Abstract
Objective
Immune checkpoint inhibitors have been widely used in the treatment of endometrial cancer (EC) with microsatellite instability-hypermutated (MSI-H). However, there is an unmet need for microsatellite stable (MSS) EC because of their modest activity. This study aimed to identify potential immune-related biomarkers in MSS EC.
Methods
One hundred and twenty-three patients with EC who underwent hysterectomy were enrolled. MSI status was determined using MSI analysis and/or immunohistochemical staining for mismatch repair proteins. Immunohistochemical analysis of programmed cell death protein 1 (PD-1), programmed death-ligand 1 (PD-L1), PD-L2, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), cluster of differentiation 3 (CD3), CD8, lymphocyte activation gene-3 (LAG-3), indoleamine 2,3-dioxygenase 1 (IDO1), phosphatase and tensin homolog (PTEN), p53, AT-rich interactive domain-containing protein 1A (ARID1A), and β-catenin was performed using tissue microarray blocks.
Results
Among 123 patients, 95 (77.2%) were classified as having MSS. Within EC with MSS, PD-L1 positivity was significantly associated with positive PD-1 (p<0.001), CTLA-4 (p<0.001), CD3 (p=0.002), CD8 (p<0.001), and LAG-3 (p<0.001). In the univariate analysis, positive PD-1 (odds ratio [OR]=9.281; 95% confidence interval [CI]=2.560–33.653; p<0.001), CTLA-4 (OR=5.33; 95% CI=1.418–19.307; p=0.005), CD3 (OR=5.571; 95% CI=1.746–17.775; p=0.004), CD8 (OR=6.909; 95% CI=2.647–18.037; p<0.001), and LAG-3 (OR=9.75; 95% CI=1.947–48.828; p=0.005) were significantly associated with PD-L1 positivity in MSS EC. In the multivariate analysis, LAG-3 demonstrated a significant association with positive PD-L1 expression in MSS EC (OR=5.061; 95% CI=1.534–16.693; p=0.023).
Conclusion
In patients with MSS EC harboring PD-L1, LAG-3 may be a potential immunotherapeutic target. Clinical trials investigating the role of anti-LAG-3 antibodies, alone or in combination with other immunotherapies, are warranted.
Keywords: Lymphocyte Activation Gene-3, Programmed Cell Death-Ligand 1, Microsatellite Stable, Endometrial Neoplasms
Synopsis
Depth of myometrial invasion, positive programmed cell death protein 1 (PD-1), cluster of differentiation 3 (CD3), CD8, and lymphocyte activation gene-3 (LAG-3) were significantly associated with programmed death-ligand 1 (PD-L1) positivity in microsatellite stable (MSS) endometrial cancer (EC). In multivariate analysis, only LAG-3 demonstrated a significant association with positive PD-L1 expression in MSS EC. In patients with MSS EC harboring PD-L1, LAG-3 might be an immunotherapeutic target.
INTRODUCTION
Endometrial cancer (EC) is the second most prevalent gynecologic cancer in Korea, showing a rapidly increasing trend, with 3,182 new cases in 2018 [1]. Approximately 80% of patients with EC are diagnosed at an early stage, with a 5-year survival rate of >95% [2]. In contrast, those with advanced or recurrent disease have very limited therapeutic options if they fail taxane-platinum-based chemotherapy, leading to detrimental outcomes. Therefore, patients with advanced or recurrent diseases require a novel therapeutic regimen, such as immune checkpoint inhibitors.
EC is classified into four molecular subgroups: polymerase epsilon (POLE)-ultramutated, microsatellite instability-hypermutated (MSI-H), copy-number low, and copy-number high [3]. Among these four clusters, patients with either POLE-ultramutated or MSI-H subtypes showed significantly higher neoantigen levels and cluster of differentiation (CD) 3+/CD8+ tumor-infiltrating lymphocyte (TIL) concentrations than those with microsatellite stable (MSS) counterparts [4]. Moreover, according to a recent study performed on over 11,000 specimens from 39 different cancer types, the prevalence of MSI-H was mostly represented by EC (31.4%), gastric adenocarcinoma (19.1%), and colorectal adenocarcinoma (16.0%) [5]. In the phase 2 KEYNOTE-158 study, in which the antitumor activity of pembrolizumab monotherapy was evaluated in patients with multiple cancer types harboring MSI-H, patients with MSI-H EC exhibited the highest objective response rate of 57.1% [6]. Pembrolizumab was subsequently approved by the Food and Drug Administration (FDA) in 2017 for the treatment of MSI-H solid tumors [7].
However, most patients with EC harbor MSS subtypes and may be poor candidates for immune checkpoint inhibitor monotherapy. In 2019, the KEYNOTE-146/STUDY-111 revealed that a pembrolizumab plus lenvatinib combination achieved a favorable response rate in patients with MSS EC [8]. This study led to another FDA approval of this doublet regimen for patients with advanced EC not harboring MSI-H. Although promising, the response rate is still much lower than that of the MSI-H subtypes (36.2% vs. 63.6%). Moreover, additional analyses regarding other immune-related markers that could affect the efficacy of immune checkpoint inhibitors were not conducted. For both low endometrioid copy number and high copy number serous-like groups, a recent study indicated that an elevated neoantigen load was predicted as a prognostic factor [9]. This association led to the assumption that particular subsets are more immunogenic and respond better to immunotherapy, despite low tumor mutational burden. Additionally, finding from the KEYNOTE-028 showing modest response rate and progression-free survival after pembrolizumab monotherapy in programmed death-ligand 1 (PD-L1) positive, MSS EC prompted us to find novel immune-related biomarkers other than PD-L1 [10].
This study aims, compared with MSI-H EC, to find the subset among patients with MSS EC who may be an appropriate candidate for immunotherapy and discover immune-related markers that could be potentially targeted.
MATERIAL AND METHODS
1. Patient selection
A retrospective review was conducted on 198 patients with EC who underwent hysterectomy at the Korea University Guro Hospital from January 2013 to May 2020. Among them, 75 patients were excluded from the analysis because of non-endometrioid EC, inadequate specimens, and inability to obtain written informed consent. A total of 123 patients were enrolled in this study. Patients were included if they had endometrioid EC and underwent MSI analysis or DNA mismatch repair (MMR) gene immunohistochemical assay. Among them, 95 patients (95/123, 77.24%) were classified as MSS, whereas the remaining 28 (28/123, 22.76%) were classified as MSI-H. To identify the subset of patients and immune-related markers, a tissue microarray (TMA) was constructed from paraffin-embedded blocks from 123 patients with EC. Clinical and pathological data, including age, parity, stage, tumor size, tumor grade, depth of myometrial invasion, lymphovascular space invasion (LVSI), and lymph node metastasis (LNM), were collected from our hospital electronic medical records and original pathology reports. This study was approved by the Institutional Review Board of the Korea University Guro Hospital (2020GR0384), and written informed consent was obtained from all patients.
2. MSI status determination
The MSI status was determined using MSI analysis and/or immunohistochemical staining for MLH1 (1:100, clone ES05, Novocastra; Leica Biosystems, Seoul, Korea), MSH2 (1:400, clone G219-1129, Novocastra; Leica Biosystems), PMS2 (1:100, clone MRQ-28; Cell Marque, Rocklin, CA, USA), and MSH6 (1:200, clone 44; Cell Marque). MSI analysis was performed using the Bethesda panel or pentaplex panel. The Bethesda panel consisted of two mononucleotide repeat markers (BAT25 and BAT26) and three dinucleotide repeat markers (D2S123, D5S346, and D17S250), whereas the pentaplex panel contained five mononucleotide repeat markers (NR21, NR24, NR27, BAT25, and BAT26). Normal and tumor allele patterns were compared for each marker. Cases with ≥2, ≥1, and ≥0 positive markers were classified as MSI-H, MSI-low (MSI-L), and MSS, respectively. MMR immunostaining results were recorded as retained or loss of nuclear expression. Retained nuclear expression was considered normal/MMR-proficient, and loss of nuclear expression was abnormal/MMR deficiency.
3. Immunohistochemistry and interpretation
TMA blocks were constructed by selecting representative tumor areas. Three 2-mm-diameter tissue cores were collected from paraffin-embedded donor blocks and embedded in TMA blocks. Immunohistochemistry was performed using the BOND-III automated staining system (Leica, Wetzlar, Germany). The antibodies used were programmed cell death protein 1 (PD-1, 1:100, clone NAT105; Cell Marque), PD-L1 (1:50, clone 22C3; DAKO, Carpinteria, CA, USA), PD-L2 (1:50, clone 176611; R&D Systems, Minneapolis, MN, USA), cytotoxic T-lymphocyte-associated protein 4 (CTLA-4, 1:500, clone CAL 49; Abcam, Cambridge, UK), CD3 (1:150, polyclonal; DAKO), CD8 (1:1, clone SP57; Roche, Basel, Switzerland), lymphocyte-activation gene (LAG)-3 (1:1,000, EPR4392(2); Abcam), indoleamine 2,3-dioxygenase 1 (IDO1, 1:2,000, EPR20374; Abcam), phosphatase and tensin homolog (PTEN, 1:200, clone 6H2-1; DAKO), p53 (1:200, clone DO-7, Novocastra; Leica Biosystems), AT-rich interactive domain-containing protein 1A (ARID1A, 1:200, polyclonal, ATLAS), and β-catenin (1:2,000, clone 6F9; Thermo Fisher Scientific, Waltham, MA, USA). TMA slides were scanned using a three-dimensional Histech Pannoramic 250 Flash III scanner (3DHistech Ltd., Budapest, Hungary). Scanned immunohistochemical staining of TMA slides was manually scored by a pathologist (YKC) who was blinded to the clinical characteristics and outcomes.
PD-1 was considered positive when >1% of TIL showed cytoplasmic staining. PD-L1 expression was defined as partial or complete membranous staining in tumor cells and membranous and/or cytoplasmic staining in immune cells (lymphocytes and macrophages). To calculate the combined positive score (CPS), we divided the total number of PD-L1-positive cells (tumor cells, lymphocytes, and macrophages) by the number of viable tumor cells multiplied by 100. The cutoff for a positive PD-L1 CPS was set at ≥1. PD-L2 staining was classified as positive when membranous and/or cytoplasmic staining was present in ≥50% of tumor cells. CTLA-4-positive TILs were calculated as the number of average positive cells in three 0.2-mm2 hotspot areas and grouped as follows: 0, negative; 1–30, low; and >30, high. CD3-positive, CD8-positive, and LAG-3-positive TILs were scored in the same way (CD3 and CD8: 1–40, low and >40, high; LAG-3: 0, negative; 1–20, low; and >20, high). IDO1 was scored by combining the proportion score (PS) (PS: 0, negative; 1, <1%; 2, 1%–10%; 3, 10%–33.3%; 4, 33.3%–66.6%; and 5, >66.6%) of positive cells and staining intensity score (IS) (IS: 0, negative; 1, weak; 2, intermediate; and 3, strong). For IDO1, a combined PS and IS score of 0 was considered negative, scores of 2 were considered low, and those of 3–8 high. PTEN staining was evaluated according to three categories: retained, loss, or heterogeneous expression. Heterogeneous expression was defined as a tumor containing a mixed portion of complete loss and retained expression. P53 immunostaining was interpreted as a mutant type if the tumor exhibited diffuse strong nuclear staining in ≥80% of the tumor cells, unequivocal cytoplasmic staining with variable nuclear staining, or a complete absence of nuclear and cytoplasmic staining. Cases were considered wild type if any degree of non-diffuse nuclear staining (<80%) was present in the tumor cells. Nuclear staining intensity for ARID1A was classified as either lost or retained. β-catenin expression was documented as either nuclear (any nuclear positivity, regardless of the positive nuclei percentage) or membranous in glandular, squamous, and corded/spindled areas.
4. POLE mutation analysis
Genomic DNA was isolated from formalin-fixed paraffin-embedded tumor tissues using a QIAamp DNA FFPE mini KIT (QIAGEN, Germantown, MD, USA). POLE exonuclease domain mutation screening of hotspots in exons 9 (c.857C>G, p.P286R; c.890C>T, p.S297F), 13 (c.1231G>C, p.V411L), and 14 (c.1366G>C, p.A456P) was performed using Sanger sequencing. The following primers were used: Ex 9F (5 to 3), GCCTAATGGGGAGTTTAGAGC; Ex 9R (5 to 3), ATGCTGCTGTAGTATGGGGA; Ex 13F (5 to 3), TGCCTGTTAGGAACTTGCAT; Ex 13R (5 to 3), CACATGTCCTCCGGGTCTA; Ex 14F (5 to 3), TCTCCTTACTGTGTGTGCGG; Ex 14R (5 to 3), TCGGGCTCCATGGGAATAA. Polymerase chain reaction (PCR) was performed with 20-ng DNA input using AmpliTaq Gold 360 Master Mix (Applied Biosystems; Thermo Fisher Scientific). Subsequently, amplified PCR products were enzymatically purified using the PureLink Quick PCR Purification Kit (Invitrogen; Thermo Fisher Scientific) and bidirectionally sequenced using a BigDye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems; Thermo Fisher Scientific). Mutation analysis was performed using the Variant Reporter Software ver. 2.0 (Applied Biosystems; Thermo Fisher Scientific) and manual inspection.
5. Statistical analysis
To compare immune-related markers (PD-1, PD-L1, PD-L2, CTLA-4, CD3, CD8, LAG-3, IDO1, ARID1A, PTEN, POLE, β-catenin, and P53) and clinicopathological parameters (age, parity, stage, grade, tumor size, depth of myometrial invasion, LVSI, low uterine segment involvement, cervical involvement, and LNM) between patients with MSS EC and MSI-H EC and between patients with the presence or absence of index immune-related markers (PD-L1, PD-1, and PD-L2) in MSS EC, chi-squared and Fisher’s exact tests were used. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using logistic regression analysis. All p-values resulting from the tests of significance were 2-sided and considered significant at p<0.05. Statistical analysis was conducted using SPSS 20.0 (IBM Corp., Armonk, NY, USA).
RESULTS
The clinicopathological characteristics and expression of immune-related markers in 123 patients with EC classified as either MSS or MSI-H were compared (Table 1). No statistically significant differences were noted in age, parity, International Federation of Gynecology and Obstetrics (FIGO) stage, grade, tumor size, depth of myometrial invasion, LVSI, lower uterine segment involvement, cervical involvement, or LNM according to MSI status. Most patients presented with FIGO stage I and grades I–II.
Table 1. Comparison of immune-related markers and clinicopathological parameters of endometrioid EC patients by MSS and MSI-H status.
| Variables | MSS (n=95) | MSI-H (n=28) | p-value | |
|---|---|---|---|---|
| PD-1 | 0.002 | |||
| Negative | 36 (37.9) | 2 (7.1) | ||
| Positive | 59 (62.1) | 26 (92.9) | ||
| PD-L1 | 0.001 | |||
| Negative | 65 (68.4) | 10 (35.7) | ||
| Positive | 30 (31.6) | 18 (64.3) | ||
| PD-L2 | 0.424 | |||
| Negative | 6 (6.3) | 3 (10.7) | ||
| Positive | 89 (93.7) | 25 (89.3) | ||
| CTLA-4 | 0.081 | |||
| Negative | 13 (13.7) | 1 (3.6) | ||
| Low | 62 (65.3) | 16 (57.1) | ||
| High | 20 (21.0) | 11 (39.3) | ||
| CD3 | 0.073 | |||
| Low | 34 (35.8) | 5 (17.9) | ||
| High | 61 (64.2) | 23 (82.1) | ||
| CD8 | 0.001 | |||
| Low | 63 (66.3) | 9 (32.1) | ||
| High | 32 (33.7) | 19 (67.9) | ||
| LAG-3 | 0.010 | |||
| Negative | 44 (46.3) | 6 (21.4) | ||
| Low | 42 (44.2) | 14 (50.0) | ||
| High | 9 (9.5) | 8 (28.6) | ||
| IDO1 | 0.998 | |||
| Negative | 31 (32.6) | 9 (32.1) | ||
| Low | 30 (31.6) | 9 (32.1) | ||
| High | 34 (35.8) | 10 (35.8) | ||
| ARID1A | 1.000 | |||
| Retained | 80 (84.2) | 24 (85.7) | ||
| Loss | 15 (15.8) | 4 (14.3) | ||
| PTEN | <0.001 | |||
| Retained | 56 (58.9) | 5 (17.9) | ||
| Loss | 37 (38.9) | 23 (82.1) | ||
| Heterogeneous | 2 (2.1) | 0 (0.0) | ||
| POLE* | 0.437 | |||
| Wild | 75 (92.6) | 22 (88.0) | ||
| Mutant | 6 (7.4) | 3 (12.0) | ||
| β-catenin | 1.000 | |||
| Membranous | 90 (94.7) | 27 (96.4) | ||
| Nuclear | 5 (5.3) | 1 (3.6) | ||
| P53 | 0.453 | |||
| Wild | 85 (89.5) | 27 (96.4) | ||
| Mutant | 10 (10.5) | 1 (3.6) | ||
| Age (yr) | 0.427 | |||
| ≤50 | 20 (21.1) | 4 (14.3) | ||
| >50 | 75 (78.9) | 24 (85.7) | ||
| Parity | 0.913 | |||
| ≤1 | 35 (36.8) | 10 (35.7) | ||
| >1 | 60 (63.2) | 18 (64.3) | ||
| Stage | 0.732 | |||
| I–II | 85 (89.5) | 26 (92.9) | ||
| III–IV | 10 (10.5) | 2 (7.1) | ||
| Grade | 0.424 | |||
| I–II | 89 (93.7) | 25 (89.3) | ||
| III | 6 (6.3) | 3 (10.7) | ||
| Tumor size (cm) | 0.883 | |||
| ≤3 | 46 (48.4) | 14 (50.0) | ||
| >3 | 49 (51.6) | 14 (50.0) | ||
| DMI | 0.809 | |||
| No myometrial invasion | 33 (34.7) | 8 (28.6) | ||
| ≤50% | 42 (44.2) | 13 (46.4) | ||
| >50% | 20 (21.1) | 7 (25.0) | ||
| LVSI | 0.273 | |||
| Negative | 77 (81.1) | 20 (71.4) | ||
| Positive | 18 (18.9) | 8 (28.6) | ||
| LUSI | 0.760 | |||
| Negative | 81 (85.3) | 25 (89.3) | ||
| Positive | 14 (14.7) | 3 (10.7) | ||
| Cervical involvement | 0.207 | |||
| Negative | 90 (94.7) | 24 (85.7) | ||
| Positive | 5 (5.3) | 4 (14.3) | ||
| LNM | 0.315 | |||
| Negative | 72 (75.8) | 24 (85.7) | ||
| Positive | 5 (5.3) | 2 (7.1) | ||
| Not submitted | 18 (18.9) | 2 (7.1) | ||
ARID1A, AT-rich interactive domain-containing protein 1A; CD, cluster of differentiation; LA-4, cytotoxic T-lymphocyte-associated protein 4; DMI, depth of myometrial invasion; EC, endometrial cancer; IDO1, indoleamine 2,3-dioxygenase 1; LAG, lymphocyte activation gene; LNM, lymph node metastasis; LUSI, low uterine segment involvement; LVSI, lymphovascular invasion; MSI-H, microsatellite instability-high; MSS, microsatellite stable; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; POLE, polymerase epsilon; PTEN, phosphatase and tensin homolog.
*Seventeen patients were excluded from POLE due to insufficient tissue or failed test.
The expression patterns of various immune-related markers were compared between patients with MSS and those with MSI-H. Expectedly, patients with MSI-H showed significantly higher PD-1 (26/28, 92.9%) and PD-L1 (18/28, 64.3%) expressions than those with MSS (p=0.002 and p=0.001, respectively). CD3-positive TILs did not show significant differences between the two groups, whereas the proportion of CD8-positive TILs in the MSS group was much smaller than that in the MSI-H group (33.7% vs. 67.9%, p=0.001). The positive expression of LAG-3 was significantly greater in the MSI-H group than that in the MSS group (78.6% vs. 53.7%, p=0.010). Furthermore, the loss of PTEN expression was much higher in the MSI-H group than that in the MSS group (82.1% vs. 38.9%, p<0.000). Nine POLE mutations were identified; however, no differences were observed between the 2 groups.
When restricted to the MSS subgroup, both clinicopathological characteristics and expression patterns of immune-related markers were compared according to the PD-L1 status (Table 2). PD-L1 was positive in 30 of the 95 patients with MSS EC (31.6%). In PD-L1-positive patients, the positive rates of PD-1 (90.0% vs. 49.2%, p<0.001), CTLA-4 (50% vs. 7.7%, p<0.001), CD3 (86.7% vs. 53.8%, p=0.001), CD8 (63.3% vs. 20%, p<0.001), and LAG-3 (16.7% vs. 6.2%, p<0.001) were significantly higher than those in PD-L1-negative patients. Within this subset, the PD-L1-positive group had a higher POLE mutation rate than the PD-L1-negative group; however, the difference was not statistically significant.
Table 2. Comparison of immune-related markers and clinicopathological parameters according to PD-L1 positivity in MSS EC.
| Variables | PD-L1 negative (n=65) | PD-L1 positive (n=30) | p-value | |
|---|---|---|---|---|
| PD-1 | <0.001 | |||
| Negative | 33 (50.8) | 3 (10.0) | ||
| Positive | 32 (49.2) | 27 (90.0) | ||
| PD-L2 | 0.661 | |||
| Negative | 5 (7.7) | 1 (3.3) | ||
| Positive | 60 (92.3) | 29 (96.7) | ||
| CTLA-4 | <0.001 | |||
| Negative | 12 (18.5) | 1 (3.3) | ||
| Low | 48 (73.8) | 14 (46.7) | ||
| High | 5 (7.7) | 15 (50.0) | ||
| CD3 | 0.001 | |||
| Low | 30 (46.2) | 4 (13.3) | ||
| High | 35 (53.8) | 26 (86.7) | ||
| CD8 | <0.001 | |||
| Low | 52 (80.0) | 11 (36.7) | ||
| High | 13 (20.0) | 19 (63.3) | ||
| LAG-3 | <0.001 | |||
| Negative | 39 (60.0) | 5 (16.7) | ||
| Low | 22 (33.8) | 20 (66.6) | ||
| High | 4 (6.2) | 5 (16.7) | ||
| IDO1 | 0.756 | |||
| Negative | 21 (32.2) | 10 (33.3) | ||
| Low | 22 (33.8) | 8 (26.7) | ||
| High | 22 (33.8) | 12 (40.0) | ||
| ARID1A | 1.000 | |||
| Retained | 55 (84.6) | 25 (83.3) | ||
| Loss | 10 (15.4) | 5 (16.7) | ||
| PTEN | 0.374 | |||
| Retained | 41 (63.1) | 15 (50.0) | ||
| Loss | 23 (35.4) | 14 (46.7) | ||
| Heterogeneous | 1 (1.5) | 1 (3.3) | ||
| POLE* | 0.080 | |||
| Wild | 53 (96.4) | 22 (84.6) | ||
| Mutant | 2 (3.6) | 4 (15.4) | ||
| β-catenin | 0.175 | |||
| Membranous | 60 (92.3) | 30 (100.0) | ||
| Nuclear | 5 (7.7) | 0 (0.0) | ||
| P53 | 1.000 | |||
| Wild | 58 (89.2) | 27 (90.0) | ||
| Mutant | 7 (10.8) | 3 (10.0) | ||
| Age (yr) | 0.476 | |||
| ≤50 | 15 (23.1) | 5 (16.7) | ||
| >50 | 50 (76.9) | 25 (83.3) | ||
| Parity | 0.372 | |||
| ≤1 | 22 (33.8) | 13 (43.3) | ||
| >1 | 43 (66.2) | 17 (56.7) | ||
| Stage | 1.000 | |||
| I–II | 58 (89.2) | 27 (90.0) | ||
| III–IV | 7 (10.8) | 3 (10.0) | ||
| Grade | 1.000 | |||
| I–II | 61 (93.8) | 28 (93.3) | ||
| III | 4 (6.2) | 2 (6.7) | ||
| Tumor size (cm) | 0.274 | |||
| ≤3 | 29 (44.6) | 17 (56.7) | ||
| >3 | 36 (55.4) | 13 (43.3) | ||
| DMI | 0.059 | |||
| No myometrial invasion | 18 (27.6) | 15 (50.0) | ||
| ≤50% | 30 (46.2) | 12 (40.0) | ||
| >50% | 17 (26.2) | 3 (10.0) | ||
| LVSI | 0.061 | |||
| Negative | 56 (86.2) | 21 (70.0) | ||
| Positive | 9 (13.8) | 9 (30.0) | ||
| LUSI | 1.000 | |||
| Negative | 55 (84.6) | 26 (86.7) | ||
| Positive | 10 (15.4) | 4 (13.3) | ||
| Cervical involvement | 0.175 | |||
| Negative | 60 (92.3) | 30 (100.0) | ||
| Positive | 5 (7.7) | 0 (0.0) | ||
| LNM | 0.546 | |||
| Negative | 47 (72.3) | 25 (83.3) | ||
| Positive | 4 (6.2) | 1 (3.3) | ||
| Not submitted | 14 (21.5) | 4 (13.4) | ||
ARID1A, AT-rich interactive domain-containing protein 1A; CD, cluster of differentiation; LA-4, cytotoxic T-lymphocyte-associated protein 4; DMI, depth of myometrial invasion; EC, endometrial cancer; IDO1, indoleamine 2,3-dioxygenase 1; LAG, lymphocyte activation gene; LNM, lymph node metastasis; LUSI, low uterine segment involvement; LVSI, lymphovascular invasion; MSI-H, microsatellite instability-high; MSS, microsatellite stable; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; POLE, polymerase epsilon; PTEN, phosphatase and tensin homolog.
*Fourteen patients were excluded from POLE due to insufficient tissue or failed test.
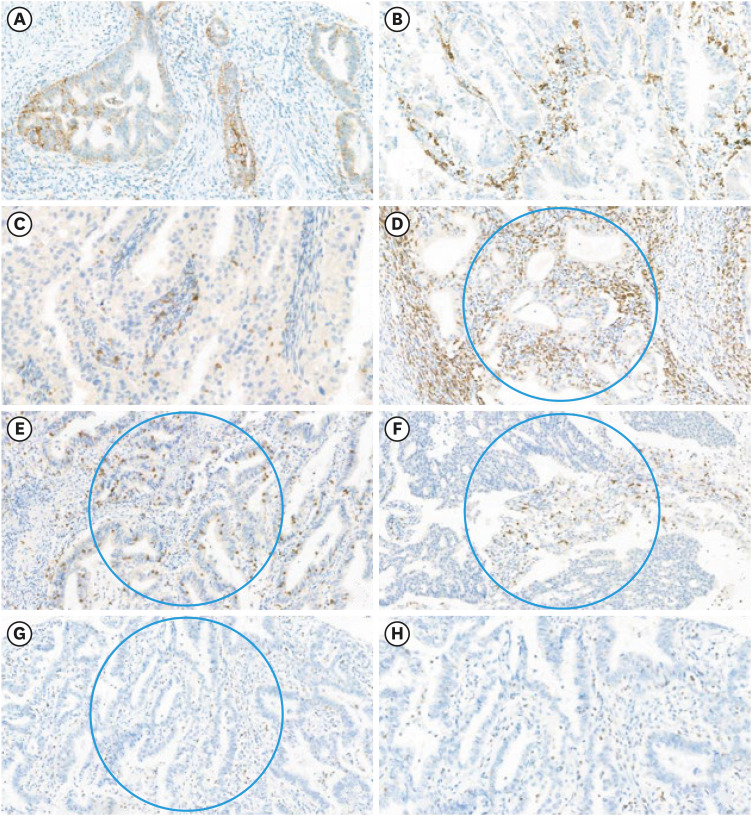
Using univariate analyses, we found that CTLA-4, PD-1, CD3, CD8, and LAG-3 expressions were significantly associated with PD-L1 positivity in MSS EC (p=0.005, p<0.001, p=0.004, p<0.001, and p=0.005, respectively) (Table 3). In contrast, the remaining clinicopathological parameters and immune-related markers were not associated with PD-L1 positivity. Representative immunohistochemical images are shown in Fig. 1. On multivariate analyses, only LAG-3 showed a statistically significant association with PD-L1 positivity in MSS EC (OR=5.061; 95% CI=1.534–16.693; p=0.023).
Table 3. Univariate and multivariate analyses of clinicopathological parameters and immune-related markers for PD-L1 positivity in MSS EC.
| Immune-related markers | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| CTLA-4 | 5.33 (1.418–19.307) | 0.005 | ||
| PD-1 | 9.281 (2.56–33.653) | <0.001 | ||
| CD3 | 5.571 (1.746–17.775) | 0.004 | ||
| CD8 | 6.909 (2.647–18.037) | <0.001 | ||
| LAG-3 | 9.75 (1.947–48.828) | 0.005 | 5.061 (1.534–16.693) | 0.023 |
CD, cluster of differentiation; CI, confidence interval; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; EC, endometrial cancer; LAG, lymphocyte activation gene; MSS, microsatellite stable; OR, odds ratio; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1.
Fig. 1. Representative images of immune-related biomarkers. (A) PD-L1 expression in tumor cells and (B) immune cells (×50 magnification). (C) PD-1 expression in TILs (×50). (D) CD3-positive TILs, (E) CD8-positive TILs, (F) CTLA-4-positive TILs, (G) LAG-3-positive TILs in a 0.2 mm2 area of hotspot (blue circle) (×40). (H) LAG-3-positive TILs showing cytoplasmic staining (×60).
CD, cluster of differentiation; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; LAG, lymphocyte activation gene; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; TIL, tumor-infiltrating lymphocyte.
For the exploratory analysis, we compared the clinicopathological parameters and immune-related markers according to PD-1 positivity in MSS EC (Supplementary Table 1). When PD-1 was positive, positive expression rates of PD-L1 (45.7% vs. 8.3%, p<0.001), CTLA-4 (33.9% vs. 0%, p<0.001), CD3 (76.3% vs. 44.4%, p=0.001), CD8 (47.5% vs. 11.1%, p<0.001), and LAG-3 (15.3% vs. 0%, p<0.001) were also significantly higher than their PD-1 negative counterparts. Univariate analyses revealed that PD-L1, CD3, CD8, and LAG-3 (p<0.001, p=0.002, p<0.001, p<0.001, respectively) were significantly associated with PD-1 positivity, whereas only LAG-3 was proven to be significantly associated with PD-1 positivity in MSS EC (OR=3.117; 95% CI=1.132–8.583; p=0.027) in the multivariate analysis (Supplementary Table 2). In contrast, PD-L2 was not associated with clinicopathological parameters or other immune-related markers (Supplementary Table 3).
DISCUSSION
In this analysis, LAG-3 was found to be a promising immunotherapeutic target among patients with MSS EC, whose response to immune checkpoint inhibitors is lacking. To the best of our knowledge, we are the first to examine the expression of bundles of immune-related biomarkers and demonstrated the possibility of the clinical usefulness of LAG-3 in poor responders to immune checkpoint inhibitor monotherapy in EC.
Tumors harboring high mutational burdens are advantageous in adopting immune checkpoint inhibitors, considering their inclination to mount adaptive mechanisms of immune evasion [11,12]. In this regard, MSI-H or MMR deficiency is a well-recognized driver of upregulated tumor mutational burden resulting in remarkable responsiveness to immune checkpoint inhibitors in various malignancies [13,14]. For example, a previous study showed that only 1 of 33 patients with colorectal cancer responded to anti-PD-1 antibody (nivolumab) and that the patient was proven to have MMR deficiency [15]. Another study including advanced cancers with MMR deficiencies across 12 different tumor types revealed an excellent objective response rate of 53% following pembrolizumab monotherapy [16]. They concluded that a large proportion of mutant neoantigens in MMR-deficient tumors make them sensitive to immune checkpoint blockade, regardless of the cancer tissue of origin. In our study, when comparing MSI-H and MSS EC, the expression of PD-1, PD-L1, and CD3/8-positive TILs, which are related to the host anti-tumor immune response, was significantly higher in the MSI-H group, which is consistent with results from previous literature [4].
However, most patients with EC present MSS phenotypes. In this regard, LAG-3, which was identified in this analysis, could be an effective alternative. LAG-3 is a co-receptor found on activated T lymphocytes, activated B lymphocytes, regulatory T cells, and natural killer cells [17]. Like CD4, LAG-3 binds to major histocompatibility complex (MHC)-II on antigen-presenting cells with high affinity [18]. By interacting with MHC-II, LAG-3 prevents the binding of the same MHC molecule to the T cell receptor and CD4, thus directly interfering with T cell signaling in the immune response [19]. TILs with high LAG-3 expression have been found in several solid tumors, including hepatocellular carcinoma, non–small cell lung cancer, melanoma, hematological cancer, and ovarian cancer [20,21,22,23,24]. LAG-3 expression causes T cell exhaustion and contributes to immune escape in carcinogenesis, similar to other immune checkpoints, including PD-1 and PD-L1 [25]. Furthermore, upregulation is likely to confer acquired resistance, which is frequently observed in patients treated with immune checkpoint inhibitors who initially respond [26].
The role of LAG-3 in ECs has not yet been clearly elucidated. Friedman et al. demonstrated that MSI-H EC had higher levels of LAG-3+ and CD3+ lymphocytes than their MSS counterparts [27]. Moreover, our study provided the same result, as shown in Table 1; however, the difference from their study was that we focused on the MSS group, which has not been evaluated in previous studies. When analyses were performed within the MSS group, we explored whether the expression of immune-related biomarkers differed according to PD-L1 expression (Table 2). There are two reasons for choosing PD-L1 as a pivotal parameter. First, in the case of advanced EC without MSI-H, PD-L1 is the most widely validated immune checkpoint marker. For example, in a multi-cohort, open-label, phase Ib basket trial (KEYNOTE-028) evaluating the safety and efficacy of pembrolizumab in 20 advanced, PD-L1-positive solid tumor cohorts, pembrolizumab was associated with a median response duration of 6 months and a 6-month progression-free survival rate of 19.0% in women with PD-L1-positive EC [10]. Second, increased PD-L1 expression rates have been reported in previous studies. Liu et al. showed positive rates of PD-L1 expression (83%, 68%, and 100% in primary, recurrent, and metastatic EC, respectively) [28]. Additionally, a study reported a tumor PD-L1 positivity rate of 48%, even in MSS endometrioid EC [29]. Compared with previous data, PD-L1 expression rate of 31.6% in our study was relatively low, probably due to the different types of antibodies used in immunohistochemistry or different calculation methods. Nevertheless, similar to a study by Crumley et al. [29], we demonstrated the increased expression of CTLA-4 and LAG-3, as well as CD3 and CD8, in a subset of MSS EC with positive PD-L1. Interestingly, the proportion of POLE-mutant patients was five-fold higher than that of POLE-wild-type patients in MSS EC with positive PD-L1; however, the difference was not statistically significant (p=0.080, Table 2). This suggests that there is a subset that may be more immunogenic and could be a suitable candidate for immunotherapy, such as anti-LAG-3 antibody treatment, as shown in our data.
In our analysis, LAG-3 expression was independently and positively associated with PD-L1 positivity in MSS EC, suggesting that strategies targeting LAG-3 could be promising in patients who cannot benefit from immune checkpoint inhibitor monotherapy. Currently, there are several ongoing LAG-3-targeted immunotherapy trials for various types of cancers using anti-LAG-3 monoclonal antibodies, mainly in conjunction with other immune checkpoint inhibitors. Some studies have demonstrated little oncologic benefit for single-agent anti-LAG antibodies; however, considerable responses are noted when anti-LAG-3 therapy is used in combination with anti-PD-1 therapy [25]. An ongoing active LAG-3-targeting trial in EC has not been conducted; however, a study of nivolumab and relatlimab (anti-LAG-3 monoclonal antibody) in patients with MSS advanced colorectal cancer is currently in progress (NCT03642067). Therefore, an urgent need to conduct clinical trials using anti-LAG-3 antibodies in EC, especially for the MSS phenotype, is warranted.
A major limitation of our study is the small sample size of MSS EC. Several reasons can be considered: first, this study was conducted in a single institution; second, we could only use those samples for which informed consent had been obtained from patients; however, there were several samples without this consent. Another limitation is that the experimental technique we adopted to find the association between LAG-3 expression and PD-L1 in MSS EC was immunohistochemistry alone. Therefore, our results needs validation study whether correlation with PD-L1 represents the effectiveness of immunotherapy. Nevertheless, the strength of our study is that, for the first time, we attempted to identify clinically useful immunotherapeutic targets in patients with MSS EC and suggested that LAG-3 could be a potential target in such patient subsets in the era of immune checkpoint inhibitor therapy.
In conclusion, LAG-3 expression was positively associated with positive PD-L1 expression in MSS ECs. These findings suggest that immunotherapy targeting LAG-3 may have an impact on the treatment of patients who can hardly anticipate benefits from immune checkpoint inhibitor monotherapy.
Footnotes
Funding: This work was supported by the Korea University Guro Hospital (Korea Research-Driven Hospitals) and a grant funded by Korea University Medicine (No. K2117211).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: H.J.H., C.H.W., L.J.K., C.Y.
- Data curation: H.J.H., O.Y.T., C.Y.
- Investigation: C.Y.
- Methodology: C.H.W.
- Writing - original draft: H.J.H., C.H.W., O.Y.T., L.J.K., C.Y.
- Writing - review & editing: H.J.H., C.H.W., O.Y.T., L.J.K., C.Y.
SUPPLEMENTARY MATERIALS
Comparison of immune-related markers and clinicopathological parameters according to PD-1 positivity in MSS EC
Univariate and multivariate analyses of clinicopathological parameters and immune-related markers for PD-1 positivity in MSS EC
Comparison of immune-related markers and clinicopathological parameters according to PD-L2 positivity in MSS EC
References
- 1.Hong S, Won YJ, Lee JJ, Jung KW, Kong HJ, Im JS, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2018. Cancer Res Treat. 2021;53:301–315. doi: 10.4143/crt.2021.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: diagnosis, treatment and follow-up. Ann Oncol. 2016;27:16–41. doi: 10.1093/annonc/mdv484. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Research Network. Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howitt BE, Shukla SA, Sholl LM, Ritterhouse LL, Watkins JC, Rodig S, et al. Association of polymerase e-mutated and microsatellite-instable endometrial cancers with neoantigen load, number of tumor-infiltrating lymphocytes, and expression of PD-1 and PD-L1. JAMA Oncol. 2015;1:1319–1323. doi: 10.1001/jamaoncol.2015.2151. [DOI] [PubMed] [Google Scholar]
- 5.Bonneville R, Krook MA, Kautto EA, Miya J, Wing MR, Chen HZ, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol. 2017;2017:PO.17.00073. doi: 10.1200/PO.17.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38:1–10. doi: 10.1200/JCO.19.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcus L, Lemery SJ, Keegan P, Pazdur R. FDA approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res. 2019;25:3753–3758. doi: 10.1158/1078-0432.CCR-18-4070. [DOI] [PubMed] [Google Scholar]
- 8.Makker V, Taylor MH, Aghajanian C, Oaknin A, Mier J, Cohn AL, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38:2981–2992. doi: 10.1200/JCO.19.02627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shukla SA, Howitt BE, Wu CJ, Konstantinopoulos PA. Predicted neoantigen load in non-hypermutated endometrial cancers: correlation with outcome and tumor-specific genomic alterations. Gynecol Oncol Rep. 2016;19:42–45. doi: 10.1016/j.gore.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ott PA, Bang YJ, Berton-Rigaud D, Elez E, Pishvaian MJ, Rugo HS, et al. Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1-positive endometrial cancer: results from the KEYNOTE-028 study. J Clin Oncol. 2017;35:2535–2541. doi: 10.1200/JCO.2017.72.5952. [DOI] [PubMed] [Google Scholar]
- 11.Liu S, Gönen M, Stadler ZK, Weiser MR, Hechtman JF, Vakiani E, et al. Cellular localization of PD-L1 expression in mismatch-repair-deficient and proficient colorectal carcinomas. Mod Pathol. 2019;32:110–121. doi: 10.1038/s41379-018-0114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C, Gong J, Tu TY, Lee PP, Fakih M. Immune profiling of microsatellite instability-high and polymerase ε (POLE)-mutated metastatic colorectal tumors identifies predictors of response to anti-PD-1 therapy. J Gastrointest Oncol. 2018;9:404–415. doi: 10.21037/jgo.2018.01.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sloan EA, Ring KL, Willis BC, Modesitt SC, Mills AM. PD-L1 expression in mismatch repair-deficient endometrial carcinomas, including Lynch syndrome-associated and MLH1 promoter hypermethylated tumors. Am J Surg Pathol. 2017;41:326–333. doi: 10.1097/PAS.0000000000000783. [DOI] [PubMed] [Google Scholar]
- 14.Boyiadzis MM, Kirkwood JM, Marshall JL, Pritchard CC, Azad NS, Gulley JL. Significance and implications of FDA approval of pembrolizumab for biomarker-defined disease. J Immunother Cancer. 2018;6:35. doi: 10.1186/s40425-018-0342-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lythgoe MP, Liu DS, Annels NE, Krell J, Frampton AE. Gene of the month: lymphocyte-activation gene 3 (LAG-3) J Clin Pathol. 2021;74:543–547. doi: 10.1136/jclinpath-2021-207517. [DOI] [PubMed] [Google Scholar]
- 18.Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, et al. LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med. 1990;171:1393–1405. doi: 10.1084/jem.171.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldberg MV, Drake CG. LAG-3 in cancer immunotherapy. Curr Top Microbiol Immunol. 2011;344:269–278. doi: 10.1007/82_2010_114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107:7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemon P, Jean-Louis F, Ramgolam K, Brignone C, Viguier M, Bachelez H, et al. MHC class II engagement by its ligand LAG-3 (CD223) contributes to melanoma resistance to apoptosis. J Immunol. 2011;186:5173–5183. doi: 10.4049/jimmunol.1002050. [DOI] [PubMed] [Google Scholar]
- 22.Keane C, Law SC, Gould C, Birch S, Sabdia MB, Merida de Long L, et al. LAG3: a novel immune checkpoint expressed by multiple lymphocyte subsets in diffuse large B-cell lymphoma. Blood Adv. 2020;4:1367–1377. doi: 10.1182/bloodadvances.2019001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He Y, Yu H, Rozeboom L, Rivard CJ, Ellison K, Dziadziuszko R, et al. LAG-3 protein expression in non-small cell lung cancer and its relationship with PD-1/PD-L1 and tumor-infiltrating lymphocytes. J Thorac Oncol. 2017;12:814–823. doi: 10.1016/j.jtho.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Guo M, Yuan F, Qi F, Sun J, Rao Q, Zhao Z, et al. Expression and clinical significance of LAG-3, FGL1, PD-L1 and CD8+ T cells in hepatocellular carcinoma using multiplex quantitative analysis. J Transl Med. 2020;18:306. doi: 10.1186/s12967-020-02469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrews LP, Marciscano AE, Drake CG, Vignali DA. LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev. 2017;276:80–96. doi: 10.1111/imr.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Friedman LA, Ring KL, Mills AM. LAG-3 and GAL-3 in endometrial carcinoma: emerging candidates for immunotherapy. Int J Gynecol Pathol. 2020;39:203–212. doi: 10.1097/PGP.0000000000000608. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Liu Y, Wang W, Wang C, Che Y. Expression of immune checkpoint molecules in endometrial carcinoma. Exp Ther Med. 2015;10:1947–1952. doi: 10.3892/etm.2015.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crumley S, Kurnit K, Hudgens C, Fellman B, Tetzlaff MT, Broaddus R. Identification of a subset of microsatellite-stable endometrial carcinoma with high PD-L1 and CD8+ lymphocytes. Mod Pathol. 2019;32:396–404. doi: 10.1038/s41379-018-0148-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of immune-related markers and clinicopathological parameters according to PD-1 positivity in MSS EC
Univariate and multivariate analyses of clinicopathological parameters and immune-related markers for PD-1 positivity in MSS EC
Comparison of immune-related markers and clinicopathological parameters according to PD-L2 positivity in MSS EC