Abstract
Since ancient times, viruses such as dengue, herpes, Ebola, AIDS, influenza, chicken meat, and SARS have been roaming around causing great health burdens. Currently, the prescribed antiviral drugs have not cured the complications caused by viruses, whereas viral replication was not controlled by them. The treatments suggested are not only ineffectual, but also sometimes inefficient against viruses at all stages of the viral cycle as well. To fight against these contagious viruses, people rely heavily on medicinal plants to enhance their innate and adaptive immune systems. In this research, the preparation of ligands and proteins was performed using the Maestro V.13.2 module tool. This software, consisting of LigPrep, Grid Generation, SiteMap, and Glide XP, has each contributed significantly to the preparation of ligands and proteins. Ultimately, the research found that (R)-(+)-rosmarinic acid was found to have significant docking scores of − 10.847 for herpes virus, of − 10.033 for NS5, and − 7.259 for NS1. In addition, the Prediction of Activity Spectra for Substances (PASS) server indicates that rosmarinic acid possesses a diverse spectrum of enzymatic activities, as probability active (Pa) values start at > 0.751, whereas it has fewer adverse effects than the drugs prescribed for viruses. Accordingly, it was found the rate of acute toxicity values of (R)-(+)-rosmarinic acid at doses LD50 log10 (mmol/g) and LD50 (mg/g) in different routes of administration, such as intraperitoneal, intravenous, oral, and subcutaneous. Ultimately, the present study concluded that (R)-(+)-rosmarinic acid would expose significant antiviral effects in in vitro and in vivo experiments, and this research would be a valuable asset for the future, especially for those who wish to discover a drug molecule for a variety of viruses.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s43450-023-00381-y.
Keywords: Antiviral drug, Biomedical profile, Acute toxicity, MM-GBSA, Pharmacophore
Introduction
Viral infections have been a major cause of morbidity and mortality worldwide since the twentieth century. In particular, viruses such as dengue, herpes virus, Ebola, AIDS, influenza, and SARS are currently roaming the world as pandemics and epidemics (Ben-Shabat et al. 2019). Of concern is that human mortality from dengue and herpes virus infections is significantly increasing (Fig. 1). The fact that the number of dengue cases has increased eightfold over the past 20 years, from 505,430 cases in 2000 to over 2.4 million in 2010 and 5.2 million in 2019, supports this alarm (WHO 2022). On the other hand, worldwide 491 million people between the ages of 15 and 49 are reported to have HSV-2 infection, while 3.7 billion people under the age of 50 are reported to have HSV-1 infection (WHO 2022). To fight the virus particles and inhibit their replication in the body, almost ninety types of antiviral drugs are available today, but one drug only controls one virus. Similar to these drugs, the classic antiviral drugs such as interferon and ribavirin have antiviral potential against most viruses in in vitro studies but often showed no effect in infected people during treatment (Ben-Shabat et al. 2019). Currently prescribed antiviral drugs are expensive, ineffective against some viruses and have harmful side effects on the human body with long-term use.
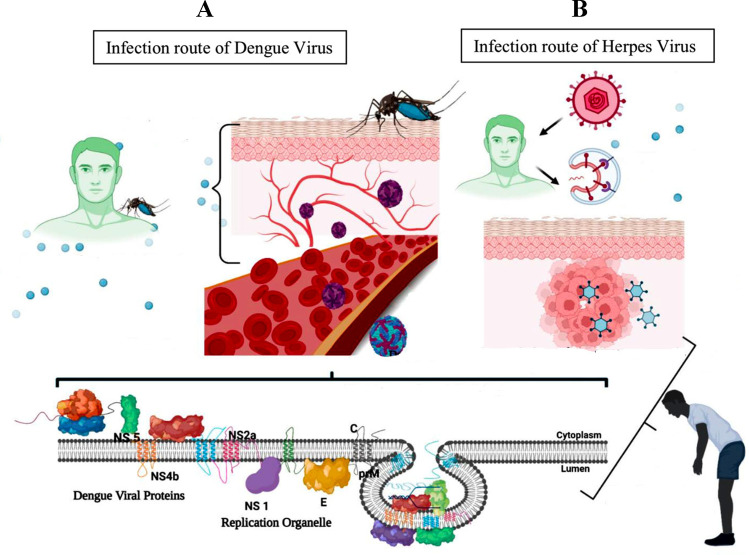
Fig. 1.
Route of viral infections and their genomes: A when a mosquito carrying the dengue virus bites a person, the virus penetrates the skin along with the mosquito’s saliva. It binds to and enters white blood cells and reproduces in the cells as they move through the body, producing several signaling proteins such as cytokines and interferons that are responsible for many of the symptoms, such as fever, flu-like symptoms, and severe pain. Once in the skin, the virus enters cells by binding between viral proteins and membrane proteins on the Langerhans cell, specifically the C-type lectins. One of the first things required for viral DNA to replicate is the suppression of the host’s cellular protein synthesis. B HSV infection occurs by binding to cells through rampant receptors that reflect a variety of host tissue infections, including sensory neurons, which leads to latency. Nuclear and cytoplasmic stages of virus replication also occur (created in BioRender.com)
Medicinal plants have a broad therapeutic potential against a wide variety of infectious diseases caused by microorganisms such as viruses, fungi, and bacteria. In India, for example, two polyherbal formulations, Nilavembu Kudineer and Kabasura Kudineer, were have used to boost the immune system during the COVID-19 lockdown period. Although viruses pose a health burden to human race, most people currently rely heavily on medicinal plants to stimulate and modulate the immune system against the infectious complications of viruses (Prabhu et al. 2022). After consuming the plant extracts, the phytochemicals unfold their therapeutic potential either alone or in combination with other constituents as synergetic effects. In order to find such a multi-targeting viral drug molecule, the present study aimed to investigate the antiviral potential of (R)-(+)-rosmarinic acid.
(R)-(+)-rosmarinic acid (1) is regarded as a promising phytochemical in the pharmaceutical industry due to its wide range of pharmacological effects (Chunxu et al. 2020). It is represented by the chemical formula C18H16O8. It has been found that there are nearly 160 species of plants, including hornworts of bryophytes and angiosperms, both dicots and monocots (Huaquan et al. 2022). However, the (R)-(+)-rosmarinic acid was first discovered and isolated in 1958 in Rosmarinus officinalis L., Lamiaceae. Therefore, this plant member of the mint family is being considered as the main source of (R)-(+)-rosmarinic acid. Later, it was found in significant amounts in other Lamiaceae species such as Thymus mastichina L., Ocimum tenuiflorum L., Hyptis pectinata Poit., and Forsythia koreana Nakai, Oleaceae (Gordo et al. 2012; Chunxu et al. 2020). Because (R)-(+)-rosmarinic acid is water soluble, previous research has explored its therapeutic potential in a variety of diseases (Fecka, 2007). Consistent with this statement, this molecule is being studied for decades in in silico, in vitro, and in vivo to screen its potential against health complications such as the central nervous system diseases, diabetes, tumors, and diseases caused by microorganisms including dengue and herpes viruses, among others.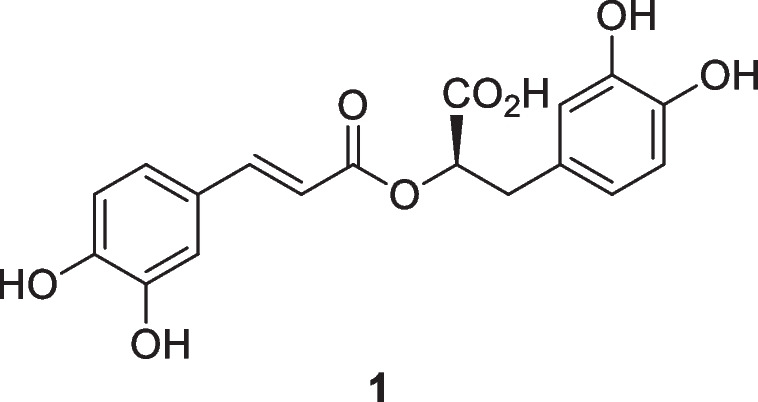
There is currently no adequate treatment for viruses, whereas the drugs available are expensive and have undesirable side effects. Therefore, given the lack of antiviral drugs and the versatile pharmacological properties of (R)-(+)-rosmarinic acid (1), the present research aimed to investigate its antiviral potential against dengue and herpes viruses.
Materials and Methods
Biological Data
Initially, (R)-(+)-rosmarinic acid was chosen as a ligand molecule and retrieved from the chemical database to find out its antiviral potential against the proteins of dengue and herpes viruses. Similar to the ligand, the viral proteins were retrieved from the protein database (www.rcsb.com) as in crystallographic form to dock with (R)-(+)-rosmarinic acid. The alphanumeric identities of the proteins were 1F5Q murine gamma herpesvirus cyclin complexed to human cyclin-dependent kinase 2 (Card et al. 2000), 2J7W dengue virus NS5 RNA-dependent RNA polymerase domain complexed with 3’dGTP (Yap et al. 2007), and 4OIG dengue virus nonstructural protein NS1 (Edeling et al. 2014).
Computational Tools
The preparation of ligands and proteins was performed using the Maestro V.13.2 module tool. This software, consisting of LigPrep, Grid Generation, SiteMap, and Glide XP, contributed significantly to the preparation of ligands and proteins. It ran on the highly configured Centox Linux operating system and was used for further investigation, in line with previous computer studies (Kalaimathi et al. 2022; Prabhu et al. 2022).
Target Preparation
With the Protein Preparation Wizard module, the proteins of dengue and herpes viruses were adjusted by removing the water molecules. In general, water molecule-containing proteins are not compatible for the molecular docking; therefore, water molecules were clean off from the proteins for further investigation. Two gears such as preparation and refinement were utilized during this process to detect water molecules and remove them from the proteins, while the workspace analyzer was aided to add the missing residues in the proteins. Later, the proteins were shifted for docking using two more gears: optimization and minimization. The entire target preparation process has been completed in accordance with our previous studies (Christy Rani et al. 2022; Kalaimathi et al. 2022).
Grid Generation
By constructing the lattice box using glide grid module, the putative binding site was stabilized as a suitable ligand site of (R)-(+)-rosmarinic acid. Prior to switching the molecular docking, the binding site was established to study the drug potential of (R)-(+)-rosmarinic acid by knowing the following parameters such as docking scores and binding affinities. With this method, a grid box was constructed to dock the chemical at the focal point of the protein within the targets. To fix the ligand binding site in the protein molecules, the grid box was constructed with X, 69.31; Y, 64.39; and Z, − 14.86 for 1F5Q, whereas the grid boxes to dengue viruses were constructed with X, 31.85; Y, 66.9; and Z, 30:43 for 2J7W and X, 38.95; Y, − 20.17; and Z, − 0.23 for 4UO5, respectively.
Ligand Preparation
Before docking with target molecules, the mole format of rosmarinic was prepared using the LigPrep (2.4) module. The Optimized Potentials for Liquid Simulations 2005 (OPLS2005) force field was also used to refine the topology of the detected ligand. Similar to protein preparation, two mechanisms such as tautomers and stereoisomers have been exploited in ligand preparation to reduce the geometric complexity of the ligand. The construction of the rosmarinic as 3D from 1D (smiles) and 2D was accomplished by the ligprep module.
Molecular Docking
The successfully prepared ligand and protein molecules were docked in the Xtra Precision Docking Mode. It was used to determine the strength of interactions between viral proteins and (R)-(+)-rosmarinic acid, specifically to know binding affinities and inhibition constants between them. To assess the effectiveness of (R)-(+)-rosmarinic acid as a potential ligand, docking metrics including docking scores, hydrophobic interactions, hydrogen bonding (side and back chains), π–π stacking, and salt bridge contacts were examined (Vijayakumar et al. 2016).
Pharmacophore Analysis
Structure-based and ligand-based techniques have been integrated into the energetic (e)-pharmacophore approach. Using the phase v 3.4 module, the pharmacophore sites of (R)-(+)-rosmarinic acid such as hydrogen bond acceptor (A), hydrogen bond donor (D), hydrophobic group (H), positively ionizable (P), negatively ionizable (N), and aromatic ring (R) were determined.
Drug Probability
The drug potential of (R)-(+)-rosmarinic acid, concerning the probabilities of active and inactive pharmacological properties, was explored, whereas the adverse effects of phytochemicals and conventional drugs were also covered. Using the Gusar, the acute toxicity of (R)-(+)-rosmarinic acid in rats was also noted at different doses of route of administration such as intravenous (i.v.), intraperitoneal (i.p.), subcutaneous (s.c.), and oral (p.o.) (Kalaimathi et al. 2021).
Results and Discussion
In the present computational research of antiviral drugs, (R)-(+)-rosmarinic acid was found to have remarkable antiviral abilities against the complications related to gamma herpes virus and NS5 RNA-dependent RNA polymerase domain complexed with 3′-dGTP and nonstructural protein 1 (NS1) of flavivirus. The present research has recognized that (R)-(+)-rosmarinic acid is an effective antiviral drug for numerous viruses like dengue and herpes virus. This statement was confirmed after evaluating the druggability of target proteins in terms of binding affinities to the tested ligand. Based on the present results, the existence of this molecule in plants and the antiviral potential of this molecule for other viruses have been clearly discussed as follows. Although the molecule was first discovered and isolated from Rosmarinus officinalis, a recent review described that (R)-(+)-rosmarinic acid has been widely isolated from many wild and cultivated plants worldwide over the past three decades. To assess the pharmacological potential of this phytochemical, it was tested on different targets in in silico, in vitro, and in vivo models.
Molecular Docking
Active Sites of Viral Proteins
This phase is critical in docking as it helps identify the correct putative binding pocket in the target molecules. Although the protein consists of an enormous number of binding sites, only one site could be used to generate a grid box. Therefore, it is chosen after its site volume and values were found to be higher than the other sites detected from this protein. Ultimately, only one site was chosen as a putative ligand binding pocket because of its potential binding metrics for ligand binding (Vijayakumar et al. 2018).
To locate the ligand binding region, the appropriate ligand site in the protein molecules was examined. Although the molecules possess numerous sites, the ligand binding sites were filtered based on their site score and volume. Here, this site analysis revealed the residue consistency of three viral proteins such as 1F5Q, 2J7W, and 4OIG (Table 1). At the radius of 3 Å, the binding site residues of murine gamma herpesvirus cyclin complexed with human cyclin-dependent kinase 2 have been identified as LYS89, ASP86, GLN85, HIE84, LEU83, PHE82, GLU81, PHE80, LEU143, ALA144, ASP145, PHE146, GLY147, GLU51, LYS33, LEU32, ALA31, LEU134, GLN131, VAL64, ILE64, and LEU55 (Table S1). Similarly, the binding site residues of NS5 RNA-dependent RNA polymerase domain complexed with 3′ dGTP have been identified as PRO319, THR317, PRO258, ASN207, ARG257, LYS211, ALA213, ASP276, PHE277, and ASP278 (Table S1). Furthermore, the binding site residues of flavivirus nonstructural protein 1 (NS1) molecule have been identified as ARG481, VAL450, THR571, LYS575, VAL577, VAL579, VAL358, LYS355, PHE354, TYR299, TRP302, ILE592, GLN602, SER600, and GLY599 (Table S1).
Table 1.
Docking scores, binding energies, and H bond interaction values of (R)-(+)-rosmarinic acid (1) with the docked viral proteins
| S. No | Ligand | PDB ID | Glide docking score | Glide emodel | Energy values | XP H bond values |
|---|---|---|---|---|---|---|
| 1 | (R)-(+)-rosmarinic acid | 1F5Q | − 10.847 | − 80.691 | − 52.949 | − 4.376 |
| 2J7W | − 10.033 | − 61.561 | − 48.078 | − 3.553 | ||
| 4OIG | − 7.259 | − 44.275 | − 47.275 | − 3.223 |
In the present in silico approach, the compatibility of (R)-(+)-rosmarinic acid with the viral proteins of dengue and herpes viruses was examined. Eventually, this tested ligand was a possible drug because it contains better docking metrics for the docked dengue protein, including docking score, electrostatic energy, and hydrogen bonding. Particularly, the docking scores revealed that it comprises potential therapeutic effects to combat these viruses. It was additionally proven by the affinities between this protein and the tested molecule such as hydrogen bonds, π–π stacking, electrostatic potential, and π-cation. The contribution and flexibility of the tested ligand were clearly comprehended with the docked viral proteins, as follows:
-
i)
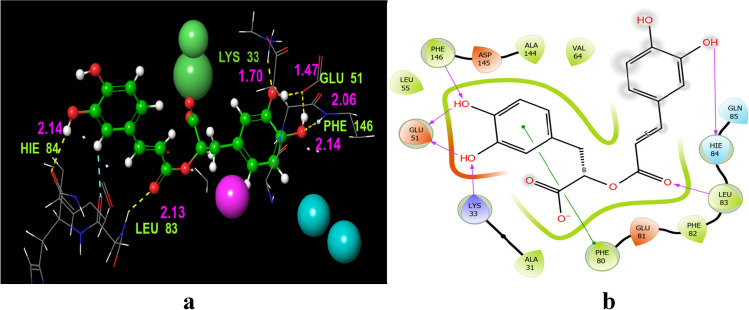
Murine gamma herpesvirus cyclin complexed to human cyclin-dependent kinase 2. (R)-(+)-rosmarinic acid was found to have a rather impressive docking score of − 10.847 with this viral protein (Table 1). In order to examine its inhibitory constant and its binding affinities with this enzyme, the docked complex was examined. This research found that (R)-(+)-rosmarinic acid established hydrogen bonding with herpes virus amino acid residues (Fig. 2a): PHE146 (2.14 Å), GLU51 (covalent bond, 1.47 and 2.06 Å), LYS33 (1.70 Å), LEU83 (2.13 Å), and HIE84 (2.14 Å), while the PHE80 was found to be forming a π–π stacking contact (Table S2). Figure 2b shows the functional group involved in the hydrogen bonding contacts with (R)-(+)-rosmarinic acid. Except residues PHE80 and LEU83, all the residues bind with the hydroxyl groups (OH). LEU83, on the other hand, was found in contact with oxygen of the carbonyl group of the ester group (C = O).
-
ii)
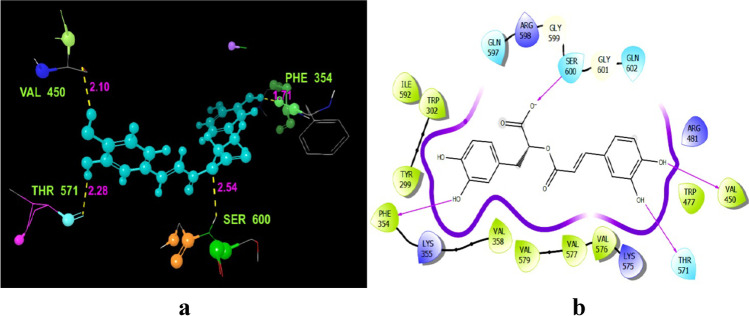
NS5 RNA-dependent RNA polymerase domain complexed with 3′ dGTP: a docking score of − 10.033 was found for (R)-(+)-rosmarinic acid with this viral protein (Table 1). (R)-(+)-rosmarinic acid displayed four hydrogen contact with viral protein amino acid residues (Fig. 3a), such as THR571 (2.28 Å), VAL450 (2.10 Å), PHE354 (1.71 Å), and SER600 (2.54 Å) (Table S2). Except the residues SER600, all the residues bind with the hydroxyl group (OH) of (R)-(+)-rosmarinic acid. LEU83, on the other hand, was found in contact with oxygen (O). The contact lines have been shown quite accurately as binding affinities in Fig. 3b.
-
iii)
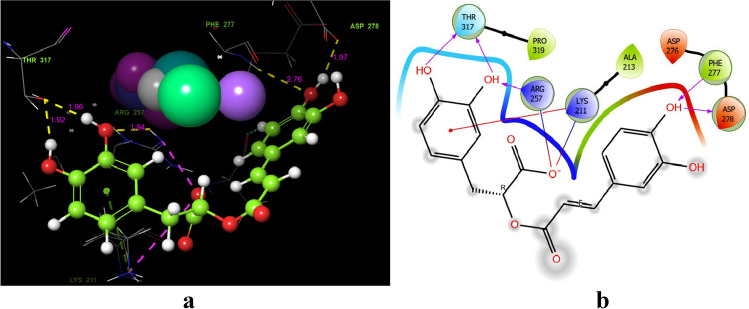
Flavivirus nonstructural protein: a docking score of − 7.259 against this viral protein (Table 1, Fig. 4). (R)-(+)-rosmarinic acid displayed five hydrogen bonding with the herpesvirus amino acid residues. Amino acid residues such as THR317 (Covalent binding), PHE277 (2.76 Å), and ASP278 (1.92 Å) and LYS211 and ARG257 were found as cation and salt bridge contacts; LYS211 in particular binds covalently with (R)-(+)-rosmarinic acid as a π-cation contact at one end and as a salt bridge contact at another (Fig. 4a). Figure 4b shows which functional group is involved in hydrogen bonding contacts with (R)-(+)-rosmarinic acid. Except residues LYS211, all the residues bind with the hydroxyl group (OH) of (R)-(+)-rosmarinic acid (Fig. 4b).
Fig. 2.
Docking of (R)-(+)-rosmarinic acid (1) to the 1F5Q murine gamma herpesvirus cyclin complexed to human cyclin-dependent kinase 2. a Residues and hydrogen bond contacts with their distance values among the ligand and catalytic pocket amino acids of the 1F5Q protein. b Revealed interactions between the hydroxy and oxygen groups of (R)-(+)-rosmarinic acid with 1F5Q
Fig. 3.
Docking of (R)-(+)-rosmarinic acid (1) to the 2J7W dengue virus NS5 RNA-dependent RNA polymerase domain complexed with 3' dGTP. a Residues and hydrogen bond contacts with their distance values among the ligand and catalytic pocket amino acids of the 2J7W protein. b Revealed interactions between the hydroxy and oxygen groups of (R)-(+)-rosmarinic acid with 2J7W
Fig. 4.
Docking of (R)-(+)-rosmarinic acid (1) to the 4OIG dengue virus dengue virus nonstructural protein NS1. a Residues and hydrogen bond contacts with their distance values among the ligand and catalytic pocket amino acids of the 4OIG protein. b Revealed interactions between the hydroxy and oxygen groups of (R)-(+)-rosmarinic acid with 4OIG
Biomedical Profiles
Since 1997 to date, it has been reported various pharmacological properties for (R)-(+)-rosmarinic acid as a novel candidate for inflammation, antioxidant potential, cancer, diabetic, and antiviral agent for zoonotic and non-zoonotic diseases, neurodegenerative, hypertensive, and antimicrobial, among other (Noor et al. 2022). In addition, it has also been used as folk medicine, cosmetics, and dietary supplements since ancient times (Baba et al. 2004). In 2011, Hsu et al. (2011) found that (R)-(+)-rosmarinic acid markedly reduced IL-1 and TNF-α release, thereby ameliorating collagen-induced arthritis in vivo. These are part of the innate immune system of the human body. The cytokinins such as interferon-1 and TNF-α play a key role in activating the innate immune system. In agreement with the research reported by Hsu et al. (2011), our results demonstrated that by inducing these two cytokinins, (R)-(+)-rosmarinic acid indirectly could activate the innate immune system to protect the human body from harmful materials.
MM-GBSA
In the MM-GBSA validation, the free energy values of (R)-(+)-rosmarinic acid are found to be − 58.65 for 1F5Q, − 52.17 for 2J7W, and − 48.52 for 4OIG (Table 2). The final results of the investigation imply that (R)-(+)-rosmarinic acid has the strong binding with the docked viral proteins.
Table 2.
Rat acute toxicity level of (R)-(+)-rosmarinic acid (1) at various doses
| Rat i.p. LD50 log10 (mmol/kg) | Rat i.v LD50 log10 (mmol/kg) | Rat p.o. LD50 log10 (mmol/kg) | Rat s.c. LD50 log10 (mmol/kg) |
| 0.359 in AD | − 0.047 in AD | 0.920 in AD | 0.360 in AD |
| Rat i.p. LD50 (mg/kg) | Rat i.v. LD50 (mg/kg) | Rat p.o. LD50 (mg/kg) | Rat s.c. LD50 (mg/kg) |
| 823.100 in AD | 323.600 in AD | 2994.000 in AD | 826.300 in AD |
Pharmacophore
The data set was divided into active, moderately active, and inactive regions by keeping the activity threshold in the 7.2 range. The (R)-(+)-rosmarinic acid binding domain in terms of its five characteristics as shown in Fig. 5 included the generic pharmacophore hypotheses due to its high survival value. Furthermore, the e-pharmacophore shows that the rosmarinic acid consists of four acceptors (A), four donors (D), one negative ionic (H), and two aromatic rings (R) that were obtained (Fig. 5).
Fig. 5.
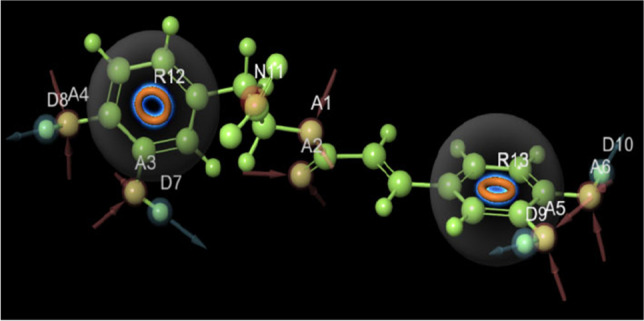
Pharmacophore hypothesis of (R)-(+)-rosmarinic acid (1). A denotes hydrogen bond acceptor in pink color, D denotes hydrogen bond donor in blue. and R denotes aromatic rings in brown color from docked phytochemicals; A–D showed the active site of docked phytochemicals developed by e-Pharmacophore
Druggability
The Prediction of Activity Spectra for Substances (PASS) server showed that (R)-(+)-rosmarinic acid has broad pharmacological possibilities as notable by the probability active (Pa) score, with a drug probability active score ranging from 0.710 to 0.956; only the most probable pharmacological activities were included in Table S3. On the other hand, the side effects of the prescribed herpesvirus drugs such as aciclovir, famciclovir, penciclovir, and valaciclovir have been shown in the supplementary data (Table S4–S7). Accordingly, the present research suggests that these drugs might lead to further complications for human health after administration as a treatment for the herpes virus. The most significant adverse effects of these drugs were shown in Figs. S4–S6. Likewise, the adverse effects of (R)-(+)-rosmarinic acid were also shown in Table S8.
Rat Acute Toxicity
The rate acute toxicity of (R)-(+)-rosmarinic acid was measured by LD50 at log10 (mmol/kg) and LD50 mg/kg levels. The applicability domain models were measured in terms of administration, including i.p., i.v., p.o., and s.c. administrations. The predicted values for acute toxicity are shown in Table 2 and Fig. 6.
Fig. 6.
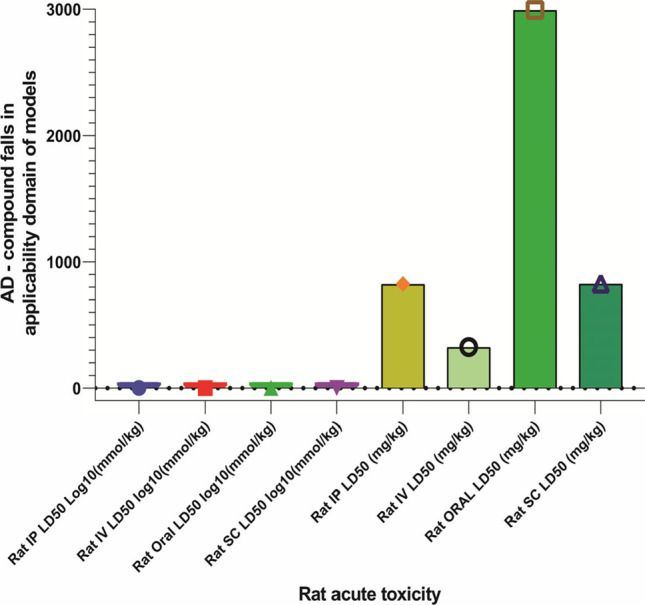
Acute toxicity for (R)-(+)-rosmarinic acid (1) at different routes of administration at LD50 dose (predicted by Gusar). Abbreviations: i.p., intraperitoneal; i.v., intravenous; s.c., subcutaneous administration
Conclusions
Viral infections have been one of the major causes of disease and death in human race worldwide. Dengue and herpes viruses in particular cause the worst health problems, sometimes leading to death. In this scenario, there is no adequate treatment to combat these viral particles and complications. Still, people around the world are trying to boost their immune systems by using plants, either alone or as polyherbal formulation, to fight off infectious agents, especially viruses. Therefore, the present research was also aimed to explore the antiviral potential from the pharmacologically versatile phytochemical (R)-(+)-rosmarinic acid. Current research shows that this polyphenol exhibits impressive hydrogen bonding interactions, strong docking values, and glide energy values. In addition, the pharmacokinetic property assessments have shown that this molecule possesses a variety of pharmacological properties by activating the enzymes of the human body. Accordingly, we believe that when tested in in vitro and in vivo experiments, (R)-(+)-rosmarinic acid would reveal drug activity against dengue and herpes virus replication. On the other hand, minimal side effects than the drugs currently prescribed for herpes viruses were predicted. Similarly, evaluation of the pharmacokinetic properties had shown that it has less toxicity to rats at doses of LD50 log10 (mmol/kg) and LD50 (mg/kg) by different routes of administration. Today’s pharmaceutical sector is trying to discover novel antiviral drugs for many viruses; so far, however, no single drug is sufficient to combat diverse viruses as a single drug.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Each author expresses their gratitude to their institutions for providing the opportunity to develop this manuscript. The corresponding author expresses his thanks to the management of Annai Vailankanni Arts and Science College, Thanjavur, for giving the time to draw this manuscript. The authors sincerely thank the anonymous reviewers and editors for the improvement in the quality of the manuscript.
Author Contribution
AC, investigation, formal analysis, original draft writing, and data maintenance. KK, conceptualization, methodology, formal analysis, written review, and editing. SJ, investigation, formal analysis, and validation. RM, investigation and formal analysis. SP, conceptualization, methodology, review, editing, and supervision. PC, data conceptualization. All authors read and approved the manuscript.
References
- Ansari Dogaheh M, Sharififar F, Arabzadeh AM, Shakibaic M, Heidarbeigi M. Inhibitory effect of a standard extract of Zhumeria majdae Rech. F and Wendelbo. against herpes simplex-1 virus. J Med Sci. 2013;13:755–760. doi: 10.3923/jms.2013.755.760. [DOI] [Google Scholar]
- Baba S, Osakabe N, Natsume M, Terao J. Orally administered rosmarinic acid is present as the conjugated and/or methylated forms in plasma and is degraded and metabolized to conjugated forms of cafeic acid, ferulic acid and m-coumaric acid. Life Sci. 2004;75:165–178. doi: 10.1016/j.lfs.2003.11.028. [DOI] [PubMed] [Google Scholar]
- Ben Shabat S, Yarmolinsky L, Porat D, Dahan A. Antiviral effect of phytochemicals from medicinal plants: applications and drug delivery strategies. Drug Deliv Transl Res. 2020;10:354–367. doi: 10.1007/s13346-019-00691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card GL, Knowles P, Laman H, Jones N, McDonald NQ. Crystal structure of a gamma-herpesvirus cyclin-cdk complex. EMBO J. 2000;19:2877–2888. doi: 10.1093/emboj/19.12.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YC, Hsieh FC, Lin YJ, Wu TY, Lin CW, Lin CT, Tang NY, Jinn TR. Magnesium lithospermate B and rosmarinic acid, two compounds present in Salvia miltiorrhiza, have potent antiviral activity against enterovirus 71 infections. Euro J Pharmacol. 2015;755:127–133. doi: 10.1016/j.ejphar.2015.02.046. [DOI] [PubMed] [Google Scholar]
- Edeling MA, Diamond MS, Fremont DH. Structural basis of flavivirus NS1 assembly and antibody recognition. Proc Natl Acad Sci. 2014;111:4285–4290. doi: 10.1073/pnas.1322036111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecka I, Turek S. Determination of water-soluble polyphenolic compounds in commercial herbal teas from Lamiaceae: peppermint, melissa, and sage. J Agric Food Chem. 2007;55:10908–10917. doi: 10.1021/jf072284d. [DOI] [PubMed] [Google Scholar]
- Gordo J, Máximo P, Cabrita E, Lourenço A, Oliva A, Almeida J, Filipe M, Cruz P, Barcia R, Santos M, Cruz H. Thymus mastichina: chemical constituents and their anti-cancer activity. Nat Prod Commun. 2012;7:1491–1494. [PubMed] [Google Scholar]
- Guan H, Luo W, Bao B, Cao Y, Cheng F, Yu S, Fan Q, Zhang L, Wu Q, Shan M. A comprehensive review of rosmarinic acid: from phytochemistry to pharmacology and its new insight. Molecules. 2022;27:3292. doi: 10.3390/molecules27103292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Cheng CP, Chang DM. Plectranthus amboinicus attenuates inflammatory bone erosion in mice with collagen-induced arthritis by downregulation of RANKL-induced NFATc1 expression. J Rheumatol. 2011;38:1844–1857. doi: 10.3899/jrheum.101223. [DOI] [PubMed] [Google Scholar]
- Kalaimathi K, Thiyagarajan G, Vijayakumar S, Bhavani K, Karthikeyan K, Rani JM, Dass K, Sureshkumar J, Prabhu S (2021) Molecular docking and network pharmacology-based approaches to explore the potential of terpenoids for Mycobacterium tuberculosis. Pharmacol Res Mod Chin Med 1:100002. 10.1016/j.prmcm.2021.100002
- Kalaimathi K, Rani JMJ, Vijayakumar S, Prakash N, Karthikeyan K, Thiyagarajan G, Bhavani K, Prabhu S, Varatharaju G. Anti-dengue potential of mangiferin: intricate network of dengue to human genes. Rev Bras Farmacogn. 2022;32:410–420. doi: 10.1007/s43450-022-00258-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WY, Yu YJ, Jinn TR. Evaluation of the virucidal effects of rosmarinic acid against enterovirus 71 infection via in vitro and in vivo study. Virol J. 2019;16:94. doi: 10.1186/s12985-019-1203-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C, Zou L, Sun H, Peng J, Gao C, Bao L, Ji R, Jin Y, Sun S. A review of the anti-inflammatory effects of rosmarinic acid on inflammatory diseases. Front Pharmacol. 2020;11:153. doi: 10.3389/fphar.2020.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolkemper S, Reichling J, Stintzing FC, Carle R, Schnitzler P. Antiviral effect of aqueous extracts from species of the Lamiaceae family against herpes simplex virus type 1 and type 2 in vitro. Planta Med. 2006;72:1378–1382. doi: 10.1055/s-2006-951719. [DOI] [PubMed] [Google Scholar]
- Noor S, Mohammad T, Rub MA, Raza A, Azum N, Yadav DK, Hassan MI, Asiri AM. Biomedical features and therapeutic potential of rosmarinic acid. Arch Pharm Res. 2022;45:205–228. doi: 10.1007/s12272-022-01378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhu S, Vijayakumar S, Manogar P, Maniam GP, Govindan N. Homology modeling and molecular docking studies on type II diabetes complications reduced PPAR γreceptor with various ligand molecules. Biomed Pharmaco. 2017;92:528–535. doi: 10.1016/j.biopha.2017.05.077. [DOI] [PubMed] [Google Scholar]
- Prabhu S, Vijayakumar S, Praseetha P. Cyanobacterial metabolites as novel drug candidatesin corona viral therapies: a review. Chronic Dis Transl Med. 2022;8:172–183. doi: 10.1002/cdt3.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani JM, Kalaimathi K, Vijayakumar S, Varatharaju G, Karthikeyan K, Thiyagarajan G, Bhavani K, Manogar P, Prabhu S (2022) Anti-viral effectuality of plant polyphenols against mutated dengue protein NS2B47-NS3: a computational exploration. Gene Rep 27:101546. 10.1016/j.genrep.2022.101546
- Swarup V, Ghosh J, Ghosh S, Basu SA, A, Antiviral and anti-inflammatory effects of rosmarinic acid in an experimental murine model of Japanese encephalitis. Antimicrob Agents Chemother. 2007;51:3367–3370. doi: 10.1128/AAC.00041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto Y, Ikeda S, Uwai K, Taguchi R, Chayama K, Sakaguchi T, Narita R, Yao W, Takeuchi F, Otakaki Y, Watashi K, Wakita T, Kato H (2018) Rosmarinic acid is a novel inhibitor for hepatitis B virus replication targeting viral epsilon RNA-polymerase interaction. PLoS One 13:197664. 10.1371/journal.pone.0197664 [DOI] [PMC free article] [PubMed]
- Vijayakumar S, Prabhu S, Rajalakhsmi S, Manogar P. Review on potential phytocompounds in drug development for Parkinson disease: a pharmacoinformatic approach. Inform Med Unlock. 2016;5:15–25. doi: 10.1016/j.imu.2016.09.00. [DOI] [Google Scholar]
- Vijayakumar S, Sathiya M, Arulmozhi P, Prabhu S, Manogar P, Vinothkannan R, Parameswari N. Molecular docking and ADME properties of bioactive molecules against human acid-beta-glucosidase enzyme, cause of Gaucher’s disease. In Silico Pharmacol. 2018;6:3. doi: 10.1007/s40203-018-0039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahab NZA, Ibrahim N, Kamarudin MKA, Lananan F, Juahir H, Ghazali A. In vitro antiviral activity of Orthosiphon stamineus extract against dengue virus type 2. J Fundam Appl Sci. 2018;10:541–551. doi: 10.4314/jfas.v10i1s.38. [DOI] [Google Scholar]
- Wang H, Zhang J, Lu Z, Dai W, Ma C, Xiang Y, Zhang Y (2021) Identification of potential therapeutic targets and mechanisms of COVID-19 through network analysis and screening of chemicals and herbal ingredients. Brief Bioinform 23:bbab373. 10.1093/bib/bbab373 [DOI] [PMC free article] [PubMed]
- Yap TL, Xu T, Chen YL, Malet H, Egloff MP, Canard B, Vasudevan SG, Lescar J. Crystal structure of the dengue virus RNA-dependent RNA polymerase catalytic domain at 1.85-angstrom resolution. J Virol. 2007;81:4753–4765. doi: 10.1128/JVI.02283-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.