Abstract
Respiration is a brain function on which our lives essentially depend. Control of respiration ensures that the frequency and depth of breathing adapt continuously to metabolic needs. In addition, the respiratory control network of the brain has to organize muscular synergies that integrate ventilation with posture and body movement. Finally, respiration is coupled to cardiovascular function and emotion. Here, we argue that the brain can handle this all by integrating a brainstem central pattern generator circuit in a larger network that also comprises the cerebellum. Although currently not generally recognized as a respiratory control center, the cerebellum is well known for its coordinating and modulating role in motor behavior, as well as for its role in the autonomic nervous system. In this review, we discuss the role of brain regions involved in the control of respiration, and their anatomical and functional interactions. We discuss how sensory feedback can result in adaptation of respiration, and how these mechanisms can be compromised by various neurological and psychological disorders. Finally, we demonstrate how the respiratory pattern generators are part of a larger and integrated network of respiratory brain regions.
Introduction
From the first cry to the last gasp, the respiratory system should never fail to supply sufficient oxygen to meet metabolic demands during every possible event throughout life (Del Negro et al., 2018). The respiratory pattern is not only determined by physical activity, but also reflects the emotional state, and volitional control of respiration can be used to alter affection and reduce stress (Suess et al., 1980; Philippot et al., 2002; Arch and Craske, 2006; Seppälä et al., 2014; Szulczewski, 2019). Indeed, multiple behaviors, such as swimming, playing musical instruments, parturition, or meditation depend on precise respiratory control (Brown and Gerbarg, 2009; Jakovljevic and McConnell, 2009; Bartlett and Leiter, 2012; Holstege, 2014; Sakaguchi and Aiba, 2016), and for many sports and arts, it is often the control of respiration that separates mediocre from top performance (Mahler et al., 1991; Phillips and Aitchison, 1997; Laczika et al., 2013; Salomoni et al., 2016). Thus, indeed, respiratory control affects all aspects of life.
Although control over respiration can be voluntary, most of it is subconscious, even during voluntary respiration. In this review, we discuss the integrated network of brain regions most involved in the control of respiration, their connections, and possible clinical consequences of their pathology. Throughout, we discuss how respiratory control and other motor and non-motor systems interact.
Respiratory muscles and their motor neurons
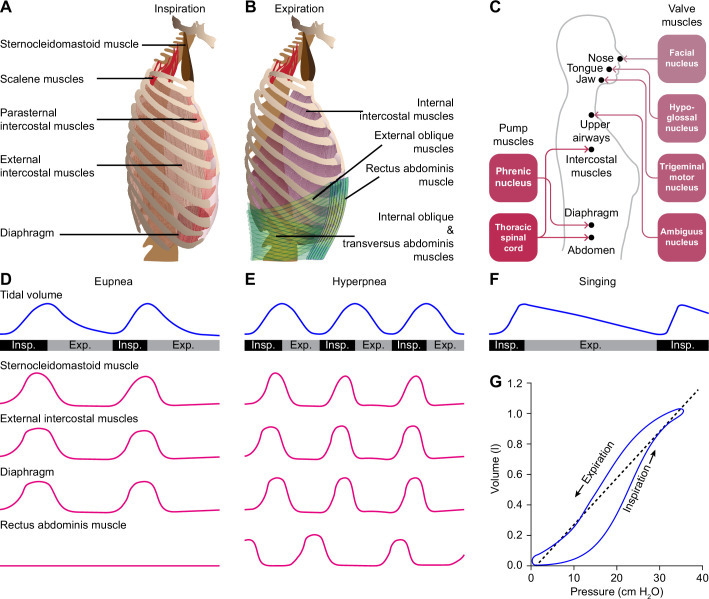
Despite their vast differences in body size and ecological niches, mammals possess similar basic mechanics of ventilation, with some variations between species or sexes (Carvalho and Gonçalves, 2011; Torres-Tamayo et al., 2018). Both lung and tidal volumes scale linearly with body weight, and the higher metabolic rate of smaller mammals is accounted for by a faster respiratory rate (Stahl, 1967; Boggs and Tenney, 1984). The force required for inspiration is delivered by so-called pump muscles that expand the rib cage. The diaphragm and external intercostal muscles are the most prominent inspiratory pump muscles, but also parasternal intercostal, sternocleidomastoid and scalene muscles can act as such (De Troyer and Estenne, 1984; De Troyer et al., 1998; De Troyer et al., 2005; Torres-Tamayo et al., 2018; Welch et al., 2019; LoMauro and Aliverti, 2021). During active expiration, expiratory pump muscles, in particular the internal intercostal and abdominal muscles, are active (De Troyer et al., 2005; Mortola, 2013; Welch et al., 2019). The diaphragm is under control of motor neurons in the phrenic nucleus located in the anterior ramus of the third to sixth cervical vertebrae (Wertheimer, 1886; Hollinshead and Keswani, 1956; Wu et al., 2017). The intercostal and abdominal muscles are innervated from the thoracic spinal cord (Figure 1A–C).
Figure 1. Respiratory muscles and their innervation.
(A) The main driving force for inspiration is delivered by the diaphragm in conjunction with the external intercostal muscles. Other muscles that can enlarge the chest, such as the parasternal intercostal, sternocleidomastoid and scalene muscles, may also contribute. (B) Active expiration involves contraction of the internal intercostal muscles together with abdominal muscles. (C) The pump muscles are innervated from the spinal cord, with the phrenic nucleus housing the motor neurons of the diaphragm, and the thoracic spinal cord those of the intercostal and abdominal muscles. (D) During regular breathing at rest (eupnea), inspiration is followed by a largely passive form of expiration termed post-inspiration or early expiration. During post-inspiration, the abdominal muscles are not (strongly) involved. (E) When the metabolic demand is higher, hyperpnea entails the activation of expiratory pump muscles during active expiration. (F) Prolonged post-inspiration, when required assisted by active expiration, ensures a longer period with constant outflow of air as exploited by professional singers. (G) Intrapleural pressure-volume curve during normal respiration in which the lung compliance is defined as the slope of the dotted line. Schematized data based on Bellani et al., 2018 and Pitts et al., 2015 (D–E), Salomoni et al., 2016 (F), and Albaiceta et al., 2005 (G). Exp.=expiration, Insp.=inspiration.
Valve muscles regulate the air flow by adjusting the resistance of the upper airways. Activity of the facial nucleus can lead to opening of the nasal valve, via the dilator naris anterior muscle and the alar part of the nasal muscle (van Dishoeck, 1937; Strohl, 1985; Vaiman et al., 2003). Contractions of these muscles do not only facilitate inspiration, but can also relate to sniffing (Welker, 1964). Just before the start of inspiration, motor neurons of the hypoglossal nucleus activate tongue muscles, reducing collapsibility of the pharynx (Fuller et al., 1999; Gestreau et al., 2005). Indeed, tongue deformation, for example as a consequence of excessive fat depositions in obesity, can be related to obstructive sleep apnea (Lowe et al., 1986; Kim et al., 2014; Yu et al., 2021). Activation of the trigeminal motor nucleus can contribute to jaw movements (Mong et al., 1988). Finally, contractions of the larynx muscles show a bimodal pattern; initially, they are dilated to allow airflow into the lungs, while at the end of inspiration these muscles contract to reduce the outflow of air, prolonging the period of gas exchange (Gesell and White, 1938; Gautier et al., 1973; Insalaco et al., 1991; Hutchison et al., 1993; Amis et al., 1995; Dutschmann et al., 2014). Laryngeal constriction is controlled by the nucleus ambiguus via the vagus nerve (Dutschmann et al., 2014). The nucleus ambiguus houses also motor neurons controlling swallowing, the control of which is strongly coupled to that of respiration (McFarland and Lund, 1993; Moore et al., 2014).
Respiratory muscles typically serve multiple functions. Rib cage muscles, for instance, control both respiration and arm movements, so that locomotion and respiration are tightly coupled during quadrupedal locomotion. Indeed, one could argue that the change from quadrupedal to bipedal locomotion during hominid evolution paved the way for the intricate breathing control required for human speech (Carrier, 1984; MacLarnon and Hewitt, 1999).
Functional anatomy of respiratory control
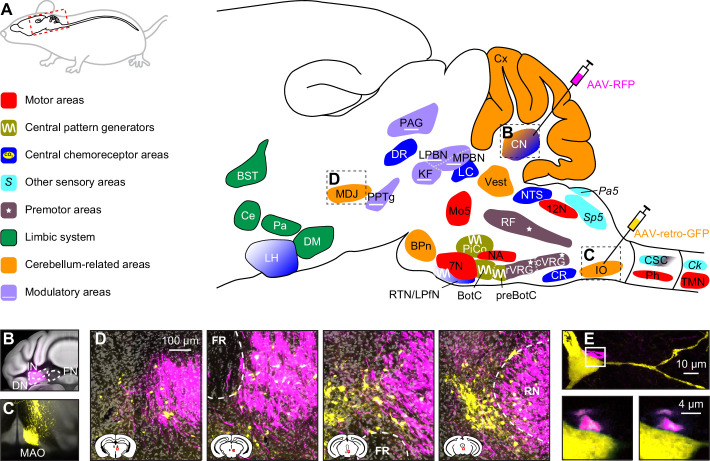
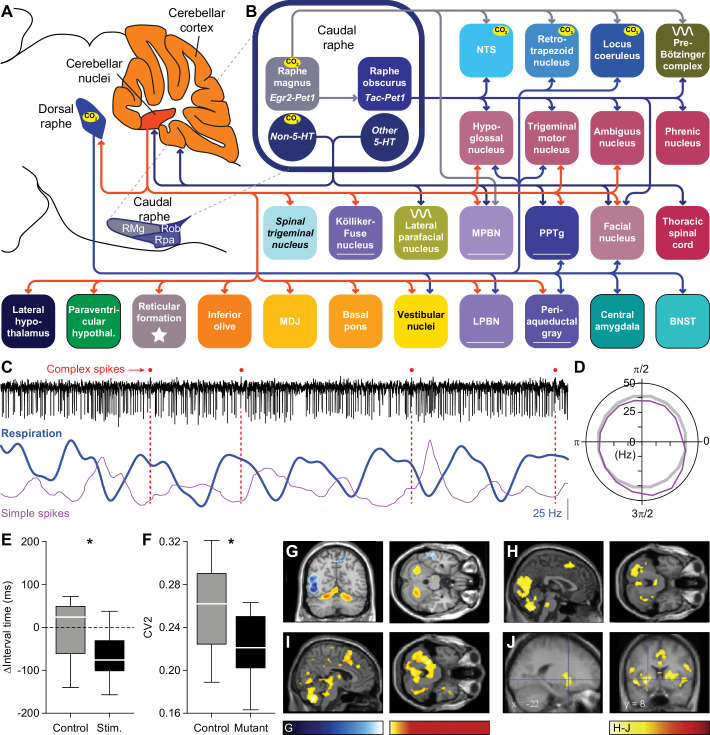
Below, we describe the brain areas most involved in subconscious control of respiration, and their main connections. Most of these pathways are bilateral, but ipsi- and contralateral projections can differ in strength or in their ratio between excitatory and inhibitory fibers, potentially introducing asymmetries in motor activity (Biancardi et al., 2021). As detailed studies on monosynaptic projections are sparse in humans, we base our summary on animal studies, with earlier descriptions mostly concerning cats, and more recent ones often performed in rats or mice (Figure 2, Supplementary file 1). Connections labeled as sparse in the original papers are not included in this overview.
Figure 2. Brain areas involved in subconscious respiratory control.
(A) The subcortical areas involved in control of respiration were classified according to their main function and plotted at their approximate location on a sagittal projection of the mouse brain. 7N = facial nucleus, 12N=hypoglossal nucleus, BotC = Bötzinger complex, BPn = basal pons, BST = bed nucleus of the stria terminalis, Ce = central amygdala, Ck = Clarke’s column, CN = cerebellar nuclei, CR = caudal raphe nucleus, CSC = cervical spinal cord, cVRG = caudal ventral respiratory group, Cx = cerebellar cortex, DM = dorsomedial hypothalamus, DR = dorsal raphe nucleus, IO = inferior olive, KF = Kölliker-Fuse nucleus, LC = locus coeruleus, LH = lateral hypothalamus, LPBN = lateral parabrachial nucleus, LPfN = lateral parafacial nucleus, MDJ = nuclei of the mesodiencephalic junction, Mo5=trigeminal motor nucleus, MPBN = medial parabrachial nucleus, NA = nucleus ambiguus, NTS = nucleus of the solitary tract, Pa5=paratrigeminal nucleus, PAG = periaqueductal gray, Pa = paraventricular hypothalamus, Ph = phrenic nucleus, PiCo = postinspiratory complex, PPTg = pedunculopontine tegmental area, preBotC = pre-Bötzinger complex, RF = reticular formation, RTN = retrotrapezoid nucleus, rVRG = rostral ventral respiratory group, Sp5 = spinal trigeminal nucleus, TMN = thoracic motor neurons, Vest = vestibular nuclei. Neural tracing can reveal monosynaptic connections between brain regions, as illustrated with an example using an anterograde tracer in the cerebellar nuclei (B; see injection needle in panel A, AAV-RFP, pseudocolored in magenta), and a retrograde tracer in the inferior olive (C; AAV-retro-GFP, pseudocolored in yellow). DN = dentate nucleus, FN = fastigial nucleus, IN = interposed nucleus, MAO = medial accessory olive. (D) Both tracers can be observed in the MDJ, indicating the presence of monosynaptic projections from the cerebellar nuclei to the MDJ and from there to the inferior olive. Sagittal sections from rostral to caudal (see schemes in the lower left corners with red rectangles indicating locations of images). FR = fasciculus retroflexus, RN = red nucleus. (E) A neuron (yellow) in the MDJ that projects to the inferior olive. Close to the soma of this neuron, a bouton (magenta) of a neuron originating from the cerebellar nuclei can be seen (insets below, imaged at two levels 0.7 µm apart). Panels B-E originate from a representative mouse and are modified from Figure 7 from Wang et al., 2022.
Many brain regions lack clear borders. In particular when different species are compared, this may lead to some variations in the interpretation of anatomical projections. On top of this, one should also take into account that anatomy and physiology do not always match. For instance, the inspiratory neurons originally considered to be located in the pre-Bötzinger complex are actually distributed around the region of the pre-Bötzinger complex and are partially intermingled with expiratory neurons originally considered to be located in more caudal nuclei (Baertsch et al., 2019). When reading this review, please note that the use of anatomical names is to help orient oneself, but in reality, borders are often fuzzy. Genetic markers may help to define more homogeneous populations of neurons, and when this information was available, we mention that in the text and figures.
Given that the neuronal mechanisms of respiratory rhythm generation are evolutionary well conserved (Cinelli et al., 2013), inter-species differences are expected to be relatively minor (Kastner and Gauthier, 2008). Important exceptions, however, are the elongation of the pharyngeal region, and the development of complex muscle control of pharynx and mouth related to human speech (Duncker, 2001). Since neural control of speech is outside the scope of this review, this will not be further discussed.
Rhythmic respiration
Quiet breathing, or eupnea, is a rhythmic alternation between inspiration and passive expiration or post-inspiration (Albaiceta et al., 2005; Figure 1G). During periods with higher metabolic demand, hyperpnea occurs, which entails also active expiration (Pitts et al., 2015; Bellani et al., 2018; Figure 1D–E). It must be noted that the different respiratory muscles cover different aspects of the respiratory cycle. As a result, a clear distinction between passive and active expiration cannot be made. For example, during passive expiration, some of the expiratory muscles can be active, and active expiration encompasses passive elastic contractions as well. In humans, post-inspiration can contribute to longer periods of relatively constant air flow as required for speech or singing (MacLarnon and Hewitt, 1999; Watson et al., 2012; Salomoni et al., 2016; Figure 1F). Here, additional muscles become active that are not active during solely post-inspiration. Thus, to what extent the respiratory cycle within the brainstem can indeed be divided into rhythmogenic phases or whether these phases are just seen at the motor output remains topic of further research. As stated, we discuss in this review multiple mechanisms that modulate the rhythmicity of respiration when confronted with respiratory challenges, such as a change in air pressure or increase in CO2 concentration (hypercapnia).
During regular breathing, the respiratory cycle is determined by brainstem central pattern generators. Inspiration is triggered by activity of neurons in the pre-Bötzinger complex, most of which fire in phase with inspiration and indirectly drive the inspiratory pump muscles (Smith et al., 1991; Guyenet and Wang, 2001; Moore et al., 2013; Del Negro et al., 2018; Yang and Feldman, 2018). Inspiration may be terminated by activation of the Kölliker-Fuse nucleus, and possibly also the postinspiratory complex can contribute to this (Dutschmann and Herbert, 2006; Anderson et al., 2016). Finally, the lateral parafacial nucleus that is silent during eupnea becomes active during active expiration (Pagliardini et al., 2011; Huckstepp et al., 2015; Del Negro et al., 2018; Figure 3). Without input from the brainstem, the spinal circuitry cannot organize respiration. It does contribute to sequential contraction of thoracic muscles to optimize the inflow of air by adjusting muscle control to body biomechanics (Marckwald, 1889; Porter, 1895; Ellenberger and Feldman, 1988; Butler et al., 2014; Shinozaki et al., 2019; Jensen et al., 2019).
Figure 3. Central pattern generators encode the respiratory rhythm.
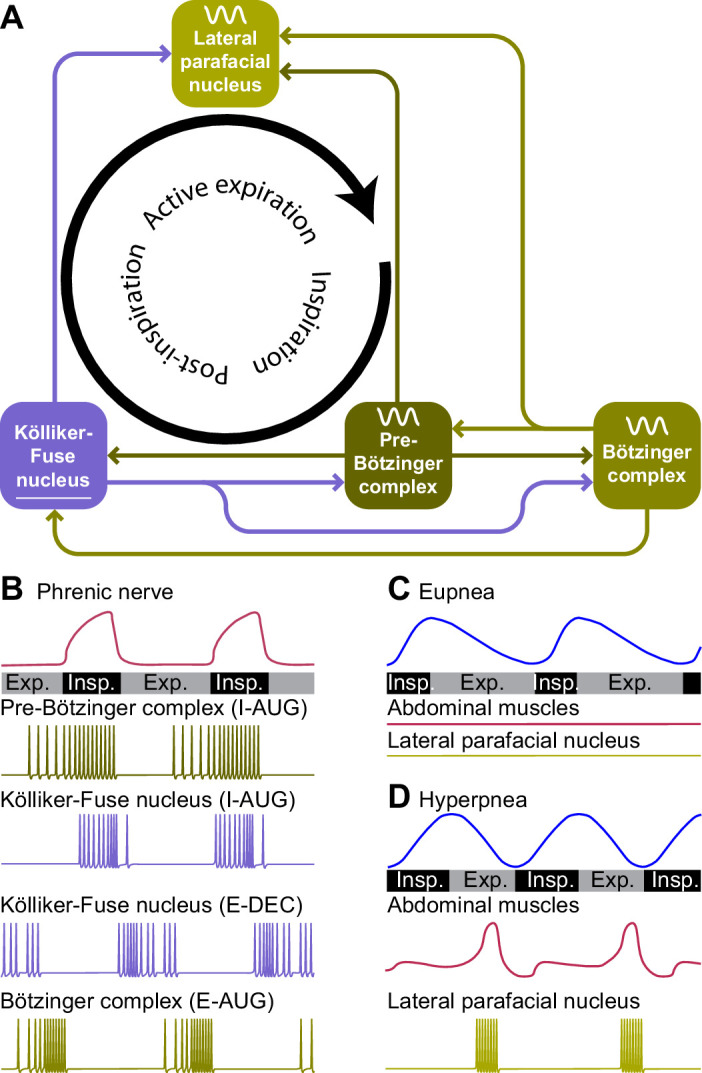
(A) Connections between the pre-Bötzinger complex that organizes inspiration, the Kölliker-Fuse nucleus that relates to the inspiration/expiration switch, the lateral parafacial nucleus that triggers active expiration, and the Bötzinger complex related to expiration. (B) Neuronal activity in the central pattern generators varies during the respiratory cycle. The schematized traces represent action potential firing of selected neuronal cell types in relation to activity of the phrenic nerve that drives the main inspiratory pump muscle, the diaphragm. From top to bottom: augmenting inspiratory neurons (I-AUG) from the pre-Bötzinger complex and the Kölliker-Fuse nucleus, a decreasing expiratory neuron (E-DEC) from the Kölliker-Fuse nucleus, and an augmenting expiratory neuron (E-AUG) from the Bötzinger complex. These representations are based on in vitro studies of Marchenko et al., 2016 (pre-Bötzinger complex), Ezure and Tanaka, 2006 (Kölliker-Fuse nucleus), and Flor et al., 2020 (Bötzinger complex). (C) During eupnea, thus in the absence of active expiration, neither the expiratory pump muscles of the abdomen, nor the neurons of the lateral parafacial nucleus are active. (D) During hyperpnea, thus when active expiration takes place, abdominal expiratory pump muscles are active when the lateral parafacial nucleus neurons produce action potentials. Schematized based on in vivo recordings of anesthetized rats by Pagliardini et al., 2011. Insp.=inspiration, Exp.=expiration.
Pre-Bötzinger and Bötzinger complexes
The pre-Bötzinger complex contains a network of neurons generating rhythmic activity that is both essential and sufficient to drive inspiration (Smith et al., 1991; Ramirez et al., 1998; Gray et al., 2001; Tan et al., 2008; Schwarzacher et al., 2011; Ashhad and Feldman, 2020; Dhingra et al., 2020; Figure 3B). In rodents, the pre-Bötzinger complex consists of around 3,000 neurons on each side of the brain, with approximately equal numbers of excitatory and inhibitory neurons (Wallen-Mackenzie et al., 2006; Tan et al., 2008; Yackle et al., 2017). Inspiratory neurons with comparable dynamics may also be found in surrounding regions of the ventral respiratory column, pointing toward a more diffuse spatiotemporal network than originally described (Baertsch et al., 2019). The rhythmogenic kernel is formed by somatostatin-negative (SST-) excitatory interneurons (Cui et al., 2016; Ashhad and Feldman, 2020). Excitatory neurons in the pre-Bötzinger complex have a refractory period that prevents them from generating bursts at a high frequency, and this refractory period can be shortened by inhibitory input (Baertsch et al., 2018). Hence, although inhibitory interneurons are not essential for generating rhythmicity, they may modulate the breathing frequency and contribute to the termination of inspiration (Janczewski et al., 2013; Sherman et al., 2015; Hülsmann et al., 2021). The output of the pre-Bötzinger complex is composed of both inhibitory and SST+ excitatory neurons.
Although there are direct projections from the pre-Bötzinger complex to multiple respiratory motor nuclei, indirect projections appear to be more common. As such, the phrenic nucleus is predominantly targeted via the rostral ventral respiratory group (rVRG) (Wu et al., 2017), and the thoracic motor neurons that control abdominal muscles via the caudal ventral respiratory group (cVRG) (Gerrits and Holstege, 1996; Yang and Feldman, 2018). In addition, the hypoglossal nucleus is principally reached via the parahypoglossal region of the reticular formation (Chamberlin et al., 2007; Tan et al., 2010; Yang and Feldman, 2018), and the facial nucleus via the intermediate reticular formation (Moore et al., 2004; Koshiya et al., 2014; Yang and Feldman, 2018; Guo et al., 2020).
In addition, there are substantial projections to other respiratory control areas: the Bötzinger complex, Kölliker-Fuse nucleus, postinspiratory complex (PiCo), and lateral parafacial nucleus (Tan et al., 2010; Koshiya et al., 2014; Yang and Feldman, 2018; Biancardi et al., 2021). Furthermore, also the retrotrapezoid nucleus, nucleus tractus solitarii (NTS), lateral and dorsomedial hypothalamus, lateral and medial parabrachial nuclei, and periaqueductal gray are targeted (Tan et al., 2010; Koshiya et al., 2014; Yang and Feldman, 2018; Biancardi et al., 2021; Trevizan-Baú et al., 2021b). A specific subset of Cdh9-neurons projects to noradrenergic neurons in the locus coeruleus (Yackle et al., 2017). Finally, there are strong projections to the contralateral pre-Bötzinger complex to promote left/right synchrony during respiration (Wu et al., 2017).
The adjacent Bötzinger complex houses mostly inhibitory neurons that either show decrementing activity during post-inspiration or incrementing activity during expiration, and both groups contribute to the inhibition of inspiratory activity in the pre-Bötzinger complex during expiration (Marchenko et al., 2016; Ausborn et al., 2018; Flor et al., 2020). These activity patterns partially reflect sensory feedback: while augmenting neurons are inhibited by lung inflation, decrementing neurons are excited by it (Manabe and Ezure, 1988; Hayashi et al., 1996). Next to the adjacent pre-Bötzinger complex, also other respiratory control centers are innervated by the Bötzinger complex: the Kölliker-Fuse and lateral parafacial nuclei (Ezure et al., 2003; Yang et al., 2020; Biancardi et al., 2021).
The Bötzinger complex also inhibits premotor areas: rVRG and to a lesser extent also cVRG (Jiang and Lipski, 1990; Bryant et al., 1993; Ezure, 2004), and directly inhibits motor neurons in the phrenic nucleus (Merrill and Fedorko, 1984; Ellenberger et al., 1990b; Tian et al., 1998). Other targets are the NTS, lateral parabrachial nucleus and periaqueductal gray (Merrill et al., 1983; Fedorko and Merrill, 1984; Livingston and Berger, 1989; Smith et al., 1989; Ezure et al., 2003; Trevizan-Baú et al., 2021b).
As the respiratory pattern has to be coordinated with ongoing behavior, the pre-Bötzinger complex receives input from many brain regions, like the Kölliker-Fuse nucleus, PiCo, cVRG, NTS, retrotrapezoid nucleus, locus coeruleus, caudal raphe, lateral and paraventricular hypothalamus, central amygdala, lateral and medial parabrachial nuclei, periaqueductal gray, spinal trigeminal nuclei, and reticular formation (Panneton et al., 2006; Rosin et al., 2006; Jones et al., 2016; Hennessy et al., 2017; Yang et al., 2020; Liu et al., 2021b; Trevizan-Baú et al., 2021b). The pre-Bötzinger complex receives also direct input from the forebrain, including several regions of the neocortex presumably involved in voluntary control of respiration, but these connections are relatively sparse (Yang et al., 2020; Trevizan-Baú et al., 2021a). The inputs of the Bötzinger complex are similar to those of the pre-Bötzinger complex, although less widespread (Gang et al., 1995; Supplementary file 1).
Kölliker-Fuse nucleus
The pre-Bötzinger complex is not the only area essential for rhythmic respiration. Selective damage to the brainstem at the level of the pons leads to impaired transition from inspiration to expiration, resulting in prolonged periods of inspiration, a condition called apneusis (Marckwald, 1890; Lumsden, 1923). This effect was later localized in parts of the parabrachial complex, initially referred to as pneumotaxic center, and later as the pontine respiratory group (Cohen and Wang, 1959; Caille et al., 1981; Ezure and Tanaka, 2006; Zuperku et al., 2017; Varga et al., 2021). The parabrachial complex is composed of the lateral and medial parabrachial nuclei, that have predominantly ascending projections carrying sensory information, and the Kölliker-Fuse nucleus that primarily targets subcortical structures (Fulwiler and Saper, 1984). Accordingly, of the parabrachial complex it is mainly the Kölliker-Fuse nucleus that influences the switch from inspiration to expiration (Damasceno et al., 2014; Dutschmann et al., 2021; Figure 3B). Neurons of the Kölliker-Fuse nucleus show activity related to specific phases of respiration, with most neurons being active during inspiration; these latter neurons abruptly stop firing at the end of inspiration, marking the inspiration-expiration transition (Dick et al., 1994; Ezure and Tanaka, 2006; Dutschmann et al., 2021). A bilateral block of activity in the Kölliker-Fuse nucleus prolonged the inspiratory activity of the phrenic nerve in an in situ preparation, but did not completely block the termination of inspiratory activity (Dutschmann et al., 2021), which would be in line with a prominent, but not exclusive role of the Kölliker-Fuse nucleus for the termination of inspiration.
The Kölliker-Fuse nucleus receives input from the pre-Bötzinger and Bötzinger complexes (Ezure et al., 2003; Tan et al., 2010; Yang and Feldman, 2018), and from several central chemoreceptor areas: the NTS (Loewy and Burton, 1978; Herbert et al., 1990; McGovern et al., 2015b), retrotrapezoid nucleus (Rosin et al., 2006; Bochorishvili et al., 2012; Silva et al., 2016b), and cerebellar fastigial nucleus (Fujita et al., 2020). In addition, the Kölliker-Fuse nucleus also receives input from the rVRG (Lipski et al., 1994; Yokota et al., 2016), cVRG (Holstege, 1989; Jones et al., 2016), periaqueductal gray (Trevizan-Baú et al., 2021b), spinal trigeminal nucleus (Panneton et al., 2006; Zhang et al., 2018), paratrigeminal nucleus (Saxon and Hopkins, 1998), pedunculopontine tegmental nucleus (PPTg) (Lima et al., 2019b), and vestibular nuclei (Shi et al., 2021). Finally, there are descending inputs from the lateral, dorsomedial and paraventricular hypothalamus (Yokota et al., 2016; Trevizan-Baú et al., 2021a).
Glutamatergic projections directly and indirectly (via the rVRG) target the phrenic nucleus (Ellenberger et al., 1990b; Yokota et al., 2004; Yokota et al., 2007; Song et al., 2012a; Geerling et al., 2017), as well as the ambiguus, hypoglossal and facial nuclei (Núñez-Abades et al., 1990; Yokota et al., 2007; Song et al., 2012a; Yokota et al., 2015; Geerling et al., 2017). The latter connections allow premotor neurons in the Kölliker-Fuse nucleus to constrict valve muscles, reducing outflow during post-inspiration (Dutschmann and Herbert, 2006).
Further excitatory projections target, in addition to the other nuclei of the parabrachial complex (Song et al., 2012a; Geerling et al., 2017), the pre-Bötzinger complex (Yang et al., 2020), PiCo (Oliveira et al., 2021), and lateral parafacial nucleus (Biancardi et al., 2021). Also the cVRG (Gerrits and Holstege, 1996; Song et al., 2012a), reticular formation (Geerling et al., 2017), retrotrapezoid nucleus, NTS, and periaqueductal gray (Fulwiler and Saper, 1984; Song et al., 2012a; Geerling et al., 2017; Trevizan-Baú et al., 2021b) receive glutamatergic input. The caudal part of the Kölliker-Fuse nucleus sends inhibitory projections mainly to the sensory trigeminal nucleus, but also to the dorsomedial hypothalamus (Geerling et al., 2017). Finally, there are projections to the raphe nuclei (Hermann et al., 1997; Peyron et al., 2018), vestibular nuclei (Shi et al., 2021), and cerebellar cortex (Fu et al., 2011).
Post-inspiratory complex
Recently, a second brain region involved in controlling post-inspiration has been identified in the ventral part of the intermediate reticular formation: the postinspiratory complex, or PiCo, consisting of cholinergic neurons (Anderson et al., 2016; Toor et al., 2019; Oliveira et al., 2021). Isolated in a tissue slice, the PiCo can generate rhythmic activity that peaks during post-inspiration, and thus potentially contributes to a biphasic (pre-Bötzinger complex – PiCo) or triphasic (pre-Bötzinger complex – PiCo – parafacial nucleus) oscillator controlling respiration (Anderson et al., 2016; Anderson and Ramirez, 2017). Optogenetic stimulation in vivo confirmed that PiCo activity can prolong post-inspiration (Oliveira et al., 2021).
The role of the PiCo in controlling post-inspiration has not yet been fully investigated (Hülsmann, 2021; Ashhad et al., 2022). Although this is not a proof against a coordinating role of the PiCo for post-inspiration, systematic recordings in a whole-brainstem preparation found widespread activity during post-inspiration, but more in the pontine region than focused in the area of the PiCo (Dhingra et al., 2020). Furthermore, inhibition of the PiCo did not affect the duration of inspiration, while in the same study the PiCo was shown to be involved in gating swallowing motor patterns to the respiratory system (Toor et al., 2019). In conclusion, it seems likely that the PiCo is involved in the neural control of post-inspiration, but probably more as part on an integrated network than as primary pattern generator.
The PiCo receives strong input from the Kölliker-Fuse nucleus and periaqueductal gray, but there are also substantial connections from the caudal and intermediate NTS and the hypothalamic paraventricular nucleus (Oliveira et al., 2021). Also the pre-Bötzinger complex projects to the PiCo (Yang and Feldman, 2018). As far as we are aware, there are no systematic studies on PiCo efferents, but projections to the pre-Bötzinger complex and retrotrapezoid nucleus have been demonstrated (Lima et al., 2019b; Yang et al., 2020).
Lateral parafacial nucleus
At the end of the respiratory cycle, the lateral parafacial nucleus can trigger active expiration by recruiting expiratory abdominal muscles via the cVRG (Janczewski and Feldman, 2006; Huckstepp et al., 2015; Silva et al., 2016a; Pisanski and Pagliardini, 2019). In the literature, the term parafacial respiratory group is sometimes used as synonym for the lateral parafacial nucleus, or for the combination of the lateral and the ventral parafacial nucleus, or even for the latter together with the retrotrapezoid nucleus (Onimaru and Homma, 2003; Huckstepp et al., 2015; Pisanski and Pagliardini, 2019; Biancardi et al., 2021).
In embryonic and newborn rodents, the lateral parafacial nucleus is rhythmically active at rest (Onimaru and Homma, 2003; Thoby-Brisson et al., 2009), but this activity wanes during early development (Oku et al., 2007; van der Heijden and Zoghbi, 2020), and in adults the lateral parafacial nucleus is generally silent during eupnea, but rhythmically active during hyperpnea (Pagliardini et al., 2011; Huckstepp et al., 2015; de Britto and Moraes, 2017; Figure 3C–D). The inactivity at rest could be due to tonic inhibition from the medial NTS (Silva et al., 2019). Excitatory input from the commissural NTS, conveying chemosensitive input from the carotid bodies, can activate neurons in the lateral parafacial nucleus during periods with elevated blood CO2 levels (Morris et al., 2018; Silva et al., 2019). In addition, the parafacial nucleus receives direct input from chemoreceptors in the adjacent retrotrapezoid nucleus (Zoccal et al., 2018; Biancardi et al., 2021). Furthermore, input comes also from the pre-Bötzinger and Bötzinger complexes, rVRG, Kölliker-Fuse nucleus, reticular formation, caudal raphe, lateral and medial parabrachial nuclei, periaqueductal gray, PPTg and vestibular nuclei (Biancardi et al., 2021).
Sensory feedback
To adapt the depth of ventilation to the metabolic state, the blood gas balance is continuously monitored. At the same time, sensory systems of the airways and lungs, consisting of thermo-, mechano-, and chemoreceptors, survey ongoing respiration and detect environmental irritants and inflammatory mediators (Sant’Ambrogio et al., 1983; Lee and Yu, 2014). Muscle spindles give feedback on posture and respiratory muscle performance (Nakayama et al., 1998). Altogether, sensory feedback affects ventilatory control, and can trigger respiratory reflexes aimed at maintaining homeostasis upon adverse events. In addition, also hormones can modulate respiratory behavior.
Chemoreception
Chemoreceptors monitor the partial pressures of O2 (pO2) and CO2 (pCO2) in blood and cerebrospinal fluid. Since CO2 reacts with water to form HCO3- and H+, increased pCO2 leads to acidosis that can cause adverse effects on tissue structures, and may result in headaches, delirium and eventually coma (Cummins et al., 2020). Regulation of pCO2 is therefore, next to that of pO2, of great importance, and a major drive for the level of ventilation (Miescher-Rüsch, 1885; Haldane and Priestley, 1905). The concentrations of both gasses are continuously measured by peripheral chemoreceptors in the carotid bodies (Gonzalez et al., 1994; Milsom and Burleson, 2007; Cummins et al., 2020; Ortega-Sáenz and López-Barneo, 2020). The carotid bodies project mainly to the NTS (Claps and Torrealba, 1988; Finley and Katz, 1992; Mifflin, 1992; Zera et al., 2019), but also to the cVRG (Finley and Katz, 1992).
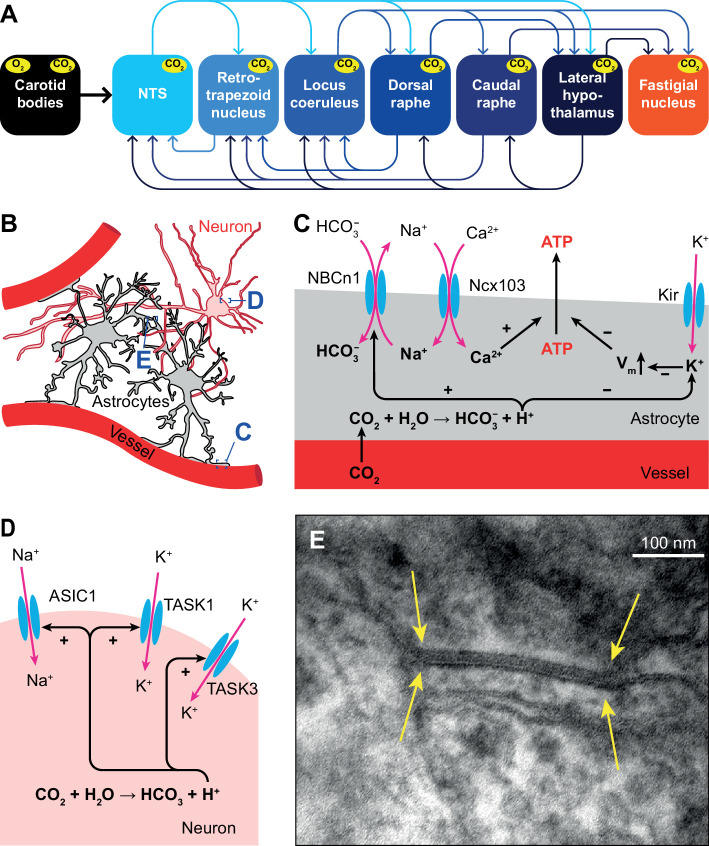
Acidosis can also be caused by inflammation, ischemia or defective acid containment. Consequently, acid sensing is not restricted to respiratory control and has evolved as an important property of neurons with unmyelinated and thinly myelinated fibers (Canning and Spina, 2009). Only those areas that sense pH changes and directly affect ventilation are considered to be central chemoreceptor areas. In this respect, most attention has been given to the retrotrapezoid nucleus, but also the NTS, locus coeruleus, raphe nuclei, lateral hypothalamus, and cerebellar fastigial nucleus are central chemoreceptor areas (Coates et al., 1993; Nattie, 1999; Nattie and Li, 2002; Xu and Frazier, 2002; Putnam et al., 2004; Guyenet et al., 2008; Dean and Putnam, 2010; Li et al., 2013; Figure 4A).
Figure 4. Respiratory chemoreception.
(A) Connections between the carotid bodies and the central chemoreceptor areas. (B) Astrocytes are in direct contact with blood vessels and neurons. (C) Chemoreceptor pathways in astrocytes triggering ATP release that can activate nearby neurons. (D) Chemoreceptor pathways in neurons, based on activation of Na+ channels and inward-rectifier K+ channels. (E) Gap junctions between glial cells, as shown here in the cerebellar cortex of a mouse (yellow arrows), can contribute to central chemoreception. In particular, the conductivity of gap junctions composed of Cx26 depends on pH. Previously unpublished electron microscopic image from our lab.
Central chemoreceptors
Several molecular mechanisms for central chemoreception have been proposed (Gourine and Dale, 2022). First, in astrocytes, increased levels of HCO3- can activate the electroneutral Na+/HCO3- co-transporter NBCn1 (SLC4A7), causing Na+ influx that in turn activates the astrocytic Na+/Ca2+ exchanger Ncx103 (SLC8A1-3; Turovsky et al., 2016). The resulting increase in intracellular Ca2+ triggers ATP release (Gourine et al., 2005; Gourine et al., 2010). Purinergic receptors on nearby neurons are activated by ATP, leading to neuronal excitation (Gourine et al., 2005; Figure 4B–C).
Second, inward-rectifier K+ (Kir) channels can be inhibited by a decrease in pH, and trigger depolarization and consequently ATP release by astrocytes (Wenker et al., 2010; Figure 4C).
A third mechanism, one that is independent of pH changes, involves the ability of CO2 to induce conformational changes in the connexin-hemichannel Cx26 located in gap junctions between glial cells, rendering Cx26 permeable to ATP (Bevans and Harris, 1999; Huckstepp et al., 2010; Meigh et al., 2013; van de Wiel et al., 2020; Figure 4E). Later studies revealed the interaction between CO2 and Cx26 to be more complicated, and probably context-dependent (Nijjar et al., 2021).
In addition to glial-mediated chemoreception, also neurons express acid-sensitive ion channels (ASICs) and receptors (Canning and Spina, 2009). Among these are some members of the family of inwardly rectifying K2P channels (Lesage and Barhanin, 2011; Sepúlveda et al., 2015), such as TASK1 and TASK3 that are co-expressed with ASIC1 in the ventrolateral medulla, and contribute to central chemoreception in rats (Wang et al., 2018). Another family member is the TASK-2 K+ leak channel, whose absence leads to impaired ventilatory responses to hypercapnia (Gestreau et al., 2010; Bayliss et al., 2015; Figure 4D).
The KCNA1 gene encodes the α subunit of Kv1.1 voltage-gated potassium channels that show particularly strong expression in hippocampus, cerebellum, neocortex and peripheral nerves (D’Adamo et al., 2014; Ovsepian et al., 2016). Mutations in the KCNA1 gene can lead to the development of episodic ataxia type 1 (EA1), an autosomal dominant disorder with multiple symptoms, most prominently episodes of cerebellar ataxia and myokymia (Browne et al., 1994; Paulhus et al., 2020). These episodes are often triggered by physical and emotional stress, which could be related to a defect in respiratory chemoreception (Kline et al., 2005). Furthermore, mutations in Kv1.1 channels have been associated with epilepsy, and Kcna1-deficient mice are considered to be a model of sudden unexpected death in epilepsy (SUDEP), while also showing progressive respiratory dysfunction (Simeone et al., 2018; D’Adamo et al., 2020; Paulhus et al., 2020). As respiratory dysfunction is hypothesized to be a primary risk factor for susceptibility to the cardiorespiratory dysfunction in epilepsy, this could reveal a new role for KCNA1 channelopathies in the regulation of basal respiratory physiology (Dhaibar et al., 2019).
Vagal sensory input
The lungs and airways, in particular the larynx, are lined with sensory receptors associated with the nervus vagus (Mortola et al., 1975; Lee and Yu, 2014; Mazzone and Undem, 2016; Kupari et al., 2019). Flow receptors sense air temperature, which is typically colder for inhaled than for exhaled air (Sant’Ambrogio et al., 1983), and drive receptors measure the laryngeal wall pressure that correlates with its conductivity (Horner, 2012). Flow and drive receptors are active during each breath (Sant’Ambrogio et al., 1983), while other mechano- and chemoreceptors are only activated during adverse conditions, such as an obstruction, the presence of irritants, or increased CO2 concentrations (Lee and Yu, 2014). Sensory information from the distal airways is transferred via the nodose ganglion to the NTS, while that of the proximal airways goes predominantly via the jugular ganglion to the paratrigeminal nucleus (McGovern et al., 2015a; McGovern et al., 2015b; Kim et al., 2020).
Vagal stretch receptors, associated with Aβ fibers, can be subdivided into slowly and rapidly adapting receptors: SARs and RARs, respectively. SARs sense inflation and can stay activated for sustained periods, while RARs are more sensitive to acute changes in pressure (Davenport et al., 1981; Kaufman et al., 1982; Mazzone and Undem, 2016). Although all RARs rapidly adapt to a persistent mechanical stimulus, a subset of them, the so-called irritant receptors, can remain activated by specific chemicals for a prolonged period (Sant’Ambrogio, 1982). Environmental irritants and inflammatory mediators can trigger activity of unmyelinated C fibers, while localized mechanical stimuli and acid can activate thinly myelinated Aδ fibers that are also known as cough receptors, and that are located in the airway epithelium and mucosa (Coleridge and Coleridge, 1984; Canning et al., 2004; Mazzone et al., 2009; Grace et al., 2012; Canning et al., 2014; Mazzone and Undem, 2016).
Cough and expiration reflexes
Coughing is a vital action to clear the airways. Coughing involves first inspiration, then obstruction of airways to build up pressure, and subsequent explosive expulsion of air. Ineffective cough reflexes, for example in patients with dementia, can lead to lethal aspiration pneumonia (Widdicombe, 1995; Mutolo, 2017; Sykes and Morice, 2021). Inversely, the relatively common condition of chronic cough, affecting more than 5% of the adult population, is thought to be caused by hypersensitivity to airway stimulation (Morice et al., 2020).
The cough reflex is typically triggered by stimulation of the cough receptors and possibly also by activation of C fibers in the airways (Ludlow, 2015; Mutolo, 2017). Mechanical stimulation of the vocal cords can trigger the expiration reflex, which resembles cough without the initial inspiration (Korpas and Jakus, 2000; Sant’Ambrogio and Widdicombe, 2001; Tatar et al., 2008; Ludlow, 2015).
Stimulation of irritant receptors in the upper airways can evoke a cough reflex via activation of the paratrigeminal nucleus, and this reflex can be suppressed by the submedial thalamic nucleus and the upstream ventrolateral orbital cortex (Mazzone et al., 2020). In the periaqueductal gray, the excitatory drive from the paratrigeminal nucleus and the inhibitory input from the ventrolateral orbital cortex come together (McGovern et al., 2015b). The periaqueductal gray is hence an important intermediate between the forebrain and the cVRG (Holstege, 1989; Subramanian et al., 2008; Chen et al., 2022). The cVRG itself is essential for the cough reflex (Mutolo, 2017; Cinelli et al., 2020), as it can activate both expiratory motor neurons in the thoracic spinal cord, controlling abdominal muscles, and in the ambiguus nucleus, controlling laryngeal muscles (Figure 5F).
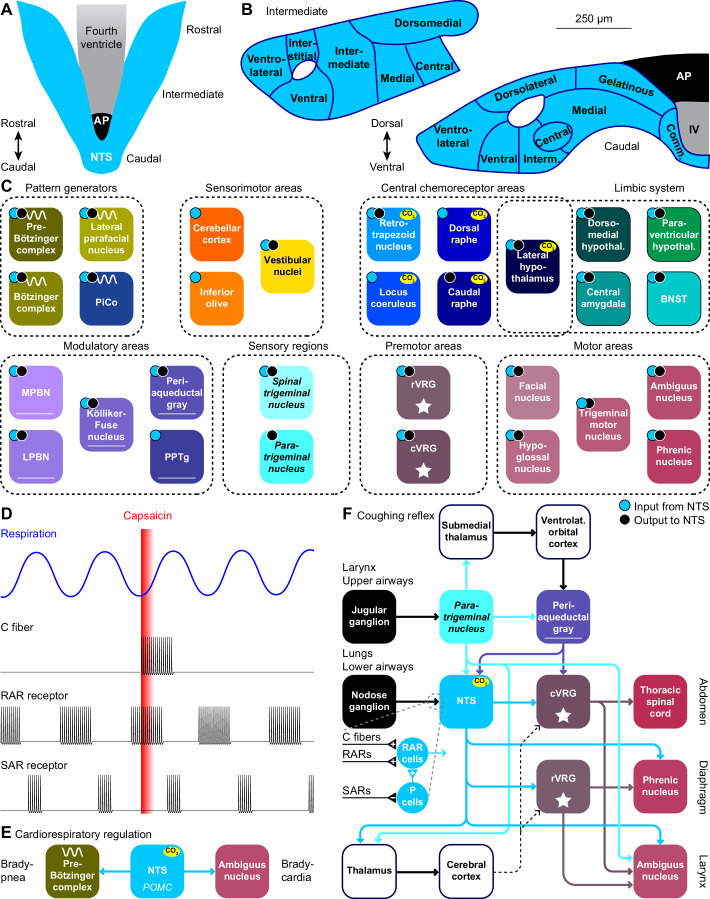
Figure 5. The nucleus of the solitary tract and vagal afferents.
Schematic drawings of the nucleus of the solitary tract (NTS) in relation to the area postrema (AP) and the fourth ventricle (IV). Dorsal (A) and coronal views at intermediate and caudal levels (B). (C) Brain regions that project to, or get input from the NTS. With most areas, bidirectional connections exist (cyan dots: areas innervated by NTS neurons; black dots: areas with neurons innervating the NTS). (D) Heterogeneity in vagal afferents. Capsaicin can, as a pulmonary irritant, evoke activity of C fibers, but not of rapidly or slowly adapting receptors (RAR and SAR, respectively). The latter two show phasic activity during regular breathing. Schematic drawing of action potential firing based on data presented in Ho et al., 2001. (E) Pro-opiomelanocortin-expressing (POMC) neurons of the NTS project to the pre-Bötzinger complex and to cardiac vagal motor neurons in the ambiguus nucleus. Via these pathways, they can reduce inspiration and cardiac function, respectively. (F) Coughing can be triggered by sensing an irritant via vagal projections from the larynx or upper airways via the jugular ganglion to the paratrigeminal nucleus, or from the lungs or lower airways via the nodose ganglion to the NTS. Distinct types of vagal fibers differentially affect RAR relay neurons, with SARs inhibiting pump neurons (P cells). The motor neurons of expiratory muscles are directly and indirectly activated from the paratrigeminal nucleus and the NTS. A specific cortical circuit for inhibiting reflexive coughing involving the submedial thalamus and the ventrolateral orbital cortex has been described, next to more general thalamo-cortical pathways that can modulate coughing. The latter pathways can use different connections to premotor nuclei (indicated with dotted lines).
Stimulation of the lower airways or the lungs activates RAR relay neurons in the NTS. These neurons receive also input from various other sources that can contribute to suppression or facilitation of coughing (Mutolo, 2017). Thalamocortical loops can further modulate coughing and exert voluntary control (Ando et al., 2016). Like the paratrigeminal nucleus, the NTS innervates the cVRG (Gerrits and Holstege, 1996). In addition, the NTS affects also the phrenic nucleus, directly as well as indirectly via the rVRG (Ezure and Tanaka, 1996; Wu et al., 2017). These connections are complemented with direct output to the ambiguus nucleus from both the paratrigeminal nucleus and the NTS (Caous et al., 2001; de Sousa Buck et al., 2001; Kawai, 2018; Figure 5F).
Sneeze reflex
In the nasal mucosa, irritant receptors expressing Trpv1, a capsaicin-sensitive cation ion channel, that can be activated by histamine H1R receptors (Shim et al., 2007), are present on thin sensory fibers of the ethmoidal nerve that terminates in the sneeze-evoking region (Lucier and Egizii, 1986; Nonaka et al., 1990; Seijo-Martínez et al., 2006; Li et al., 2021). The sneeze-evoking region is a dedicated part of the spinal trigeminal nucleus that projects to the cVRG (Li et al., 2021).
Postural feedback
The external and internal intercostal muscles assist with, respectively, expansion and contraction of the rib-cage during breathing (Figure 1A–E). In addition, the intercostal muscles exert postural control, and they combine their respiratory and postural activity in a superposed manner (Rimmer et al., 1995). Single intercostal 1a afferents project to the region of Clarke’s column, to the intercostal motor nucleus, and to the intermediate regions, conveying sensory feedback originating from muscle spindles in the intercostal muscles (Nakayama et al., 1998). From Clarke’s column, information is projected to the cerebellar cortex via the spinocerebellar tract, originating from two types of respiration-related neurons in the lower thoracic segments (T9-T12). This spinocerebellar tract contains uncrossed as well as crossed ascending axons that play different roles in transmitting signals between the spinal cord and the cerebellum (Tanaka and Hirai, 1994). Rhythmic activity in uncrossed spinocerebellar tract neurons, located in and around Clarke’s column, reflects afferent activity from the chest wall, whereas that of crossed neurons, located in laminae VII and VIII, reflect descending influence from the respiratory centers with or without peripheral influences (Matsushita et al., 1979; Tanaka and Hirai, 1994). It has been suggested that the cerebellum uses the posture-related information of the thoracic spinocerebellar tract neurons to adjust posture or coordination of whole body movements (Tanaka and Hirai, 1994).
Hormonal regulation of respiration
Several hormones can affect development and metabolism, and thus have an indirect effect on respiration. Thyroid hormones, for instance, are critical for the development of the respiratory system, and their dysfunction can lead to respiratory failure, including the respiratory distress syndrome (Pei et al., 2011; Rousseau et al., 2021). Hormones with a specific role in respiratory control are discussed below.
Sex hormones
Progesterone, estradiol and testosterone can all affect respiratory parameters (White et al., 1985), and these effects could help explain differences in respiratory behavior between males and females, as well as during different life stages (Gargaglioni et al., 2019; LoMauro and Aliverti, 2021). Indeed, sex hormone receptors are widely distributed in the brain, including central chemoreceptor areas (Gargaglioni et al., 2019; LoMauro and Aliverti, 2021), certain respiratory motor neurons, such as the hypoglossal and phrenic nuclei (Behan and Thomas, 2005), and cerebellar Purkinje cells (Perez-Pouchoulen et al., 2016). Progesterone levels correlate with the muscle tone of the genioglossus muscle, and thus with upper airway rigidity, which could play a role in the reduced occurrence of obstructive sleep apnea in females when compared to males (Popovic and White, 1998).
Leptin
Leptin is primarily secreted by adipocytes. An increase in adipose tissue therefore leads to more leptin secretion, which results in increased breathing activity (Chang et al., 2013; Bassi et al., 2016; Gauda et al., 2020). Leptin can activate a specific subset of excitatory neurons in the NTS projecting to respiratory premotor neurons in the rVRG, as well as to the dorsomedial hypothalamus (Do et al., 2020). Next to the direct projection from the NTS to the rVRG, an indirect pathway via the lateral parabrachial nucleus to the pre-Bötzinger complex is also likely to contribute to the impact of leptin on respiratory frequency (Yu et al., 2022). The dorsomedial hypothalamus, which itself also contains leptin receptors, can contribute to upper airway control during respiration (Yao et al., 2016). The impact of the leptin-mediated pathways may be compromised in obesity, as leptin resistance can contribute to the development of obesity hypoventilation syndrome and central sleep apnea (O’Donnell et al., 2000; Yao et al., 2016; Framnes and Arble, 2018).
Full-length leptin receptors (LEPRB) are not restricted to the NTS and dorsomedial hypothalamus, but can also be found in the carotid bodies, neocortex, substantia nigra and cerebellum (Guan et al., 1997; Gavello et al., 2016). Although it is not clear whether these all affect respiratory control, it has been shown that activation of leptin receptors in the carotid bodies can affect breathing and induce ventilatory response to hypoxia (Caballero-Eraso et al., 2019).
Hyperventilation
Not only increased metabolic activity, but also emotional arousal can cause an increase in the level of ventilation. While such a stress-induced reaction makes sense as preparation for a fight or flight reaction, it can derail during a panic attack (Suess et al., 1980; Meuret et al., 2017). In the absence of increased metabolic demands, hyperventilation induces a decrease in pCO2, which increases blood pH (Gardner, 1996). Hyperventilation can be associated with several symptoms of panic, including shortness of breath, heart racing, dizziness, and fear of dying (Gardner, 1996; Meuret et al., 2017). Although hyperventilation is typically triggered by stress, anxiety or panic, in rare cases it can also have a neurological cause, and central neurogenic hyperventilation is often associated with pontine damage (Plum and Swanson, 1959; Tarulli et al., 2005).
Central chemoreceptor areas
Retrotrapezoid nucleus
The retrotrapezoid nucleus is considered as the main central chemoreceptor area, and inhibition of its activity reduces the ventilatory response to hypercapnia (Mulkey et al., 2004; Marina et al., 2010; Burke et al., 2015; Ruffault et al., 2015), while optogenetic stimulation of the retrotrapezoid nucleus can increase the breathing rate by reducing the duration of expiration (Abbott et al., 2009; Abbott et al., 2011; Burke et al., 2015; Souza et al., 2020). Cholinergic input, probably from the PPTg and the PiCo, can increase the activity of chemoreceptors (Sobrinho et al., 2016; Lima et al., 2019b), while serotonergic input from the caudal and dorsal raphe can enhance the chemosensitive response in the retrotrapezoid nucleus (Rosin et al., 2006; Brust et al., 2014; Wu et al., 2019; Leirão et al., 2021).
The retrotrapezoid nucleus consists in mice of around 700 Phox2b-positive cells located ventrolateral to the facial nucleus. The locations of these neurons partially overlap with those of the lateral parafacial nucleus (Smith et al., 1989; Onimaru and Homma, 2003; Ramanantsoa et al., 2011; Shi et al., 2017). The retrotrapezoid nucleus houses also around 200 biochemically and morphologically different neurons that lack CO2-sensitivity, and that could be related to sighing via their direct projection to the pre-Bötzinger complex (Li et al., 2016; Shi et al., 2017).
Next to pCO2, also sensory input relayed via the caudal and commissural parts of the NTS (Rosin et al., 2006) affects the activity of the retrotrapezoid nucleus. In addition, direct inputs come from the pre-Bötzinger complex (Tan et al., 2010; Yang and Feldman, 2018), Kölliker-Fuse nucleus, and lateral and medial parabrachial nuclei (Rosin et al., 2006; Song et al., 2012a; Lima et al., 2019b), rVRG and cVRG (Rosin et al., 2006; Jones et al., 2016), lateral and paraventricular hypothalamus (Rosin et al., 2006; Geerling et al., 2010), and central amygdala and periaqueductal gray (Rosin et al., 2006).
The output of the retrotrapezoid is glutamatergic and affects respiration directly via projections to the pre-Bötzinger complex, rVRG and cVRG, as well as to the cervical and thoracic spinal cord (Rosin et al., 2006; Bochorishvili et al., 2012; Li et al., 2016; Silva et al., 2016a), and indirectly via the Bötzinger complex, Kölliker-Fuse nucleus, lateral parafacial nucleus, NTS, and lateral and medial parabrachial nucleus (Rosin et al., 2006; Abbott et al., 2009; Bochorishvili et al., 2012; Silva et al., 2016a).
Nucleus of the solitary tract
The NTS houses respiratory chemoreceptors (Coates et al., 1993; Nattie and Li, 2002; Nattie and Li, 2008; Fu et al., 2017), and is the prime recipient of visceral input. Especially the caudal part of the NTS receives direct input from pulmonary and cardiovascular baro-, chemo- and stretch receptors, as well as from chemoreceptors in the carotid bodies (Miura and Reis, 1972; Sant’Ambrogio, 1982; Finley and Katz, 1992; Mifflin, 1992; Lee and Yu, 2014; Zoccal et al., 2014; Mazzone and Undem, 2016; Umans and Liberles, 2018; Zera et al., 2019; Suarez-Roca et al., 2021; Figure 5A–B). These inputs allow the NTS to contribute to metabolic homeostasis, affecting not only cardiorespiratory function, but also food intake and digestion (Rinaman, 2010; Zoccal et al., 2014). The NTS is not essential for inspiration, but NTS dysfunction impairs the response to hypercapnia (Berger and Cooney, 1982; Speck and Feldman, 1982; Nattie and Li, 2008; Dean and Putnam, 2010), which could underlie congenital central hypoventilation syndrome (Fu et al., 2017). The ventrolateral NTS is also known as the dorsal respiratory group (Berger, 1977).
The NTS contains multiple types of respiratory neurons, some relate to inspiration, others to expiration or are phase-independent (Backman et al., 1984; Ezure and Tanaka, 2000; Ho et al., 2001; Kubin et al., 2006; Subramanian et al., 2007; Figure 5D). Among these are neurons, located mainly in the commissural and medial NTS, that respond to pulmonary irritant receptors, including RARs and C-fibers mediating cough reflexes, and that are suppressed by the pump neurons (P-cells; Berger, 1977; Kubin et al., 2006; Canning et al., 2014; Mutolo, 2017; Farrell et al., 2020).
P-cells of the ventrolateral and other parts of the caudal NTS respond to SARs reporting airway stretch (Kubin et al., 2006; Zoccal et al., 2014; Mazzone and Undem, 2016; Umans and Liberles, 2018). The firing rates of the P-cells relate to lung volume, possibly modulated by the respiratory rhythm (Berger, 1977; Davies et al., 1987; Bonham and McCrimmon, 1990; Miyazaki et al., 1999). Activation of these P-cells results in an inhibition of the rVRG (Ezure and Tanaka, 1996; Zheng et al., 1998) and phrenic motor nucleus (Fedorko et al., 1983; Ellenberger et al., 1990b; Boulenguez et al., 2007; Lois et al., 2009).
In addition, the NTS is involved in several other mechanisms controlling respiration. These entail a strong and direct projection to the phrenic motor nucleus (Loewy and Burton, 1978; Fedorko et al., 1983; Rikard-Bell et al., 1984; Dobbins and Feldman, 1994; Boulenguez et al., 2007; Lois et al., 2009). Furthermore, P-cells of the intermediate NTS mediate the Hering-Breuer inspiratory reflex that protects the lungs against overinflation (Breuer, 1868; Berger, 1977; Bonham and McCrimmon, 1990; Kubin et al., 2006; Lee and Yu, 2014; Chang et al., 2015; Nonomura et al., 2017; Umans and Liberles, 2018).
In addition to the extensive visceral inputs, the caudal NTS also receives central input, in particular from other regions of the NTS, lateral and paraventricular hypothalamus and central amygdala (Geerling et al., 2010; Ruyle et al., 2019; Gasparini et al., 2020), but also from the pre-Bötzinger complex (Tan et al., 2010; Koshiya et al., 2014; Yang and Feldman, 2018), Bötzinger complex (Merrill et al., 1983; Fedorko and Merrill, 1984; Livingston and Berger, 1989; Ezure et al., 2003), rVRG (Yamada et al., 1988; Ellenberger et al., 1990a; Zheng et al., 1998), Kölliker-Fuse nucleus (Fulwiler and Saper, 1984; Song et al., 2012a; Geerling et al., 2017), retrotrapezoid nucleus (Rosin et al., 2006; Bochorishvili et al., 2012), caudal raphe (Brust et al., 2014), bed nucleus of the stria terminalis (Gasparini et al., 2020), lateral and medial parabrachial nuclei (Saper and Loewy, 1980; Herbert et al., 1990; Bianchi et al., 1998), periaqueductal gray (Chen et al., 2022), spinal trigeminal nucleus (Panneton et al., 2006), paratrigeminal nucleus (Saxon and Hopkins, 1998; de Sousa Buck et al., 2001; McGovern et al., 2015b; Driessen et al., 2018), as well as from insular and infralimbic areas of the cerebral cortex (Gasparini et al., 2020; Figure 5C). Evidence for significant projections from the cerebellum to the NTS is currently lacking (Teune et al., 2000; Gasparini et al., 2020).
As mentioned above, strong and direct projections from the NTS to the phrenic nucleus have been reported, while the NTS targets also the upper airway motor nuclei (Loewy and Burton, 1978; Norgren, 1978; Beckstead et al., 1980; Núñez-Abades et al., 1990; Hayakawa et al., 2000; Kawai, 2018; Guo et al., 2020). Other target areas are the pre-Bötzinger complex (Yang et al., 2020), Bötzinger complex (Gang et al., 1995), PiCo (Oliveira et al., 2021), lateral parafacial nucleus (Biancardi et al., 2021), cVRG (Loewy and Burton, 1978; Beckstead et al., 1980; Gerrits and Holstege, 1996), retrotrapezoid nucleus (Rosin et al., 2006; Lima et al., 2019b), locus coeruleus (McGovern et al., 2015b; Kawai, 2018), dorsal raphe (Peyron et al., 2018), lateral, paraventricular and dorsomedial hypothalamus (King et al., 2012; McGovern et al., 2015b; Kawai, 2018), bed nucleus of the stria terminalis and central amygdala (Shin et al., 2008; Bienkowski and Rinaman, 2013; McGovern et al., 2015b; Ni et al., 2016; Kawai, 2018), lateral and medial parabrachial nuclei (Loewy and Burton, 1978; Beckstead et al., 1980; Herbert et al., 1990; McGovern et al., 2015b; Hashimoto et al., 2018; Kawai, 2018; Yu et al., 2022), periaqueductal gray (Herbert and Saper, 1992; Kawai, 2018), spinal trigeminal nucleus (Loewy and Burton, 1978; McGovern et al., 2015b), pedunculopontine tegmental nucleus (PPTg) (Steininger et al., 1992), cerebellum (Batini et al., 1978; Somana and Walberg, 1979b; Saigal et al., 1980b; Fu et al., 2011), and inferior olive (Loewy and Burton, 1978; McGovern et al., 2015b; Figure 5C).
Locus coeruleus
The locus coeruleus has broad impact on brain activity, affecting among others attention, motivation, memory, and the level of arousal through its widespread network of noradrenergic fibers (Schwarz et al., 2015; Breton-Provencher and Sur, 2019; Chandler et al., 2019; Poe et al., 2020). The locus coeruleus can also mediate sensory-evoked awakenings from sleep (Hayat et al., 2020) and has been proposed to be a key center in coupling brain activity with the respiratory cycle (Melnychuk et al., 2021).
Hypercapnia leads to increased activity in the locus coeruleus, which is an evolutionary conserved phenomenon observed in amphibians and mammals (Elam et al., 1981; Pineda and Aghajanian, 1997; Biancardi et al., 2008; Santin and Hartzler, 2013; Quintero et al., 2017). This increase in neural activity can lead to stronger basal ventilation (Hilaire et al., 2004; Liu et al., 2021b), although this effect was not observed in all studies (Gargaglioni et al., 2010). The impact of the locus coeruleus may therefore depend on behavioral or experimental conditions.
Stimulation of the locus coeruleus can modulate activity in the pre-Bötzinger complex via a direct projection (Liu et al., 2021b). The locus coeruleus also targets many other brain regions with three diverging pathways, with individual locus coeruleus neurons projecting to functionally related areas (Szabadi, 2013; Schwarz et al., 2015; Poe et al., 2020). The ascending pathway targets the thalamus and cerebral cortex, but also the paraventricular and lateral hypothalamus, periaqueductal gray, dorsal and caudal raphe nuclei, bed nucleus of the stria terminalis, and central amygdala (Jones and Moore, 1977; Jones and Yang, 1985; Hermann et al., 1997; Ni et al., 2016; Borodovitsyna et al., 2020). Other targets include the nuclei of the mesodiencephalic junction (MDJ) (Jones and Yang, 1985). The cerebellar pathway targets both the cerebellar nuclei and cortex (Olson and Fuxe, 1971; Saigal et al., 1980a; Nagai et al., 1981; Room et al., 1981; Loughlin et al., 1986; Dietrichs, 1988; Fu et al., 2011). The third, descending pathway targets the brainstem and spinal cord, including the ambiguus, hypoglossal and facial nuclei, dorsal raphe, and the pedunculopontine tegmental nucleus (Jones and Yang, 1985). The spinal projections are, as typical for locus coeruleus projections, widespread and involve also the phrenic nucleus, without showing a specific concentration of terminals in the latter (Bruinstroop et al., 2012).
In turn, the locus coeruleus receives widespread but relatively sparse projections from the cerebral cortex, and denser projections from the hypothalamus, central amygdala, bed nucleus of the stria terminalis, raphe nuclei, Kölliker-Fuse nucleus and adjacent parabrachial nuclei, and periaqueductal gray (Luppi et al., 1995; Schwarz et al., 2015). There is also input from the pre-Bötzinger complex (Yackle et al., 2017), NTS (McGovern et al., 2015b; Kawai, 2018), PPTg (Woolf and Butcher, 1989), and cerebellar nuclei, in addition to numerous direct projections from cerebellar Purkinje cells (Schwarz et al., 2015).
Raphe nuclei
The raphe nuclei consist of clusters of neurons along the midline of the midbrain, pons and medulla. Of these, the dorsal and caudal raphe both house CO2 chemoreceptors, and together form the main source of serotonin in the respiratory system (Wang et al., 2001; Severson et al., 2003; Teran et al., 2014; Figure 6A). Disturbances in serotonin release have been linked to respiratory dysfunction in Prader-Willi syndrome (Matarazzo et al., 2017) as well as to SIDS (Paterson et al., 2006; Kinney and Haynes, 2019). The risk of the latter may be increased by prenatal exposure to nicotine that can later cause impairments in serotonin release during hypercapnia (Avraam et al., 2020).
Figure 6. Raphe nuclei and cerebellum.
(A) The caudal raphe consists of the raphe magnus (RMg), the raphe obscurus (Rob) and the raphe pallidus (Rpa). The cerebellum consists of the cerebellar cortex and the cerebellar nuclei. (B) Different populations of raphe neurons can have different projection patterns. For example, Egr2-positive serotonergic neurons of RMg have intrinsic chemoreceptor properties and project mainly to other central chemoreceptor areas. The downstream Tac-positive serotonergic neurons of Rob lack intrinsic chemoreceptor properties and project predominantly to respiratory motor neurons. Other neurons of the caudal raphe, whether serotonergic or non-serotonergic, extend the caudal raphe projections to further respiratory regions. These projections partially overlap with those of the dorsal raphe. Indicated are also the projections from the cerebellar nuclei. Note that also the locus coeruleus receives cerebellar input via direct Purkinje cells projections. (C) At rest, Purkinje cells of the lateral cerebellum can show modulation of their complex spike and simple spike frequency in relation to the respiratory cycle. This is illustrated with a representative electrophysiological recording of a Purkinje cell in an awake mouse. Complex spikes are noted with a red dot on top of the trace. In this example, they occur preferably just after the onset of expiration (downward phase of the blue trace). Simple spike firing is increased during the last phase of expiration, as illustrated by their instantaneous frequency (magenta curve). The duration of this fragment is 3.6 s. (D) For this Purkinje cell, the simple spike frequency is upregulated at the last phase of respiration, compared to the expected frequency (grey circle). 0=start inspiration. (E) Optogenetic stimulation of Purkinje cells in the lateral cerebellum can shorten the interval until the next inspiration. (F) In the absence of excitatory output of the cerebellar nuclei, in the Atoh1-En1/2 mouse model for cerebellar neuropathology, the respiratory cycle is more regular than in control mice, as quantified by the local coefficient of variation (CV2). fMRI scans indicate that specific brain regions, including the cerebellum, are activated during respiratory challenges: (G) Increasing respiratory resistance. (H) Hypoxia (13% oxygen for 1 min). (I) Actively slowed breathing. (J) During breath holding, the cerebellum is not activated. * p<0.05 Scaling of activity levels in G: –6.7 to –3.1 (blue colors) and +3.1 to+8.5 (red colors); in H-J: 0–15. Panels C and D are modified from Figure 1 from Romano et al., 2020, E from Figure 6 from Romano et al., 2020, F from Figure 4 from Taylor et al., 2022, and H and I from Figure 4 from Critchley et al., 2015.
© 2013, Elsevier
Panel G is reproduced from Figure 2 from Raux et al., 2013, with permission from Elsevier; this panel is not covered by the CC-BY 4.0 license and further reproduction of these panels would need permission from the copyright holder.
© 2008, Elsevier
Panel J is reproduced from Figure 4 from McKay et al., 2008, with permission from Elsevier; this panel is not covered by the CC-BY 4.0 license and further reproduction of these panels would need permission from the copyright holder.
The dorsal raphe nucleus mediates the CO2 arousal reflex via its projection to the lateral parabrachial nucleus (Petrov et al., 1992; Smith et al., 2018; Kaur et al., 2020). Other projections, sometimes involving collateral fibers, target predominantly forebrain regions, including the lateral hypothalamic nucleus, bed nucleus of the stria terminalis, central amygdala, and vestibular nuclei (Vertes, 1991; Petrov et al., 1992; Halberstadt and Balaban, 2006; Vasudeva et al., 2011; McDevitt et al., 2014). Descending projections target the hypoglossal, trigeminal and facial motor nuclei (Li et al., 1993; Guo et al., 2020), retrotrapezoid nucleus (Rosin et al., 2006), locus coeruleus (Luppi et al., 1995; Schwarz et al., 2015), periaqueductal gray (Vertes, 1991), and PPTg (Vertes, 1991; Steininger et al., 1992; Figure 6B).
The dorsal raphe nucleus receives input from many areas, with relatively dense projections originating in the cerebral cortex, lateral, paraventricular and dorsomedial hypothalamus, bed nucleus of the stria terminalis, central amygdala, periaqueductal gray, lateral and medial parabrachial nuclei, Kölliker-Fuse nucleus, NTS, locus coeruleus, and vestibular nuclei (Jones and Yang, 1985; Peyron et al., 1998a; Pollak Dorocic et al., 2014; Weissbourd et al., 2014; Peyron et al., 2018; Shi et al., 2021). In addition, also the cerebellar nuclei project to the dorsal raphe nucleus, but this projection seems to be relatively sparse (Teune et al., 2000; Weissbourd et al., 2014).
The caudal, or medullary, raphe nuclei can also directly affect respiration (Lalley, 1986; Ptak et al., 2009; Depuy et al., 2011; Sabino et al., 2021). The caudal raphe nuclei exist of the raphe magnus, raphe obscurus, and raphe pallidus, and contain serotonergic, non-serotonergic and mixed neurons (Pilowsky, 2014; Figure 6A). Accordingly, neural responses of neurons in the caudal raphe are heterogeneous and hypercapnic acidosis can activate some, and inhibit other neurons of the caudal raphe (Richerson, 1995; Wang et al., 1998), the former category comprising serotonergic neurons, the latter not (Wang et al., 2001; Taylor et al., 2005).
Egr2-Pet1-expressing serotonergic neurons of the raphe magnus mediate the respiratory CO2 chemoreflex (Brust et al., 2014). These chemoreceptor neurons target predominantly other chemoreceptor areas in the brainstem: the retrotrapezoid nucleus, NTS, locus coeruleus, pre-Bötzinger complex and medial parabrachial nucleus (Brust et al., 2014). Tac1-Pet1-expressing serotonergic neurons of the raphe obscurus do not express chemoreceptor properties themselves, but are most likely downstream of Egr2-Pet1 neurons and preferentially innervate motor nuclei, including the phrenic, facial, trigeminal, hypoglossal, and ambiguus nucleus, but also the pre-Bötzinger complex and NTS (Hennessy et al., 2017).
Lateral hypothalamus
Orexinergic neurons of the lateral hypothalamus are the only neurons of the diencephalon with central chemoreceptor properties (Williams et al., 2007; Song et al., 2012b; Li et al., 2013; Fukushi et al., 2019; Wang et al., 2021). Orexins, neuropeptides exclusively produced by the lateral and posterior hypothalamus, are distributed widely throughout the brain and promote wakefulness (de Lecea et al., 1998; Peyron et al., 1998b; Hagan et al., 1999; Adamantidis et al., 2007; Berteotti et al., 2021). The activity of orexinergic neurons is largely restricted to the awake state (Mileykovskiy et al., 2005), and dysfunction of the orexinergic system can cause narcolepsy (Thannickal et al., 2000; Bassetti et al., 2019; Berteotti et al., 2021).
Orexins can also mediate respiratory chemoreflex responses and increase the tidal volume (Young et al., 2005; Zhang et al., 2005; Deng et al., 2007). To this end, orexinergic fibers innervate the phrenic (Young et al., 2005) and hypoglossal nuclei (Fung et al., 2001; Guo et al., 2020). Other targets include the pre-Bötzinger complex (Young et al., 2005; Yang et al., 2020; Trevizan-Baú et al., 2021a), Kölliker-Fuse nucleus (Peyron et al., 1998b; Yokota et al., 2016; Trevizan-Baú et al., 2021a), retrotrapezoid nucleus (Rosin et al., 2006), NTS (Peyron et al., 1998b; Gasparini et al., 2020), locus coeruleus (Luppi et al., 1995; Peyron et al., 1998b; Hagan et al., 1999; Schwarz et al., 2015), dorsal and caudal raphe nuclei (Lee et al., 2003; Lee et al., 2005; Ogawa et al., 2014; Weissbourd et al., 2014), bed nucleus of the stria terminalis (Shin et al., 2008; Ni et al., 2016), central amygdala (Peyron et al., 1998b; Fu et al., 2020), periaqueductal gray (Peyron et al., 1998b; Trevizan-Baú et al., 2021a), and PPTg (Semba and Fibiger, 1992; Steininger et al., 1992). There is also widespread innervation of the cerebellar cortex and nuclei, in particular of the fastigial nucleus (Dietrichs, 1984; Ciriello and Caverson, 2014; Çavdar et al., 2018a).
Orexinergic cells of the lateral hypothalamus receive input from other areas of the hypothalamus, including the paraventricular and dorsomedial nuclei, and from the bed nucleus of the stria terminalis, central amygdala, periaqueductal gray, dorsal raphe nucleus, and lateral parabrachial nucleus (Yoshida et al., 2006; Arima et al., 2019). Other studies revealed input from the pre-Bötzinger complex (Yang and Feldman, 2018), NTS (McGovern et al., 2015b; Kawai, 2018), locus coeruleus (Jones and Moore, 1977), medial parabrachial nucleus (Saper and Loewy, 1980; Fulwiler and Saper, 1984; Moga et al., 1990; Bianchi et al., 1998), PPTg (Woolf and Butcher, 1986), cerebellar interposed nucleus (Lu et al., 2015), and cerebellar dentate nucleus (Teune et al., 2000).
Fastigial nucleus
Electrical or chemical stimulation of the rostral part of the cerebellar fastigial nucleus can affect the respiratory pattern (Bassal and Bianchi, 1982; Lutherer and Williams, 1986; Williams et al., 1989; Xu and Frazier, 1995; Xu and Frazier, 2000; Xu et al., 2001b; Xu and Frazier, 2002; Hernandez et al., 2004). Furthermore, bilateral lesions of the fastigial nucleus suppressed spontaneous breathing in anesthetized cats (Williams et al., 1986). This effect was likely to be specific, as bilateral lesions of the cerebellar dentate nuclei had no impact of spontaneous breathing in that same study. An impact of anesthesia cannot be excluded, though, as this result could not be reproduced in awake goats (Martino et al., 2007). In contrast, the latter study describes a reduction in hypercapnia-induced increases in ventilation following bilateral lesioning of the fastigial nucleus, suggesting that the impact of the fastigial nucleus becomes especially apparent during periods with increased pCO2. Neural recordings demonstrated activity patterns in phase with respiration at rest, but in particular during respiratory challenges such as tracheal occlusion, bilateral carotid occlusion, or injection of sodium cyanide (Lutherer et al., 1989; Xu and Frazier, 1997; Xu and Frazier, 2002; Lu et al., 2013). Thus, the evidence converges on a role for the fastigial nucleus in mediating ventilatory responses to hypercapnia. The other two cerebellar nuclei lack the chemoreceptor abilities of the fastigial nucleus (Xu et al., 2001a; Xu and Frazier, 2002), and putative other roles of the interposed and dentate nuclei in respiratory control have not been extensively studied. However, electrical stimulation of these nuclei seemed to promote expiration (Farber, 1987; Huang et al., 1993), indicating a more widespread involvement of the cerebellum in respiratory control. The connections to and from the fastigial nucleus are described in the section on the cerebellum.
Other sensory areas
Paratrigeminal nucleus
The NTS and the paratrigeminal nucleus together form the main entrances of vagal sensory input, and the paratrigeminal nucleus receives mainly input from the proximal airways via the jugular ganglion (Driessen et al., 2015; McGovern et al., 2015a; McGovern et al., 2015b). The paratrigeminal nucleus also receives primary sensory input from the trigeminal, glossopharyngeal and lingual nerves, and from the upper cervical cord (Saxon and Hopkins, 2006; Panneton et al., 2017; Driessen, 2019).
The strongest projections from the paratrigeminal nucleus target the NTS and the lateral and medial parabrachial nucleus, often involving collateral projections (Menétrey et al., 1987; Saxon and Hopkins, 1998; Caous et al., 2001; de Sousa Buck et al., 2001; McGovern et al., 2015b; Driessen et al., 2018; Hashimoto et al., 2018). Other targets are the Kölliker-Fuse nucleus, ambiguus nucleus, periaqueductal gray, spinal trigeminal nucleus, and inferior olive (Caous et al., 2001; de Sousa Buck et al., 2001; McGovern et al., 2015b; Driessen et al., 2018). A projection to the cerebellum has been described (Somana and Walberg, 1979b), but this has later been questioned (Menétrey et al., 1987).
Spinal trigeminal nucleus
The trigeminal nerve conveys sensory input from the face and terminates in the sensory trigeminal nuclei that consist of a primary and a spinal nucleus. The spinal nucleus appears particularly relevant for respiratory control, receiving not only tactile information, for example, from the mystacial vibrissae (Bosman et al., 2011), but also olfactory input from the nasal mucosa, bypassing the forebrain olfactory system (Doty et al., 1978; Anton and Peppel, 1991; Schaefer et al., 2002). Stimulation of the trigeminal olfactory system can lead to sniffs and respiratory depression without involvement of the forebrain (Pérez de Los Cobos Pallares et al., 2016). Activation of the trigeminal olfactory system occurs mainly by noxious or irritable gasses, and the resultant respiratory depression is supposed to protect the lungs, and may contribute to the diving reflex (Panneton, 2013).
The anterior ethmoidal nerve, the branchlet of the trigeminal nerve that innervates the nasal mucosa, terminates in the interpolar and caudal subnuclei of the spinal trigeminal nucleus (Anton and Peppel, 1991; Panneton et al., 2006). From these regions, projections target the pre-Bötzinger complex, Kölliker-Fuse nucleus, NTS and lateral parabrachial nucleus (Panneton et al., 2006; Zhang et al., 2018), as well as the facial nucleus (Panneton et al., 2006), lateral parafacial nucleus (Biancardi et al., 2021), cVRG (Li et al., 2021), medial parabrachial nucleus (Hashimoto et al., 2018), and periaqueductal gray (Wiberg et al., 1986; Beitz, 1989). In addition, there is direct output to the cerebellum (Van Ham and Yeo, 1992; Fu et al., 2011; Henschke and Pakan, 2020) and inferior olive (Swenson and Castro, 1983b; Huerta et al., 1985; Molinari et al., 1996; Yatim et al., 1996; Panneton et al., 2006). The latter is also indirectly targeted via the MDJ (Kubo et al., 2018).
Premotor areas
Although direct projections from central pattern generators and respiratory sensory areas to motor neurons do exist, it is likely that indirect pathways via premotor areas are more prominent.
Retroambiguus nucleus
The Bötzinger and pre-Bötzinger complexes form a cell column in the ventrolateral medulla that continues caudally until the first cervical spinal segment. Caudal to the pre-Bötzinger complex is the retroambiguus nucleus that houses inspiratory premotor neurons in its rostral part and expiratory premotor neurons in its caudal part (Merrill, 1970). The respiratory neurons of the retroambiguus nucleus have later been termed the ventral respiratory group. Somewhat confusingly, some authors use the terms retroambiguus nucleus and ventral respiratory group as synonyms (Shannon and Freeman, 1981), while others refer to the retroambiguus nucleus specifically as the cVRG (Subramanian and Holstege, 2009), or consider the retroambiguus nucleus and cVRG as overlapping areas within the caudal medullary reticular formation (Jones et al., 2016). For the purpose of this review, we use the terms rVRG for the inspiratory and cVRG for the expiratory part of the ventral respiratory column.
Rostral ventral respiratory group
The rVRG provides monosynaptic input to the phrenic motor nucleus, and is the most prominent intermediate between the pre-Bötzinger complex and the diaphragmatic motor neurons (Ellenberger and Feldman, 1988; Ellenberger et al., 1990a; Tian and Duffin, 1996; Boulenguez et al., 2007; Buttry and Goshgarian, 2015). The direct pathway is complemented by a presumably weaker disynaptic pathway via premotor interneurons in the upper cervical (C1 and C2) segments (Lipski et al., 1994; Tian and Duffin, 1996; Lane et al., 2008). In addition, the rVRG projects also to the ambiguus, hypoglossal and facial motor nuclei (Yamada et al., 1988; Ellenberger et al., 1990b; Lipski et al., 1994; Zheng et al., 1998). The rVRG likely contributes to shaping the pattern of respiratory motor output, processing and transmitting sensory afferent information, coordinating ventilation with motor activity, and regulating accessory and respiratory muscle activity (Jensen et al., 2019).
The output of the rVRG is not limited to primary motor areas, but targets also the cVRG (Ellenberger and Feldman, 1990; Gerrits and Holstege, 1996) and Kölliker-Fuse nucleus (Ellenberger et al., 1990b; Lipski et al., 1994; Yokota et al., 2016), and to a lesser extent also the lateral parafacial nucleus (Biancardi et al., 2021), reticular formation (Zheng et al., 1998), retrotrapezoid nucleus (Rosin et al., 2006), NTS and lateral parabrachial nucleus (Yamada et al., 1988; Ellenberger et al., 1990a; Zheng et al., 1998), spinal trigeminal nucleus (Zheng et al., 1998), cerebellum (Gaytán and Pásaro, 1998), and inferior olive (Swenson and Castro, 1983a).
Input to the rVRG does not originate solely in the pre-Bötzinger complex, but comes also from the Bötzinger complex (Jiang and Lipski, 1990; Bryant et al., 1993; Ezure et al., 2003), Kölliker-Fuse nucleus (Ellenberger and Feldman, 1990; Zheng et al., 1998; Yokota et al., 2016), cVRG (Zheng et al., 1998), NTS (Ezure and Tanaka, 1996; Zheng et al., 1998), retrotrapezoid nucleus (Rosin et al., 2006; Bochorishvili et al., 2012; Silva et al., 2016a), and medial parabrachial nucleus (Yokota et al., 2016).
Caudal ventral respiratory group
The cVRG is home to expiratory pre-motor neurons (Merrill, 1970; Arita et al., 1987), and therefore crucial for the control of expiration-related behavior, like vocalization and expulsive reflexes such as vomiting, coughing and sneezing (Umezaki et al., 1997; Subramanian and Holstege, 2009). While vocalization depends on the projections to the nucleus ambiguus and spinal cord, expulsive reflexes use predominantly the latter only (Holstege, 1989; Umezaki et al., 1997). In particular during coughing, the cVRG may trigger also the inspiratory phase (Cinelli et al., 2020).
Contrary to the rVRG, the cVRG does not project to the phrenic nucleus, but instead innervates motor neurons of abdominal muscles in the thoracic spinal cord, and those of the upper airway muscles in the ambiguus, hypoglossal, trigeminal, and facial nuclei (Holstege, 1989; VanderHorst et al., 2001; Ezure et al., 2003; Boers et al., 2006; Holstege and Subramanian, 2016; Jones et al., 2016). Other outputs target pre-Bötzinger and Bötzinger complexes, Kölliker-Fuse nucleus, rVRG, retrotrapezoid nucleus, lateral parabrachial nucleus and periaqueductal gray (Holstege, 1989; Gang et al., 1995; Zheng et al., 1998; Rosin et al., 2006; Jones et al., 2016). Vocalization-related input to the cVRG comes largely from the periaqueductal gray, and this connection is also associated with sexual behavior (Holstege, 1989; VanderHorst et al., 2000; Oka et al., 2008; Subramanian and Holstege, 2009; Holstege and Subramanian, 2016).
The cVRG receives both excitatory and inhibitory input from the pre-Bötzinger complex (Gerrits and Holstege, 1996; Tan et al., 2010; Yang and Feldman, 2018). Also the Bötzinger complex, Kölliker-Fuse nucleus, lateral parafacial nucleus and rVRG project to the cVRG (Fedorko and Merrill, 1984; Ellenberger and Feldman, 1990; Jiang and Lipski, 1990; Bryant et al., 1993; Gerrits and Holstege, 1996; Ezure et al., 2003; Song et al., 2012a; Silva et al., 2016a). Other direct inputs come from the NTS (Loewy and Burton, 1978; Beckstead et al., 1980; Gerrits and Holstege, 1996), retrotrapezoid nucleus (Gerrits and Holstege, 1996; Rosin et al., 2006; Bochorishvili et al., 2012; Silva et al., 2016a), and lateral and medial parabrachial nuclei (Gerrits and Holstege, 1996). A direct input from the carotid bodies to the area of the cVRG has been described (Finley and Katz, 1992), but this connection did not receive as much attention as that to the NTS.
Reticular formation
The hindbrain reticular formation is a highly heterogenous region spanning from the pons to the caudal medulla, counting many subdivisions with often unclear borders. While many respiratory regions are connected with parts of the reticular formation, this connectivity is difficult to compare between different studies, especially considering that the nomenclature of the reticular subdivisions has been quite fluid. For this reason, we focus only on those connections with a clear role in respiratory control; other connections are summarized in Supplementary file 1. In particular, we mention three connections from the pre-Bötzinger complex in which the reticular formation serves as pre-motor area. First, there is the connection via the perihypoglossal area to the hypoglossal nucleus that is relevant for upper airway control (Chamberlin et al., 2007; Tan et al., 2010; Yang and Feldman, 2018). Second, two regions serve as premotor areas for the part of the facial nucleus that controls among others nose movements: the retrofacial area just caudal to the facial nucleus, and a part of the intermediate reticular formation, and both areas probably receive direct input from the pre-Bötzinger complex (Kurnikova et al., 2019). Third, next to the nose region of the intermediate reticular formation is the vibrissal region that links the pre-Bötzinger complex to the vibrissal part of the facial nucleus (Moore et al., 2013; Takatoh et al., 2022). The latter two pathways can contribute to the coupling of nose movements, whisking and respiration (Welker, 1964; Moore et al., 2013; Kurnikova et al., 2019; Romano et al., 2020; Takatoh et al., 2022). The reticular formation could serve a similar pre-motor function for the diaphragm as well, as in particular the gigantocellular part projects directly to the phrenic nucleus (Ellenberger et al., 1990b; Dobbins and Feldman, 1994; Lois et al., 2009).
Limbic system
Of the limbic system, the amygdala and hypothalamus are involved in subconscious control of respiration. The former is particularly important for handling fear responses, for example, as a consequence of high levels of CO2, while the latter is especially important for coupling with the endocrine system, as well as for peptidergic modulation of respiration. The lateral hypothalamus is a central chemoreceptor area, and – although part of the limbic system – discussed in the section on central chemoreception.
Central amygdala
The central amygdala plays a role in CO2-induced fear behavior (Ziemann et al., 2009), and electrical stimulation of the central amygdala can indeed affect breathing (Applegate et al., 1983; Harper et al., 1984; Nobis et al., 2018). In humans, amygdalar stimulation leads to hypopnea or even apnea during nasal breathing, but not during mouth breathing (Nobis et al., 2018), indicating stronger impact on the upper airways than on respiratory rhythmogenesis itself.
Renewed attention for a respiratory role of the central amygdala comes from findings in sudden unexpected death in epilepsy (SUDEP). Typically, this sequence leads to SUDEP: rapid breathing after a seizure, followed by apnea, bradycardia, and finally cardiac arrest (Ryvlin et al., 2013). Furthermore, EEG recordings in patients with epilepsy correlate amygdalar activity with seizure-induced apnea (Nobis et al., 2019).
It has been proposed that the impact of the amygdala on respiratory control is mainly exerted via its direct projection to the bed nucleus of the stria terminalis (Nobis et al., 2018). However, as the central amygdala gives rise to widespread GABAergic projections, also other connections may be relevant for respiratory control (Hopkins and Holstege, 1978; Pape and Pare, 2010; Liu et al., 2021a). Target areas include the pre-Bötzinger complex (Yang et al., 2020; Trevizan-Baú et al., 2021a), hypoglossal nucleus (Guo et al., 2020), retrotrapezoid nucleus (Rosin et al., 2006), NTS (Gasparini et al., 2020), locus coeruleus (Schwarz et al., 2015; Liu et al., 2021a), dorsal raphe (Peyron et al., 1998a; Ogawa et al., 2014; Pollak Dorocic et al., 2014; Weissbourd et al., 2014), caudal raphe (Hermann et al., 1997), lateral hypothalamus (Yoshida et al., 2006), lateral and medial parabrachial nuclei (Moga et al., 1990; Liu et al., 2021a; Yang et al., 2021), periaqueductal gray (Oka et al., 2008; Liu et al., 2021a; Trevizan-Baú et al., 2021a), and PPTg (Semba and Fibiger, 1992).
The central amygdala receives input from the NTS (Kawai, 2018), locus coeruleus (Borodovitsyna et al., 2020), dorsal raphe (Bienkowski and Rinaman, 2013), lateral and paraventricular hypothalamus (Fu et al., 2020), bed nucleus of the stria terminalis (Fu et al., 2020), lateral and medial parabrachial nuclei (Bienkowski and Rinaman, 2013), periaqueductal gray (Rizvi et al., 1991), and PPTg (Dautan et al., 2016).
Bed nucleus of the stria terminalis
The bed nucleus of the stria terminalis is part of the extended amygdala and a main output station of the central amygdala (Liu et al., 2021a). It is central to fear, aggression and stress responses (Walker et al., 2003; Lebow and Chen, 2016). In relation to respiration, the bed nucleus of the stria terminalis may be particularly relevant for anxious responses, or even panic caused by increased levels of CO2 (Taugher et al., 2014). To this end, the bed nucleus of the stria terminalis expresses relatively high levels of acid-sensing ion channel 1A (ASIC1A) (Coryell et al., 2007; Taugher et al., 2014). ASIC1A in the bed nucleus of the stria terminalis is also essential for the freezing reaction of mice, which implicates a strong reduction in breathing, upon exposure to a predator odor (Taugher et al., 2015).
Apart from the central amygdala (Dong et al., 2001a; Shin et al., 2008; Lebow and Chen, 2016; Ni et al., 2016), other areas targeting the bed nucleus of the stria terminalis are the lateral and dorsomedial hypothalamus (Shin et al., 2008), NTS (Ricardo and Tongju Koh, 1978; Shin et al., 2008; Bienkowski and Rinaman, 2013; Ni et al., 2016; Kawai, 2018), locus coeruleus (Ni et al., 2016), dorsal raphe nucleus (Vertes, 1991; Shin et al., 2008; Bienkowski and Rinaman, 2013; Ni et al., 2016), lateral parabrachial nucleus (Shin et al., 2008; Bienkowski and Rinaman, 2013; Ni et al., 2016; Jaramillo et al., 2021), and periaqueductal gray (Shin et al., 2008; Ni et al., 2016). Relating to their functional similarities, the central amygdala and bed nucleus of the stria terminalis receive input from the same brain regions, often even from collaterals, except from the substantia nigra pars compacta, that does not project to the bed nucleus of the stria terminalis (Bienkowski and Rinaman, 2013). This does not imply that all projections are equally strong. In particular, the parabrachial nuclei project stronger to the central amygdala, and the NTS stronger to the bed nucleus of the stria terminalis (Bienkowski and Rinaman, 2013).
Output reaches the central amygdala (Dong et al., 2001b; Fu et al., 2020), lateral, dorsomedial and paraventricular hypothalamus (Yoshida et al., 2006; Barbier et al., 2021), NTS (Gasparini et al., 2020), locus coeruleus (Luppi et al., 1995; Schwarz et al., 2015), dorsal raphe nuclei (Peyron et al., 1998a; Lee et al., 2003; Ogawa et al., 2014; Pollak Dorocic et al., 2014; Weissbourd et al., 2014), caudal raphe (Hermann et al., 1997), lateral and medial parabrachial nuclei (Moga et al., 1990; Luskin et al., 2021), and PPTg (Steininger et al., 1992).
Paraventricular hypothalamus
The paraventricular nucleus of the hypothalamus is vital for the hypoxia reflex: decreased heart rate and increased blood pressure and phrenic nerve activity (Kubo et al., 1997; Reddy et al., 2005; Ruyle et al., 2019). The paraventricular nucleus is reciprocally connected with the NTS (Rinaman, 1998; Geerling et al., 2010; King et al., 2012; Gasparini et al., 2020), and the NTS serves both as intermediate for the ascending input coming from the carotid bodies, and for the descending output affecting the phrenic nucleus (Ruyle et al., 2018; Ruyle et al., 2019). The impact of these NTS-projecting paraventricular fibers may be complemented by vasopressinergic connections to the phrenic nucleus (Kc et al., 2002), oxytocinergic projections to the pre-Bötzinger complex (Mack et al., 2002; Yang et al., 2020; Trevizan-Baú et al., 2021a), or by projections to the Kölliker-Fuse nucleus (Yokota et al., 2016; Trevizan-Baú et al., 2021a), PiCo (Oliveira et al., 2021), ambiguus, hypoglossal and facial motor nuclei (Mack et al., 2007; Geerling et al., 2010; Guo et al., 2020), reticular formation (Geerling et al., 2010), retrotrapezoid nucleus (Rosin et al., 2006; Geerling et al., 2010), locus coeruleus (Luppi et al., 1995; Zheng et al., 1995; Schwarz et al., 2015), dorsal and caudal raphe (Zheng et al., 1995; Hermann et al., 1997; Peyron et al., 1998a; Lee et al., 2003; Geerling et al., 2010; Ogawa et al., 2014; Pollak Dorocic et al., 2014; Weissbourd et al., 2014; Singh et al., 2022), lateral hypothalamus (Yoshida et al., 2006; Singh et al., 2022), lateral and medial parabrachial nuclei (Zheng et al., 1995; Geerling et al., 2010; Singh et al., 2022), periaqueductal gray (Zheng et al., 1995; Geerling et al., 2010; Trevizan-Baú et al., 2021a; Singh et al., 2022), bed nucleus of the stria terminalis (Singh et al., 2022), central amygdala (Fu et al., 2020), spinal trigeminal and paratrigeminal nuclei (Zheng et al., 1995; Geerling et al., 2010), PPTg (Zheng et al., 1995; Geerling et al., 2010), and cerebellum (Dietrichs, 1984; Çavdar et al., 2018a).
Apart from the NTS, the lateral and dorsomedial nucleus of the hypothalamus (Thompson et al., 1996; Singh et al., 2022), locus coeruleus (Jones and Moore, 1977; Jones and Yang, 1985; Cunningham and Sawchenko, 1988), bed nucleus of the stria terminalis (Barbier et al., 2021), and lateral parabrachial nucleus (Saper and Loewy, 1980; Fulwiler and Saper, 1984; Moga et al., 1990) project to the paraventricular nucleus.
Dorsomedial hypothalamus
The dorsomedial hypothalamus is essential for mediating respiratory effects of stressful stimuli (Bondarenko et al., 2015). To this end, it receives input from other hypothalamic regions and the bed nucleus of the stria terminalis, as well as from the periaqueductal gray, lateral parabrachial nucleus, NTS (Thompson and Swanson, 1998; Do et al., 2020), pre-Bötzinger complex (Yang and Feldman, 2018), and Kölliker-Fuse nucleus (Geerling et al., 2017).
The dorsomedial hypothalamus projects predominantly to other hypothalamic nuclei, in particular to the paraventricular nucleus, but also to the bed nucleus of the stria terminalis, periaqueductal gray, NTS (Thompson et al., 1996), Kölliker-Fuse nucleus (Trevizan-Baú et al., 2021a), dorsal and caudal raphe (Peyron et al., 2018; Trevizan-Baú et al., 2021a), and cerebellar cortex (Çavdar et al., 2018a).
Modulatory brain regions
On top of the central pattern generators, (pre)motor areas, sensory areas and limbic system, also other brain regions can affect specific aspects of respiration, often to adapt respiration to other behaviors. In fact, few brain regions are completely unrelated to respiration. In the following section, we restrict ourselves to those brain regions of which a non-voluntary respiratory function has been clearly described.
Lateral parabrachial nucleus
The parabrachial nuclei produce a tonic excitatory drive that can, in conjunction with output from the Kölliker-Fuse nucleus, contribute to setting the duration of inspiration and expiration (Navarrete-Opazo et al., 2020). In addition, the lateral parabrachial nucleus is also crucial in the hypercapnic arousal reflex that can trigger waking up during sleep (Kaur et al., 2013). It has been suggested that frequent hypercapnic arousals contribute to day-time fatigue and cardiovascular problems in patients with obstructive sleep apnea (Abbott and Souza, 2021). The lateral parabrachial nucleus has a tonic descending drive and an event-driven ascending one. The latter is consistent with other functions of the lateral parabrachial nucleus, as it responds to various aversive signals, varying from food poisoning to itch and hypercapnia, and can send a general alarm signal to the forebrain (Palmiter, 2018; Chiang et al., 2019; Jaramillo et al., 2021).
The main outputs target the lateral hypothalamus (Saper and Loewy, 1980; Fulwiler and Saper, 1984; Moga et al., 1990; Bianchi et al., 1998; Yoshida et al., 2006; Arima et al., 2019), bed nucleus of the stria terminalis (Saper and Loewy, 1980; Fulwiler and Saper, 1984; Moga et al., 1990; Bianchi et al., 1998; Shin et al., 2008; Bienkowski and Rinaman, 2013; Ni et al., 2016), central amygdala (Saper and Loewy, 1980; Moga et al., 1990; Bianchi et al., 1998; Tokita et al., 2010; Bienkowski and Rinaman, 2013), and thalamus (Jaramillo et al., 2021). Other targets are the pre-Bötzinger and Bötzinger complexes (Gang et al., 1995; Yang et al., 2020; Yu et al., 2022), lateral parafacial nucleus (Biancardi et al., 2021), cVRG (Gerrits and Holstege, 1996), ambiguus nucleus (Rübsamen and Schweizer, 1986), hypoglossal nucleus (Yokota et al., 2015), retrotrapezoid nucleus (Rosin et al., 2006), NTS (Saper and Loewy, 1980; Herbert et al., 1990; Bianchi et al., 1998), locus coeruleus (Luppi et al., 1995), dorsal and caudal raphe (Saper and Loewy, 1980; Hermann et al., 1997; Bianchi et al., 1998; Lee et al., 2003; Peyron et al., 2018), paraventricular nucleus (Saper and Loewy, 1980; Fulwiler and Saper, 1984; Moga et al., 1990; Singh et al., 2022), periaqueductal gray (Bianchi et al., 1998), and PPTg (Steininger et al., 1992).
The main ascending inputs to the lateral parabrachial nucleus come from the spinal cord and NTS (Palmiter, 2018). Other inputs come from the pre-Bötzinger and Bötzinger complexes (Tan et al., 2010; Yang and Feldman, 2018), Kölliker-Fuse nucleus (Song et al., 2012a; Geerling et al., 2017), rVRG (Holstege, 1989), NTS (Beckstead et al., 1980; Herbert et al., 1990; McGovern et al., 2015b; Kawai, 2018; Yu et al., 2022), retrotrapezoid nucleus (Rosin et al., 2006; Bochorishvili et al., 2012; Silva et al., 2016a), locus coeruleus (Robertson et al., 2013; Yang et al., 2021), dorsal raphe (Petrov et al., 1992; Quattrochi et al., 1998; Kaur et al., 2020), bed nucleus of stria terminalis (Moga et al., 1990), central amygdala (Moga et al., 1990; Yang et al., 2021), paraventricular nucleus (Zheng et al., 1995; Geerling et al., 2010; Singh et al., 2022), spinal trigeminal nucleus (Panneton et al., 2006; Zhang et al., 2018), paratrigeminal nucleus (Saxon and Hopkins, 1998; Caous et al., 2001; McGovern et al., 2015b; Driessen et al., 2018), PPTg (Quattrochi et al., 1998; Lima et al., 2019b), and fastigial nucleus (Teune et al., 2000).
Medial parabrachial nucleus
Activity of the medial parabrachial nucleus affects, like that of the lateral parabrachial nucleus, the breathing frequency by modulating the duration of expiration (von Euler et al., 1976; Zuperku et al., 2017). The medial parabrachial nucleus contains more expiratory neurons than the neighboring Kölliker-Fuse nucleus that has more inspiratory and phase-spanning neurons (Song et al., 2006). The medial parabrachial nucleus has a dense expression of µ-opioid receptors (Ding et al., 1996) and plays an important role in the mediation of respiratory depression by opioids (Miller et al., 2017; Algera et al., 2019; Saunders and Levitt, 2020). Unlike the lateral parabrachial nucleus, the medial parabrachial nucleus is not involved in the hypercapnic arousal reflex (Kaur et al., 2013).
The medial parabrachial nucleus has both excitatory and inhibitory projections to pre-inspiratory neurons in the pre-Bötzinger complex and to expiratory neurons in the Bötzinger complex (Zuperku et al., 2019). Activity of the medial parabrachial nucleus can be affected by input from pulmonary stretch receptors coming via the NTS (Zuperku et al., 2021), from which it receives direct input (Herbert et al., 1990). In addition, it receives input from chemosensitive neurons of the caudal raphe nucleus (Brust et al., 2014).
The medial parabrachial nucleus receives also input from the pre-Bötzinger complex (Tan et al., 2010; Yang and Feldman, 2018), Kölliker-Fuse nucleus (Song et al., 2012a; Geerling et al., 2017), rVRG (Holstege, 1989), NTS (Loewy and Burton, 1978; Herbert et al., 1990; McGovern et al., 2015b), retrotrapezoid nucleus (Rosin et al., 2006), locus coeruleus (Robertson et al., 2013), caudal raphe (Brust et al., 2014), bed nucleus of stria terminalis (Moga et al., 1990; Luskin et al., 2021), central amygdala (Moga et al., 1990; Liu et al., 2021a; Yang et al., 2021), paraventricular nucleus (Zheng et al., 1995; Geerling et al., 2010; Singh et al., 2022), spinal trigeminal nucleus (Hashimoto et al., 2018), paratrigeminal nucleus (Saxon and Hopkins, 1998; Caous et al., 2001; McGovern et al., 2015b; Driessen et al., 2018), Purkinje cells in the cerebellar cortex (Sugihara et al., 2009; Hashimoto et al., 2018), and fastigial nucleus (Teune et al., 2000).
The medial parabrachial nucleus projects to the pre-Bötzinger and Bötzinger complexes (Gang et al., 1995; Yang et al., 2020), lateral parafacial nucleus (Biancardi et al., 2021), rVRG and cVRG (Gerrits and Holstege, 1996; Yokota et al., 2016), ambiguus and hypoglossal nuclei (Saper and Loewy, 1980; Rübsamen and Schweizer, 1986; Núñez-Abades et al., 1990; Guo et al., 2020), retrotrapezoid nucleus (Rosin et al., 2006), NTS (Herbert et al., 1990; Bianchi et al., 1998), locus coeruleus (Luppi et al., 1995), dorsal and caudal raphe (Saper and Loewy, 1980; Hermann et al., 1997; Bianchi et al., 1998; Lee et al., 2003; Peyron et al., 2018), lateral hypothalamus (Saper and Loewy, 1980; Fulwiler and Saper, 1984; Moga et al., 1990; Bianchi et al., 1998), bed nucleus of the stria terminalis (Bianchi et al., 1998; Bienkowski and Rinaman, 2013; Ni et al., 2016), central amygdala (Fulwiler and Saper, 1984; Moga et al., 1990; Bienkowski and Rinaman, 2013), periaqueductal gray (Bianchi et al., 1998), and cerebellum (Fu et al., 2011).
Periaqueductal gray
The periaqueductal gray, also known as the central gray, contributes to maintaining the homeostatic balance of an individual. To this end it participates in controlling defensive, emotional, social, sexual and autonomic behaviors, but also in modulating unpleasant sensations, such as pain, itch or the urge to cough (Bandler et al., 2000; Linnman et al., 2012; Motta et al., 2017). In addition, the periaqueductal gray is central to the control of vocalizations (Magoun et al., 1937; Zhang et al., 1994; Esposito et al., 1999; Jürgens, 2002; Subramanian et al., 2021) via its strong projection to the cVRG that is the only brain area directly targeting all motor areas required for vocalization (VanderHorst et al., 2000; Holstege and Subramanian, 2016). This pathway is especially relevant for emotional vocalizations, like crying and laughing, that do not rely on Broca’s area, as human speech does (Holstege and Subramanian, 2016). For this reason, the periaqueductal gray can be seen as the laughing center, receiving excitatory input from emotion-related pathways originating from the basal temporal and frontal lobes, limbic system, and basal ganglia, and inhibition from voluntary systems, in particular (pre)motor cortices (Wild et al., 2003; Klingbeil et al., 2021). As to the suppression of involuntary laughter, the periaqueductal gray can also contribute to the suppression of the urge to cough. The periaqueductal gray sends predominantly GABAergic fibers to the NTS (Chen et al., 2022; Figure 5F). This can have clinical relevance, as the common condition of chronic cough is thought to be caused by hypersensitivity to airway stimulation (Morice et al., 2020).
Although the periaqueductal gray lacks clearly discernible cell groups, specific functions can be roughly attributed to four longitudinal columns that differ in their connectivity patterns (Carrive, 1993). The lateral (lPAG) and dorsolateral (dlPAG) columns are mostly involved in active coping strategies, such as fight or flight, while the ventrolateral (vlPAG) column is associated with passive coping strategies, like freezing (Bandler et al., 2000; Linnman et al., 2012; Faull et al., 2019).
The periaqueductal gray subserves several respiratory functions to support stereotypical behavioral responses. These functions are distributed over the four cell columns, largely in line with their general function (Subramanian et al., 2008; Subramanian, 2013; Faull et al., 2019). Chemical stimulation of the dlPAG triggers active breathing, as occurring during fight and flight situations, while stimulation of the dorsomedial and the medial part of the lPAG resulted in slow deep breathing and dyspnea, and inspiratory apneusis, respectively. Finally, stimulation of the lateral parts of lPAG and vlPAG induced respiratory patterns associated with vocalizations (Subramanian et al., 2008).
The periaqueductal gray is an important hub between the fore- and hindbrain, receiving input from multiple cortical areas as well as from the lateral, dorsomedial and paraventricular hypothalamus (Beitz, 1989; Zheng et al., 1995; Thompson et al., 1996; Geerling et al., 2010; Trevizan-Baú et al., 2021a; Singh et al., 2022). Ascending input to the periaqueductal gray comes directly from the spinal cord, and in particular from the upper cervical cord, to the lPAG and vlPAG (Keay et al., 1997). Also the NTS, the prime recipient of vagal sensory input, projects to the ventrolateral and medial PAG (Herbert and Saper, 1992; Kawai, 2018). Other respiratory centers in the brainstem also project to the lPAG and vlPAG: the pre-Bötzinger and Bötzinger complexes, Kölliker-Fuse nucleus (Tan et al., 2010; Yang and Feldman, 2018; Trevizan-Baú et al., 2021b), locus coeruleus (Jones and Moore, 1977; Jones and Yang, 1985), dorsal and caudal raphe (Vertes, 1991; Herbert and Saper, 1992; Trevizan-Baú et al., 2021b), lateral and medial parabrachial nuclei (Bianchi et al., 1998), paratrigeminal nucleus (McGovern et al., 2015b), and fastigial and dentate cerebellar nuclei (Teune et al., 2000; Frontera et al., 2020).
Descending output from the periaqueductal gray targets multiple respiratory centers. All four columns, but in particular the lPAG and vlPAG, target the pre-Bötzinger complex, Kölliker-Fuse nucleus and lateral parafacial nucleus (Tan et al., 2010; Yang and Feldman, 2018; Biancardi et al., 2021; Trevizan-Baú et al., 2021b). Ipsilateral connections to the parafacial nucleus are predominantly glutamatergic, while contralateral projections have a mixture of glutamatergic and GABAergic fibers (Biancardi et al., 2021). The periaqueductal gray innervates also the PiCo (Oliveira et al., 2021), retrotrapezoid nucleus (Rosin et al., 2006), NTS (VanderHorst et al., 2000; Chen et al., 2022), locus coeruleus (Luppi et al., 1995; Schwarz et al., 2015), dorsal and caudal raphe (Hermann et al., 1997; Ogawa et al., 2014; Pollak Dorocic et al., 2014; Peyron et al., 2018; Trevizan-Baú et al., 2021b), PPTg (Semba and Fibiger, 1992; Steininger et al., 1992), and inferior olive (Swenson and Castro, 1983b; VanderHorst et al., 2000). Forebrain projections include the lateral hypothalamus (Woolf and Butcher, 1986; Yoshida et al., 2006), bed nucleus of the stria terminalis (Shin et al., 2008; Ni et al., 2016), and central amygdala (Rizvi et al., 1991).
Tegmentum
The pedunculopontine tegmental nucleus (PPTg) and the adjacent laterodorsal tegmental nucleus provide important sources of cholinergic fibers, the activation of which is sufficient to induce REM sleep during non-REM sleep (Van Dort et al., 2015). To this end, the pedunculopontine and laterodorsal tegmental nuclei provide widespread innervation of the thalamus and basal ganglia (Mena-Segovia and Bolam, 2017), but also of the retrotrapezoid nucleus (Lima et al., 2019b; Silva et al., 2019). As the chemoreceptor activity in the retrotrapezoid nucleus can be modulated by acetylcholine (Sobrinho et al., 2016), this could imply a different sensitivity to pCO2 during REM sleep. Indeed, partial lesioning of the PPTg resulted in abnormal sleep patterns during intermittent hypoxia, while leaving basic respiratory parameters intact (Fink et al., 2021).
Pharmacological activation of muscarinic acetylcholine receptors in the PPTg resulted in decreased breathing rates in awake rats (Lima et al., 2019a), and also glutamate injections could suppress breathing in anesthetized rats (Saponjic et al., 2003; Topchiy et al., 2010). Inhibition of the PPTg resulted in increased inspiratory activity (Silva et al., 2019). Overall, it seems that the PPTg can inhibit the function of the retrotrapezoid nucleus, and thereby modulate the respiratory drive. This may be particularly relevant during REM sleep, given the interaction between PPTg, hypoxia and disrupted sleep patterns (Silva et al., 2019; Fink et al., 2021).
The target areas of the PPTg are not restricted to those mentioned above, but also include the Kölliker-Fuse nucleus (Lima et al., 2019b), lateral parafacial nucleus (Biancardi et al., 2021), hypoglossal, trigeminal and facial motor nuclei (Woolf and Butcher, 1989), locus coeruleus (Woolf and Butcher, 1989), dorsal and caudal raphe (Woolf and Butcher, 1989; Hermann et al., 1997; Ogawa et al., 2014; Lima et al., 2019b), lateral hypothalamus (Woolf and Butcher, 1986), bed nucleus of the stria terminalis (Ni et al., 2016), central amygdala (Dautan et al., 2016), lateral parabrachial nucleus (Quattrochi et al., 1998; Lima et al., 2019b), spinal trigeminal nucleus (Woolf and Butcher, 1989), and all three cerebellar nuclei (Woolf and Butcher, 1989; Ruggiero et al., 1997; Vitale et al., 2016).
Input to the PPTg comes from the NTS (Steininger et al., 1992), locus coeruleus (Steininger et al., 1992), dorsal and caudal raphe (Vertes, 1991; Semba and Fibiger, 1992; Steininger et al., 1992), lateral and paraventricular hypothalamus (Semba and Fibiger, 1992; Steininger et al., 1992; Zheng et al., 1998; Geerling et al., 2010), bed nucleus of the stria terminalis (Steininger et al., 1992), central amygdala (Semba and Fibiger, 1992), lateral parabrachial nucleus (Steininger et al., 1992), and cerebellar nuclei (Hazrati and Parent, 1992; Steininger et al., 1992).
Basal ganglia
Despite their widespread connectivity and importance for motor control (Roseberry et al., 2016; Lee et al., 2020; Quartarone et al., 2020), the basal ganglia are not typically considered to be part of the respiratory control network. Possibly the best-known consequence of basal gangliar dysfunction is Parkinson’s disease, caused by progressive degeneration of dopaminergic neurons in the substantia nigra (Kalia and Lang, 2015). Patients with Parkinson’s disease can be confronted with respiratory problems, but these may be attributed to degeneration of respiratory muscles, and therefore not directly to respiratory control (Guedes et al., 2012; Baille et al., 2018; Guilherme et al., 2021). Patients with Parkinson’s disease may also experience sleep-disturbed breathing or dyspnea (Docu Axelerad et al., 2021). With a prevalence of 12%, dyspnea is a relatively rare symptom of Parkinson’s disease (Barone et al., 2009), and its cause is unclear (Docu Axelerad et al., 2021). Overall, the basal ganglia, and the substantia nigra in particular, therefore, do not seem to play a major role in respiratory control.
Nevertheless, there are anatomical connections between the substantia nigra and the respiratory control centers, including a direct pathway to the pre-Bötzinger complex (Yang et al., 2020). In a rat model for Parkinson’s disease, modulation of the retrotrapezoid nucleus by the substantia nigra indirectly, via the periaqueductal gray, could contribute to respiratory control (Lima et al., 2018). Given the uncertainty of the impact of the basal ganglia on respiratory control, these outputs of the substantia nigra have been hypothesized to provide respiratory control centers with information on other ongoing movements, to facilitate respiratory control into the behavioral context of the animal (Yang et al., 2020). For this reason, we did not further consider the basal ganglia as control center for the subconscious regulation of respiration.
Cerebral cortex
Breathing is rather unique among the autonomic functions as it can be subjected to volitional control, modulated by the emotional state, adapted to other ongoing behaviors, and vital for vocalizations and human speech. It is quite likely that the cerebral cortex plays an important role in these functions (Herrero et al., 2018). Unlike lesions of the subcortical, and in particular of the medullar respiratory centers, lesions of cortical respiratory areas do not abolish respiration (Ramirez et al., 2011). The anatomical aspects of cortical modulation of respiration have received relatively little attention, and we consider the integration of the thalamocortical system into the anatomical scheme of Figure 7 an important future task.
Figure 7. Main pathways underlying subconscious control of respiration Schematic drawing representing the brain areas most involved in subconscious control of respiration and the main pathways connecting them.
Fat lines indicate relatively strong connections and thin lines moderately strong ones. Sparse connections are not included in this scheme. When an area is marked with ‘CO2’ it contains central chemoreceptor properties, while the carotid bodies sense blood levels of CO2 and of O2. The central pattern generators are indicated with a sine wave. BNST = bed nucleus of the stria terminalis; cVRG = caudal ventral respiratory group; LPBN = lateral parabrachial nucleus; MPBN = medial parabrachial nucleus; NTS = nucleus tractus solitarii; PiCo = postinspiratory complex; PPTg = peripeduncular tegmental nucleus; rVRG = rostral ventral respiratory group. See also Supplementary file 1.
There is early evidence for a direct projection from the cat motor cortex to the phrenic nucleus, bypassing the medullary respiratory circuitry (Rikard-Bell et al., 1985). In addition, a wide spectrum of projections from different cortical areas targets subcortical respiratory control areas, with the most numerous projections targeting the periaqueductal gray and the Kölliker-Fuse nucleus, and less abundant projections to the caudal raphe nucleus, Bötzinger and pre-Bötzinger complexes (Trevizan-Baú et al., 2021a). In this study, the Kölliker-Fuse nucleus was predominantly targeted from the (facial) somatosensory, endopiriform and rhinal cortices, and the periaqueductal gray from the insular, cingulate, motor, pre- and infralimbic cortices. In addition, the NTS receives a direct projection form the insular cortex (Torrealba and Müller, 1996; Gasparini et al., 2020).
In the context of the present review, especially the function of the insular cortex is relevant. It appears to be the prime cortical target area of visceral input, and it has a viscerotopic organization (Cechetto and Saper, 1987; Bagaev and Aleksandrov, 2006; Livneh and Andermann, 2021). EEG recordings of the insular cortex revealed coherence with breathing (Herrero et al., 2018). Depending on the location within the insular cortex, microstimulation could either increase or decrease the respiratory rate (Bagaev and Aleksandrov, 2006), suggesting that the insular cortex can affect respiration in response to vagal sensory input.
Cerebellum
The cerebellum is composed of a cortex surrounding the fastigial, interposed and dentate nuclei. The output of the cerebellar cortex is formed by Purkinje cells that predominantly target the cerebellar nuclei (Voogd and Glickstein, 1998), although direct connections to the vestibular nuclei, locus coeruleus, and lateral and medial parabrachial nuclei exist as well (Zeeuw and Berrebi, 1995; Sadakane et al., 2000; Sugihara et al., 2009; Schwarz et al., 2015; Hashimoto et al., 2018; Novello et al., 2022). From the cerebellar nuclei, there are projections back to the cerebellar cortex (Tolbert et al., 1977; Gao et al., 2016), inhibitory projections to the inferior olive (Voogd and Glickstein, 1998), and excitatory projections to the MDJ (De Zeeuw and Ruigrok, 1994; Wang et al., 2022) and many brain regions relevant for respiratory control.
Strikingly, there are no direct connections to the phrenic nucleus (Lois et al., 2009), nor to the pre-Bötzinger complex (Yang et al., 2020). Instead, diaphragmatic activity can be affected by a direct projection to the premotor rVRG (Gaytán and Pásaro, 1998). Although there are direct projections from the cerebellum to the trigeminal motor nucleus, affecting upper airway muscles (Judd et al., 2021), facial nucleus (Moolenaar and Rucker, 1976; Fujita et al., 2020), and hypoglossal nucleus (Guo et al., 2020; Judd et al., 2021), these direct projections to motor nuclei are typically relatively sparse so that indirect projections, for example, via the reticular formation, are probably much more abundant (Novello et al., 2022).
Other cerebellar projections that can be relevant for respiratory control target the Kölliker-Fuse nucleus (Fujita et al., 2020), locus coeruleus (Teune et al., 2000; Schwarz et al., 2015), dorsal raphe nuclei (Teune et al., 2000; Çavdar et al., 2018b), periaqueductal gray (Teune et al., 2000; Frontera et al., 2020; Fujita et al., 2020; Judd et al., 2021), lateral and paraventricular hypothalamus (Dietrichs et al., 1994; Zhu et al., 2006). Many of these areas, in particular the Kölliker-Fuse nucleus, have profound projections to medullary respiratory circuit, so that these projections can explain how the cerebellum can affect respiratory control.
Despite the absence of strong pathways from the cerebellum to the respiratory pattern generators and motor nuclei, cerebellar activity can profoundly modulate respiration. Functional brain imaging of healthy human subjects revealed that the cerebellum is particularly active during coping with respiratory challenges: broad activation during hypoxia and slow breathing (Critchley et al., 2015), and specific activation in lobules V and VI during hypercapnia (Colebatch et al., 1991; Kastrup et al., 1999). Also the cerebellar nuclei are active during hypercapnia and hypoxia (McKay et al., 2010), as well as during volitional expiration (Ramsay et al., 1993). Changes in environmental air pressure can trigger cerebellar activity as well (Isaev et al., 2002; Raux et al., 2013; Figure 6G–J).
The notion that the cerebellum is particularly relevant for deviating from rhythmic breathing is corroborated by the finding that mice suffering from a complete lack of output from the cerebellar cortex or from a partial loss of cerebellar output neurons display overly regular breathing at rest (Liu et al., 2020; Taylor et al., 2022; Figure 6F). Stimulation of the fastigial nucleus in cats can affect phrenic nerve activity, and in particular terminate expiratory activity when stimulated during expiration (Zhang et al., 1999), analogous to optogenetic stimulation experiments of Purkinje cells (Romano et al., 2020; Figure 6E). And indeed, Lurcher mice that suffer from a complete loss of Purkinje cells during their development, demonstrated impaired responses to hypercapnic and hypoxic challenges (Calton et al., 2014; Calton et al., 2016). Neuronal activity of the cerebellar cortex and the fastigial nucleus is linked to respiration (Lutherer et al., 1989; Gruart and Delgado-García, 1992; Cao et al., 2012b; Lu et al., 2013; Romano et al., 2020; Figure 6C–D). At rest, modulation of the activity of Purkinje cell occurs without phase lead or lag relative to the respiratory cycle (Romano et al., 2020), and one could speculate that the cerebellum does not only act to adapt respiration to ongoing behavior, but also facilitates adaptation of other behavior to respiration, in line with observations in cerebellar patients (Ebert et al., 1995).
The cerebellum may, in addition, have a particular role in the regulation of breathing during sleep, which includes different stages with different respiratory and oculomotor dynamics (Canto et al., 2017; De Zeeuw and Canto, 2022; Pujol et al., 2022). Indeed, when shifting from awake to subconscious behavior and vice-versa, different brain structures such as cerebellum, hippocampus and amygdala, are dynamically activated and de-activated in line with changes in breathing (Pujol et al., 2022). Accordingly, the impact of sleep on breathing and its role in the development of diurnal respiratory failure in patients suffering from hypoventilation and/or cerebellar disorders can be overlooked (Piper and Yee, 2014; Canto et al., 2017). It remains to be elucidated which sets of mechanisms are involved in this process, but it should be noted that sleep by itself does not only reduce respiratory drive, but also diminishes responsiveness to hypoxia and hypercapnia. For example, acute increases in CO2 during rapid eye movement sleep can initiate the process of bicarbonate retention, which further depresses ventilatory responsiveness (Piper and Yee, 2014) and which in turn can affect activity in the cerebellar nuclei (Parsons et al., 2001; Martino et al., 2007).
Mossy fiber inputs
Mossy fibers constitute one of the two main glutamatergic input pathways to the cerebellum. They carry ascending as well as descending information to granule cells in the cerebellar cortex, while often forming collaterals to the cerebellar nuclei (van der Want et al., 1987; Huang et al., 2013; Ruigrok et al., 2015). Cerebellar granule cells make up about halve of all neurons in the brain, and their bifurcating axons, the parallel fibers, target Purkinje cells directly, and indirectly via inhibitory interneurons (Harvey and Napper, 1991; De Zeeuw et al., 2011; Ruigrok et al., 2015).
The pontine nuclei, consisting of the basal pons and the nucleus reticularis tegmenti pontis, are the prime source of mossy fibers; not only are they the main intermediate for descending pathways from the cerebral cortex, they also receive subcortical input from among others the lateral hypothalamus, central amygdala, periaqueductal gray, spinal trigeminal nucleus, PPTg, MDJ, and cerebellar nuclei (Mihailoff et al., 1989; Brodal and Bjaalie, 1992; Liu and Mihailoff, 1999; Pijpers and Ruigrok, 2006; Bosman et al., 2011; Fu et al., 2011; Huang et al., 2013; Ruigrok et al., 2015; Henschke and Pakan, 2020).
Other important mossy fiber sources are the two vagal sensory nuclei relating respiratory visceral input to the brain, the NTS (Batini et al., 1978; Somana and Walberg, 1979a; Saigal et al., 1980a; Fu et al., 2011) and paratrigeminal nucleus (Somana and Walberg, 1979b). Strong mossy fiber connections bind also the spinal trigeminal nucleus, medial parabrachial nucleus, and several parts of the medullary reticular formation with the cerebellum (Ruigrok et al., 1995; Yatim et al., 1996; Fu et al., 2011). This is also true for multiple spinal regions (Sengul et al., 2015; Baek et al., 2019). Weaker projections come from the lateral parabrachial nucleus (Fu et al., 2011), Kölliker-Fuse nucleus (Fu et al., 2011), and rVRG (Gaytán and Pásaro, 1998). The pre-Bötzinger complex provides only sparse projections to the cerebellum (Yang and Feldman, 2018).
Climbing fiber inputs
The other main input to the cerebellum is formed by the climbing fibers that originate exclusively from the contralateral inferior olive and that form extraordinarily strong glutamatergic synapses on Purkinje cells, next to relatively weak collateral projections to the cerebellar nuclei (Szentágothai and Rajkovits, 1959; Ruigrok et al., 2015; Lu et al., 2016). Where each adult Purkinje cell can receive input from over 100,000 parallel fibers, it is typically innervated by only a single climbing fiber (Harvey and Napper, 1991; Bosman and Konnerth, 2009). Climbing fiber spikes invariably trigger complex spike firing by the postsynaptic Purkinje cells, and these consist of normal action potentials in conjunction with dendritic spikelets caused by profound influx of calcium (Llinás and Sugimori, 1980; De Zeeuw et al., 2011). Because of their impact on intracellular calcium levels, complex spikes affect the synaptic strength of parallel fiber inputs, and thereby control cerebellar learning (Ito, 2000; Coesmans et al., 2004; Ohtsuki et al., 2009; van Woerden et al., 2009; Gao et al., 2012; Yang and Lisberger, 2014; Romano et al., 2018). Accordingly, climbing fiber activity is strictly regulated, occurs at a sustained, but low rate, and can thereby serve a homeostatic function at rest, while reporting salient events when they occur (Zhou et al., 2014; Ju et al., 2019; Negrello et al., 2019; Bina et al., 2021). In general, the complex spikes therefore modulate the activity pattern of simple spikes, that organize motor output.
Apart from the cerebellum, the most important input areas to the inferior olive are the spinal cord (Brown et al., 1977), periaqueductal gray (Brown et al., 1977; Swenson and Castro, 1983b; VanderHorst et al., 2000), spinal trigeminal nucleus (Swenson and Castro, 1983b; Huerta et al., 1985; Van Ham and Yeo, 1992; Yatim et al., 1996), and MDJ (de Zeeuw et al., 1989; Wang et al., 2022). The MDJ is the main intermediate between the cerebral cortex and the inferior olive: it relays input from the rostromedial and caudal parts of the cerebral cortex to the principal olive, and from the rostrolateral parts of the cerebral cortex to the medial accessory olive (Wang et al., 2022). In addition, the MDJ receives input from the spinal trigeminal nucleus (Kubo et al., 2018) and the cerebellar nuclei (De Zeeuw and Ruigrok, 1994; Wang et al., 2022). Input to the inferior olive comes also from the two vagal sensory recipient nuclei: NTS (Loewy and Burton, 1978; McGovern et al., 2015b) and paratrigeminal nucleus (McGovern et al., 2015b), as well as from rVRG (Swenson and Castro, 1983b).
Other cerebellar inputs
Other cerebellar afferents could potentially contribute to respiratory control, although their precise impact has not yet been studied. Hypothalamocerebellar connections are evolutionary preserved, but more prominent in primates than in rodents (Dietrichs, 1984; Dietrichs et al., 1994). They originate from several hypothalamic nuclei. In the context of this review, orexinergic fibers from the lateral hypothalamus to the cerebellar nuclei seem to be the most relevant, possibly in conjunction with a projection from the paraventricular nucleus (Dietrichs, 1984; Haines et al., 1997; Zhu et al., 2006; Yu et al., 2010; Çavdar et al., 2018a).
The locus coeruleus provides widespread noradrenergic innervation of the cerebellar cortex and nuclei (Olson and Fuxe, 1971; Saigal et al., 1980a; Loughlin et al., 1986; Dietrichs, 1988; Szabadi, 2013), which could contribute to a general modulation of cerebellar output (Parfitt et al., 1988; Di Mauro et al., 2013; Lippiello et al., 2015). Also both the dorsal and caudal raphe nuclei project to the cerebellar cortex and nuclei, providing serotonergic input (Shinnar et al., 1975; Pierce et al., 1977; Bishop and Ho, 1985; Fu et al., 2011), which can have profound impact on cerebellar function (Kawashima, 2018). In mild forms of cerebellar ataxia, application of a serotonin receptor agonist could alleviate various motor symptoms (Takei et al., 2005). These, and the other anatomical projections, are summarized in Figure 7.
Cerebellar pathology
Although the role of the cerebellum in respiratory control is not yet settled in clinical settings, several lines of evidence indicate a specific role in respiratory control. For instance, in some developmental disorders, cerebellar abnormalities and respiratory dysfunction co-occur. Arguably, the most explicit example is sudden infant death syndrome (SIDS), the unexplained abrupt death of infants, typically during sleep, and most likely related to respiratory problems (Kelly et al., 1986; Kinney and Thach, 2009). Several studies link SIDS to cerebellar malformations. For instance, post-mortem analyses relate SIDS to the presence of a wide external granular layer, which is a sign of developmental delay of the cerebellum (Cruz-Sánchez et al., 1997; Lavezzi et al., 2007a). Also malformed and displaced Purkinje cells were observed in several cases (Lavezzi et al., 2013; Matschke et al., 2020). Additionally, the dentate-olivary system can be affected in SIDS (Lavezzi et al., 2007b). Note that malformations of the cerebellum and related structures have been demonstrated only in a subset of cases, as SIDS can have multiple causes (Kinney and Thach, 2009; Figure 8B).
Figure 8. Pathology.
(A) Based on the location of a lesion or structural abnormality, several types of disordered breathing can be expected. Cheyne-Stokes respiration can result from a bilateral hemispheric or diencephalic lesion. Apneustic breathing has originally been described as the consequence of a lesion at the level of the Kölliker-Fuse nucleus, but can probably also be caused by damage to surrounding tissue. A Biot’s respiration pattern can develop after a lesion in the medulla, while a respiratory arrest occurs after damage to the pre-Bötzinger complex or the upper spinal cord. Obstructive sleep apnea typically shows interrupted breathing pattern during sleep. Cerebellar dysfunction can lead to more (left) or less (right) rhythmic respiration. COPD patients show increased respiratory rates and reduced passive expiration. It should be noted however that cardiopulmonary or metabolic causes can also trigger all these respiratory patterns. Schematized traces of the tidal volume based on: Cheyne-Stokes respiration: Lorenzi-Filho et al., 1999; Apneusis: Mador and Tobin, 1990; Biot’s respiration: Farney et al., 2003: Abnormal rhythmicity: Ebert et al., 1995; COPD (and normal): Jolley et al., 2009. (B) Respiration-related brain areas demonstrating structural damage in several disorders, projected in the same notation as in Figure 2A. Note that SCA6 is mainly a cerebellar disorder. While CANVAS affects the cerebellum, the syndrome has also peripheral nerve involvement. In SIDS multiple non-cerebellar causes have been described in post mortem studies. Some post mortem brain studies, however, have shown irregularly formed Purkinje cells in the cerebellar cortex. See Supplementary file 1. (C) Apneustic breathing pattern in a mouse model of spinocerebellar ataxia type 7. Schematic representation based on Fusco et al., 2021. (D) Electrical stimulation (Stim) adjacent to the fastigial nucleus induces a fastigial pressor response in an anesthetized cat. During stimulation, the tidal volume is increased, and suppressed afterwards. Based on Miura and Takayama, 1988.
Nevertheless, respiratory deficiencies and prolonged dependency on assisted or even mechanical ventilation are frequently observed after cerebellar damage (Chen et al., 2005; Tsitsopoulos et al., 2012; Lee et al., 2013; Arnone et al., 2017). Relatively mild aberrations in respiratory functions were also noted in different types of spinocerebellar ataxia (Sriranjini et al., 2010), while in a mouse model for spinocerebellar ataxia type 7 (SCA7) abnormal respiratory patterns were recorded (Figure 8B–C). Distinct types of spinocerebellar ataxia can affect various brain regions relevant for subconscious control of respiration (Figure 8B, Supplementary file 1). Finally, chronic cough may be one of the earliest symptoms of cerebellar ataxia with neuropathy and vestibular areflexia syndrome (CANVAS), and manifests in the absence of any perceived lung abnormalities (Infante et al., 2018).
Disordered breathing
Lesions, e.g., as a consequence of hemorrhage, at different levels of the brain can result in different patterns of disordered breathing (Figure 8A).
A bilateral hemispheric or diencephalic lesion can lead to Cheyne-Stokes respiration (Brown and Plum, 1961). This is characterized by an undulating pattern of breathing with increasing depth and frequency alternated with waves of shallow, slower breathing (Lorenzi-Filho et al., 1999).
Apneustic breathing, characterized by the absence or extreme delay of the inspiration to expiration switch, has originally been described as the consequence of a lesion at the level of the Kölliker-Fuse nucleus or the surrounding area (Marckwald, 1890; Lumsden, 1923; Caille et al., 1981; Mador and Tobin, 1990).
Biot’s respiration is characterized by irregular periods of apnea alternated by several breaths of identical depth (Farney et al., 2003; Wijdicks, 2007). Although, the irregularity of this breathing pattern has led to the more common term ‘ataxic breathing’, the cerebellum is not primarily involved in this type of respiration (Summ et al., 2022). However, Biot’s respiration pattern can result after a lesion in the medulla (Summ et al., 2022). Biot’s respiration can also be a complication of long-term opioid use (Farney et al., 2003).
Finally, a respiratory arrest can result after damage to the pre-Bötzinger complex or the upper spinal cord (Ramirez et al., 1998; Schwarzacher et al., 2011; Arora et al., 2012).
Animal experiments have demonstrated that a lack of cerebellar output can lead to hyperregular respiration (Liu et al., 2020; Taylor et al., 2022). Cerebellar patients, however, can also display the opposite and present with irregular respiration (Ebert et al., 1995). Both, hyperregular as well as irregular breathing, can be the consequence of impaired coordination of respiration with ongoing behavior, as described for instance by Ebert et al., 1995.
While each of these brain areas result in a specific type of respiratory pattern. It must be noted that a specific respiratory pattern can have multiple causes such as metabolic or cardiopulmonary disorders. In addition, also peripheral diseases, like COPD (Jolley et al., 2009), can cause disordered breathing. COPD is a chronic obstructive pulmonary condition characterized by abnormalities, especially narrowing of the small airways, of the lung which leads to limitation of the airflow.
Coordination of different behaviors
Throughout this review, we have discussed several behaviors that are tightly coupled to respiration, such as those involved in airway clearance, feeding, and vocalization, as well as adjusting the rate of ventilation to metabolic demands. In addition, also postural control and cardiovascular function are linked to respiration. All pump muscles have dual functions; they do not only enable ventilation but control also posture and movements (Ebert et al., 2000). The coordination between these different tasks can be affected in cerebellar patients, displaying regular breathing at rest, but arhythmic breathing during arm movements (Ebert et al., 1995).
Brain rhythms
Global and local rhythms abound in the brain, and they have been proposed to arrange long-range synchrony and functional coupling, and thus play a vital role in motor control, sensory perception, and conscious processing (Llinás, 1988; Khoshyomn et al., 1999; Mantini et al., 2007; Fries, 2015; Lindeman et al., 2021). There is accumulating evidence that the respiratory rhythm can affect these brain rhythms.
In the olfactory system, a coupling between respiration and rhythmic activity was noticed already a long time ago (Adrian, 1942; Fontanini et al., 2003). As odorants are carried by air, each sniff brings in new information, and can be considered as the temporal unit of olfaction (Kepecs et al., 2006; Verhagen et al., 2007). Sniffing can, however, be more widely seen as patterning sensory processing, as it, for instance, can dynamically synchronize with whisking (Welker, 1964; Cao et al., 2012a; Moore et al., 2013). A hint that respiration is even more related to brain function comes from the observation that humans tend to inhale at the start of a cognitive test, even if that does not involve olfaction (Perl et al., 2019). This goes so far that a change in performance on a visuospatial task varies between inspiration and expiration was observed in line with alteration in the EEG spectrum (Perl et al., 2019). In fact, respiration-induced patterns in neural oscillations are ubiquitously observed throughout the brain (Ito et al., 2014; Yanovsky et al., 2014; Heck et al., 2016; Zelano et al., 2016; Tort et al., 2018; Kluger and Gross, 2021).
Changes in brain oscillations are by no means restricted to the frequency domain of respiration or sniffing. Gamma oscillations (30–100 Hz), which could reflect sensorimotor integration (Zagha et al., 2013; Lindeman et al., 2021), can show changes in power that are phase-locked to the respiratory rhythm (Ito et al., 2014; Kluger and Gross, 2021). Respiration can, therefore, serve as a scaffold for sensory, sensorimotor and cognitive functions.
Cardiorespiratory function
Ventilation is only one aspect of the ultimate goal of respiration: getting oxygen to mitochondria throughout the body, while maintaining balanced concentrations of O2 and CO2 in the blood. Hence, respiration has to be coordinated with cardiac and vascular regulation, a process collectively known as cardiorespiratory function. Ineffective cardiorespiratory function is an important predictor for development of averse cardiovascular events and mortality, and has a higher predictive value than for example, smoking or hypertension (Lee et al., 2010; Ross et al., 2016).
An example of the integrated control of cardiovascular and respiratory function is the fastigial pressor response that entails simultaneous increases in heart rate and arterial pressure with changes in ventilation (Miura and Reis, 1969; Achari and Downman, 1970; Lutherer and Williams, 1986; Bradley et al., 1987; Xu and Frazier, 2000; Hernandez et al., 2004; Nisimaru, 2004). The specificity of the fastigial nucleus for triggering the fastigial pressor response has been called into question, arguing that it was actually caused by activation of the passing afferents to the lateral parabrachial nucleus (Miura and Takayama, 1988). Yet, lesioning of the fastigial nuclei does lead to impairment of the fastigial pressor response (Zhuang et al., 2008; Figure 8D).
Cardiac and respiratory regulation are coupled on a cycle-by-cycle basis, as the heart rate increases during inspiration, and decreases again during post-inspiration and active expiration (Hirsch and Bishop, 1981; Elstad et al., 2018). Respiratory sinus arrhythmia is caused by respiration-related fluctuations in activity of inhibitory preganglionic parasympathetic cardiac vagal neurons that are primarily located in the nucleus ambiguus (McAllen and Spyer, 1976; Neff et al., 2003; Dergacheva et al., 2010). The Kölliker-Fuse nucleus provides the phasic excitatory drive to these cardiac premotor neurons that is required for respiratory sinus arrhythmia (Farmer et al., 2016).
Stimulation of pro-opiomelanocortin-producing neurons in the NTS can augment respiratory sinus arrhythmia, along with triggering suppressed breathing (bradypnea) and cardiac function (bradycardia) (Cerritelli et al., 2016). Pro-opiomelanocortin is a precursor of the opioid ß-endorphin, and its impact of cardiovascular function can be mimicked by administered opioids (Walker et al., 2007; Izrailtyan et al., 2018). The pro-opiomelanocortin neurons of the NTS directly target important respiratory centers as the pre-Bötzinger complex, giving rise to bradypnea, the ambiguus nucleus, giving rise to bradycardia, and the hypoglossal nucleus, rVRG, hypoglossal nucleus, and raphe obscurus nucleus (Cerritelli et al., 2016; Figure 5E).
Ideas and speculations
It is an attractive idea to have one or a few central pattern generator areas that control the respiratory rhythm (Moore et al., 2013; Anderson and Ramirez, 2017). In particular, the pre-Bötzinger complex is essential in this respect, as inspiration depends on it (Smith et al., 1991; Ramirez et al., 1998; Gray et al., 2001; Tan et al., 2008; Schwarzacher et al., 2011; Ashhad and Feldman, 2020; Dhingra et al., 2020). In this view, projections organized in a hierarchical fashion pass the respiratory control from the pattern generator(s) to the respiratory motor neurons. Throughout this review we summarize, however, that many other brain regions, including areas like the cerebellum and the limbic system that are not classically considered to be respiratory areas, can affect respiratory control (Figure 8). As all these areas are interconnected (Figure 7), the picture of an integrated network emerges. Thus, depending on specific behavioral needs, these other brain regions may modulate or even overrule the central pattern generators. This does not contradict the idea of central pattern generators being the main players that control rhythmic respiration, but postulates that these pattern generators together with their downstream areas are themselves embedded in a larger network that allows for flexibility within the respiratory control system. We therefore suggest to view the respiratory control system primarily as an integrated network, rather than a hierarchical system. Consequently, we believe that integration of behavioral conditions will add to the pathogenesis of respiratory disease.
Acknowledgements
The authors wish to thank Dr. Tom Ruigrok, Dr. Jos van der Geest and Ms. Liska Scheffers for discussions at the start of this project, and Dr. Xiaolu Wang for adapting Figure 2B–E. Financial support was provided by the Stichting Coolsingel (Grant no. 514 [RSvdG]), the Netherlands Organization for Scientific Research (NWOALW; CIDZ), the Dutch Organization for Medical Sciences (ZonMW; CIDZ; JJMP), BIG (CIDZ), Medical Neuro-Delta (CIDZ), INTENSE LSH-NWO (CIDZ), ERC-adv and ERC-POC (CIDZ), Van Raamsdonk-fonds (CIDZ), 3V-Fonds KNAW (CIDZ), Albinism Fonds NIN (CIDZ), Stichting Lijf en Leven (JJMP), and by Health Holland to promote public private partnerships (TKI-LSH EMCLSH21017 [LWJB]).
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Chris I De Zeeuw, Email: c.dezeeuw@erasmusmc.nl.
Johan JM Pel, Email: j.pel@erasmusmc.nl.
Laurens WJ Bosman, Email: l.bosman@erasmusmc.nl.
Jeannie Chin, Baylor College of Medicine, United States.
Jeannie Chin, Baylor College of Medicine, United States.
Funding Information
This paper was supported by the following grants:
Stichting Coolsingel 514 to Ruben S van der Giessen.
Nederlandse Organisatie voor Wetenschappelijk Onderzoek ALW to Chris I De Zeeuw.
ZonMw to Chris I De Zeeuw.
B.I.G. to Chris I De Zeeuw.
Medical Neuro-Delta to Chris I De Zeeuw.
INTENSE LSH-NWO to Chris I De Zeeuw.
European Research Council Advanced grant to Chris I De Zeeuw.
European Research Council POC to Chris I De Zeeuw.
Van Raamsdonk Fonds to Chris I De Zeeuw.
Koninklijke Nederlandse Akademie van Wetenschappen 3V Fonds to Chris I De Zeeuw.
Nederlands Herseninstituut Albinism Fonds to Chris I De Zeeuw.
Stichting Lijf en Leven to Johan JM Pel.
Health-Holland TKI-LSH EMCLSH21017 to Laurens WJ Bosman.
Additional information
Competing interests
No competing interests declared.
No competing interests declared.
Author contributions
Conceptualization, Investigation, Visualization, Writing – original draft, Writing – review and editing.
Investigation, Visualization, Writing – original draft, Writing – review and editing.
Conceptualization, Funding acquisition, Investigation, Visualization, Writing – original draft, Writing – review and editing.
Funding acquisition, Writing – original draft, Writing – review and editing.
Conceptualization, Funding acquisition, Investigation, Visualization, Writing – original draft, Project administration, Writing – review and editing.
Conceptualization, Supervision, Funding acquisition, Investigation, Visualization, Methodology, Writing – original draft, Project administration, Writing – review and editing.
Additional files
Connections that were identified as sparse by the authors of these studies are not listed in this table. Note that the list of neurotransmitters involved is not complete. 1 Tree shrew (Tupaia belangeri chinensis); 2 Rufous horseshoe bat (Rhinolophus rouxi); 3 Rhesus monkey (Macaca mulatta); 4 Crab-eating macaque (Macaca fascicularis); 5 Squirrel monkey (Saimiri sciureus). 5-HT = serotonin, A = anterograde, aa = amino acids, AAV = adeno associated virus, ACh = acetyl choline, BDA = biotinylated dextran amines, Biotinam = biotinamide, CRH = corticotropin-releasing hormone, CTb = cholera toxin B subunit, cVRG = caudal part of the ventral respiratory group, DA = dopamine, Degen = degeneration, DTR = dextran-Texas red, DY = diamidino yellow, EB = Evans blue, Exc = excitatory (not specified which neurotransmitter), E-phys = electrophysiology, FB = fast blue, FG = fluorogold, FR = fluoro ruby, GABA = γ-aminobutyric acid, GFP = green fluorescent protein, Glu = glutamate, Gly = glycine, HSV = herpes simplex virus, HRP = horseradish peroxidase, Inh = inhibitory (not specified which neurotransmitter), m = muscle, MDJ = nuclei of the meso-diencephalic junction, n = nucleus or nuclei, NA = noradrenaline / norepinephrine, Nbiotin = neurobiotin, NMB = neuromedin B, NT = neurotransmitter, NTS = nucleus tractus solitarii, Optogen = optogenetic stimulation, OXT = oxytocin, PHA-L = Phaseolus vulgaris leucoagglutinin, PiCo = post-inspiration complex, PPTg = pedunculopontine tegmental nucleus, PI = propidium iodide, POMC = pro-opiomelanocortin, Pseudorab = Pseudorabies, R = retrograde, rVRG = rostral part of the ventral respiratory group, term = terminalis, TMR = tetramethylrhodamine, VP = vasopressin, WGA = wheat germ agglutinin. b – Respiratory control areas affected by pathology Summary of brain regions involved in respiratory control and affected by selected diseases or syndromes. An area is listed in this table if a study reported damage to that area in a majority of subjects included in that study. Case studies with a single patient are not included in this table.
References
- Abbott SBG, Stornetta RL, Fortuna MG, Depuy SD, West GH, Harris TE, Guyenet PG. Photostimulation of retrotrapezoid nucleus phox2b-expressing neurons in vivo produces long-lasting activation of breathing in rats. The Journal of Neuroscience. 2009;29:5806–5819. doi: 10.1523/JNEUROSCI.1106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott SBG, Stornetta RL, Coates MB, Guyenet PG. Phox2b-expressing neurons of the parafacial region regulate breathing rate, inspiration, and expiration in conscious rats. The Journal of Neuroscience. 2011;31:16410–16422. doi: 10.1523/JNEUROSCI.3280-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott SBG, Souza G. Chemoreceptor mechanisms regulating CO2-induced arousal from sleep. The Journal of Physiology. 2021;599:2559–2571. doi: 10.1113/JP281305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achari NK, Downman CBB. Autonomic effector responses to stimulation of nucleus fastigius. The Journal of Physiology. 1970;210:637–650. doi: 10.1113/jphysiol.1970.sp009232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian ED. Olfactory reactions in the brain of the hedgehog. The Journal of Physiology. 1942;100:459–473. doi: 10.1113/jphysiol.1942.sp003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albaiceta GM, Luyando LH, Parra D, Menendez R, Calvo J, Pedreira PR, Taboada F. Inspiratory vs. expiratory pressure-volume curves to set end-expiratory pressure in acute lung injury. Intensive Care Medicine. 2005;31:1370–1378. doi: 10.1007/s00134-005-2746-6. [DOI] [PubMed] [Google Scholar]
- Algera MH, Kamp J, van der Schrier R, van Velzen M, Niesters M, Aarts L, Dahan A, Olofsen E. Opioid-induced respiratory depression in humans: a review of pharmacokinetic-pharmacodynamic modelling of reversal. British Journal of Anaesthesia. 2019;122:e168–e179. doi: 10.1016/j.bja.2018.12.023. [DOI] [PubMed] [Google Scholar]
- Amis TC, Brancatisano A, Tully A. Thyroid cartilage movements during breathing. Journal of Applied Physiology. 1995;78:441–448. doi: 10.1152/jappl.1995.78.2.441. [DOI] [PubMed] [Google Scholar]
- Anderson TM, Garcia AJ, Baertsch NA, Pollak J, Bloom JC, Wei AD, Rai KG, Ramirez JM. A novel excitatory network for the control of breathing. Nature. 2016;536:76–80. doi: 10.1038/nature18944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson TM, Ramirez JM. Respiratory rhythm generation: triple oscillator hypothesis. F1000Research. 2017;6:139. doi: 10.12688/f1000research.10193.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando A, Smallwood D, McMahon M, Irving L, Mazzone SB, Farrell MJ. Neural correlates of cough hypersensitivity in humans: Evidence for central sensitisation and dysfunctional inhibitory control. Thorax. 2016;71:323–329. doi: 10.1136/thoraxjnl-2015-207425. [DOI] [PubMed] [Google Scholar]
- Anton F, Peppel P. Central projections of trigeminal primary afferents innervating the nasal mucosa: A horseradish peroxidase study in the rat. Neuroscience. 1991;41:617–628. doi: 10.1016/0306-4522(91)90354-q. [DOI] [PubMed] [Google Scholar]
- Applegate CD, Kapp BS, Underwood MD, McNall CL. Autonomic and somatomotor effects of amygdala central N. stimulation in awake rabbits. Physiology & Behavior. 1983;31:353–360. doi: 10.1016/0031-9384(83)90201-9. [DOI] [PubMed] [Google Scholar]
- Arch JJ, Craske MG. Mechanisms of mindfulness: Emotion regulation following a focused breathing induction. Behaviour Research and Therapy. 2006;44:1849–1858. doi: 10.1016/j.brat.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Arima Y, Yokota S, Fujitani M. Lateral parabrachial neurons innervate orexin neurons projecting to brainstem arousal areas in the rat. Scientific Reports. 2019;9:2830. doi: 10.1038/s41598-019-39063-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita H, Kogo N, Koshiya N. Morphological and physiological properties of caudal medullary expiratory neurons of the cat. Brain Research. 1987;401:258–266. doi: 10.1016/0006-8993(87)91410-7. [DOI] [PubMed] [Google Scholar]
- Arnone GD, Esfahani DR, Wonais M, Kumar P, Scheer JK, Alaraj A, Amin-Hanjani S, Charbel FT, Mehta AI. Surgery for cerebellar hemorrhage: a national surgical quality improvement program database analysis of patient outcomes and factors associated with 30-day mortality and prolonged ventilation. World Neurosurgery. 2017;106:543–550. doi: 10.1016/j.wneu.2017.07.041. [DOI] [PubMed] [Google Scholar]
- Arora S, Flower O, Murray NPS, Lee BB. Respiratory care of patients with cervical spinal cord injury: a review. Critical Care and Resuscitation. 2012;14:64–73. [PubMed] [Google Scholar]
- Ashhad S, Feldman JL. Emergent elements of inspiratory rhythmogenesis: network synchronization and synchrony propagation. Neuron. 2020;106:482–497. doi: 10.1016/j.neuron.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashhad S, Kam K, Del Negro CA, Feldman JL. Breathing rhythm and pattern and their influence on emotion. Annual Review of Neuroscience. 2022;45:223–247. doi: 10.1146/annurev-neuro-090121-014424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausborn J, Koizumi H, Barnett WH, John TT, Zhang R, Molkov YI, Smith JC, Rybak IA. Organization of the core respiratory network: insights from optogenetic and modeling studies. PLOS Computational Biology. 2018;14:e1006148. doi: 10.1371/journal.pcbi.1006148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraam J, Wu Y, Richerson GB. Perinatal nicotine reduces chemosensitivity of medullary 5-HT neurons after maturation in culture. Neuroscience. 2020;446:80–93. doi: 10.1016/j.neuroscience.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman SB, Anders C, Ballantyne D, Röhrig N, Camerer H, Mifflin S, Jordan D, Dickhaus H, Spyer KM, Richter DW. Evidence for a monosynaptic connection between slowly adapting pulmonary stretch receptor afferents and inspiratory beta neurones. Pflugers Archiv. 1984;402:129–136. doi: 10.1007/BF00583324. [DOI] [PubMed] [Google Scholar]
- Baek M, Menon V, Jessell TM, Hantman AW, Dasen JS. Molecular logic of spinocerebellar tract neuron diversity and connectivity. Cell Reports. 2019;27:2620–2635. doi: 10.1016/j.celrep.2019.04.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baertsch NA, Baertsch HC, Ramirez JM. The interdependence of excitation and inhibition for the control of dynamic breathing rhythms. Nature Communications. 2018;9:843. doi: 10.1038/s41467-018-03223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baertsch NA, Severs LJ, Anderson TM, Ramirez JM. A spatially dynamic network underlies the generation of inspiratory behaviors. PNAS. 2019;116:7493–7502. doi: 10.1073/pnas.1900523116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagaev V, Aleksandrov V. Visceral-related area in the rat insular cortex. Autonomic Neuroscience. 2006;125:16–21. doi: 10.1016/j.autneu.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Baille G, Perez T, Devos D, Deken V, Defebvre L, Moreau C. Early occurrence of inspiratory muscle weakness in parkinson’s disease. PLOS ONE. 2018;13:e0190400. doi: 10.1371/journal.pone.0190400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandler R, Keay KA, Floyd N, Price J. Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Research Bulletin. 2000;53:95–104. doi: 10.1016/s0361-9230(00)00313-0. [DOI] [PubMed] [Google Scholar]
- Barbier M, González JA, Houdayer C, Burdakov D, Risold P-Y, Croizier S. Projections from the dorsomedial division of the bed nucleus of the stria terminalis to hypothalamic nuclei in the mouse. The Journal of Comparative Neurology. 2021;529:929–956. doi: 10.1002/cne.24988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP, Bottacchi E, Cannas A, Ceravolo G, Ceravolo R, Cicarelli G, Gaglio RM, Giglia RM, Iemolo F, Manfredi M, Meco G, Nicoletti A, Pederzoli M, Petrone A, Pisani A, Pontieri FE, Quatrale R, Ramat S, Scala R, Volpe G, Zappulla S, Bentivoglio AR, Stocchi F, Trianni G, Dotto PD, PRIAMO study group The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Movement Disorders. 2009;24:1641–1649. doi: 10.1002/mds.22643. [DOI] [PubMed] [Google Scholar]
- Bartlett D, Leiter JC. Coordination of breathing with nonrespiratory activities. Comprehensive Physiology. 2012;2:1387–1415. doi: 10.1002/cphy.c110004. [DOI] [PubMed] [Google Scholar]
- Bassal M, Bianchi AL. Inspiratory onset or termination induced by electrical stimulation of the brain. Respiration Physiology. 1982;50:23–40. doi: 10.1016/0034-5687(82)90004-4. [DOI] [PubMed] [Google Scholar]
- Bassetti CLA, Adamantidis A, Burdakov D, Han F, Gay S, Kallweit U, Khatami R, Koning F, Kornum BR, Lammers GJ, Liblau RS, Luppi PH, Mayer G, Pollmächer T, Sakurai T, Sallusto F, Scammell TE, Tafti M, Dauvilliers Y. Narcolepsy-clinical spectrum, aetiopathophysiology, diagnosis and treatment. Nature Reviews. Neurology. 2019;15:519–539. doi: 10.1038/s41582-019-0226-9. [DOI] [PubMed] [Google Scholar]
- Bassi M, Furuya WI, Zoccal DB, Menani JV, Colombari DSA, Mulkey DK, Colombari E. Facilitation of breathing by leptin effects in the central nervous system. The Journal of Physiology. 2016;594:1617–1625. doi: 10.1113/JP270308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batini C, Buisseret-Delmas C, Corvisier J, Hardy O, Jassik-Gerschenfeld D. Brain stem nuclei giving fibers to lobules VI and VII of the cerebellar vermis. Brain Research. 1978;153:241–261. doi: 10.1016/0006-8993(78)90405-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss DA, Barhanin J, Gestreau C, Guyenet PG. The role of pH-sensitive task channels in central respiratory chemoreception. Pflugers Archiv. 2015;467:917–929. doi: 10.1007/s00424-014-1633-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead RM, Morse JR, Norgren R. The nucleus of the solitary tract in the monkey: projections to the thalamus and brain stem nuclei. The Journal of Comparative Neurology. 1980;190:259–282. doi: 10.1002/cne.901900205. [DOI] [PubMed] [Google Scholar]
- Behan M, Thomas CF. Sex hormone receptors are expressed in identified respiratory motoneurons in male and female rats. Neuroscience. 2005;130:725–734. doi: 10.1016/j.neuroscience.2004.09.058. [DOI] [PubMed] [Google Scholar]
- Beitz AJ. Possible origin of glutamatergic projections to the midbrain periaqueductal gray and deep layer of the superior colliculus of the rat. Brain Research Bulletin. 1989;23:25–35. doi: 10.1016/0361-9230(89)90159-7. [DOI] [PubMed] [Google Scholar]
- Bellani G, Bronco A, Arrigoni Marocco S, Pozzi M, Sala V, Eronia N, Villa G, Foti G, Tagliabue G, Eger M, Pesenti A. Measurement of diaphragmatic electrical activity by surface electromyography in intubated subjects and its relationship with inspiratory effort. Respiratory Care. 2018;63:1341–1349. doi: 10.4187/respcare.06176. [DOI] [PubMed] [Google Scholar]
- Berger AJ. Dorsal respiratory group neurons in the medulla of cat: spinal projections, responses to lung inflation and superior laryngeal nerve stimulation. Brain Research. 1977;135:231–254. doi: 10.1016/0006-8993(77)91028-9. [DOI] [PubMed] [Google Scholar]
- Berger AJ, Cooney KA. Ventilatory effects of kainic acid injection of the ventrolateral solitary nucleus. Journal of Applied Physiology. 1982;52:131–140. doi: 10.1152/jappl.1982.52.1.131. [DOI] [PubMed] [Google Scholar]
- Berteotti C, Liguori C, Pace M. Dysregulation of the orexin/hypocretin system is not limited to narcolepsy but has far-reaching implications for neurological disorders. The European Journal of Neuroscience. 2021;53:1136–1154. doi: 10.1111/ejn.15077. [DOI] [PubMed] [Google Scholar]
- Bevans CG, Harris AL. Regulation of connexin channels by ph direct action of the protonated form of taurine and other aminosulfonates. The Journal of Biological Chemistry. 1999;274:3711–3719. doi: 10.1074/jbc.274.6.3711. [DOI] [PubMed] [Google Scholar]
- Biancardi V, Bícego KC, Almeida MC, Gargaglioni LH. Locus coeruleus noradrenergic neurons and CO2 drive to breathing. Pflugers Archiv. 2008;455:1119–1128. doi: 10.1007/s00424-007-0338-8. [DOI] [PubMed] [Google Scholar]
- Biancardi V, Saini J, Pageni A, Prashaad M H, Funk GD, Pagliardini S. Mapping of the excitatory, inhibitory, and modulatory afferent projections to the anatomically defined active expiratory oscillator in adult male rats. The Journal of Comparative Neurology. 2021;529:853–884. doi: 10.1002/cne.24984. [DOI] [PubMed] [Google Scholar]
- Bianchi R, Corsetti G, Rodella L, Tredici G, Gioia M. Supraspinal connections and termination patterns of the parabrachial complex determined by the biocytin anterograde tract-tracing technique in the rat. Journal of Anatomy. 1998;193 (Pt 3):417–430. doi: 10.1046/j.1469-7580.1998.19330417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowski MS, Rinaman L. Common and distinct neural inputs to the medial central nucleus of the amygdala and anterior ventrolateral bed nucleus of stria terminalis in rats. Brain Structure & Function. 2013;218:187–208. doi: 10.1007/s00429-012-0393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bina L, Romano V, Hoogland TM, Bosman LWJ, De Zeeuw CI. Purkinje cells translate subjective salience into readiness to act and choice performance. Cell Reports. 2021;37:110116. doi: 10.1016/j.celrep.2021.110116. [DOI] [PubMed] [Google Scholar]
- Bishop GA, Ho RH. The distribution and origin of serotonin immunoreactivity in the rat cerebellum. Brain Research. 1985;331:195–207. doi: 10.1016/0006-8993(85)91545-8. [DOI] [PubMed] [Google Scholar]
- Bochorishvili G, Stornetta RL, Coates MB, Guyenet PG. Pre-Bötzinger complex receives glutamatergic innervation from galaninergic and other retrotrapezoid nucleus neurons. The Journal of Comparative Neurology. 2012;520:1047–1061. doi: 10.1002/cne.22769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boers J, Kirkwood PA, de Weerd H, Holstege G. Ultrastructural evidence for direct excitatory retroambiguus projections to cutaneous trunci and abdominal external oblique muscle motoneurons in the cat. Brain Research Bulletin. 2006;68:249–256. doi: 10.1016/j.brainresbull.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Boggs DF, Tenney SM. Scaling respiratory pattern and respiratory “ drive. ”. Respiration Physiology. 1984;58:245–251. doi: 10.1016/0034-5687(84)90001-x. [DOI] [PubMed] [Google Scholar]
- Bondarenko E, Beig MI, Hodgson DM, Braga VA, Nalivaiko E. Blockade of the Dorsomedial Hypothalamus and the perifornical area inhibits respiratory responses to arousing and stressful stimuli. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2015;308:R816–R822. doi: 10.1152/ajpregu.00415.2014. [DOI] [PubMed] [Google Scholar]
- Bonham AC, McCrimmon DR. Neurones in a discrete region of the nucleus tractus solitarius are required for the breuer-hering reflex in rat. The Journal of Physiology. 1990;427:261–280. doi: 10.1113/jphysiol.1990.sp018171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borodovitsyna O, Duffy BC, Pickering AE, Chandler DJ. Anatomically and functionally distinct locus coeruleus efferents mediate opposing effects on anxiety-like behavior. Neurobiology of Stress. 2020;13:100284. doi: 10.1016/j.ynstr.2020.100284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman LWJ, Konnerth A. Activity-Dependent plasticity of developing climbing fiber-Purkinje cell synapses. Neuroscience. 2009;162:612–623. doi: 10.1016/j.neuroscience.2009.01.032. [DOI] [PubMed] [Google Scholar]
- Bosman LWJ, Houweling AR, Owens CB, Tanke N, Shevchouk OT, Rahmati N, Teunissen WHT, Ju C, Gong W, Koekkoek SKE, De Zeeuw CI. Anatomical pathways involved in generating and sensing rhythmic whisker movements. Frontiers in Integrative Neuroscience. 2011;5:53. doi: 10.3389/fnint.2011.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulenguez P, Gauthier P, Kastner A. Respiratory neuron subpopulations and pathways potentially involved in the reactivation of phrenic motoneurons after C2 hemisection. Brain Research. 2007;1148:96–104. doi: 10.1016/j.brainres.2007.02.060. [DOI] [PubMed] [Google Scholar]
- Bradley DJ, Pascoe JP, Paton JFR, Spyer KM. Cardiovascular and respiratory responses evoked from the posterior cerebellar cortex and fastigial nucleus in the cat. The Journal of Physiology. 1987;393:107–121. doi: 10.1113/jphysiol.1987.sp016813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton-Provencher V, Sur M. Active control of arousal by a locus coeruleus gabaergic circuit. Nature Neuroscience. 2019;22:218–228. doi: 10.1038/s41593-018-0305-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer J. Die selbststeuerung der athmung durch den nervus vagus. Sitzber Deut Akad Wiss Wien. 1868;58:1–29. [Google Scholar]
- Brodal P, Bjaalie JG. Organization of the pontine nuclei. Neuroscience Research. 1992;13:83–118. doi: 10.1016/0168-0102(92)90092-q. [DOI] [PubMed] [Google Scholar]
- Brown HW, Plum F. The neurologic basis of cheyne-stokes respiration. The American Journal of Medicine. 1961;30:849–860. doi: 10.1016/0002-9343(61)90173-5. [DOI] [Google Scholar]
- Brown JT, Chan-Palay V, Palay SL. A study of afferent input to the inferior olivary complex in the rat by retrograde axonal transport of horseradish peroxidase. The Journal of Comparative Neurology. 1977;176:1–22. doi: 10.1002/cne.901760102. [DOI] [PubMed] [Google Scholar]
- Brown RP, Gerbarg PL. Yoga breathing, meditation, and longevity. Annals of the New York Academy of Sciences. 2009;1172:54–62. doi: 10.1111/j.1749-6632.2009.04394.x. [DOI] [PubMed] [Google Scholar]
- Browne DL, Gancher ST, Nutt JG, Brunt ER, Smith EA, Kramer P, Litt M. Episodic ataxia/myokymia syndrome is associated with point mutations in the human potassium channel gene, KCNA1. Nature Genetics. 1994;8:136–140. doi: 10.1038/ng1094-136. [DOI] [PubMed] [Google Scholar]
- Bruinstroop E, Cano G, Vanderhorst V, Cavalcante JC, Wirth J, Sena-Esteves M, Saper CB. Spinal projections of the A5, A6 (locus coeruleus), and A7 noradrenergic cell groups in rats. The Journal of Comparative Neurology. 2012;520:1985–2001. doi: 10.1002/cne.23024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brust RD, Corcoran AE, Richerson GB, Nattie E, Dymecki SM. Functional and developmental identification of a molecular subtype of brain serotonergic neuron specialized to regulate breathing dynamics. Cell Reports. 2014;9:2152–2165. doi: 10.1016/j.celrep.2014.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant TH, Yoshida S, de Castro D, Lipski J. Expiratory neurons of the Bötzinger complex in the rat: a morphological study following intracellular labeling with biocytin. The Journal of Comparative Neurology. 1993;335:267–282. doi: 10.1002/cne.903350210. [DOI] [PubMed] [Google Scholar]
- Burke PGR, Kanbar R, Basting TM, Hodges WM, Viar KE, Stornetta RL, Guyenet PG. State-Dependent control of breathing by the retrotrapezoid nucleus. The Journal of Physiology. 2015;593:2909–2926. doi: 10.1113/JP270053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JE, Hudson AL, Gandevia SC. The neural control of human inspiratory muscles. Progress in Brain Research. 2014;209:295–308. doi: 10.1016/B978-0-444-63274-6.00015-1. [DOI] [PubMed] [Google Scholar]
- Buttry JL, Goshgarian HG. WGA-alexa transsynaptic labeling in the phrenic motor system of adult rats: intrapleural injection versus intradiaphragmatic injection. Journal of Neuroscience Methods. 2015;241:137–145. doi: 10.1016/j.jneumeth.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero-Eraso C, Shin MK, Pho H, Kim LJ, Pichard LE, Wu ZJ, Gu C, Berger S, Pham L, Yeung HYB, Shirahata M, Schwartz AR, Tang WYW, Sham JSK, Polotsky VY. Leptin acts in the carotid bodies to increase minute ventilation during wakefulness and sleep and augment the hypoxic ventilatory response. The Journal of Physiology. 2019;597:151–172. doi: 10.1113/JP276900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caille D, Vibert JF, Hugelin A. Apneusis and apnea after parabrachial or Kölliker-Fuse N. lesion; influence of wakefulness. Respiration Physiology. 1981;45:79–95. doi: 10.1016/0034-5687(81)90051-7. [DOI] [PubMed] [Google Scholar]
- Calton M, Dickson P, Harper RM, Goldowitz D, Mittleman G. Impaired hypercarbic and hypoxic responses from developmental loss of cerebellar purkinje neurons: implications for sudden infant death syndrome. Cerebellum. 2014;13:739–750. doi: 10.1007/s12311-014-0592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calton MA, Howard JR, Harper RM, Goldowitz D, Mittleman G. The cerebellum and SIDS: disordered breathing in a mouse model of developmental cerebellar purkinje cell loss during recovery from hypercarbia. Frontiers in Neurology. 2016;7:78. doi: 10.3389/fneur.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, Undem BJ. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. The Journal of Physiology. 2004;557:543–558. doi: 10.1113/jphysiol.2003.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ, Spina D. In: Handb Exp Pharmacol. Canning BJ, editor. Springer; 2009. Sensory nerves acid-sensitive ion channels and receptors; pp. 283–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ, Chang AB, Bolser DC, Smith JA, Mazzone SB, McGarvey L, Adams TM, Altman KW, Barker AF, Birring SS, Blackhall F, Bolser DC, Boulet LP, Braman SS, Brightling C, Callahan-Lyon P, Canning B, Chang AB, Coeytaux R, Cowley T, Davenport P, Diekemper RL, Ebihara S, El Solh AA, Escalante P, Feinstein A, Field SK, Fisher D, French CT, Gibson P, Gold P, Grant C, Harding SM, Harnden A, Hill AT, Irwin RS, Kahrilas PJ, Keogh KA, Lane AP, Lewis SZ, Lim K, Malesker MA, Mazzone P, Mazzone S, McGarvey L, Molasiotis A, Murad MH, Newcombe P, Nguyen HQ, Oppenheimer J, Prezant D, Pringsheim T, Restrepo MI, Rosen M, Rubin B, Ryu JH, Smith J, Tarlo SM, Turner RB, Vertigan A, Wang G, Weir K. Anatomy and neurophysiology of cough. CHEST. 2014;146:1633–1648. doi: 10.1378/chest.14-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto CB, Onuki Y, Bruinsma B, van der Werf YD, De Zeeuw CI. The sleeping cerebellum. Trends in Neurosciences. 2017;40:309–323. doi: 10.1016/j.tins.2017.03.001. [DOI] [PubMed] [Google Scholar]
- Cao Y, Maran SK, Dhamala M, Jaeger D, Heck DH. Behavior-related pauses in simple-spike activity of mouse purkinje cells are linked to spike rate modulation. The Journal of Neuroscience. 2012a;32:8678–8685. doi: 10.1523/JNEUROSCI.4969-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Roy S, Sachdev RNS, Heck DH. Dynamic correlation between whisking and breathing rhythms in mice. The Journal of Neuroscience. 2012b;32:1653–1659. doi: 10.1523/JNEUROSCI.4395-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caous CA, de Sousa Buck H, Lindsey CJ. Neuronal connections of the paratrigeminal nucleus: A topographic analysis of neurons projecting to bulbar, pontine and thalamic nuclei related to cardiovascular, respiratory and sensory functions. Autonomic Neuroscience. 2001;94:14–24. doi: 10.1016/s1566-0702(01)00338-1. [DOI] [PubMed] [Google Scholar]
- Carrier DR. The energetic paradox of running and hominid evolution. Current Anthropology. 1984;25:483–495. [Google Scholar]
- Carrive P. The periaqueductal gray and defensive behavior: functional representation and neuronal organization. Behavioural Brain Research. 1993;58:27–47. doi: 10.1016/0166-4328(93)90088-8. [DOI] [PubMed] [Google Scholar]
- Carvalho O, Gonçalves C. Comparative physiology of the respiratory system in the animal kingdom. The Open Biology Journal. 2011;4:35–46. doi: 10.2174/1874196701104010035. [DOI] [Google Scholar]
- Çavdar S, Özgur M, Kuvvet Y, Bay HH. The cerebello-hypothalamic and hypothalamo-cerebellar pathways via superior and middle cerebellar peduncle in the rat. Cerebellum. 2018a;17:517–524. doi: 10.1007/s12311-018-0938-1. [DOI] [PubMed] [Google Scholar]
- Çavdar S, Özgür M, Kuvvet Y, Bay H, Aydogmus E. Cortical, subcortical and brain stem connections of the cerebellum via the superior and middle cerebellar peduncle in the rat. Journal of Integrative Neuroscience. 2018b;17:609–618. doi: 10.3233/JIN-180090. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Saper CB. Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. The Journal of Comparative Neurology. 1987;262:27–45. doi: 10.1002/cne.902620104. [DOI] [PubMed] [Google Scholar]
- Cerritelli S, Hirschberg S, Hill R, Balthasar N, Pickering AE. Activation of brainstem pro-opiomelanocortin neurons produces opioidergic analgesia, bradycardia and bradypnoea. PLOS ONE. 2016;11:e0153187. doi: 10.1371/journal.pone.0153187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin NL, Eikermann M, Fassbender P, White DP, Malhotra A. Genioglossus premotoneurons and the negative pressure reflex in rats. The Journal of Physiology. 2007;579:515–526. doi: 10.1113/jphysiol.2006.121889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler DJ, Jensen P, McCall JG, Pickering AE, Schwarz LA, Totah NK. Redefining noradrenergic neuromodulation of behavior: impacts of a modular locus coeruleus architecture. The Journal of Neuroscience. 2019;39:8239–8249. doi: 10.1523/JNEUROSCI.1164-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Z, Ballou E, Jiao W, McKenna KE, Morrison SF, McCrimmon DR. Systemic leptin produces a long-lasting increase in respiratory motor output in rats. Frontiers in Physiology. 2013;4:16. doi: 10.3389/fphys.2013.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang RB, Strochlic DE, Williams EK, Umans BD, Liberles SD. Vagal sensory neuron subtypes that differentially control breathing. Cell. 2015;161:622–633. doi: 10.1016/j.cell.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ML, Witmans MB, Tablizo MA, Jubran RF, Turkel SB, Tavaré CJ, Keens TG. Disordered respiratory control in children with partial cerebellar resections. Pediatric Pulmonology. 2005;40:88–91. doi: 10.1002/ppul.20225. [DOI] [PubMed] [Google Scholar]
- Chen Z, Lin MT, Zhan C, Zhong NS, Mu D, Lai KF, Liu MJ. A descending pathway emanating from the periaqueductal gray mediates the development of cough-like hypersensitivity. IScience. 2022;25:103641. doi: 10.1016/j.isci.2021.103641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang MC, Bowen A, Schier LA, Tupone D, Uddin O, Heinricher MM. Parabrachial complex: A hub for pain and aversion. The Journal of Neuroscience. 2019;39:8225–8230. doi: 10.1523/JNEUROSCI.1162-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinelli E, Robertson B, Mutolo D, Grillner S, Pantaleo T, Bongianni F. Neuronal mechanisms of respiratory pattern generation are evolutionary conserved. The Journal of Neuroscience. 2013;33:9104–9112. doi: 10.1523/JNEUROSCI.0299-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinelli E, Iovino L, Bongianni F, Pantaleo T, Mutolo D. Essential role of the cvrg in the generation of both the expiratory and inspiratory components of the cough reflex. Physiological Research. 2020;69:S19–S27. doi: 10.33549/physiolres.934396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciriello J, Caverson MM. Hypothalamic orexin-A (hypocretin-1) neuronal projections to the vestibular complex and cerebellum in the rat. Brain Research. 2014;1579:20–34. doi: 10.1016/j.brainres.2014.07.008. [DOI] [PubMed] [Google Scholar]
- Claps A, Torrealba F. The carotid body connections: a WGA-HRP study in the cat. Brain Research. 1988;455:123–133. doi: 10.1016/0006-8993(88)90121-7. [DOI] [PubMed] [Google Scholar]
- Coates EL, Li A, Nattie EE. Widespread sites of brain stem ventilatory chemoreceptors. Journal of Applied Physiology. 1993;75:5–14. doi: 10.1152/jappl.1993.75.1.5. [DOI] [PubMed] [Google Scholar]
- Coesmans M, Weber JT, De Zeeuw CI, Hansel C. Bidirectional parallel fiber plasticity in the cerebellum under climbing fiber control. Neuron. 2004;44:691–700. doi: 10.1016/j.neuron.2004.10.031. [DOI] [PubMed] [Google Scholar]
- Cohen MI, Wang SC. Respiratory neuronal activity in pons of cat. Journal of Neurophysiology. 1959;22:33–50. doi: 10.1152/jn.1959.22.1.33. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Adams L, Murphy K, Martin AJ, Lammertsma AA, Tochon-Danguy HJ, Clark JC, Friston KJ, Guz A. Regional cerebral blood flow during volitional breathing in man. The Journal of Physiology. 1991;443:91–103. doi: 10.1113/jphysiol.1991.sp018824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleridge JC, Coleridge HM. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Reviews of Physiology, Biochemistry and Pharmacology. 1984;99:1–110. doi: 10.1007/BFb0027715. [DOI] [PubMed] [Google Scholar]
- Coryell MW, Ziemann AE, Westmoreland PJ, Haenfler JM, Kurjakovic Z, Zha X, Price M, Schnizler MK, Wemmie JA. Targeting ASIC1a reduces innate fear and alters neuronal activity in the fear circuit. Biological Psychiatry. 2007;62:1140–1148. doi: 10.1016/j.biopsych.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Nicotra A, Chiesa PA, Nagai Y, Gray MA, Minati L, Bernardi L. Slow breathing and hypoxic challenge: cardiorespiratory consequences and their central neural substrates. PLOS ONE. 2015;10:e0127082. doi: 10.1371/journal.pone.0127082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Sánchez FF, Lucena J, Ascaso C, Tolosa E, Quintò L, Rossi ML. Cerebellar cortex delayed maturation in sudden infant death syndrome. Journal of Neuropathology and Experimental Neurology. 1997;56:340–346. doi: 10.1097/00005072-199704000-00002. [DOI] [PubMed] [Google Scholar]
- Cui Y, Kam K, Sherman D, Janczewski WA, Zheng Y, Feldman JL. Defining prebötzinger complex rhythm- and pattern-generating neural microcircuits in vivo. Neuron. 2016;91:602–614. doi: 10.1016/j.neuron.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins EP, Strowitzki MJ, Taylor CT. Mechanisms and consequences of oxygen and carbon dioxide sensing in mammals. Physiological Reviews. 2020;100:463–488. doi: 10.1152/physrev.00003.2019. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. The Journal of Comparative Neurology. 1988;274:60–76. doi: 10.1002/cne.902740107. [DOI] [PubMed] [Google Scholar]
- D’Adamo MC, Gallenmüller C, Servettini I, Hartl E, Tucker SJ, Arning L, Biskup S, Grottesi A, Guglielmi L, Imbrici P, Bernasconi P, Di Giovanni G, Franciolini F, Catacuzzeno L, Pessia M, Klopstock T. Novel phenotype associated with a mutation in the KCNA1(kv1.1) gene. Frontiers in Physiology. 2014;5:525. doi: 10.3389/fphys.2014.00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Adamo MC, Liantonio A, Rolland J-F, Pessia M, Imbrici P. Kv1.1 channelopathies: Pathophysiological mechanisms and therapeutic approaches. International Journal of Molecular Sciences. 2020;21:2935. doi: 10.3390/ijms21082935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasceno RS, Takakura AC, Moreira TS. Regulation of the chemosensory control of breathing by Kölliker-Fuse neurons. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2014;307:R57–R67. doi: 10.1152/ajpregu.00024.2014. [DOI] [PubMed] [Google Scholar]
- Dautan D, Hacioğlu Bay H, Bolam JP, Gerdjikov TV, Mena-Segovia J. Extrinsic sources of cholinergic innervation of the striatal complex: A whole-brain mapping analysis. Frontiers in Neuroanatomy. 2016;10:1. doi: 10.3389/fnana.2016.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport PW, Sant’Ambrogio FB, Sant’Ambrogio G. Adaptation of tracheal stretch receptors. Respiration Physiology. 1981;44:339–349. doi: 10.1016/0034-5687(81)90028-1. [DOI] [PubMed] [Google Scholar]
- Davies RO, Kubin L, Pack AI. Pulmonary stretch receptor relay neurones of the cat: location and contralateral medullary projections. The Journal of Physiology. 1987;383:571–585. doi: 10.1113/jphysiol.1987.sp016429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. PNAS. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa Buck H, Caous CA, Lindsey CJ. Projections of the paratrigeminal nucleus to the ambiguus, rostroventrolateral and lateral reticular nuclei, and the solitary tract. Auton Neurosci. 2001;87:187–200. doi: 10.1016/S1566-0702(00)00259-9. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Estenne M. Coordination between rib cage muscles and diaphragm during quiet breathing in humans. Journal of Applied Physiology. 1984;57:899–906. doi: 10.1152/jappl.1984.57.3.899. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Legrand A, Gevenois PA, Wilson TA. Mechanical advantage of the human parasternal intercostal and triangularis sterni muscles. The Journal of Physiology. 1998;513 ( Pt 3):915–925. doi: 10.1111/j.1469-7793.1998.915ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer A, Kirkwood PA, Wilson TA. Respiratory action of the intercostal muscles. Physiological Reviews. 2005;85:717–756. doi: 10.1152/physrev.00007.2004. [DOI] [PubMed] [Google Scholar]
- de Zeeuw CI, Holstege JC, Ruigrok TJ, Voogd J. Ultrastructural study of the gabaergic, cerebellar, and mesodiencephalic innervation of the cat medial accessory olive: anterograde tracing combined with immunocytochemistry. The Journal of Comparative Neurology. 1989;284:12–35. doi: 10.1002/cne.902840103. [DOI] [PubMed] [Google Scholar]
- De Zeeuw CI, Ruigrok TJH. Olivary projecting neurons in the nucleus of darkschewitsch in the cat receive excitatory monosynaptic input from the cerebellar nuclei. Brain Research. 1994;653:345–350. doi: 10.1016/0006-8993(94)90411-1. [DOI] [PubMed] [Google Scholar]
- De Zeeuw CI, Hoebeek FE, Bosman LWJ, Schonewille M, Witter L, Koekkoek SK. Spatiotemporal firing patterns in the cerebellum. Nature Reviews. Neuroscience. 2011;12:327–344. doi: 10.1038/nrn3011. [DOI] [PubMed] [Google Scholar]
- Dean JB, Putnam RW. The caudal solitary complex is a site of central CO (2) chemoreception and integration of multiple systems that regulate expired CO (2) Respiratory Physiology & Neurobiology. 2010;173:274–287. doi: 10.1016/j.resp.2010.07.002. [DOI] [PubMed] [Google Scholar]
- de Britto AA, Moraes DJA. Non-chemosensitive parafacial neurons simultaneously regulate active expiration and airway patency under hypercapnia in rats. The Journal of Physiology. 2017;595:2043–2064. doi: 10.1113/JP273335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Negro CA, Funk GD, Feldman JL. Breathing matters. Nature Reviews. Neuroscience. 2018;19:351–367. doi: 10.1038/s41583-018-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng BS, Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. Contribution of orexin in hypercapnic chemoreflex: Evidence from genetic and pharmacological disruption and supplementation studies in mice. Journal of Applied Physiology. 2007;103:1772–1779. doi: 10.1152/japplphysiol.00075.2007. [DOI] [PubMed] [Google Scholar]
- Depuy SD, Kanbar R, Coates MB, Stornetta RL, Guyenet PG. Control of breathing by raphe obscurus serotonergic neurons in mice. The Journal of Neuroscience. 2011;31:1981–1990. doi: 10.1523/JNEUROSCI.4639-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergacheva O, Griffioen KJ, Neff RA, Mendelowitz D. Respiratory modulation of premotor cardiac vagal neurons in the brainstem. Respiratory Physiology & Neurobiology. 2010;174:102–110. doi: 10.1016/j.resp.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Zeeuw CI, Canto CB. Interpreting thoughts during sleep. Science. 2022;377:919–920. doi: 10.1126/science.add8592. [DOI] [PubMed] [Google Scholar]
- Dhaibar H, Gautier NM, Chernyshev OY, Dominic P, Glasscock E. Cardiorespiratory profiling reveals primary breathing dysfunction in Kcna1-null mice: implications for sudden unexpected death in epilepsy. Neurobiology of Disease. 2019;127:502–511. doi: 10.1016/j.nbd.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra RR, Dick TE, Furuya WI, Galán RF, Dutschmann M. Volumetric mapping of the functional neuroanatomy of the respiratory network in the perfused brainstem preparation of rats. The Journal of Physiology. 2020;598:2061–2079. doi: 10.1113/JP279605. [DOI] [PubMed] [Google Scholar]
- Dick TE, Bellingham MC, Richter DW. Pontine respiratory neurons in anesthetized cats. Brain Research. 1994;636:259–269. doi: 10.1016/0006-8993(94)91025-1. [DOI] [PubMed] [Google Scholar]
- Dietrichs E. Cerebellar autonomic function: direct hypothalamocerebellar pathway. Science. 1984;223:591–593. doi: 10.1126/science.6198719. [DOI] [PubMed] [Google Scholar]
- Dietrichs E. Cerebellar cortical and nuclear afferents from the feline locus coeruleus complex. Neuroscience. 1988;27:77–91. doi: 10.1016/0306-4522(88)90220-5. [DOI] [PubMed] [Google Scholar]
- Dietrichs E, Haines DE, Røste GK, Røste LS. Hypothalamocerebellar and cerebellohypothalamic projections--circuits for regulating nonsomatic cerebellar activity? Histology and Histopathology. 1994;9:603–614. [PubMed] [Google Scholar]
- Di Mauro M, Li Volsi G, Licata F. Noradrenergic control of neuronal firing in cerebellar nuclei: Modulation of GABA responses. Cerebellum. 2013;12:350–361. doi: 10.1007/s12311-012-0422-2. [DOI] [PubMed] [Google Scholar]
- Ding YQ, Kaneko T, Nomura S, Mizuno N. Immunohistochemical localization of mu-opioid receptors in the central nervous system of the rat. The Journal of Comparative Neurology. 1996;367:375–402. doi: 10.1002/(SICI)1096-9861(19960408)367:3<375::AID-CNE5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Do J, Chang Z, Sekerková G, McCrimmon DR, Martina M. A leptin-mediated neural mechanism linking breathing to metabolism. Cell Reports. 2020;33:108358. doi: 10.1016/j.celrep.2020.108358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. The Journal of Comparative Neurology. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- Docu Axelerad A, Stroe AZ, Arghir OC, Docu Axelerad D, Gogu AE. Respiratory dysfunctions in Parkinson’s disease patients. Brain Sciences. 2021;11:595. doi: 10.3390/brainsci11050595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Research. Brain Research Reviews. 2001a;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. The Journal of Comparative Neurology. 2001b;436:430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Doty RL, Brugger WE, Jurs PC, Orndorff MA, Snyder PJ, Lowry LD. Intranasal trigeminal stimulation from odorous volatiles: psychometric responses from anosmic and normal humans. Physiology & Behavior. 1978;20:175–185. doi: 10.1016/0031-9384(78)90070-7. [DOI] [PubMed] [Google Scholar]
- Driessen AK, Farrell MJ, Mazzone SB, McGovern AE. The role of the paratrigeminal nucleus in vagal afferent evoked respiratory reflexes: A neuroanatomical and functional study in guinea pigs. Frontiers in Physiology. 2015;6:378. doi: 10.3389/fphys.2015.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen AK, Farrell MJ, Dutschmann M, Stanic D, McGovern AE, Mazzone SB. Reflex regulation of breathing by the paratrigeminal nucleus via multiple bulbar circuits. Brain Structure & Function. 2018;223:4005–4022. doi: 10.1007/s00429-018-1732-z. [DOI] [PubMed] [Google Scholar]
- Driessen AK. Vagal afferent processing by the paratrigeminal nucleus. Frontiers in Physiology. 2019;10:1110. doi: 10.3389/fphys.2019.01110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncker H. The emergence of macroscopic complexity an outline of the history of the respiratory apparatus of vertebrates from diffusion to language production. Zoology. 2001;103:240–259. [Google Scholar]
- Dutschmann M, Herbert H. The Kölliker-fuse nucleus gates the postinspiratory phase of the respiratory cycle to control inspiratory off-switch and upper airway resistance in rat. The European Journal of Neuroscience. 2006;24:1071–1084. doi: 10.1111/j.1460-9568.2006.04981.x. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Jones SE, Subramanian HH, Stanic D, Bautista TG. The physiological significance of postinspiration in respiratory control. Progress in Brain Research. 2014;212:113–130. doi: 10.1016/B978-0-444-63488-7.00007-0. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Bautista TG, Trevizan-Baú P, Dhingra RR, Furuya WI. The pontine Kölliker-fuse nucleus gates facial, hypoglossal, and vagal upper airway related motor activity. Respiratory Physiology & Neurobiology. 2021;284:103563. doi: 10.1016/j.resp.2020.103563. [DOI] [PubMed] [Google Scholar]
- Ebert D, Hefter H, Dohle C, Freund HJ. Ataxic breathing during alternating forearm movements of various frequencies in cerebellar patients. Neuroscience Letters. 1995;193:145–148. doi: 10.1016/0304-3940(95)11674-l. [DOI] [PubMed] [Google Scholar]
- Ebert D, Rassler B, Hefter H. Coordination between breathing and forearm movements during sinusoidal tracking. European Journal of Applied Physiology. 2000;81:288–296. doi: 10.1007/s004210050045. [DOI] [PubMed] [Google Scholar]
- Elam M, Yao T, Thorén P, Svensson TH. Hypercapnia and hypoxia: Chemoreceptor-mediated control of locus coeruleus neurons and splanchnic, sympathetic nerves. Brain Research. 1981;222:373–381. doi: 10.1016/0006-8993(81)91040-4. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL. Monosynaptic transmission of respiratory drive to phrenic motoneurons from brainstem bulbospinal neurons in rats. The Journal of Comparative Neurology. 1988;269:47–57. doi: 10.1002/cne.902690104. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL, Goshgarian HG. Ventral respiratory group projections to phrenic motoneurons: electron microscopic evidence for monosynaptic connections. The Journal of Comparative Neurology. 1990a;302:707–714. doi: 10.1002/cne.903020403. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Vera PL, Haselton JR, Haselton CL, Schneiderman N. Brainstem projections to the phrenic nucleus: an anterograde and retrograde HRP study in the rabbit. Brain Research Bulletin. 1990b;24:163–174. doi: 10.1016/0361-9230(90)90201-a. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL. Brainstem connections of the rostral ventral respiratory group of the rat. Brain Research. 1990;513:35–42. doi: 10.1016/0006-8993(90)91086-v. [DOI] [PubMed] [Google Scholar]
- Elstad M, O’Callaghan EL, Smith AJ, Ben-Tal A, Ramchandra R. Cardiorespiratory interactions in humans and animals: rhythms for life. American Journal of Physiology. Heart and Circulatory Physiology. 2018;315:H6–H17. doi: 10.1152/ajpheart.00701.2017. [DOI] [PubMed] [Google Scholar]
- Esposito A, Demeurisse G, Alberti B, Fabbro F. Complete mutism after midbrain periaqueductal gray lesion. Neuroreport. 1999;10:681–685. doi: 10.1097/00001756-199903170-00004. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I. Pump neurons of the nucleus of the solitary tract project widely to the medulla. Neuroscience Letters. 1996;215:123–126. doi: 10.1016/0304-3940(96)12968-2. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I. Identification of deflation-sensitive inspiratory neurons in the dorsal respiratory group of the rat. Brain Research. 2000;883:22–30. doi: 10.1016/s0006-8993(00)02871-7. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I, Saito Y. Brainstem and spinal projections of augmenting expiratory neurons in the rat. Neuroscience Research. 2003;45:41–51. doi: 10.1016/s0168-0102(02)00197-9. [DOI] [PubMed] [Google Scholar]
- Ezure K. Respiration-related afferents to parabrachial pontine regions. Respiratory Physiology & Neurobiology. 2004;143:167–175. doi: 10.1016/j.resp.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I. Distribution and medullary projection of respiratory neurons in the dorsolateral pons of the rat. Neuroscience. 2006;141:1011–1023. doi: 10.1016/j.neuroscience.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Farber JP. Expiratory effects of cerebellar stimulation in developing opossums. The American Journal of Physiology. 1987;252:R1158–R1164. doi: 10.1152/ajpregu.1987.252.6.R1158. [DOI] [PubMed] [Google Scholar]
- Farmer DGS, Dutschmann M, Paton JFR, Pickering AE, McAllen RM. Brainstem sources of cardiac vagal tone and respiratory sinus arrhythmia. The Journal of Physiology. 2016;594:7249–7265. doi: 10.1113/JP273164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farney RJ, Walker JM, Cloward TV, Rhondeau S. Sleep-Disordered breathing associated with long-term opioid therapy. Chest. 2003;123:632–639. doi: 10.1378/chest.123.2.632. [DOI] [PubMed] [Google Scholar]
- Farrell MJ, Bautista TG, Liang E, Azzollini D, Egan GF, Mazzone SB. Evidence for multiple bulbar and higher brain circuits processing sensory inputs from the respiratory system in humans. The Journal of Physiology. 2020;598:5771–5787. doi: 10.1113/JP280220. [DOI] [PubMed] [Google Scholar]
- Faull OK, Subramanian HH, Ezra M, Pattinson KTS. The midbrain periaqueductal gray as an integrative and interoceptive neural structure for breathing. Neuroscience and Biobehavioral Reviews. 2019;98:135–144. doi: 10.1016/j.neubiorev.2018.12.020. [DOI] [PubMed] [Google Scholar]
- Fedorko L, Merrill EG, Lipski J. Two descending medullary inspiratory pathways to phrenic motoneurones. Neuroscience Letters. 1983;43:285–291. doi: 10.1016/0304-3940(83)90202-1. [DOI] [PubMed] [Google Scholar]
- Fedorko L, Merrill EG. Axonal projections from the rostral expiratory neurones of the Bötzinger complex to medulla and spinal cord in the cat. The Journal of Physiology. 1984;350:487–496. doi: 10.1113/jphysiol.1984.sp015214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink AM, Burke LA, Sharma K. Lesioning of the pedunculopontine nucleus reduces rapid eye movement sleep, but does not alter cardiorespiratory activities during sleep, under hypoxic conditions in rats. Respiratory Physiology & Neurobiology. 2021;288:103653. doi: 10.1016/j.resp.2021.103653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley JCW, Katz DM. The central organization of carotid body afferent projections to the brainstem of the rat. Brain Research. 1992;572:108–116. doi: 10.1016/0006-8993(92)90458-l. [DOI] [PubMed] [Google Scholar]
- Flor KC, Barnett WH, Karlen-Amarante M, Molkov YI, Zoccal DB. Inhibitory control of active expiration by the Bötzinger complex in rats. The Journal of Physiology. 2020;598:4969–4994. doi: 10.1113/JP280243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanini A, Spano P, Bower JM. Ketamine-xylazine-induced slow (< 1.5 hz) oscillations in the rat piriform (olfactory) cortex are functionally correlated with respiration. The Journal of Neuroscience. 2003;23:7993–8001. doi: 10.1523/JNEUROSCI.23-22-07993.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Framnes SN, Arble DM. The bidirectional relationship between obstructive sleep apnea and metabolic disease. Frontiers in Endocrinology. 2018;9:440. doi: 10.3389/fendo.2018.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. Rhythms for cognition: Communication through coherence. Neuron. 2015;88:220–235. doi: 10.1016/j.neuron.2015.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontera JL, Baba Aissa H, Sala RW, Mailhes-Hamon C, Georgescu IA, Léna C, Popa D. Bidirectional control of fear memories by cerebellar neurons projecting to the ventrolateral periaqueductal grey. Nature Communications. 2020;11:5207. doi: 10.1038/s41467-020-18953-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Tvrdik P, Makki N, Paxinos G, Watson C. Precerebellar cell groups in the hindbrain of the mouse defined by retrograde tracing and correlated with cumulative wnt1-cre genetic labeling. Cerebellum. 2011;10:570–584. doi: 10.1007/s12311-011-0266-1. [DOI] [PubMed] [Google Scholar]
- Fu C, Xue J, Wang R, Chen J, Ma L, Liu Y, Wang X, Guo F, Zhang Y, Zhang X, Wang S. Chemosensitive phox2b-expressing neurons are crucial for hypercapnic ventilatory response in the nucleus tractus solitarius. The Journal of Physiology. 2017;595:4973–4989. doi: 10.1113/JP274437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu JY, Yu XD, Zhu Y, Xie SZ, Tang MY, Yu B, Li XM. Whole-brain map of long-range monosynaptic inputs to different cell types in the amygdala of the mouse. Neuroscience Bulletin. 2020;36:1381–1394. doi: 10.1007/s12264-020-00545-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H, Kodama T, du Lac S. Modular output circuits of the fastigial nucleus for diverse motor and nonmotor functions of the cerebellar vermis. eLife. 2020;9:e58613. doi: 10.7554/eLife.58613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushi I, Yokota S, Okada Y. The role of the hypothalamus in modulation of respiration. Respiratory Physiology & Neurobiology. 2019;265:172–179. doi: 10.1016/j.resp.2018.07.003. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Williams JS, Janssen PL, Fregosi RF. Effect of co‐activation of tongue protrudor and retractor muscles on tongue movements and pharyngeal airflow mechanics in the rat. The Journal of Physiology. 1999;519:601–613. doi: 10.1111/j.1469-7793.1999.0601m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Research Reviews. 1984;7:229–259. doi: 10.1016/0165-0173(84)90012-2. [DOI] [PubMed] [Google Scholar]
- Fung SJ, Yamuy J, Sampogna S, Morales FR, Chase MH. Hypocretin (orexin) input to trigeminal and hypoglossal motoneurons in the cat: a double-labeling immunohistochemical study. Brain Research. 2001;903:257–262. doi: 10.1016/S0006-8993(01)02318-6. [DOI] [PubMed] [Google Scholar]
- Fusco AF, Pucci LA, Switonski PM, Biswas DD, McCall AL, Kahn AF, Dhindsa JS, Strickland LM, La Spada AR, ElMallah MK. Respiratory dysfunction in a mouse model of spinocerebellar ataxia type 7. Disease Models & Mechanisms. 2021;14:dmm048893. doi: 10.1242/dmm.048893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gang S, Sato Y, Kohama I, Aoki M. Afferent projections to the Bötzinger complex from the upper cervical cord and other respiratory related structures in the brainstem in cats: retrograde WGA-HRP tracing. Journal of the Autonomic Nervous System. 1995;56:1–7. doi: 10.1016/0165-1838(95)00049-x. [DOI] [PubMed] [Google Scholar]
- Gao Z, van Beugen BJ, De Zeeuw CI. Distributed synergistic plasticity and cerebellar learning. Nature Reviews. Neuroscience. 2012;13:619–635. doi: 10.1038/nrn3312. [DOI] [PubMed] [Google Scholar]
- Gao Z, Proietti-Onori M, Lin Z, Ten Brinke MM, Boele HJ, Potters JW, Ruigrok TJH, Hoebeek FE, De Zeeuw CI. Excitatory cerebellar nucleocortical circuit provides internal amplification during associative conditioning. Neuron. 2016;89:645–657. doi: 10.1016/j.neuron.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner WN. The pathophysiology of hyperventilation disorders. Chest. 1996;109:516–534. doi: 10.1378/chest.109.2.516. [DOI] [PubMed] [Google Scholar]
- Gargaglioni LH, Hartzler LK, Putnam RW. The locus coeruleus and central chemosensitivity. Respiratory Physiology & Neurobiology. 2010;173:264–273. doi: 10.1016/j.resp.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargaglioni LH, Marques DA, Patrone LGA. Sex differences in breathing. Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology. 2019;238:110543. doi: 10.1016/j.cbpa.2019.110543. [DOI] [PubMed] [Google Scholar]
- Gasparini S, Howland JM, Thatcher AJ, Geerling JC. Central afferents to the nucleus of the solitary tract in rats and mice. The Journal of Comparative Neurology. 2020;528:2708–2728. doi: 10.1002/cne.24927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauda EB, Conde S, Bassi M, Zoccal DB, Almeida Colombari DS, Colombari E, Despotovic N. Leptin: Master regulator of biological functions that affects breathing. Comprehensive Physiology. 2020;10:1047–1083. doi: 10.1002/cphy.c190031. [DOI] [PubMed] [Google Scholar]
- Gautier H, Remmers JE, Bartlett D. Control of the duration of expiration. Respiration Physiology. 1973;18:205–221. doi: 10.1016/0034-5687(73)90051-0. [DOI] [PubMed] [Google Scholar]
- Gavello D, Carbone E, Carabelli V. Leptin-mediated ion channel regulation: PI3K pathways, physiological role, and therapeutic potential. Channels. 2016;10:282–296. doi: 10.1080/19336950.2016.1164373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaytán SP, Pásaro R. Connections of the rostral ventral respiratory neuronal cell group: An anterograde and retrograde tracing study in the rat. Brain Research Bulletin. 1998;47:625–642. doi: 10.1016/s0361-9230(98)00125-7. [DOI] [PubMed] [Google Scholar]
- Geerling JC, Shin JW, Chimenti PC, Loewy AD. Paraventricular hypothalamic nucleus: Axonal projections to the brainstem. The Journal of Comparative Neurology. 2010;518:1460–1499. doi: 10.1002/cne.22283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerling JC, Yokota S, Rukhadze I, Roe D, Chamberlin NL. Kölliker-fuse gabaergic and glutamatergic neurons project to distinct targets. The Journal of Comparative Neurology. 2017;525:1844–1860. doi: 10.1002/cne.24164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrits PO, Holstege G. Pontine and medullary projections to the nucleus retroambiguus: a wheat germ agglutinin-horseradish peroxidase and autoradiographic tracing study in the cat. The Journal of Comparative Neurology. 1996;373:173–185. doi: 10.1002/(SICI)1096-9861(19960916)373:2<173::AID-CNE2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Gesell R, White F. RECRUITMENT of muscular activity and the central neurone after-discharge of hyperpnea. American Journal of Physiology-Legacy Content. 1938;122:48–56. doi: 10.1152/ajplegacy.1938.122.1.48. [DOI] [Google Scholar]
- Gestreau C, Dutschmann M, Obled S, Bianchi AL. Activation of XII motoneurons and premotor neurons during various oropharyngeal behaviors. Respiratory Physiology & Neurobiology. 2005;147:159–176. doi: 10.1016/j.resp.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Gestreau C, Heitzmann D, Thomas J, Dubreuil V, Bandulik S, Reichold M, Bendahhou S, Pierson P, Sterner C, Peyronnet-Roux J, Benfriha C, Tegtmeier I, Ehnes H, Georgieff M, Lesage F, Brunet JF, Goridis C, Warth R, Barhanin J. Task2 potassium channels set central respiratory CO2 and O2 sensitivity. PNAS. 2010;107:2325–2330. doi: 10.1073/pnas.0910059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiological Reviews. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N, Spyer KM. Atp is a mediator of chemosensory transduction in the central nervous system. Nature. 2005;436:108–111. doi: 10.1038/nature03690. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through ph-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Dale N. Brain H+/CO2 sensing and control by glial cells. Glia. 2022;70:1520–1535. doi: 10.1002/glia.24152. [DOI] [PubMed] [Google Scholar]
- Grace M, Birrell MA, Dubuis E, Maher SA, Belvisi MG. Transient receptor potential channels mediate the tussive response to prostaglandin E2 and bradykinin. Thorax. 2012;67:891–900. doi: 10.1136/thoraxjnl-2011-201443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBötzinger complex neurokinin-1 receptor-expressing neurons. Nature Neuroscience. 2001;4:927–930. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruart A, Delgado-García JM. Respiration-related neurons recorded in the deep cerebellar nuclei of the alert cat. Neuroreport. 1992;3:365–368. doi: 10.1097/00001756-199204000-00019. [DOI] [PubMed] [Google Scholar]
- Guan XM, Hess JF, Yu H, Hey PJ, van der Ploeg LH. Differential expression of mRNA for leptin receptor isoforms in the rat brain. Molecular and Cellular Endocrinology. 1997;133:1–7. doi: 10.1016/s0303-7207(97)00138-x. [DOI] [PubMed] [Google Scholar]
- Guedes LU, Rodrigues JM, Fernandes AA, Cardoso FE, Parreira VF. Respiratory changes in Parkinson’s disease may be unrelated to dopaminergic dysfunction. Arquivos de Neuro-Psiquiatria. 2012;70:847–851. doi: 10.1590/s0004-282x2012001100005. [DOI] [PubMed] [Google Scholar]
- Guilherme EM, Moreira R, de Oliveira A, Ferro AM, Di Lorenzo VAP, Gianlorenço ACL. Respiratory disorders in parkinson’s disease. Journal of Parkinson’s Disease. 2021;11:993–1010. doi: 10.3233/JPD-212565. [DOI] [PubMed] [Google Scholar]
- Guo H, Yuan XS, Zhou JC, Chen H, Li SQ, Qu WM, Huang ZL. Whole-Brain monosynaptic inputs to hypoglossal motor neurons in mice. Neuroscience Bulletin. 2020;36:585–597. doi: 10.1007/s12264-020-00468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Wang H. Pre-Bötzinger neurons with preinspiratory discharges “ in vivo ” express NK1 receptors in the rat. Journal of Neurophysiology. 2001;86:438–446. doi: 10.1152/jn.2001.86.1.438. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA. Retrotrapezoid nucleus and central chemoreception. The Journal of Physiology. 2008;586:2043–2048. doi: 10.1113/jphysiol.2008.150870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DN, Smith MI, Piper DC, Hunter AJ, Porter RA, Upton N. Orexin a activates locus coeruleus cell firing and increases arousal in the rat. PNAS. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines DE, Dietrichs E, Mihailoff GA, McDonald EF. The cerebellar-hypothalamic axis: Basic circuits and clinical observations. International Review of Neurobiology. 1997;41:83–107. doi: 10.1016/s0074-7742(08)60348-7. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Balaban CD. Serotonergic and nonserotonergic neurons in the dorsal raphe nucleus send collateralized projections to both the vestibular nuclei and the central amygdaloid nucleus. Neuroscience. 2006;140:1067–1077. doi: 10.1016/j.neuroscience.2006.02.053. [DOI] [PubMed] [Google Scholar]
- Haldane JS, Priestley JG. The regulation of the lung-ventilation. The Journal of Physiology. 1905;32:225–266. doi: 10.1113/jphysiol.1905.sp001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper RM, Frysinger RC, Trelease RB, Marks JD. State-Dependent alteration of respiratory cycle timing by stimulation of the central nucleus of the amygdala. Brain Research. 1984;306:1–8. doi: 10.1016/0006-8993(84)90350-0. [DOI] [PubMed] [Google Scholar]
- Harvey RJ, Napper RMA. Quantitative studies on the mammalian cerebellum. Progress in Neurobiology. 1991;36:437–463. doi: 10.1016/0301-0082(91)90012-p. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Yamanaka A, Kato S, Tanifuji M, Kobayashi K, Yaginuma H. Anatomical evidence for a direct projection from purkinje cells in the mouse cerebellar vermis to medial parabrachial nucleus. Frontiers in Neural Circuits. 2018;12:6. doi: 10.3389/fncir.2018.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa T, Takanaga A, Maeda S, Ito H, Seki M. Monosynaptic inputs from the nucleus tractus solitarii to the laryngeal motoneurons in the nucleus ambiguus of the rat. Anatomy and Embryology. 2000;202:411–420. doi: 10.1007/s004290000120. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Coles SK, McCrimmon DR. Respiratory neurons mediating the breuer-hering reflex prolongation of expiration in rat. The Journal of Neuroscience. 1996;16:6526–6536. doi: 10.1523/JNEUROSCI.16-20-06526.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayat H, Regev N, Matosevich N, Sales A, Paredes-Rodriguez E, Krom AJ, Bergman L, Li Y, Lavigne M, Kremer EJ, Yizhar O, Pickering AE, Nir Y. Locus coeruleus norepinephrine activity mediates sensory-evoked awakenings from sleep. Science Advances. 2020;6:eaaz4232. doi: 10.1126/sciadv.aaz4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazrati LN, Parent A. Projection from the deep cerebellar nuclei to the pedunculopontine nucleus in the squirrel monkey. Brain Research. 1992;585:267–271. doi: 10.1016/0006-8993(92)91216-2. [DOI] [PubMed] [Google Scholar]
- Heck DH, McAfee SS, Liu Y, Babajani-Feremi A, Rezaie R, Freeman WJ, Wheless JW, Papanicolaou AC, Ruszinkó M, Sokolov Y, Kozma R. Breathing as a fundamental rhythm of brain function. Frontiers in Neural Circuits. 2016;10:115. doi: 10.3389/fncir.2016.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy ML, Corcoran AE, Brust RD, Chang Y, Nattie EE, Dymecki SM. Activity of tachykinin1-expressing pet1 raphe neurons modulates the respiratory chemoreflex. The Journal of Neuroscience. 2017;37:1807–1819. doi: 10.1523/JNEUROSCI.2316-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henschke JU, Pakan JM. Disynaptic cerebrocerebellar pathways originating from multiple functionally distinct cortical areas. eLife. 2020;9:e59148. doi: 10.7554/eLife.59148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. The Journal of Comparative Neurology. 1990;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- Herbert H, Saper CB. Organization of medullary adrenergic and noradrenergic projections to the periaqueductal gray matter in the rat. The Journal of Comparative Neurology. 1992;315:34–52. doi: 10.1002/cne.903150104. [DOI] [PubMed] [Google Scholar]
- Hermann DM, Luppi PH, Peyron C, Hinckel P, Jouvet M. Afferent projections to the rat nuclei raphe magnus, raphe pallidus and reticularis gigantocellularis pars alpha demonstrated by iontophoretic application of choleratoxin (subunit B) Journal of Chemical Neuroanatomy. 1997;13:1–21. doi: 10.1016/s0891-0618(97)00019-7. [DOI] [PubMed] [Google Scholar]
- Hernandez JP, Xu F, Frazier DT. Medial vestibular nucleus mediates the cardiorespiratory responses to fastigial nuclear activation and hypercapnia. Journal of Applied Physiology. 2004;97:835–842. doi: 10.1152/japplphysiol.00134.2004. [DOI] [PubMed] [Google Scholar]
- Herrero JL, Khuvis S, Yeagle E, Cerf M, Mehta AD. Breathing above the brain stem: volitional control and attentional modulation in humans. Journal of Neurophysiology. 2018;119:145–159. doi: 10.1152/jn.00551.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilaire G, Viemari JC, Coulon P, Simonneau M, Bévengut M. Modulation of the respiratory rhythm generator by the pontine noradrenergic A5 and A6 groups in rodents. Respiratory Physiology & Neurobiology. 2004;143:187–197. doi: 10.1016/j.resp.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Hirsch JA, Bishop B. Respiratory sinus arrhythmia in humans: how breathing pattern modulates heart rate. The American Journal of Physiology. 1981;241:H620–H629. doi: 10.1152/ajpheart.1981.241.4.H620. [DOI] [PubMed] [Google Scholar]
- Ho CY, Gu Q, Lin YS, Lee LY. Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respiration Physiology. 2001;127:113–124. doi: 10.1016/s0034-5687(01)00241-9. [DOI] [PubMed] [Google Scholar]
- Hollinshead WH, Keswani NH. Localization of the phrenic nucleus in the spinal cord of man. The Anatomical Record. 1956;125:683–699. doi: 10.1002/ar.1091250403. [DOI] [PubMed] [Google Scholar]
- Holstege G. Anatomical study of the final common pathway for vocalization in the cat. The Journal of Comparative Neurology. 1989;284:242–252. doi: 10.1002/cne.902840208. [DOI] [PubMed] [Google Scholar]
- Holstege G. The periaqueductal gray controls brainstem emotional motor systems including respiration. Progress in Brain Research. 2014;209:379–405. doi: 10.1016/B978-0-444-63274-6.00020-5. [DOI] [PubMed] [Google Scholar]
- Holstege G, Subramanian HH. Two different motor systems are needed to generate human speech. The Journal of Comparative Neurology. 2016;524:1558–1577. doi: 10.1002/cne.23898. [DOI] [PubMed] [Google Scholar]
- Hopkins DA, Holstege G. Amygdaloid projections to the mesencephalon, pons and medulla oblongata in the cat. Experimental Brain Research. 1978;32:529–547. doi: 10.1007/BF00239551. [DOI] [PubMed] [Google Scholar]
- Horner RL. Neural control of the upper airway: integrative physiological mechanisms and relevance for sleep disordered breathing. Comprehensive Physiology. 2012;2:479–535. doi: 10.1002/cphy.c110023. [DOI] [PubMed] [Google Scholar]
- Huang Q, Zhou D, St John WM. Cerebellar control of expiratory activities of medullary neurons and spinal nerves. Journal of Applied Physiology. 1993;74:1934–1940. doi: 10.1152/jappl.1993.74.4.1934. [DOI] [PubMed] [Google Scholar]
- Huang CC, Sugino K, Shima Y, Guo C, Bai S, Mensh BD, Nelson SB, Hantman AW. Convergence of pontine and proprioceptive streams onto multimodal cerebellar granule cells. eLife. 2013;2:e00400. doi: 10.7554/eLife.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckstepp RTR, id Bihi R, Eason R, Spyer KM, Dicke N, Willecke K, Marina N, Gourine AV, Dale N. Connexin hemichannel-mediated CO2-dependent release of ATP in the medulla oblongata contributes to central respiratory chemosensitivity. The Journal of Physiology. 2010;588:3901–3920. doi: 10.1113/jphysiol.2010.192088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckstepp RTR, Cardoza KP, Henderson LE, Feldman JL. Role of parafacial nuclei in control of breathing in adult rats. The Journal of Neuroscience. 2015;35:1052–1067. doi: 10.1523/JNEUROSCI.2953-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta MF, Hashikawa T, Gayoso MJ, Harting JK. The trigemino-olivary projection in the cat: contributions of individual subnuclei. The Journal of Comparative Neurology. 1985;241:180–190. doi: 10.1002/cne.902410206. [DOI] [PubMed] [Google Scholar]
- Hülsmann S. The post-inspiratory complex (pico), what is the evidence? The Journal of Physiology. 2021;599:357–359. doi: 10.1113/JP280492. [DOI] [PubMed] [Google Scholar]
- Hülsmann S, Hagos L, Eulenburg V, Hirrlinger J. Inspiratory off-switch mediated by optogenetic activation of inhibitory neurons in the preBötzinger complex in vivo. International Journal of Molecular Sciences. 2021;22:2019. doi: 10.3390/ijms22042019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison AA, Wozniak JA, Choi HG, Conlon M, Otto RA, Abrams RM, Kosch PC. Laryngeal and diaphragmatic muscle activities and airflow patterns after birth in premature lambs. Journal of Applied Physiology. 1993;75:121–131. doi: 10.1152/jappl.1993.75.1.121. [DOI] [PubMed] [Google Scholar]
- Infante J, García A, Serrano-Cárdenas KM, González-Aguado R, Gazulla J, de Lucas EM, Berciano J. Cerebellar ataxia, neuropathy, vestibular areflexia syndrome (canvas) with chronic cough and preserved muscle stretch reflexes: evidence for selective sparing of afferent Ia fibres. Journal of Neurology. 2018;265:1454–1462. doi: 10.1007/s00415-018-8872-1. [DOI] [PubMed] [Google Scholar]
- Insalaco G, Kuna ST, Costanza BM, Catania G, Cibella F, Bellia V. Thyroarytenoid muscle activity during loaded and nonloaded breathing in adult humans. Journal of Applied Physiology. 1991;70:2410–2416. doi: 10.1152/jappl.1991.70.6.2410. [DOI] [PubMed] [Google Scholar]
- Isaev G, Murphy K, Guz A, Adams L. Areas of the brain concerned with ventilatory load compensation in awake man. The Journal of Physiology. 2002;539:935–945. doi: 10.1113/jphysiol.2001.012957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. Mechanisms of motor learning in the cerebellum. Brain Research. 2000;886:237–245. doi: 10.1016/s0006-8993(00)03142-5. [DOI] [PubMed] [Google Scholar]
- Ito J, Roy S, Liu Y, Cao Y, Fletcher M, Lu L, Boughter JD, Grün S, Heck DH. Whisker barrel cortex delta oscillations and gamma power in the awake mouse are linked to respiration. Nature Communications. 2014;5:3572. doi: 10.1038/ncomms4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izrailtyan I, Qiu J, Overdyk FJ, Erslon M, Gan TJ, Body S. Risk factors for cardiopulmonary and respiratory arrest in medical and surgical hospital patients on opioid analgesics and sedatives. PLOS ONE. 2018;13:e0194553. doi: 10.1371/journal.pone.0194553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovljevic DG, McConnell AK. Influence of different breathing frequencies on the severity of inspiratory muscle fatigue induced by high-intensity front crawl swimming. Journal of Strength and Conditioning Research. 2009;23:1169–1174. doi: 10.1519/JSC.0b013e318199d707. [DOI] [PubMed] [Google Scholar]
- Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. The Journal of Physiology. 2006;570:407–420. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczewski WA, Tashima A, Hsu P, Cui Y, Feldman JL. Role of inhibition in respiratory pattern generation. The Journal of Neuroscience. 2013;33:5454–5465. doi: 10.1523/JNEUROSCI.1595-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo AA, Brown JA, Winder DG. Danger and distress: parabrachial-extended amygdala circuits. Neuropharmacology. 2021;198:108757. doi: 10.1016/j.neuropharm.2021.108757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen VN, Alilain WJ, Crone SA. Role of propriospinal neurons in control of respiratory muscles and recovery of breathing following injury. Frontiers in Systems Neuroscience. 2019;13:84. doi: 10.3389/fnsys.2019.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Lipski J. Extensive monosynaptic inhibition of ventral respiratory group neurons by augmenting neurons in the Bötzinger complex in the cat. Experimental Brain Research. 1990;81:639–648. doi: 10.1007/BF02423514. [DOI] [PubMed] [Google Scholar]
- Jolley CJ, Luo YM, Steier J, Reilly C, Seymour J, Lunt A, Ward K, Rafferty GF, Polkey MI, Moxham J. Neural respiratory drive in healthy subjects and in COPD. The European Respiratory Journal. 2009;33:289–297. doi: 10.1183/09031936.00093408. [DOI] [PubMed] [Google Scholar]
- Jones BE, Moore RY. Ascending projections of the locus coeruleus in the rat. II. Autoradiographic Study. Brain Res. 1977;127:25–53. doi: 10.1016/0006-8993(77)90378-X. [DOI] [PubMed] [Google Scholar]
- Jones BE, Yang TZ. The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. The Journal of Comparative Neurology. 1985;242:56–92. doi: 10.1002/cne.902420105. [DOI] [PubMed] [Google Scholar]
- Jones SE, Stanić D, Dutschmann M. Dorsal and ventral aspects of the most caudal medullary reticular formation have differential roles in modulation and formation of the respiratory motor pattern in rat. Brain Structure & Function. 2016;221:4353–4368. doi: 10.1007/s00429-015-1165-x. [DOI] [PubMed] [Google Scholar]
- Ju C, Bosman LWJ, Hoogland TM, Velauthapillai A, Murugesan P, Warnaar P, van Genderen RM, Negrello M, De Zeeuw CI. Neurons of the inferior olive respond to broad classes of sensory input while subject to homeostatic control. The Journal of Physiology. 2019;597:2483–2514. doi: 10.1113/JP277413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd EN, Lewis SM, Person AL. Diverse inhibitory projections from the cerebellar interposed nucleus. eLife. 2021;10:e66231. doi: 10.7554/eLife.66231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens U. Neural pathways underlying vocal control. Neuroscience and Biobehavioral Reviews. 2002;26:235–258. doi: 10.1016/s0149-7634(01)00068-9. [DOI] [PubMed] [Google Scholar]
- Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- Kastner A, Gauthier P. Are rodents an appropriate pre-clinical model for treating spinal cord injury? examples from the respiratory system. Experimental Neurology. 2008;213:249–256. doi: 10.1016/j.expneurol.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Kastrup A, Krüger G, Glover GH, Neumann-Haefelin T, Moseley ME. Regional variability of cerebral blood oxygenation response to hypercapnia. NeuroImage. 1999;10:675–681. doi: 10.1006/nimg.1999.0505. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Iwamoto GA, Ashton JH, Cassidy SS. Responses to inflation of vagal afferents with endings in the lung of dogs. Circulation Research. 1982;51:525–531. doi: 10.1161/01.res.51.4.525. [DOI] [PubMed] [Google Scholar]
- Kaur S, Pedersen NP, Yokota S, Hur EE, Fuller PM, Lazarus M, Chamberlin NL, Saper CB. Glutamatergic signaling from the parabrachial nucleus plays a critical role in hypercapnic arousal. The Journal of Neuroscience. 2013;33:7627–7640. doi: 10.1523/JNEUROSCI.0173-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, De Luca R, Khanday MA, Bandaru SS, Thomas RC, Broadhurst RY, Venner A, Todd WD, Fuller PM, Arrigoni E, Saper CB. Role of serotonergic dorsal raphe neurons in hypercapnia-induced arousals. Nature Communications. 2020;11:2769. doi: 10.1038/s41467-020-16518-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai Y. Differential ascending projections from the male rat caudal nucleus of the tractus solitarius: an interface between local microcircuits and global macrocircuits. Frontiers in Neuroanatomy. 2018;12:63. doi: 10.3389/fnana.2018.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima T. The role of the serotonergic system in motor control. Neuroscience Research. 2018;129:32–39. doi: 10.1016/j.neures.2017.07.005. [DOI] [PubMed] [Google Scholar]
- Kc P, Haxhiu MA, Trouth CO, Balan KV, Anderson WA, Mack SO. Co (2) -induced c-fos expression in hypothalamic vasopressin containing neurons. Respiration Physiology. 2002;129:289–296. doi: 10.1016/s0034-5687(01)00321-8. [DOI] [PubMed] [Google Scholar]
- Keay KA, Feil K, Gordon BD, Herbert H, Bandler R. Spinal afferents to functionally distinct periaqueductal gray columns in the rat: an anterograde and retrograde tracing study. The Journal of Comparative Neurology. 1997;385:207–229. doi: 10.1002/(sici)1096-9861(19970825)385:2<207::aid-cne3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Kelly DH, Golub H, Carley D, Shannon DC. Pneumograms in infants who subsequently died of sudden infant death syndrome. The Journal of Pediatrics. 1986;109:249–254. doi: 10.1016/s0022-3476(86)80380-8. [DOI] [PubMed] [Google Scholar]
- Kepecs A, Uchida N, Mainen ZF. The sniff as a unit of olfactory processing. Chemical Senses. 2006;31:167–179. doi: 10.1093/chemse/bjj016. [DOI] [PubMed] [Google Scholar]
- Khoshyomn S, Lew S, DeMattia J, Singer EB, Penar PL. Neuronal synchrony: a versatile code for the definition of relations. Journal of Neuro-Oncology. 1999;45:111–116. doi: 10.1023/A:1006375316331. [DOI] [PubMed] [Google Scholar]
- Kim AM, Keenan BT, Jackson N, Chan EL, Staley B, Poptani H, Torigian DA, Pack AI, Schwab RJ. Tongue fat and its relationship to obstructive sleep apnea. Sleep. 2014;37:1639–1648. doi: 10.5665/sleep.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Hadley SH, Maddison M, Patil M, Cha B, Kollarik M, Taylor-Clark TE. Mapping of sensory nerve subsets within the vagal ganglia and the brainstem using reporter mice for pirt, TRPV1, 5-HT3, and tac1 expression. ENeuro. 2020;7:ENEURO.0494-19.2020. doi: 10.1523/ENEURO.0494-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King TL, Heesch CM, Clark CG, Kline DD, Hasser EM. Hypoxia activates nucleus tractus solitarii neurons projecting to the paraventricular nucleus of the hypothalamus. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2012;302:R1219–R1232. doi: 10.1152/ajpregu.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC, Thach BT. The sudden infant death syndrome. The New England Journal of Medicine. 2009;361:795–805. doi: 10.1056/NEJMra0803836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC, Haynes RL. The serotonin brainstem hypothesis for the sudden infant death syndrome. Journal of Neuropathology and Experimental Neurology. 2019;78:765–779. doi: 10.1093/jnen/nlz062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline DD, Buniel MCF, Glazebrook P, Peng Y-J, Ramirez-Navarro A, Prabhakar NR, Kunze DL. Kv1.1 deletion augments the afferent hypoxic chemosensory pathway and respiration. The Journal of Neuroscience. 2005;25:3389–3399. doi: 10.1523/JNEUROSCI.4556-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingbeil J, Wawrzyniak M, Stockert A, Brandt ML, Schneider HR, Metelmann M, Saur D. Pathological laughter and crying: insights from lesion network-symptom-mapping. Brain. 2021;144:3264–3276. doi: 10.1093/brain/awab224. [DOI] [PubMed] [Google Scholar]
- Kluger DS, Gross J. Respiration modulates oscillatory neural network activity at rest. PLOS Biology. 2021;19:e3001457. doi: 10.1371/journal.pbio.3001457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpas J, Jakus J. The expiration reflex from the vocal folds. Acta Physiologica Hungarica. 2000;87:201–215. doi: 10.1556/APhysiol.87.2000.3.1. [DOI] [PubMed] [Google Scholar]
- Koshiya N, Oku Y, Yokota S, Oyamada Y, Yasui Y, Okada Y. Anatomical and functional pathways of rhythmogenic inspiratory premotor information flow originating in the pre-Bötzinger complex in the rat medulla. Neuroscience. 2014;268:194–211. doi: 10.1016/j.neuroscience.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. Journal of Applied Physiology. 2006;101:618–627. doi: 10.1152/japplphysiol.00252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T, Yanagihara Y, Yamaguchi H, Fukumori R. Excitatory amino acid receptors in the paraventricular hypothalamic nucleus mediate pressor response induced by carotid body chemoreceptor stimulation in rats. Clinical and Experimental Hypertension. 1997;19:1117–1134. doi: 10.3109/10641969709083208. [DOI] [PubMed] [Google Scholar]
- Kubo R, Aiba A, Hashimoto K. The anatomical pathway from the mesodiencephalic junction to the inferior olive relays perioral sensory signals to the cerebellum in the mouse. The Journal of Physiology. 2018;596:3775–3791. doi: 10.1113/JP275836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupari J, Häring M, Agirre E, Castelo-Branco G, Ernfors P. An atlas of vagal sensory neurons and their molecular specialization. Cell Reports. 2019;27:2508–2523. doi: 10.1016/j.celrep.2019.04.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurnikova A, Deschênes M, Kleinfeld D. Functional brain stem circuits for control of nose motion. Journal of Neurophysiology. 2019;121:205–217. doi: 10.1152/jn.00608.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laczika K, Graber OP, Tucek G, Lohninger A, Fliri N, Berka-Schmid G, Masel EK, Zielinski CC. “ IL flauto magico ” still works: moza’t's secret of ventilation. Multidisciplinary Respiratory Medicine. 2013;8:23. doi: 10.1186/2049-6958-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley PM. Serotoninergic and non-serotoninergic responses of phrenic motoneurones to raphe stimulation in the cat. The Journal of Physiology. 1986;380:373–385. doi: 10.1113/jphysiol.1986.sp016291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, White TE, Coutts MA, Jones AL, Sandhu MS, Bloom DC, Bolser DC, Yates BJ, Fuller DD, Reier PJ. Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. The Journal of Comparative Neurology. 2008;511:692–709. doi: 10.1002/cne.21864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzi AM, Ottaviani G, Matturri L. Ontogenesis of human cerebellar cortex and biopathological characterization in sudden unexplained fetal and infant death. Virchows Archiv. 2007a;450:31–40. doi: 10.1007/s00428-006-0311-5. [DOI] [PubMed] [Google Scholar]
- Lavezzi AM, Ottaviani G, Mauri M, Matturri L. Biopathology of the dentate-olivary complex in sudden unexplained perinatal death and sudden infant death syndrome related to maternal cigarette smoking. Neurological Research. 2007b;29:525–532. doi: 10.1179/016164107X166308. [DOI] [PubMed] [Google Scholar]
- Lavezzi AM, Corna MF, Repetti ML, Matturri L. Cerebellar Purkinje cell vulnerability to prenatal nicotine exposure in sudden unexplained perinatal death. Folia Neuropathologica. 2013;51:290–301. doi: 10.5114/fn.2013.39718. [DOI] [PubMed] [Google Scholar]
- Lebow MA, Chen A. Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Molecular Psychiatry. 2016;21:450–463. doi: 10.1038/mp.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Kim MA, Valentino RJ, Waterhouse BD. Glutamatergic afferent projections to the dorsal raphe nucleus of the rat. Brain Research. 2003;963:57–71. doi: 10.1016/s0006-8993(02)03841-6. [DOI] [PubMed] [Google Scholar]
- Lee HS, Park SH, Song WC, Waterhouse BD. Retrograde study of hypocretin-1 (orexin-A) projections to subdivisions of the dorsal raphe nucleus in the rat. Brain Research. 2005;1059:35–45. doi: 10.1016/j.brainres.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Lee D, Artero EG, Sui X, Blair SN. Mortality trends in the general population: the importance of cardiorespiratory fitness. Journal of Psychopharmacology. 2010;24:27–35. doi: 10.1177/1359786810382057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Chen ML, Abeshaus S, Poliakov A, Ojemann JG. Posterior fossa tumors and their impact on sleep and ventilatory control: a clinical perspective. Respiratory Physiology & Neurobiology. 2013;189:261–271. doi: 10.1016/j.resp.2013.05.027. [DOI] [PubMed] [Google Scholar]
- Lee LY, Yu J. Sensory nerves in lung and airways. Comprehensive Physiology. 2014;4:287–324. doi: 10.1002/cphy.c130020. [DOI] [PubMed] [Google Scholar]
- Lee J, Wang W, Sabatini BL. Anatomically segregated basal ganglia pathways allow parallel behavioral modulation. Nature Neuroscience. 2020;23:1388–1398. doi: 10.1038/s41593-020-00712-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leirão IP, Colombari DSA, da Silva GSF, Zoccal DB. Lesion of serotonergic afferents to the retrotrapezoid nucleus impairs the tachypneic response to hypercapnia in unanesthetized animals. Neuroscience. 2021;452:63–77. doi: 10.1016/j.neuroscience.2020.11.005. [DOI] [PubMed] [Google Scholar]
- Lesage F, Barhanin J. Molecular physiology of ph-sensitive background K (2P) channels. Physiology. 2011;26:424–437. doi: 10.1152/physiol.00029.2011. [DOI] [PubMed] [Google Scholar]
- Li YQ, Takada M, Mizuno N. The sites of origin of serotoninergic afferent fibers in the trigeminal motor, facial, and hypoglossal nuclei in the rat. Neuroscience Research. 1993;17:307–313. doi: 10.1016/0168-0102(93)90114-6. [DOI] [PubMed] [Google Scholar]
- Li N, Li A, Nattie E. Focal microdialysis of CO₂ in the perifornical-hypothalamic area increases ventilation during wakefulness but not NREM sleep. Respiratory Physiology & Neurobiology. 2013;185:349–355. doi: 10.1016/j.resp.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Janczewski WA, Yackle K, Kam K, Pagliardini S, Krasnow MA, Feldman JL. The peptidergic control circuit for sighing. Nature. 2016;530:293–297. doi: 10.1038/nature16964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Jiang H, Shen X, Yang W, Guo C, Wang Z, Xiao M, Cui L, Luo W, Kim BS, Chen Z, Huang AJW, Liu Q. Sneezing reflex is mediated by a peptidergic pathway from nose to brainstem. Cell. 2021;184:3762–3773. doi: 10.1016/j.cell.2021.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima JC, Oliveira LM, Botelho MT, Moreira TS, Takakura AC. The involvement of the pathway connecting the substantia nigra, the periaqueductal gray matter and the retrotrapezoid nucleus in breathing control in a rat model of parkinson’s disease. Experimental Neurology. 2018;302:46–56. doi: 10.1016/j.expneurol.2018.01.003. [DOI] [PubMed] [Google Scholar]
- Lima JD, Sobrinho CR, Santos LK, Takakura AC, Moreira TS. M4-muscarinic acetylcholine receptor into the pedunculopontine tegmental nucleus mediates respiratory modulation of conscious rats. Respiratory Physiology & Neurobiology. 2019a;269:103254. doi: 10.1016/j.resp.2019.103254. [DOI] [PubMed] [Google Scholar]
- Lima JD, Sobrinho CR, Falquetto B, Santos LK, Takakura AC, Mulkey DK, Moreira TS. Cholinergic neurons in the pedunculopontine tegmental nucleus modulate breathing in rats by direct projections to the retrotrapezoid nucleus. The Journal of Physiology. 2019b;597:1919–1934. doi: 10.1113/JP277617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeman S, Hong S, Kros L, Mejias JF, Romano V, Oostenveld R, Negrello M, Bosman LWJ, De Zeeuw CI. Cerebellar purkinje cells can differentially modulate coherence between sensory and motor cortex depending on region and behavior. PNAS. 2021;118:e2015292118. doi: 10.1073/pnas.2015292118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnman C, Moulton EA, Barmettler G, Becerra L, Borsook D. Neuroimaging of the periaqueductal gray: state of the field. NeuroImage. 2012;60:505–522. doi: 10.1016/j.neuroimage.2011.11.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippiello P, Hoxha E, Volpicelli F, Lo Duca G, Tempia F, Miniaci MC. Noradrenergic modulation of the parallel fiber-Purkinje cell synapse in mouse cerebellum. Neuropharmacology. 2015;89:33–42. doi: 10.1016/j.neuropharm.2014.08.016. [DOI] [PubMed] [Google Scholar]
- Lipski J, Zhang X, Kruszewska B, Kanjhan R. Morphological study of long axonal projections of ventral medullary inspiratory neurons in the rat. Brain Research. 1994;640:171–184. doi: 10.1016/0006-8993(94)91871-6. [DOI] [PubMed] [Google Scholar]
- Liu H, Mihailoff GA. Hypothalamopontine projections in the rat: anterograde axonal transport studies utilizing light and electron microscopy. The Anatomical Record. 1999;255:428–451. doi: 10.1002/(SICI)1097-0185(19990801)255:4<428::AID-AR9>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Liu Y, Qi S, Thomas F, Correia BL, Taylor AP, Sillitoe RV, Heck DH. Loss of cerebellar function selectively affects intrinsic rhythmicity of eupneic breathing. Biology Open. 2020;9:bio048785. doi: 10.1242/bio.048785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Fu C, Yu H, Wang Y, Shi L, Hao Y, Yuan F, Zhang X, Wang S. Respiratory control by phox2b-expressing neurons in a locus coeruleus–prebötzinger complex circuit. Neuroscience Bulletin. 2021a;37:31–44. doi: 10.1007/s12264-020-00519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Hu T, Zhang MQ, Xu CY, Yuan MY, Li RX. Differential efferent projections of gabaergic neurons in the basolateral and central nucleus of amygdala in mice. Neuroscience Letters. 2021b;745:135621. doi: 10.1016/j.neulet.2020.135621. [DOI] [PubMed] [Google Scholar]
- Livingston CA, Berger AJ. Immunocytochemical localization of GABA in neurons projecting to the ventrolateral nucleus of the solitary tract. Brain Research. 1989;494:143–150. doi: 10.1016/0006-8993(89)90153-4. [DOI] [PubMed] [Google Scholar]
- Livneh Y, Andermann ML. Cellular activity in insular cortex across seconds to hours: Sensations and predictions of bodily states. Neuron. 2021;109:3576–3593. doi: 10.1016/j.neuron.2021.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. The Journal of Physiology. 1980;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás RR. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988;242:1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- Loewy AD, Burton H. Nuclei of the solitary tract: efferent projections to the lower brain stem and spinal cord of the cat. The Journal of Comparative Neurology. 1978;181:421–449. doi: 10.1002/cne.901810211. [DOI] [PubMed] [Google Scholar]
- Lois JH, Rice CD, Yates BJ. Neural circuits controlling diaphragm function in the cat revealed by transneuronal tracing. Journal of Applied Physiology. 2009;106:138–152. doi: 10.1152/japplphysiol.91125.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoMauro A, Aliverti A. Sex and gender in respiratory physiology. European Respiratory Review. 2021;30:210038. doi: 10.1183/16000617.0038-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzi-Filho G, Rankin F, Bies I, Douglas Bradley T. Effects of inhaled carbon dioxide and oxygen on cheyne-stokes respiration in patients with heart failure. American Journal of Respiratory and Critical Care Medicine. 1999;159:1490–1498. doi: 10.1164/ajrccm.159.5.9810040. [DOI] [PubMed] [Google Scholar]
- Loughlin SE, Foote SL, Bloom FE. Efferent projections of nucleus locus coeruleus: topographic organization of cells of origin demonstrated by three-dimensional reconstruction. Neuroscience. 1986;18:291–306. doi: 10.1016/0306-4522(86)90155-7. [DOI] [PubMed] [Google Scholar]
- Lowe AA, Gionhaku N, Takeuchi K, Fleetham JA. Three-dimensional CT reconstructions of tongue and airway in adult subjects with obstructive sleep apnea. American Journal of Orthodontics and Dentofacial Orthopedics. 1986;90:364–374. doi: 10.1016/0889-5406(86)90002-8. [DOI] [PubMed] [Google Scholar]
- Lu L, Cao Y, Tokita K, Heck DH, Boughter JD. Medial cerebellar nuclear projections and activity patterns link cerebellar output to orofacial and respiratory behavior. Frontiers in Neural Circuits. 2013;7:56. doi: 10.3389/fncir.2013.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu JH, Wang XQ, Huang Y, Qiu YH, Peng YP. GABAergic neurons in cerebellar interposed nucleus modulate cellular and humoral immunity via hypothalamic and sympathetic pathways. Journal of Neuroimmunology. 2015;283:30–38. doi: 10.1016/j.jneuroim.2015.04.013. [DOI] [PubMed] [Google Scholar]
- Lu H, Yang B, Jaeger D. Cerebellar nuclei neurons show only small excitatory responses to optogenetic olivary stimulation in transgenic mice: in vivo and in vitro studies. Frontiers in Neural Circuits. 2016;10:21. doi: 10.3389/fncir.2016.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucier GE, Egizii R. Central projections of the ethmoidal nerve of the cat as determined by the horseradish peroxidase tracer technique. The Journal of Comparative Neurology. 1986;247:123–132. doi: 10.1002/cne.902470108. [DOI] [PubMed] [Google Scholar]
- Ludlow CL. Laryngeal reflexes: physiology, technique, and clinical use. Journal of Clinical Neurophysiology. 2015;32:284–293. doi: 10.1097/WNP.0000000000000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden T. Observations on the respiratory centres in the cat. The Journal of Physiology. 1923;57:153–160. doi: 10.1113/jphysiol.1923.sp002052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppi PH, Aston-Jones G, Akaoka H, Chouvet G, Jouvet M. Afferent projections to the rat locus coeruleus demonstrated by retrograde and anterograde tracing with cholera-toxin B subunit and phaseolus vulgaris leucoagglutinin. Neuroscience. 1995;65:119–160. doi: 10.1016/0306-4522(94)00481-j. [DOI] [PubMed] [Google Scholar]
- Luskin AT, Bhatti DL, Mulvey B, Pedersen CE, Girven KS, Oden-Brunson H, Kimbell K, Blackburn T, Sawyer A, Gereau RW, Dougherty JD, Bruchas MR. Extended amygdala-parabrachial circuits alter threat assessment and regulate feeding. Science Advances. 2021;7:eabd3666. doi: 10.1126/sciadv.abd3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutherer LO, Williams JL. Stimulating fastigial nucleus pressor region elicits patterned respiratory responses. The American Journal of Physiology. 1986;250:R418–R426. doi: 10.1152/ajpregu.1986.250.3.R418. [DOI] [PubMed] [Google Scholar]
- Lutherer LO, Williams JL, Everse SJ. Neurons of the rostral fastigial nucleus are responsive to cardiovascular and respiratory challenges. Journal of the Autonomic Nervous System. 1989;27:101–111. doi: 10.1016/0165-1838(89)90092-1. [DOI] [PubMed] [Google Scholar]
- Mack SO, Kc P, Wu M, Coleman BR, Tolentino-Silva FP, Haxhiu MA. Paraventricular oxytocin neurons are involved in neural modulation of breathing. Journal of Applied Physiology. 2002;92:826–834. doi: 10.1152/japplphysiol.00839.2001. [DOI] [PubMed] [Google Scholar]
- Mack SO, Wu M, Kc P, Haxhiu MA. Stimulation of the hypothalamic paraventricular nucleus modulates cardiorespiratory responses via oxytocinergic innervation of neurons in pre-botzinger complex. Journal of Applied Physiology. 2007;102:189–199. doi: 10.1152/japplphysiol.00522.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLarnon AM, Hewitt GP. The evolution of human speech: the role of enhanced breathing control. American Journal of Physical Anthropology. 1999;109:341–363. doi: 10.1002/(SICI)1096-8644(199907)109:3<341::AID-AJPA5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Mador MJ, Tobin MJ. Apneustic breathing a characteristic feature of brainstem compression in achondroplasia? Chest. 1990;97:877–883. doi: 10.1378/chest.97.4.877. [DOI] [PubMed] [Google Scholar]
- Magoun HW, Atlas D, Ingersoll EH, Ranson SW. Associated facial, vocal and respiratory components of emotional expression: an experimental study. The Journal of Neurology and Psychopathology. 1937;17:241–255. doi: 10.1136/jnnp.s1-17.67.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler DA, Shuhart CR, Brew E, Stukel TA. Ventilatory responses and entrainment of breathing during rowing. Medicine and Science in Sports and Exercise. 1991;23:186–192. [PubMed] [Google Scholar]
- Manabe M, Ezure K. Decrementing expiratory neurons of the bötzinger complex. I. response to lung inflation and axonal projection. Experimental Brain Research. 1988;72:150–158. doi: 10.1007/BF00248510. [DOI] [PubMed] [Google Scholar]
- Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. PNAS. 2007;104:13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchenko V, Koizumi H, Mosher B, Koshiya N, Tariq MF, Bezdudnaya TG, Zhang R, Molkov YI, Rybak IA, Smith JC. Perturbations of respiratory rhythm and pattern by disrupting synaptic inhibition within pre-Bötzinger and Bötzinger complexes. ENeuro. 2016;3:eNeuro. doi: 10.1523/ENEURO.0011-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marckwald M. Werden de athembewegungen vom Rückenmarke beherrscht. Mitteilungen Der Naturforschenden Gesellschaft Bern. 1889;1:59–74. [Google Scholar]
- Marckwald M. Die bedeutung des mittelhirns für die athmung. Zeitschrift Fur Biologie. 1890;26:259–289. [Google Scholar]
- Marina N, Abdala AP, Trapp S, Li A, Nattie EE, Hewinson J, Smith JC, Paton JFR, Gourine AV. Essential role of phox2b-expressing ventrolateral brainstem neurons in the chemosensory control of inspiration and expiration. The Journal of Neuroscience. 2010;30:12466–12473. doi: 10.1523/JNEUROSCI.3141-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino PF, Davis S, Opansky C, Krause K, Bonis JM, Pan LG, Qian B, Forster HV. The cerebellar fastigial nucleus contributes to CO2-H+ ventilatory sensitivity in awake goats. Respiratory Physiology & Neurobiology. 2007;157:242–251. doi: 10.1016/j.resp.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarazzo V, Caccialupi L, Schaller F, Shvarev Y, Kourdougli N, Bertoni A, Menuet C, Voituron N, Deneris E, Gaspar P, Bezin L, Durbec P, Hilaire G, Muscatelli F. Necdin shapes serotonergic development and SERT activity modulating breathing in a mouse model for prader-willi syndrome. eLife. 2017;6:e32640. doi: 10.7554/eLife.32640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matschke J, Sperhake JP, Wilke N, Püschel K, Glatzel M. Cerebellar heterotopia of infancy in sudden infant death syndrome: an observational neuropathological study of four cases. International Journal of Legal Medicine. 2020;134:2143–2147. doi: 10.1007/s00414-020-02316-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita M, Hosoya Y, Ikeda M. Anatomical organization of the spinocerebellar system in the cat, as studied by retrograde transport of horseradish peroxidase. The Journal of Comparative Neurology. 1979;184:81–106. doi: 10.1002/cne.901840106. [DOI] [PubMed] [Google Scholar]
- Mazzone SB, Reynolds SM, Mori N, Kollarik M, Farmer DG, Myers AC, Canning BJ. Selective expression of a sodium pump isozyme by cough receptors and evidence for its essential role in regulating cough. The Journal of Neuroscience. 2009;29:13662–13671. doi: 10.1523/JNEUROSCI.4354-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone SB, Undem BJ. Vagal afferent innervation of the airways in health and disease. Physiological Reviews. 2016;96:975–1024. doi: 10.1152/physrev.00039.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone SB, Bautista TG, Verberne AJM, Trewella MW, Farrell MJ, McGovern AE. Descending modulation of laryngeal vagal sensory processing in the brainstem orchestrated by the submedius thalamic nucleus. The Journal of Neuroscience. 2020;40:9426–9439. doi: 10.1523/JNEUROSCI.2430-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllen RM, Spyer KM. The location of cardiac vagal preganglionic motoneurones in the medulla of the cat. The Journal of Physiology. 1976;258:187–204. doi: 10.1113/jphysiol.1976.sp011414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt RA, Tiran-Cappello A, Shen H, Balderas I, Britt JP, Marino RAM, Chung SL, Richie CT, Harvey BK, Bonci A. Serotonergic versus nonserotonergic dorsal raphe projection neurons: differential participation in reward circuitry. Cell Reports. 2014;8:1857–1869. doi: 10.1016/j.celrep.2014.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland DH, Lund JP. An investigation of the coupling between respiration, mastication, and swallowing in the awake rabbit. Journal of Neurophysiology. 1993;69:95–108. doi: 10.1152/jn.1993.69.1.95. [DOI] [PubMed] [Google Scholar]
- McGovern AE, Davis-Poynter N, Yang SK, Simmons DG, Farrell MJ, Mazzone SB. Evidence for multiple sensory circuits in the brain arising from the respiratory system: an anterograde viral tract tracing study in rodents. Brain Structure & Function. 2015a;220:3683–3699. doi: 10.1007/s00429-014-0883-9. [DOI] [PubMed] [Google Scholar]
- McGovern AE, Driessen AK, Simmons DG, Powell J, Davis-Poynter N, Farrell MJ, Mazzone SB. Distinct brainstem and forebrain circuits receiving tracheal sensory neuron inputs revealed using a novel conditional anterograde transsynaptic viral tracing system. Journal of Neuroscience. 2015b;35:7041–7055. doi: 10.1523/JNEUROSCI.5128-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay LC, Adams L, Frackowiak RSJ, Corfield DR. A bilateral cortico-bulbar network associated with breath holding in humans, determined by functional magnetic resonance imaging. NeuroImage. 2008;40:1824–1832. doi: 10.1016/j.neuroimage.2008.01.058. [DOI] [PubMed] [Google Scholar]
- McKay LC, Critchley HD, Murphy K, Frackowiak RSJ, Corfield DR. Sub-cortical and brainstem sites associated with chemo-stimulated increases in ventilation in humans. NeuroImage. 2010;49:2526–2535. doi: 10.1016/j.neuroimage.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Meigh L, Greenhalgh SA, Rodgers TL, Cann MJ, Roper DI, Dale N. CO₂directly modulates connexin 26 by formation of carbamate bridges between subunits. eLife. 2013;2:e01213. doi: 10.7554/eLife.01213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnychuk MC, Robertson IH, Plini ERG, Dockree PM. A bridge between the breath and the brain: synchronization of respiration, A pupillometric marker of the locus coeruleus, and an EEG marker of attentional control state. Brain Sciences. 2021;11:1324. doi: 10.3390/brainsci11101324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena-Segovia J, Bolam JP. Rethinking the pedunculopontine nucleus: from cellular organization to function. Neuron. 2017;94:7–18. doi: 10.1016/j.neuron.2017.02.027. [DOI] [PubMed] [Google Scholar]
- Menétrey D, Leah J, de Pommery J. Efferent projections of the paratrigeminal nucleus in the rat. Neuroscience Letters. 1987;73:48–52. doi: 10.1016/0304-3940(87)90029-2. [DOI] [PubMed] [Google Scholar]
- Merrill EG. The lateral respiratory neurones of the medulla: their associations with nucleus ambiguus, nucleus retroambigualis, the spinal accessory nucleus and the spinal cord. Brain Research. 1970;24:11–28. doi: 10.1016/0006-8993(70)90271-4. [DOI] [PubMed] [Google Scholar]
- Merrill EG, Lipski J, Kubin L, Fedorko L. Origin of the expiratory inhibition of nucleus tractus solitarius inspiratory neurones. Brain Research. 1983;263:43–50. doi: 10.1016/0006-8993(83)91198-8. [DOI] [PubMed] [Google Scholar]
- Merrill EG, Fedorko L. Monosynaptic inhibition of phrenic motoneurons: a long descending projection from Bötzinger neurons. The Journal of Neuroscience. 1984;4:2350–2353. doi: 10.1523/JNEUROSCI.04-09-02350.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret AE, Kroll J, Ritz T. Panic disorder comorbidity with medical conditions and treatment implications. Annual Review of Clinical Psychology. 2017;13:209–240. doi: 10.1146/annurev-clinpsy-021815-093044. [DOI] [PubMed] [Google Scholar]
- Miescher-Rüsch F. Bemerkungen zur lehre von den athembewegungen. Arch Anat Physiol. 1885;355:355–380. [Google Scholar]
- Mifflin SW. Arterial chemoreceptor input to nucleus tractus solitarius. The American Journal of Physiology. 1992;263:R368–R375. doi: 10.1152/ajpregu.1992.263.2.R368. [DOI] [PubMed] [Google Scholar]
- Mihailoff GA, Kosinski RJ, Azizi SA, Border BG. Survey of noncortical afferent projections to the basilar pontine nuclei: a retrograde tracing study in the rat. The Journal of Comparative Neurology. 1989;282:617–643. doi: 10.1002/cne.902820411. [DOI] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JR, Zuperku EJ, Stuth EAE, Banerjee A, Hopp FA, Stucke AG. A subregion of the parabrachial nucleus partially mediates respiratory rate depression from intravenous remifentanil in young and adult rabbits. Anesthesiology. 2017;127:502–514. doi: 10.1097/ALN.0000000000001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milsom WK, Burleson ML. Peripheral arterial chemoreceptors and the evolution of the carotid body. Respiratory Physiology & Neurobiology. 2007;157:4–11. doi: 10.1016/j.resp.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Miura M, Reis DJ. Cerebellum: a pressor response elicited from the fastigial nucleus and its efferent pathway in brainstem. Brain Research. 1969;13:595–599. doi: 10.1016/0006-8993(69)90269-8. [DOI] [PubMed] [Google Scholar]
- Miura M, Reis DJ. The role of the solitary and paramedian reticular nuclei in mediating cardiovascular reflex responses from carotid baro- and chemoreceptors. The Journal of Physiology. 1972;223:525–548. doi: 10.1113/jphysiol.1972.sp009861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M, Takayama K. The site of the origin of the so-called fastigial pressor response. Brain Research. 1988;473:352–358. doi: 10.1016/0006-8993(88)90865-7. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Tanaka I, Ezure K. Excitatory and inhibitory synaptic inputs shape the discharge pattern of pump neurons of the nucleus tractus solitarii in the rat. Experimental Brain Research. 1999;129:191–200. doi: 10.1007/s002210050889. [DOI] [PubMed] [Google Scholar]
- Moga MM, Herbert H, Hurley KM, Yasui Y, Gray TS, Saper CB. Organization of cortical, basal forebrain, and hypothalamic afferents to the parabrachial nucleus in the rat. The Journal of Comparative Neurology. 1990;295:624–661. doi: 10.1002/cne.902950408. [DOI] [PubMed] [Google Scholar]
- Molinari HH, Schultze KE, Strominger NL. Gracile, cuneate, and spinal trigeminal projections to inferior olive in rat and monkey. The Journal of Comparative Neurology. 1996;375:467–480. doi: 10.1002/(SICI)1096-9861(19961118)375:3<467::AID-CNE9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Mong FSF, Chen YC, Lu CH. Dendritic ramifications of trigeminal motor neurons innervating jaw-closing muscles of rats. Journal of the Neurological Sciences. 1988;86:251–264. doi: 10.1016/0022-510x(88)90103-7. [DOI] [PubMed] [Google Scholar]
- Moolenaar GM, Rucker HK. Autoradiographic study of brain stem projections from fastigal pressor areas. Brain Research. 1976;114:492–496. doi: 10.1016/0006-8993(76)90970-7. [DOI] [PubMed] [Google Scholar]
- Moore DB, Madorsky I, Paiva M, Barrow Heaton M. Ethanol exposure alters neurotrophin receptor expression in the rat central nervous system: effects of prenatal exposure. Journal of Neurobiology. 2004;60:101–113. doi: 10.1002/neu.20009. [DOI] [PubMed] [Google Scholar]
- Moore JD, Deschênes M, Furuta T, Huber D, Smear MC, Demers M, Kleinfeld D. Hierarchy of orofacial rhythms revealed through whisking and breathing. Nature. 2013;497:205–210. doi: 10.1038/nature12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JD, Kleinfeld D, Wang F. How the brainstem controls orofacial behaviors comprised of rhythmic actions. Trends in Neurosciences. 2014;37:370–380. doi: 10.1016/j.tins.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morice AH, Millqvist E, Bieksiene K, Birring SS, Dicpinigaitis P, Domingo Ribas C, Hilton Boon M, Kantar A, Lai K, McGarvey L, Rigau D, Satia I, Smith J, Song WJ, Tonia T, van den Berg JWK, van Manen MJG, Zacharasiewicz A. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. European Respiratory Journal. 2020;55:1901136. doi: 10.1183/13993003.01136-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris KF, Nuding SC, Segers LS, Iceman KE, O’Connor R, Dean JB, Ott MM, Alencar PA, Shuman D, Horton KK, Taylor-Clark TE, Bolser DC, Lindsey BG. Carotid chemoreceptors tune breathing via multipath routing: reticular chain and loop operations supported by parallel spike train correlations. Journal of Neurophysiology. 2018;119:700–722. doi: 10.1152/jn.00630.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortola J, Sant’Ambrogio G, Clement MG. Localization of irritant receptors in the airways of the dog. Respiration Physiology. 1975;24:107–114. doi: 10.1016/0034-5687(75)90125-5. [DOI] [PubMed] [Google Scholar]
- Mortola JP. Lung viscoelasticity: implications on breathing and forced expiration. Clinical Pulmonary Medicine. 2013;20:144–148. doi: 10.1097/CPM.0b013e31828fc9d6. [DOI] [Google Scholar]
- Motta SC, Carobrez AP, Canteras NS. The periaqueductal gray and primal emotional processing critical to influence complex defensive responses, fear learning and reward seeking. Neuroscience and Biobehavioral Reviews. 2017;76:39–47. doi: 10.1016/j.neubiorev.2016.10.012. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nature Neuroscience. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Mutolo D. Brainstem mechanisms underlying the cough reflex and its regulation. Respiratory Physiology & Neurobiology. 2017;243:60–76. doi: 10.1016/j.resp.2017.05.008. [DOI] [PubMed] [Google Scholar]
- Nagai T, Satoh K, Imamoto K, Maeda T. Divergent projections of catecholamine neurons of the locus coeruleus as revealed by fluorescent retrograde double labeling technique. Neuroscience Letters. 1981;23:117–123. doi: 10.1016/0304-3940(81)90027-6. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Niwa M, Sasaki SI, Ichikawa T, Hirai N. Morphology of single primary spindle afferents of the intercostal muscles in the cat. The Journal of Comparative Neurology. 1998;398:459–472. doi: 10.1002/(SICI)1096-9861(19980907)398:4<459::AID-CNE1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Nattie E. CO2, brainstem chemoreceptors and breathing. Progress in Neurobiology. 1999;59:299–331. doi: 10.1016/S0301-0082(99)00008-8. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. CO2 dialysis in nucleus tractus solitarius region of rat increases ventilation in sleep and wakefulness. Journal of Applied Physiology. 2002;92:2119–2130. doi: 10.1152/japplphysiol.01128.2001. [DOI] [PubMed] [Google Scholar]
- Nattie E, Li A. Muscimol dialysis into the caudal aspect of the nucleus tractus solitarii of conscious rats inhibits chemoreception. Respiratory Physiology & Neurobiology. 2008;164:394–400. doi: 10.1016/j.resp.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete-Opazo AA, Cook-Snyder DR, Miller JR, Callison JJ, McCarthy N, Palkovic B, Stuth EAE, Zuperku EJ, Stucke AG. Endogenous glutamatergic inputs to the parabrachial nucleus/kölliker-fuse complex determine respiratory rate. Respiratory Physiology & Neurobiology. 2020;277:103401. doi: 10.1016/j.resp.2020.103401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff RA, Wang J, Baxi S, Evans C, Mendelowitz D. Respiratory sinus arrhythmia: endogenous activation of nicotinic receptors mediates respiratory modulation of brainstem cardioinhibitory parasympathetic neurons. Circulation Research. 2003;93:565–572. doi: 10.1161/01.RES.0000090361.45027.5B. [DOI] [PubMed] [Google Scholar]
- Negrello M, Warnaar P, Romano V, Owens CB, Lindeman S, Iavarone E, Spanke JK, Bosman LWJ, De Zeeuw CI. Quasiperiodic rhythms of the inferior olive. PLOS Computational Biology. 2019;15:e1006475. doi: 10.1371/journal.pcbi.1006475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni RJ, Luo PH, Shu YM, Chen JT, Zhou JN. Whole-brain mapping of afferent projections to the bed nucleus of the stria terminalis in tree shrews. Neuroscience. 2016;333:162–180. doi: 10.1016/j.neuroscience.2016.07.017. [DOI] [PubMed] [Google Scholar]
- Nijjar S, Maddison D, Meigh L, de Wolf E, Rodgers T, Cann MJ, Dale N. Opposing modulation of cx26 gap junctions and hemichannels by CO2. The Journal of Physiology. 2021;599:103–118. doi: 10.1113/JP280747. [DOI] [PubMed] [Google Scholar]
- Nisimaru N. Cardiovascular modules in the cerebellum. The Japanese Journal of Physiology. 2004;54:431–448. doi: 10.2170/jjphysiol.54.431. [DOI] [PubMed] [Google Scholar]
- Nobis WP, Schuele S, Templer JW, Zhou G, Lane G, Rosenow JM, Zelano C. Amygdala-stimulation-induced apnea is attention and nasal-breathing dependent. Annals of Neurology. 2018;83:460–471. doi: 10.1002/ana.25178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobis WP, González Otárula KA, Templer JW, Gerard EE, VanHaerents S, Lane G, Zhou G, Rosenow JM, Zelano C, Schuele S. The effect of seizure spread to the amygdala on respiration and onset of ictal central apnea. Journal of Neurosurgery. 2019;132:1313–1323. doi: 10.3171/2019.1.JNS183157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka S, Unno T, Ohta Y, Mori S. Sneeze-evoking region within the brainstem. Brain Research. 1990;511:265–270. doi: 10.1016/0006-8993(90)90171-7. [DOI] [PubMed] [Google Scholar]
- Nonomura K, Woo SH, Chang RB, Gillich A, Qiu Z, Francisco AG, Ranade SS, Liberles SD, Patapoutian A. Piezo2 senses airway stretch and mediates lung inflation-induced apnoea. Nature. 2017;541:176–181. doi: 10.1038/nature20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgren R. Projections from the nucleus of the solitary tract in the rat. Neuroscience. 1978;3:207–218. doi: 10.1016/0306-4522(78)90102-1. [DOI] [PubMed] [Google Scholar]
- Novello M, Bosman LWJ, De Zeeuw CI. A systematic review of direct outputs from the cerebellum to the brainstem and diencephalon in mammals. Cerebellum. 2022;1:01499-w. doi: 10.1007/s12311-022-01499-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núñez-Abades PA, Portillo F, Pásaro R. Characterisation of afferent projections to the nucleus ambiguus of the rat by means of fluorescent double labelling. Journal of Anatomy. 1990;172:1–15. [PMC free article] [PubMed] [Google Scholar]
- O’Donnell CP, Tankersley CG, Polotsky VP, Schwartz AR, Smith PL. Leptin, obesity, and respiratory function. Respiration Physiology. 2000;119:163–170. doi: 10.1016/s0034-5687(99)00111-5. [DOI] [PubMed] [Google Scholar]
- Ogawa SK, Cohen JY, Hwang D, Uchida N, Watabe-Uchida M. Organization of monosynaptic inputs to the serotonin and dopamine neuromodulatory systems. Cell Reports. 2014;8:1105–1118. doi: 10.1016/j.celrep.2014.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuki G, Piochon C, Hansel C. Climbing fiber signaling and cerebellar gain control. Frontiers in Cellular Neuroscience. 2009;3:4. doi: 10.3389/neuro.03.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T, Tsumori T, Yokota S, Yasui Y. Neuroanatomical and neurochemical organization of projections from the central amygdaloid nucleus to the nucleus retroambiguus via the periaqueductal gray in the rat. Neuroscience Research. 2008;62:286–298. doi: 10.1016/j.neures.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Oku Y, Masumiya H, Okada Y. Postnatal developmental changes in activation profiles of the respiratory neuronal network in the rat ventral medulla. The Journal of Physiology. 2007;585:175–186. doi: 10.1113/jphysiol.2007.138180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira LM, Takakura AC, Moreira TS. Forebrain and hindbrain projecting-neurons target the post-inspiratory complex cholinergic neurons. Neuroscience. 2021;476:102–115. doi: 10.1016/j.neuroscience.2021.09.015. [DOI] [PubMed] [Google Scholar]
- Olson L, Fuxe K. On the projections from the locus coeruleus noradrealine neurons: the cerebellar innervation. Brain Research. 1971;28:165–171. doi: 10.1016/0006-8993(71)90533-6. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. The Journal of Neuroscience. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Sáenz P, López-Barneo J. Physiology of the carotid body: from molecules to disease. Annual Review of Physiology. 2020;82:127–149. doi: 10.1146/annurev-physiol-020518-114427. [DOI] [PubMed] [Google Scholar]
- Ovsepian SV, LeBerre M, Steuber V, O’Leary VB, Leibold C, Oliver Dolly J. Distinctive role of KV1.1 subunit in the biology and functions of low threshold K(+) channels with implications for neurological disease. Pharmacology & Therapeutics. 2016;159:93–101. doi: 10.1016/j.pharmthera.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Pagliardini S, Janczewski WA, Tan W, Dickson CT, Deisseroth K, Feldman JL. Active expiration induced by excitation of ventral medulla in adult anesthetized rats. The Journal of Neuroscience. 2011;31:2895–2905. doi: 10.1523/JNEUROSCI.5338-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD. The parabrachial nucleus: CGRP neurons function as a general alarm. Trends in Neurosciences. 2018;41:280–293. doi: 10.1016/j.tins.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panneton WM, Gan Q, Juric R. Brainstem projections from recipient zones of the anterior ethmoidal nerve in the medullary dorsal horn. Neuroscience. 2006;141:889–906. doi: 10.1016/j.neuroscience.2006.04.055. [DOI] [PubMed] [Google Scholar]
- Panneton WM. The mammalian diving response: an enigmatic reflex to preserve life? Physiology. 2013;28:284–297. doi: 10.1152/physiol.00020.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panneton WM, Pan B, Gan Q. Somatotopy in the medullary dorsal horn as a basis for orofacial reflex behavior. Frontiers in Neurology. 2017;8:522. doi: 10.3389/fneur.2017.00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiological Reviews. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt KD, Freedman R, Bickford-Wimer PC. Electrophysiological effects of locally applied noradrenergic agents at cerebellar purkinje neurons: receptor specificity. Brain Research. 1988;462:242–251. doi: 10.1016/0006-8993(88)90552-5. [DOI] [PubMed] [Google Scholar]
- Parsons LM, Egan G, Liotti M, Brannan S, Denton D, Shade R, Robillard R, Madden L, Abplanalp B, Fox PT. Neuroimaging evidence implicating cerebellum in the experience of hypercapnia and hunger for air. PNAS. 2001;98:2041–2046. doi: 10.1073/pnas.98.4.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA. 2006;296:2124. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- Paulhus K, Ammerman L, Glasscock E. Clinical spectrum of kcna1 mutations: new insights into episodic ataxia and epilepsy comorbidity. International Journal of Molecular Sciences. 2020;21:2802. doi: 10.3390/ijms21082802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei L, Leblanc M, Barish G, Atkins A, Nofsinger R, Whyte J, Gold D, He M, Kawamura K, Li HR, Downes M, Yu RT, Powell HC, Lingrel JB, Evans RM. Thyroid hormone receptor repression is linked to type I pneumocyte–associated respiratory distress syndrome. Nature Medicine. 2011;17:1466–1472. doi: 10.1038/nm.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez de Los Cobos Pallares F, Bautista TG, Stanić D, Egger V, Dutschmann M. Brainstem-mediated sniffing and respiratory modulation during odor stimulation. Respiratory Physiology & Neurobiology. 2016;233:17–24. doi: 10.1016/j.resp.2016.07.008. [DOI] [PubMed] [Google Scholar]
- Perez-Pouchoulen M, Toledo R, Garcia LI, Perez-Estudillo CA, Coria-Avila GA, Hernandez ME, Carrillo P, Manzo J. Androgen receptors in Purkinje neurons are modulated by systemic testosterone and sexual training in a region-specific manner in the male rat. Physiology & Behavior. 2016;156:191–198. doi: 10.1016/j.physbeh.2016.01.027. [DOI] [PubMed] [Google Scholar]
- Perl O, Ravia A, Rubinson M, Eisen A, Soroka T, Mor N, Secundo L, Sobel N. Human non-olfactory cognition phase-locked with inhalation. Nature Human Behaviour. 2019;3:501–512. doi: 10.1038/s41562-019-0556-z. [DOI] [PubMed] [Google Scholar]
- Petrov T, Krukoff TL, Jhamandas JH. The hypothalamic paraventricular and lateral parabrachial nuclei receive collaterals from raphe nucleus neurons: a combined double retrograde and immunocytochemical study. The Journal of Comparative Neurology. 1992;318:18–26. doi: 10.1002/cne.903180103. [DOI] [PubMed] [Google Scholar]
- Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998a;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. The Journal of Neuroscience. 1998b;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Rampon C, Petit JM, Luppi PH. Sub-regions of the dorsal raphé nucleus receive different inputs from the brainstem. Sleep Medicine. 2018;49:53–63. doi: 10.1016/j.sleep.2018.07.002. [DOI] [PubMed] [Google Scholar]
- Philippot P, Chapelle G, Blairy S. Respiratory feedback in the generation of emotion. Cognition & Emotion. 2002;16:605–627. doi: 10.1080/02699930143000392. [DOI] [Google Scholar]
- Phillips KH, Aitchison RE. Effects of psychomotor instruction on elementary general music students’ singing performance. Journal of Research in Music Education. 1997;45:185–196. doi: 10.2307/3345579. [DOI] [Google Scholar]
- Pierce ET, Hoddevik GH, Walberg F. The cerebellar projection from the raphe nuclei in the cat as studied with the method of retrograde transport of horseradish peroxidase. Anatomy and Embryology. 1977;152:73–87. doi: 10.1007/BF00341436. [DOI] [PubMed] [Google Scholar]
- Pijpers A, Ruigrok TJH. Organization of pontocerebellar projections to identified climbing fiber zones in the rat. The Journal of Comparative Neurology. 2006;496:513–528. doi: 10.1002/cne.20940. [DOI] [PubMed] [Google Scholar]
- Pilowsky PM. Peptides, serotonin, and breathing: the role of the raphe in the control of respiration. Progress in Brain Research. 2014;209:169–189. doi: 10.1016/B978-0-444-63274-6.00009-6. [DOI] [PubMed] [Google Scholar]
- Pineda J, Aghajanian GK. Carbon dioxide regulates the tonic activity of locus coeruleus neurons by modulating a proton- and polyamine-sensitive inward rectifier potassium current. Neuroscience. 1997;77:723–743. doi: 10.1016/s0306-4522(96)00485-x. [DOI] [PubMed] [Google Scholar]
- Piper AJ, Yee BJ. Hypoventilation syndromes. Comprehensive Physiology. 2014;4:1639–1676. doi: 10.1002/cphy.c140008. [DOI] [PubMed] [Google Scholar]
- Pisanski A, Pagliardini S. The parafacial respiratory group and the control of active expiration. Respiratory Physiology & Neurobiology. 2019;265:153–160. doi: 10.1016/j.resp.2018.06.010. [DOI] [PubMed] [Google Scholar]
- Pitts T, Gayagoy AG, Rose MJ, Poliacek I, Condrey JA, Musselwhite MN, Shen TY, Davenport PW, Bolser DC. Suppression of abdominal motor activity during swallowing in cats and humans. PLOS ONE. 2015;10:e0128245. doi: 10.1371/journal.pone.0128245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum F, Swanson AG. Central neurogenic hyperventilation in man. A.M.A. Archives of Neurology and Psychiatry. 1959;81:535–549. doi: 10.1001/archneurpsyc.1959.02340170001001. [DOI] [PubMed] [Google Scholar]
- Poe GR, Foote S, Eschenko O, Johansen JP, Bouret S, Aston-Jones G, Harley CW, Manahan-Vaughan D, Weinshenker D, Valentino R, Berridge C, Chandler DJ, Waterhouse B, Sara SJ. Locus coeruleus: a new look at the blue spot. Nature Reviews. Neuroscience. 2020;21:644–659. doi: 10.1038/s41583-020-0360-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak Dorocic I, Fürth D, Xuan Y, Johansson Y, Pozzi L, Silberberg G, Carlén M, Meletis K. A whole-brain atlas of inputs to serotonergic neurons of the dorsal and median raphe nuclei. Neuron. 2014;83:663–678. doi: 10.1016/j.neuron.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Popovic RM, White DP. Upper airway muscle activity in normal women: influence of hormonal status. Journal of Applied Physiology. 1998;84:1055–1062. doi: 10.1152/jappl.1998.84.3.1055. [DOI] [PubMed] [Google Scholar]
- Porter WT. The path of the respiratory impulse from the bulb to the phrenic nuclei. The Journal of Physiology. 1895;17:455–485. doi: 10.1113/jphysiol.1895.sp000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC. Raphé neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. The Journal of Neuroscience. 2009;29:3720–3737. doi: 10.1523/JNEUROSCI.5271-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Blanco-Hinojo L, Ortiz H, Gallart L, Moltó L, Martínez-Vilavella G, Vilà E, Pacreu S, Adalid I, Deus J, Pérez-Sola V, Fernandez-Candil J. Mapping the neural systems driving breathing at the transition to unconsciousness. NeuroImage. 2022;246:118779. doi: 10.1016/j.neuroimage.2021.118779. [DOI] [PubMed] [Google Scholar]
- Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO(2) and acid signaling in chemosensitive neurons. American Journal of Physiology. Cell Physiology. 2004;287:C1493–C1526. doi: 10.1152/ajpcell.00282.2004. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Cacciola A, Milardi D, Ghilardi MF, Calamuneri A, Chillemi G, Anastasi G, Rothwell J. New insights into cortico-basal-cerebellar connectome: clinical and physiological considerations. Brain. 2020;143:396–406. doi: 10.1093/brain/awz310. [DOI] [PubMed] [Google Scholar]
- Quattrochi J, Datta S, Hobson JA. Cholinergic and non-cholinergic afferents of the caudolateral parabrachial nucleus: a role in the long-term enhancement of rapid eye movement sleep. Neuroscience. 1998;83:1123–1136. doi: 10.1016/s0306-4522(97)00471-5. [DOI] [PubMed] [Google Scholar]
- Quintero MC, Putnam RW, Cordovez JM. Theoretical perspectives on central chemosensitivity: CO2/H+-sensitive neurons in the locus coeruleus. PLOS Computational Biology. 2017;13:e1005853. doi: 10.1371/journal.pcbi.1005853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanantsoa N, Hirsch MR, Thoby-Brisson M, Dubreuil V, Bouvier J, Ruffault PL, Matrot B, Fortin G, Brunet JF, Gallego J, Goridis C. Breathing without CO(2) chemosensitivity in conditional phox2b mutants. The Journal of Neuroscience. 2011;31:12880–12888. doi: 10.1523/JNEUROSCI.1721-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Schwarzacher SW, Pierrefiche O, Olivera BM, Richter DW. Selective lesioning of the cat pre-Bötzinger complex in vivo eliminates breathing but not gasping. The Journal of Physiology. 1998;507 ( Pt 3):895–907. doi: 10.1111/j.1469-7793.1998.895bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez J-M, Koch H, Garcia AJ, Doi A, Zanella S. The role of spiking and bursting pacemakers in the neuronal control of breathing. Journal of Biological Physics. 2011;37:241–261. doi: 10.1007/s10867-011-9214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay SC, Adams L, Murphy K, Corfield DR, Grootoonk S, Bailey DL, Frackowiak RS, Guz A. Regional cerebral blood flow during volitional expiration in man: a comparison with volitional inspiration. The Journal of Physiology. 1993;461:85–101. doi: 10.1113/jphysiol.1993.sp019503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raux M, Tyvaert L, Ferreira M, Kindler F, Bardinet E, Karachi C, Morelot-Panzini C, Gotman J, Pike GB, Koski L, Similowski T. Functional magnetic resonance imaging suggests automatization of the cortical response to inspiratory threshold loading in humans. Respiratory Physiology & Neurobiology. 2013;189:571–580. doi: 10.1016/j.resp.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Reddy MK, Patel KP, Schultz HD. Differential role of the paraventricular nucleus of the hypothalamus in modulating the sympathoexcitatory component of peripheral and central chemoreflexes. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2005;289:R789–R797. doi: 10.1152/ajpregu.00222.2005. [DOI] [PubMed] [Google Scholar]
- Ricardo JA, Tongju Koh E. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Research. 1978;153:1–26. doi: 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- Richerson GB. Response to CO2 of neurons in the rostral ventral medulla in vitro. Journal of Neurophysiology. 1995;73:933–944. doi: 10.1152/jn.1995.73.3.933. [DOI] [PubMed] [Google Scholar]
- Rikard-Bell GC, Bystrzycka EK, Nail BS. Brainstem projections to the phrenic nucleus: a HRP study in the cat. Brain Research Bulletin. 1984;12:469–477. doi: 10.1016/0361-9230(84)90162-X. [DOI] [PubMed] [Google Scholar]
- Rikard-Bell GC, Bystrzycka EK, Nail BS. Cells of origin of corticospinal projections to phrenic and thoracic respiratory motoneurones in the cat as shown by retrograde transport of HRP. Brain Research Bulletin. 1985;14:39–47. doi: 10.1016/0361-9230(85)90175-3. [DOI] [PubMed] [Google Scholar]
- Rimmer KP, Ford GT, Whitelaw WA. Interaction between postural and respiratory control of human intercostal muscles. Journal of Applied Physiology. 1995;79:1556–1561. doi: 10.1152/jappl.1995.79.5.1556. [DOI] [PubMed] [Google Scholar]
- Rinaman L. Oxytocinergic inputs to the nucleus of the solitary tract and dorsal motor nucleus of the vagus in neonatal rats. The Journal of Comparative Neurology. 1998;399:101–109. doi: 10.1002/(sici)1096-9861(19980914)399:1<101::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Rinaman L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Research. 2010;1350:18–34. doi: 10.1016/j.brainres.2010.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi TA, Ennis M, Behbehani MM, Shipley MT. Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: topography and reciprocity. The Journal of Comparative Neurology. 1991;303:121–131. doi: 10.1002/cne.903030111. [DOI] [PubMed] [Google Scholar]
- Robertson SD, Plummer NW, de Marchena J, Jensen P. Developmental origins of central norepinephrine neuron diversity. Nature Neuroscience. 2013;16:1016–1023. doi: 10.1038/nn.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano V, De Propris L, Bosman LWJ, Warnaar P, Ten Brinke MM, Lindeman S, Ju C, Velauthapillai A, Spanke JK, Middendorp Guerra E, Hoogland TM, Negrello M, D’Angelo E, De Zeeuw CI. Potentiation of cerebellar purkinje cells facilitates whisker reflex adaptation through increased simple spike activity. eLife. 2018;7:e38852. doi: 10.7554/eLife.38852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano V, Reddington AL, Cazzanelli S, Mazza R, Ma Y, Strydis C, Negrello M, Bosman LWJ, De Zeeuw CI. Functional convergence of autonomic and sensorimotor processing in the lateral cerebellum. Cell Reports. 2020;32:107867. doi: 10.1016/j.celrep.2020.107867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Room P, Postema F, Korf J. Divergent axon collaterals of rat locus coeruleus neurons: demonstration by a fluorescent double labeling technique. Brain Research. 1981;221:219–230. doi: 10.1016/0006-8993(81)90773-3. [DOI] [PubMed] [Google Scholar]
- Roseberry TK, Lee AM, Lalive AL, Wilbrecht L, Bonci A, Kreitzer AC. Cell-type-specific control of brainstem locomotor circuits by basal ganglia. Cell. 2016;164:526–537. doi: 10.1016/j.cell.2015.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. The Journal of Comparative Neurology. 2006;499:64–89. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- Ross R, Blair SN, Arena R, Church TS, Després J-P, Franklin BA, Haskell WL, Kaminsky LA, Levine BD, Lavie CJ, Myers J, Niebauer J, Sallis R, Sawada SS, Sui X, Wisløff U, American Heart Association Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health. Council on Clinical Cardiology. Council on Epidemiology and Prevention. Council on Cardiovascular and Stroke Nursing. Council on Functional Genomics and Translational Biology. Stroke Council Importance of assessing cardiorespiratory fitness in clinical practice: A case for fitness as A clinical vital sign: A scientific statement from the american heart association. Circulation. 2016;134:e653–e699. doi: 10.1161/CIR.0000000000000461. [DOI] [PubMed] [Google Scholar]
- Rousseau JP, Tenorio-Lopes L, Ghio SC, Desjardins P, Fournier S, Kinkead R. Thyroid hormones during the perinatal period are necessary to respiratory network development of newborn rats. Experimental Neurology. 2021;345:113813. doi: 10.1016/j.expneurol.2021.113813. [DOI] [PubMed] [Google Scholar]
- Rübsamen R, Schweizer H. Control of echolocation pulses by neurons of the nucleus ambiguus in the rufous horseshoe bat, rhinolophus rouxi. II. afferent and efferent connections of the motor nucleus of the laryngeal nerves. Journal of Comparative Physiology. A, Sensory, Neural, and Behavioral Physiology. 1986;159:689–699. doi: 10.1007/BF00612041. [DOI] [PubMed] [Google Scholar]
- Ruffault PL, D’Autréaux F, Hayes JA, Nomaksteinsky M, Autran S, Fujiyama T, Hoshino M, Hägglund M, Kiehn O, Brunet JF, Fortin G, Goridis C. The retrotrapezoid nucleus neurons expressing atoh1 and phox2b are essential for the respiratory response to co₂. eLife. 2015;4:e07051. doi: 10.7554/eLife.07051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero DA, Anwar M, Golanov EV, Reis DJ. The pedunculopontine tegmental nucleus issues collaterals to the fastigial nucleus and rostral ventrolateral reticular nucleus in the rat. Brain Research. 1997;760:272–276. doi: 10.1016/s0006-8993(97)00397-1. [DOI] [PubMed] [Google Scholar]
- Ruigrok TJH, Cella F, Voogd J. Connections of the lateral reticular nucleus to the lateral vestibular nucleus in the rat an anterograde tracing study with phaseolus vulgaris leucoagglutinin. The European Journal of Neuroscience. 1995;7:1410–1413. doi: 10.1111/j.1460-9568.1995.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Ruigrok TJH, Sillitoe RV, Voogd J. In: The Rat Nervous System. Paxinos G, editor. Academic Press; 2015. Chapter 9 - cerebellum and cerebellar connections; pp. 133–205. [Google Scholar]
- Ruyle BC, Klutho PJ, Baines CP, Heesch CM, Hasser EM. Hypoxia activates a neuropeptidergic pathway from the paraventricular nucleus of the hypothalamus to the nucleus tractus solitarii. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2018;315:R1167–R1182. doi: 10.1152/ajpregu.00244.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyle BC, Martinez D, Heesch CM, Kline DD, Hasser EM. The PVN enhances cardiorespiratory responses to acute hypoxia via input to the nts. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2019;317:R818–R833. doi: 10.1152/ajpregu.00135.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryvlin P, Nashef L, Lhatoo SD, Bateman LM, Bird J, Bleasel A, Boon P, Crespel A, Dworetzky BA, Høgenhaven H, Lerche H, Maillard L, Malter MP, Marchal C, Murthy JMK, Nitsche M, Pataraia E, Rabben T, Rheims S, Sadzot B, Schulze-Bonhage A, Seyal M, So EL, Spitz M, Szucs A, Tan M, Tao JX, Tomson T. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. The Lancet. Neurology. 2013;12:966–977. doi: 10.1016/S1474-4422(13)70214-X. [DOI] [PubMed] [Google Scholar]
- Sabino JPJ, Oliveira LV, Soriano RN, Kwiatkoski M, Branco LGS, da Silva GSF. Role of hydrogen sulfide in ventilatory responses to hypercapnia in the medullary raphe of adult rats. Experimental Physiology. 2021;106:1992–2001. doi: 10.1113/EP089335. [DOI] [PubMed] [Google Scholar]
- Sadakane K, Kondo M, Nisimaru N. Direct projection from the cardiovascular control region of the cerebellar cortex, the lateral nodulus-uvula, to the brainstem in rabbits. Neuroscience Research. 2000;36:15–26. doi: 10.1016/s0168-0102(99)00103-0. [DOI] [PubMed] [Google Scholar]
- Saigal RP, Karamanlidis AN, Voogd J, Mangana O, Michaloudi H. Secondary trigeminocerebellar projections in sheep studied with the horseradish peroxidase tracing method. The Journal of Comparative Neurology. 1980a;189:537–553. doi: 10.1002/cne.901890307. [DOI] [PubMed] [Google Scholar]
- Saigal RP, Karamanlidis AN, Voogd J, Michaloudi H, Mangana O. Cerebellar afferents from motor nuclei of cranial nerves, the nucleus of the solitary tract, and nuclei coeruleus and parabrachialis in sheep, demonstrated with retrograde transport of horseradish peroxidase. Brain Research. 1980b;197:200–206. doi: 10.1016/0006-8993(80)90445-x. [DOI] [PubMed] [Google Scholar]
- Sakaguchi Y, Aiba E. Relationship between musical characteristics and temporal breathing pattern in piano performance. Frontiers in Human Neuroscience. 2016;10:381. doi: 10.3389/fnhum.2016.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomoni S, van den Hoorn W, Hodges P, Larson CR. Breathing and singing: objective characterization of breathing patterns in classical singers. PLOS ONE. 2016;11:e0155084. doi: 10.1371/journal.pone.0155084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant’Ambrogio G. Information arising from the tracheobronchial tree of mammals. Physiological Reviews. 1982;62:531–569. doi: 10.1152/physrev.1982.62.2.531. [DOI] [PubMed] [Google Scholar]
- Sant’Ambrogio G, Mathew OP, Fisher JT, Sant’Ambrogio FB. Laryngeal receptors responding to transmural pressure, airflow and local muscle activity. Respiration Physiology. 1983;54:317–330. doi: 10.1016/0034-5687(83)90075-0. [DOI] [PubMed] [Google Scholar]
- Sant’Ambrogio G, Widdicombe J. Reflexes from airway rapidly adapting receptors. Respiration Physiology. 2001;125:33–45. doi: 10.1016/s0034-5687(00)00203-6. [DOI] [PubMed] [Google Scholar]
- Santin JM, Hartzler LK. Respiratory signaling of locus coeruleus neurons during hypercapnic acidosis in the bullfrog, lithobates catesbeianus. Respiratory Physiology & Neurobiology. 2013;185:553–561. doi: 10.1016/j.resp.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Research. 1980;197:291–317. doi: 10.1016/0006-8993(80)91117-8. [DOI] [PubMed] [Google Scholar]
- Saponjic J, Radulovacki M, Carley DW. Respiratory pattern modulation by the pedunculopontine tegmental nucleus. Respiratory Physiology & Neurobiology. 2003;138:223–237. doi: 10.1016/j.resp.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Saunders SE, Levitt ES. Kölliker-fuse/parabrachial complex mu opioid receptors contribute to fentanyl-induced apnea and respiratory rate depression. Respiratory Physiology & Neurobiology. 2020;275:103388. doi: 10.1016/j.resp.2020.103388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon DW, Hopkins DA. Efferent and collateral organization of paratrigeminal nucleus projections: an anterograde and retrograde fluorescent tracer study in the rat. The Journal of Comparative Neurology. 1998;402:93–110. doi: 10.1002/(SICI)1096-9861(19981207)402:1<93::AID-CNE7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Saxon DW, Hopkins DA. Ultrastructure and synaptology of the paratrigeminal nucleus in the rat: primary pharyngeal and laryngeal afferent projections. Synapse. 2006;59:220–234. doi: 10.1002/syn.20233. [DOI] [PubMed] [Google Scholar]
- Schaefer ML, Böttger B, Silver WL, Finger TE. Trigeminal collaterals in the nasal epithelium and olfactory bulb: a potential route for direct modulation of olfactory information by trigeminal stimuli. The Journal of Comparative Neurology. 2002;444:221–226. doi: 10.1002/cne.10143. [DOI] [PubMed] [Google Scholar]
- Schwarz LA, Miyamichi K, Gao XJ, Beier KT, Weissbourd B, DeLoach KE, Ren J, Ibanes S, Malenka RC, Kremer EJ, Luo L. Viral-genetic tracing of the input-output organization of a central noradrenaline circuit. Nature. 2015;524:88–92. doi: 10.1038/nature14600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzacher SW, Rüb U, Deller T. Neuroanatomical characteristics of the human pre-Bötzinger complex and its involvement in neurodegenerative brainstem diseases. Brain. 2011;134:24–35. doi: 10.1093/brain/awq327. [DOI] [PubMed] [Google Scholar]
- Seijo-Martínez M, Varela-Freijanes A, Grandes J, Vázquez F. Sneeze related area in the medulla: localisation of the human sneezing centre? Journal of Neurology, Neurosurgery, and Psychiatry. 2006;77:559–561. doi: 10.1136/jnnp.2005.068601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba K, Fibiger HC. Afferent connections of the laterodorsal and the pedunculopontine tegmental nuclei in the rat: a retro- and antero-grade transport and immunohistochemical study. The Journal of Comparative Neurology. 1992;323:387–410. doi: 10.1002/cne.903230307. [DOI] [PubMed] [Google Scholar]
- Sengul G, Fu Y, Yu Y, Paxinos G. Spinal cord projections to the cerebellum in the mouse. Brain Structure & Function. 2015;220:2997–3009. doi: 10.1007/s00429-014-0840-7. [DOI] [PubMed] [Google Scholar]
- Seppälä EM, Nitschke JB, Tudorascu DL, Hayes A, Goldstein MR, Nguyen DTH, Perlman D, Davidson RJ. Breathing-based meditation decreases posttraumatic stress disorder symptoms in U.S. military veterans: a randomized controlled longitudinal study. Journal of Traumatic Stress. 2014;27:397–405. doi: 10.1002/jts.21936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepúlveda FV, Pablo Cid L, Teulon J, Niemeyer MI. Molecular aspects of structure, gating, and physiology of ph-sensitive background k2p and kir K+-transport channels. Physiological Reviews. 2015;95:179–217. doi: 10.1152/physrev.00016.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson CA, Wang W, Pieribone VA, Dohle CI, Richerson GB. Midbrain serotonergic neurons are central ph chemoreceptors. Nature Neuroscience. 2003;6:1139–1140. doi: 10.1038/nn1130. [DOI] [PubMed] [Google Scholar]
- Shannon R, Freeman D. Nucleus retroambigualis respiratory neurons: responses to intercostal and abdominal muscle afferents. Respiration Physiology. 1981;45:357–375. doi: 10.1016/0034-5687(81)90018-9. [DOI] [PubMed] [Google Scholar]
- Sherman D, Worrell JW, Cui Y, Feldman JL. Optogenetic perturbation of preBötzinger complex inhibitory neurons modulates respiratory pattern. Nature Neuroscience. 2015;18:408–414. doi: 10.1038/nn.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Stornetta RL, Stornetta DS, Onengut-Gumuscu S, Farber EA, Turner SD, Guyenet PG, Bayliss DA. Neuromedin B expression defines the mouse retrotrapezoid nucleus. The Journal of Neuroscience. 2017;37:11744–11757. doi: 10.1523/JNEUROSCI.2055-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Wei H, Chen Z, Wang J, Qu W, Huang Z, Dai C. Whole-brain monosynaptic inputs and outputs of glutamatergic neurons of the vestibular nuclei complex in mice. Hearing Research. 2021;401:108159. doi: 10.1016/j.heares.2020.108159. [DOI] [PubMed] [Google Scholar]
- Shim WS, Tak MH, Lee MH, Kim M, Kim M, Koo JY, Lee CH, Kim M, Oh U. Trpv1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. Journal of Neuroscience. 2007;27:2331–2337. doi: 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JW, Geerling JC, Loewy AD. Inputs to the ventrolateral bed nucleus of the stria terminalis. The Journal of Comparative Neurology. 2008;511:628–657. doi: 10.1002/cne.21870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnar S, Maciewicz RJ, Shofer RJ. A raphe projection to cat cerebellar cortex. Brain Research. 1975;97:139–143. doi: 10.1016/0006-8993(75)90921-X. [DOI] [PubMed] [Google Scholar]
- Shinozaki Y, Yokota S, Miwakeichi F, Pokorski M, Aoyama R, Fukuda K, Yoshida H, Toyama Y, Nakamura M, Okada Y. Structural and functional identification of two distinct inspiratory neuronal populations at the level of the phrenic nucleus in the rat cervical spinal cord. Brain Structure and Function. 2019;224:57–72. doi: 10.1007/s00429-018-1757-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JN, Lucena EV, Silva TM, Damasceno RS, Takakura AC, Moreira TS. Inhibition of the pontine Kölliker-fuse nucleus reduces genioglossal activity elicited by stimulation of the retrotrapezoid chemoreceptor neurons. Neuroscience. 2016a;328:9–21. doi: 10.1016/j.neuroscience.2016.04.028. [DOI] [PubMed] [Google Scholar]
- Silva JN, Tanabe FM, Moreira TS, Takakura AC. Neuroanatomical and physiological evidence that the retrotrapezoid nucleus/parafacial region regulates expiration in adult rats. Respiratory Physiology & Neurobiology. 2016b;227:9–22. doi: 10.1016/j.resp.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Silva JN, Oliveira LM, Souza FC, Moreira TS, Takakura AC. Distinct pathways to the parafacial respiratory group to trigger active expiration in adult rats. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2019;317:L402–L413. doi: 10.1152/ajplung.00467.2018. [DOI] [PubMed] [Google Scholar]
- Simeone KA, Hallgren J, Bockman CS, Aggarwal A, Kansal V, Netzel L, Iyer SH, Matthews SA, Deodhar M, Oldenburg PJ, Abel PW, Simeone TA. Respiratory dysfunction progresses with age in kcna1-null mice, a model of sudden unexpected death in epilepsy. Epilepsia. 2018;59:345–357. doi: 10.1111/epi.13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh U, Jiang J, Saito K, Toth BA, Dickey JE, Rodeghiero SR, Deng Y, Deng G, Xue B, Zhu Z, Zingman LV, Geerling JC, Cui H. Neuroanatomical organization and functional roles of PVN MC4R pathways in physiological and behavioral regulations. Molecular Metabolism. 2022;55:101401. doi: 10.1016/j.molmet.2021.101401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. The Journal of Comparative Neurology. 1989;281:69–96. doi: 10.1002/cne.902810107. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HR, Leibold NK, Rappoport DA, Ginapp CM, Purnell BS, Bode NM, Alberico SL, Kim YC, Audero E, Gross CT, Buchanan GF. Dorsal raphe serotonin neurons mediate CO2-induced arousal from sleep. The Journal of Neuroscience. 2018;38:1915–1925. doi: 10.1523/JNEUROSCI.2182-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrinho CR, Kuo FS, Barna BF, Moreira TS, Mulkey DK. Cholinergic control of ventral surface chemoreceptors involves gq/inositol 1,4,5-trisphosphate-mediated inhibition of KCNQ channels. The Journal of Physiology. 2016;594:407–419. doi: 10.1113/JP271761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somana R, Walberg F. Cerebellar afferents from the nucleus of the solitary tract. Neuroscience Letters. 1979a;11:41–47. doi: 10.1016/0304-3940(79)90053-3. [DOI] [PubMed] [Google Scholar]
- Somana R, Walberg F. The cerebellar projection from the paratrigeminal nucleus in the cat. Neuroscience Letters. 1979b;15:49–54. doi: 10.1016/0304-3940(79)91528-3. [DOI] [PubMed] [Google Scholar]
- Song G, Yu Y, Poon CS. Cytoarchitecture of pneumotaxic integration of respiratory and nonrespiratory information in the rat. The Journal of Neuroscience. 2006;26:300–310. doi: 10.1523/JNEUROSCI.3029-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Wang H, Xu H, Poon CS. Kölliker–fuse neurons send collateral projections to multiple hypoxia-activated and nonactivated structures in rat brainstem and spinal cord. Brain Structure & Function. 2012a;217:835–858. doi: 10.1007/s00429-012-0384-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song N, Zhang G, Geng W, Liu Z, Jin W, Li L, Cao Y, Zhu D, Yu J, Shen L. Acid sensing ion channel 1 in lateral hypothalamus contributes to breathing control. PLOS ONE. 2012b;7:e39982. doi: 10.1371/journal.pone.0039982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza G, Stornetta RL, Stornetta DS, Abbott SBG, Guyenet PG. Differential contribution of the retrotrapezoid nucleus and C1 neurons to active expiration and arousal in rats. The Journal of Neuroscience. 2020;40:8683–8697. doi: 10.1523/JNEUROSCI.1006-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck DF, Feldman JL. The effects of microstimulation and microlesions in the ventral and dorsal respiratory groups in medulla of cat. The Journal of Neuroscience. 1982;2:744–757. doi: 10.1523/JNEUROSCI.02-06-00744.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriranjini SJ, Pal PK, Krishna N, Sathyaprabha TN. Subclinical pulmonary dysfunction in spinocerebellar ataxias 1, 2 and 3. Acta Neurologica Scandinavica. 2010;122:323–328. doi: 10.1111/j.1600-0404.2009.01306.x. [DOI] [PubMed] [Google Scholar]
- Stahl WR. Scaling of respiratory variables in mammals. Journal of Applied Physiology. 1967;22:453–460. doi: 10.1152/jappl.1967.22.3.453. [DOI] [PubMed] [Google Scholar]
- Steininger TL, Rye DB, Wainer BH. Afferent projections to the cholinergic pedunculopontine tegmental nucleus and adjacent midbrain extrapyramidal area in the albino rat. i. retrograde tracing studies. The Journal of Comparative Neurology. 1992;321:515–543. doi: 10.1002/cne.903210403. [DOI] [PubMed] [Google Scholar]
- Strohl KP. Respiratory activation of the facial nerve and alar muscles in anaesthetized dogs. The Journal of Physiology. 1985;363:351–362. doi: 10.1113/jphysiol.1985.sp015715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Roca H, Mamoun N, Sigurdson MI, Maixner W. Baroreceptor modulation of the cardiovascular system, pain, consciousness, and cognition. Comprehensive Physiology. 2021;11:1373–1423. doi: 10.1002/cphy.c190038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian HH, Chow CM, Balnave RJ. Identification of different types of respiratory neurones in the dorsal brainstem nucleus tractus solitarius of the rat. Brain Research. 2007;1141:119–132. doi: 10.1016/j.brainres.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Subramanian HH, Balnave RJ, Holstege G. The midbrain periaqueductal gray control of respiration. The Journal of Neuroscience. 2008;28:12274–12283. doi: 10.1523/JNEUROSCI.4168-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian HH, Holstege G. The nucleus retroambiguus control of respiration. The Journal of Neuroscience. 2009;29:3824–3832. doi: 10.1523/JNEUROSCI.0607-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian HH. Descending control of the respiratory neuronal network by the midbrain periaqueductal grey in the rat in vivo. The Journal of Physiology. 2013;591:109–122. doi: 10.1113/jphysiol.2012.245217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian HH, Balnave RJ, Holstege G. Microstimulation in different parts of the periaqueductal gray generates different types of vocalizations in the cat. Journal of Voice. 2021;35:804. doi: 10.1016/j.jvoice.2020.01.022. [DOI] [PubMed] [Google Scholar]
- Suess WM, Alexander AB, Smith DD, Sweeney HW, Marion RJ. The effects of psychological stress on respiration: a preliminary study of anxiety and hyperventilation. Psychophysiology. 1980;17:535–540. doi: 10.1111/j.1469-8986.1980.tb02293.x. [DOI] [PubMed] [Google Scholar]
- Sugihara I, Fujita H, Na J, Quy PN, Li BY, Ikeda D. Projection of reconstructed single purkinje cell axons in relation to the cortical and nuclear aldolase C compartments of the rat cerebellum. The Journal of Comparative Neurology. 2009;512:282–304. doi: 10.1002/cne.21889. [DOI] [PubMed] [Google Scholar]
- Summ O, Hassanpour N, Mathys C, Groß M. Disordered breathing in severe cerebral illness - towards a conceptual framework. Respiratory Physiology & Neurobiology. 2022;300:103869. doi: 10.1016/j.resp.2022.103869. [DOI] [PubMed] [Google Scholar]
- Swenson RS, Castro AJ. The afferent connections of the inferior olivary complex in rats an anterograde study using autoradiographic and axonal degeneration techniques. Neuroscience. 1983a;8:259–275. doi: 10.1016/0306-4522(83)90064-7. [DOI] [PubMed] [Google Scholar]
- Swenson RS, Castro AJ. The afferent connections of the inferior olivary complex in rats: a study using the retrograde transport of horseradish peroxidase. The American Journal of Anatomy. 1983b;166:329–341. doi: 10.1002/aja.1001660307. [DOI] [PubMed] [Google Scholar]
- Sykes DL, Morice AH. The cough reflex: the janus of respiratory medicine. Frontiers in Physiology. 2021;12:684080. doi: 10.3389/fphys.2021.684080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadi E. Functional neuroanatomy of the central noradrenergic system. Journal of Psychopharmacology. 2013;27:659–693. doi: 10.1177/0269881113490326. [DOI] [PubMed] [Google Scholar]
- Szentágothai J, Rajkovits K. Über den ursprung der kletterfasern des kleinhirns. Zeitschrift Fur Anatomie Und Entwicklungsgeschichte. 1959;121:130–141. doi: 10.1007/BF00525203. [DOI] [Google Scholar]
- Szulczewski MT. Training of paced breathing at 0.1 hz improves CO2 homeostasis and relaxation during a paced breathing task. PLOS ONE. 2019;14:e0218550. doi: 10.1371/journal.pone.0218550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatoh J, Prevosto V, Thompson PM, Lu J, Chung L, Harrahill A, Li S, Zhao S, He Z, Golomb D, Kleinfeld D, Wang F. The whisking oscillator circuit. Nature. 2022;609:560–568. doi: 10.1038/s41586-022-05144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei A, Hamada T, Yabe I, Sasaki H. Treatment of cerebellar ataxia with 5-HT1A agonist. Cerebellum. 2005;4:211–215. doi: 10.1080/14734220500222318. [DOI] [PubMed] [Google Scholar]
- Tan W, Janczewski WA, Yang P, Shao XM, Callaway EM, Feldman JL. Silencing prebötzinger complex somatostatin-expressing neurons induces persistent apnea in awake rat. Nature Neuroscience. 2008;11:538–540. doi: 10.1038/nn.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, Pagliardini S, Yang P, Janczewski WA, Feldman JL. Projections of prebötzinger complex neurons in adult rats. The Journal of Comparative Neurology. 2010;518:1862–1878. doi: 10.1002/cne.22308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Hirai N. Physiological studies of thoracic spinocerebellar tract neurons in relation to respiratory movement. Neuroscience Research. 1994;19:317–326. doi: 10.1016/0168-0102(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Tarulli AW, Lim C, Bui JD, Saper CB, Alexander MP. Central neurogenic hyperventilation: a case report and discussion of pathophysiology. Archives of Neurology. 2005;62:1632–1634. doi: 10.1001/archneur.62.10.1632. [DOI] [PubMed] [Google Scholar]
- Tatar M, Hanacek J, Widdicombe J. The expiration reflex from the trachea and bronchi. The European Respiratory Journal. 2008;31:385–390. doi: 10.1183/09031936.00063507. [DOI] [PubMed] [Google Scholar]
- Taugher RJ, Lu Y, Wang Y, Kreple CJ, Ghobbeh A, Fan R, Sowers LP, Wemmie JA. The bed nucleus of the stria terminalis is critical for anxiety-related behavior evoked by CO2 and acidosis. The Journal of Neuroscience. 2014;34:10247–10255. doi: 10.1523/JNEUROSCI.1680-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taugher RJ, Ghobbeh A, Sowers LP, Fan R, Wemmie JA. ASIC1A in the bed nucleus of the stria terminalis mediates TMT-evoked freezing. Frontiers in Neuroscience. 2015;9:239. doi: 10.3389/fnins.2015.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NC, Li A, Nattie EE. Medullary serotonergic neurones modulate the ventilatory response to hypercapnia, but not hypoxia in conscious rats. The Journal of Physiology. 2005;566:543–557. doi: 10.1113/jphysiol.2005.083873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AP, Lee AS, Goedecke PJ, Tolley EA, Joyner AL, Heck DH. Conditional loss of engrailed1/2 in atoh1-derived excitatory cerebellar nuclear neurons impairs eupneic respiration in mice. Genes, Brain, and Behavior. 2022;21:e12788. doi: 10.1111/gbb.12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teran FA, Massey CA, Richerson GB. Serotonin neurons and central respiratory chemoreception: where are we now? Progress in Brain Research. 2014;209:207–233. doi: 10.1016/B978-0-444-63274-6.00011-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teune TM, van der Burg J, van der Moer J, Voogd J, Ruigrok TJ. Topography of cerebellar nuclear projections to the brain stem in the rat. Progress in Brain Research. 2000;124:141–172. doi: 10.1016/S0079-6123(00)24014-4. [DOI] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoby-Brisson M, Karlén M, Wu N, Charnay P, Champagnat J, Fortin G. Genetic identification of an embryonic parafacial oscillator coupling to the prebötzinger complex. Nature Neuroscience. 2009;12:1028–1035. doi: 10.1038/nn.2354. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Canteras NS, Swanson LW. Organization of projections from the dorsomedial nucleus of the hypothalamus: a PHA-L study in the rat. The Journal of Comparative Neurology. 1996;376:143–173. doi: 10.1002/(SICI)1096-9861(19961202)376:1<143::AID-CNE9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Swanson LW. Organization of inputs to the dorsomedial nucleus of the hypothalamus: a reexamination with fluorogold and PHAL in the rat. Brain Research. Brain Research Reviews. 1998;27:89–118. doi: 10.1016/s0165-0173(98)00010-1. [DOI] [PubMed] [Google Scholar]
- Tian GF, Duffin J. Spinal connections of ventral-group bulbospinal inspiratory neurons studied with cross-correlation in the decerebrate rat. Experimental Brain Research. 1996;111:178–186. doi: 10.1007/BF00227296. [DOI] [PubMed] [Google Scholar]
- Tian GF, Peever JH, Duffin J. Bötzinger-complex expiratory neurons monosynaptically inhibit phrenic motoneurons in the decerebrate rat. Experimental Brain Research. 1998;122:149–156. doi: 10.1007/s002210050502. [DOI] [PubMed] [Google Scholar]
- Tokita K, Inoue T, Boughter JD. Subnuclear organization of parabrachial efferents to the thalamus, amygdala and lateral hypothalamus in C57BL/6J mice: a quantitative retrograde double labeling study. Neuroscience. 2010;171:351–365. doi: 10.1016/j.neuroscience.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert DL, Bantli H, Bloedel JR. The intracerebellar nucleocortical projection in a primate. Experimental Brain Research. 1977;30:425–434. doi: 10.1007/BF00237266. [DOI] [PubMed] [Google Scholar]
- Toor R, Sun QJ, Kumar NN, Le S, Hildreth CM, Phillips JK, McMullan S. Neurons in the intermediate reticular nucleus coordinate postinspiratory activity, swallowing, and respiratory-sympathetic coupling in the rat. The Journal of Neuroscience. 2019;39:9757–9766. doi: 10.1523/JNEUROSCI.0502-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topchiy I, Waxman J, Radulovacki M, Carley DW. Functional topography of respiratory, cardiovascular and pontine-wave responses to glutamate microstimulation of the pedunculopontine tegmentum of the rat. Respiratory Physiology & Neurobiology. 2010;173:64–70. doi: 10.1016/j.resp.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrealba F, Müller C. Glutamate immunoreactivity of insular cortex afferents to the nucleus tractus solitarius in the rat: a quantitative electron microscopic study. Neuroscience. 1996;71:77–87. doi: 10.1016/0306-4522(95)00426-2. [DOI] [PubMed] [Google Scholar]
- Torres-Tamayo N, García-Martínez D, Lois Zlolniski S, Torres-Sánchez I, García-Río F, Bastir M. 3D analysis of sexual dimorphism in size, shape and breathing kinematics of human lungs. Journal of Anatomy. 2018;232:227–237. doi: 10.1111/joa.12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tort ABL, Ponsel S, Jessberger J, Yanovsky Y, Brankačk J, Draguhn A. Parallel detection of theta and respiration-coupled oscillations throughout the mouse brain. Scientific Reports. 2018;8:6432. doi: 10.1038/s41598-018-24629-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevizan-Baú P, Dhingra RR, Furuya WI, Stanić D, Mazzone SB, Dutschmann M. Forebrain projection neurons target functionally diverse respiratory control areas in the midbrain, pons, and medulla oblongata. The Journal of Comparative Neurology. 2021a;529:2243–2264. doi: 10.1002/cne.25091. [DOI] [PubMed] [Google Scholar]
- Trevizan-Baú P, Furuya WI, Mazzone SB, Stanić D, Dhingra RR, Dutschmann M. Reciprocal connectivity of the periaqueductal gray with the ponto-medullary respiratory network in rat. Brain Research. 2021b;1757:147255. doi: 10.1016/j.brainres.2020.147255. [DOI] [PubMed] [Google Scholar]
- Tsitsopoulos PP, Tobieson L, Enblad P, Marklund N. Prognostic factors and long-term outcome following surgical treatment of 76 patients with spontaneous cerebellar haematoma. Acta Neurochirurgica. 2012;154:1189–1195. doi: 10.1007/s00701-012-1372-7. [DOI] [PubMed] [Google Scholar]
- Turovsky E, Theparambil SM, Kasymov V, Deitmer JW, Del Arroyo AG, Ackland GL, Corneveaux JJ, Allen AN, Huentelman MJ, Kasparov S, Marina N, Gourine AV. Mechanisms of CO2/H+ sensitivity of astrocytes. The Journal of Neuroscience. 2016;36:10750–10758. doi: 10.1523/JNEUROSCI.1281-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umans BD, Liberles SD. Neural sensing of organ volume. Trends in Neurosciences. 2018;41:911–924. doi: 10.1016/j.tins.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezaki T, Zheng Y, Shiba K, Miller AD. Role of nucleus retroambigualis in respiratory reflexes evoked by superior laryngeal and vestibular nerve afferents and in emesis. Brain Research. 1997;769:347–356. doi: 10.1016/s0006-8993(97)00756-7. [DOI] [PubMed] [Google Scholar]
- Vaiman M, Eviatar E, Segal S. Intranasal electromyography in evaluation of the nasal valve. Rhinology. 2003;41:134–141. [PubMed] [Google Scholar]
- van der Heijden ME, Zoghbi HY. Development of the brainstem respiratory circuit. Wiley Interdisciplinary Reviews. Developmental Biology. 2020;9:e366. doi: 10.1002/wdev.366. [DOI] [PubMed] [Google Scholar]
- VanderHorst VG, Terasawa E, Ralston HJ, Holstege G. Monosynaptic projections from the lateral periaqueductal gray to the nucleus retroambiguus in the rhesus monkey: implications for vocalization and reproductive behavior. The Journal of Comparative Neurology. 2000;424:251–268. doi: 10.1002/1096-9861(20000821)424:2<251::aid-cne5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- van der Want JJL, Gerrits NM, Voogd J. Autoradiography of mossy fiber terminals in the fastigial nucleus of the cat. The Journal of Comparative Neurology. 1987;258:70–80. doi: 10.1002/cne.902580105. [DOI] [PubMed] [Google Scholar]
- van de Wiel J, Meigh L, Bhandare A, Cook J, Nijjar S, Huckstepp R, Dale N. Connexin26 mediates CO2-dependent regulation of breathing via glial cells of the medulla oblongata. Communications Biology. 2020;3:521. doi: 10.1038/s42003-020-01248-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dishoeck HAE. Elektrogramm der nasenflügelmuskeln und nasenwiderstandskurve. Acta Oto-Laryngologica. 1937;25:285–295. doi: 10.3109/00016483709127966. [DOI] [Google Scholar]
- Van Dort CJ, Zachs DP, Kenny JD, Zheng S, Goldblum RR, Gelwan NA, Ramos DM, Nolan MA, Wang K, Weng FJ, Lin Y, Wilson MA, Brown EN. Optogenetic activation of cholinergic neurons in the PPT or LDT induces REM sleep. PNAS. 2015;112:584–589. doi: 10.1073/pnas.1423136112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ham JJ, Yeo CH. Somatosensory trigeminal projections to the inferior olive, cerebellum and other precerebellar nuclei in rabbits. The European Journal of Neuroscience. 1992;4:302–317. doi: 10.1111/j.1460-9568.1992.tb00878.x. [DOI] [PubMed] [Google Scholar]
- VanderHorst V, Terasawa E, Ralston HJ. Monosynaptic projections from the nucleus retroambiguus region to laryngeal motoneurons in the rhesus monkey. Neuroscience. 2001;107:117–125. doi: 10.1016/S0306-4522(01)00343-8. [DOI] [PubMed] [Google Scholar]
- van Woerden GM, Hoebeek FE, Gao Z, Nagaraja RY, Hoogenraad CC, Kushner SA, Hansel C, De Zeeuw CI, Elgersma Y. βCaMKII controls the direction of plasticity at parallel fiber-Purkinje cell synapses. Nature Neuroscience. 2009;12:823–825. doi: 10.1038/nn.2329. [DOI] [PubMed] [Google Scholar]
- Varga AG, Maletz SN, Bateman JT, Reid BT, Levitt ES. Neurochemistry of the Kölliker-fuse nucleus from a respiratory perspective. Journal of Neurochemistry. 2021;156:16–37. doi: 10.1111/jnc.15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudeva RK, Lin RCS, Simpson KL, Waterhouse BD. Functional organization of the dorsal raphe efferent system with special consideration of nitrergic cell groups. Journal of Chemical Neuroanatomy. 2011;41:281–293. doi: 10.1016/j.jchemneu.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Verhagen JV, Wesson DW, Netoff TI, White JA, Wachowiak M. Sniffing controls an adaptive filter of sensory input to the olfactory bulb. Nature Neuroscience. 2007;10:631–639. doi: 10.1038/nn1892. [DOI] [PubMed] [Google Scholar]
- Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. The Journal of Comparative Neurology. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- Vitale F, Mattei C, Capozzo A, Pietrantoni I, Mazzone P, Scarnati E. Cholinergic excitation from the pedunculopontine tegmental nucleus to the dentate nucleus in the rat. Neuroscience. 2016;317:12–22. doi: 10.1016/j.neuroscience.2015.12.055. [DOI] [PubMed] [Google Scholar]
- von Euler C, Marttila I, Remmers JE, Trippenbach T. Effects of lesions in the parabrachial nucleus on the mechanisms for central and reflex termination of inspiration in the cat. Acta Physiologica Scandinavica. 1976;96:324–337. doi: 10.1111/j.1748-1716.1976.tb10203.x. [DOI] [PubMed] [Google Scholar]
- Voogd J, Glickstein M. The anatomy of the cerebellum. Trends in Neurosciences. 1998;21:370–375. doi: 10.1016/s0166-2236(98)01318-6. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. European Journal of Pharmacology. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Walker JM, Farney RJ, Rhondeau SM, Boyle KM, Valentine K, Cloward TV, Shilling KC. Chronic opioid use is a risk factor for the development of central sleep apnea and ataxic breathing. Journal of Clinical Sleep Medicine. 2007;3:455–461. doi: 10.5664/jcsm.26908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen-Mackenzie A, Gezelius H, Thoby-Brisson M, Nygard A, Enjin A, Fujiyama F, Fortin G, Kullander K. Vesicular glutamate transporter 2 is required for central respiratory rhythm generation but not for locomotor central pattern generation. Journal of Neuroscience. 2006;26:12294–12307. doi: 10.1523/JNEUROSCI.3855-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Pizzonia JH, Richerson GB. Chemosensitivity of rat medullary raphe neurones in primary tissue culture. The Journal of Physiology. 1998;511 ( Pt 2):433–450. doi: 10.1111/j.1469-7793.1998.433bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Tiwari JK, Bradley SR, Zaykin RV, Richerson GB. Acidosis-stimulated neurons of the medullary raphe are serotonergic. Journal of Neurophysiology. 2001;85:2224–2235. doi: 10.1152/jn.2001.85.5.2224. [DOI] [PubMed] [Google Scholar]
- Wang X, Guan R, Zhao X, Zhu D, Song N, Shen L. Task1 and TASK3 are coexpressed with ASIC1 in the ventrolateral medulla and contribute to central chemoreception in rats. Frontiers in Cellular Neuroscience. 2018;12:285. doi: 10.3389/fncel.2018.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Guan R, Zhao X, Chen J, Zhu D, Shen L, Song N. TASK1 and TASK3 in orexin neuron of lateral hypothalamus contribute to respiratory chemoreflex by projecting to nucleus tractus solitarius. FASEB Journal. 2021;35:e21532. doi: 10.1096/fj.202002189R. [DOI] [PubMed] [Google Scholar]
- Wang X, Novello M, Gao Z, Ruigrok TJH, De Zeeuw CI. Input and output organization of the mesodiencephalic junction for cerebro-cerebellar communication. Journal of Neuroscience Research. 2022;100:620–637. doi: 10.1002/jnr.24993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AHD, Williams C, James BV. Activity patterns in latissimus dorsi and sternocleidomastoid in classical singers. Journal of Voice. 2012;26:e95–e105. doi: 10.1016/j.jvoice.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbourd B, Ren J, DeLoach KE, Guenthner CJ, Miyamichi K, Luo L. Presynaptic partners of dorsal raphe serotonergic and gabaergic neurons. Neuron. 2014;83:645–662. doi: 10.1016/j.neuron.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch JF, Kipp S, Sheel AW. Respiratory muscles during exercise: mechanics, energetics, and fatigue. Current Opinion in Physiology. 2019;10:102–109. doi: 10.1016/j.cophys.2019.04.023. [DOI] [Google Scholar]
- Welker WI. Analysis of sniffing of the albino rat 1) Behaviour. 1964;22:223–244. doi: 10.1163/156853964X00030. [DOI] [Google Scholar]
- Wenker IC, Kréneisz O, Nishiyama A, Mulkey DK. Astrocytes in the retrotrapezoid nucleus sense H+ by inhibition of a kir4.1-kir5.1-like current and may contribute to chemoreception by a purinergic mechanism. Journal of Neurophysiology. 2010;104:3042–3052. doi: 10.1152/jn.00544.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertheimer E. Recherches expérimentales sur les centres respiratoires de la moelle épinière. Journal de l’anatomie et de La Physiologie Normales et Pathologiques de l’homme et Des Animaux. 1886;22:458–507. [Google Scholar]
- White DP, Schneider BK, Santen RJ, McDermott M, Pickett CK, Zwillich CW, Weil JV. Influence of testosterone on ventilation and chemosensitivity in male subjects. Journal of Applied Physiology. 1985;59:1452–1457. doi: 10.1152/jappl.1985.59.5.1452. [DOI] [PubMed] [Google Scholar]
- Wiberg M, Westman J, Blomqvist A. The projection to the mesencephalon from the sensory trigeminal nuclei an anatomical study in the cat. Brain Research. 1986;399:51–68. doi: 10.1016/0006-8993(86)90600-1. [DOI] [PubMed] [Google Scholar]
- Widdicombe JG. Neurophysiology of the cough reflex. The European Respiratory Journal. 1995;8:1193–1202. doi: 10.1183/09031936.95.08071193. [DOI] [PubMed] [Google Scholar]
- Wijdicks EFM. Biot’s breathing. Journal of Neurology, Neurosurgery, and Psychiatry. 2007;78:512–513. doi: 10.1136/jnnp.2006.104919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild B, Rodden FA, Grodd W, Ruch W. Neural correlates of laughter and humour. Brain. 2003;126:2121–2138. doi: 10.1093/brain/awg226. [DOI] [PubMed] [Google Scholar]
- Williams JL, Robinson PJ, Lutherer LO. Inhibitory effects of cerebellar lesions on respiration in the spontaneously breathing, anesthetized cat. Brain Research. 1986;399:224–231. doi: 10.1016/0006-8993(86)91512-x. [DOI] [PubMed] [Google Scholar]
- Williams JL, Everse SJ, Lutherer LO. Stimulating fastigial nucleus alters central mechanisms regulating phrenic activity. Respiration Physiology. 1989;76:215–227. doi: 10.1016/0034-5687(89)90099-6. [DOI] [PubMed] [Google Scholar]
- Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. PNAS. 2007;104:10685–10690. doi: 10.1073/pnas.0702676104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL. Cholinergic systems in the rat brain: III. projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain. Brain Research Bulletin. 1986;16:603–637. doi: 10.1016/0361-9230(86)90134-6. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL. Cholinergic systems in the rat brain: IV. descending projections of the pontomesencephalic tegmentum. Brain Research Bulletin. 1989;23:519–540. doi: 10.1016/0361-9230(89)90197-4. [DOI] [PubMed] [Google Scholar]
- Wu J, Capelli P, Bouvier J, Goulding M, Arber S, Fortin G. A V0 core neuronal circuit for inspiration. Nature Communications. 2017;8:544. doi: 10.1038/s41467-017-00589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Proch KL, Teran FA, Lechtenberg RJ, Kothari H, Richerson GB. Chemosensitivity of phox2b-expressing retrotrapezoid neurons is mediated in part by input from 5-HT neurons. The Journal of Physiology. 2019;597:2741–2766. doi: 10.1113/JP277052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Frazier DT. Medullary respiratory neuronal activity modulated by stimulation of the fastigial nucleus of the cerebellum. Brain Research. 1995;705:53–64. doi: 10.1016/0006-8993(95)01138-2. [DOI] [PubMed] [Google Scholar]
- Xu F, Frazier DT. Respiratory-related neurons of the fastigial nucleus in response to chemical and mechanical challenges. Journal of Applied Physiology. 1997;82:1177–1184. doi: 10.1152/jappl.1997.82.4.1177. [DOI] [PubMed] [Google Scholar]
- Xu F, Frazier DT. Modulation of respiratory motor output by cerebellar deep nuclei in the rat. Journal of Applied Physiology. 2000;89:996–1004. doi: 10.1152/jappl.2000.89.3.996. [DOI] [PubMed] [Google Scholar]
- Xu F, Zhang Z, Frazier DT. Microinjection of acetazolamide into the fastigial nucleus augments respiratory output in the rat. Journal of Applied Physiology. 2001a;91:2342–2350. doi: 10.1152/jappl.2001.91.5.2342. [DOI] [PubMed] [Google Scholar]
- Xu F, Zhou T, Gibson T, Frazier DT. Fastigial nucleus-mediated respiratory responses depend on the medullary gigantocellular nucleus. Journal of Applied Physiology. 2001b;91:1713–1722. doi: 10.1152/jappl.2001.91.4.1713. [DOI] [PubMed] [Google Scholar]
- Xu F, Frazier DT. Role of the cerebellar deep nuclei in respiratory modulation. Cerebellum. 2002;1:35–40. doi: 10.1080/147342202753203078. [DOI] [PubMed] [Google Scholar]
- Yackle K, Schwarz LA, Kam K, Sorokin JM, Huguenard JR, Feldman JL, Luo L, Krasnow MA. Breathing control center neurons that promote arousal in mice. Science. 2017;355:1411–1415. doi: 10.1126/science.aai7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Ezure K, Manabe M. Efferent projections of inspiratory neurons of the ventral respiratory group a dual labeling study in the rat. Brain Research. 1988;455:283–294. doi: 10.1016/0006-8993(88)90087-x. [DOI] [PubMed] [Google Scholar]
- Yang Y, Lisberger SG. Purkinje-cell plasticity and cerebellar motor learning are graded by complex-spike duration. Nature. 2014;510:529–532. doi: 10.1038/nature13282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CF, Feldman JL. Efferent projections of excitatory and inhibitory prebötzinger complex neurons. The Journal of Comparative Neurology. 2018;526:1389–1402. doi: 10.1002/cne.24415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CF, Kim EJ, Callaway EM, Feldman JL. Monosynaptic projections to excitatory and inhibitory prebötzinger complex neurons. Frontiers in Neuroanatomy. 2020;14:58. doi: 10.3389/fnana.2020.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Sanches-Padilla J, Kondapalli J, Morison SL, Delpire E, Awatramani R, Surmeier DJ. Locus coeruleus anchors a trisynaptic circuit controlling fear-induced suppression of feeding. Neuron. 2021;109:823–838. doi: 10.1016/j.neuron.2020.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovsky Y, Ciatipis M, Draguhn A, Tort ABL, Brankačk J. Slow oscillations in the mouse hippocampus entrained by nasal respiration. The Journal of Neuroscience. 2014;34:5949–5964. doi: 10.1523/JNEUROSCI.5287-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Q, Pho H, Kirkness J, Ladenheim EE, Bi S, Moran TH, Fuller DD, Schwartz AR, Polotsky VY. Localizing effects of leptin on upper airway and respiratory control during sleep. Sleep. 2016;39:1097–1106. doi: 10.5665/sleep.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatim N, Billig I, Compoint C, Buisseret P, Buisseret-Delmas C. Trigeminocerebellar and trigemino-olivary projections in rats. Neuroscience Research. 1996;25:267–283. doi: 10.1016/0168-0102(96)01061-9. [DOI] [PubMed] [Google Scholar]
- Yokota S, Tsumori T, Ono K, Yasui Y. Glutamatergic pathways from the Kölliker-fuse nucleus to the phrenic nucleus in the rat. Brain Research. 2004;995:118–130. doi: 10.1016/j.brainres.2003.09.067. [DOI] [PubMed] [Google Scholar]
- Yokota S, Oka T, Tsumori T, Nakamura S, Yasui Y. Glutamatergic neurons in the Kölliker-Fuse nucleus project to the rostral ventral respiratory group and phrenic nucleus: a combined retrograde tracing and in situ hybridization study in the rat. Neuroscience Research. 2007;59:341–346. doi: 10.1016/j.neures.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Yokota S, Kaur S, VanderHorst VG, Saper CB, Chamberlin NL. Respiratory-Related outputs of glutamatergic, hypercapnia-responsive parabrachial neurons in mice. The Journal of Comparative Neurology. 2015;523:907–920. doi: 10.1002/cne.23720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S, Oka T, Asano H, Yasui Y. Orexinergic fibers are in contact with Kölliker-Fuse nucleus neurons projecting to the respiration-related nuclei in the medulla oblongata and spinal cord of the rat. Brain Research. 2016;1648:512–523. doi: 10.1016/j.brainres.2016.08.020. [DOI] [PubMed] [Google Scholar]
- Yoshida K, McCormack S, España RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. The Journal of Comparative Neurology. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JK, Wu M, Manaye KF, Kc P, Allard JS, Mack SO, Haxhiu MA. Orexin stimulates breathing via medullary and spinal pathways. Journal of Applied Physiology. 2005;98:1387–1395. doi: 10.1152/japplphysiol.00914.2004. [DOI] [PubMed] [Google Scholar]
- Yu L, Zhang XY, Zhang J, Zhu JN, Wang JJ. Orexins excite neurons of the rat cerebellar nucleus interpositus via orexin 2 receptors in vitro. Cerebellum. 2010;9:88–95. doi: 10.1007/s12311-009-0146-0. [DOI] [PubMed] [Google Scholar]
- Yu RB, Huang CC, Chang CH, Wang YH, Chen JW. Prevalence and patterns of tongue deformation in obstructive sleep apnea: A whole-night simultaneous ultrasonographic and polysomnographic study. Journal of Sleep Research. 2021;30:e13131. doi: 10.1111/jsr.13131. [DOI] [PubMed] [Google Scholar]
- Yu H, Shi L, Chen J, Jun S, Hao Y, Wang S, Fu C, Zhang X, Lu H, Wang S, Yuan F. A neural circuit mechanism controlling breathing by leptin in the nucleus tractus solitarii. Neuroscience Bulletin. 2022;38:149–165. doi: 10.1007/s12264-021-00742-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagha E, Casale AE, Sachdev RNS, McGinley MJ, McCormick DA. Motor cortex feedback influences sensory processing by modulating network state. Neuron. 2013;79:567–578. doi: 10.1016/j.neuron.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeuw CI, Berrebi AS. Postsynaptic targets of purkinje cell terminals in the cerebellar and vestibular nuclei of the rat. European Journal of Neuroscience. 1995;7:2322–2333. doi: 10.1111/j.1460-9568.1995.tb00653.x. [DOI] [PubMed] [Google Scholar]
- Zelano C, Jiang H, Zhou G, Arora N, Schuele S, Rosenow J, Gottfried JA. Nasal respiration entrains human limbic oscillations and modulates cognitive function. The Journal of Neuroscience. 2016;36:12448–12467. doi: 10.1523/JNEUROSCI.2586-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zera T, Moraes DJA, da Silva MP, Fisher JP, Paton JFR. The logic of carotid body connectivity to the brain. Physiology. 2019;34:264–282. doi: 10.1152/physiol.00057.2018. [DOI] [PubMed] [Google Scholar]
- Zhang SP, Davis PJ, Bandler R, Carrive P. Brain stem integration of vocalization: role of the midbrain periaqueductal gray. Journal of Neurophysiology. 1994;72:1337–1356. doi: 10.1152/jn.1994.72.3.1337. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Xu F, Frazier DT. Role of the Bötzinger complex in fastigial nucleus-mediated respiratory responses. The Anatomical Record. 1999;254:542–548. doi: 10.1002/(SICI)1097-0185(19990401)254:4<542::AID-AR9>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Zhang W, Fukuda Y, Kuwaki T. Respiratory and cardiovascular actions of orexin-A in mice. Neuroscience Letters. 2005;385:131–136. doi: 10.1016/j.neulet.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Zhang CK, Li ZH, Qiao Y, Zhang T, Lu YC, Chen T, Dong YL, Li YQ, Li JL. Vglut1 or VGLUT2 mrna-positive neurons in spinal trigeminal nucleus provide collateral projections to both the thalamus and the parabrachial nucleus in rats. Molecular Brain. 2018;11:22. doi: 10.1186/s13041-018-0362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JQ, Seki M, Hayakawa T, Ito H, Zyo K. Descending projections from the paraventricular hypothalamic nucleus to the spinal cord: anterograde tracing study in the rat. Okajimas Folia Anatomica Japonica. 1995;72:119–135. doi: 10.2535/ofaj1936.72.2-3_119. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Riche D, Rekling JC, Foutz AS, Denavit-Saubié M. Brainstem neurons projecting to the rostral ventral respiratory group (VRG) in the medulla oblongata of the rat revealed by co-application of NMDA and biocytin. Brain Research. 1998;782:113–125. doi: 10.1016/s0006-8993(97)01251-1. [DOI] [PubMed] [Google Scholar]
- Zhou H, Lin Z, Voges K, Ju C, Gao Z, Bosman LWJ, Ruigrok TJH, Hoebeek FE, De Zeeuw CI, Schonewille M. Cerebellar modules operate at different frequencies. eLife. 2014;3:e02536. doi: 10.7554/eLife.02536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JN, Yung WH, Kwok-Chong Chow B, Chan YS, Wang JJ. The cerebellar-hypothalamic circuits: potential pathways underlying cerebellar involvement in somatic-visceral integration. Brain Research Reviews. 2006;52:93–106. doi: 10.1016/j.brainresrev.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Zhuang J, Xu F, Frazier DT. Hyperventilation evoked by activation of the vicinity of the caudal inferior olivary nucleus depends on the fastigial nucleus in anesthetized rats. Journal of Applied Physiology. 2008;104:1351–1358. doi: 10.1152/japplphysiol.00824.2007. [DOI] [PubMed] [Google Scholar]
- Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard MA, Welsh MJ, Wemmie JA. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139:1012–1021. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccal DB, Furuya WI, Bassi M, Colombari DSA, Colombari E. The nucleus of the solitary tract and the coordination of respiratory and sympathetic activities. Frontiers in Physiology. 2014;5:238. doi: 10.3389/fphys.2014.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccal DB, Silva JN, Barnett WH, Lemes EV, Falquetto B, Colombari E, Molkov YI, Moreira TS, Takakura AC. Interaction between the retrotrapezoid nucleus and the parafacial respiratory group to regulate active expiration and sympathetic activity in rats. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2018;315:L891–L909. doi: 10.1152/ajplung.00011.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuperku EJ, Stucke AG, Hopp FA, Stuth EAE. Characteristics of breathing rate control mediated by a subregion within the pontine parabrachial complex. Journal of Neurophysiology. 2017;117:1030–1042. doi: 10.1152/jn.00591.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuperku EJ, Stucke AG, Krolikowski JG, Tomlinson J, Hopp FA, Stuth EA. Inputs to medullary respiratory neurons from a pontine subregion that controls breathing frequency. Respiratory Physiology & Neurobiology. 2019;265:127–140. doi: 10.1016/j.resp.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuperku EJ, Hopp FA, Stuth EAE, Stucke AG. Interaction between the pulmonary stretch receptor and pontine control of expiratory duration. Respiratory Physiology & Neurobiology. 2021;293:103715. doi: 10.1016/j.resp.2021.103715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Connections that were identified as sparse by the authors of these studies are not listed in this table. Note that the list of neurotransmitters involved is not complete. 1 Tree shrew (Tupaia belangeri chinensis); 2 Rufous horseshoe bat (Rhinolophus rouxi); 3 Rhesus monkey (Macaca mulatta); 4 Crab-eating macaque (Macaca fascicularis); 5 Squirrel monkey (Saimiri sciureus). 5-HT = serotonin, A = anterograde, aa = amino acids, AAV = adeno associated virus, ACh = acetyl choline, BDA = biotinylated dextran amines, Biotinam = biotinamide, CRH = corticotropin-releasing hormone, CTb = cholera toxin B subunit, cVRG = caudal part of the ventral respiratory group, DA = dopamine, Degen = degeneration, DTR = dextran-Texas red, DY = diamidino yellow, EB = Evans blue, Exc = excitatory (not specified which neurotransmitter), E-phys = electrophysiology, FB = fast blue, FG = fluorogold, FR = fluoro ruby, GABA = γ-aminobutyric acid, GFP = green fluorescent protein, Glu = glutamate, Gly = glycine, HSV = herpes simplex virus, HRP = horseradish peroxidase, Inh = inhibitory (not specified which neurotransmitter), m = muscle, MDJ = nuclei of the meso-diencephalic junction, n = nucleus or nuclei, NA = noradrenaline / norepinephrine, Nbiotin = neurobiotin, NMB = neuromedin B, NT = neurotransmitter, NTS = nucleus tractus solitarii, Optogen = optogenetic stimulation, OXT = oxytocin, PHA-L = Phaseolus vulgaris leucoagglutinin, PiCo = post-inspiration complex, PPTg = pedunculopontine tegmental nucleus, PI = propidium iodide, POMC = pro-opiomelanocortin, Pseudorab = Pseudorabies, R = retrograde, rVRG = rostral part of the ventral respiratory group, term = terminalis, TMR = tetramethylrhodamine, VP = vasopressin, WGA = wheat germ agglutinin. b – Respiratory control areas affected by pathology Summary of brain regions involved in respiratory control and affected by selected diseases or syndromes. An area is listed in this table if a study reported damage to that area in a majority of subjects included in that study. Case studies with a single patient are not included in this table.