Purpose of review
Combined heart and liver transplantation (CHLT) is an uncommon but increasingly performed procedure with rising need as the population who has undergone Fontan palliation for single ventricle physiology grows. This article reviews the current literature to summarize what is known about patient selection and outcomes and highlights the questions that remain.
Recent findings
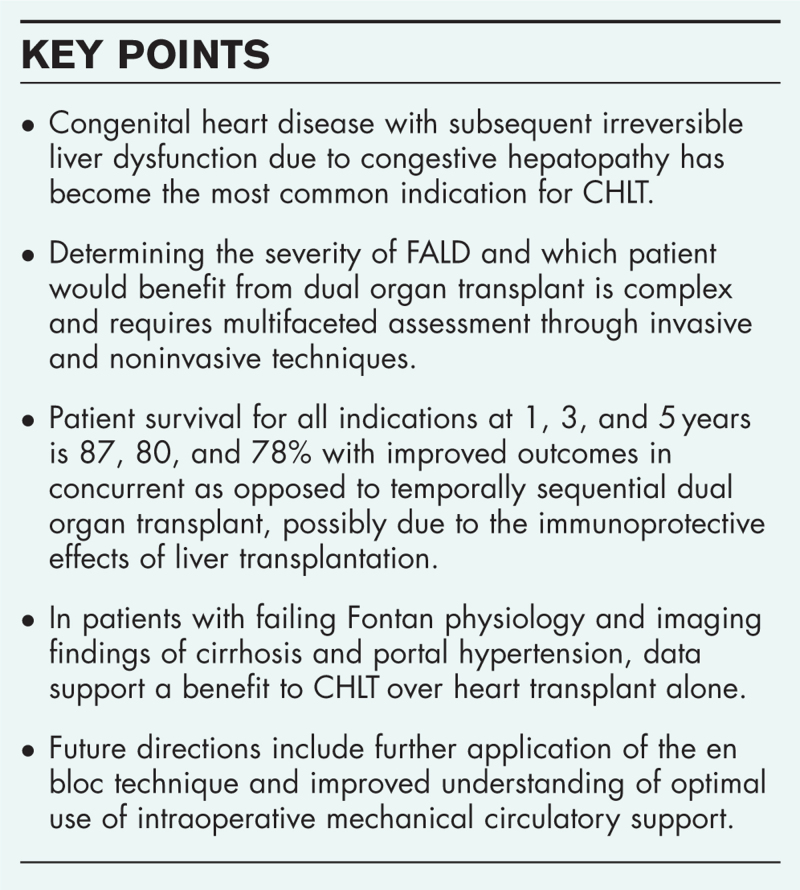
Congenital heart disease (CHD) with Fontan-associated liver disease (FALD) has surpassed noncongenital heart disease as the most common indication for CHLT. In patients with failing Fontan physiology, accurate assessment of recoverability of liver injury remains challenging and requires multifaceted evaluation to determine who would benefit from isolated versus dual organ transplantation. Patient survival has improved over time without significant differences between those with and without a diagnosis of CHD. En bloc surgical technique and best use of intraoperative mechanical circulatory support are topics of interest as the field continues to evolve.
Summary
A more refined understanding of appropriate patient selection and indication-specific outcomes will develop as we gain more experience with this complex operation and perform prospective, randomized studies.
Keywords: combined heart and liver transplant, congestive hepatopathy, Fontan-associated liver disease, mechanical circulatory support
INTRODUCTION
The first combined heart and liver transplantation (CHLT) was performed in 1984 in a 6-year-old girl to treat familial hypercholesterolemia and cardiovascular disease [1]. While implementation of this procedure has grown over time, CHLT represents a mere fraction of the total heart and liver transplants performed. To date, fewer than 500 patients have undergone CHLT since 1984 [2].
Nonetheless, CHLT is being increasingly performed, and indications for this complex procedure are evolving; in the United States, congenital, as opposed to noncongenital, heart disease has become the most common indication for CHLT [3–5,6▪,7]. With an estimated 70 000 patients worldwide who have undergone the Fontan procedure for single ventricle congenital heart disease, the incidence of Fontan-associated liver disease (FALD) necessitating CHLT will continue to rise [8]. We aim to review the major indications for CHLT, the complexity in the determination of liver dysfunction in patients with congestive hepatopathy, current outcomes of the procedure, and future directions of research to continually improve upon this rare and highly technical operation.
Box 1.
no caption available
INDICATIONS
There are three main categories of patients considered for CHLT:
-
(1)
Familial amyloid polyneuropathy
-
(2)
Primary liver disease and concurrent nonintervenable end-stage cardiac disease
-
(3)
End-stage cardiac disease and secondary liver disease
Familial amyloid polyneuropathy
Familial amyloid polyneuropathy is an inherited autosomal dominant condition characterized by cardiac and nervous system amyloid deposition, most commonly because of misfolded transthyretin protein [9]. The abnormal, amyloidogenic protein is synthesized by hepatocytes, and liver transplantation has been shown to stop progression of the disease [10]. CHLT is considered in those with moderate-to-severe restrictive cardiomyopathy or refractory conduction abnormalities [4]. Recent advances in molecular and pharmacologic therapies targeting transthyretin synthesis and tetramer stabilization are impacting this indication [11].
Primary liver disease and concurrent cardiac disease
There are multiple paths by which chronic liver disease occurs simultaneously with cardiac disease. First, diseases such as alcohol use disorder, metabolic syndrome, and less commonly hepatitis C or hemochromatosis, can simultaneously affect both the liver and heart [4,12]. Second, two unrelated disease processes, such as primary sclerosing cholangitis and ischemic cardiomyopathy, may cause dual organ failure.
Finally, liver disease alone can lead to heart disease through development of cirrhotic cardiomyopathy, present in up to 50% of patients with end-stage liver disease [13]. In these patients, cardiomyopathy occurs because of the hemodynamic and autonomic changes in cirrhosis where cardiac output increases to overcome central hypovolemia from portal hypertension and splanchnic arterial vasodilation. These changes over time can lead to end-stage diastolic and systolic dysfunction necessitating heart transplant [14,15].
End-stage cardiac disease with secondary liver disease
The pathophysiology of FALD is complex, and a multifaceted approach to determining severity of liver disease is required.
Physiology of Fontan-associated liver disease
Liver dysfunction secondary to cardiac-related congestive hepatopathy is most commonly seen in individuals with congenital univentricular physiology who have undergone a Fontan procedure. The Fontan procedure diverts systemic venous return directly to the pulmonary arteries thus isolating the single functional ventricle to supply oxygenated blood to the systemic circulation. This reconstruction results in passive, nonpulsatile hepatic venous drainage leading to hepatic congestion [13,16]. End organ damage is thought to result from a combination of rising central venous pressure, worsening chronic low cardiac output, and increasing pulmonary shunt-related hypoxia resulting from progressive failure of the Fontan [8].
Hepatic fibrosis universally develops in patients with Fontan physiology [8]. However, it is difficult to determine the degree of irreversible liver dysfunction in FALD and therefore which patient would benefit from dual organ transplant. The difficulty lies in the lack of specific laboratory markers and imaging tools to accurately assess the severity of fibrosis [8,17].
Prognostic scores
Liver function tests are typically normal or modestly abnormal, and a significant proportion of patients with advanced cardiac disease are therapeutically anticoagulated making the international normalized ratio (INR) an unreliable measure of synthetic function [4,17]. Other metrics to assess liver disease include the MELD-XI score, which excludes INR and the VAST score (Varices, Ascites, Splenomegaly, and Thrombocytopenia), which focuses on features of portal hypertension. Initially described by Elder et al.[18] in 2013, a VAST score at least 2 was associated with multifold increased risk for major adverse event in Fontan patients including need for heart transplant, development of hepatocellular carcinoma (HCC), or death [18]. Finally, a strong correlation exists between elevated central venous pressures indicative of Fontan failure and clinically significant FALD [19▪▪].
Pathologic changes associated with congestive hepatopathy
Liver biopsy remains the gold standard to assess for hepatic fibrosis and cirrhosis. In congestive hepatopathy, elevated hepatic venous pressure leads to central hepatic vein and sinusoid dilation [14,17]. Concurrently, decreased cardiac output results in reduced hepatic perfusion. The combination of these two features in failing Fontan physiology yields a re-distribution of oxygenated blood favoring periportal hepatocytes, leading to atrophy and eventually necrosis of centrilobular hepatocytes [17]. This pattern of injury leads to collagen deposition and centrilobular fibrosis, which can extend to the periportal zone [14].
To assess the accuracy of liver biopsy, Vaikunth et al. compared preoperative biopsy findings to explanted livers in patients who had undergone CHLT. They found that pretransplant liver biopsy underestimated degree of fibrosis in 40% (6 of 15) of patients, highlighting the risk of sampling error because of the heterogeneous nature of fibrosis in FALD [16].
Elastography may be a useful tool to track progression of liver stiffness over time; however, it is limited in distinguishing between hepatic congestion and fibrosis and may be affected by ascites [8,17].
Hepatocellular carcinoma
Abdominal imaging for HCC screening in patients with FALD is hampered by the presence of regenerative nodules, which can falsely mimic radiographic features of HCC [8,17]. A recent, international multicenter case series of 54 Fontan patients with HCC found a median age at time of diagnosis of 30 years old and poor overall prognosis with a 50% 1-year survival rate. At present, there are no standardized surveillance protocols in place, although twice-yearly alpha-fetoprotein (AFP) and abdominal imaging are recommended by some [20].
Pathology to inform patient selection
Experience in other disease processes, such as non-alcoholic steatohepatitis (NASH) or active hepatitis C (HCV) infection, shows that up to bridging fibrosis (F3) can regress with correction of the offending insult, such as weight loss after bariatric surgery for NASH or cure of HCV viremia [21]. As such, Izzy et al.[21] recommend caution toward dual organ transplantation for those with F3 fibrosis and would advocate for CHLT in those with pathologically confirmed cirrhosis (F4) or evidence of portal hypertension. Others argue for multidisciplinary consideration of CHLT in those with F3 fibrosis given increased risk of postoperative complications in these patients undergoing heart transplant alone [22].
OUTCOMES
To date, CHLT outcomes have been analyzed in a retrospective fashion at both the single-institution and national level. The majority of studies report data for all indications of CHLT without granularity to distinguish disease-specific outcomes.
Studies analyzing UNOS registry data through 2020 describe the current, national landscape of CHLT with improvement in patient survival over time [6▪,23,24]. Analysis of 364 recipients who underwent CHLT shows overall survival of 86.8, 80.1, and 77.9% at 1, 3, and 5 years, respectively [6▪]. Although indications for CHLT have changed over time with a predominance of congenital heart disease in the modern era, interestingly, there was no difference in survival when stratified by cardiac diagnosis.
Further multivariate analysis demonstrated that recipient diabetes and a sequential liver-first approach were independently associated with an increased risk of mortality [6▪]. Higher donor left ventricular ejection fraction was associated with decreased risk of mortality. Lee et al.[23] compared outcomes from before and after the change in cardiac allograft allocation system in 2006 finding improved allograft and overall survival in the post-2006 era. These data inform selection considerations for both the recipient and the donor and favor a heart-first intraoperative approach compared with liver-first sequence.
When specifically evaluating the outcomes of patients with CHD, Cotter et al. found no difference in 1-year or 5-year survival between patients with CHD who underwent heart transplant alone versus CHLT and no difference between patients with or without CHD who underwent CHLT. There was a trend towards improved survival for CHLT performed at high-volume versus low-volume centers with an average 9.5 and 1.6 transplants performed over the 11-year study period, respectively [24].
Sganga et al. describe their single-center experience with pediatric and young adult Fontan patients undergoing CHLT. In their cohort of 47 patients with Fontan physiology, 9 underwent CHLT and 38 underwent heart transplant alone. In this population, indication for CHLT included imaging or biopsy evidence of cirrhosis and portal hypertension; all CHLT were performed via the en bloc technique. Over a median follow-up period of 17 months, overall mortality in their cohort was 17% with no statistically significant difference in mortality or rate of postoperative complications between CHLT and heart transplant alone. To delineate, which Fontan patients would benefit from CHLT, the heart transplant-alone cohort was further analyzed. Of the 32 patients who underwent heart transplant alone and had imaging at time of listing, 10 had imaging findings of cirrhosis and did not undergo CHLT. They had a significantly lower estimated 1-year survival of 67% compared with 89% in both the heart transplant without cirrhosis and CHLT groups. These patients demonstrated worse postoperative outcomes with increased risk of an unplanned surgical procedure and a trend towards increased mortality and need for dialysis. The authors argue that these results highlight the need for strong consideration of listing for CHLT if there is evidence of cirrhosis and portal hypertension on imaging [25].
Two recent studies have compared temporally sequential versus concurrent CHLT and show a benefit to dual over single organ transplant [26,27]. Yamaguchi et al. analyzed UNOS data through 2018, which included 301 CHLT recipients and 6 sequential heart liver transplant (SHLT) recipients who underwent heart transplant followed by liver transplant a median of 331 days later. Despite a small number of patients undergoing SHLT, these patients had worse overall survival and allograft survival of both organs [26]. A similarly conducted analysis by Rucker et al. additionally included 36 individuals who were dual listed at time of heart transplant but never received a liver transplant. They found improved 10-year survival in those who underwent CHLT or liver after heart approach compared with heart transplant alone with 52.1, 53.6, and 27.5% 10-year survival, respectively (P = 0.003). Those undergoing CHLT were less likely to be treated for acute rejection within the first year after transplant compared with either those who underwent heart transplant alone or liver after heart transplant [27].
Improved outcomes with concurrent liver transplantation are hypothesized to be related in part to immunoprotection from the liver allograft. Multiple recent single-center and UNOS database studies demonstrate decreased rates of cardiac allograft rejection after CHLT [24,27,28▪,29,30]. These findings may be related to the unique ability of the liver to bind and clear donor-specific HLA class I antibodies. In one cohort, no class I DSA was observed in patients after CHLT while present in nearly 10% of a comparative heart transplant-only cohort [28▪].
In summary, these results suggest a benefit to combined, concurrent heart and liver transplant and encourage waiting for a dual organ offer rather than sequential transplant or single organ transplant.
UNIQUE CONSIDERATIONS
Two relevant topics that merit further discussion are en bloc operative technique and selective use of mechanical circulatory support.
En bloc operative technique
Transplantation of the heart and liver en bloc was initially described by Hill et al.[31] in three pediatric patients requiring CHLT with 100% 1-year patient and allograft survival. The technique begins with procurement of both organs together without division of the suprahepatic inferior vena cava. The recipient undergoes cardiectomy and hepatectomy with cardiopulmonary bypass (CPB) support. The heart and liver are then implanted simultaneously and reperfused together while on CPB [32,33▪,34▪].
The technique is performed by a minority of institutions but carries several advantages. Most notably, there is a solitary reperfusion event that occurs while the patient remains supported on CPB allowing for greater hemodynamic stability during the lability of reperfusion [33▪]. This in turn decreases stress on the cardiac allograft and decreases venous congestion in the liver allograft. Shorter cold ischemia time for the liver allograft may improve postoperative allograft function. From a technical standpoint, case series describe an overall shorter operative time and cold ischemia time [32,34▪]. Continuity of the IVC affords fewer anastomoses with a subsequently shorter warm ischemia time. Reported outcomes have been excellent with 100% 30-day [32,34▪] and 1-year survival rates [32].
Use of intraoperative mechanical support during liver transplantation
One area of particular interest is the optimal manner in which intraoperative mechanical circulatory support can be leveraged to mitigate stress on the cardiac allograft and facilitate liver transplantation during CHLT. At present, there is significant institutional heterogeneity in approach, using a variety of methods including continued CPB, conversion to venoarterial extracorporeal membrane oxygenation (VA ECMO), initiation of venovenous (VV) bypass, or in many cases, no additional mechanical support (Table 1).
Table 1.
Intraoperative use of mechanical circulatory support as described in single-center case series
| Institution | Cohort and indications | Intraoperative management practice pattern |
| Stanford University Medical Center Palo Alto, California [32] |
Nine CHLT recipients from 2006 to 2018. All patients with failing Fontan physiology and cirrhosis |
En bloc technique with patient maintained on CPB through simultaneous dual organ reperfusion. Two patients required intra-aortic balloon pump and one patient required VA ECMO to wean from CPB. |
| Mayo Clinic Rochester, Minnesota [35] |
27 CHLT recipients from 1999 to 2013. Most common indication: hereditary amyloidosis |
VV bypass utilized for all patients undergoing LT via caval interposition technique. No form of bypass used for caval preserving technique. A small fraction remained on CPB during LT. |
| The Hospital of the University of Pennsylvania Philadelphia, Pennsylvania [36] |
26 CHLT recipients from 1997 to 2013. Most common indications: heart failure from nonischemic, dilated, or congenital heart disease. |
All patients weaned from CPB without reversal of heparin and placed on VV bypass for LT. |
| University of Chicago Medical Center Chicago, Illinois [38▪] |
Seven patients undergoing combined heart liver kidney transplant from 2018 to 2020. Most common indication: noncongenital heart disease. |
CPB transitioned to VA ECMO at completion of heart transplant to proceed with LT. |
| Cedars-Sinai Medical Center Los Angeles, California [40] |
Six CHLT recipients from 1998 to 2014. Most common indication: hereditary amyloidosis. |
VV bypass with caval interposition technique used for all patients. |
| Vanderbilt University Medical Center Nashville, Tennessee (Unpublished) |
17 CHLT recipients from 2017 to 2022. Most common indication: congenital heart disease. |
VA ECMO utilized in nearly half of recipients with congenital heart disease. |
CPB, cardiopulmonary bypass; CHLT, combined heart and liver transplantation; LT, liver transplantation; VA, venoarterial; VV, venovenous; ECMO, extracorporeal membrane oxygenation.
In the largest case series published, venovenous bypass was most commonly utilized in sequential CHLT [35,36]. This approach is advantageous in minimizing cardiac allograft injury occurring during hepatectomy-related fluctuations in central venous return that are most pronounced when a caval replacement technique is performed [37▪]. Other centers advocate for use of VA ECMO after weaning CPB [38▪,39]. VA ECMO can support systemic perfusion and oxygenation that has been compromised by early cardiac allograft dysfunction, significant extracardiac shunting, or hepatic reperfusion-related injury. The addition of a femoral venous drainage catheter for a VVA configuration may also decrease preload related allograft injury [37▪].
Mechanical circulatory support after cardiac reperfusion has been utilized in nearly half of recipients at our institution. In our experience, the delayed treatment of early cardiac allograft dysfunction progressing to hepatic congestion and abdominal hypoperfusion results in a particularly poor outcome (Matsuoka LK, Alexopoulos SP, personal communication). We, therefore, advocate for early consideration of mechanical circulatory support in this scenario to assist right ventricular function and minimize central venous hypertension while ensuring systemic perfusion and oxygen delivery. Each method of intraoperative circulatory support has a unique risk–benefit profile, and the field would benefit from further comparison of current techniques and associated patient outcomes.
CONCLUSION
As the demand for CHLT rises and the procedure is increasingly performed, we continue to learn more about patient selection and patient outcomes. At present, current practices are based on small, retrospective studies and anecdotal experience without data from prospective and randomized studies. Future research will afford better understanding of the major remaining questions including assessment of the severity of FALD and best intraoperative use of mechanical circulatory support.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Starzl T, Bahnson H, Hardesty R, et al. Heart-liver transplantation in a patient with familial hypercholesterolaemia. Lancet 1984; 323:1382–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Multiple organ transplants in the U.S. by region [Internet]. Organ procurement and transplantation network [cited 3 November 2022]. Available at: https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/ [Google Scholar]
- 3.Zhao K, Mclean R, Hoteit M, Olthoff KM. Combined heart and liver transplant: indication, patient selection, and allocation policy. Clin Liver Dis 2019; 13:170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lebray P, Varnous S. Combined heart and liver transplantation: state of knowledge and outlooks. Clin Res Hepatol Gastroenterol 2019; 43:123–130. [DOI] [PubMed] [Google Scholar]
- 5.Gong T, Hall S. Considerations and experience driving expansion of combined heart-liver transplantation. Curr Opin Organ Transplant 2020; 25:496–500. [DOI] [PubMed] [Google Scholar]
- 6▪.Alexopoulos S, Wu W, Ziogas I, et al. Adult combined heart-liver transplantation: the United States experience. Transpl Int 2022; 35:10036. [DOI] [PMC free article] [PubMed] [Google Scholar]; Most recent UNOS registry analysis demonstrating improvement in CHLT outcomes over three decades with CHD as most common cardiac diagnosis in the present era.
- 7.Rizvi S, Challapalli J, Maynes E, et al. Indications and outcomes of combined heart-liver transplant: a systematic review and met-analysis. Transplant Rev 2020; 34:100517. [DOI] [PubMed] [Google Scholar]
- 8.Emamaullee J, Zaidi A, Schiano T, et al. Fontan-associated liver disease: screening, management, and transplant considerations. Circulation 2020; 142:591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ando Y, Nakamura M, Araki S. Transthyretin-related familial amyloidotic polyneuropathy. Arch Neurol 2005; 62:1057–1062. [DOI] [PubMed] [Google Scholar]
- 10.Wilczek H, Larsson M, Ericzon B. Long-term data from the Familial Amyloidotic Polyneuropathy World Transplant Registry (FAPWTR). Amyloid 2011; 18:193–195. [DOI] [PubMed] [Google Scholar]
- 11.Ruberg F, Grogan M, Hanna M, et al. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol 2019; 73:2872–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shubin A, De Gregorio L, Hwang C, MacConmara M. Combined heart-liver transplantation in a case of haemochromatosis. BMJ Case Rep 2021; 14:e241508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izzy M, VanWagner L, Lin G, et al. Redefining cirrhotic cardiomyopathy for the modern era. Hepatology 2020; 71:334–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Hadi H, Di Vincenzo A, Vettor R, Rossato M. Relationship between heart disease and liver disease: a two-way street. Cells 2020; 9:567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiese S, Hove J, Bendtsen F, Moller S. Cirrhotic cardiomyopathy: pathogenesis and clinical relevance. Nat Rev Gastroenterol Hepatol 2014; 11:177–186. [DOI] [PubMed] [Google Scholar]
- 16.Vaikunth S, Higgins J, Concepcion W, et al. Does liver biopsy accurately measure fibrosis in Fontan-associated liver disease? A comparison of liver biopsy precombined heart and liver transplant and liver explant posttransplant. Clin Transplant 2020; 34:e14120. [DOI] [PubMed] [Google Scholar]
- 17.Sessa A, Allaire M, Lebray P, et al. From congestive hepatopathy to hepatocellular carcinoma, how can we improve patient management? JHEP Rep 2021; 3:100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elder R, McCabe N, Hebson C, et al. Features of portal hypertension are associated with major adverse events in Fontan patients: The VAST study. Int J Cardiol 2013; 168:3764–3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19▪▪.Inuzuka R, Nii M, Inai K, et al. Predictors of liver cirrhosis and hepatocellular carcinoma among perioperative survivors of the Fontan operation. Heart 2022; doi: 10.1136/heartjnl-2022-320940. [DOI] [PubMed] [Google Scholar]; Large, retrospective analysis of Fontan patients identifying elevated central venous pressure as an independent predictor of development of cirrhosis and HCC.
- 20.Possner M, Gordon-Walker T, Egbe A, et al. Hepatocellular carcinoma and the Fontan circulation: clinical presentation and outcomes. Int J Cardiol 2021; 322:142–148. [DOI] [PubMed] [Google Scholar]
- 21.Izzy M, Alexopoulos S, Shingina A. Combined heart-liver transplantation for congestive hepatopathy with bridging fibrosis: is it warranted? JHEP Rep 2021; 3:100292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allaire M, Sessa A, Cadranel JF, Lebray P. Reply to: ‘Combined heart-liver transplantation for congestive hepatopathy with bridging fibrosis: is it warranted?’. JHEP Rep 2021; 3:100327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee S, Matsuoka L, Cao S, et al. Identifying predictors of outcomes in combined heart and liver transplantation. Transplant Proc 2019; 51:2002–2008. [DOI] [PubMed] [Google Scholar]
- 24.Cotter T, Wang J, Peeraphatdit T, et al. Simultaneous heart-liver transplantation for congenital heart disease in the United States: rapidly increasing with acceptable outcomes. Hepatology 2021; 73:1464–1477. [DOI] [PubMed] [Google Scholar]
- 25.Sganga D, Hollander S, Vaikunth S, et al. Comparison of combined heart–liver vs heart-only transplantation in pediatric and young adult Fontan recipients. J Heart Lung Transplant 2021; 40:298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamaguchi Y, Burrier C, Roth C, et al. Sequential versus combined heart-liver transplantation in the USA. Dig Dis Sci 2020; 65:2427–2432. [DOI] [PubMed] [Google Scholar]
- 27.Rucker A, Anderson K, Mulvihill M, et al. Simultaneous versus sequential heart-liver transplantation: ideal strategies for organ allocation. Transplant Direct 2018; 5:e415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28▪.Zhao K, Wang R, Kamoun M, et al. Incidence of acute rejection and patient survival in combined heart-liver transplantation. Liver Transpl 2022; 28:1500–1508. [DOI] [PubMed] [Google Scholar]; Retrospective comparison of CHLT to heart transplant alone demonstrating significantly reduced rate of acute cellular rejection, although no difference in patient or cardiac allograft survival at 5 years.
- 29.Daly R, Rosenbaum A, Dearani J, et al. Heart-after-liver transplantation attenuates rejection of cardiac allografts in sensitized patients. J Am Coll Cardiol 2021; 77:1331–1340. [DOI] [PubMed] [Google Scholar]
- 30.Ortega-Legaspi J, Hoteit M, Wald J. Immune benefit of combined heart and liver transplantation. Curr Opin Organ Transplant 2020; 25:513–518. [DOI] [PubMed] [Google Scholar]
- 31.Hill A, Maeda K, Bonham C, Concepcion W. Pediatric combined heart-liver transplantation performed en-bloc: a single-center experience. Pediatr Transplant 2012; 16:392–397. [DOI] [PubMed] [Google Scholar]
- 32.Vaikunth S, Concepcion W, Daugherty T, et al. Short-term outcomes of en bloc combined heart and liver transplantation in the failing Fontan. Clin Transplant 2019; 33:e13540. [DOI] [PubMed] [Google Scholar]
- 33▪.Elde S, Brubaker A, Than P, et al. Operative technique of donor organ procurement for en bloc heart-liver transplantation. Transplantation 2021; 105:2661–2665. [DOI] [PubMed] [Google Scholar]; Authors offer a detailed protocol for adoption and standardization of the en bloc procurement technique.
- 34▪.Brozzi N, Loebe M, Souki F, et al. En-Bloc simultaneous heart-liver transplantation in adult patients. Ann Surg 2021; 274:e1284–e1289. [DOI] [PubMed] [Google Scholar]; Single-center experience with en bloc CHLT detailing advantages of simultaneous organ reperfusion and shorter warm and cold ischemia times.
- 35.Barbara D, Rehfeldt K, Heimbach J, et al. The perioperative management of patients undergoing combined heart-liver transplantation. Transplantation 2015; 99:139–144. [DOI] [PubMed] [Google Scholar]
- 36.Atluri P, Gaffey A, Howard J, et al. Combined heart and liver transplantation can be safely performed with excellent short- and long-term results. Ann Thorac Surg 2014; 98:858–862. [DOI] [PubMed] [Google Scholar]
- 37▪.Smeltz A, Kumar P, Arora H. Anesthesia for combined heart and liver transplantation. J Cardiothorac Vasc Anesth 2021; 35:3350–3361. [DOI] [PubMed] [Google Scholar]; Thorough review detailing intraoperative management considerations during CHLT with attention to modalities of extracorporeal circulatory support utilized during liver transplantation.
- 38▪.Perez-Gutierrez A, Siddiqi U, Kim G, et al. Combined heart-liver-kidney transplant: the university of Chicago medicine experience. Clin Transplant 2022; 36:e14586. [DOI] [PubMed] [Google Scholar]; Single-center experience of combined heart–liver–kidney transplant advocating for use of VA ECMO during liver transplantation to manage hemodynamic instability associated with reperfusion.
- 39.Reardon L, Lin J, VanArsdell G, et al. Orthotopic heart and combined heart liver transplantation: the ultimate treatment option for failing Fontan physiology. Curr Transpl Rep 2021; 8:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reich H, Awad M, Ruzza A, et al. Combined heart and liver transplantation: the Cedars-Sinai experience. Transplant Proc 2015; 47:2722–2726. [DOI] [PubMed] [Google Scholar]