Objective:
To compare neoadjuvant chemotherapy (nCT) with CAPOX alone versus neoadjuvant chemoradiotherapy (nCRT) with capecitabine in locally advanced rectal cancer (LARC) with uninvolved mesorectal fascia (MRF).
Background Data:
nCRT is associated with higher surgical complications, worse long-term functional outcomes, and questionable survival benefits. Comparatively, nCT alone seems a promising alternative treatment in lower-risk LARC patients with uninvolved MRF.
Methods:
Patients between June 2014 and October 2020 with LARC within 12 cm from the anal verge and uninvolved MRF were randomly assigned to nCT group with 4 cycles of CAPOX (Oxaliplatin 130 mg/m2 IV day 1 and Capecitabine 1000 mg/m2 twice daily for 14 d. Repeat every 3 wk) or nCRT group with Capecitabine 825 mg/m² twice daily administered orally and concurrently with radiation therapy (50 Gy/25 fractions) for 5 days per week. The primary end point is local-regional recurrence-free survival. Here we reported the results of secondary end points: histopathologic response, surgical events, and toxicity.
Results:
Of the 663 initially enrolled patients, 589 received the allocated treatment (nCT, n=300; nCRT, n=289). Pathologic complete response rate was 11.0% (95% CI, 7.8-15.3%) in the nCT arm and 13.8% (95% CI, 10.1-18.5%) in the nCRT arm (P=0.33). The downstaging (ypStage 0 to 1) rate was 40.8% (95% CI, 35.1-46.7%) in the nCT arm and 45.6% (95% CI, 39.7-51.7%) in the nCRT arm (P=0.27). nCT was associated with lower perioperative distant metastases rate (0.7% vs. 3.1%, P=0.03) and preventive ileostomy rate (52.2% vs. 63.6%, P=0.008) compared with nCRT. Four patients in the nCT arm received salvage nCRT because of local disease progression after nCT. Two patients in the nCT arm and 5 in the nCRT arm achieved complete clinical response and were treated with a nonsurgical approach. Similar results were observed in subgroup analysis.
Conclusions:
nCT achieved similar pCR and downstaging rates with lower incidence of perioperative distant metastasis and preventive ileostomy compared with nCRT. CAPOX could be an effective alternative to neoadjuvant therapy in LARC with uninvolved MRF. Long-term follow-up is needed to confirm these results.
Keywords: locally advanced rectal cancer, neoadjuvant chemotherapy, neoadjuvant chemoradiotherapy, randomized clinical trial
The traditional standard of care for treating locally advanced rectal cancer (LARC) patients consists of neoadjuvant chemoradiotherapy (nCRT) followed by total mesorectal excision (TME) and adjuvant chemotherapy.1,2 This combined modality approach has dramatically decreased the local recurrence risk of LARC in the past 2 decades.3–5 However, most clinical trials failed to demonstrate that the addition of radiotherapy to TME improved the patients’ survival, which could probably be due to inadequate systemic control.6–8 Novel strategies have been suggested but remain currently investigational.9 Under the conventional nCRT paradigm, systemic therapy is usually administered 4 to 5 months after diagnosis, which might result in tumor progression before surgery.10 Furthermore, low compliance with adjuvant chemotherapy also has an impact, as less than 50% of patients are unable to receive the planned dose of adjuvant chemotherapy due to toxicities, surgical complications, or good response to chemoradiotherapy (CRT).11,12 Early exposure to systemic chemotherapy was expected to result in improved control of micrometastases and better tolerance to systemic chemotherapy.13 The strategy of total neoadjuvant therapy (TNT), in which chemotherapy is administered before surgery, either before or after CRT, has been widely studied recently. The RAPIDO and PRODIGE 23 trials successfully demonstrated that compared with conventional CRT, the TNT approach significantly improved the disease-free survival (DFS) and pathologic complete response (pCR) in LARC.3,4 As a result, the TNT approach has become a new treatment of choice for patients with threatened mesorectal fascia (MRF) or locally unresectable tumor. For patients with cT3 disease and clear MRF or cT1-2N1-2, both the TNT approach and conventional long-course or short-course radiotherapy are recommended in the National Comprehensive Cancer Network guideline,14 probably due to the concerns of increased toxicities with the TNT approach and lack of benefit in patients without high-risk factors. Therefore, the optimal neoadjuvant strategy for LARC patients with clear MRF is still open to future research. Since radiotherapy is associated with radiation toxicities, higher surgical complications, and worse long-term functional outcomes,15,16 the necessity for radiation in unselected LARC patients is being questioned, especially in those without high-risk factors such as stage cT4b or involved MRF.17–19
Neoadjuvant chemotherapy (nCT) seems a promising alternative. It has been shown to overcome the above-mentioned drawbacks of the conventional nCRT, shorten the treatment period and improve cost effectiveness.20–25 Previous studies exploring the approach of neoadjuvant chemotherapy have observed promising results, with a pathologic complete response (pCR) rate from 3·7% to 25·0%, a good tumor downstaging from 27·2% to 56·3%, and a local recurrence rate from 0% to 10·0%.5,17,18,20,25 The FOWARC study even demonstrated that nCT with mFOLFOX6 regimens achieved similar 3-year DFS and local recurrence rate to conventional nCRT, although the sample size was relatively small and comprised of a mixed population cohort.5 However, it should be noted that the efficacy of neoadjuvant chemotherapy alone should not be over-emphasized since the downstaging effect in unselected population was unsatisfactory.26,27 Previous studies have demonstrated that MRF invasion was associated with worse biology, the lower response rate to treatment, and worse prognosis.28–30 Thus, we hypothesized that tumors with MRF involvement might benefit less from chemotherapy alone.
In this multicenter, noninferiority, randomized trial, the CONVERT study, we compared neoadjuvant chemotherapy with CAPOX alone to standard CRT with Capecitabine for LARC in LARC patients with uninvolved MRF. Here, we report the preliminary results on their related toxicity, treatment compliance, surgical events, and other efficacy data.
METHODS
Study Design and Participants
The CONVERT trial is a phase III, open-label, multicenter, noninferiority, randomized trial performed at 21 hospitals across China from June 1, 2014 to October 1, 2020 (NCT02288195). The trial followed the Consolidated Standards of Reporting Trials reporting guideline. The protocol was approved by the central ethics committee of Sun Yat-sen University Cancer Center (Guangzhou, China), and local ethics committees of all participating hospitals. All participants provided written informed consent.
Eligibility criteria included adults aged 18-75 years with pathologically confirmed rectal cancer diagnosis and no previous treatment. All patients were also required to have an Eastern Cooperative Oncology Group performance status ≤1 and adequate hematologic, liver, and renal function. Contrast-enhanced computed tomography (CT) scan of the chest and abdomen and pelvic magnetic resonance imaging (MRI) were performed before inclusion in the trial to exclude metastases. Pelvic MRI was required for all patients unless contraindicated, in which case pelvic CT scan and endoscopic ultrasound were used for evaluation. Baseline colonoscopy and pelvic MRI were performed to confirm that the tumor had a distal edge located between 5 and 12 cm from the anal verge in consideration of the lack of data on the efficacy of nCT in low rectal cancer. From April 2019, the protocol was revised to also enroll patients with tumors within 5 cm from the anal verge because the FOWARC study reported that tumor location did not impact the response to chemotherapy. The clinical T stage was estimated based on both MRI and endoscopic ultrasound according to the AJCC seventh edition, and discrepant estimates were consulted with the surgeons and radiologists. Patients were included if their imaging suggested clinical cT2N+ or cT3-4aNany disease. Patients were ineligible if their primary tumor was staged as cT4b or adjacent to the MRF and had symptomatic bowel obstruction. Patients were also excluded if they had chemotherapy or other invasive malignancy within 5 years before registration or any prior pelvic radiation.
Random Assignment and Masking
Patients were recruited and assessed for eligibility at the center they were diagnosed and treated. A stratified randomized block design was adopted to assign patients (1:1) to the nCT (4 cycles of CAPOX followed by surgery and adjuvant chemotherapy) or nCRT (chemoradiotherapy followed by surgery and adjuvant chemotherapy; Fig. 1) group. Random assignment was conducted centrally, and patients were assigned through a phone call or internet interface hosted by the Fudan University Shanghai Cancer Center (Shanghai, China). Stratification factors included tumor location and clinical nodal staging. Investigators and participants were not masked to treatment allocations.
FIGURE 1.
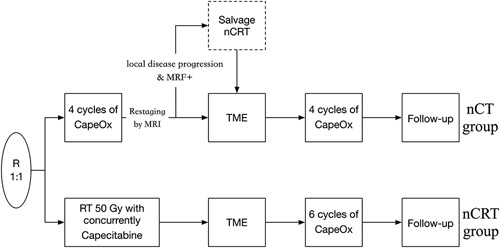
Study design. Patients were assigned (1:1) to the nCT (4 cycles of CAPOX followed by surgery and adjuvant chemotherapy) or nCRT (chemoradiotherapy followed by surgery and adjuvant chemotherapy) group.
Procedures
Neoadjuvant Chemotherapy
Patients assigned to the nCT group received 4 cycles of CAPOX regimen (Oxaliplatin, 130 mg/m2 IV day 1 plus capecitabine, 1000 mg/m2 twice daily for 14 d, Q3W). The duration of nCT is 3 weeks for each cycle and 12 weeks in total for 4 cycles of neoadjuvant chemotherapy. Treatment doses were adjusted in response to toxicities according to a predefined protocol (see the Protocol as a supplemental file, Supplemental Digital Content 1, http://links.lww.com/SLA/E394).
Neoadjuvant Chemoradiotherapy
Patients assigned to the nCRT group received 825 mg/m² of oral capecitabine twice daily with concurrent radiation therapy 5 days/week for 5 weeks. The total radiotherapy dosage was 50 Gy in 25 fractions to the gross tumor volume and 45 Gy in 25 fractions to the clinical target volume delivered by intensity-modulated radiation.
Restaging After Neoadjuvant Therapy
Restaging assessments with pelvic MRI and endoscopic ultrasound were performed 1 week after the completion of chemotherapy for the nCT group and 5 weeks after the end of chemoradiotherapy for the nCRT group. Patients with evidence of local disease progression in the nCT group underwent chemoradiation as in the nCRT group before surgery. In addition, patients with distant metastases were treated with current standard therapy. The watch-and-wait strategy was recommended only for patients who were candidates for abdominoperineal resection and achieved clinical complete response (cCR).
Surgery
Patients without disease progression were scheduled for surgery with TME 2 to 4 weeks after chemotherapy for the nCT group and 6 to 10 weeks after chemoradiotherapy for the nCRT group. Preventive diverting ileostomy was performed at the discretion of the primary surgeon.
Adjuvant Therapy
Adjuvant chemotherapy started 3 to 4 weeks following surgery in both groups, regardless of pathologic response stage. Patients in the nCT group received 4 cycles of the CAPOX regimen, and patients in the nCRT group received 6 cycles of the CAPOX regimen. For patients with microscopic (R1) or macroscopic (R2) disease in the resected specimen, postoperative chemoradiation was administered.
Post-treatment Surveillance
Post-treatment follow-up was performed every 3 months for the first 2 years and every 6 months for the next 3 years. Details of follow-up assessments, including CT scans or MRI, abdominal ultrasound, colonoscopy, carcinoembryonic antigen measurement, physical examination, and digital rectal examination, are provided in the protocol.
Safety
Laboratory and adverse event (AE) monitoring during perioperative therapy were done on day 1 of all cycles of nCT, weekly for nCRT, before and after surgery, and on day 1 of all cycles of adjuvant chemotherapy. The severity of AE and the laboratory findings were graded by the investigators according to Common Terminology Criteria for Adverse Events, version 4.
Outcomes
The primary end point is 3-year local-regional failure-free survival. Local-regional failure-free survival was defined as the time interval between the date of randomization and the date of local or regional progression/relapse or death, whichever occurred first. Secondary end points included 3-year DFS, pCR rate, tumor regression grade (TRG), pelvic R0 resection rate, overall survival, AE profiles, and rate of receiving preoperative or postoperative chemoradiation. R0 resection was defined as microscopic complete resection with adequate tumor-free margins confirmed by pathology based on a review by the study pathologist. pCR was defined as the absence of viable tumor cells in the primary tumor and lymph nodes (ypT0N0). cCR was assessed through digital rectal examination, colonoscopy, and radiographic images. TRG was assessed using the AJCC/CAP TRG system.31 The 4 categories of AJCC/CAP TRG system were classified as grade 0 (complete response), grade 1 (moderate response), grade 2 (minimal response), and grade 3 (poor response). All imaging, surgical, and pathology reports were assessed by independent masked central review.
Statistical Analysis
The use of a noninferiority margin of 1·6 for the hazard ratio and a type I error of 5 percent ensured 80 percent power to show noninferiority between the nCT and nCRT group. On the basis of the previous studies,7,32 assuming a 3-year local-regional failure-free survival of 93% for the nCRT group and allowing ~5 percent of patients to be excluded from the per-protocol population, an enrollment of 650 patients was planned.
The initial results in this report mainly focus on the pathologic findings and safety profiles of this trial. The analysis of these outcomes will be performed using the χ2 test and with 95% confidence interval of the difference between the 2 proportions. Categorical variables were compared using the χ2 or Fisher exact test, and continuous variables were compared using the t-test. A 2-sided P value <0·05 indicated statistical significance. Subgroup analysis of patients with tumors located within 5 cm from the anal verge was performed. All statistical analyses were performed using the SPSS software ( version 24·0; SPSS). A detailed description of the statistical analysis plan is provided as a supplemental file (Supplemental Digital Content 2, http://links.lww.com/SLA/E395).
RESULTS
From June 1, 2014, to October 1, 2020, 663 patients at 21 centers were recruited and randomly assigned to the nCT (n=331) or nCRT (n=332) group. Seventy-four patients were excluded, of whom 50 (7·5%) withdrew consent after enrollment, 18 (2.7%) violated the study protocol, and 6 (0·9%) did not meet the inclusion criteria. The remaining patients in the nCT (n=300) and nCRT (n=289) groups were included in the modified intention-to-treat (mITT) population (Fig. 2). Their baseline characteristics were well-balanced (Table 1).
FIGURE 2.
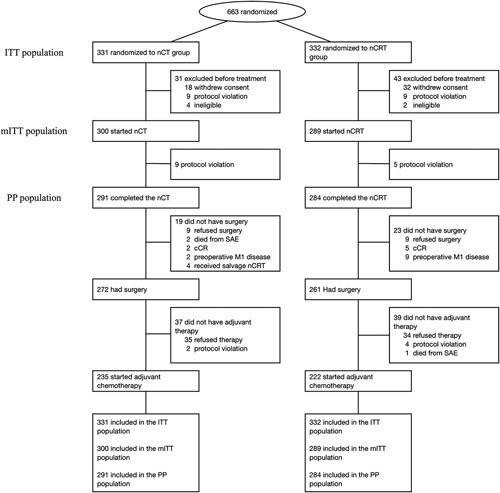
Trial profile. The ITT population comprised all patients who were were randomized to treatment. The mITT population comprised all patients who were randomized to treatment and received at least 1 dose of study treatment. The PP population comprised of patients who completed the neoadjuvant therapy without major protocol deviations. Ccr indicates clinical complete response; ITT, intention-to-treat; mITT, modified intention-to-treat; nCRT, neoadjuvant radiochemotherapy; nCT, neoadjuvant chemotherapy; PP, per-protocol; SAE, serious adverse event.
TABLE 1.
Baseline Demographic and Clinical Characteristics in the mITT Population
| Treatment group, No. (%) | ||
|---|---|---|
| Characteristics | Neoadjuvant chemotherapy (n=300) | Neoadjuvant chemoradiotherapy (n=289) |
| Age, years | ||
| Median(range) | 60 (31-75) | 60 (28-75) |
| Sex | ||
| Male | 188 (62·7) | 177 (61·2) |
| Female | 112 (37·3) | 112 (38·8) |
| Clinical T category | ||
| cT2 | 16 (5·3) | 11 (3·8) |
| cT3 | 201 (67·0) | 202 (69·9) |
| cT4a | 83 (27·7) | 76 (26·3) |
| Clinical N category | ||
| cN0 | 92 (30·7) | 77 (26·7) |
| cN1 | 147 (49·0) | 133 (46·0) |
| cN2 | 61 (20·3) | 79 (27·3) |
| Distance from the anal verge | ||
| >10 cm | 10 (3·3) | 8 (2·8) |
| 5-10 cm | 166 (55·3) | 163 (56·4) |
| ≤5 cm | 124 (41·3) | 118 (40·8) |
| EMVI by MRI | ||
| Positive | 52 (17·3) | 63 (21·8) |
| Negative | 248 (82·7) | 226 (78·2) |
| Lateral lymph node by MRI | ||
| Positive | 27 (9·0) | 36 (12·5) |
| Negative | 273 (91·0) | 253 (87·5) |
EMVI indicates extramural venous invasion; mITT, modified intention-to-treat.
In the nCT group, 300 patients received at least 1 dose of nCT, 291 (97%) completed the nCT without major protocol deviations, and 272 (90·7%) underwent TME surgery. The median time between randomization and surgery was 16 weeks (IQR 14·7–18·1). Two patients in the nCT group achieved cCR and were managed with the watch-and-wait approach. Four patients received salvage nCRT because of local disease progression. In the nCRT group, 289 patients received at least 1 dose of nCRT, 284 (98.3%) completed the nCT without major protocol deviations, and 261 (90·3%) underwent TME surgery. The median time between randomization and surgery was 16·3 weeks (IQR 14·3–19·1). Five patients in the nCRT group achieved cCR and were managed with the watch-and-wait approach.
Table 2 shows the pathologic findings of the 2 groups. The pCR rate in the nCT group and nCRT group was 11·0% (95% CI, 7·8-15·3%) and 13·8% (95% CI, 10·1-18·5%) (RR: 1.140, 95% CI, 0.8863-1.541; P=0·33). Their corresponding downstaging (ypStage 0 to 1) rates were 40.8% (95% CI, 35·1-46·7%) and 45·6% (95% CI, 39·7-51·7%) (RR: 1.101, 95% CI, 0.9305-1.310; P=0·27), and TRG 0-1 rate were 23·2% and 36·8% (P < 0·001), respectively. The perioperative distant metastases (metastases identified before or during surgery) rate of the nCT group was lower than the nCRT group (0·7% vs. 3·1%; P=0·03). Similar results were observed in the subgroup of patients with tumors located within 5 cm from the anal verge (eTable 1, Supplemental Digital Content 3, http://links.lww.com/SLA/E396).
TABLE 2.
Pathological Findings
| Treatment Group, No. (%) | |||
|---|---|---|---|
| Variable | Neoadjuvant chemotherapy (n=272) | Neoadjuvant Chemoradiotherapy (n=261) | P |
| Pathologic T category | — | — | 0·524 |
| ypT0 | 32 (11·8) | 36 (13·8) | — |
| ypTis | 1 (0·4) | 3 (1·1) | — |
| ypT1 | 15 (5·5) | 11 (4·2) | — |
| ypT2 | 73 (26·8) | 76 (29·1) | — |
| ypT3 | 110 (40·4) | 107 (41·0) | — |
| ypT4 | 41 (15·1) | 28 (10·7) | — |
| Pathologic N category | — | — | 0·038 |
| ypN0 | 200 (73·5) | 214 (82·0) | — |
| ypN1 | 62 (22·8) | 37 (14·2) | — |
| ypN2 | 10 (3·7) | 10 (3·8) | — |
| Pathologic complete response | — | — | 0·333 |
| Yes | 30 (11·0) | 36 (13·8) | — |
| No | 242 (89·0) | 225 (86·2) | — |
| ypT0-2N0M0 | — | — | 0·265 |
| Yes | 111 (40·8) | 119 (45·6) | — |
| No | 161 (59·2) | 142 (54·4) | — |
| Tumor regression grade | — | — | <0·001 |
| TRG 0 | 30 (11·0) | 36 (13·8) | — |
| TRG-1 | 33 (12·1) | 60 (23·0) | — |
| TRG-2 | 98 (36·0) | 103 (39·5) | — |
| TRG-3 | 111 (40·8) | 58 (22·2) | — |
| Missing | 0 | 4 (1·5) | — |
| TRG 0-1 | — | — | <0·001 |
| Yes | 63 (23·2) | 96 (36·8) | — |
| No | 209 (76·8) | 161 (61·7) | — |
| Missing | 0 | 4 (1·5) | — |
In regard to patients who underwent TME, their R0 resection rate was similar between the 2 treatment groups (nCT vs. nCRT, 99·6% vs. 99·6%, P>0·99; Table 3). The rate of preventive ileostomy in the nCT group was lower than in the nCRT group (52·2% vs. 63·6%; P=0·008). Similar sphincter preservation rate was observed in the 2 groups (nCT vs. nCRT, 94·9% vs. 94·3%; P= 0·76) and the subgroup of patients with tumors located within 5 cm from the anal verge (nCT vs. nCRT, 88·0% vs. 88·6%; P=0·89; eTable 2, Supplemental Digital Content 3, http://links.lww.com/SLA/E396). The difference in postoperative complications, including anastomotic leak and abscess, was of marginal significance between the 2 groups (18·8% vs. 25·7%; P=0·05).
TABLE 3.
Summary of Surgical Outcomes
| Treatment Group, No. (%) | |||
|---|---|---|---|
| Variable | Neoadjuvant chemotherapy (n=272) | Neoadjuvant chemoradiotherapy (n=261) | P |
| Surgical procedures | — | — | 0·477 |
| Low anterior resection | 251 (92·3) | 234 (89·7) | — |
| Abdominoperineal resection | 14 (5·1) | 15 (5·7) | — |
| Intersphincteric resection | 6 (2·2) | 8 (3·1) | — |
| Others | 1 (0·4) | 4 (1·5) | — |
| Sphincter preservation | — | — | 0·760 |
| Yes | 258 (94·9) | 246 (94·3) | — |
| No | 14 (5·1) | 15 (5·7) | — |
| Preventive diverting ileostomy | — | — | 0·008 |
| Yes | 142 (52·2) | 166 (63·6) | — |
| No | 130 (47·8) | 95 (36·4) | — |
| Resection limits | — | — | >0·99 |
| R0 | 271 (99·6) | 260 (99·6) | — |
| R1 | 1 (0·4) | 1 (0·4) | — |
| Postoperative morbidity (≤30 d) | 51 (18·8) | 67 (25·7) | 0·054 |
| Anastomotic leak | 16 (5·9) | 16 (6·1) | 0·913 |
| Clinical fistula | 0 | 4 (1·5) | 0·057 |
| Abscess | 5 (1·8) | 5 (1·9) | >0·99 |
| Bowel obstruction | 8 (2·9) | 5 (1·9) | 0·577 |
| Intestinal function disorder | 16 (5·9) | 19 (7·3) | 0·515 |
| Septicemia | 1 (0·4) | 2 (0·8) | 0·617 |
| Wound infection | 14 (5·1) | 23 (8·8) | 0·096 |
| Urinary complications | 3 (1·1) | 4 (1·5) | 0·720 |
| Others | 5 (1·8) | 8 (3·1) | 0·410 |
| Postoperative mortality (≤60 d) | 0 | 1 (0·4) | 0·490 |
Of the mITT patients, 259 patients (86·3%) in the nCT group and 263 patients (91·0%) in the nCRT group received the full dose of nCT or radiation (P=0·07). The incidence of adverse events during neoadjuvant therapy was similar in the 2 groups (Table 4; eTable 3 and eTable 4 in the Supplement, Supplemental Digital Content 3, http://links.lww.com/SLA/E396). Grade 3 to 4 toxicities occurred in 37 patients (12·3%) in the nCT group and 24 patients (8·3%) in the nCRT group (P=0·11). The most common grade 3 to 4 toxicities were leukopenia, thrombocytopenia and anemia. The rates of grade 3 to 4 leukopenia (nCT vs. nCRT, 3·0% vs. 4·8%), thrombocytopenia (nCT vs. nCRT, 5·3% vs. 1·0%), and anemia (nCT vs. nCRT, 2·3% vs. 1·0%) did not differ significantly between two the groups.
TABLE 4.
Safety Summary During Neoadjuvant Therapy
| Treatment Group, No. (%) | ||||
|---|---|---|---|---|
| Neoadjuvant chemotherapy (n=300) | Neoadjuvant chemoradiotherapy (n=289) | |||
| Event | Grade 1-2 | Grade 3-4 | Grade 1-2 | Grade 3-4 |
| Any event | 174 (58·0) | 37 (12·3) | 163 (56·4) | 24 (8·3) |
| Hematologic | ||||
| Leukopenia | 95 (31·7) | 9 (3·0) | 114 (39·4) | 14 (4·8) |
| Anemia | 44 (14·6) | 7 (2·3) | 43 (14·9) | 3 (1·0) |
| Thrombocytopenia | 43 (14·3) | 16 (5·3) | 18 (6·2) | 3 (1·0) |
| GI | ||||
| Nausea | 75 (25·0) | 3 (1·0) | 38 (13·1) | 0 |
| Vomiting | 43 (14·3) | 1 (0·3) | 24 (8·3) | 0 |
| Diarrhea | 12 (4·0) | 4 (1·3) | 20 (6·9) | 1 (0·3) |
| Laboratory | ||||
| Aminotransferase | 28 (9·3) | 2 (0·7) | 26 (9·0) | 0 |
| Alkaline phosphatase | 5 (1·7) | 0 | 5 (1·7) | 0 |
| Bilirubin | 8 (2·7) | 0 | 22 (7·6) | 3 (1·0) |
| Genitourinary | 0 | 0 | 10 (3·5) | 3 (1·0) |
| Neurological | 63 (21·0) | 3 (1·0) | 18 (6·2) | 0 |
| Cardiac | 3 (1·0) | 1 (0·3) | 0 | 0 |
| Mucositis | 0 | 0 | 0 | 0 |
| Allergic reaction | 0 | 0 | 0 | 0 |
| Palmar-plantar erythrodysaesthesia | 12 (4·0) | 1 (0·3) | 19 (6·6) | 1 (0·3) |
| Alopecia | 4 (1·3) | 0 | 0 | 0 |
| Other events | 11 (3·7) | 0 | 0 | 0 |
GI indicates gastrointestinal.
In the neoadjuvant phase, 2 deaths were observed in the nCT group, of whom 1 died from serve infection during neoadjuvant therapy, and the other died from unexplained sudden death during neoadjuvant therapy. One patient in the nCRT group died from multiple organ dysfunction caused by anastomotic leakage after surgery.
Among the patients who underwent surgery, 235 (86·4%) of 272 patients in the nCT group and 222 (85·0%) of 261 patients in the nCRT group received adjuvant chemotherapy (P=0·69). One patient in the nCT group with postoperative pathologically confirmed positive margins were given adjuvant radiotherapy and chemotherapy. The reasons for not undergoing adjuvant chemotherapy were as follows: 35 patients (13·0%) refused adjuvant chemotherapy and 2 patients (0·7%) did not receive the allocated adjuvant chemotherapy in the nCT group. And 34 patients (13·0%) refused the therapy, four patients (1·5%) did not receive the allocated adjuvant chemotherapy, and 1 patient (0·4%) died from SAE in the nCRT group. In addition, full-dose adjuvant chemotherapy was administered to 52·8% and 44·1% of the patients in the nCT and nCRT group (P=0·07; eTable 5, Supplemental Digital Content 3, http://links.lww.com/SLA/E396).
DISCUSSION
The preliminary results from the CONVERT trial demonstrated that for patients with MRF-negative LARC, neoadjuvant chemotherapy with CAPOX achieved similar downstaging rate and pCR rate, and was associated with lower risk of perioperative metastasis and preventive ileostomy compared with nCRT with capecitabine. These results suggest nCT with CAPOX alone as an effective alternative treatment to conventional nCRT in LARC with uninvolved MRF.
Previous studies explored the possibility of avoiding routine pelvic radiation by using systemic chemotherapy for LARC.17–19 Schrag and colleagues first reported a pilot study using nCT (FOLFOX + bevacizumab) for highly selected LARC. In their study, patients with cT4 diseases, MRF threatened, and fixed or deemed unresectable tumor before neoadjuvant therapy were excluded. They found that nCT demonstrated promising results with a pCR rate of 25%, good downstaging rate of 56·3%, 4-year local recurrence rate of 0%, and 4-year disease-free survival of 84%.18 Similarly, the GEMCAD 0801 study recruited patients with cT3 and MRF-negative LARC and observed a pCR rate of 20% with nCT (CAPOX + bevacizumab).17 The FOWARC study compared nCT with mFOLFOX6 regimens to fluorouracil-radiotherapy as neoadjuvant therapy for unselected LARC. Similar successful downstaging rate (35·5% vs. 37·1%) was observed between the 2 groups. However, the pCR rate was much lower in the chemotherapy group (6·6% vs. 14·0%).33 Likewise, the CORONA I study used the CAPOX regimen as neoadjuvant therapy for unselected LARC, and the patients achieved a pCR rate of 12% and a good downstaging rate of 29·3%. In this present study, the downstaging (40·8% vs. 45·6%) and pCR (11·0% vs. 13·8%) rates of nCT were similar to nCRT. This wide range of pCR rates in different studies could be due to the different criteria in patient selection. In a study by Schrag et al,18 their patient population was highly selected and demonstrated the highest pCR rate. Although promising, the complexity of patient selection might compromise the application of the new treatment regimens. On the other hand, the patient population in the FOWARC study was unselected, resulting in the lowest pCR rate.33 The inclusion of high-risk and aggressive patients such as MRF invasion and cT4b might inevitably increase postoperative chemoradiation and even compromise the long-term outcome.
The current study balanced the complexity of the patient selection with the likelihood of benefit from treatment by using MRF involvement as the selection criteria. Although the pCR rate was not as high as in the study of Schrag and colleagues, only a small number of patients needed salvage nCRT due to tumor progression during nCT. The favorable results were probably due to the selection of patients with uninvolved MRF, which was reported to be associated with better prognosis compared with involved MRF. Data from the MERCURY trial showed a worse DFS in patients with involved MRF than the uninvolved MRF.34 Furthermore, Yamamoto et al reported that MRF involvement was associated with increased LR after neoadjuvant chemotherapy alone, compared with neoadjuvant CRT, suggesting that omission of radiation in patients with involved MRF should be cautious.28–30 By excluding patients with MRF, we were able to select a subgroup of patients who are more likely to benefit from neoadjuvant chemotherapy. A similar design has also been implemented in the ongoing trial PROSPECT (NCT01515787) and RuCorT-02 (NCT04134897).
Of note, the perioperative distant metastasis (metastases identified before or during surgery) rate was significantly lower in nCT group (0·7% vs. 3·1%). Previous studies demonstrated that perioperative metastatic rate varied from 4·0% to 7·7% in patients receiving conventional fluorouracil-based nCRT, consistent with the rate in the nCRT group in this current trial.3,35–37 The substantial early decrease in distant metastasis supports the hypothesis that early application of systemic therapy could decrease the risk of distant metastases.
Besides the promising pathologic outcomes observed, nCT alone also resulted in similar R0 resection (99·6% vs. 99·6%, P>0·99), sphincter preservation rates (94·9% vs. 94·3%, P=0·76), lower preventive diverting ileostomy rate (52·2% vs. 63·6%, P=0·008), and similar postoperative complication rate (18·8% vs. 25·7%, P=0·05) compared with nCRT, which were consistent with previous reports.29,33 These findings provide further evidence supporting the safety and good compliance of the nCT.
Initially, patients with tumors located within 5cm from the anal verge were not enrolled in the study in consideration of the higher risk of MRF involvement if the tumor progressed to the lower rectum and the lack of data on the efficacy of nCT in low rectal cancer. After the FOWARC study reported that tumor location did not impact the response to chemotherapy, the protocol of this trial was revised to also enroll patients with tumors located within 5cm from the anal verge.5 Subgroup analysis of this current study reinforced the findings of previous reports suggesting that low rectal cancer achieved similar short-term pathologic outcomes in the upper-rectum and mid-rectum. However, it should be noted that for patients with very low rectal cancer, when the goal of treatment is organ preservation, nCT alone seems much less effective in achieving clinical complete response compared with the TNT approach and should not be considered for this subgroup of patients.
Although the TNT approach significantly improved DFS and increased organ preservation rate, its application in all patients with LARC or reserved for patients with high-risk factors or very low rectal cancer remains debatable. Since the TNT approach was associated with increased toxicities for patients without high risk of recurrence, there is still urgent need to explore novel strategies to reduce toxicities and improve long-term functional outcomes. Findings from this study and previous reports suggest that neoadjuvant chemotherapy is a promising approach, especially in patients without high-risk factors.17,18 nCT alone, with selective use of radiation reserved for nonresponders, might be a treatment of choice for LARC without high-risk factors.
The present study had several limitations. First, ~11% of the enrolled patients did not receive the assigned treatment and were excluded from the mITT population, which may have led to some unaccountable biases. However, the baseline characteristics were still well-balanced between the 2 groups. Second, it remains unclear whether our findings can be extrapolated to cT4b and MRF+ tumors because such patients were not included in this study.
In conclusion, nCT with CAPOX alone achieved similar pCR, downstaging, and R0 resection rates to conventional nCRT in LARC with uninvolved MRF. In addition, nCT was also associated with reduced risk of perioperative metastases, preventive diverting ileostomy, and postoperative complications. Thus, nCT with CAPOX could be a potential alternative to nCRT in LARC patients without MRF. Nevertheless, these findings are preliminary, and long-term follow-up is required for further confirmation.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Prof. Ji-Bin Li for providing the necessary writing assistance and statistical support for this research.
Footnotes
W.-J.M., X.-Z.W., Y.-F.L., Y.-M.S., C.-K.Y., and J.-Z.L. contributed equally to this work.
P.-R.D. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. W.-J.M., X.-Z.W., Y.-F.L., Y.-M.S., C.-K.Y., and J.-Z.L. are co–first authors. W.-J.M. and P.-R.D. had overall responsibility for the analyses and writing of the article. Z.-Z.W., Y.-F.L., Y.-M.S., C.-K.Y., J.-Z.L., Z.-Z.P., and P.-R.D. verified the data. All authors contributed to the study design, provided data and contributed to data interpretation, writing, and editing of the report, and approved the final version. All authors had access to all the anonymized data reported in the study and P.-R.D. had final responsibility for the decision to submit for publication.
The study data will be available following publication to researchers wishing to do meta-analyses or for other research proposals, subject to approval and agreement from the study sponsor (Pei-Rong Ding). Related documents (eg, study protocol and informed consent forms) will also be made available. Requests should be made to dingpr@sysucc.org.cn
Presented at the ESMO CONGRESS 2021, Paris, Virtual, France, 16 - 21 Sep 2021.
The study was funded by Sun Yat-Sen University Clinical Research 5010 Program (grant number 2014013), the National Natural Science Foundation of China (82073159 and 81871971), and the Guangdong Basic and Applied Basic Research Foundation (2020A1515110544). The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Pei-Rong Ding Honoraria: Roche, MSD, Sanofi, Medtronic, Johnson & Johnson Consulting or Advisory role: BGI Genomics. The remaining authors state that they have no proprietary interest in the products named in this article.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.annalsofsurgery.com.
Contributor Information
Wei-Jian Mei, Email: meiwj@sysucc.org.cn.
Xiao-Zhong Wang, Email: 103776710@QQ.COM.
Yun-Feng Li, Email: 13330445776@qq.com.
Yue-Ming Sun, Email: sunym@njmu.edu.cn.
Chun-Kang Yang, Email: chuck330@163.com.
Jun-Zhong Lin, Email: linjzh@sysucc.org.cn.
Zu-Guang Wu, Email: wuzg1913@163.com.
Rui Zhang, Email: 648397384@qq.com.
Wei Wang, Email: wangwei16400@163.com.
Yong Li, Email: liyong@gdph.org.cn.
Ye-Zhong Zhuang, Email: zhuangyz118@163.com.
Jian Lei, Email: 13903070123@163.com.
Xiang-Bin Wan, Email: wxbzlyy@126.com.
Ying-Kun Ren, Email: 18903839515@163.com.
Yong Cheng, Email: chengyongcq@aliyun.com.
Wen-Liang Li, Email: liwenliang@kmmu.edu.cn.
Zi-Qiang Wang, Email: wangziqiang@scu.edu.cn.
Dong-Bo Xu, Email: xdb2292388@sina.com.
Xian-Wei Mo, Email: Moxianwei@gxmu.edu.cn.
Hai-Xing Ju, Email: juhx@zjcc.org.cn.
Sheng-Wei Ye, Email: yeshengweioncolo@163.com.
Jing-Lin Zhao, Email: 403612238@qq.com.
Hong Zhang, Email: haojiubujian1203@sina.cn.
Yuan-Hong Gao, Email: gaoyh@sysucc.org.cn.
Zhi-Fan Zeng, Email: zengzhifan@sysucc.org.cn.
Wei-Wei Xiao, Email: xiaoww@sysucc.org.cn.
Xiao-Peng Zhang, Email: 15875487648@126.com.
Xuan Zhang, Email: zhangxuan66@kmmu.edu.cn.
E Xie, Email: 13502999914@139.com.
Yi-Fei Feng, Email: fengyifei1982@163.com.
Jing-Hua Tang, Email: tangjh@sysucc.org.cn.
Xiao-Jun Wu, Email: wuxj@sysucc.org.cn.
Gong Chen, Email: chengong@sysucc.org.cn.
Li-Ren Li, Email: lilr@sysucc.org.cn.
Zhen-Hai Lu, Email: luzhh@sysucc.org.cn.
De-Sen Wan, Email: wands@sysucc.org.cn.
Jin-Xin Bei, Email: beijx@sysucc.org.cn.
Zhi-Zhong Pan, Email: panzhzh@sysucc.org.cn.
Pei-Rong Ding, Email: dingpr@sysucc.org.cn.
REFERENCES
- 1.Benson AB, Venook AP, Al-Hawary MM, et al. Rectal cancer, Version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:874–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glynne-Jones R, Wyrwicz L, Tiret E, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv22–iv40. [DOI] [PubMed] [Google Scholar]
- 3.Conroy T, Bosset JF, Etienne PL, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:702–715. [DOI] [PubMed] [Google Scholar]
- 4.Bahadoer RR, Dijkstra EA, van Etten B, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:29–42. [DOI] [PubMed] [Google Scholar]
- 5.Deng Y, Chi P, Lan P, et al. Neoadjuvant modified FOLFOX6 with or without radiation versus fluorouracil plus radiation for locally advanced rectal cancer: final results of the Chinese FOWARC Trial. J Clin Oncol. 2019;37:3223–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peeters KC, Marijnen CA, Nagtegaal ID, et al. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246:693–701. [DOI] [PubMed] [Google Scholar]
- 7.van Gijn W, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575–582. [DOI] [PubMed] [Google Scholar]
- 8.Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926–1933. [DOI] [PubMed] [Google Scholar]
- 9.Silva VR, Santos LS, Dias RB, et al. Emerging agents that target signaling pathways to eradicate colorectal cancer stem cells. Cancer Commun. 2021;41:1275–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diaz Beveridge R, Akhoundova D, Bruixola G, et al. Controversies in the multimodality management of locally advanced rectal cancer. Med Oncol. 2017;34:102. [DOI] [PubMed] [Google Scholar]
- 11.Tevis SE, Kohlnhofer BM, Stringfield S, et al. Postoperative complications in patients with rectal cancer are associated with delays in chemotherapy that lead to worse disease-free and overall survival. Dis Colon Rectum. 2013;56:1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breugom AJ, van Gijn W, Muller EW, et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: a Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann Oncol. 2015;26:696–701. [DOI] [PubMed] [Google Scholar]
- 13.Diao F, Cai S. Aspirin-based chemoprevention of colorectal cancer: The role for gut microbiota. Cancer Commun. 2020;40:633–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network. Rectal cancer. Version 2; 2021. Available at: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Accessed September 10, 2021.
- 15.Kosmala R, Fokas E, Flentje M, et al. Quality of life in rectal cancer patients with or without oxaliplatin in the randomised CAO/ARO/AIO-04 phase 3 trial. Eur J Cancer. 2021;144:281–290. [DOI] [PubMed] [Google Scholar]
- 16.Kim SH, Kim JH, Jung SH. Late complications after proctectomy in rectal cancer patients who underwent radiotherapy. World J Surg. 2014;38:2471–2476. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Martos C, Brown G, Estevan R, et al. Preoperative chemotherapy in patients with intermediate-risk rectal adenocarcinoma selected by high-resolution magnetic resonance imaging: the GEMCAD 0801 Phase II Multicenter Trial. Oncologist. 2014;19:1042–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schrag D, Weiser MR, Goodman KA, et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol. 2014;32:513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bensignor T, Brouquet A, Dariane C, et al. Pathological response of locally advanced rectal cancer to preoperative chemotherapy without pelvic irradiation. Colorectal Dis. 2015;17:491–498. [DOI] [PubMed] [Google Scholar]
- 20.He F, Yu L, Ding Y, et al. Effects of neoadjuvant chemotherapy with or without intensity-modulated radiotherapy for patients with rectal cancer. Cancer Sci. 2020;111:4205–4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andre T, Meyerhardt J, Iveson T, et al. Effect of duration of adjuvant chemotherapy for patients with stage III colon cancer (IDEA collaboration): final results from a prospective, pooled analysis of six randomised, phase 3 trials. Lancet Oncol. 2020;21:1620–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomida A, Uehara K, Hiramatsu K, et al. Neoadjuvant CAPOX and bevacizumab alone for locally advanced rectal cancer: long-term results from the N-SOG 03 trial. Int J Clin Oncol. 2019;24:403–410. [DOI] [PubMed] [Google Scholar]
- 23.Nishimura J, Hasegawa J, Kato T, et al. Phase II trial of capecitabine plus oxaliplatin (CAPOX) as perioperative therapy for locally advanced rectal cancer. Cancer Chemother Pharmacol. 2018;82:707–716. [DOI] [PubMed] [Google Scholar]
- 24.Ueki T, Manabe T, Inoue S, et al. A feasibility study of neoadjuvant XELOX without radiotherapy for locally advanced lower rectal cancer. Anticancer Res. 2016;36:741–747. [PubMed] [Google Scholar]
- 25.Kamiya T, Uehara K, Nakayama G, et al. Early results of multicenter phase II trial of perioperative oxaliplatin and capecitabine without radiotherapy for high-risk rectal cancer: CORONA I study. Eur J Surg Oncol. 2016;42:829–835. [DOI] [PubMed] [Google Scholar]
- 26.Manatakis DK, Gouvas N, Souglakos J, et al. Neo-adjuvant chemotherapy alone for the locally advanced rectal cancer: a systematic review. Int J Clin Oncol. 2020;25:1570–1580. [DOI] [PubMed] [Google Scholar]
- 27.Smith CA, Kachnic LA. Evolving treatment paradigm in the treatment of locally advanced rectal cancer. J Natl Compr Canc Netw. 2018;16:909–915. [DOI] [PubMed] [Google Scholar]
- 28.de Paul TR, Augestad KM, Kiran RP, et al. Management of the positive pathologic circumferential resection margin in rectal cancer: A national cancer database (NCDB) study. Eur J Surg Oncol. 2021;47:296–303. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto T, Kawada K, Hida K, et al. Optimal treatment strategy for rectal cancer based on the risk factors for recurrence patterns. Int J Clin Oncol. 2019;24:677–685. [DOI] [PubMed] [Google Scholar]
- 30.Smith JJ, Garcia-Aguilar J. Advances and challenges in treatment of locally advanced rectal cancer. J Clin Oncol. 2015;33:1797–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edge B, Byrdholt D, Compton C, et al. AJCC cancer staging manual, 7th edition. New York: Springer: Colon and rectum; 2010. [Google Scholar]
- 32.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. [DOI] [PubMed] [Google Scholar]
- 33.Deng Y, Chi P, Lan P, et al. Modified FOLFOX6 with or without radiation versus fluorouracil and leucovorin with radiation in neoadjuvant treatment of locally advanced rectal cancer: initial results of the Chinese FOWARC multicenter, open-label, randomized three-arm phase III trial. J Clin Oncol. 2016;34:3300–3307. [DOI] [PubMed] [Google Scholar]
- 34.Taylor FG, Quirke P, Heald RJ, et al. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study. J Clin Oncol. 2014;32:34–43. [DOI] [PubMed] [Google Scholar]
- 35.Gerard JP, Azria D, Gourgou-Bourgade S, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol. 2010;28:1638–1644. [DOI] [PubMed] [Google Scholar]
- 36.Rodel C, Graeven U, Fietkau R, et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16:979–989. [DOI] [PubMed] [Google Scholar]
- 37.Aschele C, Cionini L, Lonardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29:2773–2780. [DOI] [PubMed] [Google Scholar]


