Abstract
Objective:
Executive control continues to develop throughout adolescence and is vulnerable to alcohol use. Although longitudinal assessment is ideal for tracking executive function development and onset of alcohol use, prior testing experience must be distinguished from developmental trajectories.
Method:
We used the Stroop Match-to-Sample task to examine the improvement of processing speed and specific cognitive and motor control over 4 years in 445 adolescents. The twice-minus-once-tested method was used and expanded to 4 test sessions to delineate prior experience (i.e., learning) from development. A General Additive Model evaluated the predictive value of age and sex on executive function development and potential influences of alcohol use on development.
Results:
Results revealed strong learning between the first two assessments. Adolescents significantly improved their speeded processing over 4 years. Compared with boys, girls enhanced ability to control cognitive interference and motor reactions. Finally, the influence of alcohol use initiation was tested over 4 years for development in 110 no/low, 110 moderate/heavy age- and sex-matched drinkers; alcohol effects were not detected in the matched groups.
Conclusions:
Estimation of learning effects is crucial for examining developmental changes longitudinally.
Keywords: Executive control, Development, Learning, Alcohol, Adolescence
Adolescence spans a critical period characterized by cognitive and affective changes essential for adaptation to new challenges (Steinberg et al., 2018). Central to understanding adaptive behaviors during adolescent development is executive control (Jadhav & Boutrel, 2019), which comprises distinct processes enabling goal-directed behavior and management of unexpected and complex situations (Clark et al., 2017; Luna, Marek, Larsen, Tervo-Clemmens, & Chahal, 2015). Successful goal-directed behavior involves the suppression of incongruous cognition and motor tendencies (Koziol & Lutz, 2013; Luna, Padmanabhan, & O’Hearn, 2010) requiring two executive control processes: (1) cognitive control, i.e., resisting non-relevant contexts or thoughts when executing a demanding task, and (2) motor control, i.e., stopping prepotent motor responses that may interfere with ongoing plans. The motor control component may also be related to switching abilities for flexible adaptation to changing rules (Rushworth, Hadland, Paus, & Sipila, 2002).
Executive function development is greatest from childhood to adolescence, but its enhancement continues into adulthood. Whereas the brain no longer increases in size, significant refinements occur during adolescence such as reduction of synaptic density, elaboration of dendritic arborization, and increased myelination (Brenhouse & Andersen, 2011; Selemon, 2013). This neural refining underlies learning and cognitive improvement (Luna & Sweeney, 2004). Evidence for executive control development and efficiency is observed in cognitive tasks through lower error rates and faster processing speed over time (Crone, Donohue, Honomichl, Wendelken, & Bunge, 2006; Quinzi et al., 2018). Of relevance, component processes of executive function development differ between the sexes, with girls performing better in reading comprehension and verbal fluency domains (Hurks et al., 2010; Wierenga, Bos, van Rossenberg, & Crone, 2019), and boys performing better in spatial working memory and motor domains (Krikorian & Bartok, 1998; Piper, 2011; Wierenga et al., 2019), with no strong age by sex interaction.
In addition, the maturation of executive functions during adolescence is related to the prefrontal cortex, which is the last brain region to complete development in emerging adults. This ongoing development contributes to ability to avert risky behavior in adolescence (Luna et al., 2015; Steinberg et al., 2018). By contrast, the striatum develops earlier than the prefrontal cortex, which subserves processes of cognitive control and control of reward-related behaviors, including the regulation of striatally-based motivations and urges in addiction (DePasque & Galván, 2017). Accordingly, impaired frontostriatal response in adolescence drinkers has been linked to reward sensitivity and poor executive control (Lees, Meredith, Kirkland, Bryant, & Squeglia, 2020 for a review). During adolescence, the initiation of alcohol use may adversely affect cognitive and motor development of executive functions. Indeed, adolescents are sensitive to deleterious alcohol effects, which can disrupt typical brain growth trajectories (Müller-Oehring et al., 2018; Pfefferbaum et al., 2018; Sullivan et al., 2020) with possible long-term consequences (Rohde, Lewinsohn, Kahler, Seeley, & Brown, 2001). However, much remains unknown regarding how alcohol use may alter the behavioral development of executive functions.
Failure to exert executive control is a central feature of addiction, contributing to alcohol misuse persistence and progression to binge drinking (Kwako, Momenan, Litten, Koob, & Goldman, 2016). In youth, longitudinal studies examining alcohol and other substance use disorders support the hypothesis of deleterious alcohol effects on executive control functions (Hanson, Medina, Padula, Tapert, & Brown, 2011). In several studies in adolescents, however, no relations were found between low alcohol use and either cognitive or motor control (Infante et al., 2020; Jurk, Mennigen, Goschke, & Smolka, 2018; Morin et al., 2019). Nevertheless, binge drinking, characterized by the consumption of more than 4 (women) or 5 (men) alcohol units on the same day (NIAAA, 2018), are associated with a disturbed developmental trajectory of executive control, as demonstrated in cross-sectional behavioral and longitudinal neuroimaging studies (e.g., Gil-Hernandez et al., 2017; Wetherill, Squeglia, Yang, & Tapert, 2013).
Together, the above evidence suggests a susceptibility of executive function development during adolescence and a possible association with alcohol use. However, previous works do not consider the effects of prior testing experience. These questions need to be answered with longitudinal behavioral data and assessment of improvement in processing speed and specific cognitive and motor control, distinguishing change attributable to development from change related to prior testing experience (Salthouse, 2015; Sullivan et al., 2017). Prior work showed that improvement over one year in various neuropsychological tests resulted in large part from prior testing experience (learning effects) with less contribution from developmental effects (Sullivan et al., 2017). Similarly, the relation between behavioral measures of executive functions and alcohol use must account for learning and cognitive development.
The current study posed two principal research questions: (1) What are the developmental trajectories of executive control among adolescents, and how does prior testing experience change these trajectories; and (2) Does alcohol consumption alter the developmental trajectory? We evaluated executive functions in adolescents over four years. Following previous work (e.g., Waber, Forbes, Almli, Blood, & The Brain Development Cooperative Group, 2012), we hypothesized stronger learning effects between baseline and follow-up 1 than follow-ups 2 and 3. We also hypothesized differential developmental trajectories in boys and girls, with better motor performance in boys (Piper, 2011; Wierenga et al., 2019). Finally, we expected that the initiation of alcohol use would adversely affect developmental trajectories compared to adolescents who remained no/low alcohol users.
Materials and Methods
Participants
In this study, we included adolescents who did not exceed age-adjusted drinking criteria at baseline (n = 692; Brown et al., 2015) and who completed three yearly post-baseline follow-ups (n = 210 boys and 235 girls). Participants had no history of heavy drinking (i.e., heavy drinking with moderate frequency [e.g., 2x/month] and high quantity [e.g., 3–4 drinks on average and > 4 drinks maximum] or with high frequency [e.g., 1x/week or more] and moderate quantity [e.g., 2–3 drinks on average and >4 drinks maximum]; Cahalan, Cisin, & Crosley, 1969) at the baseline assessment. Sociodemographic (age, sex, self-declared ethnicity, socioeconomic status) was assessed. The education level of parents, which served as an estimate of socioeconomic status, was between 6 and 20 years (Mean = 16.71, SD = 2.43). Participants were White (78.88%), Black (9.66%), Asian (10.56%), and 0.90% reported other self-declared ethnicities. The racial/ethnic, gender, and other demographic characteristics of the sample are described (Brown et al., 2015) and are equivalent to reported census numbers (Humes, Jones, & Ramirez, 2011). Pubertal development was evaluated using the Pubertal Development Scale (Petersen, Crockett, Richards, & Boxer, 1988), a 5-item scale with a total score coded on four points: puberty not started, beginning of puberty, advanced puberty, and puberty completed. The study included adolescents from the University of Pittsburgh (13.93%), SRI International (13.93%), Duke University Medical Center (15.28%), Oregon Health & Science University (23.37%), and University of California San Diego (33.49%), and was approved by the institutional review boards of the 5 NCANDA sites. Consent (for majors) and assent (for minors) were obtained according to the Declaration of Helsinki.
Alcohol use evaluation
Alcohol use was assessed with the Customary Drinking and Drug Use Record (Brown, Myers, Lippke, Tapert, Stewart, & Vik, 1998), a self-reported measure evaluating past-year and lifetime alcohol use, withdrawal, and hangover symptoms. At baseline, all participants included were no/low drinkers (Brown et al., 2015). Then, at follow-up, participants were categorized as remaining no/low drinkers or becoming moderate/heavy drinkers according to Cahalan’s criteria (1969). As the second aim of this paper was to assess the influence of drinking on executive development, we included drinkers who went on to initiate moderate/heavy drinking at follow-ups. At follow-up 1, 81.49% of adolescents remained no/low drinkers while 18.51% began moderate to heavy drinking (i.e., 74.39% at moderate levels and 25.61% at heavy levels). At follow-up 2, 70.59% of adolescents were identified as no/low drinkers and 29.41% as moderate/heavy drinkers (55.04% at moderate levels and 44.96% at heavy levels). Finally, at follow-up 3 3, 59.91% of adolescents were identified as no/low drinkers and 40.09% as moderate/heavy drinkers (53.37% at moderate levels and 46.63% at heavy levels).
Experimental Task: Executive Control
To assess executive control, adolescents performed the Stroop Match-to-Sample task, a validated paradigm (Schulte et al., 2011; Schulte, Müller-Oehring, Sullivan, & Pfefferbaum, 2012) previously used with adolescents (Schulte et al., 2020). This task combined Stroop and Match-to-Sample paradigms, which allowed distinguishing cognitive and motor control components of executive functioning. The task was computerized and presented with E-Prime® software (Psychology Software Tools Inc., Pittsburgh, PA). Participants matched the color of a sample (i.e. XXXX) to the font color of a color word (i.e., the Stroop stimulus: BLUE, RED, GREEN) by pressing the corresponding response key (YES for match trials, NO for non-match trials) on the keyboard. The color sample was first shown for 450 ms, followed by an inter-stimulus-interval of variable duration (200, 300, or 400 ms), and then by the color-word Stroop stimulus for 1100 ms. The task was composed of 144 trials and lasted approximately 8 minutes.
Four conditions were presented to the participants for both match and non-match trials, resulting in eight conditions. First, the Stroop stimulus was either congruent (e.g., the word RED written in red font) or incongruent (e.g., the word RED written in blue font). The incongruent condition required cognitive control to overcome the semantic interference from the Stroop word’s meaning and was compared to the congruent condition (without interference) to evaluate the Stroop effect. The Stroop effect is characterized by a prolonged response time when subjects have to name a color word printed in an incongruent color (e.g., RED written in blue) compared to naming words printed in a congruent color. Second, the task was designed in different blocks that required response switches (i.e., block with mixed match and non-match trials) or response repetitions (i.e., block with either match trials or non-match trials). The response switching blocks required control of motor response and was compared to the response repetition blocks (leading to more automatic processing) to evaluate motor control. Accuracy (number of errors) and reaction times (RTs) were collected. For more details regarding task description and procedure, see Schulte et al. (2020).
Data Preprocessing and Reduction
Exclusion of outliers was performed at both individual and group levels. First, for each participant, we computed the mean RTs and the standard deviation from the mean. We then summed the mean RTs with three standard deviations to exclude outliers with prolonged RTs according to individual performance [i.e., RTs longer than the mean + (3 * standard deviation)]. The cut-off of 3 standard deviations has been used here as a standard for individual preprocessing (i.e., participants rarely differ for more than 3 SD from themselves; see Schulte et al., 2011; 2012; 2020). Second, RTs were computed on correct answers and subjects who committed more than 33% errors were excluded from the analyses (n=31, 7%). To ensure that participants had adequate numbers of trials to compute meaningful mean RTs, we included participant with at least 67% of correct trials, which resulted in reliable performance estimations. Finally, eight participants (1.80%) were deemed group outliers having RTs larger than four standard deviations from the group’s mean. The cut-off of 4 standard deviations was used here as a standard for participants exclusion when considering the overall group’s performance (Leys et al., 2013). The final sample consisted of the 445 participants described in the Method section.
Analyses were performed on the RTs during the Stroop Match-to-Sample task performance and focused on three main dependent variables: processing speed refers as general efficiency, cognitive control, and motor control. The overall RTs were used to evaluate processing speed with correct responses as an index of general efficiency in processing an executive task. Consistent with previous studies (Schulte et al., 2011, 2012), we examined cognitive control by computing the RTs difference scores between incongruent (INC) and congruent (CON) task conditions (Diff. RTINC-CON) and motor control by computing the RTs difference between response switching (RS) and response repetition (RR) (Diff. RTRS-RR). To assess estimates of cognitive and motor component processes, we computed the cognitive control measure by subtracting congruent-match-repetition trials from incongruent-match-repetition trials, and the motor control measure by subtracting congruent-match-repetition trials from congruent-match-switching trials.
Statistical analysis
We performed analyses using Generalized Additive Models (GAMs; Wood, 2006). GAMs were used as they allow for flexible, data-driven nonlinear effects of independent variables. Nonlinear effects of age were captured via cubic spline basis functions, which encompass linear, quadratic, and cubic functions as subcases but also can capture ceiling effects or asymptotes, and hence are more appropriate to these types of data than polynomial fits (Wood, 2006).
We employed GAMs to differentiate practice from developmental effects for three measures: general efficiency (overall RT), cognitive control (Diff. RTINC-CON), and motor control. (Diff. RTRS-RR). First, separate GAMs were fit for each visit (i.e., four models per executive control outcome). In each of these four models, age was included as (smooth) nonlinear predictor. Sex was also included as a main effect and in a (smooth) age by sex interaction. As these four models were fitted to each visit separately, they characterize the between-person variation in responses as a function of age and sex, but do not capture developmental (i.e., within-person) effects. Differences in these fits across visits for a given age hence represent learning (or practice) effects, as the only difference between the fits is the number of times participants had been administered the task. Thus, practice effects (as a function of age and sex) were estimated from serial cross-sectional models by taking the difference in the fitted curves between visits 2 and 1, visits 3 and 2, and visits 4 and 3. This approach to estimating practice effects is a straightforward extension of the “once-vs.-twice tested” approach (Anderson, Reid, & Nelson, 2001; Salthouse, 2014; Salthouse et al., 2015; Sullivan et al., 2017) that allows for estimating mean practice effects by age and sex from additional testing occasions (i.e., “twice-vs.-thrice tested”, etc.).
These estimated age- and sex-dependent mean practice effects were then subtracted from each subject’s outcome as appropriate for that visit. A step-by-step description of the algorithm used in this study is available in Supplemental Materials. The practice effect adjusted longitudinal outcomes were used to determine developmental trajectories by fitting a Generalized Additive Mixed Models (GAMMs), which are extensions of linear mixed models but again allowing for flexible nonlinear effects. Age was included as a smooth effect and interacted with sex as before. Subject was included as a random effect to account for correlation in outcomes within person across all four visits (see the Supplemental Materials for more details).
Finally, we evaluated the effects of alcohol use on development by comparing no/low drinkers to those who initiated moderate/heavy drinking. To conduct these comparisons, subgroups of adolescents who remained no/low drinkers (n=110) and of those who went on to initiate moderate/heavy drinking (n=110) were matched on age, gender, and having 4 visits. The group matching was performed in R using the Matchit package (Ho et al., 2007; Ho et al., 2011). In this procedure, each drinking subject was paired with an available control subject that had the same sex and the closest age. The matching procedure led to two groups of 110 participants, all remaining control subjects were excluded from further analysis. The effects of alcohol use were then computed using GAMs including age as a smooth term and considering visit and drinking groups (Supplemental Materials). To assess significance, nominal p-values were reported for all statistical tests.
Results
The Results section comprises two parts. First, we evaluated the developmental trajectory of executive functions by 1) estimating practice effects for all participants and computing new performance scores corrected for practice effects; 2) analyzing developmental effects for all participants using the practice effect-corrected scores. Second, we assessed the alcohol use effects on development (practice effect-corrected scores) using two groups matched for age and sex. Table 1 displays the participant characteristics and executive task performance for each of the four visits (from baseline to follow-up 3).
Table 1.
Characteristics and Executive Performance of Participants in the National Consortium on Alcohol and Neurodevelopment in Adolescence (NCANDA) Study from Baseline to Follow-Up 3.
| Characteristics | Baseline | Follow-up 1 | Follow-up 2 | Follow-up 3 |
|---|---|---|---|---|
|
| ||||
| Age range | 12.00 – 21.87 | 12.91 – 22.86 | 13.95 – 23.83 | 14.92 – 24.81 |
| Age (Mean, SD) | 15.66 (2.39) | 16.69 (2.39) | 17.69 (2.38) | 18.71 (2.38) |
| Pubertal development score | 3.13 (0.72) | 3.35 (0.61) | 3.51 (0.53) | 3.63 (0.42) |
| General efficiency (ms) | 598.43 (120.26) | 546.17 (104.53) | 528.12 (103.09) | 514.78 (97.19) |
| Cognitive control (ms) | 30.45 (72.24) | 18.30 (61.45) | 14.17 (57.51) | 12.69 (56.42) |
| Motor control (ms) | 10.58 (59.96) | 6.32 (49.44) | 6.51 (51.36) | 6.31 (49.87) |
| Past year alcohol use | ||||
| Drinking days (No.) | 3.75 (4.85) | 9.36 (16.55) | 15.90 (23.12) | 23.12 (36.89) |
| Maximum drinks (No.) | 1.74 (1.14) | 4.03 (3.58) | 4.90 (3.97) | 5.46 (4.39) |
| Past month binge (No.) | – | 2.00 (1.84) | 2.26 (2.86) | 2.05 (1.76) |
Note. All values except for age = Mean (SD); Ms = milliseconds; No. = number.
The developmental trajectory of executive control
Before controlling for developmental or learning effects, we explored general efficiency cross-sectionally while performing the Stroop Match-to-Sample task for each visit. Results showed a decrease of the overall mean RTs, interpreted as greater efficiency by age and visit (Figure 1). Then, we dissociated learning from development for general efficiency, cognitive control, and motor control. Table 2 presents the main effects of the GAMM analyses.
Figure 1.
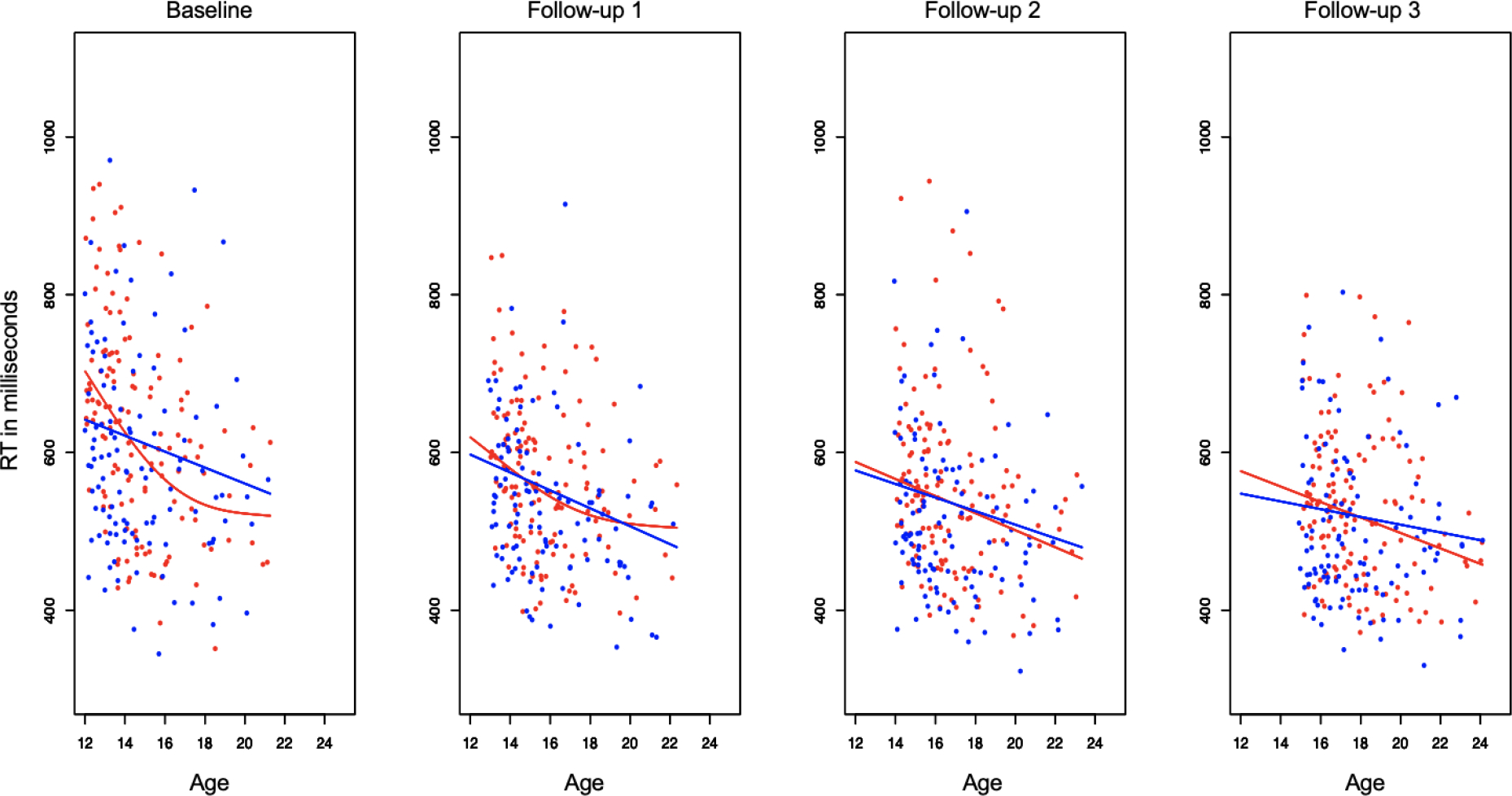
Note. Figure 1 depicts general efficiency (overall RTs) as function of age and sex (girls in red and boys in blue) for the four visits (baseline, follow-up 1, follow-up 2, follow-up 3) before differentiating trajectories for learning or development. Overall RTs to perfoming an executive function task are shown in milliseconds (Y-axis) and age (X-axis) in years.
Table 2.
Executive Function Development over Time (Learning Removed). Results of General Additive Model for Main Effects of Visit, Sex, and Age for Each Stroop Variables.
| Variables | Effects | t / F statistic | p | Adjusted R2 | Deviance explained |
|---|---|---|---|---|---|
|
| |||||
| Development of executive control over time: gam (y.adjusted ~ s(age) + visit + sex) | |||||
| General efficiency | |||||
| Overall RT | Visit | 0.65 | 0.513 | 0.085 | 8.68% |
| Sex | 3.23 | 0.001 | |||
| Age | 67.96 | < 0.001 | |||
| Cognitive control | |||||
| Diff RT INC-CON | Visit | 0.38 | 0.706 | 0.004 | 0.61% |
| Sex | 1.16 | 0.246 | |||
| Age | 8.73 | 0.003 | |||
| Motor control | |||||
| Diff. RT RS-RR | Visit | 0.65 | 0.514 | 0.005 | 0.69% |
| Sex | 2.24 | 0.025 | |||
| Age | 7.09 | 0.008 | |||
General efficiency (overall RTs)
Learning.
General efficiency learning in adolescents was strongest between baseline and follow-up 1, with RTs average improvement of 37.75 milliseconds (Mean = −37.75; p < 0.001). By contrast, learning between follow-ups 1 and 2 (Mean = −8.42; p = 0.058), and between follow-ups 2 and 3 (Mean = −5.72; p = 0.110) were not significant (Figure 2, A).
Figure 2.
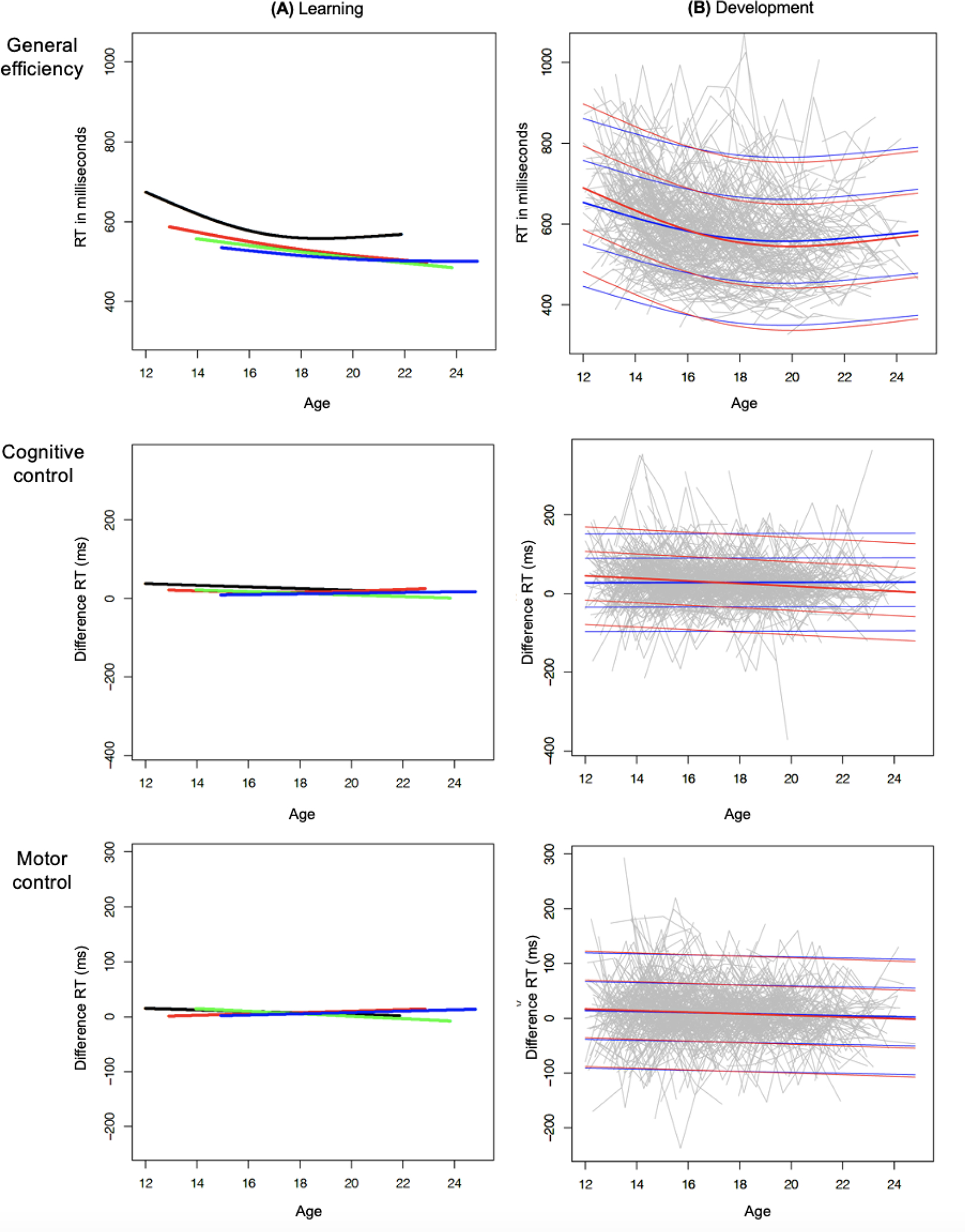
Note. Figure 2 depicts learning and developmental effects for general efficiency (overall RTs), cognitive (Diff. RTINC-CON, upper part), and motor (Diff. RTRS-RR, lower part) control (y-axes): (A) Black lines represent the predicted value for the whole group at baseline, red lines represent follow-up 1, green lines represent follow-up 2, and blue lines follow-up 3. At each visit, the learning effect from the prior visit is estimated from the difference between each sequential pair of predicted values (red-black, green-red, blue-green); (B) For development, individual RTs were plotted for each visit (baseline, follow-ups 1, 2, 3) in grey. Bold lines represent the mean RT for boys (blue) and girls (red) with 1 and 2 standard deviations (light lines above and below the bold ones). Overall RTs (general efficiency) and Diff. RTs (cognitive and motor control) are shown in milliseconds (Y-axis) and age (X-axis) in years.
Development.
After learning effects were removed (Figure 2, B), results showed no significant visit effect for general efficiency (i.e., no enhancement with development over time). There were, however, significant effects for age and sex (Table 2), indicating accelerated improvement at younger ages. Regarding sex, boys were faster than girls (i.e., had higher processing efficiency), while girls improved more over time than boys. Computing analyses by sex significantly improved the model fit when considering all visits (age by sex interaction: p = 0.04) and showed that the age effect was significant in boys (F = 18.53, p < 0.001) and girls (F = 54.0, p < 0.001).
Cognitive control (Diff. RTINC-CON)
Learning.
A learning effect was detected for cognitive control between baseline and follow-up 1 (p= 0.014), whereby adolescents improved their difference RTs on average by 9.99 milliseconds (Mean = −9.99). Learning effects between follow-ups 1 and 2 (Mean = −4.08; p = 0.449) and follow-ups 2 and 3 (Mean = −0.63; p = 0.825) were not significant (Figure 2, A).
Development.
After removing the learning effects (Figure 2, B), results showed no significant visit effect for cognitive control (i.e., no enhancement with development). There was an age effect but no sex effect (Table 2). Analyzing the model by sex significantly improved it over all visits (age by sex interaction: p = 0.002). When computing separate analyses, the age effect was significant in girls (F = 13.49, p < 0.001) but not in boys (F = 0.1, p = 0.752). As Figure 3 shows, girls improved control over cognitive interference (lower difference RTs) with age, whereas boys showed no such improvement.
Figure 3.
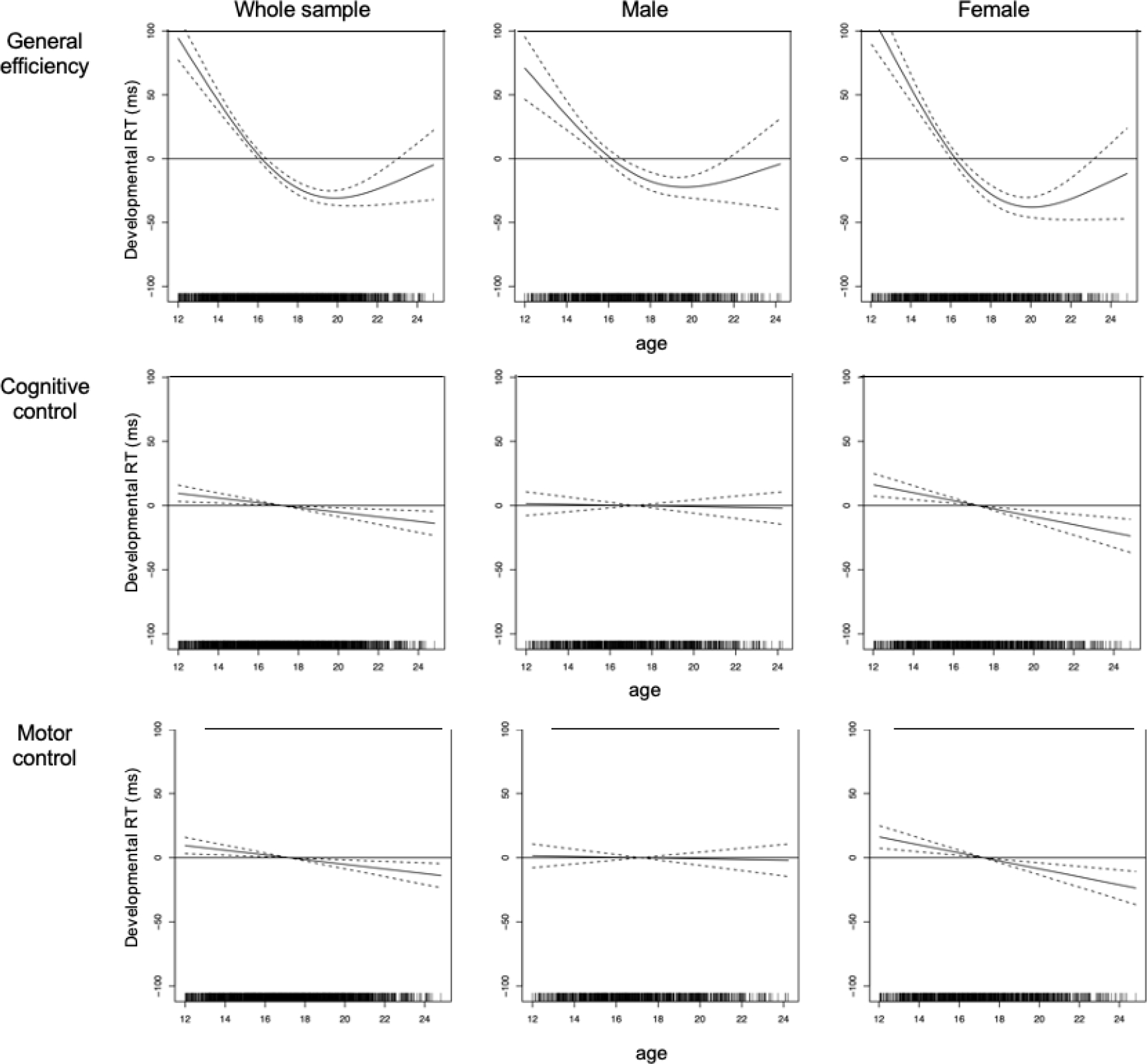
Note. Figure 3 depicts developmental effects plotted against age (x-axes) by sex (from left to right: in the whole sample [n=445], in male [n=210], in female [n=235]). Results are presented for general efficiency, cognitive control, and motor control. Lower difference RTs represent faster processing speed (general efficiency), and improved control over cognitive interference and motor responses. Solid lines represent the developmental slopes and dashed lines the confidence intervals. Y-axes represent standardized developmental RTs.
Motor control (Diff. RTRS-RR)
Learning.
The learning effect for motor control (Figure 2, A) was not significant between any pairs of testing: baseline and follow-up 1 (Mean = −2.85, p = 0.248), follow-ups 1 and 2 (Mean = −1.09; p = 0.838), and follow-ups 2 and 3 (Mean = 2.44; p = 0.836).
Development.
No significant visit effect was detected for motor control (Figure 2, B); however, we observed age and sex effects (Table 2). Results showed that the age effect was significant in girls (F = 5.68, p = 0.017), but not in boys (F = 2.20, p = 0.139), with girls showing significantly improved motor control as measured by smaller difference RTs that was greater in younger than older ages. The sex effect showed better motor control (smaller difference RTs) in boys than girls. Nonetheless, analyzing the model by sex did not improve its fit (no age by sex interaction: p = 0.582).
The influence of alcohol use
We focused our analysis on developmental effects across the four visits and compared two subgroups. These subgroups comprised 53 male and 57 female no/low drinkers (mean age=15.72 years) and 53 male and 57 female moderate/heavy drinkers (mean age=15.72 years). For general efficiency (overall RTs) results showed no main effect of visit (t = 0.39, p= 0.695) or drinking groups (t = 1.50, p = 0.134). For cognitive control (Diff. RTINC-CON), there was no main effect of visit (t = 0.39, p = 0.697) or drinking groups (t = 0.55, p = 0.582). Also, for motor control (Diff. RTRS-RR), results showed no main effect of visit (t = 1.24, p = 0.216) or drinking groups (t = 0.39, p = 0.696).
Discussion
This longitudinal study addressed two specific aims. First, we sought to distinguish developmental effects from prior testing experience (learning effects) on the evolution of general efficiency and executive control (cognitive and motor control) in adolescents over four years. Our results supported the hypothesis of strong learning between baseline and follow-up 1 and showed differential development in boys and girls. Second, we explored whether becoming a drinker affected the developmental trajectories of executive functions in adolescents; results did not support an alcohol effect.
The learning effect observed at the first repetition of the task (that is, at the first annual follow-up testing) is consistent with existing research on practice effects (e.g., Waber et al., 2012) and extends current knowledge toward cognitive and motor control learning over four years. Adolescents significantly improved their general efficiency by reducing their speed to process the Stroop Match-to-Sample task between the first two test sessions. Identifying learning effects at the first repetition but not in successive annual sessions over a four-year interval regardless of age demonstrates that practice effects are present, yet stabilize over time. This improvement was observed for cognitive control but not for motor control. Within the context of this Stroop paradigm, learning during adolescence occurs at the cognitive level but not at the motor response level. This absence of learning in motor control could be consistent with studies showing that motor learning would benefit from constant repetitions (Lelis-Torres, Ugrinowitsch, Apolinário-Souza, Benda, & Lage, 2017). In our study, participants repeated the task four times with a one-year interval between each repetition.
After controlling for learning effects, results revealed that executive function development was significantly related to age and differed by sex but not visit. For general efficiency, we observed results in line with previous studies (Luna, Garver, Urban, Lazar, & Sweeney, 2004), showing high speed improvement at the beginning of adolescence and then a stabilization between late adolescence and early adulthood. Our longitudinal findings are also in line with previous finding (Schulte et al., 2020). We consistently found that girls improved their ability to control cognitive interference with older age, whereas boys had overall better motor control at all ages studied. Indeed, numerous studies corroborate the existence of distinct pathways for executive function development in boys and girls (e.g., Boelema et al., 2014), but results vary according to component process and task characteristics (Miller & Halpern, 2014). The current analysis focused on processing speed and supported sex differences in executive processes, such that boys were more efficient than girls in controlling motor reactions at young ages (Piper, 2011), whereas girls enhanced their motor control throughout adolescence (Anderson, Anderson, Northam, Jacobs, & Catroppa, 2001; Sullivan et al., 2016). In addition, girls improved their abilities to control cognitive interference with age, while boys demonstrated no evidence of such improvement. This sex difference might be explained by childhood training and experience, gender differences in attitudes and parenting styles, and the fact that certain brain regions that are underwriting aspects of executive control mature later in boys than girls (Blakemore, 2008; Chaku & Hoyt, 2019), which is consistent with the observation that sex differences attenuate with pubertal maturation and older age (Schulte et al., 2020).
If alcohol consumption exerted any effect on the variables examined herein, it was below the level of detection. Although studies report alcohol effects on executive processes (e.g., Hanson et al., 2011), other studies did not show significant influence of low (Jurk et al., 2018) or heavy (Boelema et al., 2015) alcohol use on executive function changes with behavioral measures. It is likely that neither the cognitive nor motor measures of executive control used in our analysis had the power to detect potential alcohol effects because the mainstay of drinkers were in the older age range. Specifically, we approached our analyses conservatively by using age- and sex-matched samples, which reduced power by limiting the sample sizes and biasing the ages toward the older ages and away from the younger ages commonly showing the greatest alcohol effects. Given the disturbed brain macrostructural and microstructural developmental trajectories related to initiation of heavy drinking, especially in young drinkers (Pfefferbaum et al., 2018; Zhao, Pfefferbaum, Podhajsky, Pohl, & Sullivan, 2020), future studies are clearly warranted to seek selective cognitive or motor component processes that are concurrently disturbed with drinking.
As with most studies, ours has limitations. Among them are the focus on a single test despite its strength in testing multiple components of cognitive and motor abilities. One reason to have focused on this multifaceted test was to establish an analysis approach that would be viable for application to other neuropsychological domains. A further limitation involves the ages at drinking initiation and amounts of alcohol consumed. At the behavioral level, most studies identifying cognitive impairments in young drinkers with high drinking levels (e.g., more than 10 drinks per occasion; Nguyen-Louie et al., 2016), whereas the present study examined drinking initiation, which could explain the absence of effects. Further studies should explore genetic, familial, and environmental predisposing vulnerabilities in executive control in future drinkers and possible effects of drinking by focusing on extreme-binge drinkers. Finally, we have explored the effect of alcohol on developmental trajectories, but future studies should also explore how alcohol may affect learning abilities.
To conclude, this study shows the developmental trajectories of general efficiency, cognitive control, and motor control in adolescence over four years. Results indicated learning at the first annual visit, which was the first repetition of the task, and age-related development in task performance. By showing that improvement in performance on the Stroop Match-to-Sample task in a longitudinal study of adolescents is mainly related to learning, this research challenges previous results (Boelema et al., 2014) and confirms the relevance of taking practice effects into account. For this executive task, adolescents showed steep learning slopes across one year, from first to second testing. After considering these practice effects, our findings show evidence of age-related development for general efficiency in boys and cognitive and motor control in girls. The relevance of prior testing experience requires consideration in future longitudinal study design and analysis examining neuropsychological development of youth before and after initiating drinking or other substance use.
Supplementary Material
Key points.
Question:
This prospective study investigated the cognitive development of executive control processes (general efficiency, cognitive, and motor control) by controlling for learning effects (i.e., prior practice experience). We also evaluated whether these trajectories are affected by alcohol use initiation.
Findings:
Strong learning effects were observed between the first two repetitions of the task, cognitive development was related to age, and alcohol use initiation has no significant effects.
Importance:
This study identified prior testing experience as an essential factor to be measured in longitudinal neuropsychological and alcohol research.
Next Steps:
Larger prospective evaluations are needed and should consider additional neuropsychological processes and alcohol consumption patterns.
Funding and data release
Dr. Lannoy receives partial support through a fellowship from the Belgian American Educational Foundation. This work was made possible with support by NIH Grants AA021697, AA021695, AA021692, AA021696, AA021681, AA021690, AA021691, and AA017923. Data used here are from the data release: Pohl, K.M., Podhajsky S., Sullivan, E.V., Pfefferbaum, A.: The `NCANDA_PUBLIC_3Y_REDCAP_V02` Data Release of the National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA), Sage Bionetworks Synapse. https://dx.doi.org/10.7303/syn23524209, Retrieved: 2020-10-26, 11:45 PST.
Footnotes
Conflict of interest
All authors declare that they have no conflict of interest that could inappropriately influence, or be perceived to influence, this work.
Submission declaration and verification
We verify that the work described in the submitted manuscript has not been published previously.
References
- Anderson M, Reid C, Nelson J (2001). Developmental changes in inspection time: what a difference a year makes. Intelligence 29, 475–486. [Google Scholar]
- Anderson VA, Anderson P, Northam E, Jacobs R, & Catroppa C (2001). Development of Executive Functions Through Late Childhood and Adolescence in an Australian Sample. Developmental Neuropsychology, 20(1), 385–406. doi: 10.1207/S15326942DN2001_5 [DOI] [PubMed] [Google Scholar]
- Blakemore S-J (2008). The social brain in adolescence. Nature Reviews Neuroscience, 9(4), 267–277. doi: 10.1038/nrn2353 [DOI] [PubMed] [Google Scholar]
- Boelema SR, Harakeh Z, Ormel J, Hartman CA, Vollebergh WAM, & van Zandvoort MJE (2014). Executive functioning shows differential maturation from early to late adolescence: Longitudinal findings from a TRAILS study. Neuropsychology, 28(2), 177–187. doi: 10.1037/neu0000049 [DOI] [PubMed] [Google Scholar]
- Boelema SR, Harakeh Z, van Zandvoort MJE, Reijneveld SA, Verhulst FC, Ormel J, & Vollebergh WAM (2015). Adolescent Heavy Drinking Does Not Affect Maturation of Basic Executive Functioning: Longitudinal Findings from the TRAILS Study. PLOS ONE, 10(10), e0139186. doi: 10.1371/journal.pone.0139186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, & Andersen SL (2011). Developmental trajectories during adolescence in males and females: A cross-species understanding of underlying brain changes. Neuroscience & Biobehavioral Reviews, 35(8), 1687–1703. doi: 10.1016/j.neubiorev.2011.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Brumback T, Tomlinson K, Cummins K, Thompson WK, Nagel BJ, … Tapert SF (2015). The National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA): A Multisite Study of Adolescent Development and Substance Use. Journal of Studies on Alcohol and Drugs, 76(6), 895–908. doi: 10.15288/jsad.2015.76.895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, & Vik PW (1998). Psychometric Evaluation of the Customary Drinking and Drug Use Record (CDDR): A Measure of Adolescent Alcohol and Drug Involvement. Journal of Studies in Alcohol, 59, 427–438. [DOI] [PubMed] [Google Scholar]
- Cahalan D, Cisin IH, Crossley HM, 1969. American Drinking Practices: A National Study of Drinking Behavior and Attitudes. Rutgers Center of Alcohol Studies Monograph No 6. New Brunswick, NJ, Rutgers Center of Alcohol Studies. [Google Scholar]
- Casey BJ, & Jones RM (2010). Neurobiology of the Adolescent Brain and Behavior: Implications for Substance Use Disorders. Journal of the American Academy of Child & Adolescent Psychiatry, 49(12), 1189–1201. doi: 10.1097/00004583-201012000-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaku N, & Hoyt LT (2019). Developmental Trajectories of Executive Functioning and Puberty in Boys and Girls. Journal of Youth and Adolescence, 48(7), 1365–1378. doi: 10.1007/s10964-019-01021-2 [DOI] [PubMed] [Google Scholar]
- Clark DB, Chung T, Martin CS, Hasler BP, Fitzgerald DH, Luna B, … Nagel BJ (2017). Adolescent Executive Dysfunction in Daily Life: Relationships to Risks, Brain Structure and Substance Use. Frontiers in Behavioral Neuroscience, 11, 223. doi: 10.3389/fnbeh.2017.00223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Donohue SE, Honomichl R, Wendelken C, & Bunge SA (2006). Brain Regions Mediating Flexible Rule Use during Development. Journal of Neuroscience, 26(43), 11239–11247. doi: 10.1523/JNEUROSCI.2165-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, & Kril JJ (2014). Human alcohol-related neuropathology. Acta Neuropathologica, 127(1), 71–90. doi: 10.1007/s00401-013-1233-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePasque S, & Galván A (2017). Frontostriatal development and probabilistic reinforcement learning during adolescence. Neurobiology of Learning and Memory, 143, 1–7. doi: 10.1016/j.nlm.2017.04.009 [DOI] [PubMed] [Google Scholar]
- Gil-Hernandez S, Mateos P, Porras C, Garcia-Gomez R, Navarro E, & Garcia-Moreno LM (2017). Alcohol Binge Drinking and Executive Functioning during Adolescent Brain Development. Frontiers in Psychology, 8, 1638. doi: 10.3389/fpsyg.2017.01638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Medina KL, Padula CB, Tapert SF, & Brown SA (2011). Impact of Adolescent Alcohol and Drug Use on Neuropsychological Functioning in Young Adulthood: 10-Year Outcomes. Journal of Child & Adolescent Substance Abuse, 20(2), 135–154. doi: 10.1080/1067828X.2011.555272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho DE, Imai K, King G, & Stuart EA (2007). Matching as Nonparametric Preprocessing for Reducing Model Dependence in Parametric Causal Inference. Political Analysis 15(3), 199–236. doi: 10.1093/pan/mpl013 [DOI] [Google Scholar]
- Ho DE, Imai K, King G, & Stuart EA (2011). MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. Journal of Statistical Software, 42(8). doi: 10.18637/jss.v042.i08 [DOI] [Google Scholar]
- Humes KR, Jones NA, & Ramirez RR (2011). Overview of race and Hispanic origin: 2010. (Report Number: C2010BR-02.) Washington, D.C.: U.S. Census Bureau. Retrieved from: http://www.census.gov/library/publications/2011/dec/c2010br-02.html [Google Scholar]
- Hurks PPM, Schrans D, Meijs C, Wassenberg R, Feron FJM, & Jolles J (2010). Developmental Changes in Semantic Verbal Fluency: Analyses of Word Productivity as a Function of Time, Clustering, and Switching. Child Neuropsychology, 16(4), 366–387. doi: 10.1080/09297041003671184 [DOI] [PubMed] [Google Scholar]
- Infante MA, Nguyen-Louie TT, Worley M, Courtney KE, Coronado C, & Jacobus J (2020). Neuropsychological Trajectories Associated with Adolescent Alcohol and Cannabis Use: A Prospective 14-Year Study. Journal of the International Neuropsychological Society, 26(5), 480–491. doi: 10.1017/S1355617719001395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav KS, & Boutrel B (2019). Prefrontal cortex development and emergence of self-regulatory competence: The two cardinal features of adolescence disrupted in context of alcohol abuse. European Journal of Neuroscience, 50(3), 2274–2281. doi: 10.1111/ejn.14316 [DOI] [PubMed] [Google Scholar]
- Jurk S, Mennigen E, Goschke T, & Smolka MN (2018). Low-level alcohol consumption during adolescence and its impact on cognitive control development: Cognitive control development. Addiction Biology, 23(1), 313–326. doi: 10.1111/adb.12467 [DOI] [PubMed] [Google Scholar]
- Koziol LF, & Lutz JT (2013). From Movement to Thought: The Development of Executive Function. Applied Neuropsychology: Child, 2(2), 104–115. doi: 10.1080/21622965.2013.748386 [DOI] [PubMed] [Google Scholar]
- Krikorian R, & Bartok JA (1998). Developmental Data for the Porteus Maze Test. The Clinical Neuropsychologist, 12(3), 305–310. doi: 10.1076/clin.12.3.305.1984 [DOI] [Google Scholar]
- Kwako LE, Momenan R, Litten RZ, Koob GF, & Goldman D (2016). Addictions Neuroclinical Assessment: A Neuroscience-Based Framework for Addictive Disorders. Biological Psychiatry, 80(3), 179–189. doi: 10.1016/j.biopsych.2015.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees B, Meredith LR, Kirkland AE, Bryant BE, Squeglia LM (2020). Effect of alcohol use on the adolescent brain and behavior. Pharmacology Biochemistry and Behavior, 192, 172906. doi: 10.1016/j.pbb.2020.172906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelis-Torres N, Ugrinowitsch H, Apolinário-Souza T, Benda RN, & Lage GM (2017). Task engagement and mental workload involved in variation and repetition of a motor skill. Scientific Reports, 7(1), 14764. doi: 10.1038/s41598-017-15343-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leys C, Ley C, Klein O, Bernard P, Licata L (2013). Detecting outliers: Do not use standard deviation around the mean, use absolute deviation around the median. Journal of Experimental Social Psychology, 49, 764–766. doi: 10.1016/j.jesp.2013.03.013 [DOI] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, & Sweeney JA (2004). Maturation of Cognitive Processes From Late Childhood to Adulthood. Child Development, 75(5), 1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x [DOI] [PubMed] [Google Scholar]
- Luna B, Marek S, Larsen B, Tervo-Clemmens B, & Chahal R (2015). An Integrative Model of the Maturation of Cognitive Control. Annual Review of Neuroscience, 38(1), 151–170. doi: 10.1146/annurev-neuro-071714-034054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, & O’Hearn K (2010). What has fMRI told us about the Development of Cognitive Control through Adolescence? Brain and Cognition, 72(1), 101–113. doi: 10.1016/j.bandc.2009.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, & Sweeney JA (2004). The Emergence of Collaborative Brain Function: FMRI Studies of the Development of Response Inhibition. Annals of the New York Academy of Sciences, 1021(1), 296–309. doi: 10.1196/annals.1308.035 [DOI] [PubMed] [Google Scholar]
- Mills KL, Goddings AL, Clasen LS, Giedd JN, & Blakemore SJ (2014). The developmental mismatch in structural brain maturation during adolescence. Developmental Neuroscience, 36(3–4), 147–160. [DOI] [PubMed] [Google Scholar]
- Miller DI, & Halpern DF (2014). The new science of cognitive sex differences. Trends in Cognitive Sciences, 18(1), 37–45. doi: 10.1016/j.tics.2013.10.011 [DOI] [PubMed] [Google Scholar]
- Kathryn L Mills KL, Goddings A-L, Clasen LV, Giedd JN, Blakemore S-J (2014). The Developmental Mismatch in Structural Brain Maturation during Adolescence. Developmental Neuroscience, 36, 147–160. doi: 10.1159/000362328. [DOI] [PubMed] [Google Scholar]
- Morin J-FG, Afzali MH, Bourque J, Stewart SH, Séguin JR, O’Leary-Barrett M, & Conrod PJ (2019). A Population-Based Analysis of the Relationship Between Substance Use and Adolescent Cognitive Development. American Journal of Psychiatry, 176(2), 98–106. doi: 10.1176/appi.ajp.2018.18020202 [DOI] [PubMed] [Google Scholar]
- Müller-Oehring EM, Kwon D, Nagel BJ, Sullivan EV, Chu W, Rohlfing T, … Pohl KM (2018). Influences of Age, Sex, and Moderate Alcohol Drinking on the Intrinsic Functional Architecture of Adolescent Brains. Cerebral Cortex, 28(3), 1049–1063. doi: 10.1093/cercor/bhx014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Alcohol Abuse and Alcoholism [NIAAA], 2018. Alcohol Facts and Statistics. Report retrieved from: https://www.niaaa.nih.gov/sites/default/files/AlcoholFactsAndStats.pdf
- Nguyen-Louie TT, Tracas A, Squeglia LM, Matt GE, Eberson-Shumate S, & Tapert SF (2016). Learning and Memory in Adolescent Moderate, Binge, and Extreme-Binge Drinkers. Alcoholism: Clinical and Experimental Research, 40(9), 1895–1904. doi: 10.1111/acer.13160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, & Boxer A, (1988). A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence, 17, 117–133. doi: 10.1007/BF01537962 [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Kwon D, Brumback T, Thompson WK, Cummins K, Tapert SF, … Sullivan EV (2018). Altered Brain Developmental Trajectories in Adolescents After Initiating Drinking. American Journal of Psychiatry, 175(4), 370–380. doi: 10.1176/appi.ajp.2017.17040469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper BJ (2011). Age, handedness, and sex contribute to fine motor behavior in children. Journal of Neuroscience Methods, 195(1), 88–91. doi: 10.1016/j.jneumeth.2010.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinzi F, Perri RL, Berchicci M, Bianco V, Pitzalis S, Zeri F, & Di Russo F (2018). Weak proactive cognitive/motor brain control accounts for poor children’s behavioral performance in speeded discrimination tasks. Biological Psychology, 138, 211–222. doi: 10.1016/j.biopsycho.2018.08.014 [DOI] [PubMed] [Google Scholar]
- Rohde P, Lewinsohn PM, Kahler CW, Seeley JR, & Brown RA (2001). Natural Course of Alcohol Use Disorders From Adolescence to Young Adulthood. Journal of the American Academy of Child & Adolescent Psychiatry, 40, 83–90. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Hadland KA, Paus T, & Sipila PK (2002). Role of the Human Medial Frontal Cortex in Task Switching: A Combined fMRI and TMS Study. Journal of Neurophysiology, 87(5), 2577–2592. doi: 10.1152/jn.2002.87.5.2577 [DOI] [PubMed] [Google Scholar]
- Salthouse T (2015). Test experience effects in longitudinal comparisons of adult cognitive functioning. Developmental Psychology, 51(9), 1262–1270. doi: 10.1037/dev0000030 [DOI] [PubMed] [Google Scholar]
- Salthouse T (2014). Why are there different age relations in cross-sectional and longitudinal comparisons of cognitive functioning? Current Directions in Psychological Science, 23(4), 252–256. doi: 10.1177/0963721414535212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T, Hong J-Y, Sullivan EV, Pfefferbaum A, Baker FC, Chu W, … Müller-Oehring EM (2020). Effects of age, sex, and puberty on neural efficiency of cognitive and motor control in adolescents. Brain Imaging and Behavior, 14(4), 1089–1107. doi: 10.1007/s11682-019-00075-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T, Müller-Oehring EM, Chanraud S, Rosenbloom MJ, Pfefferbaum A, & Sullivan EV (2011). Age-related reorganization of functional networks for successful conflict resolution: A combined functional and structural MRI study. Neurobiology of Aging, 32(11), 2075–2090. doi: 10.1016/j.neurobiolaging.2009.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T, Müller-Oehring EM, Sullivan EV, & Pfefferbaum A (2012). Synchrony of Corticostriatal-Midbrain Activation Enables Normal Inhibitory Control and Conflict Processing in Recovering Alcoholic Men. Biological Psychiatry, 71(3), 269–278. doi: 10.1016/j.biopsych.2011.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD (2013). A role for synaptic plasticity in the adolescent development of executive function. Translational Psychiatry, 3(3), e238–e238. doi: 10.1038/tp.2013.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP (2014). Adolescents and alcohol: Acute sensitivities, enhanced intake, and later consequences. Neurotoxicology and Teratology, 41, 51–59. doi: 10.1016/j.ntt.2013.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Icenogle G, Shulman EP, Breiner K, Chein J, Bacchini D, … Takash HMS (2018). Around the world, adolescence is a time of heightened sensation seeking and immature self-regulation. Developmental Science, 21(2), e12532. doi: 10.1111/desc.12532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Brumback T, Tapert SF, Brown SA, Baker FC, Colrain IM, … Pfefferbaum A (2020). Disturbed Cerebellar Growth Trajectories in Adolescents Who Initiate Alcohol Drinking. Biological Psychiatry, 87(7), 632–644. doi: 10.1016/j.biopsych.2019.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Brumback T, Tapert SF, Fama R, Prouty D, Brown SA, … Pfefferbaum A (2016). Cognitive, emotion control, and motor performance of adolescents in the NCANDA study: Contributions from alcohol consumption, age, sex, ethnicity, and family history of addiction. Neuropsychology, 30(4), 449–473. doi: 10.1037/neu0000259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Brumback T, Tapert SF, Prouty D, Fama R, Thompson WK, … Pfefferbaum A (2017). Effects of prior testing lasting a full year in NCANDA adolescents: Contributions from age, sex, socioeconomic status, ethnicity, site, family history of alcohol or drug abuse, and baseline performance. Developmental Cognitive Neuroscience, 24, 72–83. doi: 10.1016/j.dcn.2017.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M, Zandbelt BB, Gladwin T, Hillegers M, Hoogendam JM, van den Wildenberg WP, ... & Kahn RS (2014). Frontostriatal activity and connectivity increase during proactive inhibition across adolescence and early adulthood. Human brain mapping, 35(9), 4415–4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waber DP, Forbes PW, Almli CR, Blood EA, & The Brain Development Cooperative Group. (2012). Four-Year Longitudinal Performance of a Population-Based Sample of Healthy Children on a Neuropsychological Battery: The NIH MRI Study of Normal Brain Development. Journal of the International Neuropsychological Society, 18(2), 179–190. doi: 10.1017/S1355617711001536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Squeglia LM, Yang TT, & Tapert SF (2013). A longitudinal examination of adolescent response inhibition: Neural differences before and after the initiation of heavy drinking. Psychopharmacology, 230(4), 663–671. doi: 10.1007/s00213-013-3198-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga LM, Bos MGN, van Rossenberg F, & Crone EA (2019). Sex Effects on Development of Brain Structure and Executive Functions: Greater Variance than Mean Effects. Journal of Cognitive Neuroscience, 31(5), 730–753. doi: 10.1162/jocn_a_01375 [DOI] [PubMed] [Google Scholar]
- Wood SN (2006). Low-Rank Scale-Invariant Tensor Product Smooths for Generalized Additive Mixed Models. Biometrics, 62, 1025–1036. doi: 10.1111/j.1541-0420.2006.00574.x [DOI] [PubMed] [Google Scholar]
- Zhao Q, Sullivan EV, Honnorat N, Adeli E, Podhajsky S, De Bellis MD, Voyvodic J, Nooner KB, Baker FC, Colrain IM, Tapert SF, Brown SA, Thompson WK, Nagel BJ, Clark DB, Pfefferbaum A, & Pohl KM (2020). Young Teens Who Initiate Heavy Drinking Risk Deviant Fiber Tract Development in Frontal Brain Systems. American Journal of Psychiatry, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


