Abstract
3D printing technology is an emerging technology. It constructs solid bodies by stacking materials layer by layer, and can quickly and accurately prepare bone tissue engineering scaffolds with specific shapes and structures to meet the needs of different patients. The field of life sciences has received a great deal of attention. However, different 3D printing technologies and materials have their advantages and disadvantages, and there are limitations in clinical application. In this paper, the technology, materials and clinical applications of 3D printed bone tissue engineering scaffolds are reviewed, and the future development trends and challenges in this field are prospected.
Keywords: 3D printing technology, 3D printing materials, Bone tissue engineering, Bone tissue engineering scaffolds
Highlights
-
•
Traditional and novel 3D printing technologies.
-
•
Bio-3D printing technology could fit bone tissue engineering scaffolds better.
-
•
4D biological printing is an emerging technology with great development prospects.
1. Introduction
Although bone has a strong ability to repair itself [1], it cannot completely repair large-volume bone defects, nor can it completely repair articular cartilage damage caused by trauma, infection and aging. Bone defect and osteoarthritis are the main reasons for clinical bone repair and transplantation [2]. At present, the main methods of bone transplantation include: autologous bone transplantation, allogeneic bone transplantation, xenograft bone transplantation and artificial bone transplantation. Allogeneic bone transplantation and artificial bone transplantation are prone to immune rejection in the above methods. Compared with other bone sources, autologous bone is the most ideal material for the treatment of bone injury, but the source of autologous bone is limited, and it will cause secondary damage to patients, resulting in poor treatment [3,4], which cannot meet clinical needs.
The proposal of bone tissue engineering provides a new idea for this problem. The basic starting point of bone tissue engineering is to achieve bone repair and regeneration using “induced osteogenesis” rather than simply using “crawling replacement”. Traditional preparation methods include solution casting/ion washing, in-situ molding, electrospinning, phase separation/lyophilization, gas pore forming, etc. [5,6], although these processes have also achieved satisfactory results. However, the precise control of the scaffold material and pore structure cannot be achieved, and the structural shape cannot be completely matched with the anatomical structure of the bone defect, so that the preparation of personalized implants cannot be realized. In recent years, with the in-depth study of bone tissue engineering, the preparation of scaffold materials and their preparation methods have gradually become the focus of research [7].
As a technology that can prepare bone tissue engineering scaffolds, 3D printing effectively makes up for these deficiencies, and has quickly been widely used in scaffold molding. 3D printing technology was first reported by Emanual Sachs of MIT in 1989. It is a kind of rapid prototyping technology, also known as additive manufacturing. Its working principle is based on discrete, accumulation molding theory combined with computer-aided design, numerical control technology, biological materials, etc. It is a new digital molding technology that can accurately and quickly manufactures materials into 1:1 models based on the principle of layered manufacturing and layer by layer superposition [8]. The process of preparing the bone scaffold is as follows: first, the three-dimensional data of the repair site is obtained by CT scan or magnetic resonance imaging, and then the three-dimensional model is “sliced” with CAD software to obtain the data of each layer and imported into the 3D printing system. Finally, the device prepares the bone scaffold by stacking the materials layer by layer according to the layered data.
At present, the research on the application of 3D printing technology in the treatment of bone defect diseases at home and abroad has achieved good results [[9], [10], [11]]. The bone tissue scaffold prepared by 3D printing technology can restore the original anatomical structure of the patient's wound to the greatest extent and precisely adjust the pore size inside the scaffold [[12], [13], [14]]. Based on 3D printing technology, the reconstruction of bone and cartilage combined with active substances such as cells and growth factors have also become available with more and more materials with good biocompatibility, strong osteoinductivity and stable mechanical properties possibly (Fig. 1) [[15], [16], [17], [18]]. This article reviews six mainstream production process, and several mainstream 3D printing materials, and their respective advantages and disadvantages are listed.
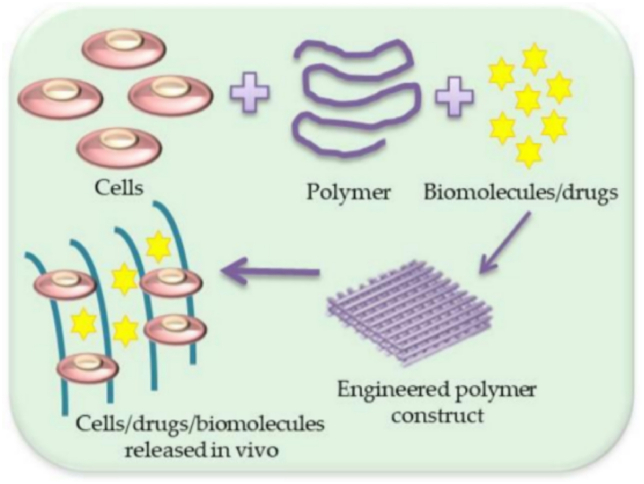
Fig. 1.
Schematic illustration of scaffold with cells/drugs or biomolecules' formation [19].
2. 3D printing bone tissue engineering scaffold technology
At present, the 3D printing technologies used in the preparation of bone tissue scaffolds mainly include Fused Deposition Modeling (FDM), Selective Laser Sintering (SLS), Stereolithography (SLA), Electron Beam Melting (EBM), 3DP technology and biological 3D printing, which has attracted a lot of attention in recent years.
2.1. Fused Deposition Modeling (FDM)
Fused Deposition Modeling, also known as Fused Lamination Modeling, is a technology that heats and melts filamentous hot-melt materials, and at the same time, under the control of a computer, the three-dimensional nozzle selectively coats the material on the workbench according to the cross-sectional profile information. After rapid cooling, a layer of the cross-section is formed. After the formation of one layer is completed, the machine table descends a height (that is, the thickness of the layer) and then forms the next layer until the entire solid shape is formed (Fig. 2).
Fig. 2.
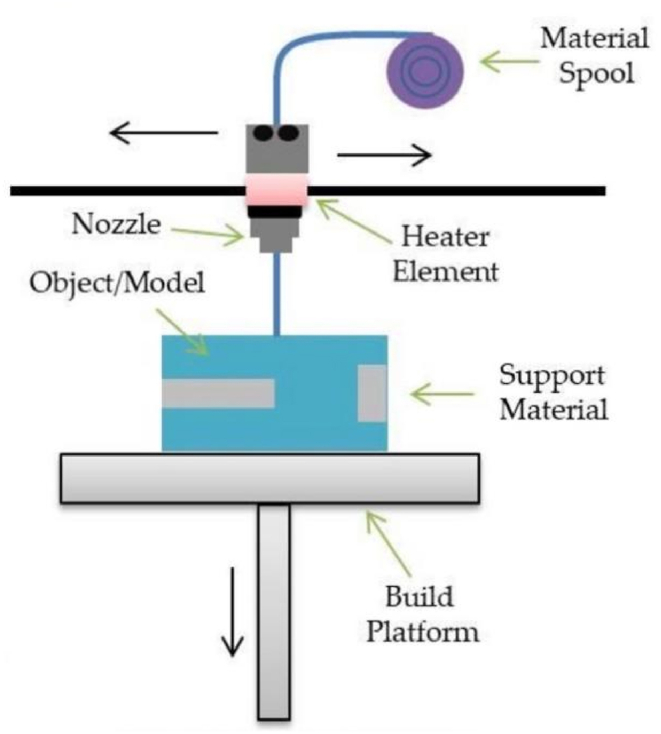
Scheme illustration of FDM [19].
The raw materials used in the fusion lamination technology are usually heat-shrinkable polymers, including ABS, polyamide, polyester, polycarbonate, polyethylene, polypropylene, and the like. Jensen et al. [20] used polycaprolactone as a material to prepare a porous 3D printed scaffold by fused deposition technology and applied it to the study of porcine calvarial defects, and found that the bone defect showed good bone continuity. Abdullah et al. [21]prepared bioceramic teeth with zirconia and β-tricalcium phosphate by fusion deposition technology, and found that the specific craniofacial implant had good mechanical strength and biocompatibility. The advantage of FDM lies in its simple manufacture and low cost, but the printed scaffold is not ideal in terms of accuracy and surface quality, and high temperature may destroy the chemical composition of the raw material. It is not stable enough, so the fusion lamination molding technology is less used in the field of rapid prototyping that requires high precision, and this technology cannot print growth factors, proteins, and cells, which limits its further application in medical scaffolds [[22], [23], [24], [25]].
In recent years, although the pure polyester scaffolds made by the fusion lamination method have a certain osteogenic effect in animal experiments, the mechanical strength and degradability of the scaffolds are still unsatisfactory [26]. Studies have shown that the scaffold made of polyester and inorganic particles has obvious advantages [27]. Schantz et al. [28]used a mixture of polycaprolactone and calcium phosphate as scaffold materials to prepare polycaprolactone-calcium phosphate scaffolds by fusion lamination technology. Compared with the simple polycaprolactone scaffold, the degradation rate and mechanical strength of the hybrid scaffold are significantly improved. Xu et al. [29]used CT-guided fused deposition modeling to prepare polycaprolactone/hydroxyapatite three-dimensional artificial bone, imitating the natural goat femur (Fig. 3). It is a simple, convenient and relatively low-cost method that suitable for making artificial bone. In addition, the polycaprolactone/hydroxyapatite artificial bone prepared by this technology is closer to the mechanics of natural bone, has good cell biocompatibility and biodegradability in vitro, and has appropriate new bone formation ability in vivo. Therefore, polycaprolactone/hydroxyapatite three-dimensional artificial bone may be used in the treatment of patients with clinical bone defects.
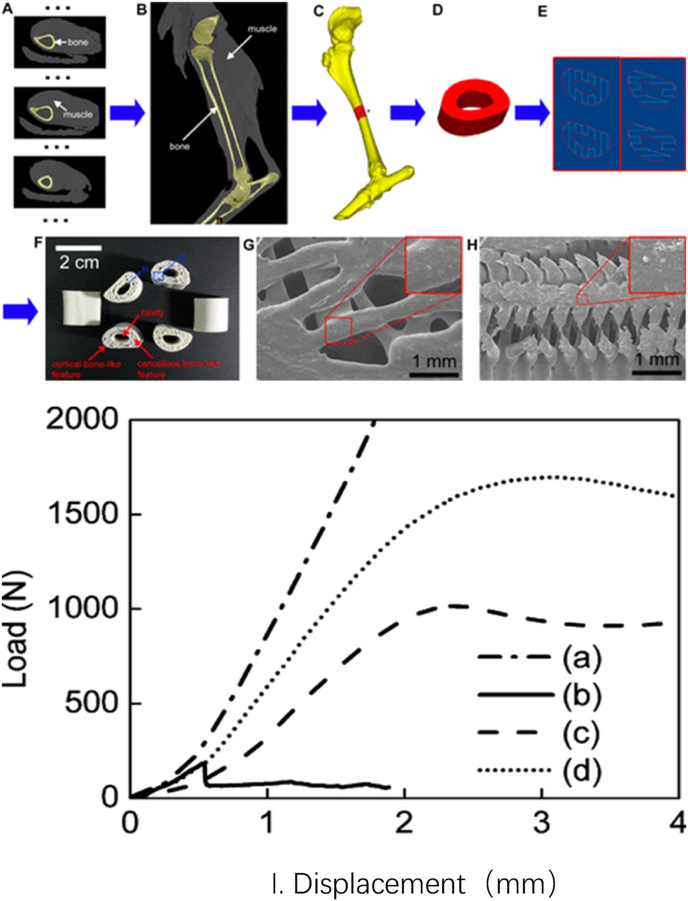
Fig. 3.
Fabrication and characterization of 3D artificial bones. (A) CT data of normal goat leg. (B) Sectional image of 3D model of normal goat leg by CT reconstruction from A. (C) Sectional image of 3D bony structure of goat femur. (D) Typical long (1.5 cm) load-bearing femur bone model. (E) Left and right images show the designed alternate slices used to fabricate 3D artificial bones. (F) The prepared PCL/HA 3D artificial bones. (G) SEM image of PCL/HA 3D artificial bone surface. The upper-right image is magnified from the corresponding area. (H) SEM image of cross-section of PCL/HA 3D artificial bones. The upper-right image is magnified from the corresponding area. (I) Typical force−displacement curves of artificial and natural bones. (a) Adult goat femur, (b) BAM artificial bone, (c) PCL 3D artificial bone, and (d) PCL/HA 3D artificial bone. Reprinted with permission from Xu, N.; Ye, X.; Wei, D.; Zhong, J.; Chen, Y.; Xu, G.; He, D. 3D Artificial Bones for Bone Repair Prepared by Computed Tomography-Guided Fused Deposition Modeling for Bone Repair. ACS Applied Materials and Interfaces2014, 6, 14,952–14963. Copyright 2014 American Chemical Society.
2.2. Selective Laser Sintering (SLS)
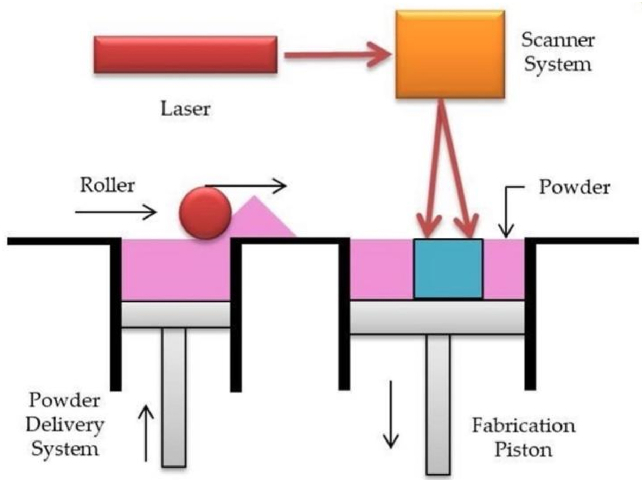
The raw materials of Selective Laser Sintering are mostly powdery substances. At present, the mature process materials are wax powder and plastic powder, and the process of sintering with metal powder or ceramic powder is still under study. During processing, the powder is first preheated to a temperature slightly lower than its melting point, and then the powder is flattened under the action of a leveling stick. Under the control of the computer, the laser beam is selectively sintered according to the information of the delamination section. After one layer is completed, the next layer is sintered. Finally, remove the excess powder. In this way, a sintered part can be obtained (Fig. 4). [[30], [31], [32]].
Fig. 4.
Scheme illustration of SLS [19].
Chen et al. [33] applied selective laser sintering technology combined with AM method to build a three-dimensional polycaprolactone scaffold with good macroscopic and microscopic features. Roskies et al. [34]used selective laser sintering technology to prepare 3D trapezoidal porous scaffolds and implanted them in the mandibular defect of New Zealand rabbits, and found that the scaffolds had a good effect on jaw reconstruction. Wanibuchi et al. [35]reconstructed a three-dimensional temporal bone model trained by skull base surgery through selective laser sintering technology. The model is highly specific to the surgical patient and has great guiding significance for the surgeon's preoperative planning.
In addition, bone tissue engineering scaffold materials should have a highly interconnected porous structure with appropriate mechanical and biological properties. In order to improve the scaffold performance, current researchers mostly use mixed raw materials to make scaffolds. Feng et al. [36]used selective laser sintering to fabricate porous tricalcium phosphate scaffolds. It was found that the doping of zinc oxide improves the mechanical and biological properties of the scaffolds. The data showed that when the content of ZnO was increased from 0 to 2.5%, the scaffold had a better ability to support cell attachment and proliferation, fracture toughness increased from 1.09MPam1/2 to 1.40MPam1/2, and compressive strength increased from 3.01 MPa to 17.89 MPa, it is speculated that the increase of ZnO will lead to the decrease of grain size and the increase of scaffold density. However, with the further increase of ZnO content, the fracture toughness and compressive strength decreased, which may be due to the sharp increase in grain size. In addition, after the simulated body fluid culture, a bone-like apatite layer was formed on the surface of the material, which had osteoinductive and osteoconductive abilities. In conclusion, the porous β-tricalcium phosphate scaffold doped with ZnO composite scaffolds showed good mechanical and biological properties and could be used for bone repair and replacement therapy.
Compared with fused deposition modeling, selective laser sintering has a wider selection of printing materials, and has fast processing speed, no support materials, high precision, and high strength. However, selective laser sintering and fused deposition modeling have the same disadvantages, that is, the properties of biological materials or growth factors will be destroyed during high processing temperature, and the surface of the molded product is rough, so it cannot be used for printing in combination with living cells, etc. [[37], [38], [39]].
2.3. Stereolithography (SLA)
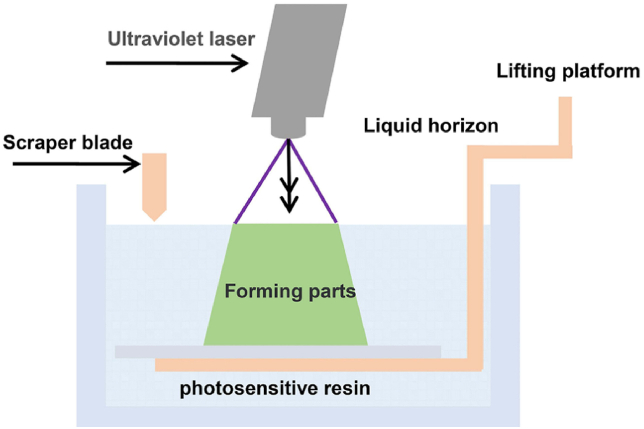
The raw material of stereolithography is photosensitive resin. It controls the laser through the computer, and scans the surface of the liquid photosensitive resin point by point according to the information of each layered section of the part. The thin resin layer in the scanned area is cured by photopolymerization to form a thin layer of the part. After the curing of one layer is completed, the worktable moves down a distance of one layer thickness. Then, a new layer of liquid resin is applied on the surface of the previously cured resin until a three-dimensional solid model is obtained. (Fig. 5). The advantages of stereolithography include short production time and a wide range of products, and high-resolution objects can be printed with complex structures. But it also has drawbacks, including printable materials are limited to liquid resins, which can be toxic, and post-processing is required to clean the impurities [42].
Fig. 5.
The schematic diagram of stereolithography appearance [98].
2.4. Electron Beam Melting (EBM)
Electron beam melting technology is a contemporary advanced technology developed by combining cutting-edge technologies such as numerical control, electronic design, and high-energy electron beams. It firstly processes the three-dimensional digital model of the part in layers to obtain its two-dimensional cross-section information, and then uses the special program for electron beam melting to import the effective information into the electron beam melting equipment to obtain the required three-dimensional products by adding materials layer by layer. Because the electron beam has the characteristics of high power, fast scanning speed, clean and pollution-free, etc., the electron beam melting technology has the advantages of high forming efficiency, safety and environmental protection, and a wide range of applicable materials, especially for the complex forming of difficult-to-process metal materials. Zhang et al. [40]applied EBM to prepare a titanium trabecular bone reconstruction system for the treatment of early femoral head necrosis. The hip joint function of the patients recovered well after the operation, and the long-term follow-up effect was satisfactory. The existence of pores in implants is conducive to the generation of bone defects, but the requirements for pores are not consistent in different parts. The “reproducible” feature of EBM makes it an excellent choice for the preparation of porous materials. Palmquist et al. [41]prepared porous implants by electron beam melting technology and implanted them into the bilateral femur and back of sheep. After 26 weeks, the implants and surrounding tissues were removed, showing excellent long-term soft tissue biocompatibility and high bone integrated effect. The problems of electron beam melting technology include high technical difficulty, high cost, and incomplete supporting software. With the development of numerical control, electronic information, new materials and other fields, electron beam melting technology will also be pushed to the grassroots hospitals for the public.
2.5. 3DP technology
3DP technology uses powder materials to shape, such as ceramic powder, metal powder. These powders are not joined by sintering, but the cross-section of the part is “printed” on top of the material powder by means of a nozzle using an adhesive such as silicone. However, the strength of the parts bonded with the adhesive is low and requires post-processing. The specific process is as follows: After the uppermost layer is bonded, the forming cylinder descends (equal to layer thickness: 0.013–0.1 mm), the cylinder supplying powder rises, pushes out some powder and is pushed to the forming cylinder by the powder spreading roller. These powders are flattened and compacted. Under the control of the computer, the spray head selectively sprays the adhesive construction layer according to the forming data of the construction section below. In this way, the bonding of a three-dimensional powder is finally completed. 3DP technology is easy to operate, the product has the advantages of high porosity, wide application range of raw materials, smooth surface of the scaffold, and cells can be directly attached. It can directly print cells, growth factors, and proteins. The disadvantage is that the mechanical strength of the product is not high [[42], [43], [44]].
Tarafder et al. [45]doped SrO and MgO in tricalcium phosphate as raw materials, and applied 3DP technology to fabricate scaffolds and fill them in rat bone defects. The fabricated scaffolds had good macroscopic pores and internal microscopic pores, and histomorphological analysis showed that new bone formation was significantly increased, which accelerated bone mineralization and accelerated early osteogenic healing. Although 3DP technology has been used to make bone tissue engineering scaffolds, the technology needs further research and improvement. Farzadi et al. [46]studied the effect of the 3DP printing delay on the scaffold performance, and the results showed that the layer printing delay had a significant effect on the compressive strength of the scaffold. This study showed that printing with a 300 m s delay was the optimal printing condition, providing the highest strength and dimensional accuracy. Tarafder et al. [47]imitated the functional properties and structure of natural bone itself, and uses 3DP technology to fabricate a porous titanium scaffold with good biocompatibility. The study of the porosity and porosity of the scaffolds showed that the pore diameter can be adjusted by changing the ratio of binder and sintering temperature. Subsequent cell culture results showed that cells proliferated more densely on 3DP titanium samples than on 2D technology titanium samples. It is foreseeable that by optimizing the ratio of combined binders and the sintering temperature, scaffolds with desired porosity and mechanical properties can be prepared, which are suitable for the intended clinical application.
3. Biological 3D printing
Although the traditional 3D printing technology mentioned above can precisely control the structure and shape of the 3D product, it cannot realize the combination of printing scaffold materials with cells, growth factors, etc. due to the high temperature or specific treatment of the materials during the printing process. In recent years, with the continuous innovation of 3D printing technology, bio-3D printing technology based on absorbable materials, cells and active factors has gradually becomes a printing method that people pay more attention to. At present, bio-3D printing mainly includes three methods: inkjet bioprinting, extrusion bioprinting and laser-assisted bioprinting.
3.1. Inkjet bioprinting
Inkjet bioprinting is one of the commonly used bioprinting technologies developed based on traditional inkjet printing. It uses heat and piezoelectric forces to eject “bioink” droplets from a printhead into a hydrogel or Petri dish under computer control (Fig. 6).
Fig. 6.
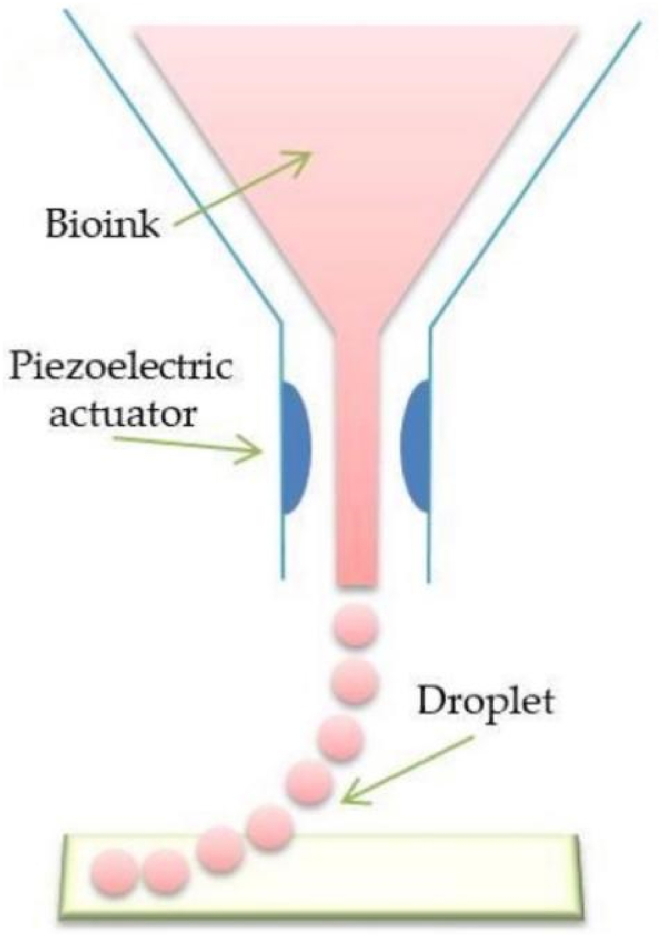
Scheme illustration of inkjet bioprinting [19].
In thermal mode, the inkjet printer uses heat-generated pressure pulses to flow " bio-ink " from the printhead to the substrate. In piezoelectric mode, the piezoelectric sensor generates a pulse that gives the " bio-ink " enough pressure to eject the droplets from the nozzle. In general, inkjet bioprinting has the advantages of high yield, low cost, simple implementation, and compatibility with low-viscosity biomaterials [48]. Therefore, inkjet bioprinting is widely used in preclinical settings and clinical practice. Cui et al. [49]developed a photopolymerization inkjet bioprinting system for 3D cartilage tissue engineering, which can achieve the printing effect of crosslinking while printing, and successfully printed photocrosslinked polymer loaded with human chondrocytes. Ethylene glycol dimethacrylate (PEGDMA)scaffold with a compressive modulus of (395.73 ± 80.40)kPa, which is close to the performance range of natural human cartilage. Quantitative PCR was performed on the scaffold to detect the gene expression of human type I collagen, type II collagen and proteoglycan. It was found that the expression levels of type II collagen and proteoglycan in the 3D printed cartilage scaffold were significantly higher than those in the injection molded cartilage scaffold. This result indicated that the 3D printed cartilage scaffold could promote the secretion of cartilage extracellular matrix and the formation of cartilage. Xu et al. [50]combined electrospinning and inkjet printing to print multi-layer cartilage structures, and the obtained hybrid scaffolds could maintain the in vivo and in vitro activities of the printed cells, while having higher mechanical strength. The results of in vivo experiments of the scaffold showed that it can promote the development of vascular membranes, the proliferation of chondrocytes and the development of lacunae without loss of integrity.
3.2. Extrusion bioprinting
At present, bioprinters generally use extrusion bioprinting technology, which has a relatively mature printing process. During the printing process, the technique utilizes a mechanical piston or air pressure to squeeze an ink-containing syringe, and then extrudes the bioink through a micro-nozzle (Fig. 7) [51].
Fig. 7.
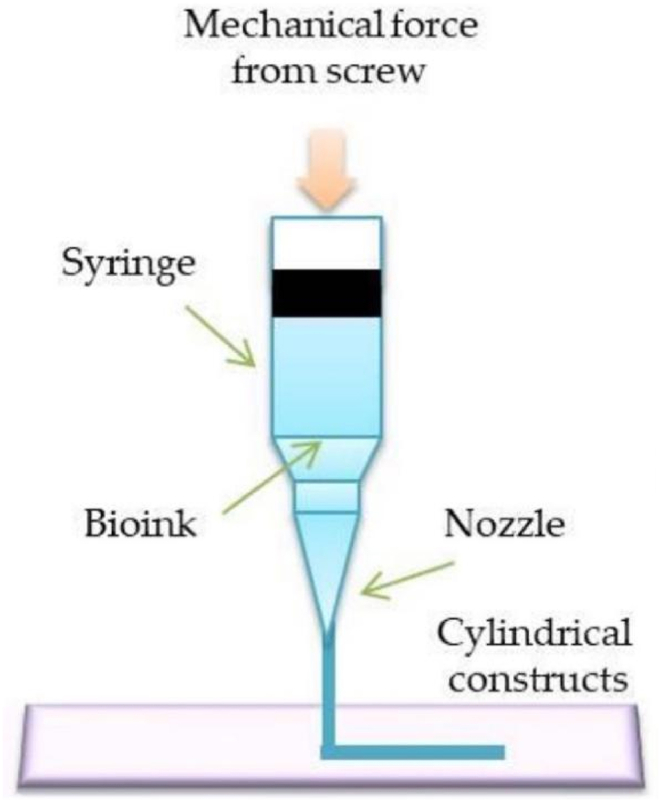
Scheme illustration of extrusion bioprinting [19].
Compared with other technologies, extrusion bioprinting technology is compatible with a variety of materials, printing speed is fast, and high precision [52]. And this technique does not involve a heating process, so cells and bioactive substances can be easily added [53]. Furthermore, compared to inkjet bioprinting, extrusion bioprinting has a wider selection of bioinks available. Therefore, extrusion bioprinting technology is the most commonly used method for cartilage bioprinting. The bioink used in extrusion bioprinting must have sufficient viscosity and cross-linking ability to maintain a good three-dimensional structure during and after printing. Kang et al. [54]developed an extruded tissue-organ printer (ITOP), which used living cells as ink for the first time to print human-sized organs and tissues. They combined 3T3 fibroblasts with PCL and used ITOP to print the mandibular and auricular cartilage structures. The cell survival rate was ≥95% 1 h after printing. The results of the cell proliferation assay of the scaffold showed that the cells proliferated normally within 15 days, which was similar to the proliferation results of the control cells encapsulated in the fibrin structure. These data show that the optimized cell printing system can maintain the viability of cells during the printing process and provide a good microenvironment for cell proliferation.
3.3. Laser assisted bioprinting
Laser assisted bioprinting relies on pulsed laser beams to generate pressure perturbations that then transport the cell-containing material to a receiving substrate. (Fig. 8). Due to the nozzleless design, laser-assisted bioprinting has never encountered nozzle-related technical difficulties such as nozzle clogging. This feature eliminates the problem of shear stress-induced cell damage and death in inkjet and extrusion bioprinting when the nozzle diameter is very small or when the bioink is very viscous. Therefore, The author believes that the outstanding advantage of this method is that it is compatible with a range of viscosities of biomaterials. Another major advantage of laser-assisted bioprinting is its high resolution. The process can also accommodate higher cell densities for better control over cell-to-cell interactions and high-definition patterns of cells. However, this method is rarely used in cartilage tissue engineering due to its high cost.
Fig. 8.
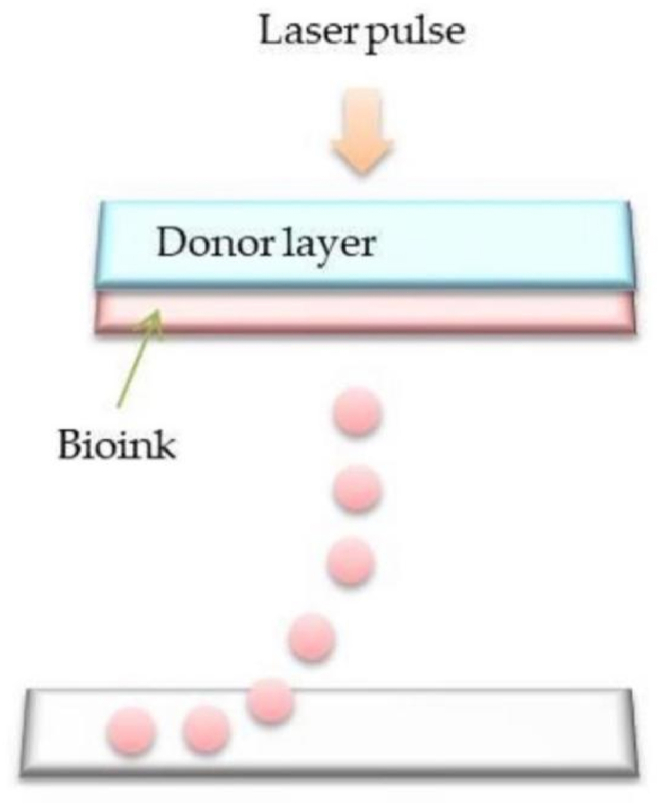
Scheme illustration of laser-assisted bioprinting [19].
In recent years, 3D bioprinting technology has entered a bottleneck stage. At the technical level, there are problems such as the lack of bioinks with high biocompatibility, and the printing accuracy still cannot fully reach the micron-level resolution of the real tissue structure. At the clinical application level, although 3D bioprinting is widely used in the manufacture of cells and even tissues and organs, most of the organs printed are structural or single-function realizations, and the printing of complex organs has not been fully realized.
However, with the development of bioprinting technology, microsphere bioprinting technology has been gradually developed and added to the method of bioprinting. The advantage of microsphere bioprinting technology is that it can provide printing of high cell density structures. Many disease states are difficult to faithfully represent when individual cells are dispersed in the gel. Prefabricated spheres can be fused into tissue chains, which are then extruded into larger 3D structures on their own. Spherical bioprinting holds great promise for developing organ and tissue models.
Table 1 compares the other differences of the above three bioprinting technologies, and Table 2 compares the advantages and disadvantages of different 3D printing technologies.
Table 1.
Unique features of the major 3D bioprinting technologies [60].
| Print methods | Advantage | Bioinks | Resolution | Cell viability | Cell density | Print speed | Target tissue |
|---|---|---|---|---|---|---|---|
| Inkjet Printing | High yield, low cost, high resolution, simple implementation and compatibility with low viscosity biomaterials | Collagen, poly (ethylene glycol) dimethacrylate (PEGDMA), fifibrinogen, alginate, GelMA | high | high | Low | high | Skin, cartilage, bone, tumor, liver |
| Extrusion Printing | Compatible with a variety of materials, high resolution, high precision | Gelatin, poly-caprolactone (PCL), polyethyleneglycol (PEG), alginate, hyaluronic acid (HA), polyamide (PA), polydimethyl-siloxane (PDMS) dECM, nanocellulose | medium | medium | Cell spheroid | Low | Skin, cartilage, vessel, bone, muscle, tumor, heart |
| Laser-assisted | Fast print speed and compatibility with a range of biomaterial viscosities | Printing Fibrinogen, collagen, GelMA | Low | high | high | medium | Skin, Vesse |
Table 2.
Different types of 3D printing techniques, and their pros and cons.
| Types | Advantage | Limitations | References |
|---|---|---|---|
| Fused Deposition Modeling (FDM) | Simple and low cost to manufacture, thermoplastic polymers are extruded without toxic organic solvents | Insufficient precision and strength, poor degradability, inability to print growth factors and cells | [[21], [22], [23], [24]] |
| Selective Laser Sintering (SLS) | Wide range of raw materials, fast processing speed, no need to use supporting materials, high precision and high mechanical strength. | The molded product has a rough surface, cannot be used for printing with living cells, and sintering can modify material properties | [[36], [37], [38], [39]] |
| Light curing molding (SLA) | Short production time and a wide range of products, and high-resolution objects can be printed with complex structures | Printable materials are limited to liquid resins, which can be toxic, and post-processing is required to clean the impurities | [42] |
| Electron Beam Melting (EBM) | High forming efficiency, safety and environmental protection, wide range of raw materials | The technical difficulty is high, the cost is high, and the supporting software is not complete | [46,47] |
| 3DP technology | Easy to operate, products with high porosity, wide range of raw materials, smooth scaffold surface, and cells can directly adhere | The mechanical strength is not high, and the product needs to be post-treated | [[48], [49], [50]] |
4. 3D printed bone tissue engineering scaffold materials
Among the 3 basic elements of bone tissue engineering (seed cells, scaffold materials and growth factors), scaffold materials undoubtedly play a pivotal role. Because on the one hand, it is a carrier of signaling molecules or target cells, and on the other hand, it also provides a scaffold for new bone growth.
In bone tissue engineering, the ideal scaffold material should have the following conditions: ①Osteoconductivity: the ability of the material to provide a channel or medium for the growth of new tissue. ②Osteoinductive: The material can stimulate the growth of bone tissue. ③Good biocompatibility: The material can promote the adhesion, proliferation and differentiation of seed cells. ④Good biodegradability. ⑤Sufficient mechanical properties. ⑥Three-dimensional porous structure: can provide space for the growth of seed cells ⑦Simple to process and sterilize [55]. And for the design of scaffolds, the following three aspects should be considered: ①It can provide the basis for cell adhesion, differentiation, proliferation and migration. The pore size and structure, porosity, and surface chemistry of scaffolds are influencing factors. ②Have suitable mechanical strength. ③Conform to the anatomical morphology of the replacement part [56,57]. In addition, it is generally believed that the porosity of 3D bone scaffolds should be greater than 40%–60% to facilitate the rapid diffusion of cells and the flow of cell nutrients, as well as the transfer of cells [58]. Therefore, when designing and preparing scaffolds, attention should be paid to the requirements of bone tissue engineering scaffolds, and the most appropriate materials should be selected.
The relationships between the properties of the scaffolds (porosity, surface area, and elastic modulus) and mechanical and biological factors are revealed in Table 3 [59].
Table 3.
Scaffold properties linked to mechanical and biological factors.
| Scaffold | Mechanical factors | biological factors |
|---|---|---|
| Porosity | Negative correlation. Increased porosity leads to decreased mechanical properties. | Positive correlation. Increased porosity improves biological activity, including cell growth and the transport and distribution of nutrients. |
| Surface area | Negative correlation. Increased surface area leads to faster degradation of scaffolds. | Positive correlation. Increased surface area can increase initial adhesion of cells. |
| Elastic modulus | Positive correlation. The increased elastic modulus makes the scaffold stronger and avoids undue deformation. | Changes in elastic modulus lead to different mechanical stimuli, which in turn alter tissue growth rate and type. |
Currently, the more commonly used 3D printing materials include metal materials, bioceramics, and composite materials composed of multiple materials [60].
4.1. Metallic material
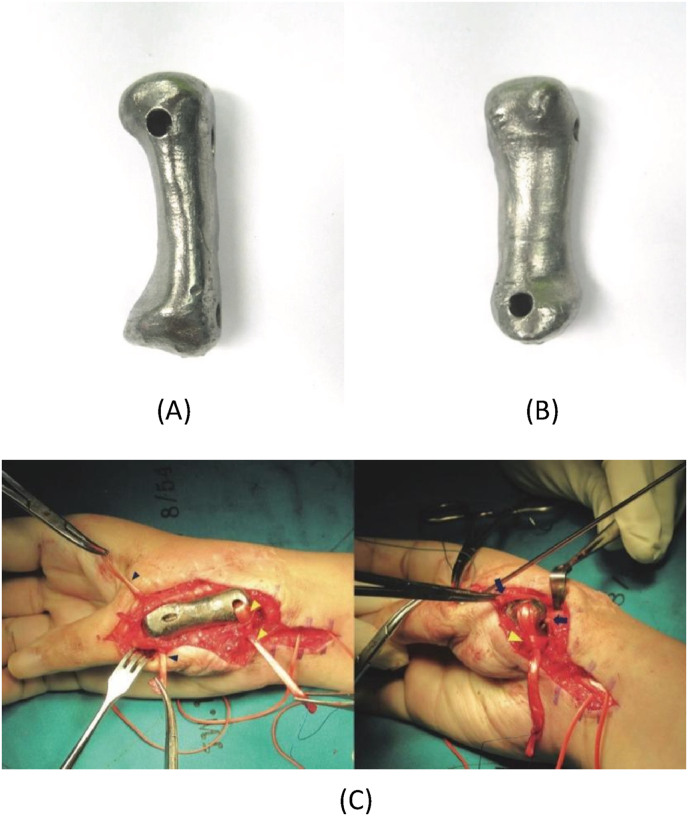
Metal materials are one of the most widely used 3D printing materials in clinical practice. The most common one is titanium alloy, which is widely used in the treatment of clinical bone defects due to its light weight and high strength. The biggest feature of medical implant materials is that there are large individual differences in size and shape, complex structure, and rich tiny details. It is difficult for metal scaffolds manufactured by traditional technology to fully match the characteristics of patients. The 3D printing technology is fast, efficient, and has a fine structure through computer simulation and direct printing according to the macro/micro features of the desired object. Such a technology is believed to have extremely broad prospects in clinical treatment. Porous bone scaffolds prepared from titanium alloy materials have good biocompatibility and have a very good effect on promoting the proliferation and differentiation of osteoblasts [61]. Choi et al. [62]constructed the patient's head model with CT scans and determined the surgical plan, and implanted 3D-printed pre-fabricated titanium implants into the defected skull and fixed them, which effectively reduced the operation time of the operation, and there was no surgical trauma during postoperative follow-up. Infection, the patient healed well. Fig. 9 shows the photos of the 3D printed titanium alloy prosthesis before and after implantation.
Fig. 9.
Photographs of the prosthesis before implantation: (A) anterior aspect. (B) Volar aspect. (C) Intraoperative photographs showing 3D printed titanium first metacarpal prosthesis with the ligament reconstruction in the proximal and distal portions: free palmaris longus tendon graft (blue arrowhead), flexor carpi radialis tendon (yellow arrowhead), and extensor pollicis brevis tendon (blue arrow) [63]. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Although metal materials are the most widely used, the printing process needs to be carried out at a high temperature, and further research is needed to maintain cell activity and function for a long time.
4.2. Non-metallic materials
4.2.1. Bioceramics
Bioceramic materials are bone repair materials with good osteoconductivity represented by tricalcium phosphate, calcium phosphate, and hydroxyapatite. The main components of these materials are similar to the inorganic components of human bone, and they have good degradability and the ability to promote new bone formation [64]. Bone scaffolds made of biphasic calcium phosphate (a mixture of hydroxyapatite and tricalcium phosphate) have an excellent ability to promote osteogenic differentiation of cells [65]. Wang et al. [66]fabricated a hydroxyapatite/chitosan composite porous scaffold by 3D printing technology after blending hydroxyapatite and chitosan, and added type I collagen to the scaffold. Through animal experiments, they found that the hydroxyapatite/chitosan composite porous scaffold added with collagen type I could greatly increase the secretion of alkaline phosphatase and promote osteogenesis.
At present, the 3D printing technologies of ceramic scaffolds mainly include sintering 3D printing and room temperature/low temperature 3D printing.
-
•
Sintered 3D printed ceramic scaffold: The most common method for preparing ceramic bone tissue scaffolds is to print scaffolds of custom shape and pore size, followed by high temperature sintering to remove all organic phases to form pure ceramic scaffolds. Dimensional shrinkage may occur after sintering, but the mechanical strength and Young's modulus can be greatly improved. Song et al. [67] used low-temperature 3D printing + sintering to fabricate bone tissue engineering scaffolds. The scaffold has a porous structure (the macroscopic pores and micropores on the surface of the scaffold are interconnected) and superior compressive strength. Chen et al. [68] constructed lithium calcium silicate crystalline biocarriers with dual bioactivity through a 3D printing composite sintering process for osteochondral interface reconstruction. The scaffold has strong mechanical strength. In the scaffold, mesenchymal stem cells can undergo osteogenic differentiation, and chondrocytes can undergo chondrogenic differentiation in vitro and in vivo.
-
•
Room temperature/low temperature 3D printed ceramic scaffold: In addition to high-temperature sintered bone tissue engineering scaffolds, more and more researchers have begun to use room temperature/low temperature 3D printing to prepare bone tissue engineering scaffolds. Song et al. [69]reported the fabrication of platelet-rich fibrin-loaded nano-biphasic calcium phosphate nBCP/PVA (nBCP: PVA = 84:16) composites by extrusion-based low-temperature 3D printing. The scaffold has better in vitro biocompatibility and biological activity, and improves the adhesion, proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells. And in the rabbit model of large segmental bone defect, more new bone formation was observed.
To further provide additional functionalities to bioceramic scaffolds, post-processing including coatings and post-adsorption of functional agents are widely employed. Kim et al. [70]used the PCL emulsion coating method to add bone morphogenetic protein (BMP-2)-loaded PLGA nanoparticles to the surface of HA scaffolds. BMP-2/PLGA nanoparticles were uniformly distributed on the scaffold surface, and BMP-2 was gradually released. In addition, the PCL coating improves the compressive strength of the scaffold. Coating scaffolds with PCL-BMP-2/PLGA nanoparticles can improve cell proliferation, adhesion, osteogenic differentiation in vitro and new bone formation in vivo.
The sintered 3D-printed ceramics exhibit superior mechanical properties, which can better support the osteogenic and chondrogenic differentiation of cells as scaffolds for bone tissue engineering. The room temperature/low temperature 3D printing ceramic scaffold makes the ceramic scaffold more acceptable to researchers. Compared with ordinary experimental conditions, more researchers can reproduce the experiment in their own laboratory, making room temperature/low temperature 3D printing. The rapid development of ceramic scaffolds, and the adsorption of agents on ceramic scaffolds also provides more possibilities for the diversification of research and applications.
4.2.2. Polymer material
Polymer materials are mainly divided into natural polymers and synthetic polymers. Natural polymer: Natural macromolecular polymers, also known as naturally derived biomaterials, are materials produced by living organisms, such as collagen, chitosan, hyaluronic acid, sodium alginate, and fibrin, which are eventually degraded into carbon dioxide and water by microorganisms. Naturally derived biomaterials have the advantages of only causing a mild inflammatory response in vivo, good biocompatibility, wide sources, easy access to materials, and good plasticity [71].
Collagen is a natural polymer found in skin, bones, tendons and ligaments. Collagen for biomedical applications is derived from animals such as pig or bovine skin, rat tail, demineralized bovine bone or rabbit bone. Collagen has high swelling properties, low antigenicity, cytocompatibility, and tissue regeneration potential, and due to these properties, collagen is considered as an ideal material for bone tissue engineering applications. However, due to the poor mechanical strength of pure collagen, it cannot be directly used as a bone substitute material, so the research on composite scaffolds of collagen and other materials has received extensive attention [72]. Cunniffe et al. [73] fabricated 3D collagen-hydroxyapatite nanocomposite scaffolds using suspension and immersion methods, and the compressive modulus was 18 and 12 times higher than that of collagen scaffolds, respectively. Four weeks after the scaffold was implanted into the rat femur, new bone tissue was seen forming in the pores, which was comparable to the biodegradability of the implant. ZHOU et al. [74]developed a collagen hydroxyapatite scaffold with a 3-layer structure. The collagen network was implanted into porous calcium phosphate ceramics by vacuum infusion method, and then encapsulated by biomimetic mineralization, which was compared with ordinary porous calcium phosphate. Compared with ceramic scaffolds, this 3-layer scaffold has better mechanical strength and faster and greater osteogenesis rate when implanted in rabbit dorsal muscle in vitro. These studies show that collagen is an important material for the modification of bone tissue engineering scaffolds and can improve the biocompatibility of composite scaffolds.
Chitosan is an abundant biological material derived from crustacean shells [75]. It is obtained by alkaline hydrolysis from the total or partial deacetylation of chitin. It is a biodegradable, biocompatible, antigen-free, non-toxic, biologically functional material, so it has been used to make various scaffolds and has been widely studied in the field of bone regeneration. However, because chitosan is insoluble in water, rapidly degraded in vivo, has poor blood compatibility and antibacterial properties, its potential for repairing bone defects is limited. The functionalization of the chitosan structure was achieved by compounding with various materials, addressing these limitations; the addition of bioceramic materials to the polymer can improve its mechanical properties. Zhang et al. [76]added nano-hydroxyapatite to chitosan, the compressive strength increased by 33.07%, and the proliferation of mouse osteoblasts (MC3T3-E1) was enhanced. Madhumathi et al. [77]combined chitosan hydrogel film with nano-hydroxyapatite, which significantly improved the crystallinity of the composite and showed good biocompatibility with MG63 cells. Wang et al. [78]treated chitosan/nano-hydroxyapatite with cold plasma, and the scaffold surface was rough with good wettability, which selectively enhanced the adsorption of fibronectin and vitamin C protein. This modification resulted in more infiltration of mesenchymal stem cells into the scaffold and increased collagen deposition and mineralization after 3 weeks. These studies show that the limitation of chitosan can be solved by compounding with other materials and become a suitable scaffold preparation material for bone tissue engineering.
Synthetic high polymer. Such materials include polylactic acid (PLA), polycaprolactone (PCL), polyglycolic acid (PGA) and their copolymers (PLGA). This type of polymer is a thermoplastic material, which can be processed into various structural shapes, and the mechanical properties and degradation speed of the material can be adjusted and controlled by adjusting and increasing the molecular weight, selecting different polymerization methods and molding methods. Because of their non-toxic degradation products and good biocompatibility, PLA and PGA have been approved by the US FDA as implants. Vacanti et al. [79]first used PGA and PLA as chondrocyte culture matrix materials in vitro, and successfully obtained new cartilage through tissue engineering. Sherwood et al. [80]prepared a cartilage-bone composite scaffold with PLGA/PLLA as the upper layer and PLGA/TCP as the lower layer by 3D printing technology. The study found that chondrocytes were more inclined to adhere to the cartilage scaffold area of the scaffold, and the formation of cartilage tissue could be seen after 6 weeks of culture. The mechanical strength of the osteogenic region of the scaffold can reach the same order of magnitude as that of human new cancellous bone. This study provides a new approach to complete joint reconstruction techniques. Tay et al. [32]used 3D printing technology to make quasi-scaffolds from the mixed powder of polycaprolactone and polyvinyl alcohol, and then used particle filtration to remove the polyvinyl alcohol to obtain porous scaffolds. The filtered scaffold is loose and soft, and the pore structure has high connectivity. Chou et al. [81]used 3D printing technology to prepare an antibiotic-embedded polylactic acid/polylactic acid-polyglycolic acid porous scaffold and used it for the reconstruction of the rabbit femoral shaft, and found that the scaffold implantation site had better cortical integrity, maximum Bending strength and cartilage proliferation effect.
The water solubility of synthetic polymers is poor, so organic solvents (such as chloroform) are needed to dissolve them. Chloroform is a toxic solvent that can cause toxic effects when left in the body. Although the use of chloroform extraction techniques can reduce chloroform, there is still a risk of chloroform remaining in the scaffold [82]. In addition, the use of organic solvents obviously increases the cost and difficulty of production, which makes it difficult for large-scale mass production of medical-grade scaffold materials.
4.2.3. Composite materials
In order to meet the requirements of making perfect 3D printed bone repair materials, composite materials combining two or more materials such as high molecular polymers, metals, and bioceramics have become a new breakthrough for 3D printing bone materials. Bone tissue scaffolds composed of high molecular polymers and bioceramics are widely used in 3D printing bone repair materials because they are similar to natural bone matrix [83]. Matsuo et al. [84]used synthetic polymer polylactic acid and hydroxyapatite as raw materials to prepare absorbable porous scaffolds for mandibular reconstruction. By comparison, they found that compared with the traditional titanium alloy scaffolds, the polymer Polylactic acid/hydroxyapatite scaffolds have a better repair effect. Some studies have also found that the materials prepared by blending tricalcium phosphate and high molecular polymers can greatly promote the proliferation and differentiation of human mesenchymal stem cells [85,86]. In addition, the composite material combined with cytokines and proteins with osteogenic induction can further enhance the effect of 3D printed bone scaffolds in bone repair. But there are still some difficulties restricting the development of composite materials. For example, there is no unified standard for 3D printing materials in China, and many materials are still imported, which makes their manufacturing costs high. Therefore, it is difficult for 3D printing technology to be widely used in primary hospitals and serve the public. Therefore, the current research and development of polymer 3D printing materials and their printing technology should gradually enter the track of systematization and standardization, and improve the application standards. In addition to improving the original materials, we must also actively develop new materials.
Table 4 summarizes the different types of 3D printing materials, and their pros and cons mentioned above. At the same time, the table illustrates their corresponding mechanical properties, animal experience and human cell test.
Table 4.
Different types of 3D printing materials, and their pros and cons.
| Types | Material example | Advantage | Limitations | Mechanical properties | Animal experiment | human cell test | clinical market | |
|---|---|---|---|---|---|---|---|---|
| metallic material | Tantalum, titanium, magnesium alloys | Light weight, high strength, and good biocompatibility [87] | Cumbersome manufacturing process and slow osteogenesis | Wang et al. [88]prepared a 3D multi-dimensional porous tantalum scaffold that simulated bone trabecular structure, with a porosity range of 60%–80%, a pore size range of 200–500 μm, and an elastic modulus range of 0.5–4.0 Gpa | Wang et al. [88] prepared a 3D multi-dimensional porous tantalum scaffold simulating trabecular bone structure, and used the scaffold to repair femoral shaft defects in dogs, proving that the new porous tantalum scaffold has excellent biocompatibility. And osteoinductive, with potential for bone tissue engineering applications. | Clainche et al. [89]studied the adhesion, growth and proliferation of human adipose stem cells on the surface of titanium-modified scaffolds. The experimental results show that adipose stem cells can not only grow, proliferate and maintain the osteogenic potential on its surface. | – | |
| -non-metallic material | Bioceramics | Tricalcium Phosphate, Calcium Phosphate, Hydroxyapatite | Similar to the mineral composition of human bone, it has good degradability, strong ability to promote new bone formation, and good osteoconductivity, compressive strength and osseointegration effect [90,91] | Slow degradation and high brittleness | Song et al. [67] used low-temperature 3D printing + sintering to fabricate a bone tissue engineering scaffold with a hierarchical porous structure (the macroscopic pores and micropores on the surface of the scaffold are interconnected) and superior compressive strength. | Song et al. [69]reported the preparation of platelet-rich fibrin-loaded nano-biphasic calcium phosphate nBCP/PVA (nBCP:PVA = 84:16) composites by extrusion-based low-temperature 3D printing. The scaffold had better in vitro biocompatibility and bioactivity, and more new bone formation was observed in the rabbit large segmental bone defect model. | Wang et al. [66] prepared hydroxyapatite/chitosan composite porous scaffolds by 3D printing technology after blending hydroxyapatite and chitosan, and added type I collagen to the scaffolds, and found through animal experiments. The hydroxyapatite/chitosan composite porous scaffold added with type I collagen can greatly increase the secretion of alkaline phosphatase and promote osteogenesis. | – |
| Natural high molecular polymer material | Collagen, Chitosan, Hyaluronic Acid, Sodium Alginate and Fibrin | It only causes a mild inflammatory response in the body, has good biocompatibility, It only causes a mild, is easy to obtain, and has good plasticity [71] | Chitosan is insoluble in water, rapidly degraded in vivo, has poor blood compatibility, and has poor antibacterial properties, and its potential to repair bone defects is limited [82] | Zhang et al. [76] added nano-hydroxyapatite to chitosan, the compressive strength increased by 33.07% | Zhang et al. [76] enhanced the proliferation of mouse osteoblasts (MC3T3-E1) after adding nano-hydroxyapatite to chitosan. | Catanzano et al. [92]fabricated macroporous alginate foam with excellent porosity, which enhanced the growth and osteogenic differentiation of human mesenchymal stem cells. | – | |
| Artificial high molecular polymer material | Polylactic acid (PLA), polycaprolactone (PCL), polyglycolic acid (PGA) and their copolymers (PLGA) | It has strong plasticity and good biocompatibility. The mechanical properties and degradation rate of the material can be adjusted and controlled by adjusting and increasing the molecular weight, selecting different polymerization methods and molding methods, and the degradation products are non-toxic [93,94]. | Relatively poor mechanical properties, poor water solubility and lack of cellular recognition sites [95] | Sherwood et al. [87] prepared a cartilage-bone composite scaffold with PLGA/PLLA as the upper layer and PLGA/TCP as the lower layer by 3D printing technology. The study found that the mechanical strength of the osteogenic region of the scaffold can reach the same order of magnitude as that of human new cancellous bone. | Chou et al. [81] used 3D printing technology to prepare an antibiotic-embedded polylactic acid/polylactic acid-polyglycolic acid porous scaffold for the reconstruction of rabbit femoral shaft, and found that the scaffold implanted site had better cortical integrity, maximum Bending strength and cartilage proliferation effect. | Vacanti et al. [79] first used PGA and PLA as chondrocyte culture matrix materials in vitro, and successfully obtained new cartilage through tissue engineering. | PLA and PGA have been approved by the US FDA as implants. | |
| composite material | PLA and hydroxyapatite | Similar to the natural bone matrix, good biocompatibility, high bioactivity, high osteoinductive activity, and low inflammatory response | High cost and high technical requirements | Feng et al. [96]used selective laser sintering to fabricate porous tricalcium phosphate and zinc oxide scaffolds, which showed good mechanical and biological properties and could be used for bone repair and replacement therapy | Zhu and Marchant [97,98]combined PCL with sodium alginate to obtain a composite scaffold with higher printability and printing resolution, and the survival rate of chondrocytes in this scaffold was 70%–85%. | Matsuo et al. [84] used synthetic polymer polylactic acid and hydroxyapatite as raw materials to prepare absorbable porous scaffoldss for mandibular reconstruction. By comparison, they found that compared with the traditional titanium alloy scaffoldss, the polymer Polylactic acid/hydroxyapatite scaffoldss have a better repair effect. | ||
5. Summarize
3D printing technology can manufacture bone scaffolds with complex structures. The prepared bone scaffolds are similar to the human body in terms of external morphology and microstructure. Based on this, the combination of cells, growth factors and other active substances makes perfect bone reconstruction possible, which is of great significance to the personalized treatment of bone defects and osteochondral regeneration, and gradually forms an emerging industry with great market potential. From a microscopic point of view, 3D printing can precisely control the porosity, pore size and geometry of the fabricated scaffolds. And the bone tissue engineering scaffold manufactured by this technology can simulate the human cell microenvironment to a large extent and accelerate the speed of bone healing after combining growth factors and osteoblasts. From a macroscopic point of view, the bone tissue engineering scaffold produced by 3D printing has controllable overall structure and mechanical properties, can simulate the multi-scale structure of human tissue, and is multifunctional and easy to use. At the same time, with the combination of 3D printing technology and multidisciplinary fields such as tissue engineering, digital medicine, and materials science, more and more 3D printing products with good biocompatibility, excellent osteogenic induction ability and stable mechanical properties have been developed.
However, several current 3D printing methods also have some disadvantages that cannot be ignored. The scaffolds made by FDM are insufficient in terms of accuracy, so it is necessary to improve the accuracy performance of the printing machine and improve the scaffold performance. SLS may destroy the chemical properties of raw materials due to laser sintering, and the surface of the molded product is rough, and needs to be improved in terms of combining cells and various factors. The scaffolds made by SLA require post-processing, during which the stents may be deformed, and the molded product contains impurities. The electron beam melting method requires high cost. It needs to be developed in combination with other fields such as numerical control, electronic information, and materials science. The disadvantage of 3DP is that the mechanical strength of the product is not high, and the product needs to be post-processed. Compared with other technologies, 3D bioprinting technology has the advantages of high yield, low cost, simple implementation, fast printing speed, high precision, compatibility with low-viscosity biological materials, and the ability to add cells and biologically active substances. It has gradually become a new direction for the development of 3D printing technology in bone tissue engineering in the future. However, in terms of technology, it lacks highly biocompatible bioinks, and the printing accuracy still cannot fully reach the micron-level resolution of real tissues. In terms of clinical applications, although 3D bioprinting is widely used in the manufacture of cells, tissues, and organs, most of the organs printed are structural or single-function realizations, and the printing of complex organs has not been fully realized.
Nevertheless, the authors believe that with the development of 3D printing technology and bone tissue engineering, 3D bioprinting technology can better meet the printing requirements for bone tissue engineering scaffolds in the future than other technologies.
In recent years, on the basis of biological 3D printing, “in situ in vivo bioprinting","4D bioprinting technology” and “microsphere printing technology” have been proposed.
The in situ in vivo bioprinting refers to a method that bioinks are directly printed at a defect site in a clinical setting to create or repair living tissues or organs. This method creates a micro robot (a micro 3D bioprinting platform which can be installed to an endoscope) to enter the human body noninvasively and carries out tissue repair inside the body. Various studies have shown the great potentials of this approach, in fields such as skin, bone and cartilage repair. It can address the existing deficiencies in conventional bioprinting. The printed cells remained a high viability and a steady proliferation, which indicated good biological functions of the cells in printed scaffolds. This method presents an innovative advance not only in the field of bioprinting but also in clinical fields.
4D bioprinting is a newly emerging technology that combines the concept of time with 3D bioprinting as the fourth dimension, which enables the fabrication of complex and functional structures. It can create dynamic three-dimensional biological structures by using special materials that can change shape in response to various stimuli. Functional transformation and maturation of printed cellular structures is also considered a form of 4D bioprinting. This technology offers unprecedented potential for tissue engineering, and although this technology has attracted much attention in the biomedical field, more research and development is required to achieve clinical application as it is still in its infancy.
At the same time, from the point of view of material selection, the performance of the scaffolds printed by simply using one raw material is still insufficient. The researchers therefore chose appropriate methods to combine several materials into composites. Composite materials have many advantages, so the bone tissue engineering scaffolds made by 3D printing obviously have better performance.
The authors believe that the application of 3D printing technology in bone tissue engineering scaffolds has great promise, but it also faces some challenges. Only by accepting the existing challenges and gradually overcoming them can they exert their greatest value and provide efficient and effective treatment methods for the treatment of bone defects in the future. With the continuous development of 3D printing technology, people's research on bone tissue engineering scaffold materials, and the continuous integration and mutual promotion of engineering and medicine, 3D printing will definitely shine in the field of bone tissue engineering scaffold construction in the near future.
Patient consent
None.
Ethical approval and informed consent
None.
Funding sources
This research was funded by Versus Arthritis UK (Grant No. 21977); European Commission via a H2020-MSCA-RISE programme (BAMOS, Grant No. 734156); Innovative UK via Newton Fund (Grant No. 102872); Engineering and Physical Science Research Council (EPSRC) via DTP CASE programme (Grant No. EP/T517793/1).
Credit author statement
Resources, Peixuan Zhi and Leixin Liu; data curation, Qiliang Zhang and Jian Zhou; writing—original draft preparation, Qiliang Zhang, Jian Zhou and Qidong Zhang; writing—review and editing, Ao Fang and Qidong Zhang; supervision, Chaozong Liu; funding acquisition, Chaozong Liu. All authors have read and agreed to the published version of the manuscript.
Data availability statement
Not applicable.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Ao Fang, Email: bxfangao@163.com.
Qidong Zhang, Email: qidongzhang0913@163.com.
References
- 1.Mouriño V., Boccaccini A.R. Bone tissue engineering therapeutics: controlled drug delivery in three-dimensional scaffolds. J R Soc Interface. 2010;7:209–227. doi: 10.1098/RSIF.2009.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao G., Schilling A.F., Yonezawa T., Wang J., Dai G., Cui X. Bioactive nanoparticles stimulate bone tissue formation in bioprinted three-dimensional scaffold and human mesenchymal stem cells. Biotechnol J. 2014;9:1304–1311. doi: 10.1002/BIOT.201400305. [DOI] [PubMed] [Google Scholar]
- 3.Li C., Liu D., Zhang Z., Wang G., Xu N. Triple point-mutants of hypoxia-inducible factor-1α accelerate in vivo angiogenesis in bone defect regions. Cell Biochem Biophys. 2013;67:557–566. doi: 10.1007/S12013-013-9541-8/FIGURES/9. [DOI] [PubMed] [Google Scholar]
- 4.Seebach C., Henrich D., Wilhelm K., Barker J.H., Marzi I. Endothelial progenitor cells improve directly and indirectly early vascularization of mesenchymal stem cell-driven bone regeneration in a critical bone defect in rats. Cell Transplant. 2012;21:1667–1677. doi: 10.3727/096368912X638937. [DOI] [PubMed] [Google Scholar]
- 5.Guarino V., Ambrosio L. Temperature-driven processing techniques for manufacturing fully interconnected porous scaffolds in bone tissue engineering. Proc Inst Mech Eng H. 2010;224:1389–1400. doi: 10.1243/09544119JEIM744. [DOI] [PubMed] [Google Scholar]
- 6.Jang J.H., Castano O., Kim H.W. Electrospun materials as potential platforms for bone tissue engineering. Adv Drug Deliv Rev. 2009;61:1065–1083. doi: 10.1016/J.ADDR.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Wang Q., Wang Q., Wan C. Preparation and evaluation of a biomimetic scaffold with porosity gradients in vitro. An Acad Bras Cienc. 2012;84:9–16. doi: 10.1590/S0001-37652012000100003. [DOI] [PubMed] [Google Scholar]
- 8.Wang X., Yan Y., Pan Y., Xiong Z., Liu H., Cheng J., et al. Generation of three-dimensional hepatocyte/gelatin structures with rapid prototyping system. Tissue Eng. 2006;12:83–90. doi: 10.1089/TEN.2006.12.83. https://HomeLiebertpubCom/Ten [DOI] [PubMed] [Google Scholar]
- 9.Martínez-Vázquez F.J., Cabañas M.v., Paris J.L., Lozano D., Vallet-Regí M. Fabrication of novel Si-doped hydroxyapatite/gelatine scaffolds by rapid prototyping for drug delivery and bone regeneration. Acta Biomater. 2015;15:200–209. doi: 10.1016/J.ACTBIO.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 10.Kumar A., Mandal S., Barui S., Vasireddi R., Gbureck U., Gelinsky M., et al. Low temperature additive manufacturing of three dimensional scaffolds for bone-tissue engineering applications: processing related challenges and property assessment. Math Sci Eng R. 2016;103:1–39. doi: 10.1016/J.MSER.2016.01.001. [DOI] [Google Scholar]
- 11.Placone J.K., Engler A.J. Recent advances in extrusion-based 3D printing for biomedical applications. Adv Healthc Mater. 2018;7 doi: 10.1002/ADHM.201701161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eltorai A.E.M., Nguyen E., Daniels A.H. Three-dimensional printing in orthopedic surgery. Orthopedics. 2015;38:684–687. doi: 10.3928/01477447-20151016-05. [DOI] [PubMed] [Google Scholar]
- 13.Kim J.W., Lee Y., Seo J., Park J.H., Seo Y.M., Kim S.S., et al. Clinical experience with three-dimensional printing techniques in orthopedic trauma. J Orthop Sci. 2018;23:383–388. doi: 10.1016/J.JOS.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Xie L., Chen C., Zhang Y., Zheng W., Chen H., Cai L. Three-dimensional printing assisted ORIF versus conventional ORIF for tibial plateau fractures: a systematic review and meta-analysis. Int J Surg. 2018;57:35–44. doi: 10.1016/J.IJSU.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Pae H.C., Kang J.H., Cha J.K., Lee J.S., Paik J.W., Jung U.W., et al. 3D-printed polycaprolactone scaffold mixed with β-tricalcium phosphate as a bone regenerative material in rabbit calvarial defects. J Biomed Mater Res B. 2019;107:1254–1263. doi: 10.1002/JBM.B.34218. [DOI] [PubMed] [Google Scholar]
- 16.Singh A.V., Dad Ansari M.H., Wang S., Laux P., Luch A., Kumar A., et al. The adoption of three-dimensional additive manufacturing from biomedical material design to 3D organ printing. Appl Sci. 2019;9 doi: 10.3390/APP9040811. [DOI] [Google Scholar]
- 17.Bittner S., Smith B., Melchiorri A., Mikos A. Fabrication of 3D-Printed, bidirectional growth factor gradient scaffolds for osteochondral tissue repair. Tissue Eng Pt A. 2017;23:S38–S39. Mary Ann Liebert, Inc 140 Huguenot Street, 3rd Fl, New Rochelle, NY 10801 USA. [Google Scholar]
- 18.Sawkins M., Brown B., Bonassar L., Cox H., Rose F., Shakesheff K. Cell, scaffold and growth factor patterning via 3D printing. J Tissue Eng Regen M. 2012;6:374. Wiley-Blackwell 111 River St, Hoboken 07030-5774, NJ USA. [Google Scholar]
- 19.Zaszczyńska A., Moczulska-Heljak M., Gradys A., Sajkiewicz P. Advances in 3D printing for tissue engineering. Materials. 2021;14 doi: 10.3390/MA14123149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen J., Rölfing J.H.D., Svend Le D.Q., Kristiansen A.A., Nygaard J.V., Hokland L.B., et al. Surface-modified functionalized polycaprolactone scaffolds for bone repair: in vitro and in vivo experiments. J Biomed Mater Res. 2014;102:2993–3003. doi: 10.1002/JBM.A.34970. [DOI] [PubMed] [Google Scholar]
- 21.Abdullah A.M., Rahim T.N.A.T., Hamad W.N.F.W., Mohamad D., Akil H.M., Rajion Z.A. Mechanical and cytotoxicity properties of hybrid ceramics filled polyamide 12 filament feedstock for craniofacial bone reconstruction via fused deposition modelling. Dent Mater. 2018;34:e309–e316. doi: 10.1016/J.DENTAL.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Nikzad M., Masood S.H., Sbarski I. Thermo-mechanical properties of a highly filled polymeric composites for fused deposition modeling. Mater Des. 2011;32:3448–3456. doi: 10.1016/J.MATDES.2011.01.056. [DOI] [Google Scholar]
- 23.Dalton P.D., Vaquette C., Farrugia B.L., Dargaville T.R., Brown T.D., Hutmacher D.W. Electrospinning and additive manufacturing: converging technologies. Biomater Sci-Uk. 2013;1:171–185. doi: 10.1039/C2BM00039C. [DOI] [PubMed] [Google Scholar]
- 24.Ilyés K., Kovács N.K., Balogh A., Borbás E., Farkas B., Casian T., et al. The applicability of pharmaceutical polymeric blends for the fused deposition modelling (FDM) 3D technique: material considerations–printability–process modulation, with consecutive effects on in vitro release, stability and degradation. Eur J Pharmaceut Sci. 2019;129:110–123. doi: 10.1016/J.EJPS.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 25.Linares V., Casas M., Caraballo I. Printfills: 3D printed systems combining fused deposition modeling and injection volume filling. Application to colon-specific drug delivery. Eur J Pharm Biopharm. 2019;134:138–143. doi: 10.1016/J.EJPB.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 26.Domingos M., Chiellini F., Gloria A., Ambrosio L., Bartolo P., Chiellini E. Effect of process parameters on the morphological and mechanical properties of 3D Bioextruded poly(1-caprolactone) scaffolds. Rapid Prototyp J. 2012;18:56–67. doi: 10.1108/13552541211193502/FULL/XML. [DOI] [Google Scholar]
- 27.Guneta V., Wang J.K., Maleksaeedi S., He Z., Wong M.T.C., Choong C. Three dimensional printing of titanium for bone tissue engineering applications: a preliminary study. J Biomim Biomater Bi. 2014;21:101–115. doi: 10.4028/WWW.SCIENTIFIC.NET/JBBBE.21.101. [DOI] [Google Scholar]
- 28.Schantz J.T., Brandwood A., Hutmacher D.W., Khor H.L., Bittner K. Osteogenic differentiation of mesenchymal progenitor cells in computer designed fibrin-polymer-ceramic scaffolds manufactured by fused deposition modeling. J Mater Sci-Mater M 2005 16:9. 2005;16:807–819. doi: 10.1007/S10856-005-3584-3. [DOI] [PubMed] [Google Scholar]
- 29.Xu N., Ye X., Wei D., Zhong J., Chen Y., Xu G., et al. 3D artificial bones for bone repair prepared by computed tomography-guided fused deposition modeling for bone repair. Acs Appl Mater Inter. 2014;6:14952–14963. doi: 10.1021/AM502716T/ASSET/IMAGES/AM502716T. SOCIAL.JPEG_V03. [DOI] [PubMed] [Google Scholar]
- 30.Chen J., Wan N., Li J., He Z. Study on the polymer material infiltrating metallic parts by selective laser sintering of 3D printing. Rapid Prototyp J. 2018;24:1539–1543. doi: 10.1108/RPJ-05-2016-0073/FULL/XML. [DOI] [Google Scholar]
- 31.Lahtinen E., Precker R.L.M., Lahtinen M., Hey-Hawkins E., Haukka M. Selective laser sintering of metal-organic frameworks: production of highly porous filters by 3D printing onto a polymeric matrix. Chempluschem. 2019;84:222–225. doi: 10.1002/CPLU.201900081. [DOI] [PubMed] [Google Scholar]
- 32.Tay B.Y., Zhang S.X., Myint M.H., Ng F.L., Chandrasekaran M., Tan L.K.A. Processing of polycaprolactone porous structure for scaffold development. J Mater Process Technol. 2007;182:117–121. doi: 10.1016/J.JMATPROTEC.2006.07.016. [DOI] [Google Scholar]
- 33.Chen C.H., Shyu V.B.H., Chen J.P., Lee M.Y. Selective laser sintered poly-ε-caprolactone scaffold hybridized with collagen hydrogel for cartilage tissue engineering. Biofabrication. 2014;6 doi: 10.1088/1758-5082/6/1/015004. [DOI] [PubMed] [Google Scholar]
- 34.Roskies M.G., Fang D., Abdallah M.N., Charbonneau A.M., Cohen N., Jordan J.O., et al. Three-dimensionally printed polyetherketoneketone scaffolds with mesenchymal stem cells for the reconstruction of critical-sized mandibular defects. Laryngoscope. 2017;127:E392–E398. doi: 10.1002/LARY.26781. [DOI] [PubMed] [Google Scholar]
- 35.Wanibuchi M., Noshiro S., Sugino T., Akiyama Y., Mikami T., Iihoshi S., et al. Training for skull base surgery with a colored temporal bone model created by three-dimensional printing technology. World Neurosurg. 2016;91:66–72. doi: 10.1016/J.WNEU.2016.03.084. [DOI] [PubMed] [Google Scholar]
- 36.Feng P., Wei P., Shuai C., Peng S. Characterization of mechanical and biological properties of 3-D scaffolds reinforced with zinc oxide for bone tissue engineering. PLoS One. 2014;9 doi: 10.1371/JOURNAL.PONE.0087755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eshraghi S., Das S. Micromechanical finite-element modeling and experimental characterization of the compressive mechanical properties of polycaprolactone–hydroxyapatite composite scaffolds prepared by selective laser sintering for bone tissue engineering. Acta Biomater. 2012;8:3138–3143. doi: 10.1016/J.ACTBIO.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang C., Zhao Q., Wang M. Cryogenic 3D printing for producing hierarchical porous and rhBMP-2-loaded Ca-P/PLLA nanocomposite scaffolds for bone tissue engineering. Biofabrication. 2017;9 doi: 10.1088/1758-5090/aa71c9. [DOI] [PubMed] [Google Scholar]
- 39.Jansen J., Melchels F.P.W., Grijpma D.W., Feijen J. Fumaric acid monoethyl ester-functionalized poly(D,L-Lactide)/N-vinyl-2- pyrrolidone Resins for the preparation of tissue engineering scaffolds by stereolithography. Biomacromolecules. 2009;10:214–220. doi: 10.1021/BM801001R/SUPPL_FILE/BM801001R_SI_001.PDF. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y., Zhang L., Sun R., Jia Y., Chen X., Liu Y., et al. A new 3D printed titanium metal trabecular bone reconstruction system for early osteonecrosis of the femoral head. Medicine. 2018;97:26. doi: 10.1097/MD.0000000000011088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palmquist A., Emanuelsson L., Thomsen P., Palmquist A., Snis A., Emanuelsson L., et al. Long-term biocompatibility and osseointegration of electron beam melted, free-form–fabricated solid and porous titanium alloy: experimental studies in sheep. J Biomater Appl. 2013;27:1003–1016. doi: 10.1177/0731684411431857. [DOI] [PubMed] [Google Scholar]
- 42.Tarafder S., Balla V.K., Davies N.M., Bandyopadhyay A., Bose S. Microwave-sintered 3D printed tricalcium phosphate scaffolds for bone tissue engineering. J Tissue Eng Regen M. 2013;7:631–641. doi: 10.1002/TERM.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trachtenberg J.E., Mountziaris P.M., Miller J.S., Wettergreen M., Kasper F.K., Mikos A.G. Open-source three-dimensional printing of biodegradable polymer scaffolds for tissue engineering. J Biomed Mater Res. 2014;102:4326–4335. doi: 10.1002/JBM.A.35108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Utela B., Anderson R.L., Kuhn H. 2006 International Solid Freeform Fabrication Symposium. 2006. Advanced ceramic materials and processes for three-dimensional printing (3DP) [Google Scholar]
- 45.Tarafder S., Davies N.M., Bandyopadhyay A., Bose S. 3D printed tricalcium phosphate bone tissue engineering scaffolds: effect of SrO and MgO doping on in vivo osteogenesis in a rat distal femoral defect model. Biomater Sci-Uk. 2013;1:1250–1259. doi: 10.1039/C3BM60132C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farzadi A., Solati-Hashjin M., Asadi-Eydivand M., Osman N.A.A. Effect of layer thickness and printing orientation on mechanical properties and dimensional accuracy of 3D printed porous samples for bone tissue engineering. PLoS One. 2014;9 doi: 10.1371/JOURNAL.PONE.0108252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tarafder S., Bose S. Polycaprolactone-coated 3D printed tricalcium phosphate scaffolds for bone tissue engineering: in vitro alendronate release behavior and local delivery effect on in vivo osteogenesis. Acs Appl Mater Inter. 2014;6:9955–9965. doi: 10.1021/AM501048N/SUPPL_FILE/AM501048N_SI_001.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gudapati H., Yan J., Huang Y., Chrisey D.B. Alginate gelation-induced cell death during laser-assisted cell printing. Biofabrication. 2014;6 doi: 10.1088/1758-5082/6/3/035022. [DOI] [PubMed] [Google Scholar]
- 49.Cui X., Breitenkamp K., Finn M.G., Lotz M., D'Lima D.D. Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng Part A. 2012;18:1304. doi: 10.1089/TEN.TEA.2011.0543. https://HomeLiebertpubCom/Tea 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu T., Binder K.W., Albanna M.Z., Dice D., Zhao W., Yoo J.J., et al. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication. 2012;5 doi: 10.1088/1758-5082/5/1/015001. [DOI] [PubMed] [Google Scholar]
- 51.Johnson B.N., Jia X. 3D printed nerve guidance channels: computer-aided control of geometry, physical cues, biological supplements and gradients. Neural Regen Res. 2016;11:1568. doi: 10.4103/1673-5374.193230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson B.N., Lancaster K.Z., Zhen G., He J., Gupta M.K., Kong Y.L., et al. 3D printed anatomical nerve regeneration pathways. Adv Funct Mater. 2015;25:6205–6217. doi: 10.1002/ADFM.201501760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ozbolat I.T., Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials. 2016;76:321–343. doi: 10.1016/J.BIOMATERIALS.2015.10.076. [DOI] [PubMed] [Google Scholar]
- 54.Kang H.W., Lee S.J., Ko I.K., Kengla C., Yoo J.J., Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol. 2016;34:312–319. doi: 10.1038/nbt.3413. 2016 34:3. [DOI] [PubMed] [Google Scholar]
- 55.Lee M., Wu B.M. Recent advances in 3D printing of tissue engineering scaffolds. Methods Mol Biol. 2012;868:257–267. doi: 10.1007/978-1-61779-764-4_15. [DOI] [PubMed] [Google Scholar]
- 56.Panetta N J., Gupta D M., Longaker M T. Bone regeneration and repair. Curr Stem Cell Res Ther. 2010;5:122–128. doi: 10.2174/157488810791268618. [DOI] [PubMed] [Google Scholar]
- 57.Brydone A.S., Meek D., MacLaine S. Bone grafting, orthopaedic biomaterials, and the clinical need for bone engineering. Proc Inst Mech Eng H. 2010;224:1329–1343. doi: 10.1243/09544119JEIM770. [DOI] [PubMed] [Google Scholar]
- 58.Will J., Melcher R., Treul C., Travitzky N., Kneser U., Polykandriotis E., et al. Porous ceramic bone scaffolds for vascularized bone tissue regeneration. J Mater Sci Mater Med. 2008;19:2781–2790. doi: 10.1007/S10856-007-3346-5/FIGURES/9. [DOI] [PubMed] [Google Scholar]
- 59.Egan P.F., Ferguson S.J., Shea K. Design of hierarchical three-dimensional printed scaffolds considering mechanical and biological factors for bone tissue engineering. J Mech Design, Transactions of the ASME. 2017;139 doi: 10.1115/1.4036396/472962. [DOI] [Google Scholar]
- 60.Zhang L., Yang G., Johnson B.N., Jia X. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019;84:16–33. doi: 10.1016/J.ACTBIO.2018.11.039. [DOI] [PubMed] [Google Scholar]
- 61.Das S., Bourell D.L., Babu S.S. Metallic materials for 3D printing. MRS Bull. 2016;41:729–741. doi: 10.1557/MRS.2016.217. [DOI] [Google Scholar]
- 62.Choi J.-W., Ahn J.-S. 3D printed titanium implant for the skull reconstruction: a preliminary case study. J Int Soc Simulat Surg. 2014;1:99–102. doi: 10.18204/JISSIS.2014.1.2.099. [DOI] [Google Scholar]
- 63.Punyaratabandhu T., Lohwongwatana B., Puncreobutr C., Kosiyatrakul A., Veerapan P., Luenam S. A Patient-matched entire first metacarpal prosthesis in treatment of giant cell tumor of bone. Case Rep Orthoped. 2017:1–6. doi: 10.1155/2017/4101346. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ma H., Feng C., Chang J., Wu C. 3D-printed bioceramic scaffolds: from bone tissue engineering to tumor therapy. Acta Biomater. 2018;79:37–59. doi: 10.1016/J.ACTBIO.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 65.Shim K.S., Kim S.E., Yun Y.P., Jeon D.I., Kim H.J., Park K., et al. Surface immobilization of biphasic calcium phosphate nanoparticles on 3D printed poly(caprolactone) scaffolds enhances osteogenesis and bone tissue regeneration. J Ind Eng Chem. 2017;55:101–109. doi: 10.1016/J.JIEC.2017.06.033. [DOI] [Google Scholar]
- 66.Wang H., Wu G., Zhang J., Zhou K., Yin B., Su X., et al. Osteogenic effect of controlled released rhBMP-2 in 3D printed porous hydroxyapatite scaffold. Colloids Surf, B. 2016;141:491–498. doi: 10.1016/J.COLSURFB.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 67.Song X., Tetik H., Jirakittsonthon T., Parandoush P., Yang G., Lee D., et al. Biomimetic 3D printing of hierarchical and interconnected porous hydroxyapatite structures with high mechanical strength for bone cell culture. Adv Eng Maters. 2019;21 doi: 10.1002/ADEM.201800678. [DOI] [Google Scholar]
- 68.Chen L., Deng C., Li J., Yao Q., Chang J., Wang L., et al. 3D printing of a lithium-calcium-silicate crystal bioscaffold with dual bioactivities for osteochondral interface reconstruction. Biomaterials. 2019;196:138–150. doi: 10.1016/J.BIOMATERIALS.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 69.Song Y., Lin K., He S., Wang C., Zhang S., Li D., et al. Nano-biphasic calcium phosphate/polyvinyl alcohol composites with enhanced bioactivity for bone repair via low-temperature three-dimensional printing and loading with platelet-rich fibrin. Int J Nanomed. 2018;13:505. doi: 10.2147/IJN.S152105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim B.S., Yang S.S., Kim C.S. Incorporation of BMP-2 nanoparticles on the surface of a 3D-printed hydroxyapatite scaffold using an ε-polycaprolactone polymer emulsion coating method for bone tissue engineering. Colloids Surf, B. 2018;170:421–429. doi: 10.1016/J.COLSURFB.2018.06.043. [DOI] [PubMed] [Google Scholar]
- 71.Aamodt J.M., Grainger D.W. Extracellular matrix-based biomaterial scaffolds and the host response. Biomaterials. 2016;86:68–82. doi: 10.1016/J.BIOMATERIALS.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Montalbano G., Borciani G., Pontremoli C., Ciapetti G., Mattioli-Belmonte M., Fiorilli S., et al. Development and biocompatibility of collagen-based composites enriched with nanoparticles of strontium containing mesoporous glass. Materials 2019. 2019;12:3719. doi: 10.3390/MA12223719. 12. 3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cunniffe G.M., Dickson G.R., Partap S., Stanton K.T., O'Brien F.J. Development and characterisation of a collagen nano-hydroxyapatite composite scaffold for bone tissue engineering. J Mater Sci Mater Med. 2010;21:2293–2298. doi: 10.1007/S10856-009-3964-1/FIGURES/5. [DOI] [PubMed] [Google Scholar]
- 74.Zhou C., Ye X., Fan Y., Ma L., Tan Y., Qing F., et al. Biomimetic fabrication of a three-level hierarchical calcium phosphate/collagen/hydroxyapatite scaffold for bone tissue engineering. Biofabrication. 2014;6 doi: 10.1088/1758-5082/6/3/035013. [DOI] [PubMed] [Google Scholar]
- 75.Lee W.K., Lim Y.Y., Leow A.T.C., Namasivayam P., Ong Abdullah J., Ho C.L. Biosynthesis of agar in red seaweeds: a review. Carbohydr Polym. 2017;164:23–30. doi: 10.1016/J.CARBPOL.2017.01.078. [DOI] [PubMed] [Google Scholar]
- 76.Zhang B.G.X., Myers D.E., Wallace G.G., Brandt M., Choong P.F.M. Bioactive coatings for orthopaedic implants—recent trends in development of implant coatings. Int J Mol Sci. 2014;15:11878–11921. doi: 10.3390/IJMS150711878. 11878–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Madhumathi K., Shalumon K.T., Rani V.V.D., Tamura H., Furuike T., Selvamurugan N., et al. Wet chemical synthesis of chitosan hydrogel–hydroxyapatite composite membranes for tissue engineering applications. Int J Biol Macromol. 2009;45:12–15. doi: 10.1016/J.IJBIOMAC.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 78.Wang M., Cheng X., Zhu W., Holmes B., Keidar M., Zhang L.G. Design of biomimetic and bioactive cold plasma-modified nanostructured scaffolds for enhanced osteogenic differentiation of bone marrow-derived mesenchymal stem cells. Tissue Eng Part A. 2013;20:71–1060. doi: 10.1089/TEN.TEA.2013.0235. https://HomeLiebertpubCom/Tea [DOI] [PubMed] [Google Scholar]
- 79.Vacanti C.A., Upton J. Tissue-engineered morphogenesis of cartilage and bone by means of cell transplantation using synthetic biodegradable polymer matrices. Clin Plast Surg. 1994;21:445–462. doi: 10.1016/S0094-1298(20)31022-1. [DOI] [PubMed] [Google Scholar]
- 80.Sherwood J.K., Riley S.L., Palazzolo R., Brown S.C., Monkhouse D.C., Coates M., et al. A three-dimensional osteochondral composite scaffold for articular cartilage repair. Biomaterials. 2002;23:4739–4751. doi: 10.1016/S0142-9612(02)00223-5. [DOI] [PubMed] [Google Scholar]
- 81.Chou Y.C., Lee D., Chang T.M., Hsu Y.H., Yu Y.H., Chan E.C., et al. Combination of a biodegradable three-dimensional (3D) – printed cage for mechanical support and nanofibrous membranes for sustainable release of antimicrobial agents for treating the femoral metaphyseal comminuted fracture. J Mech Behav Biomed. 2017;72:209–218. doi: 10.1016/J.JMBBM.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 82.Kim S.S., Sun Park M., Jeon O., Yong Choi C., Kim B.S. Poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:1399–1409. doi: 10.1016/J.BIOMATERIALS.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 83.Xia Z.Y., Hou J.Y., Li K. Composite materials and bone tissue engineering in sports injury. Adv Mater. 2012;583:91–94. doi: 10.4028/WWW.SCIENTIFIC.NET/AMR.583.91. [DOI] [Google Scholar]
- 84.Matsuo A., Chiba H., Takahashi H., Toyoda J., Abukawa H. Clinical application of a custom-made bioresorbable raw particulate hydroxyapatite/poly-l-lactide mesh tray for mandibular reconstruction. Odontology. 2010;98:85–88. doi: 10.1007/S10266-009-0111-X. 2010 98:1. [DOI] [PubMed] [Google Scholar]
- 85.Rai B., Lin J.L., Lim Z.X.H., Guldberg R.E., Hutmacher D.W., Cool S.M. Differences between in vitro viability and differentiation and in vivo bone-forming efficacy of human mesenchymal stem cells cultured on PCL–TCP scaffolds. Biomaterials. 2010;31:7960–7970. doi: 10.1016/J.Biomaterials.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 86.Kim J., McBride S., Tellis B., Alvarez-Urena P., Song Y.-H., Dean D.D., et al. Rapid-prototyped PLGA/β-TCP/hydroxyapatite nanocomposite scaffolds in a rabbit femoral defect model. Biofabrication. 2012;4 doi: 10.1088/1758-5082/4/2/025003. [DOI] [PubMed] [Google Scholar]
- 87.Jammalamadaka U., Tappa K. Recent advances in biomaterials for 3D printing and tissue engineering. J Funct Biomater. 2018;9 doi: 10.3390/JFB9010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang X., Zhu Z., Xiao H., Luo C., Luo X., Lv F., et al. Three-dimensional, multiscale, and interconnected trabecular bone mimic porous tantalum scaffold for bone tissue engineering. ACS Omega. 2020;5 doi: 10.1021/acsomega.0c03127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Martel-Frachet V., Ivanova E.P., le Clainche T., Linklater D., Wong S., Le P., et al. Mechano-bactericidal titanium surfaces for bone tissue engineering. Acs Appl Mater Inter. 2020;12 doi: 10.1021/acsami.0c11502. [DOI] [PubMed] [Google Scholar]
- 90.Dorozhkin S.v. Bioceramics of calcium orthophosphates. Biomaterials. 2010;31 doi: 10.1016/j.biomaterials.2009.11.050. [DOI] [PubMed] [Google Scholar]
- 91.Smith B.T., Shum J., Wong M., Mikos A.G., Young S. Bone tissue engineering challenges in oral & maxillofacial surgery. Adv Exp Med Biol. 2015;881 doi: 10.1007/978-3-319-22345-2_4. [DOI] [PubMed] [Google Scholar]
- 92.Catanzano O., Soriente A., la Gatta A., Cammarota M., Ricci G., Fasolino I., et al. Macroporous alginate foams crosslinked with strontium for bone tissue engineering. Carbohydr Polym. 2018;202 doi: 10.1016/j.carbpol.2018.08.086. [DOI] [PubMed] [Google Scholar]
- 93.Koupaei N., Karkhaneh A. Porous crosslinked polycaprolactone hydroxyapatite networks for bone tissue engineering. J Tissue Eng Regen M. 2016;13 doi: 10.1007/s13770-016-9061-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Frezzo J.A., Montclare J.K. Natural composite systems for bioinspired materials. Adv Exp Med Biol. 2016;940 doi: 10.1007/978-3-319-39196-0_7. [DOI] [PubMed] [Google Scholar]
- 95.Wang H., Wu G., Zhang J., Zhou K., Yin B., Su X., et al. Osteogenic effect of controlled released rhBMP-2 in 3D printed porous hydroxyapatite scaffold. Colloids Surf, B. 2016;141 doi: 10.1016/j.colsurfb.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 96.Feng P., Wei P., Shuai C., Peng S. Characterization of mechanical and biological properties of 3-D scaffolds reinforced with zinc oxide for bone tissue engineering. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhu J., Marchant R.E. Design properties of hydrogel tissue-engineering scaffolds. Expet Rev Med Dev. 2011;8 doi: 10.1586/erd.11.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cheng L., Shoma Suresh K., He H., Rajput R.S., Feng Q., Ramesh S., Wang Y., Krishnan S., Ostrovidov S., Camci-Unal G., et al. 3D printing of micro-and nanoscale bone substitutes: a review on technical and translational perspectives. Int J Nanomed. 2021;16:4289–4319. doi: 10.2147/IJN.S311001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.