Background:
Community health centers (CHCs) pivoted to using telehealth to deliver chronic care during the coronavirus COVID-19 pandemic. While care continuity can improve care quality and patients’ experiences, it is unclear whether telehealth supported this relationship.
Objective:
We examine the association of care continuity with diabetes and hypertension care quality in CHCs before and during COVID-19 and the mediating effect of telehealth.
Research Design:
This was a cohort study.
Participants:
Electronic health record data from 166 CHCs with n=20,792 patients with diabetes and/or hypertension with ≥2 encounters/year during 2019 and 2020.
Methods:
Multivariable logistic regression models estimated the association of care continuity (Modified Modified Continuity Index; MMCI) with telehealth use and care processes. Generalized linear regression models estimated the association of MMCI and intermediate outcomes. Formal mediation analyses assessed whether telehealth mediated the association of MMCI with A1c testing during 2020.
Results:
MMCI [2019: odds ratio (OR)=1.98, marginal effect=0.69, z=165.50, P<0.001; 2020: OR=1.50, marginal effect=0.63, z=147.73, P<0.001] and telehealth use (2019: OR=1.50, marginal effect=0.85, z=122.87, P<0.001; 2020: OR=10.00, marginal effect=0.90, z=155.57, P<0.001) were associated with higher odds of A1c testing. MMCI was associated with lower systolic (β=−2.90, P<0.001) and diastolic blood pressure (β=−1.44, P<0.001) in 2020, and lower A1c values (2019: β=−0.57, P=0.007; 2020: β=−0.45, P=0.008) in both years. In 2020, telehealth use mediated 38.7% of the relationship between MMCI and A1c testing.
Conclusions:
Higher care continuity is associated with telehealth use and A1c testing, and lower A1c and blood pressure. Telehealth use mediates the association of care continuity and A1c testing. Care continuity may facilitate telehealth use and resilient performance on process measures.
Key Words: care continuity, telehealth, community health centers, diabetes, hypertension
The COVID-19 pandemic led to a rapid uptake in telehealth use beginning in March 20201–3 to provide safer care to patients and reduce their exposure to the virus. While telehealth was lauded as a flexible and safe means of maintaining health care access for patients during the pandemic, care continuity’s effect on telehealth use and telehealth’s impact on quality of care is not well understood. Vulnerable populations, such as low-income patients, minoritized patients, and patients with complex care needs, patient populations often served by community health centers (CHCs), may not have experienced the same improvements as other more advantaged populations.4 Care continuity has previously been associated with improved quality of care and patient care experiences.5,6 While there is some evidence that telehealth supported care continuity during the pandemic,7 it is unclear how telehealth use impacted the relationship between care continuity and quality of care.4
Care continuity or the extent to which patient care is dispersed or concentrated among clinicians,8,9 is a key factor in providing evidence-based care to adults with diabetes and/or hypertension.10–16 Care continuity has previously been linked directly to trust in clinicians5,17–21—a key determinant of high quality patient care experiences.17,20,22,23 The link between care continuity and trust in clinicians may have important implications for telehealth adoption. Patients with limited English proficiency and trusted care team members to help with interpretation and/or deliver care can improve patient-clinician communication during a telemedicine encounter.24 In general, high levels of trust in clinicians can be especially useful in the face of external shocks when new innovations into care delivery need to be introduced.25 Limited research has been conducted on care continuity for patients with chronic conditions in CHCs. CHCs face rigorous continuous improvement expectations, and data collection and monitoring that may impact care continuity.21 CHCs also faced greater workforce loss during the pandemic compared with other health care organizations, which may have negatively impacted patient-clinician relationships for CHC patients.26
As a result of shelter-in-place ordinances, adults with diabetes and/or hypertension were vulnerable because their routine care involves close monitoring and medication management. These patients are not only likely to be at higher risk of COVID-19-related complications,27 but are at risk for exacerbations due to reduced access to, and utilization of, care.28 We analyze data from before and during COVID-19 pandemic to assess the relationship between care continuity, telehealth use, and quality of care for patients with type II diabetes and hypertension. To our knowledge, this is the first study to examine the association of care continuity and telehealth use among adult CHC patients with chronic conditions.
Recent evidence about the association of telehealth use and diabetes and hypertension care indicates telehealth helped alleviate disruptions and decreases in quality of care during the COVID-19 pandemic, although there were disparities in age, race, and income in likelihood to utilize telehealth.29–31 Based on these findings and evidence about the impact of care continuity on quality of care,11 we hypothesize that care during the COVID-19 pandemic will be associated with lower continuity of care (Hypothesis 1) due the disruption of clinician-patient relationships in CHCs and that telehealth use will be positively associated with patients with greater care continuity during the pandemic (Hypothesis 2). We also hypothesize that care continuity will be positively associated with processes and outcomes of diabetes and hypertension care during the pandemic (Hypothesis 3), and that the association between care continuity and process measures of quality will be mediated by telehealth use (Hypothesis 4).
METHODS
Data Source
We analyzed 2019 and 2020 data from California CHC members of the Oregon Community Health Information Network (OCHIN) Accelerating Data Value Across a National Community Health Center Network (ADVANCE) Collaborative.32 The goal of ADVANCE is to create a data network of CHCs to inform and disseminate research targeted at improving access, engagement, equity, and quality of care for patients of CHCs.32
Sample
The study population are CHC clinicians and adult patients from a cohort of patients with diabetes and/or hypertension (n=20,792) with ≥2 encounters/year from 2019 (March-December 2019) to 2020 (March-December 2020) among 166 California CHC sites in the OCHIN ADVANCE Collaborative’s electronic health record (EHR) data.32 We restricted the sample to adults with at least 2 encounters during each year of the study because the assessment of continuity requires multiple encounters.
Outcomes
For Hypothesis 1, the outcome measure is care continuity by year (pre-COVID-19 vs. during COVID 19) by the Modified Modified Continuity Index (MMCI), a measure of care dispersion, calculated using Equation (1):
| (1) |
where k=number of clinicians seen in a period and N=total number of encounters to all clinicians in a period. MMCI is an established measure of care continuity used commonly in published studies of care continuity33–39; scores range from 0 to 1, where 1 is perfect continuity with all encounters to a singular provider and 0 is all encounters to different clinicians.
For Hypothesis 2, the outcome measure is telehealth use, defined as at least 1 telehealth encounter by a patient in each year as established through data collected from the EHR.
For Hypothesis 3, processes of care are measured by annual blood pressure and/or A1c testing. The relationship between MMCI and the final annual systolic and diastolic blood pressure level and A1c value of patients is examined to analyze if processes of care translate to improved intermediate outcomes of care. For Hypothesis 4, we conducted mediation analysis to examine the proportion of the relationship that is mediated by telehealth use between MMCI and the outcomes that have a significant relationship with both MMCI and telehealth use.
Main Independent Variables
For Hypothesis 1, the main independent variable is the year (2019 vs. 2020) of encounter. For Hypothesis 2, 3, and 4, the main independent variable is care continuity, measured by MMCI (range: 0–1).
Control Variables
Regression models controlled for patients’ sociodemographic characteristics, health status, encounters, and clinician types seen by patients during each year, categorized based on past research,40 which include single physician only, physician and nurse practitioner/physician’s assistant or registered nurse/medical assistant, combination of physician and a nurse practitioner/physician’s assistant and registered nurse/medical assistant, 2 different physicians, and 3 or more unique physicians. Comorbidities were determined from the EHR problem list and included body mass index (BMI), congestive heart failure, cardiovascular disease, coronary heart disease, depression, anxiety/posttraumatic stress disorder, general presence of a mental health condition, diabetic retinopathy, substance abuse, alcohol abuse, tobacco use, mobility impairments.41 The Charlson Comorbidity Index (range: 1–12), a validated, weighted index of comorbidities that considers the number and severity of each condition resulting in an integer starting from zero that represents risk of mortality, was constructed and also included as a control variable.
Statistical Analysis
First, a paired t test compared average levels of care continuity (MMCI) across periods defined as before (2019) and during (2020) the COVID-19 pandemic. Next, logistic regression models estimated the association of care continuity (MMCI) with: (1) telehealth use and (2) processes of care (blood pressure, hemoglobin A1c testing), net of control variables.33 Generalized linear regression models estimated the association of MMCI and intermediate outcomes (blood pressure, A1c control). Robust SEs accounted for patients clustering within CHC sites. Models were estimated separately for 2019 and 2020. The regression model of telehealth adoption by care continuity is presented in Equation (2):
| (2) |
Where β0 is an intercept term, β1 is a term indicating the period of the analysis as described above and β1=0 for analysis in the preperiod (2019) and β1=1 for analysis in the during-period (2020), β2 is the coefficient of care continuity, β3 is the coefficient for the clinician types seen in each period, β4 is the coefficient for the control variable for the number of encounters a patient had in a given period, β5 is the coefficient for control variables related to patient characteristics, which include Charlson score, income measured by percentage of federal poverty line, BMI, sex, and age and ε is an error term.
Equation (3) exhibits the regression model for pre-COVID-19 and during COVID-19 analysis of the association of care continuity and diabetes/hypertension management and the mediating impact of telehealth use is:
| (3) |
Where β0 is an intercept term, β1 is a term indicating the period of the analysis and β1=0 for analysis in the preperiod and β1=1 for analysis in the during-period, β2 is the coefficient of care continuity, β3 is the coefficient for the control variable that controls for the different configurations of clinician types that a patient saw in each period, β4 is the coffiecient indicating telehealth use in a period, β4=0 for no telehealth use, and β4=1 for patients with at least 1 telehealth encounter in the period, β5 is the coefficient for control variables related to patient characteristics, which include the Charlson score, income measured by percentage of federal poverty line, BMI, sex, and age, β6 is the coefficient for 2019 baseline values of intermediate outcomes in 2020 regressions, and ε is an error term.
We conducted a formal mediation analysis42 to examine telehealth as a mediator, or variables that explains the relationship between care continuity and quality of care. We only examined the association of MMCI with the A1c testing during the pandemic, as it was the only significant association between telehealth use and study outcomes found in adjusted analyses. We chose this approach to enable estimation of effects described by nonlinear relationships. “PARAMED” package in STATA was used,43,44 which uses parametric regression models to estimate causal mediation effects. Percent mediation is then calculated by Equation (4) using natural indirect and direct effects45,46:
| (4) |
Mediation analyses were conducted for 2020 study outcomes for which the relationships between MMCI and the study outcome were statistically significant in adjusted analyses. Mediation analysis was not conducted for 2019 due to low uptake of telehealth during the period. All statistical analyses were performed using Stata 17.0.44
RESULTS
The analytic sample is predominantly female (58.1%) and identified as Hispanic/Latinx (52.7%). A plurality (43.5%) of the population preferred Spanish as their spoken language, 52.7% English, and 3.3% another language (Table 1). Homelessness (0.45%) were a small minority, and most patients had an assigned primary care physician (98.8%). Most (58.66%) of the sample was diagnosed with type II diabetes and 85.22% had hypertension. The average Charlson comorbidity score was 3.19 (SD=1.63).
TABLE 1.
Patient Demographics and Clinical Characteristics of the Analytic Sample, by Telehealth Exposure
| Patient demographics | n (%) | Telehealth [n (%)] | No telehealth [n (%)] | P |
|---|---|---|---|---|
| No. patients† | 20,792 (100) | 4251 (20.45) | 16,541 (79.55) | |
| Sex | 0.021* | |||
| Female | 12,069 (58.05) | 2534 (59.61) | 9535 (57.64) | |
| Male | 8723 (41.95) | 1717 (40.39) | 7006 (42.36) | |
| Race/ethnicity | 0.08 | |||
| Hispanic/Latino | 10,955 (52.69) | 2258 (53.12) | 8697 (52.58) | |
| White | 6021 (28.96) | 1191 (28.02) | 4830 (29.20) | |
| Asian | 1374 (6.61) | 288 (6.77) | 1086 (6.57) | |
| Black or African American | 1345 (6.47) | 290 (6.82) | 1055 (6.38) | |
| Native Hawaiian or Other Pacific Islander | 128 (0.66) | 20 (0.47) | 108 (0.65) | |
| American Indian or Alaskan Native | 90 (0.43) | 15 (0.35) | 75 (0.45) | |
| Multiple races | 73 (0.35) | 15 (0.35) | 58 (0.35) | |
| Unknown | 806 (3.88) | 174 (4.09) | 632 (3.82) | |
| Patient preferred spoken language | 0.132 | |||
| English | 10,960 (52.71) | 2191 (51.54) | 8769 (53.01) | |
| Spanish | 9139 (43.95) | 1904 (44.79) | 7235 (43.74) | |
| Other | 693 (3.33) | 156 (3.67) | 537 (3.25) | |
| Marital status | <0.001* | |||
| Single | 5034 (24.21) | 864 (20.32) | 4170 (25.21) | |
| Married/domestic partner | 4934 (23.73) | 887 (20.87) | 4047 (24.47) | |
| Significant other | 352 (1.69) | 64 (1.51) | 288 (1.74) | |
| Separated/divorced | 1172 (5.64) | 202 (4.75) | 970 (5.86) | |
| Widowed | 855 (4.11) | 163 (3.83) | 692 (4.18) | |
| Unknown | 8445 (40.62) | 2071 (48.72) | 6374 (38.53) | |
| Homelessness status | 0.072 | |||
| Yes | 94 (0.45) | 23 (0.54) | 71 (0.43) | |
| Insurance | <0.001* | |||
| Private | 1816 (8.73) | 376 (8.84) | 1440 (8.71) | |
| Medicaid | 7374 (35.47) | 1525 (35.87) | 5849 (35.36) | |
| Medicare | 6562 (31.56) | 1375 (32.35) | 5187 (31.36) | |
| Other public | 1734 (8.34) | 248 (5.83) | 1486 (8.98) | |
| Uninsured | 3306 (15.90) | 727 (17.10) | 2579 (15.59) | |
| Assigned primary care physician | 0.043* | |||
| Yes | 20,541 (98.79) | 4201 (98.82) | 16,340 (98.78) | |
| No | 251 (1.21) | 50 (1.18) | 201 (1.22) | |
| Comorbidities | ||||
| Type II diabetes | 12,197 (58.66) | 2887 (67.91) | 9310 (56.28) | <0.001* |
| Hypertension | 17,718 (85.22) | 3567 (83.91) | 14,151 (85.55) | 0.007* |
| Congestive heart failure | 1000 (4.81) | 231 (5.43) | 769 (4.65) | 0.033* |
| Cardiovascular disease | 1297 (6.24) | 308 (7.25) | 989 (5.98) | 0.002* |
| Congenital heart disease | 1885 (9.07) | 452 (10.63) | 1433 (8.66) | <0.001* |
| Diabetic retinopathy | 1295 (6.23) | 336 (7.90) | 959 (5.80) | <0.001* |
| Secondary diabetes | 1011 (4.86) | 273 (6.42) | 738 (4.46) | <0.001* |
| Mobility impairment | 239 (1.15) | 49 (1.15) | 190 (1.15) | 0.983 |
| Substance abuse | 2031 (9.77) | 404 (9.50) | 1627 (9.84) | 0.515 |
| Alcohol use | 1068 (5.14) | 202 (4.75) | 866 (5.24) | 0.203 |
| Tobacco use | 1836 (8.83) | 361 (8.49) | 1475 (8.92) | 0.384 |
| Depression | 5251 (25.25) | 1169 (27.50) | 4082 (24.68) | <0.001* |
| Anxiety/posttraumatic stress disorder | 4050 (19.48) | 854 (20.09) | 3196 (19.32) | 0.260 |
| Other mental health condition | 3199 (15.39) | 687 (16.16) | 2512 (15.19) | 0.116 |
| Age [mean (SD)] | 57.8 (11.9) | 57.8 (11.8) | 57.8 (11.9) | 0.703 |
| Charlson score [mean (SD)] | 3.19 (1.63) | 3.46 (1.65) | 3.12 (1.62) | <0.001* |
| Body mass index [mean (SD)] | 31.76 (7.45) | 32.16 (7.44) | 31.66 (7.44) | <0.001* |
Percentages displayed for “number of patients” are row percentages. All other percentages presented above reflect column percentages.
Significant at P<0.05 level.
Overall, encounters declined during the COVID-19 pandemic with 263,633 encounters in 2019 and 103,634 in 2020. The types of clinicians that patients had encounters with changed from 2019 to 2020, with a larger proportion of patients seeing a single physician (2019: 21.7%, 2020: 25.4%), a physician and a nurse practitioner/physician’s assistant or registered nurse/medical assistant (2019: 7.0%, 2020: 8.7%), a combination of a physician and a nurse practitioner/physician’s assistant and registered nurse/medical assistant (2019: 4.7%, 2020: 5.7%), and 2 different physicians (2019: 31.4%, 2020: 39.5%) in 2020 compared with 2019. There was a reduction in the proportion of patients seeing 3 or more unique physicians in 2020 (2019: 35.3%, 2020: 20.8%).
COVID-19 Pandemic Impact on Care Continuity
Supporting Hypothesis 1, patients experienced reduced continuity of care in 2020 (MMCI=0.63, SD=0.36) compared with 2019 (MMCI=0.71, SD=0.28, P<0.001). Almost all patients (2019: 99.99% vs. 2020: 99.75%) had their blood pressure screened annually but only 69.78% versus 63.32% of adults with diabetes had their A1c tested in 2019 versus 2020.
Telehealth Use and Care Continuity During the Pandemic
Telehealth accounted for 0.33% of encounters in 2019 and increased to 9.55% in 2020 (Fig. 1). In our sample, 14.1% of clinicians used telehealth to provide care in 2020 out of n=16,597 clinicians represented in our analytic sample (data not shown). In adjusted analyses, higher MMCI scores were associated with higher odds of telehealth use in 2020 [odds ratio (OR)=1.94, marginal effect=0.20, z=70.78, P<0.001], but not 2019, which partially supports Hypothesis 2 (Table 2). Contrary to expectations, an inverse relationship was found between 2019 MMCI scores and telehealth use in 2020 (OR=0.82, marginal effect=0.20, z=70.73, P=0.003; Table 2).
FIGURE 1.
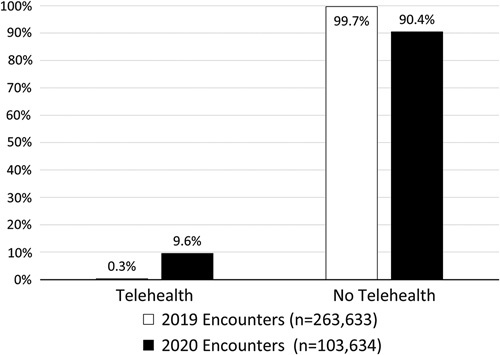
Proportion of encounters conducted via telehealth by year.
TABLE 2.
Multivariable Regression Analyses: The Association of Care Continuity and Telehealth With Quality of Hypertension and Diabetes Care
| Telehealth use (2019, n=19,385; 2020, n=19,385) | Hemoglobin A1c process (2019, n=11,373; 2020, n=11,373) | Hemoglobin A1c value (2019, n=3384; 2020, n=3625) | Systolic blood pressure (2019, n=19,360; 2020, n=17,738) | Diastolic blood pressure (2019, n=19,360; 2020, n=17,738) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | Coefficient | 95% CI | Coefficient | 95% CI | Coefficient | 95% CI | |
| 2019 | ||||||||||
| Care continuity† ‡ | 0.84 | 0.6–1.06 | 1.98*** | 1.55–2.53 | −0.57** | −0.99 to −0.15 | −1.58 | −3.17 to −0.01 | −0.67 | −1.57 to 0.23 |
| Telehealth† | — | — | 2.72*** | 1.99–3.72 | −0.15 | −0.54 to 0.24 | −0.44 | −2.31 to 1.42 | −0.88 | −1.88 to 0.11 |
| 2020 | ||||||||||
| Care continuity† ‡ | 1.94*** | 1.55–2.43 | 1.50*** | 1.23–1.82 | −0.45** | −0.78 to −0.12 | −2.90*** | −4.16 to −1.63 | −1.44*** | −2.16 to −0.72 |
| Telehealth† | — | — | 10.00*** | 7.30–13.70 | 0.03 | −0.24 to 0.30 | −0.08 | −2.06 to 1.89 | 0.84 | −0.27 to 1.94 |
Regression results control for the types of clinicians seen by the patient, the number of visits by the patient in each year, age, sex, annual income as a percentage of federal poverty line, body mass index, and the Charlson Comorbidity Index. The 2020 regressions also control for 2019 baseline values of their respective dependent variables. All regression models were estimated using robust SEs.
Care continuity is measured using the Modified Modified Continuity Index (MMCI).
P<0.01.
P<0.001.
Care Continuity, Telehealth Use, Monitoring, and Health Outcomes
Care continuity (MMCI 2019: OR=1.98, marginal effect=0.69, z=165.50, P<0.001; 2020: OR=1.50, marginal effect=0.63, z=147.73, P<0.001) and telehealth use (2019: OR=1.50, marginal effect=0.85, z=122.87, P<0.001; 2020: OR=10.00, marginal effect=0.90, z=155.57, P<0.001) were significantly associated with more consistent A1c testing in both periods (Table 2), supporting the first part of Hypothesis 3. Contrary to the second part of Hypothesis 3, MMCI, but not telehealth use, was significantly associated with lower A1c values in 2019 (β=−0.57, P=0.007) and 2020 (β=−0.45, P=0.008). Higher care continuity (MMCI) was associated with lower systolic blood pressure (β=−2.90, P<0.001) and diastolic blood pressure values (β=−1.44, P<0.001) in 2020.
Mediating Role of Telehealth in the Care Continuity and Quality Relationship
The mediation analyses found the pathway between care continuity and telehealth, telehealth and A1c testing, and care continuity and A1c testing to all be statistically significant (Fig. 2). Care continuity partially mediated the care continuity and A1c testing relationship in 2020 based on the 4 steps used to assess mediation effects.47 In 2020, 38.7% of the relationship between MMCI and A1c testing was mediated by telehealth use (direct effect: β=1.76; 95% CI: 1.45–2.12 indirect effect: β=1.11; 95% CI: 1.05–1.12), but telehealth use did not mediate the association of care continuity and other study outcomes, offering only partial support for Hypothesis 4.
FIGURE 2.
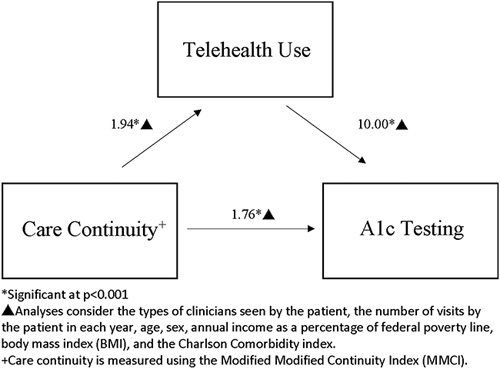
Results of causal mediation analysis with bootstrap SEs of the role of telehealth in the relationship between continuity of care and hemoglobin A1c testing.
DISCUSSION
Our analyses of care continuity, telehealth use, and quality of care among adults with diabetes and/or hypertension in CHCs before and during the pandemic reveals that care continuity and telehealth use are associated with quality of care in complex ways. Consistent with Hypothesis 1, care continuity for adults with diabetes and/or hypertension receiving care in CHCs during the early COVID-19 pandemic (2020) declined compared with prepandemic period (2019). Higher care continuity in 2020 was associated with higher telehealth use and A1c testing, as well as lower A1c scores and lower blood pressure in accordance with Hypothesis 2. Although continuity of care is generally associated with better patient outcomes, the findings of this study are consistent with the mixed effects found in the literature analyzing the impact of care continuity on quality of care for patients with diabetes and hypertension.10,11,13–15 Our findings are consistent with past research that demonstrates that care continuity improves quality of care for patients with diabetes and/or hypertension, but that processes of care do not necessarily translate to improved intermediate outcomes.10,11,13–15 However, care continuity can improve patients’ experiences of care and quality of life for patients with diabetes.15,16 Our findings that telehealth use was more common for patients that previously had low utilization of health care suggest telehealth could be a tool to enhance care continuity for patient populations that previously have low continuity of care. Further research is needed to examine how telehealth might be leveraged to enhance care continuity to improve patient outcomes to help better translate improved process outcomes into improved intermediate outcomes.
Contrary to Hypothesis 3, more frequent A1c and blood pressure testing did not translate to better intermediate outcomes. Telehealth mediated the association of care continuity with consistent A1c and blood pressure testing, indicating that care continuity facilitates telehealth use and may enable resilient performance on high priority process measures, partially supporting our Hypothesis 4. Moreover, evidence suggests there was a decline in physical activity during the COVID-19 pandemic increase in sedentary behavior that could not be addressed by care continuity and care management.48 The finding that telehealth acts as a mediator for diabetes monitoring is consistent with another recent study on a nonsafety net population highlighting the utility of telehealth in sustaining continuous care during COVID-19.2 Despite a lack of translation of telehealth use into improved intermediate outcomes of care, the finding that telehealth facilitated continuous A1c monitoring during the pandemic suggest that telehealth may be a useful tool in maintaining care continuity and processes of care during a crisis.
Long-term investment in telehealth infrastructure and information technology departments may be needed to support the resilience of CHCs during times of crises. Our result that patients experiencing lower care continuity in 2019 were more likely to use telehealth during the pandemic compared with patients with higher care continuity suggests that telehealth can support monitoring of diabetes and hypertension when in-person care is less safe. By continuing support for telehealth, policymakers can help ensure that patients are able to maintain continuous chronic care treatment and monitoring even during major shocks such as the COVID-19 pandemic. Tailoring of telehealth services to meet CHC patient needs could also increase telehealth use and support improved quality of care for adults with diabetes and/or hypertension, including ensuring that patient portals and other platforms are available in Spanish and other Medicaid threshold languages.24 Supporting audio-only telemedicine appointments may also be a key factor in meeting the needs of CHC patients,24 but more research is needed to assess whether quality of care disparities exists between audio-only and video telemedicine encounters.49
Our results should be considered in light of some limitations. First, our findings may not be reflective for all patients with diabetes and/or hypertension and may not generalize to lower utilizing patients. We could not track utilization outside of the CHCs and patients may have sought care elsewhere, but these data are not captured if they are not a member of the OCHIN ADVANCE collaborative. Another limitation is that the nature of the data used does not allow for direct measurement of team membership and collaboration. Social network analysis could be used in the future to elucidate team structure and communication patterns and to examine the relationship between care coordination and telehealth use.40 Only 1.21% of patients in our sample did not have an assigned primary care clinician, so we were unable to adequately analyze the unique effects of care continuity on this population who may be at especially high risk of exacerbations due to diabetes and/or hypertension. There were also telehealth documentation challenges for CHCs during the early pandemic and some telehealth encounters may be misclassified as “in-person” encounters in the OCHIN data. National data indicate that the proportion of overall encounters that were telehealth in outpatient settings during the study period were 30.1%,50 >14.1% documented in our analytic sample. Misclassification of telehealth encounters in our data could bias the study results. Finally, we were not able to distinguish between audio and video encounters in our dataset. The modalities may differentially impact quality of care and more evidence examining heterogenous quality effects by modality are needed.49 Given increased stress, reduced activity due to shelter-in-place, and greater isolation, care continuity and monitoring blood pressure and A1c may have been necessary, but insufficient to improve intermediate outcomes.
CONCLUSIONS
Care continuity helped maintain quality of care for adult CHC patients during the COVID-19 pandemic and may support resilient performance on high priority process measures like A1c testing during times of crises. Examining the mechanisms that connect continuity of care to increased telehealth use, including through primary care team learning, may provide additional insights about how best to implement disruptive patient-centered innovations.
Footnotes
Funded by a grant from the Center for Information Technology Research in the Interest of Society (CITRIS) and the Banatao Institute. A.A.T. received support from the Agency for Healthcare Research and Quality (T32HS022241).
The authors declare no conflict of interest.
Contributor Information
Aaron A. Tierney, Email: aat2143@berkeley.edu.
Denise D. Payán, Email: dpayan@hs.uci.edu.
Timothy T. Brown, Email: timothy.brown@berkeley.edu.
Adrian Aguilera, Email: aguila@berkeley.edu.
Stephen M. Shortell, Email: shortell@berkeley.edu.
Hector P. Rodriguez, Email: hrod@berkeley.edu.
REFERENCES
- 1.Eberly LA, Khatana SAM, Nathan AS, et al. Telemedicine outpatient cardiovascular care during the COVID-19 pandemic: bridging or opening the digital divide? Circulation. 2020;142:510–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koonin LM, Hoots B, Tsang CA, et al. Trends in the use of telehealth during the emergence of the COVID-19 pandemic—United States, January–March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1595–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyles C Fields J Lisker S, et al. Launching a toolkit for safety-net clinics implementing telemedicine during the COVID-19 pandemic. To the Point (blog). 2020. Accessed March 27, 2022. https://www.commonwealthfund.org/blog/2020/launching-toolkit-safety-net-clinics-implementing-telemedicine-during-covid-19-pandemic
- 4.Hadeed N, Fendrick AM. Enhance care continuity post COVID-19. American Journal of Managed Care. 2021;27:135–136. [DOI] [PubMed] [Google Scholar]
- 5.Donahue KE, Ashkin E, Pathman DE. Length of patient-physician relationship and patients’ satisfaction and preventive service use in the rural south: a cross-sectional telephone study. BMC Fam Pract. 2005;6:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spear SE. Reducing readmissions to detoxification: an interorganizational network perspective. Drug Alcohol Depend. 2014;137:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rust G Ye J Baltrus P, et al. Practical barriers to timely primary care access impact on adult use of emergency department services. Arch Intern Med. 2008;168:1705–1710. [DOI] [PubMed] [Google Scholar]
- 8.Magill M, Senf J. A new method for measuring continuity of care in family practice residencies. J Fam Pract. 1987;24:165–168. [PubMed] [Google Scholar]
- 9.Haggerty JL, Reid RJ, Freeman GK, et al. Continuity of care: a multidisciplinary review. BMJ. 2003;327:1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McClellan WM, Hall WD, Brogan D, et al. Continuity of care in hypertension: an important correlate of blood pressure control among aware hypertensives. Arch Intern Med. 1988;148:525–528. [DOI] [PubMed] [Google Scholar]
- 11.Chan KS, Wan EYF, Chin WY, et al. Effects of continuity of care on health outcomes among patients with diabetes mellitus and/or hypertension: a systematic review. BMC Fam Pract. 2021;22:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’connor PJ, Desai J, Rush WA, et al. Is having a regular provider of diabetes care related to intensity of care and glycemic control. J Fam Pract. 1998;47:290–297. [PubMed] [Google Scholar]
- 13.Parchman ML, Pugh JA, Hitchcock Noël P, et al. Continuity of care, self-management behaviors, and glucose control in patients with type 2 diabetes. Med Care. 2002;40:137–144. [DOI] [PubMed] [Google Scholar]
- 14.Younge R, Jani B, Rosenthal D, et al. Does continuity of care have an effect on diabetes quality measures in a teaching practice in an urban underserved community? J Health Care Poor Underserved. 2012;23:1558–1565. [DOI] [PubMed] [Google Scholar]
- 15.Gulliford MC, Naithani S, Morgan M. Continuity of care and intermediate outcomes of type 2 diabetes mellitus. Fam Pract. 2007;24:245–251. [DOI] [PubMed] [Google Scholar]
- 16.Hänninen J, Takala J, Keinänen-Kiukaanniemi S. Good continuity of care may improve quality of life in type 2 diabetes. Diabetes Res Clin Pract. 2001;51:21–27. [DOI] [PubMed] [Google Scholar]
- 17.Baker R, Mainous AG, Gray DP, et al. Exploration of the relationship between continuity, trust in regular doctors and patient satisfaction with consultations with family doctors. Scand J Prim Health Care. 2003;21:27–32. [DOI] [PubMed] [Google Scholar]
- 18.Mainous AG, Kern D, Hainer B, et al. The relationship between continuity of care and trust with stage of cancer at diagnosis. Fam Med. 2004;36:35–39. [PubMed] [Google Scholar]
- 19.Mainous A, Baker R, Love M, et al. Continuity of care and trust in one’s physician: evidence from primary care in the United States and the United Kingdom. Fam Med. 2001;33:22–27. [PubMed] [Google Scholar]
- 20.Pandhi N, Saultz JW. Patients’ perceptions of interpersonal continuity of care. J Am Board Fam Med. 2006;19:390–397. [DOI] [PubMed] [Google Scholar]
- 21.HRSA Health Center Program Bureau of Primary Health Care. Health Center Program Compliance Manual. 2018.
- 22.Wilkins CH. Effective engagement requires trust and being trustworthy. 2018. [DOI] [PMC free article] [PubMed]
- 23.Asan O, Yu Z, Crotty BH. How clinician-patient communication affects trust in health information sources: temporal trends from a national cross-sectional survey. PLoS One. 2021;16:e0247583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Payán DD, Frehn JL, Garcia L, et al. Telemedicine implementation and use in community health centers during COVID-19: clinic personnel and patient perspectives. SSM - Qualitative Research in Health. 2022;2:100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai CH. Integrating social capital theory, social cognitive theory, and the technology acceptance model to explore a behavioral model of telehealth systems. Int J Environ Res Public Health. 2014;11:4905–4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Healthcare Dive. Community health centers facing acute workforce loss. Accessed April 25, 2022. https://www.healthcaredive.com/news/community-health-centers-workforce-loss/622037/
- 27.Gregory JM, Slaughter JC, Duffus SH, et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44:526–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, mckeever Bullard K, Gregg EW, et al. Access to health care and control of ABCs of diabetes. Diabetes Care. 2012;35:1566–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel SY, McCoy RG, Barnett ML, et al. Diabetes care and glycemic control during the COVID-19 pandemic in the United States. JAMA Intern Med. 2021;181:1412–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quinton JK, Ong MK, Sarkisian C, et al. The impact of telemedicine on quality of care for patients with diabetes after March 2020. J Gen Intern Med. 2022;37:1198–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen EM, Andoh JE, Nwanyanwu K. Socioeconomic and demographic disparities in the use of telemedicine for ophthalmic care during the COVID-19 pandemic. Ophthalmology. 2022;129:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ADVANCE. Accelerating Data Value Across a National Community Health Center Network. Accessed November 28, 2022. https://advancecollaborative.org/
- 33.Blozik E, Bähler C, Näpflin M, et al. Continuity of care in Swiss cancer patients using Claims Data. Patient Prefer Adherence. 2020;14:2253–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macdonald A, Adamis D, Broadbent M, et al. Continuity of care and mortality in people with schizophrenia. BJPsych Open. 2021;7:e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung B, Cho KH, Lee DH, et al. The effects of continuity of care on hospital utilization in patients with knee osteoarthritis: analysis of nationwide insurance data. BMC Health Serv Res. 2018;18:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coma E, Mora N, Peremiquel-Trillas P, et al. Influence of organization and demographic characteristics of primary care practices on continuity of care: analysis of a retrospective cohort from 287 primary care practices covering about 6 million people in Catalonia. BMC Fam Pract. 2021;22:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dreiher J, Comaneshter DS, Rosenbluth Y, et al. The association between continuity of care in the community and health outcomes: a population-based study. Isr J Health Policy Res. 2012;1:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacDonald A, Adamis D, Craig T, et al. Continuity of care and clinical outcomes in the community for people with severe mental illness. Br J Psychiatry. 2019;214:273–278. [DOI] [PubMed] [Google Scholar]
- 39.Lee SA, Choi DW, Kwon J, et al. Association between continuity of care and type 2 diabetes development among patients with thyroid disorder. Medicine. 2019;98:e18537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuo YF, Agrawal P, Chou LN, et al. Assessing association between team structure and health outcome and cost by social network analysis. J Am Geriatr Soc. 2021;69:946–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voss RW, Schmidt TD, Weiskopf N, et al. Comparing ascertainment of chronic condition status with problem lists versus encounter diagnoses from electronic health records. J Am Med Inform Assoc. 2022;29:770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee H, Herbert RD, McAuley JH. Mediation analysis. JAMA. 2019;321:697–698. [DOI] [PubMed] [Google Scholar]
- 43.Emsley R, Liu H. PARAMED: Stata module to perform causal mediation analysis using parametric regression models. Statistical Software Components. Accessed April 25, 2022. http://econpapers.repec.org/software/bocbocode/s457581.htm [Google Scholar]
- 44.StataCorp.Stata Statistical Software: Release 17. College Station, TX:StataCorp LLC; 2021.
- 45.Ditlevsen S, Christensen U, Lynch J, et al. The mediation proportion: a structural equation approach for estimating the proportion of exposure effect on outcome explained by an intermediate variable. Epidemiology. 2005;16:114–120. [DOI] [PubMed] [Google Scholar]
- 46.Pearl J. Direct and Indirect Effects. Morgan Kaufmann; 2001. [Google Scholar]
- 47.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. [DOI] [PubMed] [Google Scholar]
- 48.Stockwell S, Trott M, Tully M, et al. Changes in physical activity and sedentary behaviours from before to during the COVID-19 pandemic lockdown: a systematic review. BMJ Open Sport Exerc Med. 2021;7:e000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Payán DD, Rodriguez HP. Telehealth disparities. Health Aff (Millwood). 2021;40:1340. [DOI] [PubMed] [Google Scholar]
- 50.Patel SY, Mehrotra A, Huskamp HA, et al. Variation in telemedicine use and outpatient care during the COVID-19 pandemic in the United States. Health Aff. 2021;40:349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]


