Abstract
The objective of this study was to develop a new heated dryer system (HDS) for high efficiency lung delivery of nebulized aerosol and demonstrate performance with realistic in vitro testing for trans-nasal aerosol administration simultaneously with high flow nasal cannula (HFNC) therapy and separately for direct oral inhalation (OI) of the aerosol. With the HDS-HFNC and HDS-OI platforms, new active synchronization control routines were developed to sense subject inhalation and coordinate drug aerosol delivery. In vitro experiments were conducted to predict regional drug loss and lung delivery efficiency in systems that included the HDS with various patient interfaces, realistic airway models, and simulated breathing waveforms. For the HDS-HFNC platform and a repeating breathing waveform, total system loss was <10%, extrathoracic deposition was approximately 6% and best-case lung delivery efficiency was 75–78% of nebulized dose. Inclusion of randomized breathing with the HFNC system decreased lung delivery efficiency by ~10% and had no impact on nasal depositional loss. For the HDS-OI platform and best-case mouthpiece, total system loss was <8%, extrathoracic deposition was <1%, and lung delivery efficiency was >90% of nebulized dose. Normal vs. deep randomized oral inhalation had little impact on performance of the HDS-OI platform and environmental aerosol loss was negligible. In conclusion, both platforms demonstrated the potential for high efficiency lung delivery of the aerosol with the HDS-OI platform having the added advantages of nearly eliminating extrathoracic deposition, being insensitive to breathing waveform, and preventing environmental aerosol loss.
Keywords: Pharmaceutical aerosols, respiratory drug delivery, non-invasive ventilation, high flow nasal cannula, high flow therapy, high efficiency aerosol delivery
INTRODUCTION
Nebulizers provide a vital option for the administration of inhaled therapeutics to the lungs (1–5). Nebulizer-based platforms are frequently selected due to their ability to operate in the humidified and positive-pressure environment encountered during non-invasive ventilation (NIV), deliver high dose therapeutics, dispense medications over extended treatment times, and administer many process-sensitive therapies including proteins, peptides, and biologicals. Furthermore, new inhaled therapies are often first delivered with nebulizers to determine efficacy in animal models and humans prior to more extensive development as dry powders or other formulations. While current nebulizers are often inefficient in terms of lung delivery (6–10), a variety of secondary devices and strategies are currently available to significantly improve the lung delivery efficiency of nebulized aerosols.
As recently reviewed by Longest et al. (11), secondary devices and strategies to improve the delivery efficiency of nebulized aerosols in a variety scenarios can be categorized as: (i) modifying the aerosol size distribution, (ii) synchronizing aerosol delivery with inhalation, (iii) reducing depositional losses at connection points, (iv) improving the patient interface and (v) guiding patient inhalation. In reviewing these technologies, it was generally found that active strategies, such as sensor-based synchronized aerosol generation during inhalation, were more effective than passive strategies (11). Moreover, each strategy listed overcomes one potential limitation commonly found with nebulized aerosol delivery. Therefore, including a majority of these strategies is typically required to improve nebulized aerosol delivery in highly challenging scenarios. While these secondary devices and strategies often make the nebulized aerosol system more complex, their use is often justified in drug delivery scenarios involving (i) poor lung delivery efficiency due to high extrathoracic depositional loss and/or high exhaled and environmental loss of the aerosol, (ii) high dose medications or medications with high cost or significant side effects, and/or (iii) targeted medications intended to deposit within a specific region of the airways to achieve proper biological activity.
The previous study of Spence et al. (12) introduced a new nebulized heated dryer system (HDS) aerosol delivery platform that includes multiple secondary devices and strategies to optimize aerosol lung delivery efficiency and targeting (12). The system implements a mesh nebulizer aerosol source (or sources) and, as a key feature, modifies the initial aerosol size through evaporation with the use of a heating section in a process referred to as active aerosol size change (12). The heating section has a narrow channel geometry with low volume (~ 40–50 mL) to evaporate the aerosol (to a dry particle state with a typical aerodynamic size of ~1 μm) and minimize depositional aerosol loss. Compared with passive aerosol size change, which often selects out the largest droplets with the use of baffles or other impaction surfaces, a key advantage of active size control is the production of a small particle aerosol at the original high mass flow rate of a nebulizer that generates much larger droplets (12). An aerosol produced by the HDS is typically expected to be small enough to pass through nasal cannula interfaces, to pass through adult and pediatric extrathoracic airways, and to provide high efficiency lung delivery of the therapeutic. As described by Spence et al. (12) and Longest et al. (11), the HDS platform (previously termed the low-volume mixer-heater) also includes synchronization of aerosol delivery with inhalation, streamlined geometrical features that minimize depositional aerosol loss, and streamlined patient interfaces that maximize aerosol transmission. Through the use of additional controlled condensational growth technologies, like excipient enhanced growth (EEG), the small particle aerosol is typically designed to increase in size within the humid airways for targeted lung deposition and reduced exhalation of the dose (13–17).
In general, the trans-nasal and oral administration of nebulized aerosols to the lungs is considered to be inefficient, even in adults, without the use of secondary devices and strategies (11). Considering nebulized aerosol administration with different mask interfaces, in vitro and in vivo studies report relatively low lung delivery efficiencies for nebulizers, typically in the range of 1.3–25% (18–20). Aerosol administration during high flow nasal cannula (HFNC) therapy is considered to be especially challenging due to elevated gas delivery velocities, increased nasal depositional loss and current nasal interface designs (6, 21–25). For example, Dugernier et al. (26) conducted in vivo gamma scintigraphy studies of radiolabeled aerosols generated from jet and mesh nebulizers through a Fisher and Paykel Optiflow® nasal cannula with a gas flow rate of 30 L/min. Lung delivery efficiency was in the range of 1.5–2.1% of the nebulizer emitted dose (using planar gamma scintigraphy) for both nebulizers with high intersubject variability. Multiple studies have demonstrated that reducing the HFNC gas flow rate down to 10 L/min and potentially removing humidity from the system typically improves lung delivery efficiency to a range of 7–30% of the nebulized dose (21, 23, 27, 28). However, flow rates below 20 L/min may not provide sufficient respiratory support and may fall outside of the typical definition of HFNC therapy or high flow therapy for adults (29–31).
Nebulizer-based systems that employ one or more secondary approaches have previously been shown to substantially improve lung delivery efficiency of administered aerosol. Considering trans-nasal aerosol delivery, the tPAD device employed passive aerosol size selection within the device that filtered larger droplets before the aerosol entered a nasal cannula interface (32). As a result, pulmonary deposition was 39% of emitted dose with only ~6% loss in the nose based on in vivo data (32). However, reported values indicate that the nebulizer production rate was reduced by a factor of 12-fold due to passive size selection and deposition in the device (32).
Considering nebulized oral drug delivery, systems that have previously employed one or more secondary techniques have also demonstrated improved lung delivery efficiency (11). For example, a vibrating mesh nebulizer employing passive synchronization with inhalation through the use of a valved holding chamber improved lung delivery efficiency to 33.3% of nominal dose, compared to 13% with a jet nebulizer and without passive synchronization (33). The combination of active synchronization with patient inhalation and guided patient inhalation have been used to achieve very high efficiency lung administrations of orally inhaled medications. As reviewed by Longest et al. (11), in a mixed population of healthy and cystic fibrosis (CF) patients, Brand et al. (34) evaluated the lung delivery of the AKITA2 APIXNEB system programmed for a deep inhalation at a low flow rate of 15 L/min. In all subjects considered, total mean lung deposition was approximately 70% with oral extrathoracic deposition in the range of 15 to 20% of the nebulized dose (34). The Dance 501 nebulizer uses visual feedback in the form of LEDs to lead subjects through a slow and deep inhalation with a target flow rate range of 7 to 14 L/min (35). In healthy subjects, >70% of the nebulized aerosol was delivered to the lungs with only 9–12% deposited in the mouth-throat region (35). While these techniques have significantly improved the lung delivery efficiency of orally inhaled nebulized aerosols, they can potentially be further enhanced with active aerosol size change for efficient aerosol administration using the oral inhalation or nose-to-lung routes.
As reviewed by Longest et al. (11), our group previously developed a large-volume nebulizer-based aerosol delivery system that implements active aerosol size change and passive synchronization with inhalation for nose-to-lung aerosol delivery (22, 36–38). With the study of Spence et al. (12) we developed a new lower-volume system, referred to in this study as the HDS, that also employed active synchronization with inhalation. Key advantages of the new HDS included a small aerosol pathway volume (< 150 mL) for rapid aerosol clearance and an optimized heating section that minimized aerosol depositional loss. In the previous study of Spence et al. (12) the HDS was developed for aerosol administration simultaneously with HFNC therapy. The resulting HDS-HFNC platform employed dual mesh nebulizers, with one nebulizer administering isotonic saline to humidify the airways and the other nebulizer administering an inhaled therapeutic, when needed, during a portion of the inhalation cycle. Based on the drug and humidity nebulizers operating in an alternating mode, system outlet temperature and relative humidity were 32 °C and ~30–40%, respectively, for HFNC gas support at a flow rate of 30 L/min (12). With cyclic actuation of the drug nebulizer, the HDS-HFNC platform emitted 80% of the nebulized dose and decreased the mass median aerodynamic diameter (MMAD) of the aerosol from 5.3 μm to 1.2 μm.
Following Spence et al. (12), a study by Dutta et al. (39) evaluated the HDS-HFNC platform using realistic in vitro administration experiments and a detailed computational fluid dynamics (CFD) analysis. The in vitro experiments indicted that >80% of the nebulized dose administered during HFNC therapy passed through an adult NT model and reached a tracheal filter during cyclic respiration (39). In the study of Dutta et al. (39), the nebulizer was triggered using a signal from the in vitro breathing simulator that indicated the start of inhalation. The CFD simulations indicated that the bolus delivery time between the start of nebulization and the aerosol reaching the nasal cannula was very short, i.e., 0.2 sec. Furthermore, using a deep nasal inhalation waveform for a short period of drug delivery indicated an optimal nebulizer actuation time of ~1 sec. Based on these parameters, lung delivery efficiency was surprisingly insensitive to subject inhalation flow rate, provided that inhalation flow was higher than the gas delivery flow rate at the time of aerosol inhalation.
The current HDS requires further development and testing prior to use in human subjects. In terms of development, a new active synchronization system is needed for use with HFNC therapy that can sense backpressure changes in the HFNC gas line and deliver the aerosol during a period of inhalation. Active synchronization of aerosol administration during HFNC therapy based on sensed backpressure has not been previously attempted to our knowledge. Furthermore, use of the HDS can be expanded if similar concepts are applied to aerosol administration during oral inhalation (OI), creating a new HDS-OI system. Considering testing, previous in vitro assessments of lung delivery efficiency were performed for one case to benchmark CFD simulations with synchronization based on a triggering signal from a breathing simulator (39). System performance across a range of extrathoracic models with variable inhalation conditions and active synchronization that senses the breathing profile is currently unknown. The new HDS-OI platform has not been previously tested for lung delivery efficiency and requires a separate inhalation sensing algorithm. Considering the patient interface, which is an additional strategy for improving nebulized aerosol delivery, previous studies have demonstrated improved lung delivery efficiency with streamlined nasal cannula interfaces. However, the need for streamlined mouthpiece (MP) designs to improve aerosol transmission and possibly further reduce mouth-throat (MT) depositional loss has not been explored previously.
The objective of this study was to develop a general HDS for high efficiency lung delivery of nebulized aerosol and demonstrate performance with realistic in vitro testing for trans-nasal aerosol administration during HFNC therapy and separately for direct oral inhalation of the aerosol. Aerosol administration during HFNC therapy may be considered one of the more difficult scenarios to achieve high efficiency lung delivery. For the HDS-HFNC platform, a new active synchronization approach was developed based on sensing upstream pressure in the HFNC gas delivery line. This new technology was evaluated for lung aerosol delivery efficiency in multiple adult nose-throat (NT) airway models with cyclic and randomly varying inhalation waveforms. Furthermore, commercial and streamlined nasal cannula results were compared to evaluate if a streamlined nasal cannula provides a substantial advantage with the small particle aerosol and should therefore be used in future human subject trials. For the HDS-OI platform, a separate active synchronization approach was developed based on negative inhalation pressure sensed within the system. Then, the lung delivery efficiency was again tested across multiple MT models, for varying waveforms, and for different MP interfaces.
MATERIALS AND METHODS
Overview of Experimental Systems
Experimental systems for testing the HDS operated simultaneously with HFNC or during OI are shown in Figures 1a and 1b, respectively. Common features in both experimental systems include the HDS, connective tubing, patient interface, airway model, tracheal filter (to approximate lung delivery efficiency), and Advanced Servo Lung 5000 (ASL 5000, IngMar Medical, Pittsburgh, PA). Components of the HDS are described in the following sections. In both HDS platforms, ~10 mm inner diameter connective tubing was used with lengths of 50 and 60 cm for the HFNC and OI platforms, respectively. Double filters (Pulmoguard II, Queset Medical, North Easton, MA) were used at the laryngeal outlet (i.e., tracheal inlet) of all extrathoracic airway geometries (nasal and oral) to estimate the lung delivered dose and to minimize filter losses of the small particles. The ASL 5000 was programmed to provide tracheal inhalation waveforms consistent with typical deep inhalation during HFNC therapy or oral inhalation administration using either a repeated or randomly varying (with set parameters) breathing waveform, as described in the Breathing Waveforms section below. A separate computer interface was used to monitor the breathing waveforms and performance of the HDS including patient interface and heating plate temperatures.
Figure 1.
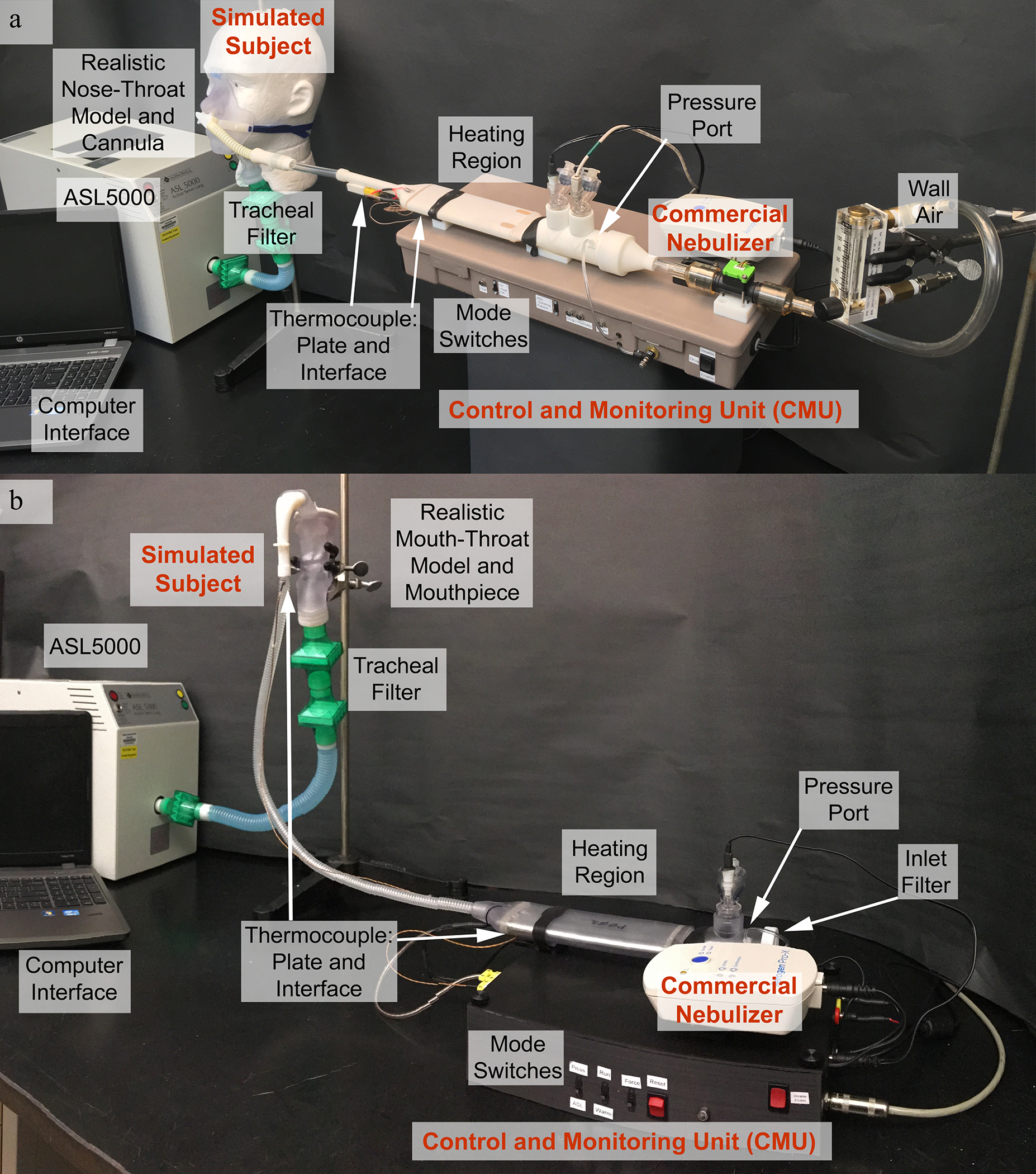
Experimental setup for in vitro performance testing of the (a) HDS-HFNC and (b) HDS-OI platforms.
Description of the HDS – Overview
For both HFNC and OI aerosol administration, common features of the HDS included commercial mesh nebulizers, a control and monitoring unit (CMU), and a flow passage containing mixing and heating regions. The commercial aerosol source in both systems was the Aerogen Solo vibrating mesh nebulizer operated with the ProX Controller (Aerogen Limited, Galway, Ireland). The CMU performed the main functions of monitoring and controlling both system temperatures and nebulizer actuation. To monitor system temperatures, thermocouples (Type-K; SA1XL-K, Omega Engineering) were placed at the patient interfaces and on the heating plates within the heating region. Feedback control was used to operate film heaters in the heating section to achieve the desired inhalation temperatures at the interfaces (~32 °C with HFNC and ~30 °C with OI) and to shut the heating system off in the event of a malfunction as a safety precaution. Separate control routines were required to activate the nebulizers during inhalation based on positive pressure feedback during HFNC therapy or negative pressure feedback during OI.
Description of the HDS – Flow Passage
The HDS flow passage was generally designed in the previous study of Spence et al. (12) based on CFD simulations, experimental tests and aerosol delivery experiments (Figures 2a and 2b). The mixing region contained an inlet flow unifier that reduced loss of the mesh nebulized aerosol. The diameter of the mixing region was sufficient to minimize depositional loss from the acoustic streaming of the mesh nebulizer aerosol jet and was smoothly connected with the heating section (12). As previously described, the heating section consisted of a narrow channel that maximized heat transfer while providing low volume and minimal depositional loss of the aerosol (Figure 2c) (12, 36). As described below, an enhanced heating section was developed for the HDS-OI platform to ensure evaporation of the aerosol in a system with a cyclic heating requirement (Figure 2d). The outlet of the heating section was smoothly connected (streamlined) with the 10 mm smoothbore spiral aerosol delivery tubing. The delivery tubing was unheated in this study to reduce system complexity considering that Dutta et al. (39) indicated heating of this tubing was unnecessary when using the narrow channel heater design with sufficient outlet temperature.
Figure 2.
Design elements of the HDS including the outer shell designs for the (a) HFNC and (b) OI platforms; as well as cross-sectional views of the heating regions of the (c) HFNC and (d) OI geometries.
Description of the HDS – Application to HFNC
The general HDS was adapted to the unique challenges of aerosol administration during HFNC therapy or direct OI. For HFNC administration, an upstream source of medical gas with a flow regulator was connected to the system and provided a positive pressure flow rate of 30 L/min. As described by Spence et al. (12), the HDS-HFNC platform employed separate humidity and drug nebulizers filled with 0.9% w/v isotonic saline and an aqueous solution of 0.25% w/v albuterol sulfate (AS), respectively (Figure 2a). The nebulizers were activated in an alternating mode where the humidity nebulizer was continuously active except for a brief period during inhalation in which the humidity nebulizer was switched off and the drug nebulizer was switched on for a defined time (Set Time). The heating section of the HDS-HFNC platform had an elliptical cross section, as illustrated in Figure 2c, with a length of 16 cm. Based on the continuous flow of the HFNC system, this heating section was found to be sufficient to produce a 32 °C outlet temperature at the nasal cannula interface and fully evaporate the aerosol without exceeding the temperature limit of the nylon material used to create the heating chamber shell. The nasal cannula interface for HFNC therapy fits loosely in the nose such that the patient exhales through the gap between the nasal prongs and nostril walls while a continuous flow rate of 30 L/min is delivered through the prongs. Additional design and construction details of the HDS-HFNC platform were provided in the previous study of Spence et al. (12).
An information flow diagram of the HDS-HFNC CMU is provided as Figure 3a. The CMU controlled all aspects of system operation including receiving and interpreting the signal from the integrated pressure measurement sensor, actuating the drug nebulizer (which alternated with the humidity nebulizer), providing feedback control of the heating section to achieve a 32 °C humidified gas temperature delivered to the patient, and shutting down the system in the event of a malfunction. The CMU was developed around an Arduino Uno microcontroller (Arduino, Somerville, MA) and custom-developed code. The code established a baseline pressure for each subject over a three-minute warmup and learning period. Additionally, the code updated this baseline pressure at defined intervals during the administration period. This baseline pressure was a function of the flow rate through the system as well as the fit between the nasal cannula interface and nostrils. Deviations in pressure measurement from this baseline pressure were then used by the code to establish the estimated start and stop of inhalation. Based on previous CFD estimates of aerosol travel time through the system (39), the drug nebulizer was triggered by the CMU to occur 0.25 sec after the estimated start of inhalation. Drug nebulization was set to last for approximately 1 sec but was ended early if exhalation was sensed during the nebulization period. The estimated baseline pressure was recalculated by the code at time intervals corresponding to approximately every 3 breaths or immediately upon sensed pressures outside the expected range to account for any changes due to shifting nasal cannula fit.
Figure 3.
Control diagrams highlighting the interactions among the control and monitoring unit (CMU), commercial nebulizer, flow path and simulated subject for the (a) HDS-HFNC and (b) HDS-OI platforms.
Description of the HDS – Application to OI
To enable synchronized aerosol administration during OI, there were multiple changes to the HDS (Figure 2b). For oral inhalation, the subject forms an airtight seal on the MP and breaths through the system to receive both respiratory gas and the aerosol. A filter, consisting of the filter media from a Pulmoguard II, was placed on the gas flow inlet/outlet of the system behind the nebulizer in order to unify the incoming air during inspiration and filter therapeutic or biological aerosol in the airstream on exhalation. The total system dead volume was 250 mL, which was deemed acceptable for oral inhalation by an adult and can be further reduced in future versions of the flow passage. For aerosol delivery during passive oral inhalation, the humidity nebulizer was not needed and only the drug nebulizer was retained. For HDS-OI testing, the drug nebulizer contained a model excipient enhanced growth (EEG) formulation with 0.25% w/v AS and 0.25% w/v NaCl. Due to the potential for higher airflow rates through the heating section with passive oral inhalation compared with the HFNC system, a more efficient heating section was required.
Computational fluid dynamic (CFD) simulations, using the methods described by Dutta et al. (39), were employed to investigate multiple heating section arrangements with the lead design including vertical fins running the length of the heating channel. As described in a subsequent section, designs with two and three longitudinal fins were compared. The best-case heat exchanger design was then 3D printed in aluminum using a Direct Metal Printing technique by 3D Systems Incorporated. This design production method was able to achieve fin thicknesses of 1.25 mm with channel surface roughness similar to cast aluminum. A sample view of the HDS-OI heating channel cross section with multiple fins is illustrated in Figure 2d. For the final design, total metal length was 163 mm with an airflow cross-sectional area of 261.3 mm2, which matched closely to the HDS-HFNC systems cross-sectional area of approximately 270 mm2. For construction of the heating section, a thin layer of high thermal conductivity paste was applied to rectangular Kapton heaters (KHLVA-105/5 Omega Engineering) before they were placed on the heat exchanger external upper and lower surfaces and the assembly was wrapped with aluminum tape. To sense heating plate temperature, a type-K thermocouple was attached to the center underside of the heat exchanger approximately 1 cm from the outlet before the aluminum wrap was applied.
Figure 3b provides an information flow diagram of the CMU operation for the HDS-OI system, which monitored temperature and breathing to control heating and nebulization via an Arduino Uno microcontroller system running custom code (40). Considering control of the heating region, the code processed temperature inputs from the mouthpiece and metal heat transfer regions along with airflow information to switch the heater input power among Full-Power, Half-Power, and No-Power states. Analytical analysis of the HFNC system found system operation requirements of a consistent power input of ~16 watts utilized for aerosol evaporation and ~5 watts utilized for heating of the air flow (12). Similar power requirements were needed for aerosol evaporation in the HDS-OI platform; however, it was expected that additional heat transfer capacity would be required due to cyclic periods of inhalation flow rate and nebulized aerosol production. The heating challenges of the OI system were addressed by utilizing a heat transfer region with a larger thermal mass, implementing the newly designed heat exchanger, and relaxing the inhalation temperature goal to allow for variations of a few degrees (~2 °C) around 30 °C. Additionally, temperature fluctuations at the start of inhalation were avoided due to the movement of ~37 °C air through the tubing and heating region during exhalation.
CMU nebulizer control was simplified for the HDS-OI platform based on the ability to assign a nominal baseline of zero gauge pressure (Figure 3b). Negative pressure readings indicated inhalation and positive pressure readings indicated exhalation. Additional details on the period of drug nebulization during active synchronization with inhalation are provided in the Nebulizer Duration Control section below.
Description of the HDS – Prototyping
The flow pathways of the HDS platforms (in addition to the metal printed heat exchanger of the OI system) were produced using 3D printing in materials that were conducive for drug quantification and, for the heating section shell, resistant to heat damage and off-gassing. Specifically, the rapid prototypes were produced using an Objet®24™ printer (Stratasys Inc, Eden Prairie, MN, USA) and VeroWhite™ (Stratasys Inc.) material. For cases where higher heat resistance was required, the heating section shell was rapid prototyped via stereolithography (SLA) in Accura ClearVue resin or via selective laser sintering in a heat resistant nylon material, DuraForm® PA (3D Systems, Rock Hill, SC, USA).
CFD Comparison of Heat Exchanger Designs
CFD simulations of the HDS-OI platform, with a focus on the heating region, were performed to develop designs that would maintain low aerosol depositional loss while boosting heat transfer efficiency. These simulations were conducted using ANSYS Fluent (v19) and included turbulent flow (Low Reynolds number k-ω turbulence model), heat and mass transfer, and aerosol transport. Two-way heat and mass transfer coupling was considered between the droplet and gas phases (12). A new polyhedral meshing strategy was implemented to maintain high mesh resolution near the walls (41). Additional details of the CFD simulations within the HDS are available in the previous study of Dutta et al. (39).
Figure 4 illustrates the HDS-OI CFD geometry and mesh. The CFD model included the OI platform inlet, nebulizer and mixing region, heating region (also referred to as the heat exchanger) and connective tubing. Thermal boundary conditions included a constant temperature metal heat exchanger surface (65 °C) and a thin wall conductive boundary applied to the connective tubing. After multiple initial iterations, longitudinal fins were selected as the best approach to increase heat transfer (including droplet evaporation) while minimizing deposition loss of the aerosol. The heating region was 16 cm long in all versions with an approximate height and width of 0.7 cm and 5 cm, respectively. A final CFD evaluation presented in this study compared the absence of a longitudinal fin with 1 and 3 fin designs. Previously measured initial droplet conditions were implemented for the Aerogen Solo mesh nebulizer (MMAD = 5.3 μm) (39), and simulations were conducted at a steady state flow rate of 30 L/min with inlet air conditions of 25 °C and 50% RH. Output metrics included temperature at the outlets of the heat exchanger and connective tubing, aerosol MMAD at these locations and deposition fraction in the mixing and heater regions of the HDS-OI platform.
Figure 4.
CFD geometry of the HDS-OI platform used to assess the impact of longitudinal fins in the heating region on aerosol evaporation. The polyhedral mesh used in the simulations is illustrated for two interior slices.
Adult Airway Models
Aerosol administration during HFNC therapy was tested with three previously developed adult NT replica geometries based on medical scans and referred to in this study as the Open, Subject A, and Subject B models (Figure 5a–c). The Open NT model has frequently been used by our group in evaluating nose-to-lung aerosol delivery using both CFD and in vitro methods (22, 37–39, 42–44) and is based on healthy adult males in an age range of 34–54 years old. The Open NT geometry was formed as an assembly of the classic nasal geometry dataset reported by Guilmette et al. (45) and an in-house matched CT scan to capture the pharynx, larynx and upper trachea (37). Subject A and Subject B NT models were previously developed by Walenga et al. (43) for testing nose-to-lung aerosol delivery with mask interfaces. Each model was extracted from the CT scan of an individual female subject with no visible nasal abnormalities and within the age range of 20–30 years old. Using a conventional NIV mask interface and vibrating mesh nebulizer, Walenga et al. (43) reported nasal depositional losses of 25.9% and 56.4% of nebulized dose in the Subject A and Subject B models, respectively, based on in vitro experiments. Considering multiple data sets, an expected ranking of nasal depositional loss in these NT models from lowest to highest was Open < Subject A < Subject B (43, 44). Further details of the selected nasal models for assessing geometric variability in nose-to-lung aerosol delivery are provided in Table I. Transverse and antero-posterior glottic dimensions were acquired by sectioning the model in the axial plane at the narrowest transverse dimension in the larynx. If the laryngeal ventricles were present in the model, the sectioning plane was angled to match the angle of the ventricles, which corresponds to the angle of the vocal folds.
Figure 5.
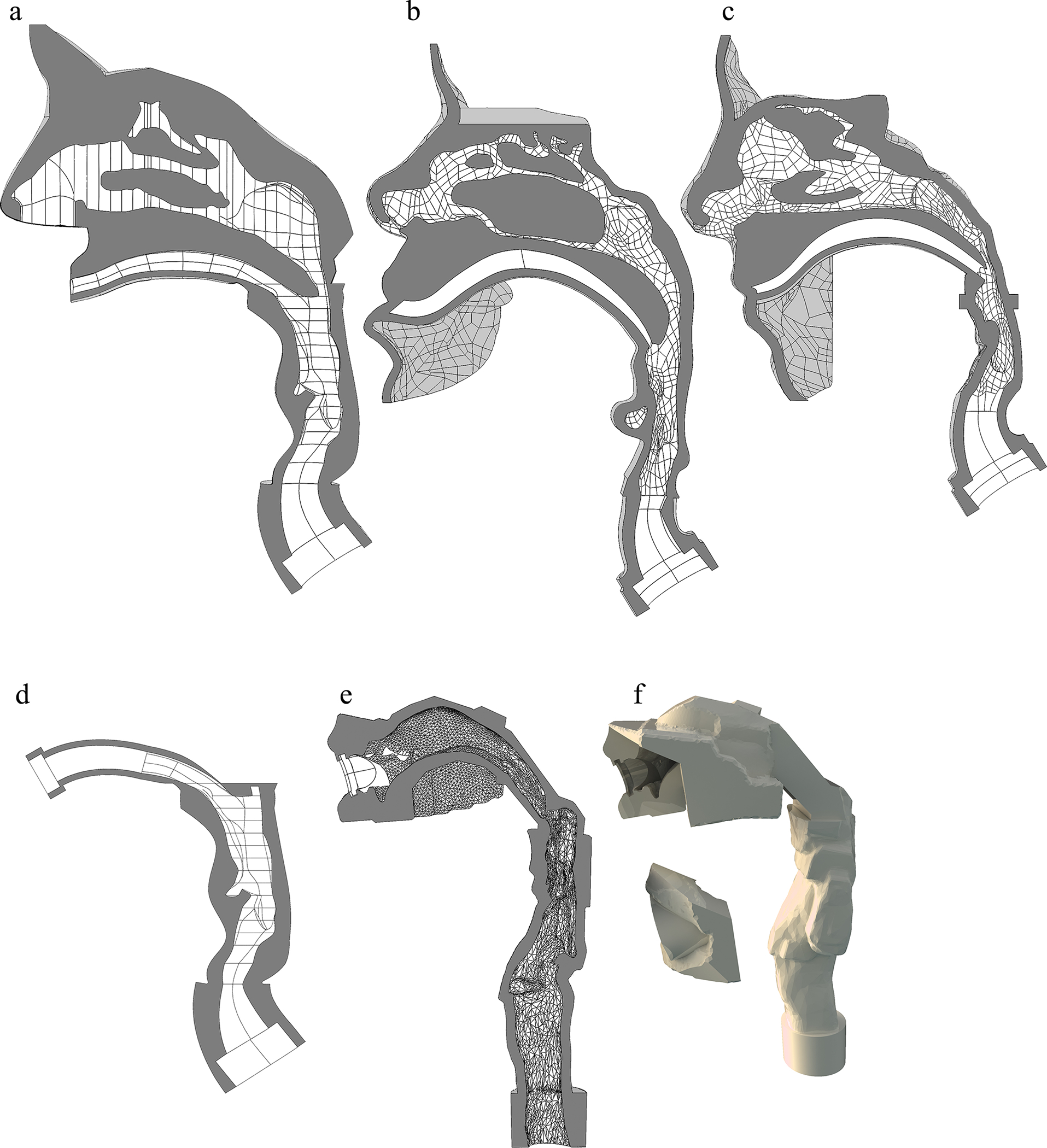
Airway geometry of the NT region illustrated with a midplane slice for the (a) Open NT, (b) Subject A NT and (c) Subject B NT models. Airway geometry of the MT region illustrated with a midplane slice for the (d) Open MT and (e) Realistic MT models; as well as the (f) outer shell of the Realistic MT model.
Table I:
Characteristics of the NT airway models
| Open NT | Subject A | Subject B | |
|---|---|---|---|
| Volume (cm3) | 10.83 | 50.19 | 38.54 |
| Surface Area (cm2) | 80.24 | 313.24 | 253.98 |
| SA/V (cm−1) | 7.41 | 6.24 | 6.59 |
| Glottic Dimensions (cm × cm) | 1.31 × 0.84 | 1.31 × 0.85 | 1.31 × 0.85 |
| Subject Age (years) | 34–54 | 30 | 20 |
| Subject Sex | Male | Female | Female |
| Source | Multiple Scans | Single Scan | Single Scan |
NT, nose-throat; SA, surface area; V, volume
For testing aerosol delivery with the HDS-OI platform, two adult MT geometries were selected (Fig. 5d–f). Both MT geometries were designed to connect with a similar MP opening, which consisted of a rounded slot profile with width and height of 26 and 9 mm, respectively. The first MT geometry was based on the Open NT model. The relatively thin oral passage of this “Open MT” model visible from the CT scan was smoothly expanded along the identified centerline to interface with the consistent MP rounded slit opening. In both MT geometries, the nasal region was excluded, which would be consistent with oral inhalation aerosol delivery without nasal inhalation, perhaps with the use of a nose clip. The second MT geometry was previously developed by Hosseini (46) based on a CT scan of a 55-year-old male without visible airway abnormalities and with an oral cavity that was well defined in the scan (Subject 5). The oral cavity of this model was expanded to accommodate the mouthpiece by rotating the jaw down and the addition of smoothly connected boundaries in the region of the cheeks. Additional modifications were made to allow insertion of different MP designs to form an airtight seal while maintaining structures representing the teeth and tongue. The resulting geometry is referred to as the Realistic MT model with designation of 5–10 and 5–14 referring to Subject 5 and the amount of additional jaw opening that was applied (10 or 14 mm, respectively). Further details on both MT geometries are provided in Table II. All airway models were rapid prototyped using stereolithography (SLA) in Accura ClearVue resin by 3D Systems On Demand Manufacturing (3D Systems Inc., Rock Hill, SC).
Table II:
Characteristics of the MT airway models
| Open MT | Realistic MT | |||
|---|---|---|---|---|
| Subject 5 | Subject 5–10 | Subject 5–14 | ||
| Volume (cm3) | 28.62 | 34.67 | 42.47 | 46.91 |
| Surface Area (cm2) | 101.03 | 141.48 | 153.97 | 160.92 |
| SA/V (cm−1) | 3.53 | 4.10 | 3.63 | 3.43 |
| Glottic Dimensions (cm × cm) | 1.31 × 0.84 | 1.62 × 0.69 | 1.62 × 0.69 | 1.62 × 0.69 |
| Subject Age (years) | 34–54 | 55 | 55 | 55 |
| Subject Sex | Male | Male | Male | Male |
| Associated MP | Base | --- | MP1 & MP3 | MP2 |
MP, mouthpiece; MT, mouth-throat; SA, surface area; V, volume
Patient Interfaces
To test aerosol delivery during HFNC therapy, a conventional nasal cannula interface was compared with a custom streamlined design. The conventional interface was the medium adult Optiflow Nasal Cannula MR850 (Fisher and Paykel, Irvine, CA) with a flexible elliptical prong outlet diameter of 4 × 5.5 mm (Figure 6a). The Optiflow cannula included a ~22 cm length of ~10 mm diameter flexible tubing that was connected to a shortened length of tubing from the HDS to maintain a total tubing length of 50 cm. Previous studies have indicated that the larger diameter Optiflow prongs generally provides the best lung delivery of aerosol administered during HFNC therapy among the commercially available options (47). The same medium prong Optiflow configuration was maintained in all three NT geometries to demonstrate flexibility in cannula size selection and to generate the real-world need for different baseline pressures in the HFNC active synchronization control routine.
Figure 6.
Patient interface nasal cannula including (a) Optiflow, (b) smallest streamlined and (c) largest streamlined versions; and MPs including the (d) Base case, (e) MP1, (f) MP2, (g) MP3 versions.
For comparison with the commercially available interface, a custom rigid nasal cannula was developed based on the previously introduced concept of streamlined designs (48, 49) for improved aerosol transmission in ventilation systems. Each streamlined cannula maintained the same cannula inlet bore diameter as the Optiflow but included prong spacing and elliptical prong outlet maximum and minimum diameters that were better matched to the NT model. The custom cannula shared design parameters of inlet angle, inward curvature, distance to diverging point, and outlet angle.
Considering oral inhalation aerosol delivery, a Base MP design with three additional iterations were considered (Figure 6d–g). The Base design consisted of a simple 90° bend and streamlined expansion from a lower approaching aerosol delivery tube to the MP outlet dimensions. The total centerline length of the Base MP design was ~7.3 cm.
The first MP iteration, denoted MP1, was similar to the Base design but include an upward angle outlet to help direct the aerosol over the tongue. The second mouthpiece, MP2, sought to decrease oral deposition by encouraging an increased oral opening, thereby lowering the tongue surface relative to the mouthpiece outlet flow. MP2 had 1 mm upper and side wall thickness but 4.2 mm lower wall thickness. As a result, MP2 required a more open mouth position but the same outlet dimensions available for aerosol flow. The third mouthpiece, MP3, utilized uniform 1 mm wall thickness but extended the lower wall along the flow path. This extension was intended to route the aerosol flow path up and over the tongue using a different approach compared with MP2. The three mouthpieces were rapid prototyped using stereolithography (SLA) in Accura ClearVue resin by 3D Systems On Demand Manufacturing (3D Systems Inc., Rock Hill, SC).
Breathing Waveforms
Both the HFNC and OI systems were tested with a variety of breathing waveforms in order to evaluate functioning of the active synchronization system and aerosol delivery through the extrathoracic models (Figure 7). For HFNC therapy, waveforms were based on instructing future subjects to inhale slowly and deeply through the nose during a period of drug delivery. Inhalation metrics were based on the in vivo study of Franca et al. (50) where subjects were supported with a different form of NIV (bi-level noninvasive ventilation) and were also instructed to inhale deeply. For HFNC therapy, a Repeating waveform was designed with a tidal volume of 1750 ml, an I:E ratio of 1:2, and a breath cycle length of 7.5 sec with all parameters held constant for each breath cycle (Table III). To test the baseline pressure adjustment algorithm and more realistic aerosol delivery, a similar HFNC Randomized waveform was also developed (Table III). This randomized waveform was comprised of a series of different breath cycles developed to have a wider parameter range. A total of 42 unique cycles all with 1225 ml tidal volume were developed with a combination of 7 different breath cycle lengths (within a range of 5.25–9.75 sec) and 6 different I:E ratios (within a range of 1:2–1:3). With the HFNC Randomized waveform, the resulting peak inhalation flow rates ranged from 36 to 88 L/min as compared to the 66 L/min of the HFNC Repeating waveform. Figure 7a illustrates three of the 42 breath cycles that comprised the HFNC Randomized waveform range (Min: all minimum parameters; Max: all maximum parameters; Mean: all mean parameters) alongside the breath cycle used for the HFNC Repeating waveform. With HFNC conditions, both the Repeating and Randomized waveforms employed sinusoidal inhalation and exhalation profiles.
Figure 7.
Breathing waveforms used with (a) HFNC and (b) OI aerosol delivery.
Table III:
Nasal breathing waveforms for testing the HDS-HFNC platform
| HFNC Repeating | HFNC Randomized | |
|---|---|---|
| Volume Tidal (mL) | 1750 | 1225 |
| Breath Cycle Length (sec) | 7.5 | 5.25 – 9.75 |
| I:E Ratio | 1:2 | 1:1.3 – 1:1.8 |
| Inhalation Length (sec) | 2.5 | 1.31 – 3.25 |
| Inhalation Peak Flow (L/min) | 65.97 | 35.52 – 87.96 |
I:E, inspiratory-to-expiratory
In testing the OI device, lower tidal volumes were employed to ensure that the system was sufficiently sensitive and that dead volume was not a significant factor for aerosol delivery efficiency. In a first waveform (OI Normal), the average resting tidal volume of adult males and females was employed (vT ~ 600 ml), based on ICRP data (51), with sinusoidal inhalation and exhalation profile shapes (Figure 7b). As with HFNC Randomized waveforms, the OI Normal waveform was comprised of a string of breath cycles created from random variations of the breath cycle length and the I:E ratio (Table IV). To test the actuation system with a different waveform, an OI Deep waveform was also developed with similar random variations but with a vT = 900 ml and trapezoidal profile shape (Table IV; Figure 7b). Peak inhalation flow rates ranged from 18 to 47 L/min for the OI Normal waveform and from 20 to 44 L/min for the OI Deep waveform. Figure 7b illustrates, for both the OI Normal and OI Deep, three (Min, Max and Mean) of the 42 breath cycles that comprised the waveforms.
Table IV:
Oral breathing waveforms for testing the HDS-OI platform
| Normal Inhalation | Deep Inhalation | |
|---|---|---|
| Waveform Shape | Sinusoidal | Trapezoidal |
| Volume Tidal (mL) | 600 | 900 |
| Breath Cycle Length (sec) | 4.2 – 7.8 | 4.2 – 7.8 |
| I:E Ratio | 1:1.5 – 1:2.5 | 1:1.3 – 1:1.8 |
| Inhalation Length (sec) | 1.20 – 3.12 | 1.50 – 3.39 |
| Inhalation Peak Flow (Min – Max) (L/min) |
18.12 – 47.12 | 19.60 – 44.31 |
| Inhalation Peak Flow Length (sec) | ----------- | 0.94 – 2.12 5/8 of Inhalation Length |
| Set Pressure of Inhalation (Pascal) | −5 | −5 |
| Set Pressure Delivery End (Pascal) | −30 | −30 |
| Set Time Delivery Length (sec) | 0.675 | 0.775 |
I:E, inspiratory-to-expiratory
Nebulizer Duration Control
For aerosol administration during HFNC therapy, previous CFD results with deep nasal inhalation identified a 1 sec optimal drug nebulization period to minimize environmental loss of the aerosol. As described, an adjustable baseline pressure was calculated by the CMU from pressure measurement readings using a Sensirion SDP800 pressure sensor with a measurement port located behind the mesh nebulizers. Pressure readings above the floating baseline pressure indicated nasal exhalation against the constant HFNC gas flow. With aerosol delivery during HFNC, the drug nebulizer was actuated (and the humidity nebulizer paused) 0.25 sec after the estimated start of inhalation for a period of 1 sec. This drug delivery period was halted early by the algorithm if nasal exhalation was sensed. Figure 8a illustrates the timing (horizontal axis) and respective flow rates (vertical axis) employed for active drug synchronization during HFNC aerosol delivery. The synchronization algorithm was designed to account for “False Inhalation Starts” using a floating baseline pressure (reset every 3 breaths) and delays arising from the CMU functioning and drug transport.
Figure 8.
Aerosol delivery timing diagram representations with time on the horizontal axis and representative flows and pressures on the vertical axis for (a) HFNC and (b) OI aerosol platforms.
With oral inhalation administration, aerosol drug delivery during a Set Pressure range and Set Time range were both explored. Set Pressure delivery administered the drug aerosol when the monitored pressure fell below −5 Pa (inhalation condition) and continued until the monitored pressure rose back above −30 Pa (near the end of inhalation). For Set Time administration, the drug nebulizer was actuated when the monitored pressure fell below −5 Pa and continued for approximately 1/3 of the average inhalation duration. In this study, the average inhalation duration was pre-set from the known breathing waveform; however, future software updates could allow for CMU determination and updating of the average inhalation duration within a specified window. In both Set Pressure and Set Time approaches, drug nebulization was halted if the monitored pressure rose above −5 Pa, which was used to indicate the end of inhalation. Extremely mild breathing profiles that do not exceed −5 Pa during inhalation would not trigger the drug nebulizer; however, setting the inhalation condition at 0 Pa would lead to false inhalation determinations due to both signal noise and false inhalation starts. To ensure that drug nebulizer was actuated, a green LED was included in the system and illuminated during the period of drug aerosol delivery. Active aerosol synchronization during inhalation with the HDS-OI platform is illustrated in Fig. 8b.
Experimental Procedure and Drug Quantification
Aerodynamic particle sizing experiments with the HDS-OI platform were performed using the Andersen Cascade Impactor (ACI; Copley Scientific, Nottingham, United Kingdom) operating at 28.3 L/min and housed in an environmental chamber (Espec, Hudsonville, MI), which was set to a temperature of 34°C and relative humidity (RH) of 30% to minimize effects of evaporation/condensation size changes during measurements. To determine the size distribution of the aerosol, the outlet of the HDS-OI delivery tubing was positioned in front of the ACI, which was oriented vertically. Drug was nebulized periodically for 1 sec intervals separated by a period of approximately 6 sec for a total of 60 cycles. For testing the OI system, room air was supplied to the device flow pathway. Airflow through the ACI was produced using a downstream vacuum pump at a constant flow rate. Aerodynamic particle sizing for the HDS-HFNC platform was previously measured to be ~1 μm at a 0.5% w/w solids concentration (12), which was consistent with analytical estimates of dried particle size and was not repeated in this study. Instead, analytic analysis was used to estimate the fully dried aerosol aerodynamic diameter (12).
In each aerosol delivery experiment from the HDS through the extrathoracic models, the nebulizers were preloaded with 750 μL of saline (humidity nebulizer) or drug solution (drug nebulizer). Connections of the full flow pathways for HFNC and OI delivery are illustrated in Figures 1a and 1b, respectively. After the breathing simulator was started with a specific inhalation waveform, the HDS was allowed to equilibrate and monitor the simulated breathing for a period of 10 breaths. After this warm-up period, the drug nebulizer was actuated (using one of the nebulizer duration control strategies) for a total of 60 cycles to determine regional drug delivery. Depositional areas of interest were the HDS flow pathway, delivery tubing, patient interface, extrathoracic model, and expected lung dose as estimated by drug capture on the tracheal filters. Drug deposited on the HDS components, interface, airway model and tracheal filter (or ACI plates for aerosol size distribution) was recovered and analyzed using a validated isocratic high-performance liquid chromatography (HPLC) method (52). Briefly, this analysis involved washing each component with known volumes of deionized water such that the drug concentration of the sample fell within the 0.5–16 μg/mL range of the AS calibration standards. The measured drug concentrations in the collected samples were then determined using quantitative HPLC analysis. The nominal dose of drug nebulized was determined by subtraction of the amount of drug remaining in the nebulizer reservoir (determined by quantitative HPLC) following each experiment from the initial mass of drug loaded into the nebulizer (determined from the known volume added and quantitative HPLC determination of its concentration). Regional drug mass deposition in the system components was calculated from the product of the measured drug concentration and the wash volume and expressed as a percentage of the nominal nebulized dose. The total recovered dose was reported as the sum of drug recovered from all deposition sites expressed as a percentage of the nominal nebulized dose. Initial experimental runs utilizing a continuous pull of air through the model, instead of the lung simulator, indicated virtually 100% recovery of drug. Therefore, in subsequent experimental runs with the lung simulator, exhaled dosages were reasonably approximated by the percentage of nebulized drug not recovered.
Statistical Analysis
Three or more measurements of each experiment were performed and used to calculate mean and standard deviation (SD) values. Statistical differences were tested using Student’s t-test and one-way or two-way ANOVA followed by Tukey’s HSD (p-value < 0.05). Evaluations were performed using JMP Pro (SAS Institute Inc., Cary, NC).
RESULTS
Aerosol Size Experiments
Cascade impaction measurements of the aerosol size exiting the HDS-OI delivery tubing indicated a mean (SD) MMAD of 1.0 (0.02) μm. Assuming an initially nebulized droplet size of 5.3 μm based on previous measurements (12) and using the HDS-OI solution formulation, the analytically predicted (12) dried geometric and aerodynamic particle diameters were 0.77 μm and 1.00 μm, respectively. Based on agreement between the measured and fully dried analytical aerodynamic diameters, the aerosol exiting the HDS-OI delivery tube was composed of almost completely dry particles. A similar analytical analysis applied to the HDS-HFNC system with the experimental drug concentration of 0.25% AS only, indicated dried geometric and aerodynamic particle diameters of 0.30 and 0.35 μm, respectively.
HDS-HFNC System Performance
Performance of the HDS-HFNC platform with the Repeating waveform is presented in Table V. Data is presented for individual NT models as well as data combined across the three NT models, which is captured in the column titled “All Models”. Considering the combined data, total recovery was high (~94%) indicating that only ~6% of the drug was lost to the environment. Combined NT depositional loss was also relatively low and in the range of 5–6%. The resulting delivery efficiency to the tracheal filter across all three geometries was 75–78% for All Models. Two-way ANOVA analysis accounting for the different NT models and cannulas indicated that the NT model had a significant impact on tracheal filter delivery. Subsequent post hoc Tukey HSD indicated that tracheal filter deposition following passage through the highly constricted Subject B Model was significantly different compared with the Open and Subject A models. The nasal cannula was not found to significantly impact tracheal filter delivery although it did affect the prong emitted dose. Additional statistical comparisons are presented in Table V.
Table V:
Performance of the HDS-HFNC platform across three NT models with the HFNC Repeating breathing waveform presented as mean (standard deviation) percentage of nebulized drug mass (n=3).
| Cannula | Open NT | Subject A NT | Subject B NT | All Modelsa | |||||
|---|---|---|---|---|---|---|---|---|---|
| Total Recovery | Optiflow | 95.0 (5.1) | 97.0 (0.7) | 91.2 (7.0) | 94.2 (4.7) | ||||
| Streamlined | 91.6 (2.5) | 92.8 (4.8) | 97.6 (2.5) | 94.0 (3.9) | |||||
| Tracheal Filter 1 | Optiflow | 76.9 (4.8) | 82.8 (1.4) | 66.1 (6.9) | 75.3 (8.0) | ||||
| Streamlined | 80.9 (2.8) | 80.9 (6.0) | 73.6 (1.5) | 78.4 (4.8) | |||||
| NT Deposition 1 | Optiflow | 4.5 (0.3) | 3.2 (0.5) | 8.8 (4.0) | 5.5 (3.1) | ||||
| Streamlined | 3.9 (0.7) | 4.8 (0.2) | 10.1 (0.1) | 6.3 (3.0) | |||||
| Prong Emitted 1,2 | Optiflow | 86.4 (1.3) | 89.0 (0.3) | 83.7 (3.7) | 86.4 (2.9) | ||||
| Streamlined | 93.1 (1.0) | 92.9 (1.4) | 86.1 (0.8) | 90.7 (3.7) | |||||
Combination of data from all three nasal models.
→ Significant effect of NT Model on Tracheal Filter, NT Deposition and Prong Emitted using two-way ANOVA with post hoc Tukey HSD showing Subject B was significantly different to Open and Subject A airway models
→ Significant effect of Cannula on Prong Emitted using two-way ANOVA with post hoc Students t-test showing Optiflow was significantly different to Streamlined
NT, nose-throat
Table VI provides performance of the HDS-HFNC platform with the Randomized breathing waveform. Considering the combined NT (“All Models”) data, randomizing the waveform increased environmental losses based on total recovery from approximately 6% with Repeated breathing to a range of 12–16% of nebulized dose. Compared with Repeating breathing, tracheal filter delivery was reduced by ~10% to a range of 65–72% for All Models. NT depositional loss remained 5–6% of the nebulized dose. With Randomized breathing, two-way ANOVA did not indicate a statistical difference in tracheal filter delivery considering the different NT models and cannula interfaces. Additional statistical comparisons are presented in Table VI.
Table VI:
Performance of the HDS-HFNC platform across three NT models with the HFNC Randomized breathing waveform presented as mean (standard deviation) percentage of nebulized drug mass (n=3).
| Open NT | Subject A NT | Subject B NT | All Modelsa | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total Recovery | Optiflow | 78.6 (10.5) | 84.9 (3.0) | 88.8 (5.3) | 83.6 (7.7) | ||||
| Streamlined | 84.3 (3.6) | 89.4 (4.4) | 90.4 (4.5) | 88.0 (4.6) | |||||
| Tracheal Filter | Optiflow | 61.4 (9.9) | 68.1 (2.9) | 67.8 (4.4) | 65.4 (6.7) | ||||
| Streamlined | 72.0 (3.0) | 78.4 (3.7) | 64.3 (6.9) | 71.6 (7.4) | |||||
| NT Deposition 1,2,3 | Optiflow | 5.7 (1.1) | 4.2 (0.4) | 7.6 (0.7) | 4.9 (1.4) | ||||
| Streamlined | 5.2 (0.5) | 3.5 (0.3) | 14.2 (2.3) | 6.6 (4.6) | |||||
| Prong Emitted 1,2,4 | Optiflow | 87.2 (1.0) | 86.8 (1.2) | 85.8 (2.7) | 87.9 (4.1) | ||||
| Streamlined | 92.1 (0.8) | 92.2 (0.6) | 86.6 (1.0) | 90.3 (2.8) | |||||
Combination of data from all three nasal models.
→ Significant effect of NT Model on NT Deposition and Prong Emitted using two-way ANOVA with post hoc Tukey HSD showing Subject B was significantly different to Open and Subject A airway models
→ Significant effect of Cannula on NT Deposition and Prong Emitted using two-way ANOVA with post hoc Students t-test showing Optiflow was significantly different to Streamlined
→ Significant interaction between Cannula and Model for NT Deposition with post hoc Tukey HSD showing an effect of Cannula for Subject B
→ Significant interaction between Cannula and Model for Prong Emitted with post hoc Tukey HSD showing an effect of Cannula for Subject A and Open airway model
NT, nose-throat
HDS-OI: CFD Design of the Heating Region
CFD simulation results of gas-phase heating and droplet transport are summarized in Figure 9 and Table VII. Enhanced heating is observed with the three fin design (Figure 9a–c), which resulted in improved droplet evaporation at the outlet of the delivery tube (Figure 9d–f). As captured in Table VII, the three fin design was required to produce an evaporated aerosol with an outlet temperature of ~36 °C at the HDS outlet. Cooling to ~30 °C at the delivery tube outlet did not increase the size of the aerosol. Furthermore, the three fin design only increased depositional loss by a marginal amount. As a result, the three fin design was selected for integration into the HDS-OI platform. Chamfering the connectors between the fins and outer surfaces (Figure 2d) was also found to be beneficial for producing very low heating region aerosol depositional loss.
Figure 9.
CFD analysis of (a-c) temperature contours and (d-f) droplet evaporation for design iterations of the HDS-OI heating region.
Table VII:
CFD predictions of HDS-OI heating region performance
| THDS_out (°C)a | TTube_out (°C)b | MMADHDS_out (μm) | MMADTube_out (μm) | Deposition Fraction (%) | |
|---|---|---|---|---|---|
| Zero Fins | 33.4 | 25.6 | 1.1 | 1.7 | 0.2 |
| One Fin | 34.5 | 26.3 | 1.1 | 1.5 | 0.7 |
| Three Fins | 36.3 | 29.6 | 1.1 | 1.1 | 1.6 |
cross-sectional mean temperature at junction of HDS heating region exit and tube entrance
cross-sectional mean temperature at junction of tube exit and mouthpiece entrance
CFD, computational fluid dynamics; HDS, heated dryer system; T, temperature
HDS-OI Platform Performance
Aerosol delivery performance for the HDS-OI platform for Normal and Deep inhalation is presented in Table VIII. It is noted that both breathing waveforms included randomized variability. Drug recovery was near 100% in all cases indicating negligible aerosol loss to the environment. Open MT model depositional loss across both breathing waveforms and both nebulizer duration controls (Set Pressure and Set Time) was ~1%. Considering tracheal filter delivery, nebulizer duration control with a Set Time was statistically better than with a Set Pressure (~85% vs. ~59%, respectively). No statistical difference was observed for tracheal filter delivery between OI Normal inhalation and OI Deep inhalation. Additional statistical comparisons are presented in Table VIII.
Table VIII.
Performance of the HDS-OI platform for Normal and Deep breathing waveforms (Randomized) using the Open MT model presented as mean (standard deviation) percentage of nebulized drug mass (n=3).
| Normal Inhalation | Deep Inhalation | ||
|---|---|---|---|
| Neb Skirt | Set Pressure | 1.47 (1.86) | 5.39 (2.58) |
| Set Time | 1.06 (0.42) | 1.17 (0.73) | |
| Inlet Filter 1,2,3 | Set Pressure | 28.39 (0.92) | 20.26 (0.36) |
| Set Time | 3.86 (0.63) | 1.79 (0.44) | |
| HDS | Set Pressure | 3.30 (1.86) | 3.96 (0.12) |
| Set Time | 4.27 (0.70) | 1.82 (0.51) | |
| Tubing 4 | Set Pressure | 5.37 (2.57) | 10.25 (1.09) |
| Set Time | 7.75 (0.28) | 7.04 (1.23) | |
| Base MP | Set Pressure | 0.65 (0.15) | 0.88 (0.15) |
| Set Time | 1.03 (0.26) | 1.00 (0.07) | |
| Open MT Deposition | Set Pressure | 1.14 (0.03) | 0.72 (0.08) |
| Set Time | 1.22 (0.44) | 1.01 (0.36) | |
| Tracheal Filter 2 | Set Pressure | 59.29 (4.46) | 58.36 (3.61) |
| Set Time | 85.34 (1.12) | 86.00 (2.23) | |
| Recovery | Set Pressure | 99.62 (2.40) | 99.83 (1.18) |
| Set Time | 104.53 (2.01) | 99.84 (3.14) | |
| Device Total 5 | Set Pressure | 10.79 (6.28) | 20.49 (3.72) |
| Set Time | 14.11 (1.08) | 11.04 (1.81) | |
→ Significant effect of Waveform on Inlet Filter using two-way ANOVA with post hoc Students t-test showing Normal Inhalation was significantly different to Deep Inhalation
→ Significant effect of Nebulizer Duration on Inlet Filter and Tracheal Filter using two-way ANOVA with post hoc Students t-test showing Set Pressure was significantly different to Set Time
→ Significant interaction between Waveform and Nebulizer Duration for Inlet Filter
→ Significant interaction between Waveform and Nebulizer Duration for Tubing
→ Significant interaction between Waveform and Nebulizer Duration for Device Total
HDS, heated dryer system; MP, mouthpiece; MT, mouth-throat
Table IX compares the effect of HDS-OI MP design on regional deposition performance for the case of Set Time nebulizer duration and the OI Deep waveform. In these experiments, the Realistic Subject 5 MT model was employed. For all MP designs, tracheal filter delivery was high with a range of 88.0% to 92.9%. Furthermore, HDS, MP and MT depositional losses were all <1%. No statistical differences were observed among the MPs for all metrics tested. Variability as quantified by SD was somewhat high for the tracheal filter delivery, which was likely due to the random nature of the breathing waveform.
Table IX.
Performance of the HDS-OI platform across three different MPs with the OI Deep breathing waveform (Randomized) using the Realistic MT model presented as mean (standard deviation) percentage of nebulized drug mass (n=3).
| MP1 | MP2 | MP3 | |
|---|---|---|---|
| Neb Skirt | 0.6 (0.3) | 0.7 (0.5) | 0.9 (0.5) |
| Inlet Filter | 2.8 (0.5) | 3.1 (1.3) | 3.5 (1.1) |
| HDS | 0.8 (0.1) | 0.7 (0.1) | 0.9 (0.2) |
| Tubing | 1.9 (0.2) | 1.4 (0.3) | 1.8 (0.3) |
| Mouthpiece | 0.2 (0.0) | 0.2 (0.0) | 0.2 (0.0) |
| MT Deposition | 0.6 (0.1) | 0.6 (0.1) | 0.6 (0.1) |
| Tracheal Filter | 88.0 (6.5) | 88.2 (10.3) | 92.9 (4.7) |
| Recovery | 94.9 (6.5) | 94.8 (10.6) | 100.9 (6.4) |
One-way ANOVA tests show no significant difference among mouthpieces for any deposition metrics
HDS, heated dryer system; MP, mouthpiece; MT, mouth-throat
DISCUSSION
A primary finding of this study is that active synchronization of the HDS-HFNC platform can be achieved through sensing changes in upstream backpressure. As illustrated in Figure 1a, the pressure port of the HDS-HFNC system is located between the gas delivery line and nebulizers. Changes in this pressure profile, arising from the downstream simulated subject breathing through and against the nasal cannula, were used to establish the period of inhalation and synchronize the nebulization of drug. Using this process with the Repeating breathing waveform and averaging across multiple nasal geometries, only ~6% of the nebulized aerosol was lost to the environment, NT loss was ~6% and lung delivery efficiency was 75–78% of nebulized dose. For comparison, the previous study of Dutta et al. (39) employed a similar HDS-HFNC platform and triggered the drug nebulizer based on an inhalation signal from the artificial lung simulator. Using this carefully controlled approach, lung delivery efficiency of 81% was achieved. The similarity between the lung delivery efficiency values in this study with backpressure sensing across multiple nasal models compared with the near ideal of artificial lung signaling reported by Dutta et al. (39) indicates that the backpressure sensing control algorithm worked well.
This study is the first to predict intersubject variability in lung aerosol delivery efficiency from the HDS-HFNC platform as well as the impact of Randomized breathing patterns. Including both multiple NT models and Randomized deep nasal breathing resulted in a lung delivery efficiency range of 61.4–78.4% of nebulized dose. While this range is lower and wider than observed with Repeated breathing (66.1–80.9%), it still appears impressive considering that the active synchronization system had to determine a different baseline pressure for each subject and apply it to a randomly occurring breathing pattern. Of the airway models, Subject B showed the most nasal depositional loss, which was consistent with our previous studies where the Subject B model had approximately 2-fold more depositional aerosol loss compared with the Subject A model (53). The Open and Subject A models showed similar amounts of nasal depositional loss. Across all breathing waveforms, cannulas and NT geometries, the total range of nasal depositional loss was 3.2–14.2%.
A surprising outcome of the HDS-HFNC testing was that the streamlined cannula did not significantly reduce cannula and nasal deposition fractions. Our previous studies have highlighted how streamlined nasal cannula improve aerosol transmission and reduce depositional loss in the nasal cavity region (11, 36, 48). One potential reason for the lack of a streamlined nasal cannula impact in the current study is the use of a low solution concentration in the drug nebulizer. As described, the 0.25% AS concentration produced an analytically predicted aerodynamic diameter of 0.35 μm. Based on the good agreement between analytical predictions and measured aerosol size for the HDS-OI platform, we did not measure the dry particle size with cascade impaction of the HDS-HFNC platform. However, it is expected that this aerosol is sufficiently small (~350 nm) such that inertial impaction is very low and streamlining the cannula is not necessary to reduce depositional loss. Furthermore, successful use of EEG lung delivery of the HDS-HFNC aerosol will require a larger initial size, which produces a larger final size, as well as the presence of a growth excipient (16, 54). These changes are not expected to largely impact the overall outcome of this study considering that in Dutta et al. (39) we considered a solution concentration of 0.25% AS and 0.25% NaCl that produced a dried 1 μm aerosol from the HDS-HFNC system. The resulting device, NT and tracheal filter deposition efficiencies were 12.2, 4.2 and 81.1%, respectively (39).
This study is the first to evaluate performance of the HDS for direct oral inhalation of EEG aerosol. Production of the aerosol with a conventional mesh nebulizer helps to maintain a high drug mass output rate. Drying this aerosol then forms micrometer or submicrometer EEG particles for direct inhalation. Inclusion of the NaCl hygroscopic excipient will increase aerosol size within the lungs through hygroscopic water uptake, thereby reducing the potential for dose exhalation and helping to target the region of deposition. Total device and interface losses were <8% of the nebulized dose. Furthermore, MT depositional loss was <1% and lung delivery efficiency was in the range of 88.0–92.9%, even with randomized breathing (Table IX). In comparison with other high-performance nebulizer systems for oral aerosol inhalation, i.e., the AKITA2 APIXNEB and Dance 501 systems, performance of the HDS-OI platform reduced MT deposition by an order of magnitude and increased lung delivery efficiency by an absolute value of 20%. By nearly eliminating MT depositional loss, intersubject variability in lung delivery efficiency is also expected to be very low (55). The current HDS-OI platform is larger than some current hand-held nebulizer devices for oral inhalation. A more compact HDS-OI platform may be possible. However, the current table-top version of the platform may be beneficial for high dose applications, extended delivery times, and radiolabeled aerosol testing where it is advantageous to have a separation distance between the subject and nebulizer source.
With the HDS-OI platform, selection of the MT model and MP design had little impact on performance. First, deposition tended to be slightly lower for the Realistic MT geometry (i.e., <1%) compared with the Open MT (i.e., 0.72 vs. 1.22%). This was somewhat surprising considering that the Realistic MT geometry appeared narrower and more tortuous than the Open model. The slight reduction in MT loss with the Realistic MT model is most likely because the three iterative MP designs tested with this geometry had streamlined profiles and helped direct the aerosol over the tongue. None of the MP iterations demonstrated a statistical advantage. However, MP3 produced >90% lung delivery efficiency and also produced the lowest standard deviation (in tracheal filter deposition). As a result, this curved upward angle design is recommended for use with the HDS-OI platform in the future. Set Time nebulizer duration control was also shown to be superior to Set Pressure. Only minor differences were observed in HDS-OI performance between Normal and Deep oral breathing.
One limitation of this study in testing the HDS-HFNC platform is the absence of head motion from the simulated subject. In human subject testing of the active synchronization control algorithm (56, 57), it was observed that subject head motion would cause the nasal cannula to shift and alter the baseline pressure. This could result in several missed breaths by the nebulizer control unit until the baseline pressure was reset automatically with each three breaths. Another potential limitation with the HFNC system is the testing of two deep nasal inhalation waveforms without testing a shallow nasal inhalation condition. Based on unchanging performance of the OI system between normal and deep inhalation, it is expected that normal (vs. deep) nasal inhalation will not significantly degrade performance of the HFNC system, but this inference needs future verification. As described earlier, the HFNC system aerosol was likely too small for EEG targeted lung deposition. However, in previous work we have demonstrated that increasing the aerosol size to a more desirable values of ~1 μm has minimal impact on device and extrathoracic losses (39).
In conclusion, it was determined that both the HDS-HFNC and HDS-OI platforms could achieve high efficiency delivery of nebulized pharmaceutical aerosols to the lungs across a range of adult extrathoracic anatomies, patient interfaces and breathing conditions. Active synchronization was effectively included in each platform to sense the subject’s breathing profile and actuate the drug nebulizer during a portion of inhalation. In both cases, pressure measurements were made away from the subject and in the HDS unit, which avoided the complexity of pressure measurement ports or velocity sensors in the patient interface. The use of upstream backpressure to actively synchronize aerosol delivery with inhalation during HFNC was demonstrated for the first time and found to work well. For the HDS-HFNC platform, total system loss was <10%, extrathoracic deposition was approximately 6% and best-case lung delivery efficiency was 75–78% of nebulized dose. Inclusion of randomized breathing with the HFNC system decreased lung delivery efficiency by ~10% and had no impact on nasal depositional loss. For the HDS-OI platform and best-case MP, total depositional system loss was <8%, extrathoracic deposition was <1%, and lung delivery efficiency was >90% of nebulized dose. Normal vs. Deep oral inhalation had little impact on performance of the HDS-OI platform. Considering aerosol release to the environment, estimates based on drug recovery for the HDS-HFNC platform with repeated and randomized breathing were ~6–15%. Environmental aerosol release was virtually eliminated with the HDS-OI platform due to filtration of the exhaled air. Considering recent successful human subject safety testing of the HDS (56), both the HDS-HFNC and IO platforms appear ready for testing lung delivery efficiency in human subjects with gamma scintigraphy or for evaluating the effect of nebulized therapeutics that require high lung delivery efficiency with low intersubject variability or regionally targeted lung deposition through the use of the EEG concept.
Funding Statement
Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number R01HL107333. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Author Disclosure Statement
Virginia Commonwealth University is currently pursuing patent protection of devices and methods described in this study, which if licensed and commercialized, may provide a future financial interest to the authors. MH is a member of the Editorial Board of AAPS PharmSciTech.
REFERENCES
- 1.Carvalho TC, McConville JT. The function and performance of aqueous aerosol devices for inhalation therapy. J Pharm Pharmacol. 2016;68(5):556–78. [DOI] [PubMed] [Google Scholar]
- 2.Laube BL. Aerosolized medications for gene and peptide therapy. Respiratory Care. 2015;60(6):806–24. [DOI] [PubMed] [Google Scholar]
- 3.Martin AR, Finlay WH. Nebulizers for drug delivery to the lungs. Expert Opin Drug Deliv. 2014;12(6):889–900. [DOI] [PubMed] [Google Scholar]
- 4.Rubin BK, Williams RW. Emerging aerosol drug delivery strategies: from bench to clinic. Advanced Drug Delivery Reviews. 2014;75:141–8. [DOI] [PubMed] [Google Scholar]
- 5.Ari A, Fink JB. Guidelines for aerosol devices in infants, children and adults: which to choose, why and how to achieve effective aerosol therapy. Expert review of respiratory medicine. 2011;5(4):561–72. [DOI] [PubMed] [Google Scholar]
- 6.Hess DR. Aerosol therapy during noninvasive ventilation or high-flow nasal cannula. Respiratory Care. 2015;60(6):880–93. [DOI] [PubMed] [Google Scholar]
- 7.Dhand R Aerosol delivery during mechanical ventilation: From basic techniques to new devices. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2008;21(1):45–60. [DOI] [PubMed] [Google Scholar]
- 8.Dhand R Aerosol therapy in patients receiving noninvasive positive pressure ventilation. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2012;25(2):63–78. [DOI] [PubMed] [Google Scholar]
- 9.Ari A, Dornelas de Andrade A, Sheard M, AlHamad B, Fink JB. Performance comparisons of jet and mesh nebulizers using different interfaces in simulated spontaneously breathing adults and children. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2015;28(4):281–9. [DOI] [PubMed] [Google Scholar]
- 10.Ari A, Fink JB. Inhalation therapy in patients receiving mechanical ventilation: an update. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2012;25(6):319–32. [DOI] [PubMed] [Google Scholar]
- 11.Longest W, Spence B, Hindle M. Devices for Improved Delivery of Nebulized Pharmaceutical Aerosols to the Lungs. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2019;32(5):317–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spence BM, Longest PW, Wei X, Dhapare S, Hindle M. Development of a high flow nasal cannula (HFNC) and pharmaceutical aerosol combination device. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2019;32(4):224–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hindle M, Longest PW. Condensational growth of combination drug-excipient submicrometer particles for targeted high efficiency pulmonary delivery: Evaluation of formulation and delivery device. J Pharm Pharmacol. 2012;64(9):1254–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Longest PW, Tian G, Li X, Son Y-J, Hindle M. Performance of combination drug and hygroscopic excipient submicrometer particles from a softmist inhaler in a characteristic model of the airways. Annals of Biomedical Engineering. 2012;40(12):2596–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Longest PW, Hindle M. Condensational growth of combination drug-excipient submicrometer particles: Comparison of CFD predictions with experimental results. Pharmaceutical Research. 2012;29(3):707–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Longest PW, Hindle M. Numerical model to characterize the size increase of combination drug and hygroscopic excipient nanoparticle aerosols. Aerosol Science and Technology. 2011;45:884–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian G, Longest PW, Li X, Hindle M. Targeting aerosol deposition to and within the lung airways using excipient enhanced growth. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2013;26(5):248–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parkes SN, Bersten AD. Aerosol kinetics and bronchodilator efficacy during continuous positive airway pressure delivered by face mask. Thorax. 1997;52(2):171–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galindo-Filho VC, Ramos ME, Rattes CSF, Barbosa AnK, Brandà DC, Brandão SCS, et al. Radioaerosol pulmonary deposition using mesh and jet nebulizers during noninvasive ventilation in healthy subjects. Respiratory Care. 2015;60(9):1238–46. [DOI] [PubMed] [Google Scholar]
- 20.Smaldone GC, Berg E, Nikander K. Variation in pediatric aerosol delivery: Importance of facemask. Journal of Aerosol Medicine-Deposition Clearance And Effects In The Lung. 2005;18(3):354–63. [DOI] [PubMed] [Google Scholar]
- 21.Perry SA, Kesser KC, Geller DE, Selhorst DM, Rendle JK, Hertzog JH. Influences of Cannula Size and Flow Rate on Aerosol Drug Delivery Through the Vapotherm Humidified High-Flow Nasal Cannula System. Pediatr Crit Care Med. 2013;14(5):E250–E6. [DOI] [PubMed] [Google Scholar]
- 22.Golshahi L, Longest PW, Azimi M, Syed A, Hindle M. Intermittent aerosol delivery to the lungs during high flow nasal cannula therapy. Respiratory Care. 2014;59(10):1476–86. [DOI] [PubMed] [Google Scholar]
- 23.Reminiac F, Vecellio L, Heuze-Vourc’h N, Petitcollin A, Respaud R, Cabrera M, et al. Aerosol therapy in adults receiving high flow nasal cannula oxygen therapy. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2016;doi: 10.1089/jamp.2015.1219. [DOI] [PubMed] [Google Scholar]
- 24.Réminiac F, Vecellio L, Loughlin RM, Le Pennec D, Cabrera M, Vourc’h NH, et al. Nasal high flow nebulization in infants and toddlers: an in vitro and in vivo scintigraphic study. Pediatric Pulmonology. 2017;52(3):337–44. [DOI] [PubMed] [Google Scholar]
- 25.Ari A, Harwood R, Sheard M, Dailey P, Fink JB. In vitro comparison of heliox and oxygen in aerosol delivery using pediatric high flow nasal cannula. Pediatric Pulmonology. 2011;46(8):795–801. [DOI] [PubMed] [Google Scholar]
- 26.Dugernier J, Hesse M, Jumetz T, Bialais E, Roeseler J, Depoortere V, et al. Aerosol delivery with two nebulizers through high-flow nasal cannula: A randomized cross-over single-photon emission computed tomography study. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2017;30(5):349–58. [DOI] [PubMed] [Google Scholar]
- 27.Bennett G, Joyce M, Sweeney L, MacLoughlin R. In Vitro Determination of the Main Effects in the Design of High-Flow Nasal Therapy Systems with Respect to Aerosol Performance. Pulmonary Therapy. 2018;4(1):73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dailey PA, Harwood R, Walsh K, Fink JB, Thayer T, Gagnon G, et al. Aerosol delivery through adult high flow nasal cannula with heliox and oxygen. Respiratory Care. 2018;62(9):1186–92. [DOI] [PubMed] [Google Scholar]
- 29.Ward JJ. High-flow oxygen administration by nasal cannula for adult and perinatal patients. Respiratory Care. 2013;58(1):98–120. [DOI] [PubMed] [Google Scholar]
- 30.Jones PG, Kamona S, Doran O, Sawtell F, Wilsher M. Randomized controlled trial of humidified high-flow nasal oxygen for acute respiratory distress in the emergency department: the HOT-ER study. Respiratory Care. 2016;61(3):291–9. [DOI] [PubMed] [Google Scholar]
- 31.Spoletini G, Alotaibi M, Blasi F, Hill NS. Heated humidified high-flow nasal oxygen in adults: mechanisms of action and clinical implications. CHEST. 2015;148(1):253–61. [DOI] [PubMed] [Google Scholar]
- 32.Zeman KL, Rojas Balcazar J, Fuller F, Donn KH, Boucher RC, Bennett WD, et al. A trans-nasal aerosol delivery device for efficient pulmonary deposition. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2017;30(4):223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dugernier J, Hesse M, Vanbever R, Depoortere V, Roeseler J, Michotte J-B, et al. SPECT-CT Comparison of Lung Deposition using a System combining a Vibrating-mesh Nebulizer with a Valved Holding Chamber and a Conventional Jet Nebulizer: a Randomized Cross-over Study. Pharmaceutical Research. 2017;34:290–300. [DOI] [PubMed] [Google Scholar]
- 34.Brand P, Schulte M, Wencker M, Herpich C, Klein G, Hanna K, et al. Lung deposition of inhaled α 1-proteinase inhibitor in CF and α 1-antitrypsin deficiency. European Respiratory Journal. 2009. [DOI] [PubMed] [Google Scholar]
- 35.Fink JB, Molloy L, Patton JS, Galindo-Filho VC, de Melo Barcelar J, Alcoforado L, et al. Good Things in Small Packages: an Innovative Delivery Approach for Inhaled Insulin. Pharmaceutical research. 2017;34(12):2568–78. [DOI] [PubMed] [Google Scholar]
- 36.Longest PW, Walenga RL, Son Y-J, Hindle M. High efficiency generation and delivery of aerosols through nasal cannula during noninvasive ventilation. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2013;26(5):266–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Golshahi L, Tian G, Azimi M, Son Y-J, Walenga RL, Longest PW, et al. The use of condensational growth methods for efficient drug delivery to the lungs during noninvasive ventilation high flow therapy. Pharmaceutical Research. 2013;30:2917–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Golshahi L, Walenga RL, Longest PW, Hindle M. Development of a transient flow aersol mixer-heater system for lung delivery of nasally administered aerosols using a nasal cannula. Aerosol Science and Technology. 2014;48:1009–21. [Google Scholar]
- 39.Dutta R, Spence B, Wei X, Dhapare S, Hindle M, Longest PW. CFD Guided Optimization of Nose-to-Lung Aerosol Delivery in Adults: Effects of Inhalation Waveforms and Synchronized Aerosol Delivery. Pharmaceutical Research. 2020;37(10):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spence BM. Nebulizer-based Systems to Improve Pharmaceutical Aerosol Delivery to the Lungs. PhD Dissertation, Virginia Commonwealth University, Richmond, VA. 2021. [Google Scholar]
- 41.Thomas ML, Longest PW. Evaluation of the polyhedral mesh style for predicting aerosol deposition in representative models of the conducting airways. Journal of Aerosol Science. 2021:105851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Longest PW, Golshahi L, Behara SRB, Tian G, Farkas DR, Hindle M. Efficient nose-to-lung (N2L) aerosol delivery with a dry powder inhaler. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2015;28(3):189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walenga RL, Longest PW, Kaviratna A, Hindle M. Aerosol drug delivery during noninvasive positive pressure ventilation: Effects of intersubject variability and excipient enhanced growth. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2017;30(3):190–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walenga RL, Tian G, Hindle M, Yelverton J, Dodson K, Longest PW. Variability in nose-to-lung aerosol delivery. Journal of Aerosol Science. 2014;78:11–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guilmette RA, Wicks JD, Wolff RK. Morphometry of human nasal airways in vivo using Magnetic Resonance Imaging. Journal of Aerosol Medicine. 1989;2(4):365–77. [Google Scholar]
- 46.Hosseini S EVALUATION OF REGIONAL EXTRATHORACIC DEPOSITION OF LOW-AND HIGH-MOMENTUM AEROSOL USING ANATOMICALLY-CORRECT IN VITRO AIRWAY MODELS. PhD Dissertation, Virginia Commonwealth University, Richmond, VA. 2021. [Google Scholar]
- 47.Dugernier J, Reychler G, Vecellio L, Ehrmann S. Nasal high-flow nebulization for lung drug delivery: theoretical, experimental, and clinical application. Journal of aerosol medicine and pulmonary drug delivery. 2019;32(6):341–51. [DOI] [PubMed] [Google Scholar]
- 48.Longest PW, Golshahi L, Hindle M. Improving pharmaceutical aerosol delivery during noninvasive ventilation: Effects of streamlined components. Annals of Biomedical Engineering. 2013;41(6):1217–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Longest PW, Azimi M, Golshahi L, Hindle M. Improving aerosol drug delivery during invasive mechanical ventilation with redesigned components. Respiratory Care. 2014;59(5):686–98. [DOI] [PubMed] [Google Scholar]
- 50.Franca EET, de Andrade AFD, Cabral G, Almeida P, Silva KC, Galindo VC, et al. Nebulization associated with Bi-level noninvasive ventilation: Analysis of pulmonary radioaerosol deposition. Respiratory Medicine. 2006;100(4):721–8. [DOI] [PubMed] [Google Scholar]
- 51.ICRP. Human Respiratory Tract Model for Radiological Protection. New York: Elsevier Science Ltd.; 1994. [Google Scholar]
- 52.Delvadia RR, Longest PW, Hindle M, Byron PR. In Vitro Tests for Aerosol Deposition. III: Effect of Inhaler insertion angle on aerosol deposition. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2013;26(3):145–56. [DOI] [PubMed] [Google Scholar]
- 53.Walenga RL, Longest PW, Kaviratna A, Hindle M. Aerosol drug delivery during noninvasive positive pressure ventilation: Effects of intersubject variability and excipient enhanced growth. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2016;DOI: 10.1089/jamp.2016.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tian G, Hindle M, Longest PW. Targeted lung delivery of nasally administered aerosols. Aerosol Science and Technology. 2014;48(4):434–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Borgstrom L, Olsson B, Thorsson L. Degree of throat deposition can explain the variability in lung deposition of inhaled drugs. Journal of Aerosol Medicine. 2006;19:473–83. [DOI] [PubMed] [Google Scholar]
- 56.Spence B, DeWilde C, Priday A, Syed A, Dhapare S, Wei X, et al. Implementation of a combination device for high efficiency aerosol delivery and high-flow nasal cannula therapy in adult human subjects. Respiratory Drug Delivery 2020. 2020;3:723–8. [Google Scholar]
- 57.Spence B, Momin M, Dhapare S, Wei X, Longest PW, Hindle M. Synchronizing high efficiency aerosol delivery with inhalation during high flow nasal cannula therapy. Respiratory Drug Delivery 2020. 2020;3:712–22. [Google Scholar]


