Objective:
Cervical cancer is a common preventable cancer among African women living with HIV (WLWH). Molecular diagnostics for high-risk human papillomavirus (HR-HPV) genotypes are standard components of cervical cancer screening in resource-rich countries but not in resource-limited settings. We evaluated HR-HPV genotypes among women with and without HIV in four African countries to inform cervical cancer preventive strategies.
Methods:
The African Cohort Study (AFRICOS) enrolled participants with and without HIV at 12 clinics in Tanzania, Kenya, Uganda, and Nigeria. Cervical cytobrush specimens from women were genotyped for 14 HR-HPV types using the multiplex Seegene Anyplex real-time PCR assay. Robust Poisson regression was used to estimate relative risks (RRs) and 95% confidence intervals (CIs) for factors associated with HR-HPV in WLWH.
Results:
From January 2015 to March 2020, 868 WLWH and 134 women living without HIV (WLWoH) were tested for HR-HPV with prevalence of 50.9 and 38.1%, respectively (P = 0.007). Among WLWH, 844 (97.4%) were antiretroviral therapy (ART)-experienced and 772 (89.7%) virally suppressed 1000 copies/ml or less. The most frequent HR-HPV types among WLWH were HPV-16 (13.5%), HPV-52 (9.5%), and HPV-35 (9.3%). HR-HPV infection was more common among Tanzanian WLWH (adjusted RR: 1.23, 95% CI 1.05–1.44, P = 0.012). Also, WLWH with CD4+ T cells of less than 200 cell/μl had 1.51-fold increased risk of having HR-HPV (95% CI 1.23–1.86, P < 0.001).
Conclusion:
HR-HPV was common in WLWH in four African countries, particularly among women with low CD4+ cell count. Scale up of HPV vaccines and development of vaccines with broader activity against less common HR-HPV types may improve cervical cancer prevention in Africa.
Keywords: AIDS, Africa, antiretroviral therapy, high-risk human papilloma virus, HIV, uterine cervical neoplasms, women living with HIV
Introduction
Cervical cancer is the leading cause of cancer-related death among women globally, with the majority of cases occurring in low-income and middle-income countries (LMICs), especially in Africa [1]. Almost all cervical cancer cases are caused by persistent high-risk human papillomavirus (HPV) infections, with carcinogenic types 16 and 18 being linked to 70% of cervical cancer cases worldwide [2]. HPV16 and HPV18 infections are largely preventable by vaccination, but such vaccines are not widely available in much of Africa [3–5].
In Africa, where HIV is often co-prevalent, 85% of women with cervical cancer are also living with HIV [6]. In fact, women living with HIV (WLWH) are at multifold increased risk of acquiring and having a persistent high-risk HPV infection, and as a consequence developing cervical cancer, compared with their counterparts without HIV [6–8]. Regular HPV-based screening has been shown to reduce cervical cancer incidence and mortality by up to 70% [9–11]. Thus, although not operational in most countries, the WHO currently recommends HPV DNA detection as the primary screening test for women (aged ≥30 years) to prevent cervical cancer [12]. Through the cervical cancer elimination initiative, WHO is aiming for 70% of women to be screened, and to effectively treat 90% of those with a positive screening test or a cervical lesion by 2030 [12]. Recognizing a high burden of HPV disease in WLWH, the US President's Emergency Plan for AIDS Relief (PEPFAR) has so far supported the integration of cervical cancer screening and precancerous treatment into PEPFAR-supported HIV care and treatment clinics in 12 African countries with high HIV prevalence [13].
Although vaccines can prevent infection with some of the most common high-risk HPV types, only 15 African countries currently include HPV vaccination in their national immunization programs [14]. Uganda, Tanzania and Kenya added HPV vaccination to their national routine immunization schedules in 2015, 2018 and 2019, respectively; while in Nigeria, HPV vaccination is still being piloted and yet to be introduced into the country's routine immunization program [14–16].
There is geographical variation in the distribution of some of the less common high-risk HPV types. For example, although HPV-35 is only linked to about 2% of global cervical cancer cases, it is more prevalent in Africa, where it occurs in about 10% of precancerous and cervical cancer cases [2,17–22]. In a recent study in East Africa, HPV-35 was the second most frequent HPV type in precancerous lesions and was still detected in 11% of cervical cancer cases in WLWH. However, HPV-35 typically occurred together with other high-risk HPV types in cervical cancer, making it difficult to understand its contribution to cancerogenesis in infected women [23]. This may have implications on the effectiveness of vaccination strategies in HPV-35 prevalent regions, as the existing HPV vaccines do not target HPV-35.
Understanding the distribution of HPV genotypes among WLWH and factors associated with high-risk HPV genotype infection and persistence in various regions is crucial for evaluating, improving, and developing cervical cancer prevention initiatives. Herein, we report on the distribution of high-risk HPV infections among WLWH attending PEPFAR-supported HIV clinics in four African countries, using a simple real-time PCR-based detection method that differentiates all 14 high-risk HPV genotypes.
Material and methods
Study population and data collection
The African Cohort Study (AFRICOS) is an ongoing multi-site observational study that enrols adults living with and without HIV aged 18 years and older at 12 PEPFAR-supported clinical care sites in Uganda, Kenya, Tanzania and Nigeria, as previously described [24]. Medical history and physical examination is conducted for every participant at enrolment and every 6 months thereafter. For individuals with HIV, assessment of CD4+ cell count, HIV-1 plasma viral load and ART history is performed. Measurements for CD4+ cell count, HIV-1 plasma viral load were done using platforms previously detailed in [25]. HIV-1 drug resistance genotyping has previously been performed for some participants as described in [26]. Additionally, demographic and clinical data including sex, age, education level, comorbidities and self-reported sexual behaviour are also collected for each participant, by using extensive questionnaires. From 2015 onward, high-risk HPV testing was offered annually as an additional optional procedure to all women participating in AFRICOS, with the test being rolled out in Tanzania first in 2015, followed by Kenya, Uganda and Nigeria in 2018. This analysis included WLWH and women living without HIV (WLWoH) enrolled in AFRICOS with HPV genotyping data.
Collection of endocervical cells
Cervical cells were collected by experienced nurses, by applying a cytobrush (Solann, Sundbyberg, Sweden) into the endocervical wall and gently rotating the brush 360°. Specimens were then stored in 5 ml PreservCyt cell collection media (Roche, Vorna Valley, South Africa). Cytobrush specimens from Tanzania were directly taken to National Institute for Medical Research-Mbeya Medical Research Center (NIMR-MMRC) HPV reference laboratory in Tanzania while those from Uganda, Kenya and Nigeria were temporarily stored at −20 or −80 °C before their transportation to Tanzania for HPV genotyping. Upon the specimens’ arrival, cells were thoroughly dislodged from the cytobrush, aliquoted and short-term (−20 °C) or long-term (−80 °C) stored prior to HPV genotyping.
Human papilloma virus genotyping
DNA was extracted from cervical cells using QIAamp DNA mini kit (Qiagen, Hilden, Germany), followed by HPV genotyping using the Anyplex II HPV HR detection test (Seegene, Seoul, Korea) with the CFX96 Real-time PCR System (Bio-Rad, Hercules, California, USA) as per manufacturer's instructions. The assay detects 14 high-risk HPV genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68), which are regarded as carcinogens. HPV infection was defined as the presence of at least one high-risk HPV genotype in the tested samples. To monitor the quality of NIMR-MMRC laboratory in HPV genotyping, the laboratory has been participating in WHO HPV genotyping proficiency testing since 2013. Of note, accuracy of the Seegene Anyplex II HPV HR detection assay at NIMR-MMRC was 100% in the most recent (2020) WHO proficiency panel.
Statistical analyses
Stata version 14 (StataCorp, College Station, Texas, USA) and Prism version 9 (GraphPad Software, San Diego, California, USA) were used for statistical analyses. Two-sided Fisher‘s exact test (or wherever applicable, Pearson's chi-squared test) was used to compare the differences in the frequency of individual HPV genotypes between groups of interest. Robust (modified) Poisson regression model was used to estimate relative risk (RRs) and associated 95% confidence intervals (CIs) for factors potentially associated with high-risk HPV in WLWH. Akaike information criteria (AIC) was used to select variables to be included in the model. The model with the lowest AIC was selected. Statistical significance was defined as P less than 0.05 for all analyses except for multiple comparison between country of residence and high-risk HPV, where Bonferroni corrections were used to adjust for the P value. High-risk HPV genotype-specific analyses included all women with data who were infected with the respective high-risk HPV genotype, meaning that women with multiple high-risk HPV infections were included in more than one of the compared groups.
Ethical consideration
All women were fully briefed on the study procedures and provided written informed consent prior to their enrolment. The study was approved by the institutional review boards of the Walter Reed Army Institute of Research (#1897) and ethics committees from all collaborating institutions. Research was performed in accordance with the Declaration of Helsinki.
Results
Study population
Between January 2015 and March 2020, a total of 1712 women were enrolled in AFRICOS and were eligible for HPV testing. Among them, there were 1002 (58.5%) women with median age of 40.8 years [interquartile range (IQR) 34.2–47.4 years] who had their endocervical specimens collected for high-risk HPV testing (Table S1). About 41.5% of eligible women opted not to participate in HPV typing. Reasons for refusal to undergo HPV testing were primarily because of invasive nature of the procedure, time limitations because of other long study procedures, participants reporting to have already received annual cervical cancer screening, menstruation, or if participants were virgo intacta. Generally, WLWH who consented to HPV testing and were included in the analyses were older (Table S1). In Tanzania, WLWH who were HPV-tested were more likely to be on ART, have CD4+ T-cell counts of above 200 cells/μl and viral load of 1000 copies/ml or less compared with those not tested (Table S2).
Table 1 details the characteristics of the 1002 AFRICOS participants who were genotyped for high-risk HPV. Of these, 98 (9.8%) were from Uganda, 554 (55.3%) from Kenya, 230 (22.9%) from Tanzania and 120 (12.0%) from Nigeria. Among 868 WLWH (86.6%), 844 (97.3%) were on ART and 772 (89.7%) had a viral load 1000 copies/ml or less (Table 1). Overall, WLWH had a median CD4+ nadir of 216 (IQR: 108–352) cells/μl, median CD4+ at the time of specimen collection of 538 (IQR: 376–732) cells/μl and median HIV-1 RNA of 0 (IQR: 0–40) copies/ml. High-risk HPV was more common among women with HIV than without (50.9 vs. 38.1%, P = 0.007). Among WLWH, high-risk HPV was more common among those not on ART, with lower CD4+ counts, and with higher viral loads (Table 1). Of note, ART exposure, CD4+ T-cell counts and viral suppression in WLWH differed by country, with Tanzania having significantly more women who were off ART and viraemic than other countries (Fig. S1 and Table S3).
Table 1.
Characteristics of the African Cohort Study women who received high-risk human papilloma virus genotyping (n = 1002).
| Characteristic | n (column %) | High-risk HPV not detected [n (column %)] | High-risk HPV detected [n (column %)] | ∗P value |
| Age (years) | 0.329 | |||
| 18–24 | 50 (5.0%) | 19 (3.7%) | 31 (6.3%) | |
| 25–39 | 430 (42.9%) | 221 (43.4%) | 209 (42.4%) | |
| 40–49 | 347 (34.6%) | 178 (35.0%) | 169 (34.3%) | |
| ≥50 | 175 (17.5%) | 91 (17.9%) | 84 (17.0%) | |
| Site, country | 0.143 | |||
| Kayunga, Uganda | 98 (9.8%) | 56 (11.0%) | 42 (8.5%) | |
| South Rift Valley, Kenya | 447 (44.6%) | 237 (46.6%) | 210 (42.6%) | |
| Kisumu West, Kenya | 107 (10.7%) | 58 (11.4%) | 49 (9.9%) | |
| Mbeya, Tanzania | 230 (22.9%) | 103 (20.2%) | 127 (25.8%) | |
| Abuja, Nigeria | 62 (6.2%) | 26 (5.1%) | 36 (7.3%) | |
| Lagos, Nigeria | 58 (5.8%) | 29 (5.7%) | 29 (5.9%) | |
| Education | 0.962 | |||
| No formal education | 36 (3.6%) | 19 (3.7%) | 17 (3.4%) | |
| ≤Primary | 256 (25.5%) | 131 (25.7%) | 125 (25.3%) | |
| ≥Secondary | 710 (70.9%) | 359 (70.5%) | 351 (71.2%) | |
| Age at 1st sexual intercourse | 0.293 | |||
| ≤18 | 701 (71.0%) | 349 (69.4%) | 352 (72.6%) | |
| >18 | 287 (29.0%) | 154 (30.6%) | 133 (27.4%) | |
| Missing | 14 | 6 | 8 | |
| Number of lifetime sexual partners | 0.541 | |||
| No partner | 323 (32.4%) | 164 (32.3%) | 159 (32.4%) | |
| One partner | 595 (59.6%) | 307 (60.6%) | 288 (58.7%) | |
| Multiple (≥two) partners | 80 (8.0%) | 36 (7.1%) | 44 (9.0%) | |
| Missing | 4 | 2 | 2 | |
| HIV status | 0.007 | |||
| Women living without HIV | 134 (13.4%) | 83 (16.3%) | 51 (10.3%) | |
| Women living with HIV | 868 (86.6%) | 426 (83.7%) | 442 (89.7%) | |
| HIV-associated parameters (among WLWH only) | ||||
| On ART | 0.010 | |||
| Yes | 844 (97.3%) | 420 (98.8%) | 424 (95.9%) | |
| No | 23 (2.7%) | 5 (1.2%) | 18 (4.1%) | |
| Missing | 1 | 1 | 0 | |
| CD4+ cell count (cells/μl) | <0.001 | |||
| ≤200 | 57 (6.7%) | 14 (3.4%) | 43 (9.9%) | |
| 201–500 | 311 (36.7%) | 142 (34.2%) | 169 (39.0%) | |
| >500 | 480 (56.6%) | 259 (62.4%) | 221 (51.0%) | |
| Missing | 20 | 11 | 9 | |
| HIV viral load (copies/ml) | 0.010 | |||
| ≤1000 | 772 (89.7%) | 391 (92.4%) | 381 (87.0%) | |
| >1000 | 89 (10.3%) | 32 (7.6%) | 57 (13.0%) | |
| Missing | 7 | 3 | 4 |
P values were calculated using Pearson's chi-squared test. Statistically significant P values (P < 0.05) are in bold.
Distribution of high-risk HPV genotypes
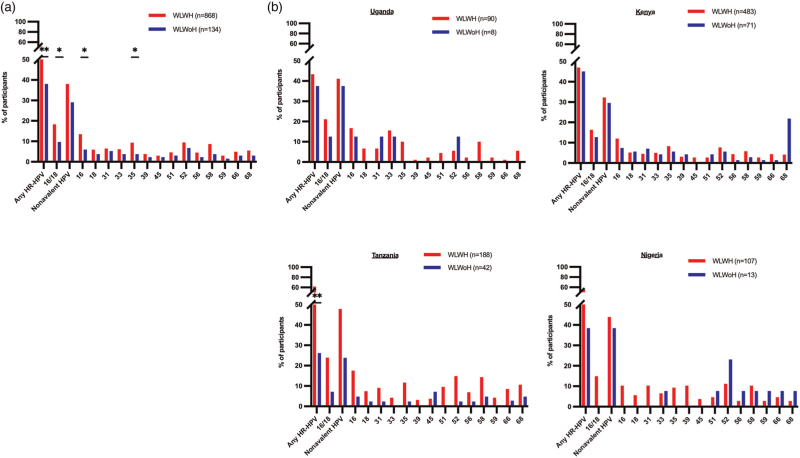
The most frequent high-risk HPV genotypes among WLWH were HPV-16 (13.5%), HPV-52 (9.4%) and HPV-35 (9.3%), whereas HPV-52 (6.7%), HPV-16 (6.0%) and HPV-31 (5.2%) were the most common HPV genotypes among WLWoH (Fig. 1a). Despite 97.4% being on ART, HIV was associated with a higher overall prevalence of infection with any high-risk HPV genotype (P = 0.007, Fig. 1a). Also, when compared with WLWoH, WLWH had higher prevalence of high-risk HPV genotypes covered by the bivalent and quadrivalent vaccine (HPV-16 and/or HPV-18) and those targeted by the nonavalent vaccine (HPV-16/18/31/33/45/52/58). Specifically, as compared with WLWoH, a higher proportion of WLWH had HPV-16 (13.5 vs. 6%, P = 0.011) and infection with the nonvaccine HPV-35 was more frequent in WLWH compared with WLWoH (9.3 vs. 3.7%, P = 0.030, Fig. 1a). When stratified by country of residence, a significant association between HIV and high-risk HPV was particularly observed for Tanzanian women (Fig. 1b).
Fig. 1.
Distribution of high-risk human papilloma virus genotypes in women living with HIV and women living without HIV in the African Cohort Study.
(a) Comparison of the frequency of each high-risk HPV genotype (shown in y-axis) between WLWH (red bars, n = 868) and WLWoH (blue bars, n = 134). Country-specific comparison of the frequency of each high-risk HPV genotype between WLWH and WLWoH is shown in (b). Fisher's exact test was used for comparison. Asterisks denote different P values: ∗P < 0.05, ∗∗P < 0.005. P values less than 0.05 are considered significant for (a) whereas P values after the results corrections (<0.003) are considered significant for (b). HPV, human papilloma virus; WLWH, women living with HIV; WLWoH, women living without HIV.
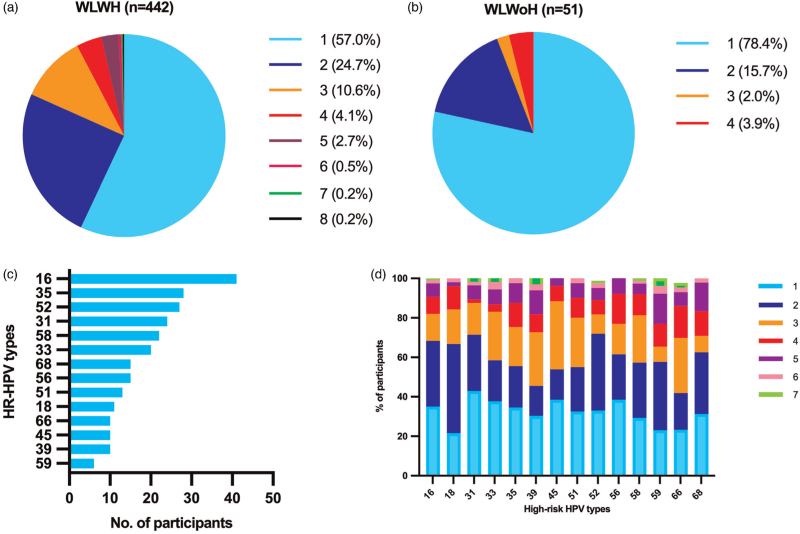
Of 442 WLWH with high-risk HPV infections, 252 (57.0%) had single high-risk HPV infection and 190 (43.0%) had two or more high-risk HPV genotypes (Fig. 2a). On the contrary, the proportion of WLWoH with multiple high-risk HPV genotypes was only 21.6% (Fig. 2b) and significantly lower than that of WLWH (P = 0.010, chi-squared test). Of 252 women with a single high-risk HPV infection, HPV-16 was the most frequent single infecting genotype, n = 41 (16.3%) followed by HPV-35, n = 28 (11.1%) and HPV-52, n = 27 (10.7%) (Fig. 2c). The proportion of women with multiple high-risk HPV co-infections varied for each HPV genotype. Although 78.4% of women with HPV-18 were also co-infected with other high-risk HPV genotypes, 57.1% of women with HPV-31 had multiple high-risk HPV genotypes (Fig. 2d).
Fig. 2.
Prevalence of multiple high-risk human papilloma virus infections in women living with HIV and women living without HIV with high-risk human papilloma virus infections in the African Cohort Study.
Proportions of number of detected HPV infections in WLWH and WLWoH diagnosed with high-risk HPV are shown in (a) and (b), respectively. (c) the distribution of high-risk HPV genotypes among WLWH with single high-risk HPV infection is shown. (D)The proportion of single and multiple HPV infections in each HPV type is shown. Colours correspond with the number of detected high-risk HPV genotypes as indicated on the legend. HPV, human papilloma virus; WLWH, women living with HIV; WLWoH, women living without HIV.
HIV-1 subtype information was available for 155 of 442 WLWH with high-risk HPV infection, of whom 68 (42.8%) had subtype A, 32 (20.1%) had C, 17 (10.7%) had D, 12 (7.5%) had G, 12 (7.5%) had CRF02_AG, whereas 18 (11.3%) had other circulating recombinant forms. The distribution of specific HIV subtypes in WLWH with detected high-risk HPV types is further detailed in Fig. S2.
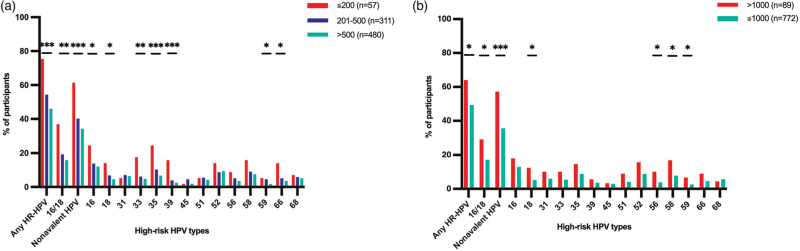
Women with CD4+ T-cell counts below 200 cells/μl were more frequently infected with a high-risk HPV type compared with those with CD4+ counts of 201–500 or greater than 500 cells/μl, especially with: HPV-16 (24.6 vs. 13.8 and 12.1%, P = 0.042), 18 (14 vs. 6.8 and 4.6%, p = 0.018) and 33 (17.5 vs. 6.1 and 4.8%, P = 0.003; Fig. 3a). Of note, the following high-risk HPV genotypes, which are not covered by the available HPV vaccines, were also significantly associated with CD4+ T-cell counts less than 200 cells/μl: HPV-35 (24.6 vs. 10.3 and 6.7%, P < 0.001), 39 (15.8 vs 3.9 and 2.5 and 2.5%, P < 0.001), 59 (5.3 vs. 4.5 and 1.7%, P < 0.025) and 66 (14 vs 5.1 and 3.5%, P < 0.006; Fig. 3a). The association of CD4+ levels with high-risk HPV genotype infections varied by country, with HPV-35 being more frequent in Tanzanian WLWH with less than 200 cells/μl CD4+ T cells (Table S4).
Fig. 3.
Distribution of high-risk human papilloma virus genotypes in women living with HIV in the African Cohort Study stratified by HIV-related parameters.
The frequency of each HPV genotype is shown for women with: (a) CD4+ T-cell counts of 200 cells/μl or less (red bars, n = 57), 201–500 cell/μl (blue bars, n = 311) and greater than 500 cell/μl (green bars, n = 480); and (b) HIV viral load of 1000 copies/ml or less (green bars, n = 772) and greater than 1000 copies/ml (red bars, n = 89). Statistical analysis was performed using Fisher's exact test. Asterisks denote different P values: ∗P < 0.05, ∗∗P < 0.005, ∗∗∗P < 0.001.
Similarly, a greater proportion of WLWH with plasma HIV-1 viral load of more than 1000 copies/ml were infected with high-risk HPV compared with WLWH with a viral load of 1000 copies/ml or less, as follows: HPV-18 (12.4 vs. 5.2%, P = 0.014), HPV-56 (10.1 vs. 3.9%, P = 0.014), HPV-58 (16.9 vs. 7.8%, P = 0.008) and HPV-59 (6.7 vs. 2.6%, P = 0.045, Fig. 3b). This difference was also observed for nonvirally suppressed women when stratified by country, but not to a significant level when statistically adjusted (Table S5).
Factors associated with high-risk human papilloma virus among women living with HIV in the African Cohort Study
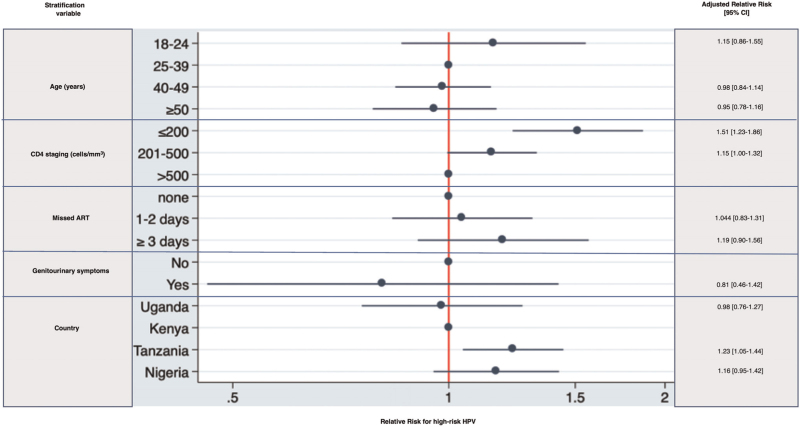
As high-risk HPV was a common outcome, Robust (modified) Poisson regression model was used to estimate RR associated with high-risk HPV [27]. A model showed that WLWH with CD4+ cell count less than 200 cells/μl and 201–500 cells/μl had a 58% (95% CI 1.31–1.91, P < 0.001) and 17% (95% CI 1.01–1.34, P = 0.030) increased RR of having high-risk HPV, respectively (Fig. 4). Moreover, residence in Tanzania was associated with an increased risk of having high-risk HPV infection in WLWH compared with Kenya (adjusted RR: 1.25, 95% CI 1.07–1.46, P = 0.004, Fig. 4).
Fig. 4.
Factors associated with high-risk human papilloma virus among women living with HIV in the the African Cohort Study.
Robust Poisson regression model with the number of high-risk HPV infections as a dependent variable for AFRICOS women living with HIV, adjusting for age (18–24 vs. 25–39, 40–49 and ≥50 years), CD4+ staging (≤200 vs. 201–500 and >500 cell/μl), ART adherence (none vs. 1–2 days and ≥3 days of missed ART dose), genitourinary symptoms (no vs. yes) and women's country of residence (Uganda vs. Kenya, Tanzania and Nigeria). The individual risk factors are indicated on the y-axis; adjusted relative risk and 95% confidence intervals (CI) are shown on the x-axis. Actual 95% CI are shown on the right side of the graph. AFRICOS, the African Cohort Study; ART, antiretroviral therapy.
Discussion
This study evaluated the distribution of clinically relevant high-risk HPV infections among WLWH attending PEPFAR-supported HIV clinics in four African countries. WLWH from this multicountry study were almost all (>97%) on ART, yet they still were more frequently infected with high-risk HPV genotypes compared with WLWoH. Women with HIV not only had a high prevalence of oncogenic HPV-16 but were also burdened with less common and moderate high-risk HPV genotypes, in particular 35 and 52. Of note, high-risk HPV infection was more common among Tanzanian WLWH. Nonetheless, high-risk HPV infection was less frequent in ‘immunocompetent’ and virally suppressed WLWH, irrespective of country of residence, emphasizing that adherence to treatment and viral suppression has a beneficial effect thereby probably increasing clearance of infection and hence reducing the risk of HPV disease progression in WLWH.
Our findings are similar to previous studies, which reported higher frequencies of HPV infection in WLWH than WLWoH [28,29], even though over 97% of women in this study were on ART. Consistent with previous findings [17,30,31], HPV-16 was the most frequently detected high-risk HPV genotype in WLWH, with infection prevalence being significantly higher in WLWH than WLWoH.
Interestingly, the high prevalence of high-risk HPV in WLWH from the studied population was in part driven by WLWH from Tanzania. This may be explained by the fact that, of the four studied countries, Tanzania had the highest proportion of WLWH who were treatment-naive, immunocompromised and viremic, perhaps because HPV testing within AFRICOS was introduced earliest in Tanzania (in 2015) before the ‘test and treat’ era [32]. This suggests that more advanced HIV disease progression in many Tanzanian study participants may contribute to the elevated prevalence of high-risk HPV infection (and persistence) and hence higher risk for cervical cancer.
Recent studies have linked HPV-35, which is currently not targeted by any of the available HPV vaccines, with cervical carcinogenesis in women of African ancestry [33]. Indeed, the prevalence of HPV-35 is higher in some African countries compared with other regions and has been detected in 4–10% of precancerous and cervical cancer cases [2,17–22]. In the present study, HPV-35 was also frequent in WLWH, where the proportion of WLWH with HPV-35 infection ranged from 8 to 11% across all countries. We and others have previously studied HPV-35 infections in WLWH with precancerous or cervical cancer [17,30,34]. Of note, in our recently published study, HPV-35 infections occurred frequently in women with precancerous lesions (27% of WLWH with such lesions), typically in the presence of other high-risk HPV genotypes with a similar pattern, yet at a lower frequency (11%) observed in cervical cancer cases [23]. In contrast, HPV-16/18 and also HPV-45 were often detected as single infections in cancers in the same study [23]. Hence, the RR of HPV-35 infections to cause cervical cancer progression in WLWH should be studied more in detail. Nonetheless, given its reported occurrence and potential contribution to cervical cancer cases in Africa, including HPV-35 in vaccines may prove beneficial, especially for African WLWH.
Infection with HPV-52 is linked to 3% of reported invasive cancer cases worldwide [2]. In our study, HPV-52 infections were prevalent in both women with and without HIV. In particular, Tanzanian WLWH had a six-fold higher prevalence of HPV-52 than WLWoH. Although HPV-52 is targeted by the nonavalent vaccine, most African countries including Tanzania use the bivalent or quadrivalent vaccine [15], which only covers the highest risk HPV genotypes 16 and 18 [2]. Robust, longitudinal research describing the persistence of high-risk HPV infections with non-HPV-16/18 genotypes, their associated risk of disease and progression to cancer will be necessary to optimize diagnostic algorithms for WLWH. Furthermore, cost-effectiveness studies on implementation of nonavalent vaccines in these settings should be considered.
Consistent with previous reports [23,31,35], we found that infections with multiple high-risk HPV genotypes were common in WLWH. We and others have also shown a predominance of multiple high-risk HPV infections in WLWH with precancerous lesions, whereas WLWH with cervical cancer often had single high-risk HPV genotype infections, especially of HPV-16, 18 and 45 [23,30]. Moreover, women aged 30 years and above with persistent high-risk HPV infection, have a high risk of developing precancerous cervical lesions and cervical cancer [36,37]. Lack of capacity for cytohistological diagnosis during our current study limited investigation of high-risk HPV genotypes associated with HPV disease in the studied WLWH. Nonetheless, the identified WLWH, with HPV-16, HPV-18 and/or HPV-45 in this study (about 18% of WLWH) could benefit from follow-up HPV testing to identify those at greatest risk for HPV disease, and thus refer them for intensive evaluation with cytohistological analyses. Importantly, prior work has similarly shown an increased risk of multiple anal HPV infections also in men living with HIV as compared with those without HIV [38].
In this study, infections with particular vaccine (16, 18 and 33) and non-vaccine (35, 39, 59 and 66) HPV genotypes were most frequent in WLWH with advanced immunosuppression. Women with less than 200 cell/μl CD4+ T cells had 1.5 increased risk of having high-risk HPV infections. These results are consistent with previous results showing that low CD4+ T-cell counts increase the risk of high-risk HPV infection, precancerous lesions and progression to cervical cancer compared with those with higher CD4+ T cells [7,29,39–44]. Low CD4+ cell counts and detectable HIV viremia in WLWH correlate with depletion of high-risk HPV-specific T-cell responses [45], which may constitute a mechanism of the increased risk for high-risk HPV persistence and associated cancerogenesis in these women [45].
Importantly, those who consented to high-risk HPV genotyping and were included in the analysis were also more likely to be on ART and be aviraemic. We would, therefore, expect that those who did not participate to have even higher risk for high-risk HPV infections compared with those included in the study, particularly in Tanzania.
One major limitation of this study is the missing cytohistological diagnosis of cervical lesions and cancer in these women. Visual inspection with acetic acid (VIA) was performed on some women as part of their cervical cancer screening procedures, but systematic interpretation of VIA results in this analysis was difficult because of methodological challenges. The AFRICOS study procedures closely reflect national cervical cancer screening and treatment algorithms for WLWH. It is, therefore, evident that more advanced cytohistological investigation for detection of precancerous lesions is typically not performed in WLWH in the participating clinical sites, preventing the possibility of early intervention in these women. Smart molecular diagnosis of high-risk HPV genotypes, which was relatively simple in this study, could, therefore, help to pre-select women with high-risk HPV infection (in particular, those above 30 years of age and with persistent infection) for referral to specialized care and treatment centres, for more advanced diagnostic work-up that require specialized medical resources (like pathologists and obstetrician/gynaecologists). Also, uptake of HPV testing was relatively low in this cohort and could be improved by increasing community awareness on the importance of cervical cancer screening, dispelling myths about cervical cancer and HPV, and introducing HPV self-collection, which has been shown to be highly acceptable among African women [46–50].
In conclusion, this study not only confirms a high prevalence of carcinogenic HPV-16 in WLWH from the four studied African countries but provides evidence of high prevalence of less common high-risk HPV genotypes 35 and 52 in these populations. Although HPV-35 is not covered in any of the currently licensed vaccines, HPV-52 is also not targeted by the currently distributed bivalent and quadrivalent vaccines in these countries, which may have implications on the effectiveness of ongoing HPV vaccination strategies in the region. Further studies should focus on better understanding the contribution of non-HPV-16/18/45 genotypes in cervical cancer development in African populations. Cost-effectiveness studies of switching to nonavalent HPV vaccines (that includes HPV- 52) in countries that have yet to introduce them at a large scale should also be considered, as this may improve cervical cancer prevention in Africa – home to the highest proportion of women burdened with cervical cancer and HIV in the world [6].
Acknowledgements
We thank the study participants, local implementing partners, and hospital leadership at Kayunga District Hospital, Kericho District Hospital, AC Litein Mission Hospital, Kapkatet District Hospital, Tenwek Mission Hospital, Kapsabet District Hospital, Nandi Hills District Hospital, Kisumu West District Hospital, Mbeya Zonal Referral Hospital, Mbeya Regional Referral Hospital, Defence Headquarters Medical Center, and the 68th Nigerian Army Reference Hospital.
We would also like to thank the AFRICOS Study Group – from the US Military HIV Research Program Headquarters Group: Danielle Bartolanzo, Alexus Reynolds, Katherine Song, Mark Milazzo, Leilani Francisco, Steven Schech, Badryah Omar, Tsedal Mebrahtu, Elizabeth Lee, Kimberly Bohince, Ajay Parikh, Jaclyn Hern, Emma Duff, Kara Lombardi, Michelle Imbach, and Leigh Anne Eller; from the AFRICOS Uganda Group: Hannah Kibuuka, Michael Semwogerere, Prossy Naluyima, Godfrey Zziwa, Allan Tindikahwa, Claire Nakazzi Bagenda, Hilda Mutebe, Cate Kafeero, Enos Baghendaghe, William Lwebuge, Freddie Ssentogo, Hellen Birungi, Josephine Tegamanyi, Paul Wangiri, Christine Nabanoba, Phiona Namulondo, Richard Tumusiime, Ezra Musingye, Christina Nanteza, Joseph Wandege, Michael Waiswa, Evelyn Najjuma, Olive Maggaga, Isaac Kato Kenoly, and Barbara Mukanza; from the AFRICOS South Rift Valley, Kenya Group: Jonah Maswai, Rither Langat, Aaron Ngeno, Lucy Korir, Raphael Langat, Francis Opiyo, Alex Kasembeli, Christopher Ochieng, Japhet Towett, Jane Kimetto, Brighton Omondi, Mary Leelgo, Michael Obonyo, Linner Rotich, Enock Tonui, Ella Chelangat, Joan Kapkiai, Salome Wangare, Zeddy Bett Kesi, Janet Ngeno, Edwin Langat, Kennedy Labosso, Joshua Rotich, Leonard Cheruiyot, Enock Changwony, Mike Bii, Ezekiel Chumba, Susan Ontango, Danson Gitonga, Samuel Kiprotich, Bornes Ngtech, Grace Engoke, Irene Metet, Alice Airo, and Ignatius Kiptoo; from the AFRICOS Kisumu, Kenya Group: John Owuoth, Valentine Sing’oei, Winne Rehema, Solomon Otieno, Celine Ogari, Elkanah Modi, Oscar Adimo, Charles Okwaro, Christine Lando, Margaret Onyango, Iddah Aoko, Kennedy Obambo, Joseph Meyo, and George Suja; from the AFRICOS Abuja, Nigeria Group: Michael Iroezindu, Yakubu Adamu, Nnamdi Azuakola, Mfreke Asuquo, Abdulwasiu Bolaji Tiamiyu, Afoke Kokogho, Samirah Sani Mohammed, Ifeanyi Okoye, Sunday Odeyemi, Aminu Suleiman, Lawrence C. Umeji, Onome Enas, Miriam Ayogu, Ijeoma Chigbu-Ukaegbu, Wilson Adai, Felicia Anayochukwu Odo, Rabi Abdu, Roseline Akiga, Helen Nwandu, Chisara Sylvestina Okolo, Ogundele Taiwo, Otene Oche Ben, Nicholas Innocent Eigege, Tony Ibrahim Musa, Juliet Chibuzor Joseph, Ndubuisi C. Okeke; from the AFRICOS Lagos, Nigeria Group: Zahra Parker, Nkechinyere Elizabeth Harrison, Uzoamaka Concilia Agbaim, Olutunde Ademola Adegbite, Ugochukwu Linus Asogwa, Adewale Adelakun, Chioma Ekeocha, Victoria Idi, Rachel Eluwa, Jumoke Titilayo Nwalozie, Igiri Faith, Blessing Irekpitan Wilson, Jacinta Elemere, Nkiru Nnadi, Francis Falaju Idowu, Ndubuisi Rosemary, Amaka Natalie Uzeogwu, Theresa Owanza Obende, Ifeoma Lauretta Obilor, Doris Emekaili, Edward Akinwale, and Inalegwu Ochai; from the AFRICOS Mbeya, Tanzania Group: Lucas Maganga, Emmanuel Bahemana, Samoel Khamadi, John Njegite, Cornelia Lueer, Abisai Kisinda, Maria Mwakatima, Wolfram Mwalongo, Lwitiho Sudi, Jaquiline Mwamwaja, Faraja Mbwayu, Gloria David, Mtasi Mwaipopo, Reginald Gervas, Dorothy Mkondoo, Nancy Somi, Paschal Kiliba, Ephrasia Mwalongo, Gwamaka Mwaisanga, Johnisius Msigwa, Hawa Mfumbulwa, Peter Edwin, Willyhelmina Olomi.
Authors’ contributions: E.B., R.M., L.M., Ja.M., R.G., H.K., Jo.Ma., V.S., M.I. and A.F. conducted clinical work and collected data. M.C., A.P.P., A.M., W.M., N.D., T.A.C., J.H., X.S. and C.P. contributed to data cleaning, management and analysis. A.P.P., A.M., Jo.Mn. and M.I. coordinated sample logistics/shipments. M.C. coordinated HPV testing and led in writing of the manuscript. A.M. and Jo.Mn. contributed to HPV genotyping analyses. C.G., M.H. and J.A.A. conceived/designed the study. All authors contributed to drafting, reading and approved the final manuscript.
Funding: this work was supported by the Military HIV Research Program and the President's Emergency Plan for AIDS Relief (PEPFAR) via cooperative agreements between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense (W81XWH-11-2-0174 and W81XWH-18-2-0040). The views expressed are those of the authors and should not be construed to represent the positions of the US Army or the Department of Defense. Optimization of high-risk HPV genotyping needed for the study was supported by Deutsche Forschungsgemeinschaft (reference number; 2128/2-1 and 2128/2-2, Project number 620615). Write-up of this work was partly supported by University of Dar es Salaam's ‘Female Leaders Academic Publishing Support Programme (FLAPS) 2022/1’. The investigators have adhered to the policies for protection of human subjects as prescribed in AR 70-25.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
J.A.K. and C.G. contributed equally to this work.
Supplemental digital content is available for this article.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71:209–249. [DOI] [PubMed] [Google Scholar]
- 2.de Sanjose S, Quint WGV, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11:1048–1056. [DOI] [PubMed] [Google Scholar]
- 3.Tsu VD, LaMontagne DS, Atuhebwe P, Bloem PN, Ndiaye C. National implementation of HPV vaccination programs in low-resource countries: Lessons, challenges, and future prospects. Prev Med (Baltim) 2021; 144:106335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mboumba Bouassa RS, Prazuck T, Lethu T, Jenabian MA, Meye JF, Bélec L. Cervical cancer in sub-Saharan Africa: a preventable noncommunicable disease. Expert Rev Anti Infect Ther 2017; 15:613–627. [DOI] [PubMed] [Google Scholar]
- 5.Black E, Richmond R. Prevention of cervical cancer in sub-Saharan Africa: the advantages and challenges of HPV vaccination. Vaccines 2018; 6:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stelzle D, Tanaka LF, Lee KK, Ibrahim Khalil A, Baussano I, Shah ASV, et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob Heal 2021; 9:e161–e169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaturvedi AK, Madeleine MM, Biggar RJ, Engels EA. Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst 2009; 101:1120–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. JNCI J Natl Cancer Inst 2000; 92:1500–1510. [DOI] [PubMed] [Google Scholar]
- 9.Sankaranarayanan R, Nene BM, Shastri SS, Jayant K, Muwonge R, Budukh AM, et al. HPV screening for cervical cancer in rural India. NEJM 2009; 360:1385–1394. [DOI] [PubMed] [Google Scholar]
- 10.Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJF, Arbyn M, et al. International HPV screening working group. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet (London, England) 2014; 383:524–532. [DOI] [PubMed] [Google Scholar]
- 11.Ogilvie GS, Krajden M, van Niekerk D, Smith LW, Cook D, Ceballos K, et al. HPV for cervical cancer screening (HPV FOCAL): Complete Round 1 results of a randomized trial comparing HPV-based primary screening to liquid-based cytology for cervical cancer. Int J Cancer 2017; 140:440–448. [DOI] [PubMed] [Google Scholar]
- 12. WHO. WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention, second edition. Geneva; 2021. Available at: https://www.who.int/publications/i/item/9789240030824 [Accessed 18 November 2021] [Google Scholar]
- 13.Godfrey C, Prainito A, Lapidos-Salaiz I, Barnhart M, Watts DH. Reducing cervical cancer deaths in women living with HIV: PEPFAR and the Go Further partnership. Prev Med (Baltim) 2021; 144:106295. [DOI] [PubMed] [Google Scholar]
- 14. Bruni L, Albero G, Serrano B, Mena M, Collado J, Gómez D, et al. Human papillomavirus and related diseases in Africa. 2021. [Google Scholar]
- 15.Runge AS, Bernstein ME, Lucas AN, Tewari KS. Cervical cancer in Tanzania: a systematic review of current challenges in six domains. Gynecol Oncol Rep 2019; 29:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mphuru A, Li AJ, Kyesi F, Mwengee W, Mazige F, Nshunju R, et al. National introduction of human papillomavirus (HPV) vaccine in Tanzania: Programmatic decision-making and implementation. Vaccine 2021; 40 Suppl:A2–A9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuguyo O, Dube Mandishora RS, Thomford NE, Makunike-Mutasa R, Nhachi CFB, Matimba A, et al. High-risk HPV genotypes in Zimbabwean women with cervical cancer: comparative analyses between HIV-negative and HIV-positive women. PLoS One 2021; 16:e0257324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan P, Howell-Jones R, Li N, Bruni L, De Sanjosé S, Franceschi S, Clifford GM. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J cancer 2012; 131:2349–2359. [DOI] [PubMed] [Google Scholar]
- 19.Clifford GM, De Vuyst H, Tenet V, Plummer M, Tully S, Franceschi S. Effect of HIV infection on human papillomavirus types causing invasive cervical cancer in Africa. J Acquir Immune Defic Syndr 2016; 73:332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denny L, Adewole I, Anorlu R, Dreyer G, Moodley M, Smith T, et al. Human papillomavirus prevalence and type distribution in invasive cervical cancer in sub-Saharan Africa. Int J cancer 2014; 134:1389–1398. [DOI] [PubMed] [Google Scholar]
- 21.Okolo C, Franceschi S, Adewole I, Thomas JO, Follen M, Snijders PJF, et al. Human papillomavirus infection in women with and without cervical cancer in Ibadan, Nigeria. Infect Agent Cancer 2010; 5:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castellsagué X, Klaustermeier JE, Carrilho C, Albero G, Sacarlal J, Quint W, et al. Vaccine-related HPV genotypes in women with and without cervical cancer in Mozambique: burden and potential for prevention. Int J cancer 2008; 122:1901–1904. [DOI] [PubMed] [Google Scholar]
- 23.Mcharo R, Lennemann T, France J, Torres L, Garí M, Mbuya W, et al. HPV type distribution in HIV positive and negative women with or without cervical dysplasia or cancer in East Africa. Front Oncol 2021; 11:4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ake JA, Polyak CS, Crowell TA, Kiweewa F, Semwogerere M, Maganga L, et al. African Cohort Study Team. Noninfectious Comorbidity in the African Cohort Study. Clin Infect Dis 2019; 69:639–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kibuuka H, Musingye E, Mwesigwa B, Semwogerere M, Iroezindu M, Bahemana E, et al. Predictors of all-cause mortality among people with HIV in a Prospective Cohort Study in East Africa and Nigeria. Clin Infect Dis 2021; 75:657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crowell TA, Danboise B, Parikh A, Esber A, Dear N, Coakley P, et al. Pre-treatment and acquired antiretroviral drug resistance among persons living with HIV in four African countries. Clin Infect Dis 2020; 73:e2311–e2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159:702–706. [DOI] [PubMed] [Google Scholar]
- 28.Liu G, Sharma M, Tan N, Barnabas RV. HIV-positive women have higher risk of HPV infection, precancerous lesions, and cervical cancer: a systematic review and meta-analysis. AIDS 2018; 32:795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adedimeji A, Ajeh R, Dzudie A, Kendowo E, Fuhngwa N, Nsame D, et al. Cervical human papillomavirus DNA detection in women living with HIV and HIV-uninfected women living in Limbe, Cameroon. J Clin Virol 2020; 128:104445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clifford GM, Tully S, Franceschi S. Carcinogenicity of human papillomavirus (HPV) types in HIV-positive women: a meta-analysis from HPV infection to cervical cancer. Clin Infect Dis 2017; 64:1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camargo M, Del Río-Ospina L, Soto-De León SC, Sánchez R, Pineda-Peña AC, Sussmann O, et al. Association of HIV status with infection by multiple HPV types. Trop Med Int Health 2018; 23:1259–1268. [DOI] [PubMed] [Google Scholar]
- 32. THE UNITED REPUBLIC OF TANZANIA. NATIONAL AIDS CONTROL PROGRAMME NATIONAL GUIDELINES FOR THE MANAGEMENT OF HIV AND AIDS. 2015. Available at: https://www.childrenandaids.org/sites/default/files/2017-04/Tanzania_National-HIV-Guidelines_2015.pdf [Accessed 30 May 2022] [Google Scholar]
- 33.Pinheiro M, Gage JC, Clifford GM, Demarco M, Cheung LC, Chen Z, et al. Association of HPV35 with cervical carcinogenesis among women of African ancestry: evidence of viral-host interaction with implications for disease intervention. Int J Cancer 2020; 147:2677–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Obiri-Yeboah D, Akakpo PK, Mutocheluh M, Adjei-Danso E, Allornuvor G, Amoako-Sakyi D, et al. Epidemiology of cervical human papillomavirus (HPV) infection and squamous intraepithelial lesions (SIL) among a cohort of HIV-infected and uninfected Ghanaian women. BMC Cancer 2017; 17:688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okoye JO, Ofodile CA, Adeleke OK, Obioma O. Prevalence of high-risk HPV genotypes in sub-Saharan Africa according to HIV status: a 20-year systematic review. Epidemiol Health 2021; 43:e2021039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elfgren K, Elfström KM, Naucler P, Arnheim-Dahlström L, Dillner J. Management of women with human papillomavirus persistence: long-term follow-up of a randomized clinical trial. Am J Obstet Gynecol 2017; 216:264.e1–264.e7. [DOI] [PubMed] [Google Scholar]
- 37.Jacobs MV, Snijders PJF, Van den Brule AJC, Helmerhorst TJM, Meijer CJLM, Walboomers JMM. A general primer GP5+/GP6(+)-mediated PCR-enzyme immunoassay method for rapid detection of 14 high-risk and 6 low-risk human papillomavirus genotypes in cervical scrapings. J Clin Microbiol 1997; 35:791–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nowak RG, Schumaker LM, Ambulos NP, Ndembi N, Dauda W, Nnaji CH, et al. TRUST/RV368 Study Group. Multiple HPV infections among men who have sex with men engaged in anal cancer screening in Abuja, Nigeria. Papillomavirus Res (Amsterdam, Netherlands) 2020; 10:100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Denny L, Boa R, Williamson AL, Allan B, Hardie D, Stan R, Myer L. Human papillomavirus infection and cervical disease in human immunodeficiency virus-1-infected women. Obstet Gynecol 2008; 111:1380–1387. [DOI] [PubMed] [Google Scholar]
- 40.Phelan DF, Gange SJ, Ahdieh-Grant L, Mehta SH, Kirk GD, Shah K, Gravitt P. Determinants of newly detected human papillomavirus infection in HIV-infected and HIV-uninfected injection drug using women. Sex Transm Dis 2009; 36:149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Konopnicki D, Manigart Y, Gilles C, Barlow P, De Marchin J, Feoli F, et al. Sustained viral suppression and higher CD4+ T-cell count reduces the risk of persistent cervical high-risk human papillomavirus infection in HIV-positive women. J Infect Dis 2013; 207:1723–1729. [DOI] [PubMed] [Google Scholar]
- 42.Ezechi OC, Ostergren PO, Nwaokorie FO, Ujah IAO, Odberg Pettersson K. The burden, distribution and risk factors for cervical oncogenic human papilloma virus infection in HIV positive Nigerian women. Virol J 2014; 11:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu E, McCree R, Mtisi E, Fawzi WW, Aris E, Lema IA, et al. Prevalence and risk factors of cervical squamous intraepithelial lesions among HIV-infected women in Dar es Salaam, Tanzania. Int J STD AIDS 2016; 27:219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guiguet M, Boué F, Cadranel J, Lang JM, Rosenthal E, Costagliola D. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol 2009; 10:1152–1159. [DOI] [PubMed] [Google Scholar]
- 45.Mbuya W, Held K, Mcharo RD, Haule A, Mhizde J, Mnkai J, et al. Depletion of human papilloma virus E6- and E7-oncoprotein-specific T-cell responses in women living with HIV. Front Immunol 2021; 12:742861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Esber A, McRee AL, Turner AN, Phuka J, Norris A. Factors influencing Malawian women's willingness to self-collect samples for HPV testing. J Fam Plan Reprod Heal care 2017; 43:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sarai Racey C, Withrow DR, Gesink D. Self-collected HPV testing improves participation in cervical cancer screening: a systematic review and meta-analysis. Can J Public Health 2013; 104:e159–e166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bansil P, Wittet S, Lim JL, Winkler JL, Paul P, Jeronimo J. Acceptability of self-collection sampling for HPV-DNA testing in low-resource settings: a mixed methods approach. BMC Public Health 2014; 14:596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mao C, Kulasingam SL, Whitham HK, Hawes SE, Lin J, Kiviat NB. Clinician and patient acceptability of self-collected human papillomavirus testing for cervical cancer screening. J Womens Health (Larchmt) 2017; 26:609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arbyn M, Smith SB, Temin S, Sultana F, Castle P. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ 2018; 363:k4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.