomatal effects and represent the photosynthetic capacity of the plant (Farquhar et al., 1980). Both V cmax and J max have their own temperature dependency. The instantaneous temperature responses of V cmax and J max each have a temperature optimum, with the optimum of J max usually occurring at a lower temperature than the optimum of V cmax (Fig. 2a). J max is more sensitive to high temperatures than V cmax because it depends on thylakoid membrane stability to function effectively (Sage & Kubien, 2007). By contrast, Rubisco‐limited photosynthesis (A c) usually declines earlier and faster at high temperatures than V cmax (Fig. 2d), as Rubisco oxygenation increases more steeply than carboxylation (Sage & Kubien, 2007; Busch & Sage, 2017). Moreover, stomatal conductance typically constrains the CO2 supply to carboxylation even further at high temperatures (Lloyd & Farquhar, 2008; Grossiord et al., 2020). As a result, declines in A net at high temperatures are usually the consequence of Rubisco limitation rather than electron transport limitation (Vårhammar et al., 2015; Busch & Sage, 2017; Dusenge et al., 2021). This is also the case at 25°C in most tree species globally (De Kauwe et al., 2016). Alternatively, reduced photosynthesis at high temperatures (> 35°C) may be limited by reduced J max rates related to reduced PSII electron flow in favour of increased cyclic electron flow by PSI (Havaux, 1996; Pastenes & Horton, 1996; Sharkey, 2005). Generally, however, at higher temperatures, there is often a rebalancing between carboxylation and electron transport in the favour of V cmax, leading to a lower J max to V cmax ratio (Bernacchi et al., 2003; Yamori et al., 2005; Kattge & Knorr, 2007).
Fitting a temperature response to V cmax and J max with a peaked Arrhenius function requires at least four different temperatures over which V cmax and J max are determined. It is modelled as:
| (Eqn 2) |
where k 25 is the value of V cmax or J max at 25°C, R is the universal gas constant (8.314 J mol−1 K−1), T k is leaf temperature in °K, E a (J mol−1) is the activation energy describing the exponential rise in enzyme activity with increasing temperature, H d (J mol−1) is the deactivation energy describing the rate of decline above the temperature optimum, and ΔS is the entropy term (J K−1). When only four different temperature points are used, H d is usually held constant at 200 kJ mol−1 (Medlyn et al., 2002; Kattge & Knorr, 2007). Even with five different temperature measurements, H d is often fixed to avoid overfitting. An in‐depth analysis by Medlyn et al. (2002) has shown that keeping H d constant does not meaningfully affect the mathematical description of the function, recently supported by Yin (2021). Because the parameters from the peaked Arrhenius curve strongly co‐vary, they cannot be evaluated independently, regardless of whether H d is fixed or not.
Based on the fitted parameters from a peaked Arrhenius function, the temperature optimum (T opt) of V cmax or J max can be calculated as:
| (Eqn 3) |
where loge represents the natural logarithm and T opt is expressed in K.
Based on biochemical theory, photosynthetic capacity at a common temperature is predicted to increase with warming in plants grown under low‐to‐moderate growth temperatures, due to faster enzyme kinetics at these warmer temperatures (Kattge & Knorr, 2007; Sage & Kubien, 2007; Fig. 2a). However, at higher growth temperatures, relatively less enzyme is needed to achieve similar rates at warmer temperatures, thereby allowing less nutrient investment in the photosynthetic apparatus (Smith & Keenan, 2020; Wang et al., 2020). Recent literature has observed decreased photosynthetic capacity (i.e. V cmax25 and J max25) with warmer growth temperatures. This observation may be related to latitudinal patterns, which have shown lower leaf N concentrations at higher growth temperatures (Reich & Oleksyn, 2004), consistent with lower rates of photosynthetic capacity in the tropics (Fig. 1). Overall, however, it remains unclear what causes reduced photosynthetic capacity at higher growth temperatures. It is expected that plants exposed to warming adjust in such a way to increase or maintain photosynthesis (Way & Yamori, 2014) including a higher temperature optimum of photosynthesis (T optA) with warmer temperatures. These physiological adjustments to warming are likely to occur through changes in one or several parameters describing the temperature response curves of photosynthesis (Eqn 2). In the following sections, we used the ESWE dataset to evaluate the adjustment of the temperature optimum of photosynthesis, and changes in photosynthetic parameters both at common and prevailing growth temperatures by their response ratio to warming (i.e. values at warming over ambient treatment) (Appendix A1).
1. Temperature optimum of net photosynthesis: a sensitive indicator
The shift in temperature optimum of net photosynthesis (T optA) is a sensitive indicator as to how much the temperature response curve of photosynthesis can adjust to maximise carbon uptake under warming (Berry & Björkman, 1980). The T optA in C3 species typically increases by 0.3–0.6°C for each 1°C shift in growth temperature (Berry & Björkman, 1980; Yamori et al., 2014). Recent studies have clarified the relationship between T optA and the underlying biochemical parameters of photosynthesis. An increased T optA with warming is usually related to increased temperature optima of V cmax and J max (Fig. 2a) (Medlyn et al., 2002; Onoda et al., 2005; Hikosaka et al., 2006; Kattge & Knorr, 2007; Crous et al., 2018; Dusenge et al., 2020). The increased optima of V cmax can reflect a more temperature tolerant form of Rubisco activase (Salvucci & Crafts‐Brandner, 2004) while increased thylakoid membrane stability at higher temperatures is important to increase the T opt of J max (Sharkey, 2005; Sage & Kubien, 2007). However, T optA can also be increased either by an increase in activation energy of V cmax or J max (Hikosaka et al., 2006) or a lower entropy term (ΔS) without a change in the activation energy parameter, E a (Kattge & Knorr, 2007). Moreover, changes in the balance between RuBP carboxylation and RuBP regeneration described by the J max : V cmax ratio (Hikosaka et al., 2006) can also affect T optA. A low J max : V cmax ratio has been related to a higher temperature optimum of photosynthesis (Hikosaka et al., 2006; Kumarathunge et al., 2019; Dusenge et al., 2020). When J max : V cmax is low, RuBP regeneration has greater control over photosynthesis (i.e. less Rubisco limitation compared with when J max : V cmax is high). As photosynthesis is usually carboxylation limited at high temperatures, this adjustment leads to a higher T optA in warmer growth temperatures (Hikosaka et al., 2006). Therefore, there are several adjustments occurring in the underlying biochemical component processes (J max : V cmax, E a and/or ΔS) that can result in a higher temperature optimum of photosynthesis, which is beneficial to plants growing in warmer temperatures (Kumarathunge et al., 2019).
We explored the T optA responses to warming in evergreen tree species in the ESWE dataset with the following hypotheses: (1) warming increases T optA as the absolute shift by the temperature difference between warming and control and (2) the relative shift (i.e. temperature shift per °C warming) in T optA with warming is similar across biomes, between 0.3 and 0.6°C per °C increase in growth temperature (Berry & Björkman, 1980). To test the effect of warming on the absolute shift in T optA, an analysis of covariance (ANCOVA) approach was used with warming amount and leaf form as factors and mean temperature of the warmest quarter as a covariate to represent the fact that measurements are usually taken under growing season conditions (see Appendix A1 for more details). Our analyses of the ESWE data indicated that T optA increased with higher growth/summer temperatures, from boreal to tropical biomes (R 2 = 0.28; P < 0.001; Figs 1f, S2, S3) consistent with Kumarathunge et al. (2019) and with higher experimental warming (Fig. 3a; R 2 = 0.28; P < 0.0001; Table 2). Further support for the sensitivity of T optA to a change in surrounding growth temperatures, is provided by temperature gradients, even within tree canopies where the T optA is often higher at the top of the canopy than in more shaded parts lower in the canopy (Carter et al., 2021).
Fig. 3.
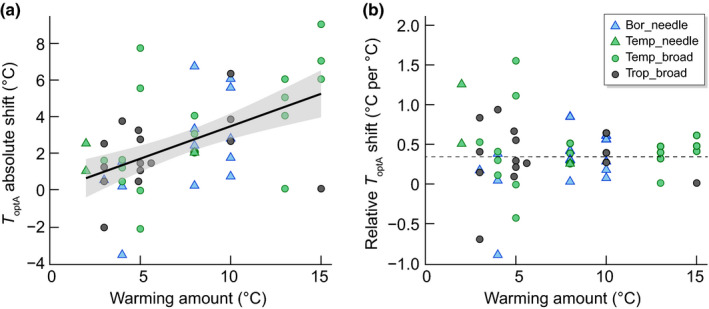
(a) Positive linear relationship with 95% confidence intervals (grey area) of the absolute temperature shift of the temperature optimum of photosynthesis (T optA) (°C, i.e. the temperature difference of T optA between warmed and control conditions) as a function of warming amount (T optA‐abs = 0.355x − 0.083, R 2 = 0.28, P < 0.0001) across biomes (Bor, boreal in skyblue; Temp, temperate in green; Trop, tropical in black) and leaf form (triangles are needleleaf and circles are broadleaf species) and (b) no relationship between the relative shift in temperature optimum (°C per °C warming) and the amount of warming, but with a significant intercept of 0.342°C per °C (P = 0.006, dashed line).
Table 2.
ANCOVA results (F‐statistic and P‐value) with warming amount, biome and leaf form as categorical variables and mean temperature of the warmest quarter as covariate (a proxy for biome) on the response ratios to warming (RR) to test the effects of warming, except for T optA for which the absolute shift and the shift per °C warming was evaluated.
| Response ratio (RR) | Total obs. |
Warming amount (binned) df = 2 |
meanTWQ (cov) df = 1 |
Leaf form df = 1 |
Warming amount × leaf form df = 2 |
||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | F | P | F | P | F | P | F | P | |
| T optA_abs. shift | 56 | 6.62 | 0.0032 | 0.79 | 0.38 | 0.09 | 0.92 | 1.36 | 0.27 |
| T optA_shift per °C | 56 | 0.06 | 0.94 | 0.24 | 0.63 | 1.18 | 0.28 | 0.54 | 0.59 |
| RR_V cmax25 | 69 | 0.13 | 0.88 | 5.93 | 0.018 | 0.02 | 0.89 | 1.95 | 0.15 |
| RR_J max25 | 68 | 0.72 | 0.49 | 1.50 | 0.23 | 0.002 | 0.99 | 1.48 | 0.24 |
| RR_J : V 25 | 53 | 0.040 | 0.96 | 6.78 | 0.013 | 0.36 | 0.55 | 0.63 | 0.54 |
| RR_A net25 | 106 | 0.03 | 0.97 | 4.76 | 0.032 | 9.93 | 0.0022 | 6.33 | 0.0027 |
| RR_A growth | 54 | 1.55 | 0.22 | 0.004 | 0.94 | 12.45 | 0.0011 | 1.96 | 0.17 |
| RR_R growth | 126 | 1.85 | 0.16 | 6.62 | 0.012 | 3.71 | 0.057 | 0.36 | 0.70 |
| RR_R d25 | 129 | 0.88 | 0.42 | 2.21 | 0.14 | 1.77 | 0.19 | 5.28 | 0.0064 |
| RR_Q 10 | 27 | 4.05 | 0.035 | 0.09 | 0.77 | 1.31 | 0.27 | 0.21 | 0.81 |
| RR_Na | 109 | 3.08 | 0.051 | 4.57 | 0.035 | 0.036 | 0.85 | 0.49 | 0.62 |
Sources of the model are the columns and variables form the rows, df is the degree of freedom for each source and the sample size for each variable is found in the second column (Total obs.). P‐values are indicated in bold when significant < 0.05.
Variables are: the absolute shift in temperature optimum of photosynthesis (T optA_abs. shift); the relative shift in T optA per °C warming (T optA_shift per °C); the maximum carboxylation rate (V cmax25); the maximum electron transport rate (J max25); the ratio of J max25 over V cmax25, (J : V 25); and net photosynthesis (A net25). All parameters were measured at a common temperature of 25°C, Photosynthesis and respiration were measured at their growth temperatures (A growth and R growth respectively), the mitochondrial dark respiration was measured at 25°C (R d25); the temperature sensitivity of respiration (Q 10); and area‐based leaf nitrogen content (Na). The response ratios of these variables are indicated with RR and all are log‐transformed to meet normality assumptions.
In contrast with an increased T optA with warming (Fig. 3a), there was no relationship between the relative shift in temperature optimum and the amount of warming applied (P = 0.94; Fig. 3b). The shift of 0.34°C per °C warming in evergreen species remains the same, regardless of the amount of warming applied and is within the range given by Berry & Björkman (1980) and Way & Yamori (2014). This consistent finding across biomes, leaf form and a broad range of experimental warming (up to +16°C) in the ESWE dataset delineates a limit to photosynthetic temperature optimum adjustment in evergreen species in a predictable manner.
2. Rates at a standard temperature: negative warming responses in warmer climates
Measurements of photosynthesis or photosynthetic capacity at a common temperature (e.g. 25°C) allowed us to compare the degree of thermal acclimation between different warming treatments or among biomes (i.e. ‘set temperature method’; Mooney, 1963; Hikosaka et al., 1999; Medlyn et al., 2002; Bernacchi et al., 2003; Atkin et al., 2005a; Onoda et al., 2005; Sage & Kubien, 2007; Ghannoum et al., 2010). The degree of thermal acclimation is interpreted as the relative change in a physiological process that a plant can make in response to a growth temperature change, expressed in the ESWE dataset by the response ratio of the values of the warmed treatment compared with the values in the control treatment for a given variable (Appendix A1). Here we review the literature and analyse the response ratio of several photosynthetic variables (V cmax25, J max25, J : V 25, Na, A net25 and A growth) to warming amount, leaf form and summer growth temperature (i.e. mean temperature of the warmest quarter) (Table 2).
The increase in T optA with warming can be accompanied by an increase or decrease in photosynthesis rates at a common, standard temperature (dotted lines in Fig. 2e). This acclimation of photosynthesis rates is often reflected in the underlying biochemical components of photosynthesis (i.e. V cmax25 and J max25). Photosynthetic capacity may increase through enhanced V cmax25 or J max25 at higher growth temperatures in some studies (Hikosaka et al., 1999; Medlyn et al., 2002; Bernacchi et al., 2003; Onoda et al., 2005; Sage & Kubien, 2007; Ghannoum et al., 2010) while other studies on evergreen trees reported decreased V cmax25 or J max25 with warming (Ferrar et al., 1989; Wertin et al., 2011; Crous et al., 2013; Aspinwall et al., 2016; Dusenge et al., 2021). It is currently unclear whether photosynthesis rates measured at a standard temperature generally increase or decrease with warming and whether there are patterns in these responses related to prevailing growth temperatures or the biome where these species grow.
Across the ESWE dataset, we found both positive (i.e. response ratio (RR) > 1, n = 26–27) and negative (i.e. RR < 1, n = 36–41) responses of V cmax25 and J max25 to warming, indicating that V cmax25 and J max25 generally acclimated to warming, although without a consistent direction. There were no significant overall effects of warming amount on the RRs of V cmax25, J max25 or J : V 25 across biomes (Table 2; Fig. 4). The same result has been reported for a broader range of species and functional groups in Way & Oren (2010). Therefore, while absolute rates of photosynthetic capacity (V cmax25, J max25) were lower in the tropical biome compared with higher latitudes (Fig. 1; Table 1), the response to experimental warming amount was similar across biomes (Table 2), suggesting that all species can acclimate to warming, regardless of where they originated.
Fig. 4.
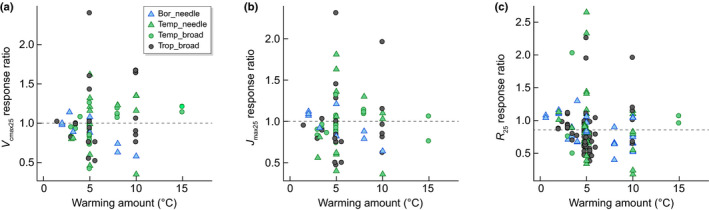
The response ratio of (a) maximum carboxylation rate at 25°C (V cmax25), (b) maximum electron transport rates at 25°C (J cmax25), and (c) mitochondrial respiration at 25°C (R 25) as a function of the amount of warming. A response ratio > 1 indicates an increased value in warming compared with control conditions, whereas a response ratio < 1 indicates a decreased value in warming compared with control conditions. There was no significant relationship with warming amount (Table 2, dashed line) but the intercept of R 25 was significantly negative (−0.858 ± 0.001; P < 0.0001), reflecting an overall 14% reduction of R 25 with warming amount across biomes (Bor, boreal in skyblue; Temp, temperate in green; Trop, tropical in black) and leaf form (triangles are needleleaf and circles are broadleaf evergreen species).
Several studies have found similar acclimation capacity of photosynthesis to warming among species within boreal (Reich et al., 2015; Sendall et al., 2015) and tropical climates (Slot & Kitajima, 2015; Slot & Winter, 2017b). However, across biomes, it has been hypothesised that tropical species would have less acclimation capacity than temperate species and therefore less ability to adjust to new temperature conditions (Janzen, 1967; Cunningham & Read, 2002). Our analyses showed reduced RRs of J : V 25 and A net25 with increasing mean temperature of the warmest quarter (Table 2; Fig. 5a,b), demonstrating that the overall photosynthetic response to warming was more negative in the tropics compared with cooler climates (despite the slightly increased response to warming of V cmax25 and Na with higher summer growth temperatures; Fig. 5c,d; Table 2). For every 10°C increase in mean temperature of the warmest quarter, the RR of A net25 decreased by 5.3% (Fig. 5a). Moreover, the rates of V cmax25 or J max25 in tropical species are often lower compared with the equivalent measure in temperate or boreal species (Fig. 1). These lower rates combined with the smaller RR observed in J : V 25 and A net25 in the tropics can contribute to seemingly small or no thermal acclimation response of photosynthesis or photosynthetic capacity in tropical species (Scafaro et al., 2017; Crous et al., 2018; Fauset et al., 2019; Carter et al., 2020; Dusenge et al., 2021). The mechanisms behind this limited thermal acclimation in the tropics need to be further explored, including limits to acclimation capacity and the potentially stronger role of stomatal conductance limitations to photosynthesis at higher temperatures (Carter et al., 2021; Slot et al., 2021). Slot & Winter (2016) indicated a possible VPD effect at higher temperatures, which may influence photosynthesis by stomatal conductance. Controlled experiments explicitly separating temperature and VPD could be valuable for improved mechanistic understanding (Slot & Winter, 2016; Grossiord et al., 2020).
Fig. 5.
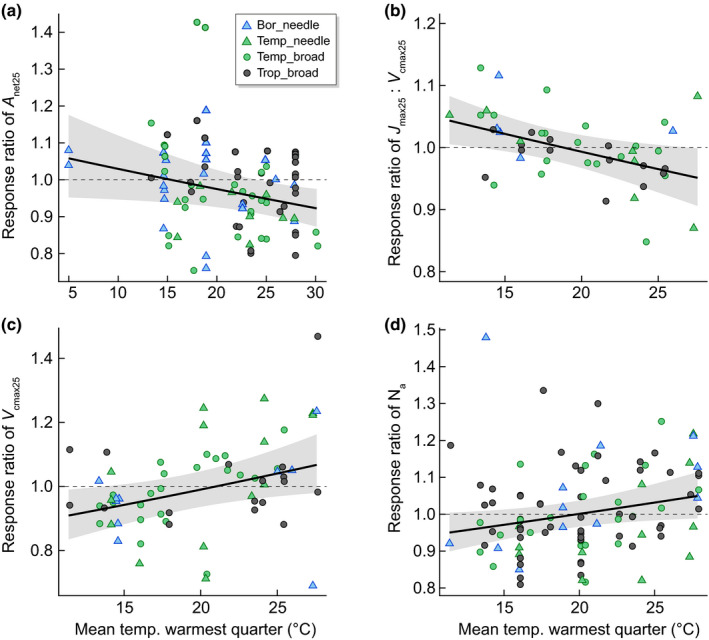
Partial residuals with 95% confidence intervals (grey area) of various response ratios (RR) as a function of mean temperature of the warmest quarter from the visreg R package to represent the effect of biome for (a) net photosynthesis at 25°C (A net25) (slope of RR_A net25 = exp(−0.0055x), (b) the ratio of maximum carboxylation rate at 25°C (V cmax) to maximum electron transport rate measured at 25°C (J max) (J max : V cmax ratio measured at 25°C: slope of RR_J : V 25 = exp(−0.0057x), (c) maximum carboxylation rate at 25°C (slope of RR_V cmax25 = exp(0.0099x)), and (d) area‐based nitrogen (Na) (slope of RR_Na = exp(0.006x). Colours indicate biome (Bor, boreal in skyblue; Temp, temperate in green; Trop, tropical in black). Leaf form is represented with triangles for needleleaf and circles for broadleaf species. A response ratio > 1 indicates an increased value in warming compared with control conditions (positive response), whereas a response ratio < 1 indicates a decreased value in warming compared with control conditions (negative response). The dashed line separates positive from negative responses to warming.
Regardless of how V cmax or J max respond to warming, one consistent response in the literature is the decrease in J max : V cmax ratio at higher growth temperatures. This response is observed in almost all studies that have investigated the shift in temperature responses of V cmax and J max (Yamori et al., 2005; Hikosaka et al., 2006; Kattge & Knorr, 2007; Sage & Kubien, 2007; Kositsup et al., 2009; Lin et al., 2012; Crous et al., 2013; Slot & Winter, 2017a; Dusenge et al., 2019; Kumarathunge et al., 2019; Bermudez et al., 2020; Slot et al., 2021). However, the magnitude of the decline in the J max : V cmax ratio often does not change in response to the amount of experimental warming (Way & Sage, 2008; Crous et al., 2018; Dusenge et al., 2020; Slot et al., 2021), which was also found in the ESWE dataset (Table 2). A reduced J max : V cmax ratio, often observed with higher growth temperatures may result from relatively less N investment in RuBP regeneration, or relatively more investment in RuBP carboxylation, or both processes at higher temperatures (Onoda et al., 2005; Hikosaka et al., 2006). However, at temperatures above 35°C, photosynthesis can be limited by a decrease in V cmax due to the heat lability of Rubisco activase (Salvucci & Crafts‐Brandner, 2004; Busch & Sage, 2017). Scafaro et al. (2017) reported a decline of Rubisco content with higher growth temperatures reducing V cmax and A net in tropical species. A similar result was found in two Eucalyptus species, E. tereticornis and E. grandis (Crous et al., 2018), for which reduced photosynthetic capacity was related to lower leaf nitrogen and lower leaf Rubisco content in tropical compared with temperate provenances of these two evergreen species. Our finding of higher J : V 25 RR with warming in cooler climates may reflect that warming stimulates carboxylation‐limited processes more than RuBP regeneration and electron transport for which plants are likely to operate below their T optA. By contrast, in warmer environments and biomes, both rapidly increasing photorespiration and declining stomatal conductance act to increase carboxylation limitations of photosynthesis, shifting N investments towards V cmax to maintain a balance between the two processes.
While we found no significant changes in responses to warming amount for V cmax25, J max25 and A net25 (Table 2), there was a significant interaction between warming amount and leaf form in the RR of A net25 (and R 25; Table 2). Needleleaf evergreen species clearly declined A net25 in response to warming, from a 5% reduction in the 5°C up to a 15% and 40% reduction with 5–10°C and > 10°C warming, respectively. Broadleaf species mostly maintained their response of A net25 and R 25 to warming with a slight increase in response to stronger warming, > 10°C (Fig. 6). A growth had a 30% lower RR in needleleaf species compared with broadleaf evergreen species (Table 2), consistent with a slight negative relationship of A growth as a function of warming in needleleaf evergreen species reported in Dusenge et al. (2019). This interaction highlights the sensitivity of needleleaf evergreen species to climate warming despite growing in cooler climates (Reich et al., 2015).
Fig. 6.
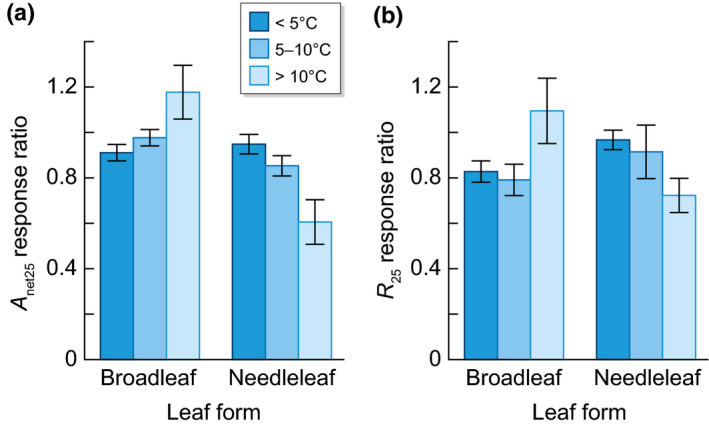
Bar plots showing the interaction between warming amount and leaf form for the mean and standard error for (a) the response ratio of net photosynthesis at 25°C (A net25) and (b) the response ratio leaf respiration at 25°C (R 25) in broadleaf and needleleaf evergreen species for three different categories of warming (< 5°C, 5–10°C, > 10°C). The interaction shows a more negative response to warming in needleleaf species with higher warming amount whereas broadleaf species had a fairly constant response and even a slight increase in R 25 with >10°C warming.
Species growing at warmer growth temperatures may benefit less from warming than species growing in cooler temperatures (Way & Oren, 2010; Ghannoum & Way, 2011). The response of A net25 to warming clearly declined with higher growth/summer temperatures (Figs 5a, S5a). Therefore, increased growth temperatures may have negative effects on photosynthesis (−8% in A growth, Fig. 7), possibly related to reduced leaf nitrogen content at higher growth temperatures (Figs 1, S2). Negative effects of warming on photosynthesis can be indirectly linked to several studies observing reduced growth rates in tropical species (Way & Oren, 2010; Drake et al., 2015). There is a need for more research conducted at higher control growth temperatures to investigate the warming responses upwards of 30°C, as many ecosystems currently experience these growth temperatures at some point in the year or will experience these conditions in the future. This includes the likely detrimental effects of warming on the balance of photosynthesis and respiration when growth temperatures are already high, as well as whole‐plant growth processes.
Fig. 7.
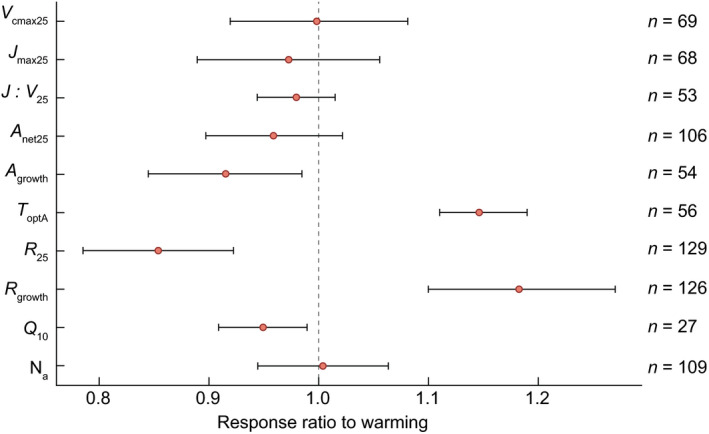
Mean (solid points) and 95% confidence interval (bars) across all evergreen species for the response ratio to warming in each of 10 variables: maximum carboxylation rate at 25°C (V cmax25), maximum electron transport rate at 25°C (J max25), the ratio of V cmax : J max at 25°C (J : V 25), net photosynthesis at 25°C (A net25) and net photosynthesis at prevailing growth temperatures (A growth), the temperature optimum of photosynthesis (T optA), mitochondrial respiration at 25°C (R 25), and dark respiration at growth temperatures (R growth), the temperature sensitivity of respiration (Q 10) and area‐based nitrogen content (Na). A response ratio > 1 indicates an increased value in warming compared with control conditions, whereas a response ratio < 1 indicates a decreased value in warming compared with control conditions (black dashed line). Overall, the response ratios of A growth (−8%), R 25 (−14%) and Q 10 (−5%) responded negatively to warming, while T optA (15%) and R growth (18%) were increased with warming. Sample sizes for each variable are indicated on the right.
IV. Acclimation of leaf respiration to warming
1. The temperature response of leaf respiration
Leaf respiration is essential for growth and survival by supporting the energetic and maintenance requirements of the cell, as well as providing C‐skeletons for biosynthesis (Penning de Vries, 1975). The temperature response of leaf respiration is described by an exponential function over a large range of temperatures (Hofstra & Hesketh, 1969; Atkin & Tjoelker, 2003; Heskel et al., 2016), indicating that leaf respiration is highly sensitive to temperature (Fig. 2c). At temperatures higher than 45°C, usually between 48°C and 60°C, leaf respiration reaches its maximum (O'Sullivan et al., 2013; Heskel et al., 2014; Weerasinghe et al., 2014) followed by a steep decline. This temperature maximum for respiration exists because, above this threshold, cell disruption or lysis of mitochondria has occurred and leaves stop respiring, signalling the point of cell death. This respiration temperature maximum varies somewhat by species, and depends on environmental factors such as growth temperatures, canopy position and species‐specific traits (O'Sullivan et al., 2017; Zhu et al., 2018).
The exponential part of the temperature response curve of respiration is used to determine the temperature sensitivity (i.e. the slope), usually across a temperature range below 45°C. While some exponential functions can fit the short‐term temperature response of respiration (Atkin et al., 2005b), the Q 10 function is most commonly used, with parameters reflecting the basal reference temperature at a given temperature and an exponential slope. The slope parameter, Q 10, reflects the proportional increase in respiration with a 10°C increase in temperature:
| (Eqn 4) |
In Eqn 4, basal respiration rate is given as R 25, but other temperatures are possible as long as they are within the measurement range to avoid extrapolation.
The Q 10 has been shown to be temperature dependent (Tjoelker et al., 2001; Atkin et al., 2005a; Heskel et al., 2016) declining with warmer temperatures (Bruhn et al., 2002; Covey‐Crump et al., 2002; Atkin & Tjoelker, 2003; Heskel et al., 2016). Therefore, the temperature sensitivity of respiration is not well represented by one value, especially in cooler biomes (Heskel et al., 2016). Heskel et al. (2016) proposed a general second order polynomial function for all biomes and PFTs to describe the temperature response of respiration:
| (Eqn 5) |
where T represents a given growth temperature and a varies by biome or PFT (Heskel et al., 2016), with a global value of a = −2.23. In contrast to the exponential Q 10 function (Eqn 4), the polynomial function allows for a peaked relationship, along with a temperature‐dependent slope parameter (i.e. Q 10 = −0.0005 × T + 0.1012) which is more appropriate for applications over large temperature ranges. However, both Q 10 and R 25 adjust to changing growth temperatures (thermal acclimation) and their responses to warming are evaluated in the next sections.
2. Acclimation of the temperature sensitivity of respiration, Q 10
The temperature response curve of respiration, described by the Q 10 and R 25 parameters in Eqn 4, changes in response to higher growth temperatures (e.g. acclimation of respiration; Fig. 2f). Atkin & Tjoelker (2003) described two types of respiratory acclimation. Type I acclimation is observed when the Q 10 (slope parameter) has changed. This enables the plant to dynamically respond to changes in surrounding growth temperatures. A reduced slope or temperature sensitivity results in lower respiration rates at higher temperatures as the upwards exponential increase is reduced compared with when acclimation did not occur (compare pink triangle with pink circle in Fig. 2f). Type II acclimation occurs when there is a change predominantly in R 25 (basal rate), often being reduced when exposed to higher growth temperatures compared with cooler temperatures, especially in new leaves developed under the new, warmer conditions. The change in R 25 is likely to be due to changes in enzyme capacity (Atkin & Tjoelker, 2003). Both types of acclimation result in reduced respiration rates at warmer temperatures compared with nonacclimated leaves.
The RR of Q 10 not only generally declined with experimental warming (−5%; Fig. 7) but also exhibited a significant negative relationship with warming amount (P = 0.0028, R 2 = 0.30; Fig. 8). This suggests that type I acclimation is important when evergreen species adjust to new growth temperatures. There was not much change in Q 10 with warming up to 5°C, but Q 10 declined up to c. 12% with warming above 5°C. Although Q 10 is reduced with warming, which results in lower respiration rates than would have occurred without acclimation, it is unclear which underlying respiratory processes drive this reduction. The temperature sensitivity of respiration is influenced by a range of internal factors, including the balance between enzyme activity, substrate availability and adenylate control over mitochondrial activity (i.e. the ratio of ATP : ADP and the concentration of ADP per se, Hoefnagel et al., 1998), each of which can change with seasonal variation, irradiance and nutrient availability (Zaragoza‐Castells et al., 2007; Crous et al., 2011). For an in‐depth review on the variation of Q 10, including the respiratory mechanism behind this variation, see (Atkin et al., 2005a,b). While a decline in Q 10 has been observed before across latitudes (Tjoelker et al., 2001), here we showed how Q 10 decreased with higher growth temperatures across a large range of warming (Fig. 8c).
Fig. 8.
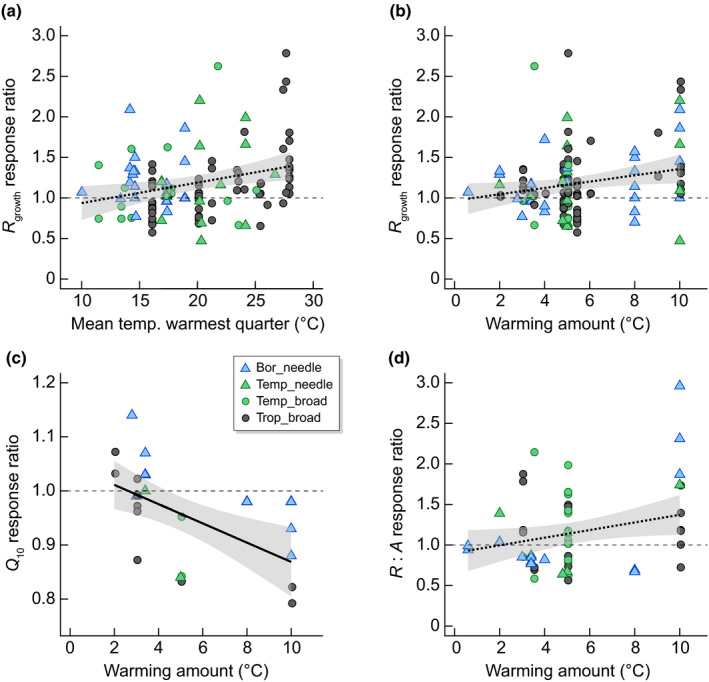
Linear regression relationships with 95% confidence intervals (grey area) between (a) dark respiration at growth temperatures (R growth) and the mean temperature of the warmest quarter (RR_R growth = 0.0252x + 0.68, R 2 = 0.05, P = 0.0083), (b) the response ratio of R growth and the amount of warming (RR_R growth = 0.039x + 0.967, R 2 = 0.03, P = 0.03), (c) the response ratio of Q 10 (where the slope parameter, Q 10, reflects the proportional increase in respiration with a 10°C increase in temperature) and the amount of warming (RR_Q 10 = 0.0178x + 1.047; R 2 = 0.30; P = 0.0028), and (d) the response ratio of R : A at growth temperatures and the amount of warming (RR_RA = 0.047x + 0.902; R 2 = 0.06; P = 0.04). The dotted lines indicate a significant but weak relationship with R 2 < 0.1. A response ratio > 1 indicates an increased value in warming compared with control conditions, whereas a response ratio < 1 indicates a decreased value in warming compared with control conditions (black dashed line separates positive from negative responses to warming). Colours indicate biome (Bor, boreal in skyblue; Temp, temperate in green; Trop, tropical in black). Leaf form is represented with different symbols with triangles for needleleaf and circles for broadleaf species.
3. Acclimation responses of leaf respiration rates to warming are universal
Thermal acclimation of respiration generally refers to processes associated with maintenance respiration, and helps to minimise carbon loss at higher temperatures while optimising ATP supply and carbon skeletons (Slot & Kitajima, 2015). As with photosynthesis, respiration measured at a common temperature determines the degree of thermal acclimation between different warming treatments or biomes. Respiration rates have been shown to adjust to new temperatures within a few days (Billings et al., 1971; Bolstad et al., 2003; Lee et al., 2005; Slot et al., 2014) although more complete thermal adjustments can be achieved over time by changes in protein density and amounts of mitochondria (Armstrong et al., 2006). Complete acclimation or homeostasis of respiration is achieved when rates of respiration in warm‐acclimated trees are the same at the higher growth temperature as rates in nonacclimated control trees at the original growth temperature. Homeostatic acclimation can be more easily achieved at moderate temperature changes, whereas larger temperature changes may not always result in complete acclimation (Campbell et al., 2007).
In the ESWE dataset, we observed reduced R 25 rates with higher mean temperatures of the warmest quarter (Fig. S2e; Table 1). Regarding the responses of respiration to warming, R 25 decreased by 14% across evergreen tree species in the ESWE dataset (Figs 4c, 7). The magnitude of R 25 reduction was independent of the amount of warming (Fig. 4; Table 2), but there was an interaction with leaf form in which needleleaf species showed a reduced RR with higher warming amounts (> 10°C), whereas broadleaf species did not. Needleleaf species reduced R 25 from a c. 6% on average up to 5–10°C warming to a 28% reduction with warming > 10°C (Fig. 6b; Table 2). A similar RR of R 25 across biomes (Table 2) implied a similar, universal degree of respiration acclimation for evergreen plants across the biogeographical domain. In a review across 103 species subjected to experimental warming, Slot & Kitajima (2015) suggested similar thermal acclimation of leaf respiration across species, which has been found in studies with boreal (Teskey & Will, 1999; Tjoelker et al., 1999) and nonevergreen species (Campbell et al., 2007), and is also apparent in the ESWE dataset. Vanderwel et al. (2015) demonstrated similar acclimation responses in respiration when examining seasonal adjustment (acclimation‐over‐time) compared with climate gradients (acclimation‐over space) based on a global analysis. Both Aspinwall et al. (2016) and Reich et al. (2016) found common underlying physiological mechanisms in respiratory acclimation responses to experimental warming and seasonal temperature changes. Therefore, a growing body of evidence suggests that respiration acclimates in a general, predictable manner to a change in growth temperature across a wide range of species and biomes (Heskel et al., 2016), resulting in the possibility of global predictions of R based on growth temperatures (Eqn 5).
The response of respiration at prevailing growth temperatures, R growth increased by 18% with warming across the entire ESWE dataset (Fig. 7). The response of R growth to warming increased both with mean temperature of the warmest quarter (R 2 = 0.05; Table 2; Fig. 8a) and warming amount (R 2 = 0.03, P = 0.03; Fig. 8b) as well as with experimental growth temperatures (Fig. S5b), although all significant relationships had low R 2 < 0.1. This increase in growth respiration to higher temperatures is consistent with expectations based on a universal respiration response to warmer growth temperatures. A similar response was observed in a global analysis in which respiration rates at prevailing growth temperatures were higher in the tropics than the Arctic (Atkin et al., 2015). Leaf form tended to affect the RR of R growth to warming; needleleaf species increased 25 ± 9%, while broadleaf evergreen species increased R growth with 15 ± 5% in response to warming (P = 0.06; Table 2).
While the processes of photosynthesis and leaf respiration have traditionally been studied separately, it has been long known that these processes are interlinked (Hoefnagel et al., 1998). In a meta‐analysis across several PFTs such as C3 herbaceous, C4 species, deciduous and woody evergreen trees, Dusenge et al. (2019) found that the ratio of respiration over photosynthesis rates, R : A, at prevailing growth temperatures was constant across a wide range of temperatures, suggesting that the processes of respiration and photosynthesis are coordinated. Our analyses found an increase in R : A with growth temperature and the amount of warming (Fig. 8d; P = 0.04; R 2 = 0.06) across 10 degrees of warming, consistent with the responses of increased R growth but not A growth with warmer temperatures (Table 2). It is likely that plants thermally acclimate photosynthesis and respiration in a coordinated fashion (Dusenge et al., 2019) although we note the scatter, which may result from variation in factors underpinning A growth, such as stomatal conductance. Respiration, being a more temperature sensitive process, can rapidly adjust to changing growth temperatures and does so in a predictable manner (Heskel et al., 2016) both in response to seasonal and experimental warming and regardless of the amount of warming (Table 2). By contrast, photosynthesis can adjust to warmer growth temperatures (Fig. 1b), but the degree of photosynthetic acclimation is more constrained with warmer temperatures (Fig. 5a; Table 2), despite increased T optA (Fig. 3a). Therefore, the adjustment of photosynthesis rates to warming is limited and factors other than growth temperatures may set additional limits on the acclimation capacity of photosynthesis.
V. Limits to the thermal acclimation capacity
While natural selection could optimise processes of carbon uptake and carbon loss, there are limits to plant thermal acclimation capabilities. What these limits are and what factors determine them is currently unclear. From our analyses, it is clear that photosynthesis can partially adjust by shifting the temperature optimum by 0.34°C per °C warming (Fig. 3b), but the warming response of net photosynthesis (A net25) also declined with higher growth temperatures (Figs 5a, S5). In a field study in which Eucalyptus globulus was moved 700 km north outside its native range and additionally exposed to 3°C warming, the species demonstrated a limited ability to acclimate to high summer temperatures, but was still able to adjust to winter temperatures (Crous et al., 2013). This evidence and others suggest that there is an upper limit of thermal tolerance for photosynthesis, which may be influenced by a species’ physiological plasticity as well as its distribution range. While changes in growth temperatures play a major role in thermal acclimation of photosynthesis (Kumarathunge et al., 2019), the RR of A net25 and R 25 declined more in needleleaf species compared with broadleaf evergreen species (Fig. 6), which may be a reflection of gymnosperm evolutionary history contrasting with angiosperms in the ESWE dataset. There is some evidence that the overall acclimation capacity in photosynthesis can be limited by genetic adaptation and evolutionary history (Read & Busby, 1990; Jump & Peñuelas, 2005), as indicated by studies on contrasting taxonomic groups with a diverse evolutionary history (i.e. PFT) (Cunningham & Read, 2002; Yamori et al., 2014; Reich et al., 2015).
While the climate at the seed of origin has been suggested to determine population responses to climate change, species generally respond to a broad range of temperatures, perhaps the temperature range experienced along a species’ native distribution. Plant species with large geographic range sizes have been indicated to cope better with climate warming than species with smaller range sizes (Thuiller et al., 2005; Aitken et al., 2008; Pacifici et al., 2015; Gonzalez‐Orozco et al., 2016). This is presumably because species with broad climatic distribution are adapted to a broader range of growth temperatures and environmental tolerances (Hamrick et al., 1992; Leimu et al., 2006; Morin & Thuiller, 2009; Slatyer et al., 2013). Gene flow among populations can also contribute to a species’ thermal acclimation as genetic adaptations to warmer temperatures can be passed on from warmer to cooler populations, which cannot occur for populations at the warmest edge of a species’ native distribution (Savolainen et al., 2007; Sexton et al., 2011).
A shift towards higher temperatures will induce large changes in most biological processes and these changes are likely to emerge at the ecosystem level by changes in species composition and shifts in distribution ranges. This has already been observed along tropical elevations (Duque et al., 2015; Fadrique et al., 2021) as well as in boreal climates (Boisvert‐Marsh et al., 2014; Lesica & Crone, 2017; Villén‐Peréz et al., 2020). Andean tropical forest plot inventories have revealed directional shifts in tree community composition over time towards greater relative abundances of species from lower, warmer elevations (Fadrique et al., 2021), often driven by enhanced mortality of higher elevation species (Duque et al., 2015). Moreover, biological processes such as competition among plants may further reduce the ultimate geographic range at which plants can occur (Ghalambor et al., 2006; Penuelas et al., 2013). For example, temperate species may be competitively excluded from tropical forests due to outshading of seedlings and slower growth rates compared with tropical species (Cunningham & Read, 2003). Other processes such as seed dispersal and germination can also contribute to competitive exclusion. Therefore, the realised geographic range of a species is often smaller compared to which geographic range is fundamentally possible based on physiological tolerances alone (Cunningham & Read, 2003; Ghalambor et al., 2006).
VI. Can spatial gradients and seasonal changes help to predict warming responses?
Current knowledge on tree physiological responses to increased temperature is primarily based on controlled warming experiments (Way & Oren, 2010; Yamori et al., 2014; Slot & Kitajima, 2015; Dusenge et al., 2019) and analyses of environmental dependencies using observational data (Ali et al., 2015; Atkin et al., 2015; Kumarathunge et al., 2019; Smith et al., 2019). While controlled experiments may have limited applicability in the field, they can provide more detailed insights into mechanisms that would not easily be derived using a field‐based approach. More ecologically relevant, field‐based observations come with inevitable uncertainties regarding the causality and origin due to co‐varying environmental factors such as light and nutrients in addition to genetic variability among different populations of a given species.
Studies along natural temperature gradients, either latitudinal or elevational, may offer a useful compromise between these two approaches to study warming responses (Malhi et al., 2010). Gradient studies still require that other co‐varying environmental variables are carefully considered. For example, the influence of temperature (and associated shifts in VPD, Amthor et al., 2010) on plant physiological traits and processes along wet tropical elevation gradients can be assessed if also potential co‐variation in nutrient availability is considered (Fyllas et al., 2017; Mujawamariya et al., 2021). Similarly, latitudinal gradients can be used to assess thermal acclimation if measurement campaigns account for variation in photoperiod and phenology (Tjoelker et al., 1999; Dillaway & Kruger, 2010; Benomar et al., 2018).
Most physiological studies using latitudinal or elevation gradients have used trees that grow naturally along the gradient (Tjoelker et al., 2009; Wertin et al., 2010; Girardin et al., 2014; Fyllas et al., 2017), which does not allow for separation between thermal acclimation and genetic adaptation. These studies are valuable for assessing the longer term (more than centuries) responses of tree community composition and population dynamics at a given location. They are perhaps less useful for assessing the impacts of rapid ongoing global warming on the physiology and growth of long‐lived and slow‐migrating organisms such as trees over the coming decades. For this, additional approaches are needed. Translocation experiments with the same genetic material planted at different sites along natural temperature gradients have been conducted to assess physiological thermal acclimation of both boreal (Dillaway & Kruger, 2010; Benomar et al., 2018) and tropical (Mujawamariya et al., 2021) trees. However, translocation experiments are mostly done on a few and/or small trees, sometimes growing in pots, compromising the ability to evaluate responses on mature trees over a longer term. By contrast, an elevation gradient approach with both multispecies plantations and established trees at different sites offers a promising approach to predict warming responses based on spatial temperature gradients (Mujawamariya et al., 2021).
Observations of seasonal changes in tree physiological processes may also offer insight into thermal acclimation. Numerous studies have explored seasonal or interannual shifts in leaf photosynthesis and respiration in relation to changes in temperature (Crous et al., 2011; Aspinwall et al., 2016; Lamba et al., 2018). In some cases, these seasonal responses agree well with long‐term warming responses, especially during the warmer part of the year (Vanderwel et al., 2015; Aspinwall et al., 2016). However, seasonal responses, even in the warmer part of the year, generally do not extend the mean growth temperatures that a tree may experience compared with long‐term climate warming. Whereas long‐term climate warming has a consistent warming response, seasonal responses are usually limited in time and vary in strength over the years. Therefore, seasonal studies offer limited insight regarding responses to climate warming. Moreover, phenology and other co‐varying environmental factors such as soil moisture or photoperiod can further confound seasonal physiological data (Bauerle et al., 2012; Lamba et al., 2018). We argue that seasonal responses are a different layer within the response to climate warming. Therefore, spatial gradients, whether elevation or latitudinal gradients, are more useful to predict climate change responses.
VII. Including acclimation capacity of photosynthesis and respiration in land surface models
Most land surface models (LSMs) have not considered physiological acclimation (discussed in Sections III and IV, above). They either rely on a single, global photosynthesis/respiration temperature response function (Leuning, 2002; Kowalczyk et al., 2006), or assume differences in the response functions of different PFTs (Clark et al., 2011; Smith & Dukes, 2017). As a consequence of assuming a fixed instantaneous temperature response, the simulated climate‐carbon feedback from such models remains a key uncertainty (Ziehn et al., 2011), particularly as the climate warms (Booth et al., 2012). Several studies have recognised the importance of accounting for acclimation in their representation of carbon fluxes (Smith & Dukes, 2013; Huntingford et al., 2017; Mercado et al., 2018). Furthermore, LSMs have separately considered acclimation in both respiration (Huntingford et al., 2017) and photosynthetic physiology (Mercado et al., 2018), but not both as a full acclimation scenario.
Given that respiration readily acclimates to warmer temperatures in a universal and predictable way, it is imperative that the thermal acclimation of respiration is considered in models. The temperature sensitivity of respiration can be predicted using growth temperatures (i.e. Q 10 = −0.0005 × T + 0.1012, Heskel et al., 2016), while the relationship between the RR of Q 10 and warming amount (Fig. 8c) can help to modify the temperature response of respiration to long‐term warming, rather than assuming a Q 10 of 2. Modelling assessments of short‐term and long‐term acclimation effects on gross primary productivity and canopy respiration have effects ranging between 9% and 20% (Atkin et al., 2008; Huntingford et al., 2017; Mercado et al., 2018) with acclimation effects on these processes largest for tropical evergreen forests. This suggests that, in spite of uncertainties in the trajectory of carbon cycle processes with warming, short‐term and long‐term aspects of physiological acclimation are important to quantify and include in LSMs.
Predicting the acclimation of photosynthesis to temperature seems less straightforward compared with the consistent response of respiration to a change in growth temperature. When thermal acclimation has been represented in LSMs (Lombardozzi et al., 2015; Smith et al., 2016; Mercado et al., 2018), there remain several knowledge gaps. First, there is uncertainty about the magnitude of species’ temperature acclimation of photosynthesis and respiration. For respiration, we found an overall acclimation of R 25 of −14% with no significant differences between biomes or warming amount (Table 2; Fig. 7). For photosynthesis, our analyses in evergreen species have shown that (1) the T optA shift per °C warming is predictable regardless of the amount of warming (Fig. 3b) and (2) the thermal acclimation response of photosynthesis is reduced with warmer growth temperatures (Figs 5a, S5), a relationship useful to represent in models. There were also effects of higher mean temperatures of the warmest quarter on the responses of V cmaxt25 and J : V 25 to warming (Fig. 5). By contrast, the response of photosynthetic capacity at a common temperature (V cmaxt25 and J max25) was not affected by warming amount, because a similar amount of observations responded either with an increased or decreased response to warming across the ESWE dataset (Fig. 4; Table 2).
When models have included acclimation mechanisms, implementation is assumed to continue indefinitely with future warming. However, it is likely that there is a limit to the acclimation potential of plants with the temperature optimum unlikely to shift much beyond this limit (Crous et al., 2013; Hogan et al., 2021). More research is needed to investigate where potential limits to the shift in T optA may lie. Regarding the timing of thermal acclimation, it is clear that the timing of acclimation of respiration is more rapid (i.e. days to 1 wk; Bolstad et al., 2003; Lee et al., 2005) than the timing of acclimation of photosynthesis. A timescale of c. 1 month (Smith & Dukes, 2017) to 8–10 wk for photosynthesis (Hogan et al., 2021) has been suggested. We need to better understand the timescale over which acclimation occurs, the mechanisms that control this timescale and how this varies amongst PFTs.
Some models such as CABLE (Kowalczyk et al., 2006) currently assume a fixed J : V 25 ratio, linking J max to the parameterisation of V cmax. However, evidence from our review, consistent with previous studies (Kattge & Knorr, 2007; Kumarathunge et al., 2019), has shown that the J : V 25 ratio declines with increasing temperature (Fig. 1), and its response to warming was reduced from boreal to tropical latitudes (Fig. 5). This implies that models that fix this ratio should consider linking the response to temperature (e.g. growing season temperature) or by leaf N changes with temperature (Fig. 1). Both J : V 25 and nitrogen decreased with warmer temperatures (Figs 1, 5a), and this relationship could be used to predict V cmax and J max rates across a range of growth temperatures.
VIII. Conclusions
The literature on evergreen species responses to warming has expanded considerably in the last 10 yr, particularly for tropical evergreen species. We found reduced acclimation of photosynthesis to warming from boreal to tropical biomes (Table 2; Fig. 5), but this response was not affected by the amount of warming (Fig. 4). While R 25 reduced with mean summer growth temperatures (Table 1), there was a universal response of respiration to warmer temperatures (Table 2; Figs 4, 7). Looking forward, there needs to be a better understanding of the role of stomatal responses in controlling the thermal acclimation response of photosynthesis, and a strengthening of the links among the thermal acclimation of leaf N, A net and R d, including physiological acclimation and plasticity for improved predictions with warming. These acclimation responses should also be linked to longer term processes such as adaptation, growth responses and carbon stocks (Sullivan et al., 2020) for parameterising large‐scale LSMs. Model experiments involving acclimation of both photosynthetic components and respiration are important to explore and elucidate key responses that influence large‐scale terrestrial carbon fluxes. As an extension from stronger climate forcing and global warming, extreme heat events are likely to occur more frequently (Meehl & Tebaldi, 2004). Heatwaves may negatively impact the persistence of species, habitat suitability and the diversity and function of ecosystems (Allen et al., 2015; Buckley & Huey, 2016). More studies simulating extreme temperature events and their impacts are needed to determine species’ upper heat tolerances and understand the mechanisms involved in adjustments at all scales, including variation among functional plant types and interactions with water availability. We argue that thermal acclimation of photosynthesis and respiration across biomes can be included with the help of the relationships and analyses from this review to enhance the understanding of how the carbon cycle at large scales will be affected by climate warming.
Supporting information
Fig. S1 Boxplots and relationship between biome, mean annual temperature and mean temperature of the warmest quarter in the ESWE dataset.
Fig. S2 Partial residuals with 95% confidence intervals of the ANCOVA as a function of mean temperature of the warmest quarter (meanTWQ) to represent the effect of biome.
Fig. S3 Linear regression relationships with 95% confidence intervals between several variables against experimental control growth temperatures across biomes.
Fig. S4 Partial residuals with 95% confidence intervals from the ANCOVA as a function of mean annual temperature (mat) to represent the effect of biome.
Fig. S5 Linear relationships with 95% confidence intervals against experimental control growth temperature for the response ratio (RR) of net photosynthesis at 25°C, A net25, and respiration rates a prevailing growth temperatures, R growth.
Table S1 Species list per biome and plant functional type, including latitude and longitude, the reference where the data originated and how controlled the study was.
Table S2 ANCOVA results with mean annual temperature as a covariate for both absolute control values and the response ratios.
Table S3 Table with sample sizes for each variable in each category (warming amount, biome and leaf form).
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Acknowledgements
We thank Professor Kouki Hikosaka and Dr Nick Smith for sharing data from their previous experiments and Dr Mirindi Eric Dusenge for sharing reference lists that facilitated the data compilation of this study. Professor Belinda Medlyn and Professor David Ellsworth are gratefully acknowledged for commenting on previous versions of this manuscript. KYC acknowledges the Australian Research Council for funding a Discovery Early Career Research Award (DE160101484) while writing this manuscript. JU acknowledges the Swedish strategic research area ‘Biodiversity and Ecosystem services in a Changing Climate’ (BECC; http://www.lu.se).
Appendix A1. Data summary and statistical analyses of the ‘Evergreen species in a warming environment’ (ESWE) dataset
The ESWE dataset focused on both field and controlled environment studies with a control and warming treatment (Appendix A2). There were 268 lines from field studies and 927 lines from glasshouse studies out of 1195 lines in the ESWE dataset across all variables. Common garden studies without warming treatment were not included. Generally, a mean and standard error (or standard deviation) of control and warming treatments were collected for each species, along with information on biome, latitude and longitude, growth temperatures of control and warmed treatments, sample size and the amount of warming applied. When studies tracked ambient data, an average temperature across the duration of the sampling was taken to represent the control growth temperature. Data were collected from tables or digitised from graphs using Datathief III (v.1.5, www.datathief.org). Latitude and longitude were added based on the coordinates mentioned in the study or the study location if using a locally grown species (Supporting Information Table S1). Mean annual temperature, the maximum temperature of the warmest month and the mean temperature of the warmest quarter based on latitude were extracted from the BIOClime database (Hijmans et al., 2005). There were clear relationships between biome and mean annual temperature, as well as mean temperature of the warmest quarter (Fig. S1).
Variables collected were measured in both control and warming treatment(s) at growth temperatures and/or at a common temperature. The variables include: photosynthesis at 25°C (A net25), photosynthesis at growth temperature (A growth), maximum carboxylation rates and maximum electron transport rates measured at 25°C (V cmax25 and J max25), the J max : V cmax ratio at that temperature (J : V 25) and mitochondrial dark respiration at a common temperature (R 25), as well as dark respiration at growth temperatures (R growth). The temperature optimum of photosynthesis (T optA) and area‐based nitrogen content (Na) were also recorded and, when available, the temperature sensitivity of the respiration temperature response, Q 10. As not all variables were recorded in each study, sample sizes and species involved varied depending on the variable investigated (Table S3). The warming amount across the ESWE dataset spanned from 0.6°C to 16°C warming, which we divided into three categories: < 5°C, 5–10°C and ≥ 10°C. For V cmax25, J max25 and J : V 25, there were no warming studies that went beyond 10°C warming in the boreal biome.
Statistical analyses were conducted in R v.3.6.1 (R Core Team, 2020). All variables were tested for a normal distribution and transformed when necessary. To evaluate how the absolute values in control conditions varied across biomes and to determine intrinsic trends across biomes and functional groups (Section II on biogeographic patterns), we used an analysis of covariance (ANCOVA) approach with leaf form as categorical factor and mean temperature of the warmest quarter as a covariate to address the collinearity between biome and leaf form. Tukey’s post hoc tests (using glht from the multcomp package; Hothorn et al., 2008) were used to determine differences between leaf form groups and warming amount categories. We chose mean temperature of the warmest quarter as our covariate to best reflect the conditions in which most experiments are measured (i.e. during the growing season). Using mean annual temperature as a covariate instead of mean temperature of the warmest quarter gave similar results (Table S2; Fig. S4). All results from this statistical analyses on control/ambient conditions are summarised in Table 1.
Similarly, a second ANCOVA was used to evaluate the response ratios to warming of several variables, summarised in Table 2 (Sections III and IV) with warming category and leaf form as categorical factors, and mean temperature of the warmest quarter as covariate to reflect trends across biomes. Data from different species were considered independent and no weighting factor for study/experiment was included due to similar variances among experiments and the small amount of studies used (Koricheva & Gurevitch, 2014). The response ratio of a given variable in warmed divided by ambient treatments assessed the degree of acclimation and was log‐transformed to meet linearity assumptions for ANCOVA. A response ratio > 1 indicated a positive warming response, while a response ratio < 1 referred to a negative response to warming. Regarding T optA, we used the absolute (in °C) as well as the relative (in °C per °C warming) temperature shifts to evaluate responses to warming instead of a response ratio.
A third approach was linear regression analyses using warming amount or bioclimatic temperature indices (e.g. mean annual temperatures, mean temperature of the warmest quarter) as a continuous factor to test for the basic functional relationships that underlay all evergreens. While the statistics were done on the logarithmic of the response ratio to satisfy linearity assumptions, graphics are displayed using the response ratio for easier interpretability or using back‐transformed partial residuals from the visreg package (Breheny & Burchett, 2017).
Appendix A2. Studies used in the analyses
- Aspinwall MJ, Drake JE, Campany C, Varhammar A, Ghannoum O, Tissue DT, Reich PB, Tjoelker MG. 2016. Convergent acclimation of leaf photosynthesis and respiration to prevailing ambient temperatures under current and warmer climates in Eucalyptus tereticornis . New Phytologist 212: 354–367. [DOI] [PubMed] [Google Scholar]
- Benomar L, Lamhamedi MS, Pepin S, Rainville A, Lambert MC, Margolis HA, Bousquet J, Beaulieu J. 2018. Thermal acclimation of photosynthesis and respiration of southern and northern white spruce seed sources tested along a regional climatic gradient indicates limited potential to cope with temperature warming. Annals of Botany 121: 443–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez R, Stefanski A, Montgomery RA, Reich PB. 2020. Short‐ and long‐term responses of photosynthetic capacity to temperature in four boreal tree species in a free‐air warming and rainfall manipulation experiment. Tree Physiology 41: 89–102. [DOI] [PubMed] [Google Scholar]
- Carter KR, Wood TE, Reed SC, Butts KM, Cavaleri MA. 2021. Experimental warming across a tropical forest canopy height gradient reveals minimal photosynthetic and respiratory acclimation. Plant, Cell & Environment 44: 2879–2897. [DOI] [PubMed] [Google Scholar]
- Carter KR, Wood TE, Reed SC, Schwartz EC, Reinsel MB, Yang X, Cavaleri MA. 2020. Photosynthetic and respiratory acclimation of understory shrubs in response to in situ experimental warming of a wet tropical forest. Frontiers in Forests and Global Change 3: 1–20. [Google Scholar]
- Cheesman AW, Winter K. 2013a. Elevated night‐time temperatures increase growth in seedlings of two tropical pioneer tree species. New Phytologist 197: 1185–1192. [DOI] [PubMed] [Google Scholar]
- Cheesman AW, Winter K. 2013b. Growth response and acclimation of CO2 exchange characteristics to elevated temperatures in tropical tree seedlings. Journal of Experimental Botany 64: 3817–3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AD, Ryan MG, Adams HD, Dickman LT, Garcia‐Forner N, Grossiord C, Powers HH, Sevanto S, McDowell NG. 2021. Foliar respiration is related to photosynthetic, growth and carbohydrate response to experimental drought and elevated temperature. Plant, Cell & Environment 44: 3853–3865. [DOI] [PubMed] [Google Scholar]
- Crous KY, Drake JE, Aspinwall MJ, Sharwood RE, Tjoelker MG, Ghannoum O. 2018. Photosynthetic capacity and leaf nitrogen decline along a controlled climate gradient in provenances of two widely distributed Eucalyptus species. Global Change Biology 24: 4626–4644. [DOI] [PubMed] [Google Scholar]
- Crous KY, Quentin AG, Lin YS, Medlyn BE, Williams DG, Barton CVM, Ellsworth DS. 2013. Photosynthesis of temperate Eucalyptus globulus trees outside their native range has limited adjustment to elevated CO2 and climate warming. Global Change Biology 19: 3790–3807. [DOI] [PubMed] [Google Scholar]
- Crous KY, Zaragoza‐Castells J, Low M, Ellsworth DS, Tissue DT, Tjoelker MG, Barton CVM, Gimeno TE, Atkin OK. 2011. Seasonal acclimation of leaf respiration in Eucalyptus saligna trees: impacts of elevated atmospheric CO2 and summer drought. Global Change Biology 17: 1560–1576. [Google Scholar]
- Cunningham SC, Read J. 2002. Comparison of temperate and tropical rainforest tree species: photosynthetic responses to growth temperature. Oecologia 133: 112–119. [DOI] [PubMed] [Google Scholar]
- Doughty CE. 2011. An in situ leaf and branch warming experiment in the Amazon. Biotropica 43: 658–665. [Google Scholar]
- Dusenge ME, Madhavji S, Way DA. 2020. Contrasting acclimation responses to elevated CO2 and warming between an evergreen and a deciduous boreal conifer. Global Change Biology 26: 3639–3657. [DOI] [PubMed] [Google Scholar]
- Dusenge ME, Wittemann M, Mujawamariya M, Ntawuhiganayo EB, Zibera E, Ntirugulirwa B, Way DA, Nsabimana D, Uddling J, Wallin G. 2021. Limited thermal acclimation of photosynthesis in tropical montane tree species. Global Change Biology 27: 4860–4878. [DOI] [PubMed] [Google Scholar]
- Fauset S, Oliveira L, Buckeridge MS, Foyer CH, Galbraith D, Tiwari R, Gloor M. 2019. Contrasting responses of stomatal conductance and photosynthetic capacity to warming and elevated CO2 in the tropical tree species Alchomea glandulosa under heatwave conditions. Environmental and Experimental Botany 158: 28–39. [Google Scholar]
- Ferrar PJ, Slatyer RO, Vranjic JA. 1989. Photosynthetic temperature acclimation in Eucalyptus species from diverse habitats, and a comparison with Nerium oleander . Australian Journal of Plant Physiology 16: 199–217. [Google Scholar]
- Ghannoum O, Phillips NG, Sears MA, Logan BA, Lewis JD, Conroy JP, Tissue DT. 2010. Photosynthetic responses of two eucalypts to industrial‐age changes in atmospheric CO2 and temperature. Plant, Cell & Environment 33: 1671–1681. [DOI] [PubMed] [Google Scholar]
- Han QM, Kawasaki T, Nakano T, Chiba Y. 2004. Spatial and seasonal variability of temperature responses of biochemical photosynthesis parameters and leaf nitrogen content within a Pinus densiflora crown. Tree Physiology 24: 737–744. [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Murakami A, Hirose T. 1999. Balancing carboxylation and regeneration of ribulose‐1,5‐bisphosphate in leaf photosynthesis: temperature acclimation of an evergreen tree, Quercus myrsinaefolia . Plant, Cell & Environment 22: 841–849. [Google Scholar]
- Hill RS, Read J, Busby JR. 1998. The temperature dependence of photosynthesis of some Australian temperate rainforest trees and its biogeographical significance. Journal of Biogeography 15: 431–449. [Google Scholar]
- Huang GM, Rymer PD, Duan HL, Smith RA, Tissue DT. 2015. Elevated temperature is more effective than elevated CO2 in exposing genotypic variation in Telopea speciosissima growth plasticity: implications for woody plant populations under climate change. Global Change Biology 21: 3800–3813. [DOI] [PubMed] [Google Scholar]
- Kellomaki S, Wang KY. 1996. Photosynthetic responses to needle water potentials in Scots pine after a four‐year exposure to elevated CO2 and temperature. Tree Physiology 16: 765–772. [DOI] [PubMed] [Google Scholar]
- Krause GH, Cheesman AW, Winter K, Krause B, Virgo A. 2013. Thermal tolerance, net CO2 exchange and growth of a tropical tree species, Ficus insipida, cultivated at elevated daytime and nighttime temperatures. Journal of Plant Physiology 170: 822–827. [DOI] [PubMed] [Google Scholar]
- Kroner Y, Way DA. 2016. Carbon fluxes acclimate more strongly to elevated growth temperatures than to elevated CO2 concentrations in a northern conifer. Global Change Biology 22: 2913–2928. [DOI] [PubMed] [Google Scholar]
- Kurepin LV, Stangl ZR, Ivanov AG, Bui V, Mema M, Huner NPA, Oquist G, Way D, Hurry V. 2018. Contrasting acclimation abilities of two dominant boreal conifers to elevated CO2 and temperature. Plant, Cell & Environment 41: 1331–1345. [DOI] [PubMed] [Google Scholar]
- Lamba S, Hall M, Rantfors M, Chaudhary N, Linder S, Way D, Uddling J, Wallin G. 2018. Physiological acclimation dampens initial effects of elevated temperature and atmospheric CO2 concentration in mature boreal Norway spruce. Plant, Cell & Environment 41: 300–313. [DOI] [PubMed] [Google Scholar]
- Li Y, Xu Y, Li Y, Wu T, Zhou G, Liu S, Meng Y, Wang J, Ling L, Liu J. 2020. Warming effects on morphological and physiological performances of four subtropical montane tree species. Annals of Forest Science 77: 1–11. [Google Scholar]
- Mujawamariya M, Wittemann M, Manishimwe A, Ntirugulirwa B, Zibera E, Nsabimana D, Wallin G, Uddling J, Dusenge ME. 2021. Complete or overcompensatory thermal acclimation of leaf dark respiration in African tropical trees. New Phytologist 229: 2548–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngugi MR, Hunt MA, Doley D, Ryan P, Dart PJ. 2003. Photosynthetic light and temperature responses of Eucalyptus cloeziana and Eucalyptus argophloia . Australian Journal of Botany 51: 573–583. [Google Scholar]
- Ow LF, Whitehead D, Walcroft AS, Turnbull MH. 2008. Thermal acclimation of respiration but not photosynthesis in Pinus radiata . Functional Plant Biology 35: 448–461. [DOI] [PubMed] [Google Scholar]
- Reich PB, Sendall KM, Stefanski A, Wei XR, Rich RL, Montgomery RA. 2016. Boreal and temperate trees show strong acclimation of respiration to warming. Nature 531: 633–636. [DOI] [PubMed] [Google Scholar]
- Scafaro AP, Xiang S, Long BM, Bahar NHA, Weerasinghe LK, Creek D, Evans JR, Reich PB, Atkin OK. 2017. Strong thermal acclimation of photosynthesis in tropical and temperate wet‐forest tree species: the importance of altered Rubisco content. Global Change Biology 23: 2783–2800. [DOI] [PubMed] [Google Scholar]
- Sheu BH, Lin CK. 1999. Photosynthetic response of seedlings of the sub‐tropical tree Schima superba with exposure to elevated carbon dioxide and temperature. Environmental and Experimental Botany 41: 57–65. [Google Scholar]
- Slot M, Rey‐Sanchez C, Gerber S, Lichstein JW, Winter K, Kitajima K. 2014. Thermal acclimation of leaf respiration of tropical trees and lianas: response to experimental canopy warming, and consequences for tropical forest carbon balance. Global Change Biology 20: 2915–2926. [DOI] [PubMed] [Google Scholar]
- Slot M, Rifai SW, Winter K. 2021. Photosynthetic plasticity of a tropical tree species, Tabebuia rosea, in response to elevated temperature and CO2 . Plant, Cell & Environment 44: 2347–2364. [DOI] [PubMed] [Google Scholar]
- Slot M, Winter K. 2017. Photosynthetic acclimation to warming in tropical forest tree seedlings. Journal of Experimental Botany 68: 2275–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot M, Winter K. 2018. High tolerance of tropical sapling growth and gas exchange to moderate warming. Functional Ecology 32: 599–611. [Google Scholar]
- Smith NG, Dukes JS. 2017. Short‐term acclimation to warmer temperatures accelerates leaf carbon exchange processes across plant types. Global Change Biology 23: 4840–4853. [DOI] [PubMed] [Google Scholar]
- Teskey RO, Will RE. 1999. Acclimation of loblolly pine (Pinus taeda) seedlings to high temperatures. Tree Physiology 19: 519–525. [DOI] [PubMed] [Google Scholar]
- Wang KY, Kellomaki S, Laitinen K. 1996. Acclimation of photosynthetic parameters in Scots pine after three years exposure to elevated temperature and CO2 . Agricultural and Forest Meteorology 82: 195–217. [Google Scholar]
- Wardlaw IF, Begg JE, Bagnall D, Dunstone RL. 1983. Jojoba – temperature adaptation as expressed in growth and leaf function. Australian Journal of Plant Physiology 10: 299–312. [Google Scholar]
- Warren CR. 2008. Stand aside stomata, another actor deserves centre stage: the forgotten role of the internal conductance to CO2 transfer. Journal of Experimental Botany 59: 1475–1487. [DOI] [PubMed] [Google Scholar]
- Way DA, Sage RF. 2008a. Elevated growth temperatures reduce the carbon gain of black spruce Picea mariana (Mill.) BSP. Global Change Biology 14: 624–636. [Google Scholar]
- Way DA, Sage RF. 2008b. Thermal acclimation of photosynthesis in black spruce Picea mariana (Mill.) BSP. Plant, Cell & Environment 31: 1250–1262. [DOI] [PubMed] [Google Scholar]
- Wertin TM, McGuire MA, van Iersel M, Ruter JM, Teskey RO. 2012. Effects of elevated temperature and CO2 on photosynthesis, leaf respiration, and biomass accumulation of Pinus taeda seedlings at a cool and a warm site within the species' current range. Canadian Journal of Forest Research 42: 943–957. [Google Scholar]
- Yin HJ, Liu Q, Lai T. 2008. Warming effects on growth and physiology in the seedlings of the two conifers Picea asperata and Abies faxoniana under two contrasting light conditions. Ecological Research 23: 459–469. [Google Scholar]
- Zhang XW, Chen LT, Wang JR, Wang MH, Yang SL, Zhao CM. 2018. Photosynthetic acclimation to long‐term high temperature and soil drought stress in two spruce species (Picea crassifolia and P. wilsonii) used for afforestation. Journal of Forestry Research 29: 363–372. [Google Scholar]
- Zhang XW, Wang JR, Ji MF, Milne RI, Wang MH, Liu JQ, Shi S, Yang SL, Zhao CM. 2015. Higher thermal acclimation potential of respiration but not photosynthesis in two alpine Picea taxa in contrast to two lowland congeners. PLoS ONE 10: e0123248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Data availability
All data used in this manuscript are available online and/or have been published before (see Appendix A2). The dataset used for this study is available from the corresponding author upon reasonable request for future collaborations.
References
- Aitken SN, Yeaman S, Holliday JA, Wang TL, Curtis‐McLane S. 2008. Adaptation, migration or extirpation: climate change outcomes for tree populations. Evolutionary Applications 1: 95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AA, Xu C, Rogers A, McDowell NG, Medlyn BE, Fisher RA, Wullschleger SD, Reich PB, Vrugt JA, Bauerle WL et al. 2015. Global‐scale environmental control of plant photosynthetic capacity. Ecological Applications 25: 2349–2365. [DOI] [PubMed] [Google Scholar]
- Allen CD, Breshears DD, McDowell NG. 2015. On underestimation of global vulnerability to tree mortality and forest die‐off from hotter drought in the Anthropocene. Ecosphere 6: 55. [Google Scholar]
- Amthor JS, Hanson PJ, Norby RJ, Wullschleger SD. 2010. A comment on “Appropriate experimental ecosystem warming methods by ecosystem, objective, and practicality” by Aronson and McNulty. Agricultural and Forest Meteorology 150: 497–498. [Google Scholar]
- Armstrong AF, Logan DC, Tobin AK, O'Toole P, Atkin OK. 2006. Heterogeneity of plant mitochondrial responses underpinning respiratory acclimation to the cold in Arabidopsis thaliana leaves. Plant, Cell & Environment 29: 940–949. [DOI] [PubMed] [Google Scholar]
- Aspinwall MJ, Drake JE, Campany C, Varhammar A, Ghannoum O, Tissue DT, Reich PB, Tjoelker MG. 2016. Convergent acclimation of leaf photosynthesis and respiration to prevailing ambient temperatures under current and warmer climates in Eucalyptus tereticornis . New Phytologist 212: 354–367. [DOI] [PubMed] [Google Scholar]
- Atkin OK, Atkinson LJ, Fisher RA, Campbell CD, Zaragoza‐Castells J, Pitchford JW, Woodward FI, Hurry V. 2008. Using temperature‐dependent changes in leaf scaling relationships to quantitatively account for thermal acclimation of respiration in a coupled global climate‐vegetation model. Global Change Biology 14: 2709–2726. [Google Scholar]
- Atkin OK, Bloomfield KJ, Reich PB, Tjoelker MG, Asner GP, Bonal D, Bönisch G, Bradford MG, Cernusak LA, Cosio EG et al. 2015. Global variability in leaf respiration in relation to climate, plant functional types and leaf traits. New Phytologist 206: 614–636. [DOI] [PubMed] [Google Scholar]
- Atkin OK, Bruhn D, Hurry VM, Tjoelker MG. 2005a. The hot and the cold: unravelling the variable response of plant respiration to temperature. Functional Plant Biology 32: 87–105. [DOI] [PubMed] [Google Scholar]
- Atkin OK, Bruhn D, Tjoelker MG. 2005b. Response of plant respiration to changes in temperature: mechanisms and consequences of variations in Q10 values and acclimation. In: Lambers H, Ribas‐Carbó M, eds. Advances in photosynthesis and respiration. Vol. 18. Plant respiration: from cell to ecosystem. Dordrecht, the Netherlands: Springer, 95–135. [Google Scholar]
- Atkin OK, Tjoelker MG. 2003. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends in Plant Science 8: 343–351. [DOI] [PubMed] [Google Scholar]
- Bauerle WL, Oren R, Way DA, Qian SS, Stoy PC, Thornton PE, Bowden JD, Hoffman FM, Reynolds RF. 2012. Photoperiodic regulation of the seasonal pattern of photosynthetic capacity and the implications for carbon cycling. Proceedings of the National Academy of Sciences, USA 109: 8612–8617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer C, Reichstein M, Tomelleri E, Ciais P, Jung M, Carvalhais N, Rödenbeck C, Arain MA, Baldocchi D, Bonan GB et al. 2010. Terrestrial gross carbon dioxide uptake: global distribution and covariation with climate. Science 329: 834–838. [DOI] [PubMed] [Google Scholar]
- Benomar L, Lamhamedi MS, Pepin S, Rainville A, Lambert MC, Margolis HA, Bousquet J, Beaulieu J. 2018. Thermal acclimation of photosynthesis and respiration of southern and northern white spruce seed sources tested along a regional climatic gradient indicates limited potential to cope with temperature warming. Annals of Botany 121: 443–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez R, Stefanski A, Montgomery RA, Reich PB. 2020. Short‐ and long‐term responses of photosynthetic capacity to temperature in four boreal tree species in a free‐air warming and rainfall manipulation experiment. Tree Physiology 41: 89–102. [DOI] [PubMed] [Google Scholar]
- Bernacchi CJ, Pimentel C, Long SP. 2003. In vivo temperature response functions of parameters required to model RuBP‐limited photosynthesis. Plant, Cell & Environment 26: 1419–1430. [Google Scholar]
- Berry J, Björkman O. 1980. Photosynthetic response and adaptation to temperature in higher plants. Annual Review of Plant Physiology and Plant Molecular Biology 31: 491–543. [Google Scholar]
- Billings WD, Godfrey PJ, Chabot BF, Bourque DP. 1971. Metabolic acclimation to temperature in Arctic and alpine ecotypes of Oxyria digyna D. Arctic and Alpine Research 3: 277–289. [Google Scholar]
- Boisvert‐Marsh L, Perie C, de Blois S. 2014. Shifting with climate? Evidence for recent changes in tree species distribution at high latitudes. Ecosphere 5: 1–33. [Google Scholar]
- Bolstad PV, Reich P, Lee T. 2003. Rapid temperature acclimation of leaf respiration rates in Quercus alba and Quercus rubra . Tree Physiology 23: 969–976. [DOI] [PubMed] [Google Scholar]
- Booth BBB, Jones CD, Collins M, Totterdell IJ, Cox PM, Sitch S, Huntingford C, Betts RA, Harris GR, Lloyd J. 2012. High sensitivity of future global warming to land carbon cycle processes. Environmental Research Letters 7: 1–9. [Google Scholar]
- Breheny P, Burchett W. 2017. Visualizing regression models using visreg . [WWW document] URL https://journal.r‐project.org/archive/2017/RJ‐2017‐046/index.html [accessed 14 October 2021]. [Google Scholar]
- Brown JH, Stevens GC, Kaufman DM. 1996. The geographic range: size, shape, boundaries, and internal structure. Annual Review of Ecology and Systematics 27: 597–623. [Google Scholar]
- Bruhn D, Mikkelsen TN, Atkin OK. 2002. Does the direct effect of atmospheric CO2 concentration on leaf respiration vary with temperature? Responses in two species of Plantago that differ in relative growth rate. Physiologia Plantarum 114: 57–64. [PubMed] [Google Scholar]
- Buckley LB, Huey RB. 2016. Temperature extremes: geographic patterns, recent changes, and implications for organismal vulnerabilities. Global Change Biology 22: 3829–3842. [DOI] [PubMed] [Google Scholar]
- Busch FA, Sage RF. 2017. The sensitivity of photosynthesis to O2 and CO2 concentration identifies strong Rubisco control above the thermal optimum. New Phytologist 213: 1036–1051. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Evans JR. 2015. Temperature responses of mesophyll conductance differ greatly between species. Plant, Cell & Environment 38: 629–637. [DOI] [PubMed] [Google Scholar]
- Campbell C, Atkinson L, Zaragoza‐Castells J, Lundmark M, Atkin O, Hurry V. 2007. Acclimation of photosynthesis and respiration is asynchronous in response to changes in temperature regardless of plant functional group. New Phytologist 176: 375–389. [DOI] [PubMed] [Google Scholar]
- Carter KR, Wood TE, Reed SC, Butts KM, Cavaleri MA. 2021. Experimental warming across a tropical forest canopy height gradient reveals minimal photosynthetic and respiratory acclimation. Plant, Cell & Environment 44: 2879–2897. [DOI] [PubMed] [Google Scholar]
- Carter KR, Wood TE, Reed SC, Schwartz EC, Reinsel MB, Yang X, Cavaleri MA. 2020. Photosynthetic and respiratory acclimation of understory shrubs in response to in situ experimental warming of a wet tropical forest. Frontiers in Forests and Global Change 3: 1–20. [Google Scholar]
- Chambers LE, Altwegg R, Barbraud C, Barnard P, Beaumont LJ, Crawford RJM, Durant JM, Hughes L, Keatley MR, Low M et al. 2013. Phenological changes in the southern hemisphere. PLoS ONE 8: e75514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H, Muraoka H, Nakamura M, Han S, Muller O, Son Y. 2013. Experimental warming studies on tree species and forest ecosystems: a literature review. Journal of Plant Research 126: 447–460. [DOI] [PubMed] [Google Scholar]
- Clark DB, Mercado LM, Sitch S, Jones CD, Gedney N, Best MJ, Pryor M, Rooney GG, Essery RLH, Blyth E et al. 2011. The Joint UK Land Environment Simulator (JULES), model description – part 2: carbon fluxes and vegetation dynamics. Geoscientific Model Development 4: 701–722. [Google Scholar]
- Covey‐Crump EM, Attwood RG, Atkin OK. 2002. Regulation of root respiration in two species of Plantago that differ in relative growth rate: the effect of short‐ and longterm changes in temperature. Plant, Cell & Environment 25: 1501–1513. [Google Scholar]
- Crous KY, Drake JE, Aspinwall MJ, Sharwood RE, Tjoelker MG, Ghannoum O. 2018. Photosynthetic capacity and leaf nitrogen decline along a controlled climate gradient in provenances of two widely distributed Eucalyptus species. Global Change Biology 24: 4626–4644. [DOI] [PubMed] [Google Scholar]
- Crous KY, Quentin AG, Lin YS, Medlyn BE, Williams DG, Barton CVM, Ellsworth DS. 2013. Photosynthesis of temperate Eucalyptus globulus trees outside their native range has limited adjustment to elevated CO2 and climate warming. Global Change Biology 19: 3790–3807. [DOI] [PubMed] [Google Scholar]
- Crous KY, Zaragoza‐Castells J, Low M, Ellsworth DS, Tissue DT, Tjoelker MG, Barton CVM, Gimeno TE, Atkin OK. 2011. Seasonal acclimation of leaf respiration in Eucalyptus saligna trees: impacts of elevated atmospheric CO2 and summer drought. Global Change Biology 17: 1560–1576. [Google Scholar]
- Cunningham SC, Read J. 2002. Comparison of temperate and tropical rainforest tree species: photosynthetic responses to growth temperature. Oecologia 133: 112–119. [DOI] [PubMed] [Google Scholar]
- Cunningham S, Read J. 2003. Comparison of temperate and tropical rainforest tree species: growth responses to temperature. Journal of Biogeography 30: 143–153. [DOI] [PubMed] [Google Scholar]
- De Kauwe MG, Lin Y‐S, Wright IJ, Medlyn BE, Crous KY, Ellsworth DS, Maire V, Prentice IC, Atkin OK, Rogers A et al. 2016. A test of the ‘one‐point method’ for estimating maximum carboxylation capacity from field‐measured, light‐saturated photosynthesis. New Phytologist 210: 1130–1144. [DOI] [PubMed] [Google Scholar]
- Dewar RC, Franklin O, Makela A, McMurtrie RE, Valentine HT. 2009. Optimal function explains forest responses to global change. BioScience 59: 127–139. [Google Scholar]
- Dillaway DN, Kruger EL. 2010. Thermal acclimation of photosynthesis: a comparison of boreal and temperate tree species along a latitudinal transect. Plant, Cell & Environment 33: 888–899. [DOI] [PubMed] [Google Scholar]
- Drake JE, Aspinwall MJ, Pfautsch S, Rymer PD, Reich PB, Smith RA, Crous KY, Tissue DT, Ghannoum O, Tjoelker MG. 2015. The capacity to cope with climate warming declines from temperate to tropical latitudes in two widely distributed Eucalyptus species. Global Change Biology 21: 459–472. [DOI] [PubMed] [Google Scholar]
- Duque A, Stevenson PR, Feeley KJ. 2015. Thermophilization of adult and juvenile tree communities in the northern tropical Andes. Proceedings of the National Academy of Sciences, USA 112: 10744–10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusenge ME, Duarte AG, Way DA. 2019. Plant carbon metabolism and climate change: elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytologist 221: 32–49. [DOI] [PubMed] [Google Scholar]
- Dusenge ME, Madhavji S, Way DA. 2020. Contrasting acclimation responses to elevated CO2 and warming between an evergreen and a deciduous boreal conifer. Global Change Biology 26: 3639–3657. [DOI] [PubMed] [Google Scholar]
- Dusenge ME, Wittemann M, Mujawamariya M, Ntawuhiganayo EB, Zibera E, Ntirugulirwa B, Way DA, Nsabimana D, Uddling J, Wallin G. 2021. Limited thermal acclimation of photosynthesis in tropical montane tree species. Global Change Biology 27: 4860–4878. [DOI] [PubMed] [Google Scholar]
- Evans JR. 1989. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78: 9–19. [DOI] [PubMed] [Google Scholar]
- Fadrique B, Gann D, Nelson BW, Saatchi S, Feeley KJ. 2021. Bamboo phenology and life cycle drive seasonal and long‐term functioning of Amazonian bamboo‐dominated forests. Journal of Ecology 109: 860–876. [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA. 1980. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149: 78–90. [DOI] [PubMed] [Google Scholar]
- Fauset S, Oliveira L, Buckeridge MS, Foyer CH, Galbraith D, Tiwari R, Gloor M. 2019. Contrasting responses of stomatal conductance and photosynthetic capacity to warming and elevated CO2 in the tropical tree species Alchomea glandulosa under heatwave conditions. Environmental and Experimental Botany 158: 28–39. [Google Scholar]
- Ferrar PJ, Slatyer RO, Vranjic JA. 1989. Photosynthetic temperature acclimation in Eucalyptus species from diverse habitats, and a comparison with Nerium oleander . Australian Journal of Plant Physiology 16: 199–217. [Google Scholar]
- Fyllas NM, Bentley LP, Shenkin A, Asner GP, Atkin OK, Díaz S, Enquist BJ, Farfan‐Rios W, Gloor E, Guerrieri R et al. 2017. Solar radiation and functional traits explain the decline of forest primary productivity along a tropical elevation gradient. Ecology Letters 20: 730–740. [DOI] [PubMed] [Google Scholar]
- Ghalambor CK, Huey RB, Martin PR, Tewksbury JJ, Wang G. 2006. Are mountain passes higher in the tropics? Janzen's hypothesis revisited. Integrative and Comparative Biology 46: 5–17. [DOI] [PubMed] [Google Scholar]
- Ghannoum O, Phillips NG, Sears MA, Logan BA, Lewis JD, Conroy JP, Tissue DT. 2010. Photosynthetic responses of two eucalypts to industrial‐age changes in atmospheric CO2 and temperature. Plant, Cell & Environment 33: 1671–1681. [DOI] [PubMed] [Google Scholar]
- Ghannoum O, Way DA. 2011. On the role of ecological adaptation and geographic distribution in the response of trees to climate change. Tree Physiology 31: 1273–1276. [DOI] [PubMed] [Google Scholar]
- Girardin CAJ, Malhi Y, Feeley KJ, Rapp JM, Silman MR, Meir P, Huaraca Huasco W, Salinas N, Mamani M, Silva‐Espejo JE et al. 2014. Seasonality of above‐ground net primary productivity along an Andean altitudinal transect in Peru. Journal of Tropical Ecology 30: 503–519. [Google Scholar]
- González‐Orozco CE, Pollock L, Thornhill A, Mishler B, Knerr N, Laffan S, Miller J, Rosauer D, Faith D, Nipperess D et al. 2016. Phylogenetic approaches reveal biodiversity threats under climate change. Nature Climate Change 6: 1110–1114. [Google Scholar]
- Grossiord C, Buckley TN, Cernusak LA, Novick KA, Poulter B, Siegwolf RTW, Sperry JS, McDowell NG. 2020. Plant responses to rising vapor pressure deficit. New Phytologist 226: 1550–1566. [DOI] [PubMed] [Google Scholar]
- Hamrick JL, Godt MJW, Shermanbroyles SL. 1992. Factors influencing levels of genetic diversity in woody plant species. New Forests 6: 95–124. [Google Scholar]
- Harrison SP, Cramer W, Franklin O, Prentice IC, Wang H, Brännström Å, Boer H, Dieckmann U, Joshi J, Keenan TF et al. 2021. Eco‐evolutionary optimality as a means to improve vegetation and land‐surface models. New Phytologist 231: 2125–2141. [DOI] [PubMed] [Google Scholar]
- Havaux M. 1996. Short‐term responses of photosystem I to heat stress – induction of a PS II‐independent electron transport through PS I fed by stromal components. Photosynthesis Research 47: 85–97. [DOI] [PubMed] [Google Scholar]
- Heskel MA, Greaves HE, Turnbull MH, O'Sullivan OS, Shaver GR, Griffin KL, Atkin OK. 2014. Thermal acclimation of shoot respiration in an Arctic woody plant species subjected to 22 years of warming and altered nutrient supply. Global Change Biology 20: 2618–2630. [DOI] [PubMed] [Google Scholar]
- Heskel MA, O’Sullivan OS, Reich PB, Tjoelker MG, Weerasinghe LK, Penillard A, Egerton JJG, Creek D, Bloomfield KJ, Xiang J et al. 2016. Convergence in the temperature response of leaf respiration across biomes and plant functional types. Proceedings of the National Academy of Sciences, USA 113: 3832–3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25: 1965–1978. [Google Scholar]
- Hikosaka K. 2005. Nitrogen partitioning in the photosynthetic apparatus of Plantago asiatica leaves grown under different temperature and light conditions: similarities and differences between temperature and light acclimation. Plant and Cell Physiology 46: 1283–1290. [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Ishikawa K, Borjigidai A, Muller O, Onoda Y. 2006. Temperature acclimation of photosynthesis: mechanisms involved in the changes in temperature dependence of photosynthetic rate. Journal of Experimental Botany 57: 291–302. [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Murakami A, Hirose T. 1999. Balancing carboxylation and regeneration of ribulose‐1,5‐bisphosphate in leaf photosynthesis: temperature acclimation of an evergreen tree, Quercus myrsinaefolia . Plant, Cell & Environment 22: 841–849. [Google Scholar]
- Hoefnagel MHN, Atkin OK, Wiskich JT. 1998. Interdependence between chloroplasts and mitochondria in the light and the dark. Biochimica et Biophysica Acta – Bioenergetics 1366: 235–255. [Google Scholar]
- Hofstra G, Hesketh JD. 1969. Effect of temperature on stomatal aperture in different species. Canadian Journal of Botany 47: 1307–1310. [Google Scholar]
- Hogan JA, Baraloto B, Ficken C, Clark MD, Weston DJ, Warren JM. 2021. The physiological acclimation and growth response of Populus trichocarpa to warming. Physiologia Plantarum 173: 1008–1029. [DOI] [PubMed] [Google Scholar]
- Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biometrical Journal 50: 346–363. [DOI] [PubMed] [Google Scholar]
- Huntingford C, Atkin OK, Martinez‐de la Torre A, Mercado LM, Heskel MA, Harper AB, Bloomfield KJ, O’Sullivan OS, Reich PB, Wythers KR et al. 2017. Implications of improved representations of plant respiration in a changing climate. Nature Communications 8: 1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen DH. 1967. Why mountain passes are higher in tropics. The American Naturalist 101: 233–249. [Google Scholar]
- Jump AS, Peñuelas J. 2005. Running to stand still: adaptation and the response of plants to rapid climate change. Ecology Letters 8: 1010–1020. [DOI] [PubMed] [Google Scholar]
- Kattge J, Knorr W. 2007. Temperature acclimation in a biochemical model of photosynthesis: a reanalysis of data from 36 species. Plant, Cell & Environment 30: 1176–1190. [DOI] [PubMed] [Google Scholar]
- Koricheva J, Gurevitch J. 2014. Uses and misuses of meta‐analysis in plant ecology. Journal of Ecology 102: 828–844. [Google Scholar]
- Kositsup B, Montpied P, Kasemsap P, Thaler P, Ameglio T, Dreyer E. 2009. Photosynthetic capacity and temperature responses of photosynthesis of rubber trees (Hevea brasiliensis Mull. Arg.) acclimate to changes in ambient temperatures. Trees‐Structure and Function 23: 357–365. [Google Scholar]
- Kowalczyk EA, Wang YP, Law RM, Davies HL, McGregor JL, Abramowitz GS. 2006. The CSIRO Atmosphere Biosphere Land Exchange (CABLE) model for use in climate models and as an offline model. Aspendale, Vic., Australia: CSIRO Marine and Atmospheric Research. [Google Scholar]
- Kumarathunge DP, Medlyn BE, Drake JE, Tjoelker MG, Aspinwall MJ, Battaglia M, Cano FJ, Carter KR, Cavaleri MA, Cernusak LA et al. 2019. Acclimation and adaptation components of the temperature dependence of plant photosynthesis at the global scale. New Phytologist 222: 768–784. [DOI] [PubMed] [Google Scholar]
- Lamba S, Hall M, Rantfors M, Chaudhary N, Linder S, Way D, Uddling J, Wallin G. 2018. Physiological acclimation dampens initial effects of elevated temperature and atmospheric CO2 concentration in mature boreal Norway spruce. Plant, Cell & Environment 41: 300–313. [DOI] [PubMed] [Google Scholar]
- Lee TD, Reich PB, Bolstad PV. 2005. Acclimation of leaf respiration to temperature is rapid and related to specific leaf area, soluble sugars and leaf nitrogen across three temperate deciduous tree species. Functional Ecology 19: 640–647. [Google Scholar]
- Leimu R, Mutikainen P, Koricheva J, Fischer M. 2006. How general are positive relationships between plant population size, fitness and genetic variation? Journal of Ecology 94: 942–952. [Google Scholar]
- Lesica P, Crone EE. 2017. Arctic and boreal plant species decline at their southern range limits in the Rocky Mountains. Ecology Letters 20: 166–174. [DOI] [PubMed] [Google Scholar]
- Leuning R. 2002. Temperature dependence of two parameters in a photosynthesis model. Plant, Cell & Environment 25: 1205–1210. [Google Scholar]
- Liang JY, Xia JY, Liu LL, Wan SQ. 2013. Global patterns of the responses of leaf‐level photosynthesis and respiration in terrestrial plants to experimental warming. Journal of Plant Ecology 6: 437–447. [Google Scholar]
- Lin YS, Medlyn BE, Ellsworth DS. 2012. Temperature responses of leaf net photosynthesis: the role of component processes Tree Physiology 32: 219–231. [DOI] [PubMed] [Google Scholar]
- Lloyd J, Farquhar GD. 2008. Effects of rising temperatures and CO2 on the physiology of tropical forest trees. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 363: 1811–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardozzi DL, Bonan GB, Smith NG, Dukes JS, Fisher RA. 2015. Temperature acclimation of photosynthesis and respiration: a key uncertainty in the carbon cycle‐climate feedback. Geophysical Research Letters 42: 8624–8631. [Google Scholar]
- Malhi Y, Silman M, Salinas N, Bush M, Meir P, Saatchi S. 2010. Introduction: elevation gradients in the tropics: laboratories for ecosystem ecology and global change research. Global Change Biology 16: 3171–3175. [Google Scholar]
- Masson‐Delmotte V, Zhai P, Pirani A, Connors SL, Péan C, Berger S, Caud N, Chen Y, Goldfarb L, Gomis MI et al. 2022. Climate change 2021: the physical science basis. In: Contribution of working group I to the sixth assessment report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Medlyn BE, Dreyer E, Ellsworth D, Forstreuter M, Harley PC, Kirschbaum MUF, Le Roux X, Montpied P, Strassemeyer J, Walcroft A et al. 2002. Temperature response of parameters of a biochemically based model of photosynthesis. II. A review of experimental data. Plant, Cell & Environment 25: 1167–1179. [Google Scholar]
- Meehl GA, Tebaldi C. 2004. More intense, more frequent, and longer lasting heat waves in the 21st century. Science 305: 994–997. [DOI] [PubMed] [Google Scholar]
- Mercado LM, Medlyn BE, Huntingford C, Oliver RJ, Clark DB, Sitch S, Zelazowski P, Kattge J, Harper AB, Cox PM. 2018. Large sensitivity in land carbon storage due to geographical and temporal variation in the thermal response of photosynthetic capacity. New Phytologist 218: 1462–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteith JL. 1995. Accommodation between transpiring vegetation and the convective boundary layer. Journal of Hydrology 166: 251–263. [Google Scholar]
- Mooney HA. 1963. Physiological ecology of coastal, subalpine and alpine populations of Polygonum bistortoides . Ecology 44: 812–816. [Google Scholar]
- Morin X, Thuiller W. 2009. Comparing niche‐ and process‐based models to reduce prediction uncertainty in species range shifts under climate change. Ecology 90: 1301–1313. [DOI] [PubMed] [Google Scholar]
- Mujawamariya M, Wittemann M, Manishimwe A, Ntirugulirwa B, Zibera E, Nsabimana D, Wallin G, Uddling J, Dusenge ME. 2021. Complete or overcompensatory thermal acclimation of leaf dark respiration in African tropical trees. New Phytologist 229: 2548–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoda Y, Hikosaka K, Hirose T. 2005. The balance between RuBP carboxylation and RuBP regeneration: a mechanism underlying the interspecific variation in acclimation of photosynthesis to seasonal change in temperature. Functional Plant Biology 32: 903–910. [DOI] [PubMed] [Google Scholar]
- O'Sullivan OS, Heskel MA, Reich PB, Tjoelker MG, Weerasinghe LK, Penillard A, Zhu L, Egerton JJG, Bloomfield KJ, Creek D et al. 2017. Thermal limits of leaf metabolism across biomes. Global Change Biology 23: 209–223. [DOI] [PubMed] [Google Scholar]
- O'Sullivan OS, Weerasinghe K, Evans JR, Egerton JJG, Tjoelker MG, Atkin OK. 2013. High‐resolution temperature responses of leaf respiration in snow gum (Eucalyptus pauciflora) reveal high‐temperature limits to respiratory function. Plant, Cell & Environment 36: 1268–1284. [DOI] [PubMed] [Google Scholar]
- Pacifici M, Foden WB, Visconti P, Watson JEM, Butchart SHM, Kovacs KM, Scheffers BR, Hole DG, Martin TG, Akçakaya HR et al. 2015. Assessing species vulnerability to climate change. Nature Climate Change 5: 215–225. [Google Scholar]
- Pastenes C, Horton P. 1996. Effect of high temperature on photosynthesis in beans. 2. CO2 assimilation and metabolite contents. Plant Physiology 112: 1253–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning de Vries FWT. 1975. Cost of maintenance processes in plant cells. Annals of Botany 39: 77–92. [Google Scholar]
- Peñuelas J, Sardans J, Estiarte M, Ogaya R, Carnicer J, Coll M, Barbeta A, Rivas‐Ubach A, Llusià J, Garbulsky M et al. 2013. Evidence of current impact of climate change on life: a walk from genes to the biosphere. Global Change Biology 19: 2303–2338. [DOI] [PubMed] [Google Scholar]
- R Core Team . 2020. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [WWW document] URL https://www.R‐project.org/ [accessed 20 November 2020]. [Google Scholar]
- Read J, Busby JR. 1990. Comparative responses to temperature of the major canopy species of Tasmanian cool temperate rainforest and their ecological significance 2. Net photosynthesis and climate analysis. Australian Journal of Botany 38: 185–205. [Google Scholar]
- Reich PB, Oleksyn J. 2004. Global patterns of plant leaf N and P in relation to temperature and latitude. Proceedings of the National Academy of Sciences, USA 101: 11001–11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich PB, Sendall KM, Rice K, Rich RL, Stefanski A, Hobbie SE, Montgomery RA. 2015. Geographic range predicts photosynthetic and growth response to warming in co‐occurring tree species. Nature Climate Change 5: 148–152. [Google Scholar]
- Reich PB, Sendall KM, Stefanski A, Wei XR, Rich RL, Montgomery RA. 2016. Boreal and temperate trees show strong acclimation of respiration to warming. Nature 531: 633–636. [DOI] [PubMed] [Google Scholar]
- Ruehr NK, Gast A, Weber C, Daub B, Arneth A. 2016. Water availability as dominant control of heat stress responses in two contrasting tree species. Tree Physiology 36: 164–178. [DOI] [PubMed] [Google Scholar]
- Sage RF, Kubien DS. 2007. The temperature response of C3 and C4 photosynthesis. Plant, Cell & Environment 30: 1086–1106. [DOI] [PubMed] [Google Scholar]
- Salvucci ME, Crafts‐Brandner SJ. 2004. Inhibition of photosynthesis by heat stress: the activation state of Rubisco as a limiting factor in photosynthesis. Physiologia Plantarum 120: 179–186. [DOI] [PubMed] [Google Scholar]
- Savolainen O, Pyhajarvi T, Knurr T. 2007. Gene flow and local adaptation in trees. Annual Review of Ecology, Evolution, and Systematics 38: 595–619. [Google Scholar]
- Saxe H, Cannell MGR, Johnsen B, Ryan MG, Vourlitis G. 2001. Tree and forest functioning in response to global warming. New Phytologist 149: 369–399. [DOI] [PubMed] [Google Scholar]
- Scafaro AP, Xiang S, Long BM, Bahar NHA, Weerasinghe LK, Creek D, Evans JR, Reich PB, Atkin OK. 2017. Strong thermal acclimation of photosynthesis in tropical and temperate wet‐forest tree species: the importance of altered Rubisco content. Global Change Biology 23: 2783–2800. [DOI] [PubMed] [Google Scholar]
- Sendall KM, Reich PB, Zhao CM, Hou JH, Wei XR, Stefanski A, Rice K, Rich RL, Montgomery RA. 2015. Acclimation of photosynthetic temperature optima of temperate and boreal tree species in response to experimental forest warming. Global Change Biology 21: 1342–1357. [DOI] [PubMed] [Google Scholar]
- Sexton JP, Strauss SY, Rice KJ. 2011. Gene flow increases fitness at the warm edge of a species' range. Proceedings of the National Academy of Sciences, USA 108: 11704–11709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD. 2005. Effects of moderate heat stress on photosynthesis: importance of thylakoid reactions, rubisco deactivation, reactive oxygen species, and thermotolerance provided by isoprene. Plant, Cell & Environment 28: 269–277. [Google Scholar]
- Slatyer RA, Hirst M, Sexton JP. 2013. Niche breadth predicts geographical range size: a general ecological pattern. Ecology Letters 16: 1104–1114. [DOI] [PubMed] [Google Scholar]
- Slot M, Kitajima K. 2015. General patterns of acclimation of leaf respiration to elevated temperatures across biomes and plant types. Oecologia 177: 885–900. [DOI] [PubMed] [Google Scholar]
- Slot M, Rey‐Sanchez C, Gerber S, Lichstein JW, Winter K, Kitajima K. 2014. Thermal acclimation of leaf respiration of tropical trees and lianas: response to experimental canopy warming, and consequences for tropical forest carbon balance. Global Change Biology 20: 2915–2926. [DOI] [PubMed] [Google Scholar]
- Slot M, Rifai SW, Winter K. 2021. Photosynthetic plasticity of a tropical tree species, Tabebuia rosea, in response to elevated temperature and CO2 . Plant, Cell & Environment 44: 2347–2364. [DOI] [PubMed] [Google Scholar]
- Slot M, Winter K. 2016. The effects of rising temperature on the ecophysiology of tropical forest trees. In: Goldstein G, Santiago LS, eds. Tropical tree physiology: adaptations and responses in a changing environment. Cham, Switzerland: Springer, 385–412. [Google Scholar]
- Slot M, Winter K. 2017a. In situ temperature relationships of biochemical and stomatal controls of photosynthesis in four lowland tropical tree species. Plant, Cell & Environment 40: 3055–3068. [DOI] [PubMed] [Google Scholar]
- Slot M, Winter K. 2017b. In situ temperature response of photosynthesis of 42 tree and liana species in the canopy of two Panamanian lowland tropical forests with contrasting rainfall regimes. New Phytologist 214: 1103–1117. [DOI] [PubMed] [Google Scholar]
- Smith NG, Dukes JS. 2013. Plant respiration and photosynthesis in global‐scale models: incorporating acclimation to temperature and CO2 . Global Change Biology 19: 45–63. [DOI] [PubMed] [Google Scholar]
- Smith NG, Dukes JS. 2017. Short‐term acclimation to warmer temperatures accelerates leaf carbon exchange processes across plant types. Global Change Biology 23: 4840–4853. [DOI] [PubMed] [Google Scholar]
- Smith NG, Keenan TF. 2020. Mechanisms underlying leaf photosynthetic acclimation to warming and elevated CO2 as inferred from least‐cost optimality theory. Global Change Biology 26: 5202–5216. [DOI] [PubMed] [Google Scholar]
- Smith NG, Keenan TF, Colin Prentice I, Wang H, Wright IJ, Niinemets Ü, Crous KY, Domingues TF, Guerrieri R, Yoko Ishida F et al. 2019. Global photosynthetic capacity is optimized to the environment. Ecology Letters 22: 506–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NG, Malyshev SL, Shevliakova E, Kattge J, Dukes JS. 2016. Foliar temperature acclimation reduces simulated carbon sensitivity to climate. Nature Climate Change 6: 407–411. [Google Scholar]
- Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM et al. 2013. Climate change 2013: the physical science basis. In: Contribution of working group I to the fifth assessment report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press, 317–382. [Google Scholar]
- Strand A, Hurry V, Henkes S, Huner N, Gustafsson P, Gardestrom P, Stitt M. 1999. Acclimation of Arabidopsis leaves developing at low temperatures. Increasing cytoplasmic volume accompanies increased activities of enzymes in the Calvin cycle and in the sucrose‐biosynthesis pathway. Plant Physiology 119: 1387–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MJP, Lewis SL, Affum‐Baffoe K, Castilho C, Costa F, Sanchez AC, Ewango CEN, Hubau W, Marimon B, Monteagudo‐Mendoza A et al. 2020. Long‐term thermal sensitivity of Earth's tropical forests. Science 368: 869–874. [DOI] [PubMed] [Google Scholar]
- Tagesson T, Schurgers G, Horion S, Ciais P, Tian F, Brandt M, Ahlström A, Wigneron J‐P, Ardö J, Olin S et al. 2020. Recent divergence in the contributions of tropical and boreal forests to the terrestrial carbon sink. Nature Ecology & Evolution 4: 202–209. [DOI] [PubMed] [Google Scholar]
- Teskey R, Wertin T, Bauweraerts I, Ameye M, McGuire MA, Steppe K. 2015. Responses of tree species to heat waves and extreme heat events. Plant, Cell & Environment 38: 1699–1712. [DOI] [PubMed] [Google Scholar]
- Teskey RO, Will RE. 1999. Acclimation of loblolly pine (Pinus taeda) seedlings to high temperatures. Tree Physiology 19: 519–525. [DOI] [PubMed] [Google Scholar]
- Thuiller W, Lavorel S, Araujo MB. 2005. Niche properties and geographical extent as predictors of species sensitivity to climate change. Global Ecology and Biogeography 14: 347–357. [Google Scholar]
- Tjoelker MG, Oleksyn J, Lorenc‐Plucinska G, Reich PB. 2009. Acclimation of respiratory temperature responses in northern and southern populations of Pinus banksiana . New Phytologist 181: 218–229. [DOI] [PubMed] [Google Scholar]
- Tjoelker MG, Oleksyn J, Reich PB. 1999. Acclimation of respiration to temperature and CO2 in seedlings of boreal tree species in relation to plant size and relative growth rate. Global Change Biology 5: 679–691. [Google Scholar]
- Tjoelker MG, Oleksyn J, Reich PB. 2001. Modelling respiration of vegetation: evidence for a general temperature‐dependent Q10 . Global Change Biology 7: 223–230. [Google Scholar]
- Togashi HF, Prentice IC, Atkin OK, Macfarlane C, Prober SM, Bloomfield KJ, Evans BJ. 2018. Thermal acclimation of leaf photosynthetic traits in an evergreen woodland, consistent with the coordination hypothesis. Biogeosciences 15: 3461–3474. [Google Scholar]
- Vanderwel MC, Slot M, Lichstein JW, Reich PB, Kattge J, Atkin OK, Bloomfield KJ, Tjoelker MG, Kitajima K. 2015. Global convergence in leaf respiration from estimates of thermal acclimation across time and space. New Phytologist 207: 1026–1037. [DOI] [PubMed] [Google Scholar]
- Vårhammar A, Wallin G, McLean CM, Dusenge ME, Medlyn BE, Hasper TB, Nsabimana D, Uddling J. 2015. Photosynthetic temperature responses of tree species in Rwanda: evidence of pronounced negative effects of high temperature in montane rainforest climax species. New Phytologist 206: 1000–1012. [DOI] [PubMed] [Google Scholar]
- Villén‐Peréz S, Heikkinen J, Salemaa M, Makipaa R. 2020. Global warming will affect the maximum potential abundance of boreal plant species. Ecography 43: 801–811. [Google Scholar]
- Wang H, Atkin OK, Keenan TF, Smith NG, Wright IJ, Bloomfield KJ, Kattge J, Reich PB, Prentice IC. 2020. Acclimation of leaf respiration consistent with optimal photosynthetic capacity. Global Change Biology 26: 2573–2583. [DOI] [PubMed] [Google Scholar]
- Way DA, Oren R. 2010. Differential responses to changes in growth temperature between trees from different functional groups and biomes: a review and synthesis of data. Tree Physiology 30: 669–688. [DOI] [PubMed] [Google Scholar]
- Way DA, Sage RF. 2008. Thermal acclimation of photosynthesis in black spruce Picea mariana (Mill.) BSP. Plant, Cell & Environment 31: 1250–1262. [DOI] [PubMed] [Google Scholar]
- Way DA, Yamori W. 2014. Thermal acclimation of photosynthesis: on the importance of adjusting our definitions and accounting for thermal acclimation of respiration. Photosynthesis Research 119: 89–100. [DOI] [PubMed] [Google Scholar]
- Weerasinghe LK, Creek D, Crous KY, Xiang S, Liddell MJ, Turnbull MH, Atkin OK. 2014. Canopy position affects the relationships between leaf respiration and associated traits in a tropical rainforest in Far North Queensland. Tree Physiology 34: 564–584. [DOI] [PubMed] [Google Scholar]
- Wertin TM, McGuire MA, Teskey RO. 2010. The influence of elevated temperature, elevated atmospheric CO2 concentration and water stress on net photosynthesis of loblolly pine (Pinus taeda L.) at northern, central and southern sites in its native range. Global Change Biology 16: 2089–2103. [Google Scholar]
- Wertin TM, McGuire MA, Teskey RO. 2011. Higher growth temperatures decreased net carbon assimilation and biomass accumulation of northern red oak seedlings near the southern limit of the species range. Tree Physiology 31: 1277–1288. [DOI] [PubMed] [Google Scholar]
- Willig MR, Kaufman DM, Stevens RD. 2003. Latitudinal gradients of biodiversity: pattern, process, scale, and synthesis. Annual Review of Ecology Evolution and Systematics 34: 273–309. [Google Scholar]
- Yamori W, Hikosaka K, Way DA. 2014. Temperature response of photosynthesis in C‐3, C‐4, and CAM plants: temperature acclimation and temperature adaptation. Photosynthesis Research 119: 101–117. [DOI] [PubMed] [Google Scholar]
- Yamori W, Noguchi K, Terashima I. 2005. Temperature acclimation of photosynthesis in spinach leaves: analyses of photosynthetic components and temperature dependencies of photosynthetic partial reactions. Plant, Cell & Environment 28: 536–547. [Google Scholar]
- Yin XY. 2021. No need to switch the modified Arrhenius function back to the old form. New Phytologist 231: 2113–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragoza‐Castells J, Sanchez‐Gomez D, Valladares F, Hurry V, Atkin OK. 2007. Does growth irradiance affect temperature dependence and thermal acclimation of leaf respiration? Insights from a Mediterranean tree with long‐lived leaves. Plant, Cell & Environment 30: 820–833. [DOI] [PubMed] [Google Scholar]
- Zhu LL, Bloomfield KJ, Hocart CH, Egerton JJG, O'Sullivan OS, Penillard A, Weerasinghe LK, Atkin OK. 2018. Plasticity of photosynthetic heat tolerance in plants adapted to thermally contrasting biomes. Plant, Cell & Environment 41: 1251–1262. [DOI] [PubMed] [Google Scholar]
- Ziehn T, Kattge J, Knorr W, Scholze M. 2011. Improving the predictability of global CO2 assimilation rates under climate change. Geophysical Research Letters 38: 1–5. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Boxplots and relationship between biome, mean annual temperature and mean temperature of the warmest quarter in the ESWE dataset.
Fig. S2 Partial residuals with 95% confidence intervals of the ANCOVA as a function of mean temperature of the warmest quarter (meanTWQ) to represent the effect of biome.
Fig. S3 Linear regression relationships with 95% confidence intervals between several variables against experimental control growth temperatures across biomes.
Fig. S4 Partial residuals with 95% confidence intervals from the ANCOVA as a function of mean annual temperature (mat) to represent the effect of biome.
Fig. S5 Linear relationships with 95% confidence intervals against experimental control growth temperature for the response ratio (RR) of net photosynthesis at 25°C, A net25, and respiration rates a prevailing growth temperatures, R growth.
Table S1 Species list per biome and plant functional type, including latitude and longitude, the reference where the data originated and how controlled the study was.
Table S2 ANCOVA results with mean annual temperature as a covariate for both absolute control values and the response ratios.
Table S3 Table with sample sizes for each variable in each category (warming amount, biome and leaf form).
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Data Availability Statement
All data used in this manuscript are available online and/or have been published before (see Appendix A2). The dataset used for this study is available from the corresponding author upon reasonable request for future collaborations.


