PURPOSE
Primary plasma cell leukemia (PCL) is the most aggressive monoclonal gammopathy. It was formerly characterized by ≥ 20% circulating plasma cells (CTCs) until 2021, when this threshold was decreased to ≥ 5%. We hypothesized that primary PCL is not a separate clinical entity, but rather that it represents ultra-high-risk multiple myeloma (MM) characterized by elevated CTC levels.
METHODS
We assessed the levels of CTCs by multiparameter flow cytometry in 395 patients with newly diagnosed transplant-ineligible MM to establish a cutoff for CTCs that identifies the patients with ultra-high-risk PCL-like MM. We tested the cutoff on 185 transplant-eligible patients with MM and further validated on an independent cohort of 280 transplant-ineligible patients treated in the GEM-CLARIDEX trial. The largest published real-world cohort of patients with primary PCL was used for comparison of survival. Finally, we challenged the current 5% threshold for primary PCL diagnosis.
RESULTS
Newly diagnosed transplant-ineligible patients with MM with 2%-20% CTCs had significantly shorter progression-free survival (3.1 v 15.6 months; P < .001) and overall survival (14.6 v 33.6 months; P = .023) than patients with < 2%. The 2% cutoff proved to be applicable also in transplant-eligible patients with MM and was successfully validated on an independent cohort of patients from the GEM-CLARIDEX trial. Most importantly, patients with 2%-20% CTCs had comparable dismal outcomes with primary PCL. Moreover, after revealing a low mean difference between flow cytometric and morphologic evaluation of CTCs, we showed that patients with 2%-5% CTCs have similar outcomes as those with 5%-20% CTCs.
CONCLUSION
Our study uncovers that ≥ 2% CTCs is a biomarker of hidden primary PCL and supports the assessment of CTCs by flow cytometry during the diagnostic workup of MM.
INTRODUCTION
Multiple myeloma (MM) is the second most common hematologic malignancy characterized by the expansion and accumulation of clonal plasma cells (PCs) in the bone marrow (BM).1 However, tumor PCs are able to egress from BM to peripheral blood (PB), where they are responsible for dissemination and extramedullary disease.2,3 Because of recent technological advances, circulating tumor plasma cells (CTCs) might be detected in the majority of patients with newly diagnosed multiple myeloma (NDMM) using highly sensitive techniques such as next-generation flow cytometry (NGF).4-6 Quantification of CTCs has been recently proposed as one of the most relevant prognostic factor in NDMM, making it the ideal novel parameter for risk stratification.4,7 Indeed, patients with NDMM may present with a variable number of CTCs, ranging from zero with exceptionally favorable outcomes as a continuum, to higher numbers of CTCs, with adverse prognoses. Nevertheless, there are virtually no published data on the prognostic significance of CTCs in transplant-ineligible patients with NDMM.8 As an extreme, on the other side of the spectrum, are patients with ≥ 20% of CTCs who are considered to be diagnosed with primary plasma cell leukemia (pPCL) as a distinct and rare clinical entity with the most aggressive behavior.9,10 In 2021, the International Myeloma Working Group decreased this threshold to ≥ 5% of CTCs evaluated by morphology to better reflect the ultra-high-risk nature of these patients.11 Here, we hypothesized that we could define new criteria to identify patients with ultra-high-risk PCL-like MM by using sensitive multiparameter flow cytometry (FC). We tested this hypothesis in a large series of transplant-ineligible patients, as well as in transplant-eligible MM.
CONTEXT
Key Objective
Primary plasma cell leukemia (PCL) is the most aggressive monoclonal gammopathy formerly characterized by ≥ 20% circulating tumor plasma cells (CTCs) until 2021, when this threshold was lowered to ≥ 5%. We hypothesized that primary PCL is not a separate clinical entity, and the lower number of CTCs would identify patients with ultra-high-risk multiple myeloma (MM) with similarly poor prognosis as primary PCL.
Knowledge Generated
Patients with newly diagnosed MM with ≥ 2% CTCs evaluated by flow cytometry have significantly inferior progression-free survival and overall survival than patients with < 2% CTCs, mimicking the prognosis of primary PCL. Patients with 2%-5% CTCs have identical outcomes as patients with 5%-20% CTCs. To our knowledge, this is for the first time that such a threshold has been defined using scientific rationale.
Relevance (S. Lentzsch)
With the 2% cutoff of CTCs, a small subset of newly diagnosed multiple myeloma with ultra-high-risk disease resembling features of primary plasma cell leukemia can be identified. Those patients need more intensive treatments and maintenance regimens.*
*Relevance section written by JCO Associate Editor Suzanne Lentzsch, MD, PhD.
METHODS
Patients and Data Collection
The assessment of CTCs was performed in 590 patients with NDMM in two Czech hematologic centers, University Hospital Ostrava and Brno, between 2012 and 2019. Patients were divided according to their eligibility to undergo autologous stem-cell transplantation to transplant-eligible (N = 185) and transplant-ineligible (N = 395; Table 1). Patients who fulfilled the criteria for pPCL (≥ 20% or ≥ 2 × 109 CTCs) were excluded from the analysis (n = 10; Data Supplement [online only]). Patients were treated in the real-world setting and clinical analysis was performed retrospectively on the basis of data from the Czech Registry of Monoclonal Gammopathies (RMG).12 The study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by each institutional ethics committee under numbers 413/2012 and EK20130724. All patients provided written informed consent.
TABLE 1.
Baseline Characteristics of Transplant-Eligible and Transplant-Ineligible Patients With Newly Diagnosed Multiple Myeloma
We extracted clinical data from the largest published real-world case series of patients with pPCL by Jurczyszyn et al13 to perform a comparison with real-world pPCL patients. We used data from transplant-ineligible (n = 40) and transplant-eligible (n = 55) patients.
For the validation of our cutoff, we used the independent cohort of transplant-ineligible patients with NDMM treated in the GEM-CLARIDEX (ClinicalTrials.gov identifier: NCT02575144) study.14 Briefly, in this phase III clinical trial, 286 patients received lenalidomide and dexamethasone with or without clarithromycin until disease progression or unacceptable toxicity. CTCs were measured at diagnosis using the EuroFlow NGF method with a median limit of detection (LOD) of 0.0002%.15
Multiparameter Flow Cytometry
The assessment of CTCs in PB was performed at the time of diagnosis using FC. PB samples were collected in EDTA anticoagulated tubes and processed within 24 hours from harvest. No cell-enrichment strategies were applied. Samples were stained using the eight-color combination of fluorescence-labeled monoclonal antibodies, then lysed and washed. The composition of monoclonal antibodies in eight-color panels as well as their modified versions during the time of the study is described in detail in the Data Supplement. Data acquisition was performed by FACSCanto II flow cytometer (Becton Dickinson Biosciences, San Jose, CA) using the FACSDiva 6.1 software. Commercial FC software program was used (Infinicyt v.1.8 or v.2.0; Cytognos SL, Salamanca, Spain) for the final analysis. Simultaneously, the BM sample from the particular patient was always processed to confirm the clonality of aberrant PCs. This strategy allowed for the detection of phenotypically aberrant PCs identified by underexpression or overexpression of particular antigens. The enumeration of CTCs was expressed as a percentage from total PB leukocytes. The median number of analyzed events was 341,918 and median LOD was 0.006%.
Morphologic Assessment of PB Smears
Wright-Giemsa–stained PB smears were used for the assessment of CTCs by morphology. Two expert hematologists experienced in PB cytology retrospectively reviewed available blood smears with a minimum of 200 nucleated cells per smear. The central reading was performed only in patients with more than 0.2% CTCs evaluated previously by FC.
Statistical Analysis
For the selection of an optimal cutoff identifying patients with ultra-high-risk PCL-like MM, we first used cubic splines from rms v6.2.0 R package to model the relationship between the level of CTCs and overall survival (OS; 222 events) and progression-free survival (PFS; 273 events) in 402 transplant-ineligible patients (N = 395), including those with pPCL (n = 7). This approach did not suggest any clear natural change point. Therefore, we decided to select a clinically relevant cutoff that would identify patients with ultra-high-risk MM with median OS resembling that of patients with pPCL (12-14 months) described in many published studies.13,16-21 We used all values of CTCs from transplant-ineligible patients with MM (N = 395) as cutoffs, computed the median OS for patients with CTC levels higher than each cutoff, and then used loess function to identify a cutoff of CTCs resulting in OS of approximately 13 months in Kaplan-Meier (KM) analysis. We used log10(x + 1) transformation in both analyses to address the skewness and zeroes in our data. The Mann-Whitney U and Kruskal-Wallis tests were used to evaluate the potentially significant clinical differences between resulting groups for categorical and continual variables. KM method and log-rank test were used to assess PFS and OS. Median follow-up was estimated by reverse KM method. Hazard ratios (HRs) were estimated by univariate Cox proportional hazard models. Multivariate analyses were performed using CTCs, International Staging System (ISS) stage, lactate dehydrogenase (LDH), and cytogenetic risk. To compare the levels of CTCs measured by morphology and FC, we used Bland-Altman plots with nonparametric limits of agreement because of a non-normal distribution of data.22 Statistical analyses were performed using R 4.0.3 and survival v3.2.11, survminer v0.4.9, lubridate v1.7.10, readxl v1.3.1, and tidyverse v1.3.1 packages (Comprehensive R Archive Network, Vienna, Austria). All plots were prepared using ggplot2 v3.3.5 R package. Manual modifications were performed with Inkscape 1.0.1. We used P = .05 as a threshold for statistical significance in all analyses.
RESULTS
Detection of CTCs in Transplant-Ineligible Patients With Newly Diagnosed MM
CTCs were detected in 75% (296/395) of transplant-ineligible patients with NDMM using FC with median LOD of 0.006%. Median percentage of CTCs was 0.03% (range, 0%-13.5%; Table 1). The median age at diagnosis was 72 years, median BM PC infiltration was 23.4%, and the majority of patients received proteasome inhibitor (PI)–based regimens (77.2%) or PI and immunomodulatory drug combination (20.5%) as first-line treatment in real-world clinical practice.
Optimal Cutoff for Identification of Ultra-High-Risk PCL-Like MM Is 2% of CTCs
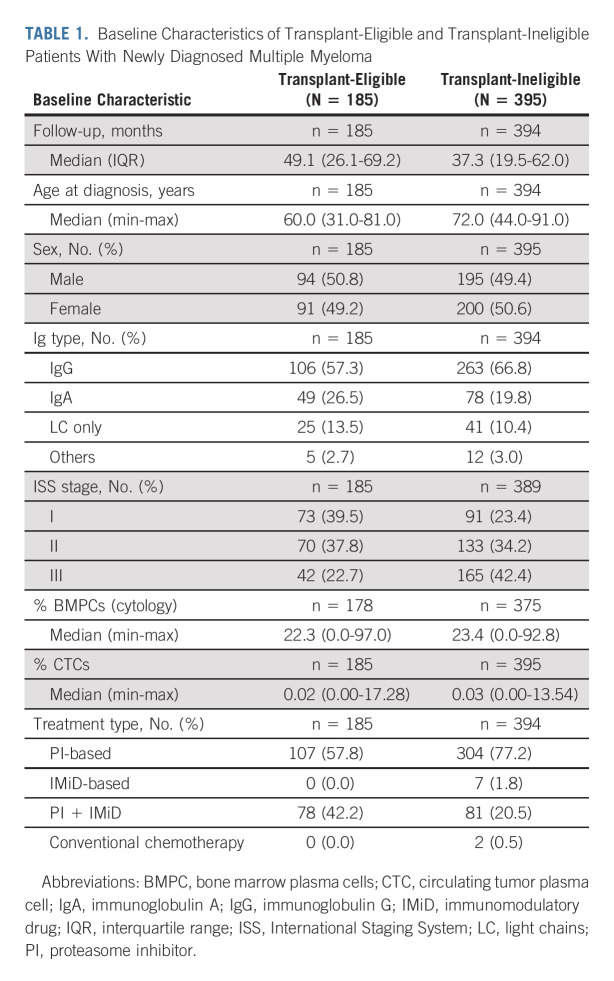
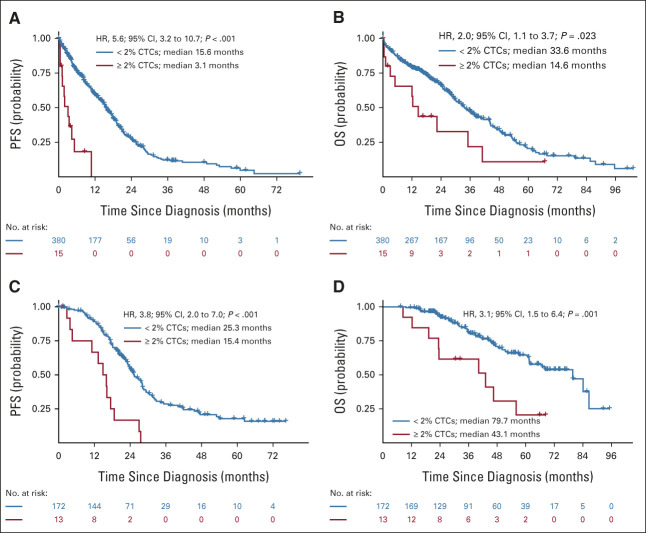
Analysis using cubic splines did not reveal any natural cutoff identifying patients with ultra-high-risk PCL-like MM (Data Supplement). Therefore, we aimed to find a clinically relevant cutoff that would separate a group of patients with comparably poor prognosis as pPCL. Using all possible cutoffs from our cohort and loess function, we identified that the cutpoint was approximately at the level of 2% CTCs (Data Supplement). Patients with 2%-20% CTCs represented approximately 4% (15/395) of the whole cohort and exhibited significantly shorter PFS compared with patients with < 2% (3.1 v 15.6 months; P < .001; HR, 5.6; Fig 1A). This was translated into significantly shorter median OS (14.6 v 33.6 months; P = .023; HR, 2.0; Fig 1B). Notably, of the 15 patients in the 2%-20% CTC group, only 20% (3/15) responded with partial response; the rest experienced stable or progressive disease. The median percentage of CTCs in the 2%-20% group was 4.9% compared with 0.03% in the < 2% group, and the median BM PC infiltration was 52.5% compared with 23.0%, yet the median level of serum M-protein was similar (26.4 v 27.1 g/L; P = .7). Patients in the 2%-20% group had a significantly higher frequency of ISS stage III (73.3 v 41.2%; P = .014) and elevated LDH level (53.3% v 9.8%; P < .001). At the same time, they had significantly lower baseline hemoglobin levels (87.2 v 105 g/L; P < .001) and worse renal functions (creatinine level 168 v 100 μmol/L; P = .039). Interestingly, more than half of the patients (5/9) had high-risk cytogenetic abnormalities in the 2%-20% group compared with less than one third of the patients (52/173) in < 2% CTCs group (Fig 2 and Data Supplement).
FIG 1.
The optimal cutoff for identification of ultra-high-risk PCL-like multiple myeloma is 2% of CTCs. Kaplan-Meier curves for (A) PFS and (B) OS for transplant-ineligible patients with NDMM (N = 395); and (C) PFS and (D) OS for transplant-eligible patients with NDMM (N = 185) with < 2% (blue line) and 2%-20% (red line) of CTCs. CTC, circulating tumor plasma cell; HR, hazard ratio; NDMM, newly diagnosed multiple myeloma; OS, overall survival; PCL, plasma cell leukemia; PFS, progression-free survival.
FIG 2.
Basic clinical characteristics and level of CTCs for the transplant-ineligible cohort: the level of CTCs, ISS stage, cytogenetic risk, LDH, sex, and median PFS. aSignificantly different distribution between multiple myeloma groups according to Fisher's exact test (Data Supplement). CTC, circulating tumor plasma cell; ISS, International Staging System; LDH, lactate dehydrogenase; PFS, progression-free survival.
Next, we used Cox proportional hazard risk models to compare the prognostic power of CTCs with other routinely used prognostic markers such as ISS stage, LDH level, and high-risk cytogenetics. Our results suggest that CTCs are an independent prognostic biomarker in the multivariate analysis for both PFS (HR, 4.3; P < .001) and OS (HR, 3.1; P = .006; Data Supplement).
Cutoff 2% of CTCs Is Applicable Also in Transplant-Eligible Patients
Next, we wanted to know whether the 2% cutoff generated in transplant-ineligible patients is applicable also for transplant-eligible MM. For this confirmation, we used the Czech cohort of 185 NDMM with the median age at diagnosis of 60 years. In total, CTCs were detected in 72% (134/185) of patients; median percentage of CTCs was 0.02% (range, 0%-17.3%). Patients with 2%-20% of CTCs represented approximately 7% (13/185) and demonstrated significantly shorter median PFS (15.4 v 25.3 months; P < .001; HR, 3.8; Fig 1C) and median OS (43.1 v 79.7 months; P = .001; HR, 3.1; Fig 1D) than patients with < 2% of CTCs.
Validation of 2% Cutoff on the Independent Cohort of Patients Treated in the GEM-CLARIDEX Trial
After demonstrating that the 2% cutoff of CTCs identifies patients with ultra-high-risk PCL-like MM, we wanted to validate the established threshold on independent cohort of transplant-ineligible patients with NDMM. Of the 286 patients enrolled in the GEM-CLARIDEX trial, 280 patients had quantified the number of CTCs by NGF. Using the established cutoff, we identified PCL-like group of 12 patients representing again approximately 4% (12/280) of NDMM. Patients with 2%-20% of CTCs had significantly shorter median PFS (11 v 29 months; P < .001; HR, 3.2) than patients with < 2% (Fig 3). Patients in the ultra-high-risk group had numerically inferior OS (2-year OS of 51% v 71%; P = .17; HR, 1.9; Data Supplement). Of the 12 patients in the 2%-20% CTC group, 58% (7/12) responded but none reached MRD negativity (Data Supplement).
FIG 3.
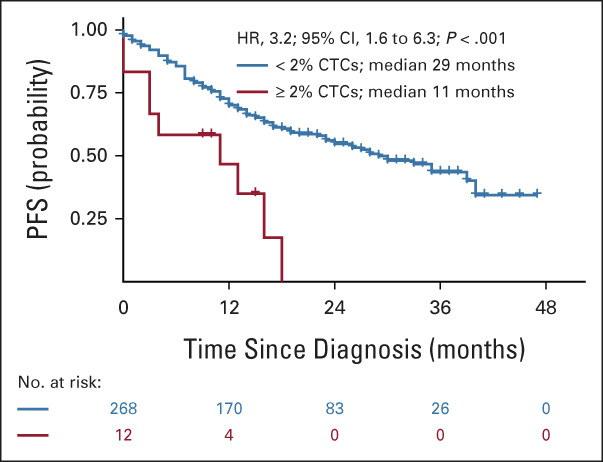
Validation of 2% cutoff on independent cohort of transplant-ineligible patients with NDMM treated in the GEM-CLARIDEX trial. Kaplan-Meier curves for PFS of transplant-ineligible patients from the GEM-CLARIDEX trial stratified according to < 2% (blue line) or 2%-20% (red line) of CTCs. CTC, circulating tumor plasma cell; NDMM, newly diagnosed multiple myeloma; PFS, progression-free survival.
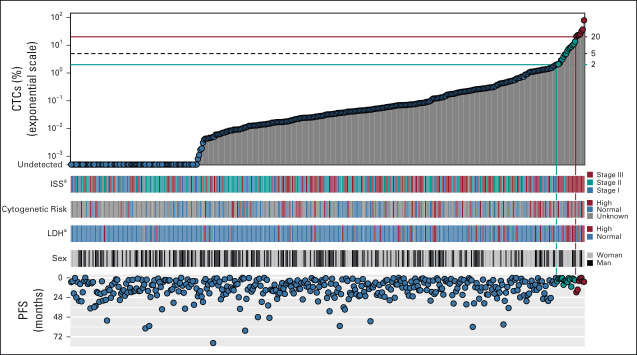
NDMM Patients With 2%-20% of CTCs Have Comparable Prognosis With Patients With Primary PCL
To demonstrate that patients with NDMM with 2%-20% of CTCs have similarly dismal outcomes as patients with pPCL, we compared our transplant-ineligible cohort with 40 transplant-ineligible patients with pPCL treated in the real-world setting published by Jurczyszyn et al (Data Supplement). The median percentage of CTCs in the pPCL cohort was 36.2%, and the majority of the patients were treated with PI-based (53%) or IMiD-based regimens (30%). The median OS for patients with pPCL was practically identical to our cohort of transplant-ineligible NDMM with 2%-20% of CTCs (13.0 v 14.6 months; P = .908) with overlapping KM curves (Fig 4A). Moreover, we compared also the outcomes of the Czech cohort of transplant-eligible patients (N = 185) with 55 transplant-eligible patients with pPCL treated in the real-world setting. Patients were treated similarly in the real world, dominantly by PI-based regimen (Czech cohort 57.8% v pPCL cohort 50.0%). Again, the median OS was comparable between the two groups and KM curves were overlapping (43.1 v 35.1 months; P = .972; Fig 4B). Finally, we compared the outcomes of 2%-20% CTCs group with Czech patients with pPCL (n = 10) in both transplant-ineligible and transplant-eligible settings and again we demonstrated comparable prognosis in terms of PFS and OS in both groups (Data Supplement).
FIG 4.
Patients with NDMM with 2%-20% of CTCs have comparable prognosis with pPCL. Kaplan-Meier curves for OS of (A) transplant-ineligible patients and (B) transplant-eligible patients with < 2% (blue line) and 2%-20% (red line) of CTCs together with patients with pPCL from the case series by Jurczyszyn et al (teal line).13 CTC, circulating tumor plasma cell; NDMM, newly diagnosed multiple myeloma; OS, overall survival; pPCL, primary plasma cell leukemia.
Comparison Between Flow Cytometry and Morphology for Quantification of CTCs
To link our data generated by FC to the most frequently used morphologic assessment of PB smears, we conducted Bland-Altman comparison between the two techniques. We retrospectively reviewed available PB smears from 70 patients with high levels of CTCs determined by FC (≥ 0.2% of CTCs) and demonstrated a low mean difference between the two methods: 0.12% CTCs; with morphology providing slightly higher results in average. Indeed, although the mean difference is minimal, the higher limits of agreement (–2.24% and +1.89%) suggest, to some extent, discordant results urging to use more accurate FC method in routine practice (Data Supplement).
Patients With 2%-5% of CTCs Have Comparable Outcomes as Patients With 5%-20% of CTCs
Finally, after revealing a low mean difference in CTC levels between FC and morphology, we wanted to challenge the currently established 5% threshold for the diagnosis of pPCL. Thus, we further dissected the cohort with 2%-20% of CTCs and compared patients with 2%-5% versus 5%-20% CTCs. To obtain sufficient numbers of patients, we merged the Czech cohort of transplant-ineligible patients with MM (N = 395) with the validation GEM-CLARIDEX cohort (N = 280). In 675 patients in total, we demonstrated that those with 2%-5% CTCs had comparable median PFS as patients with 5%-20% CTCs (3.4 v 5.1 months; P = .42), and both groups had significantly shorter PFS than patients with < 2% CTCs, with a median PFS of 18.1 months (P < .001; Fig 5).
FIG 5.
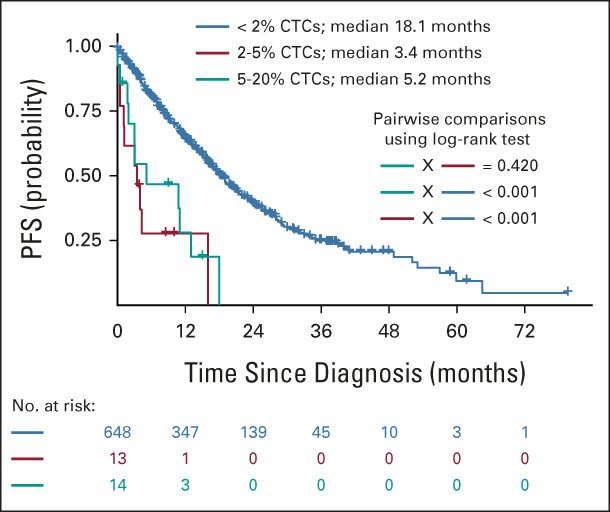
Patients with 2%-5% display similar PFS as those with 5%-20% of CTCs. Kaplan-Meier curves for PFS of patients from the transplant-ineligible cohort merged with the patients from the GEM-CLARIDEX trial stratified according to < 2% (blue line), 2%-5% (red line), and 5%-20% (teal line). CTC, circulating tumor plasma cell; PFS, progression-free survival.
DISCUSSION
Determination of prognosis or risk stratification is a routine procedure at the time of diagnosis across all blood and solid cancers. Identification of patients with exceptionally favorable outcomes or ultra-high-risk disease is of utmost importance as it guides the overall management of patients including the type of therapy. In this study, we were able to define patients with ultra-high-risk PCL-like MM using quantification of CTCs by FC instead of traditional morphology.
We established the threshold of 2% CTCs, which identifies patients with ultra-high-risk myeloma with practically identical prognosis as patients with pPCL. We demonstrated that patients with 2%-20% have significantly shorter PFS and OS than patients with < 2% CTCs in both transplant-ineligible (3.1 v 15.6 months, and 14.6 v 33.6 months) and transplant-eligible settings (15.4 v 25.3 months, and 43.1 v 79.7 months) with clinically highly relevant differences. Importantly, we successfully validated this cutoff on the independent cohort of transplant-ineligible patients with NDMM treated in the GEM-CLARIDEX trial. Finally, after revealing a low median difference in CTC numbers between FC and morphology, we showed that patients with 2%-5% CTCs have comparable outcomes as patients with 5%-20% CTCs. Besides, we performed a multivariate analysis including routinely used prognostic markers such as ISS stage, LDH level, and high-risk cytogenetics, where CTCs emerged as an independent prognostic factor for both PFS and OS. To our knowledge, this is the first time that the prognostic value of CTCs was confirmed also in transplant-ineligible patients with MM.
The incorporation of novel biomarkers into diagnostic workup is considered to be a crucial step toward personalized medicine. In the field of MM, several staging systems have been in use, such as the ISS, which was developed in 200523 and included albumin and beta-2 microglobulin. In 2015, Revised-ISS was introduced, adding LDH level and cytogenetic abnormalities,24 and in 2022, updated by R2-ISS adding chromosome 1q gain/amplification.25 Current staging systems enhanced by the level of CTCs have also been published.26-28 Nevertheless, crucial works in this field were published just recently by Bertamini et al and Garcés et al, confirming CTCs as one of the most relevant prognostic factors in transplant-eligible NDMM with the potential to become a part of staging systems. Indeed, the optimal cutoffs were determined to be 0.07%7 and 0.01%4 CTCs, respectively, in fact two orders of magnitude lower than ours, just depicting the ultra-high-risk character of patients identified by 2% CTCs. The threshold of 2% CTCs identifies a small subset (approximately 4%-7%) of NDMM with extremely poor outcomes, thus being an ideal tool for guiding therapy. Novel agents with possible activity in pPCL might be considered.29-33
The applicability of CTCs as a reliable biomarker is virtually 100% compared with cytogenetics, which is available only in 35%-50% of cases in the real-world setting (unpublished data, Czech RMG), and up to 75%-90% in randomized clinical trials.34-38 FC represents an ideal method for the quantification of CTCs as it is (1) widely available, (2) highly sensitive, (3) technically simple, and (4) a financially affordable method.39-41
Primary PCL was proposed as a separate clinical entity almost 50 years ago by Kyle et al9 because of its especially aggressive behavior and short survival. It is defined by the presence of ≥ 20% or 2 × 109/L CTCs evaluated by morphology on PB smears.42 There have been many efforts to redefine and decrease this threshold to better reflect the adverse prognosis of patients with an elevated number of CTCs, usually to 2% or 5% by morphology.16,17,43 In 2021, the International Myeloma Working Group published a position paper where the diagnostic criteria for pPCL were changed and the threshold was decreased to ≥ 5% of CTCs by morphology on the basis of two retrospective studies from the United States and Spain.11 Unfortunately, there was no scientific rationale to establish this particular threshold. Moreover, both studies provided discordant results regarding the subgroup of patients with 1%-4% of CTCs.16,17
It is remarkable to observe that OS intervals in our analysis in patients with 2%-20% CTCs agree with recently reported survival rates in pPCL.13,16-20,44,45 Moreover, the outcomes of patients with 2%-20% CTCs are comparable to those identified by the transcriptomic classifier developed by Hofste Op Bruinink et al.20 Finally, our study demonstrated that patients with 2%-5% CTCs have comparable outcomes as patients with 5%-20% of CTCs. All these findings challenge the position of pPCL as a separate clinical entity from MM.
Indeed, we do not want to establish a new threshold for pPCL being 2% of CTCs here; rather, we want to propose a novel ultra-high-risk subgroup of patients with NDMM with adverse prognosis. We believe that pPCL is not a distinct clinical entity, but rather that it represents ultra-high-risk MM. This dissection induces many practical issues in routine practice. Currently, patients with pPCL are practically always excluded from commercial as well as academic clinical trials. Moreover, in many countries across the world, there are reimbursement issues with novel and expensive agents for patients with pPCL who paradoxically need the most modern and intensive treatment. We propose that the assessment of CTCs by FC becomes a standard part of diagnostic workup. With the 2% cutoff of CTCs, we are able to identify a small subset of NDMM (approximately 4%-7% of all patients) with ultra-high-risk disease resembling features of pPCL.
ACKNOWLEDGMENT
The authors would like to thank Dr Shira Timilsina Godfrey for editing the article and Zaneta Michalikova and Martina Brillantova for central morphologic assessment of peripheral blood smears.
PRIOR PRESENTATION
Presented in part at the American Society of Hematology 2021 annual meeting, Atlanta, GA, December 12, 2021.
SUPPORT
Supported by the European Regional Development Fund—New Directions of Biomedical Research in the Ostrava Region (No. CZ.02.1.01/0.0/0.0/18_069/0010060), by the National Institute for Cancer Research (Program EXCELES, ID Project No. LX22NPO5102)—Funded by the European Union—Next Generation EU and by the Ministry of Health of the Czech Republic (AZV—NU21-03-00076), Institutional Support by MH CZ—DRO—FNOs/2019, MH CZ—DRO—FNOs/2020, Student's grant system SGS12/PrF/2022, SGS10/LF/2022 University of Ostrava and by the Ministry of Education, Youth and Sports of the Czech Republic through the e-INFRA CZ (ID:90140). The work was also supported by Centro de Investigación Biomédica en Red—Área de Oncología—del Instituto de Salud Carlos III (CIBERONC; CB16/12/00369); Instituto de Salud Carlos III/Subdirección General de Investigación Sanitaria (FIS No. PI20/00048, PI21/01816); the Cancer Research UK (C355/A26819), FCAECC and AIRC under the Accelerator Award Program (EDITOR); the European Research Council (ERC) 2015 Starting Grant (MYELOMANEXT/680200).
CLINICAL TRIAL INFORMATION
NCT02575144 (GEM-CLARIDEX)
T.J., R.B., and D.Z. contributed equally to this work and are joint first authors.
DATA SHARING STATEMENT
All data used for the development of cutoff of CTCs defining PCL-like MM are summarized in the Data Supplement and are freely available.
AUTHOR CONTRIBUTIONS
Conception and design: Tomas Jelinek, Bruno Paiva, Lucie Rihova, Roman Hajek
Provision of study materials or patients: Veronika Kapustova, Maria-Teresa Cedena, Artur Jurczyszyn, Miroslav Penka, Jakub Radocha, Maria Victoria Mateos, Ludek Pour
Collection and assembly of data: Tomas Jelinek, Renata Bezdekova, Martin Stork, Zdenka Knechtova, Ondrej Venglar, Veronika Kapustova, Tereza Popkova, Ludmila Muronova, Zuzana Chyra, Noemi Puig, Maria-Teresa Cedena, Jorge J. Castillo, Miroslav Penka, Bruno Paiva, Ludek Pour, Lucie Rihova, Roman Hajek
Data analysis and interpretation: Tomas Jelinek, Renata Bezdekova, David Zihala, Tereza Sevcikova, Anjana Anilkumar Sithara, Lenka Pospisilova, Sabina Sevcikova, Petra Polackova, Matous Hrdinka, Michal Simicek, Juan-Jose Garcés, Noemi Puig, Maria-Teresa Cedena, Artur Jurczyszyn, Jorge J. Castillo, Miroslav Penka, Jakub Radocha, Maria Victoria Mateos, Jesús F. San-Miguel, Bruno Paiva, Ludek Pour, Lucie Rihova, Roman Hajek
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
More Than 2% of Circulating Tumor Plasma Cells Defines Plasma Cell Leukemia–Like Multiple Myeloma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Maria-Teresa Cedena
Honoraria: Janssen
Tomas Jelinek
Honoraria: Pfizer, Bristol Myers Squibb/Celgene, GlaxoSmithKline, Janssen
Consulting or Advisory Role: Sanofi
Research Funding: Sanofi, Amgen
Travel, Accommodations, Expenses: Amgen
Noemi Puig
Honoraria: Amgen, Celgene, Janssen, Takeda, The Binding Site
Consulting or Advisory Role: Amgen, Celgene, Janssen, Takeda
Speakers' Bureau: Celgene
Research Funding: Celgene, Janssen, Amgen, Takeda
Travel, Accommodations, Expenses: Amgen, Celgene, Janssen, Takeda
Jorge J. Castillo
Consulting or Advisory Role: Janssen, Roche/Genentech, Beigene, AbbVie/Pharmacyclics, Cellectar
Research Funding: Pharmacyclics (Inst), AbbVie (Inst), Janssen (Inst), BeiGene (Inst), TG Therapeutics (Inst), AstraZeneca (Inst)
Jakub Radocha
Consulting or Advisory Role: Janssen, Bristol Myers Squibb/Celgene, Sanofi, GlaxoSmithKline, Amgen
Speakers' Bureau: Janssen
Travel, Accommodations, Expenses: Janssen, Bristol Myers Squibb/Celgene, Amgen, Sanofi
Maria Victoria Mateos
Honoraria: Janssen-Cilag, Celgene, Amgen, Takeda, GlaxoSmithKline, AbbVie/Genentech, Sanofi
Consulting or Advisory Role: Takeda, Janssen-Cilag, Celgene, Amgen, AbbVie, GlaxoSmithKline, Pfizer, Regeneron, Roche/Genentech
Jesús F. San-Miguel
Consulting or Advisory Role: Amgen (Inst), Celgene (Inst), Takeda (Inst), Bristol Myers Squibb (Inst), MSD (Inst), Novartis (Inst), Sanofi (Inst), Janssen (Inst), Roche (Inst), AbbVie (Inst), GlaxoSmithKline (Inst), Karyopharm Therapeutics (Inst), Secura Bio (Inst), Regeneron (Inst), Haemalogix (Inst)
Bruno Paiva
Honoraria: Amgen, Bristol Myers Squibb, Celgene, Janssen-Cilag, Takeda, Sanofi, Roche/Genentech, Adaptive Biotechnologies
Consulting or Advisory Role: Celgene, Janssen-Cilag, Sanofi, Takeda
Speakers' Bureau: Celgene
Research Funding: Celgene, Janssen-Cilag, Sanofi, Takeda, Roche/Genentech
Roman Hajek
Consulting or Advisory Role: Takeda, Amgen, Celgene, AbbVie, BMS, PharmaMar, Janssen-Cilag, Novartis, Oncopeptides, Oncopeptides, Sanofi, Janssen, GlaxoSmithKline
Speakers' Bureau: Takeda, Amgen
Research Funding: Novartis (Inst), BMS (Inst), Amgen (Inst), Celgene (Inst), Takeda (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.van de Donk NWCJ, Pawlyn C, Yong KL: Multiple myeloma. Lancet 397:410-427, 2021 [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez-Otero P, Paiva B, San-Miguel JF: Roadmap to cure multiple myeloma. Cancer Treat Rev 100:102284, 2021 [DOI] [PubMed] [Google Scholar]
- 3.Garcés J-J, Simicek M, Vicari M, et al. : Transcriptional profiling of circulating tumor cells in multiple myeloma: A new model to understand disease dissemination. Leukemia 34:589-603, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Garcés J-J, Cedena M-T, Puig N, et al. : Circulating tumor cells for the staging of patients with newly diagnosed transplant-eligible multiple myeloma. J Clin Oncol 40:3151-3161, 2022 [DOI] [PubMed] [Google Scholar]
- 5.Sanoja-Flores L, Paiva B, Flores-Montero JA, et al. : Next generation flow (NGF): A high sensitive technique to detect circulating peripheral blood (PB) clonal plasma cells (cPC) in patients with newly diagnosed of plasma cell neoplasms (PCN). Blood 126:4180, 2015 [Google Scholar]
- 6.Sanoja-Flores L, Flores-Montero J, Garcés JJ, et al. : Next generation flow for minimally-invasive blood characterization of MGUS and multiple myeloma at diagnosis based on circulating tumor plasma cells (CTPC). Blood Cancer J 8:117, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertamini L, Oliva S, Rota-Scalabrini D, et al. : High levels of circulating tumor plasma cells as a key hallmark of aggressive disease in transplant-eligible patients with newly diagnosed multiple myeloma. J Clin Oncol 40:3120-3131, 2022 [DOI] [PubMed] [Google Scholar]
- 8.Chakraborty R, Lentzsch S: Circulating tumor cell burden as a component of staging in multiple myeloma: Ready for prime time? J Clin Oncol 40:3099-3102, 2022 [DOI] [PubMed] [Google Scholar]
- 9.Kyle RA, Maldonado JE, Bayrd ED: Plasma cell leukemia. Report on 17 cases. Arch Intern Med 133:813-818, 1974 [DOI] [PubMed] [Google Scholar]
- 10.Jelinek T, Kryukov F, Rihova L, et al. : Plasma cell leukemia: From biology to treatment. Eur J Haematol 95:16-26, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Fernández de Larrea C, Kyle R, Rosiñol L, et al. : Primary plasma cell leukemia: Consensus definition by the International Myeloma Working Group according to peripheral blood plasma cell percentage. Blood Cancer J 11:192, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Registry of Monoclonal Gammopathies. https://rmg.healthregistry.org/ [Google Scholar]
- 13.Jurczyszyn A, Radocha J, Davila J, et al. : Prognostic indicators in primary plasma cell leukaemia: A multicentre retrospective study of 117 patients. Br J Haematol 180:831-839, 2018 [DOI] [PubMed] [Google Scholar]
- 14.Puig N, Hernández MT, Rosiñol L, et al. : Lenalidomide and dexamethasone with or without clarithromycin in patients with multiple myeloma ineligible for autologous transplant: A randomized trial. Blood Cancer J 11:101, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flores-Montero J, Sanoja-Flores L, Paiva B, et al. : Next Generation Flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia 31:2094-2103, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ravi P, Kumar SK, Roeker L, et al. : Revised diagnostic criteria for plasma cell leukemia: Results of a Mayo Clinic study with comparison of outcomes to multiple myeloma. Blood Cancer J 8:116, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Granell M, Calvo X, Garcia-Guiñón A, et al. : Prognostic impact of circulating plasma cells in patients with multiple myeloma: Implications for plasma cell leukaemia definition. Haematologica 102:1099-1104, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonsalves WI, Rajkumar SV, Go RS, et al. : Trends in survival of patients with primary plasma cell leukemia: A population-based analysis. Blood 124:907-912, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nahi H, Genell A, Wålinder G, et al. : Incidence, characteristics, and outcome of solitary plasmacytoma and plasma cell leukemia. Population-based data from the Swedish Myeloma Register. Eur J Haematol 99:216-222, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Hofste Op Bruinink D, Kuiper R, van Duin M, et al. : Identification of high-risk multiple myeloma with a plasma cell leukemia-like transcriptomic profile. J Clin Oncol 40:3132-3150, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Musto P, Simeon V, Martorelli MC, et al. : Lenalidomide and low-dose dexamethasone for newly diagnosed primary plasma cell leukemia. Leukemia 28:222-225, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Bland JM, Altman DG: Measuring agreement in method comparison studies. Stat Methods Med Res 8:135-160, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Greipp PR, San Miguel J, Durie BGM, et al. : International staging system for multiple myeloma. J Clin Oncol 23:3412-3420, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Palumbo A, Avet-Loiseau H, Oliva S, et al. : Revised International staging system for multiple myeloma: A Report from International Myeloma Working Group. J Clin Oncol 33:2863-2869, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Agostino M, Cairns DA, Lahuerta JJ, et al. : Second Revision of the International Staging System (R2-ISS) for overall survival in multiple myeloma: A European Myeloma Network (EMN) report within the HARMONY Project. J Clin Oncol 40:3406-3418, 2022 [DOI] [PubMed] [Google Scholar]
- 26.Gonsalves WI, Jevremovic D, Nandakumar B, et al. : Enhancing the R-ISS classification of newly diagnosed multiple myeloma by quantifying circulating clonal plasma cells. Am J Hematol 95:310-315, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galieni P, Travaglini F, Vagnoni D, et al. : The detection of circulating plasma cells may improve the Revised International Staging System (R-ISS) risk stratification of patients with newly diagnosed multiple myeloma. Br J Haematol 193:542-550, 2021 [DOI] [PubMed] [Google Scholar]
- 28.Cheng Q, Cai L, Zhang Y, et al. : Circulating plasma cells as a biomarker to predict newly diagnosed multiple myeloma prognosis: Developing nomogram prognostic models. Front Oncol 11:639528, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonsalves WI, Buadi FK, Kumar SK: Combination therapy incorporating Bcl-2 inhibition with Venetoclax for the treatment of refractory primary plasma cell leukemia with t (11;14). Eur J Haematol 100:215-217, 2018 [DOI] [PubMed] [Google Scholar]
- 30.Jelinek T, Mihalyova J, Kascak M, et al. : Single-agent venetoclax induces MRD-negative response in relapsed primary plasma cell leukemia with t(11;14). Am J Hematol 94:E35-E37, 2019 [DOI] [PubMed] [Google Scholar]
- 31.Jelinek T, Sevcikova T, Zihala D, et al. : Limited efficacy of daratumumab in multiple myeloma with extramedullary disease. Leukemia 36:288-291, 2022 [DOI] [PubMed] [Google Scholar]
- 32.Moreau P, Garfall AL, van de Donk NWCJ, et al. : Teclistamab in relapsed or refractory multiple myeloma. N Engl J Med 387:495-505, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin T, Usmani SZ, Berdeja JG, et al. : Ciltacabtagene autoleucel, an anti–B-cell maturation antigen chimeric antigen receptor T-cell therapy, for relapsed/refractory multiple myeloma: CARTITUDE-1 2-year follow-up. J Clin Oncol 41:1265-1274, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreau P, Masszi T, Grzasko N, et al. : Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 374:1621-1634, 2016 [DOI] [PubMed] [Google Scholar]
- 35.Dimopoulos MA, Oriol A, Nahi H, et al. : Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 375:1319-1331, 2016 [DOI] [PubMed] [Google Scholar]
- 36.Schjesvold FH, Dimopoulos M-A, Delimpasi S, et al. : Melflufen or pomalidomide plus dexamethasone for patients with multiple myeloma refractory to lenalidomide (OCEAN): A randomised, head-to-head, open-label, phase 3 study. Lancet Haematol 9:e98-e110, 2022 [DOI] [PubMed] [Google Scholar]
- 37.Gay F, Musto P, Rota-Scalabrini D, et al. : Carfilzomib with cyclophosphamide and dexamethasone or lenalidomide and dexamethasone plus autologous transplantation or carfilzomib plus lenalidomide and dexamethasone, followed by maintenance with carfilzomib plus lenalidomide or lenalidomide alone for patients with newly diagnosed multiple myeloma (FORTE): A randomised, open-label, phase 2 trial. Lancet Oncol 22:1705-1720, 2021 [DOI] [PubMed] [Google Scholar]
- 38.Rosiñol L, Oriol A, Rios R, et al. : Bortezomib, lenalidomide, and dexamethasone as induction therapy prior to autologous transplant in multiple myeloma. Blood 134:1337-1345, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bezdekova R, Jelinek T, Kralova R, et al. : Necessity of flow cytometry assessment of circulating plasma cells and its connection with clinical characteristics of primary and secondary plasma cell leukaemia. Br J Haematol 195:95-107, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jelinek T, Bezdekova R, Zatopkova M, et al. : Current applications of multiparameter flow cytometry in plasma cell disorders. Blood Cancer J 7:e617, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paiva B, Puig N, Cedena M-T, et al. : Measurable residual disease by next-generation flow cytometry in multiple myeloma. J Clin Oncol 38:784-792, 2020 [DOI] [PubMed] [Google Scholar]
- 42.Fernández de Larrea C, Kyle RA, Durie BGM, et al. : Plasma cell leukemia: Consensus statement on diagnostic requirements, response criteria and treatment recommendations by the International Myeloma Working Group. Leukemia 27:780-791, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.An G, Qin X, Acharya C, et al. : Multiple myeloma patients with low proportion of circulating plasma cells had similar survival with primary plasma cell leukemia patients. Ann Hematol 94:257-264, 2015 [DOI] [PubMed] [Google Scholar]
- 44.Katodritou E, Terpos E, Delimpasi S, et al. : Real-world data on prognosis and outcome of primary plasma cell leukemia in the era of novel agents: A multicenter national study by the Greek Myeloma Study Group. Blood Cancer J 8:31, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nandakumar B, Kumar SK, Dispenzieri A, et al. : Clinical characteristics and outcomes of patients with primary plasma cell leukemia in the era of novel agent therapy. Mayo Clin Proc 96:677-687, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]