PURPOSE
We aimed to study prognostic factors and efficacy of allogeneic hematopoietic stem-cell transplantation (allo-HSCT) in first remission of patients with noninfant childhood acute lymphoblastic leukemia (ALL) with 11q23/KMT2A rearrangements treated with chemotherapy regimens between 1995 and 2010.
PATIENTS AND METHODS
Data were retrospectively retrieved from 629 patients with 11q23/KMT2A-rearranged ALL from 17 members of the Ponte-di-Legno Childhood ALL Working Group. Clinical and biologic characteristics, early response assessed by minimal residual disease at the end of induction (EOI) therapy, and allo-HSCT were analyzed for their impact on outcomes.
RESULTS
A specific 11q23/KMT2A translocation partner gene was identified in 84.3% of patients, with the most frequent translocations being t(4;11)(q21;q23) (n = 273; 51.5%), t(11;19)(q23;p13.3) (n = 106; 20.0%), t(9;11)(p21_22;q23) (n = 76; 14.3%), t(6;11)(q27;q23) (n = 20; 3.8%), and t(10;11)(p12;q23) (n = 14; 2.6%); 41 patients (7.7%) had less frequently identified translocation partner genes. Patient characteristics and early response varied among subgroups, indicating large biologic heterogeneity and diversity in therapy sensitivity among 11q23/KMT2A-rearranged ALL. The EOI remission rate was 93.2%, and the 5-year event-free survival (EFS) for the entire cohort was 69.1% ± 1.9%, with a range from 41.7% ± 17.3% for patients with t(9;11)-positive T-ALL (n = 9) and 64.8% ± 3.0% for patients with t(4;11)-positive B-ALL (n = 266) to 91.2% ± 4.9% for patients with t(11;19)-positive T-ALL (n = 34). Low EOI minimal residual disease was associated with favorable EFS, and induction failure was particularly predictive of nonresponse to further therapy and relapse and poor EFS. In addition, EFS was not improved by allo-HSCT compared with chemotherapy only in patients with both t(4;11)-positive B-ALL (n = 64 v 51; P = .10) and 11q23/KMT2A-rearranged T-ALL (n = 16 v 10; P = .69).
CONCLUSION
Compared with historical data, prognosis of patients with noninfant 11q23/KMT2A-rearranged ALL has improved, but allo-HSCT failed to affect outcome. Targeted therapies are needed to reduce relapse and treatment-related mortality rates.
INTRODUCTION
Contemporary risk-adapted treatment on the basis of genetics and minimal residual disease (MRD) has improved survival rates to > 90% in childhood acute lymphoblastic leukemia (ALL).1,2 Rearrangements of the KMT2A gene (formerly MLL), involving > 100 translocation partner genes (TPGs), are detected in approximately 5% of childhood ALL.3-9 Although KMT2A rearrangements confer an inferior outcome in infant ALL, their prognostic impact in children age ≥ 1 year is less clear.9-16 In fact, there is no consensus among study groups regarding risk stratification, significance of different TPGs, and indication for allogeneic hematopoietic stem-cell transplantation (allo-HSCT) in first complete remission (CR1). In our previous study (1983-1994), we found that noninfants with KMT2A-rearranged ALL had a superior outcome compared with their infant counterparts irrespective of the type of KMT2A rearrangement.13,14 However, small numbers precluded meaningful analyses of allo-HSCT, and the impact of MRD was not assessed.13,14 To address these issues, we initiated this study of noninfants with 11q23/KMT2A-rearranged ALL, treated on contemporary, mostly MRD-based protocols, including allo-HSCT in CR1 for selected patient cohorts.17-20
CONTEXT
Key Objective
Although 11q23/KMT2A rearrangements have poor outcomes in infant acute lymphoblastic leukemia (ALL), their impact in noninfants is unclear. This international collaboration addressed outcomes and prognostic factors of noninfant 11q23/KMT2A-rearranged ALL treated with contemporary protocols, including allogeneic hematopoietic stem-cell transplantation for selected subgroups.
Knowledge Generated
This study demonstrated great clinical heterogeneity among childhood noninfant 11q23/KMT2A-rearranged ALL. Low end of induction minimal residual disease was associated with favorable event-free survival (EFS), and induction failure was predictive of resistant disease, relapse, and poor EFS. In addition, EFS was not improved by transplantation compared with chemotherapy alone in patients with both t(4;11)/KMT2A::AFF1-positive B-ALL and 11q23/KMT2A-rearranged T-ALL.
Relevance (S. Bhatia)
-
Allogeneic hematopoietic stem-cell transplantation does not influence outcomes in patients with noninfant 11q23/KMT2A-rearranged ALL, presenting the need for alternative targeted therapies.*
*Relevance section written by JCO Associate Editor Smita Bhatia, MD, MPH, FASCO.
PATIENTS AND METHODS
Data on characteristics, treatment, response including MRD, allo-HSCT, and outcomes of patients with 11q23/KMT2A-rearranged ALL age 1-18 years and treated between 1995 and 2010 were collected from 17 members of the Ponte-di-Legno Childhood ALL Group (Data Supplement, online only). 11q23/KMT2A rearrangements were defined by an 11q23-involving chromosomal aberration detected by conventional cytogenetics and/or KMT2A-split signal fluorescence in situ hybridization analyses and/or different polymerase chain reaction–based methodologies and/or Southern blots.4,5,7,21-23 The TPG was defined by the most informative method. Patients with 11q23/KMT2A deletions were excluded.13,14,24-26
Diagnosis was performed according to standard criteria.8,27 CR was defined as < 5% blasts in bone marrow and no extramedullary disease. Detection of MRD, on the basis of either immunoglobulin and T-cell receptor gene rearrangements or flow cytometry, was performed at the end of induction (EOI).28-30
Written informed consent was obtained from legal guardian(s) or patients, as appropriate. Trials and registries were conducted according to the Declaration of Helsinki and approval of local ethics committees. For nontrial/nonregistry patients, retrospective data collection was performed with institutional review board approval.
Statistical Analysis
Event-free survival (EFS) was calculated from the date of diagnosis to the date of first event (resistant disease, relapse, second malignant neoplasm, and death) and censored at the last follow-up for patients without events. Overall survival (OS) was defined as the time from diagnosis to death from any cause or last follow-up. EFS and OS curves were estimated by the Kaplan-Meier method with standard errors calculated by the Greenwood formula and compared by the log-rank test. For allo-HSCT analysis, Kaplan-Meier curves were adjusted to account for the waiting time to allo-HSCT. The influence of allo-HSCT was also analyzed with the inclusion of a time-dependent variable in Cox models and the Mantel-Byar test. Cumulative incidence functions for competing events were constructed by the method of Kalbfleisch and Prentice, including Gray's test. The Cox proportional hazard model was used for multivariable analyses.
RESULTS
Patients With 11q23/KMT2A Rearrangements
Among the 686 patients selected, 57 were excluded (Data Supplement). Therapies are shown in the Data Supplement.10,11,31-63 The male:female ratio was 1:1; the median age was 4.7 years, and the median leukocyte count was 82.9 × 109/L; 535 patients (85%) had B-ALL, and 82 (13%) had T-ALL (Table 1 and Data Supplement). EOI MRD was < 0.05% in 56%, 0.05 to < 0.5% in 23%, and ≥ 0.5% in 21% of patients (Table 1).
TABLE 1.
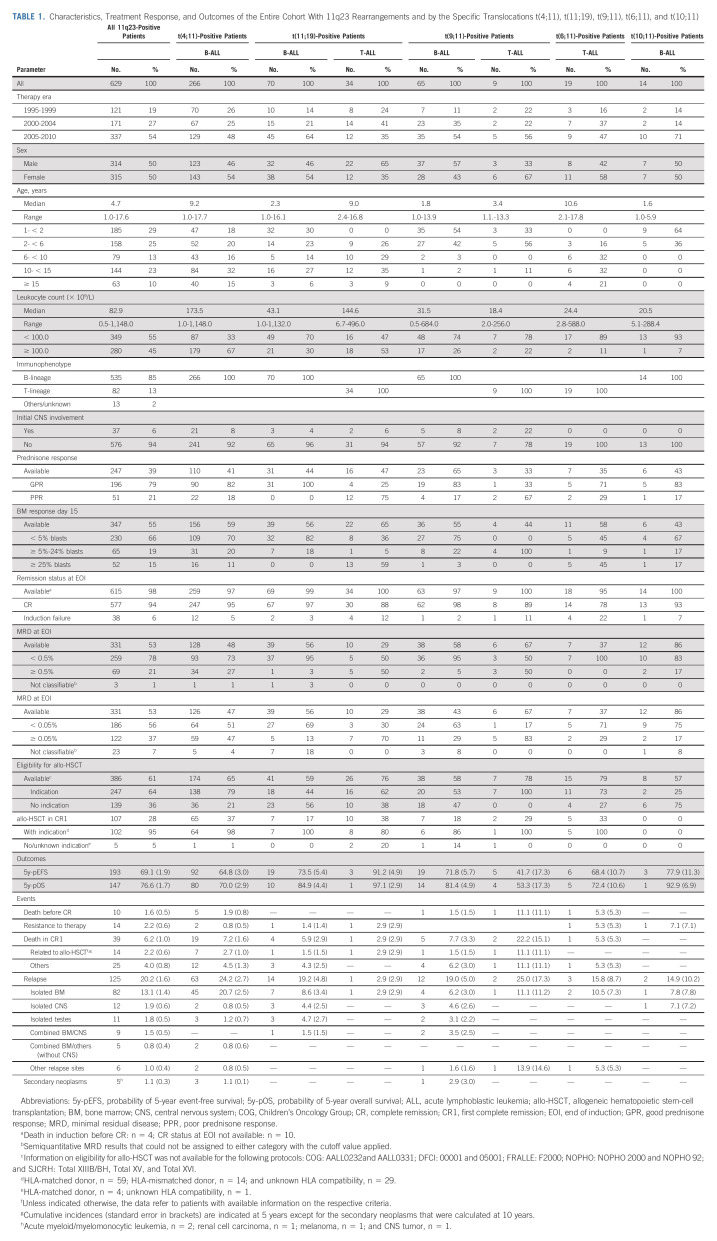
Characteristics, Treatment Response, and Outcomes of the Entire Cohort With 11q23 Rearrangements and by the Specific Translocations t(4;11), t(11;19), t(9;11), t(6;11), and t(10;11)
Among the 619 patients with EOI status data, four (0.6%) died during induction and 38 (6.1%) had induction failure (IF; 19 subsequently achieved CR); thus, the CR rate at EOI was 93.2% and the ultimate CR rate was 96.2%. With a median follow-up of 5.8 years (range, 0.1-17.5 years), the 5-year EFS probability (5y-pEFS) of the total cohort was 69.1% ± 1.9% and the 5-year OS probability (5y-pOS) was 76.6% ± 1.7%. The 5-year probability of cumulative incidence of resistance or relapse (5y-pCIRR) was 22.4% ± 1.7% (resistance, 2.2% ± 0.6%; relapse, 20.2% ± 1.6%). Two thirds of relapses were isolated bone marrow relapses (5y-CI, 13.1% ± 1.4%); 5y-CI rates of isolated testicular, isolated central nervous system, or combined central nervous system relapse were 3.6% ± 1.1%, 1.8% ± 0.5%, and 1.5% ± 0.5%, respectively. The 5y-CI of death in CR1 was 6.2% ± 1.0%; approximately one third were allo-HSCT–related (14 of 39; 36%; Table 1 and Figs 1A-1C). Five patients developed a second malignant neoplasm as a first event (10-year cumulative incidence, 1.9% ± 0.5%).
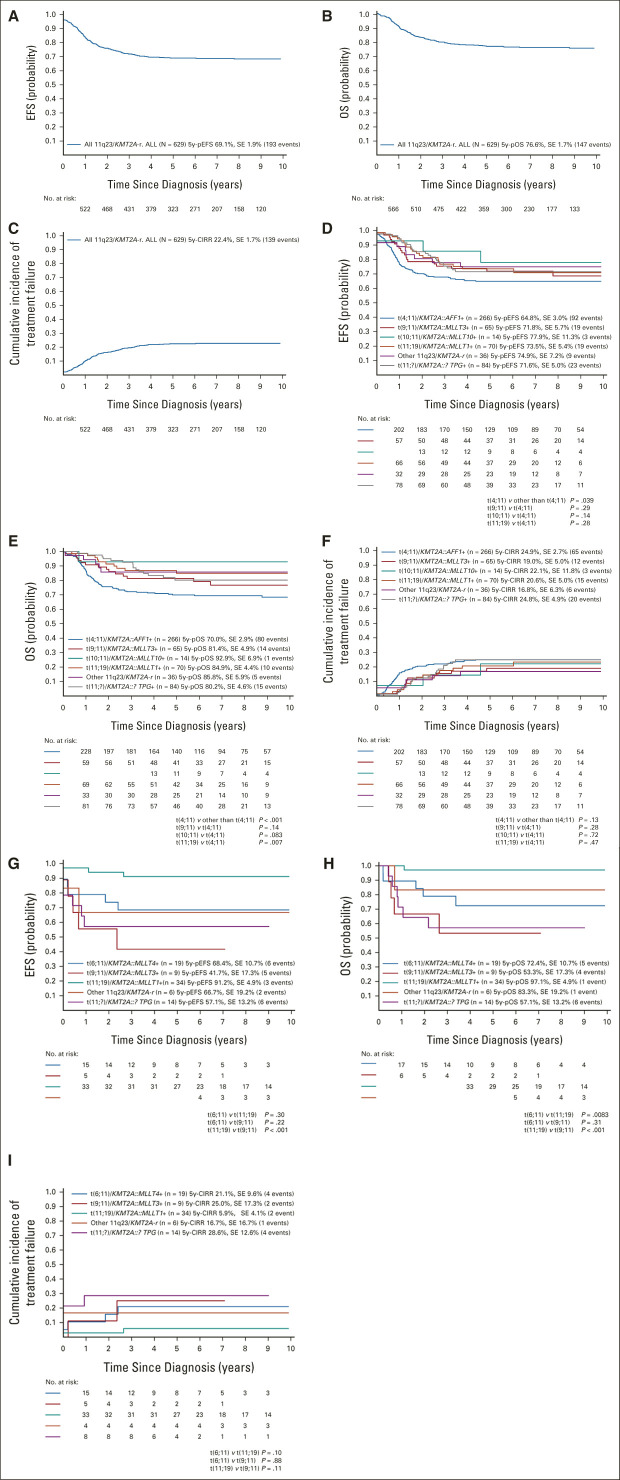
FIG 1.
Outcomes of (A-C) all patients with 11q23/KMT2A-rearranged ALL and those with (D-F) B-ALL or (G-I) T-ALL according to the type of 11q23/KMT2A rearrangement: (A, D, and G) 5-year EFS; (B, E, and H) 5-year OS; and (C, F, and I) 5-year cumulative incidence of treatment failure. 5y-pEFS, probability of 5-year event-free survival; 5y-pOS, probability of 5-year overall survival; ALL, acute lymphoblastic leukemia; EFS, event-free survival; OS, overall survival; SE, standard error.
A specific TPG was reported in 530 of 629 patients (84.3%), with the most frequent translocations being t(4;11)(51.5%), t(11;19)(20.0%), t(9;11)(14.3%), t(6;11)(3.8%), and t(10;11)(2.6%; Table 1); 7.7% had other TPGs (Data Supplement). Some TPGs were lineage-restricted (t(4;11): 266 of 267 B-ALL; t(10;11): 14 of 14 B-ALL; t(6;11): 19 of 20 T-ALL), whereas others were variable (t(11;19): 70 of 104 B-ALL; t(9;11): 65 of 74 B-ALL; Table 1).
t(4;11)(q21;q23)/KMT2A::AFF1.
Patients with t(4;11)-positive ALL were significantly older with 46.6% age ≥ 10 years compared with 13.3% of other patients with 11q23/KMT2A-rearranged B-ALL (P < .001), with age peaks in early childhood and adolescence (Table 1 and Data Supplement). The male:female ratio showed a female preponderance in younger patients (< 10 years 0.71, ≥ 10 years 1.07). Patients with t(4;11)-positive B-ALL had leukocyte counts ≥ 100 × 109/L more often than other patients with 11q23/KMT2A-rearranged B-ALL (67.3% v 26.7%; P < .001), which was more pronounced in adolescents (< 10 years 56.3%, ≥ 10 years 79.8%).
Consistent with their inferior early response, t(4;11)-positive patients had a worse 5y-pEFS (64.6% ± 2.9%) and 5y-pOS (70.0% ± 2.8%) and higher 5y-pCIRR (24.9% ± 2.7%) compared with other patients with 11q23-rearranged B-ALL (73.5% ± 3.3%, P = .039; 84.5% ± 2.7%, P < .001; 19.4% ± 3.0%, P = .13, respectively; Table 1 and Figs 1D-1F). Survival postrelapse was particularly poor in t(4;11)-positive B-ALL with a 5y-pOS of 12.9% ± 4.5% (n = 63, 53 deaths) compared with 38.1% ± 11.4% in other 11q23-rearranged B-ALL (n = 32, 16 deaths; P = .0011; Data Supplement).
t(11;19)(q23;p13.3)/KMT2A::MLLT1.
There was a higher proportion of patients with leukocyte counts ≥ 100 × 109/L at presentation in patients with t(11;19)-positive T-ALL (n = 34) compared with patients with B-ALL (n = 70; 52.9% v 30.0%; P = .023, Table 1). Age distribution also differed significantly (Table 1 and Data Supplement). Response to induction therapy was poor in patients with t(11;19)-positive T-ALL (Table 1) despite their excellent outcome: 5y-pEFS 91.2% ± 4.9%, 5y-pOS 97.1% ± 2.9%, and 5y-pCIRR 5.9% ± 4.1% v 73.5% ± 5.4% (P = .032), 84.9% ± 4.4% (P = .076), and 20.6% ± 5.0% (P = .04), respectively, in those with t(11;19)-positive B-ALL (Table 1 and Figs 1D-1I).
Comparison of patients with t(11;19)-positive B-ALL with patients with t(4;11)-positive B-ALL revealed similar age peaks in early childhood and adolescence (Data Supplement). Patients with t(11;19)-positive B-ALL had significantly less hyperleukocytosis (P < .001) and better early response than patients with t(4;11)-positive B-ALL (Table 1). Although not translating into a lower rate of resistance/relapse (P = .47), EFS tended to be higher (P = .14) and OS was significantly better (P = .007) in patients with t(11;19)-positive than in patients with t(4;11)-positive B-ALL (Figs 1D-1F).
t(9;11)(p21-22;q23)/KMT2A::MLLT3.
Nine patients with t(9;11)-positive T-ALL had inferior EOI MRD results and worse 5y-pEFS (41.7% ± 17.3%) and 5y-pOS (53.3% ± 17.3%) rates compared with the 65 patients with t(9;11)-positive B-ALL (71.8% ± 5.7%, P = .022; 81.4% ± 4.9%, P = .044; Table 1 and 1D-1E and 1G-1H). However, the 5y-pCIRR (25.0% ± 17.3% v 19.0% ± 5.0%) was not significantly different (P = .67) between the two cohorts (Table 1 and Fig 1F/1I).
The age distribution of t(9;11)-positive B-ALL was different from that of patients with t(4;11)-positive B-ALL, with 83.1% and 23.7% of patients age < 3 years, respectively (P < .001; Data Supplement). Patients with t(9;11)-positive ALL were significantly less often to have leukocyte counts ≥ 100 × 109/L compared with those with t(4;11)-positive B-ALL (26.2% v 67.3%; P < .001, Table 1). EOI MRD was significantly lower in t(9;11)-positive patients compared with patients with t(4;11)-positive B-ALL (MRD: < v ≥ 0.5%; P = .005, Table 1). EFS, OS, and CIRR of patients with t(9;11)-positive ALL were superior to those with t(4;11)-positive B-ALL although the differences were not significant (Figs 1D-1F).
t(6;11)(q27;q23)/KMT2A::MLLT4.
This small group comprised only 19 patients with T-ALL who were distributed across all ages (Data Supplement). Only 10.5% had leukocyte counts of ≥ 100 × 109/L compared with 22.2% in patients with t(9;11)-positive T-ALL and 52.9% in patients with t(11;19)-positive T-ALL (P = .006, Table 1).
The IF rate was 22.2%, the 5y-pEFS was 68.4% ± 10.7%, the 5y-pOS was 72.4% ± 10.6%, and the 5y-pCIRR was 21.1% ± 9.6% (Table 1 and Figs 1G-1I). These outcomes were significantly inferior to those with t(11;19)-positive T-ALL (P = .030, .0083, .10), but EFS and OS were superior to those with t(9;11)-positive T-ALL, although not statistically significant (P = .22, .31; Table 1 and Figs 1G-1I).
t(10;11)(p12;q23)/KMT2A::MLLT10.
All 14 t(10;11)-positive patients were age < 6 years, with 64.3% age < 2 years, and therefore, younger than patients with t(4;11)-positive B-ALL (P < .001; Data Supplement). Only 7.1% had leukocyte counts ≥ 100 × 109/L, which was lower than that in patients with t(4;11)-positive B-ALL (P < .001; Table 1). Patients with t(10;11)-positive B-ALL had a 5y-pEFS of 77.9% ± 11.3%, a 5y-pOS of 92.9% ± 6.9%, and a 5y-pCIRR of 22.1% ± 11.8%, for which EFS and OS were not significantly different from those in patients with t(4;11)-positive B-ALL (EFS: P = .14; OS: P = .083; Table 1 and Figs 1D-1F).
11q23/KMT2A rearrangements with other and unknown TPGs.
Outcomes of 41 patients with other 11q23/KMT2A translocations and 99 without a known TPG are shown in the Data Supplement and Figures 1D-1I.
Prognostic Factors
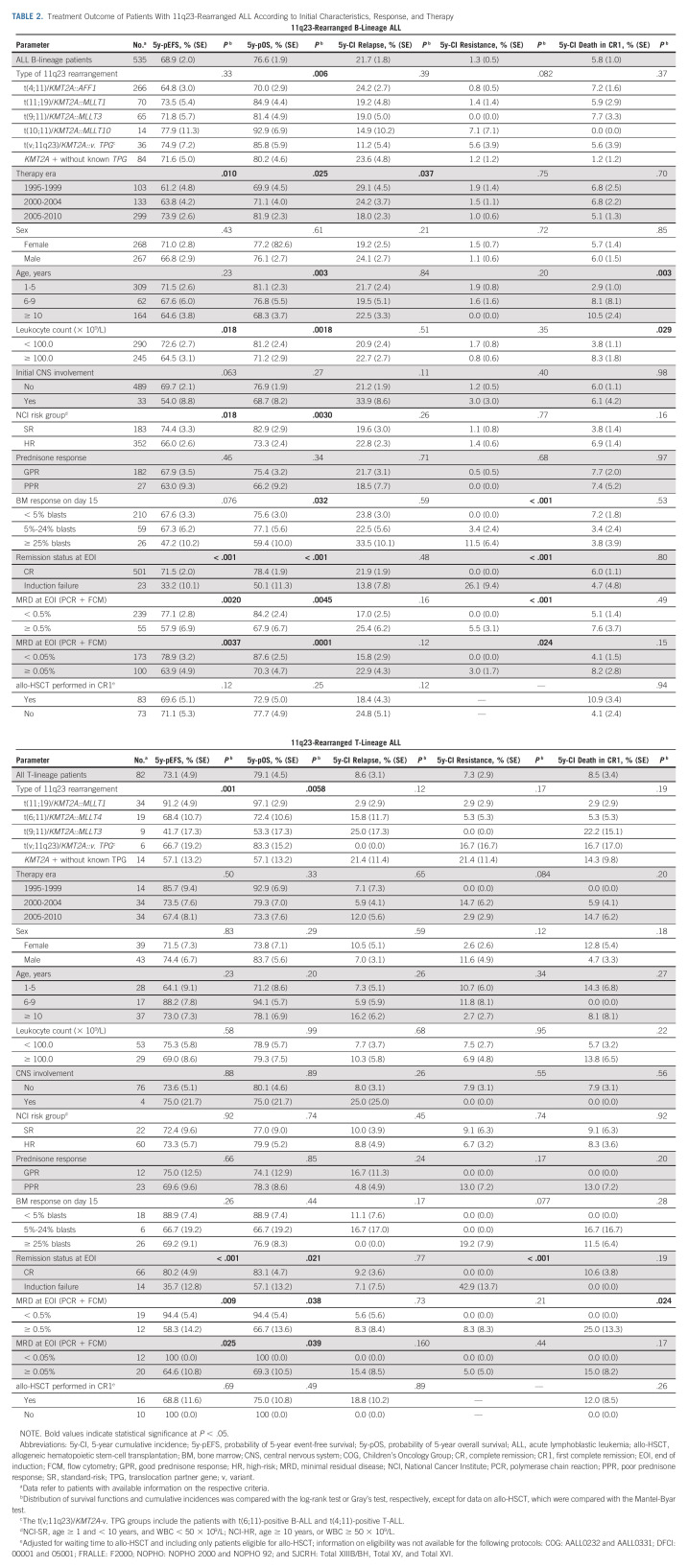
11q23/KMT2A-rearranged B-ALL.
The 5y-pOS of 76.6% ± 1.9% in 11q23/KMT2A-rearranged B-ALL was only slightly higher than the 5y-pEFS of 68.9 ± 2.0, because of not only high treatment-related mortality, accounting for 24% of the events, but also poor survival after relapse. The postrelapse 5y-pOS of the 114 patients with B-ALL who relapsed was only 24.1% ± 4.5% (81 deaths, Data Supplement). Time to relapse was prognostically significant with a 5y-pOS of 14.1% ± 4.3% for relapses occurring within 18 months of initial diagnosis (n = 69, 60 deaths) versus 41.6% ± 9.6% for later relapses (n = 45, 21 deaths; Data Supplement). The effect of time to relapse was not apparent in t(4;11)-positive patients (5y-pOS after very early v later relapse: 12.8% ± 4.9% v 12.1% ± 10.3%; P = .22; Data Supplement) but was greater in other 11q23-rearranged patients (n = 16, 12.5% ± 8.3% v n = 16, 91.7% ± 8.0%; P = .0001; Data Supplement).
Treatment during the later era (2005-2010 v 1995-2005) was associated with superior EFS and OS, mainly because of lower relapse events. Considering the different distribution of t(4;11)-positive patients in the later and earlier treatment periods (Table 1), multivariable analysis including t(4;11) status and treatment era showed independent significance for improved EFS and OS of the later era (data not shown).
Although older age did not significantly influence relapse or EFS in the whole group of 11q23-rearranged B-ALL, it was significantly associated with inferior OS. This was partly attributable to not only a significantly higher death in CR rate but also the exceptionally poor survival after relapse in patients age ≥ 10 years (postrelapse 5y-pOS: age ≥ 10 years: 9.4% ± 5.1% [n = 36, 32 deaths], < 10 years: 31.7% ± 5.8% [n = 78, 49 deaths]; P < .001; Data Supplement). This was especially true for t(4;11)-positive patients (age ≥ 10 years: postrelapse 5y-pOS: 3.3% ± 3.3% [n = 30, 29 deaths]; Data Supplement) although the number of relapses in the corresponding group with other 11q23 rearrangements was too small for evaluation (Data Supplement). Multivariable analysis for risk of reduced postrelapse survival revealed independent significance of age ≥ 10 years and a time to relapse of < 18 months with hazard ratios of 2.45 (P < .001) and 2.58 (P = .0032), respectively.
Poor response assessed morphologically on day 15, at EOI, and by MRD was predictive of resistant disease and related to poor EFS and OS (Table 2). The prognostic impact of response to induction was also evident in t(4;11)-rearranged B-ALL (Data Supplement and Figs 2C-2D) or other 11q23 rearrangements (Data Supplement) although statistical significance was not reached for all response parameters.
TABLE 2.
Treatment Outcome of Patients With 11q23-Rearranged ALL According to Initial Characteristics, Response, and Therapy
FIG 2.
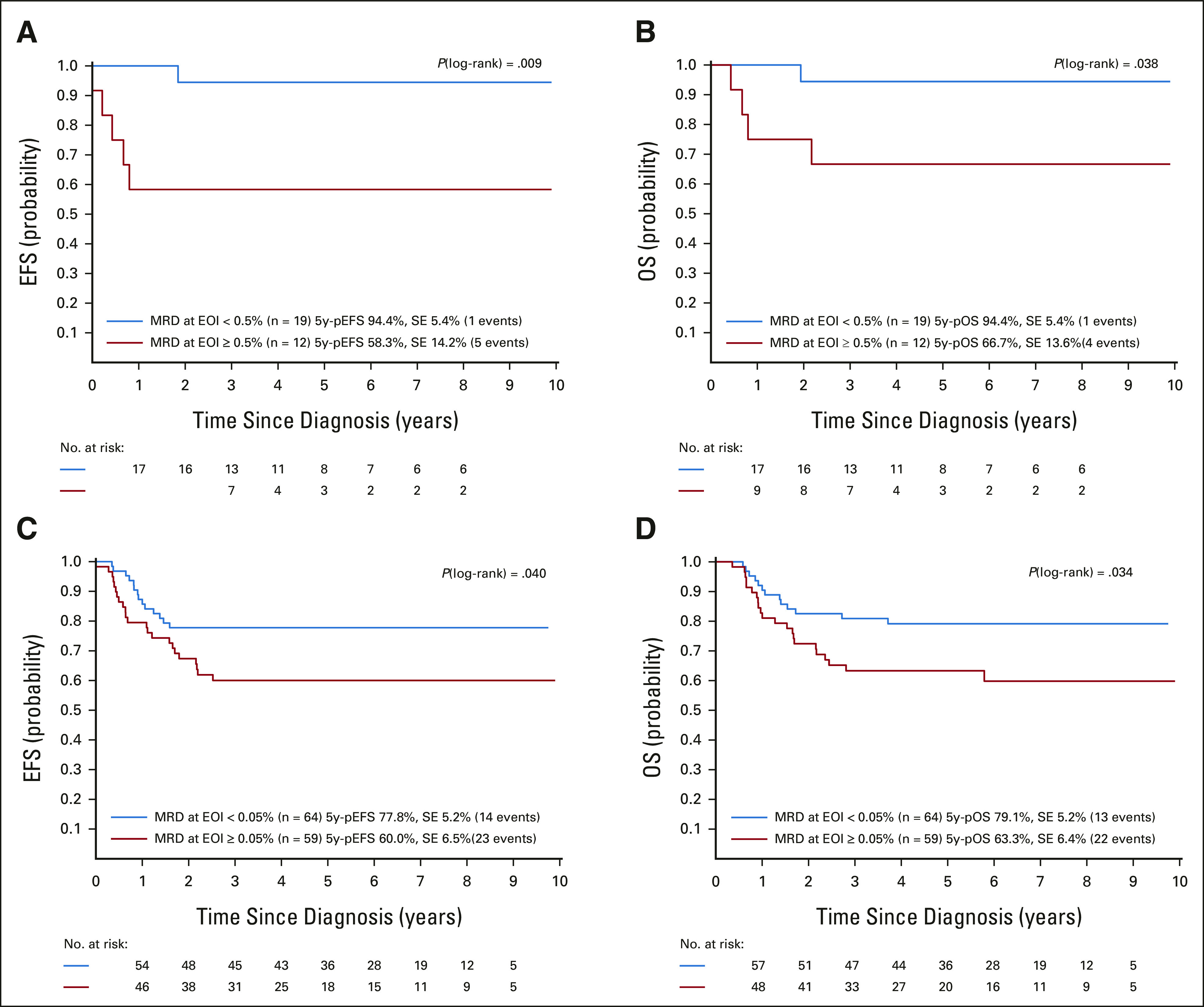
Outcomes of (A and B) patients with 11q23/KMT2A-rearranged T-ALL and (C and D) patients with t(4;11)/KMT2A-AFF1–rearranged B-ALL according to MRD levels at the EOI with a cutoff of 0.5% or 0.05% blasts, respectively. (A and C) 5-Year EFS; (B and D) 5-year OS. 5y-pEFS, probability of 5-year event-free survival; 5y-pOS, probability of 5-year overall survival; ALL, acute lymphoblastic leukemia; EFS, event-free survival; EOI, end of induction; MRD, minimal residual disease; OS, overall survival; SE, standard error.
The overall outcomes of the 535 patients with 11q23/KMT2A-rearranged B-ALL with regard to initial characteristics and response and by 11q23/KMT2A rearrangement status (t(4;11)-positive v others) are shown in the Data Supplement.
Multivariable analyses were performed with t(4;11) status, sex, age, leukocyte counts, and remission status at EOI as covariates, with and without MRD. In both models, IF remained an independent poor prognostic factor for EFS, OS, and treatment failure because of resistance/relapse, although only partly reaching statistical significance (Data Supplement, with additional analyses in the Data Supplement).
11q23/KMT2A-rearranged T-ALL.
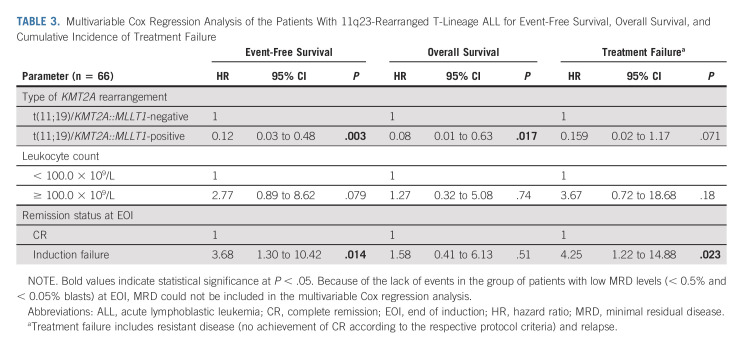
Analyses of the 82 patients with 11q23/KMT2A-rearranged T-ALL were limited by small numbers of patients and events (Table 2). Considering these limitations, no prognostic effect was evident, except for the presence of t(11;19) (Figs 1G-1I) and good morphologic and MRD response at EOI, which were associated with superior EFS and OS (Figs 2A and 2B). IF and EOI MRD ≥ 0.5% indicated a higher cumulative risk of resistance and death in CR1, respectively (Table 2).
Because of the limited number of events in patients with available MRD data, multivariable analysis was performed in a model including t(11;19) status, leukocyte counts, and remission status at EOI without MRD (Table 3). The t(11;19) showed favorable independent significance for EFS and OS, with borderline significance for treatment failure because of resistance/relapse. In addition, IF was significantly associated with inferior EFS and treatment failure.
TABLE 3.
Multivariable Cox Regression Analysis of the Patients With 11q23-Rearranged T-Lineage ALL for Event-Free Survival, Overall Survival, and Cumulative Incidence of Treatment Failure
Allogeneic Hematopoietic Stem-Cell Transplantation
The role of allo-HSCT was evaluated in patients with t(4;11)-positive B-ALL and the entire T-ALL cohort, including only those patients who were eligible for allo-HSCT according to the protocol (Data Supplement). There were no significant differences in EFS or OS between the 64 patients with t(4;11)-positive B-ALL who underwent allo-HSCT and the 51 patients who received chemotherapy alone (Figs 3A and 3B). When the analyses were stratified by EOI MRD (< 0.05% or ≥ 0.05%), there were also no differences between the groups (Data Supplement). Results were also not statistically different for the 16 and 10 patients with 11q23/KMT2A-rearranged T-ALL who received either allo-HSCT or chemotherapy only, respectively (Figs 3C and 3D). Cox regression analyses, including allo-HSCT as a time-dependent variable, did not show an advantage for allo-HSCT in t(4;11)-positive B-ALL, but rather indicated a disadvantage for 11q23/KMT2A-rearranged T-ALL (data not shown).
FIG 3.
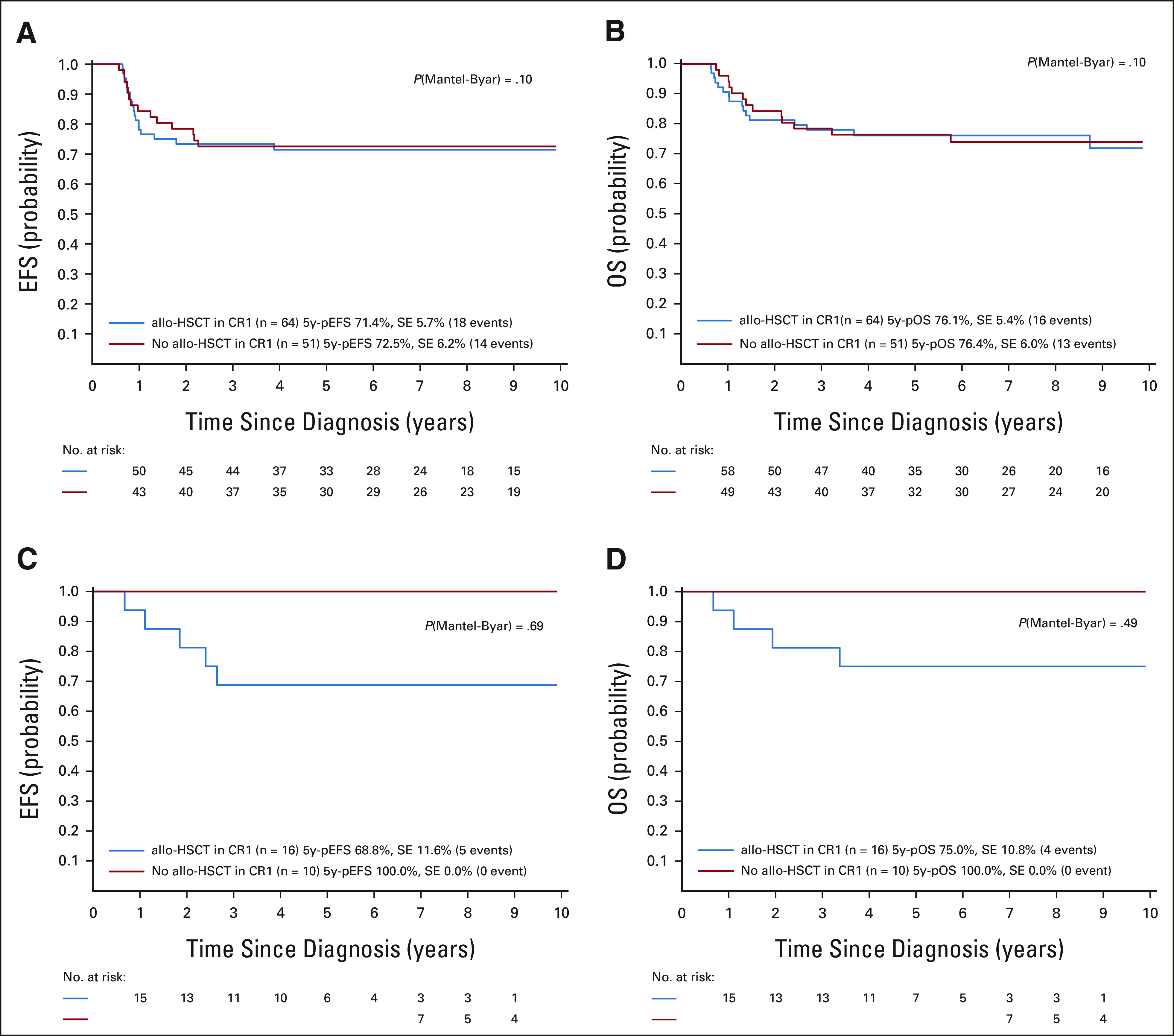
Outcomes of (A and B) patients with t(4;11)/KMT2A-AFF1–rearranged B-ALL and (C and D) patients with 11q23/KMT2A-rearranged T-ALL according to therapy performed (allo-HSCT or chemotherapy only, adjusted by waiting time to allo-HSCT [landmark of 0.54 years]): (A and C) 5-year EFS and (B and D) 5-year OS. 5y-pEFS, probability of 5-year event-free survival; 5y-pOS, probability of 5-year overall survival; ALL, acute lymphoblastic leukemia; allo-HSCT, allogeneic hematopoietic stem-cell transplantation; CR1, first complete remission; EFS, event-free survival; OS, overall survival; SE, standard error.
DISCUSSION
This study reports the largest cohort of children age ≥ 1 year with a wide variety of 11q23/KMT2A-rearranged ALL subgroups (Data Supplement). With a median presenting leukocyte count of 82.9 × 109/L, 11q23/KMT2A-positive patients had markedly higher leukocyte counts compared with the general ALL population. The 5-year EFS for the entire cohort was 69.1%, but differed according to TPGs and immunophenotype. Overall, the results were superior to the outcomes of not only noninfants in our previous study but also infant 11q23/KMT2A-rearranged B-ALL.10,13,14,16 For 11q23-rearranged B-ALL, the outcome was improved over the years, likely because of combinations of better chemotherapies (such as augmented Berlin-Frankfurt-Münster regimens, high-dose methotrexate at 5 g/m2 given repeatedly over 24 hours as a continuous infusion, and use of dexamethasone), MRD- and immunophenotype-based stratifications, and advances in supportive care.64,65 Notably, deaths in CR1 accounted for 23% of postremission events, similar to other high-risk ALL.65,66 For patients with B-ALL, survival was generally poor postrelapse, especially in adolescents, t(4;11)-positive patients, and those experiencing very early relapses. Most deaths after relapse occurred within the first few months postrelapse, indicating that high mortality from relapse therapy and failure to achieve a second remission were crucial issues. Notable exceptions were those subgroups with rearrangements other than t(4;11) and relapse ≥ 18 months after diagnosis. These 16 patients had a 5y-pOS of 91.7% ± 8.0% postrelapse.
Our data highlight the heterogeneity among childhood noninfant ALL with 11q23/KMT2A rearrangements, which has not been hitherto clearly documented.12-14,18,67-73 The heterogeneity is also reflected in the different age distributions depending on the TPG. The peak incidence in early childhood observed in all B-ALL subgroups indicates a bridge to infant ALL, in which most patients have 11q23/KMT2A rearrangements. The second peak observed in adolescence for t(4;11)- and t(11;19)-positive B-ALL is likely related to different disease biology, which is, at least in t(4;11)-positive patients, also indicated by different age-dependent patient characteristics (sex distribution and leukocyte count). Unsurprisingly, among the B-ALL cohort, t(4;11)-positive patients had the highest proportion of high EOI MRD, the lowest EFS, and highest risk of resistance and relapse. The most frequent 11q23 translocations in T-ALLs were t(11;19), t(6;11), and t(9;11). Age distribution significantly differed from that of B-ALL without an evident peak incidence in early childhood. Notably, t(11;19)-positive T-ALLs differed from their B-ALL counterpart with respect to clinical characteristics, including age and sex. As t(6;11)- and t(9;11)-positive T-ALL subgroups, response to induction therapy was particularly poor in patients with t(11;19)-positive T-ALL. However, in contrast to the t(6;11)- or t(9;11)-positive T-ALLs, t(11;19)-positive T-ALL had excellent outcomes (5y-pEFS 91.2% and 5y-pOS 97.1%). These data confirm the results of our previous study and others suggesting a good outcome for t(11;19)-positive T-ALL.16 Comparison of our cohort with published infant 11q23/KMT2A-rearranged ALL suggested that the differences and similarities between cohorts partly reflect the age-dependent incidences of the different 11q23/KMT2A rearrangements.10,11,16 Interestingly, young children age 1-2 years showed some similarities with infants, which also distinguished them from older patients, such as the well-known female predominance and the small proportion of T-ALL (2.2%). Regarding other features, however, this young age group differed from infants, showing a divergent relative distribution of various 11q23 translocations (lower proportion of t(4;11) and t(11;19) and higher proportion of t(9;11)), less frequent hyperleukocytosis, and better outcomes than those of their Interfant-06 counterparts.13 In the Interfant-99 and Interfant-06 studies, age-related prognostic differences were also observed, with increasing age being associated with improved outcomes.13,14 This effect seemed to continue beyond the first birthday. Conceivably, in 11q23/KMT2A-rearranged childhood ALL, patient age at diagnosis may reflect time from the prenatal evolution until clinical manifestation of the leukemia, therefore reflecting the aggressiveness of disease. The underlying biology of these different progressions, however, is unclear.
Our study showed no benefit of allo-HSCT in the two large subgroups of t(4;11)-positive B-ALL and 11q23/KMT2A-rearranged T-ALL. These data, however, have limitations because of presumed heterogeneity in selection of patients on the basis of different allo-HSCT eligibility criteria. In addition, analyses stratified by MRD at EOI to control for a potential MRD-related bias between patients treated with allo-HSCT or chemotherapy resulted in very small subgroups. Nevertheless, these data show that routine allo-HSCT in CR1 is not indicated for patients with 11q23/KMT2A-positive ALL. Whether pretransplant consolidation with novel immunotherapeutics can induce a deeper MRD remission for and less toxicity of a subsequent successful allo-HSCT remains to be determined.
Apart from the genetic- and phenotype-based subgroup analyses, which limited the power of the results within the minor genetic subgroups, further limitations of our study include the retrospective collection of data over a 15-year period. An unknown number of patients with 11q23/KMT2A-rearranged ALL might have been missed by the participating groups because of different screening methods. Moreover, chemotherapies, risk stratification, and allo-HSCT conduction were heterogeneous, and comparison of allo-HSCT and chemotherapy only was not based on a randomized study.
In conclusion, results from noninfants with 11q23/KMT2A-rearranged ALL in our study were superior to those of corresponding patients with infant ALL and had improved in comparison with our historical cohort.16,17 Nevertheless, outcomes remain clearly inferior to those of childhood ALL overall, and for most relapsed patients, their chances of rescue were extremely low. Despite the heterogeneity, no genetic subgroup could be identified with an excellent prognosis, with the exception of t(11;19)-positive T-ALL. Although early therapy response was shown to be prognostically important, the superior outcome of 11q23/KMT2A-rearranged patients with favorable EOI status appeared to remain below the 90%-95% level expected from other MRD-defined low-risk ALL subgroups.47 Moreover, as no benefit was shown from allo-HSCT in both t(4;11)-positive B-ALL and 11q23/KMT2A-rearranged T-ALL, alternatives to conventional treatments are required for improved outcomes.74-80 As our cohort of patients stems from a preimmunotherapy era, this may be eventually achieved through novel immunotherapeutic approaches including blinatumomab or inotuzumab ozogamicin, as well as chimeric antigen-receptor T cells and improved procedures of allo-HSCT.
ACKNOWLEDGMENT
We thank all participating institutions and physicians for their support of the study. This Ponte-di-Legno paper was written on behalf of the Berlin-Frankfurt-Münster (BFM) Study Group (Austria, Germany, Switzerland, and Czech Republic), Associazione Italiana Ematologia e Oncologia Pediatrica (AIEOP), Cooperative Study Group for ALL (CoALL; Germany), Children's Oncology Group (COG), Dutch Childhood Oncology Group (DCOG), European Organization for Research and Treatment of Cancer (EORTC), Dana-Farber Cancer Institute (DFCI), French Acute Lymphoblastic Leukemia (FRALLE) Study Group, Japan Association of Childhood Leukemia Study (JACLS), Nordic Society of Pediatric Hematology and Oncology (NOPHO), St Jude Children's Research Hospital (SJCRH), Tokyo Children's Cancer Study Group (TCCSG), United Kingdom Children's Cancer and Leukemia Study Group (CCLG), and Israel's Society of Pediatric Hematology and Oncology.
Rob Pieters
Honoraria: Jazz Pharmaceuticals, Kite, a Gilead company, Novartis, Servier/Pfizer
Consulting or Advisory Role: Kite, a Gilead company, Jazz Pharmaceuticals, Servier
Travel, Accommodations, Expenses: Kite, a Gilead company, Jazz Pharmaceuticals, Servier
Valentino Conter
Consulting or Advisory Role: Amgen
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
Kjeld Schmiegelow
Stock and Other Ownership Interests: Novo Nordisk
Consulting or Advisory Role: Illumina, Jazz Pharmaceuticals, Servier
Speakers' Bureau: Medscape
Research Funding: Novo Nordisk
Barbara De Moerloose
Uncompensated Relationships: Novartis (Inst), Gilead Sciences (Inst)
Keizo Horibe
Honoraria: Amgen
Consulting or Advisory Role: Kyowa Kirin Co, Ltd, Novartis
Speakers' Bureau: Chugai Pharma, Takeda
André Baruchel
Leadership: DBV Technologies, Lysogen, MedDay Pharmaceuticals, Ascendis Pharma
Stock and Other Ownership Interests: DBV Technologies, Ascendis Pharma
Honoraria: Novartis, Kite, a Gilead company, AstraZeneca
Consulting or Advisory Role: Jazz Pharmaceuticals, Novartis, Servier, Celgene
Research Funding: Jazz Pharmaceuticals (Inst), Servier (Inst)
Travel, Accommodations, Expenses: Jazz Pharmaceuticals, Sanofi, Servier
Stephen P. Hunger
Stock and Other Ownership Interests: Amgen, Merck
Honoraria: Jazz Pharmaceuticals, Servier/Pfizer
Lewis B. Silverman
Consulting or Advisory Role: Servier, Syndax, Jazz Pharmaceuticals
Research Funding: Servier (Inst)
Anja Möricke
Consulting or Advisory Role: Clinigen Group, BTG
Sarah Elitzur
Honoraria: Novartis, Medison
Consulting or Advisory Role: Amgen
Mignon L. Loh
Consulting or Advisory Role: MediSix Therapeutics
Martin Schrappe
Consulting or Advisory Role: Novartis, Servier, Jazz Pharmaceuticals
Speakers' Bureau: Servier, Jazz Pharmaceuticals
Research Funding: Shire (Inst), Novartis (Inst), Servier (Inst)
Travel, Accommodations, Expenses: Jazz Pharmaceuticals, Servier
Andishe Attarbaschi
Honoraria: Jazz Pharmaceuticals, Amgen, Novartis
Consulting or Advisory Role: Jazz Pharmaceuticals, Amgen, Novartis, Takeda Science Foundation
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
Ching-Hon Pui
Leadership: Adaptive Biotechnologies
Honoraria: Novartis
Consulting or Advisory Role: Adaptive Biotechnologies
Research Funding: National Cancer Institute
Meenakshi Devidas
Honoraria: Novartis
Ajay Vora
Consulting or Advisory Role: Janssen Oncology, Novartis Pharmaceuticals UK Ltd
No other potential conflicts of interest were reported.
SUPPORT
Supported by NIH grants U10 CA98543 and U10 CA180886 (COG Chair's grants; M.L.L. and S.P.H.), U10 CA98413 and U10 CA180899 (COG Statistics and Data Center grants; M.D.), St Baldrick’s Foundation (M.D., S.P.H., and M.L.L.), the National Cancer Institute (grant CA21765; C.-H.P.), and the American Lebanese Syrian Associated Charities (M.D. and C.-H.P.).
A.A., A.M., and C.J.H. are cofirst authors; M.S. and M.Z. are colast authors.
AUTHOR CONTRIBUTIONS
Conception and design: Andishe Attarbaschi, Anja Möricke, Christine J. Harrison, Georg Mann, André Baruchel, Gabriele Escherich, Stephen P. Hunger, Rob Pieters, Kjeld Schmiegelow, Ching-Hon Pui, Martin Schrappe, Martin Zimmermann
Administrative support: Andishe Attarbaschi
Provision of study materials or patients: Andishe Attarbaschi, Anja Möricke, Georg Mann, André Baruchel, Sarah Elitzur, Gabriele Escherich, Rob Pieters, Kjeld Schmiegelow, Ajay Vora, Ching-Hon Pui
Collection and assembly of data: Andishe Attarbaschi, Anja Möricke, Christine J. Harrison, André Baruchel, Valentino Conter, Meenakshi Devidas, Sarah Elitzur, Gabriele Escherich, Keizo Horibe, Atsushi Manabe, Rob Pieters, Kjeld Schmiegelow, Lewis B. Silverman, Jan Stary, Ajay Vora, Ching-Hon Pui, Martin Schrappe, Martin Zimmermann
Data analysis and interpretation: Andishe Attarbaschi, Anja Möricke, Christine J. Harrison, Georg Mann, André Baruchel, Stephen P. Hunger, Mignon L. Loh, Rob Pieters, Kjeld Schmiegelow, Jan Stary, Ajay Vora, Martin Schrappe, Martin Zimmermann
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Outcomes of Childhood Noninfant Acute Lymphoblastic Leukemia With 11q23/KMT2A Rearrangements in a Modern Therapy Era: A Retrospective International Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Rob Pieters
Honoraria: Jazz Pharmaceuticals, Kite, a Gilead company, Novartis, Servier/Pfizer
Consulting or Advisory Role: Kite, a Gilead company, Jazz Pharmaceuticals, Servier
Travel, Accommodations, Expenses: Kite, a Gilead company, Jazz Pharmaceuticals, Servier
Valentino Conter
Consulting or Advisory Role: Amgen
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
Kjeld Schmiegelow
Stock and Other Ownership Interests: Novo Nordisk
Consulting or Advisory Role: Illumina, Jazz Pharmaceuticals, Servier
Speakers' Bureau: Medscape
Research Funding: Novo Nordisk
Barbara De Moerloose
Uncompensated Relationships: Novartis (Inst), Gilead Sciences (Inst)
Keizo Horibe
Honoraria: Amgen
Consulting or Advisory Role: Kyowa Kirin Co, Ltd, Novartis
Speakers' Bureau: Chugai Pharma, Takeda
André Baruchel
Leadership: DBV Technologies, Lysogen, MedDay Pharmaceuticals, Ascendis Pharma
Stock and Other Ownership Interests: DBV Technologies, Ascendis Pharma
Honoraria: Novartis, Kite, a Gilead company, AstraZeneca
Consulting or Advisory Role: Jazz Pharmaceuticals, Novartis, Servier, Celgene
Research Funding: Jazz Pharmaceuticals (Inst), Servier (Inst)
Travel, Accommodations, Expenses: Jazz Pharmaceuticals, Sanofi, Servier
Stephen P. Hunger
Stock and Other Ownership Interests: Amgen, Merck
Honoraria: Jazz Pharmaceuticals, Servier/Pfizer
Lewis B. Silverman
Consulting or Advisory Role: Servier, Syndax, Jazz Pharmaceuticals
Research Funding: Servier (Inst)
Anja Möricke
Consulting or Advisory Role: Clinigen Group, BTG
Sarah Elitzur
Honoraria: Novartis, Medison
Consulting or Advisory Role: Amgen
Mignon L. Loh
Consulting or Advisory Role: MediSix Therapeutics
Martin Schrappe
Consulting or Advisory Role: Novartis, Servier, Jazz Pharmaceuticals
Speakers' Bureau: Servier, Jazz Pharmaceuticals
Research Funding: Shire (Inst), Novartis (Inst), Servier (Inst)
Travel, Accommodations, Expenses: Jazz Pharmaceuticals, Servier
Andishe Attarbaschi
Honoraria: Jazz Pharmaceuticals, Amgen, Novartis
Consulting or Advisory Role: Jazz Pharmaceuticals, Amgen, Novartis, Takeda Science Foundation
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
Ching-Hon Pui
Leadership: Adaptive Biotechnologies
Honoraria: Novartis
Consulting or Advisory Role: Adaptive Biotechnologies
Research Funding: National Cancer Institute
Meenakshi Devidas
Honoraria: Novartis
Ajay Vora
Consulting or Advisory Role: Janssen Oncology, Novartis Pharmaceuticals UK Ltd
No other potential conflicts of interest were reported.
REFERENCES
- 1.Mullighan CG: New strategies in acute lymphoblastic leukemia: Translating advances in genomics into clinical practice. Clin Cancer Res 17:396-400, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Pui CH, Carroll WL, Meshinchi S, et al. : Biology, risk stratification, and therapy of pediatric acute leukemias: An update. J Clin Oncol 29:551-565, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Enshaei A, O'Connor D, Bartram J, et al. : A validated novel continuous prognostic index to deliver stratified medicine in pediatric acute lymphoblastic leukemia. Blood 135:1438-1446, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Meyer C, Burmeister T, Groger D, et al. : The MLL recombinome of acute leukemias in 2017. Leukemia 32:273-284, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer C, Hofmann J, Burmeister T, et al. : The MLL recombinome of acute leukemias in 2013. Leukemia 27:2165-2176, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer C, Kowarz E, Hofmann J, et al. : New insights to the MLL recombinome of acute leukemias. Leukemia 23:1490-1499, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Schieck M, Lentes J, Thomay K, et al. : Implementation of RNA sequencing and array CGH in the diagnostic workflow of the AIEOP-BFM ALL 2017 trial on acute lymphoblastic leukemia. Ann Hematol 99:809-818, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrison CJ, Haas O, Harbott J, et al. : Detection of prognostically relevant genetic abnormalities in childhood B-cell precursor acute lymphoblastic leukaemia: Recommendations from the Biology and Diagnosis Committee of the International Berlin-Frankfurt-Munster Study Group. Br J Haematol 151:132-142, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Forgione MO, McClure BJ, Eadie LN, et al. : KMT2A rearranged acute lymphoblastic leukaemia: Unravelling the genomic complexity and heterogeneity of this high-risk disease. Cancer Lett 469:410-418, 2020 [DOI] [PubMed] [Google Scholar]
- 10.Pieters R, De Lorenzo P, Ancliffe P, et al. : Outcome of infants younger than 1 year with acute lymphoblastic leukemia treated with the Interfant-06 protocol: Results from an international phase III randomized study. J Clin Oncol 37:2246-2256, 2019 [DOI] [PubMed] [Google Scholar]
- 11.Pieters R, Schrappe M, De Lorenzo P, et al. : A treatment protocol for infants younger than 1 year with acute lymphoblastic leukaemia (Interfant-99): An observational study and a multicentre randomised trial. Lancet 370:240-250, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Pui CH: Acute leukemias with the t(4;11)(q21;q23). Leuk Lymphoma 7:173-179, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Pui CH, Chessells JM, Camitta B, et al. : Clinical heterogeneity in childhood acute lymphoblastic leukemia with 11q23 rearrangements. Leukemia 17:700-706, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Pui CH, Gaynon PS, Boyett JM, et al. : Outcome of treatment in childhood acute lymphoblastic leukaemia with rearrangements of the 11q23 chromosomal region. Lancet 359:1909-1915, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Mann G, Attarbaschi A, Schrappe M, et al. : Improved outcome with hematopoietic stem cell transplantation in a poor prognostic subgroup of infants with mixed-lineage-leukemia (MLL)-rearranged acute lymphoblastic leukemia: Results from the Interfant-99 study. Blood 116:2644-2650, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Brown PA, Kairalla JA, Hilden JM, et al. : FLT3 inhibitor lestaurtinib plus chemotherapy for newly diagnosed KMT2A-rearranged infant acute lymphoblastic leukemia: Children's Oncology Group trial AALL0631. Leukemia 35:1279-1290, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bai L, Cheng YF, Lu AD, et al. : Prognosis of haploidentical hematopoietic stem cell transplantation in non-infant children with t(v;11q23)/MLL-rearranged B-cell acute lymphoblastic leukemia. Leuk Res 91:106333, 2020 [DOI] [PubMed] [Google Scholar]
- 18.Mann G, Cazzaniga G, van der Velden VH, et al. : Acute lymphoblastic leukemia with t(4;11) in children 1 year and older: The 'big sister' of the infant disease? Leukemia 21:642-646, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Peterson JF, Baughn LB, Pearce KE, et al. : KMT2A (MLL) rearrangements observed in pediatric/young adult T-lymphoblastic leukemia/lymphoma: A 10-year review from a single cytogenetic laboratory. Genes Chromosomes Cancer 57:541-546, 2018 [DOI] [PubMed] [Google Scholar]
- 20.Tomizawa D, Kato M, Takahashi H, et al. : Favorable outcome in non-infant children with MLL-AF4-positive acute lymphoblastic leukemia: A report from the Tokyo Children's Cancer Study Group. Int J Hematol 102:602-610, 2015 [DOI] [PubMed] [Google Scholar]
- 21.van der Burg M, Beverloo HB, Langerak AW, et al. : Rapid and sensitive detection of all types of MLL gene translocations with a single FISH probe set. Leukemia 13:2107-2113, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Moorman AV, Ensor HM, Richards SM, et al. : Prognostic effect of chromosomal abnormalities in childhood B-cell precursor acute lymphoblastic leukaemia: Results from the UK Medical Research Council ALL97/99 randomised trial. Lancet Oncol 11:429-438, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Reichel M, Gillert E, Angermuller S, et al. : Biased distribution of chromosomal breakpoints involving the MLL gene in infants versus children and adults with t(4;11) ALL. Oncogene 20:2900-2907, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Attarbaschi A, Mann G, Strehl S, et al. : Deletion of 11q23 is a highly specific nonrandom secondary genetic abnormality of ETV6/RUNX1-rearranged childhood acute lymphoblastic leukemia. Leukemia 21:584-586, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Raimondi SC, Frestedt JL, Pui CH, et al. : Acute lymphoblastic leukemias with deletion of 11q23 or a novel inversion (11)(p13q23) lack MLL gene rearrangements and have favorable clinical features. Blood 86:1881-1886, 1995 [PubMed] [Google Scholar]
- 26.Harbott J, Mancini M, Verellen-Dumoulin C, et al. : Hematological malignancies with a deletion of 11q23: Cytogenetic and clinical aspects. European 11q23 Workshop participants. Leukemia 12:823-827, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Bene MC, Castoldi G, Knapp W, et al. : Proposals for the immunological classification of acute leukemias. European Group for the Immunological Characterization of Leukemias (EGIL). Leukemia 9:1783-1786, 1995 [PubMed] [Google Scholar]
- 28.Borowitz MJ, Wood BL, Devidas M, et al. : Prognostic significance of minimal residual disease in high risk B-ALL: A report from Children's Oncology Group study AALL0232. Blood 126:964-971, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maurer-Granofszky M, Schumich A, Buldini B, et al. : An extensive quality control and quality assurance (QC/QA) program significantly improves inter-laboratory concordance rates of flow-cytometric minimal residual disease assessment in acute lymphoblastic leukemia: An I-BFM-FLOW-Network report. Cancers (Basel) 13:6148-6163, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Velden VH, Panzer-Grumayer ER, Cazzaniga G, et al. : Optimization of PCR-based minimal residual disease diagnostics for childhood acute lymphoblastic leukemia in a multi-center setting. Leukemia 21:706-713, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Arico M, Valsecchi MG, Rizzari C, et al. : Long-term results of the AIEOP-ALL-95 Trial for Childhood Acute Lymphoblastic Leukemia: Insight on the prognostic value of DNA index in the framework of Berlin-Frankfurt-Muenster based chemotherapy. J Clin Oncol 26:283-289, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Bartram J, Wade R, Vora A, et al. : Excellent outcome of minimal residual disease-defined low-risk patients is sustained with more than 10 years follow-up: Results of UK paediatric acute lymphoblastic leukaemia trials 1997-2003. Arch Dis Child 101:449-454, 2016 [DOI] [PubMed] [Google Scholar]
- 33.Conter V, Bartram CR, Valsecchi MG, et al. : Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: Results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood 115:3206-3214, 2010 [DOI] [PubMed] [Google Scholar]
- 34.De Moerloose B, Suciu S, Bertrand Y, et al. : Improved outcome with pulses of vincristine and corticosteroids in continuation therapy of children with average risk acute lymphoblastic leukemia (ALL) and lymphoblastic non-Hodgkin lymphoma (NHL): Report of the EORTC randomized phase 3 trial 58951. Blood 116:36-44, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Escherich G, Troger A, Gobel U, et al. : The long-term impact of in vitro drug sensitivity on risk stratification and treatment outcome in acute lymphoblastic leukemia of childhood (CoALL 06-97). Haematologica 96:854-862, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Escherich G, Zur Stadt U, Zimmermann M, et al. : Clofarabine in combination with pegylated asparaginase in the frontline treatment of childhood acute lymphoblastic leukaemia: A feasibility report from the CoALL 08-09 trial. Br J Haematol 163:240-247, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Gandemer V, Auclerc MF, Perel Y, et al. : Impact of age, leukocyte count and day 21-bone marrow response to chemotherapy on the long-term outcome of children with Philadelphia chromosome-positive acute lymphoblastic leukemia in the pre-imatinib era: Results of the FRALLE 93 study. BMC Cancer 9:14, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasegawa D, Imamura T, Yumura-Yagi K, et al. : Risk-adjusted therapy for pediatric non-T cell ALL improves outcomes for standard risk patients: Results of JACLS ALL-02. Blood Cancer J 10:23, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horibe K, Yumura-Yagi K, Kudoh T, et al. : Long-term results of the risk-adapted treatment for childhood B-cell acute lymphoblastic leukemia: Report from the Japan Association of Childhood Leukemia Study ALL-97 trial. J Pediatr Hematol Oncol 39:81-89, 2017 [DOI] [PubMed] [Google Scholar]
- 40.Igarashi S, Manabe A, Ohara A, et al. : No advantage of dexamethasone over prednisolone for the outcome of standard- and intermediate-risk childhood acute lymphoblastic leukemia in the Tokyo Children's Cancer Study Group L95-14 protocol. J Clin Oncol 23:6489-6498, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Jeha S, Pei D, Choi J, et al. : Improved CNS control of childhood acute lymphoblastic leukemia without cranial irradiation: St Jude Total Therapy Study 16. J Clin Oncol 37:3377-3391, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamps WA, Bokkerink JP, Hakvoort-Cammel FG, et al. : BFM-oriented treatment for children with acute lymphoblastic leukemia without cranial irradiation and treatment reduction for standard risk patients: Results of DCLSG protocol ALL-8 (1991-1996). Leukemia 16:1099-1111, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Larsen EC, Devidas M, Chen S, et al. : Dexamethasone and high-dose methotrexate improve outcome for children and young adults with high-risk B-acute lymphoblastic leukemia: A report from Children's Oncology Group study AALL0232. J Clin Oncol 34:2380-2388, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maloney KW, Devidas M, Wang C, et al. : Outcome in children with standard-risk B-cell acute lymphoblastic leukemia: Results of Children's Oncology Group trial AALL0331. J Clin Oncol 38:602-612, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manabe A, Ohara A, Hasegawa D, et al. : Significance of the complete clearance of peripheral blasts after 7 days of prednisolone treatment in children with acute lymphoblastic leukemia: The Tokyo Children's Cancer Study Group study L99-15. Haematologica 93:1155-1160, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Moricke A, Reiter A, Zimmermann M, et al. : Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: Treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood 111:4477-4489, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Pieters R, de Groot-Kruseman H, Van der Velden V, et al. : Successful therapy reduction and intensification for childhood acute lymphoblastic leukemia based on minimal residual disease monitoring: Study ALL10 from the Dutch Childhood Oncology Group. J Clin Oncol 34:2591-2601, 2016 [DOI] [PubMed] [Google Scholar]
- 48.Place AE, Stevenson KE, Vrooman LM, et al. : Intravenous pegylated asparaginase versus intramuscular native Escherichia coli L-asparaginase in newly diagnosed childhood acute lymphoblastic leukaemia (DFCI 05-001): A randomised, open-label phase 3 trial. Lancet Oncol 16:1677-1690, 2015 [DOI] [PubMed] [Google Scholar]
- 49.Pui CH, Pei D, Sandlund JT, et al. : Long-term results of St Jude Total Therapy studies 11, 12, 13A, 13B, and 14 for childhood acute lymphoblastic leukemia. Leukemia 24:371-382, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pui CH, Relling MV, Sandlund JT, et al. : Rationale and design of Total Therapy Study XV for newly diagnosed childhood acute lymphoblastic leukemia. Ann Hematol 83:S124-S126, 2004. (suppl 1) [DOI] [PubMed] [Google Scholar]
- 51.Sakamoto K, Imamura T, Kihira K, et al. : Low incidence of osteonecrosis in childhood acute lymphoblastic leukemia treated with ALL-97 and ALL-02 study of Japan Association of Childhood Leukemia Study group. J Clin Oncol 36:900-907, 2018 [DOI] [PubMed] [Google Scholar]
- 52.Schmiegelow K, Forestier E, Hellebostad M, et al. : Long-term results of NOPHO ALL-92 and ALL-2000 studies of childhood acute lymphoblastic leukemia. Leukemia 24:345-354, 2010 [DOI] [PubMed] [Google Scholar]
- 53.Schramm F, Zimmermann M, Jorch N, et al. : Daunorubicin during delayed intensification decreases the incidence of infectious complications—A randomized comparison in trial CoALL 08-09. Leuk Lymphoma 60:60-68, 2019 [DOI] [PubMed] [Google Scholar]
- 54.Schramm F, Zur Stadt U, Zimmermann M, et al. : Results of CoALL 07-03 study childhood ALL based on combined risk assessment by in vivo and in vitro pharmacosensitivity. Blood Adv 3:3688-3699, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schrappe M, Valsecchi MG, Bartram CR, et al. : Late MRD response determines relapse risk overall and in subsets of childhood T-cell ALL: Results of the AIEOP-BFM-ALL 2000 study. Blood 118:2077-2084, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Stary J, Zimmermann M, Campbell M, et al. : Intensive chemotherapy for childhood acute lymphoblastic leukemia: Results of the randomized intercontinental trial ALL IC-BFM 2002. J Clin Oncol 32:174-184, 2014 [DOI] [PubMed] [Google Scholar]
- 57.Steinherz PG, Seibel NL, Sather H, et al. : Treatment of higher risk acute lymphoblastic leukemia in young people (CCG-1961), long-term follow-up: A report from the Children's Oncology Group. Leukemia 33:2144-2154, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takahashi H, Kajiwara R, Kato M, et al. : Treatment outcome of children with acute lymphoblastic leukemia: The Tokyo Children's Cancer Study Group (TCCSG) study L04-16. Int J Hematol 108:98-108, 2018 [DOI] [PubMed] [Google Scholar]
- 59.Toft N, Birgens H, Abrahamsson J, et al. : Results of NOPHO ALL2008 treatment for patients aged 1-45 years with acute lymphoblastic leukemia. Leukemia 32:606-615, 2018 [DOI] [PubMed] [Google Scholar]
- 60.Veerman AJ, Kamps WA, van den Berg H, et al. : Dexamethasone-based therapy for childhood acute lymphoblastic leukaemia: Results of the prospective Dutch Childhood Oncology Group (DCOG) protocol ALL-9 (1997-2004). Lancet Oncol 10:957-966, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Vilmer E, Suciu S, Ferster A, et al. : Long-term results of three randomized trials (58831, 58832, 58881) in childhood acute lymphoblastic leukemia: A CLCG-EORTC report. Children Leukemia Cooperative Group. Leukemia 14:2257-2266, 2000 [DOI] [PubMed] [Google Scholar]
- 62.Vrooman LM, Blonquist TM, Harris MH, et al. : Refining risk classification in childhood B acute lymphoblastic leukemia: Results of DFCI ALL Consortium Protocol 05-001. Blood Adv 2:1449-1458, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vrooman LM, Stevenson KE, Supko JG, et al. : Postinduction dexamethasone and individualized dosing of Escherichia coli L-asparaginase each improve outcome of children and adolescents with newly diagnosed acute lymphoblastic leukemia: Results from a randomized study—Dana-Farber Cancer Institute ALL Consortium Protocol 00-01. J Clin Oncol 31:1202-1210, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pui CH, Rebora P, Schrappe M, et al. : Outcome of children with hypodiploid acute lymphoblastic leukemia: A retrospective multinational study. J Clin Oncol 37:770-779, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Attarbaschi A, Mann G, Zimmermann M, et al. : Randomized post-induction and delayed intensification therapy in high-risk pediatric acute lymphoblastic leukemia: Long-term results of the international AIEOP-BFM ALL 2000 trial. Leukemia 34:1694-1700, 2020 [DOI] [PubMed] [Google Scholar]
- 66.Testi AM, Attarbaschi A, Valsecchi MG, et al. : Outcome of adolescent patients with acute lymphoblastic leukaemia aged 10-14 years as compared with those aged 15-17 years: Long-term results of 1094 patients of the AIEOP-BFM ALL 2000 study. Eur J Cancer 122:61-71, 2019 [DOI] [PubMed] [Google Scholar]
- 67.Behm FG, Raimondi SC, Frestedt JL, et al. : Rearrangement of the MLL gene confers a poor prognosis in childhood acute lymphoblastic leukemia, regardless of presenting age. Blood 87:2870-2877, 1996 [PubMed] [Google Scholar]
- 68.Huret JL, Brizard A, Slater R, et al. : Cytogenetic heterogeneity in t(11;19) acute leukemia: Clinical, hematological and cytogenetic analyses of 48 patients—Updated published cases and 16 new observations. Leukemia 7:152-160, 1993 [PubMed] [Google Scholar]
- 69.Moorman AV, Hagemeijer A, Charrin C, et al. : The translocations, t(11;19)(q23;p13.1) and t(11;19)(q23;p13.3): A cytogenetic and clinical profile of 53 patients. European 11q23 Workshop participants. Leukemia 12:805-810, 1998 [DOI] [PubMed] [Google Scholar]
- 70.Pui CH, Campana D: Age-related differences in leukemia biology and prognosis: The paradigm of MLL-AF4-positive acute lymphoblastic leukemia. Leukemia 21:593-594, 2007 [DOI] [PubMed] [Google Scholar]
- 71.Pui CH, Frankel LS, Carroll AJ, et al. : Clinical characteristics and treatment outcome of childhood acute lymphoblastic leukemia with the t(4;11)(q21;q23): A collaborative study of 40 cases. Blood 77:440-447, 1991 [PubMed] [Google Scholar]
- 72.Rubnitz JE, Camitta BM, Mahmoud H, et al. : Childhood acute lymphoblastic leukemia with the MLL-ENL fusion and t(11;19)(q23;p13.3) translocation. J Clin Oncol 17:191-196, 1999 [DOI] [PubMed] [Google Scholar]
- 73.Swansbury GJ, Slater R, Bain BJ, et al. : Hematological malignancies with t(9;11)(p21-22;q23)—A laboratory and clinical study of 125 cases. European 11q23 Workshop participants. Leukemia 12:792-800, 1998 [DOI] [PubMed] [Google Scholar]
- 74.Bhojwani D, Sposto R, Shah NN, et al. : Inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. Leukemia 33:884-892, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gore L, Locatelli F, Zugmaier G, et al. : Survival after blinatumomab treatment in pediatric patients with relapsed/refractory B-cell precursor acute lymphoblastic leukemia. Blood Cancer J 8:80, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Locatelli F, Whitlock JA, Peters C, et al. : Blinatumomab versus historical standard therapy in pediatric patients with relapsed/refractory Ph-negative B-cell precursor acute lymphoblastic leukemia. Leukemia 34:2473-2478, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maude SL, Frey N, Shaw PA, et al. : Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371:1507-1517, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maude SL, Shpall EJ, Grupp SA: Chimeric antigen receptor T-cell therapy for ALL. Hematology Am Soc Hematol Educ Program 2014:559-564, 2014 [DOI] [PubMed] [Google Scholar]
- 79.von Stackelberg A, Locatelli F, Zugmaier G, et al. : Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol 34:4381-4389, 2016 [DOI] [PubMed] [Google Scholar]
- 80.Kuhn MW, Armstrong SA: Designed to kill: Novel menin-MLL inhibitors target MLL-rearranged leukemia. Cancer Cell 27:431-433, 2015 [DOI] [PubMed] [Google Scholar]