Abstract
Light at night (LAN) has been associated with negative health consequences and metabolic risk factors. Little is known about the prevalence of LAN in older adults in the United States and its association with CVD risk factors. We tested the hypothesis that LAN in older age is associated with higher prevalence of individual CVD risk factors. Five hundred and fifty-two community-dwelling adults aged 63−84 years underwent an examination of CVD risk factor profiles and 7-day actigraphy recording for activity and light measures. Associations between actigraphy-measured LAN, defined as no light vs. light within the 5-hour nadir (L5), and CVD risk factors, including obesity, diabetes, hypertension, and hypercholesterolemia, were examined, after adjusting for age, sex, race, season of recording, and sleep variables. LAN exposure was associated with a higher prevalence of obesity (multivariable-adjusted odds ratio [OR] 1.82 [95% CI 1.26−2.65]), diabetes (OR 2.00 [1.19−3.43]), and hypertension (OR 1.74 [1.21−2.52]) but not with hypercholesterolemia. LAN was also associated with (1) later timing of lowest light exposure (L5-light) and lowest activity (L5-activity), (2) lower inter-daily stability and amplitude of light exposure and activity, and (3) higher wake after sleep onset. Habitual LAN in older age is associated with concurrent obesity, diabetes, and hypertension. Further research is needed to understand long-term effects of LAN on cardiometabolic risks.
Keywords: light at night, cardiovascular disease, obesity, diabetes, aging
Graphical Abstract
Graphical Abstract.
Statement of Significance.
Light at night (LAN) is pervasive in developed countries and has been associated with negative health consequences. Little is known about nighttime light exposure in community-dwelling older adults and its association with cardiovascular disease (CVD) risk factors. This study aims to characterize 24-h light exposure patterns in a cohort of community-dwelling older adults and to determine whether habitual LAN exposure in older age is associated with a higher prevalence of cardiovascular risk factors. The findings of this study suggest the importance of examination of light exposure in population-based cohort studies as a potentially modifiable risk factor for CVDs and negative health outcomes.
Introduction
Light at night (LAN) has been associated with poorer health, including obesity and cancer risk [1]. Cross-sectional studies have demonstrated a higher prevalence of obesity in individuals with bedroom LAN during sleep, measured objectively and subjectively [1–3]. In a large prospective study of middle-aged women followed for over 5 years, self-reported LAN while sleeping was significantly associated with an increased risk of weight gain and the development of obesity [4]. In Japanese older adults followed up over 21 months, evening or nighttime light exposure was associated with subsequent increase in obesity [5]. In addition, annual levels of outdoor LAN estimated from satellite data have been associated with a higher risk of coronary heart disease in older adults in Hong Kong over 11 years of follow-up [6].
Many factors other than obesity mediate cardiovascular disease (CVD) risk, and little is known about LAN exposure in community-dwelling older adults in the United States and its relationship with obesity and other CVD risk factors like diabetes, hypertension, and hypercholesterolemia. LAN during sleep can induce stressor-like effects on autonomic functions of heart rate and breathing in healthy individuals, which may be a potential mechanistic link between LAN and CVD [7, 8]. The objectives of this study were (1) to characterize objective light exposure patterns in a cohort of community-dwelling older adults and (2) to determine whether LAN in older age is associated with higher prevalence of individual CVD risk factors. We hypothesized that older adults with habitual LAN exposure are more likely to be obese and to have diabetes, hypertension, and hypercholesterolemia.
Materials and Methods
Study population
The Chicago Healthy Aging Study
The Chicago Healthy Aging Study (CHAS) is a study of a subset of participants from the Chicago Heart Association Detection Project in Industry (CHA), a public health program and prospective epidemiologic study conducted in 1967–1973 to identify high-risk adults in workplaces throughout the Chicago, IL, area. Details of the CHA study have been previously published elsewhere [9]. From the 11,908 potential CHAS participants identified as CHA survivors, aged 65 to 84 years during 2007–2010, and free of major ECG abnormalities or myocardial infarction (MI) at the CHA examination (baseline), 59% (n = 7090) of names were randomly selected for contact. A stratified sampling method was used to recruit CHAS participants based on their baseline CVD risk factor profile. Low-risk status (LR) was defined as having favorable levels of four major CVD risk factors: serum total cholesterol level <200 mg/dL and no use of cholesterol-lowering medication; systolic blood pressure (SBP) ≤120 mmHg, diastolic BP (DBP) ≤80 mmHg and no use of antihypertensive medication; no current smoking; and no history of diabetes or heart attack. The final CHAS sample included 1395 participants (28% women, 9.3% African American, 2.5% Hispanic or Asian, 30.2% baseline LR). LR participants were oversampled to obtain adequate numbers for between-group comparisons. Details of the CHAS study and an ancillary study involving sleep have been published [10, 11]. Both CHAS and the sleep ancillary study were approved by the institutional review board of Northwestern University.
Study procedures and measures
Measurement of CVD risk factors
In 2007–2010, all CHAS participants completed standardized questionnaires about smoking history, medical history of hypertension, high serum cholesterol, and presence or absence of diabetes. Health interviewers updated and confirmed demographic information and reviewed all prescription and nonprescription medications with their dosages. Comorbid conditions were obtained using self-reported medical history, which was verified and supplemented by Medicare claims data [11]. Height and weight were measured with participants wearing light clothing without shoes. Body mass index (BMI) was calculated as weight in kilograms per height in meter squared. With participants seated, BP was measured three times using an automatic sphygmomanometer (Omron HEM-907 XL, Omron Healthcare, Inc., Bannockburn, IL) [12]. The average of the second and third measurements was used in the analyses. Total cholesterol and glucose levels were measured with 12-hour fasting blood samples.
Definition of CVD risk factors
Diabetes was defined as a fasting glucose level >126 mg/dL or use of antihyperglycemic medication [13]. Hypertension was defined as SBP ≥140 mmHg, DBP ≥90 mmHg, or treatment with antihypertensive medication. Hypercholesterolemia was defined as total cholesterol ≥240 mg/dL or treatment with cholesterol-lowering medication. Obesity was defined as BMI ≥30 kg/m2.
Actigraphy
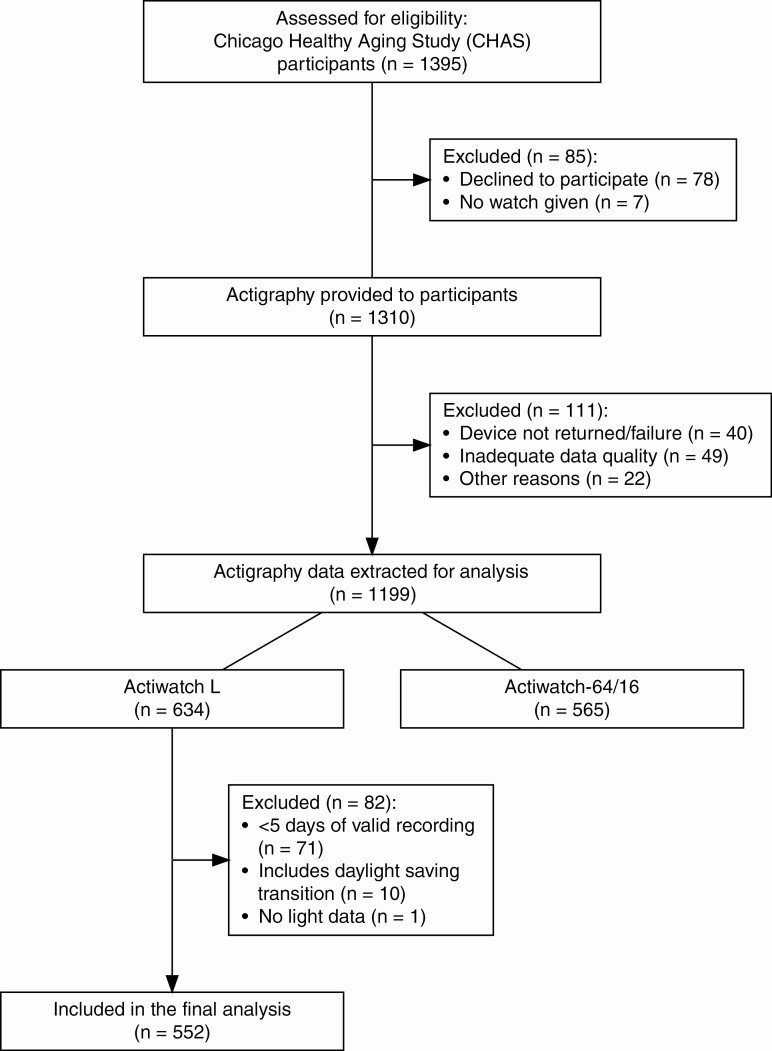
In 2007−2010, a wrist actigraphy monitor was offered to all CHAS participants and was given to 1310 participants (93.9%) who were instructed to wear it for 7 consecutive days and to maintain a daily Karolinska Sleep Diary [11, 14] (Figure 1). Participants were provided either Actiwatch-L, Actiwatch-64, or Actiwatch-16 (Phillips Respironics), based on availability. Among these three types of actigraphy monitor, only Actiwatch-L is equipped with an on-board miniature photodiode for measurement of the amount and duration of light exposure. This light sensor has a spectral sensitivity approximating that of the human eye, with wavelength range of 330−720 nm, peak spectral sensitivity at 580 nm, and illuminance range of 0.1–150 000 lux [15]. Actigraphy data were obtained in 30-s epochs. Participants were instructed to keep a log when removing the device and to keep the light sensor of the actigraphy device uncovered at all times (e.g. free of long sleeves or bedding) [16]. Ninety-two percent (n = 1199) of participants returned the actigraphy device to the study team, including 634 participants with Actiwatch-L and 565 with Actiwatch-64 or Actiwatch-16. Recordings across daylight-saving-time transitions were excluded (n = 10). Any 24-h period (12:00 pm–12:00 pm next day) with >4 h of non-wear period was removed as invalid. Since light measurements are the focus of the current analysis, only actigraphy data with minimum five valid 24-h periods of recording using Actiwatch-L were included (n = 552). Missing actigraphy data within the valid recording were imputed (see “Missing Data” for details). For analysis, season of recording (Spring, Summer, Fall, and Winter; defined by solstices and equinoxes) was defined as a covariate. We chose the astronomical over the meteorological season because it better represents light exposure [17].
Figure 1.
CONSORT flow diagram. Consolidated Standards of Reporting Trials (CONSORT) flow diagram for Chicago Healthy Aging Study and the ancillary sleep study.
Rest-activity variables
Standard nonparametric methods were applied to actigraphy data to obtain intra-daily variability (IV), inter-daily stability (IS), relative amplitude (RA), and midpoint of the most active 10-h period (M10) and the least active 5-h period (L5) computed from minute-wise averages across 24-h periods [18]. IV represents the frequency and extent of transitions between low and high values; higher IV indicates more fragmentation of the rest-activity rhythm, with IV ≈ 0 for a perfect sine wave and IV ≈ 2 for Gaussian noise. IS, which measures how similar one 24-h period is to the next, is a measure of robustness and ranges from 0 to 1; 0 indicates a complete lack of similarity from day-to-day, while 1 indicates perfect day-to-day similarity. RA is the normalized difference in activity count between M10 and L5 [19–21]. Midpoints of M10 and L5 periods are the indices of activity peak and trough timing, respectively. Analysis was conducted in R using the nparACT package [18].
Light exposure
Light data from actigraphy were used to calculate daily mean and median light exposure averaged over valid 24-h periods of recording. Log-transformed light data were analyzed using nonparametric methods to derive IV, IS, RA, L5, M10, and midpoints of L5 and M10 periods [18].
Sleep variables
Actigraphy data were manually scored as sleep or wake using a standardized hierarchical protocol and based on the sleep diary and activity/light data from the actigraphy device. Total sleep time (TST), time in bed, sleep efficiency (SE), wake after sleep onset (WASO), sleep midpoint, onset, and offset were calculated as published previously [22].
Missing data
After removing 24-h periods including >4 h of missing data, remaining actigraphy data (n = 552) contained short periods of missing data as participants were instructed to remove the device during shower or other activities where the device would be submerged in water. Since missing actigraphy data can bias estimates of nonparametric measures downwards and introduce artificial rest-activity transition [23], multiple imputation was implemented to replace missing actigraphy data prior to conducting nonparametric analyses [24, 25]. The “Multivariate Imputation by Chained Equations” (mice) R package [26] was used to characterize and impute missing actigraphy data. Based on low median proportion of missing actigraphy data and prior report suggesting insufficient power with five or less imputations [27], the number of imputations for the mice algorithm was set to 10. White light exposure in lux and activity counts in 30-s epochs were included in the imputation model with the random forest method. The quality of imputed actigraphy data were individually verified by observing the distribution of imputed versus observed data, and by visually inspecting the time series containing imputed data. There was no missing demographic and CVD risk factor data.
Statistical analysis
Descriptive statistics were summarized using means and standard deviations (SD) for continuous and normally distributed data, median and interquartile ratio (IQR) for continuous and non-normally distributed data and counts and percentages for categorical data.
L5 light value, in log-transformed lux, was selected as an objective measure of LAN (LAN), as it is less likely to be affected by season, timing and duration of sleep, or scoring bias than other measures such as light during sleep interval and light during a fixed time window (e.g. 12 pm–6 am). After inspecting the distribution of L5 light values (i.e. zero-inflated), a decision was made to categorize L5 light as 0 or >0. Participants with 0 light during the 5-h nadir (L5) were categorized as having “No-LAN,” while those with >0 light during L5 were categorized as having “LAN.” Associations between L5 light and demographic characteristics, individual CVD risk factors, sleep, activity and other light variables were examined in bivariate analyses using Wilcoxon rank-sum test, Pearson’s χ 2 test, or Fisher’s exact test, as appropriate. CVD risk factor variables that met a significance threshold (two-sided p < .05) in bivariate analyses were sequentially entered into multivariable models including a priori covariates of age, sex, race, and season.
We then conducted sensitivity analyses to test whether any significant association between LAN and CVD risk factor variables was independent of measures of sleep and physical activity. Given collinearity between measures of sleep and activity, each model included a priori covariates (age, sex, race, and season) plus only one of the following variables: WASO, SE, sleep onset, sleep midpoint, sleep offset, TST, time in bed, mean activity count during the L5 light period, mean 24-h activity count, M10 activity, L5 activity, IS activity, IV activity, RA of activity, and timing of M10 and L5 activity.
In addition, we tested whether CVD risk factor variables are associated with other measures of 24-h light exposure pattern, including 24-h mean light intensity, 24-h median light intensity, peak (M10) light intensity, IS light, IV light, RA of light, and timing of L5 and M10 light.
Additional analyses
For CVD risk factor variables that were significantly associated with LAN, potential effect modification was assessed through stratified analysis based on baseline CVD risk status (i.e. low-risk vs. not low-risk). Wolf and Breslow-Day tests of homogeneity were used to test the presence of effect modification by baseline CVD risk status, and stratified ORs were calculated using the Mantel–Haenszel formula.
In addition, we conducted exploratory analyses by dividing the LAN group to Low-LAN vs. High-LAN group based on the group median value of L5 light to test whether the associations between LAN and CVD risk factor variables are dose-dependent.
Statistical analysis was conducted in R 4.0.3.[28] and figures were produced using the package ggplot2 [29].
Results
Characteristics of the Study Population
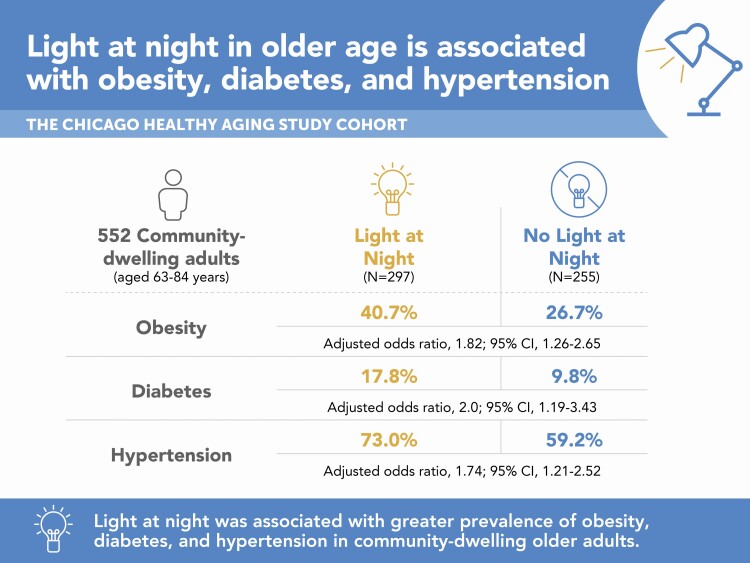
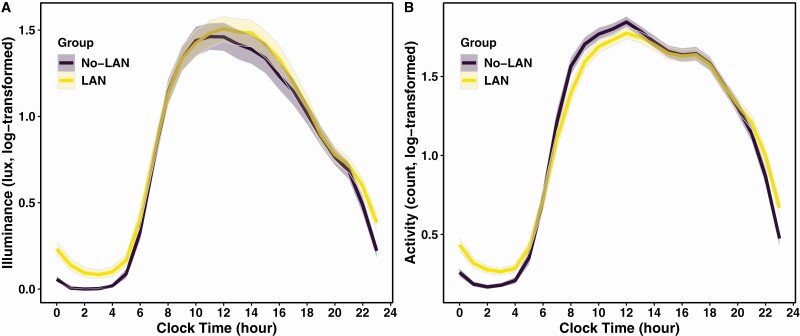
Demographic characteristics of the participants and actigraphy-derived light, sleep, and rest-activity variables are summarized in Table 1. Participants were aged 72 years on average (SD 5), with 142 (26%) women, 42 (7.6%) black, and 157 (28.4%) low-risk at baseline. Four hundred and ten (74%) participants completed 7 days of valid actigraphy recording, 128 (23%) completed 6 days, and the remainder (n = 14 (2.5%)) completed 5 days. The median missing data were 1% [IQR 0, 2.1] or 1.7 hours [IQR 0, 3.4] for each recording. Regarding LAN exposure, average light intensity in the lowest 5-h light exposure (L5 light) was zero in 255 participants (No-LAN), and greater than zero in 297 (54%) (LAN). L5 light midpoint ranged from 21:25 to 07:11, and L5 light midpoint and L5 activity midpoint were strongly correlated (ρ = 0.71, p < .001). Figure 2 demonstrates light and activity profiles across 24-h periods between LAN and No-LAN groups.
Table 1.
Sample characteristics by Light at Night (LAN)
| Characteristic | Overall, N = 552* |
No LAN, N = 255* |
LAN, N = 297* |
p-value† | q-value‡ |
|---|---|---|---|---|---|
| Age | 72 (5) | 71 (5) | 72 (5) | 0.2 | 0.3 |
| Black | 42 (7.6%) | 7 (2.7%) | 35 (12%) | <0.001 | <0.001 |
| Female | 142 (26%) | 61 (24%) | 81 (27%) | 0.4 | 0.5 |
| Body mass index (kg/m2) | 27.9 (25.1, 31.4) | 27.5 (25.0, 30.3) | 28.5 (25.3, 32.0) | 0.010 | 0.020 |
| Season | 0.2 | 0.3 | |||
| Spring | 103 (19%) | 44 (17%) | 59 (20%) | ||
| Summer | 168 (30%) | 69 (27%) | 99 (33%) | ||
| Fall | 189 (34%) | 98 (38%) | 91 (31%) | ||
| Winter | 92 (17%) | 44 (17%) | 48 (16%) | ||
| Light variables | |||||
| 24 h mean light Intensity (lux) | 342 (97, 1,034) | 322 (97, 990) | 355 (97, 1,044) | 0.7 | 0.7 |
| 24 h median light intensity (lux) | 25 (13, 46) | 26 (14, 46) | 24 (13, 44) | 0.3 | 0.4 |
| Inter-daily stability | 0.64 (0.48, 0.74) | 0.65 (0.52, 0.74) | 0.62 (0.46, 0.73) | 0.015 | 0.027 |
| Intra-daily variability | 0.50 (0.37, 0.63) | 0.50 (0.37, 0.64) | 0.50 (0.38, 0.63) | 0.8 | 0.8 |
| Relative amplitude | 0.99 (0.97, 1.00) | 1.00 (1.00, 1.00) | 0.97 (0.90, 0.99) | <0.001 | <0.001 |
| Lowest light exposure (L5) | 0.01 (0.00, 0.05) | 0.00 (0.00, 0.00) | 0.04 (0.01, 0.14) | <0.001 | <0.001 |
| Peak light exposure (M10) | 3.1 (2.1, 4.3) | 3.0 (2.1, 4.3) | 3.1 (2.1, 4.3) | 0.4 | 0.5 |
| Lowest light (L5) midpoint | 2.8 (1.8, 3.5) | 2.5 (1.6, 3.1) | 3.1 (2.1, 3.9) | <0.001 | <0.001 |
| Peak light (M10) midpoint | 13.2 (12.5, 14.1) | 13.2 (12.5, 14.0) | 13.3 (12.6, 14.2) | 0.4 | 0.5 |
| Mean activity during L5 light | 9 (6, 14) | 7 (5, 9) | 12 (8, 18) | <0.001 | <0.001 |
| Activity variables | |||||
| Mean activity count | 97 (74, 118) | 98 (75, 118) | 96 (73, 118) | 0.6 | 0.6 |
| Inter-daily stability | 0.58 (0.51, 0.65) | 0.61 (0.54, 0.67) | 0.56 (0.48, 0.64) | <0.001 | <0.001 |
| Intra-daily variability | 0.78 (0.65, 0.93) | 0.75 (0.64, 0.90) | 0.80 (0.68, 0.94) | 0.019 | 0.033 |
| Relative amplitude | 0.90 (0.85, 0.93) | 0.92 (0.90, 0.94) | 0.87 (0.82, 0.92) | <0.001 | <0.001 |
| Lowest activity (L5) | 8 (5, 12) | 6 (4, 9) | 10 (7, 15) | <0.001 | <0.001 |
| Peak activity (M10) | 158 (121, 197) | 161 (127, 199) | 152 (119, 194) | 0.069 | 0.11 |
| Lowest activity (L5) midpoint | 2.8 (1.9, 3.7) | 2.6 (1.7, 3.4) | 3.0 (2.1, 4.0) | <0.001 | <0.001 |
| Peak activity (M10) midpoint | 13.2 (12.2, 14.1) | 13.0 (12.0, 13.9) | 13.4 (12.4, 14.5) | <0.001 | <0.001 |
| Sleep variables | |||||
| Time in bed (min) | 437 (403, 478) | 450 (416, 485) | 424 (379, 467) | <0.001 | <0.001 |
| Total sleep time (min) | 394 (351, 431) | 408 (376, 442) | 383 (335, 413) | <0.001 | <0.001 |
| Wake after sleep onset (min) | 43 (31, 59) | 40 (29, 52) | 45 (34, 65) | <0.001 | <0.001 |
| Sleep efficiency (%) | 85 (79, 88) | 87 (83, 90) | 83 (76, 87) | <0.001 | <0.001 |
| Sleep midpoint | 3.0 (2.5, 3.8) | 3.0 (2.4, 3.5) | 3.1 (2.5, 3.9) | 0.023 | 0.039 |
| Sleep onset | -0.6 (-1.3, 0.1) | -0.8 (-1.5, -0.2) | -0.5 (-1.1, 0.4) | <0.001 | <0.001 |
| Sleep offset | 6.7 (5.9, 7.4) | 6.7 (5.9, 7.4) | 6.7 (5.8, 7.4) | 0.7 | 0.7 |
*Mean (SD); n (%); median (IQR).
†Wilcoxon rank-sum test; Pearson’s χ 2 test. Bold values signify <0.05.
‡False discovery rate correction for multiple testing. Bold values signify <0.05.
Figure 2.
24-Hour light and activity profiles in participants with and without LAN. Average hourly light intensity (in lux) (A) and activity counts (B) are log-transformed and plotted across 24-hour period relative to clock time. Shaded areas represent 95% confidence intervals. (A) Older adults with non-zero light during the 5-hour nadir (L5) (LAN) have greater nighttime light exposure compared to those with zero L5 light (No-LAN). Timing and intensity of peak light exposure are not different between participants with LAN and No-LAN. (B) Older adults with LAN have greater nighttime activity compared to those with No-LAN. Peak activity counts are not different between two groups. Participants with LAN have later midpoints of activity peak and nadir compared to those with No-LAN.
Associations between LAN, demographic, light, activity, and sleep variables
The LAN group was more likely to be black compared to the No-LAN group (12 vs. 2.7%, p < .001). In bivariate analyses of light exposure variables, LAN was associated with a lower IS and RA of light and later L5 light midpoint by 36 min (p = .015 for IS light, otherwise p < .001). In terms of activity variables, LAN was associated with lower IS, higher IV, lower RA due to higher L5 activity count, and later midpoints of peak (M10) and lowest activity (L5) by 24 min (p = .019 for IV activity, otherwise p < .001). Regarding sleep variables, the LAN group had shorter TST, time in bed, greater WASO, lower SE, and later sleep onset and midpoint, compared to the No-LAN group (p = 0.023 for sleep midpoint, otherwise p < .001). There were no significant differences between the LAN and No-LAN groups by age, sex, season of recording, mean or median light intensity across 24-hour period, peak light intensity and timing, mean activity count, peak activity count, and sleep offset (Table 1). In multivariable models including a priori covariates of age, sex, race, and season, all light, activity, and sleep variables remained significantly associated with LAN (all p < .05), except for IV activity (p = .06) (Supplementary Table S1)
Associations between LAN and CVD risk factors
In bivariate analyses, the LAN group was significantly more likely to have obesity (41% vs. 27%, p < .001), diabetes (18% vs. 9.8%, p = .007), and hypertension (73% vs. 59%, p < .001) compared to the No-LAN group. The prevalence of hypercholesterolemia was not different between the two groups (59% in LAN vs. 65% in No-LAN, p = .2).
In multivariable models including a priori covariates of age, sex, race, and season, all significant associations in bivariate analyses remained significant. LAN was associated with a higher prevalence of obesity (multivariable-adjusted OR 1.82 [95% CI 1.26, 2.65], p = .002), diabetes (OR 2 [1.19, 3.43], p = .01), and hypertension (OR 1.74 [1.21, 2.52], p = .003) (Table 2). Even after adjusting for sleep, mean activity count during the L5 light period, and other activity variables, the association of LAN with obesity, diabetes, and hypertension remained statistically significant (Supplementary Table S2).
Table 2.
Associations between LAN exposure and cardiovascular disease risk factors
| CVD Risk Factor | No LAN, N = 255 | LAN, N = 297 | p-value |
|---|---|---|---|
| Obesity | |||
| Prevalence (n (%)) | 68 (26.7) | 121 (40.7) | |
| Unadjusted OR (95% CI) | 1 [Reference] | 1.89 (1.32–2.72) | 0.001 |
| Multivariable-adjusted OR (95% CI)* | 1 [Reference] | 1.82 (1.26–2.65) | 0.002 |
| Diabetes | |||
| Prevalence(n (%)) | 25 (9.8) | 53 (17.8) | |
| Unadjusted OR (95% CI) | 1 [Reference] | 2 (1.21–3.37) | 0.008 |
| Multivariable-adjusted OR (95% CI)* | 1 [Reference] | 2 (1.19–3.43) | 0.010 |
| Hypertension | |||
| Prevalence (n (%)) | 151 (59.2) | 216 (73) | |
| Unadjusted OR (95% CI) | 1 [Reference] | 1.86 (1.3–2.67) | 0.001 |
| Multivariable-adjusted OR (95% CI)* | 1 [Reference] | 1.74 (1.21–2.52) | 0.003 |
LAN, light at night (defined as average light intensity during lowest 5-h light exposure); CVD, cardiovascular disease; OR, odds ratio; CI, confidence interval.
*Adjusted for age, sex, race, and season of actigraphy recording.
Regarding other measures of 24-hour light exposure pattern, no significant association was found between any of the CVD risk factors (i.e. obesity, diabetes, hypertension, and hypercholesterolemia) and 24-hour mean light intensity, 24-hour median light intensity, peak (M10) light intensity, RA, IS light, IV light, RA of light, and timing of L5 and M10 light.
Additional analysis
We conducted stratified analyses to assess whether the observed associations between LAN and CVD outcome variables (i.e. obesity, hypertension, and diabetes) are independent of the baseline CVD risk status (low-risk vs. not low-risk). Both Woolf and Breslow-Day tests of homogeneity failed to reject the null hypothesis (p > .05) for all three CVD variables, suggesting that there is no significant effect modification by baseline CVD risk status and that the ORs of CVD outcome variables are homogeneous across strata. (Data stratified by baseline risk status are presented in Supplementary Table S3.) Unstratified and stratified ORs using the Mantel–Haenszel formula for each CVD outcome variable did not differ by more than 10%, indicating that no significant confounding effect by baseline CVD risk status was present (Supplementary Figure S1).
In the exploratory analyses where the LAN group was divided to Low-LAN (n = 149) vs. High-LAN (n = 148), both Low-LAN and High-LAN groups were more likely to have obesity (aOR 1.71 and 1.94 for Low- and High-LAN, respectively, p < .02 for both), diabetes (aOR 1.87 and 2.15, p < .05 for both), and hypertension (aOR 1.77 and 1.72, p < .02 for both) compared to the No-LAN group, in multivariable models including a priori covariates of age, sex, race, and season (Table 3). These findings remained significant after further adjusting for sleep and activity variables. The risks of obesity, diabetes, and hypertension were not significantly different between Low-LAN and High-LAN groups.
Table 3.
Cardiovascular risk factors among Low-LAN and High-LAN groups compared to No-LAN group
| CVD Risk Factor | No LAN, N = 255 |
Low LAN, N = 149 |
p-value | High LAN, N = 148 |
p-value |
|---|---|---|---|---|---|
| Obesity | |||||
| Prevalence (n (%)) | 68 (26.7) | 57 (38.3) | 64 (43.2) | ||
| Unadjusted OR (95% CI) | 1 [Ref] | 1.7 (1.11–2.62) | 0.016 | 2.1 (1.37–3.22) | 0.001 |
| Multivariable-adjusted OR (95% CI)* | 1 [Ref] | 1.71 (1.1–2.67) | 0.018 | 1.94 (1.25–3.03) | 0.003 |
| Diabetes | |||||
| Prevalence (n (%)) | 25 (9.8) | 25 (16.8) | 28 (18.9) | ||
| Unadjusted OR (95% CI) | 1 [Ref] | 1.85 (1.02–3.38) | 0.042 | 2.15 (1.2–3.87) | 0.010 |
| Multivariable-adjusted OR (95% CI)* | 1 [Ref] | 1.87 (1.01–3.45) | 0.045 | 2.15 (1.17–3.97) | 0.014 |
| Hypertension | |||||
| Prevalence (n (%)) | 151 (59.2) | 108 (73) | 108 (73) | ||
| Unadjusted OR (95% CI) | 1 [Ref] | 1.86 (1.2–2.91) | 0.006 | 1.86 (1.2–2.91) | 0.006 |
| Multivariable-adjusted OR (95% CI)* | 1 [Ref] | 1.77 (1.13–2.79) | 0.013 | 1.72 (1.1–2.73) | 0.019 |
LAN, light at night (defined as average light intensity during lowest 5-hour light exposure); CVD, cardiovascular disease; OR, odds ratio; CI, confidence interval; Ref, Reference.
*Adjusted for age, sex, race, and season of actigraphy recording.
Discussion
In this study of community-dwelling older adults, we examined 24-hour light exposure patterns over 7 days. Less than half (46.2%) of the examined older adults had a 5-hour period of complete darkness per 24 hour (No-LAN), while 53.8% of participants were exposed to some light even during the 5-hour nadir (LAN). The prevalence of obesity, diabetes, and hypertension was significantly higher in older adults with LAN compared to those with No-LAN, after adjusting for a priori covariates of age, race, sex, and season as well as measures of sleep and activity.
Prior studies have documented cross-sectional associations between self-reported bedroom light during sleep and prevalence of obesity in middle-aged women in the UK [3] and United States [4]. Obayashi and colleagues have reported cross-sectional associations between measured bedroom light during sleep and obesity, diabetes, nighttime blood pressure, dyslipidemia, and subclinical atherosclerosis in Japanese older adults [2, 30, 31]. Using objective and validated measures of 24-h light exposure and cardiovascular risk factors, the present study extends the existing literature by demonstrating associations between LAN and obesity, diabetes, and hypertension in older United States adults. In contrast to the study from Japan [2], no significant association was found between LAN and hypercholesterolemia, which may be related to methodological differences (i.e., measuring room light during sleep vs. 5-hour nadir of 24-hour light exposure) and racial/ethnic differences in dyslipidemia patterns and prevalence [32].
The pathophysiology underlying development of examined CVD risk factors is complex, with both genetic predisposition and lifestyle factors contributing to an individual’s risk. Due to the study design of CHAS oversampling participants who were considered to be at low risk for all four major CVD risk factors in young adulthood (“baseline low-risk”), this cohort may have a disproportionately large number of older adults with a favorable genetic predisposition against development of CVD risk factors. However, we did not find a significant effect modification or confounding by baseline CVD risk profile in stratified analyses. Therefore, LAN may represent an under-recognized, potentially modifiable risk factor for CVD independent of CVD risk profiles at a young age.
Because of the cross-sectional study design, we are unable to determine whether LAN at older age represents chronic, habitual exposure, nor can we exclude the possibility that LAN at older age is the consequence of, rather than a casual mechanism in the development of, obesity, diabetes, and hypertension. For example, sleep disorders and nocturia [33] are frequent comorbidities in adults with diabetes [34] and hypertension [35], which may result in increased LAN exposure. However, the associations between LAN and CVD risk factors were independent of nocturnal awakening and activity level during the period of LAN measurement (i.e. a marker for bathroom visits). Alternatively, the relationship between LAN and CVD risk factors may be bi-directional. While no directionality can be drawn from this cross-sectional analysis, we postulate that LAN may increase the risk of obesity [4, 5], diabetes, and hypertension, which in turn increases the likelihood of LAN exposure, potentially creating a vicious cycle of accelerated CVD risks. Future prospective studies with longitudinal examination of light exposure are needed to better understand the relationships between LAN and health outcomes.
A few mechanisms have been postulated linking LAN and cardiometabolic dysfunction. Light is the primary synchronizer of the central circadian clock, and exposure to light during the biological night can lead to circadian disruption and dysregulation of clock-regulated physiological processes. There is evidence from animal studies that exposure to LAN can dysregulate circadian and metabolic function. For example, mice exposed to dim LAN showed increased weight gain, reduced glucose tolerance, and altered clock gene expression in the liver as compared with mice not exposed to LAN, despite equivalent caloric intake and activity counts [36–38]. Furthermore, timing of food intake was shifted in mice exposed to LAN, demonstrating a feeding behavior at an inappropriate circadian time. Therefore, LAN may increase the risk of obesity and diabetes through dysregulation of clock-controlled metabolic pathways and uncoupling of feeding behavior from biological day-night cycle, i.e. feeding at the wrong circadian time.
A second proposed mechanism through which LAN can impair cardiometabolic function is through melatonin, the “hormone of darkness.” Normally, melatonin is produced in the pineal gland and secreted into general circulation during the dark period and becomes nearly undetectable during the day in humans [39]. LAN suppresses pineal production of melatonin and weakens the circadian signal that regulates a host of cellular mechanisms in the body [40, 41]. In addition to its key function in the circadian signaling and synchronization of peripheral clocks in the body, melatonin plays important roles in metabolic and circulatory function with anti-oxidant, anti-inflammatory, and vasodilatory properties [42–44]. Lower melatonin levels have been associated with increased risk of incident diabetes in a large cohort study of women [45] and with increased risk of incident hypertension in a smaller study of young women [46].
A third potential mechanism is the effect of light exposure on autonomic nervous system activity. In a recent experimental study, healthy adults exposed to a single night of room light at 100 lux during sleep showed increased insulin resistance the next morning, compared to the those exposed to dim light (<3 lux) during sleep [8]. Sleep quality and plasma melatonin concentrations were not different between two groups. The effect of LAN on metabolic function was correlated with an increase in sympathovagal balance during sleep, suggesting that the impact of LAN on autonomic nervous system may be an important mechanism leading to cardiometabolic dysfunction.
In the present study, older adults with LAN exhibited a significantly later timing of lowest light and activity and lower IS compared to those with No-LAN, potentially indicating a later chronotype and reduced robustness of circadian rhythms, respectively. Three quarters of older adults with LAN did not enter the lowest 5-hour light period (L5) until after 23:00. Given that this is well after the timing of sunset in the Chicago area [range 16:18–20:28], these individuals likely had a significant evening exposure to artificial light leading up to their L5 light. We cannot separate independent effects of later timing and LAN on CVD risk factors due to collinearity. Nonetheless, we postulate that a later timing of lowest light and activity may increase the duration and intensity of LAN exposure, create circadian misalignment between behavioral rhythms and biological light–dark cycle, and provide opportunities for mistimed behaviors, such as later meal timing, altogether contributing to increased CVD risks. Future intervention studies are needed to test these hypotheses.
This study has a few important limitations. First, light measured by wrist actigraphy may not be the best estimate of light exposure at the eye level, and we cannot exclude the possibility of participants wearing the wrist actigraphy under blankets or sleeves and being erroneously categorized to the No-LAN group, despite the instructions to keep the device always uncovered. Prior studies utilized self-report [4] or bedroom light meter [2] to estimate light exposure, which are limited by recall bias and inability to capture relevant light exposure (e.g. bathroom visits, change in sleep location, smartphone use in bed), respectively. Developing practical methods to measure light at the eye level continuously over several days would help overcome the current methodological limitation. Second, women and nonwhite racial/ethnic groups are under-represented, reflecting the demographic composition of the original CHA cohort who was recruited from adults employed in the Chicago area in 1967–1973. Thus, the findings may not be generalizable to the population of different sex and racial/ethnic composition. Third, due to the CHAS study design of oversampling older adults who were low risk at baseline among the survivors of the original CHA participants, the study cohort may represent a “healthier” subgroup of older adults. Even though we did not find a significant effect modification or confounding by baseline risk status in our analysis, the possibility of unexamined bias cannot be excluded.
The strengths of this study include a detailed examination of cardiovascular risk factors and objective and validated measures of habitual light exposure that are simple to reproduce across different populations.
Conclusion
LAN is increasingly pervasive in developed countries where the population is aging with an alarmingly high rate of cardiometabolic disorders. The present study demonstrates a high prevalence of LAN in community-dwelling older adults and its association with obesity, diabetes, and hypertension. It will be important for future prospective population studies to include examination of light exposure patterns in young-to-middle-aged adults as a potential modifiable risk factor for CVDs and negative health outcomes. Furthermore, intervention trials will be needed to elucidate how different components of LAN, such as timing, duration, frequency, and chronicity of exposure and intensity and wavelength of light, impact human health.
Supplementary Material
Acknowledgements
This research was supported by the National Institutes of Health National Heart, Lung, and Blood Institute (grant R01 HL089695, R01 HL090873, R01 HL021010), the National Center for Advancing Translational Sciences (UL1TR001422), the National Institute on Aging (P30AG059988), and Northwestern University Feinberg School of Medicine Ken and Ruth Davee Department of Neurology. The research of the Chicago Heart Association Detection Project in Industry (CHA) was accomplished thanks to the invaluable cooperation of 84 Chicago-area companies and organizations and their officers, staff, and employees. Acknowledgement is also gratefully extended to all CHA investigators, staff members and volunteers; and the staff, investigators, and the participants of the Chicago Healthy Aging Study for their valuable contributions.
Contributor Information
Minjee Kim, Department of Neurology, Northwestern University Feinberg School of Medicine, Chicago, IL, USA; Center for Circadian and Sleep Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL, USA; Center for Applied Health Research on Aging, Institute for Public Health and Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Thanh-Huyen Vu, Department of Preventive Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Matthew B Maas, Department of Neurology, Northwestern University Feinberg School of Medicine, Chicago, IL, USA; Center for Circadian and Sleep Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Rosemary I Braun, Department of Engineering Sciences and Applied Mathematics, Northwestern University, Evanston, IL, USA.
Michael S Wolf, Center for Applied Health Research on Aging, Institute for Public Health and Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL, USA; Department of Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Till Roenneberg, Institute of Medical Psychology, Ludwig-Maximilian University, Munich, Germany.
Martha L Daviglus, Department of Preventive Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL, USA; Institute for Minority Health Research, University of Illinois Chicago, Chicago, IL, USA.
Kathryn J Reid, Department of Neurology, Northwestern University Feinberg School of Medicine, Chicago, IL, USA; Center for Circadian and Sleep Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Phyllis C Zee, Department of Neurology, Northwestern University Feinberg School of Medicine, Chicago, IL, USA; Center for Circadian and Sleep Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Disclosure statement
All authors report no conflict of interest.Financial Disclosure: none.
Nonfinancial Disclosure: none.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Cho Y, et al. Effects of artificial light at night on human health: a literature review of observational and experimental studies applied to exposure assessment. Chronobiol Int. 2015;32(9):1294–1310. [DOI] [PubMed] [Google Scholar]
- 2. Obayashi K, et al. Exposure to light at night, nocturnal urinary melatonin excretion, and obesity/dyslipidemia in the elderly: a cross-sectional analysis of the HEIJO-KYO study. J Clin Endocrinol Metab. 2013;98(1):337–344. [DOI] [PubMed] [Google Scholar]
- 3. McFadden E, et al. The relationship between obesity and exposure to light at night: cross-sectional analyses of over 100,000 women in the Breakthrough Generations Study. Am J Epidemiol. 2014;180(3):245–250. [DOI] [PubMed] [Google Scholar]
- 4. Park YM, et al. Association of exposure to artificial light at night while sleeping with risk of obesity in women. JAMA Intern Med. 2019;179(8):1061–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Obayashi K, et al. Ambient light exposure and changes in obesity parameters: a longitudinal study of the HEIJO-KYO cohort. J Clin Endocrinol Metab. 2016;101(9):3539–3547. [DOI] [PubMed] [Google Scholar]
- 6. Sun S, et al. Outdoor light at night and risk of coronary heart disease among older adults: a prospective cohort study. Eur Heart J. 2021;42(8):822–830. [DOI] [PubMed] [Google Scholar]
- 7. Yamauchi M, et al. Effects of environment light during sleep on autonomic functions of heart rate and breathing. Sleep Breath. 2014;18(4):829–835. [DOI] [PubMed] [Google Scholar]
- 8. Mason IC, et al. Light exposure during sleep impairs cardiometabolic function. Proc Natl Acad Sci USA. 2022;119(12):e2113290119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Greenland P, et al. Major risk factors as antecedents of fatal and nonfatal coronary heart disease events. JAMA 2003;290(7):891–897. [DOI] [PubMed] [Google Scholar]
- 10. Vu TH, et al. Prospective relationship of low cardiovascular risk factor profile at younger ages to ankle-brachial index: 39-year follow-up—the Chicago Healthy Aging Study. J Am Heart Assoc 2012;1(6):e001545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pirzada A, et al. Chicago Healthy Aging Study: objectives and design. Am J Epidemiol. 2013;178(4):635–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Friedman GD, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–1116. [DOI] [PubMed] [Google Scholar]
- 13. Grundy SM, et al. Prevention Conference VI: diabetes and cardiovascular disease: executive summary: conference proceeding for healthcare professionals from a special writing group of the American Heart Association. Circulation. 2002;105(18):2231–2239. [DOI] [PubMed] [Google Scholar]
- 14. Akerstedt T, et al. The subjective meaning of good sleep, an intraindividual approach using the Karolinska Sleep Diary. Percept Mot Skills. 1994;79(1 Pt 1):287–296. [DOI] [PubMed] [Google Scholar]
- 15. Respironics. Instruction Manual: Actiwatch Communication and Sleep Analysis Software. 2006. 910-0025-01 Rev B. https://johnawinegarden.files.wordpress.com/2015/03/actiwatchsoftware.pdf. [Google Scholar]
- 16. Morgenthaler T, et al. Practice Parameters for the Use of Actigraphy in the Assessment of Sleep and Sleep Disorders: An Update for 2007. Sleep 2007;30(4):519–529. [DOI] [PubMed] [Google Scholar]
- 17. MetMatters TRMS. The difference between meteorological and astronomical seasons.https://www.rmets.org/metmatters/difference-between-meteorological-and-astronomical-seasons. Accessed December 7, 2021.
- 18. Blume C, et al. “nparACT” package for R: A free software tool for the non-parametric analysis of actigraphy data . MethodsX. 2016;3:430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Witting W, et al. Alterations in the circadian rest-activity rhythm in aging and Alzheimer’s disease. Biol Psychiatry. 1990;27(6):563–572. [DOI] [PubMed] [Google Scholar]
- 20. Van Someren EJ, et al. Bright light therapy: improved sensitivity to its effects on rest-activity rhythms in Alzheimer patients by application of nonparametric methods. Chronobiol Int. 1999;16(4):505–518. [DOI] [PubMed] [Google Scholar]
- 21. Goncalves BS, et al. Nonparametric methods in actigraphy: an update. Sleep Sci (Sao Paulo, Brazil) 2014;7(3):158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim M, et al. Rest-activity rhythm disturbance in liver cirrhosis and association with cognitive impairment. Sleep 2020;44(6). doi: 10.1093/sleep/zsaa288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Catellier DJ, et al. Imputation of missing data when measuring physical activity by accelerometry. Med Sci Sports Exerc. 2005;37(11 Suppl) : S555–S562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pye J, et al. Irregular sleep-wake patterns in older adults with current or remitted depression. J Affect Disord. 2021;281:431–437. [DOI] [PubMed] [Google Scholar]
- 25. Palmer JR, et al. Rest-activity functioning is related to white matter microarchitecture and modifiable risk factors in older adults at-risk for dementia. Sleep 2021;44(7). doi: 10.1093/sleep/zsab007. [DOI] [PubMed] [Google Scholar]
- 26. Van Buuren S, et al. mice: multivariate imputation by chained equations in R. J Statist Software. 2011;45(1):1–67. [Google Scholar]
- 27. Graham JW, et al. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prevention Sci. 2007;8(3):206–213. [DOI] [PubMed] [Google Scholar]
- 28. R: A language and environment for statistical computing [computer program]. Vienna, Austria: R Foundation for Statistical Computing; 2020. [Google Scholar]
- 29. Package “ggplot2” [computer program]. 2016. [Google Scholar]
- 30. Obayashi K, et al. Independent associations of exposure to evening light and nocturnal urinary melatonin excretion with diabetes in the elderly. Chronobiol Int. 2014;31(3):394–400. [DOI] [PubMed] [Google Scholar]
- 31. Obayashi K, et al. Association between light exposure at night and nighttime blood pressure in the elderly independent of nocturnal urinary melatonin excretion. Chronobiol Int. 2014;31(6):779–786. [DOI] [PubMed] [Google Scholar]
- 32. Pu J, et al. Dyslipidemia in special ethnic populations. Cardiol Clin. 2015;33(2):325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dani H, et al. Nocturia: aetiology and treatment in adults. Nat Rev Urol. 2016;13(10):573–583. [DOI] [PubMed] [Google Scholar]
- 34. Punjabi NM, et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160(6):521–530. [DOI] [PubMed] [Google Scholar]
- 35. Sjöström C, et al. Prevalence of sleep apnoea and snoring in hypertensive men: a population based study. Thorax 2002;57(7):602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aubrecht TG, et al. Dim light at night increases body mass of female mice. Chronobiol Int. 2015;32(4):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fonken LK, et al. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci USA. 2010;107(43):18664–18669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fonken LK, et al. Dim light at night disrupts molecular circadian rhythms and increases body weight. J Biol Rhythms. 2013;28(4):262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Czeisler CA, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science (New York, NY). 1999;284(5423):2177–2181. [DOI] [PubMed] [Google Scholar]
- 40. Boivin DB, et al. Dose-response relationships for resetting of human circadian clock by light. Nature 1996;379(6565):540–542. [DOI] [PubMed] [Google Scholar]
- 41. Zeitzer JM, et al. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526(Pt 3):695–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dubocovich ML. Melatonin receptors: role on sleep and circadian rhythm regulation. Sleep Med. 2007;8(Suppl 3):34–42. [DOI] [PubMed] [Google Scholar]
- 43. Korkmaz A, et al. Role of melatonin in metabolic regulation. Rev Endocr Metab Disord. 2009;10(4):261–270. [DOI] [PubMed] [Google Scholar]
- 44. Katsi V, et al. The role of melatonin in hypertension: a brief review. Cardiovasc Endocrinol Metab. 2012;1(1):13–18. [Google Scholar]
- 45. McMullan CJ, et al. Melatonin secretion and the incidence of type 2 diabetes. JAMA 2013;309(13):1388–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Forman JP, et al. Urinary melatonin and risk of incident hypertension among young women. J Hypertens. 2010;28(3):446–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.