Summary
Background
Peripheral intravenous catheters (PIVCs) are the most commonly used invasive medical device in health care with an overall failure rate of 35–50%. Most complications are non-infectious, but local site and bloodstream infections can also occur. Even if PIVC-related infections are rare, the total number of affected patients and the preponderance of Staphylococcus aureus as related pathogen due to the frequent use of these devices are relevant arguments to implement preventive strategies. The aim of this document is to raise awareness that infections caused by PIVCs are a relevant problem that can be reduced by practice change.
Methods
A panel of experts discussed this topic based on evidence and proposed practice points by consensus.
Discussion
Despite published evidence-based guidelines, current practice concerning aseptic techniques during insertion and care of PIVCs often are substandard. These devices have become commonplace and tend to be perceived as safe. An overall lack of awareness about the true risks associated with the use of PIVCs results in limited surveillance and prevention efforts.
Conclusion
Successful insertion and maintenance bundles in central venous lines are a blueprint to the implementation of adapted bundle strategies in the prevention of PIVC-associated infections. There is a need for studies to specifically investigate infection prevention in PIVCs and to agree on effective and implementable bundles.
Keywords: Peripheral venous catheter, Complications, Bloodstream infections, Exit-site infections, Prevention, Best practice
Abbreviations: A-DIVA, Adult difficult intra venous access; BSI, Bloodstream infection; CHG, Chlorhexidine gluconate; CRBSI, Catheter-related bloodstream infection; ERPIUP, European Recommendations for Proper Indication and Use of Peripheral venous Access; IV, Intravenous; PIVC, Peripheral intravenous catheter
Introduction
Peripheral intravenous catheter (PIVC) insertion is the most common invasive hospital procedure performed worldwide [1]. In Switzerland, half of the patients in acute care have a PIVC in place on any given day [2]. The generalized use of these catheters makes PIVC insertion a routine procedure and although there are published guidelines [3], inconsistencies between policy and practice have been reported [4]. Such inconsistencies likely contribute to the high incidence of PIVC-related complications with reported overall failure rates of 35–50% [1,[4], [5], [6], [7]].
Most PIVC complications are non-infectious such as phlebitis, infiltration/extravasation, occlusion, leakage, and dislodgement. However, local site infections or even bloodstream infections (BSI) do also occur. Meta-analyses focusing on non-infectious complications of PIVCs reported that phlebitis [7,8] and infiltration/extravasation [7] are the most prevalent complications. Importantly, phlebitis (in particular if not readily diagnosed) may pave the way for infectious complications [9]. However, phlebitis does not automatically lead to BSIs [10], and phlebitis without infection is no indication for antimicrobial therapy.
Several studies have been published on the occurrence of infectious complications related to PIVCs. However, comparison is challenging because the reported infectious outcomes are different: local and systemic infections combined, local and systemic infections separate, or infections due to specific pathogens such as Staphylococcus aureus. In addition, studies report different units, either expressed as proportions by catheters or patients, or as rates by catheter- or patient-days. Incidence rates range from 0.5 [11] to 0.7 [12] PIVC-associated BSI per 1,000 catheter-days.
Reported overall 30-day mortality from PIVC-BSIs range from 11 to 13.2% [[13], [14], [15], [16], [17]]. However, mortality from BSI due to Staphylococcus aureus was 27% [13] and 35.8% [14] in two studies. Together, these reports emphasize the fact that PIVCs are not innocuous devices and support the importance of raising awareness about the potential infectious risks [7,8,18,19]. They also demonstrate the effectiveness of targeted interventions in reducing PIVC-related BSIs and mortality [15,20]. Given the success of bundles in BSI prevention of central venous lines, this strategy was also promoted for PIVC-use. A systematic review on PIVC-bundles [21] found 10 studies detailing 21 insertion bundle components, as well as 11 studies detailing 22 maintenance bundle components. For both insertion and maintenance, 2 to 7 items per bundle were described. According to this systematic review, the effect of these bundles on mechanical and infectious PIVC complications remains uncertain, although 12 studies reported reductions in phlebitis and bloodstream infection. The authors qualified the available evidence as “promising but not robust”. This is most likely due to the lack of standardization of the selected bundle components. However, even with a consistent bundle, the main challenge to confirm its effectiveness on BSI will be the fact that PIVC-associated BSI is a rare outcome and large cohorts will be needed.
The aim of this review was to discuss selected topics around PIVC-associated infections with a panel of experts and to propose practice points based on evidence and consensus. This document is intended to complement existing guidelines for awareness raising and facilitate implementation of best practice procedures.
Methods
Literature search focusing on catheter care
A literature search for articles published between 1st January 2000 and 31st October 2020 was performed using PubMed, Embase, and Medline, with the search terms “PIVC or peripheral intravenous catheters” and “warmth” or “induration” or “phlebitis” or “thrombophlebitis” or “infiltration” or “extravasation” or “dislodgement” or “occlusion” or “bleeding” or “catheter-related bacteremia” or “blood stream infection” or “dermatitis” or “pus or abscess” or “erythema” or “insertion attempts” or “complications” or “failure” or “adverse effects” or “infection”. Inclusion criteria were date of publication (not more than 20 years ago) and testing interventions on PIVC-related complications. A total of 391 articles were identified and reviewed, of which 146 were retained for data extraction: population, sample size, study arms, end points, and risk factors. Data were collected by Microsoft Power BI (https://powerbi.microsoft.com/). Six of the 146 articles were selected for the pre-reading list for the expert panel discussion based on the following criteria.
-
a.
Recent publication (not older than 5 years)
-
b.Helpful to facilitate the panel discussion on different aspects of PIVC insertion and maintenance:
Seven additional articles were selected for optional reading to expand on the core topics. [4,[6], [7], [8],15,27,28].
Expert panel meeting
Panel members were selected based on their scientific expertise and practice concerning the clinical epidemiology and the prevention of vascular catheter-related infection, and to represent a cross-section of European countries. A total of 12 experts were invited to the project. The area of expertise included infection prevention and control (WZ, JB); nursing (AB), vascular access and IV-therapy (AB, VC, DV); intensive care and vascular access (PE); infectious diseases (MP); pediatrics (AS); hygiene and environmental medicine (JT); vascular surgery (SvR); and anesthesiology (MK, MPJG). Seven experts from Germany (AS, JT), Switzerland (PE, WZ), Spain (MP), and the United Kingdom (AB, JB) participated in a discussion round using a virtual conference platform; five experts participated in the consensus statement round only. Prior to the live online meeting, panel members were provided with selected literature (mandatory pre-reading list [1,[22], [23], [24], [25], [26]] and optional reading list [4,[6], [7], [8],15,27,28]) and an agenda related to the importance and magnitude of complications related to PIVCs, followed by a discussion on interventions to best address this topic. The meeting took place virtually on 27th November 2020.
Post-meeting follow-up
A list of consensus statements was drafted by a medical writer after the meeting and reviewed by the lead author. The statements were based on the recordings from the meeting and on the pre-reading literature. They were sent in the form of an electronic survey to all expert panel participants and to additional selected healthcare providers who were not present at the meeting (listed in Acknowledgements) to broaden representation of other countries. All participants were asked to mark whether they agreed or disagreed with each statement and to provide any additional comments. The survey results were tallied and summarized to draft final consensus statements. Comments provided by the survey participants were incorporated into the final manuscript, which was reviewed by all authors.
Consensus agreement
A modified Delphi method [29,30] was used to yield consensus for each statement. In this process, consensus was defined as 80% or more of the participants in agreement with a statement. The tables below present the survey results for each statement (yes/no responses and whether agreement was achieved).
Results
Overview
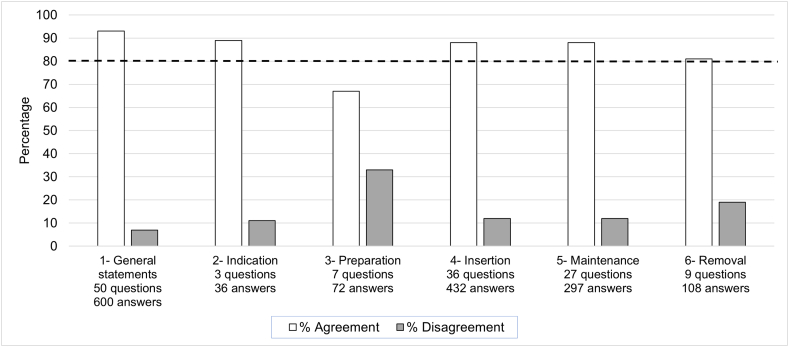
Consensus statements with > 80% agreement are outlined below. All statements (including those with less agreement, which therefore did not meet the threshold for inclusion) are summarized in the supplement (Supplement tables 1–6). Figure 1 summarizes the level of agreement for each category of statements.
Figure 1.
Level of agreement stratified by experts and category. The dotted line illustrates the 80% agreement level defined for consensus.
Consensus statements with ≥ 80% agreement
General statements
The reasons for PIVCs to fail may fall under 3 basic categories [1]:
The technology used, such as material, dressing, fixation, add-ons.
The caregiver’s skills during insertion, use, and care.
The intrinsic factors linked to the patient (body’s response, activity).
PIVCs can cause bloodstream infections/ bacteraemia.
Bloodstream infections from PIVCs are costly.
Bloodstream infections from PIVCs have a significant mortality, especially when caused by Staphylococcus aureus (about 13% [15]).
Once a bloodstream infection is present, mortality is similar for bloodstream infections due to peripheral and central catheters [13,16].
A number of intrinsic and extrinsic factors can increase the risk of infection (both insertion-site infection and catheter-related bloodstream infection - CRBSI) with PIVCs: age, severity of illness, multiple co-morbidities, and hospital length of stay.
Bacteraemia/ fungemia caused by PIVCs can lead to serious infections in other sites (e.g., endocarditis, which may lead to other septic embolic complications); this might require additional, long-term care (burden of complications).
There is a need to raise awareness of PIVC complications being a meaningful and potentially serious problem, because many healthcare providers do not currently recognize it as a such.
PIVC complications can be infectious or non-infectious.
Non-infectious complications such as catheter displacement and dressing failure increase the risk for infection.
The failure modes for peripheral IV catheters constituting frequent complications include phlebitis, infiltration, occlusion/mechanical failure, and dislodgement [1].
Smaller-gauge catheters are associated with less phlebitis [1].
Catheters composed of softer, smoother-surfaced, and less porous plastics, such as polyurethane, have improved performance, and lower phlebitis and overall failure rates compared to catheters made of other materials [1].
Documentation is key for PIVC management and should include insertion and removal dates, indication, and all complications for every PIVC.
The continuing need for each PIVC should be assessed daily and any catheter no longer needed should be removed [31].
Surveillance and audits should be used to better monitor both practice and outcomes with the aim to prevent PIVC complications.
Education on PIVC insertion and care practice is important to develop competency and proficiency in all personnel handling PIVCs.
A PIVC bundle could be beneficial to reduce complications by standardizing practice.
A PIVC bundle should be affordable.
A PIVC bundle should include protocols to address all aspects related to the use of this device:
Defining indications for PIVC.
Use of a standardized insertion set.
Defining (writing) local insertion protocols.
Use of ultrasound in difficult venous access situations.
Defining (writing) local maintenance protocols.
Daily monitoring for any signs of complications [31].
Daily monitoring to determine if the PIVC is still needed [31].
Daily monitoring to determine if the dressing needs to be changed.
Defining (writing) a local protocol for the preparation of IV medications.
Defining (writing) a local protocol for aseptic technique in PIVC care.
Defining (writing) a local protocol for catheter removal.
PIVCs are known for their overall high rate of complications leading to catheter failure. Failure ratios of 32% and 41% were reported by Marsh [32] and Rickard [33], respectively. The panel members discussed lower infection rates in PIVCs compared to central lines but given the fact that they are used at a much higher frequency, the number of affected patients is relevant. PIVCs can cause BSIs, which result in the same mortality as those caused by central lines; therefore, appropriate prevention and treatment measures are just as necessary for PIVC-associated BSI as for central line-associated BSI. There is a general need to raise awareness among health workers on PIVC-associated BSI, which might have been underestimated previously [17]. Practice matters and thus, surveillance and audits are necessary to monitor outcomes and drive prevention strategies. The expert panel concluded that a PIVC bundle could potentially reduce PIVC-associated complications by standardizing practice.
Statements on PIVC indication
PIVCs should be used for short-term IV therapies (up to 7 days).
PIVCs should not be used if oral treatment is available.
The panel members identified the practice of systematically inserting a PIVC upon admission as problematic because it leads to exposing patients to an unnecessary risk. This practice is often wrongly considered benign. Oral treatment should be the first choice if available. Interestingly, Ben Abdelaziz [34] noted that only 60% of failed PIVCs were replaced, suggesting that the other 40% were unnecessary and either could have been removed earlier or should not have been placed in the first time. Another study [19] with 614 patients reported that 83% of the patients arriving from the emergency department to the infectious diseases unit had a PIVC in place, of which 51% were judged unnecessary and removed as soon as the patient was examined. This illustrates the significant number of idle catheters that should be avoided.
Statements on preparation of PIVC insertion
Colonization of the skin at the insertion site increases the risk of infection.
Skin antisepsis at the PIVC insertion site is important to reduce the microbial load and keep it as low as possible; skin prep should be performed before PIVC insertion and at each dressing change with 2% chlorhexidine gluconate in 70% alcohol [26]. The use of iodine in alcohol is not adequate.
Phlebitis and infiltration can best be prevented by choosing the insertion site properly (hand/ wrist over forearm), providing good securement, keeping the duration of PIVC dwell time short (less than 4 days), avoiding irritating infusates, checking the insertion site daily, and using a flexible polyurethane catheter (less thrombogenic, less rigid).
The expert panel agreed that skin preparation should be performed with 2% chlorhexidine gluconate in 70% alcohol. Some members highlighted the local use of octenidine hydrochloride in isopropanol (as mentioned in the German Infection Control Guidelines for the Intensive Care Unit published in 2009 [35]), but the panel did not reach consensus whether this disinfectant is equivalent to alcohol-based 2% chlorhexidine gluconate.
Statements on PIVC insertion
All PIVC insertions should be done by a person specifically trained for this task.
PIVCs should not be inserted systematically by IV teams.
IV teams should insert PIVCs in situations where a failed cannulation on the first attempt is expected (Risk factors associated with a failed cannulation on the first attempt: target vein not palpable; target vein not visible; history of difficult peripheral intravenous cannulation; unplanned indication for surgery; vein diameter < 2 mm [36]).
Aseptic, non-touch technique should be used for insertion or reinsertion of PIVCs and during manipulation of catheter hubs, connectors, and stopcocks [1].
Extraluminal colonization can result from inadequate skin preparation, break in aseptic technique at the time of initial IV catheter insertion, attachment of normal skin flora as the insertion needle and catheter are passed through the epidermis and underlying dermal structures, inadequate catheter dressing placement at the insertion site, or inadequate technique while caring for the insertion site [1].
Intraluminal contamination of PIVC surfaces can occur at the time of catheter insertion as a result of breaches in aseptic technique during the complex and variable catheter-insertion and dressing-placement process (eg, flushing, capping, securing) [1].
Intraluminal contamination of PIVC surfaces can occur during the insertion process when the hub is touched with nonsterile gloves that have typically touched multiple nonsterile surfaces [1].
The use of an antimicrobial dressing decreases colonization of the skin at the PIVC insertion site and may offer added benefit by reducing the risk of local site infections and/or CRBSIs.
Securement of PIVCs is important to provide a barrier to infection AND to prevent dislodgement.
Strong dressing adhesion is an important aspect of catheter securement.
Securement should be done in a standardized way to reduce the risk of contamination.
Extension sets contribute to securement by helping minimize movement at the insertion site.
Extension sets contribute to securement by helping secure from an additional direction.
A specific bundle should be defined for insertion and include the following items:
Skin preparation containing alcohol and a disinfectant with residual effect.
Selection of the appropriate catheter type (catheter size).
Selection of the appropriate insertion site (hand > wrist > forearm).
Extension set.
Securement device.
The expert panel agreed that the task of PIVC insertion requires specific training. IV teams should only be involved if there is a substantial risk for a failed cannulation on first attempt (see the A-DIVA scale, a clinical predictive scale to identify difficult intravenous access in adults [36]). An aseptic, non-touch technique should be used; securement is important to prevent infection and dislodgement; and a specific bundle for PIVC insertion should be defined. Three of twelve panelists thought that sterile gloves should be used, whereas nine voted for non-sterile gloves (no consensus). The panelists agreed that an antimicrobial dressing may have an additional benefit by reducing the microbial load at the exit-site and therefore the risk of local site infections and BSI but did not reach consensus on including such a dressing in a bundle (5 in favor, 7 not in favor). Clinicians need to balance the possible advantages of adding technology against the added cost it may incur; studies would be needed to show the cost-benefit before broad adoption is considered. Local infection prevention teams should define highly vulnerable patient populations in whom the use of antimicrobial dressing may be considered.
Statements on PIVC maintenance
Hub disinfection (active or passive) must be performed in a consistent manner (with high compliance) each time the PIVC is accessed.
Disinfection of hub or connector can be done by using a disinfection cap providing continuous disinfection and replacing it after each access [1].
A disinfection cap to cover the connector when not in use may add benefit to disinfection before use (standardized disinfection, less errors) [25].
Catheter locks in PIVCs do not add benefit for CRBSI-prevention (mainly due to the short dwell time).
A specific bundle should be defined for maintenance and include some of the following items:
Non-sterile gloves for catheter care and dressing change.
Skin prep containing alcohol and a disinfectant with residual effect.
Extension line.
Securement device.
Removal of the PIVC when no longer clinically indicated.
Local infection can best be prevented by using aseptic, non-touch technique and skin antisepsis with CHG in alcohol.
Phlebitis and infiltration can best be managed by removing the catheter and choosing a new insertion site.
Occlusion can best be managed by removing the catheter and choosing a new insertion site or using pulsatile flushing with saline if left in place [3], similar to the recommended practice for central lines [37].
Catheter dislodgment can best be managed by removing the catheter and choosing a new insertion site.
Local infection can best be managed by removing the catheter and choosing a new insertion site.
Confirmed or suspected CRBSI can best be managed by removing the catheter and choosing a new insertion site.
The expert panel agreed that disinfection caps are effective to prevent hub contamination but there was no consensus about whether such devices are superior to scrubbing the hub before use. For PIVC care, gloves should be used. However, choice of glove type was not unanimous: three of eleven panelists suggested that sterile gloves should be used, but ten voted for non-sterile gloves, reaching a consensus for the latter. As noted for insertion, the panelists did not reach a consensus on including an antimicrobial dressing in a bundle based on the absence of clinical data specifically related to PIVCs (4 in favor, 7 not in favor). Emerging antimicrobial resistance and cost may be a consideration and studies showing a cost benefit would be needed to justify adoption.
Statements on PIVC removal
PIVCs inserted under emergency conditions should be replaced [38,39].
PIVCs should be removed as soon as they are no longer clinically indicated.
Phlebitis should prompt catheter removal [22].
PIVCs should be removed if signs of local or systemic infection develop.
Catheter tip culture from PIVCs should not be done routinely but may be helpful if CRBSI is suspected.
The expert panel did not reach consensus on routine replacement versus clinically indicated replacement. Some members pointed out that without routine change, PIVCs were at risk of being neglected and left in place until a problem occurred. While clinically indicated replacement is not inferior to routine replacement on phlebitis, it may be on BSI as published recently in a large study on the topic [40]. No consensus was reached on the need to culture the catheter tip if local infection is suspected. The main concern was that culturing catheter tips on the basis of local signs of infection would consistently lead to false positive cultures and may increase unnecessary use of antibiotics. This is supported by the findings from a study by Blanco-Mavillard [41], in which 297 of 711 PIVCs failed (41.8%) and where 41 of those (13.8%) had positive cultures; 22 of those 41 (53.6%) were from patients with local signs and symptoms compatible with catheter-related infections but only one patient had clinical signs improved within 48h of catheter removal (thus, being compatible with a catheter-related BSI). There were no patients diagnosed with CRBSI with concordant bacterial growth isolated in catheter tip and blood cultures, which does not support systematically performing tip cultures of PIVCs for the prediction of CRBSI. The authors concluded that the most rewarding approach for CRBSI prevention is to avoid unnecessary PIVC insertions and to remove any PIVC that is no longer needed.
Discussion
The statements presented in this scoping review and consensus exercise provide general guidance on the use of PIVCs. The recommendations on which experts agreed are based on existing guidelines, published evidence, and clinical experience. Peripheral intravenous catheters have been in use for a very long time and have become such common place that potential risks are underestimated or even ignored. In addition, outcome surveillance of PIVC-associated complications is cumbersome and time-consuming. The absence of surveillance data contributes to a lack of awareness about the potential risks associated with their use. Patient outcomes depend on consistently applying best practice procedures aligned with guidelines and recommendations and continuing education of all health workers manipulating PIVCs.
While there is agreement on most recommendations, the topic of scheduled versus clinically indicated PIVC-change is still debated. Several studies have been published advocating for PIVC replacement when clinically indicated only [27,28,42,43]. However, it is important to note that these studies used phlebitis as a primary outcome and were not powered to detect a difference in BSI rates because of the very low numbers of observed infections. On the other hand, a much larger cohort study reported a significant increase in the incidence rate ratio of BSIs associated with PIVCs when clinically indicated replacement was implemented. This study included more than 400,000 PIVCs, with a baseline period during which routine replacement was practiced, followed by an intervention period with clinically indicated replacement, and a reversion period going back to routine replacement [40]. The authors agreed that PIVC-associated BSI is a rare outcome and the results of their study may not justify practice change. On the other hand, the real life setting of the study offers a reality check on how the risk associated with the use of PIVC is trivialized. Accordingly, the Australian Commission on Safety and Quality in Health Care published a Clinical Care Standard on the management of PIVCs in May 2021 [44] and addressed this topic by suggesting routine replacement at 72h, and clinically indicated replacement only if the following three criteria are met.
-
•
Prospective surveillance of PIVC-related bloodstream infection
-
•
Comprehensive documentation of insertion, maintenance and removal of PIVCs
-
•
Compliance with competency requirements for insertion and management
This approach seems reasonable and supports a surveillance system that will allow tracking of infections to verify the impact of clinically indicated replacement on infection rates and adjust practice if needed. The European Recommendations for Proper Indication and Use of Peripheral venous Access (ERPIUP) were also published in 2021 [45]. This consensus document distinguishing between short peripheral cannula (<6 cm), long peripheral cannula (6–15 cm), and midline catheters (>15 cm) specifies that the practice of changing the site of short peripheral cannulas on a scheduled basis is not supported by evidence. The document also specifies that clinically indicated replacement requires proper surveillance of line performance and of the visual aspect of the exit site during each shift of nursing.
This expert consensus process has limitations. First, the number of experts was limited, with potential bias in panel member selection, and group member influence on the collective opinion for those members who participated in the group discussion. However, agreement was not unanimous, particularly for topics with low evidence-base, and peer-reviewed publications and recent guidelines were used to support the consensus statements. Second, patients were not represented in the panel. This is not common practice for guideline development or consensus documents, but a patient would have added valuable consumer perspectives.
In conclusion, the most important recommendations are 1) to use PIVCs only when clinically indicated and never “by default”; 2) to locally define and consistently apply standard insertion and maintenance bundles in the use of PIVCs; 3) to remove PIVCs when no longer needed; 4) to combine clinically-indicated removal with rigorous documentation of the insertion site; and 5) to establish surveillance and audit whenever possible to drive appropriate and tailored prevention strategies.
Conflict of interest
All authors and additional survey respondents are consultants for 3M and were under contract with 3M Company for the Advisory Board panel discussion and the preparation of the manuscript.
Funding source
3M funded this activity. 3M facilitated the panel, created the electronic survey used by the participants to vote on the statements, proposed to submit the article for publication, and worked with the lead author (Dr Zingg) to prepare the manuscript. All authors reviewed the final draft and provided input. Dr Zingg had the final say in the content of the publication.
Acknowledgements
The authors would like to thank the additional health care professionals who have also responded to the survey questions to provide a broader input: Mrs Maria Jesus Perez Granda (Spain), Dr Vittorio Ceroto (Italy), Dr Davide Vailati (Italy), Dr Marc Königs (Netherlands), and Mrs Simone van Rijn (Netherlands). All authors and additional survey respondents are consultants for 3M. The authors also thank Stéphanie Bernatchez, PhD, from 3M for her assistance with manuscript preparation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.infpip.2023.100271.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Helm R.E., Klausner J.D., Klemperer J.D., Flint L.M., Huang E. Accepted but unacceptable: peripheral IV catheter failure. J Infusion Nurs. 2015;38(3):189–203. doi: 10.1097/NAN.0000000000000100. [DOI] [PubMed] [Google Scholar]
- 2.Zingg W., Metsini A., Balmelli C., Neofytos D., Behnke M., Gardiol C., et al. National point prevalence survey on healthcare-associated infections in acute care hospitals, Switzerland, 2017. Euro Surveill. 2019;24(32) doi: 10.2807/1560-7917.ES.2019.24.32.1800603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorski L.A., Hadaway L., Hagle M.E., Broadhurst D., Clare S., Kleidon T., et al. Infusion therapy standards of practice. J Infusion Nurs. 2021;44(1S):1–231. doi: 10.1097/NAN.0000000000000396. [DOI] [PubMed] [Google Scholar]
- 4.Alexandrou E., Ray-Barruel G., Carr P.J., Frost S.A., Inwood S., Higgins N., et al. Use of Short Peripheral Intravenous Catheters: Characteristics, Management, and Outcomes Worldwide. J Hosp Med. 2018;13(5) doi: 10.12788/jhm.3039. [DOI] [PubMed] [Google Scholar]
- 5.Helm R.E. Accepted but Unacceptable: Peripheral IV Catheter Failure: 2019 Follow-up. J Infusion Nurs. 2019;42(3):149–150. doi: 10.1097/NAN.0000000000000324. [DOI] [PubMed] [Google Scholar]
- 6.Lim S., Gangoli G., Adams E., Hyde R., Broder M.S., Chang E., et al. Increased Clinical and Economic Burden Associated With Peripheral Intravenous Catheter-Related Complications: Analysis of a US Hospital Discharge Database. Inquiry. 2019;56 doi: 10.1177/0046958019875562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marsh N., Webster J., Ullman A.J., Mihala G., Cooke M., Chopra V., et al. Peripheral intravenous catheter non-infectious complications in adults: A systematic review and meta-analysis. J Adv Nurs. 2020;76(12):3346–3362. doi: 10.1111/jan.14565. [DOI] [PubMed] [Google Scholar]
- 8.Lv L., Zhang J. The incidence and risk of infusion phlebitis with peripheral intravenous catheters: A meta-analysis. J Vasc Access. 2020;21(3):342–349. doi: 10.1177/1129729819877323. [DOI] [PubMed] [Google Scholar]
- 9.Messika J., Roux D., Dreyfuss D., Ricard J.D. Voies veineuses périphériques et risque d’infections acquises en réanimation. Reanimation. 2015;24(3):310–317. doi: 10.1007/s13546-015-1063-5. [DOI] [Google Scholar]
- 10.Zingg W., Pittet D. Peripheral venous catheters: an under-evaluated problem. Int J Antimicrob Agents. 2009;34(Suppl 4):S38–S42. doi: 10.1016/S0924-8579(09)70565-5. [DOI] [PubMed] [Google Scholar]
- 11.Maki D.G., Kluger D.M., Crnich C.J. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81(9):1159–1171. doi: 10.4065/81.9.1159. [DOI] [PubMed] [Google Scholar]
- 12.Worth L.J., Daley A.J., Spelman T., Bull A.L., Brett J.A., Richards M.J. Central and peripheral line-associated bloodstream infections in Australian neonatal and paediatric intensive care units: findings from a comprehensive Victorian surveillance network, 2008-2016. J Hosp Infect. 2018;99(1):55–61. doi: 10.1016/j.jhin.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 13.Pujol M., Hornero A., Saballs M., Argerich M.J., Verdaguer R., Cisnal M., et al. Clinical epidemiology and outcomes of peripheral venous catheter-related bloodstream infections at a university-affiliated hospital. J Hosp Infect. 2007;67:22–29. doi: 10.1016/j.jhin.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 14.Sato A., Nakamura I., Fujita H., Tsukimori A., Kobayashi T., Fukushima S., et al. Peripheral venous catheter-related bloodstream infection is associated with severe complications and potential death: a retrospective observational study. BMC Infect Dis. 2017;17(1):434. doi: 10.1186/s12879-017-2536-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saliba P., Hornero A., Cuervo G., Grau I., Jimenez E., Berbel D., et al. Interventions to decrease short-term peripheral venous catheter-related bloodstream infections: impact on incidence and mortality. J Hosp Infect. 2018;100(3):e178–e186. doi: 10.1016/j.jhin.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Tatsuno K., Ikeda M., Wakabayashi Y., Yanagimoto S., Okugawa S., Moriya K. Clinical Features of Bloodstream Infections Associated with Peripheral Versus Central Venous Catheters. Infect Dis Ther. 2019;8(3):343–352. doi: 10.1007/s40121-019-00257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuboi M., Hayakawa K., Mezaki K., Katanami Y., Yamamoto K., Kutsuna S., et al. Comparison of the epidemiology and microbiology of peripheral line- and central line-associated bloodstream infections. Am J Infect Control. 2019;47:208–210. doi: 10.1016/j.ajic.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Nickel B. Hiding in Plain Sight: Peripheral Intravenous Catheter Infections. Crit Care Nurse. 2020;40(5):57–66. doi: 10.4037/ccn2020439. [DOI] [PubMed] [Google Scholar]
- 19.Mailhe M., Aubry C., Brouqui P., Michelet P., Raoult D., Parola P., et al. Complications of peripheral venous catheters: The need to propose an alternative route of administration. Int J Antimicrob Agents. 2020;55(3) doi: 10.1016/j.ijantimicag.2020.105875. [DOI] [PubMed] [Google Scholar]
- 20.Bhatt C.R., Meek R., Martin C., Stuart R.L., Lim Z., Bumpstead S., et al. Effect of multimodal interventions on peripheral intravenous catheter-associated Staphylococcus aureus bacteremia and insertion rates: An interrupted time-series analysis. Acad Emerg Med. 2021;28:909–912. doi: 10.1111/acem.14225. [DOI] [PubMed] [Google Scholar]
- 21.Ray-Barruel G., Xu H., Marsh N., Cooke M., Rickard C.M. Effectiveness of insertion and maintenance bundles in preventing peripheral intravenous catheter-related complications and bloodstream infection in hospital patients: A systematic review. Infect Dis Health. 2019;24(3):152–168. doi: 10.1016/j.idh.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Mermel L.A. Short-term peripheral venous catheter-related bloodstream infections: A systematic review. Clin Infect Dis. 2017;65(10):1757–1762. doi: 10.1093/cid/cix562. [DOI] [PubMed] [Google Scholar]
- 23.Olivier R.C., Wickman M., Skinner C., Ablir L. The impact of replacing peripheral intravenous catheters when clinically indicated on infection rate, nurse satisfaction, and costs in CCU, Step-Down, and Oncology units. Am J Infect Control. 2021;49(3):327–332. doi: 10.1016/j.ajic.2020.07.036. [DOI] [PubMed] [Google Scholar]
- 24.Steere L., Ficara C., Davis M., Moureau N. Reaching One Peripheral Intravenous Catheter (PIVC) Per Patient Visit With Lean Multimodal Strategy: the PIV5Rights™ Bundle. J Assoc Vasc Access. 2019;24(3):31–43. doi: 10.2309/j.java.2019.003.004. [DOI] [Google Scholar]
- 25.Duncan M., Warden P., Bernatchez S.F., Morse D. A Bundled Approach to Decrease the Rate of Primary Bloodstream Infections Related to Peripheral Intravenous Catheters. J Assoc Vasc Access. 2018;23(1):15–22. doi: 10.1016/j.java.2017.07.004. [DOI] [Google Scholar]
- 26.van Loon F.H., Leggett T., Bouwman A.R., Dierick-van Daele A.T. Cost-utilization of peripheral intravenous cannulation in hospitalized adults: An observational study. J Vasc Access. 2020;21(5):687–693. doi: 10.1177/1129729820901653. [DOI] [PubMed] [Google Scholar]
- 27.Vendramim P., Avelar A.F.M., Rickard C.M., Pedreira M. The RESPECT trial-Replacement of peripheral intravenous catheters according to clinical reasons or every 96 hours: A randomized, controlled, non-inferiority trial. Int J Nurs Stud. 2020;107 doi: 10.1016/j.ijnurstu.2019.103504. [DOI] [PubMed] [Google Scholar]
- 28.Webster J., Osborne S., Rickard C.M., Marsh N. Clinically-indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database Syst Rev. 2019;1:CD007798. doi: 10.1002/14651858.CD007798.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasson F., Keeney S., McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;32(4):1008–1015. doi: 10.1046/j.1365-2648.2000.t01-1-01567.x. [DOI] [PubMed] [Google Scholar]
- 30.McKenna H.P. The Delphi technique: a worthwhile research approach for nursing? J Adv Nurs. 1994;19(6):1221–1225. doi: 10.1111/j.1365-2648.1994.tb01207.x. [DOI] [PubMed] [Google Scholar]
- 31.Mestre G., Berbel C., Tortajada P., Alarcia M., Coca R., Fernandez M.M., et al. Successful multifaceted intervention aimed to reduce short peripheral venous catheter-related adverse events: a quasiexperimental cohort study. Am J Infect Control. 2013;41(6):520–526. doi: 10.1016/j.ajic.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 32.Marsh N., Webster J., Larson E., Cooke M., Mihala G., Rickard C.M. Observational Study of Peripheral Intravenous Catheter Outcomes in Adult Hospitalized Patients: A Multivariable Analysis of Peripheral Intravenous Catheter Failure. J Hosp Med. 2018;13(2):83–89. doi: 10.12788/jhm.2867. [DOI] [PubMed] [Google Scholar]
- 33.Rickard C.M., Marsh N., Webster J., Runnegar N., Larsen E., McGrail M.R., et al. Dressings and securements for the prevention of peripheral intravenous catheter failure in adults (SAVE): a pragmatic, randomised controlled, superiority trial. Lancet. 2018;392(10145):419–430. doi: 10.1016/s0140-6736(18)31380-1. [DOI] [PubMed] [Google Scholar]
- 34.Ben Abdelaziz R., Hafsi H., Hajji H., Boudabous H., Ben Chehida A., Mrabet A., et al. Peripheral venous catheter complications in children: predisposing factors in a multicenter prospective cohort study. BMC Pediatr. 2017;17(1):208. doi: 10.1186/s12887-017-0965-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lemmen S. Hygienemaßnahmen auf der Intensivstation (Infection control guidelines for the intensive care unit) Dt Med Wochenschr. 2009;134(41):2064–2068. doi: 10.1055/s-0029-1237556. [DOI] [PubMed] [Google Scholar]
- 36.van Loon F.H.J., Puijn L.A.P.M., Houterman S., Bouwman A.R.A. Development of the A-DIVA Scale: A Clinical Predictive Scale to Identify Difficult Intravenous Access in Adult Patients Based on Clinical Observations. Medicine. 2016;95(16):e3428. doi: 10.1097/MD.0000000000003428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boord C. Pulsatile Flushing: A Review of the Literature. J Infusion Nurs. 2019;42(1):37–43. doi: 10.1097/NAN.0000000000000311. [DOI] [PubMed] [Google Scholar]
- 38.Fakih M.G., Jones K., Rey J.E., Berriel-Cass D., Kalinicheva T., Szpunar S., et al. Sustained improvements in peripheral venous catheter care in non-intensive care units: a quasi-experimental controlled study of education and feedback. Infect Control Hosp Epidemiol. 2012;33(5):449–455. doi: 10.1086/665322. [DOI] [PubMed] [Google Scholar]
- 39.Rhodes D., Cheng A.C., McLellan S., Guerra P., Karanfilovska D., Aitchison S., et al. Reducing Staphylococcus aureus bloodstream infections associated with peripheral intravenous cannulae: successful implementation of a care bundle at a large Australian health service. J Hosp Infect. 2016;94(1):86–91. doi: 10.1016/j.jhin.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 40.Buetti N., Abbas M., Pittet D., de Kraker M.E.A., Teixeira D., Chraiti M.N., et al. Comparison of Routine Replacement With Clinically Indicated Replacement of Peripheral Intravenous Catheters. JAMA Intern Med. 2021;181:1471–1478. doi: 10.1001/jamainternmed.2021.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blanco-Mavillard I., Rodriguez-Calero M.A., de Pedro-Gomez J., Parra-Garcia G., Fernandez-Fernandez I., Castro-Sanchez E. Incidence of peripheral intravenous catheter failure among inpatients: variability between microbiological data and clinical signs and symptoms. Antimicrob Resist Infect Control. 2019;8:124. doi: 10.1186/s13756-019-0581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rickard C.M., Webster J., Wallis M.C., Marsh N., McGrail M.R., French V., et al. Routine versus clinically indicated replacement of peripheral intravenous catheters: a randomised controlled equivalence trial. Lancet. 2012;380(9847):1066–1074. doi: 10.1016/S0140-6736(12)61082-4. [DOI] [PubMed] [Google Scholar]
- 43.Tuffaha H.W., Rickard C.M., Webster J., Marsh N., Gordon L., Wallis M., et al. Cost-effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Appl Health Econ Health Pol. 2014;12(1):51–58. doi: 10.1007/s40258-013-0077-2. [DOI] [PubMed] [Google Scholar]
- 44.Australian Commission on Safety and Quality in Health Care . 2021. Management of peripheral intravenous catheters clinical care standard. [Google Scholar]
- 45.Pittiruti M., Van Boxtel T., Scoppettuolo G., Carr P., Konstantinou E., Ortiz Miluy G., et al. European recommendations on the proper indication and use of peripheral venous access devices (the ERPIUP consensus): A WoCoVA project. J Vasc Access. 2021 doi: 10.1177/11297298211023274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.