Abstract
Essential functions of mitogen-activated protein kinases (MAPKs) depend on their capacity to selectively phosphorylate a limited repertoire of substrates. MAPKs harbor a conserved groove located outside of the catalytic cleft that binds to short linear sequence motifs found in substrates and regulators. However, the weak and transient nature of these “docking” interactions poses a challenge to defining MAPK interactomes and associated sequence motifs. Here, we describe a yeast-based genetic screening pipeline to evaluate large collections of MAPK docking sequences in parallel. Using this platform, we analyzed a combinatorial library based on the docking sequences from the MAPK kinases MKK6 and MKK7, defining features critical for binding to the stress-activated MAPKs JNK1 and p38α. Our screen of a library consisting of ~12,000 sequences from the human proteome revealed multiple MAPK-selective interactors, including many that did not conform to previously defined docking motifs. Analysis of p38α/JNK1 exchange mutants identified specific docking groove residues that mediate selective binding. Finally, we verified that docking sequences identified in the screen functioned in substrate recruitment in vitro and in cultured cells. Together, these studies establish an approach to characterize MAPK docking sequences and provide a resource for future investigation of signaling downstream of p38 and JNK.
INTRODUCTION
Protein kinases function by phosphorylating a limited number of effector substrates (1). Kinases achieve specificity in part through complementarity between the catalytic cleft and residues surrounding the site of phosphorylation. However, closely related kinases with identical phosphorylation site specificity can nonetheless target distinct sets of substrates. A key example is the mitogen-activated protein kinase (MAPK) family, a group of Ser-Thr kinases conserved widely in eukaryotes (2). Canonical animal MAPKs, including the extracellular signal-regulated kinases (ERKs), c-Jun N-terminal kinases (JNKs) and p38 MAPKs (hereafter, p38), are positioned at the bottom of three-tiered kinase cascades activated in response to diverse cellular stimuli. The different MAPK subfamilies phosphorylate largely unique sets of substrates to elicit distinct cellular responses, yet each targets a minimal Ser/Thr-Pro consensus sequence. MAPK specificity is at least in part driven by docking interactions, in which regions of the kinase outside of the catalytic cleft recruit substrates through binding sites distal from their sites of phosphorylation.
A major hub for MAPK docking interactions is the D-recruitment site (DRS), a conserved region of the catalytic domain that binds to substrates, scaffold proteins, MAPK kinases (MKKs) and MAPK phosphatases (MKPs) (3-5). Several highly conserved binding partners bind to the DRS through domains with intrinsic tertiary structure (6). However, the DRS more generally recognizes short linear motifs (SLiMs) termed D-sites found in unstructured regions of MAPK interactors (7-10). D-sites bind to MAPKs with moderate affinity (~100 nM – 30 μM), promoting transient kinase-substrate interactions and dynamic remodeling of signaling networks in response to stimuli (7). DRS engagement can also impact the conformation and dynamics of the MAPK catalytic domain, which may be important in promoting activation by MKKs and inactivation by MKPs (11-13).
The MAPK DRS forms a groove consisting of three adjacent hydrophobic pockets (designated the ΦL, ΦA and ΦB pockets) and a proximal negatively charged “common docking” (CD) site (7, 8, 14, 15). D-site sequences include a cluster of basic residues complementary to the CD site, a variable linker, and two or three hydrophobic (ϕ) residues (in a ϕL-x-x-ϕA-x-ϕB, ϕL-x-ϕA-x-ϕB, or ϕA-x-ϕB arrangement) that engage the corresponding Φ pockets (10, 15). Additional sequence features within the context of this general motif appear to confer selectivity for particular MAPK subfamilies (7, 9, 16, 17). Structural studies of MAPK•D-site complexes have revealed distinct binding modes associated with these subfamily-selective motifs, driven by arrangement of the hydrophobic residues as well as the sequence composition and bound conformation of the D-site linker sequence (7, 8, 11, 18-20).
Knowledge of these MAPK-selective D-site motifs has driven computational approaches to identify previously unknown MAPK substrates by scanning protein databases for sequences similar to previously established interactors (9, 21). However, experimental approaches for the discovery of additional MAPK-selective sequence motifs are needed to better define MAPK interactomes and to understand how they assemble. Here, we describe a genetically encoded library screening platform to identify new MAPK-interacting D-sites that exploits the conservation of the core cascade from humans to budding yeast. We used this strategy to analyze a saturation mutagenesis library of MKK-derived D-sites interacting with the MAPKs JNK1 and p38α and discovered previously unknown features of their corresponding motifs. We subsequently screened these MAPKs against a large library of human proteomic sequences, which allowed us to further refine the motifs and to identify new direct interaction partners. The screen differentiated between known p38α and JNK1 interactors and identified several binding partners that are selective for either kinase. The dataset therefore provides an unbiased resource to probe selective MAPK signaling pathways and will be an important resource for future investigation into MAPK biology.
RESULTS
A yeast-based system for evaluation of MAPK docking sequences
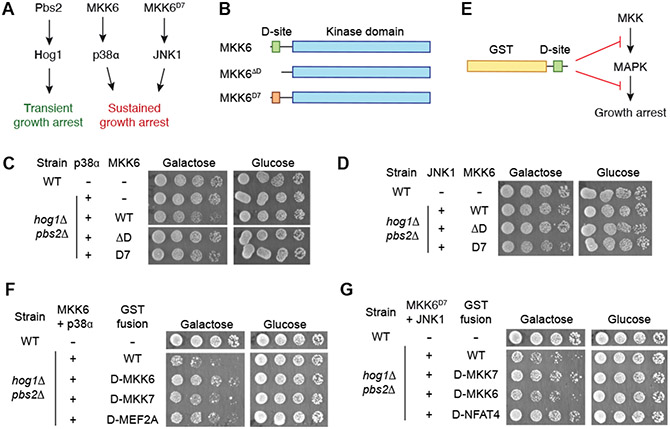
We exploited the evolutionary conservation of core MAPK cascades to enable screening complex D-site sequence libraries in budding yeast. Yeast require the p38/JNK ortholog Hog1 to adapt to osmotic stress (22). Normal activation of the Hog1 cascade by hyperosmolarity induces transient cell cycle arrest, but constitutive signaling causes stable growth suppression (23) (Fig. 1A). Presumably because it is deregulated in yeast, we found that co-expression of mammalian MKK6 with p38α was toxic to a strain lacking Hog1 and its cognate MKK Pbs2 (fig. S1). MKK6 harbors a D-site located upstream of the catalytic domain (Fig. 1B) that promotes its phosphorylation of p38α (24) . Because growth suppression in the context of wild-type (WT) p38α, which partially autophosphorylates in yeast, occurred independently of the MKK6 D-site (fig. S1), we examined a fully MKK-dependent p38α mutant (L195A) (25). Growth impairment by p38αL195A was less severe than that by WT p38α, depended on an intact MKK6 D-site, and was partly alleviated by exchanging the MKK6 D-site with the highest affinity D-site from MKK7 (MKK6D7) (26) (Fig. 1C). Furthermore, MKK6D7, but not WT MKK6 or MKK6ΔD, partly suppressed cell growth when co-expressed with the analogous JNK1L198A mutant (Fig. 1D). These results provide a system in which yeast growth is inhibited by a D-site interaction with either p38α or JNK1.
Figure 1. A system coupling MAPK D-site interactions to yeast cell growth.
(A) Scheme showing the impact of replacing yeast MAPK pathway components with human homologs on cell growth. (B) Domain structure of MKK6 and D-site variants. MKK6D7 replaces the native p38-selective D-site with that from MKK7, which binds only to JNK. (C) Growth assay for a hog1Δ pbs2Δ strain co-expressing p38α and the indicated MKK6 variants. Cells were grown in liquid culture, derepressed in raffinose media, and then spotted in 5-fold serial dilutions on solid media containing either galactose (to induce p38 expression) or glucose. Representative of 2 independent experiments. (D) Growth assay of a hog1Δ pbs2Δ strain co-expressing JNK1 and MKK6 variants conducted as in (C). Representative of 2 independent experiments. (E) Scheme showing potential mechanisms for growth rescue resulting from the expression of GST-D-site fusion proteins. (F) Effect of expressing cognate (MKK6, MEF2A) or non-cognate (MKK7) D-sites fused to GST on growth arrest mediated by p38α-MKK6 co-expression in a hog1Δ pbs2Δ strain. Cells were grown and plated as in (C). Representative of 2 independent experiments. (G) Effect of expressing cognate (MKK7, NFAT4) or non-cognate (MKK6) D-sites fused to GST on growth arrest mediated by JNK1-MKK6D7 co-expression in a hog1Δ pbs2Δ strain. Representative of 2 independent experiments.
To evaluate D-site sequences for their MAPK binding capability, we introduced a third component into this system by ectopically expressing a D-site peptide. We reasoned that engagement of the MAPK DRS by the D-site would rescue growth inhibition by blocking either MAPK-MKK or MAPK-substrate binding (Fig. 1E). We first engineered hog1Δ pbs2Δ strains harboring chromosomally integrated cassettes for constitutive expression of the MKK (WT MKK6 or MKK6D7) and for inducible expression of the MAPK (p38αL195A or JNK1L198A). We next introduced plasmids inducibly expressing D-site peptides fused to glutathione S-transferase (GST) into these strains and assessed their growth under inducing or non-inducing conditions. Expression of p38α-binding D-site peptides derived from MKK6 or MEF2A both substantially reversed growth impairment associated with co-expression of MKK6 and p38αL195A, whereas the MKK7 D-peptide improved growth to a lesser extent (Fig. 1F). Likewise, D-site peptides from MKK7 and the JNK substrate NFAT4, but not from MKK6, rescued growth of the strain expressing MKK6D7 and JNK1L198A (Fig. 1G). These experiments establish a system in which expression of a MAPK-binding D-site is coupled to cell growth, providing the basis for screens to identify sequences engaging the MAPK DRS.
Positional scanning mutagenesis of MAPK docking sites
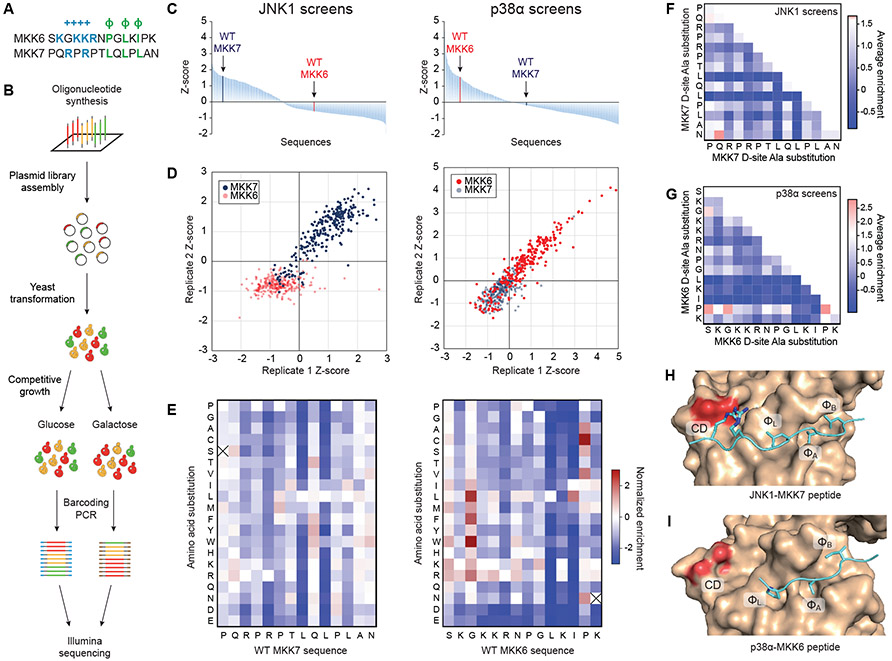
The importance of specific D-site residues in mediating selective interaction with MAPKs has been established by analysis of mutant proteins and synthetic peptides (16, 17, 27, 28), but low-throughput approaches can only analyze a limited number of variants. To analyze docking specificity more comprehensively using our yeast system, we designed a library of 715 unique sequences that included all possible single amino acid substitutions, as well as double Ala substitutions in all pairwise combinations, to D-sites from MKK6 and MKK7 (Fig. 2A, Data File S1). The MKK7 library was based on the second of its three D-sites, which has the highest affinity and has been co-crystallized with JNK1 (13, 26). Oligonucleotides encoding these sequences were custom synthesized and cloned as a pool into the yeast GST fusion vector (Fig. 2B). Plasmid pools were introduced into MAPK/MKK-expressing yeast strains, and liquid cultures were expanded in derepressing (raffinose) media. A portion of the culture was reserved for sequencing, and the remainder was split and propagated under either inducing (raffinose + galactose) or non-inducing (glucose) conditions. At various times, cultures were sampled, plasmids were extracted from cells, and the D-site regions were PCR amplified with barcoding primers. PCR samples were pooled and analyzed by Illumina sequencing, providing the relative abundance of each component of the library at each time point. Although no sequences changed substantially (>10%) in relative abundance under non-inducing conditions, library representation became skewed upon induction of either MAPK (Fig. 2C,D, fig. S2A,B, Data File S2). The change of representation of each variant over time was fit to an exponential function to provide a measure of its relative depletion or enrichment within the screen (Fig. 2C,D, Data Files S2 and S3).
Figure 2. Combinatorial library screens.
(A) MKK6 and MKK7 sequences with the core elements of the D-site motif highlighted. (B) A schematic showing the workflow of the screen. (C) Waterfall plots showing the average Z-score for the enrichment/depletion rate of each D-site sequence variant from two independent screens against JNK1 (left) or p38α (right). WT MKK6 and MKK7 D-sites are highlighted. Read counts from Illumina sequencing runs are provided in Data File S2. (D) Scatter plot showing correlation of Z-scores between two replicate screens for JNK (left) and p38α (right). Blue, MKK7 variants; red, MKK6 variants. (E) Heatmaps showing the effect of each amino acid substitution to the MKK7 D-site in the JNK screen (left) and MKK6 D-site in the p38α screen (right). Values are normalized to the respective WT sequence (white), with red indicating enrichment and blue indicating depletion of the sequence from the population. Crossmarked boxes indicate sequences missing from the screening library. (F and G) Heat maps showing mean enrichment scores of double alanine substitutions at each position in the MKK7 D-site sequence in the JNK1 screen (F) or the MKK6 D-site sequence in the p38α screen (G). In the color scheme, white indicates the enrichment score for the WT sequence. N=2 independent experiments. (H and I) Crystal structure of JNK1 (H) or p38α (I) (tan) in complex with MKK7 (H) or MKK6 (I) D-site peptides (cyan) with the CD region and three hydrophobic pockets indicated (PDB entries 4UX9 and 5ETF) (13) (29).
As anticipated, variants derived from cognate D-sites (MKK6 for p38α; MKK7 for JNK1) were generally enriched during selection in two separate experiments, indicative of a successful interaction, whereas with few exceptions non-cognate D-sites were depleted and presumably did not bind to the MAPK (Fig 2D). Most substitutions to cognate D-sites resulted in slower growth rates and thus reduced binding affinities, suggesting that the WT sequences are largely optimal, but some variants displayed improved binding to either p38α or JNK1 (Fig. 2E,F,G). In the MKK7 D-site, Leu8 and Leu10, which engage the ΦL and ΦA pockets of JNK (13), appeared to be the most critical for binding, whereas Leu12, which binds the ΦB pocket, was more tolerant to substitution (Fig 2E,F,H). As anticipated, substitutions to the N-terminally positioned basic residues Arg3 and Arg5 of the MKK7 D-site (Fig. 2A), which bind to the CD region, were also deleterious. In the JNK1 screen, the largest increase in growth was observed by aromatic substitutions to Gln9 of the MKK7 D-site. A Tyr residue located at the analogous position in the NFAT4 D-site appears to provide intra- and intermolecular van der Waals contacts in the co-crystal structure with JNK1 (fig. S3A) (7). In the p38α screen, the most deleterious single (Fig. 2E) and double (Fig. 2G) amino acid substitutions were to Leu10 and Ile12 in the MKK6 D-site, which engage the ΦA and ΦB pockets in the p38 hydrophobic groove (Fig. 2I) (7, 29). As anticipated, substitutions to basic residues in the N-terminal cluster of the D-site (Fig. 2A) were also disfavored, and incorporation of additional Lys or Arg residues in this region appeared to promote binding. Surprisingly, mutating either Lys11 or Lys14 near the D-site C-terminus, not previously reported as essential for interaction with p38α, also reduced binding. Compared to JNK1, p38α was generally less tolerant of acidic residues in the D-site sequence, particularly in the C-terminal region (positions 11 – 14, Fig. 2A). These observations suggest that p38α D-site binding may be in part driven by bulk electrostatics, rather than position-specific ionic interactions. At two sites near the D-site N-terminus, binding was apparently improved by substitution with hydrophobic residues that may access an additional hydrophobic pocket (termed the ΦU pocket) previously observed in some p38 and ERK D-site complexes (fig. S3B) (11, 29). Only three residues within the MKK6 D-site sequence appeared to be suboptimal for binding, including a Pro residue immediately downstream of the L-x-I motif. Overall, these positional scanning screens both confirmed elements of the MKK6 and MKK7 D-sites known to be critical for MAPK binding, and also identified additional features that favor or disfavor DRS interactions.
Screening the human proteome for MAPK-interacting D-sites
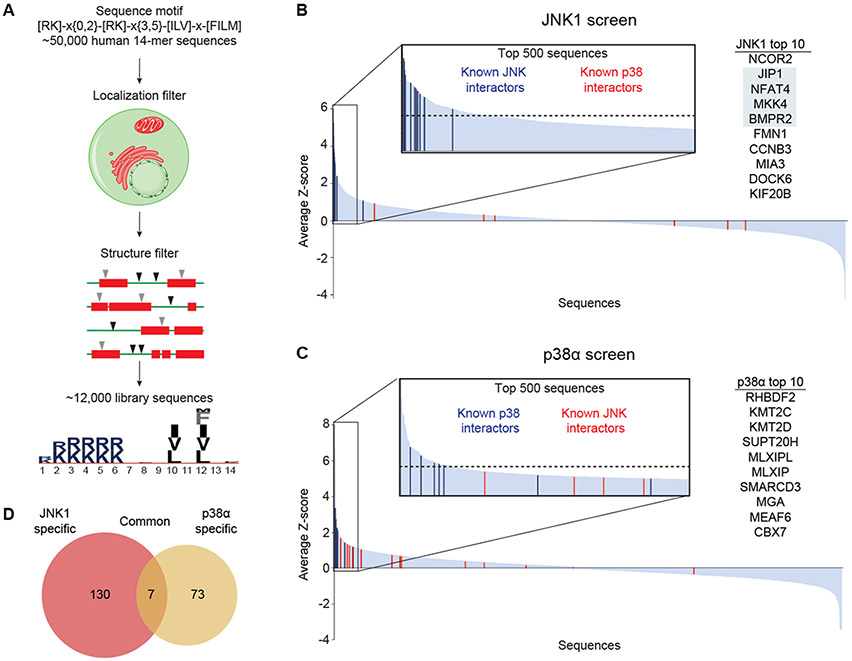
The screens described above allowed us to define key features of the MKK6 and MKK7 D-sites. However, different D-site peptides can assume distinct binding modes at the DRS, each associated with its own conformation and associated sequence motif (7, 9, 11, 13, 19). To enable discovery of multiple MAPK-targeting motifs and to discover new p38 or JNK interactors, we designed a large library consisting of candidate D-site sequences derived from the human proteome following similar criteria to those used previously for in silico screens (9, 21) (Fig. 3A). We identified ~50,000 occurrences of the general D-site motif (defined as [RK]-x0-2-[RK]-x3-5-[ILV]-x-[FILMV]) in the human proteome. To increase the likelihood that sites would be accessible to bind MAPKs, we excluded sequences that fell within folded domains and those annotated to be extracellular or located within the endoplasmic reticulum or the Golgi apparatus. The remaining sequences were incorporated into a final library of 11,756 sequences from 5426 proteins, including most established JNK- and p38-interacting D-sites (Data File S1, table S1).
Figure 3. Proteomic library screens.
(A) Schematic depicting the selection of candidate D-site sequences from the human proteome. (B) and (C) Waterfall plots for the JNK1 (B) and p38α (C) screens, respectively, ordered by decreasing average Z-score (N=3 independent experiments) for each sequence. The insets show the 500 most highly enriched sequences, with the dashed line indicating the Z=2 hit cutoff. Established functional JNK- and p38-interacting D-sites are indicated as colored bars. Proteins corresponding to the 10 most enriched sequences are shown at right with previously known JNK interactors highlighted. (D) Venn diagram showing the overlap of hits from the two screens.
Coding sequences for all components of the library were introduced as a pool into the yeast GST fusion vector and screened for interaction with p38α and JNK1 as described above for the positional scanning libraries (Data File S4, fig. S2C,D). Sequences were ranked by the average Z-score from three replicate screens (Data File S5). We observed established D-sites to be strongly enriched in screens for their respective MAPK (Fig. 3B,C, Data File S5). For example four of the five most highly ranked JNK1-interacting sequences (corresponding to JIP1, NFAT4, MKK4 and BMPR2) were previously reported docking sites (Fig. 3B). We defined “hits” as sequences enriched in all replicates with an average Z score ≥ 2. By these criteria, all save one of the known JNK-interacting D-sites present in our library scored among the 137 hits in the JNK1 screen (Fig. 3B, table S2). The 80 p38α hit sequences (Fig. 3C, table S3) included five of seven known interacting D-sites (MKK3, MKK4, MKK6, MEF2A and MEF2C). We note that the remaining D-sites (PTPN5 and PTPN7) were also enriched but did not meet our hit threshold. We observed little overlap among hits for p38α and JNK1, with only seven sequences on both lists (Fig. 3D), and no non-cognate D-sites scored as hits for either MAPK. The strong enrichment of previously known D-sites suggests that other hits are likely to include authentic MAPK-interacting sequences.
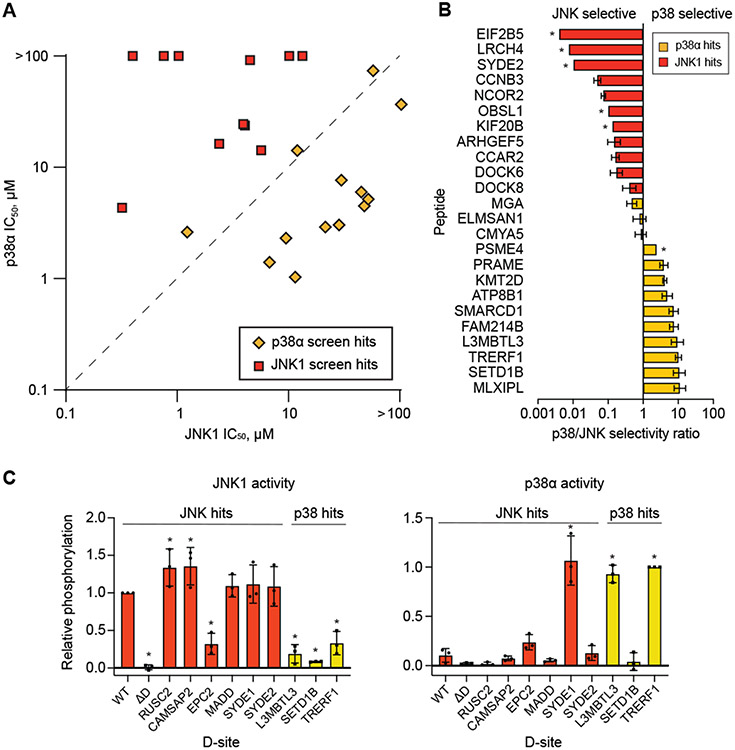
To validate results from our screens, we examined the capacity of individual enriched sequences to selectively bind p38α and JNK1. To assess MAPK binding, we examined that ability of synthetic peptides to inhibit JNK1 and p38α activity toward a fluorogenic substrate incorporating a requisite D-site (30). We chose 24 hit sequences that had not previously been established to bind the corresponding MAPKs. As a measure of relative binding affinity, we determined IC50 values for inhibition of both kinases from assays conducted at a range of competitor peptide concentrations (Fig. 4A, table S4). We found that all sequences scoring as hits in the JNK1 screen indeed bound JNK1 with higher affinity than to p38α (Fig. 4A,B). Although p38α was favored by most of its hit sequences, two of them were non-selective, and one slightly favored JNK1. We noted a tendency for JNK1 to bind D-sites more tightly than p38α, with three having sub-μM IC50 values (Fig. 4A, table S4). This phenomenon may reflect an intrinsic property of the JNK1 DRS or a more stringent affinity cutoff for enrichment in the yeast-based screen. We found a relatively weak correlation between IC50 value and Z-score among high affinity sites, particularly for JNK1-interacting sequences (fig. S4). This phenomenon could be due to variability in expression levels among D-site sequences, or alternatively that sequences above a given affinity threshold do not impart faster growth rates in the proteomic screen. Overall, these experiments confirm that most sequences identified in our screen bind selectively to p38α and JNK1 with affinities comparable to those of established D-sites.
Figure 4. Hit validation.
(A) Plot showing mean IC50 values (N=3 independent experiments) for inhibition of the indicated MAPK by each of 24 synthetic peptides. IC50 values too high to be confidently determined are indicated as being >100 μM and placed at the top or right edge. JNK1- and p38α-specific hits from the yeast screens are red squares and yellow diamonds, respectively. JNK1- and p38α-selective peptides fall respectively above and below the dotted line. (B) Graph showing the ratios of JNK1 to p38α IC50 values for the indicated peptides, ordered from most JNK1-selective at top to most p38α-selective at bottom. Peptides binding weakly to either p38α or JNK1 are indicated with an asterisk, and ratios were calculated using an IC50 value of 100 μM. Error bars show 95% confidence intervals. (C) Graphs showing the relative level of phosphorylation by JNK1 (left) and p38α (right) of chimeric NFAT4 constructs in which its D-site was replaced with the indicated hit peptides. Phosphorylation was assessed by immunoblotting with NFAT4 anti-phosphoSer165 antibody. Error bars indicate SD (N = 3 independent experiments). Values significantly different from the WT construct (p = 0.05 by Mann-Whitney non-parametric test) are indicated (*).
Because D-sites are often found in MAPK substrates, we further examined the capacity for selected p38 and JNK-targeting sequences to function in substrate recruitment. For these experiments, we used an N-terminal fragment of the JNK substrate NFAT4, which harbors a D-site positioned upstream of established phosphorylation sites, including Ser165. We generated constructs in which the native NFAT4 D-site was either eliminated or substituted with hit sequences from the yeast screens. As anticipated, the WT fragment was robustly phosphorylated at Ser165 by JNK1 in vitro, and this was significantly reduced by mutation of the docking site (Fig. 4C). Furthermore, substitution with four out of five JNK1 hit sequences tested restored Ser165 phosphorylation to that of the WT construct or higher, whereas incorporation of p38α-targeting sites resulted in significantly lower phosphorylation. By contrast, p38α poorly phosphorylated the WT fragment, and incorporating either of two of its hit sequences (L3MBTL3 and TRERF1) converted NFAT4 to a p38α substrate. However, the construct harboring one other hit (SETD1B) was not detectably phosphorylated, even though the corresponding peptide did bind to p38α in competitive kinase assays (table S4). This observation suggests that in some cases, D-site binding is insufficient to promote phosphorylation of an associated protein. D-site engagement can impact MAPK conformational dynamics to promote kinase activity (31), and our results suggest that such effects may occur in a sequence-specific manner. We also note that one D-site that was exclusively a JNK1 hit (SYDE1) promoted phosphorylation by both MAPKs and was presumably a false negative in the p38α screen.
D-site sequence motifs conferring MAPK selective binding
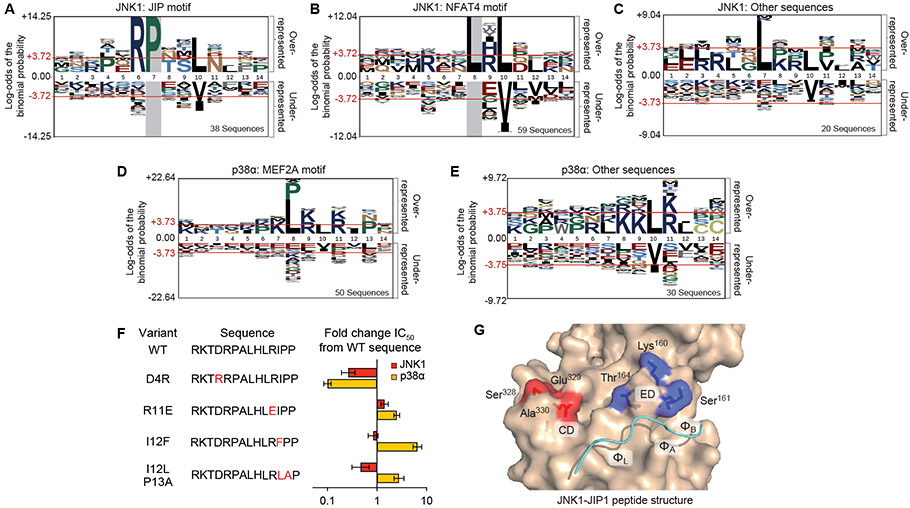
Previous structural, biochemical, and computational analyses have identified multiple distinct sequence motifs selectively targeted by JNK and p38 MAPKs. Analysis of aligned hit sequences revealed that for both MAPKs, specific amino acids were substantially overrepresented at multiple positions in comparison to the full set of sequences in the library (Fig. 5A to E). We expected that hit sequences for a given MAPK would conform to multiple distinct motifs that would not be evident from alignment of all hits. To deconvolute distinct motifs from the full dataset, we examined subsets of sequences in which a single overrepresented residue was fixed at one position. This analysis revealed two previously defined signatures within the JNK1 dataset: the “JIP class” motif (R-P-x-x-L-x-L, Fig. 5A) and the “NFAT4 class” motif (L-x-L-x-L/F, Fig. 5B) (9). Among these core motifs, we observed specific residues to be enriched at intervening positions, such as hydrophilic residues downstream of the conserved Pro in the JIP class motif and acidic/amidic residues intervening the ϕA and ϕB residues of both motifs. These two motif classes account for 85% of JNK1 hits, with the remaining sequences being enriched for a Leu residue in position 7 (producing a L-x-x-L-x-L motif, Fig. 5C) that likely occupies the ΦL pocket similar to the Pro residue in the JIP class motif. Hit sequences for p38α were most strongly enriched for the previously defined “MEF2A class” motif (P/L-x-L-x-I/L-P) (9), with 62.5% harboring a Pro or aliphatic residue in position 8 (ϕL) (Fig. 5D). Of the other sequences, 40% had an aliphatic residue in position 7, with the remainder were characterized by one or more basic residues upstream of the ϕA (position 10) Leu residue (Fig. 5E). These residual sequences generally lacked an Ile residue at the ϕB position or a Pro residue at position 13, suggestive of a distinct binding mode. In keeping with the results from the positional scanning library above, proteomic sequences selected by p38α were generally enriched for basic residues in positions proximal to the hydrophobic residues, a feature not generally considered as part of known interaction motifs. We note that Lys or Arg residues near the D-site N-terminus were not notably selected by either MAPK (Fig. 5A to E). However, because our library design included at least two basic residues in all sequences, they may still promote binding in a manner independently of their precise position.
Figure 5. MAPK-interacting sequence motifs.
(A) The subset of JNK1 hit sequences having a Pro residue at position 7 corresponding to the JIP class motif, represented by pLogo (65). (B) JNK1 hits with a Leu residue at position 8 comprising the NFAT4 class motif. (C) Sequences lacking an R-P-x-x-L or ϕ-x-L-x-L/F motif in any register. (D) pLogos corresponding to p38α MEF2A motif class hit sequences, defined as those with an Ile, Val, Leu, or Pro residue at position 8 or a Pro residue at position 13. (E) All remaining p38α hits. (F) Graph depicting ratios of the IC50 values for the indicated point-substituted KMT2D synthetic D-peptide to those of the WT peptide in competitive inhibition JNK1 or p38α in vitro kinase assays. Error bars show 95% confidence intervals from 3 independent experiments. IC50 values are provided in table S4. (G) Two patches of surface residues in the docking groove of JNK, the CD (red) and ED (blue) regions, are shown mapped on the crystal structure of JNK1 in complex with the JIP1 peptide (PDB code 1UKI) (18).
We probed the importance of key elements of these motifs by substitution analysis of a p38α hit peptide derived from KMT2D (RKTDRPALHLRIPP) in kinase inhibition assays as described above (Fig. 5F, table S4). Consistent with our screens and previous reports that the identity of hydrophobic residues can drive MAPK specificity (16), we found that substitution of the ϕB Ile residue with Phe led to a large decrease in p38α binding but did not impact binding to JNK1. Combined substitution of the Ile12-Pro13 sequence with Leu-Ala also made the peptide more JNK1 selective, suggesting a switch to an NFAT4 motif class. Substitution of Arg12 with Glu had a larger impact on binding to p38α compared to binding to JNK, consistent with results from both the proteomic and positional scanning screens. Replacement of Asp4 with Arg improved binding to both MAPKs, confirming the importance of basic residues in that region. Collectively, these assays verify key sequence features selected by the two MAPKs in our screens.
Knowledge of a protein interaction motif can be used to discover new interacting proteins by searching databases for matching sequences (32). Although our screening approach identified MAPK interacting sequences present in the human proteome, authentic MAPK D-sites that do not conform strictly to the criteria used to build the library would be excluded. To identify D-sites not present within our screening library, we scanned the human proteome for sequences similar to our hits using PSSMsearch (33). We performed four separate searches using hit sequences corresponding to the p38α (MEF2A class and non-MEF2A class) and JNK1 (JIP1 class and NFAT4 class) targeting motifs (Data File S6). As anticipated, these searches returned sequences from the yeast screens that were used to build the position-specific scoring matrix (PSSM), as well as sequences that were absent from the library. We chose 8 peptides excluded from the original library and previous studies, and we evaluated their affinities for p38α and JNK1 in the competitive kinase assay (Table 1). With one exception, peptides bound to their predicted kinases with affinities comparable to those from the yeast screens (IC50 range 0.3 - 5.6 μM). These results confirm our ability to computationally identify new docking sequences, providing additional validation for our experimentally determined motifs.
Table 1.
Inhibitory potency of peptide sequences identified by PSSMsearch.
| Protein | Sequence | JNK1 search rank |
JNK1 PSSM |
p38α search rank |
p38α PSSM |
JNK1 IC50, μM (95% CI) |
p38α IC50, μM (95% CI) |
|---|---|---|---|---|---|---|---|
| AFAP1L2 | KQVRKKEHKLKITP | - | - | 4 | Other | >100 | 40 (34 - 47) |
| RASGRF1 | SPSRRRKLSLNIPI | 74 | NFAT | 2 | MEF2A | 1.1 (0.89 - 1.3) | 2.4 (2.0 - 2.9) |
| WDR3 | KRKRKKREKLILTL | 25 | NFAT | 7 | Other | 0.90 (0.81 - 1.0) | 0.30 (0.24 - 0.36) |
| TTLL13P | RRRKRRSLAINLTN | 10 | NFAT | 48 | MEF2A | 1.8 (1.7 - 2.0) | 0.76 (0.64 - 0.90) |
| MXD4 | EKHRRAKLRLYLEQ | 1 | NFAT | 34 | MEF2A | 1.1 (0.95 - 1.3) | 5.2 (3.5 - 7.8) |
| MYH14 | GEQRRRRLELQLQE | 8 | NFAT | - | - | 5.6 (4.6 - 6.9) | 35 (26 - 49) |
| CDX1 | YPGPARPASLGLGP | 36 | JIP | - | - | 2.7 (2.4 - 3.0) | >100 |
Determinants of MAPK-selective D-site interactions
We next considered features of the p38 and JNK DRS that might mediate selective targeting of distinct motifs. As noted above, p38α was more generally selective for basic residues throughout the D-site sequence. Notably, the p38α DRS is more negatively charged than that of JNK1, having nine acidic residues in comparison to four for JNK1. Unique acidic residues in p38α cluster at two sites: the so-called Glu-Asp (ED) region proximal to the ΦL and ΦA pockets (4) and the common docking (CD) region (Fig. 5G). To examine the importance of these two regions to D-site specificity, we generated point mutants exchanging residues between p38α and JNK1 and examined binding of peptides corresponding to MAPK-selective motifs by competitive kinase assay (Table 2). Mutating either the ED or CD regions of JNK1 to the corresponding residues in p38α reduced binding of the JNK-selective NFAT4 and JIP1 peptides by an order of magnitude. Although the JNK1 CD mutant had modestly increased affinity for the p38-selective MKK6 (MEF2A motif) and SETD1B (non-MEF2A motif) peptides, a larger effect was seen with the ED mutant, which had affinities similar to that of WT p38α for the two peptides. Conversely, the p38α ED mutant decreased the affinity of both cognate peptides to levels comparable to those seen for JNK1, whereas the CD mutant did not have a substantial effect. None of the p38α mutants conferred detectable binding to the JNK1 cognate NFAT4 and JIP1 peptides. These experiments substantiate a role for a specific cluster of residues in mediating selective binding of D-sites to p38α, either through specific side chain interactions or though bulk electrostatic effects.
Table 2. Impact of p38α/JNK1 exchange mutagenesis on peptide binding affinities.
JNK1 ED mutant, K160N/S161E/T164E; JNK1 CD mutant, S328D/E329D/A330E; p38α ED mutant, N159K/E160S/E163T; p38α CD mutant, D315S/D316E/E317A. Values are the average of 3 independent replicate experiments.
| JNK1 IC50, μM (95% CI) |
p38α IC50, μM (95% CI) |
||||||
|---|---|---|---|---|---|---|---|
| Peptide | Sequence | WT | ED mutant | CD mutant | WT | ED mutant | CD mutant |
| NFAT4 | ERPSRDHLYLPLEP | 0.89 (0.76 - 1.0) | 9.5 (8.6 - 10) | 6.3 (5.0 - 7.9) | >100 | >100 | >100 |
| JIP3 | GRRKERPTSLNVFP | 0.36 (0.31 - 0.43) | 2.5 (2.2 - 2.8) | 2.9 (2.6 - 3.1) | >100 | >100 | >100 |
| MKK6 | SKGKKRNPGLKIPK | 95 (78 - 120) | 24 (21 - 27) | 75 (53 - 110) | 16 (14 - 19) | 65 (57 - 76) | 16 (14 - 18) |
| SETD1B | NQLKFRKKKLKFCK | 28 (25 - 33) | 2.0 (1.6 - 2.4) | 14 (7.4 - 28) | 7.8 (6.3 - 9.7) | 30 (25 - 37) | 8.0 (6.4 - 10) |
Discovery of docking-dependent MAPK substrates
To explore how our D-site screens might contribute to our understanding of MAPK function, we first performed gene set enrichment analysis (34) to identify potential cellular processes invovling p38 and JNK (Table 3). Hits for p38α were enriched for proteins involved in regulation of chromatin organization and gene transcription, with most (65%) localizing to the nucleus. These observations are consistent with nuclear translocalization of activated p38 and with its established roles in transcriptional regulation (35), and suggest broader control of the process than previously appreciated. Although the observed enrichment of JNK1 hits for proteins involved in the JNK MAPK cascade was expected due to the abundance of known interactors, this category included four upstream regulators not previously known to interact with JNK (TNIK, NCOR1, MAGI3, CARD9) that could constitute points for feedback regulation. In addition, JNK1 interactors were significantly enriched for the gene ontology (GO) biological process category “cytoskeleton organization” and for multiple categories related to GTPase signaling. Notably, in comparison to the full set of sequences in the library, both p38α and JNK1 hit sequences tended to be more conserved across species (fig. S5), suggesting evolutionary pressure to maintain functional sequences. We do note that the degree of conservation varied among hit sequences, consistent with prior analyses of D-site evolution (9).
Table 3. Gene set enrichment analysis of p38α and JNK1 hits.
FDR, false discovery rate.
| JNK1 screen |
p38α screen |
||
|---|---|---|---|
| GO category | FDR q-value | GO category | FDR q-value |
|
|
|
||
| Regulation of Intracellular Signal Transduction | 1.44E-10 | Chromatin Organization | 9.80E-09 |
| Nucleoside Triphosphatase Regulator Activity | 5.82E-10 | Chromosome Organization | 2.73E-08 |
| Cytoskeleton Organization | 1.65E-09 | Catalytic Complex | 1.19E-07 |
| Small GTPase Mediated Signal Transduction | 2.85E-09 | Positive Regulation of Biosynthetic Process | 3.54E-07 |
| Guanyl Nucleotide Exchange Factor Activity | 7.90E-09 | Positive Regulation of Nucleobase Containing Compound Metabolic Process | 8.98E-07 |
| Positive Regulation of Catalytic Activity | 8.41E-09 | Nuclear Protein Containing Complex | 8.98E-07 |
| Enzyme Regulator Activity | 1.94E-08 | Chromosome | 2.65E-06 |
| Positive Regulation of Molecular Function | 1.94E-08 | Positive Regulation of Transcription by RNA Pol II | 3.04E-06 |
| MAPK cascade | 2.92E-08 | Negative Regulation of Nucleobase Containing Compound Metabolic Process | 1.04E-05 |
| JNK cascade | 5.55E-08 | Covalent Chromatin Modification | 1.28E-05 |
|
|
|
||
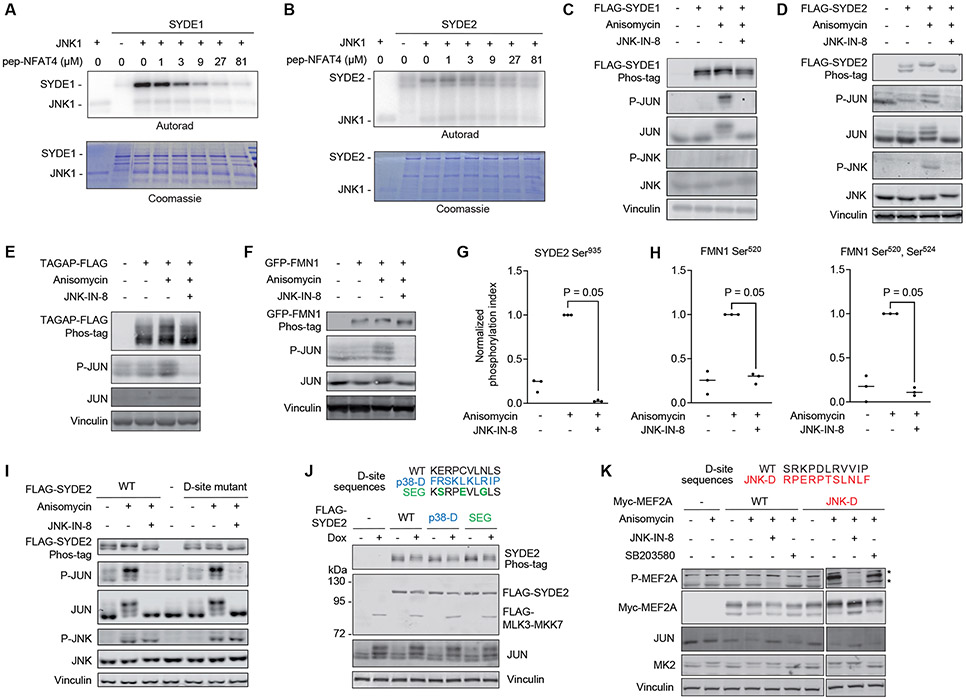
Because D-sites can play a role in MAPK substrate recruitment, we reasoned that hits from our yeast screens include previously undescribed substrates. To verify this notion, we examined a set of cytoskeletal and GTPase regulatory proteins as candidate substrates from our JNK1 screens. We found that JNK1 phosphorylated two of these proteins, the RHO family GTPase activating proteins (GAPs) SYDE1 and SYDE2 (36, 37), in kinase assays performed in vitro (Fig. 6A,B). Addition of a peptide corresponding to the NFAT4 D-site inhibited JNK1 activity toward both proteins, suggesting that phosphorylation depended on an interaction with the DRS. We next examined whether SYDE1, SYDE2, and two other hit proteins (TAGAP and FMN1) could be phosphorylated by JNK in cells. We treated HEK293T cells expressing the proteins with the protein synthesis inhibitor anisomycin to activate the JNK pathway in the presence or absence of the selective JNK inhibitor JNK-IN-8. We found that anisomycin induced phosphorylation of all four proteins as judged by partial or complete electrophoretic mobility shift on Phos-tag sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), which was reversed by treatment with the JNK inhibitor (Fig. 6C-F). Analysis of SYDE2 by liquid chromatography-tandem mass spectrometry (LC-MS/MS) revealed that a phosphorylation at a single residue (Ser935) increased with anisomycin treatment and decreased with JNK-IN-8 treatment (Fig. 6G, Data File S7). Similarly, we found that anisomycin-induced phosphorylation of multiple sites in a Ser-Pro consensus in FMN1 was also blocked by JNK inhibition (Fig. 6H, fig. S6, and Data File S8). Overall, these observations verify that our ability to identify new MAPK substrates from our D-site screens.
Figure 6. JNK substrate discovery.
(A) In vitro kinase assay measuring JNK1-mediated phosphorylation of full length SYDE1. Reactions were performed with increasing concentrations of the NFAT4 D-site competitor peptide (pep-NFAT4). Representative images from 3 separate experiments are shown. (B) In vitro kinase assay measuring JNK1-mediated phosphorylation of full length SYDE2, performed as in (A). Representative images from 3 separate experiments are shown. (C-F) Immunoblotting analysis with the indicated antibodies of HEK293T cells expressing FLAG-SYDE1, FLAG-SYDE2, FLAG-TAGAP, or GFP-FMN1 and treated with anisomycin following preincubation with or without the covalent JNK inhibitor JNK-IN-8. Lysates were subjected to either Phos-tag (top image) or standard (all others) SDS-PAGE. A representative immunoblot is shown for 2 (SYDE1), 3 (SYDE2), 5 (TAGAP) and 5 (FMN1) independent experiments. (G) LC-MS/MS analysis of tryptic peptides derived from immunopurified FLAG-SYDE2 transiently expressed in HEK293T cells treated as indicated. The graph shows the ratio of the Ser935-phosphorylated peptide to the corresponding unphosphorylated peptide. Data are normalized to the anisomycin treated sample. N=3 independent experiments. Significance was calculated using a one-tailed Mann-Whitney non-parametric test. (H) LC-MS/MS analysis of singly or doubly phosphorylated GFP-FMN1-derived tryptic phosphopeptides spanning Ser520 and Ser524 showing the ratio of phosphorylated to unphosphorylated peptides, normalized to the anisomycin treated sample. N=3 independent experiments. Significance was calculated using a one-tailed Mann-Whitney non-parametric test. (I) Immunoblotting analysis with the indicated antibodies of lysates from HEK293T cells expressing either WT SYDE2 or its D-site mutant (R1179Q/P1180Q/L1183A/L1185A) and treated with anisomycin following preincubation with or without the covalent JNK inhibitor JNK-IN-8. N=3 independent experiments. (J) Immunoblots of lysates from HEK293T cells harboring an episomal Dox-inducible MLK3-MKK7 expression plasmid and transfected with FLAG-SYDE2 variants in which the D-site was substituted with the sequence shown at top. Cells were incubated for 6 hr in the presence or absence of Dox prior to lysis. N=2 independent experiments. (K) Immunoblots of HEK293T cell lysates transfected with plasmids expressing either WT MEF2A or a mutant in which the D-site was exchanged for an optimal JNK-binding sequence shown at top (JNK-D). Cells were treated with anisomycin in the presence or absence of JNK-IN-8 or the p38α/β inhibitor SB203580 prior to lysis. Asterisk (*) indicates non-specific bands present in the untransfected control samples. N=2 independent experiments.
To determine the importance of specific features of docking motifs for phosphorylation of MAPK substrates in cells, we examined a series of SYDE2 D-site mutations. Mutation of core residues within the SYDE2 “JIP class” D-site motif (R1179Q/P1180Q/L1183A/L1185A) prevented its anisomycin-induced Phos-tag PAGE mobility shift, confirming that the docking interaction is indeed essential for phosphorylation by JNK in cells (Fig. 6I). We noted that in addition to this core R-P-x-x-L-x-L motif, the SYDE2 sequence harbored residues at other positions that were positively selected in the proteomic screen. To determine the importance of these “secondary” residues, we generated an SYDE2 mutant in which they were substituted (E1178S/C1181E/N1184G) but maintained the core motif. We expressed this mutant in cells inducibly expressing constitutively active forms of either MKK7 or MKK6 to specifically activate JNK and p38, respectively. We found that this mutation reduced, but did not eliminate, phosphorylation by JNK, confirming that residues outside of the core motif do contribute to optimal phosphorylation (Fig. 6J). We similarly exchanged the SYDE2 D-site for a sequence optimized to bind to p38α. This mutation eliminated JNK-dependent phosphorylation, despite the presence of core basic and hydrophobic residues recognized by both kinases (Fig. 6J). This substitution did not lead to detectable phosphorylation of SYDE2 by p38 in cells (fig. S7), suggesting that in additional to the essential D-site interaction, other regions of SYDE2 may contribute to its phosphorylation by MAPKs. To examine whether a JNK-selective motif could be sufficient to effect substrate recruitment in a different context, we examined the impact of exchanging the native D-site of the established p38 substrate MEF2A for a predicted JNK-targeting sequence. We were unable to observe anisomycin-induced phosphorylation of full length WT MEF2A at an ascribed p38 site (Thr319) when ectopically expressed in HEK293T cells, presumably because it was below the limit of detection (Fig. 6K). However, introduction of an optimized JNK-binding D-site led to robust anisomycin-induced phosphorylation that was eliminated by treatment with JNK-IN-8 but not affected by the p38α/β-selective inhibitor SB203580. Collectively, these results confirm the ability of specific D-site sequences to effect selective JNK recruitment in the context of full length proteins.
DISCUSSION
Here, we have described a genetic screening approach to the identification of kinase docking motifs and interacting sequences. Most prior genetic screens for docking sequences involved in substrate recruitment have focused on protein phosphatases, which are generally described as lacking dephosphorylation site specificity and hence depend on non-catalytic interactions (38-41). However, there has been growing appreciation for the importance of non-catalytic SLiM-mediated interactions to kinase substrate targeting. For MAPKs, docking interactions can enforce selective targeting to individual subfamilies and can restrict the kinase to phosphorylating specific Ser or Thr residues in a given substrate (9, 10, 42). In other systems, docking is not absolutely required for phosphorylation in cells, yet tuning the strength of such interactions can reportedly set phosphorylation rate. For example, SLiM-mediated recruitment of substrates to cyclin-dependent kinases through the cyclin subunit controls the timing of phosphorylation within the cell division cycle (43, 44). For the yeast LATS/NDR kinase Cbk1, an optimal docking sequence is not absolutely required for substrate phosphorylation but confers robustness to perturbations that attenuate kinase activity (45).
Previous approaches to identify SLiMs mediating protein-protein or protein-enzyme interactions have used libraries of synthetic peptides or genetically-encoded phage display and cell surface display libraries (40, 46-48). Our method involving reconstitution of signaling pathways in yeast involves tunable pathway inhibition through a competitive interaction between kinases in the MAPK cascade or with downstream effectors. This approach may be advantageous in that interactions occur within a eukaryotic cell while maintaining sufficiently high throughput to enable extraction of binding motifs. It also enables discovery of interaction partners that might escape detection in MS-based proteomics experiments due to low abundance or restricted distribution patterns. The expression level of D-site fusion proteins in yeast sets a relatively stringent affinity threshold providing a low false positive rate, but we may consequently fail to identify low affinity interactions. For example, although our approach selected almost all known JNK interactors in our library, it failed to identify the established D-site in the transcription factor ATF2. This is consistent with regions of ATF2 outside of its SLiM reportedly making an additional contact with MAPKs to increase binding affinity (49). Likewise, the full p38α-interacting region of the phosphatase PTPN7 (also known as HePTP) includes additional sequence flanking the canonical D-site (50, 51), and although enriched in our screen, it fell below our hit threshold.
In this study, we performed both a comprehensive mutagenesis screen of known docking sites as well as a screen of proteome-derived sequences. Screening positional scanning libraries has facilitated discovery of new kinase substrates conforming closely to the resulting motifs (52). Selection of proteomic libraries has an advantage in that it directly nominates candidate interaction partners and can discover high affinity sequences that might appear suboptimal. Furthermore, a sufficiently large set of interacting sequences will also provide key sequence features constituting the interaction motifs. Indeed, using PSSMs derived from our experimental hits, we were able to identify additional high affinity JNK1 and p38α-binding sequences absent from our screening library. We do note that the PSSMsearch scores correlated poorly with peptide binding affinity as measured in competitive kinase assays. Possible explanations for this phenomenon include compositional bias among hit sequences for reasons unrelated to binding affinity (for example to promote disorder) or that residues at distinct positions make non-additive or context-dependent contributions to binding affinity. Our PSSMs were not corrected for the background amino acid frequency of library sequences, which could have overemphasized the contribution of N-terminal basic residues to binding affinity at the expense of other residues. Determining the binding affinity of a larger set of training peptides could be used to refine input PSSMs to allow for more accurate predictions. In addition, computational approaches that can account for non-additivity, such as the use of artificial neural networks, would be expected to improve the correlations between search score and binding affinity.
Several previous studies have used computational approaches to scan proteomes for MAPK-interacting D-sites based on consensus motifs defined by alignment of known interactors and by integrating structural constraints based on crystallographic studies of MAPK-D-site complexes (9, 21). Although we did observe substantial overlap in our datasets, most of our hit sequences were not previously predicted (tables S2 and S3). For example, 38% of JNK1 and 33% of our p38α hits were also discovered in the structure-guided in silico screens conducted by Zeke et al. (9) , and 15% of JNK1 hits had been identified by Whisenant et al. through database searches for sequences similar to known D-sites (21). We found comparable overlap between these prior screens and our own in silico PSSM-based searches (45, 24, and 18 sequences from Zeke et al. were among our top 100 scoring sequences in the JIP, NFAT and MEF2A searches, respectively). We note that hits unique to our dataset were often dissimilar in sequence to those for which structural information is available. In particular, over 90% of our p38α hits lacking an ϕL residue had not been previously predicted. These observations underscore the value of unbiased screens of proteome-derived libraries in the discovery of new interactors.
JNK and p38 MAPKs were originally identified as stress-activated kinases and are now known to have diverse roles in normal and disease physiology (35, 53, 54). We found substantial enrichment for specific processes associated with proteins harboring MAPK-interacting D-sites uncovered in our studies, suggestive of expanded roles for JNK and p38. For example, p38 regulates gene expression by directly phosphorylating more than a dozen sequence-specific transcription factors (35). In addition to several previously unidentified transcription factor targets, approximately 25% of our hits were derived from chromatin-associated proteins, including multiple chromatin remodeling factors, components of lysine modification complexes, and methyllysine readers. These results suggest previously unappreciated mechanisms by which p38 may impact transcription. Furthermore, enrichment of GTPase regulators among JNK1 interactors is interesting in light of the capacity of RHO family GTPases to activate the JNK cascade (55, 56), and we verified the RHO GAPs SYDE1, SYDE2 and TAGAP to be cellular JNK substrates. JNK isoforms may therefore have general roles in crosstalk or feedback regulation between GTPase signaling pathways. Overall, these studies provide a resource for further investigation into regulation of basic cellular processes by the MAPKs p38 and JNK.
MATERIALS AND METHODS
Plasmids
The plasmid for constitutive expression of N-terminally HisMax-tagged human MKK6 in yeast was generated by insertion of the full coding sequence (polymerase chain reaction [PCR] amplified from pcDNA3-HisMax-MKK6) (57) downstream of the ACT1 promoter (PCR amplified from pGS62, a gift from Gavin Sherlock, Stanford University) in pRS416. MKK6ΔD and D-site substitution mutants on this background were generated by overlap extension PCR using oligonucleotides deleting residues Ser4 – Lys17 (SKGKKRNPGLKI) or replacing them with D-sites from MKK7 (PQRPRPTLQLPLAN), MEF2A (SRKPDLRVVIPPS) or NFAT4 (LERPSRDHLYLPLE). To generate integrative expression vectors for WT MKK6 and MKK6D7, coding sequences were subcloned into pRS416-GPD downstream of the yeast TDH3 (GPD) promoter, and the entire expression cassette was PCR amplified and cloned into the PacI and BglII sites of the plasmid HO-hisG-URA3-hisG-poly-HO (Addgene plasmid #51661). The integrating inducible yeast expression vectors for N-terminally FLAG-tagged human JNK1 (isoform α1) and rat p38α were generated by inserting the full-length coding sequences into pRS403-GAL1. The inducible yeast GST expression vector was generated by PCR amplifying the GST coding sequence from pGEX-4T1 and cloning into the SacI and SpeI sites of pRS416-GAL1. Coding sequences for individual D-sites together with the HisMax tag were PCR amplified from the corresponding pRS416-MKK6 plasmid and inserted into the SpeI and ClaI sites downstream of GST.
Bacterial expression vectors for GST-JNK1 (pGEX4T1-3xFLAG-JNK1, Addgene #47574), GST-p38α, His6-MKK4, His6-MKK6S207E/T211E (MKK6-EE), and constitutively active MEKK1 (MEKK-C) were previously reported (58, 59). Expression vectors for NFAT4 D-site variants were prepared by subcloning residues 3 – 407 of WT NFAT4 from the corresponding mammalian expression vector (Addgene #21664) into pGEX4T1, introducing ClaI and HindIII restriction sites flanking the D-site by site-directed mutagenesis, excising the native D-site coding sequence, and replacing it with synthetic oligonucleotide pairs harboring compatible ends.
The mammalian expression vectors for human SYDE1 (UniProt isoform 2, Q6ZW31-2) and human TAGAP (UniProt isoform 1, Q8N103-1) were generated by Gateway recombination from pDONR223-SYDE1 (human ORFeome collection) into the C-terminal 3xFLAG epitope tagged plasmid pV1900. The expression vector for N-terminally FLAG-tagged mouse SYDE2 was generated by PCR amplification of the coding sequence from pNICE HA-mSYD1B (36) (Addgene #59362) and insertion into pcDNA3-FLAG by Gibson assembly. The plasmid expressing Myc epitope and 6xHis-tagged MEF2A in the pcDNA3.1 vector (60) and N-terminally GFP-tagged mouse FMN1 isoform 2 were obtained from Addgene (#118354 and #19320).
Point mutations in all plasmids were introduced by QuikChange site-directed mutagenesis. Sequences of all primers used for cloning, mutagenesis and Illumina sequencing are provided in Data File S9.
Design and generation of D-site libraries
The positional scanning libraries consisted of all possible single amino acid substitutions and all double Ala mutations to the MKK6 (SKGKKRNPGLKIPK) and second MKK7 (PQRPRPTLQLPLAN) D-site. To design the proteomic library, we identified all sequences matching the regular expression [RK]-x0-2-[RK]-x3-5-[ILV]-x-[FILMV] in human proteins in the UniProt database. Sequences were extended at both termini to include two residues downstream of the motif and to bring the total length to 14 residues, and overlapping sequences were removed. With R and custom Perl scripts, we parsed the reviewed UniProt KnowledgeBase to filter sequences based on localization and to exclude motifs falling within annotated domains. Only proteins labeled as “cytoplasm” or “nucleus” were included, and non-cytosolic sequences in transmembrane proteins were excluded. Sequences were reverse translated in silico to yeast optimized codons, and silent mutations were introduced to remove restriction sites used for cloning. Common flanking sequences were added for separate PCR amplification of the positional scanning (5’: GCTTCAGGTGGACAACAATCACAA, 3’: GAAGCTTCACTCTGTGTTGAAGTTCCGTCAG) and proteomic (5’: GGTCGCGGATCTATGTCTCAG, 3’: GAAGCTTTTGAACAACCTCAGCAC) libraries. Core DNA and protein sequences are provided in Data File S1. Oligonucleotides were commercially synthesized as a pool (CustomArray), PCR-amplified, and restriction enzyme cloned into the NheI and HindIII sites downstream of the GST coding sequence of the pRS416-GAL1-GST plasmid (fig. S8). DH10B cells (Invitrogen ElectroMAX) were transformed by electroporation with ligation products to produce at least 1000 transformants per library variant, and plasmid library DNA was prepared from pooled colonies. To ensure representation of all components of the library, the variable region was PCR-amplified and sequenced on an Illumina HiSeq 4000 instrument. Read counts for the proteomic library are provided in Data File S10.
Yeast growth assays
Liquid cultures of the indicated strains transformed with the indicated plasmids were grown to mid-logarithmic phase in the appropriate selective media containing 2% raffinose. Aliquots of five-fold dilution series were spotted onto agar plates containing either 2% glucose or 2% raffinose + 1% galactose as indicated. Plates were incubated at 30°C for 48 – 96 hours.
Yeast-based screens
The S. cerevisiae hog1Δ pbs2Δ strain was generated by PCR-based replacement of the HOG1 open reading frame with the LEU2 marker in a pbs2Δ::KanMX strain from the yeast knockout collection (BY4741 strain background, Open Biosystems). The genotype was confirmed by diagnostic PCR of both deletion arms from genomic DNA. Strains used for screening were generated by subsequent integration of cassettes for galactose-inducible expression of p38αL195A or JNK1L198A (at the HIS3 locus) and constitutive GPD promoter-driven expression of His-tagged WT MKK6 or MKK6D7 (at the HO locus). Expression of MAPK and MKK6 alleles were confirmed by immunoblotting lysates from galactose-treated cells with antibodies to the FLAG and His6 tags, respectively.
Libraries of plasmids expressing D-site GST fusion proteins were introduced into yeast by LiOAc high-efficiency transformation (61) and selection on synthetic complete (SC)-Ura agar plates to produce at least 200 transformants per component. Transformed yeast were scraped from plates, pooled, diluted to an OD600 of 0.1 in SC-Ura liquid media with 2% glucose, and grown to saturation at 30°C. Cells were diluted into SC-Ura with 2% raffinose and grown for 6 hours to derepress the GAL1 promoter. A starting time (T0) sample (20 OD600 units) was reserved, and the remaining cells were split and diluted to an OD600 of 0.1 in either SC-Ura + 2% raffinose + 1% galactose (inducing conditions) or SC-Ura + 2% glucose (control conditions). Cultures were subjected to four growth and dilution cycles in which they were propagated until the induced culture reached an OD600 of ~1.5, a portion (20 OD600 units) reserved, and remaining cells diluted in fresh pre-warmed media to an OD600 of 0.1. Reserved cells were pelleted, washed once with sterile dH2O, snap-frozen on dry ice/EtOH and stored at −80°C. Plasmids were extracted from each cell pellet and the D-site regions were PCR amplified, incorporating barcodes specific to each condition and time point and adaptors for sequencing. PCR products were agarose gel-purified, pooled, and subjected to Illumina sequencing (HiSeq 4000). The positional scanning library was screened twice, and the human proteomic library was screened three times against each MAPK. Data for each sequence were fit to an exponential function in Microsoft Excel, in which the inverse time constant λ was calculated as the slope of the line of the log2 transformed fold-change in normalized read counts as a function of time, with the y-intercept set to zero. Z-scores for each sequence within an individual screen were calculated from (λsequence - λmean)/SD. In each of the proteomic screens, a small number of components were not detected in the time zero sample. To allow comparisons across all screens, we performed Z-score analyses for only those components present in the starting time point samples from every replicate. In total, 53 sequences (0.45% of the library) were excluded from the analysis.
Database searches
For PSSM searching, hit sequences were binned into four categories, accounting for sites to occur in a different register from our original definition. The JIP class comprised JNK1 hits containing a R-P-x-x-ϕ-x-ϕ sequence starting at either position 4 or position 6. The NFAT4 class comprised JNK1 hits with an ϕ-x-ϕ-x-ϕ sequence starting at either position 8 or position 10 that were not included in the JIP class. The MEF2 class comprised p38α hit sequences with either a Leu or Pro residue at position 8 or a Pro residue at position 13. The “other p38α” class comprised p38α hit sequences with a Lys or Arg residue at either position 8 or 9, excluding sequences defined in the MEF2 motif. Sequences belonging to each motif class were entered into the program PSSMsearch (http://slim.icr.ac.uk/pssmsearch/) (33) and the resulting PSSM used to searched the human proteome with default settings (disorder cutoff = 0.4, p-value cutoff = 0.001). Search results were ranked based on the PWM score, and the top 1000 sequences are shown in Data File S6. Gene set enrichment analysis for GO categories associated with hit sequences was performed using the Broad Institute web interface (https://www.gsea-msigdb.org/) (34).
Protein Expression and Purification
GST-tagged (JNK1, p38α, and NFAT43-407 variants) and His6-tagged (MKK6-EE and active MKK4 prepared by co-expression with MEKK-C) were expressed in BL21(DE3) E. coli and purified as described (58, 59). FLAG epitope-tagged SYDE1 and SYDE2 were expressed in and purified from polyethyleneimine-transfected HEK293T cells (ATCC #CRL-3216) as previously described (62). The concentration and purity of protein preparations were assessed by SDS-PAGE and Coomassie Brilliant Blue R250 staining alongside bovine serum albumin (BSA) standards.
GST-p38α and GST-JNK1 (50 μM) were activated in vitro by incubation with 500 nM His6-MKK6-EE or 50 nM active His6-MKK4, respectively, in reaction buffer (50 mM Tris [pH 8.0], 100 mM NaCl, 10 mM MgCl2, 1mM DTT, 0.012% Brij-35, 300 μM ATP) at 30°C for 1.5 hours. Phosphorylation was confirmed by immunoblotting with anti-p38 pThr180/pTyr182 and JNK pThr183/pTyr185 antibodies as appropriate.
Immunoblotting
The following primary antibodies used for immunoblotting were obtained from Cell Signaling Technology: p38 pThr180/pTyr182 (#9211), JNK pThr183/pTyr185 (#9251 and #9255), JNK (#3708), c-JUN (#2315), c-JUN pSer63 (#9261), MK2 (#3042), GST (#2624), and Myc tag (#2276). Other antibodies used were: NFAT4 pSer165 (Sigma-Aldrich SAB4503947), FLAG M2 (Sigma-Aldrich F3165), vinculin (Sigma-Aldrich V9131), penta-His (Qiagen), MEF2A pThr319 (Origene #TA325686). Fluorophore-conjugated secondary antibodies (Invitrogen #A21109 goat anti-rabbit and Li-Cor #926-32210 goat anti-mouse) were diluted 1:20,000 in TBS-T and 5% BSA. Blotting membranes were analyzed using an Odyssey CLx imaging system (LI-COR Biosciences) and quantified using Image Studio Lite software.
MAPK D-site peptide inhibition assays
D-site peptides were commercially synthesized (GenScript) incorporating a fixed Tyr-Ala sequence upstream of 14 residues corresponding to the yeast library sequence. Peptides were dissolved in dimethyl sulfoxide (DMSO) to 10 mM and stored at −20°C. MAPK assays were performed with a sulfonamido-oxine (SOX) containing substrate peptide (AssayQuant AQT0376). Kinase assays were performed in technical duplicate in black 384 well plates in reactions containing 50 mM HEPES (pH 7.5), 10 mM MgCl2, 0.012% Brij-35, 1% glycerol, 0.2 mg/mL BSA, 1 mM ATP, 1.2 mM DTT and 4 μM SOX peptide substrate. Competitor D-site peptides were titrated in two-fold increments over a range from 31 nM – 64 μM. Reactions were initiated by adding activated p38α or JNK1 to final concentrations of 3 nM and 60 nM, respectively, and fluorescence (excitation 360 nm, emission 485 nm) was read every min over 1 hour in a Molecular Devices SpectraMax M5 plate reader. Initial velocities were calculated from the linear portions of the reaction progress curves. IC50 values and 95% confidence intervals were calculated by fitting data collected from three biological replicates to a sigmoidal dose-response curve using Prism 8.2.0 (GraphPad).
Protein kinase assays
GST-NFAT43-407 and its variants (0.5 μM) were incubated with p38α (14 nM) or JNK1 (7 nM) in reaction buffer (50mM HEPES [pH 7.4], 100mM NaCl, 10mM MgCl2, 0.012% Brij-35, 1mM DTT, 1mM Na3VO4, 5mM β-glycerophosphate, 100 μM ATP) at 30°C for 20 min. Reactions were quenched by adding SDS-PAGE loading buffer and analyzed by immunoblotting with antibodies to GST and NFAT4 pSer165.
Purified SYDE1 (100 nM) or SYDE2 (120 nM) was incubated with or without active JNK1 (70 nM) in kinase reaction buffer containing 50 mM HEPES (pH 7.4), 100 mM NaCl, 10 mM MgCl2, 0.012% Brij-35, 1 mM DTT, 1 mM Na3VO4, 1 mM β-glycerophosphate and 50 nM staurosporine (to suppress background phosphorylation in the control reaction). Reactions were initiated by adding [γ-32P]ATP to a final concentration of 20 μM at 0.1 μCi/μL. Reactions were incubated at 30°C for 20 min and then quenched with the addition of 5 μL 4x SDS-PAGE loading buffer. Samples were separated by SDS-PAGE (10% acrylamide) and gels were stained with Coomassie, destained, and exposed to a phosphor screen. Exposures were analyzed by phosphorimager and quantified using QuantityOne software (BioRad). Experiments were performed at least three times.
Analysis of protein phosphorylation in cultured cells
HEK293T cells were transiently transfected with the indicated expression plasmids using polyethyleneimine. After 48 hr, cells were treated with either 5 μM JNK-IN-8 (SelleckChem, S4901), 50 μM SB203580, or vehicle (0.1% DMSO) for 1 hour followed by either 10 μg/mL anisomycin or vehicle (0.1% DMSO) for an additional hour at 37°C. Lysates were prepared as described (62), and a portion was subjected to either standard or Phos-tag SDS-PAGE (63) and immunoblotting with the indicated antibodies (all used at 1:1000 dilution). Phos-tag gels included 4% or 7.5% acrylamide, 50 μM Phos-tag reagent (Nard Institute AAL-107), and 100 μM MnCl2.
Phosphorylation of SYDE2 D-site mutants was analyzed in cells inducibly expressing constitutively active forms of MKK7 (MLK3-MKK7 fusion protein) or MKK6 (MKK6-EE) to activate the JNK and p38 pathways, respectively (64). HEK293T cells were stably transfected with episomal plasmids expressing active MKKs from a doxycycline (Dox)-inducible promoter and selected for at least one week in the appropriate antibiotic (5 μg/mL puromycin and 100 μg/mL hygromycin for MLK3-MKK7 and MKK6-EE, respectively). Cells were transfected as above, and two days later were treated for 6 hr with 2 μg/mL Dox before lysis and immunoblotting as described above.
For MS analysis, FLAG-SYDE2 was isolated from 10 cm plates as described above, and GFP-FMN1 was isolated similarly using GFP nanotrap beads. Proteins were fractionated by SDS-PAGE, and gels were stained briefly with Coomassie Brilliant Blue and de-stained. Protein bands were excised and in-gel digested with trypsin (MS grade, Promega) overnight at 37°C overnight, and peptides extracted with 80% acetonitrile/0.1% formic acid. Peptides were desalted with C18 spin columns (Nest Group, Inc.) and dried. For SYDE2 only, phosphopeptides were enriched by dissolving in 70 mM glutamic acid in 65% acetonitrile with 2% TFA, and subjected to TiO2 phosphopeptide enrichment; the flowthrough fraction was reserved, and the enriched phosphopeptide fraction was eluted with 28% NH4OH and dried. Peptides were dissolved in 10 μL 70% formic acid plus 30 μL 50 mM sodium phosphate. Flowthrough and enriched SYDE2 samples and total FMN1 samples (all 0.3 μg in 5 μL) were subjected to LC-MS/MS analysis on a Q-Exactive Plus mass spectrometer (ThermoFisher Scientific) equipped with a Waters nanoAcquity ultra-performance liquid chromatography (UPLC) system (Waters) with a C18 (180 μm x 20 mm) trap column and a 1.7 μm, 75 μm x 250 mm nanoAcquity UPLC column. Trapping was done using 99% Buffer A (100% water, 0.1% formic acid) and peptide separation was undertaken using a linear gradient of solvents A (0.1% formic acid in water) and B (0.075% formic acid in acetonitrile) over 90 minutes, at a flow rate of 300 nL/min. Raw LC-MS/MS data files were processed with Progenesis QI Software (Waters, Inc.) with exported .mgf files searched against a custom database for the SYDE2 construct with MASCOT 2.7 (Matrix Science). Sites of phosphorylation were validated manually. Search results were imported into Progenesis QI and matched with the spectral features, and where relevant normalized enriched and flowthrough data were merged. The exported peptide lists with normalized abundances are provided in Data Files S7 and S8. Data are available through ProteomeXchange with identifier PXD028694.
Data and materials availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium through the PRIDE partner repository with the dataset identifier PXD028694. All other data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials. Plasmids and yeast strains generated for this study are available through an MTA with Yale University.
Supplementary Material
ACKNOWLEDGMENTS
We thank Elias Lolis, Anton Bennett and Joel Sexton (Yale University), and Erik Schaefer (AssayQuant), for advice and suggestions regarding this work, and we thank Titus Boggon (Yale University) for advice and feedback on the manuscript. We thank Karl Barber (Yale University) for assistance with oligonucleotide library design, and Florine Collin and Jean Kanyo (Yale Keck Mass Spectrometry and Proteomics Resource) for assistance with proteomics sample preparation and LC MS/MS data collection. We thank Ana Thevenin (Moravian College), Gavin Sherlock (Stanford University) and Attila Reményi (Research Center for Natural Sciences, Budapest) for providing plasmids, and the following investigators from whom plasmids were obtained through Addgene: Anjana Rao, Peter Scheiffele, David Stillman, Lea Sistonen, Philip Leder, and Kevin Janes.
Funding
This work was supported by National Institutes of Health grant R01 GM135331 to B.E.T. Additional support was provided by the China Scholarship Council to G.S., a National Science Foundation Graduate Research Fellowship to J.T.R. and NIH T32 GM007324 to C.S. The Q-Exactive Plus mass spectrometer located at the Keck MS & Proteomics Resource was supported in part by NIH SIG grant S10 OD018034 and Yale School of Medicine.
Footnotes
Competing interests: The authors declare that they have no competing interests
REFERENCES AND NOTES
- 1.Miller CJ, Turk BE, Homing in: Mechanisms of Substrate Targeting by Protein Kinases. Trends Biochem. Sci 43, 380–394 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raman M, Chen W, Cobb MH, Differential regulation and properties of MAPKs. Oncogene 26, 3100–3112 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Peti W, Page R, Molecular basis of MAP kinase regulation. Protein. Sci 22, 1698–1710 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanoue T, Maeda R, Adachi M, Nishida E, Identification of a docking groove on ERK and p38 MAP kinases that regulates the specificity of docking interactions. EMBO J. 20, 466–479 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldsmith EJ, Akella R, Min X, Zhou T, Humphreys JM, Substrate and docking interactions in serine/threonine protein kinases. Chem. Rev 107, 5065–5081 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang YY, Wu JW, Wang ZX, A distinct interaction mode revealed by the crystal structure of the kinase p38α with the MAPK binding domain of the phosphatase MKP5. Sci. Signal 4, ra88 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Garai A, Zeke A, Gogl G, Toro I, Fordos F, Blankenburg H, Barkai T, Varga J, Alexa A, Emig D, Albrecht M, Remenyi A, Specificity of linear motifs that bind to a common mitogen-activated protein kinase docking groove. Sci. Signal 5, ra74 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang CI, Xu BE, Akella R, Cobb MH, Goldsmith EJ, Crystal structures of MAP kinase p38 complexed to the docking sites on its nuclear substrate MEF2A and activator MKK3b. Mol. Cell 9, 1241–1249 (2002). [DOI] [PubMed] [Google Scholar]
- 9.Zeke A, Bastys T, Alexa A, Garai A, Meszaros B, Kirsch K, Dosztanyi Z, Kalinina OV, Remenyi A, Systematic discovery of linear binding motifs targeting an ancient protein interaction surface on MAP kinases. Mol. Syst. Biol 11, 837 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharrocks AD, Yang SH, Galanis A, Docking domains and substrate-specificity determination for MAP kinases. Trends Biochem. Sci 25, 448–453 (2000). [DOI] [PubMed] [Google Scholar]
- 11.Zhou T, Sun L, Humphreys J, Goldsmith EJ, Docking interactions induce exposure of activation loop in the MAP kinase ERK2. Structure 14, 1011–1019 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Kumar GS, Zettl H, Page R, Peti W, Structural basis for the regulation of the mitogen-activated protein (MAP) kinase p38α by the dual specificity phosphatase 16 MAP kinase binding domain in solution. J. Biol. Chem 288, 28347–28356 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kragelj J, Palencia A, Nanao MH, Maurin D, Bouvignies G, Blackledge M, Jensen MR, Structure and dynamics of the MKK7-JNK signaling complex. Proc. Natl. Acad. Sci. U. S. A 112, 3409–3414 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu B, Stippec S, Robinson FL, Cobb MH, Hydrophobic as well as charged residues in both MEK1 and ERK2 are important for their proper docking. J. Biol. Chem 276, 26509–26515 (2001). [DOI] [PubMed] [Google Scholar]
- 15.Tanoue T, Adachi M, Moriguchi T, Nishida E, A conserved docking motif in MAP kinases common to substrates, activators and regulators. Nat. Cell Biol 2, 110–116 (2000). [DOI] [PubMed] [Google Scholar]
- 16.Bardwell AJ, Bardwell L, Two hydrophobic residues can determine the specificity of mitogen-activated protein kinase docking interactions. J. Biol. Chem 290, 26661–26674 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barsyte-Lovejoy D, Galanis A, Sharrocks AD, Specificity determinants in MAPK signaling to transcription factors. J. Biol. Chem 277, 9896–9903 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Heo YS, Kim SK, Seo CI, Kim YK, Sung BJ, Lee HS, Lee JI, Park SY, Kim JH, Hwang KY, Hyun YL, Jeon YH, Ro S, Cho JM, Lee TG, Yang CH, Structural basis for the selective inhibition of JNK1 by the scaffolding protein JIP1 and SP600125. EMBO J. 23, 2185–2195 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haar ET, Prabakhar P, Liu X, Lepre C, Crystal structure of the p38 alpha-MAPKAP kinase 2 heterodimer. J. Biol. Chem 282, 9733–9739 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Xin F, Wu J, Crystal structure of the p38α MAP kinase in complex with a docking peptide from TAB1. Sci. China Life Sci 56, 653–660 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Whisenant TC, Ho DT, Benz RW, Rogers JS, Kaake RM, Gordon EA, Huang L, Baldi P, Bardwell L, Computational prediction and experimental verification of new MAP kinase docking sites and substrates including Gli transcription factors. PLoS Comput. Biol 6, e1000908 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saito H, Posas F, Response to hyperosmotic stress. Genetics 192, 289–318 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wurgler-Murphy SM, Maeda T, Witten EA, Saito H, Regulation of the Saccharomyces cerevisiae HOG1 mitogen-activated protein kinase by the PTP2 and PTP3 protein tyrosine phosphatases. Mol. Cell. Biol 17, 1289–1297 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grewal S, Molina DM, Bardwell L, Mitogen-activated protein kinase (MAPK)-docking sites in MAPK kinases function as tethers that are crucial for MAPK regulation in vivo. Cell. Signal 18, 123–134 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tzarum N, Komornik N, Ben Chetrit D, Engelberg D, Livnah O, DEF pocket in p38alpha facilitates substrate selectivity and mediates autophosphorylation. J. Biol. Chem 288, 19537–19547 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho DT, Bardwell AJ, Grewal S, Iverson C, Bardwell L, Interacting JNK-docking sites in MKK7 promote binding and activation of JNK MAP kinases. J. Biol. Chem 281, 13169–13179 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bardwell AJ, Frankson E, Bardwell L, Selectivity of docking sites in MAPK kinases. J. Biol. Chem 284, 13165–13173 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ho DT, Bardwell AJ, Abdollahi M, Bardwell L, A docking site in MKK4 mediates high affinity binding to JNK MAPKs and competes with similar docking sites in JNK substrates. J. Biol. Chem 278, 32662–32672 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pellegrini E, Palencia A, Braun L, Kapp U, Bougdour A, Belrhali H, Bowler MW, Hakimi MA, Structural basis for the subversion of MAP kinase signaling by an intrinsically disordered parasite secreted agonist. Structure 25, 16–26 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peterson LB, Yaffe MB, Imperiali B, Selective mitogen activated protein kinase activity sensors through the application of directionally programmable D domain motifs. Biochemistry 53, 5771–5778 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tokunaga Y, Takeuchi K, Takahashi H, Shimada I, Allosteric enhancement of MAP kinase p38α's activity and substrate selectivity by docking interactions. Nat. Struct. Mol. Biol 21, 704–711 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Ehrenberger T, Cantley LC, Yaffe MB, Computational prediction of protein-protein interactions. Methods Mol. Biol 1278, 57–75 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krystkowiak I, Manguy J, Davey NE, PSSMSearch: a server for modeling, visualization, proteome-wide discovery and annotation of protein motif specificity determinants. Nucleic Acids Res. 46, W235–W241 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP, Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U. S. A 102, 15545–15550 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Canovas B, Nebreda AR, Diversity and versatility of p38 kinase signalling in health and disease. Nat. Rev. Mol. Cell Biol 22, 346–366 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wentzel C, Sommer JE, Nair R, Stiefvater A, Sibarita JB, Scheiffele P, mSYD1A, a mammalian synapse-defective-1 protein, regulates synaptogenic signaling and vesicle docking. Neuron 78, 1012–1023 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lo HF, Tsai CY, Chen CP, Wang LJ, Lee YS, Chen CY, Liang CT, Cheong ML, Chen H, Association of dysfunctional synapse defective 1 (SYDE1) with restricted fetal growth - SYDE1 regulates placental cell migration and invasion. J Pathol. 241, 324–336 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Hertz EPT, Kruse T, Davey NE, Lopez-Mendez B, Sigurethsson JO, Montoya G, Olsen JV, Nilsson J, A conserved motif provides binding specificity to the PP2A-B56 phosphatase. Mol. Cell 63, 686–695 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Ueki Y, Kruse T, Weisser MB, Sundell GN, Larsen MSY, Mendez BL, Jenkins NP, Garvanska DH, Cressey L, Zhang G, Davey N, Montoya G, Ivarsson Y, Kettenbach AN, Nilsson J, A consensus binding motif for the PP4 protein phosphatase. Mol. Cell 76, 953–964 e956 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wigington CP, Roy J, Damle NP, Yadav VK, Blikstad C, Resch E, Wong CJ, Mackay DR, Wang JT, Krystkowiak I, Bradburn DA, Tsekitsidou E, Hong SH, Kaderali MA, Xu SL, Stearns T, Gingras AC, Ullman KS, Ivarsson Y, Davey NE, Cyert MS, Systematic discovery of short linear motifs decodes calcineurin phosphatase signaling. Mol. Cell 79, 342–358 e312 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kataria M, Mouilleron S, Seo MH, Corbi-Verge C, Kim PM, Uhlmann F, A PxL motif promotes timely cell cycle substrate dephosphorylation by the Cdc14 phosphatase. Nat. Struct. Mol. Biol 25, 1093–1102 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fantz DA, Jacobs D, Glossip D, Kornfeld K, Docking sites on substrate proteins direct extracellular signal-regulated kinase to phosphorylate specific residues. J. Biol. Chem 276, 27256–27265 (2001). [DOI] [PubMed] [Google Scholar]
- 43.Faustova I, Bulatovic L, Matiyevskaya F, Valk E, Örd M, Loog M, A new linear cyclin docking motif that mediates exclusively S-phase CDK-specific signaling. EMBO J. 40, e105839 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bandyopadhyay S, Bhaduri S, Ord M, Davey NE, Loog M, Pryciak PM, Comprehensive analysis of G1 cyclin docking motif sequences that control CDK regulatory potency in vivo. Curr. Biol 30, 4454–4466 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gógl G, Schneider KD, Yeh BJ, Alam N, Nguyen Ba AN, Moses AM, Hetényi C, Reményi A, Weiss EL, The structure of an NDR/LATS kinase-Mob complex reveals a novel kinase-coactivator system and substrate docking mechanism. PLoS Biol. 13, e1002146 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davey NE, Seo MH, Yadav VK, Jeon J, Nim S, Krystkowiak I, Blikstad C, Dong D, Markova N, Kim PM, Ivarsson Y, Discovery of short linear motif-mediated interactions through phage display of intrinsically disordered regions of the human proteome. FEBS J. 284, 485–498 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Ivarsson Y, Arnold R, McLaughlin M, Nim S, Joshi R, Ray D, Liu B, Teyra J, Pawson T, Moffat J, Li SS, Sidhu SS, Kim PM, Large-scale interaction profiling of PDZ domains through proteomic peptide-phage display using human and viral phage peptidomes. Proc. Natl. Acad. Sci. U. S. A 111, 2542–2547 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen HQ, Roy J, Harink B, Damle NP, Latorraca NR, Baxter BC, Brower K, Longwell SA, Kortemme T, Thorn KS, Cyert MS, Fordyce PM, Quantitative mapping of protein-peptide affinity landscapes using spectrally encoded beads. eLife 8, e40499 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kirsch K, Zeke A, Tőke O, Sok P, Sethi A, Sebő A, Kumar GS, Egri P, Póti Á L, Gooley P, Peti W, Bento I, Alexa A, Reményi A, Co-regulation of the transcription controlling ATF2 phosphoswitch by JNK and p38. Nat. Commun 11, 5769 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Francis DM, Różycki B, Koveal D, Hummer G, Page R, Peti W, Structural basis of p38α regulation by hematopoietic tyrosine phosphatase. Nat. Chem. Biol 7, 916–924 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piserchio A, Francis DM, Koveal D, Dalby KN, Page R, Peti W, Ghose R, Docking interactions of hematopoietic tyrosine phosphatase with MAP kinases ERK2 and p38α. Biochemistry 51, 8047–8049 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mok J, Kim PM, Lam HY, Piccirillo S, Zhou X, Jeschke GR, Sheridan DL, Parker SA, Desai V, Jwa M, Cameroni E, Niu H, Good M, Remenyi A, Ma JL, Sheu YJ, Sassi HE, Sopko R, Chan CS, De Virgilio C, Hollingsworth NM, Lim WA, Stern DF, Stillman B, Andrews BJ, Gerstein MB, Snyder M, Turk BE, Deciphering protein kinase specificity through large-scale analysis of yeast phosphorylation site motifs. Sci. Signal 3, ra12 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeke A, Misheva M, Reményi A, Bogoyevitch MA, JNK signaling: Regulation and functions based on complex protein-protein partnerships. Microbiol. Mol. Biol. Rev 80, 793–835 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kyriakis JM, Avruch J, Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol. Rev 92, 689–737 (2012). [DOI] [PubMed] [Google Scholar]
- 55.Minden A, Lin A, Claret FX, Abo A, Karin M, Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell 81, 1147–1157 (1995). [DOI] [PubMed] [Google Scholar]
- 56.Coso OA, Chiariello M, Yu JC, Teramoto H, Crespo P, Xu N, Miki T, Gutkind JS, The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell 81, 1137–1146 (1995). [DOI] [PubMed] [Google Scholar]
- 57.Goldberg AB, Cho E, Miller CJ, Lou HJ, Turk BE, Identification of a Substrate-selective Exosite within the Metalloproteinase Anthrax Lethal Factor. J. Biol. Chem 292, 814–825 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sheridan DL, Kong Y, Parker SA, Dalby KN, Turk BE, Substrate discrimination among mitogen-activated protein kinases through distinct docking sequence motifs. J. Biol. Chem 283, 19511–19520 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thévenin AF, Zony CL, Bahnson BJ, Colman RF, Activation by phosphorylation and purification of human c-Jun N-terminal kinase (JNK) isoforms in milligram amounts. Protein Expr. Purif 75, 138–146 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hietakangas V, Anckar J, Blomster HA, Fujimoto M, Palvimo JJ, Nakai A, Sistonen L, PDSM, a motif for phosphorylation-dependent SUMO modification. Proc. Natl. Acad. Sci. U. S. A 103, 45–50 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gietz RD, Schiestl RH, High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat. Protoc 2, 31–34 (2007). [DOI] [PubMed] [Google Scholar]
- 62.Miller CJ, Lou HJ, Simpson C, van de Kooij B, Ha BH, Fisher OS, Pirman NL, Boggon TJ, Rinehart J, Yaffe MB, Linding R, Turk BE, Comprehensive profiling of the STE20 kinase family defines features essential for selective substrate targeting and signaling output. PLoS Biol. 17, e2006540 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kinoshita-Kikuta E, Aoki Y, Kinoshita E, Koike T, Label-free kinase profiling using phosphate affinity polyacrylamide gel electrophoresis. Mol. Cell. Proteomics 6, 356–366 (2007). [DOI] [PubMed] [Google Scholar]
- 64.Kirsch K, Zeke A, Toke O, Sok P, Sethi A, Sebo A, Kumar GS, Egri P, Poti AL, Gooley P, Peti W, Bento I, Alexa A, Remenyi A, Co-regulation of the transcription controlling ATF2 phosphoswitch by JNK and p38. Nat. Commun 11, 5769 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O'Shea JP, Chou MF, Quader SA, Ryan JK, Church GM, Schwartz D, pLogo: a probabilistic approach to visualizing sequence motifs. Nat. Methods 10, 1211–1212 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.