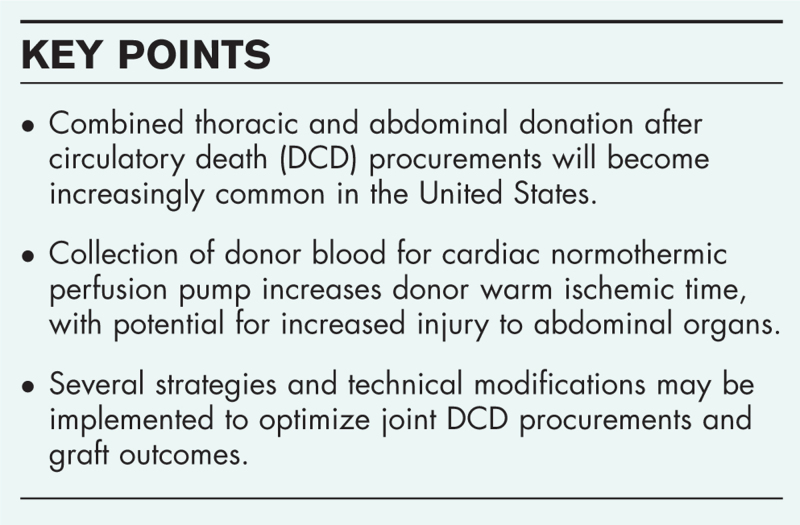
Purpose of the review
To summarize the international experience with heart-liver (joint) donation after circulatory death (DCD) procurements and to explore the technical challenges in joint abdominal and thoracic DCD procurement.
Recent findings
Following completion of the Donors After Circulatory Death Heart Trial in the US, combined thoracic and abdominal DCD is poised to become the standard of care, expanding access to life-saving heart and lung allografts. DCD heart procurement relies on collection of donor blood for priming of the normothermic perfusion pump, which delays cooling of abdominal organs and increases risk of ischemic injury. We review the effect of donor ischemia time on abdominal organs, with several proposed technical solutions to optimize transplant outcomes for all organs.
Summary
The strategies reviewed in this manuscript may inform clinical decision-making, preoperative coordination between thoracic and abdominal procurement teams, and surgical technique for joint DCD procurements. Several approaches to organ procurement organization (OPO) and national policy, as well as future areas of focus for research are proposed.
Keywords: donation after circulatory death, heart donor, kidney donor, liver donor, organ procurement
INTRODUCTION
Although cadaveric donation was first achieved by cardiopulmonary declaration criteria, donation after circulatory death (DCD) procurement fell out of practice during the late 1970 s due to poor recipient outcomes, developments in neurosciences, and a shifting ethical understanding of the definition of death [1]. Interest in DCD procurement was renewed in the 1990s as the demand for organs and wait times for transplant surged. The University of Pittsburgh Medical Center developed a DCD protocol in 1992 and the Institute of Medicine authored reports on DCD donation in 1997 and 2000 [2–4]. Since 2007, the number of DCD procurements in the United States steadily increased [5]; in 2019, DCD donors comprised 22% of deceased organ procurements [5].
Heart allografts from DCD donors were historically not utilized due to concerns of organ injury and ethical considerations. In the United States, this practice changed with the initiation of the Donors After Circulatory Death Heart Trial in 2019, a prospective, randomized trial comparing 6-month survival for recipients of brain-death donor (DBD) hearts preserved in standard cold storage versus DCD hearts preserved with the TransMedics Organ Care System (OCS) Heart pump. Other studies have employed normothermic regional perfusion in DCD heart procurement [6]. DCD heart transplant outcomes using these approaches are promising [6,7].
Given increasing utilization of abdominal organs from DCD donors and the emerging use of DCD hearts, joint thoracic-abdominal organ procurements are standard practice in the United Kingdom and Australia, and are becoming increasingly common in the United States. Such joint procurements pose unique operative and logistical challenges. We have discussed some of the ethical considerations raised by joint DCD procurements elsewhere [8▪]. In this article, we explore ways abdominal transplant teams, cardiac transplant teams, and organ procurement organizations (OPOs) can adapt current procurement practices to optimize outcomes for recipients of all of these organs.
Box 1.
no caption available
CHANGES TO THE ABDOMINAL ORGAN DCD PROCEDURE REQUIRED FOR JOINT DCD PROCUREMENTS
The abdominal organ DCD procurement is finely choreographed to minimize the time to initiation of cold aortic flush, thereby decreasing abdominal organ warm ischemia time. The team begins by making a midline exploratory laparotomy for immediate cannulation of the common iliac artery or distal abdominal aorta. The expected time from incision to cold flush is 1–3 min, followed by rapid external cooling with ice. In the absence of a thoracic team, abdominal surgeons perform a median sternotomy immediately after cannulation, providing thoracic access to drain the venous blood at the cavo-atrial junction and cross-clamp the descending thoracic aorta. The organs are then removed, and the hepatectomy time is typically under 30 min, with an additional 5–10 min for bilateral nephrectomy.
The addition of a heart or lung procurement alters this DCD approach. DCD heart procurements require collection of approximately 1.5 l of donor whole blood to prime the cardiac pump. While donor blood is collected, initiation of abdominal flush is postponed, adding to warm ischemia time. In the US experience during the Donors After Circulatory Death Heart Trial, blood was collected via cannulation of the right atrial appendage. Some authors estimate that approximately 90–120 s are required for passive drainage of the requisite blood volume [9▪▪], although in practice, collection of donor blood can require up to 5 min to collect sufficient volume for the perfusion pump [7,9▪▪].
IMPACT OF JOINT DONATION AFTER CIRCULATORY DEATH PROCUREMENTS ON ABDOMINAL ORGANS
The modifications to successfully accomplish joint DCD procurements raise a theoretical risk for increasing organ ischemia through a combination of delay in initiation of abdominal flush and prolonged hepatectomy and nephrectomy times. Although the impact on abdominal organ quality and utilization are not currently known, the effects of ischemia should be considered as joint DCD procurements become standard of care in the United States.
Warm ischemic time
The overall risk of organ ischemic injury during a procurement is a combination of procurement time, cold ischemia time (from flush to out of ice), and warm ischemic time (from out-of-ice to reperfusion). DCD organs also have an initial donor warm ischemic period between withdrawal of donor care and initiation of cold flush. The effects of warm ischemic time and cold storage in DCD procurement are well studied [10,11,12▪,13]. Similarly, there is a growing body of evidence that ‘lukewarm’ ischemia time during donor extraction time has a negative impact on both liver and kidney allograft function following transplant, particularly in early allograft function [14,15▪].
Liver graft dysfunction
Livers from DCD donors already have significantly higher rates of primary graft nonfunction, hepatic artery stenosis and ischemic cholangiopathy relative to those from DBD donors [16–19]. DCD donor functional warm ischemic time was the second most significant predictor or graft failure due to ischemic cholangiopathy or primary nonfunction [20]. A 90–120 s delay in initiating cold perfusion during a joint DCD procurement increases the risk of ischemic cholangiopathy by 20–30% [21,22].
Renal graft dysfunction
Unlike kidneys from DBD donors, time to nephrectomy increases the risk of graft loss in kidneys from DCD donors. Every 10 min of additional nephrectomy time increases the risk of graft loss by the approximately same amount as an additional hour of cold ischemic time [15▪]. Delayed graft function has also been correlated to donor extraction time, with a nearly 20% increased risk of delayed graft function for every 5 min extraction time over 60 min [23]. The increased rate of DGF among DCD kidney recipients is associated with longer postoperative hospital stays [24].
Organ discard rate
Abdominal organ discard is another significant potential consequence of joint DCD procurements. For donors that are pronounced near the acceptable upper limit of functional warm ischemia time, a several minute delay in flush resulting from the collection of donor blood can lead to liver and heart discard [9▪▪]. Although data from the UK and Australia suggests that this may be a rare occurrence, the overall effect of widespread adaptation of joint DCD procurements on organ discard in the US remains to be seen.
TECHNICAL CONSIDERATIONS TO MINIMIZE DELAY IN INITIATION OF FLUSH
The primary focus in optimizing joint DCD procurements should be to minimize ischemic time for all organs. Potential approaches fall in three categories: initiation of abdominal flush independent of cardiac team blood collection, expediting blood collection, and improved team-based coordination. We outline several options below; some may be used in combination.
Initiation of abdominal flushing independent of cardiac team blood collection
Cross-clamp location: The optimal site of aortic cross clamp should be easily accessible and pose low risk for organ injury. It has been suggested the abdominal procurement team clamp the suprahepatic inferior vena cava (IVC) and the supraceliac aorta to allow for early aortic flushing without dilution of the blood collected for the cardiac pump [9▪▪]. Although this does isolate thoracic blood collection from abdominal flush, it requires some liver mobilization with risk of injury to the suprahepatic cava or an aberrant left hepatic artery and adds time prior to flush. Instead, we propose thoracic control of the aorta and vena cava. The cardiac team can complete the sternotomy, then clamp the descending thoracic aorta and occlude the suprahepatic vena cava above the diaphragm with a Bainbridge clamp or a vessel loop.
Use of a balloon catheter: Many European centers routinely use double balloon catheters for thoracic aortic occlusion during DCD procurements [25]. This technique can be modified to the joint DCD procurement. A double balloon triple lumen catheter can be introduced via the right common femoral artery with inflation of the upper balloon above the diaphragm and the lower balloon at the aortic bifurcation. The abdominal organs may then be flushed in isolation from the thoracic organs and lower extremities. If rapid cannulation from the iliac artery is not feasible, the cardiac team could pass a large Foley catheter down the aortic arch to the descending thoracic aorta to allow for aortic ‘crossclamp’ on inflation.
Either of the above maneuvers will require modifications to venting. Sufficient venting is key to creating a low resistance circuit that allows for a good flush. During joint DCD procurements, the traditional vent via the supra-diaphragmatic IVC at the cavo-atrial junction is not an option until the donor blood is obtained. One option is venting infra-renal IVC at the iliac bifurcation, either by placing a large Foley catheter in the iliac vein or by creating a venotomy.
Normothermic regional perfusion
Abdominal in situ normothermic regional perfusion (NRP) was developed in Spain in a porcine model as an adaptation of extra-corporeal membrane oxygenation (ECMO) to the DCD context [26–28] and is now commonly used in Europe. Use of NRP during DCD procurements decreases rates of early allograft dysfunction and ischemic cholangiopathy for livers [29] and delayed graft function for kidneys [30]. Intra-abdominal NRP protocols vary slightly. One common variation entails placement of a balloon catheter via the donor's femoral artery with inflation of the balloon in the descending thoracic aorta, as well as femoral artery and femoral vein cannulation in the contralateral groin. This vascular access can achieved percutaneously [31] or during a rapid laparotomy [32]. Heparin is administered, either directly to the donor or via the primed ECMO circuit. Blood passes from the patient into the femoral vein catheter, through a pump and ECMO circuit and heat exchanger, and is returned oxygenated via the arterial line. The procurement dissection then proceeds as it would for a brain-dead donor.
The abdominal NRP procedure may be adapted for joint pulmonary-abdominal procurements in one of two ways, procurement of the thoracic organs with abdominal NRP or thoraco-abdominal NRP (TA-NRP). The first involves initiation of abdominal NRP as previously described, followed by sternotomy, clamping the thoracic aorta above the aortic occlusion balloon, cannulation of the pulmonary artery for pneumoplegia, then clamping the superior vena cava (SVC) in the chest to allow continued abdominal NRP [33]. This approach can be adapted for cardiac procurement [34]. In TA-NRP the sternotomy is performed with arterial cannulation of the distal ascending aorta and venous cannulation of the right atrial appendage and attached to the ECMO circuit. After the aortic arch branches are clamped to prevent cerebral reperfusion, ECMO is initiated. A laparotomy is performed expeditiously with ligation of the limb vessels to occlude distal circulation [35].
Enhanced blood availability or collection
Preprocurement donor transfusion: Some studies using the TransMedics OCS required the donor to be transfused to a hemoglobin of 10 g/dl to facilitate blood collection during the procurement [36]. While this is feasible, it may complicate the critical care management of donors, who may already be volume overloaded following extensive resuscitation efforts.
Suction-assisted blood collection: In the UK, some groups place a large cannula into the right atrium with connection to a cell-saver device to drain blood via suction aspiration. This technique reduces blood collection to under a minute [37]. This technique should be strongly considered, as it accomplishes the necessary aims of both teams with minimal procurement delay. Availability at donor hospitals and cost of equipment may limit wider application.
Use of washed banked blood to prime cardiac perfusion pump: Priming the TransMedics OCS machine requires 4–5 units of packed red blood cells. This completely eliminates any delay for blood collection. However, banked blood is hypocalcemic due to chelation with the citrate used for anticoagulation and hyperkalemic; both can adversely affect cardiac function. Washing the blood eliminates these electrolyte concerns but increases cost.
Patient positioning: Placing the donor in steep Trendelenburg can facilitate blood collection. Unfortunately, this also significantly impairs ergonomics and visualization for the abdominal team during this critical time. The plan for changing patient position during the blood collection should be discussed in advance, as the abdominal surgeons will often require footstool(s) to allow them to continue operating.
Systems-based improvements
Team learning: An alternative way to minimize donor warm ischemic time would be to develop joint DCD heart and liver procurement teams. Numerous studies demonstrate that learning curve associated with any new procedure is best addressed by utilization of a consistent team [38,39]. Instead of the current reliance on random pairings of teams that meet for the first time a the donor hospital, cardiac teams that regularly procure DCD hearts could work with selected abdominal procurement surgeons, either from their center or from the OPO. These teams could then be deployed together for all joint heart liver transplants. With increasing frequency of joint DCD procurements, training sessions could be offered to all procurement teams at the OPO or national level.
Creation of national or OPO-based standard protocols: Ideally, standardized parameters for the procedure, order of operations, and timing of joint procurements should be addressed by United Network for Organ Sharing (UNOS) to optimize all organ outcomes. Potential areas of focus include:
-
(1)
Recommend the use of one or more methods to speed the collection of blood for the cardiac pump;
-
(2)
Establish an upper limit for delay in aortic flush resulting from blood collection;
-
(3)
Standardize a protocol for aortic and caval control for all joint DCD procurements;
-
(4)
Consider the extent to which allowable delays should be modified in the case of marginal organ donors (e.g. increased age, higher BMI).
-
(5)
Establish whether or not the abdominal team must delay the hepatectomy to allow lung recovery.
However, given the extant wide variation in DCD procurement practices [40], such consensus may be difficult to achieve. In the absence of an overriding policy, the logistics of a joint DCD procurement should be discussed between teams as early as possible, preferably at the time of organ offer.
AVENUES FOR FURTHER RESEARCH
Further data is needed to accurately assess the impact of joint DCD procurement on organ quality and usage. Time to initiation of flush in joint DCD procurements should be tracked and compared to that in abdominal DCD procurements at a national level. Likewise, the organ discard rate and reasons for nonuse in joint procurements should be reported.
Though the Donors After Circulatory Death Heart Trial is monitoring outcomes for DCD heart recipients, we believe that it is equally essential to follow the long-term outcomes of individuals who receive kidneys and livers procured during combined DCD liver and heart procurements. Long-term graft survival, patient survival, and complications rates should be compared to matched recipients who received livers form standard DCD procurements. The comparative outcomes should inform policy decisions regarding acceptable delays in initiation of cold perfusion.
In addition, the use of machine perfusion for livers merits further consideration, particularly in the context of joint DCD procurements. In the DHOPE-DCD study, hypothermic machine perfusion of DCD livers reduced the nonanastomotic biliary stricture rate from 18% to 6% [41]. This suggests that machine perfusion for DCD livers could mitigate risk of ischemic cholangiopathy secondary to prolonged donor warm ischemic time.
CONCLUSION
The increasing utilization of DCD heart and lung grafts is an excellent example of how our field continues to evolve, and the goal of helping more wait listed patients is omnipresent. Expansion of joint DCD procurements will increase organ availability, and national UNOS guidelines should be established to optimize outcomes for all waitlisted patients. Early and frequent communication between the procurement teams is paramount to ensure a successful joint procurement.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
Footnotes
These authors contributed equally to this work.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Abt PL, Fisher CA, Singhal AK. Donation after cardiac death in the US: history and use. J Am Coll Surg 2006; 203:208–225. [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine. Non-heart-beating organ transplantation: practice and protocols. Washington DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- 3.Potts JT, Herdman R. Non-heart-beating organ transplantation: medical and ethical issues in procurement. Washington DC: National Academy Press; 1997. [PubMed] [Google Scholar]
- 4.University of Pittsburgh Medical Center policy and procedure manual. Management of terminally ill patients who may become organ donors after death. Kennedy Inst Ethics J 1993; 3:A1–15. [PubMed] [Google Scholar]
- 5.Israni AK, Zaun D, Rosendale JD, et al. OPTN/SRTR 2019 annual data report: deceased organ donors. Am J Transplant 2021; 21: (Suppl 2): 521–558. [DOI] [PubMed] [Google Scholar]
- 6.Messer S, Page A, Axell R, et al. Outcome after heart transplantation from donation after circulatory-determined death donors. J Heart Lung Transplant 2017; 36:1311–1318. [DOI] [PubMed] [Google Scholar]
- 7.Chew HC, Iyer A, Connellan M, et al. Outcomes of donation after circulatory death heart transplantation in Australia. J Am Coll Cardiol 2019; 73:1447–1459. [DOI] [PubMed] [Google Scholar]
- 8▪.Wisel SA, Thiessen C, Day R, et al. Setting rules for the sandbox: a response to ‘Successfully sharing the sandbox: a perspective on combined DCD liver and heart donor procurement’. Am J Transplant 2020; 21:1981–1982. [DOI] [PubMed] [Google Scholar]; This commentary in response to Croome and Daneshmand (below) proposes several additional modifications to the combined approach to thoracic and abdominal DCD organ procurement.
- 9▪▪.Croome KP, Daneshmand MA. Successfully sharing the sandbox: a perspective on combined DCD liver and heart donor procurement. Am J Transplant 2021; 21:484–487. [DOI] [PubMed] [Google Scholar]; This report documents one transplant center's early experience with simultaneous thoracic and abdominal DCD organ procurement in the United States, and identifies several key factors in successful coordination between thoracic and abdominal surgical teams.
- 10.Coffey JC, Wanis KN, Monbaliu D, et al. The influence of functional warm ischemia time on DCD liver transplant recipients’ outcomes. Clin Transplant 2017; 31:e13068. [DOI] [PubMed] [Google Scholar]
- 11.Kalisvaart M, Schlegel A, Umbro I, et al. The impact of combined warm ischemia time on development of acute kidney injury in donation after circulatory death liver transplantation: stay within the golden hour. Transplantation 2018; 102:783–793. [DOI] [PubMed] [Google Scholar]
- 12▪.Paterno F, Guarrera JV, Wima K, et al. Clinical implications of donor warm and cold ischemia time in donor after circulatory death liver transplantation. Liver Transpl 2019; 25:1342–1352. [DOI] [PubMed] [Google Scholar]; This review of SRTR data for DCD liver allografts investigates graft and patient outcomes for over 2000 DCD liver transplants, identifying donor and recipient risk factors for adverse graft outcomes.
- 13.Heylen L, Jochmans I, Samuel U, et al. The duration of asystolic ischemia determines the risk of graft failure after circulatory-dead donor kidney transplantation: a Eurotransplant cohort study. Am J Transplant 2018; 18:881–889. [DOI] [PubMed] [Google Scholar]
- 14.Adelmann D, Roll GR, Kothari R, et al. The impact of deceased donor liver extraction time on early allograft function in adult liver transplant recipients. Transplantation 2018; 102:e466–e471. [DOI] [PubMed] [Google Scholar]
- 15▪.Heylen L, Pirenne J, Samuel U, et al. Effect of donor nephrectomy time during circulatory-dead donor kidney retrieval on transplant graft failure. Br J Surg 2020; 107:87–95. [DOI] [PubMed] [Google Scholar]; This study, investigating over 13,000 kidney transplants in the Eurotransplant cohort, highlights the impact of prolonged organ extraction time on renal allograft outcomes following DCD organ donation.
- 16.Jay CL, Lyuksemburg V, Ladner DP, et al. Ischemic cholangiopathy after controlled donation after cardiac death liver transplantation: a meta-analysis. Ann Surg 2011; 253:259–264. [DOI] [PubMed] [Google Scholar]
- 17.Skaro AI, Jay CL, Baker TB, et al. The impact of ischemic cholangiopathy in liver transplantation using donors after cardiac death: the untold story. Surgery 2009; 146:543–552. discussion 552–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foley DP, Fernandez LA, Leverson G, et al. Donation after cardiac death: the University of Wisconsin experience with liver transplantation. Ann Surg 2005; 242:724–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Vera ME, Lopez-Solis R, Dvorchik I, et al. Liver transplantation using donation after cardiac death donors: long-term follow-up from a single center. Am J Transplant 2009; 9:773–781. [DOI] [PubMed] [Google Scholar]
- 20.Schlegel A, Kalisvaart M, Scalera I, et al. The UK DCD Risk Score: a new proposal to define futility in donation-after-circulatory-death liver transplantation. J Hepatol 2018; 68:456–464. [DOI] [PubMed] [Google Scholar]
- 21.Taner CB, Bulatao IG, Perry DK, et al. Asystole to cross-clamp period predicts development of biliary complications in liver transplantation using donation after cardiac death donors. Transpl Int 2012; 25:838–846. [DOI] [PubMed] [Google Scholar]
- 22.Taner CB, Bulatao IG, Willingham DL, et al. Events in procurement as risk factors for ischemic cholangiopathy in liver transplantation using donation after cardiac death donors. Liver Transpl 2012; 18:100–111. [DOI] [PubMed] [Google Scholar]
- 23.Osband AJ, James NT, Segev DL. Extraction time of kidneys from deceased donors and impact on outcomes. Am J Transplant 2016; 16:700–703. [DOI] [PubMed] [Google Scholar]
- 24.Nunez-Nateras R, Reddy KS, Aqel BA, et al. Simultaneous liver-kidney transplantation from donation after cardiac death donors: an updated perspective. Am J Transplant 2020; 20:3582–3589. [DOI] [PubMed] [Google Scholar]
- 25.Domínguez-Gil B, Haase-Kromwijk B, Van Leiden H, et al. Current situation of donation after circulatory death in European countries. Transpl Int 2011; 24:676–686. [DOI] [PubMed] [Google Scholar]
- 26.Johnson LB, Plotkin JS, Howell CD, et al. Successful emergency transplantation of a liver allograft from a donor maintained on extracorporeal membrane oxygenation. Transplantation 1997; 63:910–911. [DOI] [PubMed] [Google Scholar]
- 27.Fondevila C, Hessheimer AJ, Ruiz A, et al. Liver transplant using donors after unexpected cardiac death: novel preservation protocol and acceptance criteria. Am J Transplant 2007; 7:1849–1855. [DOI] [PubMed] [Google Scholar]
- 28.Valero R, García-Valdecasas JC, Tabet J, et al. Hepatic blood flow and oxygen extraction ratio during normothermic recirculation and total body cooling as viability predictors in nonheart-beating donor pigs. Transplantation 1998; 66:170–176. [DOI] [PubMed] [Google Scholar]
- 29.Watson CJE, Hunt F, Messer S, et al. In situ normothermic perfusion of livers in controlled circulatory death donation may prevent ischemic cholangiopathy and improve graft survival. Am J Transplant 2019; 19:1745–1758. [DOI] [PubMed] [Google Scholar]
- 30.Padilla M, Coll E, Fernández-Pérez C, et al. Improved short-term outcomes of kidney transplants in controlled donation after the circulatory determination of death with the use of normothermic regional perfusion. Am J Transplant 2021; 21:3618–3628. [DOI] [PubMed] [Google Scholar]
- 31.Foss S, Nordheim E, Sørensen DW, et al. First Scandinavian protocol for controlled donation after circulatory death using normothermic regional perfusion. Transplant Direct 2018; 4:e366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oniscu GC, Randle LV, Muiesan P, et al. In situ normothermic regional perfusion for controlled donation after circulatory death—the United Kingdom experience. Am J Transplant 2014; 14:2846–2854. [DOI] [PubMed] [Google Scholar]
- 33.Miñambres E, Suberviola B, Dominguez-Gil B, et al. Improving the outcomes of organs obtained from controlled donation after circulatory death donors using abdominal normothermic regional perfusion. Am J Transplant 2017; 17:2165–2172. [DOI] [PubMed] [Google Scholar]
- 34.Mohite PN, García Sáez D, Butler AJ, et al. Direct procurement of donor heart with normothermic regional perfusion of abdominal organs. Ann Thorac Surg 2019; 108:597–600. [DOI] [PubMed] [Google Scholar]
- 35.Tsui SSL, Oniscu GC. Extending normothermic regional perfusion to the thorax in donors after circulatory death. Curr Opin Organ Transplant 2017; 22:245–250. [DOI] [PubMed] [Google Scholar]
- 36.García Sáez D, Zych B, Sabashnikov A, et al. Evaluation of the organ care system in heart transplantation with an adverse donor/recipient profile. Ann Thorac Surg 2014; 98:2099–2105. discussion 2105–6. [DOI] [PubMed] [Google Scholar]
- 37.Smail H, Garcia-Saez D, Stock U, et al. Direct heart procurement after donation after circulatory death with ex situ reperfusion. Ann Thorac Surg 2018; 106:e211–e214. [DOI] [PubMed] [Google Scholar]
- 38.Edmondson AC, Bohmer RM, Pisano GP. Disrupted routines: team learning and new technology implementation in hospitals. Admin Sci Q 2001; 46:685–716. [Google Scholar]
- 39.Okamura A, Watanabe M, Fukudome I, et al. Surgical team proficiency in minimally invasive esophagectomy is related to case volume and improves patient outcomes. Esophagus 2018; 15:115–121. [DOI] [PubMed] [Google Scholar]
- 40.Choubey AP, Siskind EJ, Ortiz AC, et al. Disparities in DCD organ procurement policy from a national OPO survey: a call for standardization. Clin Transplant 2020; 34:e13826. [DOI] [PubMed] [Google Scholar]
- 41.van Rijn R, Schurink IJ, de Vries Y, et al. Hypothermic machine perfusion in liver transplantation – a randomized trial. N Engl J Med 2021; 384:1391–1401. [DOI] [PubMed] [Google Scholar]