Abstract
Background
Multiple regimens have been widely used in the eradication treatment of Helicobacter pylori infection in children. However, there is a lack of comparison and evaluation of their effectiveness in different regions of the world.
Methods
Randomized controlled trials were retrieved. Review Manager 5.4, Stata SE 15 and R 4.0.4 statistical software were used to analyze date. The ranking probability is assessed according to the surfaces under cumulative ranking (SUCRA).
Results
163 studies were eligible for this study, involving 336 arms and 18,257 children, and 10 different interventions. The results showed that the eradication rates of sequential therapy with probiotics (SP), bismuth-containing quadruple (Quadruple) therapy, concomitant therapy and PCN therapy were at least 90%. Cumulative ranking showed that SP therapy had the best eradication effect (SUCRA 92.7%) whereas Bismuth-containing triple therapy (B) had the worst (SUCRA 3.5%). Subgroup analysis suggested that SP therapy ranked first in China and other regions, and the ranking of Triple therapy with probiotics therapy (TP) was equally stable (SUCRA 72.0% vs 76.4% respectively). The security of the SP and TP therapy had great advantages.
Conclusions
As for the eradication treatment of Helicobacter pylori infection in children, SP therapy ranks highest. SP and TP therapies are most safe.
Keywords: Helicobacter pylori, children, treatment regimens, efficacy, network meta-analysis
1. Introduction
Helicobacter pylori infection in children is a common pediatric disease. Although an epidemiology investigation has showed that the prevalence of H. pylori is decreasing in developed countries (Burucoa and Axon, 2017), it remains a serious problem in developing and less developed regions (Daugule et al., 2016; Hooi et al., 2017). H. pylori was discovered by Robin Warren and Barry Marshall during the study of gastric biopsy specimens in 1982 (Goodwin et al., 1989). It usually parasitizes in the human gastric mucosa and spreads through the oral-oral and fecal-oral routes. Most children infected with the bacteria are asymptomatic or show mild symptoms, such as common regular epigastric pain, loss of appetite, nausea and vomiting. Thereby they are often easily overlooked by their parents, and the disease is aggravated without timely diagnosis and treatment. A few patients with H. pylori infection have symptoms of recurrent headache (Shivalingaiah et al., 2019; Cavestro et al., 2022). Severe cases may also suffer from gastritis, peptic ulcer, gastric mucosa-associated lymphoid tissue (MALT) lymphoma, and even gastric cancer (Plummer et al., 2015), which have a serious impact on children’s quality of life and growth. H. pylori gastritis was officially recognized as an infectious disease in 2015 (Sugano et al., 2015). Compared with adults, children have poorer digestive tract mucosal resistance and self-repair function, so the gastric mucosal damage caused by H. pylori infection is more serious, and the clinical symptoms are often more obvious. Therefore, children from families with low economic level and poor living environment often become a high-risk group of H. pylori infection (Czinn, 2005).
The North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) recommend in their guidelines for the treatment of children and adolescents that a 14-day triple therapy of different antibiotics should be developed for children infected with H. pylori according to the results of drug sensitivity test, while children with unknown antibiotic sensitivity were given 14 days of high-dose triple therapy or bismuth-containing quadruple therapy (Jones et al., 2017). However, with the abuse of antibiotics and the enhancement of drug resistance, the effect of simple standard triple therapy eradicating H. pylori is on the wane, and the recurrence rate of H. pylori also has showed an upward trend in recent years (Zhao et al., 2021). This has become a great challenge for clinicians. With the continuous exploration of the disease, a variety of eradication methods have been introduced, including metronidazole replacement triple therapy, bismuth triple therapy (excluding PPI) and quadruple therapy, probiotic quadruple therapy, sequential therapy, concomitant therapy (Dong and Zhang, 2022). Nevertheless, most of them are empirical treatments in clinical application, and there is no unified drug use standard for children up to now. At present, evaluating the eradication rate of each treatment mostly relies on RCT and pairwise meta-analysis (Zhou et al., 2016), and there is a lack of comprehensive comparison of their efficacy. Compared with traditional meta-analysis, network meta-analysis (NMA) can collect a wide variety of interventions under the same conditions and rank them according to their efficacy (Liu et al., 2014). The purpose of this study is to summarize and compare the eradication rate and safety of treatments of H. pylori in children around the world through NMA, hoping to provide reference for future guidelines and clinical medication for the treatment of H. pylori infection in children.
2. Materials and methods
The protocol for this Network Meta-analysis was prospectively registered with PROSPERO (CRD42022342787).
2.1. Inclusion criteria
2.1.1. Object
H. pylori infection was diagnosed by histopathological examination, urea breath test (UBT), fecal HP antigen test (SAT), or rapid urease test; the age was less than 18 years old; there was no restriction in race, sex, and course of disease; patients had no other serious gastrointestinal diseases; the subjects did not use related antibiotics to treat other diseases at the same time.
2.1.2. Type
Eligible studies were randomized controlled trials (RCTs). The language was limited to Chinese and English.
2.1.3. Interventions
All the children were randomly assigned to at least 2 groups. The intervention measures in each group were in line with the standards of the guidelines, and the course of treatment was at least 7 days.
2.1.4. Outcomes
The eradication rate reexamined after at least 2 weeks of treatment was used as the outcome index of effectiveness, and the total side effects rate was used as the safety evaluation index.
2.2. Exclusion criteria
Retrospective studies, cohort studies, case reports, commentaries, conference abstracts, animal experiments, reviews, and repeatedly published articles; articles with inappropriate intervention measures, such as RCTs in which the two groups have the same regimen but different doses or courses of treatment; articles with unavailable or missing data; articles for which outcome measures can’t be combined.
2.3. Publication search strategy
Relevant trials were searched in 8 databases, including PubMed, Embase, Web of Science, the Cochrane Library, China National Knowledge Infrastructure (CNKI), Chinese Biomedical Literature database (CBM), Wanfang Database, and VIP Information (VIP). The following search phrases were used: “Helicobacter pylori”, “Campylobacter pylori”, “children”, “randomized controlled trial” and so on. If there are duplicate publications, only the one published earlier will be retained. In order to ensure the comprehensiveness of the search results, the references in important articles were screened manually. The specific retrieval methods are shown in Supplementary Table S1 .
2.4. Literature screening, data extraction and quality evaluation
Two researchers (LMM, ZCB) independently screened and cross checked the literature. Any dissents were solved by discussing together or consulting a third researcher for assistance. All the searched articles were imported into EndnoteX9.1 for management. The extracted data include the first author, year of publication, country, total sample size, study design, regimens compared, course of treatment. The data were imported into the Excel2007 and cross-checked by the aforesaid two researchers. According to the bias risk assessment scale of randomized controlled trials recommended by Cochrane system evaluator manual 5.1.0, the bias risk quality assessment tool with 6 items in ReviewManager5.4 was used to evaluate the quality of included literature (Cumpston et al., 2019).
2.5. Statistical analysis
Stata SE 15 was used for data processing and a random-effects model was used to draw a network evidence diagram. When there was a closed loop, a heterogeneity test was further performed for both direct evidence and indirect evidence. A P > 0.05 indicated a good consistency and therefore a consistency model was applied. The gemtc program package called by R 4.0.4 statistical software and the Markov Chain Monte Carlo were used for Bayesian network meta-analysis. The Markov chain was set to 4 with iterations set to 50,000 and annealing set to 20,000, in order to eliminate the influence of the initial values. The outcome measures in the present study were binary variables. Therefore, the odds ratio (OR) with 95% confidence intervals (CI) was used as the effect size of this statistical analysis. When P < 0.05, the difference was statistically significant. To explore a more effective and safer treatment, Stata SE 15 was also used to calculate and visualize the surface under the cumulative ranking (SUCRA) of the cumulative probability according to the matrix table of ranking probability obtained from R 4.0.4 software. The larger the SUCRA value was, the more effective and safer the treatment was. And then, the difference of eradication rate between different regions was examined by subgroup analysis. Finally, an inverted funnel plot was drawn to assess publication bias and determine whether publication bias or small-sample effects existed (Dias et al., 2010).
3. Results
3.1. Literature screening
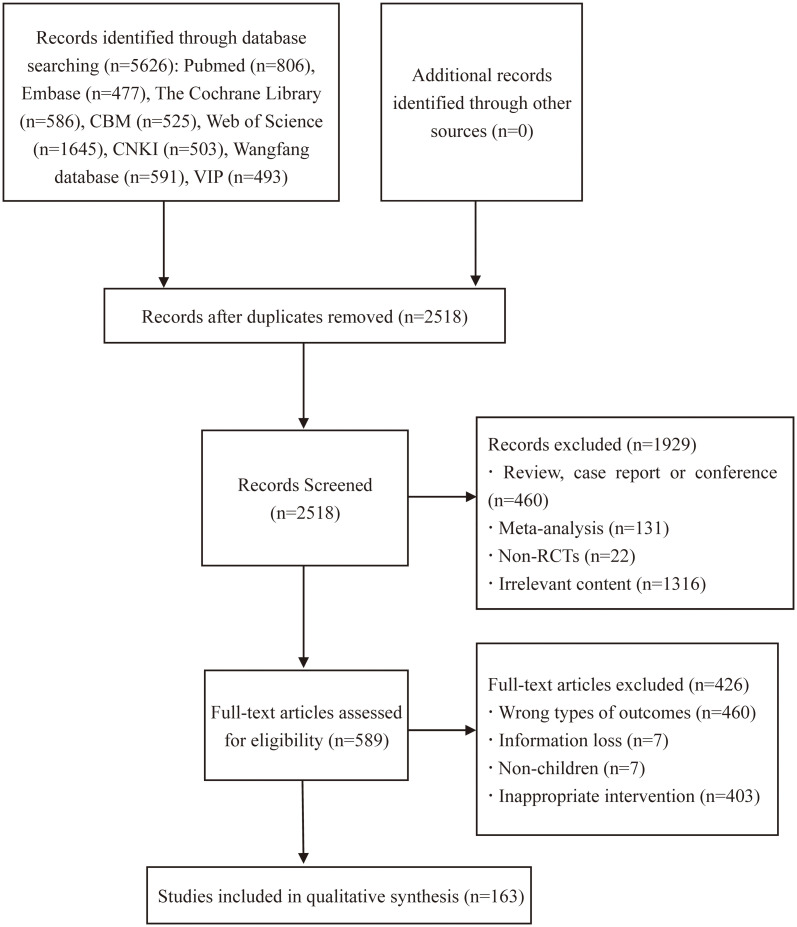
Initially 5626 related articles were searched and strictly screened in Endnote X9.1 software. After the removal of 3108 duplicates, 1929 articles were excluded based on topics and abstracts. After full-text review, 426 articles were excluded. Finally, 163 articles (Li, 2003; Francavilla et al., 2005; Sýkora et al., 2005; Bahremand et al., 2006; Goldman et al., 2006; Lionetti et al., 2006; Huang, 2007; Tang, 2008; Wan and Liao, 2008; Hurduc et al., 2009; Lei, 2009; Li, 2009; Worona-Dibner, 2009; Xiong et al., 2009; Zhang, 2009; Li, 2010; Liu, 2010; Pan et al., 2010; Zhu, 2010; Albrecht et al., 2011; Bontems et al., 2011; Li and Yu, 2011; Li et al., 2011; Liu F. et al., 2011; Liu Y. et al., 2011; Wang et al., 2011; Wu, 2011; Chu, 2012; Ji, 2012; Li and Lin, 2012; Lie, 2012; Liu, 2012; Rong, 2012; Tolone et al., 2012; Wu et al., 2012; Zhang, 2012; Zhang and Li, 2012; Ahmad et al., 2013; Baysoy et al., 2013; Chen, 2013; Chen et al., 2013; Huang et al., 2013; Laving et al., 2013; Pan, 2013; Sun et al., 2013; Xu et al., 2013; Yang et al., 2013; Zhang, 2013; Zhao et al., 2013; Zhong, 2013; Chen et al., 2014; He, 2014; Hou, 2014; Ke, 2014; Ku et al., 2014; Kutluk et al., 2014; Li and Zhou, 2014; Liu, 2014; Wang, 2014; Wang and Feng, 2014; Wang and Huang, 2014; Yang, 2014; Akcam et al., 2015; Chen, 2015; Gao et al., 2015; Islek et al., 2015; Liu et al., 2015; Luo et al., 2015; Sun, 2015; Wang and Liu, 2015; Xiao et al., 2015; Zhang, 2015a; Zhang, 2015b; Zhang, 2015c; Zhang, 2015d; Zhao and Zhou, 2015; Zhong et al., 2015; Zhou and Li, 2015; Zhou and Li, 2015; Cong and Hu, 2016; Fan et al., 2016; Gong, 2016; Han, 2016; Huang, 2016; Li et al., 2016; Qin, 2016; Shahraki et al., 2016; Xu et al., 2016; Zhang, 2016; Zhou, 2016; Zhu and Li, 2016; Fang and Li, 2017; He, 2017; Li, 2017; Tang and Liu, 2017; Urganci, 2017; Ustundag et al., 2017; Wang, 2017; Wu, 2017; Xiang et al., 2017; Zhu et al., 2017; Chen et al., 2018; Fan, 2018; Han, 2018; Huang, 2018; Li, 2018; Li and Shi, 2018; Li J., 2018; Li, 2018; Luo, 2018; Qiu et al., 2018; Wu et al., 2018; Xie, 2018; Zhang, 2018; Zhou, 2018; Zhou and Shi, 2018; He et al., 2019; He et al., 2019; Lhamo and Tinley, 2019; Liang, 2019; Lin and Huang, 2018; Liu and Fang, 2019; Liu et al., 2019; Mei et al., 2019; Wang, 2019; Wang et al., 2019; Zhang, 2019; Zhang et al., 2019b; Zhang et al., 2019a; Zhao, 2019; Zhou et al., 2019; Zhu, 2019; Chen et al., 2020; Chen X. et al., 2020; Lai and Liang, 2020; Li, 2020; Liu, 2020; Liu and Zhang, 2020; Rong and Xiao, 2020; Su, 2020; Sun et al., 2020; Yang et al., 2020; Yao and Luo, 2020; Zhao, 2020; Zheng et al., 2020; Gao, 2021; Han, 2021; Hu, 2021; Ke et al., 2021; Li, 2021a; Li, 2021b; Li and Qi, 2021; Liu, 2021; Liu and Wu, 2021; Shi et al., 2021; Xiao and Shao, 2021; Yang, 2021; Yuan, 2021; Yuan and Ning, 2021; Zhang, 2021; Zhou, 2021; Liu et al., 2022; Zhou, 2022) were adopted, including 21 English articles and 142 Chinese studies. The detailed search strategy is described in Figure 1 .
Figure 1.
Flow diagram of the study search process.
3.2. Basic characteristics
163 studies were included, with a total of 336 therapeutic arms, including 156 dual arm trials, 4 three-arm trials (Zhang, 2009; Liu et al., 2011; Li, 2017; Luo, 2018) and 3 four-arm trials (Rong, 2012; Zhu et al., 2017; Chen et al., 2020). The total sample size is 18257 cases, with the largest sample size of 541 cases and the minimum sample size of 15 cases of per arm. The 10 treatment regimens implemented by these researchers and the number of subjects around the world are as follows (in alphabetical order):
1. B (Bismuth-containing triple therapy), n=348;
2. Concomitant (Concomitant therapy), n=50;
3. PAC (PPI, amoxicillin and clarithromycin), n=7448;
4. PAF (PPI, amoxicillin and nitrofuran drugs), n=196;
5. PAN (PPI, amoxicillin and nitroimidazoles), n=2291;
6. PCN (PPI, clarithromycin and nitroimidazoles), n=908;
7. Quadruple (Bismuth-containing quadruple therapy), n=1537;
8. Sequential (Sequential therapy), n=1964;
9. SP (Sequential therapy with probiotics), n=189;
10. TP (Triple therapy with probiotics), n=3326.
Basic information is provided in Supplementary Table 2 .
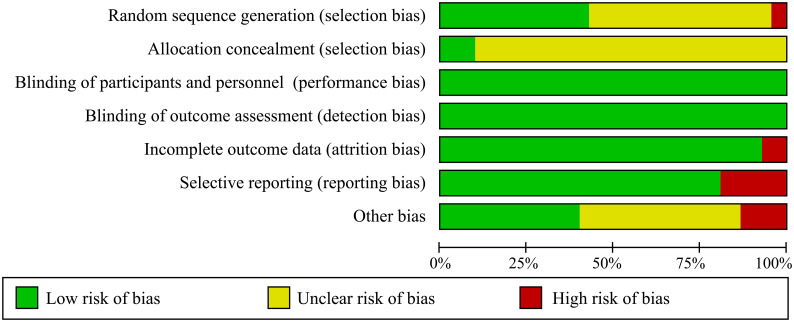
3.3. Risk assessment of bias
All studies mentioned randomness in the allocation, but some were considered “unclear” or “high risk” for unspecific allocation schemes or nonstandard allocation schemes. Eradication rate is an objective detection index and does not represent the subjective will of researchers. Therefore, performance bias and detection bias were regarded as “low risk”. Any study with drop-out cases is considered as “high risk” in selective reporting. In most comparisons, the certainty of the evidence was moderate. The overall evaluation results are shown in Figure 2 .
Figure 2.
Risk of bias graph of methodological bias.
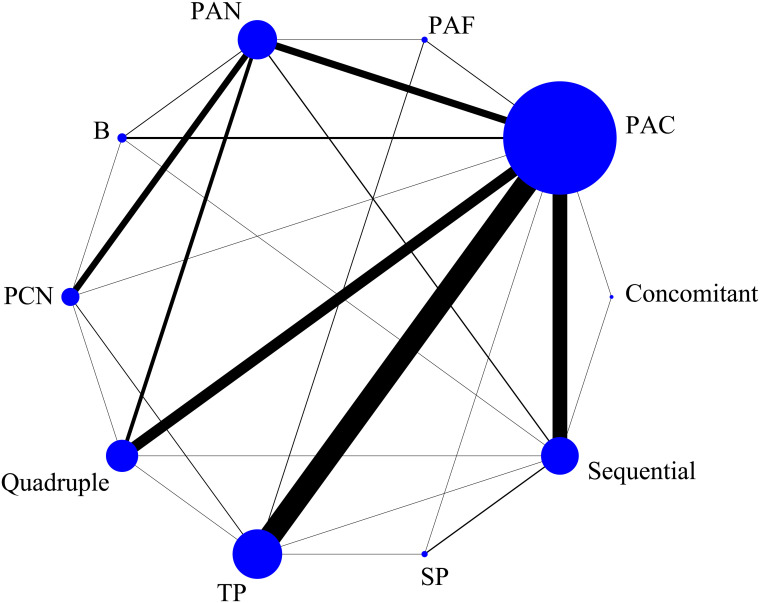
3.4. Network map
The eradication rate of H. pylori was used as the outcome measure. There are 26 direct comparisons, including closed loops, among the 10 treatment regimens in the network map. Each node in the map represents an intervention, and the lines between nodes represent a direct comparison between the two interventions. In addition, the node size represents the total sample size of the intervention, and the line thickness represents the number of studies directly compared. The results showed the standard triple therapy composed of proton-pump inhibitor (PPI), amoxicillin and clarithromycin was the most studied. The network map is shown in Figure 3 .
Figure 3.
Network graph of eradication rate. The size of the blue node is positively correlated with the sample size involved in the intervention, and the thickness of the connection between two nodes represents the number of studies. Regimen labels: B, bismuth-containing triple therapy; Concomitant, concomitant therapy; PAC, PPI, amoxicillin and clindamycin; PAF, PPI, amoxicillin and furanes; PAN, PPI, amoxicillin and nitroimidazoles; PCN, PPI, clindamycin and nitroimidazoles; Quadruple, quadruple therapy; Sequential, sequential therapy; SP, sequential therapy with probiotics; TP, triple therapy with probiotics.
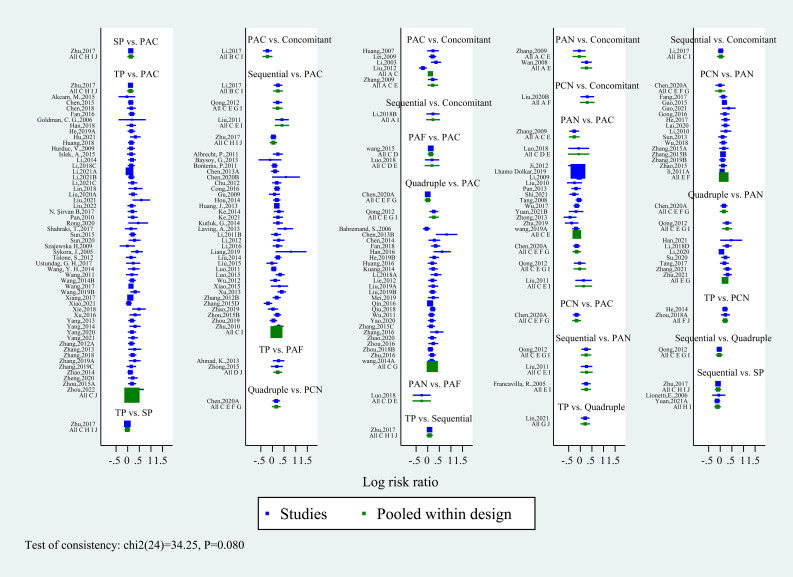
3.5. Inconsistency test
Due to multiple closed-loop structures formed by 10 intervention nodes, the inconsistency test was carried out. The results showed that the inconsistency is not significant (P=0.08), which could be analyzed by the consistency model. The inconsistency test forest map is shown in Figure 4 .
Figure 4.
Forest plot of inconsistency test for 10 eradication regimens. The results show that the inconsistency is not significant (P>0.05). Regimen labels: B, bismuth-containing triple therapy; Concomitant, concomitant therapy; PAC, PPI, amoxicillin and clindamycin; PAF, PPI, amoxicillin and furanes; PAN, PPI, amoxicillin and nitroimidazoles; PCN, PPI, clindamycin and nitroimidazoles; Quadruple, quadruple therapy; Sequential, sequential therapy; SP, sequential therapy with probiotics; TP, triple therapy with probiotics.
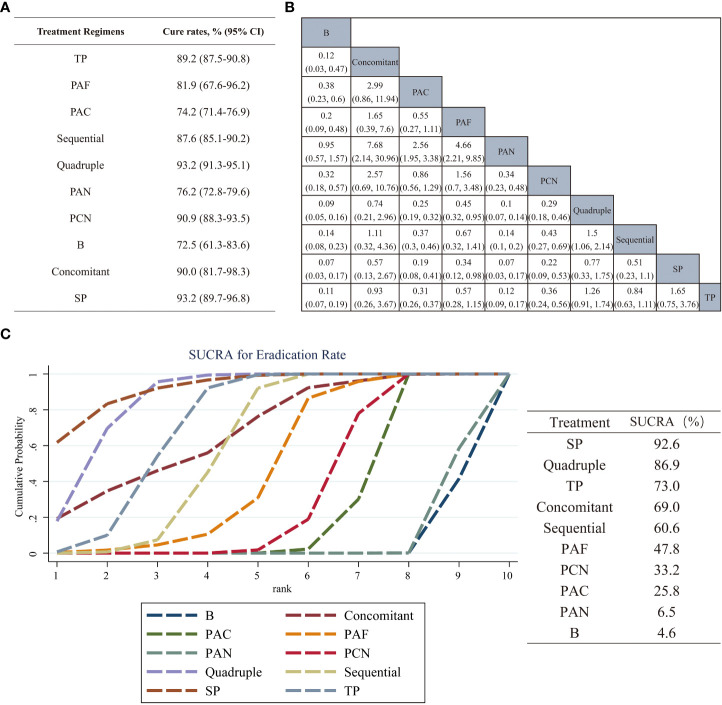
3.6. Overall cure rates and SUCRA probability ranking
A Bayesian network meta-analysis of eradication rates was performed, and the overall cure rates for each intervention were calculated and compared with each other. Figure 5A shows the overall regimen cure rates of 10 regimens. The results signified that the eradication rates of SP, quadruple, concomitant and PCN therapies in children with H. pylori were at least 90%. The eradication rates of Sequential, TP and PAF therapies were more than 80%.
Figure 5.
(A) Statistical results of the overall cure rates of 10 eradication regimens for Helicobacter pylori infection in children. (B) The comparison league table of 10 eradication programs. The values in the table are the effect sizes and 95% confidence intervals of the treatment measures in the column and the row. (C) The SUCRA was used to rank the efficacy of the 10 eradication regimens included. The larger the area under the curve, the better the therapeutic effect. Regimen labels: B, bismuth-containing triple therapy; Concomitant, concomitant therapy; PAC, PPI, amoxicillin and clindamycin; PAF, PPI, amoxicillin and furanes; PAN, PPI, amoxicillin and nitroimidazoles; PCN, PPI, clindamycin and nitroimidazoles; Quadruple, quadruple therapy; Sequential, sequential therapy; SP, sequential therapy with probiotics; TP, triple therapy with probiotics.
There was no difference in the efficacy in the pairwise comparison of PAF therapy, PCN therapy and PAC therapy. However, the effectiveness of SP therapy and quadruple therapy was significantly higher than that of the above three therapies. The comparisons of PCN therapy vs sequential therapy (OR, 0.43; 95% CI, 0.27–0.69), PCN therapy vs TP therapy (OR, 0.36; 95% CI, 0.24–0.56), PAC therapy vs sequential therapy (OR, 0.37; 95% CI, 0.3–0.46), PAC therapy vs TP therapy (OR, 0.31; 95% CI, 0.26–0.37) yielded significant results that sequential therapy and TP therapy were more effective. In contrast, the comparisons of sequential therapy vs PAF therapy, TP therapy vs PAF therapy revealed insignificant results. In terms of other comparisons, only the comparison between quadruple therapy and sequential therapy showed differences (OR, 1.5; 95% CI, 1.06 2.14). Pairwise comparison results are shown in Figure 5B .
The efficacy of the above 10 regimens was ranked, and the SUCRA values are shown in Figure 5C . The probability of different interventions was ranked as follows: SP (SUCRA 92.6%) > Quadruple (SUCRA 86.9%) > TP (SUCRA 73.0%) > Concomitant (SUCRA 69.0%) > Sequential (SUCRA 60.6%) > PAF (SUCRA 47.8%) > PCN (SUCRA 33.2%) > PAC (SUCRA 25.8%) > PAN (SUCRA 6.5%) > B (SUCRA 4.6%).
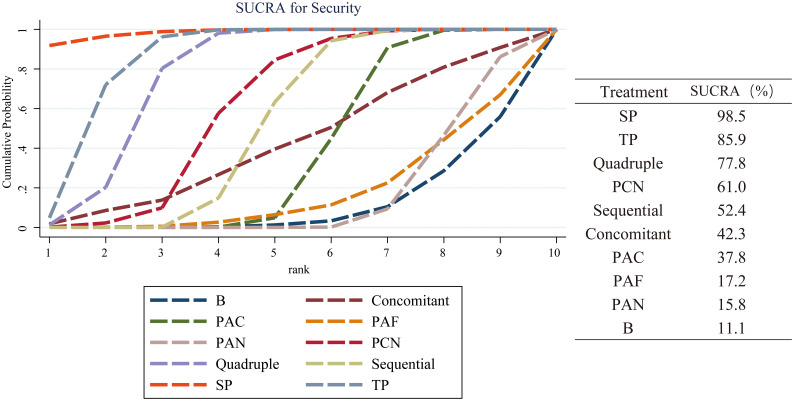
3.7. Safety outcomes
Total side effects rate was used as the outcome measure to evaluate the safety of the 10 regimens. 116 articles reported the total cases of adverse reactions in each group, including constipation, diarrhea, rash, nausea, vomiting. All reactions were at a mild level, posing no influence on the therapies. The SUCRA is shown in Figure 6 . The security ranking is as follows: SP (SUCRA 98.5%) > TP (SUCRA 85.9%) > Quadruple (SUCRA 77.8%) > PCN (SUCRA 61.0%) > Sequential (SUCRA 52.4%) > Concomitant (SUCRA 42.3%) > PAC (SUCRA 37.8%) > PAF (SUCRA 17.2%) > PAN (SUCRA 15.8%) > B (SUCRA 11.1%).
Figure 6.
Safety evaluation of 10 regimens in children with H. pylori Infection. Taking total side effects rate as the safety index of the eradication scheme, the larger the SUCRA value and the area under the curve, the higher the security of the regimens. Regimen labels: B, bismuth-containing triple therapy; Concomitant, concomitant therapy; PAC, PPI, amoxicillin and clindamycin; PAF, PPI, amoxicillin and furanes; PAN, PPI, amoxicillin and nitroimidazoles; PCN, PPI, clindamycin and nitroimidazoles; Quadruple, quadruple therapy; Sequential, sequential therapy; SP, sequential therapy with probiotics; TP, triple therapy with probiotics.
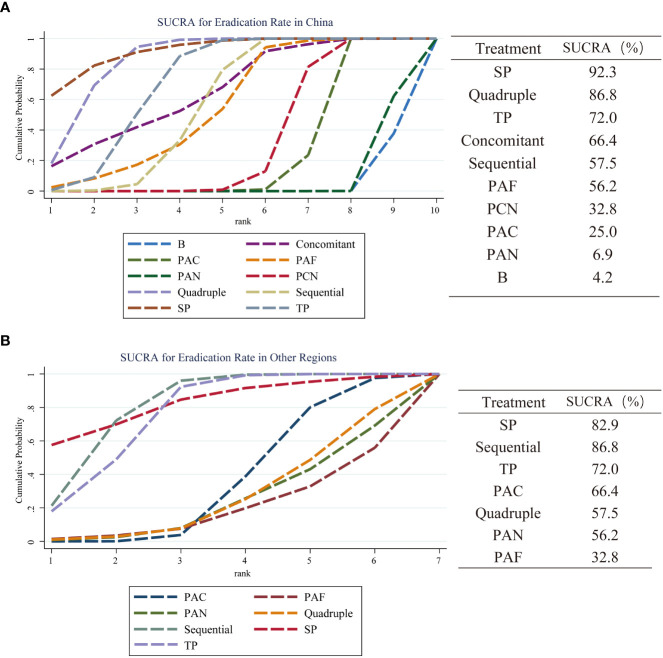
3.8. Subgroup analysis
The literature included in this study has a clear regional distribution. Therefore, a subgroup analysis was performed according to the region where eligible studies were published (China and other regions) so as to identify the most effective eradication therapy for H. pylori. The results show that SP therapy (SUCRA 92.3%) has the best curative effect in China. The ranking of other options is as follows: Quadruple (SUCRA 86.8%), TP (SUCRA 72.0%), Concomitant (SUCRA 66.4%), Sequential (SUCRA 57.5%), PAF (SUCRA 56.2%), PCN (SUCRA 32.8%), PAC (SUCRA 25.0%), PAN (SUCRA 6.9%), B (SUCRA 4.2%). In other areas, only seven regimens are mentioned, and their curative effects are ranked as follows: SP (SUCRA 82.9%) > Sequential (SUCRA 81.5%) > TP (SUCRA 76.4%) > PAC (SUCRA 36.7%) > Quadruple (SUCRA 27.4%) > PAN (SUCRA 24.9%) > PAF (SUCRA 20.2%). The results of subgroup analysis are shown in Figures 7A, B .
Figure 7.
A subgroup analysis of the eradication rate of 10 treatment regimens in different regions. (A) SUCRA value of eradication rate and ranking of curative effect in China; (B) The performance of these results in other regions. Regimen labels: B, bismuth-containing triple therapy; Concomitant, concomitant therapy; PAC, PPI, amoxicillin and clindamycin; PAF, PPI, amoxicillin and furanes; PAN, PPI, amoxicillin and nitroimidazoles; PCN, PPI, clindamycin and nitroimidazoles; Quadruple, quadruple therapy; Sequential, sequential therapy; SP, sequential therapy with probiotics; TP, triple therapy with probiotics.
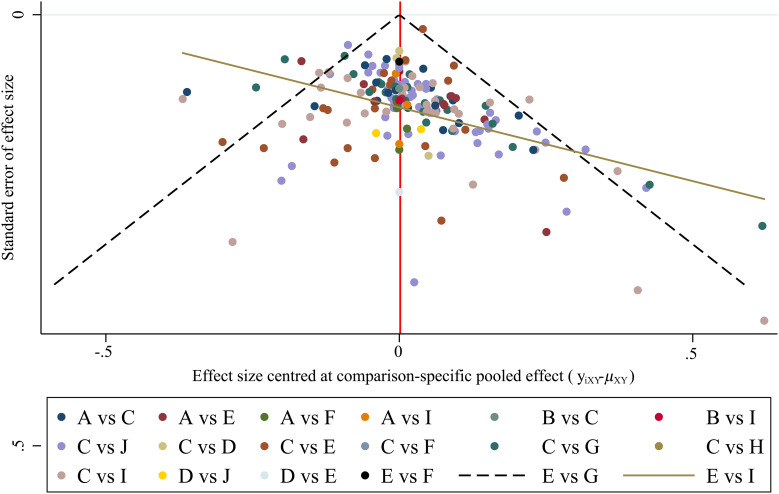
3.9. Risk of bias assessment
The bias funnel diagram is shown in Figure 8 . As can be seen, most of the studies are at the top of the funnel chart, and roughly symmetrically distributed on both sides of the vertical line, so the possibility of publication bias and small-study effect is low.
Figure 8.
The funnel plots for assessing publication bias. The bias of the included literature is tested, and the result shows that research spots are symmetrically distributed. It means that the risk of the existence of publication bias or small-study effects in this network is low.
4. Discussion
With the in-depth study of gastrointestinal diseases in recent years, Helicobacter pylori infection in children has been brought into focus, and the correlation between H. pylori infection and other systemic diseases has been gradually found (Zhang and Zhu, 2015). Due to unique characteristics of children’s growth and their own drug metabolism, H. pylori infection guidelines based on adults are not fully applicable. Since 2015, China, Occident, and Japan have successively published consensus and guidelines on the diagnosis and treatment of Helicobacter pylori infection in children, but there are differences in the recommended treatment regimens (Huang and Gong, 2015; Jones et al., 2017; Kato et al., 2020). Pediatric clinicians around the world try to use a variety of different solutions to treat Helicobacter pylori infection in children, but the eradication rate in some areas is not ideal. The present study summarized relevant published RCTs around the world, and compared and ranked the efficacy and safety of 10 reported eradication regimens for H. pylori infection in children. The overall results of these schemes show that SP therapy (SUCRA 92.6%) is the best. Quadruple therapy (SUCRA 86.9%), TP therapy (SUCRA 73.0%) and concomitant therapy (SUCRA 69.0%) are at least effective regimens. The results of the remaining regimens such as sequential therapy (SUCRA 60.6%), PAF therapy (SUCRA 47.8%), PCN therapy (SUCRA 33.2%), PAC therapy (SUCRA 25.8%), PAN therapy (SUCRA 6.5%) and B therapy (SUCRA 4.6%) are not satisfactory in this comparison. The ranking results of safety evaluation show that SP therapy and TP therapy have advantages in reducing side effects.
In the treatment of H. pylori infection in children, clinicians need to consider not only the eradication rate, but also medication compliance. Therefore, probiotics with rich tastes are widely accepted. Studies have shown that probiotics can inhibit H. pylori through a variety of immune and non-immune mechanisms, such as reducing the level of pro-inflammatory factors, increasing local IgA concentration, secreting antibacterial substances, and increasing gastric surface mucin to prevent the adhesion of pathogenic bacteria (Homan and Orel, 2015). A previous meta-analysis of 484 children found that triple therapy with Lactobacillus increased the eradication rate of H. pylori by 13% (Fang et al., 2019). The results of our analysis reveal that the addition of probiotics to sequential or triple therapy can increase the effectiveness.
Sequential therapy was initially proposed by Zullo (Zullo et al., 2000; Zullo et al., 2003), and in the subsequent prospective study, new sequential treatment was used and obtained fairly good results (eradication rate > 90%). However, the complicated medication mode of sequential therapy may lead to a decrease in children’s compliance. In contrast, the advantages of concomitant therapy on simplicity and easy operation make it successful in Korea (Lee et al., 2015). The results of the present study show that concomitant therapy merely ranks fourth (SUCRA69.0%). As a rescue treatment plan for adults after the failure of Helicobacter pylori eradication in the ACG guidelines (Chey et al., 2017), whether concomitant therapy can be used as the first treatment for children still needs careful consideration (Graham and Lee, 2015). The SUCRA values show that although the efficacy of bismuth-containing triple therapy (B, SUCRA 4.6%) is poor, the result of bismuth-containing quadruple therapy (Quadruple, SUCRA 86.9%) is unexpected, and we speculate that it is due to the addition of PPIs to this therapy. Sakurai et al. (2015) suggested that the eradication of H. pylori depended on a near-neutral gastric pH. PPIs have long been proved to effectively reduce gastric acid secretion and enhance antibiotic activity, which is conducive to the eradication of H. pylori (Miehlke et al., 1997). In the studies included in this analysis, metronidazole was used in almost all bismuth-containing triple therapy, but Graham pointed out that it needs to be combined with antisecretory drugs in the cases of metronidazole resistance (Graham et al., 2019). Judging from the results (eradication rate 93.2% vs 73.5%), quadruple therapy seems to be a good choice. However, it must be emphasized that: (1) Bismuth agents are unpalatable, such as colloidal bismuth citrate (De-Nol) has a very pungent ammonia smell, so it’s difficult for children to accept, which may eventually lead to insufficient dose or course of treatment and affect the effect. (2) The most suitable pH for bismuth to form bismuth salts and dissolve in the stomach is between 4 and 7. Concomitant administration of bismuth and antacids (PPIs such as omeprazole) will affect the formation of bismuth salts and reduce its absorption (Lambert and Midolo, 1997). (3) At present, the mechanism of bismuth to eradicate H. pylori is not completely clear. As a semi-metal or metalloid, bismuth salts have toxicity——although perhaps low toxicity——which may be one of the reasons for pediatricians to use it cautiously.
A Japanese study found that the prevalence of clarithromycin-resistant strains increased from 8.7% to 34.5% between 1997 and 2008, and the H. pylori eradication rate of triple therapy decreased from 90.6% to 74.8% (Sasaki et al., 2010). This phenomenon is still growing. Our pooled results also show that the overall eradication rate of PAC therapy is only 74%. In addition, the effect of PAN therapy is poor (eradication rate <80%), which is undoubtedly related to the rapid rise of drug resistance in this kind of drugs, especially metronidazole. The resistance rate of metronidazole in the Asia-Pacific region increased from 36% before 2000 to 45% in 2011-2015 (Savoldi et al., 2018). A new study in Chongqing, China found that the resistance rate of metronidazole in 156 children was as high as 88.4% (P = 0.0003) (Geng et al., 2022). This suggests that this therapy is likely to be abandoned by most clinicians. PAF therapy (SUCRA 47.8%) was initially ignored by us due to its low SUCRA values, but the statistical results exceeded our expectations because its overall eradication rate reached 81.9%. However, this is not a reason for us to give it a direct affirmation, because of its small sample size in this study.
In terms of safety outcomes, although not all researchers in eligible RCTs provided data of total side effects, we compared all regimens in the results of this study. According to the SUCRA values, the SP and TP therapies rank highest, suggesting that probiotics can significantly reduce the side effects of H. pylori eradication therapy. Daelemans (Daelemans et al., 2022) and Dargenio (Dargenio et al., 2022) supported our view in the latest review. Moreover, Wen (Wen et al., 2017) also found that some probiotics could effectively reduce specific side effects, which was helpful for clinical workers to adjust the medication in children.
The results of subgroup analysis show that SP therapy is the most effective regimen, and several different triple therapy regimens are the least effective regimens, whether in China or other regions. TP therapy ranks higher in both subgroups (surfaces under cumulative ranking 72.0% vs 76.4% respectively), so it may be an efficacious candidate for the treatment of H. pylori infection in children.
Generally speaking, the most important factors for successful eradication of H. pylori include resistance rate of antimicrobial, course of treatment, therapeutic regimen and patient compliance. It has been shown that eradication effects are associated with longer duration, regardless of first- or second-line treatment regimens (Yuan et al., 2013; Li et al., 2015; Muñoz et al., 2018; Yeo et al., 2018; Yeo et al., 2019). This reminds clinicians that it may be a better regimen to try to extend the dosing time rather than rush to change another one when the first formula has not achieved desired effects.
In short, the eradication rates of quadruple therapy, concomitant therapy and PCN therapy are at least 90% for empirical treatment of H. pylori infection in children. However, the results of standard triple therapy and Bismuth-containing triple therapy show that they are not completely reliable. The ranking results show that SP therapy is the most effective regimen in both China and other regions, and the ranking stability and approximately 90% eradication rate of TP therapy may be worthy of our attention. Safety evaluation shows that SP therapy and TP therapy can reduce side effects during the course of treatment in children.
The funnel plot suggests a low potential for bias, but there are still some limitations. On the one hand, only different regimens were compared, and no detailed distinction was made between different courses of treatment or doses of the same regimen. On the other hand, the number of literatures on probiotic-containing therapy accounts for a high proportion, which may have a certain impact on the analysis. In order to improve the stability and reliability of this study, in-depth research with large samples, multiple regions, and high-quality literature is still needed to optimize our results.
5. Conclusion
SP therapy is the most efficient and safest regimen for H. pylori infection in children, and TP therapy also has advantages in this network meta-analysis. Eradication rate of standard triple therapy is not exceptional.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Author contributions
ML and CZ, concept, design, research collection, data analysis, manuscript drafting. PZ, XZ, JS, data analysis, cross-checking, manuscript drafting, research collection. BY, concept, design, research collection, data analysis, manuscript review. The final submitted version has been confirmed by all authors. All authors contributed to the article and approved the submitted version.
Acknowledgments
We are grateful to Nanjing University of Chinese Medicine Library for the help in systematic search.
Glossary
| SUCRA | Surface Under the Cumulative Ranking Curve |
| H. pylori | Helicobacter pylori |
| NASPGHAN | North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition |
| ESPGHAN | European Society for Pediatric Gastroenterology, Hepatology, and Nutrition |
| MALT | mucosa-associated lymphoid tissue |
| NMA | network meta-analysis |
| PPI | Proton-pump inhibitor |
| RCT | Randomized Controlled Trial |
| UBT | urea breath test |
| SAT | ELISA stool antigen test |
| OR | odds ratio |
| CI | confidence intervals |
| B | Bismuth-containing triple therapy |
| Concomitant | Concomitant therapy |
| PAC | PPI, amoxicillin and clarithromycin |
| PAF | PPI, amoxicillin and nitrofuran drugs |
| PAN | PPI, amoxicillin and nitroimidazoles |
| PCN | PPI, clarithromycin and nitroimidazoles |
| Quadruple | Bismuth-containing quadruple therapy |
| Sequential | Sequential therapy |
| SP | Sequential therapy with probiotics |
| TP | Triple therapy with probiotics |
Funding Statement
This work is supported by TCM evidence-based capacity building project (2019XZZX-ek003) and National Natural Science Foundation, China (82174436).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2023.1068809/full#supplementary-material
References
- Ahmad K., Fatemeh F., Mehri N., Maryam S. (2013). Probiotics for the treatment of pediatric helicobacter pylori infection: A randomized double blind clinical trial. Iran J. Pediatr. 23 (1), 79–84. [PMC free article] [PubMed] [Google Scholar]
- Akcam M., Koca T., Salman H., Karahan N. (2015). The effects of probiotics on treatment of helicobacter pylori eradication in children. Saudi Med. J. 36 (3), 286–290. doi: 10.15537/smj.2015.3.10124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht P., Kotowska M., Szajewska H. (2011). Sequential therapy compared with standard triple therapy for helicobacter pylori eradication in children: A double-blind, randomized, controlled trial. J. Pediatr. 159 (1), 45–49. doi: 10.1016/j.jpeds.2011.01.023 [DOI] [PubMed] [Google Scholar]
- Bahremand S., Nematollahi L. R., Fourutan H., Tirgari F., Nouripour S., Mir E., et al. (2006). Evaluation of triple and quadruple helicobacter pylori eradication therapies in Iranian children: A randomized clinical trial. Eur. J. Gastroenterol. Hepatol. 18 (5), 511–514. doi: 10.1097/00042737-200605000-00009 [DOI] [PubMed] [Google Scholar]
- Baysoy G., Saltık Temızel İN., Uslu N., Balamtekın N., Demır H., Gürkan F., et al. (2013). Ornidazole-based sequential therapy is not effective in helicobacter pylori eradication in children. Turkish J. gastroenterol: Off. J. Turkish Soc. Gastroenterol. 24 (5), 382–386. doi: 10.4318/tjg.2013.0575 [DOI] [PubMed] [Google Scholar]
- Bontems P., Kalach N., Oderda G., Salame A., Muyshont L., Miendje D. Y., et al. (2011). Sequential therapy versus tailored triple therapies for helicobacter pylori infection in children. J. Pediatr. Gastroenterol. Nutr. 53 (6), 646–650. doi: 10.1097/MPG.0b013e318229c769 [DOI] [PubMed] [Google Scholar]
- Burucoa C., Axon A. (2017). Epidemiology of helicobacter pylori infection. Helicobacter 22 (Suppl 1). doi: 10.1111/hel.12403 [DOI] [PubMed] [Google Scholar]
- Cavestro C., Prandi G., Manildo M., Martini S., Genovesi C., Premoli A., et al. (2022). A cross-sectional study on the association between helicobacter pylori infection and headache. Neurolog sciences: Off. J. Ital. Neurolog Soc. Ital. Soc. Clin. Neurophysiol. 43 (10), 6031–6038. doi: 10.1007/s10072-022-06153-1 [DOI] [PubMed] [Google Scholar]
- Chen C. (2013). Comparison of curative effects of different therapies in the treatment of helicobacter pylori-associated gastritis in children. Chin. J. Mod Drug Appl. 7 (23), 27–28. doi: 10.14164/j.cnki.cn11-5581/r.2013.23.188 [DOI] [Google Scholar]
- Chen L. (2015). The research of drug treatment for children infected with hp. Chin. J. Integr. Trad West Med. Dig. 23 (12), 865–867. doi: 10.3969/j.issn.1671-038X.2015.12.10 [DOI] [Google Scholar]
- Chen Q., Li Y., Cen Y. (2013). Efficacy of quadruple therapy in the treatment of peptic ulcer in children with helicobacter pylori infection. Youjiang Med. J. 41 (03), 354–355. [Google Scholar]
- Chen Z., Liu L., You J., Ouyang H. (2014). Efficacy observation of omeprazole combined with antibiotics in the treatment of peptic ulcer in children. J. Clin. Res. 5, 1008–1009. [Google Scholar]
- Chen J., Xu X., Yang H., Liu X. (2018). Observation on the effect of bifidobacterium triple viable bacteria powder synergistically treating peptic ulcer in children. People’s Military Surgeon 61 (6), 532–535. [Google Scholar]
- Chen X., Zheng X., Lin G., Du B. (2020). Effects of triple therapy, sequential therapy and reversed sequential therapy on helicobacter pylori infection among children. Chin. J. Exp. Clin. Infect. Dis. 14 (06), 490–495. [Google Scholar]
- Chen J., Zhuo Z., Wang X., Wang M., Su Y., Hong S. (2020). Drug resistance of helicobacter pylori infection in school-age children in xiamen and effects of four treatment options. Chin. J. Nosocomiol 30 (19), 3031–3035. [Google Scholar]
- Chey W. D., Leontiadis G. I., Howden C. W., Moss S. F. (2017). Acg clinical guideline: Treatment of helicobacter pylori infection. Am. J. Gastroenterol. 112 (2), 212–239. doi: 10.1038/ajg.2016.563 [DOI] [PubMed] [Google Scholar]
- Chu X. (2012). Clinical observation of 10-day sequential therapy with omeprazole, clarithromycin, amoxicillin-clavulanate potassium and standard triple therapy for eradication of helicobacter pylori. Health Required 7 (367), 230. [Google Scholar]
- Cong X., Hu C. (2016). Curative effect analysis of sequential therapy and traditional therapy in treatment of anti-helicobacter pylori. J. China Med. Pharm. 6 (17), 205–207. [Google Scholar]
- Cumpston M., Li T., Page M. J., Chandler J., Welch V. A., Higgins J. P., et al. (2019). Updated guidance for trusted systematic reviews: A new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database systemat Rev. 10, Ed000142. doi: 10.1002/14651858.Ed000142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czinn S. J. (2005). Helicobacter pylori infection: Detection, investigation, and management. J. Pediatr. 146 (3 Suppl), S21–S26. doi: 10.1016/j.jpeds.2004.11.037 [DOI] [PubMed] [Google Scholar]
- Daelemans S., Deseck V., Levy E. I., Vandenplas Y. (2022). Are pro- and/or synbiotics beneficial in helicobacter pylori eradication therapy in children? a narrative review. Eur. J. Pediatr. 181 (9), 3225–3234. doi: 10.1007/s00431-022-04523-7 [DOI] [PubMed] [Google Scholar]
- Dargenio V. N., Cristofori F., Dargenio C., Giordano P., Indrio F., Celano G., et al. (2022). Use of limosilactobacillus reuteri dsm 17938 in paediatric gastrointestinal disorders: An updated review. Beneficial Microbes 13 (3), 221–242. doi: 10.3920/bm2021.0151 [DOI] [PubMed] [Google Scholar]
- Daugule I., Karklina D., Rudzite D., Remberga S., Rumba-Rozenfelde I. (2016). Prevalence of helicobacter pylori infection among preschool children in Latvia: No significant decrease in prevalence during a ten year period. Scand J. Public Health 44 (4), 418–422. doi: 10.1177/1403494816631861 [DOI] [PubMed] [Google Scholar]
- Dias S., Welton N. J., Caldwell D. M., Ades A. E. (2010). Checking consistency in mixed treatment comparison meta-analysis. Stat. Med. 29 (7-8), 932–944. doi: 10.1002/sim.3767 [DOI] [PubMed] [Google Scholar]
- Dong Y., Zhang Z. (2022). Interpretation of the guidelines for the management of helicobacter pylori infection in Eastern and Western children. Chin. J. Gastroenterol. Hepatol. 31 (03), 336–342. [Google Scholar]
- Fan X. (2018). Comparison of the clinical efficacy of different omeprazole therapy on children with peptic ulcer complicated with helicobacter pylori infection. J. Anti-Infect Pharm. 15 (10), 1790–1791. doi: 10.13493/j.issn.1672-7878.2018.10-048 [DOI] [Google Scholar]
- Fan J., Li M., Liao J. (2016). Clinical effect of probiotics combined with triple therapy in the treatment of helicobacter pylori infection in children. J. J. Modern Clin. Med. 42 (2), 114–116. doi: 10.11851/j.issn.1673-1557.2016.02.011 [DOI] [Google Scholar]
- Fang Z., Li Z. (2017). Clinical effect of different components of triple therapy in the treatment of peptic ulcer in children. J. J. Shenyang Med. Coll. 19 (4), 333–335. doi: 10.16753/j.cnki.1008-2344.2017.04.011 [DOI] [Google Scholar]
- Fang H. R., Zhang G. Q., Cheng J. Y., Li Z. Y. (2019). Efficacy of lactobacillus-supplemented triple therapy for helicobacter pylori infection in children: A meta-analysis of randomized controlled trials. Eur. J. Pediatr. 178 (1), 7–16. doi: 10.1007/s00431-018-3282-z [DOI] [PubMed] [Google Scholar]
- Francavilla R., Lionetti E., Castellaneta S. P., Magistà A. M., Boscarelli G., Piscitelli D., et al. (2005). Improved efficacy of 10-day sequential treatment for helicobacter pylori eradication in children: A randomized trial. Gastroenterology 129 (5), 1414–1419. doi: 10.1053/j.gastro.2005.09.007 [DOI] [PubMed] [Google Scholar]
- Gao L., Wang J., Yan F., Zhang X., Wang J., Feng L., et al. (2015). Clarithromycin and amoxicillin combined with omeprazole and metronidazole, respectively comparison of the effects of treating peptic ulcer in children. Chin. J. Clin. Rational Drug Use 8 (01), 104–105. doi: 10.15887/j.cnki.13-1389/r.2015.01.061 [DOI] [Google Scholar]
- Gao M. (2021). Clinical observation of clarithromycin triple therapy in the treatment of helicobacter pylori infection of peptic ulcer in children. Sci. Regimen 24 (3), 159. [Google Scholar]
- Geng T., Yu Z. S., Zhou X. X., Liu B., Zhang H. H., Li Z. Y. (2022). Antibiotic resistance of helicobacter pylori isolated from children in chongqing, China. Eur. J. Pediatr. 181 (7), 2715–2722. doi: 10.1007/s00431-022-04456-1 [DOI] [PubMed] [Google Scholar]
- Goldman C. G., Barrado D. A., Balcarce N., Rua E. C., Oshiro M., Calcagno M. L., et al. (2006). Effect of a probiotic food as an adjuvant to triple therapy for eradication of helicobacter pylori infection in children. Nutr. (Burbank Los Angeles County Calif) 22 (10), 984–988. doi: 10.1016/j.nut.2006.06.008 [DOI] [PubMed] [Google Scholar]
- Gong Y. (2016). Clinical effect of triple therapy on gastritis caused by helicobacter pylori in children. China Health Care Nutr. 26 (19), 167–168. [Google Scholar]
- Goodwin C. S., Armstrong J. A., Chilvers T., Peters M., Collins M. D., Sly L., et al. (1989). Transfer of campylobacter pylori and campylobacter mustelae to helicobacter gen. nov. as helicobacter pylori comb. nov. and helicobacter mustelae comb. nov., respectively. Int. J. Syst. Bacterial 39 (4), 397–405. doi: 10.1099/00207713-39-4-397 [DOI] [Google Scholar]
- Graham D. Y., Lee S. Y. (2015). How to effectively use bismuth quadruple therapy: The good, the bad, and the ugly. Gastroenterol. Clinics North America 44 (3), 537–563. doi: 10.1016/j.gtc.2015.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D. Y., Lu H., Dore M. P. (2019). Relative potency of proton-pump inhibitors, helicobacter pylori therapy cure rates, and meaning of double-dose PPI. Helicobacter. Feb 24 (1), e12554. doi: 10.1111/hel.12554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H. (2016). Observation on the curative effect of quadruple therapy containing bismuth in the treatment of peptic ulcer in children. Modern Digestion Intervent 21 (2), 334–335. doi: 10.3969/j.issn.1672-2159.2016.02.063 [DOI] [Google Scholar]
- Han Z. (2018). Eradication rate and development of probiotics in children with hp infection. J. J. Clin. Med. Literature 5 (44), 924. doi: 10.3877/j.issn.2095-8242.2018.44.051 [DOI] [Google Scholar]
- Han D. (2021). Efficacy of bismuth quadruple therapy in the treatment of hp positive children with gastritis. J. Chin. J. Modern Drug Appl. 15 (22), 180–182. doi: 10.14164/j.cnki.cn11-5581/r.2021.22.071 [DOI] [Google Scholar]
- He A. (2014). Observation of curative effect of probiotics combined with triple therapy in radical treatment of helicobacter pylori infection in children. World Health Digest. 19 (2), 122. [Google Scholar]
- He S. (2017). Therapeutic methods and effect evaluation of helicobacter pylori-associated gastritis in children. J. Guide China Med. 15 (29), 189. [Google Scholar]
- He R., Yu D., He Y., Li W., Song X. (2019). Clinical effect of bismuth potassium citrate combined with omeprazole quadruple therapy in the treatment of upper peptic ulcer in children and its correlation with serum mda, lpo and no. J. Clin. Misdiagnosis Misther 32 (6), 23–27. doi: 10.3969/j.issn.1002-3429.2019.06.007 [DOI] [Google Scholar]
- Homan M., Orel R. (2015). Are probiotics useful in helicobacter pylori eradication? World J. Gastroenterol. 21 (37), 10644–10653. doi: 10.3748/wjg.v21.i37.10644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooi J. K. Y., Lai W. Y., Ng W. K., Suen M. M. Y., Underwood F. E., Tanyingoh D., et al. (2017). Global prevalence of helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology 153 (2), 420–429. doi: 10.1053/j.gastro.2017.04.022 [DOI] [PubMed] [Google Scholar]
- Hou F. (2014). Clinical efficacy of four kinds drug oral medicine for 10 days sequential therapy for eradication of helicobacter pylori infection of children. Clin. Med. China, 1220–1222. [Google Scholar]
- Hu W. (2021). Study on the therapeutic value of bifidobacterium quadruple viable tablets combined with triple therapy on children with helicobacter pylori infection. J. Chin. Community Doctors 37 (16), 24–25. doi: 10.3969/j.issn.1007-614x.2021.16.010 [DOI] [Google Scholar]
- Huang Z. (2007). A comparative observation on the curative effect of triple therapy in the treatment of peptic ulcer in children. J. Modern Pract. Med. 19 (6), 457–458. doi: 10.3969/j.issn.1671-0800.2007.06.016 [DOI] [Google Scholar]
- Huang F. (2016). Effect of bismuth potassium citrate capsules on eradication rate of helicobacter pylori in children with peptic ulcer. J. Heilongjiang Med. J. 40 (3), 252–253. doi: 10.3969/j.issn.1004-5775.2016.03.028 [DOI] [Google Scholar]
- Huang H. (2018). Clinical study on the eradication of helicobacter pylori by lactobacillus acidophilus in children with abdominal pain. J. Jilin Med. J. 39 (4), 681–682. doi: 10.3969/j.issn.1004-0412.2018.04.036 [DOI] [Google Scholar]
- Huang Y., Gong S. (2015). Expert consensus on the diagnosis and treatment of helicobacter pylori infection in children. J. Chin. J. Pediatr. 53 (7), 492–495. doi: 10.3760/cma.j.issn.0578-1310.2015.07.005 [DOI] [PubMed] [Google Scholar]
- Huang J., Zhou L., Geng L., Yang M., Xu X. W., Ding Z. L., et al. (2013). Randomised controlled trial: Sequential vs. standard triple therapy for helicobacter pylori infection in Chinese children-a multicentre, open-labelled study. Alimentary Pharmacol. Ther. 38 (10), 1230–1235. doi: 10.1111/apt.12516 [DOI] [PubMed] [Google Scholar]
- Hurduc V., Plesca D., Dragomir D., Sajin M., Vandenplas Y. (2009). A randomized, open trial evaluating the effect of saccharomyces boulardii on the eradication rate of helicobacter pylori infection in children. Acta Paediatrica 98 (1), 127–131. doi: 10.1111/j.1651-2227.2008.00977.x [DOI] [PubMed] [Google Scholar]
- He J., Wang W., Gao C. (2019). Treatment of helicobacter pylori infection in children with saccharomyces boulardii powder combined with triple therapy. J. Chin. J. Microecol 31 (2), 171–173. [Google Scholar]
- Islek A., Sayar E., Yilmaz A., Artan R. (2015). Bifidobacterium lactis B94 plus inulin for treatment of helicobacter pylori infection in children: Does it increase eradication rate and patient compliance? Acta Gastro-enterolog Belgica 78 (3), 282–286. [PubMed] [Google Scholar]
- Ji X. (2012). Comparative analysis of two treatment regimens in children with helicobacter pylori infection with peptic ulcer. J. Chin. Foreign Med. Res. 10 (13), 20–21. doi: 10.3969/j.issn.1674-6805.2012.13.011 [DOI] [Google Scholar]
- Jones N. L., Koletzko S., Goodman K., Bontems P., Cadranel S., Casswall T., et al. (2017). Joint Espghan/Naspghan guidelines for the management of helicobacter pylori in children and adolescents (Update 2016). J. Pediatr. Gastroenterol. Nutr. 64 (6), 991–1003. doi: 10.1097/mpg.0000000000001594 [DOI] [PubMed] [Google Scholar]
- Kato S., Shimizu T., Toyoda S., Gold B. D., Ida S., Ishige T., et al. (2020). The updated jspghan guidelines for the management of helicobacter pylori infection in childhood. Pediatr. international: Off. J. Japan Pediatr. Soc. 62 (12), 1315–1331. doi: 10.1111/ped.14388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Y. (2014). Comparing the efficacy of sequential therapy and triple therapy in children with helicobacter pylori infection. J. Chin. J. Postgraduates Med. 37 (10), 68–69. doi: 10.3760/cma.j.issn.1673-4904.2014.10.025 [DOI] [Google Scholar]
- Ke Y., Wu J., Gu Q. (2021). Cost-effectiveness evaluation of sequential therapy and standard triple therapy in eradication of helicobacter pylori infection in children. J. Int. J. Pathol. Clin. Med. 41 (11), 2632–2637. doi: 10.3978/j.issn.2095-6959.2021.11.022 [DOI] [Google Scholar]
- Ku C., Cai X., Ou S., Zou C., Chen T., Fang J. (2014). An efficacy and safety study on the quadruple therapy of peptic ulcer in chlidren. J. J. Bethune Military Med. Coll. 3, 222–223. [Google Scholar]
- Kutluk G., Tutar E., Bayrak A., Volkan B., Akyon Y., Celikel C., et al. (2014). Sequential therapy versus standard triple therapy for helicobacter pylori eradication in children: Any advantage in clarithromycin-resistant strains? Eur. J. Gastroenterol. Hepatol. 26 (11), 1202–1208. doi: 10.1097/meg.0000000000000190 [DOI] [PubMed] [Google Scholar]
- Lai W., Liang B. (2020). Clinical effect of clarithromycin triple therapy in the treatment of peptic ulcer in children with helicobacter pylori infection. J. Jilin Med. J. 41 (5), 1145–1146. doi: 10.3969/j.issn.1004-0412.2020.05.046 [DOI] [Google Scholar]
- Lambert J. R., Midolo P. (1997). The actions of bismuth in the treatment of helicobacter pylori infection. Alimentary Pharmacol. Ther. 27–33,11 Suppl 1. doi: 10.1046/j.1365-2036.11.s1.13.x [DOI] [PubMed] [Google Scholar]
- Laving A., Kamenwa R., Sayed S., Kimang’a A. N., Revathi G. (2013). Effectiveness of sequential v. standard triple therapy for treatment of helicobacter pylori infection in children in Nairobi, Kenya. South Afr. Med. J. = Suid-Afrikaanse tydskrif vir geneeskunde 103 (12), 921–924. doi: 10.7196/samj.7012 [DOI] [PubMed] [Google Scholar]
- Lhamo D., Tinley W. (2019). Clinical effect of traditional triple therapy on helicobacter pylori (Hp) positive children with gastritis. World Latest Med. Inf. 67–68. [Google Scholar]
- Lee H. J., Kim J. I., Lee J. S., Jun E. J., Oh J. H., Cheung D. Y., et al. (2015). Concomitant therapy achieved the best eradication rate for helicobacter pylori among various treatment strategies. World J. Gastroenterol. 21 (1), 351–359. doi: 10.3748/wjg.v21.i1.351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X. (2009). Observation of curative effect of triple therapy on helicobacter pylori infection. J. modern J. integrated traditional Chin. Western Med. 18 (11), 1221–1223. doi: 10.3969/j.issn.1008-8849.2009.11.008 [DOI] [Google Scholar]
- Li Z. (2018). Treatment of helicobacter pylori in children by different first-line therapy. J China Medical University. [Google Scholar]
- Li Y. (2003). Treatment of helicobacter pylori infection in children with different drugs. J. J. Heze Med. Coll. 15 (4), 33–34. doi: 10.3969/j.issn.1008-4118.2003.04.014 [DOI] [Google Scholar]
- Li F. (2009). Clinical analysis of two kinds of triple therapy in the treatment of helicobacter pylori infection. J. modern Med. Health 25 (14), 2143. [Google Scholar]
- Li J. (2010). Observation on the curative effect of different triple therapy in the treatment of peptic ulcer in children. J. China Foreign Med. Treat 29 (23), 118–119. doi: 10.3969/j.issn.1674-0742.2010.23.085 [DOI] [Google Scholar]
- Li G. (2017). Comparison of curative effect of three kinds of therapies for eradication of helicobacter pylori in children. J. Tianjin Pharm. 29 (2), 45–46. doi: 10.3969/j.issn.1006-5687.2017.02.015 [DOI] [Google Scholar]
- Li M. (2018). Comparative analysis of curative effect of different treatment regimens in the treatment of children with helicobacter pylori infection with peptic ulcer. J. World Latest Med. 96, 327–328. [Google Scholar]
- Li J. (2018). Evaluation of efficacy and safety of triple therapy combined with probiotics in treatment of hp related gastritis in children. J. J. Snake 30 (4), 632–633. doi: 10.3969/j.issn.1001-5639.2018.04.028 [DOI] [Google Scholar]
- Li H. (2020). Comparison of the efficacy of quadruple therapy containing bismuth and traditional triple therapy in the treatment of children with gastritis caused by helicobacter pylori infection. J. Contemp. Med. Forum 18 (17), 135–136. doi: 10.3969/j.issn.2095-7629.2020.17.101 [DOI] [Google Scholar]
- Li X. (2021. a). Clinical observation of probiotic-assisted triple standard regimen in the treatment of children with helicobacter pylori infection. J. J. Huaihai Med. 39 (6), 636–639. doi: 10.14126/j.cnki.1008-7044.2021.06.026 [DOI] [Google Scholar]
- Li X. (2021. b). Clinical efficacy of bifidobacterium triple viable bacteria powder synergistically treating helicobacter pylori (Hp) positive peptic ulcer in children. J. Strait Pharm. J. 33 (2), 143–145. doi: 10.3969/j.issn.1006-3765.2021.02.055 [DOI] [Google Scholar]
- Li L., Lin Q. (2012). Clinical analysis of 10-day sequential therapy for helicobacter pylori infection children. modern preventive medicine. J. Modern Prev. Med. 39 (10), 2440–2441. [Google Scholar]
- Li X., Qi H. (2021). Analysis of effect of adjuvant treatment with bifidobacterial quadruple viable tablets in child patients with hp positive of peptic ulcer. J. J. Med. Forum 42 (18), 17–21. [Google Scholar]
- Li J., Shi Y. (2018). Comparative analysis of the effect of bismuth quadruple therapy and traditional triple therapy on helicobacter pylori positive children with gastritis. J. Med. Diet Health 9, 52. [Google Scholar]
- Li B. Z., Threapleton D. E., Wang J. Y., Xu J. M., Yuan J. Q., Zhang C., et al. (2015). Comparative effectiveness and tolerance of treatments for helicobacter pylori: Systematic review and network meta-analysis. BMJ 351, h4052. doi: 10.1136/bmj.h4052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Yu J. (2011). Efficacy of sequential therapy with furazolidone for eradication of helicobacter pylori. J. J. Clin. Med. Pract. 15 (17), 132–133. doi: 10.3969/j.issn.1672-2353.2011.17.048 [DOI] [Google Scholar]
- Li X., Zhang X., Huang L., Zou S. (2011). Clinical study of triple therapy in the treatment of children with hp-related gastritis. J. Contemp. Med. Forum 17 (34), 5–6. doi: 10.3969/j.issn.1009-4393.2011.34.003 [DOI] [Google Scholar]
- Li J., Zhang G., Sun D., Liu J. (2016). Comparison of curative effect of two therapies for helicobacter pylori infection in children. Modern Med. J. China 18 (3), 50–52. doi: 10.3969/j.issn.1672-9463.2016.03.012 [DOI] [Google Scholar]
- Li M., Zhou X. (2014). Observation on the efficacy of triple therapy combined with probiotics in the treatment of children with helicobacter pylori infection. J. Modern J. Integrated Traditional Chin. Western Med. 26, 2883–2884. doi: 10.3969/j.issn.1008-8849.2014.26.013 [DOI] [Google Scholar]
- Liang M. (2019). Clinical efficacy of sequential therapy in the treatment of helicobacter pylori infection in children. J. J. Clin. Med. Pract. 23 (5), 56–59. doi: 10.7619/jcmp.201905015 [DOI] [Google Scholar]
- Lie Y. (2012). Efficacy and safety of quadruple therapy in the treatment of peptic ulcer with helicobacter pylori infection in children. J. Chin. J. Pract. Med. 39 (23), 9–10. doi: 10.3760/cma.j.issn.1674-4756.2012.23.004 [DOI] [Google Scholar]
- Lin L., Huang Y. (2018). Clinical efficacy of probiotics combined with triple therapy in the treatment of chronic gastritis associated with helicobacter pylori in children. J Clin Rational Drug Use 667, 64–65. [Google Scholar]
- Lionetti E., Miniello V. L., Castellaneta S. P., Magistá A. M., de Canio A., Maurogiovanni G., et al. (2006). Lactobacillus reuteri therapy to reduce side-effects during anti-helicobacter pylori treatment in children: A randomized placebo controlled trial. Alimentary Pharmacol. Ther. 24 (10), 1461–1468. doi: 10.1111/j.1365-2036.2006.03145.x [DOI] [PubMed] [Google Scholar]
- Liu G. (2010). Clinical analysis of helicobacter pylori infection in children. J. Chin. J. misdiagnostics 10 (33), 8174. [Google Scholar]
- Liu Y. (2012). Observation on the effect of triple therapy in the treatment of helicobacter pylori-associated gastritis in children. J. Chin. J. Misdiagnostics 12 (13), 3195–3196. [Google Scholar]
- Liu X. (2014). Clinical value of sequential therapy and standard triple therapy in the treatment of children with helicobacter pylori. World Health Digest 27, 102–690. [Google Scholar]
- Liu X. (2021). Comparison of clinical effects of probiotic-assisted triple therapy and bismuth quadruple therapy in children with chronic gastritis. J. Chin. J. Clin. Rational Drug Use 14 (35), 141–143. doi: 10.15887/j.cnki.13-1389/r.2021.35.057 [DOI] [Google Scholar]
- Liu J., Chen Q., Li X., Chen J. (2019). Analysis of the clinical effect of different omeprazole therapy on children with peptic ulcer complicated with helicobacter pylori infection. J. J. Med. Aesthetice Cosmetology 28 (1), 88. [Google Scholar]
- Liu C., Fang Z. (2019). A comparative study on the clinical efficacy of quadruple therapy and traditional triple therapy in the treatment of children with gastritis complicated with peptic ulcer. J. Modern Digestion Intervent 24 (3), 268–270. doi: 10.3969/j.issn.1672-2159.2019.03.013 [DOI] [Google Scholar]
- Liu Y., Hu C., Lu M., Wang L. (2011). Effects of sequential therapy for helicobacter pylori eradication in children. J. Chin. J. Gen. Pract. 09 (6), 90675. [Google Scholar]
- Liu J., Li W., Jia L., Zhao X., Jia B. (2022). Clinical effect of triple therapy combined with probiotics on children with helicobacter pylori infection and its influence on children’s growth and development. China Med. Pharm. 17 (01), 76–79. [Google Scholar]
- Liu H., Wu W. (2021). Observation on the effect of triple therapy combined with yeast in the treatment of peptic ulcer in children. J. Shenzhen J. Integrated Traditional Chin. Western Med. 31 (21), 116–118. doi: 10.16458/j.cnki.1007-0893.2021.21.046 [DOI] [Google Scholar]
- Liu Y., Xie L., Du J. (2015). Observation on the curative effect of three therapies for eradication of helicobacter pylori infection in children. J. Chin. J. Clin. 6, 79–82. doi: 10.3969/j.issn.2095-8552.2015.06.033 [DOI] [Google Scholar]
- Liu G., Yu L., Jia H., Gu X. (2014). Progress of indirect comparison and network meta-analysis method research in systematic reviews. Chin. J. Evid-based Med. 14 (10), 1276–1280. [Google Scholar]
- Liu C., Zhang Z. (2020). Efficacy and safety of colloidal bismuth potassium subcitrate and omeprazole combined with antibiotics in the treatment of children with peptic ulcer. J. China Pharm. 29 (13), 90–92. doi: 10.3969/j.issn.1006-4931.2020.13.025 [DOI] [Google Scholar]
- Liu Y. (2020). Clinical observation of triple therapy combined with saccharomyces boulardii powder in the treatment of helicobacter pylori infection in children. Chin. J. Clin. Rational Drug Use, 55–56. [Google Scholar]
- Liu F., Zhen L., Zhao Y., Xia Z., Situ A., Zhang S. (2011). The efficacy of sequential therapy and standard triple therapy for eradication of helicobacter pylori infection in children. J. J. Clin. Pediatr. 29 (10), 925–928. doi: 10.3969/j.issn.1000-3606.2011.10.007 [DOI] [Google Scholar]
- Luo C. (2018). The clinical research on eradicating Helicobacter pylori . Children by Fuarzolidone-Contained Triple Therapy (Chongqing Medical University; ). [Google Scholar]
- Luo W., Liu Z., He Y., He X., Peng S. (2015). Comprehensive curative effect evaluation on sequential therapy for eradication of helicobacter pylori infection in children. Clin. Medication J. 13 (06), 48–51. [Google Scholar]
- Mei L., Jia Z., Yang Y., Su L. (2019). Efficacy of bismuth-containing quadruple therapy on peptic ulcer in children with helicobacter pylori positive. J. Modern Digestion Intervent 24 (4), 395–398. doi: 10.3969/j.issn.1672-2159.2019.04.017 [DOI] [Google Scholar]
- Miehlke S., Mannes G. A., Lehn N., Hele C., Stolte M., Bayerdörffer E. (1997). An increasing dose of omeprazole combined with amoxycillin cures helicobacter pylori infection more effectively. Aliment Pharmacol. Ther. Apr 11 (2), 323–329. doi: 10.1046/j.1365-2036.140316000.x [DOI] [PubMed] [Google Scholar]
- Muñoz N., Sánchez-Delgado J., Baylina M., Puig I., López-Góngora S., Suarez D., et al. (2018). Systematic review, meta-analysis, and meta-regression: Successful second-line treatment for helicobacter pylori. Helicobacter 23 (3), e12488. doi: 10.1111/hel.12488 [DOI] [PubMed] [Google Scholar]
- Pan T., Zhu H., Lu H. (2010). Efficacy observation of probiotic-assisted triple therapy in the treatment of children with helicobacter pylori infection. J. strait Pharm. J. 22 (2), 109–110. doi: 10.3969/j.issn.1006-3765.2010.02.054 [DOI] [Google Scholar]
- Pan Y. (2013). Efficacy observation of clarithromycin triple therapy in the treatment of hp positive peptic ulcer in children. Natl. Med. Front. China 8 (08), 66. [Google Scholar]
- Plummer M., Franceschi S., Vignat J., Forman D., de Martel C. (2015). Global burden of gastric cancer attributable to helicobacter pylori. Int. J. Cancer 136 (2), 487–490. doi: 10.1002/ijc.28999 [DOI] [PubMed] [Google Scholar]
- Qin P. (2016). Study on drug treatment of children with helicobacter pylori infection with peptic ulcer. J. Chin. J. Trauma Disability Med. 24 (2), 111–112. doi: 10.13214/j.cnki.cjotadm.2016.02.084 [DOI] [Google Scholar]
- Qiu X., Li H., Lin J. (2018). Clinical observation of quadruple therapy in the treatment of peptic ulcer in children with helicobacter pylori infection. Chin. J. Clin. Rational Drug Use 11 (23), 72–73. doi: 10.15887/j.cnki.13-1389/r.2018.23.044 [DOI] [Google Scholar]
- Rong H. (2012). Analysis of the effect of sequential therapy in the treatment of children with helicobacter pylori infection. J. Clin. Misdiagnosis Misther 25 (12), 78–80. doi: 10.3969/j.issn.1002-3429.2012.12.031 [DOI] [Google Scholar]
- Rong J., Xiao X. (2020). Clinical study of probiotic-assisted triple therapy in the treatment of children with chronic gastritis complicated with helicobacter pylori infection. Med. Forum 24 (14), 1959–1960. doi: 10.19435/j.1672-1721.2020.14.020 [DOI] [Google Scholar]
- Sakurai Y., Mori Y., Okamoto H., Nishimura A., Komura E., Araki T., et al. (2015). Acid-inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects–a randomised open-label cross-over study. Aliment Pharmacol. Ther. Sep 42 (6), 719–730. doi: 10.1111/apt.13325 [DOI] [PubMed] [Google Scholar]
- Sasaki M., Ogasawara N., Utsumi K., Kawamura N., Kamiya T., Kataoka H., et al. (2010). Changes in 12-year first-line eradication rate of helicobacter pylori based on triple therapy with proton pump inhibitor, amoxicillin and clarithromycin. J. Clin. Biochem. Nutr. 47 (1), 53–58. doi: 10.3164/jcbn.10-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoldi A., Carrara E., Graham D. Y., Conti M., Tacconelli E. (2018). Prevalence of antibiotic resistance in helicobacter pylori: A systematic review and meta-analysis in world health organization regions. Gastroenterology 155 (5), 1372–82.e17. doi: 10.1053/j.gastro.2018.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahraki T., Shahraki M., Shafighi Shahri E., Mohammadi M. (2016). No significant impact of lactobacillus reuteri on eradication of helicobacter pylori in children (Double-blind randomized clinical trial). Iran Red Crescent Med. J 19 (3), e42101. doi: 10.5812/ircmj.42101 [DOI] [Google Scholar]
- Shi C., Wang R., Zhou F., Li X. (2021). Clinical effect of clarithromycin triple therapy in the treatment of helicobacter pylori positive peptic ulcer in children. Clin. Med. Eng. 28 (01), 35–36. [Google Scholar]
- Shivalingaiah M., Kariyappa M., Hanumanna A. K. (2019). Helicobacter pylori infection presenting as childhood recurrent headache: A case report. Aust Fam Physician 6, 7, 397–399. doi: 10.32677/IJCH.2019.v06.i07.017 [DOI] [Google Scholar]
- Su Q. (2020). Efficacy and safety analysis of bismuth quadruple therapy and traditional triple therapy in the treatment of helicobacter pylori positive children with gastritis. Med. Forum. 24 (31), 4480–4481. doi: 10.19435/j.1672-1721.2020.31.021 [DOI] [Google Scholar]
- Sugano K., Tack J., Kuipers E. J., Graham D. Y., El-Omar E. M., Miura S., et al. (2015). Kyoto Global consensus report on helicobacter pylori gastritis. Gut 64 (9), 1353–1367. doi: 10.1136/gutjnl-2015-309252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B. (2015). Clinical efficacy of probiotics combined with triple therapy in children with helicobacter pylori infection. China Med. Herald 12 (20), 125–128. [Google Scholar]
- Sun T., Gong T., Zhang L. (2013). Treatment of 30 children with helicobacter pylori positive peptic ulcer by clarithromycin triple therapy. J. China Pharm. 22 (15), 91–92. doi: 10.3969/j.issn.1006-4931.2013.15.052 [DOI] [Google Scholar]
- Sun H., Liu S., Liu Y. (2020). Efficacy and safety of probiotics in children with chronic gastritis infected by helicobacter pylori. J. Shenzhen J. Integrated Traditional Chin. Western Med. 30 (14), 123–124. doi: 10.16458/j.cnki.1007-0893.2020.14.063 [DOI] [Google Scholar]
- Sýkora J., Valecková K., Amlerová J., Siala K., Dedek P., Watkins S., et al. (2005). Effects of a specially designed fermented milk product containing probiotic lactobacillus casei dn-114 001 and the eradication of H. pylori in children: A prospective randomized double-blind study. J. Clin. Gastroenterol. 39 (8), 692–698. doi: 10.1097/01.mcg.0000173855.77191.44 [DOI] [PubMed] [Google Scholar]
- Tang H. (2008). Clinical study of eradication and rescue regimens in the treatment of children with helicobacter pylori infection. J. shandong Med. J. 48 (14), 111–112. doi: 10.3969/j.issn.1002-266X.2008.14.064 [DOI] [Google Scholar]
- Tang X., Liu T. (2017). Comparison of bismuth quadruple therapy and traditional triple therapy in the treatment of helicobacter pylori positive children with gastritis. J. China Modern Med. 24 (21), 78–80. doi: 10.3969/j.issn.1674-4721.2017.21.026 [DOI] [Google Scholar]
- Tolone S., Pellino V., Vitaliti G., Lanzafame A., Tolone C. (2012). Evaluation of helicobacter pylori eradication in pediatric patients by triple therapy plus lactoferrin and probiotics compared to triple therapy alone. Ital. J. Pediatr. 38, 63. doi: 10.1186/1824-7288-38-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urganci N. (2017). Are synbiotics added to the standard therapy to eradicate helicobacter pylori in children beneficial? a randomized controlled study. Euroasian J. hepato-gastroenterology 7 (1), 17–22. doi: 10.5005/jp-journals-10018-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustundag G. H., Altuntas H., Soysal Y. D., Kokturk F. (2017). The effects of synbiotic “Bifidobacterium lactis B94 plus inulin” addition on standard triple therapy of helicobacter pylori eradication in children. Can. J. Gastroenterol. Hepatol. 2017, 8130596. doi: 10.1155/2017/8130596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan D., Liao P. (2008). Contrastive observation of combination drug treatment for peptic ulcer in children. J. Med. Theory Pract. 07, 797–799. doi: 10.19381/j.issn.1001-7585.2008.07.035 [DOI] [Google Scholar]
- Wang D. (2014). Efficacy and safety of quadruple therapy in the treatment of peptic ulcer with hp infection in children. Contemp. Med. Forum 20 (18), 58–59. [Google Scholar]
- Wang J. (2017). Efficacy observation of bifidobacteria triple viable bacteria capsule combined with triple therapy in the treatment of children with helicobacter pylori. J. China Health Care Nutr. 27 (10), 152. doi: 10.3969/j.issn.1004-7484.2017.10.223 [DOI] [Google Scholar]
- Wang H. (2019). Clinical analysis of clarithromycin triple therapy in the treatment of helicobacter pylori positive peptic ulcer in children. Chin. J. Modern Drug Appl. 13 (04), 104–106. doi: 10.14164/j.cnki.cn11-5581/r.2019.04.065 [DOI] [Google Scholar]
- Wang H., Feng D. (2014). Observation on the effect of multi-drug combination in the treatment of children with active gastric ulcer. J. China Med. Herald 26, 58–61. [Google Scholar]
- Wang Y. H., Huang Y. (2014). Effect of lactobacillus acidophilus and bifidobacterium bifidum supplementation to standard triple therapy on helicobacter pylori eradication and dynamic changes in intestinal flora. World J. Microbiol. Biotechnol. 30 (3), 847–853. doi: 10.1007/s11274-013-1490-2 [DOI] [PubMed] [Google Scholar]
- Wang C., Liu X. (2015). Clinical observation of furazolidone triple therapy in the treatment of children with helicobacter pylori infection. Strait Pharm. J. 27 (10), 198–199. [Google Scholar]
- Wang W., Luo H., Wei J., Zheng Y. (2011). Effects of probiotics on eradication of helicobacter pylori in children with abdominal pain. J. Med. Theory Pract. 23 (10), 916–917+920. [Google Scholar]
- Wang X., Yang Z., Ji X. (2019). Probiotics combined with triple therapy on the eradication rate of helicobacter pylori infection in children and the effect on nutrition and development of children. World Latest Med. Inf. 19 (86), 148+53. doi: 10.19613/j.cnki.1671-3141.2019.86.079 [DOI] [Google Scholar]
- Wen J., Peng P., Chen P., Zeng L., Pan Q., Wei W., et al. (2017). Probiotics in 14-day triple therapy for Asian pediatric patients with helicobacter pylori infection: A network meta-analysis. Oncotarget 8 (56), 96409–96418. doi: 10.18632/oncotarget.21633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worona-Dibner L. B. (2009). [Randomized, double-blind, placebo-controlled trial: Effect of lactobacillus gg supplementation on helicobacter pylori eradication rates and side effects during treatment in children]. Rev. gastroenterologia Mexico 74 (2), 142–143. [PubMed] [Google Scholar]
- Wu G. (2011). Observation on the effect of quadruple therapy on 41 cases of peptic ulcer in children with hp infection. J. shandong Med. J. 51 (23), 74–75. doi: 10.3969/j.issn.1002-266X.2011.23.051 [DOI] [Google Scholar]
- Wu M. (2017). Evaluation of the effect of clarithromycin-based triple therapy in the treatment of helicobacter pylori-positive peptic ulcer in children. J. China Med. Pharm. 7 (18), 62–64. doi: 10.3969/j.issn.2095-0616.2017.18.019 [DOI] [Google Scholar]
- Wu C., Cai D., Zhang H. (2012). 10-day sequential therapy in the treatment of chronic gastritis of helicobacter pylori infection in children clinical analysis. J. J. qiqihar Univ. Med. 33 (5), 584–585. doi: 10.3969/j.issn.1002-1256.2012.05.013 [DOI] [Google Scholar]
- Wu Y., Li W., Peng B. (2018). Clinical value of triple therapy in the treatment of peptic ulcer in children. Strait Pharm. J. 30 (01), 110–111. [Google Scholar]
- Xiang M., Di M., Deng X., Shang L. (2017). Clinical value of triple therapy combined with saccharomyces boulardii in the treatment of helicobacter pylori infection in children. J. Pract. J. Clin. Med. 14 (5), 98–101. doi: 10.3969/j.issn.1672-6170.2017.05.032 [DOI] [Google Scholar]
- Xiao C., Peng H., Zhou L. (2015). Observation on the clinical efficacy of modified guanotherapy in the treatment of children with helicobacter pylori infection. J. Med. Inf. 17, 203–204. doi: 10.3969/j.issn.1006-1959.2015.17.278 [DOI] [Google Scholar]
- Xiao Q., Shao Z. (2021). Observation on the effect of saccharomyces boulardii assisted eradication of helicobacter pylori. Med. Forum 25 (20), 2861–2863. doi: 10.19435/j.1672-1721.2021.20.026 [DOI] [Google Scholar]
- Xie W. (2018). Clinical observation of bifidobacteria quadruple viable bacteria tablets combined with triple therapy in the treatment of children with helicobacter pylori infection. Modern Digestion Intervent 23 (01), 60–62. [Google Scholar]
- Xiong Z., Gu S., Liu Y., Zhong W. (2009). Clinical observation of sequential therapy in the treatment of helicobacter pylori infection in children. J. Int. Med. Health guidance News 15 (16), 64–65. doi: 10.3760/cma.j.issn.1007-1245.2009.16.025 [DOI] [Google Scholar]
- Xu L., Liu Z., Xu J. (2013). Comparison of the efficacy of sequential therapy and traditional triple therapy for eradication of helicobacter pylori in children. China Modern Med. 20 (08), 49–51. [Google Scholar]
- Xu L., Yang X., Guo J., Sun M., Mao Z. (2016). A randomized controlled clinical trial of clostridium butyricum dual viable adjuvant treatment of helicobacter pylori infection in children. J. Chin. J. Microecol 28 (4), 413–416. doi: 10.13381/j.cnki.cjm.201604010 [DOI] [Google Scholar]
- Yang X. (2014). Clinical observation of probiotics combined with standard triple therapy for eradication of helicobacter pylori in children (China Medical University; ). [Google Scholar]
- Yang M. (2021). Observation on the effect of triple therapy combined with probiotics in the treatment of children with chronic gastritis complicated with helicobacter pylori infection. J. Contemp. Med. Forum 19 (2), 103–104. doi: 10.3969/j.issn.2095-7629.2021.02.073 [DOI] [Google Scholar]
- Yang Y., Huang J., Shao S. (2013). Efficacy analysis of probiotics combined with triple therapy in the treatment of helicobacter pylori infection in children. Chin. J. Nosocomiol 23 (23), 5757–8+61. [Google Scholar]
- Yang Y., Tian Y., Hong S. (2020). Eradication rate of probiotics against helicobacter pylori infection in children and the effect on nutrition and development of children. J. China Foreign Med. Treat 39 (10), 105–107. doi: 10.16662/j.cnki.1674-0742.2020.10.105 [DOI] [Google Scholar]
- Yao P., Luo Y. (2020). Effects of omeprazole, amoxicillin, clarithromycin combined with potassium bismuth citrate on peptic ulcer and hp eradication rate in children. Clin. Res. Pract. 5 (08), 116–118. doi: 10.19347/j.cnki.2096-1413.202008048 [DOI] [Google Scholar]
- Yeo Y. H., Hsu C. C., Lee C. C., Ho H. J., Lin J. T., Wu M. S., et al. (2019). Systematic review and network meta-analysis: Comparative effectiveness of therapies for second-line helicobacter pylori eradication. J. Gastroenterol. Hepatol. 34 (1), 59–67. doi: 10.1111/jgh.14462 [DOI] [PubMed] [Google Scholar]
- Yeo Y. H., Shiu S. I., Ho H. J., Zou B., Lin J. T., Wu M. S., et al. (2018). First-line helicobacter pylori eradication therapies in countries with high and low clarithromycin resistance: A systematic review and network meta-analysis. Gut 67 (1), 20–27. doi: 10.1136/gutjnl-2016-311868 [DOI] [PubMed] [Google Scholar]
- Yuan J. (2021). Efficacy of clarithromycin triple therapy in the treatment of helicobacter pylori-positive peptic ulcer in children. J. Healthmust-Readmagazine 16, 121. [Google Scholar]
- Yuan Y., Ford A. C., Khan K. J., Gisbert J. P., Forman D., Leontiadis G. I., et al. (2013). Optimum duration of regimens for helicobacter pylori eradication. Cochrane Database systemat Rev. 12, Cd008337. doi: 10.1002/14651858.CD008337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan N., Ning P. (2021). Effect of probiotics combined with omeprazole sequential therapy in treatment of helicobacter pylori-positive peptic ulcer and its influence on mda, tgf-P1 protein levels and intestinal flora in children. Clin. Res. Pract. 50 (4), 472–475. doi: 10.3969/j.issn.1000-7377.2021.04.023 [DOI] [Google Scholar]
- Zhang H. (2012). Comparison of therapeutic effects of different methods in the treatment of helicobacter pylori infection in children. Contemp. Med. Forum 18 (11), 49–50. [Google Scholar]
- Zhang Y. (2009). Efficacy analysis of triple therapy in the treatment of helicobacter pylori infection. J. Chin. J. Of Pract. Med. 36 (7), 558. doi: 10.3760/cma.j.issn.1674-4756.2009.07.023 [DOI] [Google Scholar]
- Zhang H. (2013). Clinical effect about boulardii powder combined triple therapy treating helicobacter-pylori infection in children. J. Med. J. Of Chin. People’s Health 25 (1), 38–40. doi: 10.3969/j.issn.1672-0369.2013.01.012 [DOI] [Google Scholar]
- Zhang H. (2015. a). Children with helicobacter pylori positive peptic ulcer with reported clinical effect of treatment of clarithromycin triple therapy. China Foreign Med. Treat 4, 126–127. doi: 10.3969/j.issn.1674-0742.2015.04.063 [DOI] [Google Scholar]
- Zhang H. (2015. b). Observation of curative effect of triple therapy on peptic ulcer in children. J. Modern Diagnosis Treat 26 (16), 3676–3678. [Google Scholar]
- Zhang Y. (2015. c). Clinical application and observation of quadruple therapy in treating peptic ulcer with hp infection in children 26 (21), 4845–4846. [Google Scholar]
- Zhang Y. (2015. d). Effect analysis application of triple therapy for treating children helicobacter pylori infection. China Continuing Med. Educ. 7 (30), 134–135. [Google Scholar]
- Zhang Y. (2018). Clinical efficacy, hp eradication rate and recurrence of probiotics combined with triple therapy on helicobacter pylori infection in children. Med. Innovation China 15 (02), 29–33. [Google Scholar]
- Zhang Y. (2019). Clinical observation of clarithromycin triple therapy in the treatment of helicobacter pylori positive peptic ulcer in children. Health For Everyone 13, 205. [Google Scholar]
- Zhang L. (2021). Comparison of the effect of traditional triple therapy and bismuth-containing quadruple therapy in the treatment of helicobacter pylori-associated gastritis in children. Clin. Med. Eng. 41 (08), 62–63. doi: 10.19528/j.issn.1003-3548.2021.08.024 [DOI] [Google Scholar]
- Zhang Y., Li J. (2012). He effect of addition of saccharomyces boulardii to the triple therapy on helicobacter pylori infection in children. J. J. Of Clin. Pediatr. 30 (10), 928–931. doi: 10.3969/j.issn.1000-3606.2012.10.009 [DOI] [Google Scholar]
- Zhang Y. (2016). Clinical effect of quadruple therapy in the treatment of peptic ulcer in children with helicobacter pylori infection. China J. Pharm. Econom 11 (08), 51–53. [Google Scholar]
- Zhang X., Ma W., Chen X., Nie L., Guo Y. (2019. a). Eradication rate of probiotics against helicobacter pylori infection in children and the effect on nutrition and development of children. J. J. North Sichuan Med. Coll. 34 (1), 130–133. doi: 10.3969/j.issn.1005-3697.2019.01.036 [DOI] [Google Scholar]
- Zhang S., Zhu X. (2015). Advances in helicobacter pylori infection in children. J. Clin. Pediatr. 33 (04), 391–395. [Google Scholar]
- Zhang X., Zhu M., Jiang Y. (2019. b). Efficacy observation of triple therapy combined with probiotics in the treatment of children with chronic gastritis complicated with helicobacter pylori infection. Modern Digestion Intervent 24 (07), 777–780. [Google Scholar]
- Zhao J. (2019). Observation of curative effect of sequential therapy in the treatment of helicobacter pylori infection in children. Chin. J. Modern Drug Appl. 13 (10), 100–101. doi: 10.14164/j.cnki.cn11-5581/r.2019.10.057 [DOI] [Google Scholar]
- Zhao X. (2020). Clinical observation of bismuth quadruple combination in the treatment of gastric helicobacter pylori-positive children with chronic gastritis. Capital Food Med. 27 (06), 74. [Google Scholar]
- Zhao H., Ouyang H., Duan B., Xu B., Chen Z., Tang J., et al. (2013). Clinical effect of triple therapy combined with saccharomyces boulardii in the treatment of helicobacter pylori infection in children. Chin. J. Contemp. Pediatr. 16 (03), 230–233. [PubMed] [Google Scholar]
- Zhao H., Yan P., Zhang N., Feng L., Chu X., Cui G., et al. (2021). The recurrence rate of helicobacter pylori in recent 10 years: A systematic review and meta-analysis. Helicobacter 26 (6), e12852. doi: 10.1111/hel.12852 [DOI] [PubMed] [Google Scholar]
- Zhao X., Zhou H. (2015). Observation on the effect of triple therapy in the treatment of children with helicobacter pylori-associated gastritis. Chin. Pediatr. Integrated Traditional Western Med. 7 (02), 144–146. [Google Scholar]
- Zheng L., Yu J., Wang X., Li X., Huang J. (2020). Clinical effect of triple therapy combined with saccharomyces boulardii in the treatment of helicobacter pylori infection in children. J. Clin. Res. Pract. 5 (1), 99–100. doi: 10.19347/j.cnki.2096-1413.202001040 [DOI] [Google Scholar]
- Zhong L. (2013). Clinical analysis of 98 children with peptic ulcer associated with helicobacter pylori infection. J. Modern Diagnosis Treat 24 (4), 770–771. doi: 10.3969/j.issn.1001-8174.2013.04.028 [DOI] [Google Scholar]
- Zhong H., Ye Y., Lv B. (2015). Clinical observation of probiotics combined with triple therapy in the treatment of children with helicobacter pylori infection. China Med. Eng. 23 (11), 144+6. [Google Scholar]
- Zhou S. (2016). Effect observation of omeprazole quadruple therapy in children with peptic ulcer combined with helicobacter pylori infection. J. China Modern Med. 23 (27), 82–84. [Google Scholar]
- Zhou D. (2018). Effects of drug combination on treatment of peptic ulcer in children and helicobacter pylori eradication rate. J. Chin. J. Woman Child Health Res. 29 (10), 1296–1299. doi: 10.3969/j.issn.1673-5293.2018.10.019 [DOI] [Google Scholar]
- Zhou J. (2021). Comparison of clinical effects of bismuth quadruple therapy and traditional triple therapy in the treatment of helicobacter pylori-positive children with gastritis. Chin. J. Clin. Rational Drug Use 14 (18), 124–126. doi: 10.15887/j.cnki.13-1389/r.2021.18.046 [DOI] [Google Scholar]
- Zhou G. (2022). Efficacy and incidence of adverse reactions of probiotics combined with triple therapy in the treatment of helicobacter pylori-related chronic gastritis in children. Jilin Med. J. 43 (01), 180–181. [Google Scholar]
- Zhou F., Li X. (2015). Study on the curative effects of saccharomyces boulardii combined with triple therapy on eradication of pediatric helicobacter pylori infection. J. China Modern Doctor 13, 71–73. [Google Scholar]
- Zhou C., Shi G. (2018). Study on the short-term effect of quadruple regimen on children with helicobacter pylori-positive gastritis. Chin. J. Postgraduates Med. 41 (11), 964–967. [Google Scholar]
- Zhou F., Li X. (2015). Analysis of clinical efficacy of two therapeutic regimens for eradication of helicobacter pylori. China Pract. Med. 10 (07), 169–170. doi: 10.14163/j.cnki.11-5547/r.2015.07.120 [DOI] [Google Scholar]
- Zhou Y., Yan Y., Li M., Liu N., Huang X., Chen Y. (2019). Clinical analysis of different methods for the treatment of helicobacter pylori infection in children. Guide China Med. 17 (21), 12+16. doi: 10.15912/j.cnki.gocm.2019.21.009 [DOI] [Google Scholar]
- Zhou T., Zhou X., Wan S., Luo Y. (2016). Meta analysis method and its application in the field of health care. Chongqing Med. 45 (07), 985–988. [Google Scholar]
- Zhu L. (2010). Efficacy and safety of amoxicillin and clavulanate for oral suspension combined with rabeprazole for helicobacter pylori eradication in children. Chin. J. Pract. Pediatr. 25 (07), 545–548. [Google Scholar]
- Zhu D. (2019). Study on the treatment of clarithromycin triple therapy in children with helicobacter pylori positive peptic ulcer. J. Heilongjiang J. Traditional Chin. Med. 48 (2), 98–99. [Google Scholar]
- Zhu Y., Li H. (2016). Efficacy and safety evaluation of quadruple therapy in the treatment of peptic ulcer in children with helicobacter pylori infection. Anti-Infect Pharm. 13 (01), 157–159. doi: 10.13493/j.issn.1672-7878.2016.01-057 [DOI] [Google Scholar]
- Zhu L., Liu Z., Wu Z., Li D., Jiang A., Yu G. (2017). Evaluation of clinical efficacy of different treatment regimens for children with helicobacter pylori infection. Chin. J. Contemp. Pediatr. 19 (06), 672–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zullo A., Rinaldi V., Winn S., Meddi P., Lionetti R., Hassan C., et al. (2000). A new highly effective short-term therapy schedule for helicobacter pylori eradication. Alimentary Pharmacol. Ther. 14 (6), 715–718. doi: 10.1046/j.1365-2036.2000.00766.x [DOI] [PubMed] [Google Scholar]
- Zullo A., Vaira D., Vakil N., Hassan C., Gatta L., Ricci C., et al. (2003). High eradication rates of helicobacter pylori with a new sequential treatment. Alimentary Pharmacol. Ther. 17 (5), 719–726. doi: 10.1046/j.1365-2036.2003.01461.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.