Objective:
To assesses the prevalence and severity of CAS in patients undergoing PD/total pancreatectomy and its association with major postoperative complications after PD.
Summary of background data:
CAS may increase the risk of ischemic complications after PD. However, the prevalence of CAS and its relevance to major morbidity remain unknown.
Methods:
All patients with a preoperative computed tomography with arterial phase undergoing partial PD or TP between 2014 and 2017 were identified from a prospective database. CAS was assessed based on computed tomography and graded according to its severity: no stenosis (<30%), grade A (30%–<50%), grade B (50%–≤80%), and grade C (>80%). Postoperative complications were assessed and uni- and multivariable risk analyses were performed.
Results:
Of 989 patients, 273 (27.5%) had CAS: 177 (17.9%) with grade A, 83 (8.4%) with grade B, and 13 (1.3%) with grade C. Postoperative morbidity and 90-day mortality occurred in 278 (28.1%) patients and 41 (4.1%) patients, respectively. CAS was associated with clinically relevant pancreatic fistula (P=0.019), liver perfusion failure (P=0.003), gastric ischemia (P=0.001), clinically relevant biliary leakage (P=0.006), and intensive care unit (P=0.016) and hospital stay (P=0.001). Multivariable analyses confirmed grade B and C CAS as independent risk factors for liver perfusion failure; in addition, grade C CAS was an independent risk factor for clinically relevant pancreatic fistula and gastric complications.
Conclusions:
CAS is common in patients undergoing PD. Higher grade of CAS is associated with an increased risk for clinically relevant complications, including liver perfusion failure and postoperative pancreatic fistula. Precise radiological assessment may help to identify CAS. Future studies should investigate measures to mitigate CAS-associated risks.
Keywords: celiac axis stenosis, complications, liver perfusion failure, morbidity, pancreatectomy, pancreatoduodenectomy, postoperative pancreatic fistula
Pancreatoduodenectomy (PD) is the standard surgical treatment for patients with benign or malignant pancreatic head lesions. 1–3 With substantial advances in surgical techniques, perioperative care, and interdisciplinary management, both perioperative and long-term outcomes of pancreatic surgery have improved considerably in recent years and mortality had decreased to under 5%. 4–7 However, despite these advances, complication rates after partial PD and total pancreatectomy (TP) remain high, with major morbidity up to 40%. 6,8,9 The most common and serious complication after PD is postoperative pancreatic fistula (POPF). 10,11 Clinically relevant POPF occurs in up to 30% of cases after PD and is associated with increased hospital stay, costs, reintervention rates, and mortality. 11 Moreover, a clinically relevant POPF can trigger further serious complications, such as sepsis, postpancreatectomy hemorrhage or intestinal perforation. 10,11 Several studies have evaluated and defined pre- and intraoperative factors associated with increased risk of POPF. These include pancreatic texture, pancreatic duct size, blood loss, pathology, and body mass index (BMI). 12–14 Other less frequent complications that occur after PD or TP include liver ischemia, gastric complications, and leakage of the biliodigestive anastomosis. 15–18
During PD, the blood supply to the pancreatic head is dissected, particularly the gastroduodenal artery (GDA) and the inferior pancreaticoduodenal artery. Dissecting these collaterals between the celiac artery and the superior mesenteric artery may affect the blood flow in both vessels. Under normal conditions these changes have no clinically relevant consequences. However, in the presence of celiac axis stenosis (CAS), the liver, stomach, spleen, and remnant pancreas are at risk of ischemia after pancreatic head resection with division of arterial collaterals because their blood supply is entirely dependent on blood flow from the celiac artery. 19–21
Several case reports and small case series have focused on identifying and managing CAS in the context of PD/TP. 20,22–24 Sugae et al have classified CAS based on severity of the stenosis caused by a median arcuate ligament. 20 However, the association between CAS and the typical complications after PD/TP has not been systematically assessed.
The present study systematically assesses the prevalence and severity of CAS in patients undergoing PD/TP and its association with major postoperative complications after PD.
Patients And Methods
Study Design and Patient Cohort
This study is based on an analysis of prospectively collected clinical data from an institutional database of consecutive pancreatic operations. The study was approved by the institutional ethics committee (S-011/2015) and follows the STrengthening the Reporting of OBservational studies in Epidemiology recommendations for observational studies. 25
All consecutive patients undergoing partial PD or TP between January 2014 and December 2017 at the Department of General, Visceral and Transplantation Surgery, Heidelberg University Hospital, were screened for inclusion in the study. Patients without a contrast-enhanced computed tomography (CT) with arterial phase and sagittal reconstruction were excluded.
Data Collection
Clinical parameters extracted from the prospectively maintained database included age, sex, BMI, American Society of Anesthesiologists class, alcohol consumption, and smoking. Data pertaining to previous surgical procedures on the pancreas, liver, and stomach, was also collected to determine the role of previous abdominal surgeries on postoperative outcomes. In addition, data on preoperative stenting, percutaneous transhepatic cholangio-drainage, and neoadjuvant chemoor radiotherapy were collected.
Surgery-related parameters were also extracted from the database, including indication for surgery, type of pancreatic re section,extendedand vascular resections according to the International Study Group on Pancreatic Surgery, 26 blood loss, and duration of the operation. In addition, Michel classification was used to determine the variants of the celiac axis, hepatic artery, and superior mesenteric artery, 27 to distinguish the distribution of the blood supply of upper abdominal organs in patients with CAS.
Assessment of CAS
To evaluate the presence of CAS, CT scans with arterial phase and sagittal reconstruction were retrospectively reviewed by 3 observers who were blinded to the surgical outcome parameters. According to the radiologic features, the cause of CAS was divided into either extrinsic stenosis (eg, median arcuate ligament, compression by the celiac ganglion or surrounding fibrotic transformations) or intrinsic stenosis caused by atherosclerosis (Fig. 1).
Figure 1.
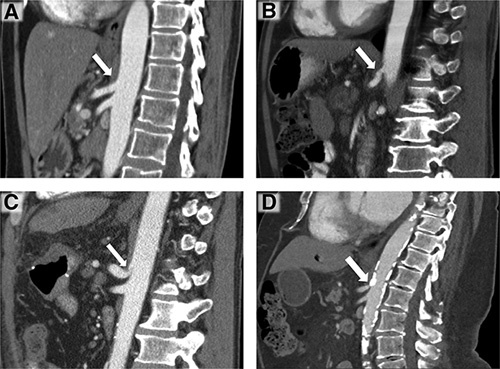
Examples of celiac axis without stenosis (A) and of CAS of different grades (B–D) in sagittal reconstructions of contrast-enhanced computed tomography during arterial phase. (A) No evidence of celiac axis stenosis (white arrow). (B) Grade A (30%–<50%) extrinsic CAS (white arrow). (C) Grade B (50%–≤80%) extrinsic CAS due to median arcuate ligament with the characteristic hooked appearance (white arrow). (D) Grade C (>80%) intrinsic CAS due to severe atherosclerosis (white arrow). CAS indicated celiac axis stenosis.
CAS was graded according to the severity of luminal stenosis regardless of its length (Fig. 1). According to the classification system proposed by Sugae et al, ( 20 CAS was classified into 3 grades: A (30%–<50%), B (50%–≤80%), and C (>80%). Patients without stenosis or with a stenosis <30% without hemodynamic relevance were included in the group “no stenosis.”
Postoperative complications, including POPF, 11 postpancreatectomy hemorrhage, 13 and postoperative bile leakage 28 were assessed and classified using the definition of the International Study Groups of Pancreatic Surgery and Liver Surgery. Further procedure-related complications, including completion pancreatectomy rate, gastroenterostomy leakage, gastric perforation, and gastric ischemia were also recorded. Liver perfusion failure was categorized according to Hackert et al, 16 as follows: mild liver perfusion failure: peak serum alanine transaminase and aspartate transaminase levels in day 1 and 3 below 500 units per liter and 250 units per liter, respectively; moderate perfusion failure, enzyme levels between 500 units per liter and 1000 units per liter; severe perfusion failure with enzyme levels higher than 1000 units per liter for both markers. The composite endpoint was defined by combining POPF, postpancreatectomy hemorrhage, liver perfusion failure, postoperative bile leakage, and gastric complications. The durations of intensive care unit (ICU) and intermediate care unit (IMCU) stay, and hospital stay, were recorded. Mortality was reported as all causes of death occurring within the first 30 and 90 days after pancreas surgery.
Statistical Analysis
International Business Machines Corporation (IBM) Superior Performing Software System (SPSS) Statistics Version 25.0 (IBM Corp. Released 2017. Armonk, NY) was used for statistical analyses. Continuous data are presented as a mean with standard deviation and categorical data as frequencies with proportions. Univariate analyses were performed using the Kruskal–Wallis test for continuous data and Chi-square or Fisher exact test for categorical data to define the factors associated with liver perfusion failure, gastric complications and POPF after PD/TP. A P value < 0.05 was considered statistically significant. To define the association between CAS severity and postoperative complications, a post hoc analysis was carried out by calculating the standardized residuals of crosstabulation [(frequency-expected)/standard error]. 29,30 The 2-sided α level (P value) was adjusted to 0.006 [=0.05/(2*4)] by Bonferroni correction for multiple comparisons.
Variables with a P value < 0.1 in the univariate analyses were included in the multivariate binary logistic regression analysis to determine the independent predictors of POPF, gastric complications, and liver perfusion failure after PD/TP. Cases with missing values were excluded from the multivariable analyses. Patients with TP were excluded from the analysis regarding POPF and completion pancreatectomy because they were not at risk. Results were reported as odds ratios and 95% confidence intervals. Factors that were significantly associated with pancreatic and gastric complications were used in binominal logistic regression analysis to identify independent predictors. Bonferroni correction was applied at accepted significance levels.
Results
Demographic and Preoperative Data
Between January 2014 and December 2017, a total of 1429 patients underwent PD or TP. Of these, 440 (30.8%) patients were excluded from the study because they had no CT scan (117; 8.2%), a CT scan without arterial phase and sagittal reconstruction (314; 22%), or insufficient postoperative documentation (9; 0.6%). Some 989 (69.2%) patients with complete data were included in the analysis.
The most common indication for PD/TP was ductal adenocarcinoma (544 patients; 55%), followed by intraductal papillary mucinous neoplasia (113 patients; 11.4%), chronic pancreatitis (83 patients; 8.3%), periampullary carcinoma (38 patients; 3.8%), and pancreaticoduodenal neuroendocrine neoplasia (30 patients; 3.0%). The demographic and preoperative data of the study cohort are summarized in Table 1. The median patient age was 65 years (range: 20–89) and 551 patients (55.7%) were male.
Table 1.
Demographic Characteristics and Preoperative Data of the Patients
| Celiac Axis Stenosis | ||||||
|---|---|---|---|---|---|---|
| Total Median (IQR)/n (%) (n = 989) | None (n = 716) | 30% – <50% (n = 177) | 50% – ≤80% (n = 83) | >80% (n = 13) | P Value | |
| Demographic data | ||||||
| Age | 65 (55–73) | 63 (54–72) | 71 (63–75) | 68 (61 –75) | 69 (57–76) | <0.001 |
| BMI | 24.8 (22.4–27.8) | 24.9 (22.4–27.9) | 24.7 (22.2–27.5) | 23.9 (21.6–27.6) | 23.7 (23.3–28.6) | 0.171 |
| Sex | ||||||
| Female | 438 (44.3%) | 313 (43.7%) | 79 (44.6%) | 39 (47.0%) | 7 (53.8%) | 0.881 |
| Male | 551 (55.7%) | 403 (56.3%) | 98 (55.4%) | 44 (53.0%) | 6 (46.2%) | |
| Smoking | 267 (27.0%) | 197 (27.5%) | 41 (23.1%) | 22 (26.5%) | 7 (53.8%) | 0.090 |
| Alcohol abuse | 165 (16.7%) | 124 (17.3%) | 24 (13.6%) | 13 (15.7%) | 4 (30.8%) | 0.338 |
| ASA classification | ||||||
| I | 31 (3.1%) | 25 (3.5%) | 5 (2.8%) | 1 (1.2%) | 0 (0.0%) | 0.227 |
| II | 559 (56.5%) | 423 (59.1%) | 87 (49.2%) | 44 (53%) | 5 (38.5%) | |
| III | 391 (39.5%) | 263 (36.7%) | 83 (46.9%) | 37 (44.6%) | 8 (51.5%) | |
| IV | 8 (0.9%) | 5 (0.7%) | 2 (1.1%) | 1 (1.2%) | 0 (0.0%) | |
| Previous procedures | ||||||
| Previous abdominal surgery | 54 (5.5%) | 40 (5.6%) | 8 (4.5%) | 5 (6.0%) | 1 (7.7%) | 0.918 |
| Pancreatic duct stent | 9 (0.9%) | 7 (1.0%) | 1 (0.6%) | 1 (1.2%) | 0 (0.0%) | 0.928 |
| Bile duct stent | 244 (24.7%) | 168 (23.5%) | 47 (26.6%) | 28 (33.7%) | 1 (7.7%) | 0.064 |
| PTCD | 23 (2.3%) | 17 (2.4%) | 4 (2.3%) | 2 (2.4%) | 0 (0.0%) | 0.956 |
| Neoadjuvant therapy | ||||||
| None | 849 (85.9%) | 600 (83.8%) | 162 (91.5%) | 76 (91.6%) | 11 (84.6%) | 0.102 |
| Chemotherapy | 125 (12.6%) | 102 (14.2%) | 14 (7.9%) | 7 (8.4%) | 2 (15.4%) | |
| Chemoradiotherapy | 15 (1.5%) | 14 (2.0%) | 1 (0.6%) | 0 (0.0%) | 0 (0.0%) | |
ASA indicates American Society of Anesthesiologists; PTCD, percutaneous transhepatic cholangiodrainage.
Celiac Axis Stenosis
According to retrospective assessments of preoperative CT scans, CAS was identified in 273 (27.6%) patients, including extrinsic CAS in 153 (15.4%) patients, intrinsic CAS in 96 (9.7%) patients, and combined extrinsic/intrinsic in 24 (2.4%) patients. With regard to the stenosis grade, 17.9% (n = 177) of the patients had grade A, 8.4% (n = 83) had grade B, and 1.3% (n = 13) had grade C CAS. Patients with CAS were significantly older without any other differences in further demographic and preoperative parameters (Table 1).
During treatment, CAS was documented in only 91 (9.2%) patients. CAS was recognized before or during surgery in 32 (3.2%) patients and after surgery in 59 (5.9%) patients. In addition, GDA test was also routinely performed in patients. No preoperative management of CAS was carried out. Intraoperative diagnosis of CAS was reported in thirteen patients who developed an abnormal GDA test during the operation and underwent dissection of median arcuate ligament (Table 2). The rate of ligament dissection was significantly higher in patients with CAS grade C (P <0.001). Celiac trunk resection and arterial reconstruction was inevitable in 19 patients due to trunk infiltration (sixteen patients) or intraoperative bleeding (2 patients). Arterial reconstruction of the celiac trunk was performed due to CAS only in 1 patient.
Table 2.
Intraoperative Data of the Patients
| Total Median (IQR)/n (%) (n = 989) | Celiac Axis Stenosis | P Value | ||||
|---|---|---|---|---|---|---|
| None (n = 716) | 30% – <50% (n = 177) | 50% – ≤80% (n = 83) | >80% (n = 13) | |||
| PD | 735 (74.3%) | 525 (73.3%) | 134 (75.7%) | 65 (78.3%) | 11 (84.6%) | 0.566 |
| TP | 254 (25.7%) | 191 (26.7%) | 43 (24.3%) | 18 (21.7%) | 2 (15.4%) | 0.566 |
| Arcuate ligament dissection | 13 (1.3%) | 2 (0.3%) | 3 (1.7%) | 6 (7.2%) | 2 (15.4%) | <0.001 |
| Extended resection | 398 (40.2%) | 293 (40.9%) | 70 (39.5%) | 31 (37.3%) | 4 (30.8%) | 0.814 |
| Liver resection | 47 (4.7%) | 40 (5.6%) | 4 (2.3%) | 3 (3.6%) | 0 (0.0%) | 0.235 |
| Colon resection | 69 (6.9%) | 51 (7.1%) | 11 (6.2%) | 7 (8.4%) | 0 (0.0%) | 0.705 |
| Vascular resection | 294 (29.7%) | 188 (26.3%) | 48 (27.1%) | 11 (13.2%) | 5 (38.5%) | 0.788 |
| Venous resection | 281 (28.4%) | 202 (28.2%) | 54 (30.5%) | 23 (27.7%) | 2 (15.4%) | 0.636 |
| Arterial resection | 47 (4.7%) | 37 (5.2%) | 7 (3.9%) | 2 (2.4%) | 1 (7.7%) | 0.620 |
| Operation time (min) | 330 (280–386) | 335 (284–335) | 315 (269.3–379) | 315 (263–363) | 315 (290–381) | 0.031 |
| Blood loss (mL)* | 1274 ± 1087 | 1283 ± 1044 | 1304 ± 1332 | 1210 ± 922 | 808 ± 456 | 0.147 |
mean ± SD.
PD indicates partial pancreatoduodenectomy; SD, standard deviation; TP, total pancreatectomy.
Intraoperative Data
An overview of surgery-related parameters is provided in Table 2. The majority of patients (74.3%) underwent PD and 25.7% underwent TP. Almost 30% had vascular resections and 40% had extended resections. The mean intraoperative blood loss was 1.3 liters. There were no major differences in intraoperative data between patients without CAS and those with CAS of different grades. According to the Michel classification, no significant correlation was observed between CAS grade and anatomy of the blood supply to upper abdominal organs (Supplemental Digital Content Table 1, http://links.lww.com/SLA/D622).
Postoperative Outcomes
Postoperative complications are summarized in detail in Table 3. The most relevant complications after PD and TP were clinically relevant POPF (122 patients, 16.6%), grade B/C postpancreatectomy hemorrhage (92 patients, 9.3%), reoperation with completion pancreatectomy (38 patients, 5.1%), moderate or severe liver perfusion failure (135 patients, 13.6%), biliary leakage (62 patients, 6.3%), and gastric complications (60 patients, 6.1%). The median ICU/IMCU stay was 2 days and the median hospital stay was 14 days.
Table 3.
Postoperative Morbidity and Mortality Rate and Celiac Axis Stenosis
| CAS | P Value | |||||
|---|---|---|---|---|---|---|
| Total n (%) (n = 989) | None (n = 716) | 30% – <50% (n = 177) | 50% – ≤80% (n = 83) | >80% (n = 13) | ||
| POPF | ||||||
| Grade B | 72 (9.8%)* | 51 (9.7%)* | 16 (11.9%)* | 3 (4.6%)* | 2 (18.1%)* | 0.314 |
| Grade C | 50 (6.8%)* | 26 (4.9%)* | 10 (7.5%)* | 11 (16.9%)* | 3 (27.2%)* | <0.001 |
| Grade B/C | 122 (16.6%)* | 77 (14.6%)* | 26 (19.4%)* | 14 (21.5%)* | 5 (45.3%)* | 0.019 |
| Completion pancreatectomy* | 38 (5.1%)* | 25 (4.8%)* | 4 (3.0%)* | 7 (10.8%)* | 2 (18.1%)* | 0.024 |
| Postpancreatectomy hemorrhage | ||||||
| Grade B | 45 (4.5%) | 31 (4.3%) | 7 (3.9%) | 7 (8.4%) | 0 (0.0%) | 0.293 |
| Grade C | 47 (4.7%) | 31 (4.3%) | 8 (4.5%) | 7 (8.4%) | 1 (7.7%) | 0.386 |
| Grade B/C | 92 (9.3%) | 62 (8.6%) | 15 (8.5%) | 14 (16.9%) | 1 (7.7%) | 0.104 |
| Liver perfusion failure | ||||||
| Moderate/severe | 135 (13.6%) | 93 (12.9%) | 23 (13.0%) | 16 (19.2%) | 6 (46.2%) | 0.003 |
| Biliary leakage | ||||||
| Grade B/C | 62 (6.3%) | 35 (4.9%) | 17 (9.6%) | 7 (8.4%) | 3 (23.1%) | 0.006 |
| Gastric complications | 60 (6.1%) | 36 (5.0%) | 14 (7.9%) | 5 (6.0%) | 3 (23.1%) | 0.036 |
| Composite endpoint (POPF, PPH, LPF, BL, Gastric complications) | ||||||
| Grade B/C or Moderate/severe | 218 (22.0%) | 146 (20.4%) | 41 (23.2%) | 24 (28.9%) | 7 (53.8%) | 0.011 |
| 90-day mortality | 41 (4.1%) | 25 (3.5%) | 11 (6.2%) | 4 (4.8%) | 1 (7.7%) | 0.346 |
| ICU/IMCU stay (days) | 2 (1–5) | 2 (1–4) | 2 (1–7) | 3 (1–7) | 7 (1 –23) | 0.016 |
| Hospital stay (days) | 14 (10–22) | 13 (10– 21) | 14 (10–24) | 16 (12–25) | 30 (19–50) | 0.001 |
Only patients who underwent PD (n = 735) were at risk and included in this analysis.
BL indicates biliary leakage; CAS, celiac axis stenosis; ICU, intensive care unit; IMCU, intermediate care unit; LPF, liver perfusion failure; POPF, postoperative pancreatic fistula; PPH, postpancreatectomy hemorrhage.
Association of CAS With Postoperative Outcomes
The analysis of the association of CAS with postoperative outcomes is presented in Table 3. CAS was significantly associated with clinically relevant POPF after PD. In addition, patients with CAS had significantly higher rates of completion pancreatectomy after primary PD (P=0.024). In contrast, there was no association between postpancreatectomy hemorrhage and CAS. Furthermore, the overall rate of moderate and severe liver perfusion failure was significantly associated with the presence of CAS (P=0.003). Patients with CAS had significantly higher rates of clinically relevant biliary leakage and gastric complications (P=0.006, P=0.036, respectively). As shown in Figure 2, the rate of PD-specific complications rise gradually with higher grades of CAS. In addition, the presence of CAS was significantly associated with the rate of the composite endpoint (P¼0.011) as this increased gradually among patients with CAS, as shown in Figure 3.
Figure 2.
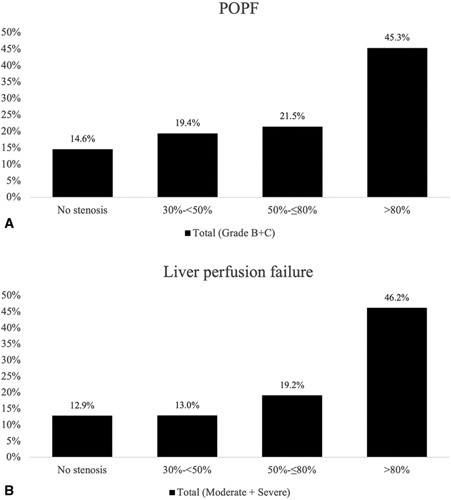
Frequency of clinically relevant complications associated with the severity of CAS after PD/TP. (A) POPF (grade B/C): the rate of clinically relevant POPF is significantly associated with CAS (P = 0.019);(B) Liver perfusion failure (moderate/severe): the overall rate of moderate and severe liver perfusion failure is significantly associated with CAS (P = 0.003). CAS indicates celiac axis stenosis; POPF, postoperative pancreatic fistula.
Figure 3.
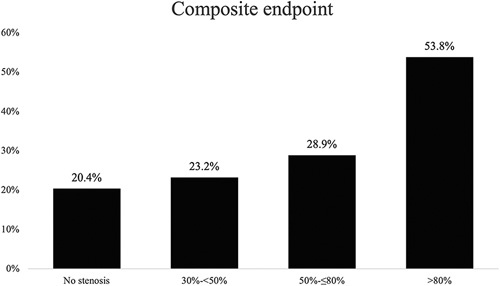
Rate of composite endpoint (POPF, PPH, LPF, BL, and gastric complications) is significantly associated with CAS (P = 0.011). BL indicates biliary leakage; CAS, celiac axis stenosis; LPF, liver perfusion failure; POPF, postoperative pancreatic fistula; PPH, postpancreatectomy hemorrhage.
Consistent with the increased risk for major morbidity in patients with CAS, the presence and the severity of CAS was significantly associated with prolonged ICU/IMCU and hospital stay. CAS was not associated with 30- and 90-day mortality after PD/TP.
CAS as Independent Risk Factor for POPF, Gastric Complications, and Liver Perfusion Failure
To further assess the relevance of CAS in postoperative morbidity after PD/TP, univariable and multivariable analyses were performed for the most relevant complications (Table 4). POPF was associated with BMI, preoperative endoscopic retrograde cholangiopancreatography, vascular resection, and CAS >80% in univariate analysis. A binomial logistic regression was performed to identify independent predictive factors of POPF. Of the 8 predictor variables, BMI, vascular resection, and CAS >80% were significant independent predictors of POPF after PD. Univariable analysis showed that liver perfusion failure was significantly associated with preoperative chemotherapy, blood loss, operation time, vascular resection, extended resection, and severe CAS (grade B and C). Binomial logistic regression defined previous abdominal surgery, operation time, vascular resection, and CAS grade B and C as predictive factors of liver perfusion failure. Gastric complications after PD/TP were associated with a history of smoking, preoperative bile duct stenting, intraoperative blood loss, and CAS >80%. Of these, smoking and CAS >80% were independent predictors of gastric complications after PD/TP. Overall, the multivariate analyses revealed that severe CAS was an independent risk factor for clinically relevant POPF, liver perfusion failure, and gastric complications.
Table 4.
Univariate and Multivariate Analysis of Factors Associated With Clinically Relevant (Grade B and C) POPF and Gastric Complications After PD/TP
| POPF (Grade B/C) | Gastric Complications | Liver Perfusion Failure (Moderate/Severe) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate P Value | Multivariate OR (95%CI) | P Value | Univariate P Value | Multivariate OR (95%CI) | P Value | Univariate P Value | Multivariate OR (95%CI) | P value | |
| Age | 0.082 | 0.400 | 0.408 | ||||||
| Sex | 0.427 | 0.378 | 0.310 | ||||||
| BMI | <0.001 | 1.058 (1.018–1.099) | 0.004 | 0.883 | 0.667 | ||||
| ASA classification (I/II vs III/IV) | 0.984 | 0.124 | 0.298 (0.069–1.040) | 0.092 | 0.351 | ||||
| Alcohol abuse | 0.218 | 0.500 | 0.106 | ||||||
| Smoking | 0.694 | 0.005 | 0.349 (0.193–0.631) | 0.001 | 0.153 | ||||
| Previous abdominal surgery | 0.492 | 0.102 | 0.004 | 2.655 (1.347–5.231) | 0.005 | ||||
| Preoperative ERCP | 0.045 | 0.363 (0.106–1.241) | 0.106 | 0.075 | 0.435 | ||||
| Pancreatic duct stent | 0.059 | 0.569 | 0.258 | ||||||
| Preoperative PTCD | 0.364 | 0.250 | 0.584 | ||||||
| Neoadjuvant therapy | 0.686 | 0.144 | 0.279 | ||||||
| Neoadjuvant chemotherapy | 0.243 | 0.507 | 0.009 | 1.163 (0.691–1.958) | 0.570 | ||||
| Operation time | 0.431 | 0.686 | 1.003 (0.999–1.006) | 0.141 | <0.0001 | 1.007 (1.003–1.008) | <0.0001 | ||
| Blood loss | 0.482 | 0.025 | 1.000 (0.990–1.005) | 0.148 | <0.0001 | 1.000 (0.998–1.005) | 0.701 | ||
| Vascular resection | <0.001 | 1.709 (1.168–2.501) | 0.006 | 0.052 | 1.162 (0.621–2.175) | 0.638 | <0.0001 | 2.546 (1.660–3.904) | 0.001 |
| Extended resection | 0.738 | 0.292 | <0.0001 | 1.478 (0.999–2.186) | 0.051 | ||||
| CAS | |||||||||
| 30% –<50% | 0.311 | 0.157 | 0.379 | ||||||
| 50% –≤80% | 0.250 | 0.615 | 0.087 | 2.019 (1.082–3.766) | 0.027 | ||||
| >80% | 0.016 | 2.830 (1.721–4.654) | <0.001 | 0.039 | 2.145 (0.613–7.503) | <0.001 | 0.005 | 9.1274 (2.771–31.045) | 0.005 |
ASA indicates American Society of Anesthesiologists; BMI, body mass index; CAS, celiac axis stenosis; CI, confidence intervals; ERCP, endoscopic retrograde cholangiopancreatography; OR, odds ratios; POPF, postoperative pancreatic fistula; PTCD, percutaneous transhepatic cholangiography drainage.
Discussion
This study is the first to systematically analyze the prevalence of CAS in patients undergoing PD or TP and its relevance to postoperative complications in a large cohort. The associations between CAS incidence, CAS grade, and postoperative complications with potential ischemic origin, in particular POPF, liver perfusion failure, and gastric complication, were investigated. The previous literature is mainly restricted to case reports or small case series that focused on the identification and management of CAS in patients undergoing PD. 20,23,24 In a recent systematic review on the impact of CAS on surgical outcome, Giovanradi et al identified 30 articles that investigated the outcome after PD in the presence of CAS in a total number of 87 patients. 22 In the present study, 989 patients, including 273 patients with CAS, were investigated. The analysis showed that CAS was significantly associated with POPF, rate of completion pancreatectomy, moderate to severe liver perfusion failure, gastric complications, and biliary leakage after PD/TP.
Based on its high morbidity, PD/TP are considered a high-risk surgical procedure. Centralization of pancreatic surgery in highvolume centers and the increased standardization of perioperative management based on evidence-based principles have significantly improved the surgical outcome. 9 However, the complication rates remain high (up to 40%), even in specialized high-volume centers. 9 Although complications such as delayed gastric emptying and intraabdominal abscesses are more common, clinically relevant POPF is the most central and serious complication after PD; it has an incidence of up to 20% to 30% 31 and increases the risk of postoperative mortality, reoperation, prolonged hospital stays, and increased costs. 7,32–34
Several randomized controlled trials have investigated surgical techniques that aim to reduce the incidence of POPF. However, no technique has been found to effectively mitigate POPF risk so far. 2,35,36 Several risk factors, such as pancreatic texture, pancreatic duct size, and BMI, were identified and these can predict the risk of POPF. 12–14,16,37 Identification of further factors may improve the prediction of POPF and may even mitigate the risk of POPF if these risk factors can be modified.
Impaired blood flow to and from the celiac trunk can lead to life-threatening liver complications. 38,39 The same mechanism might also result in pancreas- and stomach-related complications by impairing blood supply and anastomotic healing. This study has identified CAS as a risk factor, not only for liver perfusion failure, but also for POPF and gastric ischemia. Moderate CAS has already been associated with an increased risk of clinical complications, emphasizing the need to diagnose CAS before surgery. Postpancreatectomy acute pancreatitis is receiving increasing attention; 40 it has been associated with POPF and may have an ischemic origin. Although postpancreatectomy acute pancreatitis could not be retrospectively assessed in this study, it may play a role in the higher rates of POPF and the higher rates of completion pancreatectomies after PD among patients with severe CAS. 41
Previous small series have suggested that CAS increases postoperative morbidity, ischemic complications, and anastomosis-related complications 21,22 and that CAS can prolong hospital stay and increase the risk of reoperation after PD. 24,42 Based on the systematic assessment of a large cohort of patients treated in a highvolume center, the present study revealed thatCAS increases the risk of the most relevant surgical complications after PD/TP. Uni- and multivariate analyses revealed that CAS >80% is an independent predictive factor for POPF, gastric ischemia, and liver perfusion failure. Furthermore, CAS is associated with significantly higher rates of a combined endpoint of clinically relevant or moderate and severe complications after pancreatic surgery. The rate of liver perfusion failure increased among patients with CAS >50%; however, the rate of severe perfusion failure was significantly higher in patients with CAS >80%. Bile leakage also increased in patients with CAS > 80%, suggesting that severe liver perfusion failure leads to biliary tract ischemia and an increased the rate of biliodigestive anastomosis leakage in these patients. POPF is a complex and multifactorial complication after pancreatic surgery, even in patients without CAS. This multifactorial origin of POPF probably explains why a CAS severity of less than 80% was not an independent risk factor for POPF in multivariate analysis. The rate of gastric complications was significantly higher in patients with CAS >80%. However, patients with intraoperative signs of malperfusion may have undergone preemptive subtotal gastrectomy, which might have been a source of bias in the occurrence of gastric complications in this study. Although CAS may increase the risk of rare complications and higher mortality after PD/TP, this study lacked the power to assess these associations.
Severe complications such as POPF, liver perfusion failure, and gastric ischemia reduce overall survival by delaying adjuvant therapies. 43,44 Identifying modifiable factors that are associated with these complications after PD/TP, such as CAS in this study, may offer the opportunity to mitigate these risks and improve surgical and oncological outcomes. This is particularly important in centers with a high number of pancreatoduodenectomies. The morbidity and mortality rates in this study are consistent with those of other studies, indicating that these results are applicable to other pancreatic surgery centers.
In this study, 55% of CAS were only detected after surgery or during this retrospective analysis, highlighting that CAS is underestimated and heavily underdiagnosed even in a specialized highvolume center. This is most likely because the clinical relevance of less severe cases is often underestimated; CAS is usually detected during surgery, when blood flow to the hepatic artery is found to be insufficient after the GDA has been divided.
The retrospective design is the main limitation of this study. Another relevant limitation is the small number of patients with higher grades of CAS. Further prospective studies are needed to evaluate the relevance of CAS and to assess the efficacy and safety of different measures to manage CAS in reducing postoperative complications after PD/TP.
In conclusion, CAS is common in patients undergoing PD or TP and is an underestimated and neglected risk factor for the most relevant surgical complications, particularly POPF after PD. The composite endpoint with the clinically relevant postpancreatectomy complications were significantly associated with higher grades of CAS. Based on these findings, we recommend a thorough preoperative assessment of CAS so that mitigation strategies can be implemented to reduce the risk of postoperative complications, including POPF. These mitigation strategies, which have to be evaluated in future studies, may include preoperative radiologic intervention in severe cases of intrinsic CAS, intraoperative division of the median arcuate ligament in cases of extrinsic CAS, and even vascular reconstruction to avoid ischemia after pancreatic head resection.
Footnotes
Reprints: Requests for reprints should be addressed to the corresponding author.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.annalsofsurgery.com.
References
- 1.Diener MK, Hüttner FJ, Kieser M, et al. Partial pancreatoduodenectomy versus duodenum-preserving pancreatic head resection in chronic pancreatitis: the multicentre, randomised, controlled, double-blind ChroPac trial. Lancet. 2017;390:1027–1037. [DOI] [PubMed] [Google Scholar]
- 2.Shrikhande SV, Sivasanker M, Vollmer CM, et al. Pancreatic anastomosis after pancreatoduodenectomy: a position statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2017;161:1221–1234. [DOI] [PubMed] [Google Scholar]
- 3.Schnelldorfer T, Ware AL, Sarr MG, et al. Long-term survival after pancreatoduodenectomy for pancreatic adenocarcinoma: is cure possible? Ann Surg. 2008;247:456–462. [DOI] [PubMed] [Google Scholar]
- 4.Hackert T, Sachsenmaier M, Hinz U, et al. Locally advanced pancreatic cancer: neoadjuvant therapy with folfirinox results in resectability in 60% of the patients. Ann Surg. 2016;264:457–463. [DOI] [PubMed] [Google Scholar]
- 5.Hartwig W, Gluth A, Hinz U, et al. Outcomes after extended pancreatectomy in patients with borderline resectable and locally advanced pancreatic cancer. Br J Surg. 2016;103:1683–1694. [DOI] [PubMed] [Google Scholar]
- 6.Strobel O, Neoptolemos J, Jäger D, et al. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol. 2019;16:11–26. [DOI] [PubMed] [Google Scholar]
- 7.Hank T, Sandini M, Ferrone CR, et al. Association between pancreatic fistula and long-term survival in the era of neoadjuvant chemotherapy. JAMA Surg. 2019;154:943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernández-del Castillo C, Morales-Oyarvide V, McGrath D, et al. Evolution of the Whipple procedure at the Massachusetts General Hospital. Surgery. 2012;152:S56–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartwig W, Werner J, Jäger D, et al. Improvement of surgical results for pancreatic cancer. Lancet Oncol. 2013;14:e476–e485. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad SA, Edwards MJ, Sutton JM, et al. Factors influencing readmission after pancreaticoduodenectomy: a multi-institutional study of 1302 patients. Ann Surg. 2012;256:529–537. [DOI] [PubMed] [Google Scholar]
- 11.Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017;161:584–591. [DOI] [PubMed] [Google Scholar]
- 12.Cameron JL, He J. Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg. 2015;220:530–536. [DOI] [PubMed] [Google Scholar]
- 13.Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH) – an international study group of pancreatic surgery (ISGPS) definition. Surgery. 2007;142:20–25. [DOI] [PubMed] [Google Scholar]
- 14.Callery MP, Pratt WB, Kent TS, et al. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. 2013;216:1–14. [DOI] [PubMed] [Google Scholar]
- 15.Stauffer JA, Bridges MD, Turan N, et al. Aberrant right hepatic arterial anatomy and pancreaticoduodenectomy: recognition, prevalence and management. HPB. 2009;11:161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hackert T, Stampfl U, Schulz H, et al. Clinical significance of liver ischaemia after pancreatic resection. Br J Surg. 2011;98:1760–1765. [DOI] [PubMed] [Google Scholar]
- 17.Winter JM, Cameron JL, Yeo CJ, et al. Duodenojejunostomy leaks after pancreaticoduodenectomy. J Gastrointest Surg. 2008;12:263–269. [DOI] [PubMed] [Google Scholar]
- 18.Javed AA, Mirza MB, Sham JG, et al. Postoperative biliary anastomotic strictures after pancreaticoduodenectomy. HPB (Oxford). 2021;23:1716–1721. [DOI] [PubMed] [Google Scholar]
- 19.Bong JJ, Karanjia ND, Menezes N, et al. Total gastric necrosis due to aberrant arterial anatomy and retrograde blood flow in the gastroduodenal artery: a complication following pancreaticoduodenectomy. HPB. 2007;9:466–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugae T, Fujii T, Kodera Y, et al. Classification of the celiac axis stenosis owing to median arcuate ligament compression, based on severity of the stenosis with subsequent proposals for management during pancreatoduodenectomy. Surgery. 2012;151:543–549. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y, Wang W, Shi Y, et al. Substantial atherosclerotic celiac axis stenosis is a new risk factor for biliary fistula after pancreaticoduodenectomy. Int J Surg. 2018;49:62–67. [DOI] [PubMed] [Google Scholar]
- 22.Giovanardi F, Lai Q, Garofalo M, et al. Collaterals management during pancreatoduodenectomy in patients with celiac axis stenosis: a systematic review of the literature. Pancreatology. 2018;18:592–600. [DOI] [PubMed] [Google Scholar]
- 23.Harb S-G., O’sullivan AW, Marangoni G, et al. Managing arterial collaterals due to coeliac axis stenosis during pancreaticoduodenectomy. Pancreas. 2009;10:547–549. [PubMed] [Google Scholar]
- 24.Nara S, Sakamoto Y, Shimada K, et al. Arterial reconstruction during pancreatoduodenectomy in patients with celiac axis stenosis—utility of Doppler ultrasonography. World J Surg. 2005;29:885–889. [DOI] [PubMed] [Google Scholar]
- 25.Von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495–1499. [DOI] [PubMed] [Google Scholar]
- 26.Hartwig W, Vollmer CM, Fingerhut A, et al. Extended pancreatectomy in pancreatic ductal adenocarcinoma: definition and consensus of the International Study Group for Pancreatic Surgery (ISGPS). Surgery. 2014;156:1–14. [DOI] [PubMed] [Google Scholar]
- 27.Michels NA. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg. 1966;112:337–347. [DOI] [PubMed] [Google Scholar]
- 28.Koch M, Garden OJ, Padbury R, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680–688. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Perez MA, Nunez-Anton V. Cellwise residual analysis in two-way contingency tables. Educ Psychol Meas. 2003;63:825–839. [Google Scholar]
- 30.Beasley TM, Schumacker RE. Multiple regression approach to analyzing contingency tables: post hoc and planned comparison procedures. J Exp Educ. 1995;64:79–93. [Google Scholar]
- 31.Callery MP, Pratt WB, Vollmer CM., Jr. Prevention and management of pancreatic fistula. J Gastrointest Surg. 2009;13:163–173. [DOI] [PubMed] [Google Scholar]
- 32.Yuan F, Essaji Y, Belley-Cote EP, et al. Postoperative complications in elderly patients following pancreaticoduodenectomy lead to increased postoperative mortality and costs. A retrospective cohort study. Int J Surg. 2018;60:204–209. [DOI] [PubMed] [Google Scholar]
- 33.Jiang J, Upfill-Brown A, Dann AM, et al. Association of hospital length of stay and complications with readmission after open pancreaticoduodenectomy. JAMA Surg. 2019;154:88–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Pastena M, Paiella S, Marchegiani G, et al. Postoperative infections represent a major determinant of outcome after pancreaticoduodenectomy: results from a high-volume center. Surgery. 2017;162:792–801. [DOI] [PubMed] [Google Scholar]
- 35.Diener MK, Seiler CM, Rossion I, et al. Efficacy of stapler versus hand-sewn closure after distal pancreatectomy (DISPACT): a randomised, controlled multicentre trial. Lancet. 2011;377:1514–1522. [DOI] [PubMed] [Google Scholar]
- 36.Keck T, Wellner UF, Bahra M, et al. Pancreatogastrostomy versus pancreatojejunostomy for RECOnstruction after PANCreatoduodenectomy (RECOPANC, DRKS 00000767): perioperative and long-term results of a multicenter randomized controlled trial. Ann Surg. 2016;263:440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mungroop TH, van Rijssen LB, van Klaveren D, et al. Alternative fistula risk score for pancreatoduodenectomy (a-FRS): design and international external validation. Ann Surg. 2019;269:937–943. [DOI] [PubMed] [Google Scholar]
- 38.Kurosaki I, Hatakeyama K, Nihei KE, et al. Celiac axis stenosis in pancreaticoduodenectomy. J Hepatobiliary Pancreat Surg. 2004;11:119–124. [DOI] [PubMed] [Google Scholar]
- 39.Sakorafas GH, Sarr MG, Peros G. Celiac artery stenosis: an underappreciated and unpleasant surprise in patients undergoing pancreaticoduodenectomy. J Am Coll Surg. 2008;206:349–356. [DOI] [PubMed] [Google Scholar]
- 40.Loos M, Strobel O, Dietrich M, et al. Hyperamylasemia and acute pancreatitis after pancreatoduodenectomy: two different entities. Surgery. 2021;169:369–376. [DOI] [PubMed] [Google Scholar]
- 41.Marchegiani G, Perri G, Burelli A, et al. High-risk pancreatic anastomosis vs. total pancreatectomy after pancreatoduodenectomy: postoperative outcomes and quality of life analysis. Ann Surg. 2021. [DOI] [PubMed]
- 42.Gaujoux S, Sauvanet A, Vullierme M-P, et al. Ischemic complications after pancreaticoduodenectomy: incidence, prevention, and management. Ann Surg. 2009;249:111–117. [DOI] [PubMed] [Google Scholar]
- 43.Xia BT, Ahmad SA, Al Humaidi AH, et al. Time to initiation of adjuvant chemotherapy in pancreas cancer: a multi-institutional experience. Ann Surg Oncol. 2017;24:2770–2776. [DOI] [PubMed] [Google Scholar]
- 44.Merath K, Mehta R, Tsilimigras DI, et al. In-hospital mortality following pancreatoduodenectomy: a comprehensive analysis. J Gastrointest Surg. 2020;24:1119–1126. [DOI] [PubMed] [Google Scholar]


