Objective:
To examine whether depression status before metabolic and bariatric surgery (MBS) influenced 5–year weight loss, diabetes, and safety/utilization outcomes in the PCORnet Bariatric Study.
Summary of Background Data:
Research on the impact of depression on MBS outcomes is inconsistent with few large, long–term studies.
Methods:
Data were extracted from 23 health systems on 36,871 patients who underwent sleeve gastrectomy (SG; n=16,158) or gastric bypass (RYGB; n=20,713) from 2005–2015. Patients with and without a depression diagnosis in the year before MBS were evaluated for % total weight loss (%TWL), diabetes outcomes, and postsurgical safety/utilization (reoperations, revisions, endoscopy, hospitalizations, mortality) at 1, 3, and 5 years after MBS.
Results:
27.1% of SG and 33.0% of RYGB patients had preoperative depression, and they had more medical and psychiatric comorbidities than those without depression. At 5 years of follow-up, those with depression, versus those without depression, had slightly less %TWL after RYGB, but not after SG (between group difference = 0.42%TWL, P = 0.04). However, patients with depression had slightly larger HbA1c improvements after RYGB but not after SG (between group difference = – 0.19, P = 0.04). Baseline depression did not moderate diabetes remission or relapse, reoperations, revision, or mortality across operations; however, baseline depression did moderate the risk of endoscopy and repeat hospitalization across RYGB versus SG.
Conclusions:
Patients with depression undergoing RYGB and SG had similar weight loss, diabetes, and safety/utilization outcomes to those without depression. The effects of depression were clinically small compared to the choice of operation.
Keywords: bariatric surgery, cohort, depression, longitudinal, outcomes, psychiatric, psychosocial
Metabolic and bariatric surgery (MBS) is the most effective treatment for severe obesity and its associated medical comorbidities, including type 2 diabetes. 1 – 3 Sleeve gastrectomy (SG) and Roux-en-Y gastric bypass (RYGB) are the most commonly performed bariatric operations worldwide. 4 RYGB results in greater weight loss and improvements in medical comorbidity, particularly for higher-risk individuals 2 and those with BMI ≥50kg/m 2 . Although outcomes vary, maximum weight loss is typically achieved in the first 2 postsurgical years, and most patients experience durable weight and diabetes improvements. 2 ,3 ,5 ,6 However, it is common to see some weight regain and recurrence of diabetes in the years after surgery. 6 ,7 For example, using data on 46,510 patients from 41 health systems in the National Patient Centered Clinical Research Network (PCORnet) Bariatric Study (PBS), we previously found that the average percent total weight loss (%TWL) at 1-, 3- and 5-years following surgery was 31.2%, 29.0%, and 25.5% for RYGB, and 25.2%, 21.0%, and 18.8% for SG. 8 Furthermore, relapse rates among patients with initial diabetes remission increased over time from 8.4% and 11.0% at 1 year to 33.1% and 41.6% at 5 years after RYGB and SG, respectively. 9
An often elusive goal has been identifying patient characteristics that may be associated with less favorable long-term MBS outcomes. Psychological factors, including psychiatric diagnoses such as depression, have been associated with less postsurgical weight loss, however, some studies have not demonstrated an association between depressive disorders and surgical outcomes. 10 – 14 Large studies with long-term outcomes are lacking. Because psychiatric comorbidity, particularly depression, is common among those seeking MBS-with almost 70% of MBS candidates having a lifetime history of psychiatric illness and 40% having a lifetime major depressive disorder-it is important to understand more clearly whether psychiatric comorbidity influences surgical out- comes 13 ,15 – 17
The goal of the current study was to use existing data from the PBS to examine whether a depression diagnosis in the year before MBS was an effect modifier of weight loss, diabetes, and adverse health outcomes at 1-, 3- and 5- years after SG and RYGB. We hypothesized that those with depression at baseline would have less weight loss and diabetes improvement and more adverse health events compared to those not diagnosed with depression, regardless of their choice of bariatric operation.
Methods
Study Setting and Population
The PBS cohorts and protocol were previously described. 8 ,9 ,18 – 20 Three PBS cohorts were created, each corresponding to 1 of the 3 primary aims of the study assessing long-term weight loss, diabetes, and safety/utilization outcomes. The analysis described in this manuscript was added during an extension of the project. All 41 sites that agreed to participate in the original study were invited to participate in the depression-related analysis, and 25 agreed, which required them to update their data sharing agreements and IRB approvals (see Supplemental Digital Content Table 1, http:// links.lww.com/SLA/D612 for a list of sites for weight loss, diabetes and safety/utilization cohorts).
Table 1.
Baseline Characteristics of the PBS Cohort, Stratified by Baseline Depression Status and Metabolic and Bariatric Operation (N=36, 871)
| No Depression | Depression | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RYGB | SG | Overall | RYGB | SG | Overall | |||||||
| (n=13885 | [54.1%]) | (n=11773 | [45.9%]) | (n=25658 | [100%]) | (n=6828 | [60.9%]) | (n=4385 | [39.1%]) | (n=11213 | [100%]) | |
| Mean Age (SD) | 45.2 | (11.7) | 43.4 | (11.7) | 44.4 | (11.7) | 46.7 | (11.2) | 45.9 | (11.7) | 46.4 | (11.4) |
| Age Category, n (%) | ||||||||||||
| 12–19 | 92 | (0.7) | 149 | (1.3) | 241 | (0.9) | 28 | (0.4) | 50 | (1.1) | 78 | (0.7) |
| 20–44 | 6507 | (46.9) | 6286 | (53.4) | 12793 | (49.9) | 2878 | (42.2) | 1920 | (43.8) | 4798 | (42.8) |
| 45–64 | 6627 | (47.7) | 4887 | (41.5) | 11514 | (44.9) | 3583 | (52.5) | 2196 | (50.1) | 5779 | (51.5) |
| 65–80 | 659 | (4.8) | 451 | (3.8) | 1110 | (4.3) | 339 | (5.0) | 219 | (5.0) | 558 | (5.0) |
| Female, n (%) | 10694 | (77.0) | 9234 | (78.4) | 19928 | (77.7) | 5952 | (87.2) | 3868 | (88.2) | 9821 | (87.6) |
| Not recorded | 1 | (0.0) | 0 | (0.0) | 1 | (0.0) | 0 | (0.0) | 1 | (0.0) | 1 | (0.0) |
| Mean BMI (SD) | 49.9 | (8.2) | 49.2 | (8.1) | 49.6 | (8.2) | 49.5 | (8.2) | 48.2 | (7.8) | 49.0 | (8.0) |
| BMI Category | ||||||||||||
| 35–39.9 kg/m2 | 940 | (6.8) | 788 | (6.7) | 1728 | (6.7) | 516 | (7.6) | 446 | (10.2) | 962 | (8.6) |
| 40–49.9 kg/m2 | 7165 | (51.6) | 6572 | (55.8) | 13737 | (53.5) | 3565 | (52.2) | 2469 | (56.3) | 6034 | (53.8) |
| 50–59.9 kg/m2 | 4220 | (30.4) | 3207 | (27.2) | 7427 | (28.9) | 2048 | (30.0) | 1096 | (25.0) | 3144 | (28.0) |
| ≥60 kg/m2 | 1560 | (11.2) | 1206 | (10.2) | 2766 | (10.8) | 699 | (10.2) | 374 | (8.5) | 1073 | (9.6) |
| Mean Weight (SD), kg | 129.3 | (26.4) | 125.8 | (25.8) | 127.7 | (26.2) | 125.7 | (24.5) | 120.9 | (23.5) | 123.8 | (24.2) |
| Procedure Year | ||||||||||||
| 2005–2009 | 2724 | (19.6) | 351 | (3.0) | 3075 | (12.0) | 1460 | (21.4) | 106 | (2.4) | 1566 | (14.0) |
| 2010 | 2029 | (14.6) | 868 | (7.4) | 2897 | (11.3) | 1070 | (15.7) | 344 | (7.8) | 1414 | (12.6) |
| 2011 | 2761 | (19.9) | 2348 | (19.9) | 5109 | (19.9) | 1222 | (17.9) | 756 | (17.2) | 1978 | (17.6) |
| 2012 | 2405 | (17.3) | 2624 | (22.3) | 5029 | (19.6) | 1098 | (16.1) | 898 | (20.5) | 1996 | (17.8) |
| 2013 | 2030 | (14.6) | 2609 | (22.2) | 4639 | (18.1) | 942 | (13.8) | 996 | (22.7) | 1938 | (17.3) |
| 2014 | 1719 | (12.4) | 2659 | (22.6) | 4378 | (17.1) | 903 | (13.2) | 1123 | (25.6) | 2026 | (18.1) |
| 2015 | 217 | (1.6) | 314 | (2.7) | 531 | (2.1) | 133 | (2.0) | 162 | (3.7) | 295 | (2.6) |
| Race | ||||||||||||
| Black | 2351 | (16.9) | 3287 | (27.9) | 5638 | (22.0) | 640 | (9.4) | 783 | (17.9) | 1423 | (12.7) |
| All Other Races* | 706 | (5.1) | 697 | (5.9) | 1403 | (5.5) | 233 | (3.4) | 144 | (3.3) | 377 | (3.4) |
| White | 9050 | (65.2) | 5764 | (49.0) | 14814 | (57.7) | 5472 | (80.1) | 2981 | (68.0) | 8453 | (75.4) |
| Not Recorded | 1778 | (12.8) | 2025 | (17.2) | 3803 | (14.8) | 483 | (7.1) | 477 | (10.9) | 960 | (8.6) |
PBS, National Patient Centered Clinical Research Network (PCORnet) Bariatric Study; RYGB, Roux-en-Y gastric bypass; SG, Sleeve gastrectomy; BMI, Body Mass Index; BP, blood pressure; DVT, deep vein thrombosis; GERD, Gastroesophageal reflux disease; NAFLD, Non-alcoholic fatty liver disease; PE, pulmonary embolism.
All other races includes: American Indian/Alaskan Native, Asian/Asian American, Native Hawaiian, Pacific Islander, multiracial, and “Other” races.
Weight Loss Cohort
We identified all patients who underwent a primary (first, non- revisional) bariatric operation at health systems affiliated with 23 PCORnet sites (Supplemental Digital Content Table 1, http://links.lww.com/SLA/D612) from January 1, 2005 through September 30, 2015 using ICD-9, Current Procedure Terminology (CPT-4), and Healthcare Common Procedure Coding System (HCPCS) codes (available from authors) extracted from the PCORnet common data model at each site). 21 ,22 We then excluded: 1) patients age <12 years or ≥80 years at the index (first) bariatric operation; 2) individuals with multiple conflicting bariatric procedure codes on same day; 3) any revision bariatric procedure code, gastrointestinal cancer diagnosis code, or fundoplasty procedure in the year before the index operation; 4) any emergency room encounter on the day of index operation; and 5) patients without documented BMI ≥35 kg/m 2 in the year before their operation. We also excluded patients who did not have any BMI measurements, or height and weight data to determine BMI, in the 5 years after surgery (n=5). For the depression-related analysis, we applied 2 additional exclusions. First, we excluded 2628 patients that underwent adjustable gastric banding surgery. Finally, we excluded 7555 patients from 16 PBS health systems that did not agree to participate in the depression- related analyses.
Diabetes Cohort
For the diabetes cohort, we included 23 PBS health systems that provided data on HbA1c and use of diabetes medications (Supplemental Digital Content Table 1, http://links.lww.com/SLA/ D612). We applied all the criteria listed in the weight cohort above, except patients were not required to have postoperative BMI data. We defined patients with diabetes as having HbA1c ≥6.5% or at least 1 diabetes medication prescription in the year before surgery. Patients taking only metformin, thiazolidinedione, or liraglutide needed an ICD-9 or Systematized Nomenclature of Medicine code for diabetes or HbA1c ≥6.5% in the year before surgery to be included.
Safety/Utilization Cohort
For the safety/utilization cohort, we only included patients from those 9 PBS health systems that were able to link their electronic health record databases to both a) insurance claims and b) state or national death indices, to support more complete capture of major events such as death, reoperation, and hospitalization.
Depression Status
Baseline depression was defined as having 1 or more ICD-9 diagnosis code (296.2x, 296.3x, 300.4, 311) 24 for depression in the year before the bariatric procedure (index date). Of note, most MBS patients undergo a psychosocial evaluation 25 in the preoperative evaluation process, thus screening rates and case detection are likely high. Unfortunately, data on depression symptoms and antidepressant medication use were not collected for the original PBS.
Outcomes
Weight Loss
Our weight loss outcome was percent total weight loss (%TWL) calculated as [(weight [kg] at 1, 3, and 5 years-weight [kg] at surgery)/weight at surgery [kg] x100]. 26 We compared %TWL between RYGB and SG stratified by depression status at each time point following surgery among patients with at least 1 weight measurement at that time point, defined as: 1 year (weight measurement within 6–18 months post-surgery); 3 years (3042 months); and 5 years (54–66 months).
Diabetes Outcomes
Diabetes remission was defined as the first post-surgical occurrence of HbA1c <6.5% following at least 6 months without diabetes medication prescription orders. The occurrence of HbA1c ≥6.5% and/or a prescription for diabetes medication after remission defined relapse. Absolute change in HbA1c at 1, 3, and 5 years after surgery was calculated.
Safety and Utilization Outcomes
The primary outcome of interest was the occurrence of a post- bariatric gastrointestinal operation or intervention (O/I) and was identified using ICD-9 and CPT-4 procedure codes (Supplement 2, http://links.lww.com/SLA/D613). 18 Additional secondary outcomes included the occurrence of endoscopy, hospitalization, allcause mortality, and 30-day major adverse events. 27
Statistical Analyses
Weight Loss Analysis
Each operations’ adjusted mean weight at 1, 3, and 5 years was estimated using a linear mixed effects (random effects) model with a cubic polynomial b-spline basis with 4 knots for flexible curves over time. 28 ,29 The model estimated a population-level curve for the mean weight from the time of surgery to the end of study and included random effect terms for individual (intercept) and follow up time (slope). For clinical relevance in presentation, model-based mean weight and standard error estimates were used to compute mean %TWL and corresponding 95% confidence intervals and P-values. 8 We examined variability in treatment effects across subgroups defined as having depression or not at baseline by including a 3way interaction between operation type, baseline depression status and time since bariatric surgery. Potential confounders included in the models as adjustment variables were baseline weight, demographic variables (Table 1), Charlson/Elixhauser comorbidity score (range: -2 to 20; higher score generally indicates worse health), 23 all comorbidities listed in Supplemental Digital Content Table 3, http://links.lww.com/SLA/D614 number of days between baseline weight measurement and bariatric operation, number of hospitalized days in the year before surgery, diastolic and systolic blood pressure and year and site of surgery.
Table 3.
Adjusted Hazard Ratios Among Adults With Diabetes (N=8407) Comparing Time to Remission Since Surgery and Time to Relapse Since Remission for Gastric Bypass and Sleeve Gastrectomy, Stratified by Baseline Depression Status
| Estimated Cumulative Percentage of Patients With T2DM Who Had Experienced an Initial Remission (95% CI) at Different Times of Follow-up | ||||||
|---|---|---|---|---|---|---|
| T2DM Remission | Adjusted HR* (95% CI) | P-value | 1 yr | 3 yr | 5 yr | |
| RYGB vs SG - No Depression | 1.09 (1.02, 1.15) | 0.025 | RYGB | 63.3 (61.5, 65.0) | 86.4 (85.0, 87.7) | 88.0 (86.6, 89.3) |
| SG | 60.3 (58.0, 62.5) | 84.1 (82.1, 85.8) | 85.8 (83.9, 87.5) | |||
| RYGB vs SG - Depression | 1.12 (1.03, 1.23) | 0.028 | RYGB | 61.1 (58.7, 63.3) | 84.7 (82.8, 86.4) | 86.4 (84.5, 88.1) |
| SG | 56.8 (53.3, 60.0) | 81.2 (78.1, 83.8) | 83.1 (80.0, 85.6) | |||
| Interaction P-Value = | 0.571 | |||||
| Estimated Cumulative Percentage of Patients With Initial T2DM Remission Who Had Experienced Relapse (95% CI) at Different Times of Follow-up | ||||||
| T2DM Remission | Adjusted HR† (95% CI) | P-value | 1 yr | 3 yr | 5 yr | |
| RYGB vs SG – No Depression | 0.77 (0.68, 0.87) | <0.001 | RYGB | 9.1 (8.0, 10.1) | 23.7 (21.3, 26.0) | 37.2 (33.3, 40.9) |
| SG | 11.7 (10.1, 13.2) | 29.6 (26.2, 32.9) | 45.4 (40.1, 50.2) | |||
| RYGB vs SG – Depression | 0.67 (0.56, 0.80) | <0.001 | RYGB | 8.0 (6.9, 9.2) | 21.1 (18.4, 23.8) | 33.6 (29.1, 37.8) |
| SG | 11.8 (9.6, 13.9) | 29.8 (24.9, 34.4) | 45.7 (38.5, 52.0) | |||
| Interaction P-value = | 0.282 | |||||
Remission of diabetes defined as HbAlc <6.5% after б months without any prescription order for a diabetes medication; Covariates included: age, sex, race, Hispanic ethnicity, BMI, HbA1c, blood pressure, days from BMI measurement to baseline, number of inpatient hospital days in the year before surgery, number of diabetes medications excluding insulin, insulin use, Charlson/Elixhauser comorbidity score, year of procedure, having a code for diabetes, smoking, having a code for other comorbidities (hypertension, dyslipidemia, sleep apnea, osteoarthritis, NAFLD, GERD, depression, anxiety, eating disorder, substance use, psychosis, kidney disease, infertility, polycystic ovaries, deep vein thrombosis, pulmonary embolism), having codes for specific diabetes medications (biguanides, GLP-1 agonists, sulfonylureas, thiazolidinediones, and other) and site.
Relapse of diabetes defined as occurrence of any HbA1c ≥6.5% and/or prescription order for a diabetes medication Covariates included: age, sex, race, Hispanic ethnicity, BMI, HbA1c, blood pressure, days from BMI measurement to baseline, number of in patient hospital days in the year before surgery, number of diabetes medications excluding insulin, insulin use, Charlson/Elixhauser comorbidity score, year of procedure, having a code for diabetes, smoking, having a code for other comorbidities (hypertension, dyslipidemia, sleep apnea, osteoarthritis, NAFLD, GERD, depression, anxiety, eating disorder, substance use, psychosis, kidney disease, infertility, polycystic ovaries, deep vein thrombosis, pulmonary embolism), having codes for specific diabetes medications (biguanides, GLP-1 agonists, sulfonylureas, thiazolidinediones, and other) and site.
CI, Confidence interval; HR, hazard ratios; RYGB, Roux-en-Y gastric bypass; SG, Sleeve gastrectomy.
Diabetes Analysis
Cox proportional hazards models calculated the adjusted hazard ratio (HR) for remission and estimated the adjusted cumulative proportion of individuals remitting at 1-, 3-, and 5-years for RYGB and SG with an interaction term between operation type and baseline depression status. 30 Similar analyses were conducted for diabetes relapse. Additional details on the diabetes analysis are provided in Supplement 2, http://links.lww.com/SLA/D613.
Safety and Utilization Analysis
A Cox proportional hazards model was used to estimate the adjusted hazards ratio (HR) for time-to-operation/intervention (O/I) for the comparison of RYGB vs. SG with an interaction term between operation type and baseline depression status. Additional modeling details are provided in Supplement 2, http://links.lww.com/SLA/D613. Analyses of time-to-event for all-cause mortality, hospitalization, endoscopy, and revision followed the same approach as the primary analysis.
All analyses were conducted using R version 3.4.3.
Results
Characteristics of the PBS Weight Loss, Diabetes, and Safety/Utilization Cohorts
The PBS weight loss cohort included 25,658 patients without depression and 11,213 with depression. RYGB was the more common operation, with 54% of those without depression and 61% with depression having undergone RYGB, while the others had SG. Baseline characteristics of these groups are presented in Table 1. Patients with depression were older when they underwent RYGB or SG than those without depression. Patients were predominantly female and White; 78% of those without depression and 88% with depression were female, and 58% without depression and 75% with depression were White. As shown in Supplemental Digital Content Table 1, http://links.lww.com/SLA/D612 mean BMI at baseline was similar across groups, ranging from 48 to 50 kg/m 2 . RYGB patients with depression spent more days in the hospital in the year before surgery than RYGB patients without depression (mean 0.43 and 0.29 days, respectively) (Supplemental Digital Content Table 3, http://links.lww.com/SLA/D614). However, mean hospital days in the previous year were similar for SG patients with and without depression (0.40 and 0.33, respectively). Regardless of depression diagnosis, the 3 most common comorbidities were hypertension, dyslipidemia, and sleep apnea, and prevalence of each was greater in the depression group. Anxiety was much more prevalent among patients with depression (42%) than those without (11%), as cooccurrence of anxiety with depression is common.
The diabetes cohort included 2769 patients with depression and 5638 patients without depression at baseline (Supplemental Digital Content Table 4, http://links.lww.com/SLA/D615). Patients with diabetes were older, with slightly higher BMI and more comorbidity than seen in the general weight loss cohort. The mean baseline HbA1c (7.1 to 7.3) and number of diabetes medications prescribed (1.6 to 1.7) were similar across depression groups. The 3 most common diabetes medications in both depression groups were metformin (biguanides), insulin, and sulfonylureas. The safety/utilization cohort had similar characteristics to the weight loss cohort (Supplemental Digital Content Table 5, http://links.lww.com/SLA/D616).
Table 4.
Adjusted Hazard Ratios for Comparison of Time to Different Safety Events for Gastric Bypass and Sleeve Gastrectomy, Stratified by Patients' Baseline Depression Status
| Outcome: Operation or Intervention | ||||||
|---|---|---|---|---|---|---|
| Estimated Cumulative Percentage of Patients With an Operation or Intervention (95% CI) at Different Times of Follow-up | ||||||
| Depression Status | Adjusted HR Comparing RYGB vs SG* (95% CI) | P-value | 1 yr | 3 yr | 5 yr | |
| No Depression | 1.38 (1.22, 1.56) | <0.001 | RYGB | 3.7 (3.4, 4.0) | 8.5 (7.8, 9.1) | 11.9 (11.0, 12.8) |
| SG | 2.7 (2.4, 3.0) | 6.2 (5.6, 6.8) | 8.8 (7.9, 9.6) | |||
| Depression | 1.36 (1.15, 1.60) | <0.001 | RYGB | 3.9 (3.5, 4.3) | 9.0 (8.1, 9.8) | 12.6 (11.4, 13.7) |
| SG | 2.9 (2.5, 3.3) | 6.7 (5.8, 7.6) | 9.4 (8.1, 10.7) | |||
| Interaction P-Value = | 0.856 | |||||
| Outcome: Revision | ||||||
| Estimated Cumulative Percentage of Patients With a Revision (95% CI) at Different Times of Follow-up | ||||||
| Depression Status | Adjusted HR Comparing RYGB vs SG* (95% CI) | P-value | 1 yr | 3 yr | 5 yr | |
| No Depression | 0.79 (0.63, 0.98) | 0.031 | RYGB | 0.9 (0.7, 1.0) | 1.6 (1.4, 1.9) | 2.7 (2.3, 3.1) |
| SG | 1.1 (0.9, 1.3) | 2.1 (1.8, 2.4) | 3.4 (2.9, 4.0) | |||
| Depression | 0.72 (0.54, 0.96) | 0.027 | RYGB | 0.9 (0.7, 1.1) | 1.7 (1.4, 2.0) | 2.8 (2.2, 3.3) |
| SG | 1.3 (1.0, 1.6) | 2.3 (1.8, 2.8) | 3.8 (2.9, 4.7) | |||
| Interaction P-value — | 0.605 | |||||
| Outcome: Endoscopy | ||||||
| Estimated Cumulative Percentage of Patients With an Endoscopy (95% CI) at Different Times of Follow-up | ||||||
| Depression Status | Adjusted HR Comparing RYGB vs SG* (95% CI) | P-value | 1 yr | 3 yr | 5 yr | |
| No Depression | 2.40 (2.12, 2.72) | <0.001 | RYGB | 6.2 (5.8, 6.6) | 10.8 (10.1, 11.5) | 14.8 (13.8, 15.7) |
| SG | 2.6 (2.4, 2.9) | 4.6 (4.2, 5.1) | 6.5 (5.8, 7.1) | |||
| Depression | 1.82 (1.57, 2.11) | <0.001 | RYGB | 7.1 (6.5, 7.6) | 12.2 (11.3, 13.1) | 16.7 (15.4, 17.9) |
| SG | 3.9 (3.4, 4.4) | 6.9 (6.0, 7.8) | 9.5 (8.3, 10.7) | |||
| Interaction P-value = | 0.003 | |||||
Covariates included: site, age, sex, race, Hispanic ethnicity, baseline BMI, HbAlc, and blood pressure categories, days from BMI measurement to bariatric surgery, number of inpatient hospital days in the year before surgery, Charlson/Elixhauser comorbidity category, year of surgery, smoking, and presence of codes for comorbidities (diabetes, hypertension, dyslipidemia, sleep apnea, osteoarthritis, NAFLD, GERD, anxiety, eating disorder, substance use, psychosis, kidney disease, infertility, polycystic ovaries, deep vein thrombosis, pulmonary embolism).
CI, Confidence interval; HR, hazard ratios; RYGB, Roux-en-Y gastric bypass; SG, Sleeve gastrectomy.
Weight Loss
Follow-up rates for weight/BMI at years 1, 3, and 5 are provided in Supplemental Digital Content Table 6, http://links.lww.com/SLA/ D617. Overall, %TWL was greater for RYGB than SG, and %TWL was slightly larger for patients without depression than those with depression for both operations at 1 and 3 years post-surgery (Table 2, Fig. 1A). However, by 5 years, patients with and without depression had no significant differences in weight loss. The interaction between depression status and operation type was not significant in years 1 or 3, but was significant at year 5 (interaction P = 0.04). However, the between group difference at year 5 was small (0.42%TWL).
Table 2.
Comparative Effectiveness of Gastric Bypass and Sleeve Gastrectomy for Percentage of Total Weight Loss (%TWL) and Absolute Difference in Hemoglobin A1c Level Among Adults with Diabetes With 1, 3, and 5 Years of Follow−up, Stratified by Baseline Depression Status
| 1 yr | 3 yr | 5 yr | ||||||
|---|---|---|---|---|---|---|---|---|
| Total Weight Loss, % | N Patients | %TWL | 95% CI | %TWL | 95% CI | %TWL | 95% CI | |
| SG | No Depression | 11522 | −25.2 | (−25.3,−25.0) | −21.3 | (−21.4,−21.1) | −19.2 | (−19.4,−19.0) |
| Depression | 4283 | −24.2 | (−24.5,−24.0) | −20.7 | (−20.9,−20.5) | −19.3 | (−19.6,−19.0) | |
| Difference | 0.97 | (0.7, 1.2) | 0.58 | (0.3, 0.9) | −0.18 | (−0.5, 0.2) | ||
| P-value | <0.001 | <0.001 | 0.300 | |||||
| RYGB | No Depression | 13327 | −30.8 | (−30.9,−30.7) | −28.4 | (−28.6,−28.3) | −25.7 | (−25.8,−25.5) |
| Depression | 6520 | −30.0 | (−30.1,−29.8) | −28.0 | (−28.2,−27.8) | −25.4 | (−25.6,−25.2) | |
| Difference | 0.83 | (0.6, 1.1) | 0.46 | (0.2, 0.7) | 0.23 | (0.0, 0.5) | ||
| P-value | <0.001 | <0.001 | 0.050 | |||||
| Between Group Differences | −0.14 | (−0.47, 0.19) | −0.13 | (−0.47, 0.21) | 0.42 | (0.01, 0.82) | ||
| Interaction P-value | 0.399 | 0.466 | 0.044 | |||||
| 1 yr | 3 yr | 5 yr | ||||||
| Hemoglobin A1c mean difference (95% CI), % | N Patients | Avg. Chg. | 95% CI | Avg. Chg. | 95% CI | Avg. Chg. | 95% CI | |
| SG | No Depression | 1936 | −0.97 | (−1.0,−0.9) | −0.57 | (−0.6,−0.5) | −0.25 | (−0.4,−0.2) |
| Depression | 834 | −0.96 | (−1.0,−0.9) | −0.47 | (−0.6,−0.4) | −0.22 | (−0.4,−0.1) | |
| Difference | 0.01 | (−0.1, 0.1) | 0.10 | (0.0, 0.2) | 0.03 | (−0.1, 0.2) | ||
| P-value | 0.901 | 0.048 | 0.730 | |||||
| RYGB | No Depression | 3612 | −1.12 | (−1.2,−1.1) | −0.76 | (−0.8,−0.7) | −0.54 | (−0.6,−0.5) |
| Depression | 1909 | −1.09 | (−1.1,−1.0) | −0.87 | (−0.9,−0.8) | −0.70 | (−0.8,−0.6) | |
| Difference | 0.03 | (0.0, 0.1) | −0.11 | (−0.2,−0.1) | −0.16 | (−0.2,−0.1) | ||
| P-value | 0.333 | <0.001 | <0.001 | |||||
| Between Group Differences | 0.02 | (−0.08, 0.12) | −0.21 | (−0.32,−0.10) | −0.19 | (−0.37,−0.01) | ||
| Interaction P-value | 0.658 | <0.001 | 0.038 | |||||
Covariates included: age, sex, race, Hispanic ethnicity, baseline BMI, maximum BMI in the prior year, baseline HbA1c, baseline blood pressure, days from most recent BMI measurement to baseline, days from most recent HbA1c measurement to baseline, number of in patient hospital days in the year prior to surgery, Charlson/Elixhauser comorbidity score, year of operation, smoking, and presence of codes for comorbidities (diabetes, hypertension, dyslipidemia, sleep apnea, osteoarthritis, NAFLD, GERD, anxiety, eating disorder, substance use, psychosis, kidney disease, infertility, polycystic ovaries, deep vein thrombosis, pulmonary embolism). RYGB, Roux-en-Y gastric bypass; SG, Sleeve gastrectomy; CI, Confidence interval; Avg Chg, Average Change.
Figure 1.
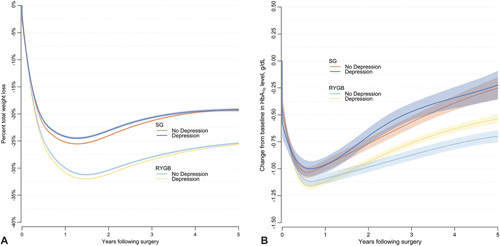
A. Estimated percentage of total weight loss through 5years after bariatric surgery for gastric bypass and sleeve gastrectomy in patients with and without depression at baseline. B. Estimated change in HbA1c level through 5 years after bariatric surgery for gastric bypass and sleeve gastrectomy patients with type 2 diabetes, with and without depression at baseline.
Change in Hemoglobin A1c
For both operations, change in HbA1c was not significantly different 1 year after surgery between those with and without a depression at baseline (Fig. 1B, Table 2). At 3 years follow-up, HbAlc declined more, on average, for SG patients without depression than those with depression (-0.57 and -0.47 percentage points, respectively); however, at 5 years follow-up, the mean HbA1c decline was similar for SG patients with and without depression. For RYGB patients, HbA1c declined more, on average, for those with depression than those without at 3 years (–0.87 and –0.76 percentage points, respectively) and 5 years (–0.70 and –0.54 percentage points, respectively). Baseline depression status was a significant moderator of glycemic control across operation types in years 3 and 5 (interaction P < 0.001 and P = 0.038, respectively), where patients with depression had significantly better glycemic control after RYGB but not after SG.
Diabetes Remission and Relapse
Results for time to remission since surgery and time to relapse since remission in the diabetes cohort are presented in Table 3, and the cumulative incidence rates of remission and relapse for 5 years following surgery are in Fig. 2. The rate of remission was higher for RYGB than for SG among those with and without depression (hazard ratios 1.12 and 1.09, respectively), and the relapse rate was lower for RYGB than for SG in patients with and without depression (hazard ratios 0.67 and 0.77, respectively). There was no significant interaction between baseline depression status and operation type for either the remission or relapse outcomes (interaction P = 0.571 for remission; interaction P = 0.282 for relapse).
Figure 2.
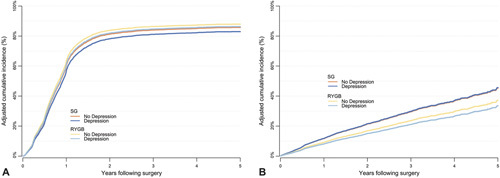
A. Cumulative incidence rates of T2DM remission across 5 years in the PBS diabetes cohort comparing gastric bypass and sleeve gastrectomy stratified by baseline depression status. B. Cumulative incidence rates of T2DM relapse (across 5 years in the PBS diabetes cohort comparing gastric bypass and sleeve gastrectomy stratified by baseline depression status. RYGB indicates Roux-en-Y gastric bypass;SG, sleeve gastrectomy.
Safety and Utilization Outcomes
In Table 4 and Supplemental Digital Content Table 7, http:// links.lww.com/SLA/D618 we present hazard ratios for time to safety-related events, including reoperation, revision, endoscopy, hospitalization, and mortality stratified by operation type and depression status. Cumulative incidence of these outcomes at 1, 3 and 5 years post-surgery are also included in Table 4/Supplemental Digital Content Table 7, http://links.lww.com/SLA/D618 and depicted in Fig. 3 A-E. Patients who had RYGB had greater risk of reoperation than those who underwent SG for patients with and without depression (hazard ratios 1.36 and 1.38, respectively). Baseline depression status was not a significant moderator of time to reoperation between the 2 operation types (interaction P = 0.856). Risk of revision was lower for RYGB than SG for patients with and without depression at baseline (hazard ratios 0.72 and 0.79, respectively). However, baseline depression status did not significantly impact risk of revision by operation type (interaction P = 0.605).
Figure 3.
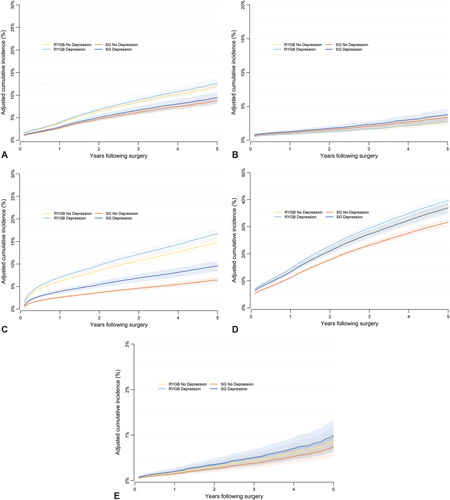
Cumulative Incidence Rates of operation or Intervention (Figure A), Revision (Figure B), Endoscopy (Figure C), Hospitalization (Figure D) and Mortality (Figure E). RYGB indicates Roux-en-Y gastric bypass;SG, sleeve gastrectomy; Depr = Depression.
Risk of endoscopy was greater for RYGB patients than SG patients both with and without baseline depression (hazard ratios 1.82 and 2.40, respectively). Those with depression had greater cumulative incidence of endoscopy than those without depression for each procedure (Fig. 3C). The interaction between operation type and baseline depression status was significant (P = 0.003). Compared to those without baseline depression, patients with depression had more of an increase in risk of endoscopy if they had SG than RYGB, even though the risk of endoscopy was lower for SG than RYGB overall.
Rates of hospitalization were greatest for RYGB patients with depression and lowest for SG patients without depression (Fig. 3D). Risk of hospitalization was greater for RYGB than SG patients with and without baseline depression (hazard ratios 1.10 and 1.21, respectively), but depression status was a significant moderator of time to re-hospitalization between operation type (interaction P = 0.046).
For all-cause mortality, results were not significant, suggesting no difference in risk between operation type and no impact of depression status. Rates of any 30-day Major Adverse Event (AE) were lower for SG than RYGB, and for both operations, AE rates were greater for those with depression than without (Supplemental Digital Content Table 8, http://links.lww.com/SLA/D619). Depression status was not a significant moderator of AE rates between operation type (interaction P = 0.415).
Discussion
In this large, multicenter, cohort study, we found that having a depression diagnosis recorded in the year before undergoing MBS was associated with small differences in weight, diabetes, and safety/ utilization outcomes in the 5 years after surgery. Patients with depression had slightly lower %TWL at 1 and 3 years for both RYGB and SG, and at 5 years for those undergoing RYGB, although the subgroup differences were clinically small (all <1%TWL). Patients with depression who underwent RYGB had greater improvements in HbA1c at both 3 and 5 years compared to those without depression, but this too was clinically small (<0.2% HbA1c) and depression was not a moderator of diabetes remission or relapse outcomes. Finally, depression was a significant moderator of the association between bariatric operation type and the risk of endoscopy and hospitalization, where depression was associated with a greater increased risk of those outcomes after SG than after RYGB. Taken as a whole, patients with depression generally seem to fare quite well after MBS. These findings are promising, considering that depression is common in this population and is associated with more medical and psychiatric comorbidities.
Our finding of little to no clinically-meaningful impact of depression on 5-year weight loss outcomes is consistent with prior research. In a review of the relationship between depression and weight outcomes, 4 out of 5 studies found that depression did not significantly predict weight outcomes at 6 months to 2 years after surgery, while 1 study found that depression and anxiety predicted weight loss at 4 years. 11,31 – 33 One challenge to interpreting the prior literature is that the studies were heterogeneous in their designs: some used measures of depressive symptoms while others used diagnoses as markers of depression; the outcomes examined (e.g., weight loss vs. regain) differed; and rates of follow-up were low. While our study only examined the impact of baseline depression status, it is possible that depression status after surgery may be more predictive of weight outcomes. 34 A recent study identified 6 distinct postsurgical depression change trajectories and found that those who experienced initial decreases in depression over the first 2 postsurgical years lost the most weight at 7 years in comparison to other depression subgroups. 35
Our HbA1c findings are more paradoxical, as those with depression undergoing RYGB had significantly better glycemic control at 3 and 5 years than patients without depression. This finding was counter to our hypothesis and may reflect closer adherence and followup among those with depression having RYGB. It may also be a result of changes in pharmacokinetic properties (e.g., decreased absorption) of psychotropic medications that worsen glycemic control. While further research is needed to explore potential explanations, it is important to note that the HbA1c finding is likely only of minor clinical significance given that the difference between those with and without depression having RYGB was -0.11 and -0.16 at 3 and 5 years.
When examining safety/utilization outcomes, rates of reoperations, hospitalizations, and endoscopy procedures were higher for those who underwent RYGB as compared to SG, regardless of their baseline depression status. 34 However, patients with depression were more likely to undergo postoperative endoscopy compared to those without depression, and this risk was particularly true for those with depression undergoing SG. Findings were similar for hospitalizations; that is, patients with depression, particularly those having SG, had an increased risk of hospitalization compared to those without depression. Overall, the choice of operation (RYGB vs SG) seems to be the strongest driver of these outcomes-the impact of depression is relatively small. The finding of greater risk of endoscopy and hospitalization for patients with depression may be explained by prior research showing that post-bariatric patients with mental health conditions were more likely to present to the emergency department than patients without mental health conditions, 33 which may lead to increased use of endoscopy and hospitalizations to evaluate gastrointestinal complaints. As expected, risk of a revisional/conversion MBS was higher for those who had undergone SG vs RYGB, and is consistent with prior literature, 36 ,37 however, depression was not a moderator of this relationship.
A limitation of the current study is loss-to-follow-up of patients without complete data capture in the electronic health record of outcomes during the 5 years of follow-up, including potential differential attrition by depression status. The greatest differential seems to be in Year 5, particularly for SG, thus our 3-year results may provide the least bias. Another limitation is our lack of data on depressive symptoms, severity, treatment, and remission rates at time of surgery. It is possible that patients with more severe depression could have differential outcomes. Likewise, unmeasured changes in depression treatment may impact the outcomes analyzed here. Furthermore, as has been shown with other psychosocial factors, such as maladaptive eating, 38 ,39 it may be that postsurgical symptoms are more relevant to surgical outcomes than presurgical levels and that ongoing monitoring and intervention are as important, if not more important, after bariatric surgery than presurgically. Another limitation of this study is the non-randomized design, which precludes causal inference and may be subject to residual unmeasured confounding. Finally, there is likely variation in each health system related to operation choice/recommendation, and thus there may be residual unmeasured confounding due to operation selection that was not accounted for in our models.
Conclusions
Presurgical psychosocial evaluation is a standard component of MBS and has become a valued part of assessing patients’ understanding and expectations of surgery, readiness, social support, and behavioral/psychiatric functioning. While many factors must be considered from a psychosocial standpoint when evaluating the MBS candidate, particularly in regards to assessing potential mental health related outcomes (eg, elevated postsurgical suicide and substance abuse risk), it seems that a diagnosis of depression should not preclude patients from undergoing MBS when anticipating weight, diabetes, and safety outcomes. Patients with depression, though losing slightly less weight, tended to lose within 1% of their counterparts without depression and, in the case of glycemic control, may do slightly better than those without depression when undergoing RYGB. While hospitalizations and endoscopic procedures seem to be more common for those with baseline depression, particularly for those who have SG, the differences in these outcomes across depression status were much smaller than the differences across bariatric operations. Taken as a whole, it seems that patients’ decision between RYGB and SG is a more important driver of outcomes than their preoperative depression status, and it does not seem that shared decision making discussions about choosing between RYGB and SG need to be carefully tailored to patients with vs. without baseline depression. Additional research is needed to examine whether baseline depression severity and treatment patterns or post-operative depression treatment trajectories are more significant predictors of long-term MBS outcomes.
Footnotes
For a list of full collaborative authors, see Supplement 1.
Funding: The PCORnet Study reported in this publication was conducted using PCORnet, the National Patient-Centered Clinical Research Network. PCORnet has been developed with funding from the Patient-Centered Outcomes Research Institute (PCORI).
This study was funded by the PCORI via contract OBS-1505-30683.
The views expressed in this manuscript are solely the responsibility of the authors and do not necessarily represent the views of other organizations participating in, collaborating with, or funding PCORnet or of the PCORI.
Dr. Arterburn received travel support from the World Congress for Interventional Therapy for Diabetes and the IFSO Latin America Chapter during the conduct of the study. Dr Courcoulas reports grants from Covidien/Ethicon Johnson & Johnson, during the conduct of the study. Dr Jones reports personal fees from Allurion. Dr. Apovian reports personal fees from Nutrisystem, Zafgen, Sanofi- Aventis, Orexigen, Novo Nordisk, GI Dynamics, Takeda, Scientific Intake, Xeno Biosciences, Rhythm Pharmaceuticals, Eisai, EnteroMedics, and Baria- trix Nutrition outside the submitted work; grants from Orexigen, Aspire Bariatrics, GI Dynamics, Myos, Takeda, the Vela Foundation, the Dr. Robert C. and Veronica Atkins Foundation, Coherence Lab, Energesis, and the National Institutes of Health outside the submitted work; and past ownership of stock in Science-Smart LLC. Dr. Fitzpatrick reports financial support from WW (formerly Weight Watchers) outside the submitted work. Dr. Coleman receives funding from Janssen and the Food and Drug Administration outside the submitted work.
The other authors report no conflicts of interest.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.annalsofsurgery.com.
Contributor Information
Collaborators: Janelle W. Coughlin, Wendy L. Bennett, Elizabeth Nauman, Robert Wellman, R. Yates Coley, Jane Anau, Andrea J. Cook, David Arterburn, Kathleen M. McTigue, Anita Courcoulas, Sengwee Toh, Jessica L Sturtevant, Casie E. Horgan, Jeffrey S. Brown, Karen J. Coleman, Daniel B. Jones, Christina C. Wee, Kristina H Lewis, Jonathan N. Tobin, Stephanie L. Fitzpatrick, Jay R. Desai, Sameer Murali, Ellen H. Morrow, Molly B. Conroy, Ann M. Rogers, Jennifer L. Kraschnewski, G. Craig Woods, Christopher D. Still, David J. Schlundt, James C. McClay, Corrigan L. McBride, Rohit Soans, Meredith C. Duke, Cynthia Blalock, Rabih Nemr, Neely Williams, Ana B. Emiliano, Stavra A. Xanthakos, Thomas Inge, Timothy S Carey, Marc Michalsky, Matthew F. Daley, Howard S. Gordon, Kirk W. Reichard, Caroline M. Apovian, Ali Tavakkoli, William G. Adams, and John H. Holmes
References
- 1.Pareek M, Schauer PR, Kaplan LM, et al. Metabolic surgery: weight loss, diabetes, and beyond. J Am Coll Cardiol. 2018;71:670–687. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed B, King WC, Gourash W, et al. Long-term weight change and health outcomes for sleeve gastrectomy (SG) and matched Roux-en-Y gastric bypass (RYGB) participants in the Longitudinal Assessment of Bariatric Surgery (LABS) study. Surgery. 2018;164:774–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376:641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welbourn R, Hollyman M, Kinsman R, et al. Bariatric surgery worldwide: baseline demographic description and one-year outcomes from the fourth IFSO Global registry report 2018. Obes Surg. 2019;29:782–795. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien PE, Hindle A, Brennan L, et al. Long-term outcomes after bariatric surgery: a systematic review and meta-analysis of weight loss at 10 or more years for all bariatric procedures and a single-centre review of 20-year outcomes after adjustable gastric banding. Obes Surg. 2019;29:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Courcoulas AP, King WC, Belle SH, et al. Seven-year weight trajectories and health outcomes in the longitudinal assessment of bariatric surgery (LABS) study. JAMA Surg. 2018;153:427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Courcoulas AP, Christian NJ, Belle SH, et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. 2013;310:2416–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arterburn D, Wellman R, Emiliano A, et al. Comparative effectiveness and safety of bariatric procedures for weight loss: a PCORnet cohort study. Ann Intern Med. 2018;169:741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McTigue KM, Wellman R, Nauman E, et al. Comparing the 5-year diabetes outcomes of sleeve gastrectomy and gastric bypass: the national patientcentered clinical research network (PCORNet) bariatric study. JAMA Surg. 2020;155:e200087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalarchian MA, Marcus MD, Levine MD, et al. Relationship of psychiatric disorders to 6-month outcomes after gastric bypass. Surg Obes Relat Dis. 2008;4:544–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pedro J, Neves JS, Ferreira MJ, et al. Impact of depression on weight variation after bariatric surgery: a three-year observational study. Obesity facts. 2020;13:213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Zwaan M, Enderle J, Wagner S, et al. Anxiety and depression in bariatric surgery patients: a prospective, follow-up study using structured clinical interviews. J Affect Disord. 2011;133:61–68. [DOI] [PubMed] [Google Scholar]
- 13.White MA, Kalarchian MA, Levine MD, et al. Prognostic significance of depressive symptoms on weight loss and psychosocial outcomes following gastric bypass surgery: a prospective 24-month follow-up study. Obes Surg. 2015;25:1909–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheets CS, Peat CM, Berg KC, et al. Post-operative psychosocial predictors of outcome in bariatric surgery. Obes Surg. 2015;25:330–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith CE, Hawkins MAW, Williams-Kerver GA, et al. Depression subtypes, binge eating, and weight loss in bariatric surgery candidates. Surg Obes Relat Dis. 2020;16:690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalarchian MA, King WC, Devlin MJ, et al. Psychiatric disorders and weight change in a prospective study of bariatric surgery patients: a 3-year follow-up. Psychosom Med. 2016;78:373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell JE, Selzer F, Kalarchian MA, et al. Psychopathology before surgery in the longitudinal assessment of bariatric surgery-3 (LABS-3) psychosocial study. Surg Obes Relat Dis. 2012;8:533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Courcoulas A, Coley RY, Clark JM, et al. Interventions and operations 5 years after bariatric surgery in a cohort from the US National Patient-Centered Clinical Research Network Bariatric Study. JAMA Surg. 2020;155:194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toh S, Rasmussen-Torvik LJ, Harmata EE, et al. The national patient-centered clinical research network (PCORnet) bariatric study cohort: rationale, methods, and baseline characteristics. JMIR Res Protoc. 2017;6:e222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forrest CB, McTigue KM, Hernandez AF, et al. PCORnet® 2020: current state, accomplishments, and future directions. J Clin Epidemiol. 2021;129:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleurence RL, Curtis LH, Califf RM, et al. Launching PCORnet, a national patientcentered clinical research network. J Am Med Inform Assoc. 2014;21:578–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qualls LG, Phillips TA, Hammill BG, et al. Evaluating foundational data quality in the National Patient-Centered Clinical Research Network (PCOR-net®). EGEMS (Washington DC). 2018;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gagne F, Glynn RJ, Avorn J, et al. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64:749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiest KM, Jette N, Quan H, et al. Systematic review and assessment of validated case definitions for depression in administrative data. BMC Psychiatry. 2014;14:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sogg S, Lauretti J, West-Smith L. Recommendations for the presurgical psychosocial evaluation of bariatric surgery patients. Surg Obes Relat Dis. 2016;12:731–749. [DOI] [PubMed] [Google Scholar]
- 26.Brethauer SA, Kim J, el Chaar M, et al. , ASMBS Clinical Issues Committee. Standardized outcomes reporting in metabolic and bariatric surgery. Surg Obes Relat Dis. 2015;11:489–506. [DOI] [PubMed] [Google Scholar]
- 27.Flum DR, Belle SH, King WC, et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361:445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 29.Chambers JM, Hastie TJ. (Ed.). Statistical Models in S (1st ed.). First ed: Routledge; 1992. https://www.routledge.com/Statistical-Models-in-S/Cham-bers-Hastie/p/book/9780412830402. [Google Scholar]
- 30.Efron B. The efficiency of cox’s likelihood function for censored data. J Am Stat Ass. 1977;72:557–565. [Google Scholar]
- 31.Dawes AJ, Maggard-Gibbons M, Maher AR, et al. Mental health conditions among patients seeking and undergoing bariatric surgery: a meta-analysis. JAMA. 2016;315:150–163. [DOI] [PubMed] [Google Scholar]
- 32.Legenbauer T, De Zwaan M, Benecke A, et al. Depression and anxiety: their predictive function for weight loss in obese individuals. Obesity facts. 2009;2:227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisher D, Coleman KJ, Arterburn DE, et al. Mental illness in bariatric surgery: a cohort study from the PORTAL network. Obesity (Silver Spring). 2017;25:850–856. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell JE, King WC, Chen JY, et al. Course of depressive symptoms and treatment in the longitudinal assessment of bariatric surgery (LABS-2) study. Obesity (Silver Spring). 2014;22:1799–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith KE, Mason TB, Cao L, et al. Trajectories of depressive symptoms and relationships with weight loss in the seven years after bariatric surgery. Obes Res Clin Pract. 2020;14:456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brethauer SA, Kothari S, Sudan R, et al. Systematic review on reoperative bariatric surgery: American Society for Metabolic and Bariatric Surgery Revision Task Force. Surg Obes Relat Dis. 2014;10:952–972. [DOI] [PubMed] [Google Scholar]
- 37.Altieri MS, Yang J, Nie L, et al. Rate of revisions or conversion after bariatric surgery over 10 years in the state of New York. Surg Obes Relat Dis. 2018;14:500–507. [DOI] [PubMed] [Google Scholar]
- 38.Kalarchian MA, Marcus MD. Psychosocial concerns following bariatric surgery: current status. Curr Obes Rep. 2019;8:1–9. [DOI] [PubMed] [Google Scholar]
- 39.Brode CS, Mitchell JE. Problematic eating behaviors and eating disorders associated with bariatric surgery. Psychiatr Clin North Am. 2019;42:287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]


