Abstract
Background
Monitoring the effectiveness of COVID-19 vaccines against SARS-CoV-2 infections remains important to inform public health responses. Estimation of vaccine effectiveness (VE) against serological evidence of SARS-CoV-2 infection might provide an alternative measure of the benefit of vaccination against infection.
Methods
We estimated mRNA COVID-19 vaccine effectiveness (VE) against development of SARS-CoV-2 anti-nucleocapsid antibodies in March–October 2021, during which the Delta variant became predominant. Participants were enrolled from four participating healthcare systems in the United States, and completed electronic surveys that included vaccination history. Dried blood spot specimens collected on a monthly basis were analyzed for anti-spike antibodies, and, if positive, anti-nucleocapsid antibodies. We used detection of new anti-nucleocapsid antibodies to indicate SARS-CoV-2 infection, and estimated VE by comparing 154 case-participants with new detection of anti-nucleocapsid antibodies to 1,540 seronegative control-participants matched by calendar period. Using conditional logistic regression, we estimated VE ≥ 14 days after the 2nd dose of an mRNA vaccine compared with no receipt of a COVID-19 vaccine dose, adjusting for age group, healthcare worker occupation, urban/suburban/rural residence, healthcare system region, and reported contact with a person testing positive for SARS-CoV-2.
Results
Among individuals who completed a primary series, estimated VE against seroconversion from SARS-CoV-2 infection was 88.8% (95% confidence interval [CI], 79.6%–93.9%) after any mRNA vaccine, 87.8% (95% CI, 75.9%–93.8%) after BioNTech vaccine and 91.7% (95% CI, 75.7%–97.2%) after Moderna vaccine. VE was estimated to be lower ≥ 3 months after dose 2 compared with < 3 months after dose 2, and among participants who were older or had underlying health conditions, although confidence intervals overlapped between subgroups.
Conclusions
VE estimates generated using infection-induced antibodies were consistent with published estimates from clinical trials and observational studies that used virologic tests to confirm infection during the same period. Our findings support recommendations for eligible adults to remain up to date with COVID-19 vaccination.
Keywords: COVID-19, SARS-CoV-2, Serology, Vaccine effectiveness, COVID-19 vaccines, Adults
1. Background
Following the introduction of mRNA COVID-19 vaccines, estimated vaccine effectiveness (VE) against symptomatic SARS-CoV-2 infection (COVID-19) based on observational studies was similar to vaccine efficacy reported from the original clinical trials [1]. However, VE has declined over time, because of a combination of waning vaccine-induced immunity within individuals, and reduced protection against SARS-CoV-2 variants [1], [2]. Continued monitoring of VE is needed to assess the ongoing benefit of COVID-19 vaccination, including the benefit of additional or booster doses, and among population subgroups.
Although protection against severe illness is most critical for individuals, monitoring VE against any SARS-CoV-2 infection remains important because even mild infections can result in transmission to others [3], and result in post-acute sequelae [4]. However, accurately assessing VE against any infection depends on testing practices, which may change over time and can vary by vaccination status, and reliable estimates of VE against asymptomatic infection are generally limited to cohort studies that include routine testing of asymptomatic persons [5]. Assessment of asymptomatic infection generally requires consistent routine testing of asymptomatic persons in the population, or cohort studies with intensive follow-up testing.
The use of infection-induced seroconversion is a potential additional approach to virological testing in estimating VE against SARS-CoV-2 infection. Since mRNA COVID-19 vaccines elicit antibodies against the SARS-CoV-2 spike protein but not the nucleocapsid protein, serologic assays for anti-nucleocapsid antibodies can be used to distinguish serologic evidence of SARS-CoV-2 infection from vaccination. Several platforms have been established as part of responses to COVID-19 to monitor SARS-CoV-2 seroprevalence in the community [6]. We estimated VE against new SARS-CoV-2 infection using data from a cohort study of healthcare system users who participated in serial serology testing.
2. Methods
2.1. Study design
We performed a nested case-control analysis of data collected in four healthcare systems as part of a prospective cohort study cohort. Case-participants with serological evidence of SARS-CoV-2 infection during March–October 2021 were matched 1:10 to control-participants without serologic evidence of infection during the same period. We restricted the analysis to March 2021 onwards to include a period in which second mRNA doses were widely available. To estimate VE against SARS-CoV-2 infection using serologic data, we compared cases (with serologic evidence of new SARS-CoV-2 infection) to controls (without serologic evidence of any SARS-CoV-2 infection) by previous vaccination status, accounting for differences in other characteristics. We opted for a case-control rather than cohort-based analysis to allow simplification of assumptions about person-time in the context of uncertain timing of infection as ascertained by seroconversion. By conditioning on serology results, this approach was also designed to limit potential selection bias related to use and return of serology kits by study participants [7].
2.2. Study participants
Participants were enrolled in the COVID-19 Community Research Partnership (COVID-19 CRP), a multi-site cohort study in the United States [8], [9]. Persons affiliated with 10 participating healthcare systems were invited to enroll in the study, including patients and staff based at clinical facilities; other community members were invited to participate via patient portals, public websites, or community outreach [8]. Participant follow-up included a daily electronic survey, and permission to access electronic health record (EHR) data. At six participating healthcare systems during November 2020–October 2021, adults aged ≥ 18 years from a demographically representative subgroup of participants were invited to contribute monthly dried blood spot (DBS) specimens for serology testing. The current analysis was limited to participants enrolled at the four of these six healthcare systems: University of Maryland and Medstar Health (defined as ‘Mid-Atlantic’ sites), or Atrium Health and Wake Forest Baptist Health (defined as ‘Southeast’ sites). The other two sites included <2% of participants in the serology component, and were excluded from the analysis due to small sample size.
2.3. Data collection
Daily electronic surveys solicited for presence of any symptoms of COVID-19-like illness, contact with a person with COVID-19, and receipt of any COVID-19 vaccination (Supplementary Box 1). Vaccination status was ascertained using a combination of self-reported data from the daily survey and a supplemental survey that replaced vaccine questions in the daily survey during September 2021, and included date, dose, and product of any COVID-19 vaccine received (Supplementary Box 2). For a subset of participants with vaccination information available in the EHR, EHR information concerning a particular dose was used instead if that dose was reported in the EHR but not in the survey. An enrollment survey was used to collect additional information concerning demographic factors (Supplementary Box 3); underlying health conditions were determined if reported from electronic surveys and/or EHR data (Supplementary Table 1 , Supplementary Table 2). We classified counties of residence as urban, rural, or suburban based on population density from various sources (Supplementary Box 4). For serology testing, participants were asked to perform at-home specimen collection using Whatman 5-spot DBS card, which were requested to be returned monthly for laboratory analysis. All viable specimens received were evaluated for anti-spike antibody using a Euroimmun qualitative assay for SARS-CoV-2 anti-spike IgG [10]; any specimen with a positive result was reflexed to test for anti-nucleocapsid antibody using a qualitative Roche pan-Ig assay [11]. Both serologic assays used were internally validated for use with DBS cards, consistent with others’ evaluation of the Euroimmun assay [12].
Table 1.
Characteristics of participants with and without serologic evidence of SARS-CoV-2 infection.
|
Serologic evidence of new SARS-CoV-2 infection (case-participants, N = 154)a |
No serologic evidence of new SARS-CoV-2 infection (control-participants, N = 1,540)a |
p-valueb | |
|---|---|---|---|
| Age in years | 0.001 | ||
| 18–34 | 24 (15.6%) | 130 (8.4%) | |
| 35–49 | 50 (32.5%) | 353 (22.9%) | |
| 50–64 | 41 (26.6%) | 434 (28.2%) | |
| ≥65 | 39 (25.3%) | 623 (40.5%) | |
| Sex | 0.698 | ||
| Female | 98 (63.6%) | 950 (61.7%) | |
| Male | 56 (36.4%) | 590 (38.3%) | |
| Race and ethnicityc | 0.502 | ||
| Non-Hispanic, White | 129 (83.8%) | 1,312 (85.2%) | |
| Non-Hispanic, Black | 10 (6.5%) | 94 (6.1%) | |
| Hispanic | 8 (5.2%) | 47 (3.1%) | |
| Non-Hispanic, Other | 7 (4.5%) | 87 (5.6%) | |
| Education Level | 0.236 | ||
| College Degree | 115 (75.7%) | 1,210 (80.1%) | |
| No College Degree | 37 (24.3%) | 301 (19.9%) | |
| Healthcare worker | <0.001 | ||
| No | 102 (66.2%) | 1,278 (83.0%) | |
| Yes | 52 (33.8%) | 262 (17.0%) | |
| County classificationd | 0.051 | ||
| Rural | 42 (27.3%) | 359 (23.3%) | |
| Suburban | 52 (33.8%) | 423 (27.5%) | |
| Urban | 60 (39.0%) | 758 (49.2%) | |
| Healthcare system regione | <0.001 | ||
| Mid-Atlantic | 64 (41.6%) | 893 (58.0%) | |
| Southeast | 90 (58.4%) | 647 (42.0%) | |
| Month of ‘previous negative’ testf | 0.4091 | ||
| March 2021 | 20 (13.0%) | 189 (12.3%) | |
| April 2021 | 14 (9.1%) | 119 (7.7%) | |
| May 2021 | 10 (6.5%) | 138 (9.0%) | |
| June 2021 | 8 (5.2%) | 106 (6.9%) | |
| July 2021 | 24 (15.6%) | 311 (20.2%) | |
| August 2021 | 71 (46.1%) | 637 (41.4%) | |
| September 2021 | 7 (4.5%) | 40 (2.6%) | |
| Underlying health conditionsg | 0.851 | ||
| 0 | 39 (25.3%) | 358 (23.3%) | |
| 1 | 40 (26.0%) | 412 (26.8%) | |
| >1 | 75 (48.7%) | 767 (49.9%) | |
| Recent close contact with COVID-19?h | <0.0011 | ||
| No | 73 (47.4%) | 1,360 (89.9%) | |
| Yes | 81 (52.6%) | 152 (10.1%) | |
| Vaccination statusi | |||
| Unvaccinated | 40 (26.0%) | 65 (4.2%) | <0.001 |
| ≥14 days after dose 2 | 114 (74.0%) | 1,475 (95.8%) | |
| Pfizer-BioNTech | 91 (79.8%) | 935 (63.4%) | <0.001 |
| Moderna | 23 (20.2%) | 540 (36.6%) | |
| Median days (interquartile range) between two included serology tests | 31 (27, 37) | 29 (26,36) | 0.047 |
a. No. (%) are shown unless otherwise stated. New serologic evidence of SARS-CoV-2 infection was defined as a positive anti-nucleocapsid antibody following a negative serology test result; the absence of SARS-CoV-2 infection was indicated by a negative anti-spike antibody, or negative anti-nucleocapsid antibody.
b. P-values from Pearson’s Chi-squared tests for categorical variables and Welch’s two sample t-test for continuous variables.
c. ‘Other’ included ‘American Indian or Alaska Native’, and ‘Asian or Pacific Islander’.
d. Counties were categorized as rural, urban or suburban based on estimated population density (see Supplementary Box 4).
e. University of Maryland and Medstar Health (based in Maryland and the District of Columbia) were defined as ‘Mid-Atlantic’ sites; Atrium Health and Wake Forest Baptist Health (based in North Carolina) were defined as ‘Southeast’ sites.
f. ‘Previous negative’ test defined as a serology test indicating no evidence of SARS-CoV-2 infection that was followed by a subsequent positive test (case-participants) or a further negative test (control-participants).
g. Underlying health conditions were defined using electronic health information or self-reported conditions as part of survey responses.
h. Self-reported contact with a person with COVID-19 during the 14 days before the previous negative test was based on the daily survey.
i. Vaccination status was defined on the date of the ‘previous negative’ serology test. Among 114 case-participants who received dose 2 ≥ 14 days previously, 35 (30.7%) received dose 2 < 3 months previously, 59 (51.8%) received dose 2 4–6 months previously, and 20 (17.5%) received dose 2 > 6 months previously; among 1,490 control-participants who received dose 2 ≥ 14 days previously, 609 (40.9%) received dose 2 < 3 months previously, 804 (54.0%) received dose 2 4–6 months previously, and 77 (5.2%) received dose 2 > 6 months previously.
2.4. Analysis definitions
Participants were eligible for inclusion in the analysis if all of the following criteria were met: 1) age ≥ 18 years; 2) there was no self-reported previous SARS-CoV-2 infection from the electronic survey on or before the day of the first available study serology test result; 3) a SARS-CoV-2 serology result indicating no serologic evidence of SARS-CoV-2 infection was collected during March 1, 2021–October 20, 2021; and 4) a subsequent serology test result was available for a specimen collected during the 90 days after the specimen collection date of the initial serology test. We considered results to indicate no serologic evidence of SARS-CoV-2 infection if negative for anti-spike antibody (indicating no evidence of infection or vaccination), or positive for anti-spike antibody but negative for anti-nucleocapsid antibody (indicating no evidence of infection). We defined seroconversion from SARS-CoV-2 infection as new detection of anti-nucleocapsid antibody following a previous result that indicated no serologic evidence of infection.
To account for changes in the probability of SARS-CoV-2 infection over calendar time, each case-participant was matched to 10 control-participants with a serology specimen collected within 14 days of the ‘previous negative’ date for the case-participants, and with at least one subsequent serology result (indicating that seroconversion had not occurred, Supplementary Box 5). Participants were eligible to be controls only if there was serologic evidence of no SARS-CoV-2 infection, and no self-reported positive viral test, at any point during follow-up. To account for multiple negative serology tests available for matching for each control-participant, we used an optimal matching algorithm that selected each control with the closest available calendar date for a test, without replacement [13]. For analysis purposes, we considered the ‘previous negative’ serology result that preceded a subsequent positive serology result to represent the approximate date of infection for case-participants; we used the date of the equivalent ‘previous negative’ result as the index date for control-participants.
Vaccination status was defined on this index date as ‘two doses’ if a second mRNA vaccine dose1 was administered ≥14 days previously, and ‘unvaccinated’ if no vaccine dose had been received. Participants were excluded if they had received a vaccine dose <14 days previously, they had received a non-mRNA vaccine dose, vaccination status changed between the ‘previous negative’ and subsequent serology, they had received only 1 vaccine dose, or if they had received more than 2 vaccine doses. Participant characteristics were also defined based on the ‘previous negative’ date; ‘reported COVID-19 exposure’ was defined as any known close contact with a person with COVID-19 in the 14 days before the ‘previous negative’ date. We considered seroconversion to be ‘symptomatic’ if between 14 days before the date of the previous negative test and the date of the subsequent positive serology test result, participants reported any of the following symptoms: fever, chills, cough, shortness of breath, fatigue, muscle pain, headache, loss of taste/smell, sore throat, congestion/runny nose, nausea/vomiting, or diarrhea.
2.5. Estimation of VE
After matching case- and control-participants, we estimated VE as 100% multiplied by the reciprocal of the odds ratio comparing the odds of having received 2 doses by case/control status, using conditional logistic regression to account for matching by calendar time. In multivariable models, we adjusted for several additional factors in the models that were hypothesized to lead to differential risk of exposure to SARS-CoV-2, and that also differed between case and control participants: age group (18–49, >50 years), health care worker occupation (yes, no), healthcare system region (Mid-Atlantic, Southeast), county type (rural, suburban, urban), and reported close contact with a person with COVID-19 in the 14 days before the ‘previous negative’ date. We estimated VE overall, and by product, age group, and months since dose 2 (<3 months or ≥3 months). We also estimated VE by whether infection occurred during the pre-Delta period (defined as March 1, 2021–June 30, 2021) or the Delta period (defined as July 1, 2021–October 31, 2021).
For our primary analysis we assumed that infection occurred on the date of the negative test that preceded a serology result indicating new infection. Since some participants might have had infection even earlier than the ‘previous negative’ date, we assessed robustness of our definition by performing a sensitivity analysis using an alternative definition of infection as 14 days before the ‘previous negative’ collection date (with exposure periods redefined accordingly). We also repeated VE estimates limited to symptomatic infection. We performed supportive analyses to assess the performance of serology compared with self-reported infection between 14 days before the previous negative serology test and more than 14 days before the subsequent serology test. We assessed whether eligible control-participants had self-reported a positive swab, and whether seropositivity after self-reported infection differed by vaccination status. To estimate the effect on VE of misclassification of SARS-CoV-2 infection by using serologic evidence as a proxy, we calculated hypothetical VE assuming different values of specificity and sensitivity for serology as a proxy for SARS-CoV-2 infection. Finally, we assessed whether case-participants remain seropositive on subsequent serology results. All statistical analyses were performed using R Statistical Software (version 4.0.3; R Foundation for Statistical Computing, Vienna, Austria).
The study was reviewed and approved by the Wake Forest Institutional Review Board (IRB), which served as the central IRB for this study (See 45C.F.R. part 46; 21C.F.R. part 56).
3. Results
3.1. Study participants
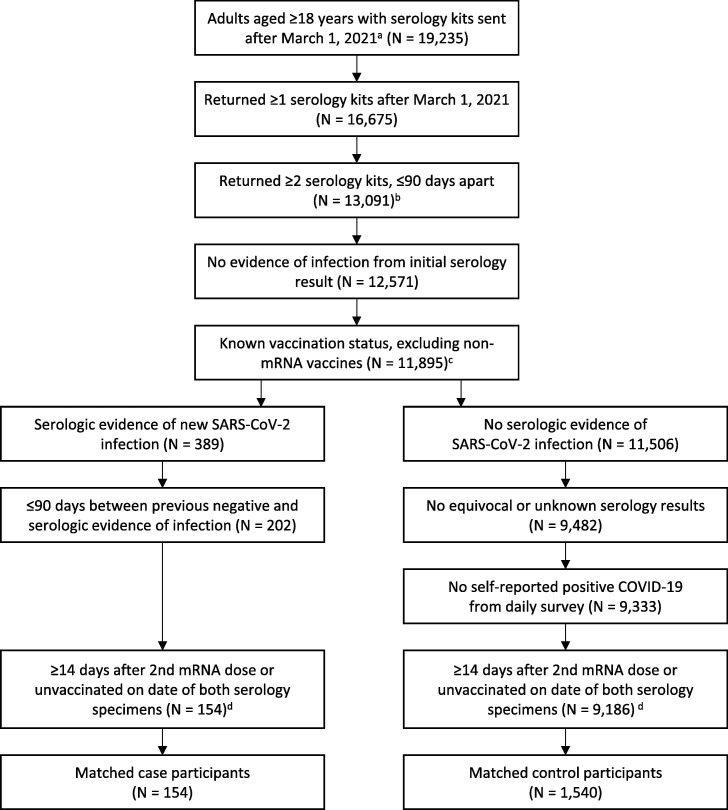
Among 19,235 participants who were allocated serology test kits from March 1 to October 20, 2021, 14,255 had at least two serology test results from specimens collected ≤ 90 days apart. Participants with at least 2 serology test results were more likely to be older, male, non-Hispanic White, to live in a suburban county, and less likely to be a healthcare worker (Supplementary Table 3). After applying exclusion criteria, 154 eligible case-participants were matched to 1,540 of 9,186 (16.7%) eligible control-participants (Fig. 1 ). Among the 1,694 case- and control-participants, 1691 (99.8%) had vaccination information from either survey, and 261 (15.4%) had EHR information. For three participants, vaccination status was based on EHR information alone (Supplementary Table 4).
Fig. 1.
Participants of the COVID-19 Community Research Partnership Study included in analysis of vaccine effectiveness using serologic data. a. Among 29,187 adults enrolled in the study via one of the 4 included health systems in this analysis, 27,535 did not report a previous COVID-19 diagnosis at the time of study enrollment. Of these, 19,235 were sent at least one serology kit between March 1, 2021 and October 31, 2021, and 17,235 returned one or more serology dried blood spot specimens. b. Among 16,675 participants who returned ≥1 serology kits after March 1, 2021, 15,125 returned at least 2 serology kits. c. Among the 676 participants excluded, 625 were excluded because they only received one dose or a non-mRNA vaccine; the remaining 51 participants had an unknown vaccination status. d. Test kit results were excluded based on the timing of vaccination status if a participant was categorized as “partially vaccinated” or having received a third dose at the collection date for the previous negative test.
3.2. Characteristics of case- and control-participants
Participants with seroconversion from SARS-CoV-2 infection were more likely to be younger, to be a healthcare worker, to reside in a county classified as rural or suburban, and to use a healthcare system in the southeast region, compared with control-participants without seroconversion from SARS-CoV-2 infection (Table 1). Case-participants were also more likely than control-participants to report close contact with a person with SARS-CoV-2. Several other characteristics were similar, including the prevalence of underlying health conditions (Table 1, Supplementary Table 2).
3.3. Estimation of vaccine effectiveness
Overall, 114 (74.0%) of all case-participants had received dose 2 of an mRNA vaccine ≥ 14 days earlier, of whom 91 (79.8%) had received Pfizer-BioNTech and 23 (20.2%) received Moderna vaccine. Among control-participants, 1,475 (95.8%) received dose 2 of an mRNA vaccine ≥ 14 days earlier, of whom 935 (63.4%) had received Pfizer-BioNTech and 540 (36.6%) had received Moderna vaccine (Table 1). Using conditional logistic regression, overall VE against seroconversion from SARS-CoV-2 infection by any mRNA vaccine was 91.0 (85.0–94.6) unadjusted, and 88.8% (95% CI, 79.6%–93.9%) when adjusted for covariates. Adjusted VE was 87.8% (95% CI, 75.9%–93.8%) with Pfizer-BioNTech vaccine, and 91.7% (95% CI, 75.7%–97.2%) with Moderna vaccine. VE estimates were slightly higher during the pre-Delta period (93.2%, 95% CI 80.9%–97.5%) compared with the Delta period (89.0%, 95% CI 72.0%–95.6%). Estimated VE was lower if there was ≥3 months since dose 2 (81.6%, 95% CI 56.6%–92.2%) compared with <3 months since dose 2 (93.5%, 95% CI 83.8%–97.4%); if age ≥50 (75.9%, 95% CI 36.3%–90.9%) compared with younger age groups (95.2%, 95% CI 83.9%–98.6%); and if >1 underlying health condition (85.9%, 95% CI 52.2%–95.9%) compared with no underlying health condition (93.9%, 95% CI 45.1–99.3) or one underlying health condition (89.7%, 95% CI 36.3%–98.3%). However, all of these estimates were similar, with overlapping confidence intervals (Table 2 ).
Table 2.
Estimated effectiveness ≥ 14 days after 2nd mRNA COVID-19 vaccination against new detection of SARS-CoV-2 anti-nucleocapsid antibody, March–October 2021.
| Characteristic | Case-participantsa | Control-participantsa |
VE (95% CI) Unadjustedb |
Case-participantsa | Control-participantsa |
VE (95% CI) Adjustedb |
|---|---|---|---|---|---|---|
| By vaccine product | ||||||
| Any mRNA | 154 | 1540 | 91.0 (85.0–94.6) | 154 | 1512 | 88.8 (79.6–93.9) |
| Pfizer-BioNTech | 131 | 1000 | 89.0 (81.0–93.6) | 131 | 982 | 87.8 (75.9–93.8) |
| Moderna | 63 | 605 | 95.9 (89.4–98.4) | 63 | 595 | 91.7 (75.7–97.2) |
| By calendar periodc | ||||||
| Pre-Delta | 52 | 552 | 93.0 (84.9–96.8) | 52 | 541 | 93.2 (80.9–97.5) |
| Delta | 102 | 988 | 92.5 (83.7–96.5) | 102 | 971 | 89.0 (72.0–95.6) |
| By time since dose 2 | ||||||
| <3 months | 75 | 686 | 92.5 (85.1–96.2) | 75 | 675 | 93.5 (83.8–97.4) |
| ≥3 months | 119 | 919 | 88.0 (75.5–94.2) | 119 | 902 | 81.6 (56.6–92.2) |
| By age group (years) | ||||||
| 18–49 | 74 | 483 | 93.6 (83.1–97.6) | 74 | 471 | 95.2 (83.9–98.6) |
| ≥50 | 80 | 1057 | 81.0 (55.7–91.8) | 80 | 1041 | 75.9 (36.3–90.9) |
| By underlying health conditiond | ||||||
| 0 | 39 | 358 | 92.6 (65.3–98.4) | 39 | 352 | 93.9 (45.1–99.3) |
| 1 | 40 | 412 | 84.4 (49.2–95.2) | 40 | 403 | 89.7 (36.3–98.3) |
| >1 | 75 | 767 | 85.0 (63.8–93.8) | 75 | 757 | 85.9 (52.2–95.9) |
a. Case-participants had new serologic evidence of SARS-CoV-2 infection, defined as a positive anti-nucleocapsid antibody following a negative serology test result; control-participant had serologic evidence of no infection, indicated by a negative anti-spike antibody, or negative anti-nucleocapsid antibody.
b. Vaccine effectiveness (VE) was estimated as 100- (100 × odds ratio) with the unvaccinated group as the referent. Conditional logistic regression models accounted for matching by calendar time and adjusted for region of enrollment site, age, healthcare worker occupation, population density of county of residence, and recent close contact with COVID-19.
c. Pre-Delta period defined as March 1, 2021–June 30, 2021; Delta period defined as July 1, 2021–October 31, 2021.
d. Defined using self-report and electronic health record information as autoimmune disease, cardiovascular disease, diabetes, immunocompromised state, liver disease, obesity, pulmonary disease, renal disease, substance abuse, mental health conditions, neurologic disease, sickle cell disease.
3.4. Additional analyses
Sensitivity analyses assessing VE against symptomatic seroconversion or assuming infection occurred 14 days before the ‘previous negative’ date produced similar estimates of VE (Supplementary Table 5). We also examined the relationship between the serologic endpoint of infection and the timing of self-reported positive virologic test results. Among case-participants who reported a positive virologic test between 28 days before the ‘previous negative’ serology result and the subsequent positive result, 92/92 (100%) participants reported that the virologic test was on or after 14 days before the ‘previous negative’, and 91/94 (97%) participants reported that the test was >14 days before the ‘subsequent positive’ serology test (Supplementary Table 6). Among eligible control-participants with at least one self-reported virologic test result that was between 14 days before a negative serology test and more than 14 days before the subsequent serology test, 2596/2604 (99%) had only negative virologic test results during this time; those with a positive self-reported result were excluded from the analysis.
Among participants with at least one self-reported positive swab during the period from 14 days before a negative serology result to more than 14 days before a subsequent serology result, 72/91 (79%) had a subsequent serology result consistent with SARS-CoV-2 infection 30–90 days after the positive swab. Among participants who were ≥14 days after a 2nd mRNA vaccine dose on the day of the initial negative serology, 49/64 (77%) had a subsequent positive serology result; among unvaccinated participants, 23/27 (85%) had a subsequent positive serology result (Supplementary Table 7).
Under hypothetical conditions, VE against seroconversion from SARS-CoV-2 infection was similar to unobserved VE against SARS-CoV-2 infection under the assumption that serology was 99% specific for self-reported infection and 80% sensitive. VE against seroconversion would be higher than VE against infection if vaccinated persons were less likely to seroconvert. However, the simulation indicated a minimal difference between unobserved VE against infection and VE against seroconversion under values obtained in this study, and modest differences at relatively high VE values even with substantial differences in seroconversion by vaccination status (Supplementary Table 8). For participants with subsequent serology tests after an initial positive anti-nucleocapsid antibody, approximately 80% remained positive during the next 0–60 days (Supplementary Table 9).
4. Discussion
VE of a two-dose mRNA COVID-19 vaccine series against seroconversion from SARS-CoV-2 infection was approximately 89% against any SARS-CoV-2 infection during March–October 2021, during periods of predominance by pre-Delta and Delta variants. VE estimates of 88% after Pfizer BioNTech vaccine and 92% after Moderna vaccine were similar to estimates against symptomatic infection reported in respective clinical trials [14], [15], and to estimates against any infection or asymptomatic infection from several observational studies during the same period [1], [16], [17]. We found that overall VE estimates were slightly lower (82% vs. 94%) at least 3 months after dose 2 compared with<3 months after dose 2, consistent with other studies indicating some waning of VE during this period [1]. Previous studies have shown lower VE during the Delta period; lower VE has also been estimated among older age groups and those with more comorbidities [1], [18]. Our findings are broadly consistent with other studies, although overlapping confidence intervals preclude definitive comparisons between subgroups.
To infer VE against SARS-CoV-2 infection from serologic evidence, several potential limitations need to be addressed. First, the use of serologic endpoints to estimate VE against SARS-CoV-2 infection could result in misclassification of case- or control-participants. Seroprevalence studies have used anti-nucleocapsid antibody as evidence of SARS-CoV-2 infection in the population [19], and the assays selected are highly specific—99.5% specificity was estimated for the Roche assay used in the current analysis [20]. Therefore, seroconversion to anti-nucleocapsid antibody is unlikely to include false-positive results among case-participants. Misclassification of seronegative control-participants might also occur if SARS-CoV-2 infections are not detected by serology. Sensitivity of the Roche anti-nucleocapsid assay is estimated to be approximately 96%, although effective sensitivity might be lower in this study because the anti-nucleocapsid assay was only performed after a positive Euroimmun anti-spike assay, which has an estimated sensitivity of 91% [20]. Even with a slightly lower sensitivity, few individuals with SARS-CoV-2 infection are likely to have been misclassified as control-participants. Among participants with self-reported virologic test results, <1% of control-participants reported a positive virologic test result between 14 days before one negative serology test and more than 14 days before a subsequent negative serology test, and these participants were excluded from the analysis.
A second potential challenge in using serologic evidence of infection as an endpoint for VE arises if vaccination is associated with decreased seroconversion, which would result in over-representation of vaccinated individuals among the control-participants, and an overestimation of VE. This phenomenon has been reported for influenza vaccines, with less seroconversion after receipt of an inactivated influenza vaccine, although this was less of a problem for live attenuated vaccines [21]. For SARS-CoV-2, a lower rate of seroconversion might be expected after vaccination since infections after vaccination are generally milder [22], and milder infections are associated with lower levels of antibody response [23]. Since neutralizing anti-spike antibodies mediate vaccine-induced immunity it is also plausible that VE is higher after seroconversion because individuals who tend to produce more antibody after infection might also tend to produce more protective antibodies after vaccination [24]. A previous study demonstrated a modest decrease in anti-nucleocapsid antibody titers following vaccination, but this did not result in a substantial difference in seropositivity [25]. By contrast, a subsequent study that included analysis of Moderna vaccine trial data noted seroconversion after confirmed infection in only 40% of intervention-recipients compared with 93% of placebo-recipients. This difference appeared to be mediated by differences in viral load that might reflect milder infection after vaccination [26].
However, the impact of such a difference might be tempered if there is limited variation in severity of infection, or if a high proportion of infections result in seroconversion. In the current study, approximately 79% of participants with a positive virologic test result seroconverted to a positive anti-nucleocapsid antibody—77% among vaccinated participants and 85% among unvaccinated participants. The high ratio of control- to case-participants in the current analysis also limits the impact of sensitivity on misclassification by limiting the proportion of potential ‘false positives’ among control-participants. We estimated that these values would have minimal effect on estimates under the conditions of this analysis, and that using parameters from the Moderna vaccine trial data would also have a modest impact on overall findings.
Third, compared with endpoints using virus detection, serologic endpoints can present an additional challenge because the timing of infection is uncertain. In the current study, this uncertainty was mitigated by collection of serial serology samples, and by assessing seroconversion rather than only seropositivity. We used a case-control approach to limit assumptions about person-time at risk and found that VE estimates were similar under an alternative assumption that infection occurred 14 days earlier. This also limited the potential for participants to have been vaccinated after the date of infection. A related potential limitation is loss of antibody detection over time due to waning antibody levels. However, the sensitivity of many SARS-CoV-2 serologic assays remains stable over several months, including those used in this study [20]. In our analysis the time between serologic tests used was usually about 30 days.
Lastly, our analysis is subject to limitations common to other analyses of VE [27], [28]. Estimation of VE assumes that differences in infection by vaccination status reflect differences in vaccine-induced immunity rather than exposure or ascertainment that could otherwise confound the observed association [29]. In addition to matching by calendar time we were able to account for measured differences in location, demographic factors and known contact with a person with COVID-19. Estimates were similar before and after adjustment, although residual or unmeasured confounding could still lead to bias. High vaccination coverage among participants might have led to bias if the unvaccinated group were unrepresentative of the population [28]; the limited number of unvaccinated participants also limited statistical power to compare subgroups. This might have also affected estimates over time. A separate analysis of the study cohort indicated that during the Delta period, unvaccinated persons were less likely to wear a mask [30]—a higher risk of exposure among unvaccinated participants compared with vaccinated participants might have inflated estimated VE estimates during this period. Conditioning on serology results from returned tests limited potential selection bias from differences in return rates. However, lack of representativeness among participants who returned specimens for serology tests could limit generalizability to other settings. Other differences between study participants and the general U.S. population might limit national representativeness, although VE estimates during the study period were generally similar among different subgroups [18]. We also relied on self-reported vaccination status, although an analysis of study data indicated more than 90% concordance for reported vaccination timing between EHR and self-report, and more than 98% concordance for vaccine product information [31]. Lastly, our analysis was limited to data collected until October 2021, and does not include estimates of VE since the emergence of the Omicron variant.
After considering the potential limitations above, the use of serology to estimate VE provides an additional method to estimate VE against SARS-CoV-2 infection. Compared with ascertainment of SARS-CoV-2 infection using data from virologic testing performed as part of routine care, the current study is not prone to access bias or diagnostic bias, which can lead to differential access to healthcare or thresholds for clinical testing by vaccination status [27], [28], [32]. Serology can also exclude persons with previous infection, limiting possible bias from infection-induced immunity from undetected prior infection at baseline or from accrual of infection-induced immunity among unvaccinated persons during the study period [27], [28], [33]. Use of quantitative assays might also enable detection of SARS-CoV-2 reinfections using serology because of boosted antibody levels, and thereby enable the inclusion of persons with previous infection [34], [35].
Compared with prospective cohort studies that include regular virologic testing, the use of serologic data also has some logistical advantages. First, notwithstanding the need to assess vaccination status before infection, detection of SARS-CoV-2 infection using serology requires less frequent testing. Although some serologic assays for SARS-CoV-2 lose sensitivity over time, others remain highly sensitive over several months [20]. Second, we demonstrated the feasibility of collecting specimens in this study using DBS via at-home collection. At-home testing has the benefits of convenience, reduced costs and minimized infection control risks while enabling longitudinal surveillance. Third, many countries have established national or regional serologic surveillance programs which might be leveraged to assess VE against seroconversion [6], [36].
In addition to the use of serology to ascertain SARS-CoV-2 infection, seroconversion from SARS-CoV-2 infection might itself be considered an important outcome to prevent. Detection of anti-nucleocapsid antibodies in the blood indicates a systemic immune response to SARS-CoV-2 infection that includes both cellular and humoral components [37], [38]. Consistent with this, seroconversion is generally reflective of a higher viral load and more severe illness [23], [39]; higher viral load might also lead to increased risk of transmission to others [40]. Vaccine-induced protection against SARS-CoV-2 infection leading to seroconversion therefore represents an important public health benefit.
To apply serologic surveillance data for estimation of ongoing effectiveness of COVID-19 vaccines, it will be best to ensure linkage to validated vaccination data, to use serologic assays that retain sensitivity over time, and to validate or test analysis assumptions by comparing estimates with those from other data sources [20], [28]. In this study we have demonstrated VE estimates using serologic data that are consistent with other studies that analyzed data during predominant circulation by the Delta variant [1], and with an analysis of serologic data from repeat blood donors [41]. Other studies of vaccine effectiveness have indicated that, despite waning of VE over time and lower protection since the emergence of the Omicron variant, COVID-19 vaccines continue to offer substantial protection against severe disease [42], [43]. Protection can be maintained by remaining up to date with COVID-19 vaccines, and persons aged ≥ 16 years are recommended to receive a bivalent booster dose when eligible [44].
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [Roberto P. Santos has received honoraria and/or travel grants from the American Board of Pediatrics (2020–2025), the American Academy of Pediatrics (2022), and the Mississippi Osteopathic Medicine Association (2022) 2022. Dr. Santos is also site principal investigator/coordinator for a contract awarded to the University of Mississippi Medical Center (UMMC) with Eli Lilly, Janssen Pharmaceutical Companies of Johnson & Johnson, Merck Sharp & Dohme (MSD), and the Health Resources and Services Administration (2020–2021).].
Acknowledgments
Acknowledgements
Jefferson Jones, Meredith McMorrow, Tamara Pilishvili (CDC)
Funding source
This publication was supported by the Centers for Disease Control and Prevention (CDC) [Contract #75D30120C08405] and the CARES Act of the U.S. Department of Health and Human Services (HHS) [Contract # NC DHHS GTS #49927].
Additional statements
All authors attest they meet the ICMJE criteria for authorship.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
An mRNA COVID-19 vaccine dose was defined as receipt of Pfizer-BioNTech COVID-19 vaccine or Moderna COVID-19 vaccine. For recommended administration of these vaccines, see https://www.fda.gov/emergency-preparedness-and-response/counterterrorism-and-emerging-threats/coronavirus-disease-2019-covid-19.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2023.03.006.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Feikin D.R., Higdon M.M., Abu-Raddad L.J., Andrews N., Araos R., Goldberg Y., et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399:924–944. doi: 10.1016/S0140-6736(22)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tartof S.Y., Slezak J.M., Fischer H., Hong V., Ackerson B.K., Ranasinghe O.N., et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayek S., Shaham G., Ben-Shlomo Y., Kepten E., Dagan N., Nevo D., et al. Indirect protection of children from SARS-CoV-2 infection through parental vaccination. Science. 2022;375:1155–1159. doi: 10.1126/science.abm3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dooling K., Gargano J.W., Moulia D., Wallace M., Rosenblum H.G., Blain A.E., et al. Use of Pfizer-BioNTech COVID-19 vaccine in persons aged ≥16 years: recommendations of the advisory committee on immunization practices - United States, September 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1344–1348. doi: 10.15585/mmwr.mm7038e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.NIH. COVID-19 SeroHub. https://covid19serohub.nih.gov/ (accessed March 8, 2023).
- 7.Vandenbroucke J.P., Pearce N. Test-negative designs: differences and commonalities with other case-control studies with “Other Patient” controls. Epidemiology. 2019;30:838–844. doi: 10.1097/EDE.0000000000001088. [DOI] [PubMed] [Google Scholar]
- 8.The COVID-19 Community Research Partnership: a multistate surveillance platform for characterizing the epidemiology of the SARS-CoV-2 pandemic. Biol Methods Protoc 2022; 7: bpac033. [DOI] [PMC free article] [PubMed]
- 9.Wake Forest University Health S. A Multicenter, Prospective Study of COVID-19 Using Real-Time Syndromic Surveillance, Scheduled At-home Serologic Testing, and Electronic Health Records. clinicaltrials.gov; 2022.
- 10.FDA. EUROIMMUN Anti-SARS-CoV-2 ELISA (IgG). 2020. https://www.fda.gov/media/137609/download (accessed April 16, 2022)
- 11.FDA. Elecsys Anti-SARS-CoV-2. 2020. https://www.fda.gov/media/137605/download (accessed April 16, 2022)
- 12.Walker Gregory J, Davis R, Naing Z, McEntee B, Lu Y, Denadija T, et al. Serological detection of SARS-CoV-2 IgG using commercially available enzyme immunoassays on dried blood spots collected from patients. Microbiol Spectrum. 9:e01245-21. [DOI] [PMC free article] [PubMed]
- 13.Mamouris P., Nassiri V., Molenberghs G., van den Akker M., van der Meer J., Vaes B. Fast and optimal algorithm for case-control matching using registry data: application on the antibiotics use of colorectal cancer patients. BMC Med Res Method. 2021;21:62. doi: 10.1186/s12874-021-01256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Sahly H.M., Baden L.R., Essink B., Doblecki-Lewis S., Martin J.M., Anderson E.J., et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med. 2021;385:1774–1785. doi: 10.1056/NEJMoa2113017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas S.J., Moreira E.D., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med. 2021;385:1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CDC. Grading of recommendations, assessment, development, and evaluation (GRADE): moderna COVID-19 vaccine; 2022. https://www.cdc.gov/vaccines/acip/recs/grade/bla-covid-19-moderna-vaccine.html (accessed March 8, 2023).
- 17.Wallace M., Collins J.P., Moline H., Plumb I.D., Godfrey M., Morgan R.L., et al. Effectiveness of Pfizer-BioNTech COVID-19 vaccine as evidence for policy action: a rapid systematic review and meta-analysis of non-randomized studies. PLoS One. 2022;17:e0278624. doi: 10.1371/journal.pone.0278624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilishvili T., Gierke R., Fleming-Dutra K.E., Farrar J.L., Mohr N.M., Talan D.A., et al. Effectiveness of mRNA Covid-19 vaccine among U.S. Health Care Personnel. N Engl J Med. 2021;385:e90. doi: 10.1056/NEJMoa2106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones J.M., Stone M., Sulaeman H., Fink R.V., Dave H., Levy M.E., et al. Estimated US infection- and vaccine-induced SARS-CoV-2 seroprevalence based on blood donations, July 2020-May 2021. JAMA. 2021;326:1400–1409. doi: 10.1001/jama.2021.15161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stone M., Grebe E., Sulaeman H., Di Germanio C., Dave H., Kelly K., et al. Evaluation of commercially available high-throughput SARS-CoV-2 serologic assays for serosurveillance and related applications. Emerg Infect Dis. 2022;28:672–683. doi: 10.3201/eid2803.211885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrie J.G., Ohmit S.E., Johnson E., Cross R.T., Monto A.S. Efficacy studies of influenza vaccines: effect of end points used and characteristics of vaccine failures. J Infect Dis. 2011;203:1309–1315. doi: 10.1093/infdis/jir015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scobie HM, Johnson AG, Suthar AB, Severson R, Alden NB, Balter S, et al. Monitoring Incidence of COVID-19 Cases, Hospitalizations, and Deaths, by Vaccination Status - 13 U.S. Jurisdictions, April 4-July 17, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1284–90. [DOI] [PMC free article] [PubMed]
- 23.Peluso Michael J, Takahashi S, Hakim J, Kelly JD, Torres L, Iyer Nikita S, et al. SARS-CoV-2 antibody magnitude and detectability are driven by disease severity, timing, and assay. Sci Adv. 7:eabh3409. [DOI] [PMC free article] [PubMed]
- 24.Gilbert Peter B., Montefiori David C., McDermott Adrian B., Fong Y., Benkeser D., Deng W., et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375:43–50. doi: 10.1126/science.abm3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitaker HJ, Gower C, Otter AD, Simmons R, Kirsebom F, Letley L, et al. Nucleocapsid antibody positivity as a marker of past SARS-CoV-2 infection in population serosurveillance studies: impact of variant, vaccination, and choice of assay cut-off. medRxiv. 2021:2021.10.25.21264964.
- 26.Follmann D., Janes H.E., Buhule O.D., Zhou H., Girard B., Marks K., et al. Antinucleocapsid antibodies after SARS-CoV-2 infection in the blinded phase of the randomized, placebo-controlled mRNA-1273 COVID-19 vaccine efficacy clinical trial. Ann Intern Med. 2022;175:1258–1265. doi: 10.7326/M22-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewnard J.A., Patel M.M., Jewell N.P., Verani J.R., Kobayashi M., Tenforde M.W., et al. Theoretical framework for retrospective studies of the effectiveness of SARS-CoV-2 vaccines. Epidemiology. 2021;32:508–517. doi: 10.1097/EDE.0000000000001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO. Evaluation of COVID-19 vaccine effectiveness: Interim Guidance. https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccine_effectiveness-measurement-2021.1 (accessed March 5, 2022).
- 29.Loughlin AM, Strathdee SA, editors. Vaccines: Past, Present, and Future. Infectious disease epidemiology. 3rd ed. Jones & Bartlett Learning; Burlington: 2014. pp. 273–300. [Google Scholar]
- 30.Calamari L.E., Tjaden A.H., Edelstein S.L., Weintraub W.S., Santos R., Gibbs M., et al. Self-reported mask use among persons with or without SARS CoV-2 vaccination —United States, December 2020–August 2021. Prev Med Rep. 2022;28 doi: 10.1016/j.pmedr.2022.101857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tjaden A.H., Fette L.M., Edelstein S.L., Gibbs M., Hinkelman A.N., Runyon M., et al. Self-reported SARS-CoV-2 vaccination is consistent with electronic health record data among the COVID-19 community research partnership. Vaccines. 2022;10 doi: 10.3390/vaccines10071016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dean N.E., Hogan J.W., Schnitzer M.E. Covid-19 vaccine effectiveness and the test-negative design. N Engl J Med. 2021;385:1431–1433. doi: 10.1056/NEJMe2113151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kahn R., Schrag S.J., Verani J.R., Lipsitch M. Identifying and alleviating bias due to differential depletion of susceptible people in postmarketing evaluations of COVID-19 vaccines. Am J Epidemiol. 2022:kwac015. doi: 10.1093/aje/kwac015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siddiqui Sameed M, Bowman Kathryn A, Zhu Alex L, Fischinger S, Beger S, Maron Jenny S, et al. Serological markers of SARS-CoV-2 reinfection. mBio.13:e02141-21. [DOI] [PMC free article] [PubMed]
- 35.Edridge A.W.D., Kaczorowska J., Hoste A.C.R., Bakker M., Klein M., Loens K., et al. Seasonal coronavirus protective immunity is short-lasting. Nat Med. 2020;26:1691–1693. doi: 10.1038/s41591-020-1083-1. [DOI] [PubMed] [Google Scholar]
- 36.Arora R.K., Joseph A., Van Wyk J., Rocco S., Atmaja A., May E., et al. SeroTracker: a global SARS-CoV-2 seroprevalence dashboard. Lancet Infect Dis. 2021;21:e75–e76. doi: 10.1016/S1473-3099(20)30631-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cromer D., Juno J.A., Khoury D., Reynaldi A., Wheatley A.K., Kent S.J., et al. Prospects for durable immune control of SARS-CoV-2 and prevention of reinfection. Nat Rev Immunol. 2021;21:395–404. doi: 10.1038/s41577-021-00550-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poland G.A., Ovsyannikova I.G., Kennedy R.B. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet. 2020;396:1595–1606. doi: 10.1016/S0140-6736(20)32137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Follmann D, Janes HE, Buhule OD, Zhou H, Girard B, Marks K, et al. Anti-nucleocapsid antibodies following SARS-CoV-2 infection in the blinded phase of the mRNA-1273 Covid-19 vaccine efficacy clinical trial. medRxiv. 2022:2022.04.18.22271936. [DOI] [PMC free article] [PubMed]
- 40.Marks M., Millat-Martinez P., Ouchi D., Roberts C.h., Alemany A., Corbacho-Monné M., et al. Transmission of COVID-19 in 282 clusters in Catalonia, Spain: a cohort study. Lancet Infect Dis. 2021;21:629–636. doi: 10.1016/S1473-3099(20)30985-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grebe E., Yu E.A., Bravo M.D., Welte A., Bruhn R.L., Stone M., et al. Coronavirus disease 2019 vaccine effectiveness against severe acute respiratory syndrome coronavirus 2 infection in the united states before the delta- and omicron-associated surges: a retrospective cohort study of repeat blood donors. J Infect Dis. 2022;226:1556–1561. doi: 10.1093/infdis/jiac318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferdinands J.M., Rao S., Dixon B.E., Mitchell P.K., DeSilva M.B., Irving S.A., et al. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance - VISION Network, 10 States, August 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:255–263. doi: 10.15585/mmwr.mm7107e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tartof S.Y., Slezak J.M., Puzniak L., Hong V., Xie F., Ackerson B.K., et al. Durability of BNT162b2 vaccine against hospital and emergency department admissions due to the omicron and delta variants in a large health system in the USA: a test-negative case–control study. Lancet Respir Med. 2022;10:689–699. doi: 10.1016/S2213-2600(22)00101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.CDC Recommends the First Updated COVID-19 Booster. Centers for Disease Control and Prevention 2022. https://www.cdc.gov/media/releases/2022/s0901-covid-19-booster.html (accessed September 18, 2022)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.