SUMMARY
Background
Superinfections acquired during the hospital course represent common complications in COVID-19 patients. Several studies reported an increasing incidence of COVID-19 associated pulmonary aspergillosis (CAPA) and candidaemia. The aim of this study is to describe fungal superinfections in a large cohort of hospitalized patients with COVID-19 and identify factors independently associated with the risk of fungal superinfections.
Methods
Observational study including patients with COVID-19 admitted to the tertiary-care, University Hospital of Pisa, Italy from April 2020 to May 2021. Patients with pneumonia and laboratory confirmed SARS-CoV-2 infection with a RT-PCR test on a nasopharyngeal swab, were eligible for the study. Patients who died within 24 hours from admission and those with missing data were excluded. Data about fungal superinfections were collected. To identify factors independently associated with the development of fungal superinfections, a multivariate regression analysis was performed.
Results
Among 983 patients with COVID-19, 52 (5.3%) fungal superinfections were detected. Fungal superinfections included: 24/52 (46%) CAPA, 27/52 (51.9%) episodes of candidaemia and 1 case of pulmonary pneumocystosis in a haematological patient. All patients with CAPA were cared for in intensive care unit (ICU). The majority of patients received liposomal amphotericin B as antifungal treatment (83.3%). In-hospital mortality was 41.7%. Among 27 episodes of candidaemia, 16 (59.3%) occurred in ICU while 11 (40.7%) in medical wards. In-hospital mortality was 14.8%. Overall, patients with fungal superinfections had a median age of 73 (IQRs 59–77) years and a median length of ICU stay of 40 (17–50) days. In-hospital mortality among all patients with superinfections was 28.8%. On multivariable analysis, ICU stay (OR 17.63, 95% CI 8.3–37.41, p<0.001), high-dose steroids (OR 13.48, 95% CI 6.68–27.26, p<0.001), and diabetes mellitus (OR 2.14, 95% CI 1.09–4.17, p=0.026) were factors independently associated with the risk of developing a fungal superinfection.
Conclusions
Fungal superinfections may complicate the hospital course of COVID-19 patients, especially of those admitted to ICU. Surveillance with detection of galactomannan on bronchoalveolar lavage in patients with clinical deterioration should be performed. A rational use of steroids is essential to avoid the risk of developing a fungal superinfection.
Keywords: Fungal infections, pulmonary aspergillosis, candidemia, COVID-19, SARS-CoV-2
INTRODUCTION
The COVID-19 pandemic has overwhelmed healthcare facilities and raised new concern about the management of hospitalized patients. Superinfections acquired during the hospital course represent common complications in COVID-19 patients and are prevalent, but not limited to, intensive care settings [1–4]. It has been demonstrated that bacterial superinfections lead to prolonged length of hospital stay as well as concern about nosocomial transmission of resistant organisms [1]. However, there is raising concern about an increase in fungal infections among COVID-19 patients.
Since the start of the pandemic, coronavirus associated pulmonary aspergillosis (CAPA) has been reported as a severe complication of patients undergoing invasive mechanical ventilation [5]. Several studies reported heterogeneous data about CAPA with a prevalence ranging from less that 5% up to 30% according to disease severity, setting of care, use of surveillance programs and different diagnostic criteria [6, 7].
Not only CAPA but also other fungal infections may complicate the course of COVID-19. Several recent studies reported an increasing incidence of candidaemia among COVID-19 patients [8–12]. Finally, other less common fungal infections, including pneumocystosis and mucormycosis, have been described in patients with severe COVID-19 [13–15].
The aim of this study was to describe clinical characteristics and outcome of hospitalized patients with COVID-19 who developed fungal superinfections.
PATIENTS AND METHODS
Ethics
The research was conducted in accordance the Declaration of Helsinki and national and institutional standards. The Internal Review Board (IRB) of the Comitato Etico Area Nord-Ovest (CEAV-NO) approved the study (approval number 17681) and a written informed consent was obtained from study participants.
Study design and definitions
This is a single-centre observational study including patients with COVID-19 admitted to the tertiary-care, University Hospital of Pisa, Italy from April 2020 to May 2021. Patients with pneumonia and laboratory confirmed SARS-CoV-2 infection with a RT-PCR test on a nasopharyngeal swab, were eligible for the study. Only patients with available data about study variables were included in the final analysis. We excluded patients who died within 24 hours from hospital admission and those who did not provide informed consent.
Since during the study period there was limited evidence for any anti-COVID-19 treatment, the patients were treated according to an internal guide for the management of COVID-19 patients elaborated by a panel of experts (infectious diseases physicians, pneumologists, microbiologists, emergency medicine experts, intensivists). Treatment strategies have been previously detailed [16]. Immunomodulant agents used for patients with severe COVID-19 were tocilizumab and baricitinib. The decision to prescribe one of the 2 immunosuppressants was made by the attending physician. Tocilizumab was used at the dosage of 400 mg ev, followed by a second administration of 400 mg ev after 24 hours. Baricitinib was administered at a dose of 4 mg/day for 14 days [17]. The use of steroids was classified in low and high dose based on the cut-off of 1 mg/kg/day of methylprednisolone or equivalents [18].
All patients were prospectively followed-up: a dedicated staff of research fellows identified patients with SARS-CoV-2 pneumonia as soon as they arrived at the Emergency Department, followed the patients during the hospital stay and collected all data prospectively without interfering with the therapeutic decisions. Epidemiological and demographic information, medical history, co-morbidities, information on clinical symptoms at admission, treatments received during the hospital course were collected.
All fungal superinfections were described. Superinfections were defined as infections occurred 48 hours after admission for COVID-19 [1]. CAPA was diagnosed according to proposed criteria [19,20]. A diagnostic workup was adopted in clinically deteriorating patients with no other explanation or with cavitary and/or nodular lesions on CT scan. In these cases, bronchoscopy with bronchoalveolar lavage was performed according to judgment of infectious disease consultant. Microbiological investigations including culture, GM and Aspergillus PCR were performed on BAL samples. CAPA was defined according to the recently proposed CAPA definition consisting in COVID-19 positive patients with pulmonary infiltrates (entry criterion) who had positive GM index (serum GM index >0.5 or BAL GM index >1.0) [20]. The galactomannan antigen index was measured with a sandwich enzyme-linked immunosorbent assay (ELISA) in BALs and serum specimens. Candidaemia was defined by at least 1 positive blood culture yielding Candida spp. in a patient with fever and/or other clinical signs of infection [21, 22].
Fungal identification on positive blood cultures was performed by microscopic examination of lactophenol cotton-blue stained slides and by MALDI-TOF Mass Spectrometry Instrument (Bruker, Italy), following manufacturer’s instructions.
Study outcome
The primary objective was to describe clinical characteristics of fungal superinfections in hospitalized patients with COVID-19.
The secondary objective was to identify factors independently associated with the development of fungal superinfections.
Statistical analysis
Continuous and categorical variables were presented as median (IQR) and absolute number (percentage), respectively. The Mann-Whitney U-test, χ2 test and Fisher’s exact test were used to compare differences between groups.
According to the study outcome, we performed a comparison between patients who developed fungal superinfections during the hospital stay and those who did not. To identify factors independently associated with the development of fungal superinfections, a multivariable regression analysis was performed. The multivariable analysis using logistic regression prediction models was constructed using a forward stepwise procedure, entering all variables with univariate p<0.05. Collinearity among the variables was calculated on the basis of the variance inflation factor (VIF). Variables with collinearity and those clinically correlated each other were not entered. The final multivariate model was chosen according to the Akaike information criterion and to parsimony and clinical interpretability of data. Statistical significance was established at p<0.05. All reported p values are two tailed. The results obtained were analyzed using a commercially available statistical software package (SPSS 27.0; IBM, Armonk, NY, USA).
RESULTS
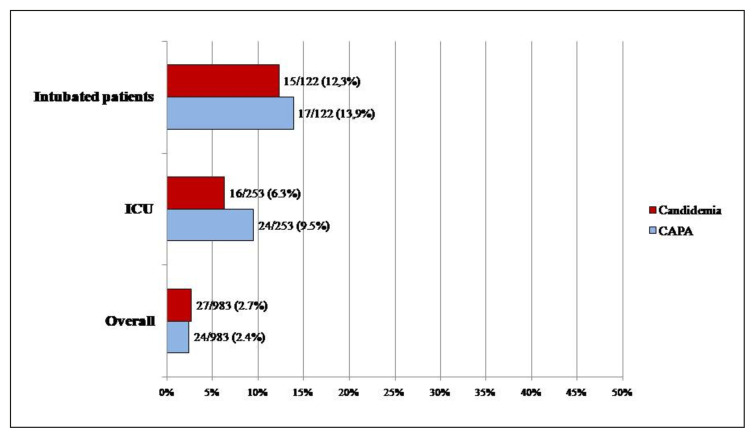
Among 983 patients with COVID-19, 52 (5.3%) fungal superinfections were detected. Fungal superinfections included: 24/52 (46%) CAPA, 27/52 (51.9%) episodes of candidaemia and 1 case of pulmonary pneumocystosis in a haematological patient. Among patients hospitalized in ICU (n=253), CAPA occurred in 9.5% (24/253) and candidaemia in 6.3% (16/253) of patients (Figure 1). Among intubated patients (n=122), CAPA occurred in 13.9% (17/122) and candidaemia in 12.3% (15/122) of patients.
Figure 1.
Distribution of fungal superinfections in COVID-19 patients hospitalized in all type of wards and in ICU.
Characteristics of the 24 patients with CAPA and COVID-19 associated candidaemia are reported in Table 1. All patients were cared for in ICU. Five (20.8%) patients underwent extracorporeal membrane oxygenation support (ECMO). The majority of patients received liposomal amphotericin B as antifungal treatment (83.3%). In-hospital mortality was 41.7%.
Table 1.
Clinical characteristics and outcomes of patients with CAPA and candidaemia.
| Characteristics |
CAPA
N=24 |
Covid-19 associated candidemia
N=27 |
|---|---|---|
|
| ||
| Demographics | ||
| Age, median (IQRs) | 59.5 (55–75.5) | 76 (68–79) |
| Male sex | 20 (83.3%) | 19 (70.4%) |
|
| ||
| Underlying diseases | ||
| Hypertension | 2 (8.3%) | 15 (55.6%) |
| Diabetes mellitus | 18 (75%) | 5 (18.5%) |
| Cardiovascular disease | 8 (33.3%) | 12 (44.4%) |
| COPD | 6 (25%) | 6 (22.2%) |
| CKD | 4 (16.7%) | 3 (11.1%) |
| Solid cancer | 0 | 3 (11.1%) |
|
| ||
| ICU stay | 24 (100%) | 16 (59.3%) |
|
| ||
| Non-invasive mechanical ventilation | 5 (20.8%) | 4 (14.8%) |
| Invasive mechanical ventilation | 17 (70.8%) | 15 (55.6%) |
|
| ||
| Covid-19 treatment | ||
| Steroids | 24 (100%) | 18 (66.7%) |
| High dose steroids | 18 (75%) | 11 (40.7%) |
| IL-6 or JAK inhibitors | 20 (83.3%) | 7 (25.9%) |
|
| ||
| ECMO | 5 (20.8%) | 0 |
|
| ||
| Length of hospital stay | 49 (25–57.5) | 25 (17–31) |
| Length of ICU stay after CAPA diagnosis | 45 (45 (28–49) | 19 (17–25) |
|
| ||
| In-hospital mortality | 10 (41.7%) | 4 (14.8%) |
|
| ||
| CAPA Treatment | ||
| Azoles* | 4 (16.7%) | 7 (25.9%) |
| Liposomal amphotericin B | 20 (83.3%) | 6 (22.2%) |
| Echinocandins | 14 (51.8%) | |
Patients with CAPA were treated with isavuconazole.
CAPA: COVID-19 associated pulmonary aspergillosis; COPD: chronic obstructive pulmonary disease; CKD: chronic kidney disease; ECMO extracorporeal membrane oxygenation support; ICU: intensive care unit.
Among 27 episodes of candidaemia, 16 (59.3%) occurred in ICU while 11 (40.7%) in medical wards. The most common Candida species was Candida albicans (13/27, 48.1%), followed by Candida parapsilosis (12/27, 44.4%), Candida glabrata (1/27, 3.7%) and Candida tropicalis (1/27, 3.7%). Overall, 15 (55.6%) episodes were CVC-related candidaemia, while in the remaining 12 (44.4%) source of candidaemia remained unknown. The majority of patients were intubated (55.6%). In-hospital mortality was 14.8%.
Among all patients with fungal superinfection, median age was 73 (IQRs 59–77) years and the majority of patients were males (76.9%). Median length of ICU stay was 40 (17–50) days. In-hospital mortality among patients with superinfections was 28.8% (15/52 patients).
Comparison of patients with fungal superinfections and those without is reported in Table 2. Patients who developed fungal superinfections were more frequently cared for in ICU and underwent more commonly invasive mechanical ventilation. Immunomodulant agents, including IL-6 and JAK inhibitors, were more common in patients who developed fungal infections. Length of ICU stay was longer in patients with fungal superinfections than in controls.
Table 2.
Comparison of COVID-19 patients who developed fungal superinfections and those who did not.
| Characteristics |
Controls
N=931 |
Fungal superinfections
N=52 |
p |
|---|---|---|---|
|
| |||
| Demographics | |||
| Age, median (IQRs) | 68 (57–79) | 73 (59–77) | 0.637 |
| Male sex | 40 (76.9%) | 599 (64.3%) | 0.064 |
|
| |||
| Underlying diseases | |||
| Hypertension | 430 (46.2%) | 17 (32.7%) | 0.057 |
| Diabetes mellitus | 214 (23%) | 23 (44.2%) | <0.001 |
| Cardiovascular disease | 287 (30.8%) | 20 (38.5%) | 0.248 |
| COPD | 100 (10.8%) | 12 (23.1%) | 0.007 |
| CKD | 67 (7.2%) | 7 (13.5%) | 0.096 |
| Solid cancer | 100 (10.8%) | 3 (5.8%) | 0.254 |
|
| |||
| ICU stay | 213 (22.9%) | 40 (76.9%) | <0.001 |
|
| |||
| High flow nasal cannula | |||
| Non-invasive mechanical ventilation | 222 (23.8%) | 9 (17.3%) | 0.279 |
| Invasive mechanical ventilation | 90 (9.7%) | 32 (61.5%) | <0.001 |
|
| |||
| Covid-19 treatment | |||
| Steroids | 673 (72.3%) | 43 (82.7%) | 0.101 |
| High dose steroids | 129 (13.9%) | 29 (55.8%) | <0.001 |
| IL-6 or JAK inhibitors | 153 (16.4%) | 27 (51.9%) | <0.001 |
|
| |||
| ECMO | 25 (2.7%) | 5 (9.6%) | 0.005 |
|
| |||
| Length of hospitalization | 14 (9–22) | 45 (27.5–49) | <0.001 |
| Length of ICU stay | 0 (0–5) | 40 (17–50) | <0.001 |
|
| |||
| In-hospital mortality | 89 (9.6%) | 15 (28.8%) | <0.001 |
COPD: chronic obstructive pulmonary disease; CKD: chronic kidney disease; ECMO extracorporeal membrane oxygenation support, ICU: intensive care unit.
On multivariate analysis, ICU stay (OR 17.63, 95% CI 8.3–37.41, p<0.001), high-dose steroids (OR 13.48, 95% CI 6.68–27.26, p<0.001), and diabetes mellitus (OR 2.14, 95% CI 1.09–4.17, p=0.026) were factors independently associated with the risk of developing a fungal superinfection (Table 3).
Table 3.
Multivariable analysis of factors independently associated with fungal superinfections.
| OR (95% CI) | p value | |
|---|---|---|
| ICU stay | 17.63 (8.3–37.41) | <0.001 |
| High-dose steroids | 13.48 (6.68–27.26) | <0.001 |
| Diabetes mellitus | 2.14 (1.09–4.17) | 0.026 |
DISCUSSION
In this observational study, we described clinical characteristics and outcomes of COVID-19 patients who developed fungal superinfections during their hospital stay. Of importance, we found that the majority of fungal superinfections occurred in ICU and length of stay may be prolonged in patients with fungal infections. Co-morbidities, including diabetes mellitus, and the use of high-dose steroids are factors increasing the risk of fungal superinfections. Since the start of the pandemic, there was concern about the development of CAPA among patients with severe COVID-19 [7]. Although invasive aspergillosis has been traditionally considered an infection occurring in patients with well established risk factors, such as neutropenia, hematologic malignancies, organ transplantation, or HIV, some cases have been reported in special patients’ populations [23]. Pulmonary aspergillosis has been described as a potential complication of influenza [24]. In fact, it is known that viruses, like influenza, may induce alveolar epithelial and endothelial damage, impaired muco-ciliary function and immune cell dysregulation favoring the development of fungal superinfections [25]. In presence of a systematic surveillance program, CAPA was diagnosed in 27.7% of patients with COVID-19 [7]. However, there is a great heterogeneity in the prevalence of this clinical entity. This heterogeneity is also due to several reasons. First, disease severity affects the risk of CAPA. A recent review highlighted that pulmonary aspergillosis may complicate COVID-19 in 8.9% of patients admitted to the ICU and in 20.1% of those requiring invasive ventilation [5]. Second, the prevalence rates varied widely due to the fact that CAPA was, and still remains, challenging to diagnose in patients with COVID-19. There was reluctance to perform bronchoscopies and some studies relied on unspecific mycological evidence, such as culture growth or non-validated GM detection in tracheal aspirate. These circumstances, combined with the unspecific clinical and radiological presentation of CAPA in patients with COVID-19, contributed to the high variability observed across studies. Third, absence of uniform consensus criteria in the early phases of the pandemic impacted on the reported prevalence data. More recent diagnostic criteria underline the role of validated GM on BAL to discriminate between airway colonization and pulmonary aspergillosis. Thus, a better diagnostic approach is essential in guiding the clinician to an evidence-based decision between early treatment or a “wait and see” strategy [5]. Clinical suspicion based on risk factors together with GM detection on BAL should guide the therapeutic strategy in patients with COVID-19. In our study, we found that 9.5% of patients hospitalized in ICU and 13.9% of intubated patients developed CAPA based on current diagnostic criteria. This may be due to the lack of systematic surveillance on BAL, but our data reflect the real-world practice in which patients with clinical suspicion of CAPA and deterioration of respiratory function undergo GM detection on BAL. We observed a high in-hospital mortality rates in patients with CAPA, but it should be considered that patients with CAPA had a high disease severity.
Candidaemia episodes have been increasingly reported in COVID-19 patients. In a recent observational study including 275 hospitalized patients with COVID-19, 101 Candida isolates identified in the 91 case patients with candidaemia were reported [8]. Of importance, in-hospital mortality was significantly higher in patients who developed candidaemia than in those who did not [8]. This led some authors to develop a score specifically derivated in patients with COVID-19 (CAC-Score) to help clinicians in the early identification of patients with candidaemia [9]. In our cohort of patients, there was a consistent prevalence of C. parapsilosis isolates. A recent study conducted in Spain showed that the incidence of candidaemia caused by fluconazole-resistant C. parapsilosis increased significantly in the pandemic period [12]. Although this increasing prevalence may reflect the local epidemiology, it should be acknowledged that C. parapsilosis are associated with indwelling catheters due to their ability to form biofilm on the surfaces of intravascular devices and on the hands of healthcare workers [26]. These findings highlight the need of implementation of infection control procedures in COVID-19 wards.
Of importance, the use of high dose of steroids increase the risk of fungal superinfections. There is controversy about the use of high-dose of steroids. Dexamethasone 20 mg/day from day 1 to 5 and 10 mg/day from day 6 to 10 in severe COVID-19 showed no clinical benefit [27]. Thus, high dose of steroids should be avoided in patients with severe COVID-19 and attention should be paid to patients who received both steroids and immunomodulant agents.
This study has some limitations. First, the decision to perform or not BAL was based on clinical judgment by attending physician together with the suggestion of the infectious diseases’ consultant. This may have led to an underestimation of CAPA, but our diagnostic workup represents a real-world approach that combined clinical suspicion and GM detection on BAL in selected cases. The role of infectious diseases’ consultant may have a pivotal role in selecting patients who need BAL to exclude CAPA. Second, our experience may depend on local epidemiology and might not be generalizable to all ICU settings in other countries. Finally, our study has been conducted before the spread of new variants of concern and include also patients before the vaccination campaign. The prevalence and the outcomes of fungal superinfections in patients affected by the new variants may vary from those reported.
In conclusion, we reported that about 5.3% of COVID-19 patients developed a fungal superinfection. This prevalence is higher if we consider only patients cared for in ICU. CAPA and COVID-19 associated candidaemia represent the most frequent fungal superinfections in COVID-19 patients. A rational use of steroids is essential to avoid the risk of developing a fungal superinfection.
Funding Statement
This work was supported by Gilead that provided an unconditional support for data collection.
Footnotes
Declaration of interest statement
MF received speakers’ honoraria from Angelini, MSD, Pfizer, and Nordic Pharma. FM has participated in advisory boards and/or received speaker honoraria from Angelini, Correvio, MSD, Pfizer, Astellas, Gilead, BMS, Jansenn, ViiV, bioMérieux, Biotest, Becton-Dickinson, Nordic Pharma, Pfizer, Shionogi. All COI are outside the submitted study.
Funding
This work was supported by Gilead that provided an unconditional support for data collection.
REFERENCES
- 1. Falcone M, Tiseo G, Giordano C, et al. Predictors of hospital-acquired bacterial and fungal superinfections in COVID-19: a prospective observational study. J Antimicrob Chemother. 2021;76:1078–1084. doi: 10.1093/jac/dkaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Falcone M, Suardi LR, Tiseo G, et al. Superinfections caused by carbapenem-resistant Enterobacterales in hospitalized patients with COVID-19: a multicentre observational study from Italy (CREVID Study) JAC Antimicrob Resist. 2022;4:dlac064. doi: 10.1093/jacamr/dlac064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Falcone M, Tiseo G, Arcari G, et al. Spread of hypervirulent multidrug-resistant ST147 Klebsiella pneumoniae in patients with severe COVID-19: an observational study from Italy, 2020–21. J Antimicrob Chemother. 2022;77:1140–1145. doi: 10.1093/jac/dkab495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Antinori S, Galimberti L, Milazzo L, Ridolfo AL. Bacterial and fungal infections among patients with SARS-CoV-2 pneumonia. Infez Med. 2020;28:29–36. [PubMed] [Google Scholar]
- 5. Egger M, Bussini L, Hoenigl M, Bartoletti M. Prevalence of COVID-19-associated pulmonary aspergillosis: critical review and conclusions. J Fungi (Basel) 2022;8:390. doi: 10.3390/jof8040390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alanio A, Dellière S, Fodil S, Bretagn S, Mégarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir Med. 2020;8:e48–e49. doi: 10.1016/S2213-2600(20)30237-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bartoletti M, Pascale R, Cricca M, et al. Epidemiology of invasive pulmonary aspergillosis among intubated patients with COVID-19: a prospective study. Clin Infect Dis. 2021;73:e3606–e3614. doi: 10.1093/cid/ciaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dixit D, Jen P, Maxwell TD, et al. Risk Factors and clinical outcomes of candidemia associated with severe COVID-19. Crit Care Explor. 2022;4:e0762. doi: 10.1097/CCE.0000000000000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kayaaslan B, Eser F, Asilturk D, et al. Development and validation of COVID-19 associated candidemia score (CAC-Score) in ICU patients. Mycoses. 2022 doi: 10.1111/myc.13531. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blaize M, Raoelina A, Kornblum D, et al. Occurrence of candidemia in patients with COVID-19 admitted to five ICUs in France. J Fungi (Basel) 2022;8:678. doi: 10.3390/jof8070678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kamali Sarvestani H, Mahmoudi S, et al. Epidemiology, risk factors, species distribution, and antifungal susceptibility of candidemia among hospitalized patients with COVID-19. Curr Med Mycol. 2021;7:12–18. doi: 10.18502/cmm.7.4.8406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ramos-Martínez A, Pintos-Pascual I, Guinea J, et al. Impact of the COVID-19 pandemic on the clinical profile of candidemia and the incidence of fungemia due to fluconazole-resistant Candida parapsilosis. J Fungi (Basel) 2022;8:451. doi: 10.3390/jof8050451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gentile I, Viceconte G, Lanzardo A, et al. Pneumocystis jirovecii Pneumonia in Non-HIV patients recovering from COVID-19: a single-center experience. Int J Environ Res Public Health. 2021;18:11399. doi: 10.3390/ijerph182111399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kyuno D, Kubo T, Tsujiwaki M, et al. COVID-19-associated disseminated mucormycosis: An autopsy case report. World J Clin Cases. 2022;10:10358–10365. doi: 10.12998/wjcc.v10.i28.10358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nagalli S, Kikkeri NS. Mucormycosis in COVID-19: A systematic review of literature. Infez Med. 2021;29:504–512. doi: 10.53854/liim-2904-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Falcone M, Tiseo G, Barbieri G, et al. Role of low-molecular-weight heparin in hospitalized patients with severe acute respiratory syndrome Coronavirus 2 pneumonia: a prospective observational study. Open Forum Infect Dis. 2020;7:ofaa563. doi: 10.1093/ofid/ofaa563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stebbing J, Sánchez Nievas G, et al. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci Adv. 2021;7:eabe4724. doi: 10.1126/sciadv.abe4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tiseo G, Yahav D, Paul M, et al. What have we learned from the first to the second wave of COVID-19 pandemic? An international survey from the ESCMID Study Group for Infection in the Elderly (ESGIE) group. Eur J Clin Microbiol Infect Dis. 2022;41:281–288. doi: 10.1007/s10096-021-04377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Verweij PE, Brüggemann RJM, Azoulay E, et al. Taskforce report on the diagnosis and clinical management of COVID-19 associated pulmonary aspergillosis. Intensive Care Med. 2021;47:819–834. doi: 10.1007/s00134-021-06449-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koehler P, Bassetti M, Chakrabarti A, et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2021;21:e149–e162. doi: 10.1016/S1473-3099(20)30847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Falcone M, Tiseo G, Gutiérrez-Gutiérrez B, et al. impact of initial antifungal therapy on the outcome of patients with candidemia and septic shock admitted to medical wards: a propensity score-adjusted analysis. Open Forum Infect Dis. 2019;6:ofz251. doi: 10.1093/ofid/ofz251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Falcone M, Tiseo G, Tascini C, et al. Assessment of risk factors for candidemia in non-neutropenic patients hospitalized in Internal Medicine wards: A multicenter study. Eur J Intern Med. 2017;41:33–38. doi: 10.1016/j.ejim.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 23. Falcone M, Massetti AP, Russo A, Vullo V, Venditti M. Invasive aspergillosis in patients with liver disease. Med Mycol. 2011;49(4):406–413. doi: 10.3109/13693786.2010.535030. [DOI] [PubMed] [Google Scholar]
- 24. Schauwvlieghe AFAD, Rijnders BJA, Philips N, et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6:782–789. doi: 10.1016/S2213-2600(18)30274-1. [DOI] [PubMed] [Google Scholar]
- 25. Short KR, Kasper J, van der Aa S, et al. Influenza virus damages the alveolar barrier by disrupting epithelial cell tight junctions. Eur Respir J. 2016;47(3):954–966. doi: 10.1183/13993003.01282-2015. [DOI] [PubMed] [Google Scholar]
- 26. Chen CY, Sheng WH, Huang SY, et al. Clinical characteristics and treatment outcomes of patients with candidaemia due to Candida parapsilosis sensu lato species at a medical centre in Taiwan, 2000–12. J Antimicrob Chemother. 2015;70:1531–1538. doi: 10.1093/jac/dku540. [DOI] [PubMed] [Google Scholar]
- 27. Jamaati H, Hashemian SM, Farzanegan B, et al. No clinical beneft of high dose corticosteroid administration in patients with COVID-19: A preliminary report of a randomized clinical trial. Eur J Pharmacol. 2021;897:173947. doi: 10.1016/j.ejphar.2021.173947. [DOI] [PMC free article] [PubMed] [Google Scholar]