1. Introduction
To perform multi-step functional tasks, one must choose a specific action sequence from an unbounded set of movement options, often described as a degrees-of-freedom problem (Rosenbaum, Chapman, Coelho, Gong, & Studenka, 2013). One’s choice of an action sequence relies on multiple constraints such as object affordances, task goals, and individual capability (Scharoun, Gonzalez, Roy, & Bryden, 2018; Seegelke, Hughes, Schütz, & Schack, 2012). In particular, choice of an initial action in the sequence relies on goal-related planning constraints or anticipatory planning. Such planning is often carried out to optimize comfort at the end of task accomplishment, a second-order planning strategy described as the “end-state comfort (ESC) effect” (Rosenbaum, Marchak, Barnes, Vaughan, & Slotta, 1990). For example, to pick up an inverted wine glass, it is reliably observed that one reaches for and grasps the glass with an uncomfortable thumb-down posture, and then supinates to achieve a more comfortable thumb-up posture with the upright glass. Multiple experiments have demonstrated that the choice and kinematic performance of an initial action in a multi-step task reflects anticipatory planning for functional tasks (Alt Murphy, Baniña, & Levin, 2017; Rosenbaum et al., 2013; Tan, Tretriluxana, Pitsch, Runnarong, & Winstein, 2012).
Unilateral stroke impairs the ability to plan and execute functional tasks. The nature and severity of such impairment depends on the side of the hemispheric damage (Sainburg, Maenza, Winstein, & Good, 2016). Hermsdörfer and colleagues (1999) investigated planning for ESC in individuals with left hemisphere damage (LHD) and right hemisphere damage (RHD) compared to neurotypical controls (Hermsdörfer, Laimgruber, Kerkhoff, Mai, & Goldenberg, 1999). A bar-presented in different initial orientations-was grasped and placed into a target under two conditions: an unspecified condition, where participants could place any end of the bar into a target; and a specified condition, where participants had to place a specific end of the bar into a target. Performance differences were observed between LHD and RHD groups compared to controls in the specified condition-the condition that necessitated anticipatory planning of initial grasp to ensure a comfortable end-state. Specifically, the LHD group showed slower and less coordinated performance of the initial grasp, suggesting that anticipatory planning of initial grasp for ESC may be impaired after LHD. This finding suggested that the left hemisphere may subserve motor planning and coordination of multi-step object manipulation.
Deficits in motor planning and coordination after LHD have gathered further support (Mani, Przybyla, Good, Haaland, & Sainburg, 2014; Mutha, Haaland, & Sainburg, 2012; Poole, Sadek, & Haaland, 2009; Schaefer, Haaland, & Sainburg, 2007, 2009); however, factors that might contribute to or rehabilitate such deficits remain underexplored. Work in neurotypical adults demonstrates that the focus of one’s attention during a motor task impacts performance and learning (Wulf, Höß, & Prinz, 1998). Studies of attentional focus consistently demonstrate performance and learning benefits when one attends to the task-relevant external effects of intended action, described as the external focus of attention (e.g., place the red end of a dowel into a red target). These benefits are not observed when one attends to their body movements or the mechanics of their actions, described as the internal focus of attention (e.g., focus on your palm as you reach for the dowel) (Wulf, McConnel, Gärtner, & Schwarz, 2002; Wulf, Shea, & Lewthwaite, 2010; Wulf, Shea, & Park, 2001; Wulf & Prinz, 2001).
The facilitative motor performance effects of external focus instruction-as observed in neurotypical adults-seem to extend to the affected arm of stroke survivors, albeit with some nuance. Fasoli and colleagues (2002) found that external focus instruction benefitted motor performance (evidenced as shorter movement times and higher peak velocities) of the affected arm during three functional reaching actions; however, there were no consistent effects of instructional focus (internal or external) on motor planning variables (e.g., time-to-peak velocity or number of movement subunits across all tasks). Durham and colleagues (2014) similarly demonstrated improvements in the motor performance of the affected arm with external focus instruction; however, internal and external focus instruction often lacked clear delineation. (For example, to emphasize finger opening during grasping, internal and external focus instruction included “open wider”.) Also, the benefit of external focus instruction was particularly augmented when preceded by internal focus instruction, suggesting an order effect. Because of these limitations, the authors judged that external focus instruction “may be of some benefit” to reaching performance in stroke survivors. Interestingly, observational studies of clinical practice have reported that therapists most often rely on instruction, feedback, and demonstration strategies that direct patients’ attention to a specific body part (i.e., internal focus of attention) (Johnson, Burridge, & Demain, 2013; E. Kal et al., 2018). The popularity of internal focus instruction in stroke rehabilitation suggests it might assist motor performance; however, this has not been empirically demonstrated.
In summary, prior work comparing the effects of internal and external focus instruction on motor performance in stroke survivors has assessed performance of the affected hand. As a result, planning deficits cannot be dissociated from execution deficits related to hemiparesis and sensory loss. Prior work has also not considered the impact of lesion side on anticipatory planning and performance of ESC with use of internal and external focus instruction. Use of internal focus instruction may be particularly impacted after left hemisphere damage. Neuroimaging studies in neurotypical adults suggest that the left hemisphere implements body-centered information to assist motor performance (Goldenberg, 2001; Mengotti, Ripamonti, Pesavento, & Rumiati, 2015; Wong, Jax, Smith, Buxbaum, & Krakauer, 2019). When performing a finger tapping sequence, for example, neurotypical adults showed left-lateralized activation in the left somatosensory cortex and intraparietal lobule when shifting their focus to their fingers (i.e., internal focus) from the keys of the response box (Zimmermann et al., 2012). If left-lateralized, the effects of internal focus instruction on anticipatory planning for ESC in stroke survivors with LHD may differ from stroke survivors with RHD and neurotypical individuals.
Our preliminary study had two aims. First, to replicate the findings of Hermsdörfer and colleagues (1999), we aimed to determine if the side of hemispheric damage affected planning and performance of the initial action for ESC in the less affected arm in individuals with unilateral stroke compared to age-matched controls. We hypothesized that individuals with LHD will demonstrate greater deficits in planning and performance of the optimal initial grasp for ESC during a two-step functional task. Second, we aimed to test the effects of internal and external focus instruction on anticipatory planning and performance in individuals with RHD compared to LHD. In line with our review of the literature, we hypothesized internal focus instruction will preferentially impair planning and performance of the optimal initial grasp during a two-step functional task in patients with LHD.
2. Methods
2.1. Participants
Twenty-one individuals with chronic unilateral stroke (10 left hemisphere-damaged; 11 right hemisphere-damaged; 14 male, 7 female; mean upper extremity Fugl-Meyer (UEFM) score = 15.95, range: 0–46; mean age = 57.86 years, range: 38–74) and 20 control subjects (6 male, 14 female; mean age = 59.45 years, range: 38–76) consented to participate in the experimental protocol approved by the Institutional Review Board of Albert Einstein Medical Center (Table 1). Inclusion criteria were as follows: (1) at least 6 months after stroke; (2) unilateral, first ischemic or hemorrhagic anterior circulation stroke; (3) a score of 24 or higher on the Standardized Mini-Mental State Examination (SMMSE) or, for individuals with LHD-related aphasia, a score of 4 or higher on the Western Aphasia Battery (WAB) Auditory Comprehension subtest; (4) ability to transfer 10 blocks in one minute with the less affected hand during the Box and Blocks Test (BBT); and (5) no pain or musculoskeletal problems. Exclusion criteria were as follows: (1) hemineglect as assessed by the line bisection test; (2) a painful upper extremity joint condition at rest or motion; (3) an active medical, neurological, or psychiatric condition that would interfere with the ability to perform upper extremity tasks; (4) inability to follow instructions or perform the task; (5) bilateral, cerebellar, or brainstem stroke; (6) use of pacemakers, defibrillators, or similar medical implants; and (7) pregnancy.
Table 1.
Demographic and clinical characteristics of participants.
| Stroke (n = 21) | Controls (n = 20) | ||||
|---|---|---|---|---|---|
| Factor | Total Sample | Right Hemisphere Damage (n = 11) | Left Hemisphere Damage (n = 10) | Active Right Hand (n = 11) | Active Left Hand (n = 9) |
| Sex | |||||
| Male (n) | 21 | 7 | 7 | 5 | 2 |
| Female (n) | 20 | 4 | 3 | 6 | 7 |
| Age in years (SD; Range) | 58.63 (10.06; 38 – 76) | 56.00 (8.44; 45 – 74) | 59.90 (10.17; 38 – 70) | 60.55 (11.63; 43 – 76) | 58.11 (10.73; 38 – 73) |
| Handedness | |||||
| Right (n) | 36 | 9 | 8 | 11 | 8 |
| Left (n) | 4 | 1 | 2 | 0 | 1 |
| Ambidextrous (n) | 1 | 1 | 0 | 0 | 0 |
| Years Post-Stroke | 7.00 (4.58) | 10.00 (3.68) | --- | --- | |
| Line Bisection (T-score) a | 0.91 (50.18) | 0.85 (55.70) | --- | --- | |
| Cognitive Tests | |||||
| WAB (SD) b | --- | 8.81 (1.16) | --- | --- | |
| SMMSE (SD) c | 28.00 (2.28) | --- | 29.36 (0.67) | 29.22 (1.30) | |
| TMT Part A (SD) d | 48.25 (31.07) | 57.85 (28.22) | 80.65 (31.07) | 26.35 (7.42) | 28.35 (11.08) |
| TMT Part B (SD) e | 95.25 (63.25) | 116.18 (65.76) | 161.77 (72.84) | 59.36 (16.77) | 56.72 (15.20) |
| Sensorimotor Tests | |||||
| Monofilament Test (SD; Range) f | 3.63 (0.36; 2.83 – 4.56) | 3.75 (0.48; 2.83 – 4.56) | 3.67 (0.41; 2.83 – 4.31) | 3.54 (0.24; 2.83 – 3.61) | 3.52 (0.26; 2.83 – 3.61) |
| UEFM (SD) g | 15.95 (13.47) | 20.09 (14.75) | 11.40 (10.84) | --- | --- |
| BBT (SD) h | 52.63 (13.72) | 44.64 (19.48) | 50.80 (8.75) | 53.91 (7.11) | 57.00 (8.81) |
| Grip strength (SD) i | 69.04 (24.06) | 62.78 (21.29) | 82.43 (27.19) | 65.40 (27.26) | 66.28 (27.19) |
Abbreviations: Western Aphasia Battery (WAB); Standardized Mini Mental State Examination (SMMSE); TMT (Trail Making Test); UEFM (Upper Extremity Fugl-Meyer); Box and Blocks Test (BBT).
Raw scores and T-scores on the line bisection test. Cut-off score is ± 2.5 for neglect.
Participants with left hemisphere damage were screened using the WAB. The range of possible scores is 0 to 10 with lower scores indicative of greater verbal comprehension deficits.
Participants with right hemisphere damage and control subjects were screened using the SMMSE. The range of possible scores is 0 to 30 with lower scores indicative of greater cognitive impairment.
Time in seconds to complete the task. Average score = 29 seconds. Scores greater than 78 seconds indicate impairment. One participant with RHD damage was unable to complete the task.
Time in seconds to complete the task. Average score = 75 seconds. Scores greater than 273 seconds indicate impairment. One participant with RHD and 2 participants with LHD were unable to complete the task.
Score of 2.83 indicates normal hand sensation; 3.61 – 4.31 indicates diminished light touch; and 4.56 indicates loss of protective sensation. All group means within the normal to diminished light touch range.
The range of possible scores is 0 to 66 with lower scores indicative of greater upper extremity motor impairment.
Total blocks transported in one minute with the test hand (ipsilesional hand in subjects with stroke).
Grip strength measured using hand-held dynamometer
2.2. Experimental setup
As shown in Figure 1, a dowel, 20.25 cm long and 4.9 cm in diameter, rested horizontally on a cradle of a custom-made support stand (inner width = 9 cm; outer width = 15 cm; height = 23.5 cm; depth = 5 cm) secured to the table. The dowel, positioned at 75% of the participant’s maximum arm reach, was centered to align with the participant’s acromion process. A target hole, 5.3 cm in diameter, was centered in front of the support stand at about 50% of the participant’s maximum reach. A taped line, positioned at 25% of the participant’s maximum reach, designated the starting position of the tested hand before each trial. To assess reach-to-grasp kinematics, three electromagnetic markers of the motion-tracking system, 3D Guidance trakSTAR NDI, were secured to the radial styloid and dorsal surface of the distal phalanges of the thumb and index fingers of the tested hand. An opaque screen occluded the participant’s view of the dowel prior to the start of each trial. Audio and video were recorded of the experiment. The video camera was positioned to capture each participant’s side view, with the tested hand and eye level in the frame. Video recordings were stored for offline analyses.
Figure 1. Participant and motor task setup.

A dowel rested horizontally on a cradle of a stand secured to the table. The dowel, positioned at 75% of the participant’s maximum arm reach, was centered to align with the participant’s acromion process. A target hole was centered in front of the apparatus at about 50% of the participant’s maximum reach. A taped line, positioned at 25% of the participant’s maximum reach, designated the starting position of the tested hand. Three electromagnetic markers were secured to the radial styloid and dorsal surface of the distal phalanges of the thumb and index fingers of the tested hand. An opaque screen (not pictured) occluded the participant’s view of the dowel. The video camera framed the tested hand and side view of the eyeline.
2.3. Procedure
Control participants completed the experiment with their left or right hand and participants with stroke, their less affected (i.e., ipsilesional) hand. Participants sat at a table in a straight-backed chair with two adjustable straps crossed at the chest to constrain the trunk. For all conditions, participants rested their forearm in the mid-prone (neutral) position with their thumb and index fingers clasped at the starting line (Figure 1). Before the start of each trial, the opaque screen was placed between the dowel and the starting line. Participants were instructed to move as soon as they saw the object after the screen lift, and complete the task as fast as possible. Following a “ready” command, the opaque screen was lifted. After trial completion, the screen was reinstated between the dowel and the starting line. Then, the examiner placed the dowel in position for the subsequent trial. At the beginning of the experiment, one to two practice trials were provided to ensure task comprehension.
Three conditions were tested in the following order (Figure 2):
Figure 2.
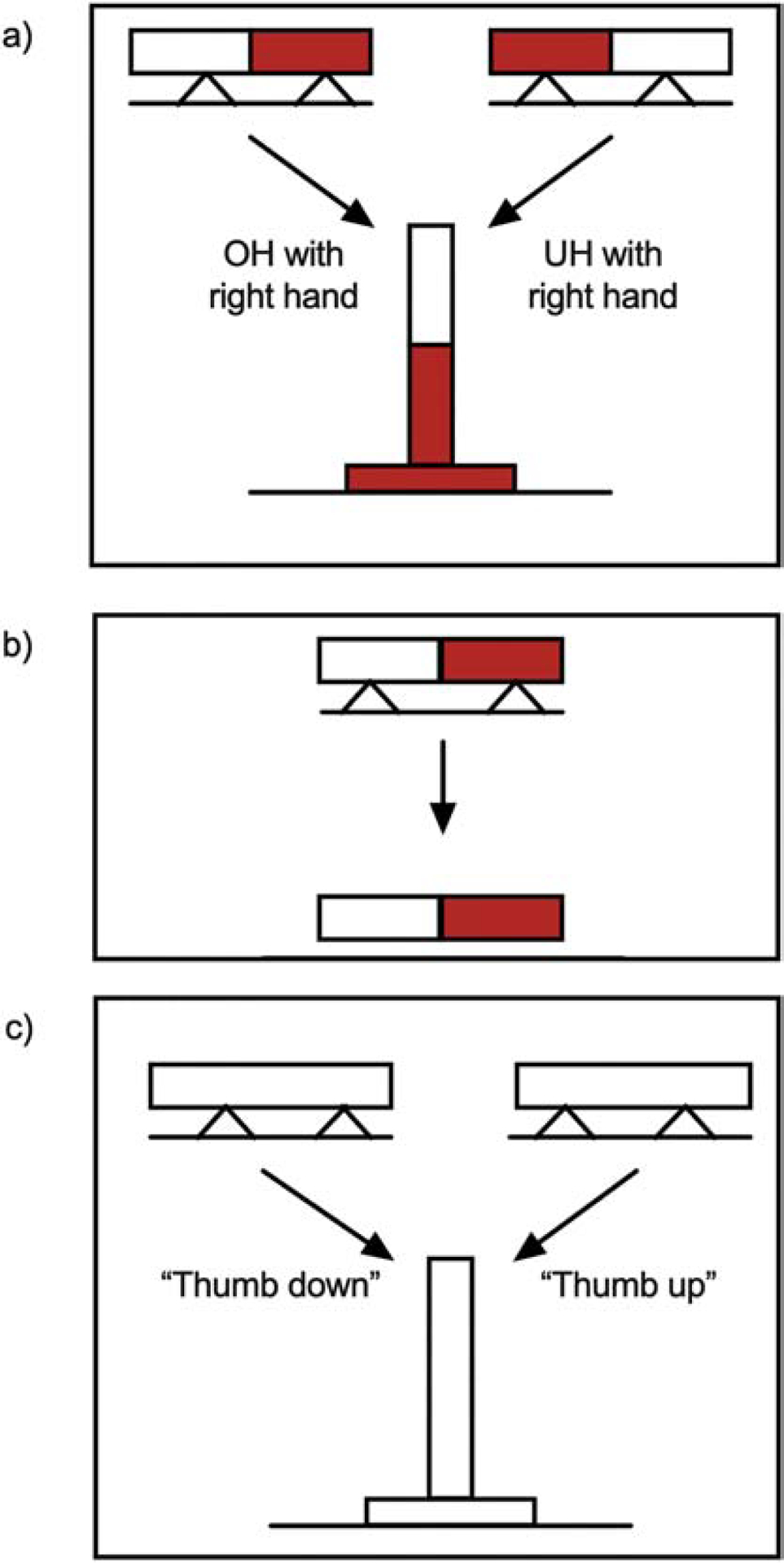
Experimental design. (a) External Focus Condition: Participants were instructed to reach for and grasp the center of a red and white dowel with their tested hand and then place the red end of the dowel vertically into a red target hole. As illustrated above, for participants executing the task with their right hand, the red-right condition required an overhand (OH) grasp for ESC and the red-left condition, an underhand (UH) grasp for ESC. (b) Control Condition: Participants were asked to move the colored dowel from the cradle to another horizontal location without the need to rotate the dowel to a vertical position or place it into a target hole. (c) Internal Focus Condition: Participants were instructed to reach for and grasp the center of a neutral-colored dowel with their “thumb up” (underhand) or “thumb down” (overhand) and then place the dowel vertically into a neutral target.
Condition 1: External focus instruction
Similar to classic ESC experiments, participants were tested for their ability to plan for and execute a grasp that ensured a more comfortable position at the end of task accomplishment. For this condition, the dowel was painted half red and half white. At the beginning of the trial-block, participants were instructed to reach for, grasp, and place the red end of the dowel vertically into the red target hole. The provision of color matching aimed participants’ focus on the task-relevant external effects of the movement. Neither demonstration nor explicit instruction was provided regarding grasp strategy. The experimenter oriented the dowel on the cradle to accord with the experimental condition (overhand or underhand) for the forthcoming trial. The dowel was presented in two orientations in the frontal plane such that the red end pointed right or left. For participants using the right hand, the red-right orientation required an overhand grasp for ESC and the red-left orientation, an underhand grasp for ESC (Figure 2a). For participants using the left hand, the red-right orientation required an underhand grasp for ESC and the red-left orientation, an overhand grasp for ESC. Eight overhand trials (i.e., trials requiring an overhand grasp for ESC) and 8 underhand trials (i.e., trials requiring an underhand grasp for ESC) were randomly presented, thus affording equal opportunity for overhand and underhand grasps (16 total trials). After each trial, the experimenter noted whether the initial grasp posture conformed to ESC expectations.
Condition 2: Control condition
Participants were instructed to reach for, grasp, and transport the red and white dowel from the cradle to another horizontal location as fast as possible (Figure 2b). Rotation of the dowel to a vertical position, target placement, and choice of an optimal initial posture was not required. Control condition performance was used to account for general differences in motor performance across groups.
Condition 3: Internal focus instruction
Participants were instructed to reach for and grasp a neutral-colored dowel with their “thumb down” (an overhand grasp) or “thumb up” (an underhand grasp), and then rotate and place the dowel vertically into a neutral-colored target as fast as possible (Figure 2c). For each trial, thumb-down or thumb-up instruction was given before the opaque screen was lifted. The provision of thumb posture compelled participants to focus on the mechanics of the grasp action. At the beginning of the trial-block, the experimenter supplemented instruction with demonstration of the two postures. Demonstration may be distinct from oral internal focus instruction, but it was included to ensure task comprehension. The experimenter also asked participants to demonstrate the thumb-down and thumb-up grasp postures prior to testing to further ensure task comprehension. The neutral-colored dowel replaced the red and white dowel to eliminate external cues that may have assisted task performance. In other words, participants had to rely entirely on the oral instruction provided at the beginning of each trial. Eight trials of each strategy were randomly instructed (16 total trials). For each trial, the experimenter ensured the initial grasp posture aligned with the specified posture.
2.4. Clinical testing
UEFM (Sullivan et al., 2011) was administered to all participants with stroke. All participants underwent the Trail Making Test (Bowie & Harvey, 2006; Muir et al., 2015) to determine their capacity for task-switching. Grip strength was measured using a hand-held Jamar dynamometer for the test hand in all participants. For LHD patients, we tested for deficits in finger identification and right-left discrimination as well as measures of apraxia. Five items testing finger identification and seven items testing right-left discrimination, derived from the WAB (Shewan & Kertesz, 1980), were measured in all stroke survivors with LHD. For 7 of the 10 participants with LHD, we retrieved apraxia scores available in the Moss Rehabilitation Research Institute (MRRI) research registry. Apraxia assessment tested the following: semantic gesture recognition, the ability to associate an audio-visually presented action verb (e.g., hammering) with its associated gesture; spatial gesture recognition, the ability to discriminate the correct arm or hand posturing, amplitude, and timing of the gesture associated with a specific action verb; and meaningless gesture imitation, the ability to observe and imitate 10 meaningless movements or analogues of realistic gestures with slight changes (Buxbaum, 2005).
2.5. Lesion data
Nine of 10 participants with LHD who participated in the Brain Behavior Relationship Research Group at MRRI consented to the use of research quality MRI scans acquired at the Hospital of the University of Pennsylvania. Research MRI scans included whole-brain T1-weighted MR images collected on a 3T (Seimens Trio, Erlangen, Germany: repetition time = 1620 msec, echo time = 3.87 msec, field of view = 192 × 256 mm, 1×1×1 mm voxels) scanner and were manually segmented to produce a 3-D lesion mask of 0s and 1s, with 1 indicating a lesioned voxel. Segmentation included both gray and white matter voxels. As a result, the analysis potentially reveals both gray and white matter damage associated with behavioral impairments (Schwartz, Faseyitan, Kim, & Coslett, 2012; Watson & Buxbaum, 2015). Thresholded, binarized lesion drawings were then warped to a 1mm×1mm×1mm common template brain (Montreal Neurological Institute “Colin27”) using a symmetric diffeomorphic registration algorithm (Avants, Epstein, Grossman, & Gee, 2008, www.picsl.upenn.edu/ANTS) to translate manual lesion segmentations to standardized space.
2.6. Data analyses
Analyses focused on the first movement in the two-sequence task: reaching to grasp the dowel. We disregarded the subsequent movement of the task (i.e., transporting or placing the dowel) because we were concerned with anticipatory planning and performance of initial grasp in the two-sequence task.
Video data were analyzed to compute reaction time and the percentage of overhand and underhand trials conforming to ESC expectations. Percentage of optimal posture trials was calculated for the external focus condition as the number of trials (for both overhand and underhand trial conditions) executed with optimal posture divided by total trials multiplied by 100. Reaction time was manually inspected from video recordings. For each trial, we marked the frame number in which the screen no longer impeded the participant’s eye line (i.e., the dowel was within view) and the subsequent frame number in which the participant’s hand initiated the reaching movement. Reaction time was calculated as the difference between the two marked frame numbers divided by the frame rate (59.94 frames per second).
All kinematic position data recorded using the electromagnetic motion-tracking system were captured at 200 Hz and filtered using a zero-phase lag, low-pass fourth-order Butterworth filter with a 10 Hz cutoff frequency (Kantak, Zahedi, & McGrath, 2016; Winter, 2004). Reach path was derived from position coordinates of the wrist sensor. Three-dimensional displacement was calculated from the wrist sensor position and then tangential velocity of reach was derived using a finite-difference technique (Winter, 2004). Grasp aperture was derived from the distance between the thumb and index finger sensors. Movement onset was defined as the time at which the tangential reach velocity exceeded 10% of the peak velocity (Stewart, Gordon, & Winstein, 2014; Tretriluxana, Gordon, & Winstein, 2008). Movement offset - defined as the time at which the object was grasped - was identified as the first point of smallest grasp aperture after peak grasp aperture that remained stable for the subsequent 1000 data samples.
Outcome measures:
The following measures were extracted from the kinematic data for all conditions: (a) total movement time (TMT) for reach-to-grasp was calculated from movement onset to movement offset and provided an index of global motor performance for the initial grasp; (b) peak reach velocity (pRV) was the maximum tangential velocity of reaching (wrist sensor) after movement onset; (c) time-to-peak reach velocity (TpRV) characterized the planning of the reach component and was calculated as the time from movement onset to point of peak tangential reach velocity; (d) time-to-peak grasp aperture (TpGA) characterized the planning of initial grasp and was calculated as the time from movement onset to the point of maximum grasp aperture; (e) the highest cross-correlation coefficient (R-Max) characterized the spatial coordination between reach and grasp components and was quantified using cross-correlation analyses between tangential reach velocity and aperture displacement; and (f) the associated time-lag (T-Lag) of cross-correlation characterized the temporal coordination between reach and grasp components.
2.7. Statistical analyses
Differences in clinical measures (UEFM, BBT, grip strength, Trail Making Test) between LHD and RHD groups were determined using independent samples t-test. Group differences in the percentage of optimal grasp for overhand and underhand trials during the external focus condition were analyzed nonparametrically using Kruskal-Wallis analysis of variance and the Mann-Whitney U-test for pairwise comparisons. Reaction time and kinematic data during external and internal focus conditions were first normalized to the control condition to account for differences in general motor performance.
First, we tested our hypothesis that planning and performance of the optimal initial grasp during the two-step functional task will be impaired in patients with LHD compared to other groups. To that end, we analyzed the external focus condition separately to determine if patients demonstrated planning deficits in a classic ESC experiment. We used a 4 group (CON-R, CON-L, RHD, LHD) X 2 optimal grasp posture (overhand, underhand) repeated measures ANOVA with group as the between-subjects factor and the optimal grasp condition as the repeated measures factor to assess for group differences in performance with external focus instruction.
Next, we tested our hypothesis that compared to external focus, internal focus instruction will impair planning and performance of the initial grasp during a two-step functional task in the LHD group. We analyzed reaction time and kinematic data using separate 4 (group) X 2 (instruction condition) X 2 (optimal grasp posture) analyses of variance (ANOVA) with repeated measures on the last two factors. Bonferroni’s multiple comparisons were conducted for post-hoc analyses to identify the locus of significance. In all comparisons, p-values for Bonferroni comparison were adjusted (p < 0.025) to correct for testing different optimal posture and instruction conditions. We further calculated effect size using Cohen’s d and conducted post-hoc power analyses using G-Power (Erdfelder, Faul, & Buchner, 1996).
Exploratory analyses were conducted to evaluate the relationships between clinical impairments and motor performance changes under internal and external focus conditions. First, spearman rank order correlations (rho) were used to determine if the severity of affected arm motor impairment (characterized by UEFM score) was related to performance changes with internal compared to external focus instruction in participants with stroke. Participants with LHD were subdivided into equal subgroups on the basis of grasp performance with internal relative to external focus instruction. Scores of finger agnosia, right-left discrimination, and apraxia were compared between the two LHD subgroups and effect sizes were calculated keeping in mind the limited sample size and exploratory nature of these analyses.
Finally, we conducted exploratory lesion analyses in individuals with LHD to determine the neural substrates in the left hemisphere, which when lesioned, lead to poorer performance with internal compared to external focus instruction. Lesions from research scans were drawn onto a template brain in MRIcron for lesion subtraction analyses. We conducted lesion subtraction analyses for LHD participants to identify lesions to specific brain structures putatively associated with poor performance in the internal compared to external focus condition. To do so, we grouped LHD participants according to grasp performance during internal relative to external focus instruction. Lesion overlap maps for each subgroup were generated using MRIcron. The low-performing subgroup (i.e., LHD participants with greater deficits in grasp performance using internal focus instruction) was chosen as the group of interest to which the high-performing subgroup (i.e., LHD participants with less impaired grasp performance using internal focus instruction) was compared. The participant whose performance sat in the middle of the data was omitted to delineate performance between groups. Voxelwise subtractions between subgroups were then performed by calculating the difference between the percentages of lesions at each voxel in the two subgroups (n = 4 in each subgroup).
3. Results:
3.1. Clinical measures:
Table 1 summarizes the clinical characteristics of all participants. There were no significant differences in UEFM (t(19) = 1.52; p = 0.144), BBT (t(19) = 0.92; p = 0.370), grip strength (t(19)= 1.85; p = 0.079) and Trail Making Test (t(19) = 1.44; p = 0.167) between RHD and LHD groups. Three participants with stroke (2 RHD and 1 LHD) demonstrated contralesional neglect (Table 1).
3.2. Reach-to-grasp performance of controls and stroke in the external focus condition
Hypothesis 1:
Individuals with LHD will demonstrate greater deficits in the planning and performance of the optimal initial grasp for ESC during a two-step functional task.
3.2.1. Choice of optimal initial grasp posture:
All participants grasped the dowel and placed the red end into the red target as instructed. Each dowel orientation (i.e., red end oriented to the left or right) afforded two potential grasp strategies with the tested hand: overhand or underhand. As Figure 3 illustrates, the combination of tested hand (i.e., left or right) and dowel orientation (i.e., red end pointed left or right) permitted four grasp postures congruent with the ESC expectations of the two conditions (i.e., overhand or underhand). Figure 3 further shows individual (red triangles) and group (black bars) averages for the percentage of overhand and underhand trials in which the initial grasp posture conformed to ESC expectations. Most of the participants selected an overhand posture when it was optimal. Relatively fewer ones selected an underhand posture when it was optimal (significant main effect of optimal grasp posture, p < 0.05). However, the choice for optimal grasp did not significantly differ among groups for overhand (Kruskal Wallis H test, X = 4.49; p = 0.217) and underhand (Kruskal Wallis H test, X = 2.67; p = 0.445) trials.
Figure 3.
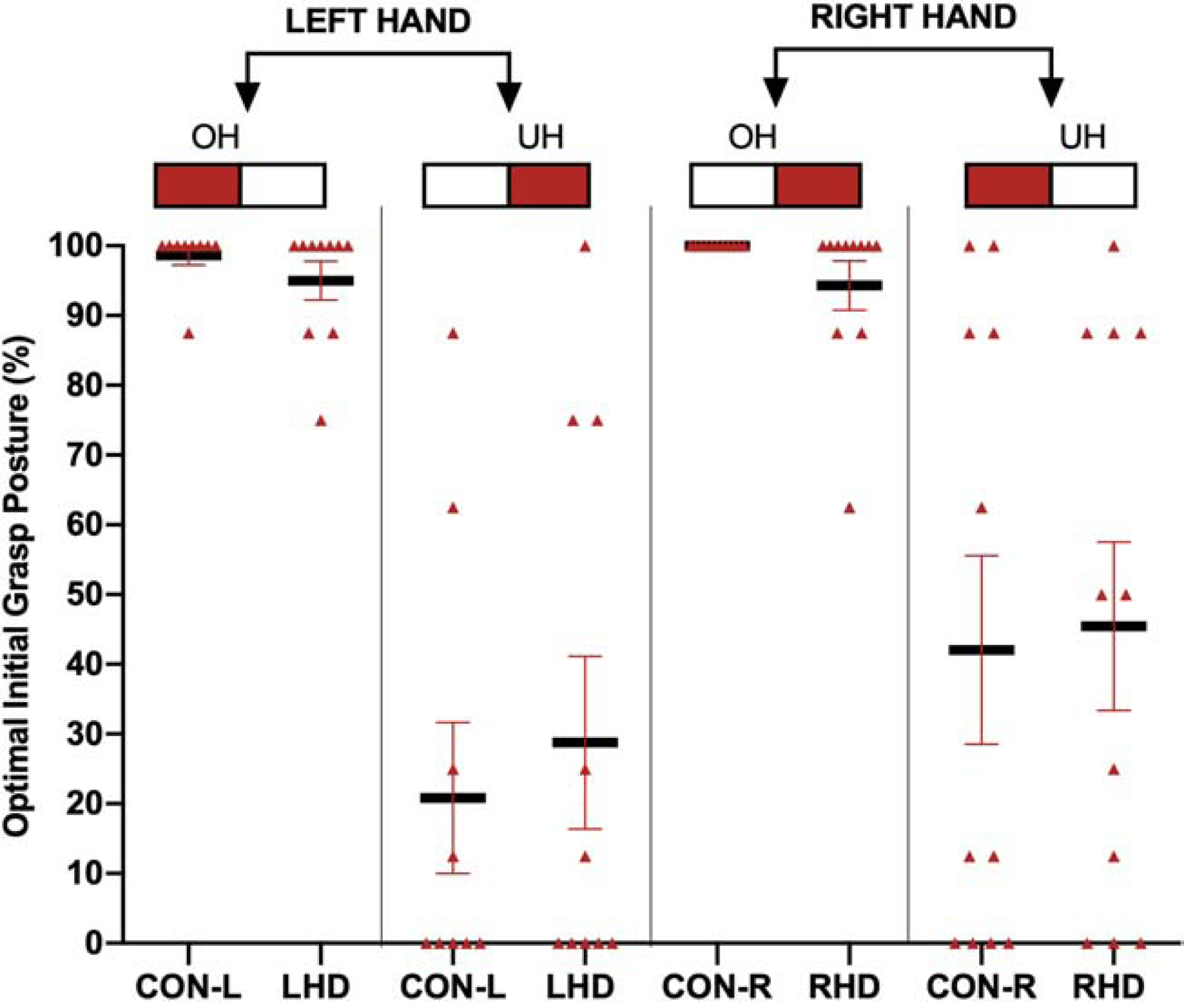
Percentage of trials performed with an optimal initial grasp for each participant organized by tested hand (left or right), condition (underhand [UH] and overhand [OH]), and group. The top part of the figure illustrates the dowel orientation at the start and the hand used. Red triangles represent individual participant averages; black bold bars represent group means with SEM in red.
3.2.2. Kinematics of initial grasp posture:
Because performance was often inconsistent with ESC expectations for overhand and underhand trials, we used paired t tests to determine if there were significant differences in the kinematic variables between actual postures within each condition. We observed no significant differences in the kinematic variables (TMT, TpGA, pRV, and TpRV) between the trials with actual overhand and underhand grasps within each specified condition. Therefore, all trials, irrespective of the chosen posture, were clasped and included within each optimal posture condition for the main analyses.
There was a significant main effect of optimal grasp posture for normalized TMT (F(1,37) = 13.49; p = 0.001, Figure 4a), pRV (F(1,37) = 25.49; p < 0.001, Figure 4b), and normalized TpGA (F(1,37) = 10.06; p = 0.003, Figure 4c), suggesting that global performance as well as reaching and planning of grasping were slower for underhand compared to overhand trials. Further, we observed a significant group X optimal grasp posture interaction for normalized TpGA (F(3,37) = 4.65; p = 0.007, Figure 4c). Bonferroni’s multiple comparisons revealed a significant difference in the normalized TpGA between overhand and underhand trials in the LHD group (p = 0.013; Cohen’s d = 0.85; calculated post-hoc power = 0.88), suggesting significant delays in planning for grasp aperture during underhand compared to overhand trials in the LHD group. There were no significant effects of group or optimal grasp posture on reaction time or TpRV (F(3,37) = 1.63; p = 0.2).
Figure 4.
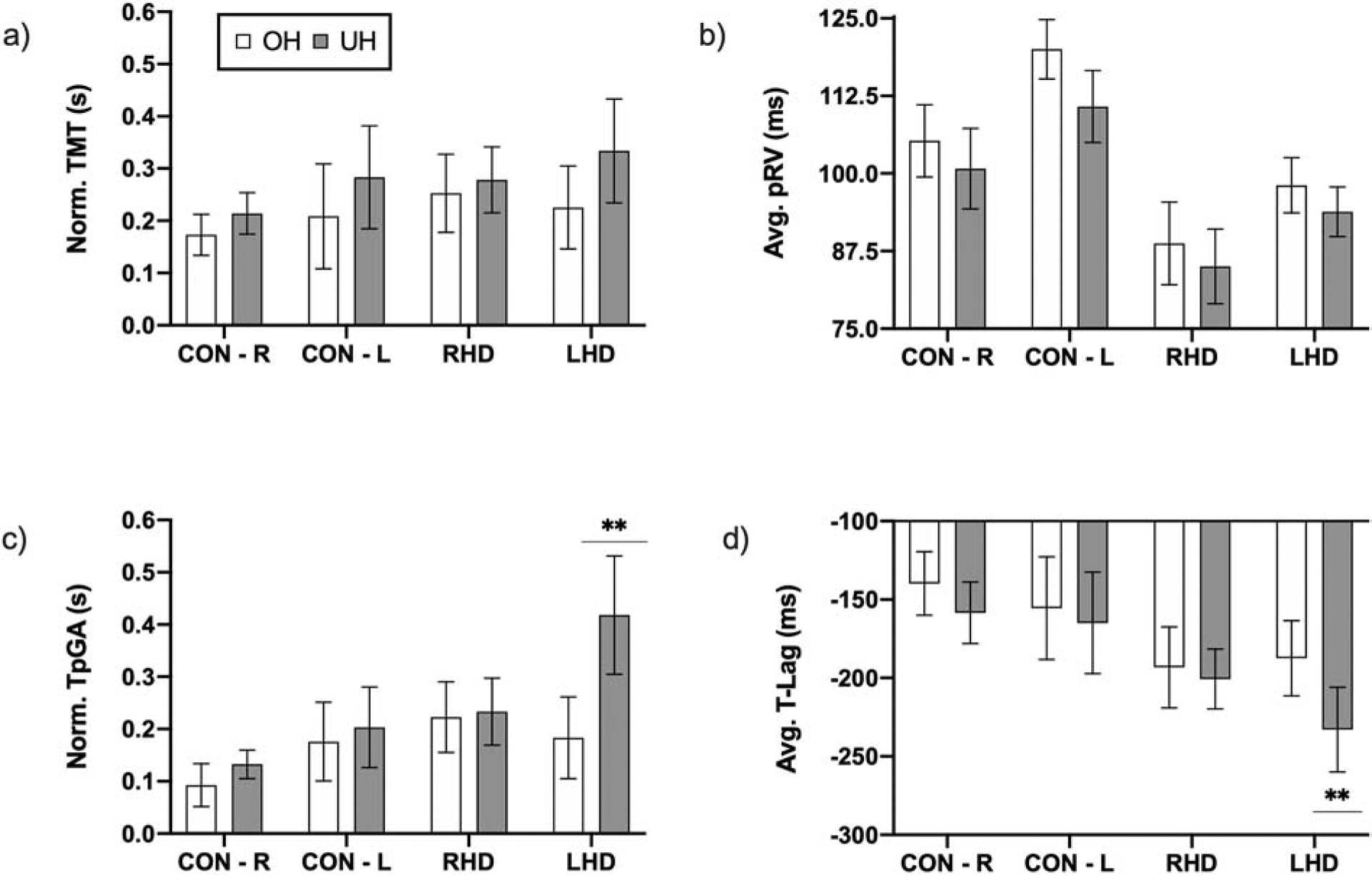
Kinematics and coordination of initial grasp performance in the external focus condition. Graphs show mean and SEM for (a) normalized total movement time (TMT), (b) peak reach velocity (pRV), (c) normalized time-to-peak grasp aperture (TpGA), and (d) time-lag (T-Lag), the temporal coordination between reach and grasp components of the initial grasping action. ** Indicates significant group X optimal grasp posture interaction for normalized TpGA and T-Lag indicating that during the initial grasp posture, participants with LHD showed significant delays in the initial planning phase compared to participants with RHD and controls, particularly for underhand trials.
Spatial coordination (characterized by R-Max) was significantly higher for overhand compared to underhand trials (Related samples Wilcoxon Signed rank test, 5.58; p < 0.001); however, there were no significant between-group differences for overhand (Kruskal Wallis H test, X = 2.51; p = 0.473) and underhand (Kruskal Wallis H test, X = 4.75; p = 0.191) trials. Cross-correlation time-lags between reach and grasp were significantly longer for the underhand compared to overhand trials (significant effect of optimal grasp condition; F(1, 37) = 22.15; p < 0.001). Further, a significant group X optimal grasp condition interaction (F(3,37) = 4.09; p = 0.013) suggested differential effects of optimal grasp posture for each group. Post-hoc Bonferroni’s comparisons indicated significantly longer time-lags during underhand relative to overhand trials for the LHD group (p = 0.018, Figure 4d), but not for the RHD group (p = 0.438).
3.3. Effect of instruction condition on reach-to-grasp kinematics and coordination
Hypothesis 2: Internal focus instruction will impair planning and performance of the optimal initial grasp during a two-step functional task, particularly in patients with LHD.
Figure 5 shows the effects of internal relative to external focus instruction on initial grasp kinematics and coordination in control and stroke participants. There was a significant main effect of instruction on normalized TMT (F(1,37) = 6.97; p = 0.012), pRV (F(1,37) = 98.05; p < 0.001), TpRV (F(1,37) = 4.30; p = 0.045), and TpGA (F(1,37) = 16.86; p < 0.001), suggesting that internal focus instruction deteriorated motor performance. There was a significant group X instruction interaction effect on TpGA (F(3,37) = 5.61; p = 0.003), suggesting a differential effect of instruction on planning of initial grasp aperture between groups. As evident in Figure 5, the LHD group had significantly longer TpGA during both overhand and underhand trials when using internal compared to external focus instruction. TpGA detriments were not observed for the other three groups. Post-hoc comparison indicated that the TpGA delay with internal focus instruction was significantly greater for the LHD group compared to control groups using their right (p = 0.007; Cohen’s d = 0.99; calculated post-hoc power = 0.73) and left (p = 0.001; Cohen’s d = 1.2; calculated post-hoc power = 0.93) hand. While post-hoc comparisons did not yield a significant difference in TpGA with internal focus instruction between LHD and RHD groups, power calculations yielded a large effect size (Cohen’s d = 0.8) indicating that a sample larger than 21 per group would be needed to demonstrate statistically significant differences between the LHD and RHD groups.
Figure 5.
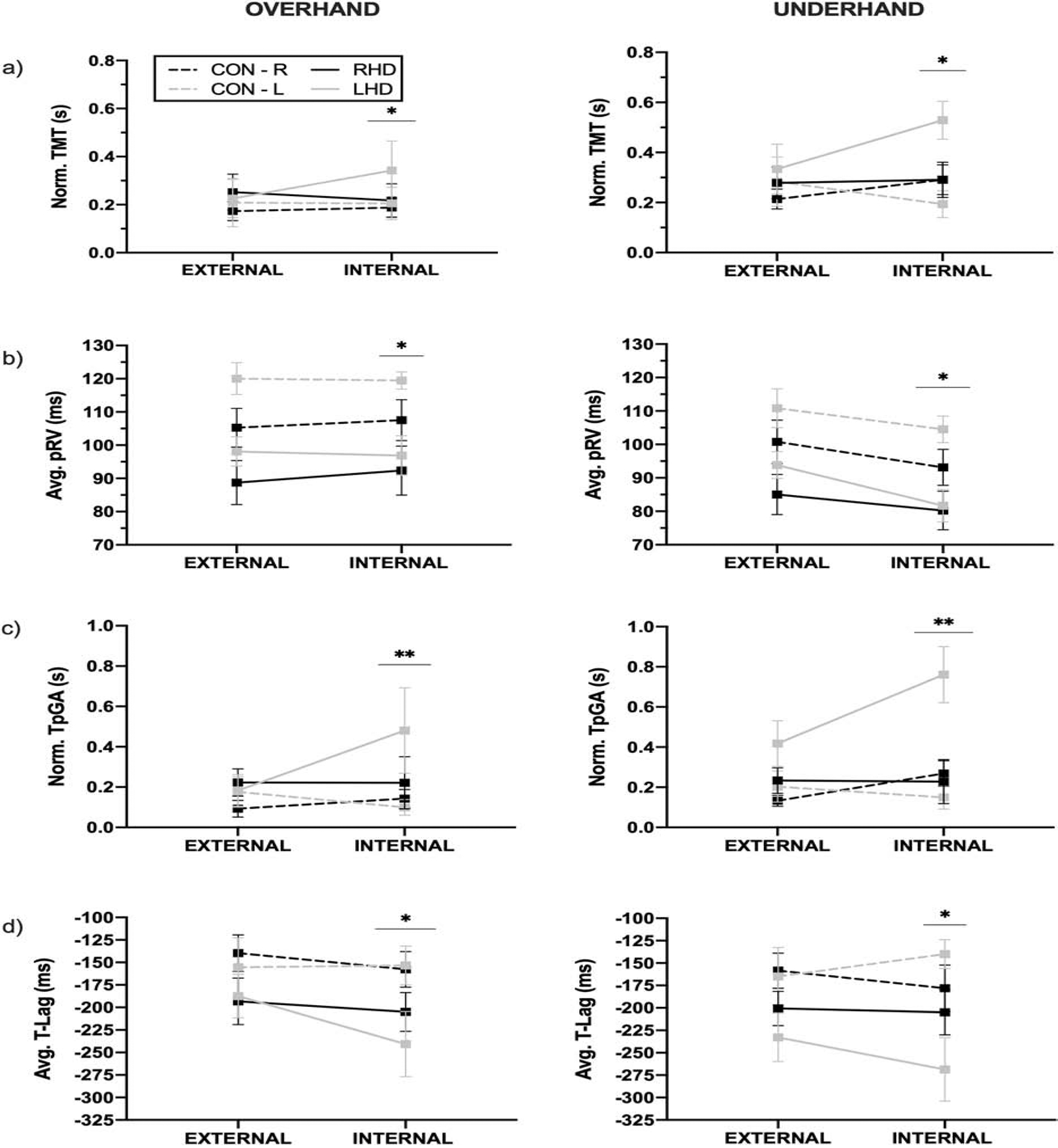
Effect of external versus internal focus instruction on reach-to-grasp kinematics and coordination in overhand and underhand trials. Graphs show mean and SEM for (a) normalized total movement time (TMT), (b) average peak reach velocity (pRV), (c) normalized time-to-peak grasp aperture (TpGA), and (d) average time-lag (T-Lag). *Results show a significant main effect of instruction, indicating a detrimental effect of internal focus instruction. **Results show a significant main effect and interaction between instruction condition and group, indicating that the TpGA delay with internal focus instruction was significantly greater for individuals with LHD.
As seen in Figure 5, temporal coordination (i.e., T-Lag) between reach and grasp during overhand and underhand trials was significantly delayed with internal relative to external focus instruction (main effect of instruction condition; F(1,37) = 8.92; p = 0.005); this detriment was significantly greater for the LHD group (F(1,37) = 8.92; p = 0.038). There was no significant main or interaction effects on spatial coordination (i.e., R-Max).
3.4. Relationship with clinical measures and neuroanatomy (exploratory):
Detriments in TpGA with internal compared to external focus instruction did not show a statistically significant relationship with UEFM scores in participants with stroke (Spearmann’s rho r = −0.021; p = 0.929).
The subgroup of LHD participants (n = 5) whose motor performance was more impaired with internal focus instruction identified less finger items (64%) compared to the less impaired subgroup (92%). The low-performing subgroup also scored lower on right-left discrimination items (68.6%) compared to the high-performing subgroup (91.4%). We similarly compared apraxia scores for LHD subgroups with prior apraxia testing (n = 7). The low-performing LHD subgroup scored lower (80%) than the high-performing LHD subgroup (94%) on semantic gesture recognition items. No deficits were found in meaningless gesture imitation and spatial gesture recognition in the low-performing subgroup.
Preliminary lesion analyses:
Results of the subtraction analyses indicated that lesion to the left premotor cortex, secondary somatosensory area, superior temporal cortex, and anterior inferior parietal cortex was particularly associated with poor performance during internal relative to external focus instruction (Figure 6).
Figure 6.
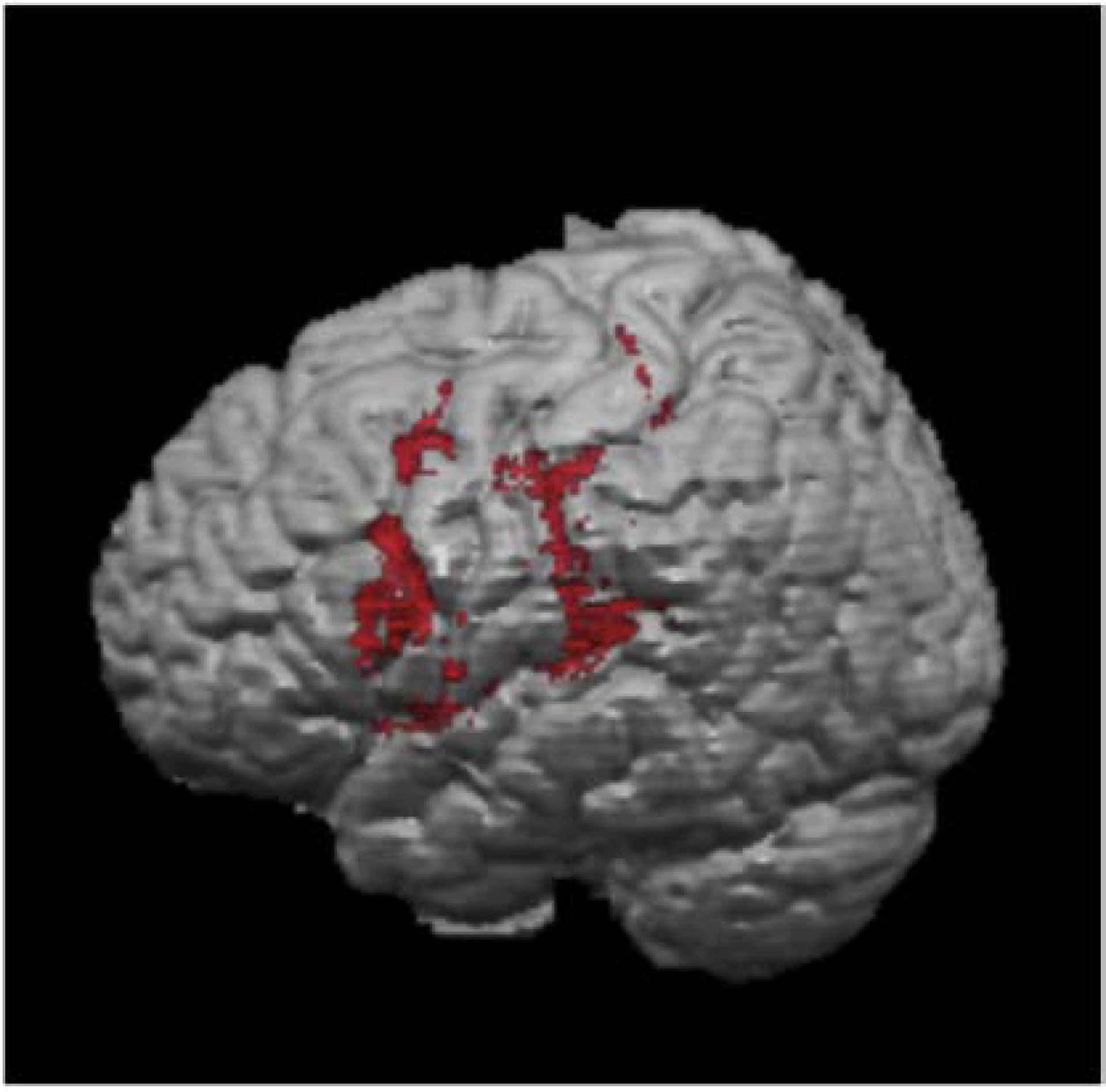
Results of the subtraction analyses. Brain regions associated with greater deterioration of normalized TpGA with internal focus instruction in LHD participants included: left premotor cortex, left secondary somatosensory area, superior temporal cortex, and anterior inferior parietal cortex.
4. Discussion:
In this study, we investigated the effects of internal and external focus instruction on planning and performance of initial grasp posture for ESC in individuals with LHD and RHD secondary to stroke. In agreement with our first hypothesis, when planning for ESC, participants with LHD demonstrated deficits in initial grasp performance and temporal coordination between reach and grasp compared to RHD and control groups. Further, as predicted by our second hypothesis, internal focus instruction disproportionately impaired planning of grasp performance and temporal coordination for reach-to-grasp actions in the LHD group, but not in the RHD or control groups. Presence of planning and performance deficits in the less affected arm of LHD participants provide support for a left-lateralized system for anticipatory planning and use of internal focus instruction. Our exploratory analyses suggested that detriments in TpGA with internal focus instruction in LHD survivors may be greater with finger agnosia, right-left discrimination deficits, and semantic gesture recognition deficits. Finally, preliminary lesion analyses indicated that poor performance with internal focus instruction in participants with LHD was associated with lesion to the left premotor cortex, secondary somatosensory area, superior temporal cortex, and anterior inferior parietal cortex; this finding needs confirmation in a larger sample.
4.1. Selection of end-state comfort in controls and stroke
Studies in neurotypical younger adults consistently indicate that participants choose an initial grasp to ensure ESC at the end of task accomplishment (Rosenbaum et al., 1990). Contrary to our expectations, control participants in the present study infrequently chose an initial grasp to ensure ESC, particularly for trials that required an underhand grasp. However, they conformed to ESC behavior for trials that required an overhand grasp. ESC behavior in stroke groups was not significantly different from age-matched controls. Like controls, individuals with RHD and LHD conformed to ESC behavior for trials requiring an overhand but not underhand grasp. Given the comparable infrequent selection of underhand grasp actions amongst groups, the inability to consistently choose an underhand initial grasp for ESC cannot be attributed to stroke-related motor deficits. All participants with stroke moved with their less affected arm and showed no overt motor deficits. Our findings parallel those of Hermsdörfer et al. (1999) who demonstrated that participants with stroke chose an overhand grasp more frequently than an underhand grasp irrespective of the optimal grasp required for ESC (Hermsdörfer et al., 1999). Our findings also corroborate those of Wunsch and colleagues (2015) who reported decreased ESC sensitivity for underhand but not overhand trials in neurotypical adults (Seegelke et al., 2012; Wunsch, Weigelt, & Stöckel, 2015). Thus, we confirm that the choice of initial grasp posture in older adults with or without stroke may defy ESC expectations, particularly in conditions that require an underhand grasp. How these findings generalize to the affected arm in stroke survivors is unknown. One may hypothesize that grasp planning deficits may be further augmented given the well-reported deficits in active range of motion and control of supination in the affected arm of stroke survivors.
The observed preponderance of overhand grasping may be consequent to age-related cognitive decline or other competing task priorities. Control participants in the present study were considerably older (mean age: 59.45 years) than those in previous studies (e.g., university students in Rosenbaum, 1990) investigating the ESC phenomenon. Reduced cognitive and executive function capacities associated with aging may interfere with higher-order motor planning. As a result, older adults may be more likely to employ habitual overhand postures as observed in our study. Defaulting to the overhand posture may also relate to precrastination-a phenomenon whereby the performer chooses habitual postures to hasten task performance at the expense of ESC (Rosenbaum & Sauerberger, 2019). Since our instructions emphasized movement speed, participants may have used habitual postures to hasten task performance. Future studies are needed in older adults and stroke survivors to disentangle factors that favor ESC over precrastination.
4.2. Kinematic deficits of initial grasp with external focus instruction
Performance of initial reach-to-grasp was slower when the optimal initial posture required underhand grasping for ESC compared to overhand grasping for ESC. Prior work suggests that the statistical preponderance of natural everyday movements influences motor performance on standardized laboratory tasks (Howard, Ingram, Körding, & Wolpert, 2009). Given that overhand grasping actions are more prevalent in our everyday repertoire; overhand actions may require less stringent higher-order planning. On the contrary, underhand grasping likely requires additional planning to override the habitual overhand default. The additional processing may slow planning of the initial grasp and reach during underhand grasp conditions evidenced as longer TpGA, slower pRV, and overall slower performance.
Our results suggest that the planning required for initial grasp action during underhand trials may be more compromised in individuals with LHD. Participants with LHD demonstrated significantly longer TpGA compared to RHD participants during underhand but not overhand trials. TpGA putatively encompasses processes involved in the feedforward planning of grasp aperture characteristics required to manipulate objects. In addition to planning, temporal coordination between reach and grasp components was also impaired for underhand trials in individuals with LHD. Our findings partially accord with those of Hermsdörfer and colleagues (1999) who demonstrated slower motor performance (longer movement times and lower peak reach velocity) in individuals with LHD compared to RHD (Hermsdörfer et al., 1999). Although we did not find differences in movement times and peak reach velocity in those with LHD, we found that our LHD group had slower planning of the grasp component during underhand trials. These differences may be due to differences in the tasks and participant characteristics between the two studies. Hermsdörfer et al. (1999) tested multiple hand orientations as the participants reached to grasp a bar of 2 cm in diameter. We only tested two hand orientations using a dowel of 4.9 cm in diameter. Further, the Hermsdörfer (1999) study included a mix of mild, moderate, and severely impaired patients. Our study included more severely impaired patients (mean UEFM = 15.95). Previous studies have evidenced left hemispheric specialization for motor planning of relatively simpler two-dimensional reaching tasks and pantomiming postures for tool use (Buxbaum & Randerath, 2018; Schaefer et al., 2007; Wong et al., 2019). Our present work lends additional support for a specialized role of the left hemisphere in the performance of initial actions that require higher-order motor planning to ensure ESC.
4.3. Impact of side of hemispheric damage with internal focus instruction
We questioned if we could improve task performance by specifying the initial grasp posture. In effect, our internal focus instruction provided subjects with the optimal grasp “solution.” Provision of the “solution” deteriorated grasp performance and temporal coordination in the LHD group; however, performance in the RHD and control groups was spared. The distinct performance deficits observed in the LHD group suggest that neural substrates within the left hemisphere may implement processes to transform internal focus instruction into actions.
The fixed order of testing - external focus, control, and internal focus - may have confounded the internal focus condition due to the absence of color cues. Having relied on color cues for goal accomplishment with external focus instruction, the novelty of neutral colors may have confused the task goal. We do not think this contributed to the results as the testers ensured that participants understood the goal of the motor task and posture specification for initial grasp. Further, deterioration of motor performance with internal focus instruction despite understanding the task and having prior practice with external focus instruction indicates that internal focus instruction was indeed detrimental to motor performance, particularly for those with LHD.
Another explanation for the detrimental effects of internal focus instruction may relate to impairments in specific cognitive processes that are implemented by the left hemisphere. The LHD subgroup with greater deterioration in grasp performance during internal compared to external focus instruction had poorer scores on subsets of WAB that tested finger identification and right-left discrimination. Use of internal focus instruction relied on the ability to identify one’s fingers (i.e., the thumb) and to process and integrate auditory cues about spatial orientation (i.e., up versus down) to achieve the instructed grasp posture. Right-left disorientation might indicate a more global directional or spatial orientation deficit, which may impede the ability to readily assume thumb-down or thumb-up instruction. Our exploratory analyses suggest that individuals with LHD who demonstrate finger agnosia and/or right-left discrimination deficits may be more likely to show significant performance deficits with internal focus instruction. Future studies need to directly test and confirm these findings.
Poor grasp performance with internal focus instruction was strongly associated with poor semantic gesture recognition-the ability to pair an action verb (e.g., cutting) with the correct gesture (e.g., hand action when using scissors). Semantic gesture recognition deficits, commonly observed in LHD individuals with apraxia, have been associated with the inability to pantomime and imitate actions (Buxbaum & Coslett, 2001; Buxbaum, Kyle, & Menon, 2005). Pantomiming and imitating actions may share similar processes needed to transform internal focus instruction into actions. Pantomiming relies on a higher-order action representation that allows mapping of a verbal command (e.g., how would you use a scissor?) to an action in an internal frame of reference (i.e., pantomiming action of using a scissor). Similar mapping of verbal commands to appropriate actions using an internal frame of reference is required for processing internal focus instruction. Thus, use of internal focus instruction may be affected in individuals with apraxia. More extensive investigations in a larger sample are required to confirm this speculation.
The specific neural substrates within the left hemisphere involved in motor performance with internal focus instruction is largely unknown. Our exploratory subtraction analyses indicated that LHD participants with lesions involving the left premotor cortex, secondary somatosensory area, superior temporal cortex, and anterior inferior parietal cortex may be disproportionately disadvantaged when using internal focus instruction to assist motor performance. Our observations align with previous functional imaging work that showed activation of the left somatosensory cortex and intraparietal area when switching from external to internal focus of attention, as conducted in the present experiment (Zimmermann et al., 2012). It thus follows that internal focus of attention may require amplification of afferent information to plan and execute an appropriate motor response, thereby necessitating processing in the left somatosensory cortex. In addition, lesion to the anterior inferior parietal area - an area associated with finger agnosia - may further impair performance during internal focus instruction (Rusconi et al., 2014). Finally, ventral premotor cortex has been implicated in selection of action (Kantak, Stinear, Buch, & Cohen, 2012). Though we cannot draw any specific conclusions based on a preliminary subtraction method in a small sample, our exploratory findings encourage more sophisticated lesion-symptom mapping in a larger sample of stroke survivors to identify the influence of lesion location on motor performance with use of internal focus instruction.
4.4. Limitations
This preliminary study has potential limitations. First, we only included individuals with stroke who had moderate-to-severe motor impairment in the affected arm. We cannot ascertain if patients with smaller lesions or mild-to-moderate motor impairments will demonstrate similar deficits with internal focus instruction. Second, the order of external and internal focus conditions was not randomized. Because of this, it is possible that the removal of visual cues with internal focus instruction may have confused the task goal. However, we ensured that participants understood the task. Empirical observations indicated that participants with LHD would often initiate the movement with the instructed posture, but with slower movements and/or hesitation in the choice of optimal grasp. Third, we did not perform a comprehensive cognitive exam of visual perception and executive function that may be critical in grasping actions as tested here. We had limited cognitive testing for our small sample, which limits the interpretation and generalizability of our preliminary findings. Finally, the present study focused on short-term performance; hence, these findings cannot be generalized to learning and recovery. Various studies have investigated the effects of attentional focus during learning and rehabilitation with conflicting results (Durham et al., 2014; Durham, Van Vliet, Badger, & Sackley, 2009; Kal et al., 2015; Kim, Hinojosa, Rao, Batavia, & O’Dell, 2017). Our findings suggest that future studies account for the potential impacts of lesion location and cognitive and perceptual deficits on learning.
Conclusions
This is the first study to demonstrate that use of internal focus instruction may disadvantage performance of initial actions in multi-step tasks in stroke survivors with LHD. From a theoretical perspective, this study provides initial support for a left-lateralized system for motor planning and motor performance with use of internal focus instruction. These findings have crucial implications for how instructions might be individualized during arm rehabilitation to improve motor performance after stroke. Our findings suggest that compared to internal focus, external focus instruction may better assist motor performance in stroke survivors with LHD. We also identify putative cognitive processes and brain areas that may be necessary to implement internal focus instruction. Specifically, the relationship of finger agnosia, right-left discrimination, and apraxia with motor performance under distinct attentional focus conditions warrants further study. Finally, our results implore us to investigate the effects of attentional focus on motor performance and learning using a randomized controlled design with a larger sample of RHD and LHD patients with detailed cognitive and neuroimaging characterizations.
Funding sources:
NIH R03 HD091881-01A1 and Albert Einstein Society Research grant to SK
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alt Murphy M, Baniña MC, & Levin MF (2017). Perceptuo-motor planning during functional reaching after stroke. Experimental Brain Research. 10.1007/s00221-017-5058-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avants BB, Epstein CL, Grossman M, & Gee JC (2008). Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Medical Image Analysis, 12(1), 26–41. 10.1016/j.media.2007.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowie CR, & Harvey PD (2006). Administration and interpretation of the Trail Making Test. Nature Protocols, 1(5), 2277–2281. 10.1038/nprot.2006.390 [DOI] [PubMed] [Google Scholar]
- 4.Buxbaum LJ, & Branch Coslett H (2001). Specialised structural descriptions for human body parts: Evidence from autotopagnosia. Cognitive Neuropsychology, 18(4), 289–306. 10.1080/02643290126172 [DOI] [PubMed] [Google Scholar]
- 5.Buxbaum LJ, Kyle KM, & Menon R (2005). On beyond mirror neurons: Internal representations subserving imitation and recognition of skilled object-related actions in humans. Cognitive Brain Research, 25(1), 226–239. 10.1016/j.cogbrainres.2005.05.014 [DOI] [PubMed] [Google Scholar]
- 6.Buxbaum LJ, & Randerath J (2018). Limb apraxia and the left parietal lobe. Handbook of Clinical Neurology, 151, 349–363. 10.1016/B978-0-444-63622-5.00017-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durham KF, Sackley CM, Wright CC, Wing AM, Edwards MG, & van Vliet P (2014). Attentional focus of feedback for improving performance of reach-to-grasp after stroke: a randomised crossover study. Physiotherapy, 100(2), 108–115. 10.1016/j.physio.2013.03.004 [DOI] [PubMed] [Google Scholar]
- 8.Durham K, Van Vliet PM, Badger F, & Sackley C (2009). Use of information feedback and attentional focus of feedback in treating the person with a hemiplegic arm. Physiotherapy Research International : The Journal for Researchers and Clinicians in Physical Therapy, 14(2), 77–90. 10.1002/pri.431 [DOI] [PubMed] [Google Scholar]
- 9.Erdfelder E, Faul F, & Buchner A (1996). GPOWER: A general power analysis program. Behavior Research Methods, Instruments, and Computers, 28(1), 1–11. 10.3758/BF03203630 [DOI] [Google Scholar]
- 10.Goldenberg G (2001). Imitation and matching of hand and finger postures. NeuroImage, 14(1 Pt 2), S132–6. 10.1006/nimg.2001.0820 [DOI] [PubMed] [Google Scholar]
- 11.Hermsdörfer J, Laimgruber K, Kerkhoff G, Mai N, & Goldenberg G (1999). Effects of unilateral brain damage on grip selection, coordination, and kinematics of ipsilesional prehension. Experimental Brain Research, 128(1–2), 41–51. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10473738 [DOI] [PubMed] [Google Scholar]
- 12.Howard IS, Ingram JN, Körding KP, & Wolpert DM (2009). Statistics of natural movements are reflected in motor errors. Journal of Neurophysiology, 102(3), 1902–1910. 10.1152/jn.00013.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson L, Burridge JH, & Demain SH (2013). Internal and External Focus of Attention During Gait Re-Education: An Observational Study of Physical Therapist Practice in Stroke Rehabilitation. Physical Therapy, 93(7), 957–966. 10.2522/ptj.20120300 [DOI] [PubMed] [Google Scholar]
- 14.Kal EC, van der Kamp J, Houdijk H, Groet E, van Bennekom CAM, & Scherder EJA (2015). Stay Focused! The Effects of Internal and External Focus of Attention on Movement Automaticity in Patients with Stroke. PloS One, 10(8), e0136917. 10.1371/journal.pone.0136917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kal E, van den Brink H, Houdijk H, van der Kamp J, Goossens PH, van Bennekom C, & Scherder E (2018). How physical therapists instruct patients with stroke: an observational study on attentional focus during gait rehabilitation after stroke. Disability and Rehabilitation, 40(10), 1154–1165. 10.1080/09638288.2017.1290697 [DOI] [PubMed] [Google Scholar]
- 16.Kantak SS, Stinear JW, Buch ER, & Cohen LG (2012). Rewiring the brain: Potential role of the premotor cortex in motor control, learning, and recovery of function following brain injury. Neurorehabilitation and Neural Repair, 26(3). 10.1177/1545968311420845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kantak S, Zahedi N, & McGrath RL (2016). Task-Dependent Bimanual Coordination After Stroke: Relationship With Sensorimotor Impairments. Archives of Physical Medicine and Rehabilitation, 97(5), 798–806. 10.1016/j.apmr.2016.01.020 [DOI] [PubMed] [Google Scholar]
- 18.Kim GJ, Hinojosa J, Rao AK, Batavia M, & O’Dell MW (2017). Randomized Trial on the Effects of Attentional Focus on Motor Training of the Upper Extremity Using Robotics With Individuals After Chronic Stroke. Archives of Physical Medicine and Rehabilitation, 98(10), 1924–1931. 10.1016/j.apmr.2017.06.005 [DOI] [PubMed] [Google Scholar]
- 19.Mani S, Przybyla A, Good DC, Haaland KY, & Sainburg RL (2014). Contralesional Arm Preference Depends on Hemisphere of Damage and Target Location in Unilateral Stroke Patients. Neurorehabilitation and Neural Repair, 28(6), 584–593. 10.1177/1545968314520720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mengotti P, Ripamonti E, Pesavento V, & Rumiati RI (2015). Anatomical and spatial matching in imitation: Evidence from left and right brain-damaged patients. Neuropsychologia, 79, 256–271. 10.1016/j.neuropsychologia.2015.06.038 [DOI] [PubMed] [Google Scholar]
- 21.Muir RT, Lam B, Honjo K, Harry RD, McNeely AA, Gao F-Q, … Black SE (2015). Trail Making Test Elucidates Neural Substrates of Specific Poststroke Executive Dysfunctions. Stroke, 46(10), 2755–2761. 10.1161/STROKEAHA.115.009936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mutha PK, Haaland KY, & Sainburg RL (2012). The effects of brain lateralization on motor control and adaptation. Journal of Motor Behavior, 44(6), 455–469. 10.1080/00222895.2012.747482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poole JL, Sadek J, & Haaland KY (2009). Ipsilateral deficits in 1-handed shoe tying after left or right hemisphere stroke. Archives of Physical Medicine and Rehabilitation, 90(10), 1800–1805. 10.1016/j.apmr.2009.03.019 [DOI] [PubMed] [Google Scholar]
- 24.Rosenbaum David A;Marchak Frank; Barnes Heather J; Vaughan, & Slotta JDJMJ (1990). (PDF) Constraints for action selection: Overhand versus underhand grips. In Constraints for action selection: overhand versus underhand grips (pp. 321-). Retrieved from https://www.researchgate.net/publication/232555677_Constraints_for_action_selection_Overhand_versus_underhand_grips [Google Scholar]
- 25.Rosenbaum DA,A, Marchak F, Barnes HJ, Vaughan J, Slotta JD, & Jorgensen MJ (1990). Constraints for Action Selection - Overhand Versus Underhand Grips. Attention and Performance, (13), 321–342. [Google Scholar]
- 26.Rosenbaum DA, Chapman KM, Coelho CJ, Gong L, & Studenka BE (2013). Choosing actions. Frontiers in Psychology, 4, 273. 10.3389/fpsyg.2013.00273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenbaum DA, & Sauerberger KS (2019). End-state comfort meets precrastination. Psychological Research, 83(2), 205–215. 10.1007/s00426-018-01142-6 [DOI] [PubMed] [Google Scholar]
- 28.Rusconi E, Tamè L, Furlan M, Haggard P, Demarchi G, Adriani M, … Schwarzbach J (2014). Neural correlates of finger gnosis. Journal of Neuroscience, 34(27), 9012–9023. 10.1523/JNEUROSCI.3119-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sainburg RL, Maenza C, Winstein C, & Good D (2016). Motor Lateralization Provides a Foundation for Predicting and Treating Non-paretic Arm Motor Deficits in Stroke. Advances in Experimental Medicine and Biology, 957, 257–272. 10.1007/978-3-319-47313-0_14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaefer SY, Haaland KY, & Sainburg RL (2007). Ipsilesional motor deficits following stroke reflect hemispheric specializations for movement control. Brain : A Journal of Neurology, 130(Pt 8), 2146–2158. 10.1093/brain/awm145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaefer SY, Haaland KY, & Sainburg RL (2009). Hemispheric specialization and functional impact of ipsilesional deficits in movement coordination and accuracy. Neuropsychologia, 47(13), 2953–2966. 10.1016/j.neuropsychologia.2009.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scharoun SM, Gonzalez DA, Roy EA, & Bryden PJ (2018). End-State Comfort Across the Lifespan: A Cross-Sectional Investigation of How Movement Context Influences Motor Planning in an Overturned Glass Task. Motor Control, 22(2), 211–230. 10.1123/mc.2016-0064 [DOI] [PubMed] [Google Scholar]
- 33.Schwartz MF, Faseyitan O, Kim J, & Coslett HB (2012). The dorsal stream contribution to phonological retrieval in object naming. Brain, 135(12), 3799–3814. 10.1093/brain/aws300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seegelke C, Hughes CML, Schütz C, & Schack T (2012). Individual differences in motor planning during a multi-segment object manipulation task. Experimental Brain Research, 222(1–2), 125–136. 10.1007/s00221-012-3203-8 [DOI] [PubMed] [Google Scholar]
- 35.Shewan CM, & Kertesz A (1980). Reliability and validity characteristics of the Western Aphasia Battery (WAB). Journal of Speech and Hearing Disorders, 45(3), 308–324. 10.1044/jshd.4503.308 [DOI] [PubMed] [Google Scholar]
- 36.Stewart JC, Gordon J, & Winstein CJ (2014). Control of reach extent with the paretic and nonparetic arms after unilateral sensorimotor stroke: kinematic differences based on side of brain damage. Experimental Brain Research, 232(7), 2407–2419. 10.1007/s00221-014-3938-5 [DOI] [PubMed] [Google Scholar]
- 37.Sullivan KJ, Tilson JK, Cen SY, Rose DK, Hershberg J, Correa A, … Duncan PW (2011). Fugl-Meyer assessment of sensorimotor function after stroke: standardized training procedure for clinical practice and clinical trials. Stroke; a Journal of Cerebral Circulation, 42(2), 427–432. 10.1161/STROKEAHA.110.592766 [DOI] [PubMed] [Google Scholar]
- 38.Tan C, Tretriluxana J, Pitsch E, Runnarong N, & Winstein CJ (2012). Anticipatory planning of functional reach-to-grasp: A pilot study. Neurorehabilitation and Neural Repair, 26(8), 957–967. 10.1177/1545968312437938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tretriluxana J, Gordon J, & Winstein CJ (2008). Manual asymmetries in grasp pre-shaping and transport-grasp coordination. Experimental Brain Research, 188(2), 305–315. 10.1007/s00221-008-1364-2 [DOI] [PubMed] [Google Scholar]
- 40.Watson CE, & Buxbaum LJ (2015). A distributed network critical for selecting among tool-directed actions. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 65, 65–82. 10.1016/j.cortex.2015.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winter DA (2004). Biomechanics and Motor Control of Human Movement - David A. Winter. Retrieved from https://books.google.com/books?hl=en&lr=&id=_bFHL08IWfwC&oi=fnd&pg=PA2&dq=Winter+DA+(2004)+Biomechanics+and+motor+control+of+human+movement.+Wiley,+New+York&ots=Jmoreu8bS6&sig=2F4I7IJfip1ce36Np2K9BibMng0#v=onepage&q&f=false
- 42.Wong AL, Jax SA, Smith LL, Buxbaum LJ, & Krakauer JW (2019). Movement Imitation via an Abstract Trajectory Representation in Dorsal Premotor Cortex. The Journal of Neuroscience, 39(17), 3320–3331. 10.1523/JNEUROSCI.2597-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wulf G, Shea C, & Park JH (2001). Attention and motor performance: preferences for and advantages of an external focus. Research Quarterly for Exercise and Sport, 72(4), 335–344. 10.1080/02701367.2001.10608970 [DOI] [PubMed] [Google Scholar]
- 44.Wulf Gabriele, Höß M, & Prinz W (1998). Instructions for motor learning: Differential effects of internal versus external focus of attention. Journal of Motor Behavior, 30(2), 169–179. 10.1080/00222899809601334 [DOI] [PubMed] [Google Scholar]
- 45.Wulf Gabriele, McConnel N, Gärtner M, & Schwarz A (2002). Enhancing the learning of sport skills through external-focus feedback. Journal of Motor Behavior, 34(2), 171–182. 10.1080/00222890209601939 [DOI] [PubMed] [Google Scholar]
- 46.Wulf Gabriele, & Prinz W (2001). Directing attention to movement effects enhances learning: A review. Psychonomic Bulletin and Review, Vol. 8, pp. 648–660. 10.3758/BF03196201 [DOI] [PubMed] [Google Scholar]
- 47.Wulf Gabriele, Shea C, & Lewthwaite R (2010, January). Motor skill learning and performance: A review of influential factors. Medical Education, Vol. 44, pp. 75–84. 10.1111/j.1365-2923.2009.03421.x [DOI] [PubMed] [Google Scholar]
- 48.Wunsch K, Weigelt M, & Stöckel T (2015). Anticipatory Motor Planning in Older Adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 72(3), gbv078. 10.1093/geronb/gbv078 [DOI] [PubMed] [Google Scholar]
- 49.Zimmermann KM, Bischoff M, Lorey B, Stark R, Munzert J, & Zentgraf K (2012). Neural Correlates of Switching Attentional Focus during Finger Movements: An fMRI Study. Frontiers in Psychology, 3, 555. 10.3389/fpsyg.2012.00555 [DOI] [PMC free article] [PubMed] [Google Scholar]


