SUMMARY
Intestinal helminthiasis are a common public health problem in developed and developing countries. It is thought that they can influence pregnancy by causing gestational anemia. The aim of this study was to determine if there is a relationship between helminth infection and gestational anemia. A structured review of scientific literature was conducted through active search in the electronic databases MEDLINE® and LILACS® until December 2021, following 2020 PRISMA statement. The studies were reviewed independently by two authors, extracting the most relevant information from each study. Cross-sectional studies, case-control and ecological studies were included, with no date or language limit. Randomized clinical trials were excluded. A total of 38 studies were included in the systematic review. The study populations of all studies belonged to low- and middle-income countries: 28 studies from Africa, 6 from Asia, 3 from Latin America and 1 from Oceania. Overall, the average prevalence of gestational anemia among the included studies was 40% (95% CI 34–46%). Hookworm was the predominant species detected in most studies (19/38; 50%), followed by Ascaris lumbricoides (15/38; 39.5%). Gestational anemia was positively associated with A. lumbricoides (OR 1.86, 95% CI 1.12–3.08) and hookworms (OR 3.09, 95% CI 1.99–4.78). Prevalence of malaria was not associated with the magnitude of the effect of hookworm on anemia risk during meta-regression (p=0.5182). The results of this review indicate that there is a statistically significant association between helminthiasis and gestational anemia. Although hookworm is the main species associated with the outcome, prevalence of malaria was not associated with the magnitude of the effect of hookworm on anemia risk. The impact of other species needs to be defined given the expected bias that arises from polyparasitism when defining comparison groups.
Keywords: Helminths, anemia, pregnancy, ascariasis, haemoglobin
INTRODUCTION
Anemia is the most common haematologic complication diagnosed during pregnancy [1]. It is a public health problem that affects developed and developing countries. The World Health Organization (WHO) states that, between 1993 and 2005, 41.8% [95% CI 39.9–43.8] (56 million) of pregnant women were affected by this condition [2]. Gestational anaemia (GA) has progressively decreased since 1995 (42.59%), with a global prevalence of 38.17% in 2011. In Latin America, countries such as Brazil (32%), Chile (25%), Ecuador (29%), and Colombia (30%) had prevalence below the average in 2011.
In Colombia, according to the National Survey of Nutritional Situation (Encuesta Nacional de la Situación Nutricional, ENSIN) 2010, one in every six pregnant women had anemia and 7.6% of women of fertile age had this haematological disorder [3].
Helminthiasis, specifically geohelminthiases caused by intestinal parasites, are the most common infections worldwide and they affect the poorest and most vulnerable populations. These infections are more common in women and children, and it is estimated that one in three people are infected; women of childbearing age, particularly pregnant women during the second and third trimesters and lactating women are at permanent risk [4, 5].
Helminthiasis affect a large part of the population and have a significant disease burden given their chronicity and association with malnutrition. Due to these associations, delays in physical and cognitive development can also be seen [6–8]. Surprisingly, there are not agreements upon screening guidelines for helminthiases in pregnant women in Colombia.
Several studies have shown that the prevalence of helminthiasis in pregnancy is considerable in countries of Latin America and Africa, and that it has a strong association with anemia in pregnant woman [6].
There are a few systematic reviews of the literature assessing the impact of these infections on pregnancy. This study sought to systematically collect and evaluate information regarding the association between intestinal helminthiases (IH), including several species, and GA from scientific databases.
MATERIALS AND METHODS
Study design
This review was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement [9]. The protocol of this systematic review was registered in the PROSPERO registry of systematic reviews (registration number: CRD42022312384). The primary outcome of this review was to estimate the association between intestinal helminthiasis (IH) and GA. Other outcomes were to determine the prevalence of GA and the helminths most frequently associated with GA.
Eligibility criteria
Women who were examined with a stool examination during their pregnancy and were also tested for anemia were included. Definition of anemia was based on the measurement of haemoglobin. Women whose anemic state was evaluated in the puerperium were excluded. Clinical trials were excluded. Studies comparing haemoglobin values as a continuous variable in regard to the infection status, but without definition of anemia status were neither included. Interventional studies that recruit women for iron supplementation were neither included.
Search strategy
In this study, an active search of information was done in two electronic databases MEDLINE® and LILACS®. Studies were selected from January 2000 to December 2021 using MeSH and DECS terms: “anaemia”, “anemia”, “pregnancy”, “hemoglobin”, “helminths”, “helminth”, “helminthiasis”, “ascariasis”, “schistosomiasis”, “hookworm” and “trichuriasis” without language restrictions. Studies that reported anemia during pregnancy as outcome measures and the detection of intestinal helminths by direct fecal microscopy as a variable of interest were included. The odds ratios (OR) with 95% confidence intervals (CI) to develop anaemia were estimated independently for each infection type using absolute numbers extracted from the publications. The available evidence and the internal validity of the evaluations were examined [10].
Data collection
There were two delegated investigators (JZ and JA) to perform the search, one of them in charge of identifying records through database searching. The other investigator screened the identified records by title and abstract, removing those whose full text was not available, and determine eligible studies according to select criteria. Finally, both researchers did a qualitative synthesis of the included articles. Individual judgements about specific issues were discussed, including inadequate population, intervention, or publication type.
Data from the final included studies was extracted using a form designed in Kobo Toolbox [11]. This form included the following fields: first author, year of publication, geographic location, publication type, age, number of cases with anemia, number of cases with parasite infection in anaemic and non-anaemic subjects, prevalence of anemia, rates of infection for each helminth species (i.e. ascaris, schistosoma, hookworm and trichuris) as well as the general rate of intestinal parasite or helminth infection (when available), adjusted odds ratio for anemia/parasite infection. Information of other covariates that may influence associations was recorded: Human immunodeficiency virus (HIV) prevalence if (available), infection with Plasmodium (if available). Ferritin levels in parasitized and non-parasitized pregnant women and mean haemoglobin levels in parasite-infected and non-infected groups was extracted, but not analyzed due to few detected data.
Quality measure
The quality of the articles included in this study was evaluated using the “Strengthening the Reporting of Observational studies in Epidemiology” (STROBE) tool, which consists of a 22-item evaluation form, designed to examine the quality of every component of population-based descriptive and analytical observational studies [22].
Target population, sampling frame, random selection, census collects and response rate of the study, are the evaluated criteria for external validity, otherwise, case definition, data collection, study instrument, prevalence period and error calculation, are the evaluated criteria for internal validity of the article. The final score is calculated using a dichotomous method, in which, an affirmative answer for each item sums 1 point, while negative answer results in 0 points. The final grade is obtained out of a total of 22 and is considered as an adequate result for those studies that reach 60% of the criteria evaluated, which is, a score greater than 13.
Statistical analysis
The meta-analyses were conducted only for exposures (helminth type) assessed by three or more studies. OR for the association of GA presentation with:
helminthiasis
ascariasis
trichuriasis
hookworm infection
schistosomiasis.
Crude OR were calculated based on the number of subjects exposed or not and presenting or not the outcome [13]. Microsoft excel sheets were used for data synthesis [14]. For meta-analysis, pooled OR were calculated by using the inverse of variance method [15]. Heterogeneity of estimates across studies was assessed using the I2 statistic in each analysis, as previously described [16]. A value of over 50% was indicative of significant heterogeneity and random-effects model was chosen; otherwise, a fixed-effect model was used. The Restricted Maximum-Likelihood method was used for tauw2 estimation. Prevalence of GA was metanalyzed using the inverse variance method.
To handle with heterogeneity, different strategies were applied: subgroup analysis considering as geographical region, study type and crude/adjusted odds ratio) as group variables. Meta-regression (considering co-infection with plasmodium, year of publication) as covariates were considered. We additionally generated a contour improved funnel plot to visually examine asymmetry in the plot and give an explanation for the underlying cause, such as, time lag or pipeline bias [17]. All analyses were performed using R statistical software ver. 4.1.1. (2021-08-10) (R Core Team, R Foundation for Statistical Computing) with the “meta” package [18].
RESULTS
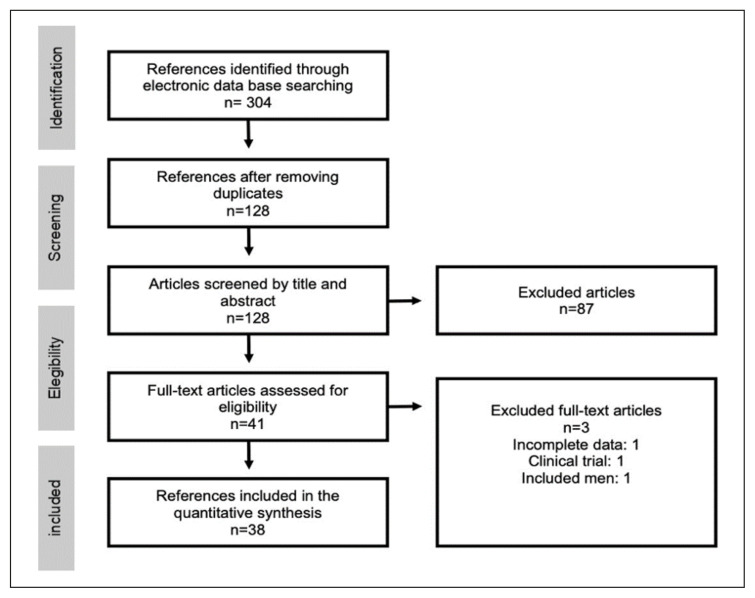
Three-hundred and four publications were identified through database search. After duplicate removal, we screened 128 articles and excluded 87 references based on title and abstract. Forty-one full-text publications were assessed for eligibility. Two studies were excluded in this phase due to unsatisfactory reporting of exposure status or inclusion of male individuals [19, 20]. Moreover, a study by Gyorkos et al was also excluded because it was a clinical trial [21]. As a result, data from 38 articles were eligible and assessed for quality evidence and synthesis [6, 7, 22–57]. The algorithm for the selection of the articles for the quantitative synthesis is shown in Figure 1. All included studies were considered of appropriate quality.
Figure 1.
Flowchart of studies included in the review.
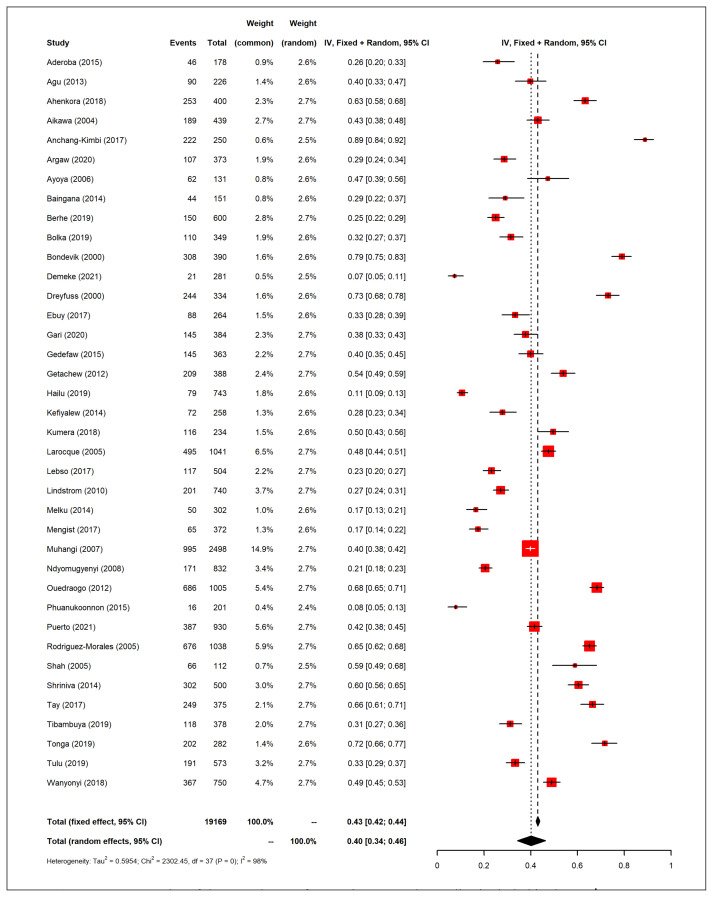
A summary of the included studies is presented in Table 1. Most studies had a cross-sectional design (n=29), 5 of them were prospective cohorts and 4 were case-control studies. The study populations of all studies belonged to low- and middle-income countries: 28 studies from Africa, 6 from Asia, 3 from Latin America and 1 from Oceania. Most studies did not report HIV status (21/38; 55%) or excluded HIV patients in the recruitment phase (11/38; 29%). HIV prevalence was 1–12% among the studies that did report HIV status (6/38; 16%). We evaluated the prevalence of GA reported among the studies included in this review, which was high in most studies (Figure 2). Overall, the average prevalence of GA among the included studies was 40% (95% CI 34–46%). The lowest prevalence was reported by Demeke et al (7%, 95% CI 5–11%) in Ethiopia, whereas the highest was reported by Anchang-Kimbi (89%, CI 95% 84–92%) in Cameroon [25, 32]. Hookworm was the predominant species detected in most studies (19/38; 50%), followed by Ascaris lumbricoides (15/38; 39.5%).
Table 1.
Summary of the studies included in the meta-analysis of anaemia prevalence.
| Author | Year | Population | Prevalence of anaemia | Predominant helminth | Malaria status | HIV status | Ref. |
|---|---|---|---|---|---|---|---|
| Aderoba | 2015 | Nigeria | 26% | Ascaris lumbricoides | Excluded | 8% | [6] |
| Agu | 2013 | Nigeria | 40% | Hookworm | 53% | NR | [22] |
| Ahenkora | 2018 | Ghana | 63% | Ascaris lumbricoides | 19% | Excluded | [23] |
| Aikawa | 2006 | Vietnam | 43% | Ascaris lumbricoides | NR | NR | [24] |
| Anchang-Kimbi | 2017 | Cameroon | 88% | Schistosoma haematobium | 39% | NR | [25] |
| Argaw | 2020 | Ethiopia | 29% | Trichuris trichiura | NR | NR | [26] |
| Ayoya | 2006 | Mali | 63% | Hookworm | 11% | NR | [27] |
| Baingana | 2015 | Uganda | 29% | Hookworm | 5% | Excluded | [28] |
| Berhe | 2019 | Ethiopia | 25% | Hookworm | NR | NR | [29] |
| Bolka | 2019 | Ethiopia | 32% | Hookworm | NR | NR | [30] |
| Bondevik | 2000 | Nepal | 79% | Ascaris lumbricoides | NR | Excluded | [31] |
| Demeke | 2021 | Ethiopia | 7% | Hookworm | NR | Excluded | [32] |
| Dreyfuss | 2000 | Nepal | 67% | Hookworm | 20% | NR | [33] |
| Ebuy | 2017 | Ethiopia | 37% | Hookworm | 7% | 1% | [34] |
| Gari | 2020 | Ethiopia | 38% | Hookworm | 5% | Excluded | [35] |
| Gedefaw | 2015 | Ethiopia | 40% | Hookworm | 4% | NR | [36] |
| Getachew | 2012 | Ethiopia | 54% | Hookworm | 41% | NR | [37] |
| Hailu | 2019 | Ethiopia | 11% | Hookworm | NR | NR | [38] |
| Kefiyalew | 2014 | Ethiopia | 28% | Ascaris lumbricoides | 3% | NR | [39] |
| Kumera | 2018 | Ethiopia | 12% | Hookworm | NR | 1% | [40] |
| Larocque | 2005 | Peru | 47% | Hookworm | 0% | NR | [41] |
| Lebso | 2017 | Ethiopia | 23% | Ascaris lumbricoides | NR | NR | [42] |
| Lidstrom | 2010 | Bangladesh | 28% | Ascaris lumbricoides | NR | NR | [43] |
| Melku | 2014 | Ethiopia | 17% | Ascaris lumbricoides | 5% | 10% | [44] |
| Mengist | 2017 | Ethiopia | 17% | Hookworm | NR | NR | [45] |
| Muhangi | 2007 | Uganda | 40% | Hookworm | 11% | 12% | [46] |
| Ndyomugyenyi | 2008 | Uganda | 21% | Hookworm | 35% | NR | [47] |
| Ouédraogo | 2012 | Benin | 68% | Hookworm | 15% | Excluded | [48] |
| Phuanukoonnon | 2015 | New Guinea | 8% | Hookworm | NR | NR | [49] |
| Puerto | 2021 | Colombia | 42% | Trichuris trichiura | NR | Excluded | [50] |
| Rodriguez-Morales | 2006 | Venezuela | 65% | Ascaris lumbricoides | 0% | Excluded | [7] |
| Shah | 2005 | Nepal | 59% | Ascaris lumbricoides | NR | NR | [51] |
| Shrinivas | 2014 | India | 60% | Ascaris lumbricoides | NR | Excluded | [52] |
| Tay | 2017 | Ghana | 66% | Ascaris lumbricoides | 17% | NR | [53] |
| Tibambuya | 2019 | Ghana | 31% | Ascaris lumbricoides | 21% | Excluded | [54] |
| Tonga | 2019 | Cameroon | 72% | Schistosoma haematobium | NR | 2% | [55] |
| Tulu | 2019 | Ethiopia | 33% | Ascaris lumbricoides | 2% | Excluded | [56] |
| Wanyonyi | 2018 | Kenya | 49% | Ascaris lumbricoides | 22% | NR | [57] |
Figure 2.
Forest plot of the prevalence of anaemia among the studies included in the review.
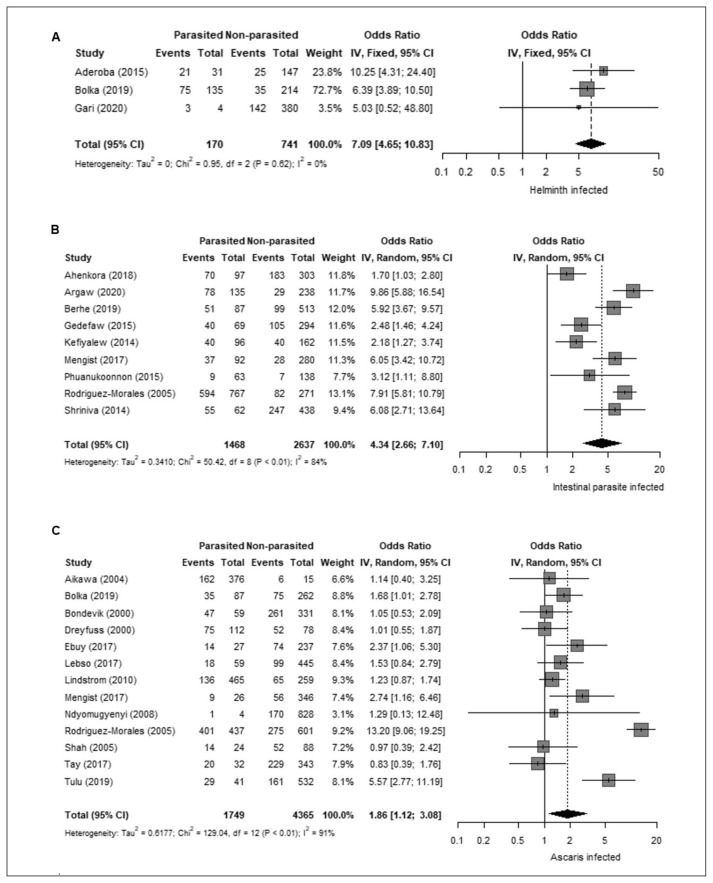
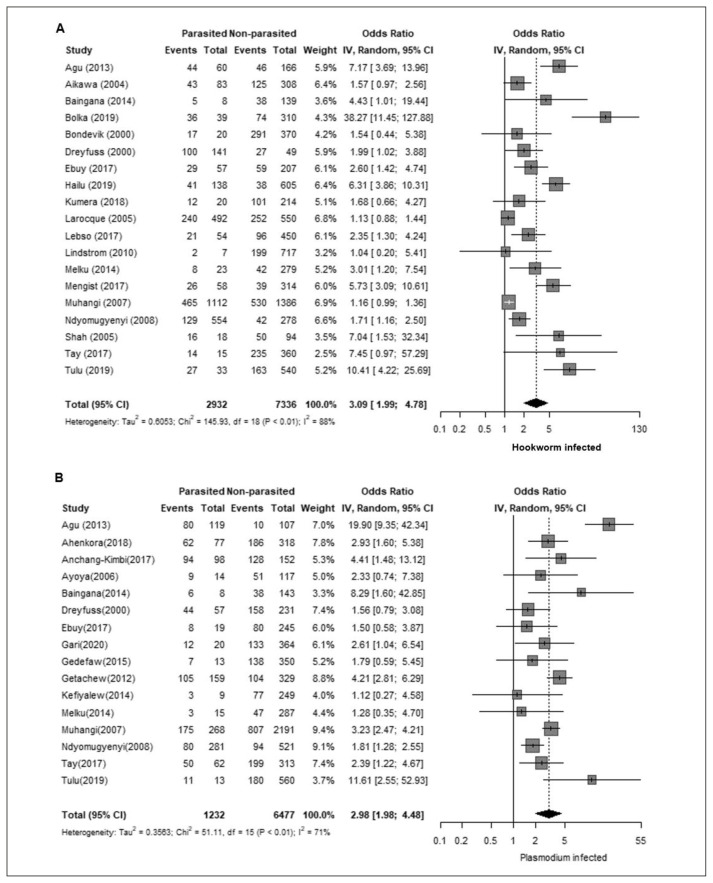
Next, we analyzed the association between GA and parasitic infections. GA was strongly associated with infection by any helminth (OR 7.09, 95% CI 4.65–10.83; Figure 3-A). We also evaluated the relation between GA and infection by intestinal parasites (including both helminths and protozoa), which also showed a positive association (OR 4.34, 95% CI 2.66–7.10; Figure 3-B). Regarding the association with specific helminth species, GA was positively associated with A. lumbricoides (OR 1.86, 95% CI 1.12 – 3.08; Figure 3-C) and hookworm (OR 3.09, 95% CI 1.99–4.78; Figure 4-A). In addition, GA showed non-significant associations with Schistosoma mansoni (OR 1.36, 95% CI 0.85–2.17) and Trichuris trichiura (OR 1.33, 95% CI 0.84–2.10). Due to the predominant co-infection between malaria and helminthiasis, we consider this condition as a potential bias for the study. To address this situation, we also analyzed the malaria prevalence data reported among the included studies. Malaria prevalence was reported in 21 out of the 38 studies (55%), with no detected cases in two studies and a range of 2–53% among those with positive cases (average: 16%). Sixteen studies (42%) did not report malaria prevalence and in one study (3%) malaria was used as an exclusion criterion. Moreover, the protozoan parasite Plasmodium, the causal agent of malaria, showed a positive association with GA (OR 2.98, 95% CI 1.98–4.48; Figure 4-B).
Figure 3.
A. Association between gestational anaemia and any helminth. B. Association between gestational anaemia and intestinal parasites. C. Association between gestational anaemia and Ascaris lumbricoides.
Figure 4.
A. Association between gestational anaemia and Hookworm. B. Association between gestational anaemia and Plasmodium.
To assess risk of bias, we performed meta-regression between hookworm or Ascaris lumbricoides OD and malaria prevalence or year of publication as covariates (Table 2). However, only six studies reported simultaneously Ascaris risk estimates and malaria prevalence, which precluded meta-regression analysis for this pair of variables. Year of publication was associated with the magnitude of the effect of hookworm on anemia risk. This covariate was not associated with Ascaris OR. Prevalence of malaria was not associated with the magnitude of the effect of hookworm on anemia risk.
Table 2.
Subgroups and meta-regression analysis of the factors associated with risk of bias.
| Covariate | Coefficient | SE | p-value | Model |
|---|---|---|---|---|
| Year of publication | 0.0200 | 0.0331 | 0.5586 | Ascaris |
| Year of publication | 0.0831 | 0.0255 | 0.0046 | Hookworm |
| Malaria | 1.0478 | 1.5502 | 0.5182 | Hookworm |
Furthermore, asymmetry in funnel plots of hookworms and plasmodium were observed during meta-analyses. Ascaris lumbricoides and Trichuristrichuria in opposition showed a symmetric figure plot, which corresponds to no intervention effect in most of studies.
DISCUSSION
Intestinal helminthiasis continue to be a public health problem in areas with low socioeconomic status and in the most vulnerable groups of children and pregnant women [58, 59]. Although the management of these infections depends primarily on the improvement of health services and hygiene conditions in the population, it is important to actively treat populations at risk to reduce their impact on health and the transgenerational cycle of this disease. No clear guidelines exist on the management of intestinal parasitosis in pregnancy, especially in the most affected areas in the world. This can be due to a couple of reasons such as the adoption of protocols from developed countries where these infections are not frequent and also the lack of attention that has been placed on some intestinal parasitosis in populations other than children under 5 years of age. Current recommendations on deworming in pregnancy are not clear due to the lack of studies to determine their consequences. There is a small quantity of meta-regression analysis of literature on evaluating the impact of helminthiases on GA. In this systematic review and meta-analysis, a significant association between IH and GA in pregnant women was found.
As the review shows, IH are a common problem in tropical countries. This is partially explained by the climatic conditions favoring the life cycle of the parasites. Furthermore, the absence of effective hygienic measures (i.e., correct sanitary handling of stools) favor the transmission of the infection and the economic constraints of the affected countries contribute to the problem.
Only three studies in Latin America have evaluated the impact of these infections in pregnancy [7, 41, 50]. Two of them showed a high prevalence of helminth infection during pregnancy and its association with GA. Interestingly, despite having data about the impact of intestinal helminthiasis in pre-kinder population from the National Survey of Parasitism, there is no data regarding the prevalence of this infection in the gestational age in Colombia [60]. Thereby, national studies are needed to evaluate if this problem is similar to neighboring countries.
A. lumbricoides is the most common helminthiasis on the planet and it is estimated that almost two billion humans are infected by this parasite. The disease burden of the infection is driven by its high prevalence. However, it is estimated that its effect on health is lower than other infections such as hookworm infection (Necator americanus or Anchylostomaduodenalis) which cause blood loss and have a stronger association with anemia and nutritional deficiencies. This review showed that geohelminthiases, specifically hookworm infection, are associated with GA. However, since polyparasitism is common, evaluating each helminth infection individually could lead to result bias because control groups (non-infected) could include individuals infected with different species [61]. Furthermore, we have to consider that hookworm is the predominant helminth in most of the studies that evaluated the relationship between IH and the outcome in this meta-analysis. Conducting studies in populations where these helminths are more common (e.g., Colombia) will help clarify the association between each helminth infection and anemia. It will be important to define if pregnant women with helminth infections require treatment due to the potential negative effects of an untreated infection on fetal development [62]. In addition, it is important to note that infection with multiple parasites has an additive effect on health outcome. Although hookworm is the main species associated with the outcome, the impact of other species needs to be defined with better epidemiological studies given the expected bias that arises from polyparasitism when defining comparison groups. Moreover, year of publication was associated with the magnitude of the effect of hookworm on anemia risk (p-value 0.0046); recent publications show a larger effect than previous ones. This could be attributed to better quality of study design and increase sensitivity of instruments that measure parasitic infection.
Meanwhile the most common parasitic co-infection in the world is malaria and helminths, we found prevalence of malaria was not associated with the magnitude of the effect of hookworm on anemia risk (p-value 0.5182); contrasting in the results showed in a study in children, which reported higher mean haemoglobin concentration in coinfected group with P. falciparum and S. haematobium than those with only malaria infection, and also a study, which reported that the odds of anemia were higher in children who were co-infected with malaria and helminths than those infected with Plasmodium alone [63, 64]. These different findings are consistent with the proposed mechanism that modulates anemia during parasitic coinfection [65]. However, target population was variable in most of studies. This is the first study on pregnant women that shows a negative association between prevalence of malaria and anemia risk during co-infection, supporting that single helminthic infestation is associated with the outcome of (GA), as shown in A. lumbricoides (OR 1.86, 95% CI 1.12–3.08) and hookworms (OR 3.09, 95% CI 1.99–4.78).
Another bias of the results is the heterogeneity of the cut off points to define anemia as each study used different haemoglobin levels relevant for their target population. Nonetheless, we consider that the risk of this variability is lower than choosing a fixed haemoglobin cut off point as it varies depending on the target population (e.g., oxygen concentration varies based on altitude) [66].
In conclusion, the results of this review indicate that there is a statistically significant association between intestinal parasitosis (helminthiasis) and (GA). Although hookworm is the main species associated with the outcome, prevalence of malaria was not associated with the magnitude of the effect of hookworm on anemia risk. The impact of other species needs to be defined given the expected bias that arises from polyparasitism when defining comparison groups.
Footnotes
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1. Hill CC, Pickinpaugh J. Physiologic changes in pregnancy. Surg Clin North Am. 2008;88(2):391–401. doi: 10.1016/j.suc.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Worldwide prevalence of anemia 1993–2005: WHO global database on anemia. Geneve: WHO Library; 2008. p. 51. Available in: https://apps.who.int/iris/handle/10665/43894. [Google Scholar]
- 3.Colombian Institute of Family Welfare - Ministry of Health of Colombia. National Survey of Nutritional Situation 2010 [Internet] [Consulted 27 Jul 2022]. Available in: https://www.minsalud.gov.co/salud/publica/epidemiologia/Paginas/encuesta-nacional-de-situacion-nutricional-ensin.aspx.
- 4.Pan American Health Organization. Geohelmintiasis [Internet] [Consulted 27 Jul 2022]. Available in: https://www3.paho.org/hq/index.php?option=com_topics&view=rdmore&cid=3957&Itemid=41001&lang=es.
- 5.World Health Organization. Geohelmintiasis [Internet] [Consulted 27 Jul 2022]. Available in: https://www.who.int/es/news-room/fact-sheets/detail/soiltransmitted-helminth-infections#:~:text=Las%20helmintiasis%20transmitidas%20por%20elsufren%20deterioro%20nutricional%20y%20f%C3%ADsico.
- 6. Aderoba AK, Iribhogbe OI, Olagbuji BN, Olokor OE, Ojide CK, Ande AB. Prevalence of helminth infestation during pregnancy and its association with maternal anemia and low birth weight. Int J Gynaecol Obstet. 2015;129(3):199–202. doi: 10.1016/j.ijgo.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 7. Rodríguez-Morales A, Barbella R, Case C, et al. Intestinal Parasitic Infections Among Pregnant Women in Venezuela. Infect Dis Obstet Gynecol . 2006:1–5. doi: 10.1155/IDOG/2006/23125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mpairwe H, Tweyongyere R, Elliott A. Pregnancy and helminth infections. Parasite Immunol. 2014;36(8):328–337. doi: 10.1111/pim.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Page M, Moher D, Bossuyt P, et al. BMJ. 2021. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews; p. n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hernández Sampieri R, Fernández Collado C, Baptista Lucio P. Research methodology. McGraw Hil: 2010. [Google Scholar]
- 11.KoBoToolbox. Data Collection Tools for Challenging Environments. n.d. [accessed March 10, 2022]. https://www.kobotoolbox.org/
- 12. Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4(10):e297. doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 14.Katz A. Style (DeKalb, IL) 2010. Microsoft Excel 2010; pp. 21–39. [Google Scholar]
- 15.Schwarzer G, Carpenter JR, Rücker G. Meta-Analysis with R. Springer International Publishing; Switzerland: 2015. Meta-analysis with binary outcomes; pp. 55–83. [Google Scholar]
- 16. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. The Ottawa Hospital Research Institute. 2013:1–4. [Google Scholar]
- 17. Tatsioni A, Ioannidis J. Meta-analysis. International Encyclopedia of Public Health. 2017:117–124. [Google Scholar]
- 18. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baidoo SE, Tay SCK, Obiri-Danso K, Abruquah HH. Intestinal helminth infection and anemia during pregnancy: A community-based study in Ghana. Afr J Microbiol Res. 2010;4(16):1713–1718. [Google Scholar]
- 20. Massawe S, Ronquist G, Nyström L, Lindmark G. Iron status and iron deficiency anemia in adolescents in a Tanzanian Suburban Area. Gynecol Obstet Invest. 2002;54(3):137–144. doi: 10.1159/000067879. [DOI] [PubMed] [Google Scholar]
- 21. Gyorkos T, Gilbert N, Larocque R, Casapía M. Trichuris and hookworm infections associated with anemia during pregnancy. Trop Med Int Health. 2011;16(4):531–537. doi: 10.1111/j.1365-3156.2011.02727.x. [DOI] [PubMed] [Google Scholar]
- 22. Agu PU, Ogboi JS, Akpoigbe K, Okeke T, Ezugwu E. Impact of Plasmodium falciparum and hookworm infections on the frequency of anemia in pregnant women of rural communities in Enugu, South East Nigeria. Pan Afr Med J. 2013;14:27. doi: 10.11604/pamj.2013.14.27.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ahenkorah B, Nsiah K, Baffoe P, Anto EO. Biochemical and hematological changes among anemic and non-anemic pregnant women attending antenatal clinic at the Bolgatanga regional hospital, Ghana. BMC Hematol. 2018;18(1):1–7. doi: 10.1186/s12878-018-0121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aikawa R, Ngyen CK, Sasaki S, Binns CW. Risk factors for iron-deficiency anemia among pregnant women living in rural Vietnam. Public Heal Nutr. 2006;9(4):443–8. doi: 10.1079/phn2005851. [DOI] [PubMed] [Google Scholar]
- 25. Anchang-Kimbi JK, Elad DM, Sotoing GT, Achidi EA. Coinfection with Schistosoma haematobium and Plasmodium falciparum and anemia severity among pregnant women in Munyenge, Mount Cameroon Area: A Cross-Sectional Study. J Parasitol Res. 2017;2017:6173465. doi: 10.1155/2017/6173465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Argaw D, Kabthymer RH, Birhane M. Magnitude of anemia and its associated factors among pregnant women attending antenatal care in Southern Ethiopia: A Cross-Sectional Study. J Blood Med. 2020;11:335–344. doi: 10.2147/JBM.S264369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ayoya MA, Spiekermann-Brouwer GM, Traoré AK, Stoltzfus RJ, Garza C. Determinants of anemia among pregnant women in Mali. Nutr Bull. 2006;27(1):3–11. doi: 10.1177/156482650602700101. [DOI] [PubMed] [Google Scholar]
- 28. Baingana RK, Enyaru JK, Tjalsma H, Swinkels DW, Davidsson L. The aetiology of anemia during pregnancy: A study to evaluate the contribution of iron deficiency and common infections in pregnant Ugandan women. Public Health Nutr. 2015;18(8):1423–1435. doi: 10.1017/S1368980014001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Berhe K, Fseha B, Gebremariam G, et al. Risk factors of anemia among pregnant women attending antenatal care in health facilities of eastern zone of Tigray, Ethiopia, case-control study, 2017/18. Pan Afr Med J. 2019;34:1–10. doi: 10.11604/pamj.2019.34.121.15999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bolka A, Gebremedhin S. Prevalence of intestinal parasitic infection and its association with anemia among pregnant women in Wondo Genet district, Southern Ethiopia: A cross-sectional study. BMC Infect Dis. 2019;19(1):1–8. doi: 10.1186/s12879-019-4135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bondevik GT, Eskeland B, Ulvik RJ, et al. Anemia in pregnancy: possible causes and risk factors in Nepali women. Eur J Clin Nutr. 2000;54(1):3–8. doi: 10.1038/sj.ejcn.1600883. [DOI] [PubMed] [Google Scholar]
- 32. Demeke G, Mengistu G, Abebaw A, et al. Effects of intestinal parasite infection on hematological profiles of pregnant women attending antenatal care at Debre Markos Referral Hospital, Northwest Ethiopia: Institution based prospective cohort study. PLoS One. 2021;16(5):e0250990. doi: 10.1371/journal.pone.0250990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dreyfuss ML, Stoltzfus RJ, Shrestha JB, et al. Hookworms, malaria and vitamin A deficiency contribute to anemia and iron deficiency among pregnant women in the plains of Nepal. J Nutr. 2000;130(10):2527–2536. doi: 10.1093/jn/130.10.2527. [DOI] [PubMed] [Google Scholar]
- 34. Ebuy Y, Alemayehu M, Mitiku M, Goba G. Determinants of severe anemia among laboring mothers in Mekelle city public hospitals, Tigray region, Ethiopia. PLoS One. 2017;12(11):e0186724. doi: 10.1371/journal.pone.0186724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gari W, Tsegaye A, Ketema T. Magnitude of anemia and its associated factors among pregnant women attending antenatal care at Najo General Hospital, northwest Ethiopia. Anemia. 1997;2020:885. doi: 10.1155/2020/8851997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gedefaw L, Ayele A, Asres Y, Mossie A. Anemia and Associated Factors Among Pregnant Women Attending Antenatal Care Clinic in Wolayita Sodo Town, Southern Ethiopia. Ethiop J Health Sci. 2015;25(2):155–162. doi: 10.4314/ejhs.v25i2.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Getachew M, Yewhalaw D, Tafess K, Getachew Y, Zeynudin A. Anemia and associated risk factors among pregnant women in Gilgel Gibe dam area, Southwest Ethiopia. Parasit Vectors. 2012;5:296. doi: 10.1186/1756-3305-5-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hailu T, Kassa S, Abera B, Mulu W, Genanew A. Determinant factors of anemia among pregnant women attending antenatal care clinic in Northwest Ethiopia. Trop Dis Travel Med Vaccines. 2019;5:13. doi: 10.1186/s40794-019-0088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kefiyalew F, Zemene E, Asres Y, Gedefaw L. Anemia among pregnant women in Southeast Ethiopia: prevalence, severity and associated risk factors. BMC Res Notes. 2014;7:771. doi: 10.1186/1756-0500-7-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kumera G, Haile K, Abebe N, Marie T, Eshete T. Anemia and its association with coffee consumption and hookworm infection among pregnant women attending antenatal care at Debre Markos Referral Hospital, Northwest Ethiopia. PLoS One. 2018;13(11):e0206880. doi: 10.1371/journal.pone.0206880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Larocque R, Casapia M, Gotuzzo EGT. Relationship between intensity of soil-transmitted helminth infections and anemia during pregnancy. Am J Trop Med Hyg. 2005;73(4):783–789. [PubMed] [Google Scholar]
- 42. Lebso M, Anato A, Loha E. Prevalence of anemia and associated factors among pregnant women in Southern Ethiopia: A community based cross-sectional study. PLoS One. 2017;12(12):e0188783. doi: 10.1371/journal.pone.0188783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lindström E, Hossain MB, Lönnerdal B, Raqib R, El Arifeen S, Ekström EC. Prevalence of anemia and micronutrient deficiencies in early pregnancy in rural Bangladesh, the MINIMat trial. Acta Obs Gynecol Scand. 2011;90(1):47–56. doi: 10.1111/j.1600-0412.2010.01014.x. [DOI] [PubMed] [Google Scholar]
- 44. Melku M, Addis Z, Alem M, Enawgaw B. Prevalence and Predictors of Maternal Anemia during Pregnancy in Gondar, Northwest Ethiopia: An Institutional Based Cross-Sectional Study. Anemia. 2014:108593. doi: 10.1155/2014/108593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mengist HM, Zewdie O, Belew A. Intestinal helminthic infection and anemia among pregnant women attending ante-natal care (ANC) in East Wollega, Oromia, Ethiopia. BMC Res Notes. 2017;10(1):440. doi: 10.1186/s13104-017-2770-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Muhangi L, Woodburn P, Omara M, et al. Associations between mild-to-moderate anemia in pregnancy and helminth, malaria and HIV infection in Entebbe, Uganda. Trans R Soc Trop Med Hyg. 2007;101(9):899–907. doi: 10.1016/j.trstmh.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ndyomugyenyi R, Kabatereine N, Olsen A, Magnussen P. Malaria and hookworm infections in relation to haemoglobin and serum ferritin levels in pregnancy in Masindi district, western Uganda. Trans R Soc Trop Med Hyg. 2008;102(2):130–136. doi: 10.1016/j.trstmh.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 48. Ouédraogo S, Accrombessi M, Massougbodji A, Koura G, Bodeau-Livinec F, Cot M. Maternal anemia at first antenatal visit: prevalence and risk factors in a malaria-endemic area in Benin. Am J Trop Med Hyg. 2012;87(3):418–424. doi: 10.4269/ajtmh.2012.11-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Phuanukoonnon S, Michael A, Kirarock WS, Pomat WS, van den Biggelaar AH. Intestinal parasitic infections and anemia among pregnant women in the highlands of Papua New Guinea. P N G Med J. 2013;56(3–4):119–125. [PubMed] [Google Scholar]
- 50. Puerto A, Trojan A, Alvis-Zakzuk N, et al. Iron status in late pregnancy is inversely associated with birth weight in Colombia. Public Health Nutr. 2021;24(15):5090–5100. doi: 10.1017/S136898002100166X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shah B, Baig L. Association of anemia with parasitic infestation in pregnant Nepalese women: results from a hospital-based study done in eastern Nepal. J Ayub Med Coll Abbottabad. 2005;17(1):5–9. [PubMed] [Google Scholar]
- 52. Shrinivas K, Radhika, Sreelathar R, Katvitha K. Study of Helminthiasis in Pregnancy and its Correlation with Haemoglobin Level. Obs Gynecol. 2014;8(10):OC07–0C09. doi: 10.7860/JCDR/2014/10148.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tay SC, Nani EA, Walana W. Parasitic infections and maternal anemia among expectant mothers in the Dangme East District of Ghana. BMC Res Notes. 2017;10(1):3. doi: 10.1186/s13104-016-2327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tibambuya BA, Ganle JK, Ibrahim M. Anemia at antenatal care initiation and associated factors among pregnant women in West Gonja District, Ghana: a cross-sectional study. Pan Afr Med J. 2019;33:325. doi: 10.11604/pamj.2019.33.325.17924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tonga C, Ngo Bayoi C, Tchanga FC, et al. Schistosomiasis among pregnant women in Njombe-Penja health district, Cameroon. J Infect Dev Ctries. 2019;13(12):1150–1158. doi: 10.3855/jidc.11767. [DOI] [PubMed] [Google Scholar]
- 56. Tulu BD, Atomssa EM, Mihiretie H, Id M. Determinants of anemia among pregnant women attending antenatal care in Horo Guduru Wollega Zone, West Ethiopia: Unmatched case-control study. PLoS One. 2019;14(10):e0224514. doi: 10.1371/journal.pone.0224514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wanyonyi WA, Mulambalah CS, Mulama DH, Omukunda E, Siteti DI. Malaria and geohelminthiasis coinfections in expectant women: effect on maternal health and birth outcomes in a malaria endemic region in Kenya. J Parasitol Res. 2018;2018:2613484. doi: 10.1155/2018/2613484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. WHO Expert Committee. Public health significance of intestinal parasitic infections. WHO Expert Committee. Bull World Health Organ. 1998;65(5):575–588. [PMC free article] [PubMed] [Google Scholar]
- 59. McDevitt MA, Xie J, Gordeuk VBR. The anemia of malaria infection: role of inflamatory cytokines. Curr Hematol Rep. 2004;3(2):97–106. [PubMed] [Google Scholar]
- 60.Ministry of Health of Colombia. National survey of intestinal parasitism in the school population Colombia, 2012–2014 [Internet] 2015. [Consulted 28 Jul 2022]. Available in: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/VS/ED/GCFI/Encuesta-de-Parasitismo.pdf.
- 61. Pullan R, Brooker S. The health impact of polyparasitism in humans: are we under-estimating the burden of parasitic diseases? Parasitology. 2008;135(7):783–794. doi: 10.1017/S0031182008000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Haider BA, Humayun Q, Bhutta ZA. Effect of administration of antihelminthics for soil transmitted helminths during pregnancy. Cochrane Database Syst Rev. 2009;2:CD005547. doi: 10.1002/14651858.CD005547.pub2. [DOI] [PubMed] [Google Scholar]
- 63. Degarege A, Degarege D, Veledar E, et al. Plasmodium falciparum Infection Status among children with Schistosoma in Sub-Saharan Africa: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2016;10(12):e0005193. doi: 10.1371/journal.pntd.0005193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Naing C, Whittaker MA, Nyunt-Wai V, et al. Malaria and soil-transmitted intestinal helminth co-infection and its effect on anemia: a meta-analysis. Trans R Soc Trop Med Hyg. 2013;107(11):672–683. doi: 10.1093/trstmh/trt086. [DOI] [PubMed] [Google Scholar]
- 65. Roussilhon C, Brasseur P, Agnamey P, Pérignon J-L, Druilhe P. Understanding human-Plasmodium falciparum immune interactions uncovers the immunological role of worms. PLoS One. 2010;5(2):e9309-e. doi: 10.1371/journal.pone.0009309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gonzales GF. Mother’s hemoglobin in perinatal and mother health in the highlands: implications in the Andean region. Rev Peru Med Exp Salud Publica. 2012;29(4):570–574. doi: 10.1590/s1726-46342012000400025. [DOI] [PubMed] [Google Scholar]