Abstract
Background/Aims:
To investigate the association of macular optical coherence tomography (OCT)/OCT angiography (OCTA) parameters with visual acuity (VA) in glaucoma.
Methods:
144 pseudophakic primary open angle glaucoma eyes were included. Foveal (fVD), parafoveal (pfVD), perifoveal (perifVD), and whole-image vessel densities (wiVD) of superficial and deep layers, and their corresponding GCC thicknesses were obtained from OCTA 6×6mm2 macula scans. Foveal avascular zone (FAZ) area, FAZ circumference, and foveal density-300 (FD300) were measured. Correlation between OCT/OCTA parameters and logMAR VA in early and moderate-advanced glaucoma was evaluated with age and signal strength index-adjusted mixed models. Area under receiver-operating-characteristic (AUC) was used to evaluate discriminative power of OCT/OCTA for decreased VA (<20/25).
Results:
In early glaucoma (80 eyes), no parameter correlated with VA. In moderate-advanced glaucoma (64 eyes), greater FAZ area (β=0.228) and circumference (β=0.063) correlated with worse VA (P<0.05), but not FD300. Thinner sectoral and global GCC was associated with worse VA (β=0.002–0.003, P<0.05), except for inferior hemifield perifGCC and wiGCC. For VD, lower superior hemifield superficial perifVD and wiVD (β=0.007–0.008) and deep fVD (β=0.004) correlated with worse VA (P<0.05). OCT/OCTA parameters showed modest ability to discriminate decreased VA, with the superior hemifield performing better than the inferior hemifield. In early glaucoma, GCC and VD showed similar discrimination (AUC=0.67–0.77). In moderate-advanced glaucoma, fGCC and pfGCC yielded higher AUC (0.75–0.81) than VD (AUC=0.63–0.72).
Conclusions:
Some macular OCT/OCTA parameters were associated with VA in moderate-advanced, but not early glaucoma. These structural parameters may help identify glaucoma patients with impaired vision and reduced quality of life.
Keywords: glaucoma, vessel density, ganglion cell complex, visual acuity, OCT, OCTA
INTRODUCTION
An accurate and timely evaluation of glaucomatous damage relies on both functional and structural examinations, and optical coherence tomography (OCT) and OCT angiography (OCTA) are increasingly utilized for diagnosing and monitoring glaucoma.[1 2] Since macular damage directly affects central vision,[3] clinical relevance of macular structural parameters have been particularly studied. Both macular ganglion cell complex (GCC) thinning and macular vessel density (VD) loss were found to correlate with glaucomatous visual field (VF) loss.[4–8] Similar association has been found between some metrics of the foveal avascular zone (FAZ) and central VF defect,[9 10] indicating these macular OCT and OCTA parameters are useful in evaluating glaucomatous vision impairment.
While VF loss is more characteristic of glaucoma, visual acuity (VA) is another important functional parameter affecting our vision-related quality of life (VrQOL).[11–13] Since significant VA impairment usually does not present until later stages in glaucoma,[14] it is often overlooked in the management of glaucoma. Nevertheless, prior studies have shown even mild-moderate decline in VA can result in decreased VrQOL and daily function,[11 12 15] indicating the clinical relevance of this measure. Additionally, VA was found to have a significant effect on VrQOL in patients with mild glaucomatous damage in a recent study.[12] This finding further suggests a possible role of VA in the early stages of glaucoma, which was not attended to previously.
Information about the relationship between structural changes and VA decline in glaucoma is scarce, although some studies have reported a correlation between VA and central VF.[16 17] One study investigated the correlation between retinal thicknesses and VA,[18] and found a stronger association in glaucoma eyes with more severe disease. Another study examined the relationship between VA and OCTA parameters, and found macular VD to be most promising in discriminating decreased VA; however, only advanced glaucoma eyes were evaluated.[19] Since OCTA has demonstrated a good ability to detect glaucomatous change in early glaucoma that is non-inferior to OCT, whether this holds true for the detection of VA decline is also of interests.
Considering that decreased VA directly affects the patients’ VrQOL, it is possible that macular OCT and OCTA parameters may help identify glaucoma eyes at risk of impaired VA. In the current study, we investigated the association between macular OCT and OCTA parameters with VA in eyes with different severities of glaucomatous damage.
METHODS
This study was approved by the University of California San Diego Human Research Protection Program (NCT00221897) and adhered to the tenets of the Declaration of Helsinki and the Health Insurance Portability and Accountability Act.
Participants from the Diagnostic Innovations in Glaucoma Study (DIGS, details described previously[20 21]) meeting the below inclusion criteria were included in this cross-sectional study. Written informed consent was obtained from all participants. Briefly, all DIGS subjects underwent annual comprehensive ophthalmic examination in both eyes with slit-lamp biomicroscopy, dilated fundus examination, best-corrected visual acuity (BCVA), Goldmann applanation tonometry, and stereoscopic optic disc photography, and semi-annual examination of standard automated perimetry (SAP), intraocular pressure (IOP) measurement, and OCTA/OCT imaging. Gonioscopy and ultrasound pachymetry were performed at the first visit. Other demographic information, including age, race, systemic medical history, blood pressure, and medication use, was also collected.
Inclusion criteria for the current study were: (1) age older than 18 years, (2) a diagnosis of primary open angle glaucoma (POAG), (3) pseudophakic status. Exclusion criteria were: (1) history of ocular trauma, (2) coexisting retinal pathologies, (3) non-glaucomatous optic neuropathy, (4) uveitis, (5) axial length >27 mm. Participants diagnosed with Parkinson’s disease, Alzheimer’s disease, dementia, or a history of stroke were also ineligible.
POAG was defined as eyes showing repeatable and reliable abnormal SAP results (fixation losses and false negatives ≤ 33% and false positives ≤ 33%) using the 24–2 Swedish Interactive Thresholding Algorithm with either a pattern standard deviation outside the 95% normal limits or a glaucoma hemifield test result outside the 99% normal limit. Glaucoma severity was classified as early if the 24–2 VF mean deviation (MD) was greater than −6 dB, and moderate-severe if < −6 dB.
Optical Coherence Tomography Angiography and Spectral-Domain Optical Coherence Tomography
OCTA and spectral domain-OCT imaging with the Avanti Angiovue system (Optovue, Inc. Fremont, CA) was performed on all patients,[22] and non-HD 6mm × 6mm (304-A scans in each B-scan and 304-B scans acquired) macula scans centered on the fovea were acquired. The OCT/OCTA images were acquired simultaneously, and the OCT-based thickness analyses and OCTA-based vascular analyses were calculated from the same scan slab. The Angiovue software (version 2018.1.0.43) performed automatic segmentation with registration of the analyzed regions,[23] with the superficial VD measured from the internal limiting membrane to 10 μm above the inner plexiform layer, and the deep VD measured from 10 μm above the IPL to 10 μm below the outer plexiform layer. Automated projection artifacts removal was performed for calculation of VD in the deep layer.
The VD was calculated as the percentage of measured area occupied by flowing blood vessels. The following VD parameters calculated from the macula scans centered on the fovea were analyzed in this study (Figure 1A): (1) foveal VD (fVD), calculated from the area of a 1-mm-diameter circle centered on the fovea; (2) parafoveal VD (pfVD), calculated from the annular region with an inner diameter of 1 mm and an outer diameter of 3 mm centered on the fovea; (3) perifoveal VD (perifVD), calculated the annular region with an inner diameter of 3 mm and an outer diameter of 6 mm centered on the fovea; (4) whole-image VD (wiVD), calculated from the entire macula scan.
Figure 1.
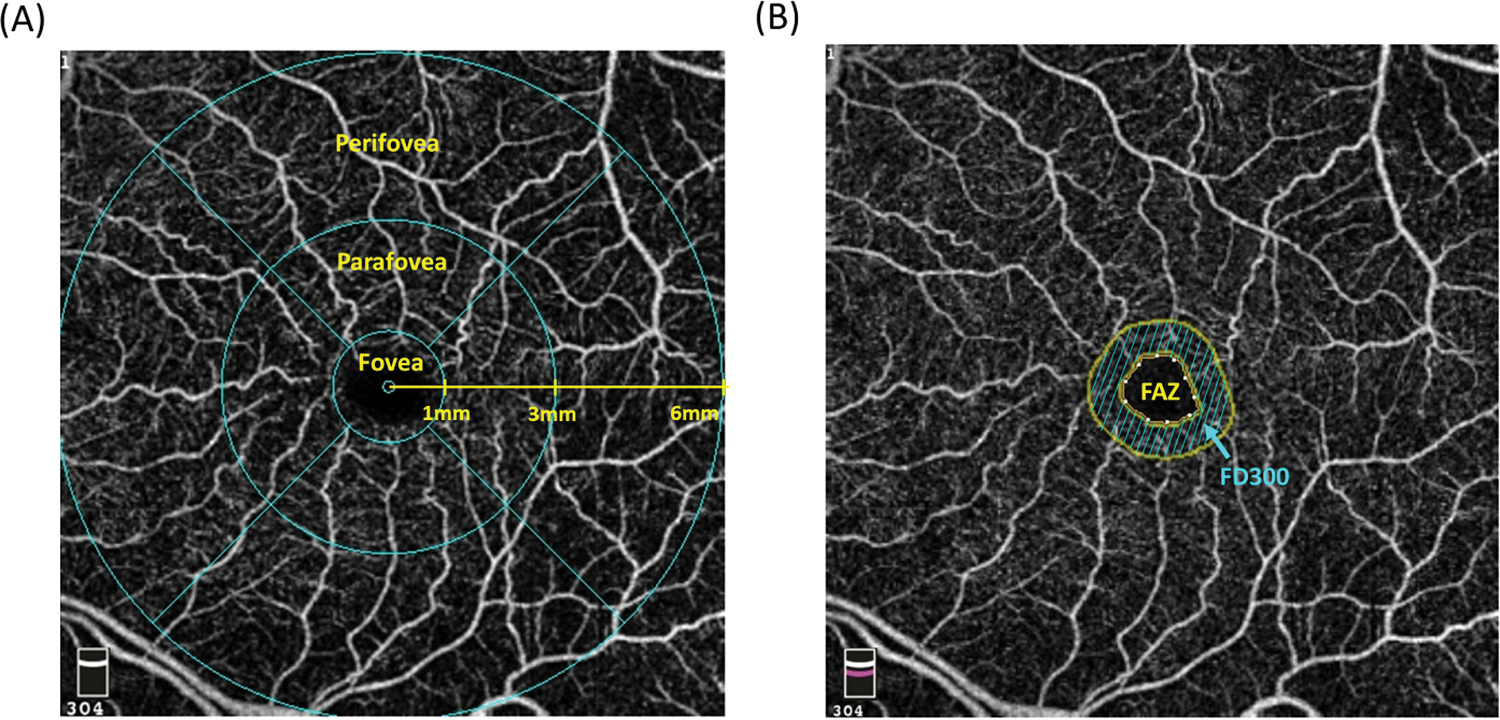
Regions corresponding to the (A) foveal, parafoveal, and perifoveal measurements and (B) foveal avascular zone (FAZ) and foveal density 300 (FD300) on the 6 mm*6 mm macula scans.
Thickness measurements of GCC, consisting of the ganglion cell layer, internal plexiform layer, and retinal nerve fiber layer (RNFL), was calculated using Angiovue from the macular cube image acquired from the OCTA scan. The foveal GCC (fGCC), parafoveal GCC (pfGCC), perifoveal GCC (perifGCC), and whole-image GCC (wiGCC) thicknesses were calculated from the same fovea-centered regions where fVD, pfVD, perifVD, and wiVD were obtained.
For measurement of metrics associated with FAZ, the methods used in this study followed that used in a prior publication.[24] Briefly, FAZ was defined as the region that is enclosed by the innermost macular arcade, and the Avanti Angiovue software automatically detected capillary-free area and calculated FAZ metrics based on the retinal slab. The following parameters were evaluated in this study: (1) FAZ area, (2) FAZ circumferences, (3) foveal density 300 (FD300), defined as the superficial VD of the 300 μm width ring surrounding the FAZ (Figure 1B). For calculation of FAZ area, correction to consider the magnification effect was performed using the Littman formula (corrected FAZ area = FAZ area × 3.462 × 0.0130622 × [axial length − 1.82]2), which was derived from on the default axial length in the Avanti systems (23.95 mm).
OCTA image quality review was performed by trained graders according to the University of California, San Diego, Imaging Data Evaluation and Analysis Reading Center standard protocol. An image was considered poor-quality and excluded if any of the following was presented: (1) scan quality <4, (2) poor clarity, (3) residual motion artifacts visible as irregular vessel pattern on the en-face angiogram, (4) image cropping or local weak signal, (5) off-centered fovea, (6) severe segmentation errors that could not be corrected.
Statistical analysis
Continuous variables were presented as mean (95% confidence interval (CI)) and categorical variables as count (%). Categorical variables were compared using the chi-square test. Eye characteristics were compared using linear mixed-effects models to account for within-participant variability. Age and signal strength index (SSI)-adjusted mixed modeling was performed to characterize the correlation between macular OCT/OCTA parameters, including their hemifield measurements, and logMAR VA. A positive β coefficient indicated worse logMAR VA. Axial length was additionally adjusted for FAZ area in the mixed model analysis. Receiver operating characteristic analysis was performed, and area under receiver operating characteristic (AUC) was calculated to examine the discriminative power of OCT/OCTA parameters for decreased VA (defined as LogMAR VA > 0.10 or Snellen VA < 20/25) due to glaucoma.[18 25] Adjustment for possible within-subject correlation between eyes obtained from the same patients was performed in all analysis. Statistical analyses were performed using Stata version 16.0 (StataCorp, College Station, TX), and statistical significance was defined as a P value < 0.05 for all analyses.
RESULTS
Clinical characteristics of the subjects are summarized in Table 1. A total of 144 POAG eyes of 100 participants were included. The eyes were divided into early glaucoma group (80 eyes of 62 participants) and moderate-advanced glaucoma group (64 eyes of 53 participants), with the mean age (95% CI) of 78.7 (76.7, 80.6) and 79.8 (77.8, 81.7) years, respectively. There were significant differences in mean VF MD (early = −2.7 [95% CI: −3.1, −2.3] dB, moderate-advanced = −13.4 [95% CI: −14.5, −11.5] dB, P < 0.001) and mean logMAR VA (early = −0.05 [95% CI: 0.03, 0.07], moderate-advanced = 0.11 [95% CI: 0.08, 0.13], P = 0.003). A greater percentage of eyes (38%) had decreased VA in the moderate-advanced glaucoma group (P < 0.001). Most OCT/OCTA parameters were significantly different between the two groups (P < 0.05 for all), except for FAZ area, FAZ circumference, and foveal measurements of superficial VD, deep VD, and GCC.
Table 1.
Demographics and Baseline Clinical Characteristics of the Subjects
| POAG eyes (n=144 eyes of 100 patients) | |||
|---|---|---|---|
| Early glaucoma | Moderate-advanced glaucoma | P value | |
| Characteristic | n= 80 eyes of 62 patients | n= 64 eyes of 53 patients | |
| Age (years) | 78.7 (76.7, 80.6) | 79.8 (77.8, 81.7) | 0.213 |
| Gender (Female/ Male) | 34/28 | 25/28 | 0.697 |
| Race (African American/ non-African American) | 12/50 | 8/45 | 0.635 |
| Hypertension (Hypertensive/ non-hypertensive) | 38/24 | 36/17 | 0.303 |
| Diabetes (Diabetic/ non-diabetic) | 7/55 | 9/44 | 0.412 |
| IOP (mmHg) | 14.6 (13.5, 15.6) | 13.2 (11.6, 14.5) | 0.089 |
| VF MD (dB) | −2.7 (−3.1, −2.3) | −13.4 (−14.5, −11.5) | <0.001 |
| VF PSD (dB) | 4.0 (3.5, 4.5) | 9.7 (9.0, 10.4) | <0.001 |
| LogMAR VA | 0.05 (0.03, 0.07) | 0.11 (0.08, 0.13) | 0.003 |
| Decreased VA (%) | 9 (11%) | 24 (38%) | <0.001 |
| Corrected FAZ area (mm2) | 0.30 (0.27, 0.33) | 0.30 (0.27, 0.33) | 0.979 |
| FAZ circumference (mm) | 2.06 (1.94, 2.17) | 2.10 (1.99, 2.21) | 0.661 |
| FD300 (%) | 49.2 (47.8, 50.5) | 45.8 (44.2, 47.4) | 0.003 |
| fGCC (μm) | 53.0 (50.4, 55.6) | 49.0 (45.6, 52.4) | 0.055 |
| pfGCC (μm) | 87.5 (84.6, 90.5) | 79.1 (75.2, 83.0) | 0.001 |
| perifGCC (μm) | 79.9 (77.7, 82.0) | 74.1 (71.4, 76.7) | 0.001 |
| wiGCC (μm) | 81.0 (78.9, 83.1) | 74.7 (72.1, 77.3) | <0.001 |
| Superficial fVD (%) | 17.6 (16.0, 19.1) | 18.0 (16.0, 19.9) | 0.803 |
| Superficial pfVD (%) | 43.8 (42.6, 45.0) | 40.5 (39.2, 41.8) | 0.002 |
| Superficial perifVD (%) | 40.2 (39.3, 41.1) | 36.0 (34.9, 37.1) | <0.001 |
| Superficial wiVD (%) | 40.1 (39.3, 41.0) | 36.2 (35.1, 37.3) | <0.001 |
| Deep fVD (%) | 32.7 (30.8, 34.5) | 31.6 (29.7, 33.5) | 0.489 |
| Deep pfVD (%) | 50.0 (49.0, 51.1) | 47.4 (46.2, 48.5) | 0.003 |
| Deep perifVD (%) | 44.5 (43.3, 45.8) | 41.3 (39.8, 42.7) | 0.003 |
| Deep wiVD (%) | 44.1 (42.9, 45.2) | 41.1 (39.8, 42.4) | 0.003 |
| OCT/OCTA SSI | 59.0 (57.5, 60.5) | 57.4 (55.9, 58.9) | 0.192 |
Values are shown in mean (95% confidence interval), unless otherwise indicated.
Abbreviations: f = foveal; FAZ = foveal avascular zone; FD300 = foveal density 300; GCC = ganglion cell complex; IOP = intraocular pressure; MD = mean deviation; OCT = optical coherence tomography; OCTA = optical coherence tomography angiography; No. = number; PSD = pattern standard deviation; perif = perifoveal; pf = parafoveal; SSI = signal strength index; VA = visual acuity; VD = vessel density; VF = visual field; wi = whole-image
Table 2 presents the age and SSI-adjusted associations of OCT/OCTA parameters with VA stratified by glaucoma severity. In the early glaucoma group, none of the OCT/OCTA parameters, including their hemifield measurements, demonstrated significant association with logMAR VA (P > 0.05 for all). In moderate-advanced glaucoma eyes, greater FAZ area (β [95% CI] = 0.023 [0.002, 0.043], P = 0.035, R2 = 0.11) and FAZ circumference (β [95% CI] = 0.063 [0.006, 0.120], P = 0.032, R2 = 0.11) were associated with worse logMAR VA, but FD300 was not. Lower measurements of almost all GCC thicknesses, including their superior hemifield thicknesses, were significantly associated with worse logMAR VA (range of β = 0.002–0.003, P < 0.05 for all, range of R2 = 0.11–0.33), except for the inferior hemifields of perifGCC and wiGCC (P > 0.05). For superficial VD parameters, lower superior hemifield perifVD (β [95% CI] = 0.007 [0.000, 0.014], P = 0.045, R2 = 0.11) and lower superior hemifield wiVD (β [95% CI] = 0.008 [0.000, 0.016], P = 0.042, R2 = 0.12) were associated with worse logMAR VA. For deep VD, only fVD was associated with worse logMAR VA (β [95% CI] = 0.004 [0.000, 0.007], P = 0.049, R2 = 0.11). A supplemental analysis was performed with IOP included as an additional covariate in the mixed model (Supplemental Table 1). Overall, the results were similar to those of the main analysis, and no significant effect of IOP on VA measurement was observed.
Table 2.
Mixed model analysis of OCT and OCTA parameters associated with logMAR visual acuity based on glaucoma severity
| Early glaucoma | Moderate-advanced glaucoma | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Superficial VD (per 1% lower) | Deep VD (per 1% lower) | GCC thickness (per 1 um thinner) | Superficial VD (per 1% lower) | Deep VD (per 1 % lower) | GCC thickness (per 1 um thinner) | ||||||
| β, 95 % CI | P value | β, 95 % CI | P value | β, 95 % CI | P value | β, 95 % CI | P value | β, 95 % CI | P value | β, 95 % CI | P value | |
| Corrected FAZ area, per 0.1 mm2 increase | 0.006 (−0.013, 0.025) | 0.533 | 0.023 (0.002, 0.043) | 0.035 | ||||||||
| FAZ circumference, per 1mm increase | 0.016 (−0.025, 0.058) | 0.437 | 0.063 (0.006, 0.120) | 0.032 | ||||||||
| FD300 | 0.002 (−0.002, 0.005) | 0.250 | 0.001 (−0.004, 0.006) | 0.676 | ||||||||
| Foveal | 0.001 (−0.002, 0.003) | 0.626 | 0.002 (−0.000, 0.004) | 0.060 | 0.001 (−0.002, 0.003) | 0.552 | 0.002 (−0.001, 0.005) | 0.167 | 0.004 (0.000, 0.007) | 0.049 | 0.003 (0.002, 0.005) | 0.001 |
| Parafoveal | 0.002 (−0.001, 0.006) | 0.163 | 0.003 (−0.002, 0.007) | 0.308 | 0.001 (−0.001, 0.002) | 0.502 | 0.005 (−0.002, 0.012) | 0.160 | 0.002 (−0.007, 0.015) | 0.634 | 0.003 (0.001, 0.004) | <0.001 |
| Superior hemifield | 0.003 (−0.001, 0.007) | 0.180 | 0.004 (−0.002, 0.010) | 0.210 | 0.001 (−0.001, 0.003) | 0.382 | 0.007 (−0.000, 0.014) | 0.061 | 0.003 (−0.007, 0.013) | 0.516 | 0.003 (0.001, 0.005) | <0.001 |
| Inferior hemifield | 0.001 (−0.001, 0.004) | 0.253 | 0.003 (−0.004, 0.005) | 0.897 | 0.000 (−0.001, 0.002) | 0.693 | 0.002 (−0.004, 0.008) | 0.566 | 0.001 (−0.007, 0.008) | 0.889 | 0.002 (0.001, 0.003) | 0.012 |
| Perifoveal | 0.001 (−0.004, 0.006) | 0.778 | 0.003 (−0.002, 0.007) | 0.238 | −0.001 (−0.004, 0.002) | 0.386 | 0.006 (−0.001, 0.013) | 0.090 | 0.006 (−0.001, 0.013) | 0.115 | 0.002 (0.001, 0.005) | 0.015 |
| Superior hemifield | 0.001 (−0.005, 0.006) | 0.770 | 0.003 (−0.001, 0.008) | 0.154 | −0.001 (−0.004, 0.002) | 0.491 | 0.007 (0.000, 0.014) | 0.045 | 0.006 (−0.002, 0.013) | 0.120 | 0.003 (0.001, 0.005) | 0.001 |
| Inferior hemifield | 0.000 (−0.004, 0.005) | 0.822 | 0.001 (−0.003, 0.006) | 0.523 | −0.001 (−0.003, 0.001) | 0.366 | 0.003 (−0.002, 0.009) | 0.224 | 0.003 (−0.003, 0.009) | 0.260 | 0.001 (−0.001, 0.003) | 0.284 |
| Whole-image | 0.001 (−0.004, 0.006) | 0.628 | 0.002 (−0.003, 0.007) | 0.371 | −0.001 (−0.004, 0.001) | 0.336 | 0.006 (−0.001, 0.014) | 0.099 | 0.007 (−0.001, 0.015) | 0.090 | 0.003 (0.001, 0.005) | 0.008 |
| Superior hemifield | 0.002 (−0.004, 0.007) | 0.582 | 0.003 (−0.002, 0.008) | 0.232 | −0.001 (−0.004, 0.002) | 0.549 | 0.008 (0.000, 0.016) | 0.042 | 0.007 (−0.002, 0.015) | 0.119 | 0.003 (0.001, 0.005) | 0.001 |
| Inferior hemifield | 0.001 (−0.003, 0.005) | 0.690 | 0.001 (−0.003, 0.006) | 0.538 | −0.001 (−0.003, 0.001) | 0.283 | 0.003 (−0.003, 0.009) | 0.273 | 0.005 (−0.002, 0.011) | 0.160 | 0.001 (−0.001, 0.003) | 0.195 |
Values are shown in β coefficient (95% CI). A positive β coefficient indicated worse logMAR VA. Statistically significant P values are shown in bold.
All models were adjusted for age and signal strength index. The FAZ area was additionally adjusted for axial length.
Abbreviations: CI = confidence interval; FAZ = foveal avascular zone; FD300 = foveal density 300; GCC = ganglion cell complex; OCT = optical coherence tomography; OCTA = optical coherence tomography angiography; POAG = primary open angle glaucoma; VD = vessel density
The AUC of OCT/OCTA parameters for discriminating between eyes with and without decreased VA are summarized in Table 3. In early glaucoma, FAZ area yielded the highest AUC (0.72 [95% CI: 0.50, 0.90]) among the FAZ metrics. For superficial and deep VD, parafoveal, perifoveal and whole-image measurements perfomed similarly across all sectors (AUC range = 0.68–0.77). As for GCC, the parafoveal region showed the best discrimination (AUC range = 0.72–0.76). In moderate-advanced glaucoma, all FAZ metrics showed similar discrimination (AUC range = 0.66–0.67). For superficial and deep VD, measurements obtained from different regions again performed similarly (AUC range = 0.63–0.72). While no regional differences in AUC were found among VD parameters, GCC thickness tended to perform better with foveal (AUC = 0.79 [95% CI: 0.68, 0.90]) and parafoveal measurements (AUC range = 0.75–0.81). Figure 2 showed the AUC of foveal and parafoveal OCT/OCTA measurements in moderate-advanced glaucoma. The discriminative power to detect decreased VA of most OCTA parameters was slightly weaker in the moderate-advanced group. While for GCC thicknesses, most parameters seemed to show a slightly higher AUC in moderate-advanced glaucoma. In both severity groups, the superior hemifield yielded higher AUC as compared to the inferior hemifield for most OCT/OCTA parameters.
Table 3.
Receiver operating characteristic analysis of the discriminative power of OCT/OCTA parameters to detect decreased visual acuity
| Early glaucoma | Moderate-advanced glaucoma | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Superficial VD | Deep VD | GCC thickness | Superficial VD | Deep VD | GCC thickness | ||||||
| AUC | 95% CI | AUC | 95% CI | AUC | 95% CI | AUC | 95% CI | AUC | 95% CI | AUC | 95% CI | |
| Corrected FAZ area | 0.72 | (0.50, 0.90) | 0.66 | (0.52, 0.80) | ||||||||
| FAZ circumference | 0.65 | (0.39, 0.92) | 0.67 | (0.53, 0.81) | ||||||||
| FD300 | 0.67 | (0.45, 090) | 0.66 | (0.52, 0.80) | ||||||||
| Foveal | 0.71 | (0.49, 0.92) | 0.72 | (0.50, 0.93) | 0.73 | (0.54, 0.93) | 0.65 | (0.51,0.79) | 0.66 | (0.51, 0.80) | 0.79 | (0.68, 0.90) |
| Parafoveal | 0.74 | (0.53, 0.95) | 0.73 | (0.52, 0.94) | 0.75 | (0.55, 0.94) | 0.71 | (0.58, 0.83) | 0.70 | (0.55, 0.85) | 0.80 | (0.68, 0.91) |
| Superior hemifield | 0.75 | (0.53, 0.97) | 0.74 | (0.54, 0.94) | 0.76 | (0.54, 0.97) | 0.72 | (0.59, 0.85) | 0.65 | (0.51, 0.79) | 0.81 | (0.69, 0.92) |
| Inferior hemifield | 0.73 | (0.53, 0.93) | 0.71 | (0.48, 0.93) | 0.72 | (0.52, 0.93) | 0.67 | (0.54, 0.81) | 0.68 | (0.52, 0.83) | 0.75 | (0.62, 0.88) |
| Perifoveal | 0.70 | (0.48, 0.91) | 0.74 | (0.53, 0.94) | 0.67 | (0.44, 0.91) | 0.67 | (0.54, 0.81) | 0.69 | (0.55, 0.83) | 0.68 | (0.55, 0.82) |
| Superior hemifield | 0.71 | (0.47, 0.94) | 0.75 | (0.54, 0.95) | 0.68 | (0.45, 0.91) | 0.71 | (0.58, 0.84) | 0.70 | (0.56, 0.83) | 0.71 | (0.58, 0.84) |
| Inferior hemifield | 0.68 | (0.45, 0.91) | 0.72 | (0.50, 0.93) | 0.68 | (0.44, 0.91) | 0.63 | (0.50, 0.77) | 0.66 | (0.51, 0.80) | 0.64 | (0.50, 0.78) |
| Whole-image | 0.73 | (0.53, 0.93) | 0.72 | (0.52, 0.92) | 0.72 | (0.50, 0.93) | 0.68 | (0.55, 0.82) | 0.70 | (0.56, 0.84) | 0.68 | (0.55, 0.82) |
| Superior hemifield | 0.73 | (0.52, 0.94) | 0.77 | (0.59, 0.96) | 0.68 | (0.44, 0.92) | 0.71 | (0.58, 0.84) | 0.71 | (0.57, 084) | 0.73 | (0.60, 0.86) |
| Inferior hemifield | 0.70 | (0.49, 0.92) | 0.71 | (0.49, 0.92) | 0.71 | (0.48, 0.93) | 0.65 | (0.52, 0.79) | 0.68 | (0.54, 0.82) | 0.64 | (0.51, 0.78) |
All models were adjusted for age and signal strength index. The FAZ area was additionally adjusted for axial length.
Abbreviations: AUC = area under the curve; CI = confidence interval; FAZ = foveal avascular zone; FD300 = foveal density 300; GCC = ganglion cell complex; OCT = optical coherence tomography; OCTA = optical coherence tomography angiography; VD = vessel density.
Figure 2.
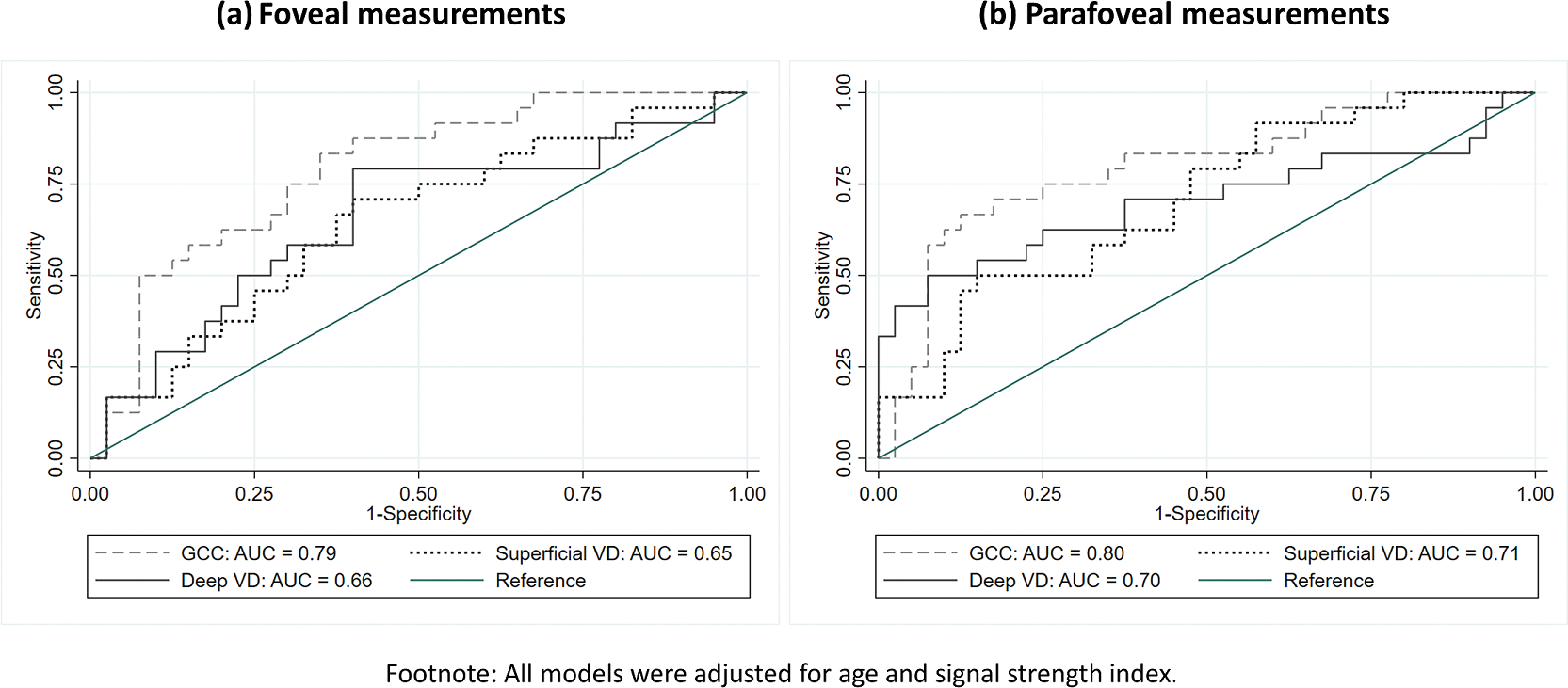
Area under the receiver operating characteristic of (a) foveal and (b) parafoveal measurements for discrimination between eyes with and without decreased visual acuity in moderate-advanced glaucoma group. (Abbreviation: GCC = ganglion cell complex, VD = vessel density)
DISCUSSION
This study investigated the relationship between macular OCT and OCTA structural parameters with VA across different glaucoma severities. For early glaucoma eyes, none of the parameters were associated with VA. However, in moderate-advanced glaucoma eyes, lower values of most GCC thickness parameters were associated with worse VA. In contrast, most VD parameters were not significantly associated with VA. For discrimination between eyes with and without decreased VA, GCC and VD showed similar discriminatory ability in early glaucoma group. For moderate-advanced glaucoma, foveal and parafoveal GCC thickness overall and in the superior hemifield had the greatest power, whereas OCTA parameters had lower AUC.
These findings are consistent with prior studies reporting the association between macular OCT parameters and VA.[18 26] In an earlier study, circumpapillary RNFL and macular GCC thicknesses were evaluated for correlation with VA in severity-stratified analysis.[18] While the VF cut-off (MD = −12 dB) used for stratification in that study was different from ours, similar to our results, a significantly stronger association between VA and OCT parameters was found for the more severe group. Examining only advanced and severe (MD < −12 and −20 dB) glaucoma eyes, a recent OCTA study suggested macular VD, particularly deep nasal VD, as a promising indicator of VA.[19] Macular GCC, however, was not significantly associated with VA in their study population, which might be due to the OCT measurement floor effect. As compared to functional parameters, structural parameters are more subjected to floor effect in late-stage glaucoma.[26 27] Furthermore, the floor is detected earlier in OCT than in OCTA, particularly with a VF MD worse than −14 dB.[28] In their study, a mean VF MD around −20 dB was reported. Whereas in our analysis, most moderate-advanced glaucoma eyes had a VF better than −14dB, which may explain in part the stronger association observed between OCT and VA in the current study.
Some other findings in the current study also differ from prior results. The association between FAZ metrics and central VF has been shown previously.[24 29] In the aforementioned OCTA study,[19] both FAZ area and FD300 were significantly associated with VA. However, a similar association was not found for FD300 in our analysis. Without available evidence from other similar studies, it is most likely the discrepancy is also related to the differences in the severity of glaucoma in the study participants. Additionally, in the current analysis, deep VD was associated with VA only in the foveal area and did not outperform superficial VD. While in their study, deep VD was more strongly associated with VA as compared to superficial VD, with significant results obtained from the foveal, parafoveal, and whole-image measurements.[19] Since they examined only advanced-severe glaucoma, the authors hypothesized the findings may have resulted from an increased impairment of deep capillary perfusion (DCP) with glaucoma progression, as DCP is usually preserved until the later stages due to the anastomoses supply from superficial plexus.[30 31]
In most past reports, deep VD was reported to be less strongly associated with VA than superficial VD in glaucoma, particularly during the early-moderate stages,[32–35] and whether the involvement of deep VD in glaucomatous damage alters as the disease progresses is unclear. Nevertheless, some studies which investigated other ocular conditions have also found a stronger correlation between VA and deep VD as compared to superficial VD,[36–38] although the mechanism remains unknown and the association might be more attributed to the underlying pathologies. Overall, more studies are needed to clarify if there a relationship between deep VD and VA in glaucoma, and if the role of DCP truly varies across different glaucoma severities.
Another intriguing finding of the present study is the region-dependent association of VD obtained from different vascular layers, with deep VD demonstrating better correlation at the central/foveal region and superficial VD at the more peripheral and superior region. This is supported by a prior study that showed a better association between central VF and deep VD as compared to superficial VD in glaucoma,[39] suggesting a primary effect of central/foveal DCP on central vision, which is related to VA. However, an association between VA and superficial VD obtained from the foveal and parafoveal regions has also been found in a previous OCTA study, in which a larger number of advanced glaucoma eyes were included.[25]
In the current study, a modest discriminative power of superficial and deep VD was found for both severity groups, with a slightly weaker discrimination in the moderate-advanced glaucoma group. In contrast, OCT-measured GCC thicknesses, which performed similarly to OCTA in early glaucoma group, showed better discrimination than OCTA in moderate-advanced glaucoma. In addition, the parafoveal measurements yielded the best results for superficial VD and GCC parameters, indicating this region may be more helpful for this task. Results in a prior study examining VD also support the greater discriminative power of parafoveal measurements, although perifVD was not specifically examined.[19] As mentioned previously, even mild-to-moderate decline in VA can cause impairment to VrQOL and daily function, and this should not be overlooked in glaucoma.[11 12 15] Therefore, to identify structural parameters that may facilitate discrimination of patients at risk of decreased VA is clinically beneficial.
There are several limitations of this study. First, although all images went through quality review, the VD measurements are more variable the OCT thickness measurements.[40] Analysis of deep VD, in particular, might be affected by projection artifacts that were not completely removed.[41] Nevertheless, our result may serve as the basis for future investigation on the long-term association between VA and OCT/OCTA in glaucoma. Second, due to the limited number of available eyes, a detailed severity-stratified analysis (early/moderate/advanced) could not be performed, and some patients had both eyes included. Third, since data of the Early Treatment Diabetic Retinopathy Study letter score was not available, Snellen VA-converted LogMAR VA was used in the current analysis, which was also the approach adopted by most prior studies.[18 19] Last, to exclude the possible confounding effect of cataract on VA,[42] only pseudophakic eyes were included, and most patients demonstrated mild-moderate VA decline. Whether the results would differ with inclusion of non-pseudophakic eyes or more eyes with severe VA decline is a subject for future study. However, the current study still provides insights into the relationship between local structures and VA performance in its earlier course.
In summary, some macular OCT and OCTA parameters, particularly GCC thickness, FAZ area and FAZ circumference, showed statistically significant associations with VA in moderate-advanced, but not early glaucoma. The association of superficial and deep VD with VA varied by region, indicating the potentially differential involvement of local vasculatures in the decline of VA. Parafoveal measurements of GCC, especially in the superior hemifield, showed a greater ability to discriminate glaucoma eyes with decreased VA from those without it. These structural parameters may help to identify glaucoma patients at risk of impaired vision and reduced quality of life.
Supplementary Material
KEY MESSAGE.
• What is already known on this topic –
Although pertinent to our quality of life, VA is often overlooked in glaucoma, and information about its relationship with other structural parameters is scarce.
• What this study adds –
Some macular OCT/OCTA parameters were associated with VA in moderate-advanced, but not early glaucoma. Most OCT/OCTA parameters showed modest discriminative power for glaucoma eyes with decreased VA, with parafoveal GCC showing the best overall discrimination.
• How this study might affect research, practice or policy –
Through structural examination by OCT/OCTA, clinicians might be able to identify glaucoma patients at risk of impaired VA.
SYNOPSIS.
In moderate-advanced glaucoma, some macular parameters from both OCT and OCTA showed association with VA and modest ability to discriminate glaucoma eyes with decreased VA.
Grant information/Funding/Support:
This work is supported by National Institutes of Health/National Eye Institute Grants R01EY029058, R01EY011008, R01EY019869, R01EY027510, R01EY026574, R01EY018926, P30EY022589; University of California Tobacco Related Disease Research Program (T31IP1511), and an unrestricted grant from Research to Prevent Blindness (New York, NY). The sponsor or funding organization had no role in the design or conduct of this research.
Footnotes
Commercial Disclosures: Linda Zangwill reported grants from the National Eye Institute; grants and nonfinancial support from Heidelberg Engineering, nonfinancial support from Carl Zeiss Meditec, Optovue, and Topcon. Robert N. Weinreb reported nonfinancial support from Heidelberg Engineering, Carl Zeiss Meditec, Konan Medical, Optovue, Centervue, and Topcon; grants from the National Eye Institute; personal fees from Abbvie, Aerie Pharmaceuticals, Allergan, Equinox, Nicox, and Topcon; all outside the submitted work. No other disclosures were reported.
Meeting presentation: Paper presentation at ARVO 2022
Data availability:
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1.Rao HL, Pradhan ZS, Suh MH, et al. Optical Coherence Tomography Angiography in Glaucoma. J Glaucoma 2020;29(4):312–21 doi: 10.1097/ijg.0000000000001463[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bussel II, Wollstein G, Schuman JS. OCT for glaucoma diagnosis, screening and detection of glaucoma progression. British Journal of Ophthalmology 2014;98(Suppl 2):ii15–ii19 doi: 10.1136/bjophthalmol-2013-304326[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans K, Law SK, Walt J, et al. The quality of life impact of peripheral versus central vision loss with a focus on glaucoma versus age-related macular degeneration. Clin Ophthalmol 2009;3:433–45 doi: 10.2147/opth.s6024[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le PV, Tan O, Chopra V, et al. Regional Correlation Among Ganglion Cell Complex, Nerve Fiber Layer, and Visual Field Loss in Glaucoma. Investigative Ophthalmology & Visual Science 2013;54(6):4287–95 doi: 10.1167/iovs.12-11388[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scuderi G, Fragiotta S, Scuderi L, et al. Ganglion Cell Complex Analysis in Glaucoma Patients: What Can It Tell Us? Eye Brain 2020;12:33–44 doi: 10.2147/eb.S226319[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Renard JP, Fénolland JR, Giraud JM. Glaucoma progression analysis by Spectral-Domain Optical Coherence Tomography (SD-OCT). J Fr Ophtalmol 2019;42(5):499–516 doi: 10.1016/j.jfo.2019.03.001[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 7.Penteado RC, Zangwill LM, Daga FB, et al. Optical Coherence Tomography Angiography Macular Vascular Density Measurements and the Central 10–2 Visual Field in Glaucoma. J Glaucoma 2018;27(6):481–89 doi: 10.1097/ijg.0000000000000964[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Peripapillary and Macular Vessel Density in Patients with Glaucoma and Single-Hemifield Visual Field Defect. Ophthalmology 2017;124(5):709–19 doi: 10.1016/j.ophtha.2017.01.004[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon J, Choi J, Shin JW, et al. Alterations of the Foveal Avascular Zone Measured by Optical Coherence Tomography Angiography in Glaucoma Patients With Central Visual Field Defects. Investigative Ophthalmology & Visual Science 2017;58(3):1637–45 doi: 10.1167/iovs.16-21079[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 10.Kwon J, Choi J, Shin JW, et al. Glaucoma Diagnostic Capabilities of Foveal Avascular Zone Parameters Using Optical Coherence Tomography Angiography According to Visual Field Defect Location. J Glaucoma 2017;26(12):1120–29 doi: 10.1097/ijg.0000000000000800[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 11.Nickels S, Schuster AK, Elflein H, et al. Vision-related quality of life considering both eyes: results from the German population-based Gutenberg Health Study (GHS). Health and Quality of Life Outcomes 2019;17(1):98 doi: 10.1186/s12955-019-1158-1[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chun YS, Sung KR, Park CK, et al. Factors influencing vision-related quality of life according to glaucoma severity. Acta Ophthalmologica 2019;97(2):e216–e24 doi: 10.1111/aos.13918[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 13.Brown GC. Vision and quality-of-life. Trans Am Ophthalmol Soc 1999;97:473–511 [DOI] [PubMed] [Google Scholar]
- 14.Law SK, Nguyen AM, Coleman AL, et al. Severe Loss of Central Vision in Patients With Advanced Glaucoma Undergoing Trabeculectomy. Archives of Ophthalmology 2007;125(8):1044–50 doi: 10.1001/archopht.125.8.1044[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 15.Chan EW, Chiang PP, Liao J, et al. Glaucoma and associated visual acuity and field loss significantly affect glaucoma-specific psychosocial functioning. Ophthalmology 2015;122(3):494–501 doi: 10.1016/j.ophtha.2014.09.030[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 16.Asaoka R The relationship between visual acuity and central visual field sensitivity in advanced glaucoma. British Journal of Ophthalmology 2013;97(10):1355–56 doi: 10.1136/bjophthalmol-2013-303431[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 17.Pickett JE, Terry SA, O’Connor PS, et al. Early loss of central visual acuity in glaucoma. Ophthalmology 1985;92(7):891–6 doi: 10.1016/s0161-6420(85)33938-6[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 18.Kim JH, Lee HS, Kim NR, et al. Relationship Between Visual Acuity and Retinal Structures Measured by Spectral Domain Optical Coherence Tomography in Patients With Open-Angle Glaucoma. Investigative Ophthalmology & Visual Science 2014;55(8):4801–10 doi: 10.1167/iovs.13-13052[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 19.Hsia Y, Wang TH, Huang JY, et al. Relationship Between Macular Microvasculature and Visual Acuity in Advanced and Severe Glaucoma. Am J Ophthalmol 2021;236:154–63 doi: 10.1016/j.ajo.2021.10.005[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 20.Sample PA, Girkin CA, Zangwill LM, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol 2009;127(9):1136–45 doi: 10.1001/archophthalmol.2009.187[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Girkin CA, Sample PA, Liebmann JM, et al. African Descent and Glaucoma Evaluation Study (ADAGES): II. Ancestry differences in optic disc, retinal nerve fiber layer, and macular structure in healthy subjects. Arch Ophthalmol 2010;128(5):541–50 doi: 10.1001/archophthalmol.2010.49[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia Y, Tan O, Tokayer J, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express 2012;20(4):4710–25 doi: 10.1364/oe.20.004710[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L, Jia Y, Takusagawa HL, et al. Optical Coherence Tomography Angiography of the Peripapillary Retina in Glaucoma. JAMA Ophthalmol 2015;133(9):1045–52 doi: 10.1001/jamaophthalmol.2015.2225[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishida T, Oh WH, Moghimi S, et al. Central macular OCTA parameters in glaucoma. British Journal of Ophthalmology 2021:bjophthalmol-2021–319574 doi: 10.1136/bjophthalmol-2021-319574[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsia Y, Wang T-H, Huang J-Y, et al. Relationship Between Macular Microvasculature and Visual Acuity in Advanced and Severe Glaucoma: Macular Microvasculature and Visual Acuity. American Journal of Ophthalmology 2021. doi: 10.1016/j.ajo.2021.10.005[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki Y, Kiyosawa M. Visual Acuity in Glaucomatous Eyes Correlates Better with Visual Field Parameters than with OCT Parameters. Current Eye Research 2021;46(11):1717–23 doi: 10.1080/02713683.2021.1924384[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 27.Mwanza JC, Budenz DL, Warren JL, et al. Retinal nerve fibre layer thickness floor and corresponding functional loss in glaucoma. Br J Ophthalmol 2015;99(6):732–7 doi: 10.1136/bjophthalmol-2014-305745[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moghimi S, Bowd C, Zangwill LM, et al. Measurement Floors and Dynamic Ranges of OCT and OCT Angiography in Glaucoma. Ophthalmology 2019;126(7):980–88 doi: 10.1016/j.ophtha.2019.03.003[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwon J, Choi J, Shin JW, et al. Alterations of the Foveal Avascular Zone Measured by Optical Coherence Tomography Angiography in Glaucoma Patients With Central Visual Field Defects. Invest Ophthalmol Vis Sci 2017;58(3):1637–45 doi: 10.1167/iovs.16-21079[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 30.Hormel TT, Jia Y, Jian Y, et al. Plexus-specific retinal vascular anatomy and pathologies as seen by projection-resolved optical coherence tomographic angiography. Prog Retin Eye Res 2021;80:100878 doi: 10.1016/j.preteyeres.2020.100878[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell JP, Zhang M, Hwang TS, et al. Detailed Vascular Anatomy of the Human Retina by Projection-Resolved Optical Coherence Tomography Angiography. Sci Rep 2017;7:42201 doi: 10.1038/srep42201[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Nimri NW, Manalastas PIC, Zangwill LM, et al. Superficial and Deep Macula Vessel Density in Healthy, Glaucoma Suspect, and Glaucoma Eyes. J Glaucoma 2021;30(6):e276–e84 doi: 10.1097/ijg.0000000000001860[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamalipour A, Moghimi S, Hou H, et al. Multilayer Macula Vessel Density and Visual Field Progression in Glaucoma. Am J Ophthalmol 2021. doi: 10.1016/j.ajo.2021.11.018[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee J, Park CK, Park H-YL. Determinants of vessel defects in superficial and deep vascular layers in normal-tension glaucoma using optical coherence tomography angiography. Scientific Reports 2021;11(1):9941 doi: 10.1038/s41598-021-89428-5[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu J-H, Moghimi S, Nishida T, et al. Correlation of ganglion cell complex thinning with baseline deep and superficial macular vessel density in glaucoma. British Journal of Ophthalmology 2022:bjophthalmol-2021–320663 doi: 10.1136/bjophthalmol-2021-320663[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 36.Dupas B, Minvielle W, Bonnin S, et al. Association Between Vessel Density and Visual Acuity in Patients With Diabetic Retinopathy and Poorly Controlled Type 1 Diabetes. JAMA Ophthalmol 2018;136(7):721–28 doi: 10.1001/jamaophthalmol.2018.1319[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ng H, La Heij EC, Andrinopoulou ER, et al. Smaller Foveal Avascular Zone in Deep Capillary Plexus Is Associated with Better Visual Acuity in Patients after Macula-off Retinal Detachment Surgery. Transl Vis Sci Technol 2020;9(10):25 doi: 10.1167/tvst.9.10.25[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye J, Wang M, Shen M, et al. Deep Retinal Capillary Plexus Decreasing Correlated With the Outer Retinal Layer Alteration and Visual Acuity Impairment in Pathological Myopia. Investigative Ophthalmology & Visual Science 2020;61(4):45–45 doi: 10.1167/iovs.61.4.45[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeon SJ, Park H-YL, Park CK. Effect of Macular Vascular Density on Central Visual Function and Macular Structure in Glaucoma Patients. Scientific Reports 2018;8(1):16009 doi: 10.1038/s41598-018-34417-4[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manalastas PIC, Zangwill LM, Saunders LJ, et al. Reproducibility of Optical Coherence Tomography Angiography Macular and Optic Nerve Head Vascular Density in Glaucoma and Healthy Eyes. J Glaucoma 2017;26(10):851–59 doi: 10.1097/ijg.0000000000000768[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El-Nimri NW, Manalastas PIC, Zangwill LM, et al. Superficial and Deep Macula Vessel Density in Healthy, Glaucoma Suspect, and Glaucoma Eyes. Journal of Glaucoma 2021;30(6):e276–e84 doi: 10.1097/ijg.0000000000001860[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shandiz JH, Derakhshan A, Daneshyar A, et al. Effect of cataract type and severity on visual acuity and contrast sensitivity. J Ophthalmic Vis Res 2011;6(1):26–31 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.


