Abstract
The use of plant growth regulators has led to environmental contamination of water bodies that occur adjacent to agricultural areas. Some of these chemicals are bioactive, not only to plants, but also to non-target exposed biota, namely of the aquatic compartment. Previous work demonstrated the establishment of hepato- and nephrotoxic effects in juvenile tilapia (Oreochromis niloticus) exposed via aquatic media to gibberellic acid (GA3), which is among the most used plant growth regulators, in agricultural practices. Here, we investigated the effect of GA3 on hematological indices, poikilocytosis, nuclear abnormalities, and genotoxic indices measured in Nile tilapia (Oreochromis niloticus), as well as the putative protective effects of dietary supplementation of Spirulina (Arthrospira platensis). Fish were evenly assorted into 5 groups: group I served as a control, and groups II–V were fed diets supplemented with Spirulina at rates of 0 g/kg, 5 g/kg, 20 g/kg, and 100 g/kg, respectively, for 2 months before being exposed to 150 mg/L GA3. The results revealed that GA3 exposure decreased significantly all hematological indices (P < 0.05), except leucocytes and mean corpuscular hemoglobin concentration (MCHC), compared to the control group (P > 0.05). GA3 exposure increased significantly the percentage of nuclear abnormalities, altered erythrocytes and the percentages of tail DNA, compared to the control group (P < 0.05). Spirulina supplementation restored the hematological, poikilocytosis, nuclear abnormalities, and the percentages of tail DNA to near normal levels. The 100 g/kg SP treatment was the most effective in attaining such effect, showing concentration-dependency. The present study reinforces our findings of the toxicity of GA3 on O. niloticus and suggests that the addition of Spirulina to fish diet can mitigate the hemotoxic effects of GA3.
Keywords: GA3, Comet assay, Poikilocytosis, Tilapia, Spirulina
Introduction
Gibberellic acid (GA3) is a widely used plant growth regulator (PGR) in agriculture, as it regulates fruit ripening and shoot growth (Taiz & Zeige 1991; Gianfagna 1995). However, agricultural companies often provide more GA3 than crops can uptake (Mickel 1978). When GA3 is applied by conventional air-assisted spraying, it can drift from the application location(Wei et al. 2016), thereby contaminating bodies of water via run-off or off-target spraying. Despite this possibility, and of its significant agricultural use, GA3 ecotoxicological profile has not been thoroughly studied so far, and a very limited amount of data on its final environmental fate, concentrations in the aquatic compartment, and specially, toxicological effects are still extremely scarce (EFSA 2012).
However, previous studies have determined that GA3 is not exempt of toxicity. The hematotoxicity of GA3 has been studied using animal models such as rats and mice (Tuluce and Çelik 2006; Muthu et al. 2011; Troudi et al. 2012a; Ali et al. 2021, 2022; Ahmed and Nofal 2021; Galal et al. 2021; Soliman et al. 2021a, b, 2022a, b; Bushra and Shenouda 2022), hens and quail hens (Elsebai et al. 2003b; Elkomy et al. 2008; Ismail 2009), and rabbits (El-Sebai 2004). Nevertheless, the impacts of GA3 on the hematological parameters and erythron profile of fish have not been investigated (Sayed et al. 2020). In fish, direct contact between highly vascularized structures, such as gills and polluted water represents one of the main exposure routes for contaminants to enter the bloodstream (Barboza et al. 2020). Accordingly, erythrocytes may provide a unique tool for cytotoxicity assessment in fish (Fazio 2019; Soliman & Sayed 2020). The hematological and erythron profiles (poikilocytosis, nuclear abnormalities) of fish erythrocytes are valuable biomarkers for assessing the toxicity of chemicals and pharmaceutical residues ( Sayed et al. 2013; Sayed et al. 2016). Comet assay or alkaline single-cell gel electrophoresis (SCGE) is a simple, fast, and sensitive technique for assessing genotoxicity by quantifying the amount of DNA damage in individual cells. Thus, SCGE is an important tool for environmental monitoring, and for assessing the health of aquatic species (Hamed et al. 2019a). Toxicological effects caused by exposure to GA3 may have a significant importance when considering fish species of high economic importance that are frequently cultured in the vicinity of agricultural areas. Due to this factor, some fish species (e.g., Nile tilapia, Oreochromis niloticus) are particularly prone to have their physiology challenged, following exposure to chemicals used in agricultural practices, such as PGRs.
The blue-green alga Spirulina platensis (SP) is a suitable and cost-effective natural antioxidant and immune-stimulant for humans and animals, with fewer side effects than synthetic products (Belay 2020). Spirulina contains vital compounds, including protein (50–70% on dry mass basis) with all essential amino acids (Farag et al. 2016), carotenoids, chlorophyll, pigments, essential fatty acids (alpha-linolenic, gamma-linolenic, and linoleic acid (Mendes et al. 2003, Peiretti and Giorgia 2011), photosynthetic pigments (Bermejo et al. 2008), vitamins (thiamine, nicotinamide, riboflavin, folic acid, pyridoxine, vitamins A, D, and E) (Hosseini et al. 2013) and minerals (such as Ca, K, Cr, Cu, Mn, Fe, P, Mg, Na, Zn, and Se) (Babadzhanov et al. 2004), making Spirulina an efficient feed supplement (Yousefi et al. 2019). Nevertheless, the potential remediation effects of Spirulina against toxicity of phytohormones such as GA3 have not been investigated, especially in fish of high economic importance (Sayed et al. 2020).
The Nile tilapia (O. niloticus) is one of the most economically important freshwater fishes of Egypt and has recently been used as a toxicological model in bioremediation studies (Sayed et al. 2015). Considering that the culture of Nile tilapia is often subjected to anthropogenic contamination, namely by agricultural xenobiotics, it is extremely important to understand the toxic effects deriving from such exposure and also how to prevent and revert the toxic effects caused by such chemicals. This is important, not only in purely economic terms (by preventing losses due to intoxication of cultured fish), but also ecologically. In this study, we investigated the protective effect of Spirulina supplementation on hematological and comet assay indices, as well as poikilocytosis, and nuclear abnormalities in late juvenile Nile tilapia exposed to gibberellic acid.
Materials and methods
Chemical and microalgae
Gibberellic acid (GA3) 10% was purchased from Jiangxi New Reyphon Biochemical Co., China. Spirulina tablets were bought from Japan Algae Co., Ltd. Spirulina tablets were dissolved in water, and their bioactive ingredients became available in the water column for fish to absorb via the gastrointestinal canal.
Fish exposure
Early juveniles of tilapia (O. niloticus) (1 month old; weight 4.68 ± 0.1 g, length 3.45 ± 0.14 cm) were donated from an aquaculture unit (Al-Azhr University, Assiut) and transported to a laboratory in the Zoology Department of Assiut University, Egypt. The fish were kept in ≈200-L glass tanks (92 cm × 46 cm × 46 cm) containing 100 L dechlorinated tap water, with continuous aeration, under laboratory conditions for 4 weeks for acclimatization, and were fed 30% protein (Skerting Company®) diets.
The physicochemical properties of test water were recorded as follows: conductivity 269.5 μ M cm−1, pH 7.4, dissolved oxygen 6.85 mg L−1, temperature 20.9 °C, photoperiod 12 h:12 h light:dark. Five groups (30 fish/group) were assigned in three replicates for each treatment group (10 fish/glass aquarium) during the experimental period; group I served as a control, and groups II–V were fed 30% protein (Skerting Company®) diets supplemented with SP at rates of 0 g/kg, 5 g/kg, 20 g/kg, and 100 g/kg, respectively, for 2 months. These levels of supplementation were based on previously obtained data (Sayed & Fawzy 2014). Fish were subjected to these conditions before being exposed to 150 mg/L GA3 (Boeri 1991). Animals were physically separated from each other, in independent aquaria.
After 15 days, 6 fish from each group were randomly selected and anesthetized using ice to reduce the stress during processing. This procedure has been proved to be rapid, effective, and less stressful for animals, not causing significant changes in the structures of sampled tissues (Wilson et al. 2009). Blood was collected from the caudal vein in heparinized tube for hematological, comet assay, poikilocytosis, and nuclear abnormality analyses.
Hematological parameters
The hematological parameters, red blood cells (RBCs), white blood cells (WBCs), platelets, hematocrit (Ht), hemoglobin (Hb), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and MCH concentration (MCHC), were assessed according to Fijan (2002).
Erythron profile (poikilocytosis and nuclear abnormalities of RBCs)
Blood smears were prepared, stained with hematoxylin-eosin, selected, coded, randomized, and blindly scored for erythrocyte alterations and nuclear abnormalities, according to the criteria of al-Sabti and Metcalfe (1995) and Schmid (1975).
Comet assay
At the end of the experiment, blood (50 μL) was collected from the caudal veins, according to Sayed et al. (2017), then immediately placed on ice to prevent endogenous DNA damage and repair in the unfixed cells. Cell viability was assessed using a hemocytometer. A neutral comet assay was conducted using a previously described protocol (Hidaka et al. 2010) with minor modifications (Sayed et al. 2017). Cells were observed under a Zeiss Axioplan 2 fluorescence microscope (200×) with a digital 3 CCD color video camera (Sony, AVT-Horn). CASP software was used to analyze the comet image and calculate the tail moment score for each cell (Końca et al. 2003).
Statistical analyses
Data were analyzed using SPSS (version 25) at a significance level of 0.05. Briefly, data were
tested for normality using the Shapiro–Wilk test. Then, data were tested for homogeneity of variances (Levene’s test) using one-way analysis of variance. In the case of variance equality, Fisher’s LSD post hoc test was used to compare treatment groups against the control; in cases of variance inequality, Dunnett’s post hoc test was used.
Ethical statement
Experimental design and fish treatment were approved by the Committee of the Faculty of Science, Assuit University.
Results
Hematological parameters
The results of the current work revealed that the hematological indices, erythrocyte count (RBCs), Hb, Ht, MCH, MCV, and platelet levels, were significantly decreased in group II (exposed to GA3), compared to the control group. However, these levels were significantly increased in groups treated with SP (group III, IV, and V), compared to group II (Table 1). Conversely, there was an increase in MCHC and WBC levels in group II (exposed to GA3), compared with the control group; these parameters significantly decreased in groups treated with SP, compared with group II, reaching normal levels.
Table 1.
Hematological indices of juvenile Nile tilapia (Oreochromis niloticus) exposed to 150 mg/L gibberellic acid and previously fed diets supplemented with Spirulina platensis
| 1st group | 2nd group | 3rd group | 4th group | 5th group | |
|---|---|---|---|---|---|
| (RBC) (million/mm3) | 1.9 ± 0.0a | 1.7 ± 0.0b | 1.8 ± 0.0c | 1.9 ± 0.0d | 1.9 ± 0.0a |
| Hemoglobin (Hb) (g/dL) | 8.7 ± 0.1a | 8.1 ± 0.0b | 8.1 ± 0.0b | 8.4 ± 0.0c | 8.5 ± 0.1c |
| Ht (PCV) (%) | 26.3 ± 0.3a | 23.3 ± 0.2b | 24.7 ± 0.2c | 24.7 ± 0.2c | 26.5 ± 0.1a |
| MCV (µm3) | 135.1 ± 1.2ab | 134.2 ± 2.5ab | 136.03 ± 1.2ab | 133.5 ± 1.2a | 138.7 ± 1.4b |
| MCH (Pg) | 44.5 ± 0.5a | 46.3 ± 0.5b | 45 ± 0.1a | 45.03 ± 0.1a | 44.4 ± 0.6a |
| MCHC (%) | 33 ± 0.4ac | 34.5 ± 0.3b | 33 ± 0.3a | 34 ± 0.3b | 32.01 ± 0.3c |
| Platelets (Thousands/mm3) | 317.7 ± 0.5a | 311.5 ± 0.2b | 313 ± 0.4c | 314 ± 0.4c | 315.7 ± 0.4d |
| (WBC) (thousands/mm3) | 856 ± 1.9a | 861.5 ± 2.1b | 850.2 ± 1.4 cd | 853.5 ± 0.9ac | 848 ± 1.8d |
Data are represented as mean ± SE. Values with different superscript letters in the same row are significantly different (P < 0.05). Treatments consisted of exposure to 150 mg/L gibberellic acid (GA3) and/or diet supplementation with Spirulina platensis (SP) for 2 months prior. Group I, control group; group II, 150 mg/L GA3; group III, 150 mg/L GA3 and 5 g/kg SP diet; group IV, 150 mg/L GA3 and 20 g/kg SP diet; group V, 150 mg/L GA3 and 100 g/kg SP diet. RBC red blood cells; Ht hematocrit; PCV packed-cell volume; Hb hemoglobin; MCV mean corpuscularvolume; MCH mean corpuscular hemoglobin; MCHC mean corpuscular hemoglobin concentration; WBC white blood cells
Poikilocytosis (morphological changes of erythrocytes)
Our results showed that, compared to the control, exposure to GA3 resulted in a significant increase in the percentage of RBC morphological changes, as well as the appearance of some pathologic cells (Table 2; Fig. 1b and c), including tear-drop cells, crenated cells, acanthocytes, eccentric nuclei, hemolyzed cells, bionuclei, loped cells, elliptocytes, schistocytes, and kidney cells. The prevalence of these changes was significantly reduced in groups pre-fed SP, compared to group II (Table 2; Fig. 1d, e, and f).
Table 2.
Percentage of altered erythrocytes of juvenile Nile tilapia (Oreochromis niloticus) exposed to 150 mg/L gibberellic acid and previously fed diets supplemented with Spirulina platensis
| 1st group | 2nd group | 3rd group | 4th group | 5th group | |
|---|---|---|---|---|---|
| Hemolyzed cell | 2.33 ± 0.33a | 12.33 ± 0.67b | 8.33 ± 0.33c | 7.67 ± 0.88c | 4.67 ± 0.33d |
| Sickle cell | 0.67 ± 0.33a | 5.66 ± 0.33b | 4.33 ± 0.33b | 2.66 ± 0.33c | 1.66 ± 0.33ac |
| Irregular cell | 0 ± 0.00a | 16.33 ± 1.20b | 12.33 ± 0.33c | 7.33 ± 0.33d | 3.33 ± 0.88e |
| Schistocyte | 0.33 ± 0.33a | 13.66 ± 0.88b | 11.33 ± 0.88c | 7.33 ± 0.67d | 5.33 ± 0.33d |
| Acanthocyte | 1.33 ± 0.33a | 13 ± 1.15b | 10.33 ± 0.66b | 8 ± 0.57b | 3.66 ± 0.33c |
| Tear drop | 0.33 ± 0.33a | 12.33 ± 0.67b | 8.33 ± 0.67bc | 5 ± 0.58 cd | 3.33 ± 0.33d |
| Heinz bodies | 0.67 ± 0.67a | 5.67 ± 1.2bc | 3.67 ± 0.33c | 2.67 ± 0.67ac | 1.33 ± 0.33a |
| Elliptocyte | 1.33 ± 0.33a | 12.33 ± 0.88b | 10.33 ± 0.67c | 6.67 ± 0.33d | 3.33 ± 0.67e |
| Heart shape | 0 ± 00a | 4.67 ± 0.88b | 2.33 ± 0.33c | 0.67 ± 0.33a | 0.33 ± 0.33a |
| Eccentric nucleus | 2 ± 0.57a | 14 ± 0.57b | 12 ± 0.57c | 8.67 ± 0.33d | 3.67 ± 0.33e |
| Crenated cell | 2.33 ± 0.33a | 23.33 ± 0.88b | 17 ± 0.57c | 12.33 ± 0.33d | 5.67 ± 0.33e |
| Kidney-shape | 0 ± 0.00a | 4 ± 0.57b | 1.67 ± 0.33c | 1.33 ± 0.33c | 0.67 ± 0.33ac |
| Total malformed | 11.33 ± 3.30a | 137 ± 1.50b | 102 ± 1.00c | 70.33 ± 2.90d | 37 ± 2.60e |
Data are represented as mean ± SE. Values with different superscript letters in the same row are significantly different (P < 0.05). Treatments consisted of exposure to 150 mg/L gibberellic acid (GA3) and/or diet supplementation with Spirulina platensis (SP) for 2 months prior. Group I, control group; group II, 150 mg/L GA3; group III, 150 mg/L GA3 and 5 g/kg SP diet; group IV, 150 mg/L GA3 and 20 g/kg SP diet; group V, 150 mg/L GA3 and 100 g/kg SP diet
Fig. 1.
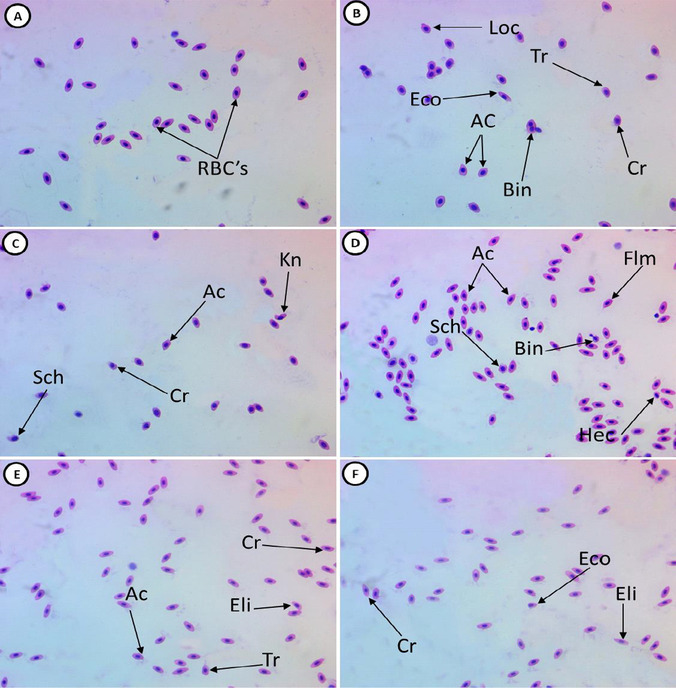
Representative hematoxylin–eosin-stained blood smears collected from juvenile Nile tilapia (Oreochromis niloticus) showing normal erythrocytes (A), deformed erythrocytes after fish exposure to 150 mg/L gibberellic acid (GA3) for 15 days (B, C), deformed erythrocytes after fish exposure to 150 mg/L GA3 for 15 days and feed supplemented with 5 g/kg Spirulina platensis (SP) for 2 months (D), deformed erythrocytes after fish exposure to 150 mg/L GA3, and 20 g/kg SP diet (E), and deformed erythrocytes after exposure to 150 mg/L GA3, and 100 g/kg SP diet (F). Tr, tear-drop cell; Cr, crenated cell; Ac, acanthocyte; Eco, eccentric nucleus; Hec, hemolyzed cells; Bin, bionuclei; Loc, loped cell; Eli, elliptocyte; Shc, schistocyte; Kn, kidney cell (1000 × magnification)
Erythrocyte nuclei alterations
Compared to the control group, fish exposed to GA3 exhibited a significant increase in the percentage of erythrocyte nuclei alterations (Table 3; Fig. 1b and c), including micronuclei, binucleated, blebbed nuclei, notched nuclei, lobed nuclei, and hemolyzed nuclei. These alterations were significantly reduced in groups pre-fed SP, compared to group II (Table 3; Fig. 1d, e, and f).
Table 3.
Percentage of altered erythrocyte nuclei of juvenile Nile tilapia (Oreochromis niloticus) exposed to 150 mg/L gibberellic acid and previously fed diets supplemented with Spirulina platensis
| 1st group | 2nd group | 3rd group | 4th group | 5th group | |
|---|---|---|---|---|---|
| Micronuclei | 0.67 ± 0.33a | 11.33 ± 0.33b | 8.66 ± 0.33c | 5.66 ± 0.88d | 3.33 ± 0.33e |
| Binucleated | 0.67 ± 0.33a | 10 ± 0.58b | 7.33 ± 0.33c | 4.33 ± 0.33d | 3.33 ± 0.33d |
| Blebbed nuclei | 0 ± 0.00a | 2.33 ± 0.33b | 1.67 ± 0.33bc | 1.33 ± 0.33c | 0.67 ± 0.33ac |
| Notched nuclei | 0.33 ± 0.33a | 4.66 ± 0.67b | 2.33 ± 0.33c | 1.67 ± 0.33c | 1.33 ± 0.33ac |
| Lobed nuclei | 0 ± 0.00a | 2.33 ± 0.67b | 1.33 ± 0.33bc | 1 ± 0.00c | 0.33 ± 0.33ac |
| Hemolyzed nuclei | 0.67 ± 0.33a | 6.67 ± 0.33b | 5 ± 0.58c | 3.67 ± 0.33d | 2.33 ± 0.33e |
| Total malformed | 2.33 ± 0.33a | 37.33 ± 0.33b | 26.33 ± 0.88c | 17.66 ± 1.45d | 11.33 ± 0.33e |
Data are represented as mean ± SE. Values with different superscript letters in the same row are significantly different (P < 0.05). Treatments consisted of exposure to 150 mg/L gibberellic acid (GA3) and/or diet supplementation with Spirulina platensis (SP) for 2 months prior. Group I, control group; group II, 150 mg/L GA3; group III, 150 mg/L GA3 and 5 g/kg SP diet; group IV, 150 mg/L GA3 and 20 g/kg SP diet; group V, 150 mg/L GA3 and 100 g/kg SP diet
Comet assay
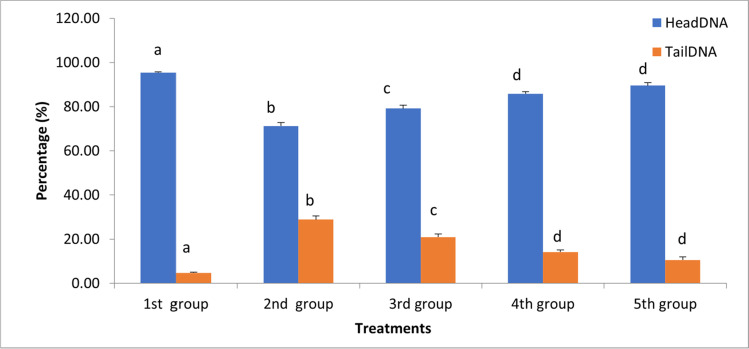
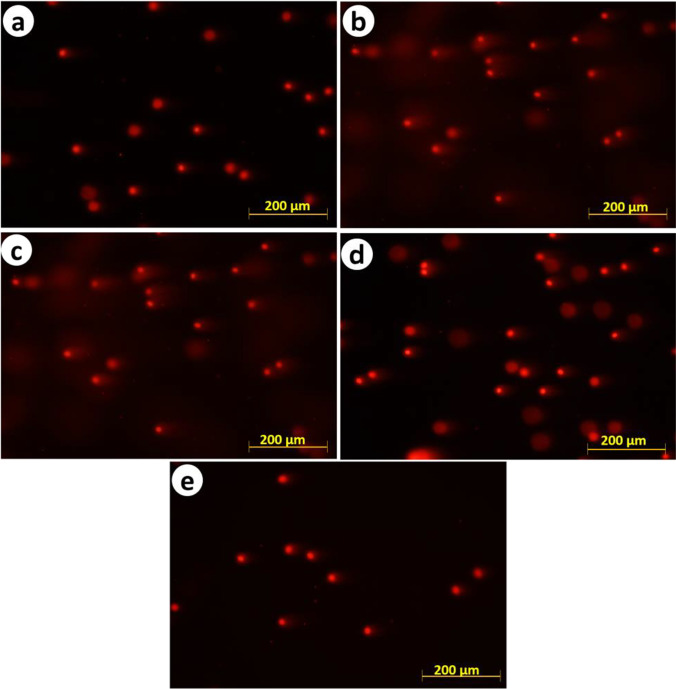
In comparison with the control group, a clear and statistically significant increase in the percentages of tail DNA and decrease in head DNA was found in group II, treated with GA3 (Fig. 2). Undamaged control erythrocytes showed comets with an intact head (Fig. 3a), while the results of the comet assay of fish from group II showed an increase in tail length, compared to the control group (Fig. 3b). Pre-feeding with SP attenuated the changes observed in group II, but the number of alterations in groups III–V was still higher than those observed for the control group (Figs. 2; 3c, d, and e).
Fig. 2.
Percentages of head DNA and tail DNA in blood cell comet assays of juvenile Nile tilapia (Oreochromis niloticus). Treatments consisted of exposure to 150 mg/L gibberellic acid (GA3) and/or diet supplementation with Spirulina platensis (SP) for 2 months prior. Group I, control group; group II, 150 mg/L GA3; group III, 150 mg/L GA3 and 5 g/kg SP diet; group IV, 150 mg/L GA3 and 20 g/kg SP diet; group V, 150 mg/L GA3 and 100 g/kg SP diet.
Fig. 3.
Blood cells stained with ethidium bromide after comet assay from control and treated groups of juvenile Nile tilapia (Oreochromis niloticus). Treatments consisted of exposure to 150 mg/L gibberellic acid (GA3) and/or diet supplementation with Spirulina platensis (SP) for 2 months prior. A Group I, control group; B group II, 150 mg/L GA3; C group III, 150 mg/L GA3 and 5 g/kg SP diet; D group IV, 150 mg/L GA3 and 20 g/kg SP diet; E group V, 150 mg/L GA3 and 100 g/kg SP diet. Scale bar 25 μm
Discussion
Although gibberellic acid is extensively used as a plant growth regulator in Egypt and other countries, little is known about the toxic effects of GA3 on fish (Sayed et al. 2020). Hematological parameters are important measures that reflect fish health status (Thummabancha et al. 2016). The results of the current work revealed that erythrocyte count (RBCs), Hb, Ht, MCH, MCV, and platelet levels, were significantly decreased in GA3-exposed fish group. Additionally, El-Okazy (2008) observed significant dose-dependent decreases in total erythrocyte counts and Hb levels in mice treated with gibberellic acid; total leukocyte counts showed a highly significant dose-dependent increase. Elkomy et al. (2008) reported that GA3 doses caused a significant increase in Hb concentration and packed-cell volume (PCV), but RBC count was not significantly increased in aged female fowl. Furthermore, Troudi et al. (2012a) stated that GA3 reduced the RBC count, Hb concentration, and Ht in suckling rats, while these parameters remained unchanged in their mothers. However, an increase in WBC count was observed in mothers but not their pups. In addition, Abdou et al. (2017) observed that direct exposure to GA3 for 1 month in rams induced a significant decrease in blood picture.
In contrast, Zilva and Pannall (1983) noted that GA3 affected PCV in birds by increasing hematopoiesis and subsequently increasing erythrocyte counts. Similarly, Abdelhamid et al. (1994) reported that GA3 treatment increased the plasma iron concentration in chicks; the improved Hb concentration in chicks seems to be due to increased iron absorption rates from the diet. In addition, Ozmen et al. (1995) demonstrated that GA3 treatment significantly increased erythrocyte counts in laboratory mice. Elsebai et al. (2003a) reported significant dose-dependent increases in quail hen RBC, Hb, and PCV% after GA3 exposure. Elsebai (2004) revealed that GA3 significantly increased RBC count, WBC count, PCV, and Hb in rabbits in a dose-dependent manner. Srikumar et al. (2009) reported that GA3 influenced changes in RBC and WBC content and increased Hb in rats. Ismail (2009) found that RBCs and Hb were significantly increased in laying hens injected with GA3, compared with the control group. In male albino rats, RBC, WBC, and neutrophil counts significantly increased at all doses of GA3 treatment, possibly due to the effect of GA3 on hematopoiesis, whereas lymphocyte counts decreased (Muthu et al. 2011). Askar and IsmaeI (2012) found that WBCs, RBCs, PCV, and lymphocytes improved significantly in hens injected with GA3.
Despite the lack of knowledge concerning the mechanisms by which GA3, and PGRs in general, exert their adverse effects, it may be suggested that oxidative stress may be a decisive event. PGRs may induce oxidative stress, leading to generation of free radicals and cause lipid peroxidation, which causes changes in fluidity and an increase in the permeability to different ions leading to hemolysis of RBCs (Tuluce & Çelik 2006). The data obtained by Tuluce and Celike (2006) showed that GA3 induced alterations in antioxidant defense systems and lipid peroxidation level in erythrocytes in rats exposed to 75 ppm of GA3 in drinking water for 50 days. In addition, Troudi et al. (2011a,b; 2012b) showed that GA3 was able to induce significant oxidative changes in rodents, with the activation of antioxidant defensive mechanisms, followed also by an increase in lipid peroxidation. Oxidative stress was again the main outcome resulting from GA3 exposure, in the insect species Galleria mellonella L., with the activation of a series of antioxidant mechanisms, such as superoxide dismutase, glutathione S-transferase, and catalase, as demonstrated by Altuntas (2015). This effect of favoring oxidative stress seems not be limited to animals, since sorghum (Sorghum bicolor), after being exposed to GA3, also had changes in the levels of antioxidant enzymes, as described by Forghani et al. (2020). However, the role of GA3 in the modulation of antixodiant responses in plants is not absolutely straightforward and will depend on the existence of other sources of physiological stress. The administration of GA3 to plants (maize, Zea mays) subjected to salinity stress resulted in the increase of the efficacy of antioxidant defense, namely by augmenting the activity of antioxidant enzymes (Shahzad et al. 2021). In addition, antioxidant enzymes (SOD, and CAT) were significantly elevated in GA3-treated tilapia (Sayed et al. 2020).
The results of the present study demonstrated that fish exposure to GA3 resulted in significant increases in poikilocytosis (RBC morphological changes) and the appearance of some pathologic cells and nuclei. Similarly, Troudi et al. (2012a) used blood smear analyses to assess GA3-treated rat pups and observed empty red cells, which is a sign of anemia; the mothers’ blood smears showed polynuclear blood cell infiltration. Also, Ayoub and El Aalem (2016) observed that GA3-exposed groups evoked a significant increase in the total aberrated cells and total chromosomal aberrations of bone marrow cells in rabbit.
To the best of our knowledge, this is the first study to determine the effect of GA3 on RBC poikilocytosis and nuclear abnormalities. A growing amount of evidence indicates that GA3 alters the antioxidative systems as it induces oxidative stress, leading to generation of free radicals and causes lipid peroxidation which may trigger RBC membrane damage, such as a decreased membrane transporter activity and alterations in membrane permeability (Troudi et al. 2012a). This effect may be the source of cellular damage that ultimately results in morphological changes on blood cells. In fact, previously published data have established the linkage between the occurrence of oxidative stress (and of its concomitant adverse changes, including oxidation of membrane lipids and proteins) and morphological changes in erythrocytes (Gyawali et al. 2015a), which in turn, alters blood viscosity parameters (Gyawali and Richards 2015b). In fact, oxidative stress seems to be a major force in deleterious changes to the morphology of erythrocytes, as evidenced by the review by Bissinger et al. (2018).
Comet assays are used to detect genetic damage in the form of DNA strand breaks, providing a sensitive indicator of changes in the overall health of an organism. Comet assays have been applied to assess and monitor the health and genetic condition of both vertebrate and invertebrate organisms (Hamed et al. 2019b). Blood is readily available and easy to collect from fish; RBCs comprise 97% of fish blood. Hence, fish RBCs are frequently used to evaluate DNA damage using comet assay (Tasneem &Yasmeen 2018). The DNA damage observed in the blood cells in the present study was similar to that of Hosseinchi et al. (2013) who stated that GA3 increased the immature sperms and sperms with damaged chromatin. Also, DNA isolated from control and tadpoles treated with gibberellin-A3 showed degradation into oligonucleotide fragments forming a clear laddering pattern of apoptosis (Sakr and Shalaby 2012). Substances that induce genotoxicity have been shown also to produce reactive oxygen species, as well as electrophilic free-radical metabolites that interact with DNA and lead to DNA damage (Sayed et al. 2020). It is likely that GA3 metabolism may result in the overproduction of ROS. This outcome has been already reported, and for a considerable number of animal models, namely rodents (Troudi et al. 2012a; Tuluce and Çelik 2006), as already described. Sakr et al. (2009) stated that by increasing the concentration of gibberellin A3, the number of damage cells and the damage DNA spots increase. Also, DNA fragmentation was markedly increased in the retina of treated mother rats and their offspring’s after intra-gastric administration of gibberellic and indole acetic acids (El-Sayyad et al. 2015). Consequently, it is possible to suggest that the here-observed DNA damage may be associated to the occurrence of oxidative stress, which is a common outcome of GA3 exposure in vertebrates.
The addition of S. platensis to the fish diets significantly restored hematological parameters and erythron profiles to their normal levels and shapes. These results are attributed to the bioactive components of S. platensis. Among these, it is possible to suggest that the combination of iron and vitamins, is particularly effective in preventing the hematological adverse effects caused by GA3. On one hand, iron is detrimental for the control of the production of red blood cells and decrease the RBC destruction that results from O. niloticus exposure to GA3 (Hemalatha et al. 2012). S. platensis also contains polysaccharides, which induce RBC regeneration (Mohamed et al. 2014). Furthermore, S. platensis contains phycocyanin pigment, which induces erythropoietin (EPO) hormone and contributes to erythropoiesis (Zhang 1994), and beta-carotene, which enhances RBC recovery to reduce cell lysis (Stivala et al. 1996). Also, the addition of SP to fish diet decreased the level of DNA damage induced by GA3 exposure. This could be ascribed to the antioxidant components of Spirulina, which scavenge free radicals to reduce oxidative DNA damage (Chu et al. 2010). Spirulina has been long known for its antioxidant activity (Piñero Estrada et al. 2001; Dartsch 2008) and has been shown to exert antioxidant activity on several animals model exposed to known oxidant chemicals, such as cadmium (Amin et al. 2006), sodium arsenite (Bashandy et al. 2016), deltamethrim (Abdel-Daim et al. 2013), glyphosate (Wided et al. 2021), and γ-irradiation and thioacetamide (Salem and Ismail 2021). Spirulina has also been implicated in the prevention of oxidative damage and inflammation resulting from excessive physical activity (Brito et al. 2020). In addition, S. platensis contains polysaccharides, which improve both the repair activity of damaged DNA excision and unscheduled DNA synthesis (Bhat and Madyastha 2001).
In conclusion, supplementing fish diets with SP can mitigate the adverse effects of gibberellic acid.
Author contribution
Experimental design: AHS; experiment and analysis: AHS, MH, and HAMS. Data interpretation: AHS, MH, and HAMS; writing and revision: AHS, MH, HAMS, and BN. All the authors read and approved the final manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
All the data generated or analyzed during this study are included in the research article.
Declarations
Consent to participate
It is not applicable.
Consent for publication
It is not applicable.
Conflict of interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdelhamid AM, Dorra TM, Ali MA, Abou-Egla EH. Effect of gibberellic acid on broiler chickens performance and some metabolic parameters. Arch Tierernahr. 1994;46:269–276. doi: 10.1080/17450399409381776. [DOI] [PubMed] [Google Scholar]
- Abdel-Daim MM, Abuzead SMM, Halawa SM. Protective role of Spirulina platensis against acute deltamethrin-induced toxicity in rats. PLoS ONE. 2013;8(9):e72991. doi: 10.1371/journal.pone.0072991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdou M, Nour E, Hussein E, Zaabal E. Biochemical and reproductive studies on the effect of gibberellic acid on rams. Suez Canal Vet Med J SCVMJ. 2017;22(1):205–217. [Google Scholar]
- Ahmed RAE, Nofal AE (2021) Ameliorating impact of Phoenix dactylifera L. leaves oil extract on testicular toxicity induced by gibberellic acid: histomorphometric and immunohistochemical studies. Egypt J Histol 44(1):128-143
- Ali AF, Ghoneim NS, Salama R. Light and electron microscopic study on the effect of Gibberellic acid on the renal cortex of adult male albino rats and the possible protective role of coenzyme Q10. Egypt J Histol. 2021;44(2):368–383. [Google Scholar]
- Ali S, Moselhy, WA, Mohamed HM, Nabil TM, Abo El-Ela FI, Abdou K (2022) Ameliorative effects of Dictyota dichotoma on hepatotoxicity induced by gibberellic acid in albino rats. Toxicol Res 38(3):379–392. 10.1007/s43188-022-00122-8 [DOI] [PMC free article] [PubMed]
- al-SabtiMetcalfe KCD. Fish micronuclei for assessing genotoxicity in water. Mutat Res. 1995;343:121–135. doi: 10.1016/0165-1218(95)90078-0. [DOI] [PubMed] [Google Scholar]
- Altuntaş H. Determination of gibberellic acid (GA3)-induced oxidative stress in a model organism Galleria mellonella L. (Lepidoptera: Pyralidae) Environ Entomol. 2015;44(1):100–105. doi: 10.1093/ee/nvu020. [DOI] [PubMed] [Google Scholar]
- Amin A, Hamza AA, Daoud S, Hamza W. Spirulina protects against cadmium-induced hepatotoxicity in rats. Am J Pharmacol Toxicol. 2006;1(2):21–25. [Google Scholar]
- Askar AA, IsmaeIl EI. Effect of gibberellic acid on some physiological, reproductive and hatchability parameters of laying hens during winter and summer seasons. Egypt J Anim Prod. 2012;49:77–86. [Google Scholar]
- Ayoub MA, El Aalem MM. Cytogenetic and pathological studies on the effect of gibberellic acid in rabbit. Egypt J Chem Environ Health. 2016;2(2):566–579. [Google Scholar]
- Babadzhanov AS, Abdusamatova N, Yusupova FM, Faizullaeva N, Mezhlumyan LG, Malikova MK. Chemical composition of Spirulina platensis cultivated in Uzbekistan. Chem Nat Compd. 2004;40:276–279. [Google Scholar]
- Barboza LGA, Lopes C, Oliveira P, Bessa F, Otero V, Henriques B, Raimundo J, Caetano M, Vale C, Guilhermino L. Microplastics in wild fish from North East Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci Total Environ. 2020;717:14. doi: 10.1016/j.scitotenv.2019.134625. [DOI] [PubMed] [Google Scholar]
- Bashandy SAE, El Awdan SA, Ebaid H, Alhazza IM (2016) Antioxidant potential of Spirulina platensis mitigates oxidative stress and reprotoxicity induced by sodium arsenite in male rats. Oxidative Med Cell Longev 2016, Article ID 7174351, 8 pages. 10.1155/2016/7174351 [DOI] [PMC free article] [PubMed]
- Belay A. The potential application of Spirulina (Arthrospira) as a nutritional health and therapeutic supplement in health management. J Am Nutraceutical Assoc. 2020;5:27–48. [Google Scholar]
- Bermejo P, Piñero E, Villar ÁM. Iron-chelating ability and antioxidant properties of phycocyanin isolated from a protean extract of Spirulinaplatensis. Food Chem. 2008;110:436–445. doi: 10.1016/j.foodchem.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Bhat VB, Madyastha KM. Scavenging of peroxynitrite by phycocyanin and phycocyanobilin from Spirulina platensis: protection against oxidative damage to DNA. Biochem Biophys Res Commun. 2001;285:262–266. doi: 10.1006/bbrc.2001.5195. [DOI] [PubMed] [Google Scholar]
- Bissinger R, Bhuyan AAM, Qadri SM, Lang F. Oxidative stress, eryptosis and anemia: a pivotal mechanistic nexus in systemic diseases. FEBS J. 2018;286(2019):826–854. doi: 10.1111/febs.14606. [DOI] [PubMed] [Google Scholar]
- Boeri R. Acute toxicity of gibberellic acid to the rainbow trout. Oncorhynchus mykiss: Lab Project Number. Resource Analysts Inc; 1991. p. 41. [Google Scholar]
- Brito AF, Silva AS, Oliveira CVC, Souza AA, Ferreira PB, Souza ILL, Araujo LCC, Félix GS, Sampaio RS, Tavares RL, Pereira RA, Neto MM, Silva BA. Spirulina platensis prevents oxidative stress and infammation promoted by strength training in rats: dose-response relation study. Sci Rep. 2020;10:6382. doi: 10.1038/s41598-020-63272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushra R, Shenouda M (2022) The possible protective role of N-acetylcysteine against gibberellic acid-induced lung structural changes of the adult albino rat. Egypt J Histol. 10.21608/ejh.2022.102176.1578
- Chu WL, Lim YW, Radhakrishnan AK, Lim PE. Protective effect of aqueous extract from Spirulina platensis against cell death induced by free radicals. BMC Complement Altern Med. 2010;10:1472–6882. doi: 10.1186/1472-6882-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartsch PC. Antioxidant potential of selected Spirulina platensis preparations. Phytother Res. 2008;22(5):627–633. doi: 10.1002/ptr.2310. [DOI] [PubMed] [Google Scholar]
- El-Okazy AM. The effects of combination of gibberellic acid - 3 (GA3) and ethephon (2-chloroethyl phosphonic acid) (plant growth regulators) on some physiological parameters in mice. J Egypt Public Health Assoc. 2008;83:67–86. [PubMed] [Google Scholar]
- Elkomy AE, El-Shaarrawi G, El-Ansary E, Elnagar AA. Evaluation of estrogenic response to subcutaneously injection of gibberellic acid (GA3) in aged female fowl. Egypt Poult Sci. 2008;28:1265–1286. [Google Scholar]
- El-Sayyad HIH, Ramadan MM, Abou-Egla MH, Asiri AM, El-Beeh ME. Role of gibberellic and indole acetic acid in altering ocular structure and function of mother rats and their offspring. Br J Med Med Res. 2015;10(12):1. [Google Scholar]
- Elsebai A, Abaza M, Elnagar S. Physiological effects of gibberellic acid (GA3) on female Japanese quail production and reproduction. Egypt Poult Sci. 2003;23:977. [Google Scholar]
- El-Sebai A. Gibberellic acid (GA3) as growth promoter for growing rabbits. Egypt Poult Sci. 2004;24:1033–1047. [Google Scholar]
- Farag MR, Alagawany M, El-Hac MEA, Dhama K. Nutritional and Healthical Aspects of Spirulina (Arthrospira) for Poultry, Animals and Human. Int J Pharmacol. 2016;12:36–51. [Google Scholar]
- Fazio F. Fish hematology analysis as an important tool of aquaculture: a review. Aquaculture. 2019;500:237–242. [Google Scholar]
- Fijan N. Composition of main haematopoietic compartments in normal and bled channel catfish. J Fish Biol. 2002;60:1142–1154. [Google Scholar]
- Forghani AH, Almodares A, Ehsanpour AA. The role of gibberellic acid and paclobutrazol on oxidative stress responses induced by in vitro salt stress in sweet sorghum. Russ J Plant Physiol. 2020;67:555–563. doi: 10.1134/S1021443720030073. [DOI] [Google Scholar]
- Gianfagna T (1995) Natural and synthetic growth regulators and their use in horticultural and agronomic crops. In: Davies PJ (ed) Plant hormones. Springer, Dordrecht, pp 751–773. 10.1007/978-94-011-0473-9_34
- Galal HM, Gomea M, Sayed AM. Possible ameliorative effects of silymarin on gibberellic acid-induced pancreatic dysfunction in adult female rats and their pups. Bull Egypt Soc Physiol Sci. 2021;41(4):484–499. [Google Scholar]
- Gyawali P, Richards RS, Bwititi PT, Nwose EU. Association of abnormal erythrocyte morphology with oxidative stress and inflammation in metabolic syndrome. Blood Cells Mol Dis. 2015;54(4):360–363. doi: 10.1016/j.bcmd.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Gyawali P, Richards RS. Association of altered hemorheology with oxidative stress and inflammation in metabolic syndrome. Redox Rep. 2015;20(3):139–144. doi: 10.1179/1351000214Y.0000000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamed M, Soliman HAM, Osman AGM, Sayed AH. Assessment the effect of exposure to microplastics in Nile tilapia (Oreochromis niloticus) early juvenile: I. Blood Biomark Chemosphere. 2019;228:345–350. doi: 10.1016/j.chemosphere.2019.04.153. [DOI] [PubMed] [Google Scholar]
- Hamed M, Soliman HAM, Sayed AH. Ameliorative effect of Spirulina platensis against lead nitrate-induced cytotoxicity and genotoxicity in catfish Clarias gariepinus. Environ Sci Pollut Res Int. 2019;26:20610–20618. doi: 10.1007/s11356-019-05319-3. [DOI] [PubMed] [Google Scholar]
- Hidaka M, Oda S, Kuwahara Y, Fukumoto M, Mitani H. Cell lines derived from a medaka radiation-sensitive mutant have defects in DNA double-strand break responses. J Radiat Res. 2010;51:165–171. doi: 10.1269/jrr.09107. [DOI] [PubMed] [Google Scholar]
- Hosseini SM, Khosravi-Darani K, Mozafari MR. Nutritional and medical applications of spirulina microalgae. Mini Rev Med Chem. 2013;13:1231–1237. doi: 10.2174/1389557511313080009. [DOI] [PubMed] [Google Scholar]
- Hosseinchi M, Soltanalinejad F, Najafi G, Roshangar L. Effect of gibberellic acid on the quality of sperm and in vitro fertilization outcome in adult male rats. Vet Res Forum: Int Q J. 2013;4:259–264. [PMC free article] [PubMed] [Google Scholar]
- Ismail EI (2009) Physiological and reproductive studies on laying hens. Ph. D. Thesis, Faculty of Agric, Zagazig. Uni., Zagazig, Egypt
- Końca K, Lankoff A, Banasik A, Lisowska H, Kuszewski T, Góźdź S, Koza Z, Wojcik A. A cross-platform public domain PC image-analysis program for the comet assay. Mutat Res. 2003;534:15–20. doi: 10.1016/s1383-5718(02)00251-6. [DOI] [PubMed] [Google Scholar]
- Mendes RL, Nobre B, Cardoso M, Pereira A, Palavra A. Supercritical carbon dioxide extraction of compounds with pharmaceutical importance from microalgae. Inorg Chim Acta. 2003;356:328–334. [Google Scholar]
- Mickel L. Plant growth regulators controlling biological behavior with chemicals. Chem Eng News. 1978;56:18. [Google Scholar]
- Mohamed WA, Ismail S, El-Hakim YA. Spirulina platensis ameliorative effect against GSM 900-MHz cellular phone radiation-induced genotoxicity in male Sprague-Dawley rats. Comp Clin Pathol. 2014;23:1719–1726. [Google Scholar]
- Muthu S, Muthuraman P, Muthuviveganandavel V, Srikumar K. Acute effect of gibberellic acid on serum enzymes and blood markers in male albino rats. Int J Drug Deliv. 2011;3:340–347. [Google Scholar]
- Ozmen, Fatih TS, Suna B, AN Z (1995). Effect of abscises acid and gibberellic acid on sexual differentiation and some physiological parameters of laboratory mice. Turk-J Biology 19:357-364
- Peiretti PG, Giorgia M. Effects of diets with increasing levels of Spirulina platensis on the carcass characteristics, meat quality and fatty acid composition of growing rabbits. Livest Sci. 2011;140:218–224. [Google Scholar]
- Piñero Estrada JE, Bermejo Bescós P, Villar del Fresno AM. Antioxidant activity of different fractions of Spirulina platensis protean extract. Farmaco. 2001;56(5–7):497–500. doi: 10.1016/s0014-827x(01)01084-9. [DOI] [PubMed] [Google Scholar]
- Sakr SA, Shalaby SY. Effect of gibberellin-A3 on metamorphosis in the Egyptian toad Bufo regularis. J Basic Appl Zool. 2012;65:153–156. [Google Scholar]
- Sakr SA, Hassab-Elnabi SE, El-Ghonaimy DA (2009) Effect of green tea on cytogenetic changes induced by gibberellin a3 in human lymphocyte culture. Can J Pure Appl Sci 3(3):917–924
- Salem AA, Ismail AFM. Protective impact of Spirulina platensis against γ-irradiation and thioacetamide-induced nephrotoxicity in rats mediated by regulation of micro-RNA 1 and micro-RNA 146a. Toxicol Res. 2021;10(3):453–466. doi: 10.1093/toxres/tfab037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayed AH, Fawzy M. Effect of dietary supplementation of Spirulina platensis on the growth and haematology of the catfish Clarias gariepinus. J Adv Biology. 2014;5:625–635. [Google Scholar]
- Sayed AEH, Igarashi K, Watanabe-Asaka T, Mitani H. Double strand break repair and γ-H2AX formation in erythrocytes of medaka (Oryzias latipes) after γ-irradiation. Environ Pollut. 2017;224:35–43. doi: 10.1016/j.envpol.2016.11.050. [DOI] [PubMed] [Google Scholar]
- Sayed AH, AbdAllah EA, Hamed M, Soliman HAM. Hepato-nephrotoxicity in late juvenile of Oreochromis niloticus exposed to gibberellic acid: ameliorative effect of Spirulina platensis. Pestic Biochem Physiol. 2020;167:11. doi: 10.1016/j.pestbp.2020.104600. [DOI] [PubMed] [Google Scholar]
- Sayed A AH, Elbaghdady HA, Zahran E. Arsenic-induced genotoxicity in Nile tilapia (Orechromis niloticus); the role of Spirulina platensis extract. Environ Monit Assess. 2015;187:015–4983. doi: 10.1007/s10661-015-4983-7. [DOI] [PubMed] [Google Scholar]
- Sayed AH, Watanabe-Asaka T, Oda S, Mitani H. Apoptosis and morphological alterations after UVA irradiation in red blood cells of p53 deficient Japanese medaka (Oryzias latipes) J Photochem Photobiol B. 2016;161:1–8. doi: 10.1016/j.jphotobiol.2016.05.004. [DOI] [PubMed] [Google Scholar]
- Sayed AH, Abdel-Tawab HS, Abdel Hakeem SS, Mekkawy IA. The protective role of quince leaf extract against the adverse impacts of ultraviolet–a radiation on some tissues of Clarias gariepinus (Burchell, 1822) J Photochem Photobiol B. 2013;119:9–14. doi: 10.1016/j.jphotobiol.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Schmid W. The micronucleus test. Mutat Res. 1975;31:9–15. doi: 10.1016/0165-1161(75)90058-8. [DOI] [PubMed] [Google Scholar]
- Shahzad K, Hussain S, Arfan M, Hussain S, Waraich EA, Zamir S, Saddique M, Rauf A, Kamal KY, Hano C, El-Esawi MA. Exogenously applied gibberellic acid enhances growth and salinity stress tolerance of maize through modulating the morpho-physiological, biochemical and molecular attributes. Biomolecules. 2021;2021(11):1005. doi: 10.3390/biom11071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikumar K, Vikramathithan J, Gautami G, Ganesh I. Differences in rat tissue lactate dehydrogenase activity caused by giberellic acid and homobrassinolide. Turk J Biochem. 2009;34:57–61. [Google Scholar]
- Soliman HAM, Sayed AH. Poikilocytosis and tissue damage as negative impacts of tramadol on juvenile of tilapia (Oreochromis niloticus) Environ Toxicol Pharmacol. 2020;78:7. doi: 10.1016/j.etap.2020.103383. [DOI] [PubMed] [Google Scholar]
- Soliman MM, Aldhahrani A, Gaber A, Alsanie WF, Shukry M, Mohamed WA, Metwally MM. Impacts of n‐acetyl cysteine on gibberellic acid‐induced hepatorenal dysfunction through modulation of pro‐inflammatory cytokines, antifibrotic and antioxidant activity. J Food Biochem. 2021;45(4):e13706. doi: 10.1111/jfbc.13706. [DOI] [PubMed] [Google Scholar]
- Soliman MM, Aldhahrani A, Gaber A, Alsanie WF, Shukry M, Mohamed WA, Mohamed AA. Impacts of n‐acetyl cysteine on gibberellic acid‐induced testicular dysfunction through regulation of inflammatory cytokines, steroid and antioxidant activity. Andrologia. 2021;53:5–e14036. doi: 10.1111/and.14036. [DOI] [PubMed] [Google Scholar]
- Soliman MM, Gaber A, Alsanie WF, Mohamed WA, Metwally MM, Abdelhadi AA, Shukry M. Gibberellic acid‐induced hepatorenal dysfunction and oxidative stress: mitigation by quercetin through modulation of antioxidant, anti‐inflammatory, and antiapoptotic activities. J Food Biochem. 2022;46(2):e14069. doi: 10.1111/jfbc.14069. [DOI] [PubMed] [Google Scholar]
- Soliman MM, Aldhahrani A, Gaber A, Alsanie WF, Mohamed WA, Metwally MM, Shukry M. Ameliorative impacts of chrysin against gibberellic acid-induced liver and kidney damage through the regulation of antioxidants, oxidative stress, inflammatory cytokines, and apoptosis biomarkers. Toxicol Res. 2022;11(1):235–244. doi: 10.1093/toxres/tfac003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stivala L, Savio M, Cazzalini O, Pizzala R, Rehak L, Bianchi L, Vannini V, Prosperi E. Effect of carotene on cell cycle progression of human fibroblasts. Carcinogenesis. 1996;17:2395–2401. doi: 10.1093/carcin/17.11.2395. [DOI] [PubMed] [Google Scholar]
- Taiz Z. Plant physiology. Redwood City: CA. The Bejamin/Cumming Publishing Company; 1991. Gibberellins; pp. 426–451. [Google Scholar]
- Tasneem S, Yasmeen R. Evaluation of genotoxicity by comet assay (single-cell gel electrophoresis) in tissues of the fish Cyprinus carpio during sub-lethal exposure to Karanjin. J Basic Appl Zool. 2018;79:19. [Google Scholar]
- Thummabancha K, Onparn N, Srisapoome P. Analysis of hematologic alterations, immune responses and metallothionein gene expression in Nile tilapia (Oreochromis niloticus) exposed to silver nanoparticles. J Immunotoxicol. 2016;13:909–917. doi: 10.1080/1547691X.2016.1242673. [DOI] [PubMed] [Google Scholar]
- Troudi A, Soudani N, Amara IB, Bouaziz H, Ayadi FM, Zeghal N. Oxidative damage in erythrocytes of adult rats and their suckling pups exposed to gibberellic acid. Toxicol Ind Health. 2012;28:820–830. doi: 10.1016/j.etp.2010.11.017. [DOI] [PubMed] [Google Scholar]
- Troudi A, Bouaziz H, Soudani N, Amara IB, Boudawara T, Touzani H, Lyoussi B, Zeghal N. Neurotoxicity and oxidative stress induced by gibberellic acid in rats during late pregnancy and early postnatal periods: biochemical and histological changes. Exp Toxicol Pathol. 2012;64(6):583–590. doi: 10.1016/j.etp.2010.11.017. [DOI] [PubMed] [Google Scholar]
- Troudi A, Amara IB, Soudani N, Samet AM, Zeghal N. Oxidative stress induced by gibberellic acid on kidney tissue of female rats and their progeny: biochemical and histopathological studies. J Physiol Biochem. 2011;67(3):307–316. doi: 10.1007/s13105-011-0076-4. [DOI] [PubMed] [Google Scholar]
- Troudi A, Amara IB, Samet AM, Fetoui H, Soudani N, Guermazi F, Boudawara T, Zeghal N. Oxidative stress and thyroid impairment after gibberellic acid treatment in pregnant and lactating rats and their offspring. BioFactors. 2011;37:429–438. doi: 10.1002/biof.178. [DOI] [PubMed] [Google Scholar]
- Tuluce Y, Çelik I. Influence of subacute and subchronic treatment of abcisic acid and gibberellic acid on serum marker enzymes and erythrocyte and tissue antioxidant defense systems and lipid peroxidation in rats. Pestic Biochem Physiol. 2006;86:85–92. doi: 10.1002/jbt.20134. [DOI] [PubMed] [Google Scholar]
- Wei Q, Sanqin Z, Wei-min D, Chengda S, Jiang L, Yi-nian L, Jiabing G. Effects of fan speed on spray deposition and drift for targeting air-assisted sprayer in pear orchard. Int J Agric Biol Eng. 2016;9:53–62. [Google Scholar]
- Wided O, Abdelhafidh K, Ali M, Touaylia S. Protective role of Spirulina platensis against glyphosate induced toxicity in marine mussel Mytilus galloprovincialis. J Environ Sci Health C. 2021 doi: 10.1080/26896583.2021.1954833. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Bunte RM, Carty AJ. Evaluation of rapid cooling and tricaine methanesulfonate (MS222) as methods of euthanasia in zebrafish (Danio rerio) J Am Assoc Lab Anim Sci. 2009;48:785–789. [PMC free article] [PubMed] [Google Scholar]
- Yousefi R, Saidpour A, Mottaghi A. The effects of Spirulina supplementation on metabolic syndrome components, its liver manifestation and related inflammatory markers: a systematic review. Complement Ther Med. 2019;42:137–144. doi: 10.1016/j.ctim.2018.11.013. [DOI] [PubMed] [Google Scholar]
- Zhang C (1994) The effects of polysaccharide and phycocyanin from Spirulina platensis variety on peripheral blood and hematopoietic system of bone marrow in mice. Second Asia Pac Conf Alga Biotechnol 25–27
- Zilva JF, Pannall PR (1983) Clinical chemistry in diagnosis and treatment, 3rd edn. PG Pub, Singapore, p 492
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data generated or analyzed during this study are included in the research article.