PURPOSE
Patients with advanced pancreatic neuroendocrine tumors (NETs) have few treatment options that yield objective responses. Retrospective and small prospective studies suggest that capecitabine and temozolomide are associated with high response rates (RRs) and long progression-free survival (PFS).
PATIENTS AND METHODS
E2211 was a multicenter, randomized, phase II trial comparing temozolomide versus capecitabine/temozolomide in patients with advanced low-grade or intermediate-grade pancreatic NETs. Key eligibility criteria included progression within the preceding 12 months and no prior temozolomide, dimethyl-triazeno-imidazole-carboxamide or dacarbazine, capecitabine or fluorouracil. The primary end point was PFS; secondary endpoints were overall survival, RR, safety, and methylguanine methyltransferase (MGMT) by immunohistochemistry and promoter methylation.
RESULTS
A total of 144 patients were enrolled between April 2013 and March 2016 to temozolomide (n = 72) or capecitabine and temozolomide (n = 72); the primary analysis population included 133 eligible patients. At the scheduled interim analysis in January 2018, the median PFS was 14.4 months for temozolomide versus 22.7 months for capecitabine/temozolomide (hazard ratio = 0.58), which was sufficient to reject the null hypothesis for the primary end point (stratified log-rank P = .022). In the final analysis (May 2021), the median overall survival was 53.8 months for temozolomide and 58.7 months for capecitabine/temozolomide (hazard ratio = 0.82, P = .42). MGMT deficiency was associated with response.
CONCLUSION
The combination of capecitabine/temozolomide was associated with a significant improvement in PFS compared with temozolomide alone in patients with advanced pancreatic NETs. The median PFS and RR observed with capecitabine/temozolomide are the highest reported in a randomized study for pancreatic NETs. MGMT deficiency was associated with response, and although routine MGMT testing is not recommended, it can be considered for select patients in need of objective response (ClinicalTrials.gov identifier: NCT01824875).
BACKGROUND
Patients with advanced pancreatic neuroendocrine tumors (NETs) have few treatment options that yield objective radiographic tumor regression. Prospective studies evaluating everolimus1 and sunitinib2 in this patient population have demonstrated prolonged progression-free survival (PFS) compared with placebo; however, response rates (RRs) with these agents are < 10%. 177Lu-DOTATATE, a novel radiopeptide, is approved for use in gastroenteropancreatic NETs on the basis of both the randomized NETTER-1 trial in midgut NETs3 and retrospective studies in pancreatic NETs.4 Retrospective studies, which often overestimate efficacy, report RRs for pancreatic NETs around 50%.
CONTEXT
Key Objective
There are few treatments for advanced pancreatic neuroendocrine tumors that both prolong survival and yield objective responses and even fewer predictive and prognostic biomarkers. This randomized phase II clinical trial examines single-agent temozolomide versus the combination of capecitabine and temozolomide and, to our knowledge, is the first randomized trial to examine methylguanine methyltransferase (MGMT) as a biomarker for temozolomide-based chemotherapy.
Knowledge Generated
The combination of capecitabine and temozolomide demonstrated a significant improvement in progression-free survival compared with temozolomide alone and a high response rate compared with most approved therapies. There is also a clinically meaningful improvement in overall survival although it did not reach statistical significance. MGMT deficiency is significantly associated with response to temozolomide.
Relevance (A.H. Ko)
-
The combination of capecitabine plus temozolomide represents an appropriate standard-of-care option for the treatment of advanced low-grade and intermediate-grade pancreatic neuroendocrine tumors. MGMT deficiency may predict a higher likelihood of objective response to this regimen and can be considered for select patients in whom cytoreduction is a primary goal of treatment.*
*Relevance section written by JCO Associate Editor Andrew H. Ko, MD, FASCO.
Historical studies reporting the highest RRs and longest PFS intervals for pancreatic NETs include regimens with cytotoxic chemotherapy, in particular, the alkylating agent streptozocin.5,6 The RRs associated with these older clinical trials were reported as high as 69%; however, the true objective radiologic RRs were likely lower given the use of older response criteria. Retrospective series have reported an overall RR of 6%-39% and a PFS of 4-18 months for streptozocin-based regimens in pancreatic NETs.7-9
Recent retrospective series and small, prospective phase II studies suggest that temozolomide is similarly active but less toxic than streptozocin-based therapy in patients with pancreatic NETs.10-12 In addition, temozolomide has been investigated prospectively in patients with NETs in small phase II combination studies.13-15 Studies evaluating single-agent capecitabine are limited to retrospective or small prospective studies and show little to modest activity.16,17 However, preclinical evidence and early clinical evidence suggest that capecitabine may be synergistic with temozolomide, perhaps by downregulating the DNA repair enzyme, methylguanine methyltransferase (MGMT).18,19 The most promising combination data are from a single-institution retrospective study of 30 patients with advanced pancreatic NETs treated with capecitabine and temozolomide, demonstrating a RR of 70% and a median PFS of 18 months.20 The 2009 NCI Neuroendocrine Tumor Clinical Trials Planning Meeting recommended evaluating temozolomide in pancreatic NETs by comparing single-agent versus combination therapy.21
Furthermore, temozolomide, like streptozocin, is an alkylating agent that induces DNA methylation at the O6 position of guanine, leading to DNA damage and cell death, usually repaired by MGMT. In glioblastoma, MGMT is silenced by promoter methylation, rendering cells more sensitive to alkylating agents. However, in pancreatic NETs, other mechanisms may be involved with MGMT downregulation since promoter methylation seems to be less common, yet MGMT is still lost. In glioblastoma, MGMT promoter methylation testing has become routine as methylation confers a survival advantage and predicts response to temozolomide-based treatment. However, data from retrospective studies in pancreatic NETs have been mixed.22-24 The 2009 NET Clinical Trials Planning Meeting also recommended identification of biomarkers in prospective trials.
To date, no prospective studies have evaluated the antitumor activity of temozolomide alone or in combination with capecitabine in pancreatic NETs. Temozolomide alone is considered a reference arm in this study on the basis of the strength of previous retrospective and limited prospective data, demonstrating activity with temozolomide-containing regimens in NETs, and the inclusion of temozolomide in treatment compendia. In addition, this study explored whether MGMT deficiency, as determined by promoter methylation and/or immunohistochemistry (IHC), is associated with response to temozolomide-based therapy in patients with pancreatic NETs. As both arms contain temozolomide, this study was not designed to test MGMT as a predictive biomarker.
PATIENTS AND METHODS
Patients
This multicenter, phase II trial was led by the ECOG-ACRIN Cancer Research Group (ECOG-ACRIN) and conducted within the National Clinical Trial Network (NCTN). Eligible patients were adults with histologically or pathologically confirmed, locally unresectable or metastatic, low-grade or intermediate-grade pancreatic NETs. Patients were required to have measurable disease by the Response Evaluation Criteria in Solid Tumors (RECIST) guideline (version 1.1) and radiographic disease progression within 12 months from the date of random assignment (progression not defined by RECIST 1.1). Patients may not have received prior temozolomide, dimethyl-triazeno-imidazole-carboxamide or dacarbazine, capecitabine, or fluorouracil therapy. Other prior therapies, including somatostatin analogs, everolimus, and/or sunitinib therapy, were allowed. Concurrent somatostatin analogs were allowed. Additional eligibility criteria are outlined in the Protocol (online only).
Trial Design
In this open-label, multicenter, phase II trial, patients were randomly assigned 1:1 to receive either temozolomide alone or capecitabine and temozolomide. Random assignment was stratified by prior everolimus, prior sunitinib, and concurrent octreotide. Allowing for 5% ineligibility, 145 patients were required to obtain 138 eligible patients to detect a difference in median PFS of 9 versus 14 months (hazard ratio [HR] of 0.64), using a two-sided log-rank test at an overall significance level of 0.20 with a power of 81%. The assumption of 9 months for the temozolomide arm was based on historical data from temozolomide-based regimens. The assumption of 14 months for the combination arm was based on the goal of improving treatment by 55% (which conservatively approximated the 18-month PFS from the study by Strosberg et al3). One interim analysis of PFS was planned at 76% information time (80 PFS events, projected to occur at a study time of 26 months) at which time a stricter P value of .119 was required. The overall type I error was controlled using an O'Brien-Fleming boundary function. This interim analysis served to inform whether the trial should be stopped for futility if negative or reported early if positive.
Arm A consisted of temozolomide 200 mg/m2 by mouth once daily on days 1-5, repeated every 28 days. Arm B consisted of capecitabine 750 mg/m2 by mouth twice a day for days 1-14 and temozolomide 200 mg/m2 by mouth once daily on days 10-14, repeated every 28 days. Ondansetron was prescribed 8 mg by mouth once daily 30-60 minutes before temozolomide. In both arms, temozolomide was capped at 400 mg once daily, and the maximum duration of treatment was 13 cycles. Patients could remain on study treatment beyond 13 cycles at investigator discretion, and these patients were followed until their treatment was ended. Dose modifications for toxicity were allowed per protocol, but if the dose was held for > 4 weeks from the next planned cycle start date, the patient was required to discontinue protocol treatment. Tumor assessments by computed tomography or magnetic resonance imaging were performed every 12 weeks, and confirmatory scans were obtained at least 4 weeks after initial documentation of objective response. Patients were treated until withdrawal of consent, unacceptable toxicity, receipt of nonprotocol cancer therapies, disease progression per protocol criteria, death, or completion of 13 cycles of therapy. All patients provided written informed consent according to institutional guidelines. The trial was approved by relevant review boards, performed in accordance with the Declaration of Helsinki and applicable local regulatory requirements and laws.
End Points and Assessment
The primary end point was PFS by local radiology review, defined as the time from random assignment to progression or death without evidence of progression within 4 months of the date last known to be progression-free; cases without progression or death were censored at the last disease assessment. Secondary end points were overall survival (OS), RR, safety, central pathology review (not in real time), and evaluation of MGMT by IHC and promoter methylation. OS was defined as the time from random assignment to death from any cause, and patients without death were censored at the date last known alive. RR was defined as the proportion of patients with complete response or partial response. Response and progression were evaluated by local radiology review using the revised RECIST 1.1. Definitions of all response categories are provided in the study protocol. Safety was assessed monthly in both arms on the basis of adverse events (AEs), which were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. A protocol-defined, planned interim analysis of the primary PFS end point was reviewed by the ECOG-ACRIN Data and Safety Monitoring Committee.
For the correlative end points, tissue use was prioritized in order of (1) central pathology review, (2) MGMT by IHC, and (3) MGMT by promoter methylation, which were performed blinded to the study end points. Central pathology review included WHO grade (1 v 2); Ki-67 and mitotic index were determined if tissue was available. The degree of differentiation was not assessed as it was not part of the WHO classification at the time of protocol development. MGMT expression was quantified by IHC H-score calculated from staining intensity and percentage of positive cells.22 MGMT promoter methylation was scored as positive (methylated) or negative (unmethylated). MGMT testing methodology is provided in the Data Supplement (online only).
Statistical Analysis
Descriptive statistics were used to characterize patients at baseline. Survival curves were obtained with 95% CIs using the Kaplan-Meier method,4 and stratified log-rank tests and Cox proportional hazards models were used to compare survival distributions between treatment groups, for which the proportional hazards assumption was examined using Schoenfeld residuals. The incidence of treatment-related grade 3 or higher AEs was summarized for each arm using binomial proportions and exact 90% CIs. Rates of toxicity and response were compared using Fisher's exact tests. In addition to the primary and secondary end points, the best percentage change in size of target lesions (from baseline) was calculated for each patient, and the duration of response was calculated for patients with confirmed objective response. The association between MGMT deficiency and RR was assessed using a two-sided Fisher's exact test, and associations between PFS and OS were also explored via Kaplan-Meier curves and log-rank tests.
RESULTS
Patients
From April 2013 to March 2016, a total of 144 patients were randomly assigned to receive temozolomide (n = 72) or capecitabine and temozolomide (n = 72), among whom 136 were eligible (68 on each arm). An additional three patients (all on the temozolomide arm) were excluded on the basis of central pathology review, leading to the primary analysis population of 133 eligible patients. Reasons for ineligibility and pathology exclusion are given in the Data Supplement. The interim analysis (data updated through January 24, 2018) and final analysis (data updated through May 26, 2021) were included in this report. The median follow-up was 59.9 months. Accrual by the NCTN group is provided in the Data Supplement. The disposition of all randomly assigned patients is shown in the CONSORT diagram (Fig 1).
FIG 1.
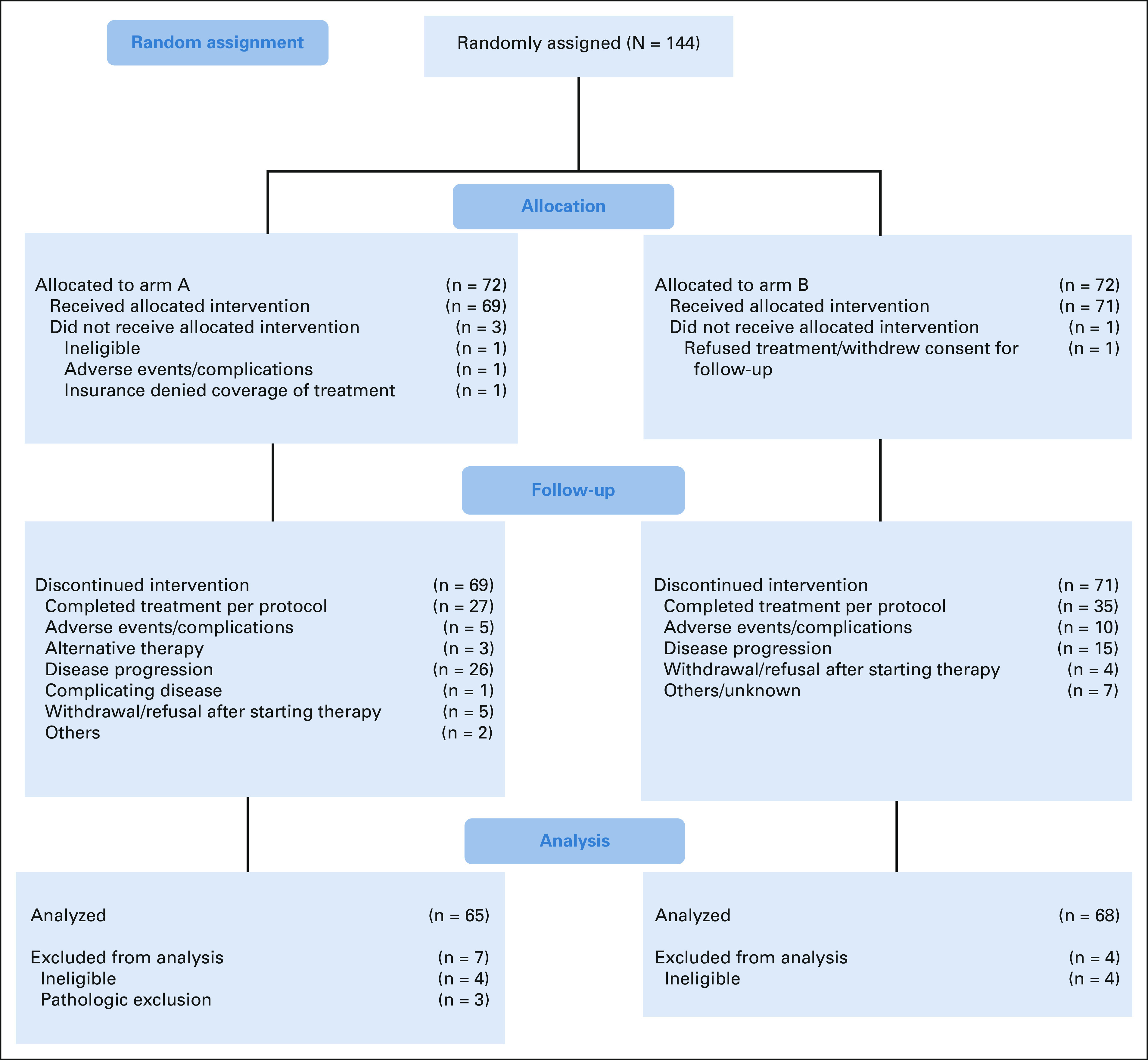
CONSORT diagram.
Patient demographics and disease characteristics of eligible patients by treatment arm are shown in Table 1. Characteristics were balanced between treatment groups except for Black race (higher percentage in the temozolomide arm) and time from diagnosis (longer in the combination arm). The patients were predominantly male, White, and non-Hispanic. In both arms, most patients had not received prior everolimus or sunitinib, whereas approximately half of patients received concurrent octreotide. There were more WHO grade 2 tumors at the time of central pathology review compared with the eligibility pathology review. The most common metastatic sites were liver and regional lymph nodes. The most common prior treatments were somatostatin analogues and surgery. Data on poststudy treatment were not collected.
TABLE 1.
Patient Demographics and Disease Characteristics (eligible population)

Treatment Administration
The mean number of treatment cycles completed was 10.3 (± 7.9) in the temozolomide arm and 12.2 (± 8.2) in the capecitabine and temozolomide arm. The most common off-treatment reasons were treatment completion per protocol criteria (41.3% in the temozolomide arm and 47.8% in the capecitabine and temozolomide arm) and disease progression (34.9% in the temozolomide arm and 22.4% in the capecitabine and temozolomide arm). Fourteen patients in the temozolomide arm and 17 patients in the capecitabine and temozolomide arm received protocol therapy beyond the intended 13 cycles at the discretion of the treating physician. For these patients, the mean number of off-treatment cycles was 8.9 (± 11) in the temozolomide arm and 11.4 (± 7.6) in the capecitabine and temozolomide arm. Treatment administration is shown in Table 2.
TABLE 2.
Treatment Administration (eligible population)
Efficacy
Progression-free survival.
This study met its primary end point of PFS at the interim analysis in January 2018, scheduled at 76% information time (80 PFS events; O'Brien-Fleming boundary = 2.43). This corresponded to an adjusted two-sided significance level of 0.119. Of the 80 PFS events, 74 were documented progressions and six were deaths without documented progression. The median PFS was 14.4 months in the temozolomide arm and 22.7 months in the capecitabine and temozolomide arm, corresponding to an HR of 0.58 (95% CI, 0.36 to 0.93; stratified log-rank test statistic = 5.28, P = .022). When adjusting for grade, this treatment effect remained significant (HR = 0.61, P = .04). In the final analysis on the basis of a data cutoff in May 2021, the median PFS was 15.1 months (95% CI, 10.5 to 21.0) for the temozolomide arm and 23.2 months (95% CI, 16.6 to 32.2) for the capecitabine and temozolomide arm, corresponding to an HR of 0.71 (95% CI, 0.46 to 1.07). When adjusting for grade, the treatment effect on PFS is strengthened (HR, 0.63; 95% CI, 0.39 to 1.01; Data Supplement). Kaplan-Meier curves of PFS by treatment arm and by WHO grade are shown in Figure 2. A forest plot demonstrates the effect of treatment on PFS by subgroup (Data Supplement).
FIG 2.
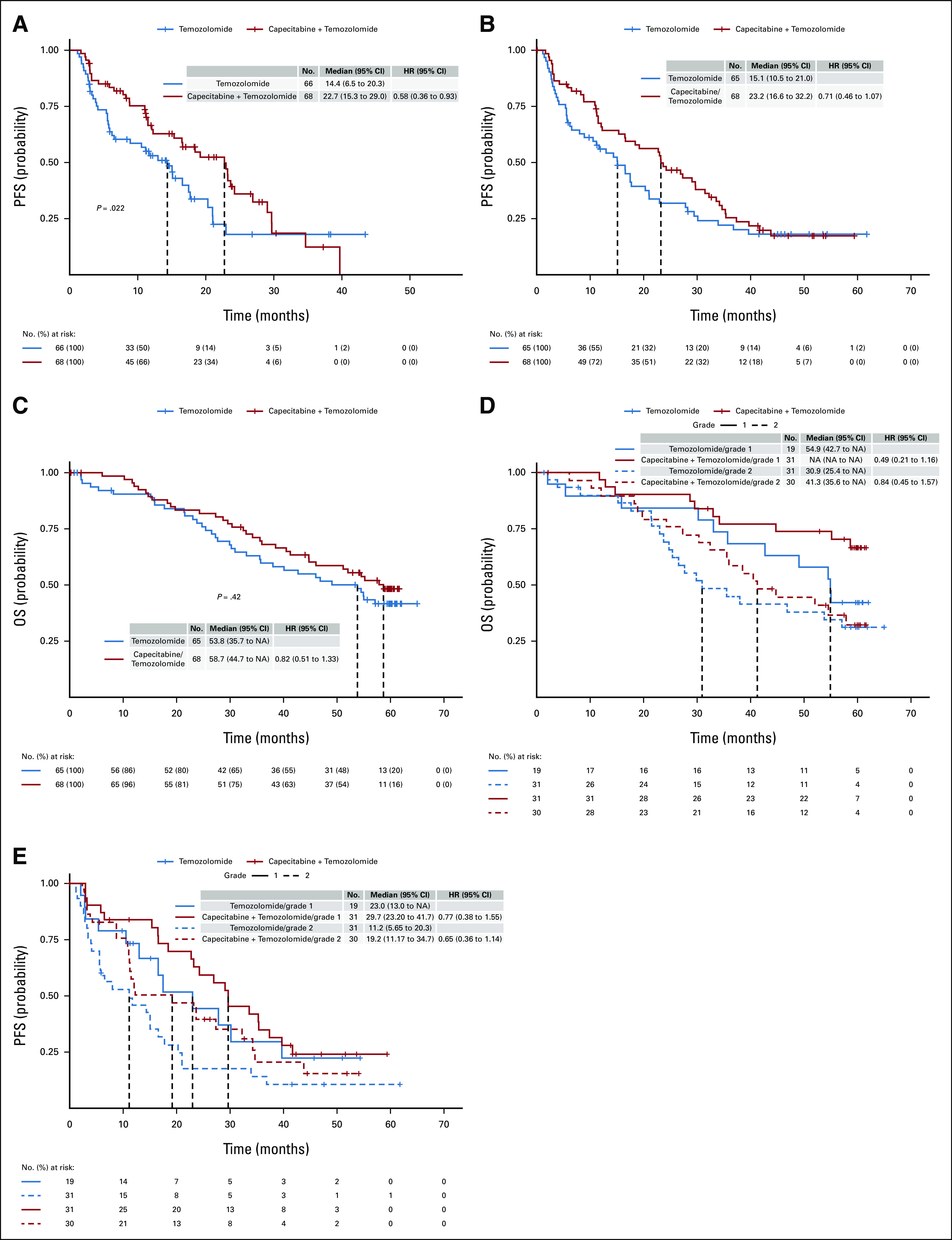
Kaplan-Meier survival curves. (A) PFS by treatment arm at interim analysis. (B) PFS by treatment arm at final analysis. (C) OS by treatment arm at final analysis. (D) PFS by histologic grade at final analysis (central review). (E) OS by histologic grade at final analysis (central review). NA, not applicable; OS, overall survival; PFS, progression-free survival.
Overall survival.
There was a statistically significant difference in OS at the interim analysis (median OS = 38 months for temozolomide and not reached for the combination arm; HR, 0.41; 95% CI, 0.21 to 0.82; P = .012). However, a trend toward improved OS did not reach statistical significance in the final analysis (Fig 2). The median OS was 53.8 months (95% CI, 35.7 to NA) for the temozolomide arm and 58.7 months (95% CI, 44.7 to NA) for the capecitabine and temozolomide arm, corresponding to an HR of 0.82 (95% CI, 0.51 to 1.33; stratified log-rank P = .42). When adjusting for grade, the treatment effect on OS remained nonsignificant (HR, 0.65; 95% CI, 0.38 to 1.11; P = .11; Data Supplement).
Response rate.
Among the 133 eligible patients, seven were deemed unevaluable for response, with reasons shown in the Data Supplement. There was no significant difference in RR between the temozolomide arm and the capecitabine and temozolomide arm (33.7% v 39.7%, respectively; Fisher's exact P = .59); however, the study was not powered for a RR end point. The median duration of response in the temozolomide arm was 12.6 (interquartile range, [8.6-30.7]) months, and in the capecitabine and temozolomide arm, it was 16.6 (interquartile range, [11.4-24.7]) months. Best response by treatment arm is shown in Table 3; best response by treatment arm and grade is shown in the Data Supplement.
TABLE 3.
Best Responses (eligible population)
Safety
The primary safety analysis includes all patients who had > one cycle of treatment (139 patients). Safety analysis is typically conducted among all patients who received any protocol treatment (n = 140). However, one patient refused treatment after cycle 1 and withdrew consent, and no further disease or toxicity assessments were obtained. A minority of patients discontinued treatment because of AEs although it was higher in the combination arm (6.3% in the temozolomide arm and 14.9% in the capecitabine/temozolomide arm). Table 4 shows the treatment related AEs that occurred in ≥ 5% of patients; the most common AEs were hematologic and gastrointestinal. The capecitabine and temozolomide arm showed double the grade 3-4 toxicity rates compared with the temozolomide arm (45% v 22%, OR [95% CI] = 2.69 [1.28 to 5.68]; Fisher's exact P = .005). Seven second primary cancers were reported (three on the temozolomide arm and four on the capecitabine and temozolomide arm), including one case of myelodysplastic syndrome in the capecitabine and temozolomide arm. There were no treatment-related deaths. Data on duration and recovery of cytopenias were not collected.
TABLE 4.
Treatment-Related Adverse Events Observed in ≥ 5% Patients (safety population)
Correlative Analyses
MGMT deficiency (defined as low IHC or positive promoter methylation) is predictive of response to temozolomide in glioblastoma but is a matter of debate for pancreatic NETs. Table 5 shows response by MGMT as measured via IHC and promoter methylation. Of the 133 patients included in the efficacy analysis, 97 (73%) had tissue available for IHC, and 57 (43%) for promoter methylation. The RR in patients whose tumors had low IHC expression was 33 of 63 (52%), whereas in those whose tumors had high IHC expression, it was 5 of 34 (15%), corresponding to an OR [95% CI] of 6.38 [2.19 to 18.60] (P = .0004). The RR in patients whose tumors had evidence of promoter methylation was 6 of 7 (85%), and in patients whose tumors had no evidence of promoter methylation, it was 19 of 50 (38%), corresponding to an OR [95% CI] of 9.79 [1.09 to 87.71] (P = .04). Response by arm and MGMT status is shown in the Data Supplement. Of note, all patients (n = 7) with positive promoter methylation also had low IHC (Data Supplement). PFS and OS by MGMT status are included in the Data Supplement. Demographics of patients who had MGMT testing are presented in the Data Supplement. Most characteristics have similar patterns of distribution when compared with the overall study population, except sex. In the overall study population, there were more males; in the cohort of patients who underwent MGMT by promoter methylation or by both methods, there was a predominance of females.
TABLE 5.
RECIST Response by MGMT
DISCUSSION
This randomized, multicenter, phase II trial of patients with advanced progressive pancreatic NETs met its primary end point as treatment with capecitabine and temozolomide resulted in a prolonged median PFS compared with temozolomide alone (22.7 v 14.4 months; HR, 0.58; 95% CI, 0.36 to 0.93). This clinically and statistically significant improvement in PFS with capecitabine and temozolomide is encouraging when surveying the treatment landscape for patients with progressive pancreatic NETs. Approved therapies include everolimus and sunitinib, both of which were associated with a median PFS of < 12 months in randomized clinical trials. 177Lu-DOTATATE has not been formally evaluated in prospective randomized clinical trials in patients with pancreatic NETs, therefore limiting an accurate estimate of PFS with that treatment. In addition, the RR of 39.7% in the capecitabine and temozolomide arm that we reported is higher than with other available therapies, and therefore, this combination treatment represents an important option for patients in need of clinically meaningful tumor shrinkage. RRs for other approved therapies for advanced pancreatic NETs are considerably lower: lanreotide (2%),25 everolimus (5%),1 and sunitinib (9%).2 The high RR in this trial therefore sets temozolomide-based regimens apart from the others.
A trend toward improved OS did not reach statistical significance in the final analysis; however, there was a 5-month clinically meaningful difference. This lack of OS benefit is a finding common to other NET clinical trials attributable to relatively long survival durations, the effect of poststudy treatments, and lack of statistical power to detect differences in OS. However, in the capecitabine and temozolomide arm, time from diagnosis was longer (34.3 v 25.5 months), which could have dampened the efficacy results in the combination arm.
In terms of safety, no new toxicity signals emerged. The rate of second primary cancers was 4% on the temozolomide arm and 5% on the combination arm. This may be explained by the long duration of follow-up (median 59.9 months) and known association of NETs with second primary cancers (as high as 16% in one study26). Future studies should better characterize these toxicities, develop predictors of toxicity, and explore opportunities to mitigate them, a topic especially important for patients with indolent cancers and chronic survivorship challenges like pancreatic NETs.
Finally, the role of MGMT as a biomarker in pancreatic NETs has been a matter of debate, with some studies indicating an association with objective response and other studies showing none. To our knowledge, this study represents the first prospective analysis of MGMT by both IHC and promoter methylation in pancreatic NETs and demonstrates that MGMT deficiency, as defined by either low IHC or promoter methylation, is associated with tumor response. The absence of a nontemozolomide control arm precludes a definitive conclusion regarding whether MGMT deficiency is predictive. In addition, MGMT by promoter methylation is not sufficient to fully explain MGMT downregulation, as many more patients had low MGMT expression by IHC. In summary, there is insufficient evidence to support the routine use of MGMT testing for all patients with metastatic pancreatic NETs. However, MGMT testing can be considered in select patients receiving temozolomide when response is a primary goal of treatment. Additional investigation in future studies is warranted.
In conclusion, to our knowledge, this is the first prospective, multicenter, randomized trial of capecitabine and temozolomide in advanced pancreatic NETs. We found that the combination of temozolomide and capecitabine is associated with improved PFS compared with temozolomide alone. Treatment with this combination is associated with the longest PFS and highest RR reported in randomized trials of pancreatic NETs. In addition, MGMT deficiency is associated with tumor response. These results suggest that the combination of capecitabine and temozolomide should be included as a standard treatment option for patients with advanced pancreatic NETs and is a reasonable comparator arm in future randomized studies. To this point, two follow-up NCTN studies have been developed to examine the role of capecitabine and temozolomide in other indications: a randomized phase II trial of postoperative adjuvant capecitabine and temozolomide versus observation in high-risk pancreatic NETs (SWOG 2104, ClinicalTrials.gov identifier: NCT05040360) and a phase II randomized trial of 177Lu-DOTATATE versus capecitabine and temozolomide in advanced well-differentiated pancreatic NETs (A022001).
ACKNOWLEDGMENT
We would like to acknowledge funding from the Goldhirsh-Yellin Foundation for the correlative analyses.
Pamela L. Kunz
This author is an ASCO Meeting Abstracts Editor. Journal policy recused the author from having any role in the peer review of this manuscript.
Stock and Other Ownership Interests: Guardant Health
Consulting or Advisory Role: Ipsen, Lexicon, Sun Pharma, Acrotech Biopharma, Advanced Accelerator Applications/Novartis, Genentech/Roche, Amgen, Crinetics Pharmaceuticals, RayzeBio, Natera, HUTCHMED
Research Funding: Lexicon (Inst), Ipsen (Inst), Xencor (Inst), Brahms (Inst), Advanced Accelerator Applications/Novartis (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/191388
Paul J. Catalano
Research Funding: Regeneron (Inst), Astellas Pharma (Inst), AstraZeneca (Inst)
Halla S. Nimeiri
Employment: Northwestern Medicine, Tempus
Stock and Other Ownership Interests: Roche, AbbVie
George A. Fisher
Stock and Other Ownership Interests: Seattle Genetics
Honoraria: eChina Health
Consulting or Advisory Role: Merck, Taiho Pharmaceutical, Ipsen, Genentech/Roche
Research Funding: Genentech/Roche (Inst), Merck (Inst)
Travel, Accommodations, Expenses: Merck, Roche/Genentech
Other Relationship: CytomX Therapeutics, Silenseed, AstraZeneca, Terumo Clinical Supply, Taiho Pharmaceutical, Hutchison MediPharma
Teri A. Longacre
Consulting or Advisory Role: Merck, Astellas Pharma, Mersana
Expert Testimony: Johnson & Johnson/Janssen
James C. Yao
Consulting or Advisory Role: Ipsen, Hutchison MediPharma, Crinetics Pharmaceuticals, Amgen, Chiasma, Advanced Accelerator Applications
Research Funding: Novartis, Advanced Accelerator Applications
Matthew H. Kulke
Research Funding: Bristol Myers Squibb (Inst)
Andrew E. Hendifar
Consulting or Advisory Role: Novartis, Ipsen, Perthera, Celgene, AbbVie, Eisai, Valar Labs
Research Funding: Ipsen, NGM Biopharmaceuticals (Inst)
Travel, Accommodations, Expenses: Halozyme
Other Relationship: FibroGen
Manisha H. Shah
Research Funding: Merck (Inst), Lilly (Inst)
Mark M. Zalupski
Research Funding: AstraZeneca/MedImmune, Seattle Genetics, BioMed Valley Discoveries
Edmond L. Schmulbach
Stock and Other Ownership Interests: Pfizer, AstraZeneca, Gilead Sciences
Diane L. Reidy-Lagunes
Honoraria: Novartis
Consulting or Advisory Role: Lexicon, Advanced Accelerator Applications, ITM Oncologics, Chiasma
Research Funding: Novartis, Ipsen, Merck
Jonathan R. Strosberg
Consulting or Advisory Role: TerSera
Speakers' Bureau: Ipsen
Research Funding: Novartis (Inst), ITM Solucin (Inst)
Peter J. O'Dwyer
Research Funding: Bristol Myers Squibb (Inst), Pfizer (Inst), Novartis (Inst), Genentech (Inst), Mirati Therapeutics (Inst), Celgene (Inst), GlaxoSmithKline (Inst), BBI Healthcare (Inst), Pharmacyclics (Inst), Five Prime Therapeutics (Inst), Forty Seven (Inst), Amgen (Inst), H3 Biomedicine (Inst), Taiho Pharmaceutical (Inst), Array BioPharma (Inst), Lilly/ImClone (Inst), Syndax (Inst), Minneamrita Therapeutics (Inst)
Expert Testimony: Daiichi Sankyo
Al B. Benson
Consulting or Advisory Role: Merck Sharp & Dohme, Array BioPharma, Bristol Myers Squibb, Samsung Bioepis, Pfizer, HalioDx, AbbVie, Janssen Oncology, Natera, Apexigen, Artemida Pharma, Xencor, TheraBionic, Mirati Therapeutics, Boston Scientific, HUTCHMED
Research Funding: Infinity Pharmaceuticals (Inst), Merck Sharp & Dohme (Inst), Taiho Pharmaceutical (Inst), Bristol Myers Squibb (Inst), Celgene (Inst), Rafael Pharmaceuticals (Inst), MedImmune (Inst), Xencor (Inst), Astellas Pharma (Inst), Amgen (Inst), SynCoreBio (Inst), Elevar Therapeutics (Inst), Tyme Inc (Inst), ST Pharm (Inst), ITM Solucin (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the 2018 ASCO annual meeting, June 1-5, 2018, Chicago, IL, and at the 2022 ASCO annual meeting, June 3-7, Chicago, IL.
SUPPORT
This study was coordinated by the ECOG-ACRIN Cancer Research Group (P.J.O., MD, and Mitchell D. Schnall, MD, PhD, Group cochairs) and supported by the National Cancer Institute of the National Institutes of Health under award Nos. U10CA180820, U10CA180794, UG1CA233337, UG1CA233320, UG1CA233253, UG1CA233329, UG1CA233180, U10CA180888, UG1CA189863, U10CA180821, UG1CA233331, UG1CA233160, UG1CA189821, and UG1CA233290. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
CLINICAL TRIAL INFORMATION
NCT01824875 E2211
AUTHOR CONTRIBUTIONS
Conception and design: Pamela L. Kunz, George A. Fisher, James C. Yao, Matthew H. Kulke, Al B. Benson III
Financial support: Pamela L. Kunz, Peter J. O'Dwyer, Al B. Benson III
Administrative support: Pamela L. Kunz
Provision of study materials or patients: Halla S. Nimeiri, George A. Fisher, James C. Yao, Matthew H. Kulke, Andrew E. Hendifar, James C. Shanks, Manisha H. Shah, Mark M. Zalupski, Edmond L. Schmulbach, Diane L. Reidy-Lagunes, Jonathan R. Strosberg, Peter J. O'Dwyer, Al B. Benson III
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized Study of Temozolomide or Temozolomide and Capecitabine in Patients With Advanced Pancreatic Neuroendocrine Tumors (ECOG-ACRIN E2211)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Pamela L. Kunz
This author is an ASCO Meeting Abstracts Editor. Journal policy recused the author from having any role in the peer review of this manuscript.
Stock and Other Ownership Interests: Guardant Health
Consulting or Advisory Role: Ipsen, Lexicon, Sun Pharma, Acrotech Biopharma, Advanced Accelerator Applications/Novartis, Genentech/Roche, Amgen, Crinetics Pharmaceuticals, RayzeBio, Natera, HUTCHMED
Research Funding: Lexicon (Inst), Ipsen (Inst), Xencor (Inst), Brahms (Inst), Advanced Accelerator Applications/Novartis (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/191388
Paul J. Catalano
Research Funding: Regeneron (Inst), Astellas Pharma (Inst), AstraZeneca (Inst)
Halla S. Nimeiri
Employment: Northwestern Medicine, Tempus
Stock and Other Ownership Interests: Roche, AbbVie
George A. Fisher
Stock and Other Ownership Interests: Seattle Genetics
Honoraria: eChina Health
Consulting or Advisory Role: Merck, Taiho Pharmaceutical, Ipsen, Genentech/Roche
Research Funding: Genentech/Roche (Inst), Merck (Inst)
Travel, Accommodations, Expenses: Merck, Roche/Genentech
Other Relationship: CytomX Therapeutics, Silenseed, AstraZeneca, Terumo Clinical Supply, Taiho Pharmaceutical, Hutchison MediPharma
Teri A. Longacre
Consulting or Advisory Role: Merck, Astellas Pharma, Mersana
Expert Testimony: Johnson & Johnson/Janssen
James C. Yao
Consulting or Advisory Role: Ipsen, Hutchison MediPharma, Crinetics Pharmaceuticals, Amgen, Chiasma, Advanced Accelerator Applications
Research Funding: Novartis, Advanced Accelerator Applications
Matthew H. Kulke
Research Funding: Bristol Myers Squibb (Inst)
Andrew E. Hendifar
Consulting or Advisory Role: Novartis, Ipsen, Perthera, Celgene, AbbVie, Eisai, Valar Labs
Research Funding: Ipsen, NGM Biopharmaceuticals (Inst)
Travel, Accommodations, Expenses: Halozyme
Other Relationship: FibroGen
Manisha H. Shah
Research Funding: Merck (Inst), Lilly (Inst)
Mark M. Zalupski
Research Funding: AstraZeneca/MedImmune, Seattle Genetics, BioMed Valley Discoveries
Edmond L. Schmulbach
Stock and Other Ownership Interests: Pfizer, AstraZeneca, Gilead Sciences
Diane L. Reidy-Lagunes
Honoraria: Novartis
Consulting or Advisory Role: Lexicon, Advanced Accelerator Applications, ITM Oncologics, Chiasma
Research Funding: Novartis, Ipsen, Merck
Jonathan R. Strosberg
Consulting or Advisory Role: TerSera
Speakers' Bureau: Ipsen
Research Funding: Novartis (Inst), ITM Solucin (Inst)
Peter J. O'Dwyer
Research Funding: Bristol Myers Squibb (Inst), Pfizer (Inst), Novartis (Inst), Genentech (Inst), Mirati Therapeutics (Inst), Celgene (Inst), GlaxoSmithKline (Inst), BBI Healthcare (Inst), Pharmacyclics (Inst), Five Prime Therapeutics (Inst), Forty Seven (Inst), Amgen (Inst), H3 Biomedicine (Inst), Taiho Pharmaceutical (Inst), Array BioPharma (Inst), Lilly/ImClone (Inst), Syndax (Inst), Minneamrita Therapeutics (Inst)
Expert Testimony: Daiichi Sankyo
Al B. Benson
Consulting or Advisory Role: Merck Sharp & Dohme, Array BioPharma, Bristol Myers Squibb, Samsung Bioepis, Pfizer, HalioDx, AbbVie, Janssen Oncology, Natera, Apexigen, Artemida Pharma, Xencor, TheraBionic, Mirati Therapeutics, Boston Scientific, HUTCHMED
Research Funding: Infinity Pharmaceuticals (Inst), Merck Sharp & Dohme (Inst), Taiho Pharmaceutical (Inst), Bristol Myers Squibb (Inst), Celgene (Inst), Rafael Pharmaceuticals (Inst), MedImmune (Inst), Xencor (Inst), Astellas Pharma (Inst), Amgen (Inst), SynCoreBio (Inst), Elevar Therapeutics (Inst), Tyme Inc (Inst), ST Pharm (Inst), ITM Solucin (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Yao JC, Shah MH, Ito T, et al. : Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 364:514-523, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raymond E, Dahan L, Raoul JL, et al. : Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 364:501-513, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Strosberg J, El-Haddad G, Wolin E, et al. : Phase 3 trial of 177Lu-DOTATATE for midgut neuroendocrine tumors. N Engl J Med 376:125-135, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brabander T, van der Zwan WA, Teunissen JJM, et al. : Long-term efficacy, survival, and safety of [177Lu-DOTA0,Tyr3]octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors. Clin Cancer Res 23:4617-4624, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Moertel CG, Hanley JA, Johnson LA: Streptozocin alone compared with streptozocin plus fluorouracil in the treatment of advanced islet-cell carcinoma. N Engl J Med 303:1189-1194, 1980 [DOI] [PubMed] [Google Scholar]
- 6.Moertel CG, Lefkopoulo M, Lipsitz S, et al. : Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med 326:519-523, 1992 [DOI] [PubMed] [Google Scholar]
- 7.Kouvaraki MA, Ajani JA, Hoff P, et al. : Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol 22:4762-4771, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Cheng PN, Saltz LB: Failure to confirm major objective antitumor activity for streptozocin and doxorubicin in the treatment of patients with advanced islet cell carcinoma. Cancer 86:944-948, 1999 [PubMed] [Google Scholar]
- 9.McCollum AD, Kulke MH, Ryan DP, et al. : Lack of efficacy of streptozocin and doxorubicin in patients with advanced pancreatic endocrine tumors. Am J Clin Oncol 27:485-488, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Ekeblad S, Sundin A, Janson ET, et al. : Temozolomide as monotherapy is effective in treatment of advanced malignant neuroendocrine tumors. Clin Cancer Res 13:2986-2991, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Kulke MH, Hornick JL, Frauenhoffer C, et al. : O6-methylguanine DNA methyltransferase deficiency and response to temozolomide-based therapy in patients with neuroendocrine tumors. Clin Cancer Res 15:338-345, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maire F, Hammel P, Faivre S, et al. : Temozolomide: A safe and effective treatment for malignant digestive endocrine tumors. Neuroendocrinology 90:67-72, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Kulke MH, Stuart K, Enzinger PC, et al. : Phase II study of temozolomide and thalidomide in patients with metastatic neuroendocrine tumors. J Clin Oncol 24:401-406, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Chan JA, Stuart K, Earle CC, et al. : Prospective study of bevacizumab plus temozolomide in patients with advanced neuroendocrine tumors. J Clin Oncol 30:2963-2968, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan JA, Blaszkowsky L, Stuart K, et al. : A prospective, phase 1/2 study of everolimus and temozolomide in patients with advanced pancreatic neuroendocrine tumor. Cancer 119:3212-3218, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medley L, Morel AN, Farrugia D, et al. : Phase II study of single agent capecitabine in the treatment of metastatic non-pancreatic neuroendocrine tumours. Br J Cancer 104:1067-1070, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bongiovanni A, Riva N, Calpona S, et al. : Metronomic capecitabine in gastroenteropancreatic neuroendrocrine tumors: A suitable regimen and review of the literature. Onco Targets Ther 7:1919-1926, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fine R, Fogelman D, Schreibman S: Effective treatment of neuroendocrine tumors with temozolomide and capecitabine. J Clin Oncol 23:4216, 2005. (suppl) [Google Scholar]
- 19.Murakami J, Lee YJ, Kokeguchi S, et al. : Depletion of O6-methylguanine-DNA methyltransferase by O6-benzylguanine enhances 5-FU cytotoxicity in colon and oral cancer cell lines. Oncol Rep 17:1461-1467, 2007 [PubMed] [Google Scholar]
- 20.Strosberg JR, Fine RL, Choi J, et al. : First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer 117:268-275, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulke MH, Siu LL, Tepper JE, et al. : Future directions in the treatment of neuroendocrine tumors: Consensus report of the National Cancer Institute Neuroendocrine Tumor clinical trials planning meeting. J Clin Oncol 29:934-943, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cros J, Hentic O, Rebours V, et al. : MGMT expression predicts response to temozolomide in pancreatic neuroendocrine tumors. Endocr Relat Cancer 23:625-633, 2016 [DOI] [PubMed] [Google Scholar]
- 23.Girot P, Dumars C, Mosnier JF, et al. : Short article: Evaluation of O6-methylguanine-DNA methyltransferase as a predicting factor of response to temozolomide-based chemotherapy in well-differentiated metastatic pancreatic neuroendocrine tumors. Eur J Gastroenterol Hepatol 29:826-830, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Kulke M, Frauenhoffe C, Hooshmand S, et al. : Prediction of response to temozolomide (TMZ)-based therapy by loss of MGMT expression in patients with advanced neuroendocrine tumors (NET). J Clin Oncol 25:4505, 2007. (18_suppl)17906217 [Google Scholar]
- 25.Caplin ME, Pavel M, Cwikla JB, et al. : Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 371:224-233, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Low SK, Giannis D, Bahaie NS, et al. : Competing mortality in patients with neuroendocrine tumors. Am J Clin Oncol 42:668-674, 2019 [DOI] [PubMed] [Google Scholar]






