Objectives:
We sought to better understand factors associated with ovarian aging in women with HIV (WWH).
Design:
HIV has been associated with diminished fertility, younger age at menopause, and shorter leukocyte telomere length (LTL), a marker of cellular aging. We herein examine cross-sectional and longitudinal associations between LTL, anti-Müllerian hormone (AMH), and HIV.
Methods:
We included WWH and HIV-negative women 12–50 years of age in the CARMA cohort with one or more study visit(s). LTL and AMH were measured by qPCR and ELISA, respectively. Women were analyzed in peak reproductive (<35 years) vs. late reproductive (≥35 years) life phases. Using multivariable mixed-effect linear or logistic regressions, we assessed factors associated with AMH and ΔAMH/year while adjusting for relevant confounders.
Results:
WWH had shorter LTL and lower AMH levels compared to HIV-negative controls despite being of similar age. After adjusting for relevant factors, HIV was associated with 20% lower AMH levels in women under 35 years of age and shorter LTL was associated with AMH levels below 2 ng/ml among women aged 35 years or older. Longitudinally, ΔAMH/year was largely related to initial AMH level among older women, and to age in younger women.
Conclusions:
Factors associated with AMH change across women's reproductive lifespan. Lower AMH among peak reproductive aged WWH suggests that HIV may have an initial detrimental effect on ovarian reserve, an observation that may warrant counseling around pregnancy planning. In women aged 35 years or older, the association between shorter LTL and lower AMH suggests that the immune and reproductive aging connections are more important in this age group.
Keywords: anti-Müllerian hormone, HIV, leukocyte telomere length, ovarian aging, women with HIV
Introduction
Women and girls comprise over half of people with HIV (PWH) worldwide [1]. Evidence suggests that women with HIV (WWH) experience more rapid ovarian aging [2,3] with associated adverse health outcomes. For example, WWH appear to have diminished fertility compared to the general population [4–6] and may experience an earlier onset of menopause [7–9]. This stresses the need to understand the impact of HIV on the reproductive health of WWH.
The degree to which WWH experience more rapid ovarian aging is important for their health and family planning. Many WWH indicate a desire to have children [10], thus, understanding the impact of HIV on fertility is important for appropriate counseling. Additionally, women who experience an earlier onset of menopause are at an increased risk of type 2 diabetes, cardiovascular disease, neurological disease, osteoporosis, and other age-related morbidities [11–13]. It is well recognized that PWH are at an increased risk of age-related morbidities [12,14]. Understanding any additional risk conferred by more rapid ovarian aging is especially important for the care of WWH. That understanding may be improved by measurement of biomarkers of immune aging and ovarian reserve, such as telomere length and anti-Müllerian hormone (AMH).
Telomeres are nucleoprotein complexes that protect the ends of chromosomes. Telomere length shortens with age in the general population and is a marker of cellular aging [15]. Shorter leukocyte telomere length (LTL) has been observed in PWH compared to controls, and potentially represents an expression of accentuated aging [16–19]. Studies have also reported associations between shorter LTL and earlier age at menopause in WWH [20–23].
AMH is a glycoprotein produced by granulosa cells in developing ovarian follicles [24]. Throughout a woman's lifetime, AMH initially increases into the mid-twenties, then plateaus and begins to decline in the early to mid-thirties [25,42]. It is a sensitive marker of ovarian reserve with lower AMH indicating diminished ovarian reserve [25–29,42]. Owing to its consistency as a marker, lack of menstrual cycle phase variation and advances in assays in serum, AMH provides a rapid and simple biochemical evaluation of ovarian reserve that was previously difficult to achieve [30]. Studies that have examined AMH in WWH [2,31–36], have found that AMH is a reliable marker of ovarian reserve in WWH [34], and is predictive of the menopausal transition [32]. However, there is conflicting evidence regarding the impact of HIV on AMH when WWH are compared with controls. Some studies found lower AMH among WWH [2,31] and others reported that HIV does not impact AMH [33,34].
HIV infection has been associated with decreased ovarian reserve [2,31] and shorter LTL [16–19], and both are indicators of ovarian aging. However, to our knowledge no studies have examined the impact of HIV on both LTL and AMH values in WWH. To better understand ovarian aging in WWH, we sought to compare AMH levels and predictors of AMH between WWH and HIV-negative controls across the reproductive lifespan. Further, we proposed to examine the association between LTL and AMH, to better characterize the relative contribution of clinical and sociodemographic factors to ovarian aging in WWH.
Materials and methods
Study sample
Study participants were WWH and HIV-negative controls 12–50 years of age who had intact ovaries and were enrolled between December 2008 and April 2017 in the Children and Women: AntiRetrovirals and the Markers of Aging (CARMA) cohort (previously described [16,37]). Study inclusion criteria are described in Figure S1, Supplemental Digital Content. For cross-sectional analysis, all participants with available blood and plasma for at least a single study visit were included. For longitudinal analysis, participants with available blood and plasma for at least two study visits were included unless they turned 35 years old between study visits, thereby excluding them from analysis in either the under 35 or 35 years or older age groups. This study was approved by the University of British Columbia Children's and Women's Research Ethics Board (H08-02018).
Demographic and clinical data
Demographic data were collected by self-report, including age, ethnicity, household annual income, and highest level of education. For participants under 19 years of age, income and education data were not collected.
Substance use data were collected by self-report. Tobacco use was trichotomized as current, past, or never. Current methadone use, other prescription opioid use, and heroin use were collected as binary variables (yes/no), then combined into a single current opioid use variable.
HIV clinical data were collected from medical records, including HIV plasma viral load (pVL), highest HIV pVL ever recorded, CD4+ cell count, and CD4+ nadir. HCV infection ever was self-reported for all participants and confirmed via medical chart review for WWH.
Leukocyte telomere length and anti-Müllerian hormone
Whole blood relative LTL was measured by multiplex quantitative polymerase chain reaction (MMqPCR) as previously described [38,39]. AMH levels were quantified using the picoAMH ELISA kit (product ID: AL-124, Ansh Labs, Webster, Texas, USA) as per manufacturer instructions. The linear range of this assay is between 25–6000 pg/ml. AMH measurements that fell below the limit of detection are reported as at the limit of detection. More information is found in the supplement.
Statistical analysis
First visit univariate comparisons of demographic variables between groups were performed using Mann–Whitney U or chi-squared tests. Among all participants, a polynomial model was created to describe AMH vs. age univariately, as this relationship was not linear. For more in-depth analyses, participants were separated into women aged under 35 and those aged 35 years or oder to evaluate predictors of AMH among reproductive and late reproductive age groups. Among women aged 35 years or older, many AMH measurements fell below the limit of detection, rendering a linear model inappropriate. Therefore, AMH was categorized as ≥2 or <2 ng/ml for this age group, a common threshold in the fertility literature used to assess a woman's odds of achieving a live birth [40]. To assess factors associated with the rate of change of AMH over time, ΔAMH/year, was calculated as: (AMH at latest visit – AMH at initial visit)/years between visits. Cross-sectional AMH levels were natural log-transformed to approximate a normal distribution in all analyses, whereas ΔAMH/year remained untransformed.
In bivariate analyses, models were adjusted for age in the cross-sectional analysis, and for initial AMH level longitudinally. Then, variables from Table 1 that were univariably associated with AMH or ΔAMH/year (P <0.1) were considered for multivariable mixed-effects linear regressions or logistic regressions. For these models, an a priori decision was made to include HIV status in each multivariable model because the purpose of this study was to evaluate the relationship between AMH and HIV. The influence of collinearities between certain demographic and substance use variables were evaluated with sensitivity models. All analyses used R (4.0.3; R Foundation for Statistical Computing, Vienna, Austria).
Table 1.
Participant demographics.
| WWH (N = 256) | HIV-negative (N = 206) | P-value | |
| Age, years | 34.5 [25.5–41.1] (12.0–49.3) | 32.3 [24.8–41.9] (12.3–49.9) | 0.62 |
| Relative LTL | 7.2 [6.4–7.9] (4.7–10.5) | 7.5 [6.9–8.3] (5.3–11.3) | 0.0002 |
| AMH, ng/ml | 1.5 [0.6–3.4] (0.0–85.0) | 2.3 [0.5–4.7] (0.0–31.6) | 0.032 |
| Amenorrhea (N = 452) | 0.86 | ||
| Yes | 37 (15) | 29 (14) | |
| No | 212 (85) | 174 (86) | |
| BMI, kg/m2 (N = 441) | 24.0 [21.4–28.6] (15.0–46.5) | 22.9 [20.6–27.5] (14.0–47.6) | 0.067 |
| Ethnicity (N = 456) | <0.0001 | ||
| Indigenous | 59 (23) | 53 (26) | |
| Asian/Southeast Asian | 21 (8) | 30 (15) | |
| African/Caribbean/Black | 72 (29) | 21 (10) | |
| White | 95 (38) | 91 (45) | |
| Other | 5 (2) | 9 (4) | |
| Household Income (N = 378) | 0.015 | ||
| <$15 000/year | 104 (53) | 73 (40) | |
| >$15 000/year | 93 (47) | 108 (60) | |
| Education (N = 378) | <0.0001 | ||
| Grade school | 9 (5) | 4 (2) | |
| Some high school | 62 (32) | 37 (20) | |
| High school graduate | 46 (23) | 18 (10) | |
| Any college | 77 (39) | 121 (66) | |
| Other | 2 (1) | 2 (1) | |
| Tobacco smoking (N = 459) | <0.0001 | ||
| Never | 113 (44) | 130 (64) | |
| Past | 27 (11) | 25 (12) | |
| Current | 115 (45) | 49 (24) | |
| Current opioid (N = 426) | <0.0001 | ||
| Yes | 67 (28) | 20 (11) | |
| No | 172 (72) | 167 (89) | |
| Detectable HIV VL (N = 250) | |||
| Yes | 106 (42) | ||
| No | 144 (58) | ||
| HIV peak VL >100 000 copies/ml (N = 253) | |||
| Yes | 117 (46) | ||
| No | 136 (54) | ||
| Perinatal HIV acquisition (N = 249) | |||
| Yes | 49 (20) | ||
| No | 200 (80) | ||
| On ART at visit (N = 240) | |||
| Yes | 182 (76) | ||
| No | 58 (24) | ||
Data are presented as number (%) of individuals or median [interquartile range] (range). Number of participants with available data are indicated when applicable. Comparisons were done using chi-squared or Mann–Whitney U tests. Opioid use includes any prescribed or illicit use.
AMH, anti-Müllerian hormone; AR, antiretroviral therapy; BMI, body mass index; LTL, leukocyte telomere length; VL, viral load; WWH, women with HIV.
Results
Participant characteristics
The study included a total of 462 women aged 12–50 years, of whom 256 were WWH and 206 were HIV-negative controls (Table 1). Among them, two had four visits, 39 had three visits, 173 had two visits, and 248 had one visit for a total of 719 visits (WWH = 478 visits, HIV-negative controls = 241 visits) (Figure S1, Supplemental Digital Content).
Age, BMI, and the number of participants who had experienced amenorrhea for at least one year were well balanced between WWH and controls. WWH had shorter relative LTL (7.2 vs. 7.5) and lower AMH (1.5 vs. 2.3 ng/ml) than controls. The WWH group had a greater proportion of participants who were of African/Caribbean/Black ethnicity (29 vs. 10%), currently smoked (45 vs. 24%), and currently used opioids (28 vs. 11%), a lower proportion of participants who were of East Asian/South East Asian ethnicity (8 vs. 15%), had >$15 000 annual household income (47 vs. 60%), or had completed high school (63 vs. 77%). Among WWH, at the initial visit, 76% were on ART, 42% had a detectable HIV pVL, and 46% had a peak pVL > 100 000 copies/ml (Table 1). Almost all WWH in our study under 19 years of age acquired HIV perinatally (N = 43/46), and the majority of WWH aged 19 years or older acquired HIV after puberty (N = 204/210).
Anti-Müllerian hormone and age
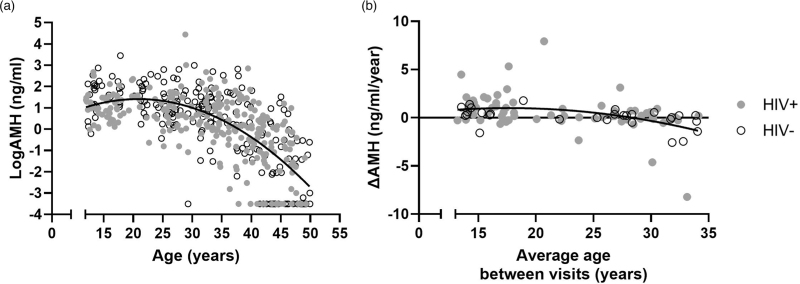
Among all participants AMH increased until the mid-twenties, then declined in the mid-thirties. A univariate curvilinear regression demonstrated a large proportion of variance in AMH was explained by age alone (R2 = 0.49) (Fig. 1a). A similar pattern was observed in longitudinal data within participants under 35 years old, in whom a small gain in AMH was detected among those in their twenties and loss among those in their thirties (Fig. 1b, R2 = 0.17). The rate of ΔAMH/year could not be plotted for those aged 35 years or older due to frequent below-detection-limit values.
Fig. 1.
Curvilinear relationships between AMH, ΔAMH/year, and age.
(a) Among all participants (N = 462), relationship between AMH and age from cross-sectional sample at earliest visit. (b) Among women under 35 years old (N = 96), relationship between ΔAMH/year and average age between visits. Black lines represent polynomial best fit (a, R2 = 0.49; b, R2 = 0.17). AMH, anti-Müllerian hormone.
Cross-sectional analysis
Women under 35 years old
For the cross-sectional analysis of reproductive aged women under 35 years old, we analyzed 367 visits (WWH = 233 visits, HIV-negative control = 134 visits) from 259 women (WWH = 150, HIV-negative controls = 109). When modeled linearly, older age was univariately associated with higher AMH, such that one year older corresponded to a 14% AMH increase. After adjusting for age, other variables bivariately associated with lower AMH included HIV, ethnicity, household income <$15 000 per year, and current opioid use (Table 2). LTL and HIV-specific variables were not associated with AMH.
Table 2.
Bivariate analysis of cross-sectional AMH.
| <35 years old (eβ) | P-value | ≥35 years old (odds ratio) | P-value | |
| Age, years | 1.14 (1.02–1.27) | 0.02 | 0.78 (0.71–0.85) | <0.0001 |
| Age2 | 1.00 (0.99–1.00) | 0.004 | ||
| LTL | 1.05 (0.95–1.15) | 0.32 | 1.33 (0.98–1.81) | 0.07 |
| HIV | 0.79 (0.64–0.98) | 0.03 | 0.61 (0.32–1.14) | 0.12 |
| Ethnicity (ref. White) | 0.02 | 0.006 | ||
| Indigenous | 0.70 (0.51–0.95) | 0.34 (0.13–0.89) | ||
| Asian/Southeast Asian | 1.31 (0.93–1.85) | 2.28 (0.89–5.85) | ||
| African/Caribbean/Black | 1.10 (0.82–1.47) | 1.01 (0.40–2.53) | ||
| Other | 0.92 (0.55–1.55) | 2.41 (0.57–10.19) | ||
| BMI, kg/m2 | 1.01 (0.99–1.03) | 0.48 | 0.96 (0.91–1.00) | 0.06 |
| Income (ref. <$15 000 CAD) | 0.01 | 0.0002 | ||
| ≥$15,000 CAD | 1.40 (1.08–1.81) | 3.29 (1.61–6.73) | ||
| N/A | 1.79 (1.13–2.85) | |||
| Education (ref. some high school or less) | 0.09 | 0.02 | ||
| High school graduate or more | 1.23 (0.94–1.61) | 2.41 (1.10–5.26) | ||
| N/A | 1.61 (1.02–2.52) | |||
| Ever infected with HCV | 0.78 (0.57–1.07) | 0.12 | 0.48 (0.25–0.93) | 0.02 |
| Current opioid use | 0.70 (0.53–0.94) | 0.02 | 0.21 (0.08–0.51) | <0.0001 |
| Tobacco smoking (ref. never) | 0.07 | 0.009 | ||
| Current | 0.79 (0.62–1.01) | 0.35 (0.16–0.75) | ||
| Past | 0.70 (0.48–1.02) | 0.77 (0.35–1.67) | ||
| Current psychoactive medication use | 0.93 (0.69–1.25) | 0.63 | 0.83 (0.46–1.51) | 0.54 |
| CD4+ nadir | 1.0005 (0.9999–1.001) | 0.10 | 0.998 (0.996–1.001) | 0.21 |
| Peak HIV VL >100 000 | 0.96 (0.71–1.30) | 0.80 | 0.71 (0.35–1.42) | 0.33 |
| HIV detectable VL | 0.86 (0.69–1.07) | 0.18 | 0.70 (0.31–1.59) | 0.40 |
The relationship between age and AMH levels was nonlinear, as demonstrated by the markedly improved fit between AMH and the quadratic term for age compared to the linear term. As such, the quadratic age term was considered for these models. Age was univariately associated with AMH levels for women under 35 years old and the likelihood of AMH levels ≥2 ng/ml for women aged 35 years or older. For all other potential explanatory variables, bivariate models of AMH are shown, adjusting for age.
AMH, anti-Müllerian hormone; BMI, body mass index; CAD, Canadian dollar; HCV, hepatitis C virus; LTL, leukocyte telomere length; VL, viral load; N/A, not collected, usually because of participant's young age.
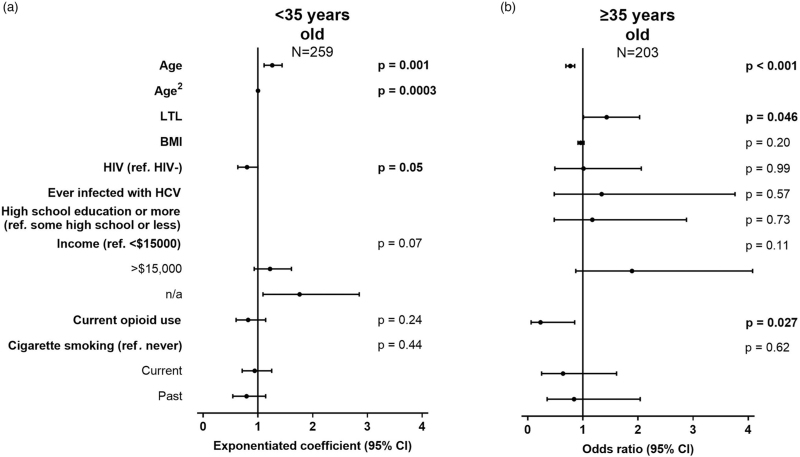
In the multivariable analysis older age and HIV were independently associated, respectively, with higher and lower AMH levels. Ethnicity was not included in the multivariable model due to collinearity with other variables that may have a more direct biological impact on AMH. Each year of increase in age was associated with a 26% AMH increase, although WWH had 20% lower AMH levels overall. Participants with no income data also had higher AMH levels, but they were mostly girls under 19 years old for whom income data were not collected. None of the remaining variables were independently associated with AMH (Fig. 2a, Table S1, Supplemental Digital Content).
Fig. 2.
Multivariable analysis of cross-sectional AMH levels.
Models among women: (a) under 35 (R2 = 0.13) and (b) at least 35 years old (R2 = 0.38), showing unstandardized exponentiated coefficients of log transformed AMH levels and odds ratios of AMH levels ≥2 ng/ml, respectively. Significant confidence intervals do not cross 1. AMH, anti-Müllerian hormone.
A sensitivity analysis was performed to address the collinearity among income, smoking, and opioid use (Table S2, Supplemental Digital Content). In this analysis, the effect of HIV status remained the same regardless of which collinear variable was included, indicating that the relationship between HIV and AMH was independent of these variables.
Women aged 35 years or older
The cross-sectional analysis of women aged 35 years or older analyzed 352 visits (WWH = 245 visits, HIV-negative controls = 107 visits) from 244 women (WWH = 155, HIV-negative controls = 80). Age was univariately associated with the odds of having AMH ≥2 ng/ml such that per year past 35 years of age, the odds of having AMH ≥2 ng/ml declines by 22%. After adjusting for age, other variables bivariately associated with lower odds of AMH ≥2 ng/ml included Indigenous ethnicity, HCV infection ever, current opioid use, and smoking. Variables bivariately associated with higher odds of an AMH ≥2 ng/ml included Asian ethnicity, household income >$15 000 per year, and having a high school education or more. HIV status, HIV specific variables, and LTL were not associated with AMH (Table 2).
In the multivariable analysis, younger age and longer LTL were independently associated with higher AMH levels, such that each year was associated with 23% lower odds of AMH ≥2 ng/ml and every unit of relative LTL with 43% higher odds of AMH ≥2 ng/ml. Currently using opioids was independently associated with a 77% decrease in the odds of AMH ≥2 ng/ml. Ethnicity was not included in the multivariable model as described above. HIV status was not associated with AMH levels (Fig. 2b, Table S1, Supplemental Digital Content).
In the sensitivity analysis for collinear variables including income, education, smoking, and opioid use, the independent effects of age and LTL remained stable (Table S3, Supplemental Digital Content).
Longitudinal analysis
In the longitudinal analysis, 184 participants were included. Younger women tended to gain AMH (positive ΔAMH/year) and older women tended to lose AMH (negative ΔAMH/year) (Fig. 1b).
Women under 35 years old
We analyzed 96 women (WWH = 73, HIV-negative controls = 23) under 35 years of age. The median time between earliest and latest visits was 2.5 years (interquartile range [IQR] = 1.7–5.0 years). Age was univariately associated with ΔAMH/year such that the rate of AMH increase slowed by 10%/year. Higher initial AMH, ethnicity, HCV infection ever, and smoking were also bivariately associated with slower AMH gain, whereas longer LTL, and being a participant with no income and education data were bivariately associated with faster AMH gain. As above, missing education and income data represent girls. HIV was not bivariately associated with ΔAMH/year (Table 3).
Table 3.
Bivariate analysis of longitudinal ΔAMH/year.
| <35 years old β | P-value | ≥35 years old β | P-value | |
| Age, years | −0.1 (−0.15 to −0.05) | <0.0001 | −0.01 (−0.05 to 0.04) | 0.77 |
| Initial AMH, ng/ml | −0.13 (−0.23 to −0.03) | 0.01 | −0.12 (−0.16 to −0.08) | <0.0001 |
| LTL | 0.32 (0.02 to 0.62) | 0.04 | 0.02 (−0.09 to 0.13) | 0.68 |
| HIV | 0.38 (−0.35 to 1.10) | 0.31 | 0.08 (−0.22 to 0.37) | 0.62 |
| Ethnicity (ref. White) | 0.03 | 0.74 | ||
| Indigenous | −0.40 (−1.50 to 0.69) | −0.16 (−0.43 to 0.11) | ||
| Asian/Southeast Asian | 0.45 (−0.73 to 1.64) | −0.07 (−0.49 to 0.35) | ||
| African/Caribbean/Black | 1.05 (0.31 to 1.79) | −0.11 (−0.43 to 0.21) | ||
| Other | 0.47 (−1.20 to 2.14) | −0.25 (−0.79 to 0.29) | ||
| BMI, kg/m2 | −0.03 (−0.08 to 0.03) | 0.40 | −0.01 (−0.02 to 0.01) | 0.47 |
| Income (ref. <$15 000 CAD) | 0.009 | 0.96 | ||
| ≥$15 000 CAD | 0.49 (−0.39 to 1.37) | 0.01 (−0.19 to 0.20) | ||
| N/A | 1.22 (0.42 to 2.02) | |||
| Education (ref. some high school or less) | 0.003 | 0.99 | ||
| High school graduate or more | 0.68 (−0.29 to 1.66) | −0.0001 (−0.23 to 0.23) | ||
| N/A | 1.53 (0.58 to 2.48) | |||
| Ever infected with HCV | −1.75 (−2.83 to −0.66) | 0.002 | −0.01 (−0.24 to 0.22) | 0.93 |
| Current opioid use | −0.45 (−1.73 to 0.83) | 0.48 | 0.05 (−0.19 to 0.29) | 0.66 |
| Tobacco Smoking (ref. never) | 0.04 | 0.79 | ||
| Current | −1.03 (−1.84 to −0.22) | −0.04 (−0.28 to 0.20) | ||
| Past | −0.39 (−1.87 to 1.09) | −0.11 (−0.44 to 0.22) | ||
| Current psychoactive medication use | −1.15 (−2.34 to 0.04) | 0.06 | −0.13 (−0.36 to 0.10) | 0.25 |
| CD4+ nadir | 0.002 (−0.0004 to 0.003) | 0.13 | 0.00004 (−0.0009 to 0.001) | 0.93 |
| Peak HIV VL >100 000 | −0.09 (−1.03 to 0.86) | 0.85 | −0.05 (−0.30 to 0.20) | 0.70 |
| HIV detectable VL | −0.09 (−1.01 to 0.82) | 0.84 | 0.19 (−0.06 to 0.44) | 0.14 |
Age was univariately associated with ΔAMH/year longitudinally among women under 35 years old. For all other potential explanatory variables, bivariate models of ΔAMH/year are shown, adjusting for initial AMH level.
AMH, anti-Müllerian hormone; BMI, body mass index; CAD, Canadian dollar; HCV, hepatitis C virus; LTL, leukocyte telomere length; VL, viral load; N/A not collected, usually because of participant's young age.
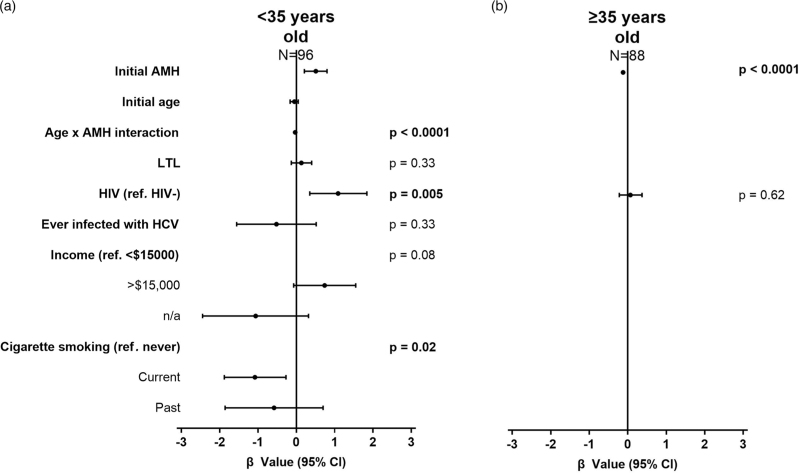
In the multivariable analysis, HIV was forced into the model as per our a priori analysis plan and was independently associated with faster AMH gain, whereas current and past smoking were independently associated with slower AMH gain (Fig. 3a, Table S4, Supplemental Digital Content). An interaction between age and initial visit AMH level was detected, showing that the initial AMH relationship with ΔAMH/year depends on age. This is illustrated in Figure S2, Supplemental Digital Content showing that higher initial AMH is associated with slower rate of AMH gain among young women and faster AMH attrition among older women.
Fig. 3.
Multivariable analysis of longitudinal AMH rate of change.
Models among women: (a) under 35 (R2 = 0.51) and (b) at least 35 years old (R2 = 0.27), showing unstandardized effect sizes. Significant confidence intervals do not cross 1. AMH, anti-Müllerian hormone.
A sensitivity analysis was done to parse the effects of collinear variables income and smoking and showed that the Age × AMH interaction term remained independently associated with ΔAMH/year. HIV remained independently associated with ΔAMH/year in models adding either income or smoking (Table S5, Supplemental Digital Content).
Women aged 35 years or older
Eighty-eight women (WWH = 74, HIV-negative controls = 14) at least 35 years of age were analyzed longitudinally. The median time between visits was 4.6 years (IQR = 2.5–6.2 years). Only initial AMH was univariately associated with ΔAMH/year (Table 3). In the multivariable model, initial AMH remained the only variable independently associated with ΔAMH/year, and showed a similar effect size, (Fig. 3b, Table S4, Supplemental Digital Content), suggesting that no other variables were associated with longitudinal change in AMH among women at least 35 years old.
Discussion
Our investigation of AMH across the reproductive lifespan showed a nonlinear pattern of AMH levels with age, whereby it rose in young women but fell sharply beginning in the mid-thirties. This dynamic has been observed in WWH and in the general population [41] and suggests that predictors of AMH vary across reproductive stages. Indeed, we observed that lower AMH levels were independently predicted by HIV status during peak reproductive years (<35 years old) but this was not seen in those aged 35 years or older where shorter LTL became the independent predictor. It is possible that as an immunologic stressor, HIV may acutely influence AMH levels during the reproductive stage of life following which, the accumulation of many immunologic stresses, as reflected by LTL attrition, shape AMH dynamics during the late reproductive years. The longitudinal analysis supported the cross-sectional pattern of AMH levels with age, showing that participants’ AMH rose early in life and fell later in life. However, the rates of AMH gain and loss were largely driven by AMH level itself, and therefore indirectly influenced by factors identified in the cross-sectional analysis.
Among women under 35 years of age, the longitudinal analysis showed that ΔAMH/year was driven by an interaction between initial AMH levels and age. Higher initial AMH levels were associated with slower AMH rise among younger women and faster AMH decline among older women. This interaction can be explained by the function of AMH. In early life, rising AMH levels reflect follicle recruitment, and eventually a high AMH inhibits follicle growth, leading to AMH decline later in life [42]. Thus, younger women with higher AMH levels have a limited margin for growth before peak AMH occurs. In older women however, higher initial AMH levels may indicate proximity to the AMH peak and are thus accompanied by faster AMH decline due to maximum follicle inhibition. This is reflected in the agreement between our cross-sectional and longitudinal data as seen in Fig. 1, wherein the age at which longitudinal decline of AMH begins generally occurs after the age of peak AMH cross-sectionally.
Given that the rate of AMH rise among women under 35 years old is related to initial AMH level, it follows that the AMH rise itself may be related to the predictors of AMH identified in our cross-sectional analysis, namely HIV. Lower AMH in WWH has been previously reported [43], although never in a focused analysis in women of reproductive age. In our analysis, the effect of HIV was not accompanied by associations between AMH and HIV-related variables. This deviates from previous reports, wherein both lower CD4+ cell count [43] and detectable viral load [2] were associated with lower AMH. A study of 2621 WWH and 941 controls found an association between lower CD4+ cell count and lower AMH levels, even among controls with healthy CD4+ cell counts [43]. In our study, we did not detect an association between CD4+ cell count and AMH. It is possible that this effect exists predominantly among older women. In the aforementioned study, only 8% of WWH were under 30 years old, compared to 36% in our study. Indeed, a more recent study of WWH in Denmark with a younger study sample (21% <30 years old) also reported no association between CD4+ cell count and AMH among WWH [44]. Taken together, this suggests that the mechanism explaining lower AMH in WWH does not necessarily involve CD4+ depletion in women of reproductive age.
The evidence for HIV viral load driving lower AMH among WWH is likewise unclear. Higher HIV viral load has been associated with lower AMH in a study of WWH requesting assisted reproductive technology [2]. However, such a sample cannot be generalized to the population at large. Furthermore, HIV has been associated with lower AMH, even when the virus is fully suppressed [44], again suggesting that uncontrolled viremia does not necessarily drive lower AMH among WWH. While it would be difficult to demonstrate a causal relationship between HIV and decreased AMH, the available data are consistent with a mechanism that does not necessarily depend on either CD4+ cell count or viral load.
Immune aging could be a conceptual framework within which the causal relationship between HIV and decreased AMH levels can be further investigated. CD4+ depletion is a hallmark of immune aging, which is associated with waning immune competence and diminishing ovarian reserve. Shorter LTL is a widely accepted marker of immune aging and is associated with both earlier menopause [20] and primary ovarian insufficiency [45]. However, prior to our study, an association between LTL and AMH levels had yet to be detected in women [46]. In a recent study of 35 egg donors aged 18–33, no relationship was found between AMH and TL in leukocytes, cumulus cells, or granulosa cells. The authors posit that the lack of signal was due to a young and homogenous sample, something that could also explain our own observations wherein lower AMH was associated with HIV among women under 35 years old and shorter LTL among women at least 35 years old. Our observations ostensibly suggest that ovarian age is more strongly predicted by HIV status during peak reproductive years and by immune aging markers during late reproductive years. Given the relationship observed between HIV and shorter LTL [47] our data would support a model whereby accelerated immune aging plays a role in the mechanism by which HIV may mediate ovarian aging in younger women. However, in women at least 35 years old, immune age may have been affected by any number of stresses that have accrued over time. It follows that LTL would be the more robust predictor in this older age group, as it would better reflect the total burden of cumulative immune aging. Taken together, although our analyses of AMH levels point to different explanatory variables in different reproductive phases, it remains an observational study. Future studies are required to further explore the potential causal relationships between HIV, immune aging, and ovarian reserve. Furthermore, telomere biology in cumulus cells differ considerably from leukocytes [48], and it is possible that telomere length in cumulus and/or granulosa cells are better indicators of ovarian age than LTL.
The wide age range of our study sample is a primary strength of this analysis, which allowed us to interpret the relationship between HIV and AMH in different reproductive stages. This is critically important when studying ovarian health, given the nonlinear dynamic of AMH levels and functional ovarian reserve throughout life. Our analysis also benefited from the use of an FDA-approved assay with a limit of detection more than an order of magnitude lower than previous generation techniques [49]. Furthermore, we were able to consider potentially confounding variables of both ovarian health and immune aging in our multivariable analyses. However, our study was limited by the collinearity between some of these confounding variables. Nonetheless, the effect size of age and HIV remained stable in sensitivity models indicating that the effects of age and HIV on AMH levels are independent of these confounders, even if we could not isolate their individual effects. Indeed, smoking has been associated with lower AMH levels [50] as well as earlier menopause [32]. Future studies are needed to characterize the effect of smoking among WWH.
Taken together, our study shows that HIV is associated with lower AMH in women who are in the reproductive stage of life and shorter LTL during late reproductive years. For young WWH, this has important clinical implications as a more rapidly diminishing ovarian reserve may mean that delaying pregnancy could lead to more difficulty conceiving and/or an earlier onset of menopause. This highlights the importance of counseling young WWH on pregnancy planning as a key component of their HIV care. Our analysis of the older age group suggests that cumulative immune aging may influence ovarian reserve through an unknown mechanism, prompting future studies to investigate the potential impact of factors contributing to immune aging on ovarian health in WWH.
Acknowledgements
All authors contributed to the conceptualization of this study. C.E.V.H. conducted data extraction and synthesis. A.Y.Y.H. and E.A.K. conducted laboratory analysis. A.Y.A. and A.Y.Y.H. conducted statistical analysis. A.Y.Y.H. and C.E.V.H. prepared the draft of the manuscript. All authors reviewed and edited the manuscript.
Funding: Canadian Institute for Health Research (CIHR) Canadian HIV Trials Network (CTN) CARMA-Endo Study – Study Number 277.
CIHR team grant: Children and Women: AntiRetrovirals and the Markers of Aging (CARMA).
Michael Smith Foundation for Health Research Health Professional Investigator Salary Award, for M.C.M.M.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Denotes co-first authors: C.E.V.O, and A.Y.Y.H.
Supplemental digital content is available for this article.
References
- 1.UNAIDS. Women and girls and HIV. UN Joint Programme on HIV/AIDS (UNAIDS). Geneva, Switzerland: UNAIDS; 2018. [Google Scholar]
- 2.Santulli P, de Villardi D, Gayet V, Pillet ML, Marcellin L, Blanchet V, et al. Decreased ovarian reserve in HIV-infected women. AIDS 2016; 30:1083–1088. [DOI] [PubMed] [Google Scholar]
- 3.Van Ommen CE, King EM, Murray MC. Age at menopause in women living with HIV: a systematic review. Menopause 2021; 28:1428–1436. [DOI] [PubMed] [Google Scholar]
- 4.Hunter S, Isingo R, Boerma JT, Urassa M, Mwaluko GM, Zaba B. The association between HIV and fertility in a cohort study in rural Tanzania. J Biosoc Sci 2003; 35:189–199. [DOI] [PubMed] [Google Scholar]
- 5.Gray RH, Wawer MJ, Serwadda D, Sewankambo N, Li C, Wabwire-Mangen F, et al. Population-based study of fertility in women with HIV-1 infection in Uganda. Lancet 1998; 351:98–103. [DOI] [PubMed] [Google Scholar]
- 6.Zaba B, Gregson S. Measuring the impact of HIV on fertility in Africa. AIDS 1998; 12: (Suppl 1): 41. [PubMed] [Google Scholar]
- 7.Calvet GA, Grinsztejn BGJ, Quintana MDSB, Derrico M, Jalil EM, Cytryn A, et al. Predictors of early menopause in HIV-infected women: a prospective cohort study. Obstet Gynecol 2015; 212:765.765.e1-765.e13. [DOI] [PubMed] [Google Scholar]
- 8.de Pommerol M, Hessamfar M, Lawson-Ayayi S, Neau D, Geffard S, Farbos S, et al. Menopause and HIV infection: age at onset and associated factors, ANRS CO3 Aquitaine cohort. Int J STD AIDS 2011; 22:67–72. [DOI] [PubMed] [Google Scholar]
- 9.Ferreira CE, PintoNeto AM, Conde DM, CostaPaiva L, Morais SS, Magalhaes J. Menopause symptoms in women infected with HIV: prevalence and associated factors. Gynecol Endocrinol 2007; 23:198–205. [DOI] [PubMed] [Google Scholar]
- 10.Ogilvie GS, Palepu A, Remple VP, Maan E, Heath K, MacDonald G, et al. Fertility intentions of women of reproductive age living with HIV in British Columbia. Canada AIDS 2007; 21: (Suppl 1): 83. [DOI] [PubMed] [Google Scholar]
- 11.Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: long-term health consequences. Maturitas 2010; 65:161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deeks SG, Phillips AN. Clinical review: HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ 2009; 338:288–292. [DOI] [PubMed] [Google Scholar]
- 13.Gold EB, Bromberger J, Crawford S, Samuels S, Greendale GA, Harlow SD, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol 2001; 153:865–874. [DOI] [PubMed] [Google Scholar]
- 14.Guaraldi G, Orlando G, Zona S, Menozzi M, Carli F, Garlassi E, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011; 53:1120–1126. [DOI] [PubMed] [Google Scholar]
- 15.Müezzinler A, Zaineddin AK, Brenner H. A systematic review of leukocyte telomere length and age in adults. Ageing Res Rev 2013; 12:509–519. [DOI] [PubMed] [Google Scholar]
- 16.Zanet DL, Thorne A, Singer J, Maan EJ, Sattha B, Le Campion A, et al. Association between short leukocyte telomere length and HIV infection in a cohort study: No evidence of a relationship with antiretroviral therapy. Clin Infect Dis 2014; 58:1322–1332. [DOI] [PubMed] [Google Scholar]
- 17.Pathai S, Lawn SD, Gilbert CE, McGuinness D, McGlynn L, Weiss HA, et al. Accelerated biological ageing in HIV-infected individuals in South Africa: a case–control study. AIDS 2013; 27:2375–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rickabaugh TM, Kilpatrick RD, Hultin LE, Hultin PM, Hausner MA, Sugar CA, et al. The dual impact of HIV-1 infection and aging on naïve CD4 T-cells: additive and distinct patterns of impairment. PLoS One 2011; 6:e16459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu JC, Leung JM, Ngan DA, Nashta NF, Guillemi S, Harris M, et al. Absolute leukocyte telomere length in HIV-infected and uninfected individuals: evidence of accelerated cell senescence in HIV-associated chronic obstructive pulmonary disease. PLoS One 2015; 10:e0124426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray KE, Schiff MA, Fitzpatrick AL, Kimura M, Aviv A, Starr JR. Leukocyte telomere length and age at menopause. Epidemiology 2014; 25:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aydos SE, Elhan AH, Tükün A. Is telomere length one of the determinants of reproductive life span?. Arch Gynecol Obstet 2005; 272:113–116. [DOI] [PubMed] [Google Scholar]
- 22.Lahme AM, Stern R, Cooper D. Factors impacting on menstrual hygiene and their implications for health promotion. Global health promotion 2018; 25:54–62. [DOI] [PubMed] [Google Scholar]
- 23.Keefe DL, Marquard K, Liu L. The telomere theory of reproductive senescence in women. Curr Opin Obstet Gynecol 2006; 18:280–285. [DOI] [PubMed] [Google Scholar]
- 24.Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update 2014; 20:370–385. [DOI] [PubMed] [Google Scholar]
- 25.Lie Fong S, Visser JA, Welt CK, De Rijke YB, Eijkemans M, Broekmans FJ, et al. Serum antimüllerian hormone levels in healthy females: a nomogram ranging from infancy to adulthood. J Clin Endocrinol Metab 2012; 97:4650–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelsey TW, Wright P, Nelson SM, Anderson RA, Wallace WHB. A validated model of serum anti-Müllerian hormone from conception to menopause. PLoS One 2011; 6:e22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tremellen K, Zander-Fox D. Serum anti-Mullerian hormone assessment of ovarian reserve and polycystic ovary syndrome status over the reproductive lifespan. Austr N Z J Obstet Gynaecol 2015; 55:384–389. [DOI] [PubMed] [Google Scholar]
- 28.van Disseldorp J, Faddy MJ, Themmen A, De Jong FH, Peeters P, Van der Schouw YT, et al. Relationship of serum anti-Mullerian hormone concentration to age at menopause. J Clin Endocrinol Metab 2008; 93:2129–2134. [DOI] [PubMed] [Google Scholar]
- 29.Seifer DB, MacLaughlin DT. Mullerian inhibiting substance is an ovarian growth factor of emerging clinical significance. Fertil Steril 2007; 88:539–546. [DOI] [PubMed] [Google Scholar]
- 30.Fleming R, Seifer DB, Frattarelli JL, Ruman J. Assessing ovarian response: antral follicle count versus anti-Müllerian hormone. Reprod Biomed 2015; 31:486–496. [DOI] [PubMed] [Google Scholar]
- 31.Fanchin R, Taieb J, Lozano DH, Ducot B, Frydman R, Bouyer J. High reproducibility of serum anti-Mullerian hormone measurements suggests a multistaged follicular secretion and strengthens its role in the assessment of ovarian follicular status. Hum Reprod 2005; 20:923–927. [DOI] [PubMed] [Google Scholar]
- 32.Wessman M, Korsholm A, Bentzen JG, Andersen AN, Ahlström MG, Katzenstein TL, et al. Antimüllerian hormone levels are reduced in women living with human immunodeficiency virus compared to control women: a case–control study from Copenhagen, Denmark. J Virus Eradic 2018; 4:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scherzer R, Greenblatt RM, Merhi ZO, Kassaye S, LambertMesserlian G, Maki PM, et al. Use of anti-Mullerian hormone to predict the menopausal transition in HIV-infected women. Obstet Gynecol 2017; 216:46.e1–46.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seifer DB, Golub ET, Lambert-Messerlian G, Benning L, Anastos K, Watts DH, et al. Variations in serum mullerian inhibiting substance between white, black, and Hispanic women. Fertil Steril 2009; 92:1674–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seifer DB, Golub ET, Lambert-Messerlian G, Springer G, Holman S, Moxley M, et al. Biologic markers of ovarian reserve and reproductive aging: application in a cohort study of HIV infection in women. Fertil Steril 2007; 88:1645–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merhi ZO, Seifer DB, Weedon J, Adeyemi O, Holman S, Anastos K, et al. Circulating vitamin D correlates with serum anti-Mullerian hormone levels in late-reproductive-aged women: women's Interagency HIV study. Fertil Steril 2012; 98:228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zanni MV, Currier JS, Kantor A, Smeaton L, Rivard C, Taron J, et al. Correlates and timing of reproductive aging transitions in a global cohort of midlife women with human immunodeficiency virus: insights from the REPRIEVE trial. J Infect Dis 2020; 222:S20–S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sokalski KM, Mai A, Chu J, Cote H, Maan EJ, Pick N, et al. Endocrinopathy and leukocyte telomere length in HIV+ individuals in the CARMA cohort. Can J Infect Dis Med Microbiol. 22nd Annual Canadian Conference on HIV/AIDS Research, CAHR 2013.Vancouver, BC Canada. Conference Publication: (var.pagings) 2013; 24:12A. [Google Scholar]
- 39.Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res 2009; 37:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsieh AY, Saberi S, Ajaykumar A, Hukezalie K, Gadawski I, Sattha B, et al. Optimization of a relative telomere length assay by monochromatic multiplex real-time quantitative PCR on the LightCycler 480: sources of variability and quality control considerations. J Mol Diagn 2016; 18:425–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lukaszuk K, Liss J, Kunicki M, Jakiel G, Wasniewski T, Woclawek-Potocka I, et al. Anti-Müllerian hormone (AMH) is a strong predictor of live birth in women undergoing assisted reproductive technology. Reprod Biol 2014; 14:176–181. [DOI] [PubMed] [Google Scholar]
- 42.Themmen AP. Anti-Müllerian hormone: its role in follicular growth initiation and survival and as an ovarian reserve marker. JNCI Monogr 2005; 2005:18–21. [DOI] [PubMed] [Google Scholar]
- 43.Scherzer R, Bacchetti P, Messerlian G, Goderre J, Maki PM, Seifer DB, et al. Impact of CD 4 lymphocytes and HIV infection on anti-Müllerian hormone levels in a large cohort of HIV-infected and HIV-uninfected women. Am J Reprod Immunol 2015; 73:273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wessman M, Korsholm A, Bentzen JG, Andersen AN, Ahlström MG, Katzenstein TL, et al. Antimüllerian hormone levels are reduced in women living with human immunodeficiency virus compared to control women: a case–control study from Copenhagen, Denmark. J Virus Eradic 2018; 4:123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Butts S, Riethman H, Ratcliffe S, Shaunik A, Coutifaris C, Barnhart K. Correlation of telomere length and telomerase activity with occult ovarian insufficiency. J Clin Endocrinol Metab 2009; 94:4835–4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gu D, Li J, Little J, Li H, Zhang X. Associations between serum sex hormone concentrations and telomere length among US adults, 1999–2002. J Nutr Health Aging 2020; 24:48–54. [DOI] [PubMed] [Google Scholar]
- 47.Pathai S, Lawn SD, Gilbert CE, McGuinness D, McGlynn L, Weiss HA, et al. Accelerated biological ageing in HIV-infected individuals in South Africa: a case–control study. AIDS 2013; 27:2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lara-Molina EE, Franasiak JM, Marin D, Tao X, Díaz-Gimeno P, Florensa M, et al. Cumulus cells have longer telomeres than leukocytes in reproductive-age women. Fertil Steril 2020; 113:217–223. [DOI] [PubMed] [Google Scholar]
- 49.Moolhuijsen LM, Visser JA. Anti-Müllerian hormone and ovarian reserve: update on assessing ovarian function. J Clin Endocrinol Metab 2020; 105:3361–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Plante BJ, Cooper GS, Baird DD, Steiner AZ. The impact of smoking on antimüllerian hormone levels in women aged 38 to 50 years. Menopause 2010; 17:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.