Abstract
Rationale: Ambient air pollution exposure is associated with respiratory morbidity among individuals with chronic obstructive pulmonary disease (COPD), particularly among those with concomitant obesity. Although people with COPD report high incidence of poor sleep quality, no studies have evaluated the association between air pollution exposure, obesity, and sleep disturbances in COPD.
Methods: We analyzed data collected from current and former smokers with COPD enrolled in the Subpopulations and Intermediate Outcome Measures in COPD -Air Pollution ancillary study (SPIROMICS AIR). Socio-demographics and anthropometric measurements were collected, and 1-year mean historical ambient particulate matter (PM2.5) and ozone concentrations at participants’ residences were estimated by cohort-specific spatiotemporal modeling. Sleep quality was assessed with the Pittsburgh Sleep Quality Index (PSQI), and regression models were constructed to determine the association of 1-year PM2.5 (1Yr-PM2.5) and 1-year ozone (1Yr-ozone) with the PSQI score, and whether obesity modified the association.
Results: In 1308 participants (age: 65.8±7.8 years, 42% women), results of regression analyses suggest that each 10µg/m3 increase in 1Yr-PM2.5 was associated with a 2.1-point increase in PSQI (P=0.03). Obesity modified the association between 1Yr-PM2.5 and PSQI (P=0.03). In obese and overweight participants, a 10µg/m3 increase in 1Yr-PM2.5 was associated with a higher PSQI (4.0 points, P<0.01, and 3.4 points, P<0.01, respectively); but no association in lean-normal weight participants (P=0.51). There was no association between 1 Yr-ozone and PSQI.
Conclusion: Overweight and obese individuals with COPD appear to be susceptible to the effects of ambient PM2.5 on sleep quality. In COPD, weight and ambient PM2.5 may be modifiable risk factors to improve sleep quality.
Keywords: copd, air pollution, sleep quality, obesity
Introduction
Air pollution exposure is a major public health concern thought to be an important determinant of lung and sleep health.1-3 More than 4 in 10 Americans live in counties across the nation with unhealthy levels of ozone or particulate matter pollution.4 Air pollution exposure may have implications for sleep quality and health outcomes, especially in patients with respiratory disorders.
Chronic obstructive pulmonary disease (COPD) is a highly prevalent respiratory disorder characterized by poor sleep quality.5-7 Sleep disturbance in COPD patients is largely attributed to nocturnal breathing difficulties, hypoxemia, and medications.6-8 It is unclear, however, if ambient air pollution exposure is also a risk factor for poor sleep quality among COPD patients. Several studies conducted in the general population have revealed that those who live in areas with greater ambient air pollution have poorer sleep quality, particularly those with concomitant sleep apnea.9,10 Given the compromise in lung function, COPD patients may be uniquely vulnerable to the effect of air pollution exposure on sleep quality. In addition, factors such as obesity and smoking may modulate the relationship between air pollution exposure and sleep quality in COPD, since they predispose to sleep apnea,11-13 and are known to be associated with inflammation.14-17
Our goal was to examine the association between ambient air pollution exposure and sleep quality in COPD patients. We analyzed ambient air and sleep quality data collected from the Subpopulations and InteRmediate Outcome Measures In COPD Study (SPIROMICS)18 and SPIROMICS Air Pollution (SPIROMICS AIR) ancillary study. We hypothesized that: (1) ambient air pollution exposure would be associated with worse sleep quality in patients with COPD, and that (2) the association of ambient air pollution exposure and sleep quality would be modified by obesity, such that obese patients are more susceptible to the adverse effects of ambient pollution exposure. We also hypothesized that (3) the association of ambient air pollution exposure and sleep quality would be modified by smoking status, such that current smokers are more susceptible to the adverse effects of ambient pollution exposure.
Methods
Study Population
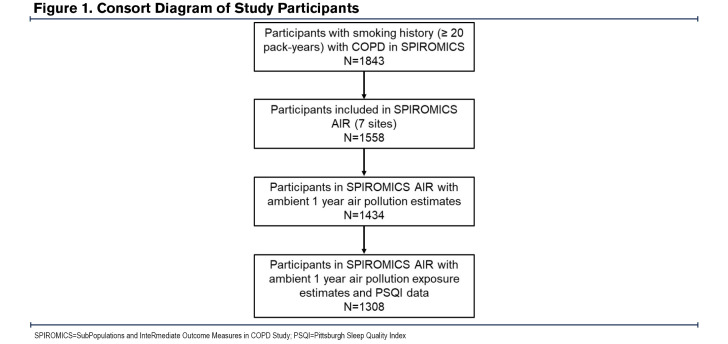
The current cross-sectional analysis was conducted on data collected from current and former smokers (≥20 pack years) with COPD enrolled in the SPIROMICS AIR study.18 Participants were enrolled at 7 (New York City, New York; Baltimore, Maryland; Los Angeles, California; Ann Arbor, Michigan; San Francisco, California; Salt Lake City, Utah; and Winston-Salem, North Carolina) of the 12 SPIROMICS clinical sites.18
SPIROMICS participants who were current or former smokers with COPD (postbronchodilator forced expiratory volume in 1 second [FEV1] to forced vital capacity [FVC] ratio < 70%) and who had available air pollution exposure and sleep quality data were included in our analyses (Figure 1). SPIROMICS was approved by the institutional review board at each of the clinical centers. All study participants provided written informed consent.
Assessment of Ambient Particulate Matter and Ozone
Participant addresses were obtained and geocoded with a geographic information system (ArcGIS 10.3; ESRI). Two-week mean outdoor concentrations of fine particulate matter (PM2.5) and ozone outside each participant’s home were predicted using spatiotemporal modeling methods.19,20 The 2-week air pollution exposures were used to calculate the mean 1-year PM2.5 (1 Yr-PM2.5) and 1-year ozone (1 Yr-ozone) levels,19,20 which we used for our analyses.
Assessment of Sleep Quality
We assessed sleep quality with the Pittsburgh Sleep Quality Index (PSQI). The PSQI is a validated self-rated survey that evaluates sleep quality and disturbances over a 4-week period. It is an 18-item questionnaire comprising 7 sub-sections, each scored 0-3, allowing for total scores ranging from 0-21, with higher scores representing poorer sleep quality.21
Statistical Analyses
Participant characteristics were described using the median with interquartile range (IQR) for continuous variables and n (%) for the categorical variables. We modeled 1 Yr-PM2.5 and 1 Yr-ozone as continuous variables by increments of 10 µg/m3 and 5 parts per billion (ppb) respectively. PSQI was examined as a continuous composite score and also as a dichotomous outcome based on PSQI total scores above and below the threshold for poor sleep quality (> 5 and ≤ 5).21 To address our hypotheses, we examined the association of 1 Yr-PM2.5 and 1 Yr-ozone as the independent variables and PSQI as the dependent variable using linear and logistic regression in unadjusted and adjusted models. In adjusted models, we adjusted for age, sex, race, education, income, current smoking status, smoking pack years, FEV1% predicted, body mass index (BMI) (continuous), and occupational exposure, which refers to self-reported exposure to vapor, gases, dust, and fumes in the longest held job. The adjusted model also included study site and neighborhood poverty rate, which represents the percentage of families below the poverty level in a census tract based on 2010 American Community Survey 5-year estimates.22 We performed sequential covariate adjustments to check against potential over-adjustment and checked for multicollinearity in the fully adjusted model using the variance inflation factor (VIF).23,24 We examined whether BMI category (lean/normal weight, < 25kg/m2; overweight, ≥25kg/m2 and < 30kg/m2; and obese, ≥30 kg/m2) and smoking status modified the association of ambient air pollution exposure and PSQI. Effect modification was tested by introducing an interaction term of categorical BMI and smoking status in the adjusted models.
Since sex-related differences in fat distribution can impact tidal volumes25-27 and potential air pollutant deposition in the lungs, in post-hoc analyses we examined whether sex modified the association of ambient air pollution exposure and PSQI in the 3 BMI categories (lean/normal weight, overweight, and obese).
All analyses were performed using STATA (version 15.1).
Results
Participant Characteristics
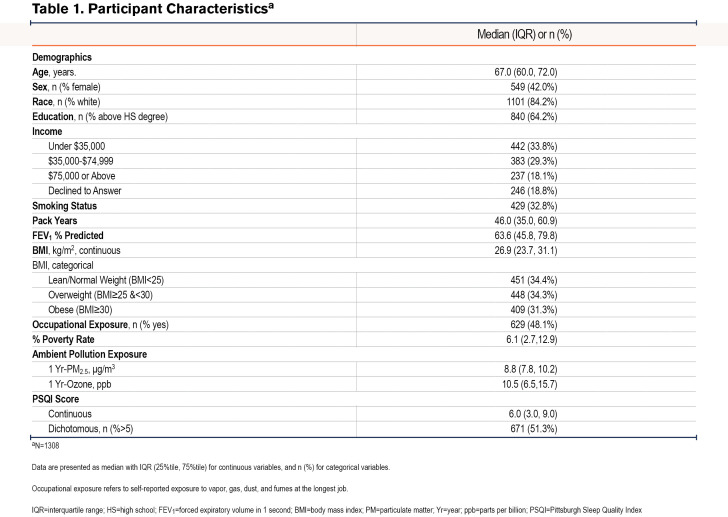
A total of 1308 SPIROMICS AIR participants with COPD had available PSQI scores as well as 1-Yr historical PM2.5 and ozone exposure data. As shown in Table 1, the median age was 67 years and the median BMI was 26.9 kg/m2, with women comprising 42% of the participants. The group had a median 1-Yr PM2.5 exposure of 8.8 μg/m3 and a median 1-Yr ozone exposure of 10.5 ppb. The median PSQI score was 6.0 and approximately half (51.3%) of the participants had PSQI scores greater than 5, which is the validated threshold for poor sleep.21
Association of Ambient Air Pollution Exposure and Pittsburgh Sleep Quality Index
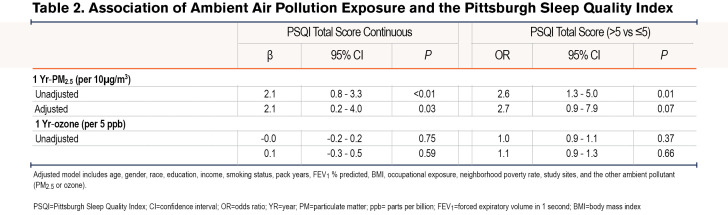
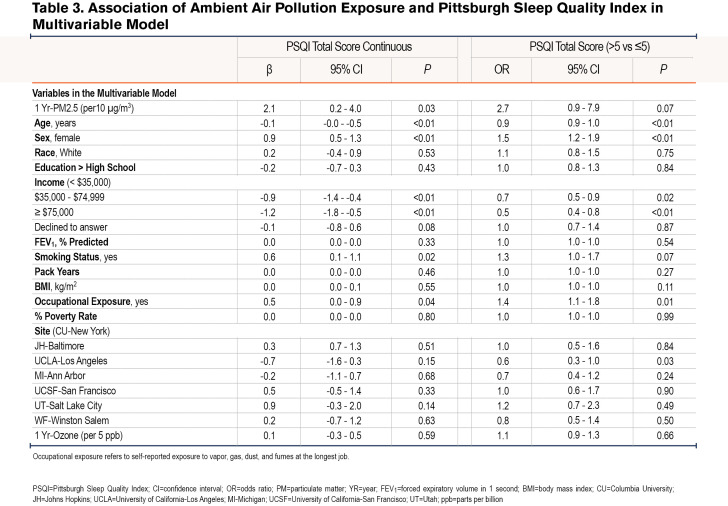
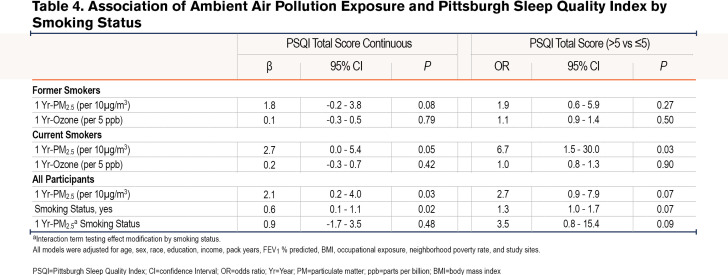
Linear regression analyses revealed a positive association between 1 Yr-PM2.5 and PSQI, such that a 10µg/m3 increase in 1 Yr-PM2.5 was associated with a 2.1 point (95% confidence interval [CI]: 0.8-3.3, P<0.01) increase in PSQI in the unadjusted model, and a 2.1-point increase (95% CI: 0.2-4.0, P=0.03) in the adjusted models. Logistic regression also revealed a positive association between 1 Yr-PM2.5 and the odds of poor sleep quality (PSQI>5) in the unadjusted (OR=2.6, 95% CI: 1.3-5.0, P=0.01), and adjusted (OR=2.7, 95% CI: 0.9-7.9, p=0.07) models. 1 Yr-Ozone was not associated with PSQI either as continuous or categorical (Table 2 and Table 3).
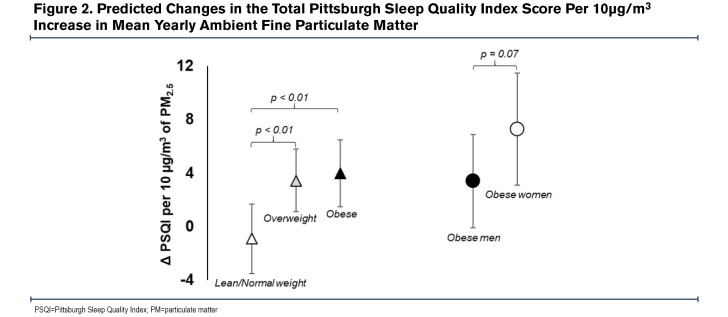
Additionally, in comparison to lean-normal weight, the linear association between 1-Yr PM2.5 and PSQI was amplified among overweight (βinteraction=4.2, 95% CI: 1.5-7.0, P<0.01) and obese participants (βinteraction=4.8, 95% CI: 1.9-7.7, P<0.01). For example, a 10µg/m3 increase in 1 Yr-PM2.5 was associated with a 3.4 point (95% CI: 1.0-5.8, P=0.01) increase among the overweight, a 4.0 point (95% CI: 1.5 - 6.5, P<0.01) increase in PSQI among the obese but was not associated with PSQI among the lean-normal weight (β=-0.8, 95% CI: -3.4-1.7, P=0.52). Similarly, the relationship between 1-Yr PM2.5 and the odds of poor sleep quality was amplified among current smokers compared to former smokers (OR interaction=3.6, 95% CI: 0.8-15.4, P = 0.09), as a 10µg/m3 increase in 1-Yr PM2.5 was associated with a 6 times higher odds of poor sleep quality among current smokers (OR=6.7, 95% CI: 1.5-30.0, P=0.01) but not associated with the odds of poor sleep quality among former smokers (OR=1.9, 95% CI: 0.6-5.9, P=0.27) (Table 4). Within obese participants (n=409), the adverse linear association of 1 Yr-PM2.5 with PSQI tended to be greater among women than among men (βinteraction=3.9, 95% CI: -0.4-8.2, P=0.07), with a 10µg/m3 increase in 1 Yr-PM2.5 associated with a 7.3 point (95% CI: 3.1-11.5, P<0.01) increase in PSQI among women and a 3.4 point (95% CI: -0.1-6.9, P=0.06) increase in PSQI in men. There was no sex difference in the relationship between 1 Yr-PM2.5 and PSQI among overweight (βinteraction=-1.1, 95% CI: -5.1-2.8, P=0.57) or lean-normal participants (βinteraction=1.3, 95% CI: -3.2-5.7, P=0.57). The modifying effect of weight and sex on the relationship between 1 Yr-PM2.5 and PSQI is shown in Figure 2.
The sequential covariate adjustment for checking against potential over-adjustment showed that our results were robust across inclusion of different covariates; and, in our final fully adjusted model, none of our predictors’ VIF exceeded the conventional benchmark23,24 of 4, suggesting no serious multicollinearity issue.
Discussion
In this cross-sectional analysis of ambient air pollution exposure and subjective sleep quality in the SPIROMICS cohort, we found that an increased ambient 1 Yr-PM2.5 but not 1 Yr-ozone was associated with a higher PSQI, indicating worse sleep quality. We also found that the association of 1 Yr-PM2.5 and PSQI was modified by obesity and smoking status, such that overweight-obese participants were more susceptible to the adverse effects of air pollution exposure compared to normal weight adults and current smokers more susceptible compared to former smokers. Furthermore, female obese participants seemed to be more susceptible than male obese participants.
A recent systematic review by Liu et al showed that in a general population cohort, ambient pollution exposure was associated with poor sleep quality.2 Our study confirms this finding in the SPIROMICS cohort, a nationally diverse group of COPD patients. Our results indicate that a 10 µg/m3 increase in 1 Yr-PM2.5 is associated with a 2.1 unit increase in PSQI, a self-reported measure of poor sleep quality, and a 3 times higher odds of having poor sleep quality defined21 by a PSQI score greater than 5. Previous studies have reported a minimum clinically important difference of 3 units for PSQI,28,29 suggesting that an increase of 15 µg/m3 in PM2.5 exposure can result in clinically significant worsening in sleep quality. Although poor sleep quality is prevalent in COPD, our results imply that ambient PM2.5 is a potential contributor.
Associations between ambient PM2.5 and sleep quality in COPD may be driven by several mechanisms. The deposition of particulate matter in the lungs could lead to alveolar inflammation and a worsening of the inefficient gas exchange3 already present in COPD. Inefficient gas exchange will promote hypercapnia and hypoxia increasing respiratory drive and sympathetic activation, which would culminate in micro-arousals and sleep disruption. Air pollutants may also irritate the nasal and pharyngeal mucosa leading to upper airway inflammation, exacerbating sleep apnea, and yielding more breathing difficulties that can disrupt sleep. Alteration of CNS control of sleep, increased depression, and anxiety are other mechanisms by which air pollution exposure may contribute to poor sleep.30,31
Our findings further suggested interactions between weight and ambient PM2.5, sex and ambient PM2.5, and between smoking status and ambient PM2.5. For example, ambient PM2.5 was associated with sleep quality only in overweight-obese participants, suggesting that this COPD subgroup is more vulnerable to the effect of air pollution exposure on sleep. In the overweight-obese patients who may have COPD-obstructive sleep apnea overlap, increased risk for sleep- disordered breathing due to air pollution exposure32 may explain poorer sleep quality. It is also possible that the inflammation provoked by air pollution exposure is synergistic with the chronic inflammatory state of obesity,14-16 thereby promoting sleep-disordered breathing. Overweight-obese persons also have greater minute ventilation given the greater metabolic demand,33,34 possibly increasing the dose of inhaled air pollutants over time.27 Increased minute ventilation may also explain why there was a stronger association of ambient PM2.5 and sleep quality in obese women compared to obese men, since obesity in men is associated with visceral fat deposition unlike subcutaneous adiposity in women,25,26 which is more likely to allow for lung expansion and air pollutant deposition in the lungs.35,36 The lack of an interaction between sex and ambient PM2.5 in the lean/normal weight and overweight BMI categories suggests that differential fat distribution is most likely to impact the relationship of air pollution exposure and sleep quality at BMI levels above 30 kg/m2. On the other hand, increased upper airway inflammation and susceptibility to sleep apnea might explain the stronger association of ambient PM2.5 and sleep quality observed in the current smokers compared to former smokers. Our results also show that current smokers have worse sleep quality compared to former smokers (Table 3 and Table 4), which implies that smoking cessation might improve sleep quality.
Of note, there was no association between ambient ozone and sleep quality, suggesting that the effect of air pollution exposure on sleep may be dependent on the type of air pollutant. The lack of an association could mean that ozone does not irritate the upper airway mucosa, nor does it persist in the alveoli long enough to significantly alter gas exchange nocturnally. It also suggests that ozone does not provoke inflammation as much as PM2.5. In addition, it is possible that ambient ozone levels were not substantial enough to disrupt sleep.
We acknowledge, however, several limitations in interpreting our findings. First, our study lacked objective assessment of sleep and diagnostic validation of sleep-disordered breathing. Recognizing this limitation, it is pertinent that future projects investigating the effect of air pollution on sleep quality, characterize sleep and breathing with gold standard polysomnography. It will also be useful to employ rigorously tested patient-reported sleep assessment tools such as the PROMIS Sleep-Related Impairment and Sleep Disturbance questionnaires that capture unique psychometric domains of sleep quality.37,38 Furthermore, focused assessments like home-based actigraphy may reveal movement disorders and changes in circadian rhythm as potential links between air pollution exposure and sleep health disruption. Second, we did not account for the level of indoor air pollution exposure, which could have been different between participants and, therefore, confounded our results. Finally, we realize that even though environmental air pollution measurement provides an index of patient exposure, it does not represent the dose or volume of air pollutants inhaled. Future studies should, therefore, include methods to estimate the amount of air pollutants deposited in the airways and alveoli, and also quantify tidal volume and minute ventilation. Nevertheless, there are several strengths of this study. Our analysis is based on a large, well-defined, nationally diverse cohort with fine spatiotemporal modeling of ambient air pollution exposure. In addition, this is the first study, to our knowledge, which has evaluated the association between air pollution exposure, obesity, and sleep disturbances in COPD.
Implication
Air pollution exposure may be an important predisposing factor for poor sleep in COPD, with smokers, overweight-obese, and in particular, obese female subgroups, being the most vulnerable. Hence, adopting strategies to mitigate air pollution exposure may improve sleep quality in these vulnerable patient groups. Identification of vulnerable groups in our study also highlights potential mechanisms by which air pollution exposure might affect sleep quality. Future work should, therefore, adopt a multi-dimensional approach to sleep health assessment39,40 to improve the characterization of sleep quality, while investigating the possible role of inflammation, sleep-disordered breathing, and lung mechanics on the effect of air pollution exposure on sleep quality.
Abbreviations
Abbreviations: BMI=body mass index; COPD=chronic obstructive pulmonary disease; CI=confidence interval; FEV1=forced expiratory volume in 1 second; FVC=forced vital capacity; HS=high school; IQR=interquartile range; OR=odds ratio: PMSQ=Pittsburgh Sleep Quality Index, PM2.5=fine particulate matter; ppb=parts per billion; SPIROMICS AIR=SubPopulations and InteRmediate Outcome Measures In COPD Study, Air Pollution ancillary study; VIF=variance inflation factor; Yr=year
Funding Statement
SPIROMICS was supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI), (HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN268200900019C, HHSN268200900020C, U01 HL137880 and U24 HL141762), and grants from the National Institutes of Environmental Health Sciences (NIEHS) (R01ES023500). SPIROMICS was supplemented by contributions made through the Foundation for the National Institutes of Health (NIH) and the COPD Foundation from AstraZeneca/MedImmune; Bayer; Bellerophon Therapeutics; Boehringer-Ingelheim Pharmaceuticals, Inc.; Chiesi Farmaceutici S.p.A.; Forest Research Institute, Inc.; GlaxoSmithKline; Grifols Therapeutics, Inc.; Ikaria, Inc.; Novartis Pharmaceuticals Corporation; Nycomed GmbH; ProterixBio; Regeneron Pharmaceuticals, Inc.; Sanofi; Sunovion; Takeda Pharmaceutical Company; Theravance Biopharma, and Mylan.
References
- 1.Minos D,Butzlaff I,Demmler KM,Rischke R. Economic growth, climate change, and obesity. Curr Obes Rep. 2016;5(4):441-448. doi: https://doi.org/10.1007/s13679-016-0234-7 [DOI] [PubMed] [Google Scholar]
- 2.Liu J,Wu T,Liu Q,Wu S,Chen JC. Air pollution exposure and adverse sleep health across the life course: a systematic review. Environ Pollut. 2020;262:114263. doi: https://doi.org/10.1016/j.envpol.2020.114263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Losacco C,Perillo A. Particulate matter air pollution and respiratory impact on humans and animals. Environ Sci Pollut Res Int. 2018;25(34):33901-33910. doi: https://doi.org/10.1007/s11356-018-3344-9 [DOI] [PubMed] [Google Scholar]
- 4.American Lung Association (ALA). Key findings - state of the air. ALA website. Published 2022. Accessed December 30, 2021. doi: https://www.lung.org/research/sota/key-findings [Google Scholar]
- 5.Shorofsky M,Bourbeau J,Kimoff J,et al. Impaired sleep quality in COPD is associated with exacerbations: the CanCOLD cohort study. Chest. 2019;156(5):852-863. doi: https://doi.org/10.1016/j.chest.2019.04.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McSharry DG,Ryan S,Calverley P,Edwards JC,McNicholas WT. Sleep quality in chronic obstructive pulmonary disease. Respirology. 2012;17(7):1119-1124. doi: https://doi.org/10.1111/j.1440-1843.2012.02217.x [DOI] [PubMed] [Google Scholar]
- 7.Zohal MA,Yazdi Z,Kazemifar AM,Mahjoob P,Ziaeeha M. Sleep quality and quality of life in COPD patients with and without suspected obstructive sleep apnea. Sleep Disord. 2014;2014:e508372. doi: https://doi.org/10.1155/2014/508372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scharf SM,Maimon N,Simon-Tuval T,Bernhard-Scharf BJ,Reuveni H,Tarasiuk A. Sleep quality predicts quality of life in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2011;6:1-12. doi: https://doi.org/10.1186/1465-9921-12-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanobetti A,Redline S,Schwartz J,et al. Associations of PM10 with sleep and sleep-disordered breathing in adults from seven U.S. urban area. Am J Respir Crit Care Med. 2010;182(6):819-825. doi: https://doi.org/10.1164/rccm.200912-1797OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang SC,Schwartz J,Yang M,Yaggi HK,Bliwise DL,Araujo AB. Traffic-related air pollution and sleep in the Boston area community health survey. J Expo Sci Environ Epidemiol. 2015;25(5):451-456. doi: https://doi.org/10.1038/jes.2014.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz AR,Patil SP,Laffan AM,Polotsky V,Schneider H,Smith PL. Obesity and obstructive sleep apnea: pathogenic mechanisms and therapeutic approaches. Proc Am Thorac Soc. 2008;5(2):185-192. doi: https://doi.org/10.1513/pats.200708-137MG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz AR,Patil SP,Squier S,Schneider H,Kirkness JP,Smith PL. Obesity and upper airway control during sleep. J Appl Physiol. 2010;108(2):430-435. doi: https://doi.org/10.1152/japplphysiol.00919.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim KS,Kim JH,Park SY,et al. Smoking induces oropharyngeal narrowing and increases the severity of obstructive sleep apnea syndrome. J Clin Sleep Med. 2012;8(4):367-374. doi: https://doi.org/10.5664/jcsm.2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellulu MS,Patimah I,Khaza'ai H,Rahmat A,Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;13(4):851-863. 8928. doi: https://doi.org/10.5114/aoms.2016.58928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang BY,Qian ZM,Vaughn MG,et al. Overweight modifies the association between long-term ambient air pollution and prehypertension in Chinese adults: the 33 communities Chinese health study. Environ Health. 2018;17(1):57. doi: https://doi.org/10.1186/s12940-018-0401-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCormack MC,Belli AJ,Kaji DA,et al. Obesity as a susceptibility factor to indoor particulate matter health effects in COPD. Eur Respir J. 2015;45(5):1248-1257. doi: https://doi.org/10.1183/09031936.00081414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan S,Ma Z,Jiao M,Wang Y,Li A,Ding S. Effects of smoking on inflammatory markers in a healthy population as analyzed via the gut microbiota. Front Cell Infect Microbiol. 2021;11:633242. doi: https://doi.org/10.3389/fcimb.2021.633242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansel NN,Paulin LM,Gassett AJ,et al. Design of the subpopulations and intermediate outcome measures in COPD (SPIROMICS) AIR study. BMJ Open Respir Res. 2017;4(1):e000186. doi: https://doi.org/10.1136/bmjresp-2017-000186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirwa K,Szpiro AA,Sheppard L,et al. Fine-scale air pollution models for epidemiologic research: insights from approaches developed in the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air). Curr Environ Health Rep. 2021;8(2):113-126. doi: https://doi.org/10.1007/s40572-021-00310-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang M,Keller JP,Adar SD,et al. Development of long-term spatiotemporal models for ambient ozone in six metropolitan regions of the United States: the MESA air study. Atmospheric Environ Oxf Engl. 2015;123(A):79-87. doi: https://doi.org/10.1016/j.atmosenv.2015.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buysse DJ,Hall ML,Strollo PJ,et al. Relationships between the Pittsburgh sleep quality index (PSQI), Epworth Sleepiness Scale (ESS), and clinical/polysomnographic measures in a community sample. J Clin Sleep Med. 2008;4(6):563-571. doi: https://doi.org/10.5664/jcsm.27351 [PMC free article] [PubMed] [Google Scholar]
- 22.Social Explorer. ACS 2010 (5-Year Estimates). Social Explorer website. Published 2010. Accessed August 8, 2022. https://www.socialexplorer.com/tables/ACS2010_5yr [Google Scholar]
- 23.Craney TA,Surles JG. Model-dependent variance inflation factor cutoff values. Qual Eng. 2002;14(3):391-403. doi: https://doi.org/10.1081/QEN-120001878 [Google Scholar]
- 24.Salmerón R,García CB,García J. Variance inflation factor and condition number in multiple linear regression. J Stat Comput Simul. 2018;88(12):2365-2384. doi: https://doi.org/10.1080/00949655.2018.1463376 [Google Scholar]
- 25.Frank AP,de Souza Santos R,Palmer BF,Clegg DJ. Determinants of body fat distribution in humans may provide insight about obesity-related health risks. J Lipid Res. 2019;60(10):1710-1719. doi: https://doi.org/10.1194/jlr.R086975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor RW,Grant AM,Williams SM,Goulding A. Sex differences in regional body fat distribution from pre- to postpuberty. Obesity. 2010;18(7):1410-1416. doi: https://doi.org/10.1038/oby.2009.399 [DOI] [PubMed] [Google Scholar]
- 27.Afshar-Mohajer N,Wu TD,Shade R,et al. Obesity, tidal volume, and pulmonary deposition of fine particulate matter in children with asthma. Eur Respir J. 2022;59(3):13993003. doi: https://doi.org/10.1183/13993003.00209-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinberg M,Mollon B,Kaplan D,Zuckerman J,Strauss E. Improvement in sleep quality after total shoulder arthroplasty. Phys Sportsmed. 2020;48(2):194-198. doi: https://doi.org/10.1080/00913847.2019.1671142 [DOI] [PubMed] [Google Scholar]
- 29.McDonnell LM,Hogg L,McDonnell L,White P. Pulmonary rehabilitation and sleep quality: a before and after controlled study of patients with chronic obstructive pulmonary disease. NPJ Prim Care Respir Med. 2014;24:14028. doi: https://doi.org/10.1038/npjpcrm.2014.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kampa M,Castanas E. Human health effects of air pollution. Environ Pollut. 2008;151(2):362-367. doi: https://doi.org/10.1016/j.envpol.2007.06.012 [DOI] [PubMed] [Google Scholar]
- 31.Pekkanen J,Peters A,Hoek G,et al. Particulate air pollution and risk of ST-segment depression during repeated submaximal exercise tests among subjects with coronary heart disease. Circulation. 2002;106(8):933-938. doi: https://doi.org/10.1161/01.CIR.0000027561.41736.3C [DOI] [PubMed] [Google Scholar]
- 32.Billings ME,Gold D,Szpiro A,et al. The association of ambient air pollution with sleep apnea: the multi-ethnic study of atherosclerosis. Ann Am Thorac Soc. 2019;16(3):363-370. doi: https://doi.org/10.1513/AnnalsATS.201804-248OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chlif M,Keochkerian D,Choquet D,Vaidie A,Ahmaidi S. Effects of obesity on breathing pattern, ventilatory neural drive and mechanics. Respir Physiol Neurobiol. 2009;168(3):198-202. doi: https://doi.org/10.1016/j.resp.2009.06.012 [DOI] [PubMed] [Google Scholar]
- 34.Littleton SW. Impact of obesity on respiratory function. Respirology. 2012;17(1):43-49. doi: https://doi.org/10.1111/j.1440-1843.2011.02096.x [DOI] [PubMed] [Google Scholar]
- 35.Behazin N,Jones SB,Cohen RI,Loring SH. Respiratory restriction and elevated pleural and esophageal pressures in morbid obesity. J Appl Physiol. 2010;108(1):212-218. doi: https://doi.org/10.1152/japplphysiol.91356.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peters U,Dixon AE. The effect of obesity on lung function. Expert Rev Respir Med. 2018;12(9):755-767. doi: https://doi.org/10.1080/17476348.2018.1506331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanish AE,Lin-Dyken DC,Han JC. PROMIS sleep disturbance and sleep-related impairment in adolescents: examining psychometrics using self-report and actigraphy. Nurs Res. 2017;66(3):246-251. doi: https://doi.org/10.1097/NNR.0000000000000217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu L,Buysse DJ,Germain A,et al. Development of short forms from the promis sleep disturbance and sleep-related impairment item banks. Behav Sleep Med. 2011;10(1):6-24. doi: https://doi.org/10.1080/15402002.2012.636266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buysse DJ. Sleep health: can we define it? does it matter? Sleep. 2014;37(1):9-17. doi: https://doi.org/10.5665/sleep.3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung J,Goodman M,Huang T,Bertisch S,Redline S. Multidimensional sleep health in a diverse, aging adult cohort: concepts, advances, and implications for research and intervention. Sleep Health. 2021;7(6):699-707. doi: https://doi.org/10.1016/j.sleh.2021.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]