Purpose of review
Three years into the coronavirus disease 2019 (COVID-19) pandemic, data on pediatric COVID-19 from African settings is limited. Understanding the impact of the pandemic in this setting with a high burden of communicable and noncommunicable diseases is critical to implementing effective interventions in public health programs.
Recent findings
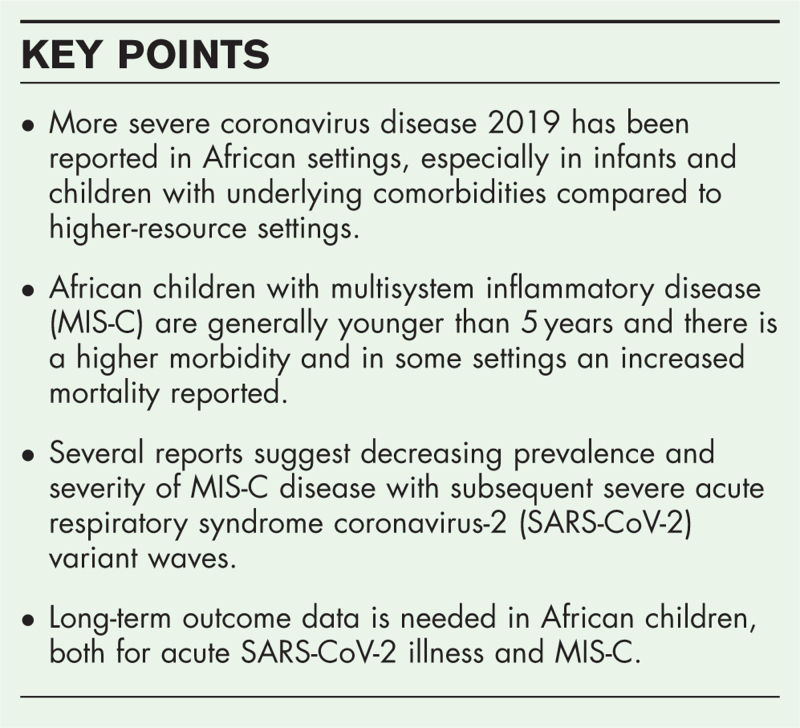
More severe COVID-19 has been reported in African settings, with especially infants and children with underlying comorbidities at highest risk for more severe disease. Data on the role of tuberculosis and HIV remain sparse. Compared to better resourced settings more children with multisystem inflammatory disease (MISC) are younger than 5 years and there is higher morbidity in all settings and increased mortality in some settings. Several reports suggest decreasing prevalence and severity of MIS-C disease with subsequent severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) variant waves. Whether this decrease continues remains to be determined. Thus far, data on long-COVID in African settings is lacking and urgently needed considering the severity of the disease seen in the African population.
Summary
Considering the differences seen in the severity of disease and short-term outcomes, there is an urgent need to establish long-term outcomes in children with COVID-19 and MIS-C in African children, including lung health assessment.
Keywords: Africa, coronavirus disease 2019, pediatrics, severe acute respiratory syndrome coronavirus-2
INTRODUCTION
As of the 1 January 2023, more than 664 million cases and 6.7 million deaths associated with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) are reported globally, of which more than 57 million cases and 43 000 deaths are estimated among children and adolescents aged 0–19 years [1]. Of all World Health Organization (WHO) Regions, Africa contributes 1.4% of all reported confirmed cases, which is the lowest of all regions. There are many speculations about why Africa has been relatively spared, including underreporting, limited testing/ capacity and that children younger than 18 constitute almost 50% of people living in Africa [2,3]. Despite the high proportion of children and adolescents living in Africa and the unprecedented volume of coronavirus disease 2019 (COVID-19) research done over the past three years, data on the epidemiology, clinical features and outcomes of African children and adolescents remain sparse [4]. This review will discuss recent literature on COVID-19 in children and adolescents living in Africa, including multisystem inflammatory syndrome (MIS-C) and long-COVID.
Box 1.
no caption available
ACUTE CORONAVRUS DISEASE 2019
Available literature from the last 3 years consistently shows that children and adolescents are relatively protected from severe COVID-19 disease, with the vast majority having either asymptomatic or mild disease [5,6]. When severe disease does occur in young children and adolescents, case fatality rates generally remain low. However, the majority of this data comes from high-resource settings. A systematic review of global literature on severe pediatric COVID-19 illness by Kitano et al.[7▪▪] report that the overall case fatality rate was significantly higher in low-and middle-income countries (LMICs) (0.24%) than in high-income countries (HICs) (0.01%). Reports from African settings subsequently confirmed this finding [8▪▪]. This may be explained by the fact that coincident infections with tuberculosis, other respiratory viruses, and HIV may synergistically impact outcomes. Although data is limited on the role of tuberculosis and HIV, adult data shows that these are risk factors for more severe COVID-19 disease [9,10,11]. The high prevalence of concurrent communicable diseases, such as tuberculosis and HIV and noncommunicable diseases, including malnutrition, are expected to play a role in the differences.
Most publications on clinical experiences from African settings describe either case series or small cohorts of mostly asymptomatic children found through contact tracing [12–16]. Table 1 summarizes clinical data of four hospital-based studies in Africa, of which three are from Sub-Saharan Africa and one from North Africa (Tunisia) [8▪▪,17▪,18▪,19]. The Tunisian study only includes children admitted to the pediatric intensive care unit (PICU). The first South African study was done during the ancestral wave [18▪], and the second was done during the first Omicron wave [17▪]. The vast majority of children with SARS-CoV-2 illness requiring hospitalization were under five years of age, with a large proportion of children being infants. The two South African studies reported incidental SARS-CoV-2 detection of 16 and 36%. Underlying comorbidities were seen in about half of the children, with 3–4% living with HIV or tuberculosis.
Table 1.
Summary of findings of published clinical hospital cohorts in Africa
| vd Zalm et al.[18▪] | Nachega et al.[8▪▪] | Cloete et al.[17▪] | Borgi et al.[19] | |
| Design | Prospective single-center observational study of SARS-CoV-2 PCR positive children | Multicenter retrospective record study of SARS-CoV-2 PCR positive children | Prospective multicenter observational study of SARS-CoV-2 positive children | Retrospective record study of SARS-CoV-2 PCR positive children in PICU |
| Number included, N | 62 | 469 | 183 COVID positive hospital admissions | 20 |
| Setting | Hospital, single center | Hospital, multiple center | Hospital, multiple center (n = 38) | PICU, single center |
| Country of study | South Africa, Cape Town | 25 healthcare facilities in 6 different Sub-Saharan countriesa | South Africa, Tshwane district | Tunisia |
| Inclusion start date | 17 April 2020 | 1 March 2020 | 31 October 2021 | 23 June 2021 |
| Inclusion end date | 24 July 2020 | 31 December 2020 | 11 December 2021 | 16 August 2021 |
| Period covered, months | 3 | 10 | 1.5 | 2 |
| Age children included | 0–13 years | 0–19 years | 0–13 years detailed clinical data | 0–15 years |
| Age, years • <1 • 1 to 5 • 5–9 • 10 to ≤ 13 |
4.0 (0.2–3.6) 48.4 33.9 17.7c |
5.9 (1.7–11.1) | 4.2 ± 4.1 35% 28% 25% 12% |
47 days (6.5–77) |
| Male, % | 56.5% | 52.4% | 56% | 46% |
| Black African ethnicity | Not reported | >90% | 89% | Not reported |
| SARS-CoV-2 test positive • Incidental • Contributory • Causative |
16.1% Na 82.3% |
Not reported |
36% 20% 44% |
Not applicable |
| Comorbidities TB HIV exposure HIV infection |
46.8% 3.6% 40.6% 3.2% |
Not reported 4.1% Not reported 3.2% |
51% Not specified 5% 4% |
None |
| Clinical symptoms | Not reported | |||
| • Fever | 35.5% | 46% | 95% | |
| • Cough | 35.5% | 40% | 25% | |
| • Diarrhea | 19.4% | 20% | 40% | |
| • Shortness of breath or difficulty breathing | 32.2% | 22% | Not reported | |
| • Seizures | 8.1% | 20% | Not reported | |
| • Skin rash | 9.7% | 3% | Not reported | |
| Laboratory findings | Only reported in COVID illness | Not reported | ||
| • CRP, normal range <10 mg/L | 13.5 (2.0–43.8) | 40.3 (0–502.0) | 12.5 (3.5–54.7) | |
| • White blood cell count, normal range 3.90–10.20 × 109/L | 12.5 (9.5–20.8) | 10.5 (0.1–29.0) | ||
| • Platelets, normal range 180–440 × 109/L | 406 (227–544) | 318.0 (38.0–795.0) | 272 (239–360) | |
| PICU admission (%) | 23.5% | 15.0% | 5% | 100% |
| Ventilated (%) | 9.8% | 7.8% | 5% | 85% |
| Oxygen therapy (%) | 49.0% | 34.6% | 20% | 30% |
| Duration of hospitalization, days | 5.0 (2.0–9.0) | 9.0 (5–16)d | 2 (1–3) | 10 days (5–19)e |
| Mortality, % | 2.0% | 7.9% | 3%b | 20% |
CRP, C-reactive protein; PCR, polymerase chain reaction; PICU, pediatric intensive care unit; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; TB, tuberculosis.
Part of data vd Zalm et al.
Considered due to complex underlying copathology.
For children >5 years (groups combined).
For children recovered (not died).
In PICU.
Fever, respiratory and gastrointestinal symptoms were most common. Seizures were reported in both South African studies but were more frequently reported during the Omicron wave. Laboratory findings were similar between studies and showed an increased C-reactive protein (CRP), high white blood cell count and raised platelets. During the ancestral wave in South Africa, children more often required oxygen therapy, mechanical ventilation and PICU admission. The mortality ranged from 2 to 7.9% in the non-PICU studies. Overall the clinical data from African studies show a high proportion of especially young children being affected by more severe COVID-19 disease, requiring oxygen and respiratory support. In addition, the mortality rates were higher compared to other high-resource settings [7▪▪]. Infants and children with underlying comorbidities are at highest risk for more severe disease and poor outcomes. To date, limited data is available on the role of tuberculosis and HIV on disease severity. Adult studies have shown that both active tuberculosis and a past history of tuberculosis are associated with more severe disease and death [10]. For HIV, results are conflicting; a recent analysis by du Bruyn et al. showed that the immune response to SARS-CoV-2 is adversely affected by co-existent HIV-1 and tuberculosis [9]. More data is needed on the role of tuberculosis and HIV on the severity of the disease in children with COVID-19. Furthermore, considering the high proportion of young children affected who require respiratory support long-term lung health consequences need to be considered.
MULTISYSTEM INFLAMMATORY SYNDROME IN CHILDREN
In April 2020, the first reports of MIS-C emerged in the United Kingdom as a serious pediatric manifestation of SARS-CoV-2 [20]. Since then, there have been multiple reports of MIS-C in other parts of the world; however, despite MIS-C being more common in black children worldwide, there is limited published data on MIS-C in Africa. The incidence of MIS-C is uncertain, as it has been difficult to accurately estimate the number of children exposed to SARS-CoV-2 globally, especially when different testing strategies are used. In general, it is considered a relatively rare complication of COVID-19 in children, occurring in <1 percentage of children with confirmed SARS-CoV-2 infection. Several reports suggest decreasing prevalence and severity of MIS-C with subsequent SARS-CoV-2 variant waves likely explained by increasing seroprevalence and vaccination [21▪▪,22].
The case definitions used by the United States Centers for Disease Control and Prevention (CDC) and the WHO vary slightly but the CDC case definition requires the child to have severe symptoms requiring hospitalization [23]. Both case definitions include fever, elevated inflammatory markers, at least two signs of multisystem involvement, evidence of SARS-CoV-2 infection or exposure, and exclusion of other potential causes. These case definitions are likely to change as high seroprevalence of SARS-CoV-2 will not allow for differentiation between children.
Five cohort studies describe 251 children with MIS-C from four countries, South Africa, Nigeria, Kenya and Egypt, with most data coming from South Africa (Table 2) [21▪▪,24▪▪–27▪▪]. The median age of children across the studies was 4.0–7.5 years as opposed to 9 years in the USA [28]. In the USA, 24.5% of children were <5 years old age. Two cohorts reported the number of children <5 years, 59% and 39.3% in Durban and Nigerian, respectively. A decline in the age of children diagnosed with MIS-C was seen over time, with the youngest children presenting during the Omicron wave. A male majority was noted in four of the studies. The clinical presentation of MIS-C was generally similar in African children compared to other reports with rash, abdominal pain, conjunctivitis, tachycardia and hypotension being common. Altered levels of consciousness were reported in 34% of children from Durban, South Africa and coma in 44.4% of Egyptian children, which was associated with shock.
Table 2.
Summary of findings of published MIS-C cohorts in Africa
| South Africa, Cape Town [21▪▪] | South Africa, Durban [24▪▪] | Nigeria [25▪▪] | Kenya [26▪▪] | Egypt [27▪▪] | |
| Number included, N | 129 | 29 | 28 | 20 | 45 |
| Inclusion start date | 1 May 2020 | 1 June 2020 | 10 July 2020 | 1 August 2020 | February 2021 |
| Inclusion end date | 31 March 2022 | 30 April 2021 | 30 July 2021 | 31 August 2021 | 31 January 2022 |
| Period covered, months | 22 | 10 | 12 | 12 | 11 |
| Age children included | 0–13 years | 0–13 years | 0–13 years | 0–18 years | 0–16 years |
| Age, years | 6.85 (3.39–9.28) | 4.6 ± 3.8 [1] | 7.5 (2.3–9.4) | 4.0 (1.7–7.4) | 4.0 (1.3–10.0) |
| Male, % | 53.5% | 45.0% | 64.5% | 70.0% | 53.3% |
| Black African ethnicity | 51.2% | 86% | Not explicitly reported | 85.5% | Not explicitly reported |
| SARS-CoV-2 PCR positive (%) | 13.3% | 55% | 39.3% | 20% (1/5 tested) | 62.2% |
| Comorbidities | 21.5% | 28% | Not reported | 25% | 0% |
| Clinical symptoms | |||||
| • Fever | 100% | 100% | 100% | 90% | 100% |
| • Mucocutaneus | 83.7% | 65% | 89.3% | 65.0% | 17.8% |
| • Gastrointestinal | 59.7% | 59% | 75% | Not reported | 31.1% |
| • Respiratory | Not reported | 41% | 60.7% | Not reported | 64.4% |
| • Neurological | 28.7% | 34% | 32.1% | Not reported | 48.9% |
| • Renal | 29.5% | 41% | 32.1% | 15.0% | 11.1% |
| ECHO performed | 100% | 69% | 100% | 30% | 100% |
| ECHO abnormal | 70.9% | 50% | 46.4% | 85% | 37.7% |
| • Coronary artery abnormality | 7.8% | 0 | 46.4% | 33.3% | 2.2% |
| • Ventricle dysfunction | Median ejection fraction 59% (IQR, 47–65)a | Not reported | 39.3% | 66.7% | 33.3%, not specified |
| Laboratory findings | |||||
| • CRP, normal range <10 mg/l | 213 (127–302) | 181 (106–233) | 142 (87.8) | 68.2 (19.9–143.0) | 45 (15–93) |
| • Ferritin, normal range 7–84 ng/l | 552 (287- 1020) | 631 (226–1593) | Only 2 patients tested | Not reported | 500 (327–599) |
| • White blood cell count, normal range 3.90–10.20 × 109/l | 16.5 (12.0–23.5) | 12 (9.1–21.3) | 25.2 (5.9–55.0) | 9.8 (8.1–13.2) | 73 (67–77) |
| • Neutrophil count, normal range 1.7–5.0 × 109/l | 11.7 (7.4–17.9) | Not reported | Not reported | 5.3 (3.9–8.4) | 6.7 (4.2–9.1) |
| • Neutrophil ratio | Not reported | Not reported | 77.5% neutrophils | Not reported | Not reported |
| • Lymphocytes, normal range 1.90–4.30 × 109/l | 1.3 (0.8–2.6) | 1.4 (1.1–2.1) | 25.2 (range 5.9–55.0) | 3.3 (1.8–518) | 1.8 (1.3–2.7) |
| • Platelets, normal range 180–440 × 109/l | 193 (144. 296) | 133 (78–312) | 581 (range of 218–1200) | 325.5 (236.0–426.0) | 230 (141–413) |
| • Hemoglobin, normal range 11.2–14.5 g/dl (6–12 years) | Not reported | Not reported | Not reported | 11.5 (9.6–12.2) | 10.3 ± 1.8 [1] |
| • Anemia as per national reference ranges | Not reported | Not reported | 17/28 (60.7%) HB <10 g/dl | Not reported | Not reported |
| Treated with IVIG (%) | Not reported | 72.0% | 60.7% | 10.0% | 100% |
| Ventilated (%) | Not reported | 34.0% | 3.5% | 5.0% | 51.1% |
| Inotrope support (%) | 33.3% | 52.0% | 17.8% | 5.0% | 57.8% |
| Duration of hospitalization, days | 7 (6–10) | 11 (7–19) | 11.1 ± 5.7 [1] | Not reported | 18 (11–23) |
| Mortality, % | 0.8% | 20.6% | 0 | 0 | 33.3% |
Data are presented as median values with interquartile range (IQR), or proportions with percentage, unless stated otherwise in the Table [mean with standard deviation (SD)].
CRP, C-reactive protein; IVIG, intravenous immunoglobulins; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2.
Where multiple symptoms and features in the same system was mentioned the most prevalent is referenced.
Similarly, CRP, ferritin, hemoglobin, neutrophil count, cardiac markers and coagulation markers are markedly deranged in African children with MIS-C.
Cardiac abnormalities, particularly ventricular dysfunction, were common, but echocardiography was not performed in all children. Hospital stay was longer than the reported five days from the USA, with the shortest median stay from Cape Town (7 days) and the longest, 18 days from Egypt. Children from Durban and Egypt had high mortality, 20.6 and 33.3%, respectively. In both studies, children that died had higher serum ferritin, and liver enzymes were higher in Egyptian children. Data from high-resource countries have estimated mortality of 2% and overall good long-term outcomes [29,30].
No prospective treatment data is available for MIS-C, and it is unclear whether intravenous immune globulins (IVIGs) work better alone or in conjunction with glucocorticoids. In addition, IVIG is expensive, and availability is limited in many African regions. Therapy in African studies did not always include IVIGs, with remarkably low use in the report from Kenya [26▪▪]. The best available treatment study did not show the superiority of IVIGs alone compared to glucocorticoids alone [31]. Prospective randomized treatment studies designed for LMICs are urgently needed. The Nigerian study showed normal echocardiogram and electrocardiogram findings in all patients assessed at six months follow-up; however, only 28.5% of children were assessed [25▪▪]. None of the other African studies reported long-term outcomes.
LONG-CORONAVIRUS DISEASE IN CHILDREN
Although during the first part of the pandemic, the attention of public authorities and scientific committees was focused on the acute phase of SARS-CoV-2 infection. In the last year, after the massive vaccination campaign and the emergence of clinically milder variants, the evaluation and characterization of the emerging long-COVID condition have become more critical. Long-COVID is a heterogeneous multisystemic condition with signs and symptoms that persist, develop, or fluctuate after SARS-CoV-2 infection [32,33▪▪]. In October 2021, the WHO proposed a clinical definition for post-COVID-19 through a Delphi consensus that requires symptoms lasting at least two months, cannot be explained by an alternative diagnosis, affects everyday functions, and occurs three months from the onset of COVID-19 [34]. Differently the National Institutes of Health (NIH) also define long-COVID as postacute symptoms with a different time frame of four weeks [35]. In February 2022, the National Institute for Health and Care Excellence (NICE) published a guideline defining long-COVID as signs and symptoms that continue or develop after acute COVID-19—dividing by time in ongoing symptomatic (from 4 to 12 weeks) and post-COVID-19 syndrome (12 weeks or more) [36]. The definition encompasses a broad range of symptoms, including anatomical complications of COVID-19 (pulmonary fibrosis, myocardial dysfunction), mental health conditions, and more subjective, nonspecific symptoms such as postviral chronic fatigue syndrome [37].
To our knowledge, no reports have been published on long-COVID in African countries. Most published research on long-COVID comes from adult literature, with limited data on pediatric populations. Estimates of the prevalence of long-COVID vary, partly because these definitions encompass a broad range of symptoms, including anatomical complications of COVID-19 (pulmonary fibrosis, myocardial dysfunction), mental health conditions, and more subjective, nonspecific symptoms such as postviral chronic fatigue syndrome. The importance of lower respiratory tract infections on child lung health is increasingly acknowledged, but little is still known about the impact of early-in-life SARS-CoV-2 infection on long-term lung health in children, especially in children with other underlying illnesses affecting the lungs.
In a recent retrospective cohort study of electronic health records of 659 286 children <21 years of age (59 893 with a SARS-CoV-2 infection), the incidence of ≥1 symptom or syndrome/condition or use of medication between COVID- positive and COVID- negative groups was 3.7% [95% confidence interval (CI) 3.2–4.2], suggesting a minor difference between postviral illness in children that had COVID-19 and other respiratory infections [38▪▪,39▪]. These findings underline the importance of appropriate control groups (including children with other infections) and, simultaneously, the challenge of distinguishing persistent symptoms due to the pandemic restrictions or COVID-19.
Whether symptoms reported as part of long-COVID are specific to SARS-CoV-2, potentially part of postviral illnesses or are related to the pandemic is unclear. Therefore, the CDC analyzed an extensive medical claims database assessing nine potential post-COVID signs and symptoms and 15 potential post-COVID conditions among 781 419 United States children and adolescents <18 years of age with documented COVID-19 compared with 2 344 257 children without the infection. Symptoms and conditions reported slightly more likely in the COVID-19 group were rare or uncommon such as acute pulmonary embolism, myocarditis and cardiomyopathy, venous thromboembolic event, acute and unspecified renal failure, and type 1 diabetes [40▪▪]. Conversely, symptoms and conditions that were less likely in children with COVID-19 included respiratory signs and symptoms, mental health symptoms and conditions, neurological conditions, muscle disorders, and sleeping disorders. Furthermore, a large retrospective cohort study that evaluated neurologic and psychiatric trajectories in COVID-19 patients of any age-matched patients affected by other respiratory infections showed that the increased incidence of mood and anxiety disorders in COVID-19 patients (adults and children) was transient, with no difference with the group of non-COVID respiratory illnesses. However, in the six months after the SARS-CoV-2 infection, children had an increased risk of cognitive deficit, insomnia, intracranial hemorrhage, ischemic stroke, nerve, nerve root, and plexus disorders, psychotic disorders, and seizures compared with children any other respiratory infection [41▪▪]. Taken together, the data suggest that long-COVID and long-non-COVID illness is similar with different clinical features compared to adults. However, even if rare, the possible consequences can profoundly impact children's quality of life.
Understanding the predisposing factors for this condition represents another critical point. The main risk factors associated with long-COVID in the pediatric population have been identified include older age, female gender, comorbidities (including mostly body mass index ≥85th percentile for age and sex and allergic diseases), the number of symptoms at presentation and organs involved [42▪▪,43▪▪]. However, further research is needed to determine the prevalence and identify risk factors for long-COVID in African children and adolescents.
The pathogenesis of long-COVID is unknown. So far, different mechanisms have been proposed, including SARS-CoV-2's direct and indirect effects. The hypotheses of the virus's direct effect include a possible viral latency, persistent immune system activation and inflammation with microvascular or endothelial damage [44–46]. As suggested by Lopez-Leon et al.[47▪], since many of the most common long-COVID symptoms are also associated with dysautonomia, it has been hypothesized that these direct infection effects could lead to the dysfunction of the sympathetic and/or parasympathetic autonomic nervous system. The hypothesis for the indirect effects of the pandemic is linked to posttraumatic stress and social isolation [33▪▪]. Clarifying this point will help to understand the difference between long-COVID and other postviral syndromes and will allow for developing targeted intervention, prevention and management guidelines. Understanding how pediatric vaccinations and the emergence of new variants will influence the prevalence and the burden of post-COVID sequelae is pivotal. Recent studies in adults suggest that vaccines are associated with reducing the risk of long-COVID in adults [48]. However, only a few studies on children reported the percentage of vaccinations [47▪]. Since COVID-19 remains predominantly an asymptomatic or mild infection in the pediatric population understanding the incidence and burden of sequelae is essential to balance risks and benefits for decisions on COVID-19 vaccines for children, especially in more vulnerable groups [37,49▪,50]. This is especially important in Africa as vaccine uptake is low, and not all vaccines have been approved for use in young children by African regulating authorities.
CONCLUSION
Overall, the presentation and outcomes of acute SARS-CoV-2 and MIS-C differ in African children, with higher morbidity and mortality rates than in other high-resource settings. In general, access to diagnostic tests, specific drugs, and intensive care is limited in most African countries due to high costs and the availability of resources. This, in combination with a high prevalence of underlying comorbidities and concurrent endemic infections, might explain worse outcomes. Considering the differences seen in the severity of the disease and short-term outcomes, there is an urgent need to establish long-term outcomes in children with COVID-19 and MIS-C in African children, including lung health assessment.
Despite the increasing literature about long-COVID in children, there is still a significant gap in knowledge in Africa, which accounts for a large proportion of pediatric COVID-19 cases globally. More resources and funding are needed in Africa and other LMICs, to fill this important gap. The limited healthcare system resources, high prevalence of infectious diseases, and malnutrition limit the application of interventions already developed in HICs [51]. Future research perspectives on long-term outcomes must consider a standardized approach, scalable in different socio-economic settings and populations, to compare the effect of these sequelae in different populations and the outcomes of different interventions.
Acknowledgements
The authors would like to thank Professor Mark Cotton for his support.
Financial support and sponsorship
This work is part of the VERDI project (101045989), which is funded by the European Union. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or the European Health and Digital Executive Agency. Neither the European Union nor the granting authority can be held responsible for them.
M.V.D.Z. was supported by a career development grant from the EDCTP2 program supported by the European Union (grant number TMA2019SFP-2836 TB- Lung FACT2), by the Fogarty International Center of the National Institutes of Health under Award Number K43TW011028 and funding from a researcher-initiated grant from the South African Medical Research Council.
Funding received by NIH, EDCTP, Horizon Europe.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1. World Health Organization (WHO). WHO coronavirus (COVID-19) dashboard. Available at: https://covid19.who.int/ [cited 2023 Jan 24]. [Google Scholar]
- 2.Nguimkeu P, Tadadjeu S. Why is the number of COVID-19 cases lower than expected in Sub-Saharan Africa? A cross-sectional analysis of the role of demographic and geographic factors. World Dev 2021; 138:105251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobia F, Gitaka J. COVID-19: are Africa's diagnostic challenges blunting response effectiveness? AAS Open Res 2020; 3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sam-Agudu NA, Rabie H, Pipo MT, et al. The critical need for pooled data on COVID-19 in African children: an AFREhealth call for action through multi-country research collaboration. Clin Infect Dis 2021; 73:1913–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Götzinger F, Santiago-García B, Noguera-Julián A, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Heal 2020; 4:653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui X, Zhao Z, Zhang T, et al. A systematic review and meta-analysis of children with coronavirus disease 2019 (COVID-19). J Med Virol 2021; 93:1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7▪▪.Kitano T, Kitano M, Krueger C, et al. The differential impact of pediatric COVID-19 between high-income countries and low- and middle-income countries: a systematic review of fatality and ICU admission in children worldwide. PLoS One 2021; 16:e0246326. [DOI] [PMC free article] [PubMed] [Google Scholar]; This global systematic review includes data of 216 countries. Among the 3,788 global pediatric COVID-19 deaths, 91.5% of deaths were reported from low- and middle-income countries (LMIC), while 83.5% of pediatric population from all included countries were from LMIC. The overall case fatality rate was significantly higher in LMICs (0.24%) than in high-income countries (0.01%).
- 8▪▪.Nachega JB, Sam-Agudu NA, Machekano RN, et al. Assessment of clinical outcomes among children and adolescents hospitalized with COVID-19 in 6 sub-Saharan African countries. JAMA Pediatr 2022; 176:e216436. [DOI] [PMC free article] [PubMed] [Google Scholar]; This multicentre retrospective cohort study included data from 25 hospitals in the Democratic Republic of the Congo, Ghana, Kenya, Nigeria, South Africa, and Uganda from March 1 to December 31, 2020, and included 469 hospitalized patients aged 0 to 19 years with SARS-CoV-2 infection. The study showed that in children and adolescents hospitalized with COVID-19 in sub-Saharan Africa, high rates of morbidity and mortality were observed among especially infants and patients with noncommunicable disease comorbidities.
- 9.du Bruyn E, Stek C, Daroowala R, et al. Effects of tuberculosis and/or HIV-1 infection on COVID-19 presentation and immune response in Africa. Nat Commun 2023; 14:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boulle A, Davies M-A, Hussey H, et al. Risk factors for COVID-19 death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis 2021; 73: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertagnolio S, Thwin SS, Silva R, et al. Clinical features of, and risk factors for, severe or fatal COVID-19 among people living with HIV admitted to hospital: analysis of data from the WHO Global Clinical Platform of COVID-19. Lancet HIV 2022; 9:e486–e495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Kassas M, Asem N, Abdelazeem A, et al. Clinical features and laboratory characteristics of patients hospitalized with COVID-19: single centre report from Egypt. J Infect Dev Ctries 2020; 14:1352–1360. [DOI] [PubMed] [Google Scholar]
- 13.Isabelle M, David C, Eugene E, et al. Pediatric COVID-19 in sub-Saharan Africa: a series of cases report in Cameroon. Open J Pediatr 2020; 10:363–368. [Google Scholar]
- 14.Fakiri KEl, Nassih H, Sab IA, et al. Epidemiology and clinical features of coronavirus disease 2019 in Moroccan children. Indian Pediatr 2020; 57:808–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ibrahim OR, Suleiman BM, Sanda A, et al. COVID-19 in children: a case series from Nigeria. Pan Afr Med J 2020; 35: (Suppl 2): 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adedeji IA, Abdu YM, Bashir MF, et al. Profile of children with COVID-19 infection: a cross sectional study from North-East Nigeria. Pan Afr Med J 2020; 35: (Suppl 2): 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17▪.Cloete J, Kruger A, Masha M, et al. Paediatric hospitalisations due to COVID-19 during the first SARS-CoV-2 omicron (B.1.1.529) variant wave in South Africa: a multicentre observational study. Lancet Child Adolesc Heal 2022; 6:294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]; This multicentre observational study reported the clinical manifestations and outcomes of paediatric patients (aged ≤19 years) who had tested positive for SARS-CoV-2 and were admitted to hospital for any reason during the first Omicron wave in South Africa.
- 18▪.van der Zalm MM, Lishman J, Verhagen LM, et al. Clinical experience with severe acute respiratory syndrome coronavirus 2-related illness in children: hospital experience in Cape Town, South Africa. Clin Infect Dis 2021; 72:e938–e944. [DOI] [PMC free article] [PubMed] [Google Scholar]; This single-centre observational study presented the clinical presentation and outcomes of children hospitalized with SARS-CoV-2 infection in South Africa during the Ancestral wave. This study showed more severe COVID-19 respiratory illness in especially in infants and children with underlying comorbidities. This data is part of reference [8▪▪] Nachega et al..
- 19.Borgi A, Louati A, Miraoui A, et al. Critically ill infants with SARS-COV-2 delta variant infection. Pediatr Neonatol 2022; [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 2020; 324:259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21▪▪.Abraham DR, Butters C, Abdulbari Yunis N, et al. The impact of SARS-CoV-2 variants on the clinical phenotype and severity of multisystem inflammatory syndrome in children in South Africa. Pediatr Infect Dis J 2022; 41:e510–e512. [DOI] [PMC free article] [PubMed] [Google Scholar]; This report from South Africa describes the largest group of African children with MIS-C. The authors report that SARS-CoV-2 variance had no effect on the clinical presentation or outcomes of children with MIS-C. The description of these children is mostly similar to those in high resource settings, and nearly all children responded to IVIG and or steriods.
- 22.Levy N, Koppel JH, Kaplan O, et al. Severity and incidence of multisystem inflammatory syndrome in children during 3 SARS-CoV-2 pandemic waves in Israel. JAMA 2022; 327:2452–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organization (WHO). Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19; Scientific Brief. May 2020. Available at: https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19. [Google Scholar]
- 24▪▪.Chinniah K, Bhimma R, Naidoo KL, et al. Multisystem inflammatory syndrome in children associated with SARS-CoV-2 infection in KwaZulu-Natal, South Africa. Pediatr Infect Dis J 2023; 42:e9–e14. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this report of 29 children from KwaZulu Natal in South Africa the age of patient was mean age was only 55 months. Markedly elevated biomarkers and critical organ involvement were associated with severe disease. Mortally was high (20.6%) and risk factors for poor outcomes include higher ferritin levels and the need for mechanical ventilation.
- 25▪▪.Sokunbi O, Akinbolagbe Y, Akintan P, et al. Clinical presentation and short-term outcomes of multisystemic inflammatory syndrome in children in Lagos, Nigeria during the COVID-19 pandemic: a case series. EClinicalMedicine 2022; 49:101475. [DOI] [PMC free article] [PubMed] [Google Scholar]; This report from Nigeria describes 28 children and adolescents and is the only cohort with outcomes after discharge at 3 months and 6 months. Coronary artery dilatation was seen in 46.4% of children and 16,0% had a first degree heart block. All children survived, though 7.1% of children still had coronary artery abnormalities at 3 months. All abnormalities resolved by 6 months, although long-term data was only available of a small group.
- 26▪▪.Migowa A, Samia P, Del Rossi S, et al. Management of multisystem inflammatory syndrome in children (MIS-C) in resource limited settings: the Kenyan experience. Pediatr Rheumatol Online J 2022; 20:110. [DOI] [PMC free article] [PubMed] [Google Scholar]; This report form Kenya describes 20 children with 100% survival despite only 2 receiving IVIG and 3 children receiving systemic steroids.
- 27▪▪.Abdelaziz TA, Abdulrahman DA, Baz EG, et al. Clinical and laboratory characteristics of multisystem inflammatory syndrome in children associated with COVID-19 in Egypt: a tertiary care hospital experience. J Paediatr Child Health 2022; [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]; This report of 45 MIS-C cases is the only cohort from North Africa. This cohort reported the highest mortality with 33.3%. Children where young and almost half of them presented with coma. Seizures and stroke were common in children that died.
- 28.Melgar M, Lee EH, Miller AD, et al. Council of state and territorial epidemiologists/CDC surveillance case definition for multisystem inflammatory syndrome in children associated with SARS-CoV-2 infection - United States. Morb Mortal Wkly Rep 2022; 71:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fremed MA, Farooqi KM. Longitudinal outcomes and monitoring of patients with multisystem inflammatory syndrome in children. Front Pediatr 2022; 10:820229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmed M, Advani S, Moreira A, et al. Multisystem inflammatory syndrome in children: a systematic review. EClinicalMedicine 2020; 26:100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McArdle AJ, Vito O, Patel H, et al. Treatment of multisystem inflammatory syndrome in children. N Engl J Med 2021; 385:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iqbal FM, Lam K, Sounderajah V, et al. Characteristics and predictors of acute and chronic post-COVID syndrome: a systematic review and meta-analysis. EClinicalMedicine 2021; 36:100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33▪▪.Groff D, Sun A, Ssentongo AE, et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw open 2021; 4:e2128568. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this systematic review of 57 studies comprising more than 250 000 survivors of COVID-19, most sequelae included mental health, pulmonary, and neurologic disorders, which were prevalent longer than six months after SARS-CoV-2 exposure.
- 34.Soriano JB, Murthy S, Marshall JC, et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis 2022; 22:e102–e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nalbandian A, Sehgal K, Gupta A, et al. Postacute COVID-19 syndrome. Nat Med 2021; 27:601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Clements W, Joseph T, Koukounaras J. UK NICE guidelines for EVAR: cost implications for post-COVID Australian Public Health. Vol. 44, Cardiovascular and Interventional Radiology. United States; 2021. p. 1286–8. [DOI] [PubMed] [Google Scholar]
- 37.Zimmermann P, Pittet LF, Curtis N. How common is long COVID in children and adolescents? Pediatr Infect Dis J 2021; 40:e482–e487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38▪▪.Rao S, Lee GM, Razzaghi H, et al. Clinical features and burden of postacute sequelae of SARS-CoV-2 infection in children and adolescents. JAMA Pediatr 2022; 176:1000–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a large retrospective cohort study comparing 59 893 children with a SARS-CoV-2 infection with Children with 599 393 tested negative to identify diagnosed symptoms, diagnosed health conditions, and medications.
- 39▪.Radtke T, Ulyte A, Puhan MA, Kriemler S. Long-term symptoms after SARS-CoV-2 infection in children and adolescents. JAMA 2021; 326:869–871. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a longitudinal cohort study investigating SARS-CoV-2 seroprevalence in 55 randomly selected schools in the canton of Zurich in Switzerland.
- 40▪▪.Kompaniyets L, Bull-Otterson L, Boehmer TK, et al. Post-COVID-19 symptoms and conditions among children and adolescents – United States, March 1, 2000–January 31. MMWR Morb Mortal Wkly Rep 2022; 71:993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]; Study on an extensive medical claims database, CDC assessed nine potential post-COVID signs and symptoms (symptoms) and 15 potential post-COVID conditions among 781,419 U.S. children and adolescents aged 0–17 years with laboratory-confirmed COVID-19 (patients with COVID-19) compared with 2 344 257 US children and adolescents without recognized COVID-19 (patients without COVID-19).
- 41▪▪.Taquet M, Sillett R, Zhu L, et al. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients. lancet Psychiatry 2022; 9:815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this 2-year retrospective cohort study, a cohort of patients of any age with COVID-19 diagnosed was identified and propensity-score matched (1:1) to a contemporaneous cohort of patients with any other respiratory infection. Matching was done on the basis of demographic factors, risk factors for COVID-19 and severe COVID-19 illness, and vaccination status. Analyses were stratified by age group and date of diagnosis. The authors assessed the risks of 14 neurological and psychiatric diagnoses after SARS-CoV-2 infection and compared these risks with the matched comparator cohort.
- 42▪▪.Maddux AB, Berbert L, Young CC, et al. Health impairments in children and adolescents after hospitalization for acute COVID-19 or MIS-C. Pediatrics 2022; 150:e2022057798. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a large prospective multicenter prospective cohort study of children hospitalized with acute COVID-19 and MIS-C in 25 US pediatric hospitals.
- 43▪▪.Funk AL, Kuppermann N, Florin TA, et al. Post-COVID-19 conditions among children 90 days after SARS-CoV-2 infection. JAMA Netw open 2022; 5:e2223253. [DOI] [PMC free article] [PubMed] [Google Scholar]; To estimate the proportion of SARS-CoV-2-positive children with long-COVID, this prospective large cohort study was conducted in 36 emergency departments in 8 countries, including1884 SARS-CoV-2-positive children who completed 90-day follow-up; 1686 of these children were matched by hospitalization status, country, and recruitment date with 1701 SARS-CoV-2-negative controls.
- 44.Zollner A, Koch R, Jukic A, et al. Postacute COVID-19 is characterized by gut viral antigen persistence in inflammatory bowel diseases. Gastroenterology 2022; 163:495–506. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Buonsenso D, Di Giuda D, Sigfrid L, et al. Evidence of lung perfusion defects and ongoing inflammation in an adolescent with postacute sequelae of SARS-CoV-2 infection. Vol. 5. The Lancet. Child & adolescent health. England; 2021. p. 677–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phetsouphanh C, Darley DR, Wilson DB, et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol 2022; 23:210–216. [DOI] [PubMed] [Google Scholar]
- 47▪.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep 2021; 11:16144. [DOI] [PMC free article] [PubMed] [Google Scholar]; This systematic review and meta-analysis evaluated 55 long-term effects of COVID-19 through 21 meta-analyses and 47,910 included patients (age 17–87 years).
- 48.Kuodi P, Gorelik Y, Zayyad H, et al. Association between BNT162b2 vaccination and reported incidence of post-COVID-19 symptoms: cross-sectional study 2020-21, Israel. NPJ Vaccines 2022; 7:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49▪.Borch L, Holm M, Knudsen M, et al. Long COVID symptoms and duration in SARS-CoV-2 positive children – a nationwide cohort study. Eur J Pediatr 2022; 181:1597–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a nationwide cohort study of 37 522 children aged with COVID-19 (response rate 44.9%) and a control group of 78 037 children (response rate 21.3%) evaluating symptoms and duration of ’long COVID’ in children.
- 50.Kikkenborg Berg S, Dam Nielsen S, Nygaard U, et al. Long COVID symptoms in SARS-CoV-2-positive adolescents and matched controls (LongCOVIDKidsDK): a national, cross-sectional study. Lancet Child Adolesc Heal 2022; 6:240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Erinoso O. Post-COVID-19 condition: current evidence and unanswered questions. Lancet Glob Heal 2022; 10:e1210–e1211. [DOI] [PMC free article] [PubMed] [Google Scholar]