Abstract
Insulin stimulates glucose uptake into skeletal muscle tissue mainly through the translocation of glucose transporter 4 (GLUT4) to the plasma membrane. The precise mechanism involved in this process is presently unknown. In the cascade of events leading to insulin-induced glucose transport, insulin activates specific protein kinase C (PKC) isoforms. In this study we investigated the roles of PKCζ in insulin-stimulated glucose uptake and GLUT4 translocation in primary cultures of rat skeletal muscle. We found that insulin initially caused PKCζ to associate specifically with the GLUT4 compartments and that PKCζ together with the GLUT4 compartments were then translocated to the plasma membrane as a complex. PKCζ and GLUT4 recycled independently of one another. To further establish the importance of PKCζ in glucose transport, we used adenovirus constructs containing wild-type or kinase-inactive, dominant-negative PKCζ (DNPKCζ) cDNA to overexpress this isoform in skeletal muscle myotube cultures. We found that overexpression of PKCζ was associated with a marked increase in the activity of this isoform. The overexpressed, active PKCζ coprecipitated with the GLUT4 compartments. Moreover, overexpression of PKCζ caused GLUT4 translocation to the plasma membrane and increased glucose uptake in the absence of insulin. Finally, either insulin or overexpression of PKCζ induced serine phosphorylation of the GLUT4-compartment-associated vesicle-associated membrane protein 2. Furthermore, DNPKCζ disrupted the GLUT4 compartment integrity and abrogated insulin-induced GLUT4 translocation and glucose uptake. These results demonstrate that PKCζ regulates insulin-stimulated GLUT4 translocation and glucose transport through the unique colocalization of this isoform with the GLUT4 compartments.
One of the fundamental actions of insulin is to stimulate the uptake of glucose from blood into tissues. This uptake occurs via facilitated diffusion by specific proteins known as glucose transporters (GLUT), which are translocated from intracellular pools to the plasma membrane (19). The most important GLUT in insulin action is GLUT4, which is localized in endosomal vesicles and is induced by insulin to translocate within the vesicle to the plasma membrane (11, 17, 26, 27). The mechanisms underlying the translocation and fusion of GLUT4 vesicles (GLUT4 compartment) to the plasma membrane are unclear, but it has been shown that phosphorylation on serine/threonine residues may be important in regulation of the docking and fusion proteins (14, 23). Several proteins have been identified in association with the GLUT4 compartment and appear to cycle with GLUT4 to the plasma membrane in an insulin-dependent manner. These include the SNAP receptor (v-SNARE) protein, vesicle-associated membrane protein 2 (VAMP2), which interacts with the target membrane SNAP receptor (t-SNARE) and has been shown to mediate insulin-induced GLUT4 translocation to the plasma membrane, and VAMP3 and phosphatidylinositol 4-kinase (PI 4-kinase), which are involved in vesicle trafficking and fusion (9, 12, 32).
Skeletal muscle is one of the most important insulin-responsive tissues and is responsible for most of the clearance of glucose from the plasma. The preparation of primary skeletal muscle cultures obtained from neonatal rat pups is a useful model for the study of blood glucose regulation by insulin. The mature fibers display resting membrane action potentials that are nearly identical to those seen in vivo, and the physiological expression of a number of membrane proteins in these cells, in contrast to muscle cell lines, closely resembles that observed in vivo (7, 8).
The first step of insulin-induced glucose transport is the binding of insulin to its receptor and the initiation of receptor tyrosine kinase activity. Tyrosine phosphorylation of the insulin receptor (IR) leads to activation of the IR substrate family and a number of downstream signaling pathways (34). While several of the key proteins participating in this cascade have been identified, the precise steps between IR activation and GLUT4 translocation have not been entirely delineated. One component of this cascade is protein kinase C (PKC), a family of serine/threonine kinases. PKC isoforms play an important regulatory role in a variety of biological phenomena (15, 18, 24), and some have been shown to participate in insulin action in a variety of tissues (2, 6, 10).
Researchers have shown that insulin increases the activity of PKCζ via a PI 3-kinase-dependent pathway and that this isoform plays a role in insulin-induced glucose uptake in a number of different tissues (2, 6, 13). Moreover, it has been shown that insulin induces translocation of PKCζ and -λ to the GLUT4 compartments in rat adipocytes (30). The precise role of PKCζ in GLUT4 translocation, however, is as yet unclear. We show here that translocation of GLUT4 compartments in skeletal muscle in response to insulin stimulation involves PKCζ-induced serine phosphorylation of VAMP2 in the GLUT4 compartment.
(This study represents a portion of the thesis to be submitted by L. Braiman in partial fulfillment of the Ph.D. degree at Bar-Ilan University.)
MATERIALS AND METHODS
Materials.
Tissue culture media and serum were purchased from Biological Industries (Beit HaEmek, Israel). Antiphosphatases and antiproteases were purchased from Sigma Chemical Co. (St. Louis, Mo.). An enhanced-chemiluminescence kit was purchased from Bio-Rad (Rishon le Zion, Israel). Antibodies against various proteins were obtained from the following sources: anti-GLUT1 and anti-GLUT4 (polyclonal antibodies) were a gift from S. Cushman (Diabetes Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health); anti-PKC antibodies were purchased from Santa Cruz Biotechnology (polyclonal; Santa Cruz, Calif.) and from Transduction Laboratories (monoclonal; Lexington, Ky.); anti-PI 4-kinase, anti-VAMP2, and anti-syntaxin 4 were purchased from UBI; antiphosphoserine was purchased from Zymed (South San Francisco, Calif.); and horseradish peroxidase and anti-rabbit and anti-mouse immunoglobulin G were obtained from Bio-Rad.
Preparation of rat muscle cell cultures.
Skeletal muscle cultures were prepared from thigh muscles obtained from 1- to 2-day-old neonatal rats as described elsewhere (4, 6). On day 5 in culture, myotubes were transferred to low glucose (4.5 mM), serum-free Dulbecco's modified Eagle medium containing 1% bovine serum albumin and were incubated 24 h prior to study.
Immunoprecipitation.
Muscle cell cultures grown in dishes (90 mm; Nunc) were washed with Ca2+/Mg2+-free phosphate-buffered saline (PBS) and were then mechanically detached in radioimmunoprecipitation assay buffer containing a cocktail of protease inhibitors. After scraping, the preparation was centrifuged at 20,000 × g for 20 min at 4°C. The supernatant was used for immunoprecipitation as described elsewhere (6). Briefly, 25 μl of A/G Sepharose was added to 0.3 ml of cell lysate and the suspension was rotated continuously for 30 min at 4°C. The preparation was then centrifuged at 2,000 × g at 4°C for 2 min. Specific antibodies to individual PKC isoforms (dilution 1:100) were added to the supernatant, and the mixture was rotated continuously for 60 min at room temperature. Next, 30 μl of A/G Sepharose was added to the suspension, which was rotated overnight at 4°C. The suspension was then centrifuged at 2,000 × g for 2 min at 4°C, and the pellet was washed twice with HNTG (HEPES, 20 mM; NaCl, 80 mM; Triton X-100, 0.1%; and glycerol, 10%). The immunoprecipitate was mixed with 25 μl of sample buffer (0.5 M Tris HCl, pH 6.8; 10% sodium dodecyl sulfate [SDS]; 10% glycerol; 4% 2-β-mercaptoethanol; and 0.05% bromophenol blue). The suspension was again centrifuged at 500 × g (4°C for 2 min), boiled for 5 min, and then subjected to SDS-polyacrylamide gel electrophoresis (PAGE).
Cell fractionation.
Crude membrane preparations were isolated from muscle cell cultures as described earlier (4, 20). Culture dishes (90 mm; Nunc) containing the muscle cells were washed with Ca2+/Mg2+-free PBS, and the cells were then mechanically detached with a rubber policeman in Ca2+/Mg2+-free PBS containing 2 mM EDTA. The cells were pelleted by centrifugation at 500 × g for 10 min at 4°C. The cells were resuspended in sonication buffer (Tris HCl, pH 7.4, 50 mM; NaCl, 150 mM; EDTA, 2 mM; EGTA, 1 mM; and sucrose, 25 mM) containing antiproteases and antiphosphatases. The suspension was homogenized in a Dounce glass homogenizer (30 strokes) and was centrifuged at 1,100 × g for 5 min. The supernatant was centrifuged at 31,000 × g for 60 min. The supernatant from this centrifugation was then centrifuged at 190,000 × g for 60 min to collect the light-density microsome fraction. The 31,000 × g pellet was resuspended in homogenization buffer to a final volume of 500 μl and was placed on a discontinuous sucrose gradient of 500 μl each of 32% (wt/wt), 40% (wt/wt), and 50% (wt/wt) sucrose solution in 5 mM Tris, pH 7.5. This gradient was centrifuged at 210,000 × g for 50 min. The plasma membranes banded above the 32% layer; the 32/40% and 40/50% interfaces were collected by puncture with a syringe. In initial studies in which dominant-negative PKCζ (DNPKCζ) was expressed in the cells, the cytosolic fraction was found to contain GLUT4. Therefore, in subsequent experiments, this fraction was centrifuged at 260,00 × g for 60 min to collect the very-light-density microsome fraction. All fractions were diluted in homogenization buffer containing 1% Triton X-100, freeze-thawed 4 times, and centrifuged at 30,000 × g for 30 min, and the supernatant was designated the membrane protein. All membrane fractions were stored at −70°C until use. The purity of the membrane preparations was confirmed by identification of the α1 subunit of the Na+/K+ pump (6).
Isolation of GLUT4 compartments.
The GLUT4 compartments were immunoprecipitated with anti-GLUT4 antibodies from nonsolubilized internal membrane (light-density microsome) fractions. Aliquots of the immunoprecipitated fraction were run on SDS-PAGE and were probed with anti-GLUT4 antibodies (see “Western blot analysis” below) to verify the purity of the isolated vesicles.
Western blot analysis.
Western blots were performed as described elsewhere (6).
PKC recombinant adenoviruses and viral infection of cultures.
The recombinant adenoviruses were constructed as described earlier (25). Following differentiation of cultured rat myoblasts into myotubes, the culture medium was aspirated and cultures were infected with medium containing PKCζ or PKCα recombinant adenoviruses as recently described (4).
PKC activity.
Specific PKC activity was determined in freshly prepared immunoprecipitates from mature muscle cultures following appropriate treatments. These lysates were prepared in radioimmunoprecipitation assay buffer without NaF. PKCζ and PKCα were immunoprecipitated using specific PKC antibodies as described above. Activity of the specific PKC isoforms was measured using the SignaTECT Protein Kinase C Assay System (Promega, Madison, Wis.).
In vitro phosphorylation of VAMP2.
Synaptobrevin-2 fragments were obtained from Bachem (Bubendorf, Switzerland) and biotinylated by standard techniques (ENCO, Ltd., Petah Tikva, Israel). This fragment contains two serine sites and one threonine site. PKCζ was immunoprecipitated from appropriate cultures, and its activity was measured utilizing the biotinylated synaptobrevin-2 fragments as a PKC substrate for the above-described PKC activity assay.
Glucose uptake.
The total and nonspecific rates of glucose transport were measured in triplicate samples in 24-well plates with the use of [3H]2-deoxyglucose (2-DG) as described earlier (4).
RESULTS
Insulin increases activity of PKCζ in internal membrane fractions.
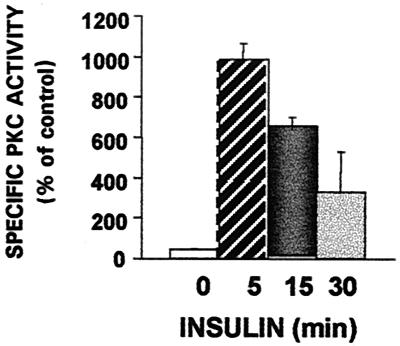
We have previously shown that insulin stimulation of skeletal muscle in primary culture is associated with the selective translocation and activation of PKCδ, -β2, and -ζ and with the translocation of GLUT4 to membrane fractions (6). PKCα, -ɛ, and -θ, which are expressed in this muscle cell preparation, are not translocated, tyrosine phosphorylated, or activated by insulin. To further examine the activation of PKCζ, we immunoprecipitated PKCζ from cytosolic, internal membrane, and plasma membrane fractions of myotubes before and at different times after stimulation with insulin (10−7 M). We found that insulin induced an increase in PKCζ activity selectively associated with the internal membrane fraction of cultured skeletal myotubes (Fig. 1). PKCζ immunoprecipitated from other cellular fractions did not display activity (not shown).
FIG. 1.
Effects of insulin on activity of PKCζ in internal membrane fraction. Myotubes after 6 days in culture were transferred to a serum-free, low-glucose medium for 24 h. PKCζ was immunoprecipitated from different cell fractions of control cells and cells stimulated with 100 nM insulin for the times indicated. The immunoprecipitates were then assayed for PKC activity as described in Materials and Methods. Each bar represents the mean ± standard error of duplicate measurements in each of three experiments (P < 0.005).
Overexpression of PKCζ increases glucose transport.
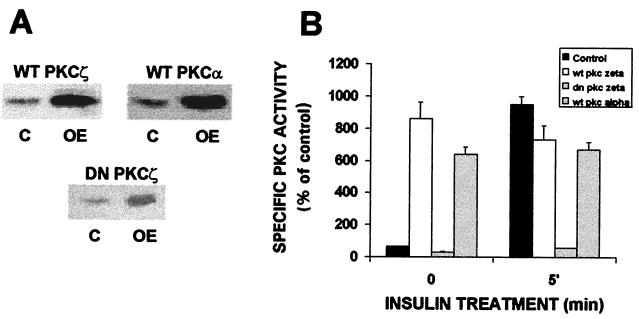
The translocation and activation of PKCζ in response to insulin indicate that this isoform may help mediate or regulate insulin-induced glucose uptake, as suggested for atypical PKC isoforms (1–3, 6). To examine this more directly, we used recombinant adenovirus constructs to overexpress specific PKC isoforms in the myotube cultures. As shown in Fig. 2, infection of myotubes with adenovirus constructs containing cDNAs for wild-type PKCα (WTPKCα) or WTPKCζ or kinase-inactive, dominant-negative PKCζ (DNPKCζ) resulted in a marked increase (about 10-fold) in expression of the respective PKC isoforms. This was associated with a strong increase in kinase activity of each overexpressed WT isoform, approximately eightfold greater than that in control cells. Insulin did not further increase the activity of PKCζ in these cells. As expected, overexpression of DNPKCζ, which is kinase inactive (25), was not associated with increased kinase activity and insulin failed to stimulate PKCζ activity in these cells. Moreover, overexpression of each isoform resulted in an increase in activity of that isoform without altering the activity of other isoforms (4).
FIG. 2.
Overexpression of PKC isoforms α and ζ in skeletal muscle cells by recombinant adenovirus constructs. (A) Western blots of PKCs α and ζ in control cells (C) and in cells overexpressing (OE) either one of these isoforms. Myotubes after 6 days in culture were infected with PKCα or PKCζ recombinant adenovirus (as described in Materials and Methods). Whole-cell lysates were prepared from control and infected cells 16 h postinfection, and the samples were subjected to SDS-PAGE and immunoblotted with anti-PKC antibodies. The blot is representative of five separate experiments. (B) Comparison of PKC activity in control and PKC-overexpressing myotubes. Myotubes were treated as for Fig. 2A, after which PKC isoforms α and ζ were immunoprecipitated from control cells and cells overexpressing PKCα or -ζ with specific anti-PKC antibodies. The specific PKC immunoprecipitates were then assayed for PKC activity as described in Materials and Methods. Each bar represents the mean ± standard error of duplicate measurements in each of three experiments (P < 0.005).
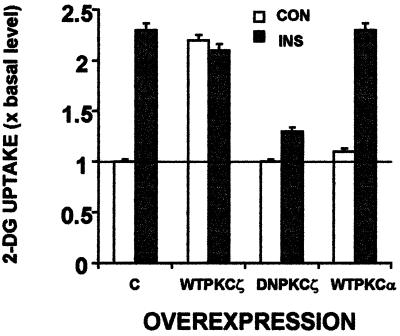
Because insulin-stimulated PKC activity is associated with an increase in glucose uptake (6), we next measured glucose uptake in cells overexpressing WTPKCζ, DNPKCζ, or WTPKCα. Figure 3 shows that overexpression of PKCζ resulted in a twofold elevation in basal glucose transport, quantitatively similar to that produced by insulin. Addition of insulin to cells overexpressing PKCζ did not further increase glucose uptake. PKCα, though active in its overexpressed form (Fig. 2), did not affect either basal or insulin-induced glucose uptake. Overexpression of DNPKCζ did not alter basal glucose uptake but completely abrogated the insulin-induced increase in glucose uptake.
FIG. 3.
Effects of PKCζ or PKCα overexpression on basal and insulin-induced glucose uptake. PKC isoforms were overexpressed as for Fig. 2A. Sixteen hours postinfection, control cells and cells overexpressing PKCζ or PKCα were either untreated (empty bars) or treated with insulin for 30 min (filled bars) and were assayed for 2-DG uptake as described in Materials and Methods. Data are expressed as fold increase over the basal level determined in control, non-insulin-stimulated cultures. Each bar represents the mean ± standard error of duplicate measurements in each of three experiments (P < 0.005).
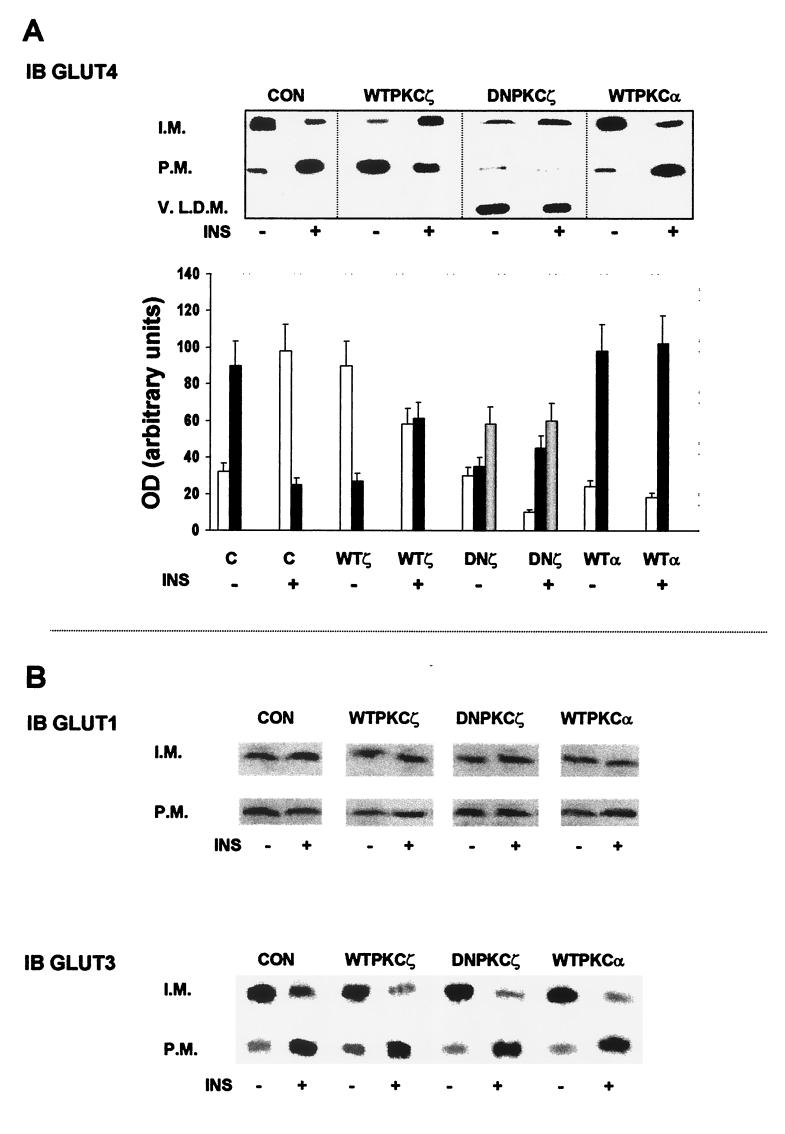
Insulin stimulation of glucose transport is due in large part to the translocation and activation of GLUT4. Accordingly, we examined distribution of GLUT4 as well as GLUT1 and GLUT3 in different cellular fractions prepared from lysates of control and insulin-stimulated noninfected or WTPKCζ- or DNPKCζ-overexpressing cells (Fig. 4A and B). Under basal conditions, GLUT4 was located primarily in the internal membrane fraction, and insulin induced translocation of GLUT4 from the internal membrane fraction to the plasma membrane (6). In cells overexpressing WTPKCζ, GLUT4 was found to be located primarily in the plasma membrane in the absence of insulin stimulation. Addition of insulin did not further increase the amount of GLUT4 in the plasma membrane. In fact, insulin stimulation of WTPKCζ-expressing cells caused GLUT4 to translocate from the plasma membrane to the internal membrane fraction (Fig. 4A). Infection of cells with adenoviruses containing PKCα cDNA neither altered the basal distribution of GLUT4 nor interfered with the ability of insulin to translocate GLUT4 to the plasma membrane. Overexpression of DNPKCζ caused translocation of GLUT4 into the very-light-density microsome fraction and completely blocked insulin-induced translocation of GLUT4. This fraction did not contain other elements associated with the GLUT4 compartments, such as PI 4-kinase, VAMP3, and VAMP2 (not shown). As shown in Fig. 4B, overexpression of either PKCζ (WT or DN) or PKCα caused no detectable change in the distribution of GLUT1 or GLUT3. GLUT1 was not translocated by insulin under any of the conditions (6), whereas GLUT3 was translocated to the plasma membrane by insulin in control cells and in cells overexpressing WT- or DNPKCζ. In fact, insulin-induced translocation of GLUT3 appeared to be slightly greater in cells overexpressing WT- or DNPKCζ than in control, noninfected cells.
FIG. 4.
Effects of overexpression of PKCζ on distribution of GLUTs. (A) GLUT4 distribution. PKC isoforms were overexpressed as for Fig. 2A. Sixteen hours postinfection, control (CON) cells and cells overexpressing PKCζ or PKCα were either untreated or treated with insulin (INS) for 30 min and were fractionated to plasma membrane (P.M.), internal membrane (I.M.), and very-light-density microsome (V.L.D.M.) fractions. The samples were subjected to SDS-PAGE and were immunoblotted (IB) with anti-GLUT4 antibody. The blot shown is representative of five separate experiments. The graph represents the densitometry measurements (mean ± standard error) of five Western blot analyses. Empty bars, plasma membrane; black-bars, internal membrane (I.M.); gray bars, very-light-density micosomes. (B) Western blots of the distribution of GLUT1 (upper set) and GLUT3 (lower set). PKC isoforms were overexpressed and the cells were treated as for panel A. Following treatment, cells were fractionated to plasma membrane (P.M.) or internal membrane (I.M.) fractions.
Overexpression induces selective association of PKCζ with GLUT4 compartments.
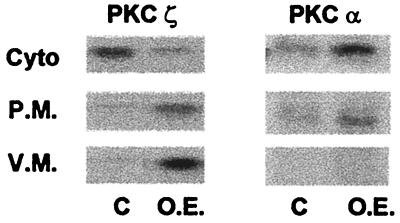
The translocation of GLUT4 to the plasma membrane induced by overexpression of WTPKCζ suggests that PKCζ may directly interact with the intracellular pool of GLUT4. In order to test this possibility, we examined the differential translocation of PKC isoforms into different cellular fractions. We separated vesicular and plasma membranes by immunoprecipitation of GLUT4 compartments and further sucrose gradient centrifugation. In agreement with other studies from this and other laboratories, PKCζ under basal conditions is found primarily in the cytosolic fraction. Figure 5 shows that PKCζ, when overexpressed in mature myotubes, was found to be associated primarily with the GLUT4 compartment. Indeed, the distribution of WTPKCζ was remarkably similar to that of the native PKCζ following insulin stimulation (6). In contrast, PKCα is located primarily in the cytosolic fraction in both noninfected and PKCα-overexpressing cells. The localization of activated PKCζ with the GLUT4 compartment indicates that function of this isoform may be directly associated with the GLUT4 compartment. This suggests further that activation of PKCζ by insulin may direct the association of PKCζ with the GLUT4 compartment.
FIG. 5.
PKCζ distribution in cytosolic (Cyto) and membrane fractions of skeletal myotubes. Cells were infected as for Fig. 2A. Control (C) cells and cells overexpressing (O.E.) PKCζ were fractionated into vesicular (V.M.) and plasma (P.M.) fractions, and the samples were subjected to SDS-PAGE and immunoblotted with anti-PKCζ antibody. The blot shown is representative of three separate experiments.
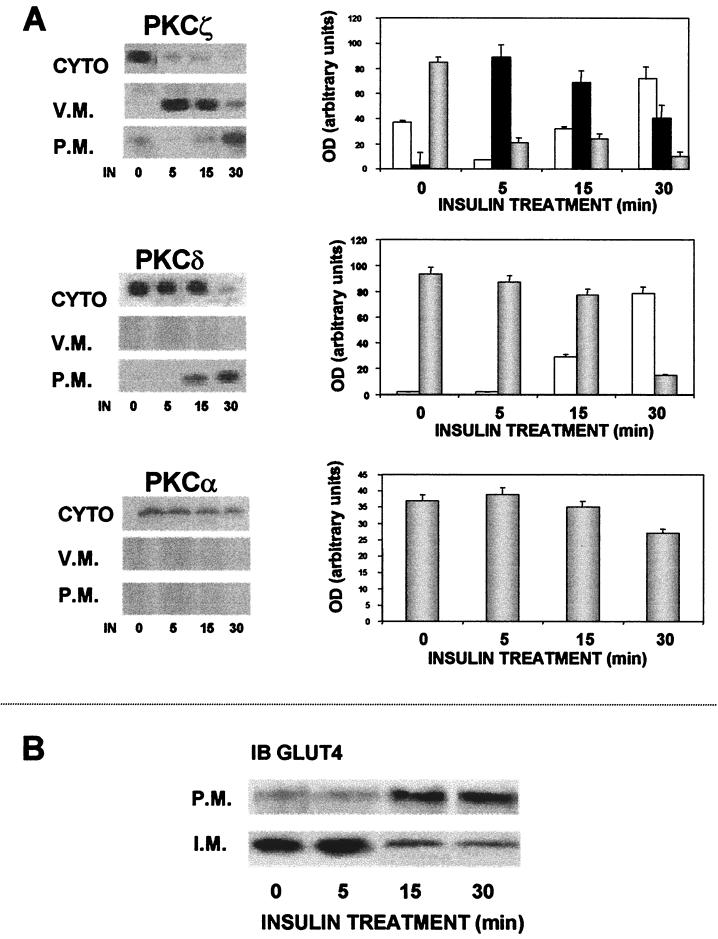
Insulin induces PKCζ to associate with GLUT4 compartments.
We have shown so far that PKCζ, when overexpressed in skeletal myotubes, is constitutively active and is physically associated with the GLUT4 compartments. These findings raise the possibility that activation of PKCζ by insulin may induce this isoform to associate with the GLUT4 compartments as an important early step in stimulation of glucose transport. To investigate this possibility, we analyzed the time course of insulin-induced translocation of PKCζ and other isoforms and of GLUT4 into the different cellular fractions. We studied the effects of insulin on PKC isoforms α and δ in addition to those on PKCζ. PKCα is not activated by insulin in this preparation (6), and PKCδ is activated upstream early in IR signaling independently of PI 3-kinase (4). The results of these studies are shown in Fig. 6A. Insulin caused a unique pattern of translocation of PKCζ compared to PKCδ. Thus, insulin induced PKCζ to first associate with the GLUT4 compartment fraction, so that within 5 min virtually all of the isoform was found in this fraction. The amount of PKCζ in the vesicular fraction subsequently decreased and by 30 min was translocated to the plasma membrane. In contrast, PKCδ, which is also translocated by insulin, appeared to be translocated exclusively to the plasma membrane without association with the GLUT4 compartment. This isoform was clearly detectable in the plasma membrane by 15 min and reached a maximal level 30 min after insulin treatment. No PKCδ was detected in the GLUT4 compartment. Finally, PKCα, which is not affected by insulin in this system, did not translocate either to the vesicular or to the plasma membrane. As seen in Fig. 6B, insulin induced translocation of GLUT4 from the GLUT4 compartment to the plasma membrane. GLUT4 remained in the vesicular membrane fraction until about 15 min after insulin addition, at which time the transporter was translocated to the plasma membrane.
FIG. 6.
Western blots showing effects of insulin (IN) on distribution of PKC isoforms and GLUT4 in cytosolic (CYTO), vesicular (V.M.), and plasma (P.M.) membrane fractions of skeletal muscle. Cells were infected with PKC viral constructs and 16 to 20 h postinfection were either untreated or stimulated with insulin for the designated times (in minutes) and fractionated on sucrose gradient (as described in Materials and Methods). The cytosolic, vesicular, and plasma membrane fractions were subjected to SDS-PAGE and immunoblotted (IB) with specific anti-PKC (A) or anti-GLUT4 (B) antibodies. The blots shown are representative of three separate experiments. Graphs of densitometry measurements (mean ± standard error) of the appropriate Western blot of PKC isoform distribution are shown to the right of each blot. OD, optical density. White bars, plasma membranes; black bars, vesicular membranes; gray bars, cytosol.
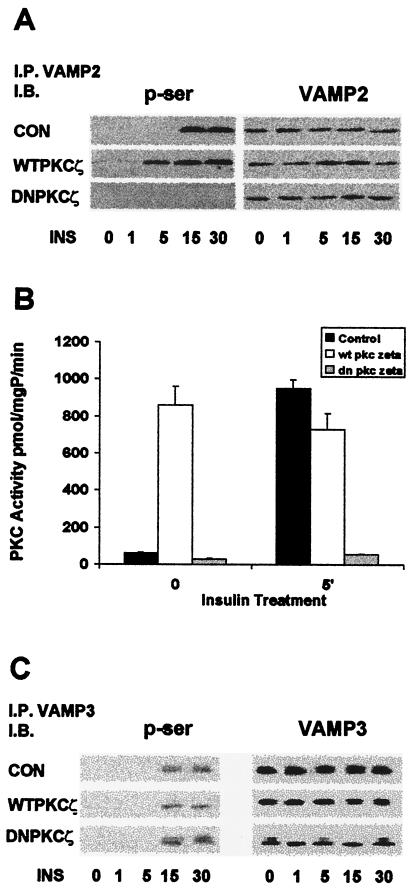
Insulin induces serine phosphorylation of GLUT4-associated VAMP2 via PKCζ.
These results demonstrate that insulin stimulation results in physical association of PKCζ with the GLUT4 compartment and that this association appears to be important in the translocation of the vesicles to the plasma membrane. As PKCζ is a serine/threonine kinase, it may be proposed that this physical linkage results in serine phosphorylation of GLUT4-compartment-associated proteins. It was recently shown that the vesicle-associated protein VAMP2 is involved in mediating insulin-induced incorporation of GLUT4 into the plasma membrane of L6 myoblasts (29). Accordingly, in the next series of experiments, we investigated the possible serine phosphorylation of proteins associated with the GLUT4 compartment. In preliminary studies we determined that VAMP2, VAMP3, and PI 4-kinase were all associated with the GLUT4 compartment (not shown). Accordingly, we immunoprecipitated these proteins from lysates prepared from noninfected and PKCζ-overexpressing (WT and DN) myotubes before and at various times after insulin stimulation; the separated proteins were then immunoblotted with antiphosphoserine antibodies (Fig. 7). The findings with regard to VAMP2 are illustrated in Fig. 7A. Prior to insulin stimulation, no detectable serine phosphorylation was seen on VAMP2. Within 15 min of insulin stimulation, there was strong serine phosphorylation, which remained for at least 30 min. In cells that had been infected with recombinant adenoviruses containing cDNA for WTPKCζ (but not WTPKCα), slight serine phosphorylation of VAMP2 was detectable under basal conditions. Serine phosphorylation was strongly increased by insulin within 5 min and remained elevated without a noticeable decrease for at least 30 min. Thus, overexpression of WTPKCζ hastened insulin-induced phosphorylation on serine residues of VAMP2. In contrast, insulin did not cause any detectable serine phosphorylation of VAMP2 in cells expressing DNPKCζ. Whereas serine phosphorylation of VAMP3 was also induced by insulin, this effect was not altered in cells overexpressing either WTPKCζ or DNPKCζ (Fig. 7C). Similar results were obtained regarding PI 4-kinase (not shown).
FIG. 7.
Induction of VAMP2 serine phosphorylation by insulin and PKCζ. Cells were infected as for Fig. 2A, and 16 h postinfection, control (CON) and infected cells were treated with insulin for the times indicated. (A) Western blots of serine phosphorylation of VAMP2 by insulin (INS) and overexpression of PKCζ. Whole-cell lysates were immunoprecipitated (I.P.) with anti-VAMP2 antibody and immunoblotted (I.B.) with antiphosphoserine (p-ser) and anti-VAMP3. The blots shown are representative of three separate experiments; time is given in minutes below blots. (B) PKC activity assay showing serine phosphorylation of synaptobrevin-2 fragments by immunoprecipitated PKCζ. Cells were infected as for Fig. 2A, and, 16 h postinfection, control and infected cells were treated with insulin for 5 min. Whole-cell lysates were immuno-precipitated with anti-PKCζ antibody and were added to an activity assay in which the synaptobrevin-2 fragments served as the substrate. (C) Western blots of serine phosphorylation of VAMP3 by insulin (INS) and PKCζ. Whole-cell lysates were immunoprecipitated with anti-VAMP3 antibody and immunoblotted with antiphosphoserine and anti-VAMP3. The blots shown are representative of three separate experiments. Time is given in minutes below blots.
In order to demonstrate more directly that VAMP2 is a substrate for insulin-activated PKCζ, we performed an in vitro kinase assay on VAMP2 utilizing PKCζ immunoprecipitated from untreated and insulin-stimulated cells infected with WTPKCζ or DNPKCζ on noninfected myotubes. We measured the incorporation of 32P into VAMP2 peptide by PKCζ from the variously treated cells. Results are shown in Fig. 7B. PKCζ from noninfected cells stimulated by insulin caused the amount of 32P incorporated into VAMP2 to increase by 100%. This indicates that activated PKCζ phosphorylates VAMP2 on serine/threonine residues. Similarly, PKCζ immunoprecipitated from cells overexpressing WTPKCζ, which is constitutively active, also displayed an increase in 32P incorporation, even in the absence of insulin stimulation. Serine phosphorylation was further increased slightly but not significantly by insulin. DNPKCζ, which is kinase inactive, did not phosphorylate VAMP2 and abrogated the effect of insulin.
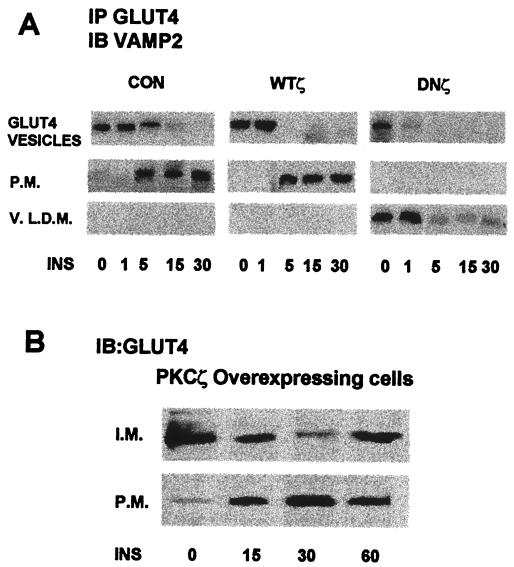
Finally, we examined the effects of PKCζ on the physical association between the GLUT4 compartment and VAMP2. GLUT4 was immunoprecipitated from cytosolic, internal membrane, and plasma membrane fractions of noninfected and adenovirus-infected myotubes before and at various times after insulin stimulation. Separated proteins were then probed with anti-VAMP2 antibodies. Figure 8 shows that prior to insulin stimulation of control, non-infected myotubes, VAMP2 was detected exclusively in the GLUT4 compartments in the internal membrane fraction. Within 5 min of insulin stimulation, VAMP2 could be identified in the plasma membrane, where it increased with time for at least 30 min in parallel with GLUT4. Overexpression of PKCζ appeared to increase the amount of VAMP2 associated with the GLUT4 compartments but did not alter the distribution or time course of VAMP2 translocation in response to insulin. On overexpression of DNPKCζ, the basal expression and distribution of VAMP2 were unchanged. However, VAMP2 translocation in response to insulin was totally blocked. Moreover, following stimulation of the cells by insulin, VAMP2 dissociated from the GLUT4 compartment and could be detected only associated with GLUT4 from the very-light-density microsome fraction. Thus, PKCζ appears to regulate insulin-induced translocation of GLUT4 through direct interaction with VAMP2.
FIG. 8.
(A) Insulin-(INS)-induced translocation of VAMP2 in noninfected (CON) and WTPKCζ- and DNPKCζ-overexpressing cells. PKC isoforms were overexpressed as for Fig. 2A. Sixteen hours postinfection, noninfected cells and cells overexpressing WTPKCζ or DNPKCζ were either untreated or treated with insulin for the designated times. Whole-cell lysates were fractionated on sucrose gradient (as described in Materials and Methods). The cytosolic, vesicular, and plasma membrane (P.M.) fractions were then immunoprecipitated (IP) with anti-GLUT4 antibody, subjected to SDS-PAGE and immunoblotted (IB) with specific anti-VAMP2 antibody. V.L.D.N., very-light-density membrane. Time is given in minutes below blots. (B) Recycling of GLUT4 following insulin stimulation of WTPKCζ-overexpressing cells. WTPKCζ was overexpressed as for Fig. 2A. Sixteen hours postinfection, cells were either untreated or treated with insulin for the designated times. Whole-cell lysates were fractionated on sucrose gradient (as described in Materials and Methods). The internal membrane (I.M.) and plasma membrane fractions were then subjected to SDS-PAGE and immunoblotted with specific anti-GLUT4 antibody.
These results demonstrate differences between the translocation and recycling of VAMP2 and GLUT4. Thus, overexpression of PKCζ translocated GLUT4 directly to the plasma membrane without insulin stimulation; indeed, insulin stimulation appeared to down regulate plasma membrane GLUT4 (Fig. 4A). Following PKCζ overexpression, VAMP2 translocation did not parallel GLUT4 translocation (Fig. 8A). This may be due to differences in recycling of GLUT4 and VAMP2. Thus, as shown in Fig. 8B, GLUT4 was found in maximal levels in the plasma membrane in PKCζ-overexpressing cells. VAMP2 had already recycled to the internal membrane fraction (Fig. 8A).
DISCUSSION
PKCζ is activated by insulin and appears to be involved in insulin-stimulated glucose transport in a variety of cell types, including skeletal muscle (in vivo and in culture) cells, fat cells, and 3T3-L1 adipocytes (2, 3, 6). Prior attention has been devoted almost exclusively to the mechanism of PKCζ activation by insulin, which has been shown to occur via a PI 3-kinase-dependent pathway (6, 31). However, the precise role of PKCζ in insulin signaling and glucose transport has not been clarified. The main GLUTs expressed in skeletal muscle are GLUT4, GLUT3, and GLUT1. Insulin-induced glucose transport is accomplished primarily by activation of GLUT4 and does not acutely alter GLUT1 distribution (6). In addition, it was shown that infection of animals with an adenovirus vector containing PKCζ cDNA lowers blood glucose concentration (13). Effects on GLUT4 translocation, however, were not reported. According to our results in mature myotubes, overexpressed PKCζ was found both to be active in internal membrane fractions, in particular after insulin induction, and to associate with the GLUT4 compartment. The specific localization of activated PKCζ to the GLUT4 compartments, whether by insulin stimulation or by overexpression, indicates that this isoform has an important function in translocation of the GLUT4 compartment. This situation is analogous to that related to PKCδ, which was shown when activated by insulin stimulation or by overexpression to associate with IR in the plasma membrane (5). In both cases, the activated isoform associates with a membrane component indicative of the site of action. Thus, activated PKCδ associates with and regulates the IR phosphorylation state and internalization. In the studies reported here, activated PKCζ physically associated with VAMP2 in the GLUT4 compartment and increased GLUT4 translocation and glucose transport.
The distribution of the insulin-sensitive GLUT4 was altered by PKCζ activation, while the distribution of GLUT1, which is not regulated by insulin, was not affected by PKCζ. Overexpression of kinase-inactive DNPKCζ prevented the translocation of GLUT4 to the plasma membrane fraction in response to insulin stimulation and completely blocked insulin-induced glucose uptake. These findings indicate that PKCζ plays a unique role in insulin-induced trafficking of the GLUT4 compartment to the plasma membrane. Alternatively, PKCζ could be involved in biogenesis of an insulin-responsive GLUT4 compartment, as indicated by the decrease in GLUT4 in the internal membrane fraction and in insulin-induced translocation of GLUT4 to the plasma membrane in cells expressing DNPKCζ. The GLUT4 remaining in the internal membrane fraction might be responsive to other stimuli, such as exercise. The reduction of GLUT4 in the internal membrane fraction and its appearance in higher-speed, low-density membranes in cells overexpressing DNPKCζ suggest that GLUT4 was dissociated from the vesicles by an inactive PKCζ. This indicates that PKCζ may also be important for stabilizing the GLUT4 compartment.
GLUT-containing vesicles have been suggested to represent a unique, specialized population of intracellular membranes which are translocated to the plasma membrane upon insulin stimulation (11, 17). Recently several proteins have been identified in association with the GLUT4 compartment and appear to cycle with GLUT4 to the plasma membrane in an insulin-dependent manner (9, 12, 32). PI 4-kinase, VAMPs, and SNARE proteins (syntaxin and SNAP) colocalize with the GLUT4 compartments and may be involved in trafficking and docking; certain of these proteins may serve as receptors or attachment sites for binding between the GLUT4 compartments and the plasma membrane (14, 16, 26). Thus, the mechanism by which the GLUT4 compartments translocate, dock, and fuse to the plasma membrane under insulin-stimulated conditions is unclear, but it appears to involve a tightly regulated system mediated by chaperon, docking, and fusion proteins.
Our results reveal some differences between the translocation and recycling of VAMP2 and GLUT4. Thus, overexpression of PKCζ translocated GLUT4 directly to the plasma membrane without insulin stimulation; indeed, insulin stimulation appeared to down regulate plasma membrane GLUT4. This may be explained by the observations that overexpressed PKCζ is constitutively active and that insulin actually decreases its tyrosine phosphorylation and activity (unpublished observations). Following PKCζ overexpression, VAMP2 translocation did not parallel GLUT4 translocation. Thus, in PKCζ-overexpressing cells, VAMP2 was identified in the GLUT4 compartments associated with the internal membrane fraction, in contrast to GLUT4, and was translocated to the plasma membrane in response to insulin. This discrepancy may be explained by differences in recycling of GLUT4 and VAMP2. We propose that with PKCζ overexpression, GLUT4 is found in maximal levels in the plasma membrane, while VAMP2 has already recycled to the internal membrane fraction. Following insulin stimulation, plasma membrane GLUT4 is down regulated to recycle to the internal membrane while VAMP2 is translocated with new GLUT4 compartment to the plasma membrane. At this stage more GLUT4 is recycling than translocating to the plasma membrane (Fig. 8). Other studies show that the kinetics of GLUT4 and VAMP2 recycling differ (22, 28, 33). Our results demonstrate that PKCζ plays an essential role in this process of GLUT4 translocation and recycling.
It has recently been shown that phosphorylation of certain docking and fusion proteins, possibly on serine/threonine residues (23), may be important in their regulation (14). In this regard, it has been reported that PKC phosphorylates certain SNARE proteins, such as syntaxin and SNAP-23 (14). In addition, it was reported that VAMP2 is an important element in insulin-induced incorporation of GLUT4 into the plasma membrane (29). Our results support and extend this concept. We specifically investigated the effect of PKCζ on several proteins associated with the GLUT4 compartment. We found that overexpression and subsequent activation of PKCζ resulted both in its association with the GLUT4 compartment and in insulin-induced serine phosphorylation on VAMP2 (synaptobrevin) but had no effect on insulin-induced serine phosphorylation of VAMP3 or PI 4-kinase, even though these proteins were found associated with the GLUT4 compartments. Both immunoblotting with antiphosphoserine antibodies and the in vitro kinase assay demonstrated that PKCζ directly phosphorylates VAMP2. The apparent quantitative difference in results between the two experiments may be explained by the fact that in vitro assays measured the effects of PKCζ specifically. In contrast, serine phosphorylation determined by immunoblotting reveals the effect of all serine kinases, including PKB, which was shown to serine phosphorylate VAMP2 in rat adipocytes (21). The role of PKB in insulin signaling in skeletal muscle cells in primary culture is currently under study.
The results of this report thus demonstrate that PKCζ is functionally colocalized with GLUT4 in the GLUT4 compartments. The additional findings that overexpression of DNPKCζ blocked insulin-induced VAMP2 serine phosphorylation and caused disruption of vesicular proteins into a small particulate fraction that does not display characteristics of the GLUT4 compartments lend further support to the important roles of both PKCζ and VAMP2 in insulin-stimulated glucose transport.
ACKNOWLEDGEMENTS
This work was supported in part by the Sorrell Foundation, the Ben and Effie Raber Research Fund, the Harvett-Aviv Neuroscience Research Fund, and a grant from the Israel Science Foundation (founded by the Israel Academy of Sciences and Humanities). S.R.S. is the incumbent of the Louis Fisher Chair in Cellular Pathology.
REFERENCES
- 1.Bandyopadhyay G, Kanoh Y, Sajan M P, Standaert M L, Farese R V. Effects of adenoviral gene transfer of wild-type, constitutively active, and kinase-defective protein kinase C-lambda on insulin-stimulated glucose transport in L6 myotubes. Endocrinology. 2000;141:4120–4127. doi: 10.1210/endo.141.11.7766. [DOI] [PubMed] [Google Scholar]
- 2.Bandyopadhyay G, Standaert M L, Galloway L, Moscat J, Farese R V. Evidence for involvement of protein kinase C (PKC)-zeta and noninvolvement of diacylglycerol-sensitive PKCs in insulin-stimulated glucose transport in L6 myotubes. Endocrinology. 1997;138:4721–4731. doi: 10.1210/endo.138.11.5473. [DOI] [PubMed] [Google Scholar]
- 3.Bandyopadhyay G, Standaert M L, Kikkawa U, Ono Y, Moscat J, Farese R V. Effects of transiently expressed atypical (zeta, lambda), conventional (alpha, beta) and novel (delta, epsilon) protein kinase C isoforms on insulin-stimulated translocation of epitope-tagged GLUT4 glucose transporters in rat adipocytes: specific interchangeable effects of protein kinases C-zeta and C-lambda. Biochem J. 1999;337:461–470. [PMC free article] [PubMed] [Google Scholar]
- 4.Braiman L, Alt A, Kuroki T, Ohba M, Bak A, Tennenbaum T, Sampson S R. Protein kinase Cdelta mediates insulin-induced glucose transport in primary cultures of rat skeletal muscle. Mol Endocrinol. 1999;13:2002–2012. doi: 10.1210/mend.13.12.0393. [DOI] [PubMed] [Google Scholar]
- 5.Braiman L, Alt A, Kuroki T, Ohba M, Bak A, Tennenbaum T, Sampson S R. Insulin induces specific interaction between insulin receptor and PKC in primary cultured skeletal muscle. Mol Endocrinol. 2001;15:565–574. doi: 10.1210/mend.15.4.0612. [DOI] [PubMed] [Google Scholar]
- 6.Braiman L, Sheffi-Friedman L, Bak A, Tennenbaum T, Sampson S R. Tyrosine phosphorylation of specific protein kinase C isoenzymes participates in insulin stimulation of glucose transport in primary cultures of rat skeletal muscle. Diabetes. 1999;48:1922–1929. doi: 10.2337/diabetes.48.10.1922. [DOI] [PubMed] [Google Scholar]
- 7.Brodie C, Bak A, Shainberg A, Sampson S R. Role of Na-K ATPase in regulation of resting membrane potential of cultured rat skeletal myotubes. J Cell Physiol. 1987;130:191–198. doi: 10.1002/jcp.1041300204. [DOI] [PubMed] [Google Scholar]
- 8.Brodie C, Brody M, Sampson S R. Characterization of the relation between sodium channels and electrical activity in cultured rat skeletal myotubes: regulatory aspects. Brain Res. 1989;488:186–194. doi: 10.1016/0006-8993(89)90708-7. [DOI] [PubMed] [Google Scholar]
- 9.Cain C C, Trimble W S, Lienhard G E. Members of the VAMP family of synaptic vesicle proteins are components of glucose transporter-containing vesicles from rat adipocytes. J Biol Chem. 1992;267:11681–11684. [PubMed] [Google Scholar]
- 10.Chalfant C E, Ohno S, Konno Y, Fisher A A, Bisnauth L D, Watson J E, Cooper D R. A carboxy-terminal deletion mutant of protein kinase C beta II inhibits insulin-stimulated 2-deoxyglucose uptake in L6 rat skeletal muscle cells. Mol Endocrinol. 1996;10:1273–1281. doi: 10.1210/mend.10.10.9121494. [DOI] [PubMed] [Google Scholar]
- 11.Cushman S W, Wardzala L J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980;255:4758–4762. [PubMed] [Google Scholar]
- 12.Del Vecchio R L, Pilch P F. Phosphatidylinositol 4-kinase is a component of glucose transporter (GLUT 4)-containing vesicles. J Biol Chem. 1991;266:13278–13283. [PubMed] [Google Scholar]
- 13.Etgen G J, Valasek K M, Broderick C L, Miller A R. In vivo adenoviral delivery of recombinant human protein kinase C-zeta stimulates glucose transport activity in rat skeletal muscle. J Biol Chem. 1999;274:22139–22142. doi: 10.1074/jbc.274.32.22139. [DOI] [PubMed] [Google Scholar]
- 14.Foster L J, Yeung B, Mohtashami M, Ross K, Trimble W S, Klip A. Binary interactions of the SNARE proteins syntaxin-4, SNAP23, and VAMP-2 and their regulation by phosphorylation. Biochemistry. 1998;37:11089–11096. doi: 10.1021/bi980253t. [DOI] [PubMed] [Google Scholar]
- 15.Haller H, Lindschau C, Luft F C. Role of protein kinase C in intracellular signaling. Ann NY Acad Sci. 1994;733:313–320. doi: 10.1111/j.1749-6632.1994.tb17281.x. [DOI] [PubMed] [Google Scholar]
- 16.Holman G D, Cushman S W. Subcellular localization and trafficking of the GLUT4 glucose transporter isoform in insulin-responsive cells. Bioessays. 1994;16:753–759. doi: 10.1002/bies.950161010. [DOI] [PubMed] [Google Scholar]
- 17.Kandror K V, Coderre L, Pushkin A V, Pilch P F. Comparison of glucose-transporter-containing vesicles from rat fat and muscle tissues: evidence for a unique endosomal compartment. Biochem J. 1995;307:383–390. doi: 10.1042/bj3070383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kazanietz M G, Blumberg P M. Protein kinase C and signal transduction in normal and neoplastic cells. In: Sirica A E, editor. Cellular and molecular pathogenesis. New York, N.Y: Raven Press; 1996. pp. 389–402. [Google Scholar]
- 19.Klip A, Ramlal T, Bilan P J, Marette A, Liu Z, Mitsumoto Y. What signals are involved in the stimulation of glucose transport by insulin in muscle cells? Cell Signal. 1993;5:519–529. doi: 10.1016/0898-6568(93)90047-p. [DOI] [PubMed] [Google Scholar]
- 20.Klip A, Ramlal T, Young D A, Holloszy J O. Insulin-induced translocation of glucose transporters in rat hindlimb muscles. FEBS Lett. 1987;224:224–230. doi: 10.1016/0014-5793(87)80452-0. [DOI] [PubMed] [Google Scholar]
- 21.Kupriyanova T A, Kandror K V. Akt-2 binds to Glut4-containing vesicles and phosphorylates their component proteins in response to insulin. J Biol Chem. 1999;274:1458–1464. doi: 10.1074/jbc.274.3.1458. [DOI] [PubMed] [Google Scholar]
- 22.Lee W J, Ryu, Spangler R A, Jung C Y. Modulation of GLUT4 and GLUT1 recycling by insulin in rat adipocytes: kinetic analysis based on the involvement of multiple intracellular compartments. Biochemistry. 2000;39:9358–9366. doi: 10.1021/bi0007021. [DOI] [PubMed] [Google Scholar]
- 23.Linial M. SNARE proteins—why so many, why so few? J Neurochem. 1997;69:1781–1792. doi: 10.1046/j.1471-4159.1997.69051781.x. [DOI] [PubMed] [Google Scholar]
- 24.McGraw K, McKay R, Miraglia L, Boggs R T, Pribble J P, Muller M, Geiger T, Fabbro D, Dean N M. Antisense oligonucleotide inhibitors of isozymes of protein kinase C: in vitro and in vivo activity, and clinical development as anti-cancer therapeutics. Anticancer Drug Des. 1997;12:315–326. [PubMed] [Google Scholar]
- 25.Ohba M, Ishino K, Kashiwagi M, Kawabe S, Chida K, Huh N-H, Kuroki T. Induction of differentiation in normal human keratinocytes by adenovirus-mediated introduction of the η and δ isoforms of protein kinase C. Mol Cell Biol. 1998;18:5199–5207. doi: 10.1128/mcb.18.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pessin J E, Thurmond D C, Elmendorf J S, Coker K J, Okada S. Molecular basis of insulin-stimulated GLUT4 vesicle trafficking. Location! Location! Location! J Biol Chem. 1999;274:2593–2596. doi: 10.1074/jbc.274.5.2593. [DOI] [PubMed] [Google Scholar]
- 27.Ploug T, van Deurs B, Ai H, Cushman S W, Ralston E. Analysis of GLUT4 distribution in whole skeletal muscle fibers: identification of distinct storage compartments that are recruited by insulin and muscle contractions. J Cell Biol. 1998;142:1429–1446. doi: 10.1083/jcb.142.6.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rampal A L, Jhun B H, Kim S, Liu H, Manka M, Lachaal N, Spangler R A, Jung C Y. Okadaic acid stimulates glucose transport in rat adipocytes by increasing the externalization rate constant of GLUT4 recycling. J Biol Chem. 1995;270:3938–3943. doi: 10.1074/jbc.270.8.3938. [DOI] [PubMed] [Google Scholar]
- 29.Randhawa V K, Bilan P J, Khayat Z A, Daneman N, Liu Z, Ramlal T, Volchuk A, Peng X R, Coppola T, Regazzi R, Trimble W S, Klip A. VAMP2, but not VAMP3/cellubrevin, mediates insulin-dependent incorporation of GLUT4 into the plasma membrane of L6 myoblasts. Mol Biol Cell. 2000;11:2403–2417. doi: 10.1091/mbc.11.7.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Standaert M L, Bandyopadhyay G, Perez L, Price D, Galloway L, Poklepovic A, Sajan M P, Cenni V, Sirri A, Moscat J, Toker A, Farese R V. Insulin activates protein kinases C-zeta and C-lambda by an autophosphorylation-dependent mechanism and stimulates their translocation to GLUT4 vesicles and other membrane fractions in rat adipocytes. J Biol Chem. 1999;274:25308–25316. doi: 10.1074/jbc.274.36.25308. [DOI] [PubMed] [Google Scholar]
- 31.Standaert M L, Galloway L, Karnam P, Bandyopadhyay G, Moscat J, Farese R V. Protein kinase C-zeta as a downstream effector of phosphatidylinositol 3-kinase during insulin stimulation in rat adipocytes. Potential role in glucose transport. J Biol Chem. 1997;272:30075–30082. doi: 10.1074/jbc.272.48.30075. [DOI] [PubMed] [Google Scholar]
- 32.Volchuk A, Sargeant R, Sumitani S, Liu Z, He L, Klip A. Cellubrevin is a resident protein of insulin-sensitive GLUT4 glucose transporter vesicles in 3T3–L1 adipocytes. J Biol Chem. 1995;270:8233–8240. doi: 10.1074/jbc.270.14.8233. [DOI] [PubMed] [Google Scholar]
- 33.Watson R T, Pessin J E. Intracellular organization of insulin signaling and GLUT4 translocation. Recent Prog Horm Res. 2001;56:175–193. doi: 10.1210/rp.56.1.175. [DOI] [PubMed] [Google Scholar]
- 34.White M F, Kahn C R. The insulin signaling system. J Biol Chem. 1994;269:1–4. [PubMed] [Google Scholar]