Objective:
Limited data exist regarding the immune benefits of fourth COVID-19 vaccine doses in people with HIV (PWH) receiving antiretroviral therapy (ART), particularly now that most have experienced a SARS-CoV-2 infection. We quantified wild-type, Omicron-BA.5 and Omicron-BQ.1-specific neutralization up to 1 month post-fourth COVID-19 vaccine dose in 63 (19 SARS-CoV-2-naive and 44 SARS-CoV-2-experienced) PWH.
Design:
A longitudinal observational cohort.
Methods:
Quantification of wild-type-, Omicron-BA.5, and Omicron-BQ.1-specific neutralization using live virus assays.
Results:
Participants received monovalent (44%) and bivalent (56%) mRNA fourth doses. In COVID-19-naive PWH, fourth doses enhanced wild-type and Omicron-BA.5-specific neutralization modestly above three-dose levels (P = 0.1). In COVID-19-experienced PWH, fourth doses enhanced wild-type specific neutralization modestly (P = 0.1) and BA.5-specific neutralization substantially (P = 0.002). Consistent with humoral benefits of ’hybrid’ immunity, COVID-19-experienced PWH exhibited the highest neutralization post-fourth dose, wherein those with Omicron-era infections displayed higher wild-type specific (P = 0.04) but similar BA.5 and BQ.1-specific neutralization than those with pre-Omicron-era infections. Nevertheless, BA.5-specific neutralization was significantly below wild-type in everyone regardless of COVID-19 experience, with BQ.1-specific neutralization lower still (both P < 0.0001). In multivariable analyses, fourth dose valency did not affect neutralization magnitude. Rather, an mRNA-1273 fourth dose (versus a BNT162b2 one) was the strongest correlate of wild-type specific neutralization, while prior COVID-19, regardless of pandemic era, was the strongest correlate of BA.5 and BQ.1-specific neutralization post-fourth dose.
Conclusion:
Fourth COVID-19 vaccine doses, irrespective of valency, benefit PWH regardless of prior SARS-CoV-2 infection. Results support recommendations that all adults receive a fourth COVID-19 vaccine dose within 6 months of their third dose (or their most recent SARS-CoV-2 infection).
Keywords: COVID-19, fourth dose, HIV, hybrid immunity, Omicron BA.5, Omicron BQ.1, vaccine, viral neutralization
Introduction
A third COVID-19 vaccine dose substantially boosts humoral responses in people with HIV (PWH) [1–4], but antibody concentrations and activities decline rapidly thereafter, particularly in individuals who remain SARS-CoV-2-naive [4]. This, combined with the observation that vaccine (and infection-) induced antibodies neutralize newer Omicron variants even more poorly than the original Omicron-BA.1 variant [4–7], led to the recommendation that all adults receive fourth COVID-19 vaccine doses. Moreover, bivalent vaccine formulations (initially featuring wild-type and Omicron-BA.1 variants [8], and more recently wild-type and Omicron-BA.4/5 variants [9]), were approved in Fall 2022. Questions remain however, including the following: In PWH who remain COVID-19-naive, does a fourth dose simply boost humoral responses to post-third dose levels, or does it enhance them? Do triple-vaccinated PWH who have had COVID-19 further benefit from a fourth dose, and does it matter whether the infection was Omicron? and How well do fourth doses induce responses to newer Omicron variants, and does valency matter? To address these questions, we quantified wild-type, Omicron-BA.5, and Omicron-BQ.1-specific virus neutralization 1 month post-fourth COVID-19 vaccine dose in 63 PWH, including 19 COVID-19-naive and 44 COVID-19-experienced (12 pre-Omicron-era and 32 Omicron-era) participants.
Materials and methods
Ethics approval
This study was approved by the University of British Columbia/Providence Healthcare and Simon Fraser University Research Ethics Boards. All participants provided written informed consent.
Participants
The cohort has been described previously [1]. This analysis included all 63 PWH who completed their 1 month post-fourth dose visit as of December 2022. SARS-CoV-2 infections were identified through self-reported PCR or rapid-antigen test results and/or the presence of anti-Nucleocapsid antibodies using the Elecsys Anti-SARS-CoV-2 assay (Roche Diagnostics (Laval, Quebec, Canada)).
Live virus neutralization
Virus neutralizing activity in plasma was assessed using live wild-type (USA-WA1/2020; BEI Resources, Manassas, Virginia, USA), Omicron-BA.5 (GISAID Accession# EPI_ISL_15226696), and Omicron-BQ.1 (GISAID Accession # EPI_ISL_15967292) viruses on VeroE6-TMPRSS2 (JCRB-1819) target cells [10]. Virus stocks were diluted to 50 TCID50/200 μl in the presence of serial two-fold plasma dilutions (1/20 to 1/2560) and added to target cells in triplicate. Viral cytopathic effects were recorded 3 days post-infection. Neutralization was reported as the highest reciprocal dilution able to prevent cytopathic effects in all three wells. Partial or no neutralization at 1/20 dilution was considered below the limit of quantification.
Statistical analyses
As untransformed neutralization reciprocal dilution values are not normally distributed, all comparisons shown in the figures use nonparametric statistics. Specifically, the Mann–Whitney U-test was used to compare neutralization distributions between independent groups (e.g., between COVID-19 naive and experienced participants at a given time point), while the Wilcoxon matched-pairs signed rank test was used to compare neutralization distributions on paired measurements (e.g., within a given group across time points). Multiple linear regression was used to investigate the relationship between sociodemographic, health and vaccine-related variables, and SARS-CoV-2 variant-specific neutralization 1 month following the fourth dose. Here, neutralization values were log2 transformed prior to analysis. The variables explored were recent and nadir CD4+ T-cell counts (per cells/μl), age (per year), sex at birth (female as reference), ethnicity (non-white as reference), number of chronic conditions (per additional), dual ChAdOx1 as the initial regimen (with mRNA or mixed ChAdOx1/mRNA regimen as the combined reference group) [1,11], interval between the first and second doses (per day), third COVID-19 mRNA vaccine brand (BNT162b2 as reference), interval between second and third doses (per day), fourth COVID-19 mRNA vaccine brand (BNT162b2 as reference), fourth dose valency (monovalent as reference), and interval between third and fourth doses (per day). Prior COVID-19 was also explored, two ways: any prior infection (naive as reference) and any Omicron-era infection (naive and pre-Omicron-era infections as the combined reference group). All variables with univariable P value less than 0.1 were included in the multivariable model, except for prior COVID-19, where only one of the definitions of this variable (the one with the smallest P value) was included in the model to avoid collinearity. We tested for variable multicollinearity using Variance Inflation Factors (VIFs), which are reported in the table footnotes. Briefly, a VIF of 1 indicates that there is no correlation between a given independent variable and any others, VIFs between 1 and 5 suggest moderate correlation, while VIFs greater than 5 represent strong correlation. No variables exhibited VIFs greater than 5. All tests were two-tailed, with P value less than 0.05 considered statistically significant. P values were not corrected for multiple comparisons. All statistical analyses were performed in Prism for Mac OS v9.3.1 (GraphPad Software).
Results
Participant characteristics
Characteristics of the 63 PWH are summarized in Table 1. All had suppressed plasma HIV on ART, median CD4+ T-cell counts of 720 [interquartile range (IQR) 540–920] cells/μl, and median nadir CD4+ T-cell counts of 280 (IQR 90–530) cells/μl, at enrolment. PWH were a median of 57 (IQR 44–65) years old, 86% men, 73% of white ethnicity, and had a median 1 (IQR 0–1) chronic conditions. A total of 10% received two doses of the recombinant viral vector ChAdOx1 vaccine as their primary series, where second doses were administered a median 59 days following the first, due to initially limited vaccine supply in Canada [12,13]. All third doses were monovalent mRNA vaccines, where 33% received BNT162b2 and 67% received mRNA-1273, administered approximately 6 months after the second dose. For third doses, PWH who met one or more of the following criteria were eligible for a 100 μg mRNA-1273 third dose (instead of the standard 50 μg): age at least 65 years, prior AIDS-defining illness, prior CD4+ cell count less than 200 cells/μl, prior CD4+ fraction 15% or less, any plasma HIV load more than 50 copies/ml in 2021, or perinatally acquired HIV [14]. Fourth doses, received an average of 8.5 months post-third, were BNT162b2 monovalent (14%), BNT162b2 bivalent (14%), mRNA-1273 monovalent (30%), and mRNA-1273 bivalent (41%). Overall, 30% of participants remained SARS-CoV-2-naive, whereas 19 and 51% experienced COVID-19 during the pre-Omicron and Omicron eras, respectively, as estimated using local molecular epidemiology trends [15].
Table 1.
Participant characteristics.
| Characteristica | n = 63 |
| HIV-related variables | |
| Receiving antiretroviral therapy, n (%) | 63 (100%) |
| Recent plasma viral load, copies HIV RNA/ml, median [IQR] | <50 [<50 to <50] |
| Recent CD4+ T-cell count in cells/μl, median [IQR] | 720 [540–920] |
| Nadir CD4+ T-cell count in cells/μl, median [IQR] | 280 [90–530] |
| Sociodemographic and health variables | |
| Age in years, median [IQR] | 57 [44–65] |
| Female sex at birth, n (%) | 9 (14.2%) |
| White ethnicity, n (%) | 46 (73%) |
| Number of chronic conditions, median [IQR]b | 1 [0–1] |
| Vaccine details | |
| Initial regimen | |
| Two mRNA doses, n (%) | 51 (81%) |
| Two ChAdOx1 doses, n (%) | 6 (9.5%) |
| Heterologous (ChAdOx1 + mRNA), n (%) | 6 (9.5%) |
| Days between first and second doses, median [IQR] | 59 [53–67] |
| Third dose | |
| BNT162b2, n (%) | 21 (33%) |
| mRNA-1273, n (%) | 42 (67%) |
| Days between second and third doses, median [IQR] | 182 [134–192] |
| Fourth dose | |
| BNT162b2 monovalent, n (%) | 9 (14%) |
| BNT162b2 bivalent, n (%) | 9 (14%) |
| mRNA-1273 monovalent, n (%) | 19 (30%) |
| mRNA-1273 bivalent, n (%) | 26 (41%) |
| Days between third and fourth doses, median [IQR] | 258 [217–282] |
| SARS-CoV-2 infection historyc | |
| SARS-CoV-2 naive, n (%) | 19 (30%) |
| SARS-CoV-2 infection pre-Omicron era, n (%) | 12 (19%) |
| SARS-CoV-2 infection Omicron era, n (%) | 32 (51%) |
Sociodemographic, health, and vaccine data were collected by self-report and confirmed through medical records where available.
Chronic conditions were defined as hypertension, diabetes, asthma, obesity, chronic diseases of lung, liver, kidney, heart or blood, cancer, and immunosuppression due to chronic conditions or medication.
Two COVID-19-experienced participants had SARS-CoV-2 infection twice. These participants were classified according to the pandemic era of their first infection (one was in the ‘pre-Omicron era’ group, and the other was in the ‘post-Omicron era’ group).
Longitudinal viral neutralization following COVID-19 vaccination
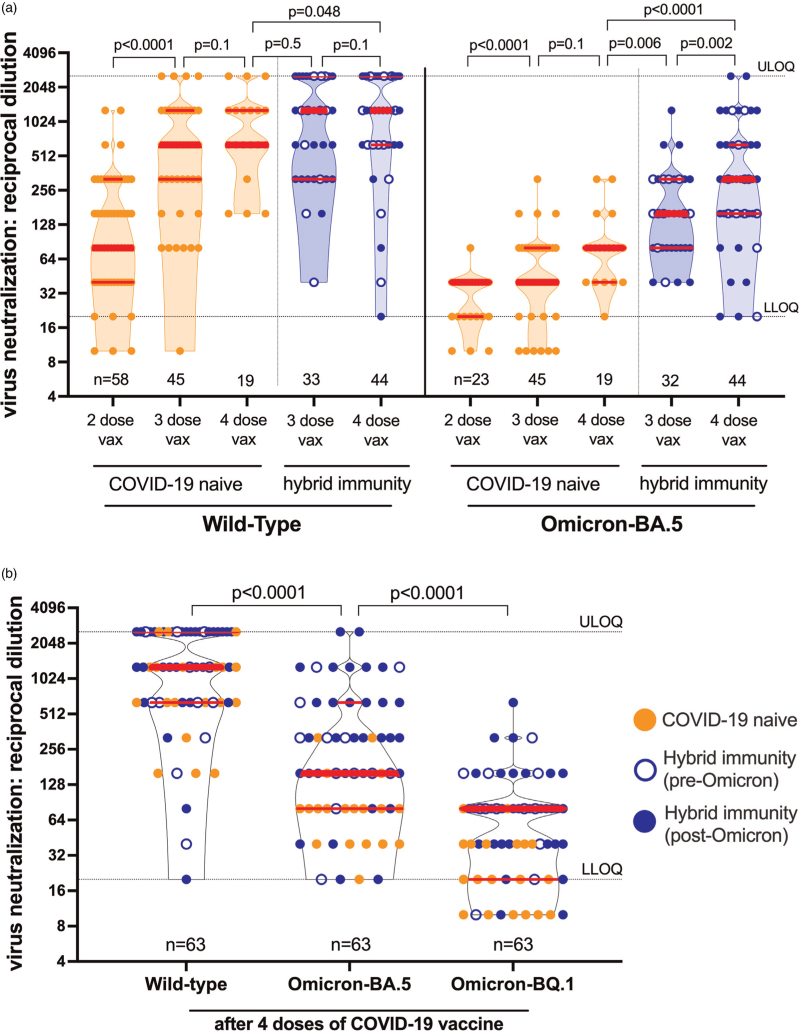
In SARS-CoV-2-naive PWH, a fourth COVID-19 vaccine dose boosted both wild-type and BA.5-specific neutralization to levels that were modestly higher than those observed 1 month after three-dose vaccination (Fig. 1a). Specifically, one month post-third dose, wild-type specific neutralization was achieved at a median reciprocal dilution of 640 (IQR 320–1280), whereas one month post-fourth dose, the median remained at 640, but the IQR rose to 640–1280. Moreover, no low or nonresponders to the wild-type virus remained following four doses. Applying a Wilcoxon matched pairs signed rank test to the 19 paired measurements across the post-third and post-fourth dose time points yielded P = 0.1. Similarly, one month post-third dose, BA.5-specific neutralization was achieved at a median reciprocal dilution of 40 (IQR 40–80), whereas one month post-fourth dose, this rose to a median 80 (IQR 40–80), again yielding P = 0.1 (Wilcoxon matched pairs signed rank test; 19 pairs). For context, these increases were less pronounced than those observed between two-dose and three-dose vaccination: wild-type specific neutralization for example rose from a median 80 after two doses to a median 640 after three doses (Wilcoxon matched pairs signed rank test; 45 pairs; P < 0.0001; Fig. 1a).
Fig. 1.
SARS-CoV-2 neutralization following COVID-19 vaccination.
(a) Longitudinal live virus neutralization specific to wild-type (left side) and Omicron BA.5 (right side) one month following two, three and four COVID-19 vaccine (vax) doses in people with HIV (PWH) receiving suppressive antiretroviral therapy. Data points reflect the highest reciprocal plasma dilution at which neutralization was observed in all wells of a triplicate assay, where serial two-fold dilutions between 1/20 (lower limit of quantification; LLOQ) to 1/2560 (upper limit of quantification; ULOQ) were tested. Plasma samples showing neutralization in fewer than three wells at a 1/20 dilution are displayed as a reciprocal dilution of ‘10’. COVID-19-naive PWH are in orange, while COVID-19-experienced PWH (labeled ‘hybrid immunity’) are in blue, with pre-Omicron-era infections in open blue circles and Omicron-era infections in closed blue circles. Thick red bars indicate the median and thinner bars indicate the IQR. Occasionally, the median and one of the quartiles are superimposed. Comparisons between independent groups (e.g., between COVID-19 naive and experienced participants at a given time point) were performed using the Mann–Whitney U-test. Longitudinal paired comparisons (e.g., within a specific group across time points) were performed using the Wilcoxon matched pairs signed rank test, where P values are only computed on the subset of data that constitute linked pairs. To avoid clutter, P values are rounded to the nearest single digit except P = 0.048 (as our predefined significance threshold was P < 0.05). P values are not corrected for multiple comparisons. Omicron BA.5-specific neutralization following two vaccine doses was assessed on a subset of samples only. (b) Wild-type, BA.5, and BQ.1-specific neutralization 1 month after four COVID-19 vaccine doses. Legend as in (a). P values computed using the Wilcoxon matched pairs signed rank test.
A fourth dose also boosted neutralization in COVID-19-experienced PWH (Fig. 1a). For example, one month post-third dose, wild-type specific neutralization was achieved at a median reciprocal dilution of 1280 (IQR 320–2560) in COVID-19 experienced PWH, whereas one month post-fourth dose, the median remained at 1280 but the IQR rose to 640–2560 (Wilcoxon matched pairs signed rank test; 33 pairs; P = 0.1). Similarly, one month post-third dose, BA.5-specific neutralization was achieved at a median reciprocal dilution of 160 (IQR 80–320), whereas post-fourth dose, this rose to a median 320 (IQR 160–640) (Wilcoxon matched pairs signed rank test; 32 pairs; P = 0.002). As a result, COVID-19-experienced PWH displayed significantly higher wild-type and BA.5-specific neutralization post-fourth dose compared with COVID-19-naive individuals (Mann--Whitney U-test; P = 0.048 for WT; P < 0.0001 for BA.5). In fact, wild-type specific neutralization in naive PWH post-fourth dose was comparable to COVID-19-experienced individuals post-third dose (Mann--Whitney U-test; P = 0.5), whereas BA.5-specific neutralization was significantly higher in COVID-19-experienced PWH compared with COVID-19-naive individuals at these time points (Mann--Whitney U-test; P = 0.006).
Our results also demonstrated that Omicron-specific neutralization remained significantly weaker than wild-type even after four vaccine doses. The median BA.5-specific neutralization post-fourth dose across the entire cohort was 160, a value that was eight-fold lower than the median wild-type specific neutralization value of 1280 (Mann--Whitney U-test, P < 0.0001, Fig. 1b; note that all WT/BA.5 comparisons in Fig. 1a are also P < 0.0001; not shown). Moreover, the cohort median BQ.1-specific neutralization post-fourth dose was 80, which was two-fold lower than the cohort median 160 for BA.5 (Mann--Whitney U-test; P < 0.0001, Fig. 1b). Nevertheless, COVID-19-experienced PWH exhibited significantly higher ability to neutralize all tested SARS-CoV-2 variants post-fourth dose compared with COVID-19-naive individuals (Mann–Whitney U-test; all P < 0.05; Figure S1). In COVID-19-experienced PWH for example, BQ.1-specific neutralization was a median of 80, a value that was four-fold higher than the median of 20 observed in COVID-19-naive PWH (Mann–Whitney U-test; P < 0.0001; Figure S1). Stratification of infections by pandemic era further revealed that, while COVID-19-experienced PWH with Omicron-era infections exhibited significantly higher wild-type specific neutralization post-fourth dose compared with those with pre-Omicron era infections (median >2560 versus 640, respectively, Mann–Whitney U-test; P = 0.04), BA.5, and BQ.1-specific neutralization were comparable regardless of infection era (P ≥ 0.5; Figure S2).
Finally, we explored sociodemographic, clinical, and vaccine-related correlates of SARS-CoV-2 variant-specific neutralization post-fourth dose. In univariable analyses, having received mRNA-1273 as a fourth dose, a longer interval between third and fourth doses, and having experienced an Omicron-era SARS-CoV-2 infection were significantly associated with higher wild-type specific neutralization (all P < 0.05; Supplementary Table 1). Fourth dose valency, however, was not associated with wild-type specific neutralization magnitude, nor was CD4+ T-cell count (neither recent nor nadir), nor prior COVID-19 regardless of pandemic era (all P > 0.2). The multivariable model included these three significant variables along with dual ChAdOx1 as the primary vaccine series (which yielded a univariable P = 0.09). After adjustment, only two variables were associated with wild-type specific neutralization: an mRNA-1273 fourth dose (P = 0.02) and a longer interval between third and fourth doses (P = 0.04; Supplementary Table 1).
The same factors were associated with BA.5-specific neutralization in univariable analyses, though for this outcome, any prior COVID-19 was more strongly associated with BA.5-specific neutralization than Omicron-era infections specifically (Supplementary Table 2). In multivariable analyses, the strongest correlate of BA.5-specific neutralization post-fourth dose was prior COVID-19 (P = 0.0003), with a longer interval between third and fourth doses representing an additional correlate (P = 0.047; Supplementary Table 2). Factors significantly associated with higher BQ.1-specific neutralization in univariable analyses were a higher nadir CD4+ T-cell count, younger age, a longer interval between second and third doses, having received a bivalent fourth dose (regardless of vaccine brand), a longer interval between third and fourth doses, and prior COVID-19 (all P < 0.05; Supplementary Table 3). In multivariable analyses, the strongest correlate of BQ.1-specific neutralization was prior COVID-19 (P = 0.0003), with longer intervals between second/third (P = 0.02) and third/fourth doses (P = 0.01) representing additional correlates (Supplementary Table 3).
Discussion
Our results indicate that PWH, regardless of SARS-CoV-2 infection history, are likely to benefit from a fourth COVID-19 vaccine dose, as the additional dose improved neutralization activity towards ancestral as well as newer virus variants. Neutralization is a strong correlate of vaccine efficacy [16,17]. In COVID-19-naive PWH, a fourth dose induced modest (but not statistically significant) increases in both wild-type and Omicron-BA.5-specific neutralization above three-dose levels and also reduced the frequency of low responders. Somewhat in contrast, in COVID-19-experienced PWH, a fourth dose induced a statistically significant increase in BA.5-specific neutralization (P = 0.002) above three-dose levels, but only a modest increase in wild-type specific neutralization. To our knowledge, only one study to date has investigated post-fourth dose responses in PWH, in eight participants [18]. Overall, our findings are consistent with the slightly higher wild-type specific neutralization observed post-fourth dose (compared with post-third dose) in the original open-label, nonrandomized clinical study in the general adult population [19], as well as studies of monovalent and bivalent boosters in context of more recent Omicron variants [7,8].
Our results also underscore the benefits of ’hybrid’ immunity gained through a combination of vaccination and infection [7,20,21], and further suggest that hybrid immunity may enhance Omicron variant neutralization in particular. This is supported by our observation that BA.5-specific neutralization post-third dose in COVID-19-experienced PWH was significantly higher than that observed post-fourth dose in COVID-19-naive individuals, while wild-type specific neutralization was comparable between these groups. Moreover, and consistent with reports in individuals without HIV [7,8], the highest neutralization was observed in COVID-19-experienced PWH after four vaccine doses. We further demonstrate that, while pandemic era appeared to influence wild-type specific neutralization post-fourth dose, with the highest neutralization occurring in PWH with Omicron-era infections, it did not influence BA.5 and BQ.1-specific neutralization. Rather, an infection from any era enhanced neutralization of these variants. Multivariable analyses also confirmed the differential correlates of variant-specific neutralization post-fourth dose: whereas an mRNA-1273 fourth dose (regardless of valency) was the strongest correlate of wild-type specific neutralization, prior COVID-19, regardless of pandemic era, was the strongest correlate of BA.5 and BQ.1-specific neutralization. Nevertheless, and consistent with recent reports [22–24], Omicron-BA.5-specific neutralization remained significantly weaker than wild-type in all participants regardless of COVID-19 experience, and BQ.1-specific neutralization was even lower than BA.5 [25,26].
Our study has some limitations. The number of participants studied (N = 63) was relatively modest. There was no control group of people without HIV, though our group and others have previously shown that PWH with preserved CD4+ T-cell counts receiving ART mounted equivalent responses to people without HIV after two and three COVID-19 vaccine doses [1–4]. Our study was not designed nor powered to investigate differences between the various bivalent fourth dose formulations, though recent large studies of the general population indicate that bivalent mRNA booster doses provide additional protection against symptomatic Omicron-lineage infections [27] and severe Omicron infections [28]. Cellular immunity was not assessed. Finally, our observations may not be generalizable to PWH with low CD4+ T-cell counts or who are not receiving ART, who may mount weaker responses to immunization [29–36], so additional studies in these populations are warranted.
In conclusion, fourth COVID-19 vaccine doses provide immune benefits to PWH regardless of SARS-CoV-2 infection history, supporting public health recommendations that all adults receive this additional immunization within 6 months of their third dose (or their most recent SARS-CoV-2 infection) [37]. As neutralization postfourth dose will likely decline rapidly [38], durability studies are required to inform the timing of additional booster doses.
Acknowledgements
The COVID-19 vaccine immunity study team comprises: Aslam Anis, Rolando Barrios, Laura Burns, Curtis Cooper, Cecilia T. Costiniuk, Sneha Datwani, Mari L. DeMarco, Maggie C. Duncan, Bruce Ganase, Silvia Guillemi, Marianne Harris, Malcolm Hedgcock, Daniel Holmes, Mark Hull, Rebecca Kalikawe, Victor Leung, Julio S.G. Montaner, Nadia Moran-Garcia, F. Harrison Omondi, Natalie Prystajecky, Paul Sereda, Junine Toy, Gisele Umviligihozo, Fatima Yaseen, and Landon Young.
This work is dedicated to the memory of our friend and colleague Hesham Ali who sadly passed away in July 2022. The authors thank the phlebotomists and laboratory staff at the BC Centre for Excellence in HIV/AIDS, the Hope to Health Research and Innovation Centre, St. Paul's Hospital, and Simon Fraser University for assistance. Above all, they also thank the participants, without whom this study would not have been possible.
This work was supported by funding from Genome BC, Michael Smith Health Research BC and the BCCDC Foundation for Public Health through a rapid SARS-CoV-2 vaccine research initiative in BC award (VAC-009 to Z.L.B., M.A.B.). It was also supported by the Public Health Agency of Canada (PHAC) through a COVID-19 Immunology Task Force (CITF) COVID-19 Award (2020-HQ-000120 to Z.L.B., M.G.R., M.A.B.). Additional funding was received from the Canadian Institutes for Health Research (GA2–177713; to M.A.B.), the Coronavirus Variants Rapid Response Network (FRN-175622; to M.A.B.), the Canada Foundation for Innovation through two Exceptional Opportunities Fund COVID-19 awards (the first to C.J.B. and C.F.L., and the second to M.N., M.A.B., Z.L.B.), a British Columbia Ministry of Health–Providence Healthcare Research Institute COVID-19 Research Priorities Grant (to C.J.B. and C.F.L.). FM is supported by a fellowship from the CIHR Canadian HIV Trials Network. E.B. was supported by an SFU Undergraduate Research Award. Z.L.B. holds a Scholar Award from Michael Smith Health Research BC.
M.A.B. and Z.L.B. are co-principal investigators and conceived the study, with M.G.R. additionally contributing to study design and development. M.A.B., Z.L.B., and M.G.R. obtained project funding. M.A.B., Z.L.B., and P.K.C. designed experiments. P.K.C., H.R.L., Y.S., S.E., F.M., S.S., and E.B. contributed to specimen and data collection, curation, and/or analysis. H.R.L., Y.S., J.S., M.N., M.G.R., M.A.B., and Z.L.B. supervised the research, laboratory assays, and/or contributed to project or cohort management. Z.L.B. performed statistical analyses with support from R.L. C.F.L., W.D., C.J.B., and M.N. provided, generated, and/or validated local Omicron isolates. Z.L.B. wrote the manuscript.
Conflicts of interest
None.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Peter K. Cheung, Hope R. Lapointe, Mark A. Brockman, and Zabrina L. Brumme denote equal contribution.
Supplemental digital content is available for this article.
References
- 1.Lapointe HR, Mwimanzi F, Cheung PK, Sang Y, Yaseen F, Umviligihozo G, et al. People with HIV receiving suppressive antiretroviral therapy show typical antibody durability after dual COVID-19 vaccination, and strong third dose responses. J Infect Dis 2022; jiac229.doi:10.1093/infdis/jiac229. [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vergori A, Cozzi Lepri A, Cicalini S, Matusali G, Bordoni V, Lanini S, et al. Immunogenicity to COVID-19 mRNA vaccine third dose in people living with HIV. Nat Commun 2022; 13:4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fidler S, Fox J, Tipoe T, Longet S, Tipton T, Abeywickrema M, et al. Booster vaccination against SARS-CoV-2 induces potent immune responses in people with HIV. Clin Infect Dis 2023; 76:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lapointe HR, Mwimanzi F, Cheung PK, Sang Y, Yaseen F, Speckmaier S, et al. Antibody response durability following three-dose COVID-19 vaccination in people with HIV receiving suppressive ART. AIDS 2023; 37:709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graham C, Lechmere T, Rehman A, Seow J, Kurshan A, Huettner I, et al. The effect of Omicron breakthrough infection and extended BNT162b2 booster dosing on neutralization breadth against SARS-CoV-2 variants of concern. PLoS Pathog 2022; 18:e1010882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kliker L, Zuckerman N, Atari N, Barda N, Gilboa M, Nemet I, et al. COVID-19 vaccination and BA.1 breakthrough infection induce neutralising antibodies which are less efficient against BA.4 and BA.5 Omicron variants, Israel, March to June 2022. Euro Surveill 2022; 27:2200785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmann M, Behrens GMN, Arora P, Kempf A, Nehlmeier I, Cossmann A, et al. Effect of hybrid immunity and bivalent booster vaccination on omicron sublineage neutralisation. Lancet Infect Dis 2023; 23:25–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalkias S, Harper C, Vrbicky K, Walsh SR, Essink B, Brosz A, et al. A bivalent Omicron-containing booster vaccine against Covid-19. N Engl J Med 2022; 387:1279–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.COVID-19 update: bivalent Pfizer and Moderna COVID-19 vaccines for booster immunization. Med Lett Drugs Ther 2022; 64:159–160. [PubMed] [Google Scholar]
- 10.Mwimanzi F, Lapointe HR, Cheung PK, Sang Y, Yaseen F, Umviligihozo G, et al. Older adults mount less durable humoral responses to two doses of COVID-19 mRNA vaccine, but strong initial responses to a third dose. J Infect Dis 2022; 226:983–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brumme ZL, Mwimanzi F, Lapointe HR, Cheung PK, Sang Y, Duncan MC, et al. Humoral immune responses to COVID-19 vaccination in people living with HIV receiving suppressive antiretroviral therapy. NPJ Vaccines 2022; 7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Advisory Committee on Immunization. NACI rapid response: extended dose intervals for COVID-19 vaccines to optimize early vaccine rollout and population protection in Canada. National Advisory Committee on Immunization (NACI). 2021. [Google Scholar]
- 13.National Advisory Committee on Immunization. Extended dose intervals for COVID-19 vaccines to optimize early vaccine rollout and population protection in Canada in the context of limited vaccine supply. National Advisory Committee on Immunization (NACI). 2021. [Google Scholar]
- 14. BC Ministry of Health. COVID-19 vaccines. Immunize BC. https://immunizebc.ca/covid-19. [Accessed 3 January 2023] [Google Scholar]
- 15. BC Centre for Disease Control. Weekly update on variants of concern. http://www.bccdc.ca/health-info/diseases-conditions/covid-19/data. [Accessed 3 January 2023] [Google Scholar]
- 16.Gilbert PB, Montefiori DC, McDermott AB, Fong Y, Benkeser D, Deng W, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022; 375:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021; 27:1205–1211. [DOI] [PubMed] [Google Scholar]
- 18.Lamacchia G, Salvati L, Kiros ST, Mazzoni A, Vanni A, Capone M, et al. Fourth dose of mRNA COVID-19 vaccine transiently reactivates spike-specific immunological memory in people living with HIV (PLWH). Biomedicines 2022; 10:3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Regev-Yochay G, Gonen T, Gilboa M, Mandelboim M, Indenbaum V, Amit S, et al. Efficacy of a fourth dose of Covid-19 mRNA vaccine against Omicron. N Engl J Med 2022; 386:1377–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, Muecksch F, Schaefer-Babajew D, Finkin S, Viant C, Gaebler C, et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature 2021; 595:426–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaballa ME, Perez-Saez J, de Mestral C, Pullen N, Lamour J, Turelli P, et al. Seroprevalence of anti-SARS-CoV-2 antibodies and cross-variant neutralization capacity after the Omicron BA.2 wave in Geneva, Switzerland: a population-based study. Lancet Reg Health Eur 2022; 24:100547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan K, Karim F, Ganga Y, Bernstein M, Jule Z, Reedoy K, et al. Omicron BA.4/BA. 5 escape neutralizing immunity elicited by BA 1 infection Nat Commun 2022; 13:4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aggarwal A, Akerman A, Milogiannakis V, Silva MR, Walker G, Stella AO, et al. SARS-CoV-2 Omicron BA.5: evolving tropism and evasion of potent humoral responses and resistance to clinical immunotherapeutics relative to viral variants of concern. EBioMedicine 2022; 84:104270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao Y, Yisimayi A, Jian F, Song W, Xiao T, Wang L, et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature 2022; 608:593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qu P, Evans JP, Faraone JN, Zheng YM, Carlin C, Anghelina M, et al. Enhanced neutralization resistance of SARS-CoV-2 Omicron subvariants BQ.1, BQ.1.1, BA.4.6, BF.7, and BA.2.75.2. Cell Host Microbe 2023; 31:9–17.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang XL, Zhu KL, Wang XJ, Wang GL, Li YK, He XJ, et al. Omicron BQ.1 and BQ.1.1 escape neutralisation by omicron subvariant breakthrough infection. Lancet Infect Dis 2023; 23:28–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Link-Gelles R, Ciesla AA, Roper LE, Scobie HM, Ali AR, Miller JD, et al. Early estimates of bivalent mRNA booster dose vaccine effectiveness in preventing symptomatic SARS-CoV-2 infection attributable to Omicron BA.5- and XBB/XBB.1.5-related sublineages among immunocompetent adults: increasing community access to Testing Program, United States, December 2022-January. MMWR Morb Mortal Wkly Rep 2023; 72:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin DY, Xu Y, Gu Y, Zeng D, Wheeler B, Young H, et al. Effectiveness of bivalent boosters against severe Omicron infection. N Engl J Med 2023; doi: 10.1056/NEJMc2215471. [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frater J, Ewer KJ, Ogbe A, Pace M, Adele S, Adland E, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: a single-arm substudy of a phase 2/3 clinical trial. Lancet HIV 2021; 8:e474–e485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woldemeskel BA, Karaba AH, Garliss CC, Beck EJ, Wang KH, Laeyendecker O, et al. The BNT162b2 mRNA vaccine elicits robust humoral and cellular immune responses in people living with human immunodeficiency virus (HIV). Clin Infect Dis 2022; 74:1268–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madhi SA, Koen AL, Izu A, Fairlie L, Cutland CL, Baillie V, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in people living with and without HIV in South Africa: an interim analysis of a randomised, double-blind, placebo-controlled, phase 1B/2A trial. Lancet HIV 2021; 8:e568–e580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hassold N, Brichler S, Ouedraogo E, Leclerc D, Carroue S, Gater Y, et al. Impaired antibody response to COVID-19 vaccination in advanced HIV infection. AIDS 2022; 36:F1–F5. [DOI] [PubMed] [Google Scholar]
- 33.Spinelli MA, Peluso MJ, Lynch KL, Yun C, Glidden DV, Henrich TJ, et al. Differences in Post-mRNA vaccination severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) immunoglobulin G (IgG) concentrations and surrogate virus neutralization test response by human immunodeficiency virus (HIV) status and type of vaccine: a matched case-control observational study. Clin Infect Dis 2022; 75:e916–e919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuan JJ, Zapata H, Critch-Gilfillan T, Ryall L, Turcotte B, Mutic S, et al. Qualitative assessment of anti-SARS-CoV-2 spike protein immunogenicity (QUASI) after COVID-19 vaccination in older people living with HIV. HIV Med 2022; 23:178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antinori A, Cicalini S, Meschi S, Bordoni V, Lorenzini P, Vergori A, et al. Humoral and cellular immune response elicited by mRNA vaccination against severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) in people living with human immunodeficiency virus receiving antiretroviral therapy based on current CD4 T-lymphocyte count. Clin Infect Dis 2022; 75:e552–e563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jedicke N, Stankov MV, Cossmann A, Dopfer-Jablonka A, Knuth C, Ahrenstorf G, et al. Humoral immune response following prime and boost BNT162b2 vaccination in people living with HIV on antiretroviral therapy. HIV Med 2022; 23:558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ismail SJ, Young K, Tunis MC, Killikelly A, Stirling R, Baclic O, et al. COVID-19 vaccine: Canadian Immunization Guide. Health Canada; 2022. [Google Scholar]
- 38.Canetti M, Barda N, Gilboa M, Indenbaum V, Mandelboim M, Gonen T, et al. Immunogenicity and efficacy of fourth BNT162b2 and mRNA1273 COVID-19 vaccine doses; three months follow-up. Nat Commun 2022; 13:7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.