Abstract
Future personalized approaches to weight management are likely to include consideration of genetic influences on eating behaviors. This study explores whether genetic beliefs about eating behaviors influence dietary self-efficacy and confidence. In a survey of 261 individuals of various weight statuses, we find that endorsing genetic causes of two specific eating behaviors (taste preference and disinhibition) predicts poorer dietary self-efficacy for people who exhibit these eating behaviors. This suggests there may be utility to considering eating behaviors individually when it comes to predicting the influence of genetic information provision in the service of precision medicine interventions. Individuals with high disinhibited eating and/or bitter taster status may be particularly sensitive to interpreting genetic predisposition information in ways that undercut self-efficacy and confidence.
Keywords: Eating Behavior, Diet, Self-efficacy, Confidence, Obesity, Weight, Disinhibition
Healthy eating and weight management are central in the prevention of chronic diseases such as cardiovascular disease, diabetes, osteoarthritis, and some cancers (Pi-Sunyer, 2015). However, controlling diet and managing weight is difficult for individuals to carry out, with few able to manage their weight and maintain weight loss in the long term. Part of this difficulty relates to the fact that the effectiveness of weight management approaches varies greatly between individuals (Salas, 2015). Individual differences in preferences, habits, and predispositions likely underlie the ability or inability of individuals to adhere to various diets, which is a key factor for weight loss success (Thom & Lean, 2017). As such, there is a need to find ways of matching individuals with dietary regimens that they will find manageable and where they will feel success is possible.
Developing treatment plans for weight management is made difficult by the complex biopsychosocial determinants of obesity. Precision medicine may offer a potential solution by considering individual level factors when deciding treatment type and intensity as opposed to traditional approaches that apply treatment modalities broadly without accounting for individual, patient-level differences (Bomberg et. al, 2019). Precision nutrition in particular aims to prevent and manage chronic diseases by tailoring dietary interventions to an individual’s genetic background, metabolic profile, and or environmental exposures (Wang, & Hu, 2018).
To advance this cause, some researchers have examined eating behavior traits and tendencies to arrive at common phenotypes that can be targeted with dietary modification approaches. Bouhlal et. al. (2017) examined characteristics including higher food reward sensitivity, disinhibition, satiety responsiveness, and bitter taste sensitivity. They found that eating drive characteristics were most highly related to dietary self-efficacy. The current examination explores the potential role of individuals’ beliefs in modifying these relationships.
Specifically, to better understand the potential of precision medicine to promote dietary self-efficacy, it’s important to consider the role of genetic beliefs. The relationship between eating behaviors and dietary self-efficacy is undoubtedly complex. A potentially relevant factor is the extent to which individuals believe that their eating behaviors have genetic underpinnings. There is evidence of reduced confidence in one’s ability to manage weight related to the general belief that eating behaviors are influenced by genetics (Persky, Bouhlal, Goldring & McBride 2017; Persky & Yaremych, 2020). It is likely that responses to the notion that eating behaviors have genetic underpinnings are variable depending upon the specific eating behavior in question, although this has not been assessed. Such responses may also depend upon beliefs about one’s own behaviors. Previous research indicates that the relationship between genetic beliefs and diet depends on an individual’s weight status (Knerr, Bowen, Beresford, & Wang, 2017). It is similarly likely that responses to the notion that genetic factors influence specific eating behaviors will depend upon self-relevance, in this case, whether an individual perceives that they personally exhibit that eating behavior. In addition, according to several theoretical models (e.g., Theory of Planned Behavior), low perceived controllability is related to reduced engagement in goal-directed behavior (McVay et al., 2015). This might explain why individuals would experience reduced self-efficacy or a reduced sense of control when a ‘condition’ an individual has is believed to be genetic in nature (and thus intrinsic and unmodifiable).
The current study assesses whether participant beliefs about the genetic underpinnings of four specific eating behaviors (disinhibition, food reward sensitivity, satiety responsiveness, and taste preference), as well as participant reports about their own eating behavior tendencies on these four dimensions, predict dietary self-efficacy and confidence. We chose these outcome variables due to the link between genetic causal attributions and reduced self-efficacy and behavior change confidence (Hoyt et al., 2014). We further examine whether the influence of participants’ genetic beliefs depends upon whether participants perceive that they personally exhibit the relevant behavioral tendency. Finally, we explore what, if any, demographic variables are associated with genetic beliefs about specific eating behaviors.
Method
Participants
Data included in this analysis were collected as part of a larger survey on eating behavior phenotypes (Bouhlal et al., 2017). A random sample of participants from a National Institutes of Health database of individuals interested in research participation were contacted by email with an introduction to the study and an opportunity to opt out of further contact. Those who had a mailing address on file and who did not opt out were mailed a packet of study materials a week later. Participants were incentivized for participation by check or gift card. Participants completed questionnaires online and administered the bitter taster assessment using materials in the packet (see below).
Two hundred and sixty-one participants returned the survey (150 women, 110 men). The response rate for the survey was 23%. Participants represented a variety of ages from 18–69 (Mean = 34.29, SD = 11.40), and racial backgrounds (38% Black, 43% White, 19% other). 45% of participants had a BMI <25–30 (not overweight or obese), 30% had a BMI >25 – <30 (overweight), and 25% had a BMI < 30 (obese). Participants were generally highly educated with 83% attending at least some college.
Measures
Eating Behavior Traits
Food reward sensitivity was assessed with The Power of Food Scale (Lowe et al., 2009) which measures the extent to which participants are attracted to and have difficulty resisting palatable foods. This scale contains 15 items and measures the appetite for palatable foods on a 5-point Likert-type scale from “I don’t agree” to “I strongly agree”. Disinhibition was measured using The Three Factor Eating Questionnaire (Stunkard, & Messick, 1985) which measures a tendency to eat in response to social or emotional cues and includes 16 items to which participants rate as “true” or “false”. Satiety responsiveness was assessed using an adapted version of the Child Eating Behavior Questionnaire for adults (Wardle, Guthrie, Sanderson, & Rapoport, 2001) to measure the extent to which participants experience feelings of fullness or satiety. Participants completed this on a 5-point scale from “never” to “always”. Finally, Bitter taster status was assessed using a taste test strip containing PROP/6-n-propylthiouracil (Zhao, Kirkmeyer, & Tepper, 2003), included in the mailed packet of materials which was self-administered. Participants reported whether they perceived no taste, bitter, or extremely bitter, coded on a 1–3 scale.
Genetic Beliefs about Eating Behaviors
Single items were used to assess genetic beliefs about specific eating behaviors. These assessed on a 4-point scale the extent to which each eating behavior is “influenced by a person’s genes” from “not at all” to “a lot”. Genetic beliefs were measured regarding disinhibition, taste preference1, satiety responsiveness, and food reward sensitivity. (See supplemental Table 1).
Dietary Self-Efficacy and Confidence
Dietary Self-efficacy was assessed using the Self-Efficacy and Eating Habits Survey (Sallis, Pinski, Grossman, Patterson & Nader, 1988), specifically the subscales related to ability to stick to a diet and to reduce calories. These were assessed on a 5-point scale from “I know I cannot” to “I know I can”. Dietary Confidence was assessed with the average of two items related to confidence in controlling weight and controlling diet on a 5-point scale from “not at all confident” to “extremely confident”.
Demographics
Demographics included participants’ self-reported height and weight (used to calculate BMI), gender (male/female), race (collapsed into categories Black, White, “other”), and education (collapsed into college graduate vs. less than a college degree).
Data Analysis
Multiple regression analyses were conducted to determine which genetic beliefs predicted participants’ dietary self-efficacy and explore any interaction with participants’ ratings of their own eating behavior. Separate regression analyses were conducted for each measure of confidence and self-efficacy. In each model, all perceived eating behaviors were entered simultaneously (disinhibition, food reward sensitivity, satiety responsiveness, and bitter taste preferences) and demographic variables (gender, BMI, age, and race) were also controlled for.
Results
Direct Effects of Genetic Beliefs and Perceived Eating Behaviors
Genetic beliefs about two specific eating behaviors predicted participants’ dietary self-efficacy, namely, disinhibition and taste preference (see Table 1). Higher endorsement of genetic causes of these eating behaviors predicted lower dietary self-efficacy and confidence. Participant’s own perceived eating behavior was directly predictive of dietary self-efficacy for disinhibition and food reward sensitivity (see Table 1).
Table 1.
Predictors of dietary self-efficacy and confidence.
| Control Weight | Stick to Diet | Reduce Calories | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| β | p | ηp2 | β | p | ηp2 | β | p | ηp2 | |
|
| |||||||||
| Genetic Beliefs about Eating Behaviors | |||||||||
| Genetic Beliefs about Disinhibition | −0.10 | .343 | .004 | 0.20 | .118 | .011 | 0.27 | .029 | .021 |
| Genetic Beliefs about Food Reward Sensitivity | −0.21 | .150 | .010 | −0.6 | .743 | .001 | −0.04 | .804 | <.001 |
| Genetic Beliefs about Satiety Responsiveness | −0.19 | .376 | .004 | 0.18 | .497 | .002 | 0.43 | .101 | .012 |
| Genetic Beliefs about Taste Preferences | 0.61 | .018 | .024 | 0.10 | .738 | .001 | −0.22 | .481 | .002 |
| Perceived Eating Behaviors | |||||||||
| Disinhibition | −0.36 | <.001 | .100 | −0.26 | .003 | .039 | −0.37 | <.001 | .074 |
| Food Reward Sensitivity | −0.14 | .035 | .019 | −0.16 | .048 | .017 | 0.00 | .965 | <.001 |
| Satiety Response | −0.06 | .272 | .005 | 0.02 | .772 | <.001 | 0.05 | .457 | .002 |
| Bitter Taster Status | −0.06 | .238 | .006 | −0.08 | .212 | .007 | 0.02 | .743 | .001 |
| Genetic Beliefs * Eating Behaviors | |||||||||
| Disinhibition * Genetic Beliefs about Disinhibition | −0.04 | .847 | <.001 | −0.43 | .093 | .013 | −0.52 | .042 | .018 |
| Food Reward Sensitivity * Genetic Beliefs about Food Reward Sensitivity | 0.37 | .124 | .011 | 0.12 | .681 | .001 | 0.17 | .551 | .002 |
| Satiety Responsiveness * Genetic Beliefs about Satiety Responsiveness | 0.12 | .639 | .001 | −0.13 | .664 | .001 | −0.10 | .206 | .007 |
| Taster Status * Genetic Beliefs about Taste Preferences | −0.82 | .008 | .030 | −0.09 | .804 | <.001 | 0.36 | .336 | .004 |
Note: Covariate demographic variables (gender, BMI, age, and race) were included in each regression.
Interactions between Genetic Beliefs and Perceived Eating Behaviors
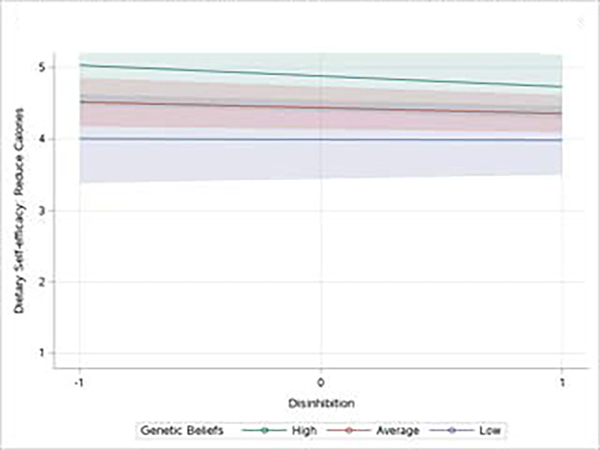
There was a significant interaction between genetic beliefs about disinhibited eating and participant’s perceived disinhibited eating (β = −0.52, p = 0.042, see figure 1). Simple slopes analysis revealed that when participants endorsed genetic explanations for disinhibition at an average or above average level their dietary self-efficacy was predicted by their own level of disinhibited eating (ps < 0.001). Participants with a high level of disinhibited eating had poorer dietary self-efficacy when combined with the endorsement of genetic causes. Participants with below average endorsement of genetic causes of disinhibited eating did not differ in their self-efficacy regardless of whether they report this eating behavior or not (p = 0.773).
Figure 1.
The Effect of Disinhibition on Self-efficacy to Control Calorie Intake Depends Upon Genetic Beliefs
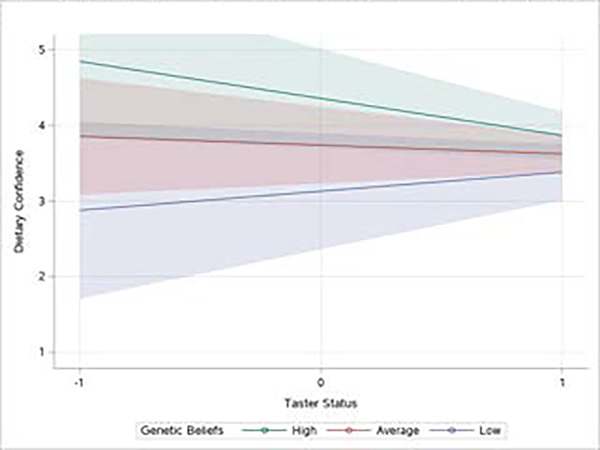
Similarly, there was a significant interaction between genetic beliefs about taste preferences and participant’s bitter taste preference (β = −0.82, p = 0.008, see figure 2). Simple slopes analysis revealed that when participants endorsed genetic explanations for taste preferences at an above average level their confidence was predicted by their own perceived bitter taste preferences (p = 0.008). Participants who reported high sensitivity to bitter taste had poorer dietary confidence when combined with endorsement of genetic causes of taste preferences. Participants with average or below average endorsement of genetic causes of taste preferences did not differ in their dietary confidence regardless of whether they reported bitter taster status or not (ps > 0.05). All other interaction analyses were nonsignificant (all ps > 0.05; see Table 1).
Figure 2.
The Effect of Taster Status on Dietary Confidence Depends Upon Genetic Beliefs
Predicting Genetic Beliefs About Eating Behavior
We found no evidence that demographic variables consistently predicted participants’ genetic beliefs about eating behaviors (see Table 2). Although there was some evidence that endorsement of genetic beliefs may increase with age, this was constrained only to genetic beliefs about food reward sensitivity (β = −0.15, p = 0.041); all other demographic predictors were nonsignificant (all ps > 0.05). There was also no evidence that participants’ eating behaviors influenced their beliefs about the genetic basis of those eating behaviors. Participants that reported high disinhibition, food reward sensitivity, satiety response, or had a positive bitter taster status were no more likely to endorse genetic causes of these behaviors (all ps > 0.05).
Table 2.
Predictors of genetic beliefs about eating behaviors
| Genetic beliefs about Disinhibition | Genetic beliefs about Food Reward Sensitivity | Genetic beliefs about Satiety Response | Genetic beliefs about Taste Preferences | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| β | p | ηp2 | β | p | ηp2 | β | p | ηp2 | β | p | ηp2 | |
|
| ||||||||||||
| Demographics | ||||||||||||
| Age | −0.05 | .457 | .002 | −0.15 | .041 | .018 | <0.01 | .995 | <.001 | −0.10 | .198 | .007 |
| Gender | 0.07 | .315 | .004 | 0.09 | .194 | .007 | 0.02 | .804 | <.001 | 0.01 | .834 | <.001 |
| Race | −0.11 | .086 | .012 | 0.01 | .797 | <.001 | 0.03 | .685 | .001 | 0.05 | .452 | .002 |
| BMI | 0.04 | .576 | .001 | −0.01 | .922 | <.001 | 0.05 | .510 | .002 | −0.07 | .325 | .004 |
| Parenthood | −0.06 | .455 | .002 | 0.02 | .796 | <.001 | −0.09 | .238 | .006 | −0.04 | .572 | .001 |
| Family History Overweight | −0.06 | .370 | .003 | <0.01 | .975 | <.001 | −0.07 | .290 | .005 | 0.06 | .414 | .003 |
| Eating Behaviors | ||||||||||||
| Disinhibition | 0.09 | .191 | .007 | |||||||||
| Food Reward Sensitivity | 0.12 | .086 | .013 | |||||||||
| Satiety Response | −0.12 | .106 | .011 | |||||||||
| Bitter Taster Status | −0.08 | .226 | .006 | |||||||||
Discussion
The current analysis revealed that for two specific eating behaviors (disinhibition and taste preference) genetic attributions influenced dietary confidence and self-efficacy. Participant’s perceptions are in line with the scientific literature wherein disinhibition has shown strong links with dietary weight management processes (Bryant, King, & Blundell, 2008). Taste preference and dietary weight relationships, however, are more mixed (Cox, Hendrie, & Carty, 2016). For both eating behaviors, the influence of participants’ genetic beliefs on self-efficacy and confidence to control diet were dependent upon whether or not they believed they exhibited this eating behavior. Indeed, beliefs about the general influence of genetics on a given trait or behavior should be much more likely to influence one’s self-relevant health beliefs to the extent that individual believes they exhibit the trait in question.
It is unclear at present why genetic causal beliefs moderate the influence of disinhibition and taste preference but not satiety responsiveness and food reward sensitivity. All these eating behaviors have been linked with dietary behavior and in particular with weight loss (Boutelle, Manzano, & Eichen, 2020). The unique status of disinhibition and taste preference may be due to the common genetic influence they share. For example, TAS2R38, a bitter taste receptor, has been linked to disinhibited eating in a cohort of Amish women (Dotson et al., 2020). Further research is needed to ascertain why genetic beliefs influence dietary self-efficacy specifically for these eating behaviors.
This suggests there may be utility to considering eating behaviors individually when it comes to predicting the influence of genetic information provision in the service of precision medicine interventions. Individuals with high disinhibited eating or positive taster status may be particularly sensitive to interpreting genetic predisposition information in ways that undercut self-efficacy and confidence.
We found that few demographic variables influenced individuals’ beliefs about the genetic underpinnings of specific eating behaviors. Most surprisingly, perhaps, is that genetic beliefs were unrelated to individuals’ perceptions of whether or not they, themselves, exhibited the eating behavior in question. This deviates from previous research showing that whether an individual is affected by a condition influences the extent to which they attribute that condition to genetic factors (Rose et. al, 2019; Haider-Markel, 2018). This may be because eating behaviors are more of a continuum than a binary disease diagnosis, and unlike diseases, individuals are not typically made aware of their status or ‘level’ of eating behaviors.
There are several important limitations to our research, including a reliance on self-reported eating behaviors and the use of dietary self-efficacy and confidence as outcomes, as opposed to behavioral measures. However, mounting research supports that dietary self-efficacy is a key component of a healthy diet and weight loss maintenance (Byrne, Barry, & Petry, 2012). In addition, we asked participants for their beliefs about the role of genetics in specific eating behaviors which they may be considering for the very first time. These beliefs and their relationships with other constructs may change over time once they have been made salient. Although we asked participants about their beliefs about the causes of taste preferences in general, we only tested participant’s bitter taste preference. Beliefs about the genetic causes of other taste preferences may therefore have impacted this relationship. Finally, the effects we found were relatively small. Although we had reasonable power to detect interactions, our sample size prevents us from doing more stratified analyses.
In all, our results suggest that people who endorse genetic causes of disinhibited eating and taste preferences may struggle to feel control and self-efficacy over their diet and their weight when they, themselves, exhibit these eating behaviors. From this perspective, discussing eating behaviors may influence self-efficacy and confidence differently depending upon the extent to which the genesis of these behaviors feels like it is under personal control. Education on how environmental changes can modulate the outcome of genetic predispositions may be beneficial for those who endorse genetic causes of disinhibition and taste preferences as a way of increasing dietary self-efficacy. This may be a fruitful avenue for mitigating any negative influences of learning about how genes underpin eating behaviors in the context of future personalized medicine or personalized nutrition intervention approaches.
Footnotes
Participants were asked about genetic causes of taste preferences in general, not bitter taste preferences specifically.
Declarations
This project was funded by the Intramural Research Program of the National Human Genome Research Institute. The authors have no conflicts or competing interests to report. This project was approved by the relevant IRB and all participants indicated their consent for participation. Data from the current report are available upon request. All authors contributed substantially to this report, MOG planned the study, conducted data analysis, and drafted the manuscript for publication; AJM conducted data analysis and drafted the manuscript for publication; SP planned the study, was involved in data collection, conducted data analysis, and drafted the manuscript for publication.
References
- Bomberg EM, Ryder JR, Brundage RC, Straka RJ, Fox CK, Gross AC, Oberle MM, Bramante CT, Sibley S, & Kelly AS (2019). Precision medicine in adult and pediatric obesity: a clinical perspective. Therapeutic Advances in Endocrinology and Metabolism, 10, 1–25. https://doi.org/10.1177%2F2042018819863022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhlal S, McBride CM, Trivedi NS, Agurs-Collins T, & Persky S (2017). Identifying eating behavior phenotypes and their correlates: A novel direction toward improving weight management interventions. Appetite, 111, 142–150. 10.1016/j.appet.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutelle KN, Manzano MA, & Eichen DM (2020). Appetitive traits as targets for weight loss: the role of food cue responsiveness and satiety responsiveness. Physiology & Behavior, 224. https://doi-org.proxy-um.researchport.umd.edu/10.1016/j.physbeh.2020.113018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant EJ, King NA, & Blundell JE (2008). Disinhibition: its effects on appetite and weight regulation. Obesity Reviews, 9(5), 409–419. https://doi-org.proxy-um.researchport.umd.edu/10.1111/j.1467-789X.2007.00426.x [DOI] [PubMed] [Google Scholar]
- Byrne S, Barry D, & Petry NM (2012). Predictors of weight loss success. Exercise vs. dietary self-efficacy and treatment attendance. Appetite, 58(2), 695–698. 10.1016/j.appet.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary N, Kumar V, Sangwan P, Pant NC, Saxena A, Joshi S, & Yadav AN (2021). Personalized Nutrition and -Omics. Comprehensive Foodomics, 495–507. 10.1016/B978-0-08-100596-5.22880-1 [DOI] [Google Scholar]
- Cox DN, Hendrie GA, & Carty D (2016). Sensitivity, hedonics and preferences for basic tastes and fat amongst adults and children of differing weight status: a comprehensive review. Food Quality and Preference, 48, 359–367. https://doi-org.proxy-um.researchport.umd.edu/10.1016/j.foodqual.2015.01.006 [Google Scholar]
- Dotson CD, Shaw HL, Mitchell BD, Munger SD, & Steinle NI (2010). Variation in the gene TAS2R38 is associated with the eating behavior disinhibition in Old Order Amish women. Appetite, 54(1), 93–99. 10.1016/j.appet.2009.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider-Markel DP, & Joslyn MR (2018). “Nanny State” Politics: Causal Attributions About Obesity and Support for Regulation. American Politics Research, 46(2), 199–216. 10.1177/1532673X17691493 [DOI] [Google Scholar]
- Hoyt CL, Burnette JL, & Auster-Gussman L (2014). “Obesity is a disease”: examining the self-regulatory impact of this public-health message. Psychological Science, 25(4), 997–1002 [DOI] [PubMed] [Google Scholar]
- Knerr S, Bowen D, Beresford S, & Wang C (2016). Genetic causal beliefs about obesity, self-efficacy for weight control, and obesity-related behaviors in a middle-aged female cohort. Psychology and Health, 31(4), 420–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe MR, Butryn ML, Didie ER, Annunziato RA, Thomas JG, Crerand CE, Ochner CN, Coletta MC, Bellace D, Wallaert M & Halford J (2009). The Power of Food Scale. A new measure of the psychological influence of the food environment. Appetite, 53(1), 114–118. 10.1016/j.appet.2009.05.016 [DOI] [PubMed] [Google Scholar]
- Madden CE, Leong SL, Gray A, & Horwath CC (2012). Eating in response to hunger and satiety signals is related to BMI in a nationwide sample of 1601 mid-age New Zealand women. Public Health Nutrition, 15(12), 2272–2279. 10.1017/S1368980012000882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeier HM, Phelan S, Fava JL, & Wing RR (2007) Internal disinhibition predicts weight regain following weight loss and weight loss maintenance. Obesity. 15(10), 2485-24-94. 10.1038/oby.2007.295 [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer X (2009). The medical risks of obesity. Postgraduate medicine, 121(6), 21–33. 10.3810/pgm.2009.11.2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persky S, Bouhlal S, Goldring MR, & McBride CM (2017). Beliefs about genetic influences on eating behaviors: Characteristics and associations with weight management confidence. Eating Behaviors, 26, 93–98. https://dx.doi.org/10.1016%2Fj.eatbeh.2017.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persky S & Yaremych HE (2020) Parents’ genetic attributions for children’s eating behaviors: Relationships with beliefs, emotions, and food choice behavior. Appetite, 155, 104824. 10.1016/j.appet.2020.104824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliner P, & Hobden K (1992). Development of a scale to measure the trait of food neophobia in humans. Appetite, 19(2), 105–120. 10.1016/0195-6663(92)90014-W [DOI] [PubMed] [Google Scholar]
- Rose MK, Costabile KA, Boland SE, Cohen R, Persky S. (2019). Diabetes causal attributions among affected and unaffected individuals. BMJ Open Diabetes Research and Care doi: 10.1136/bmjdrc-2019-000708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas XR (2015). The ineffectiveness and unintended consequences of the public health war on obesity. Canadian Journal of Public Health, 106(2), 79–81. 10.17269/cjph.106.4757 [DOI] [PubMed] [Google Scholar]
- Sallis JF, Pinski RB, Grossman RM, Patterson TL, & Nader PR (1988). The development of self-efficacy scales for health related diet and exercise behaviors. Health Education Research, 3(3), 283–292. 10.1093/her/3.3.283 [DOI] [Google Scholar]
- Stunkard AJ, & Messick S (1985). The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. Journal of Psychosomatic Research, 29(1), 71–83. 10.1016/0022-3999(85)90010-8 [DOI] [PubMed] [Google Scholar]
- Thom G, & Lean M (2017). Is There an Optimal Diet for Weight Management and Metabolic Health? Gastroenterology, 152(7), 1739–1751. 10.1053/j.gastro.2017.01.056 [DOI] [PubMed] [Google Scholar]
- Wang DD, & Hu FB (2018). Precision nutrition for prevention and management of type 2 diabetes. Lancet Diabetes Endocrinol, 6 (5), 416–426. 10.1016/S2213-8587(18)30037-8 [DOI] [PubMed] [Google Scholar]
- Wardle J, Guthrie CA, Sanderson S, & Rapoport L (2001). Development of the children’s eating behaviour questionnaire. Journal of Child Psychology and Psychiatry, 42(7), 963–970. 10.1111/1469-7610.00792 [DOI] [PubMed] [Google Scholar]
- Zhao L, Kirkmeyer SV, & Tepper BJ (2003). A paper screening test to assess genetic taste sensitivity to 6-n-propylthiouracil. Physiology & Behavior, 78(4–5), 625–633. 10.1016/S0031-9384(03)00057-X [DOI] [PubMed] [Google Scholar]