Abstract
OBJECTIVE:
To evaluate whether the use of inhaled nitric oxide (iNO)200 improves respiratory function.
METHODS:
This retrospective cohort study used data from pregnant patients hospitalized with severe bilateral coronavirus disease 2019 (COVID-19) pneumonia at four teaching hospitals between March 2020 and December 2021. Two cohorts were identified: 1) those receiving standard of care alone (SoC cohort) and 2) those receiving iNO200 for 30 minutes twice daily in addition to standard of care alone (iNO200 cohort). Inhaled nitric oxide, as a novel therapy, was offered only at one hospital. The prespecified primary outcome was days free from any oxygen supplementation at 28 days postadmission. Secondary outcomes were hospital length of stay, rate of intubation, and intensive care unit (ICU) length of stay. The multivariable-adjusted regression analyses accounted for age, body mass index, gestational age, use of steroids, remdesivir, and the study center.
RESULTS:
Seventy-one pregnant patients were hospitalized for severe bilateral COVID-19 pneumonia: 51 in the SoC cohort and 20 in the iNO200 cohort. Patients receiving iNO200 had more oxygen supplementation–free days (iNO200: median [interquartile range], 24 [23–26] days vs standard of care alone: 22 [14–24] days, P =.01) compared with patients in the SoC cohort. In the multivariable-adjusted analyses, iNO200 was associated with 63.2% (95% CI 36.2–95.4%; P<.001) more days free from oxygen supplementation, 59.7% (95% CI 56.0–63.2%; P<.001) shorter ICU length of stay, and 63.6% (95% CI 55.1–70.8%; P<.001) shorter hospital length of stay. No iNO200-related adverse events were reported.
CONCLUSION:
In pregnant patients with severe bilateral COVID-19 pneumonia, iNO200 was associated with a reduced need for oxygen supplementation and shorter hospital stay.
Pneumonia related to coronavirus disease 2019 (COVID-19) is particularly threatening during pregnancy, because it may quickly progress to hypoxic respiratory failure requiring hospitalization and cardiopulmonary support.1,2 According to the Centers for Disease Control and Prevention, when compared with nonpregnant female patients with COVID-19 pneumonia, pregnant patients are more likely to develop severe hypoxemic respiratory failure requiring prolonged hospitalization.1 The risk for pregnant individuals requiring intensive care unit (ICU) admission, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO) is about threefold that of nonpregnant patients with COVID-19, approximately quadrupling the risk of death.1,3 Moreover, the risk of obstetric complications, including preeclampsia, preterm delivery, and stillbirth, is also increased with COVID-19.4
To date, no respiratory therapies to complement supplemental oxygenation have been tested in randomized clinical trials targeting pregnant patients with COVID-19.5 Steroids and remdesivir are commonly used in pregnant patients with severe forms of the disease, as they have shown benefit in trials of nonpregnant populations with COVID-19.6
Inhaled nitric oxide (iNO) is a therapeutic gas that is used as rescue therapy for severe hypoxemia in intubated patients with respiratory failure. It is typically delivered at a dose of 10–80 ppm to produce selective pulmonary vasodilatation and improve ventilation-perfusion matching, improving systemic oxygenation.7 It has been used in the context of adult respiratory distress syndrome, in particular that caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), as a rescue treatment for hypoxia.8 Nitric oxide has also shown antiviral properties against SARS-CoV-2. In in-vitro studies, S-nitroso-N-acetylpenicillamine, a nitric oxide donor, was able to reduce SARS-CoV-2 viral replication in a dose-dependent fashion. High-dose nitric oxide directly and nonspecifically nitrosates the SARS-CoV-2 3CL cysteine protease, causing a reduction of viral replication.9
During the first wave of COVID-19 in Boston, Massachusetts, we treated six nonintubated pregnant patients admitted to Massachusetts General Hospital for severe COVID-19 pneumonia with up to 200 ppm of iNO (iNO200) delivered by a snug-fitting mask for 30 minutes, twice daily.10,11 Thirty-nine treatments with iNO200 were administered safely, which resulted in increased systemic oxygenation and decreased respiratory rate.12 Based on these results, iNO200 was implemented as a treatment for COVID-19 pneumonia in pregnancy at Massachusetts General Hospital.
To further investigate the safety and efficacy of iNO200 in pregnant patients with COVID-19, we designed this study to assess whether iNO200 treatment improves respiratory function and outcomes among pregnant patients hospitalized with severe COVID-9 pneumonia.
METHODS
This is a retrospective cohort study that was designed to assess the safety efficacy of iNO200 in the treatment of pregnant patients hospitalized for severe bilateral COVID-19 pneumonia. We created a multidisciplinary network between four academic hospitals in Boston, Massachusetts (Beth Israel Deaconess Medical Center, Boston Medical Center, Massachusetts General Hospital, and Tufts Medical Center) called the “DELivery oF iNO network for COVID-19 pregnant patients” (the DELFiNO network). All institutions in the DELFiNO network have similar access to advanced techniques in the management of respiratory failure, including ECMO. Treatment with iNO200 is a novel and unlabeled use of iNO therapy and was available only at one hospital (Massachusetts General Hospital).
Institutional review board approval was obtained, together with a data-use agreement allowing sharing of data with the coordinating center (Mass General Brigham IRB# 2020P001362). A waiver of written informed consent was obtained due to the observational nature of the data collection. Inclusion criteria and data collection are presented in Appendix 2, available online at http://links.lww.com/AOG/C775.
Based on beneficial and lasting effects on oxygenation reported in recent data on intermitted high-dose iNO in patients with COVID-19 pneumonia, the prespecified primary endpoint of this study was the number of days free from oxygen supplementation at 28 days postadmission. Oxygen supplementation was defined as oxygen delivery by any mode, including nasal cannula, facemask, noninvasive ventilation, and mechanical ventilation. Oxygen delivery was targeted at maintaining SpO2 within the levels suggested by the Society for Maternal-Fetal Medicine guidelines (SpO2 95% or higher).6 The prespecified secondary endpoints were hospital length of stay, ICU length of stay, intubation rate, and maternal and fetal survival rates. The safety of iNO200 was also assessed (Appendix 2, http://links.lww.com/AOG/C775).
The option to receive iNO200 therapy was available only at one center (Massachusetts General Hospital), and the decision to use iNO200 was at the discretion of the maternal–fetal medicine team caring for the patient, as was the case for other pharmacologic therapy (eg, remdesivir, antibiotics, and steroids). The iNO200 protocol and the delivery system have been previously published (Appendix 2, http://links.lww.com/AOG/C775).11–14
Continuous data are presented as mean±SD or median (interquartile range) according to the data distribution. Categorical values are presented as absolute number (%). Normality of distribution was assessed using the Shapiro-Wilk test. Student’s t test, Wilcoxon rank-sum and Fisher exact test (two-tail) were used to compare groups for continuous and categorical variables, respectively. We did not apply any imputation for missing values. Cohorts were compared for variables with less than 20% missing data.
The two cohorts were compared for differences at baseline for potential confounding factors. Associations of primary and secondary outcomes with iNO200 were evaluated with generalized linear regression models. Covariates included in the multivariable models were factors known to be associated with COVID-19 severity15 and therapies associated with improved outcome.16,17 Differences in vital parameters recorded before, during and after iNO administration were analyzed using a linear mixed model. The statistical methods are presented in greater detail in Appendix 2 (http://links.lww.com/AOG/C775). Two-sided P<.05 was considered statistically significant.
RESULTS
Between March 2020 and December 2021, 71 pregnant patients were treated for bilateral severe or critical COVID-19 pneumonia within the DELFiNO network. Of the 71 pregnant patients, 20 received iNO200 therapy (iNO200 cohort) and 51 received standard of care alone (SoC cohort) (Fig. 1). A total of 30 out of 71 patients were treated at Massachusetts General Hospital, 20 with iNO200 and 10 with standard of care alone. The characteristics of the patients are presented in Table 1. The two cohorts did not show statistical differences in baseline characteristics, past medical history, gestational age, and clinical presentation at the time of hospital admission. With the exception of ferritin (P=.04), white blood cell count (P=.03), and neutrophil count (P=.04), the laboratory results at the time of admission were similar. The pharmacologic interventions for COVID-19 did not differ between the two cohorts (Table 1 and Appendix 3 [Appendix 3 is available online at http://links.lww.com/AOG/C775]). Moreover, only one patient (in the SoC cohort) in the whole study had received a COVID-19 vaccination.
Fig. 1.
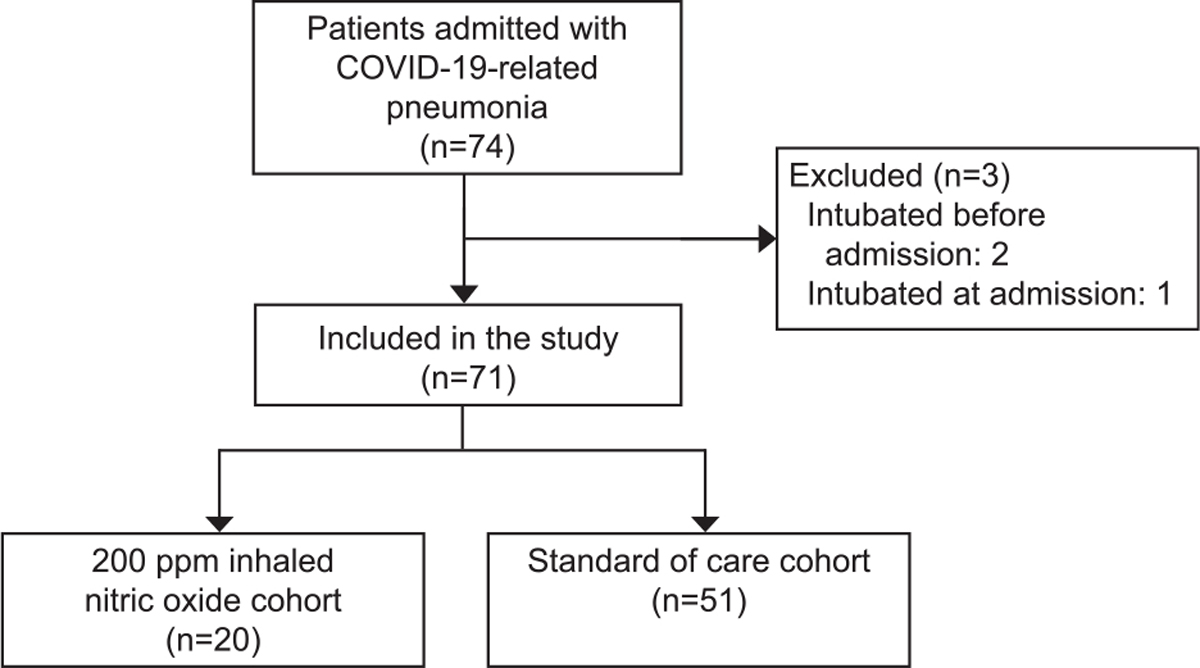
Flow chart. COVID-19, coronavirus disease 2019.
Valsecchi. High-Dose Nitric Oxide in Severe COVID-19. Obstet Gynecol 2022.
Table 1.
Main Demographic Information, Vital Signs, Laboratory Results, and Coronavirus Disease 2019 (COVID-19) Therapies
| iNO200 Cohort (n=20) | SoC Cohort (n=51) | P | |
|---|---|---|---|
|
| |||
| Demographics | |||
| Age (y) | 30.30±4.43 | 32.33±5.76 | .12 |
| BMI (kg/m2) | 31.84±4.85 | 33.58±7.02 | .26 |
| Multiple gestation | 1 (5) | 5 (10) | .67 |
| Gestational age (wk) | 29.77±6.03 | 29.93±5.25 | .92 |
| 2nd trimester | 7 (35) | 12 (25) | |
| 3rd trimester | 13 (65) | 36 (75) | |
| Comorbidity | |||
| Hypertension | 1 (5) | 5 (10) | .67 |
| Diabetes | 1 (5) | 3 (6) | >.99 |
| Malignancy | 1 (5) | 1 (2) | .49 |
| Asthma | 2 (10) | 7 (14) | >.99 |
| Others* | 9 (45) | 16 (31) | .41 |
| Hospital admission vital signs and laboratory results | |||
| Respiratory rate (bpm) | 28 (20–32) | 24 (20–30) | .38 |
| Mean arterial pressure (mm Hg) | 82±10 | 83±11 | .67 |
| SpO2 (%) | 96 (94–98) | 96 (94–98) | .87 |
| FiO2 (%) | 0.21 (0.21–0.32) | 0.23 (0.21–0.29) | .97 |
| Creatinine (mg/dL) | 0.52 (0.44–0.57) | 0.55 (0.5–0.61) | .11 |
| WBC count (109/L) | 6.05 (4.50–8.00) | 7.41 (6.00–8.80) | .03 |
| Lymphocytes (109/L) | 0.80±0.26 | 0.94±0.29 | .08 |
| Neutrophil (109/L) | 4.64 (3.38–5.92) | 5.62 (4.47–7.20) | .04 |
| CRP (mg/dL) | 7.27 (5.15–11.12) | 9.23 (5.95–12.24) | .25 |
| Ferritin (ng/mL) | 132.50 (78.00–397.00) | 93.00 (59.00–133.00) | .04 |
| Other COVID-19 therapies | |||
| Steroids | 16 (80) | 32 (63) | .26 |
| Remdesivir | 15 (75) | 28 (55) | .18 |
| Chloroquine | 3 (15) | 8 (16) | >.99 |
iNO, inhaled nitric oxide; SoC, standard of care alone; BMI, body mass index; bpm, breaths per minute; WBC, white blood cell; CRP, C-reactive protein; COVID-19, coronavirus disease 2019.
Data are mean±SD, n (%), or median (interquartile range) unless otherwise specified.
Other comorbidities: anemia, prediabetes, congenital single kidney, liver lesion, depression, anxiety, hyperthyroidism, hypothyroidism, celiac disease, congenital latent tuberculosis, previous pulmonary embolism, previous pancreatitis, previous hepatitis, hyperlipidemia, previous heart valvular replacement, autoimmune disease. No events for coronary heart disease, cerebrovascular disease, chronic kidney disease, and immune deficiency.
Within the iNO200 cohort, the administration of nitric oxide gas was initiated very close to the time of hospital admission (median 1 [interquartile range 0.75–1] day), with the exception of one patient who began treatment 3 days after admission. The median (interquartile range) number of days free from oxygen supplementation within 28 days postadmission was 24 (23–26) in the iNO200 cohort and 22 (14–24) in the SoC cohort (P=.01, Table 2).
Table 2.
Coronavirus Disease 2019 (COVID-19) Pneumonia Severity and Hospital Outcomes
| iNO200 Cohort (n=20) | SoC Cohort (n=51) | P | |
|---|---|---|---|
|
| |||
| Severity | .78 | ||
| Severe* | 15 (75) | 35 (69) | |
| Critical† | 5 (25) | 16 (31) | |
| Oxygen supplementation-free days at 28 d | 24 (23–26) | 22 (14–24) | .01 |
| ICU admission | 5 (25) | 21 (41) | .28 |
| ICU length of stay (h) | 77 (66–274) | 354 (189–485) | .09 |
| Intubation | 2 (10) | 16 (31) | .08 |
| Ventilation time (h) | 171 (30–311) | 332 (185–515) | .21 |
| ECMO | 0 | 2 (4) | >.99 |
| Hospital length of stay (d) | 7 (5–9) | 9 (6–20) | .03 |
| Death | 0 | 1 (2) | >.99 |
iNO, inhaled nitric oxide; SoC, standard of care alone; ICU, intensive care unit; ECMO, extracorporeal membrane oxygenation.
Data are n (%) or median (interquartile range) unless otherwise specified.
Bilateral lung opacities on chest radiography and dyspnea, with either tachypnea (respiratory rate 30 breaths per minute or greater), hypoxemia (SpO2 93% or lower on room air at rest [95% or lower with oxygen]), PaO2/FiO2 ratio less than 300 mm Hg, or a combination of these.
In addition to the signs and symptoms of severe pneumonia, the patient required mechanical ventilation or ECMO, developed shock (mean arterial pressure less than 70 mm Hg, systolic blood pressure less than 90 mm Hg, or inotrope or vasopressor requirement), or developed multiple organ failure requiring ICU admission.
The patients in the iNO200 cohort had significantly shorter hospital length of stay (iNO200 cohort: median [interquartile range] 7 [5–9] days vs SoC cohort: 9 [6–20] days, P=.03) and showed a trend toward lower rates of intubation (iNO200 10% vs standard of care alone 31%, P=.08) and toward shorter ICU length of stay (iNO200 cohort: median [interquartile range] 77 [66–274] hours, SoC cohort 354 [189–485] hours, P=.09, Table 2).
Multivariable-adjusted analyses noted that, compared with the SoC cohort, iNO200 was associated with 63.2% (95% CI 36.2–95.4%; P<.001) more oxygen supplementation-free days within 28 days postadmission. It also correlated with 59.7% (95% CI 56.0–63.2%; P<.001) fewer hours in the ICU and 63.6% (95% CI 55.1–70.8%; P<.001) fewer days in the hospital (Appendices 4 and 5, available online at http://links.lww.com/AOG/C775). The only two patients requiring ECMO were in the SoC cohort (Table 2); one patient in the SoC cohort died. All patients in the iNO200 cohort did not require ECMO and were discharged. The two cohorts were similar regarding delivery and neonatal outcomes (Appendix 6, http://links.lww.com/AOG/C775).
Breathing iNO200 transiently improved oxygenation in patients with hypoxemia (Fig. 2A). The respiratory rate of patients with tachypnea decreased and remained lower during and after treatment with iNO200 (Fig. 2B), confirming the ability of iNO200 to relieve the signs and symptoms of respiratory distress.
Fig. 2.

Effect of inhaled nitric oxide (iNO)200 on respiratory rate, oxygenation (measured as SpO2/FiO2 ratio), and hemodynamics (data are mean, minimum–maximum). Linear mixed-model fit by maximum likelihood. A. Effect of iNO200 on SpO2/FiO2 ratio in patients with hypoxemia (defined as SpO2/FiO2 ratio 315 or lower) at the commencement of administration (n=56). B. Effect of iNO200 on respiratory rate, measured in breaths per minute (bpm) in patients with tachypnoea (defined as respiratory rate 24 bpm or higher) at the commencement of administration (n=94). C. Effect of iNO200 on mean arterial pressure, measured in mm Hg (n=139). D. Effect of iNO200 on heart rate, measured as beats per minute (bpm) (n=144) *P<.05 in the post hoc analysis (Tuckey’s range test) vs before (for additional statistical methods, see Appendix 2, available online at http://links.lww.com/AOG/C775).
Valsecchi. High-Dose Nitric Oxide in Severe COVID-19. Obstet Gynecol 2022.
A total of 144 treatments of iNO were administered during this study. No iNO200-related adverse events occurred. The median (interquartile range) dose of nitric oxide per treatment was 190 (170–200) ppm administered for a median of 30 minutes. The mean (minimum–maximum) levels of methemoglobin and nitrogen dioxide (NO2) during breathing iNO200 were 2.8% (0.4–5.3%) and 1.8 ppm (1.14–2.5 ppm), respectively. The mildly elevated levels of methemoglobin promptly dropped after completion or discontinuation of iNO session in all patients (Fig. 3) and always returned to baseline levels before the initiation of the next treatment. No methemoglobin or NO2 levels in any patient rose to exceed cutoff values mandating treatment cessation. One patient reported claustrophobia associated with the use of a snug-fitting mask. The mask was replaced with a mouthpiece, which the patient tolerated well.
Fig. 3.
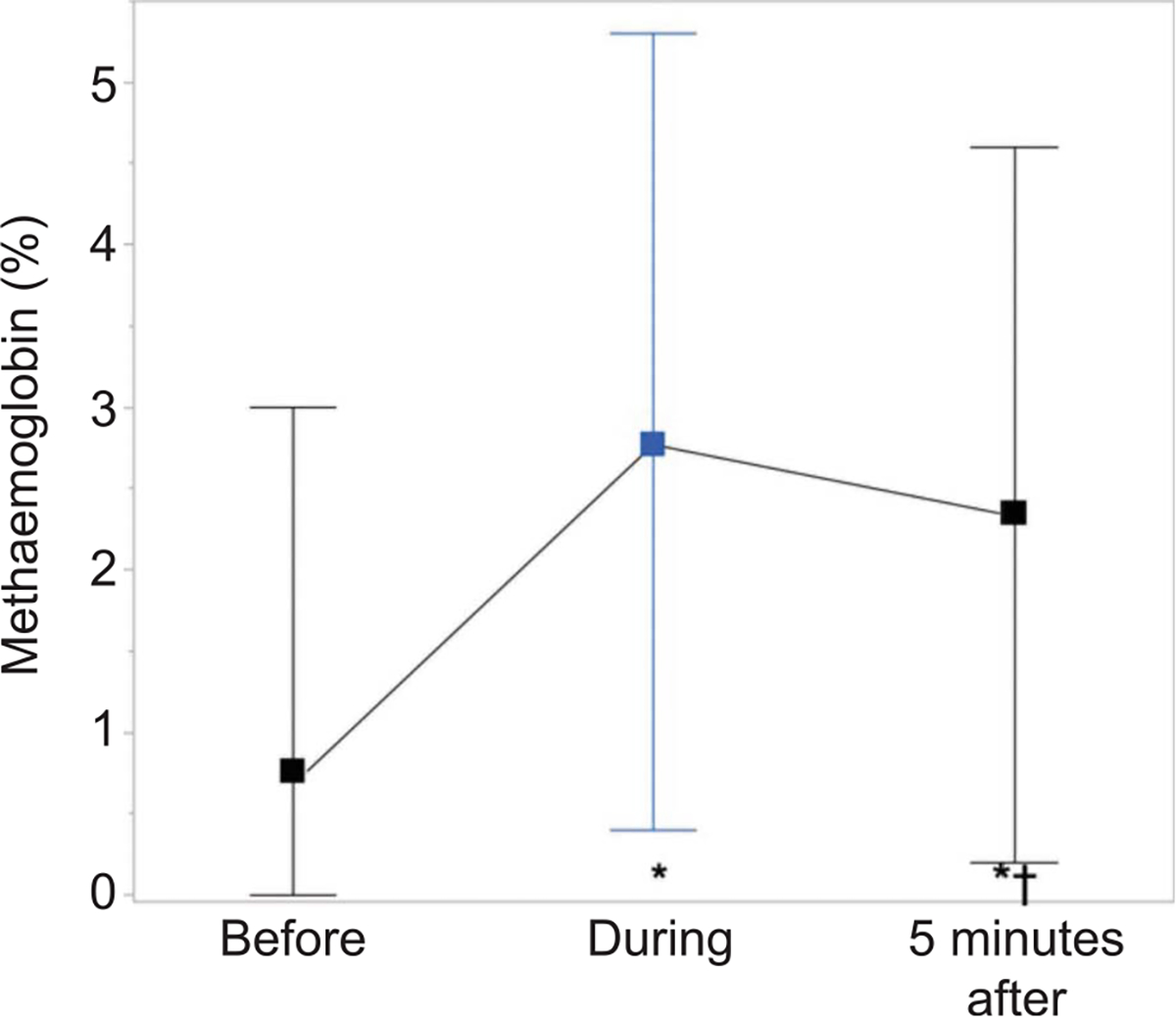
Methemoglobin levels (data are mean, minimum–maximum) during the administration of inhaled nitric oxide (iNO200). Linear mixed-model fit by maximum likelihood (n = 136). *P<.05 in the post hoc analysis (Tuckey’s range test) vs before; †P<.05 vs maximum levels during iNO. For additional statistical methods, see Appendix 2, available online at http://links.lww.com/AOG/C775.
Valsecchi. High-Dose Nitric Oxide in Severe COVID-19. Obstet Gynecol 2022.
All pregnant patients were hemodynamically stable for the entire duration of the treatments (Figs. 2C, D). Heart rate and systemic arterial pressure did not change before, during, or after the treatment. Transthoracic echocardiography was performed in six patients before, during, and after iNO treatment. There was no echocardiographic evidence of rebound pulmonary hypertension or right ventricle dysfunction associated with iNO use (Appendix 7, available online at http://links.lww.com/AOG/C775). The levels of serum creatinine remained unchanged after the start of the iNO treatment, suggesting that breathing iNO200 twice daily did not impair kidney function (Appendix 8, available online at http://links.lww.com/AOG/C775).
DISCUSSION
Pregnant patients are at risk of developing hypoxic respiratory failure as a consequence of pregnancy-associated physiologic, immune, and hormonal changes and increased oxygen needs. At present, there are no targeted respiratory interventions to treat severe bilateral COVID-19 pneumonia in pregnancy other than supportive care with supplemental oxygen and, eventually, mechanical ventilation. We collected data from 71 pregnant patients with severe bilateral COVID-19 pneumonia admitted to four large academic hospitals. We assessed the safety and efficacy of the addition of twice-daily treatment with iNO200 to standard of care alone and found that iNO200 was associated with improved oxygenation and decreased dyspnea. When compared with standard of care alone, iNO200 was associated with a reduction of the duration of oxygen supplementation and hospital and ICU length of stay. This innovative respiratory intervention was feasible in pregnant patients with COVID-19 pneumonia, and no adverse events occurred in mothers or infants.
The use of iNO was initially approved by the U.S. Food and Drug Administration in 1999 as inhalation therapy for the treatment of intubated and mechanically ventilated neonates with hypoxic respiratory failure associated with pulmonary hypertension. To date, the U.S. Food and Drug Administration–approved dosage for iNO is not greater than 20 ppm. However, several clinical trials have been conducted under widely varying dosages (up to 80 ppm).18
The decision to deliver iNO200 for 30 minutes twice daily by a snug-fitting mask to patients with severe COVID-19 pneumonia was based on prior reports showing antimicrobial effects of high-dose nitric oxide.9,10,13,19–24
Thanks to its unique selective pulmonary vasodilatory properties, since its introduction to neonatal practice, iNO has been used to improve oxygenation and has been shown to reduce ECMO use, need for supplemental oxygen, length of hospital stay, rate of bronchopulmonary dysplasia, and, ultimately, mortality in infants.25–27 Due to its role in reducing ventilation-perfusion mismatching, iNO is used as a rescue therapy for hypoxemia in intubated and mechanically ventilated patients with hypoxic respiratory failure, both from COVID-19 and not.8
Maternal hypoxemia is associated with an increased risk of miscarriage and fetal demise; thus, the Society for Maternal-Fetal Medicine COVID-19 task force recommends prompt reversal of hypoxemia and maintenance of an oxygen saturation greater than 95% in pregnant patients.6 In a previous study, iNO was delivered to six nonintubated pregnant patients with severe COVID-19 pneumonia with the intent of increasing oxygenation.12 In the current study, we confirmed that breathing iNO200 improved oxygenation and reduced tachypnea. The improved respiratory function is of particular clinical interest during pregnancy due to increased oxygen demand and the lower oxygen tension within the placental circulation.
Akaberi et al have previously demonstrated that nitric oxide also has an antiviral effect against SARS-CoV-2. The demonstrated mitigation of viral replication, measured as a decrease in viral copy number and by cellular cytopathic effect, was correlated with the reduction of the activity of SARS-CoV-2 3CL cysteine protease by direct S-nitrosation.9 In an in vitro model, nitric oxide reduced the replication of SARS-CoV-1 by interfering with the regular palmitoylation of the spike protein, ultimately hindering the ability of the spike protein to interact with the receptor, and reduced viral RNA production by affecting the cysteine proteases.28 Thus, it is reasonable to speculate that the antiviral activity of nitric oxide may also be effectively exerted on possible variants of the virion proteins involved in viral replication.
To assess the efficacy of this innovative respiratory intervention during pregnancy, we created a network of hospitals to compare the outcomes among patients treated with iNO200 against a similar population treated with standard care alone. The disease severity at hospital admission was comparable between the iNO200 and SoC cohorts. Our findings suggest that iNO200 correlated with faster recovery from respiratory support, including more rapid weaning from oxygen therapy. The improved oxygenation could have resulted in the reduction of the rate of intubation and shorter ICU and hospital stay in the iNO200 cohort. The implications of a reduced disease acuity and length of hospital stay likely include reduced stress for the patient and family and reduced risk of nosocomial infection for the mother and the fetus-neonate. Furthermore, shorter hospitalizations reduce the strain on health systems in the setting of a global pandemic and reduce cost for both the institution and the patient. An additional cost reduction effect was shown in a recent trial among nonintubated patients hospitalized for COVID-19 pneumonia.29 In that study, high-dose iNO also reduced hospital readmission compared with standard of care alone.
From a safety perspective, it is important to note that no adverse event occurred during the 144 iNO200 treatments. The highest recorded value of methemoglobin was 5.3%, which decreased promptly after completion of the treatment. Inhaled nitric oxide delivery requires continuous monitoring of methemoglobin by serial blood tests, more practically, by a noninvasive digital co-oximeter. An additional safety consideration is formation of NO2, an oxidative product of nitric oxide. In combination with water (or water vapor in the alveolar space), NO2 will form nitric acid, a highly corrosive agent. Patients with hypoxemic respiratory failure who are breathing a high concentration of oxygen are at highest risk of nitric acid formation within the lower respiratory tract. Filters to minimize NO2 breathing should be inserted in the nitric oxide delivery system and continuous monitoring of NO2 is of paramount importance.10,11 Rebound pulmonary hypertension has been implicated as a possible adverse effect after continuous and prolonged (multiple days) breathing of nitric oxide, possibly related to inhibition of the endothelial nitric oxide synthetase. In our study, echocardiographic and clinical evaluation did not reveal evidence of rebound pulmonary hypertension, hypotension, or cardiac failure, probably due to the short (30-minute) exposure of iNO200 treatments. Lastly, nephrotoxicity has historically been a concern among patients with acute respiratory distress syndrome treated with nitric oxide. A meta-analysis from four trials involving critically ill, mechanically ventilated patients with septic respiratory failure suggested an increased risk of acute kidney injury associated with iNO therapy.30 The explanation for this remains unknown. In contrast, a nephroprotective effect of nitric oxide treatment was observed in a separate meta-analysis that studied 579 patients who underwent cardiac surgery.31 Notably, none of the patients receiving iNO200 in our study developed evidence of acute kidney injury based on serum creatinine levels. Based on the present findings, we believe that administration of iNO200 for 30 minutes twice daily with close monitoring is a safe respiratory therapy for pregnant patients, as we observed no obstetric or neonatal adverse events. Future studies on dose-limiting toxicity should be performed to establish the highest levels of iNO that can be safely administered to pregnant patients.
There are several limitations of our study. First, the retrospective study design warrants some caution in the interpretation of the results, as residual, unaccounted confounding factors in the adjusted generalized multivariable model may affect the results (eg, unaccounted variables in the baseline status, differences between clinical management among sites and their effect on the study outcomes and association with iNO). Subsequent randomized controlled trials are warranted to test our hypothesis generating results. Second, iNO200 was delivered only in one center, adding possible bias related to the hospital setting. However, 10 of the 51 patients in the SoC cohort were treated at the only institution delivering iNO200. Third, the significance and generalizability of the secondary outcomes may be hindered by the small size of our study cohorts. Fourth, no systematic viral tests (ie, time to negative swab) were performed in the two study cohorts due to supply constraints. Finally, it is possible that the extra time spent at the bedside by the iNO200 delivering clinicians could have improved the patients’ well-being. Of note, the physician delivering iNO was not involved in clinical decisions, in particular with regards to oxygen delivery strategy, therapies, and discharge dispositions.
In conclusion, iNO200 may be considered an effective respiratory therapy to improve oxygenation and decrease respiratory rate in hypoxemic and tachypneic pregnant patients hospitalized with severe bilateral COVID-19 pneumonia. The treatment was associated with a reduced need for oxygen supplementation and a shorter ICU and hospital length of stay. Based on our present findings and the absence of therapeutic trials in pregnant patients with severe pneumonia, randomized controlled trials are warranted to test improved outcomes with iNO200.
Supplementary Material
Acknowledgments
This study was supported by the Reginald Jenney Endowment Chair at Harvard Medical School to Lorenzo Berra, by Lorenzo Berra Sundry Funds at Massachusetts General Hospital, and by laboratory funds of the Anesthesia Center for Critical Care Research of the Department of Anesthesia, Critical Care and Pain Medicine at Massachusetts General Hospital.
This work is dedicated to the recently deceased Prof. Warren M. Zapol, MD, and Prof. Robert M. Kacmarek, PhD. The authors thank Prof. Zapol, Director of the Anesthesia Center for Critical Care Research, who pioneered the work on inhaled nitric oxide more than 30 years ago at the Massachusetts General Hospital in Boston. Prof. Zapol and colleagues presented the first physiological results on inhaled nitric oxide in newborns with persistent pulmonary hypertension in 1992 (Roberts et al. Inhaled nitric oxide in persistent pulmonary hypertension of the newborn. Lancet 1992;239:818–9). Over the past decades, Prof. Zapol taught and discovered groundbreaking therapeutic implications of inhaled nitric oxide and provided intellectual insight during the first phases of the present work. The authors also thank Prof. Kacmarek, Director of the Respiratory Care Department at the Massachusetts General Hospital. Prof. Kacmarek was pivotal in performing the first studies on inhaled nitric oxide, teaching our respiratory care personnel and creating and coordinating the novel nitric oxide delivery device systems in the present work.
Footnotes
Financial Disclosure:
Lorenzo Berra receives salary support from K23 HL128882/NHLBI NIH as principal investigator for his work on hemolysis and nitric oxide. Lorenzo Berra receives technologies and devices from iNO Therapeutics LLC, Praxair Inc., and Masimo Corp. Lorenzo Berra receives grants from iNO Therapeutics LLC. Jamel Ortoleva received a compensation for a one-time meeting for La Jolla Pharmaceutical for angiotensin II. Ai-ris Y. Collier disclosed that money was paid to their institution from the Reproductive Scientist Development Program at the Eunice Kennedy Shriver National Institute of Child Health & Human Development and Burroughs Wellcome Fund HD000849. Carolyn La Vita disclosed receiving payment from the Orange Medical Nihon Kohden for honorariums for lectures to AARC. Jeanne B. Ackman disclosed that money was paid to her from Elsevier for royalties for associate editorship of a textbook and Celgene (a single consultancy fee in 2019). She also disclosed that this article discusses off-label use of nitric oxide to treat hypoxic pregnant patients with COVID-19. Ryan Carroll receives salary support from UNITAID for tuberculosis research in Uganda. The other authors did not report any potential conflicts of interest.
Each author has confirmed compliance with the journal’s requirements for authorship.
A list of DELFiNO Network Collaborators is available in Appendix 1 online at http://links.lww.com/AOG/C775.
REFERENCES
- 1.Zambrano LD, Ellington S, Strid P, Galang RR, Oduyebo T, Tong VT, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–October 3, 2020. Morbidity Mortal Wkly Rep 2020;69:1641–7. doi: 10.15585/mmwr.mm6944e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villar J, Ariff S, Gunier RB, Thiruvengadam R, Rauch S, Kholin A, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection. Jama Pediatr 2021;175:817–26. doi: 10.1001/jamapediatrics.2021.1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kasehagen L, Byers P, Taylor K, Kittle T, Roberts C, Collier C, et al. COVID-19–associated deaths after SARS-CoV-2 infection during pregnancy—Mississippi, March 1, 2020–October 6, 2021. Morbidity Mortal Wkly Rep 2021;70:1646–8. doi: 10.15585/mmwr.mm7047e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeSisto CL, Wallace B, Simeone RM, Polen K, Ko JY, Meaney-Delman D, et al. Risk for stillbirth among women with and without COVID-19 at delivery hospitalization—United States, March 2020–September 2021. Morbidity Mortal Wkly Rep 2021;70:1640–5. doi: 10.15585/mmwr.mm7047e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor MM, Kobeissi L, Kim C, Amin A, Thorson AE, Bellare NB, et al. Inclusion of pregnant women in COVID-19 treatment trials: a review and global call to action. Lancet Glob Heal 2021;9:e366–71. doi: 10.1016/S2214-109X(20)30484-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Society for Maternal-Fetal Medicine. Management considerations for pregnant patients with COVID-19. Accessed February 1, 2022. https://s3.amazonaws.com/cdn.smfm.org/media/2734/SMFM_COVID_Management_of_COVID_pos_preg_patients_2-2-21_(final).pdf
- 7.Ichinose FJDR Jr, Zapol WM. Inhaled nitric oxide. Circulation 2004;109:3106–11. doi: 10.1161/01.CIR.0000134595.80170.62 [DOI] [PubMed] [Google Scholar]
- 8.Kamenshchikov NO, Berra L, Carroll RW. Therapeutic effects of inhaled nitric oxide therapy in COVID-19 patients. Biomed 2022;10:369. doi: 10.3390/biomedicines10020369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akaberi D, Krambrich J, Ling J, Luni C, Hedenstierna G, Järhult JD, et al. Mitigation of the replication of SARS-CoV-2 by nitric oxide in vitro. Redox Biol 2020;37:101734. doi: 10.1016/j.redox.2020.101734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gianni S, Morais CCA, Larson G, Pinciroli R, Carroll R, Yu B, et al. Ideation and assessment of a nitric oxide delivery system for spontaneously breathing subjects. Nitric Oxide 2020;104:29–35. doi: 10.1016/j.niox.2020.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinciroli R, Traeger L, Fischbach A, Gianni S, Morais CCA, Fakhr BS, et al. A novel inhalation mask system to deliver high concentrations of nitric oxide gas in spontaneously breathing subjects. J Vis Exp 2021. May 4. doi: 10.3791/61769 [DOI] [PubMed] [Google Scholar]
- 12.Fakhr BS, Wiegand SB, Pinciroli R, Gianni S, Morais CCA, Ikeda T, et al. High concentrations of nitric oxide inhalation therapy in pregnant patients with severe coronavirus disease 2019 (COVID-19). Obstet Gynecol 2020;136:1109–13. doi: 10.1097/AOG.0000000000004128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gianni S, Fenza RD, Morais CCA, Fakhr BS, Mueller AL, Yu B, et al. High-dose nitric oxide from pressurized cylinders and nitric oxide produced by an electric generator from air. Respir Care 2021;67:201–8. doi: 10.4187/respcare.09308 [DOI] [PubMed] [Google Scholar]
- 14.Bartley BL, Gardner KJ, Spina S, Hurley BP, Campeau D, Berra L, et al. High-dose inhaled nitric oxide as adjunct therapy in cystic fibrosis targeting burkholderia multivorans. Case Rep Pediatr 2020;2020:1536714. doi: 10.1155/2020/1536714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalafat E, Prasad S, Birol P, Tekin AB, Kunt A, Fabrizio CD, et al. An internally validated prediction model for critical COVID-19 infection and intensive care unit admission in symptomatic pregnant women. Am J Obstet Gynecol 2022;226:403.e1–13. doi: 10.1016/j.ajog.2021.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Institutes of Health. COVID-19 treatment guidelines: corticosteroids. Accessed March 21, 2022. https://www.covid-19treatmentguidelines.nih.gov/therapies/immunomodulators/corticosteroids/
- 17.National Institutes of Health. COVID-19 treatment guidelines: remdesivir. Accessed March 21, 2022. https://www.covid19treatmentguidelines.nih.gov/therapies/antiviral-therapy/remdesivir/ [PubMed]
- 18.Stewart DL, Vogel PA, Jarrett B, Potenziano J. Effect of inhaled nitric oxide on oxygen therapy, mechanical ventilation, and hypoxic respiratory failure. Minerva Pediatr 2018;70:51–8. doi: 10.23736/S0026-4946.17.04944-1 [DOI] [PubMed] [Google Scholar]
- 19.Goldbart A, Golan-Tripto I, Pillar G, Livnat-Levanon G, Efrati O, Spiegel R, et al. Inhaled nitric oxide therapy in acute bronchiolitis: a multicenter randomized clinical trial. Sci Rep 2020;10:9605. doi: 10.1038/s41598-020-66433-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiegand SB, Traeger L, Nguyen HK, Rouillard KR, Fischbach A, Zadek F, et al. Antimicrobial effects of nitric oxide in murine models of Klebsiella pneumonia. Redox Biol 2020;39:101826. doi: 10.1016/j.redox.2020.101826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keyaerts E, Vijgen L, Chen L, Maes P, Hedenstierna G, Ranst MV. Inhibition of SARS-coronavirus infection in vitro by S-nitroso-N-acetylpenicillamine, a nitric oxide donor compound. Int J Infect Dis 2004;8:223–6. doi: 10.1016/j.ijid.2004.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L, Liu P, Gao H, Sun B, Chao D, Wang F, et al. Inhalation of nitric oxide in the treatment of severe acute respiratory syndrome: a rescue trial in Beijing. Clin Infect Dis 2004;39:1531–5. doi: 10.1086/425357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tal A, Greenberg D, Av-Gay Y, Golan-Tripto I, Feinstein Y, Ben-Shimol S, et al. Nitric oxide inhalations in bronchiolitis: a pilot, randomized, double-blinded, controlled trial. Pediatr Pulm 2018;53:95–102. doi: 10.1002/ppul.23905 [DOI] [PubMed] [Google Scholar]
- 24.Wiegand SB, Fakhr BS, Carroll RW, Zapol WM, Kacmarek RM, Berra L. Rescue treatment with high-dose gaseous nitric oxide in spontaneously breathing patients with severe coronavirus disease 2019. Crit Care Explor 2020;2:e0277. doi: 10.1097/CCE.0000000000000277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts JD, Fineman JR, Morin FC, Shaul PW, Rimar S, Schreiber MD, et al. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. New Engl J Med 1997;336:605–10. doi: 10.1056/NEJM199702273360902 [DOI] [PubMed] [Google Scholar]
- 26.Ballard RA, Truog WE, Cnaan A, Martin RJ, Ballard PL, Merrill JD, et al. Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. New Engl J Med 2006;355:343–53. doi: 10.1056/NEJMoa06108 [DOI] [PubMed] [Google Scholar]
- 27.Schreiber MD, Gin-Mestan K, Marks JD, Huo D, Lee G, Srisuparp P. Inhaled nitric oxide in premature infants with the respiratory distress syndrome. New Engl J Med 2003;349:2099–107. doi: 10.1056/NEJMoa031154 [DOI] [PubMed] [Google Scholar]
- 28.Åkerström S, Gunalan V, Keng CT, Tan Y-J, Mirazimi A. Dual effect of nitric oxide on SARS-CoV replication: viral RNA production and palmitoylation of the S protein are affected. Virology 2009;395:1–9. doi: 10.1016/j.virol.2009.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fakhr BS, Fenza RD, Gianni S, Wiegand SB, Miyazaki Y, Morais CCA, et al. Inhaled high dose nitric oxide is a safe and effective respiratory treatment in spontaneous breathing hospitalized patients with COVID-19 pneumonia. Nitric Oxide 2021;116:7–13. doi: 10.1016/j.niox.2021.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gebistorf F, Karam O, Wetterslev J, Afshari A. Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults. The Cochrane Database of Systematic Reviews 2016, Issue 6. Art. No.: CD002787. doi: 10.1002/14651858.CD002787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu J, Spina S, Zadek F, Kamenshchikov NO, Bittner EA, Pedemonte J, et al. Effect of nitric oxide on postoperative acute kidney injury in patients who underwent cardiopulmonary bypass: a systematic review and meta-analysis with trial sequential analysis. Ann Intensive Care 2019;9:129. doi: 10.1186/s13613-019-0605-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


