Abstract
Traditionally, smoking has been the predominant method for administering cannabis, but alternative routes of administration have become more prevalent. Additionally, research examining urinary cannabinoid excretion profiles has primarily focused on 11-nor-9-carboxy-∆9-tetrahydrocannabinol (∆9-THC-COOH), a metabolite of ∆9-tetrahydrocannabinol (∆9-THC), as the primary analyte. The aim of the current study was to characterize the urinary excretion profile of ∆9-THC-COOH, ∆9-THC, ∆8-tetrahydrocannabinol (∆8-THC), 11-hydroxy-∆9-tetrahydrocannabinol (11-OH-∆9-THC), ∆9-tetrahydrocannabivarin (THCV), 11-nor-∆9-tetrahydrocannabivarin-9-carboxlic acid (THCV-COOH), cannabidiol (CBD), cannabinol (CBN) and 8,11-dihydroxytetrahydrocannabinol (8,11-diOH-∆9-THC) following controlled administration of both oral and vaporized cannabis. Participants (n = 21, 11 men/10 women) who were infrequent cannabis users ingested cannabis-containing brownies (0, 10 and 25 mg ∆9-THC) and inhaled vaporized cannabis (0, 5 and 20 mg ∆9-THC) across six double-blind outpatient sessions. Urinary concentrations of ∆9-THC analytes were measured at baseline and for 8 h after cannabis administration. Sensitivity, specificity and agreement between the three immunoassays (IAs) for ∆9-THC-COOH (cutoffs of 20, 50 and 100 ng/mL) and liquid chromatography–tandem mass spectrometry (LC–MS-MS) analyses (confirmatory cutoff concentrations of 15 ng/mL) were assessed. Urinary concentrations for ∆9-THC-COOH, ∆9-THC, 11-OH-∆9-THC, THCV, CBN and 8,11-diOH-∆9-THC all peaked at 5–6 h and 4 h following oral and vaporized cannabis administration, respectively. At each active dose, median maximum concentrations (Cmax) for detected analytes were quantitatively higher after oral cannabis administration compared to vaporized. Using current recommended federal workplace drug-testing criteria (screening via IA with a cutoff of ≥50 ng/mL and confirmation via LC–MS-MS at a cutoff of ≥15 ng/mL), urine specimens tested positive for ∆9-THC-COOH in 97.6% of oral sessions and 59.5% of vaporized sessions with active ∆9-THC doses. These data indicate that while ∆9-THC-COOH may serve as the most consistent confirmatory analyte under the current drug-testing guidelines, future work examining 11-OH-∆9-THC under similar parameters could yield an alternative analyte that may be helpful in distinguishing between licit and illicit cannabis products.
Introduction
The regulation of cannabis is experiencing a rapid and global shift throughout the developed world, with widespread policy reforms toward legalization for medicinal and/or non-medicinal purposes. Coincident with such policy reforms, the perceived risks associated with cannabis use have decreased while overall use and availability have increased (1, 2). Moreover, the retail cannabis market has expanded with novel, diverse cannabis products that vary with respect to chemical composition, route of administration and formulation [for review, see (3)]. Although smoked cannabis remains the most popular method of administration (4), novel methods such as oral cannabis products (‘edibles’, e.g., brownies, candies and beverages) and cannabis vaporizers have emerged as popular alternatives (3, 5, 6).
The rise in novel, non-smoked cannabis products presents new challenges for drug-testing programs (7). Despite the expansion of cannabis legalization, cannabis drug testing is still prevalent in many sectors, including safety-related occupations, treatment or criminal justice settings and workplace environments. Within these sectors, urine drug testing remains the primary method for detecting cannabis use. Typically, urine specimens are collected under observation and tested for 11-nor-9-carboxy-∆9-tetrahydrocannabinol (∆9-THC-COOH), a metabolite of ∆9-tetrahydrocannabinol (∆9-THC), the principal psychoactive constituent of cannabis. While most research on cannabinoid detection has focused on smoked cannabis, the pharmacokinetic profiles of ∆9-THC-COOH and other cannabinoid metabolites from other routes of administration can have markedly different outcomes relative to smoked cannabis. Thus, there is growing interest in understanding how novel cannabis formulations like oral and vaporized cannabis products uniquely impact the validity and interpretation of current urine drug testing methods.
Controlled laboratory studies have evaluated the urinary excretion profile of ∆9-THC-COOH following acute administration of oral (8–14) and vaporized cannabis (11–13, 15). Collectively, these studies revealed several important insights. Relative to vaporized cannabis administration containing 25 mg ∆9-THC (15), ingestion of a cannabis-containing brownie with a 25 mg ∆9-THC dose yielded a 10-fold higher peak ∆9-THC-COOH concentration in urine, as well as a longer time to last-positive urine drug test (14). However, inter-participant variability highlights the need to evaluate both oral and vaporized cannabis using a within-subjects design. One study evaluated the urinary excretion profile of ∆9-THC-COOH in both frequent and occasional cannabis users following oral, vaporized or smoked cannabis administration (13). However, this study was limited to a single ∆9-THC dose (50.6 mg) and study participants were routine cannabis users, resulting in high baseline concentrations of ∆9-THC-COOH in urine specimens. No prior studies have administered oral and vaporized cannabis to the same individuals using multiple doses of ∆9-THC. This gap in knowledge is especially noteworthy considering the range of ∆9-THC doses available in retail cannabis products (6). Another limitation is that prior studies did not investigate other ∆9-THC metabolites or other cannabinoids and their metabolites in urine. Analyses of other cannabinoids may yield alternate analytes that could be useful in differentiating botanical cannabis products from dronabinol or between the use of THC-rich cannabis and the use of hemp or CBD-rich cannabis.
The present study evaluated the urinary cannabinoid concentrations of ∆9-THC, ∆9-THC-COOH, ∆8-tetrahydrocannabinol (∆8-THC), 11-nor-9-carboxy-∆8-tetrahydrocannabinol (∆8-THC-COOH), 8-hydroxy-∆9-tetrahydrocannabinol (8-OH-∆9-THC), 11-hydroxy-∆9-tetrahydrocannabinol (11-OH-∆9-THC), 8,11-dihydroxytetrahydrocannabinol (8,11-diOH-∆9-THC), ∆9-tetrahydrocannabivarin (THCV), 11-nor-∆9-tetrahydrocannabivarin-9-carboxlic acid (THCV-COOH), cannabidiol (CBD) and cannabinol (CBN) following administration of oral (0, 10 and 25 mg ∆9-THC) and vaporized cannabis (0, 5 and 20 mg ∆9-THC) by male and female infrequent cannabis users (no cannabis use in the month prior to enrollment). This report provides detailed urinary pharmacokinetics of ∆9-THC-COOH, including maximum concentrations (Cmax) and time to maximum concentrations (Tmax). Sensitivity, specificity and agreement between immunoassay (IA) and liquid chromatography–tandem mass spectrometry (LC–MS-MS) are also presented.
Methods
Participants
Participants were recruited using media advertisements and word-of-mouth communication. Study volunteers completed screening assessments that included medical history interview, electrocardiogram (EKG), routine blood testing (chemistry, hematology and serology), physical examination and assessment of recent drug/alcohol use via urine drug screen, alcohol breathalyzer and completion of the Timeline Follow-Back (16). All participants provided written informed consent prior to study participation and received monetary compensation following each completed visit. Experimental procedures were approved by the Institutional Review Board of Johns Hopkins University School of Medicine and were conducted in accordance with the Declaration of Helsinki.
Key inclusion criteria were as follows: (i) aged 18–45 years; (ii) good health status as determined by in-person screening; (iii) no self-reported cannabis use for at least 1 month prior to the first experimental session; (iv) self-reported prior experience inhaling cannabis (e.g., smoking and vaporizing); (v) negative urine test for cannabis and other illicit drugs and a negative breath test for alcohol at the screening visit and before each session; (vi) body mass index (BMI) between 19 and 36 kg/m2; (vii) negative pregnancy test (assessed via serum at screening and via urine before study sessions) and not breastfeeding; (viii) no food allergies related to cannabis brownies (e.g., chocolate, eggs, etc.) and (ix) not donated blood for 30 days prior to screening.
Study drug
∆9-THC-dominant and placebo cannabis were obtained from the National Institute on Drug Abuse Drug Supply Program. ∆9-THC-dominant cannabis contained 10.3% ∆9-THC, 0.05% CBD and 0.85% CBN. The placebo cannabis contained 0.001% ∆9-THC, 0.003% CBD and 0.005% CBN. For acute dosing sessions with vaporized cannabis, 194.2 mg of placebo cannabis, ∆9-THC-dominant cannabis or a combination of the two were placed into the Volcano Medic vaporizer to achieve ∆9-THC doses of 0, 5 or 20 mg. The doses for CBD were 0.05 and 0.12 mg, and 0.03 and 0.1 mg CBD, and the doses for CBN were 0.8 and 2.1 mg, and 0.4 and 1.7 mg CBN for oral and vaporized routes of administration, respectively. For the purposes of the results, all results will be discussed in the context of ∆9-THC doses. For acute oral dosing sessions, placebo and active cannabis brownies were prepared by mixing 242.7 mg of placebo cannabis, ∆9-THC-dominant cannabis or a combination of the two to achieve ∆9-THC doses of 0, 10 or 25 mg. Detailed cannabis brownie preparation protocols from our laboratory were previously published (8, 14, 15, 17, 18). Justification for the ∆9-THC doses used in this study has been described previously (19). All cannabis was prepared and dispensed by the Johns Hopkins Behavioral Pharmacology Research Unit (BPRU) Pharmacy.
Study design and procedure
A total of 21 participants (11 male and 10 female) completed six double-blind acute dosing sessions, each lasting about 10 hours, during which they orally ingested cannabis-containing chocolate brownies (0, 10 and 25 mg ∆9-THC) or inhaled vaporized cannabis (0, 5 and 20 mg ∆9-THC). Both participants and research staff were blind to ∆9-THC dose but not route of administration. Sessions were conducted at the Johns Hopkins BPRU and separated by at least 1 week to ensure adequate drug washout between doses. The 1-week washout period was selected based on prior work conducted in our laboratory using identical orally administered doses (i.e., 10 and 25 mg ∆9-THC), indicating that pharmacodynamic and pharmacokinetic effects would subside in a population similar to the current study (14). For each participant, sessions were clustered by route of administration; for example, participants completed the three oral cannabis sessions first and the three vaporized cannabis sessions second or vice versa. ∆9-THC doses were counterbalanced within each cluster, and session clusters were counterbalanced across participants.
Before each session, participants self-reported their use of cannabis, alcohol, tobacco and other drugs since the previous study visit. Urine specimens were provided to assess drug use/pregnancy, and an alcohol breathalyzer was performed. Participants consumed a standardized low-fat breakfast (toast with jam) and received an intravenous catheter to permit repeated blood sampling. Next, baseline vital signs were obtained, baseline pharmacodynamic assessment (i.e., Drug Effect Questionnaire, Digit Serial Substitution Task, Divided Attention Task, Paced Serial Addition Task and DRiving Under the Influence of Drugs; DRUID®) were administered, and baseline biospecimens (urine, blood and oral fluid) were collected. Outcomes of the pharmacodynamic analyses are reported (19).
After baseline assessments, participants either orally ingested a cannabis-containing brownie or inhaled vaporized cannabis. In oral dosing sessions, participants consumed the entire brownie within 5 min. Participants inhaled vaporized cannabis using the Volcano Medic (Storz and Bickel, Tuttlingen, Germany). The Volcano heated cannabis at 204°C (400°F) and captured the vapor in a ‘balloon’; participants inhaled three full balloons within 10 min ad libitum. To avoid contamination from prior doses, a new balloon was used for each session. Balloons were covered with an opaque bag to reduce vapor visibility to participants and study staff. Following drug administration, outcome measures were collected at regular timepoints for 8 h (see below). The session concluded after the 8-h timepoint as this time exceeded the time course of intoxicating effects associated with oral (17) and vaporized (20) cannabis.
Urine specimen collection
Individual urine specimens were collected at baseline and repeatedly for 8 h after exposure to the study drug (i.e., 1, 2, 3, 4, 5, 6 and 8 h). These timepoints were selected to capture the full timecourse of pharmacodynamic outcomes and thus were not expected to capture the full urinary pharmacokinetic profile of each cannabinoid; indeed, other studies have demonstrated longer urinary excretion profiles for some of these analytes [e.g., ∆9-THC-COOH (14)]. For each urine specimen, two 30-mL aliquots were transferred into polypropylene tubes and wrapped with parafilm. All samples were stored at −20°C until they were shipped overnight (on dry ice) to the Clinical Reference Laboratory (CRL; Lenexa, KS).
IA and creatinine
Urine specimens were analyzed with the DRI Cannabinoid Assay via the manufacturer’s procedure (Thermo Fisher Scientific, Fremont, CA) utilizing 20, 50 and 100 ng/mL cutoff concentrations. IA methods and cross-reactivity data were previously described (21). Creatinine was determined with the Siemens modified Jaffe reagent.
Hydrolysis and extraction methods for confirmatory LC–MS-MS
In this study, it was anticipated that cannabinoids would be excreted primarily as ether-linked and acid-linked metabolites. The ether-linked metabolites were enzymatically hydrolyzed, and the acid-linked metabolites were cleaved by base hydrolysis. Both enzyme and base hydrolyzed samples were extracted according to previously published methods (21).
LC–MS-MS analyses
Extracts from the base hydrolyzed samples were analyzed by LC–MS-MS for the following cannabinoids: ∆9-THC-COOH, ∆8-THC-COOH, 8-OH-∆9-THC and THCV-COOH. Extracts from the ether hydrolyzed samples were analyzed by LC–MS-MS for the following cannabinoids: ∆9-THC, ∆8-THC, 11-OH-∆9-THC, THCV, CBD, CBN and 8,11-diOH-∆9-THC. Analyses were conducted with an API6500 QTrap by electrospray ionization (in positive or negative mode) with a source temperature of 450°C.
The linearity was determined by five replicate analyses of the analytes with a single point calibrator at 10 ng/mL for all analytes. The analytical range was verified with four levels below the calibrator and five levels above the calibrator. The limit of quantification (LOQ) for ∆9-THC-COOH, 8-OH-∆9-THC, THCV-COOH and 8,11-diOH-THC was 1.0 ng/mL; the LOQ for other analytes was 0.25 ng/mL. The upper limit of linearity and carry-over limit for all analytes was 1,000 ng/mL. The criteria for acceptance of results were based on the ion ratio of ±20% for the analyte and internal standard, relative retention time of ±2%, internal standard response of 20–200%, asymmetry of peak from ≥0.5 to ≤3.0 and resolution of a co-eluting peak at ≥90%. The analytical range for the low control (40% of calibrator) and positive control (125% of calibrator) was ±20% of target. Analytes were reported as not detected if the calculated concentration was less than the LOQ. A further detailed explanation of the LC–MS-MS methods and validation was previously published (21).
Data presentation and analysis
Descriptive statistics were used to summarize participant demographics and LC–MS-MS urine results. As the doses for each route of administration were selected based on the ability to produce discriminable drug effects with or without marked cognitive/psychomotor impairments, no formal comparisons were made across routes of administration (i.e., direct comparison of urinary cannabinoid concentrations is not possible). Urinary analyte concentrations are presented both in absolute form and normalized by creatinine. Absolute analyte concentrations for each specimen were divided by the specimen’s corresponding creatinine concentration as in ref. (22). Creatinine normalization was conducted to reduce variability in analyte concentrations attributable to differences in the degree of dilution between urine samples (22). However, one of the primary objectives of this study was to examine the analyte concentrations under conditions that would be representative of real-world assessments (e.g., workplace drug testing). Therefore, all analytes are analyzed and presented as absolute analyte concentrations (i.e., non-creatinine normalized). Planned contrasts were conducted to compare absolute analyte Cmax values between each dose within a route of administration. Analytes that were quantified at greater than their respective LOQ by LC–MS-MS are designated as positive analytes. As such, for each positive analyte (∆9-THC-COOH, ∆9-THC, 11-OH-∆9-THC, THCV-COOH, CBN and 8,11-diOH-∆9-THC), two separate one-way repeated-measures analysis of variances (ANOVAs) were employed to compare Cmax across the three oral dosing conditions (0, 10 and 25 mg) and across the three vaporized dosing conditions (0, 5 and 20 mg). For all ANOVAs, Dunnett’s multiple comparisons were used to compare each dose of ∆9-THC to the control (i.e., 0) and to compare both the lower and higher doses to each other within the drug route of administration. The following analytes were not detected in any specimens and, thus, were excluded from analyses: THCV, CBD, ∆8-THC, ∆8-THC-COOH and 8-OH-∆9-THC. Within the oral and vaporized dosing conditions, paired-samples t-tests were employed to compare Tmax values for the positive analytes (∆9-THC-COOH, ∆9-THC, 11-OH-∆9-THC, THCV-COOH, CBN and 8,11-diOH-∆9-THC) between the low ∆9-THC dose (10 or 5 mg) and the high ∆9-THC dose (25 or 20 mg). Between oral and vaporized dosing conditions, the Cmax and Tmax at low doses (10 mg vs 5 mg) and high doses (25 mg vs 20 mg) of ∆9-THC were compared using paired-samples t-tests for the positive analytes (∆9-THC-COOH, ∆9-THC, 11-OH-∆9-THC, THCV-COOH, CBN and 8,11-diOH-∆9-THC). Finally, for both oral and vaporized dosing conditions, male versus female differences in Cmax and Tmax were assessed at each dose using independent-samples t-tests for the positive analytes (∆9-THC-COOH, ∆9-THC, 11-OH-∆9-THC, THCV-COOH, CBN and 8,11-diOH-∆9-THC). Statistical analyses were conducted using Prism 8 for macOS (Version 8.3.0, GraphPad Software, LLC); the α level was set at 0.05 for all analyses.
Sensitivity, specificity and agreement between IA and LC–MS-MS results were conducted for urinary ∆9-THC-COOH for the active oral (10 and 25 mg ∆9-THC) and vaporized (5 and 20 mg ∆9-THC) doses. Three IA screening cutoffs were employed for these analyses: 20, 50 and 100 ng/mL. A confirmatory LC–MS-MS cutoff of 15 ng/mL was used for all analyses, which is consistent with mandatory guidelines for federal workplace drug testing established by the Substance Abuse and Mental Health Services Administration (23). Urinary ∆9-THC-COOH test results were categorized as either true positive (TP; IA response ≥ cutoff concentration and LC–MS-MS positive), true negative (TN; IA response <cutoff concentration and LC–MS-MS negative), false positive (FP; IA response ≥ cutoff concentration and LC–MS-MS negative) or false negative (FN; IA response < cutoff concentration and LC–MS-MS positive). Sensitivity, specificity and agreement were calculated as follows: sensitivity (100 × [TP/(TP + FN)]), specificity (100 × [TN/(TN + FP)]) and agreement (100 × [(TP + TN)/(TP + TN + FP + FN)]).
Results
Participants
Participant race/ethnicity was predominantly white (n = 9) or black/African-American (n = 8). Their mean (SD) BMI was 26 kg/m2 (4), weight was 77 kg (20) and age was 29 years old (6). On average, participants had last used cannabis 259 days (SD = 365 days; range: 30–1,278) prior to their first session. No participants experienced unanticipated or serious adverse events during the study.
LC–MS-MS results
IA and LC–MS-MS results for absolute and creatinine-normalized ∆9-THC-COOH for each individual participant and time point are presented in Supplementary Table S1; LC–MS-MS results for all analytes are provided in Supplementary Table S2. Creatinine-normalized values generally had lower peak values, but the timecourse was the same as the absolute values. Additionally, creatinine concentrations were variable across participants as the result of variability in sample dilution (e.g., due to the variability in participants’ degree of hydration). However, this level of variability in creatinine concentrations is in line with prior studies of this nature [e.g., (14, 15)]. Table I presents the maximum concentration (Cmax), time to maximum concentration (Tmax) and ranges for the total cannabinoid profiles for ∆9-THC-COOH, ∆9-THC, 11-OH-∆9-THC, THCV-COOH, CBN and 8,11-diOH-∆9-THC in urine following oral and vaporized administration.
Table I.
Maximum Concentration (Cmax), Time to Maximum Concentration (Tmax) and Ranges for Cannabis Analytes in Urine following Oral and Vaporized Cannabis Administration
| Dose (mg) | Mean (SD) | Median | Range | Mean (SD) | Median | Range |
|---|---|---|---|---|---|---|
| ∆9-THC-COOH Cmax (ng/mL) | ∆9-THC-COOH Tmax (h) | |||||
| Oral | ||||||
| 10 | 52.0 (37.9) | 42.6 | 6.1–163.3 | 4.9 (1.5) | 5.0 | 2.0–8.0 |
| 25 | 155.4 (144.9) | 131.2 | 27.0–599.7 | 5.6 (1.1) | 6.0 | 4.0–8.0 |
| Vaporized | ||||||
| 5 | 18.2 (20.8) | 12.0 | 1.7–90.8 | 4.2 (1.9) | 4.0 | 2.0–8.0 |
| 20 | 54.3 (50.4) | 24.9 | 5.7–192.1 | 3.9 (1.7) | 4.0 | 2.0–8.0 |
| ∆9-THC Cmax (ng/mL) | ∆9-THC Tmax (h) | |||||
| Oral | ||||||
| 10 | 1.4 (1.4) | 1.1 | 0.0–4.5 | 2.7 (1.7) | 3.0 | 0.0–6.0 |
| 25 | 5.0 (4.4) | 3.7 | 0.6–14.3 | 3.6 (1.6) | 3.0 | 2.0–8.0 |
| Vaporized | ||||||
| 5 | 2.9 (4.2) | 1.2 | 0.0–15.5 | 1.0 (0.6) | 1.0 | 0.0–3.0 |
| 20 | 18.2 (20.7) | 11.1 | 0.0–80.8 | 1.1 (0.5) | 1.0 | 0.0–2.0 |
| 11-OH-∆9-THC Cmax (ng/mL) | 11-OH-∆9-THC Tmax (h) | |||||
| Oral | ||||||
| 10 | 73.3 (65.6) | 50.6 | 8.2–245.4 | 3.3 (1.2) | 4.0 | 1.0–5.0 |
| 25 | 222.0 (205.5) | 159.8 | 29.0–863.0 | 4.0 (1.6) | 4.0 | 2.0–8.0 |
| Vaporized | ||||||
| 5 | 24.9 (35.1) | 11.3 | 2.1–160.8 | 1.7 (1.2) | 1.0 | 1.0–6.0 |
| 20 | 115.9 (133.7) | 65.6 | 7.8–507.1 | 1.7 (1.3) | 1.0 | 1.0–6.0 |
| THCV-COOH Cmax (ng/mL) | THCV-COOH Tmax (h) | |||||
| Oral | ||||||
| 10 | 8.6 (5.2) | 8.0 | 1.4–21.9 | 3.9 (1.5) | 4.0 | 2.0–8.0 |
| 25 | 27.4 (17.8) | 25.6 | 4.4–74.5 | 4.2 (1.4) | 5.0 | 2.0–8.0 |
| Vaporized | ||||||
| 5 | 2.4 (2.2) | 1.5 | 0.0–7.5 | 2.0 (2.3) | 2.0 | 0.0–8.0 |
| 20 | 10.8 (6.6) | 10.4 | 0.0–24.2 | 2.3 (1.9) | 2.0 | 0.0–8.0 |
| CBN Cmax (ng/mL) | CBN Tmax (h) | |||||
| Oral | ||||||
| 10 | 10.5 (10.4) | 7.5 | 1.1–42.8 | 3.3 (1.3) | 4.0 | 1.0–6.0 |
| 25 | 25.2 (26.7) | 14.0 | 3.3–110.5 | 3.7 (1.5) | 3.0 | 2.0–8.0 |
| Vaporized | ||||||
| 5 | 0.6 (0.8) | 0.3 | 0–2.5 | 0.6 (0.5) | 0.3 | 0.0–1.0 |
| 20 | 1.4 (1.4) | 1.1 | 0.0–4.5 | 1.4 (0.5) | 1.1 | 0.0–2.0 |
| 8,11-diOH-∆9-THC Cmax (ng/mL) | 8,11-diOH-∆9-THC Tmax (h) | |||||
| Oral | ||||||
| 10 | 28.4 (22.4) | 21.9 | 4.6–103.3 | 3.9 (1.3) | 4.0 | 2.0–6.0 |
| 25 | 76.3 (55.3) | 60.0 | 20.2–202.2 | 4.2 (1.6) | 5.0 | 2.0–8.0 |
| Vaporized | ||||||
| 5 | 2.4 (3.5) | 2.0 | 0–13.8 | 2.0 (2.1) | 2.0 | 0.0–8.0 |
| 20 | 10.8 (8.4) | 10.4 | 1.3–25.7 | 2.3 (1.5) | 2.0 | 1.0–6.0 |
Notes: SD = standard deviation, h = hour, range indicates the Cmax and Tmax range across participants. n = 21 across all analytes. LOQ for ∆9-THC-COOH, THCV-COOH and 8,11-diOH-∆9-THC was 1.0 ng/mL; the LOQ for ∆9-THC, 11-OH-∆9-THC and CBN was 0.25 ng/mL. The upper limit of linearity and carry-over limit for all analytes was 1,000 ng/mL.
Figure 1 illustrates the mean (±SEM) total cannabinoid urinary concentrations for ∆9-THC-COOH, ∆9-THC, 11-OH-∆9-THC, THCV-COOH, CBN and 8,11-diOH-∆9-THC following oral cannabis administration. In general, all of the positive analytes produced three similar patterns following oral cannabis ingestion: (i) both the 10-mg and 25-mg doses of ∆9-THC produced significantly higher Cmax relative to placebo (all P < 0.05), (ii) the Cmax concentrations were dose dependent (all P < 0.05) and (iii) the mean time to Tmax for the 10-mg and 25-mg doses of ∆9-THC was not significantly different for any of the analytes (all P > 0.05). There were sex differences observed with the mean Cmax and Tmax values for certain analytes (Supplementary Table S3). Specifically, the mean Cmax values were significantly greater in males versus females at both the 10-mg and 25-mg doses of ∆9-THC for 11-OH-∆9-THC and ∆9-THC (P < 0.05). Additionally, the mean Tmax values were significantly shorter in men relative to women for 8,11-diOH-∆9-THC at the 10-mg dose of ∆9-THC and for 8,11-diOH-∆9-THC, CBN and THCV-COOH at the 25-mg dose of ∆9-THC (P < 0.05).
Figure 1.
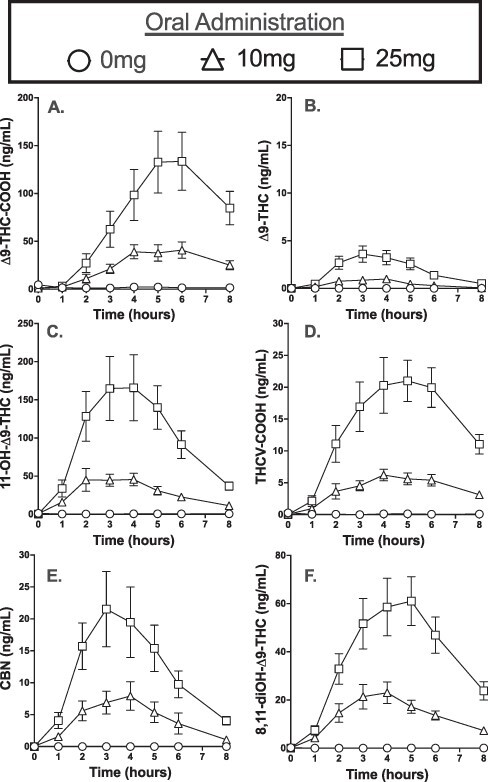
Mean urine concentrations (±SEM) for (A) ∆9-THC-COOH, (B) ∆9-THC, (C) 11-OH-∆9-THC, (D) THCV-COOH, (E) CBN and (F) 8,11-diOH-∆9-THC before and for 8 h after oral ingestion of a cannabis-containing chocolate brownie (0, 10 or 25 mg ∆9-THC).
Figure 2 illustrates the mean total cannabinoid urinary concentrations for ∆9-THC-COOH, ∆9-THC, 11-OH-∆9-THC, THCV-COOH, CBN and 8,11-diOH-∆9-THC following vaporized cannabis administration. Consistent with oral administration, all of the positive analytes produced the same three patterns following vaporized administration: (i) both the 5-mg dose and the larger 20-mg dose of ∆9-THC produced significantly higher Cmax relative to the placebo (all P < 0.05), (ii) the 25-mg dose produced a significantly higher Cmax relative to the smaller dose (all P < 0.05) and (iii) the mean time to Tmax for the 5-mg and 20-mg doses was not significantly different for any of the analytes (all P > 0.05).
Figure 2.
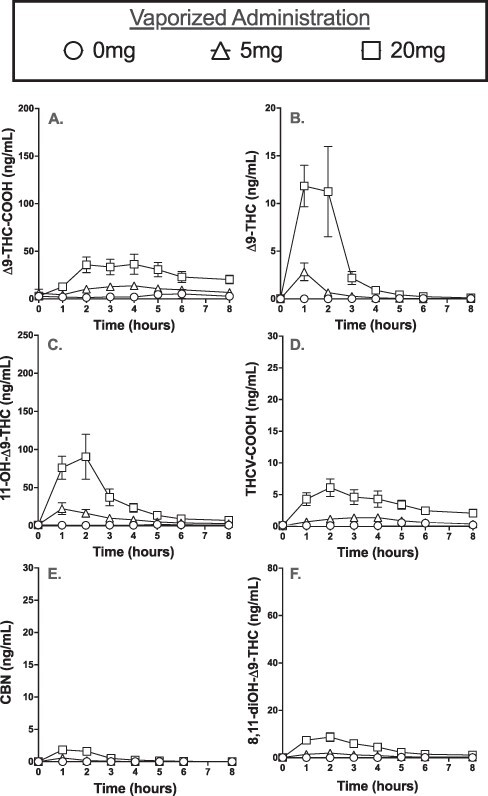
Mean urine concentrations (±SEM) for (A) ∆9-THC-COOH, (B) ∆9-THC, (C) 11-OH-∆9-THC, (D) THCV-COOH, (E) CBN and (F) 8,11-diOH-∆9-THC before and for 8 h after inhalation of vaporized cannabis (0, 5 or 20 mg ∆9-THC).
Additionally, sex differences were observed with the mean Cmax and Tmax values for certain analytes (Supplementary Table S3). Specifically, the mean Cmax values were significantly higher in males versus females for CBN and ∆9-THC at the 5-mg dose and ∆9-THC-COOH at the 20-mg dose of ∆9-THC (P < 0.05). Further, the mean Tmax value was significantly later in males versus females for CBN at the 5-mg dose of ∆9-THC (P < 0.05).
All positive urinary analytes produced significantly greater Cmax following oral administration relative to vaporized ∆9-THC (all P < 0.05), with one exception; vaporized ∆9-THC produced significantly greater ∆9-THC Cmax at both lower and higher doses relative to oral ∆9-THC (all P < 0.05). Additionally, in all cases but one [∆9-THC-COOH at lower doses (10 mg vs 5 mg)], the Tmax was significantly shorter following vaporized administration relative to oral ∆9-THC (all P < 0.05).
Mean detection times and individual ranges to first and last positive for ∆9-THC-COOH (cutoff = 15 ng/mL) are displayed in Table II. Positive ∆9-THC-COOH specimens (i.e., ≥15 ng/mL) were observed for qualitatively longer in the high ∆9-THC dose (20–25 mg) conditions compared with the low dose (5–10 mg) conditions. Specifically, the time to last positive sample (Tlast) for vaporized 5 mg (mean Tlast = 5.7 h; median Tlast = 5.5 h) and oral 10 mg (mean Tlast = 6.5 h; median Tlast = 6 h) ∆9-THC was shorter than the vaporized 20 mg (mean Tlast = 6.3 h; median Tlast = 7 h) and oral 25 mg (mean Tlast = 7.9 h; median Tlast = 8 h) ∆9-THC, respectively, although these values were not quantitatively compared. Specimens also tested positive (i.e., ≥15 ng/mL) for ∆9-THC-COOH for longer after oral cannabis administration relative to vaporized cannabis (Table II). Specimens with urinary ∆9-THC-COOH concentrations ≥15 ng/mL were observed in 97.6% of oral sessions (10 mg: n = 20; 25 mg: n = 21) and 59.5% of vaporized sessions (5 mg: n = 9; 20 mg: n = 16). Additionally, under the same ≥15 ng/mL concentration parameter, 11-OH-∆9-THC was observed in 95% of oral sessions (10 mg: n = 19; 25 mg: n = 21) and 62% of vaporized sessions (5 mg: n = 8; 20 mg: n = 18), and 8,11-diOH-∆9-THC was observed in 83% of oral sessions (10 mg: n = 15; 25 mg: n = 20) and 21% of vaporized sessions (5 mg: n = 0; 20 mg: n = 9).
Table II.
Mean Detection Times and Ranges of Positive ∆9-THC-COOH (≥15 ng/mL) Urine Specimens following Oral and Vaporized Cannabis Administration
| Dose (mg) | Time (h) | n |
|---|---|---|
| Oral: detection time (h) to first positive | ||
| 10 | 3.8 (1.0–6.0) | 20 |
| 25 | 2.9 (1.0–4.0) | 21 |
| Oral: detection time (h) to last positive | ||
| 10 | 6.5 (4.0–8.0) | 20 |
| 25 | 7.9 (6.0–8.0) | 21 |
| Vaporized: detection time (h) to first positive | ||
| 5 | 2.3 (1.0–5.0) | 9 |
| 20 | 1.8 (1.0–4.0) | 16 |
| Vaporized: detection time (h) to last positive | ||
| 5 | 5.9 (4.0–8.0) | 9 |
| 20 | 6.3 (2.0–8.0) | 16 |
Using current federal workplace drug-testing criteria established by Substance Abuse and Mental Health Services Administration (SAMSHA) (IA cutoff of 50 ng/mL plus LC–MS-MS concentration ≥15 ng/mL), urine specimens tested positive for ∆9-THC-COOH in 97.6% of oral sessions and 54.8% of vaporized sessions with active ∆9-THC doses. Urine specimens that tested positive at the last timepoint (i.e., at hour 8) were 43% for the 10-mg dose and 95% for the 25-mg dose of ∆9-THC following oral administration. Interestingly, 100% of the positive specimens that tested positive at hour 8 at the 10-mg dose of ∆9-THC were from males. Following vaporized cannabis administration, 0% of the urine specimens at 5 mg and 38% of the samples at 20 mg tested positive at the 8-h timepoint. Finally, at the last time point (hour 8) following oral administration, 50% of participants had urinary concentrations of 11-OH-∆9-THC (n = 6 for 10 mg dose; n = 15 for 25 mg) at ≥15 ng/mL, and 33% of participants (n = 2 for 10 mg; n = 12 for 25 mg) had concentrations of 8,11-diOH-∆9-THC at ≥15 ng/mL.
Sensitivity, specificity and agreement
Results of sensitivity, specificity and agreement analyses between IA and LC–MS-MS for ∆9-THC-COOH in urine are summarized in Supplementary Table S4. Three separate IA cutoffs (20, 50 and 100 ng/mL) were compared to the LC–MS-MS results (confirmation of positive test was always: ≥15 ng/mL). For both oral and vaporized cannabis, sensitivity was highest at the 20 ng/mL cutoff and decreased at the 50 and 100 ng/mL cutoffs while the opposite trend was observed for specificity results (i.e., highest specificity observed at the 100 ng/mL cutoff). For oral conditions, agreement was similar at the 50 and 100 ng/mL cutoff, but for vaporized conditions, the highest agreement was observed at the 50 ng/mL cutoff. At the 100 ng/mL cutoff, higher sensitivity was observed in oral versus the vaporized sessions. At the 20 ng/mL cutoff, higher specificity was observed in vaporized conditions compared to oral conditions.
Discussion
Improving the understanding of the urinary excretion profile following cannabis administration has implications across a variety of domains. Prior controlled studies have focused on the pharmacokinetic profile for urinary ∆9-THC-COOH concentrations following smoked cannabis administration. However, characterizing the urinary profiles of multiple cannabinoids and their metabolites using different routes of administration is important because it assists with interpreting drug test results and informing regulatory standards (e.g., identifying confirmatory cutoff ranges for other cannabinoids for high-∆9-THC cannabis). For example, distinguishing CBD-dominant hemp cannabinoid profiles, which display trace or no amounts of cannabinoids like THCV-COOH, 11-OH-∆9-THC and ∆9-THC (24, 25), from ∆9-THC-dominant cannabis is critical considering the legality surrounding widely used hemp-based products. Therefore, the purpose of the present study was to conduct a more comprehensive analysis of the urinary cannabinoids (i.e., beyond just ∆9-THC-COOH) after oral and vaporized ∆9-THC administration by infrequent cannabis users.
In previous studies conducted by our group, the same dose of cannabis produced a 10-fold higher Cmax for ∆9-THC-COOH in urine when administered orally (14) relative to vaporized administration (15); these outcomes were mirrored in a sample of occasional cannabis users who administered 50.6 mg ∆9-THC via both oral and vaporized routes (13). Similarly, in the present study, oral cannabis administration produced a higher Cmax for ∆9-THC-COOH relative to vaporized administration, while urine specimens tested positive for ∆9-THC-COOH in 97.6% of oral sessions versus 54.8% of vaporized sessions with active ∆9-THC doses. This data suggests that oral cannabis administration elicits a greater Cmax and longer Tmax relative to vaporized cannabis.
Following 5 mg of vaporized ∆9-THC cannabis administration, 42.9% of participants excreted ∆9-THC-COOH in urinary concentrations ≥15 ng/mL, the confirmatory cutoff concentration listed in the mandatory guidelines for federal workplace drug testing (23). These data have important public health implications due to the widespread popularity of ∆9-THC ‘microdosing’, which is described by popular cannabis websites as using the lowest dose of ∆9-THC to achieve desired effects without intoxication. In particular, two popular cannabis websites (i.e., Leafly, WeedMaps) have published recommendations for using low doses of ∆9-THC (typically described as 1–5 mg THC) while ‘working from home’ (26, 27). Such website articles substantiate claims about ∆9-THC microdosing utilizing published research. As cannabis-focused media continues to promote the use of low dose ∆9-THC, especially in workplace settings, it is important to acknowledge that even low doses (5 mg) of ∆9-THC, which are unlikely to be associated with impairment (19), are capable of producing a cannabis-positive drug test.
Notably, urinary pharmacokinetic profiles for the cannabinoid metabolites were markedly different across routes of administration and dose. Following oral ingestion of cannabis, 11-OH-∆9-THC, THCV-COOH, ∆9-THC-COOH, CBN and 8,11-diOH-∆9-THC all had greater Cmax and longer Tmax values relative to vaporized cannabis administration. Urinary concentrations for THCV, CBD, ∆8-THC, ∆8-THC-COOH and 8-OH-∆9-THC were <15 ng/mL for any of the tested conditions. Additionally, a similar number of biospecimens were above the positive cutoff for 11-OH-∆9-THC compared to ∆9-THC-COOH (defined by ≥15 ng/mL LC–MS-MS concentrations) for each condition. Further, the results presented for 11-OH-∆9-THC, CBN and THCV-COOH contrast a previous study that was unable to detect these cannabinoids in urine following vaporized or oral cannabis administration in frequent and infrequent cannabis users (13). In addition, other reports evaluating smoked cannabis also failed to detect quantifiable concentrations of 11-OH-∆9-THC (28, 29). One key difference between this and previous studies is that in the present study metabolites were hydrolyzed prior to LC–MS-MS analysis, which can increase metabolite concentration, including 11-OH-∆9-THC (30, 31). One explanation for this observed difference is that 11-OH-∆9-THC is metabolized into ∆9-THC-COOH (32), and following administration of higher potency cannabis, 11-OH-∆9-THC would be quantifiable and present for longer. Another explanation would be that this increase in 11-OH-∆9-THC concentrations following oral ingestion is, in part, due to the first-pass metabolism of ∆9-THC in the liver, which results in higher accumulation of 11-OH-∆9-THC relative to both smoked and vaporized cannabis (32). Thus, 11-OH-∆9-THC could serve as an additional metabolite for distinguishing between hemp-based and THC-dominant cannabis products and more specifically orally administered products. Finally, while sex differences were observed, these trends were not systematic across all analytes. Additional research is needed to understand whether the pharmacokinetic profiles for these urinary analytes are affected by other various factors (e.g., age).
There were some limitations to the present study. First, within each route of administration, the selected low doses were intended to produce discriminable drug effects without eliciting significant impairment of cognitive/psychomotor functioning while the high doses were intended to produce discriminable drug effects as well as marked cognitive/psychomotor impairment. However, the different ∆9-THC doses employed for oral (10 and 25 mg) versus vaporized (5 and 20 mg) administration precluded formal comparison of urinary pharmacokinetics between these routes of administration. Future studies comparing cannabinoid urinary pharmacokinetics following oral versus vaporized cannabis administration using a within-subjects design should employ the same doses across routes of administration to allow for a more formal comparison. Second, only one type of cannabis (THC-dominant), vaporizer (Volcano Medic®) and edible matrix (chocolate brownie) were included in this study. Future studies should evaluate urinary cannabinoid pharmacokinetics using different chemovars (e.g., CBD-dominant), vaporizers (e.g., handheld vaporizer) and edible product formulations (e.g., gummy candies and beverages). Third, the collection window for urine in this study (i.e., 8 h) was shorter than other studies that have measured urinary excretion of ∆9-THC-COOH. For example, in this study, 95.2% of participants voided positive urine specimens at the 8-h timepoint following ingestion of the high oral cannabis dose (25 mg ∆9-THC). Future studies should compare the urinary pharmacokinetics of oral and vaporized cannabis over longer periods of time in order to fully characterize the excretion profiles. Fourth, the study sample was relatively homogenous in certain ways that limited our ability to examine how some potentially relevant individual characteristics (e.g., age, body fat percentage and user experience level with cannabis) may impact urinary cannabinoid pharmacokinetics.
Conclusion
In summary, the present study characterized the urinary pharmacokinetics of ∆9-THC-COOH and cannabinoid metabolites (∆9-THC, 11-OH-∆9-THC, THCV-COOH, CBN and 8,11-diOH-∆9-THC) across two routes of administration (i.e., oral and vaporized), at three doses—placebo, low (5–10 mg ∆9-THC) and high (20–25 mg ∆9-THC)—in people that infrequently use cannabis. This study found that both ingestion and vaporization of cannabis produced dose-dependent increases in the concentrations (Cmax) of each positive ∆9-THC analyte, and the Tmax was considerably longer following oral administration relative to vaporized cannabis. Under the described federal workplace drug-testing criteria (IA cutoff of 50 ng/mL plus LC–MS-MS concentration ≥15 ng/mL), urine specimens following oral ingestion tested positive for ∆9-THC-COOH at much higher rates relative to vaporized cannabis. Although not confirmed using IA screening, 11-OH-∆9-THC produced a similar amount of positive LC–MS-MS samples compared to ∆9-THC-COOH under both routes of administration. With the expansion of cannabis legalization, and the ever-increasing popularity of cannabis use among individuals for medical and recreational purposes, understanding the urinary pharmacokinetic profiles of emergent cannabis products will be critical for influencing and shaping future drug-testing policies.
Supplementary Material
Acknowledgments
We thank the support staff of the Johns Hopkins University Behavioral Pharmacology Research Unit for outstanding contributions to the implementation of this study. We also thank Dr David Kuntz at the Clinical Reference Laboratory (CRL) and support staff at RTI International, the National Institute on Drug Abuse Drug Supply Program, and Storz and Bickel for critical services and material support.
Contributor Information
Dennis J Sholler, Behavioral Pharmacology Research Unit, Johns Hopkins University School of Medicine, 5510 Nathan Shock Dr., Baltimore, MD 21224, USA.
C Austin Zamarripa, Behavioral Pharmacology Research Unit, Johns Hopkins University School of Medicine, 5510 Nathan Shock Dr., Baltimore, MD 21224, USA.
Tory R Spindle, Behavioral Pharmacology Research Unit, Johns Hopkins University School of Medicine, 5510 Nathan Shock Dr., Baltimore, MD 21224, USA.
Erin L Martin, Department of Neuroscience, Medical University of South Carolina, 125 Doughty St., Charleston, SC 29403, USA.
David Kuntz, Clinical Reference Laboratory, 8433 Quivira Rd, Lenexa, KS 66214, USA.
Ryan Vandrey, Behavioral Pharmacology Research Unit, Johns Hopkins University School of Medicine, 5510 Nathan Shock Dr., Baltimore, MD 21224, USA.
Megan Grabenauer, Center for Forensic Sciences, RTI International, 3040 East Cornwallis Rd., Research Triangle Park, NC 27709, USA.
Supplementary Data
Supplementary Data are available at Journal of Analytical Toxicology.
Funding
This research was supported by the National Institute of Justice (NIJ; Award Number 2016-DN-BX-0193) and the National Institute on Drug Abuse (NIDA; T32DA007209, T32-DA007288 and R44 DA046272-01A1). The opinions, findings, conclusions and recommendations expressed in this publication are those of the authors and do not necessarily reflect those of the NIJ or NIDA.
Data availability
The data underlying this article are available in the article and its supplementary materials.
References
- 1. Berg C.J., Stratton E., Schauer G.L., Lewis M., Wang Y., et al. (2015) Perceived harm, addictiveness, and social acceptability of tobacco products and marijuana among young adults: marijuana, hookah, and electronic cigarettes win. Substance Use & Misuse, 50, 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. SAMHSA . (2018) Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health. Vol. HHS Publication No. PEP19-5068, NSDUH Series H-54. 2019. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration: Rockville, MD. [Google Scholar]
- 3. Russell C., Rueda S., Room R., Tyndall M., Fischer B. (2018) Routes of administration for cannabis use—basic prevalence and related health outcomes: a scoping review and synthesis. International Journal of Drug Policy, 52, 87–96. [DOI] [PubMed] [Google Scholar]
- 4. Spindle T.R., Bonn-Miller M.O., Vandrey R. (2019) Changing landscape of cannabis: novel products, formulations, and methods of administration. Current Opinion in Psychology, 30, 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schauer G.L., King B.A., Bunnell R.E., Promoff G., McAfee T.A. (2016) Toking, vaping, and eating for health or fun: marijuana use patterns in adults, U.S., 2014. American Journal on Preventitive Medicine, 50, 1–8. [DOI] [PubMed] [Google Scholar]
- 6. Steigerwald S., Wong P.O., Khorasani A., Keyhani S. (2018) The form and content of cannabis products in the United States. Journal of General Internal Medicine, 33, 1426–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Macdonald S., Hall W., Roman P., Stockwell T., Coghlan M., Nesvaag S. (2010) Testing for cannabis in the work-place: a review of the evidence. Addiction, 105, 408–416. [DOI] [PubMed] [Google Scholar]
- 8. Vandrey R., Herrmann E.S., Mitchell J.M., Bigelow B.E., Flegel R., LoDico C., et al. (2017) Pharmacokinetic profile of oral cannabis in humans: blood and oral fluid disposition and relation to pharmacodynamic outcomes. Journal of Analyticial Toxicology, 41, 83–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Niedbala R.S., Kardos K.W., Fritch D.F., Kardos S., Fries T., et al. (2001) Detection of marijuana use by oral fluid and urine analysis following single-dose administration of smoked and oral marijuana. Journal of Analytical Toxicology, 25, 289–303. [DOI] [PubMed] [Google Scholar]
- 10. Cone E.J., Johnson R.E., Paul B.D., Mell L.D., Mitchell J. (1988) Marijuana-laced brownies: behavioral effects, physiologic effects, and urinalysis in humans following ingestion. Journal of Analytical Toxicology, 12, 169–175. [DOI] [PubMed] [Google Scholar]
- 11. Newmeyer M.N., Swortwood M.J., Barnes A.J., Abulseoud O.A., Scheidweiler K.B., Huestis M.A. (2016) Free and glucuronide whole blood cannabinoids’ pharmacokinetics after controlled smoked, vaporized, and oral cannabis administration in frequent and occasional cannabis users: identification of recent cannabis intake. Clinical Chemistry, 62, 1579–1592. [DOI] [PubMed] [Google Scholar]
- 12. Huestis M.A., Blount B.C., Milan D.F., Newmeyer M.N., Schroeder J., Smith M.L. (2019) Correlation of creatinine- and specific gravity-normalized free and glucuronidated urine cannabinoid concentrations following smoked, vaporized, and oral cannabis in frequent and occasional cannabis users. Drug Testing and Analysis, 11, 968–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huestis M.A., Sempio C., Newmeyer M.N., Andersson M., Barnes A.J., et al. (2020) Free and glucuronide urine cannabinoids after controlled smoked, vaporized and oral cannabis administration in frequent and occasional cannabis users. Journal of Analyticial Toxicology, 44, 651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schlienz N.J., Cone E.J., Herrmann E.S., Lembeck N.A., Mitchell J.M., et al. (2018) Pharmacokinetic characterization of 11-nor-9-carboxy-∆9-tetrahydrocannabinol in urine following acute oral cannabis ingestion in healthy adults. Journal of Analytical Toxicology, 42, 232–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spindle T.R., Cone E.J., Schhlienz N.J., Mitchell J.M., Bigelow G.E., et al. (2020) Urinary excretion profile of 11-nor-9-carboxy-∆9-tetrahydrocannabinol (THCCOOH) following smoked and vaporized cannabis administration in infrequent cannabis users. Journal of Analytical Toxicology, 44, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sobell L.C., Sobell M.B.. Timeline follow-back. In: Litten, R.Z., Allen, J.P. (eds.). Measuring Alcohol Consumption. Totowa, NJ: Humana Press, 1992; pp 41–72. [Google Scholar]
- 17. Schlienz N.J., Spindle T.R., Cone E.J., Herrmann E.S., Bigelow G.E., et al. (2020) Pharmacodynamic dose effects of oral cannabis ingestion in healthy adults who infrequently use cannabis. Drug and Alcohol Dependence, 211, 107969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spindle T.R., Cone E.J., Herrmann E.S., Mitchell J.M., Flegel R., et al. (2020) Pharmacokinetics of cannabis brownies: a controlled examination of ∆9-tetrahydrocannabinol and metabolites in blood and oral fluid of healthy adult males and females. Journal of Analytical Toxicology, 44, 661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Spindle T.R., Martin E.L., Grabenauer M., Woodward T., Milburn M.A., Vandrey R. (2021) Assessment of cognitive and psychomotor impairment, subjective effects, and blood THC concentrations following acute administration of oral and vaporized cannabis. Journal of Psychopharmacology, 35, 786–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spindle T.R., Cone E.J., Schlienz N.J., Mitchell J.M., Bigelow G.E., Flegel R., et al. (2018) Acute effects of smoked and vaporized cannabis in healthy adults who infrequently use cannabis: a crossover trial. JAMA Network Open, 1, e184841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spindle T.R., Cone E.J., Kuntz D., Mitchell J.M., Bigelow G.E., et al. (2020) Urinary pharmacokinetic profile of cannabinoids following administration of vaporized and oral cannabidiol and vaporized CBD-dominant cannabis. Journal of Analytical Toxicology, 44, 109–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huestis M.A., Cone E.J. (1998) Differentiating new marijuana use from residual drug excretion in occasional marijuana users. Journal of Analytical Toxicology, 39, 1–12. [DOI] [PubMed] [Google Scholar]
- 23. SAMHSA . (2017) Mandatory guidelines for federal workplace drug testing programs. Federal Register, 82, 7920–7970. [Google Scholar]
- 24. Sholler D.J., Spindle T.R., Cone E.J., Goffi E., Kuntz D., Mitchell J.M., et al. (2022) Urinary pharmacokinetic profile of cannabidiol (CBD), ∆9-tetrahydrocannabinol (THC) and their metabolites following oral and vaporized CBD and vaporized CBD-dominant cananbis administration. Journal of Analytical Toxicology, 46, 494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dahlgren M.K., Sagar K.A., Lambros A.M., Smith R.T., Gruber S.A. (2021) Urinary tetrahydrocannabinol after 4 weeks of a full-spectrum, high-cannabidiol treatment in an open-label clinical trial. JAMA Psychiatry, 78, 335–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leafy . (2017) Microdosing Cannabis: Benefits without the Buzz. https://www.leafly.com/news/cannabis-101/microdosing-weed-guide (accessed May 25, 2017).
- 27. Cannigma . (2019) What is Microdosing Cannabis and Why Should you do it? https://cannigma.com/products/what-is-microdosing-cannabis-and-why-do-it/ (accessed Nov 19, 2019).
- 28. Manno J.E., Manno B.R., Kemp P.M., Alford D.D., Abukhalaf I.K., et al. (2001) Temporal indication of marijuana use can be estimated from plasma and urine concentrations of delta9-tetrahydrocannabinol, 11-hydroxy-delta9-tetrahydrocannabinol, and 11-nor-delta9-tetrahydrocannabinol-9-carboxylic acid. Journal of Analytical Toxicology, 25, 538–549. [DOI] [PubMed] [Google Scholar]
- 29. Desrosiers N.A., Lee D., Concheiro-Guisan M., Scheidweiler K.B., Gorelick D.A., Huestis M.A. (2014) Urinary cannabinoid disposition in occasional and frequent smokers: is THC-glucuronide in sequential urine samples a marker of recent use in frequent smokers? Clinical Chemistry, 60, 361–372. [DOI] [PubMed] [Google Scholar]
- 30. Abraham T.T., Lowe R.H., Pirnary S.O., Darwin W.D., Huestis M.A. (2007) Simultaneous GC-EI-MS determination of delta9-tetrahydrocannabinol, 11-hydroxy-Delta9-tetrahydrocannabinol, and 11-nor-9-carboxy-delta9-tetrahydrocannabinol in human urine following tandem enzyme-alkaline hydrolysis. Journal of Analytical Toxicology, 31, 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lowe R.H., Abraham T.T., Darwin W.D., Herning R., Cadet J.L., Huestis M.A. (2009) Extended urinary delta9-tetrahydrocannabinol excretion in chronic cannabis users precludes use as a biomarker of new drug exposure. Drug and Alcohol Dependence, 105, 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schwilke E.W., Schwope D.M., Karschner E.L., Lowe R.H., Darwin D.L., et al. (2009) ∆9-Tetrahydrocannabinol (THC), 11-hydroxy-THC, and 11-nor-9-carboxy-THC plasma pharmacokinetics during and after continuous high-dose oral THC. Clinical Chemistry, 55, 2180–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and its supplementary materials.


