Abstract
Objective
To conduct a systematic review and meta-analysis of studies evaluating the oncological and fertility outcomes of early-stage endometrial cancer (EC) treated with the levonorgestrel-releasing intrauterine system (LIUS)-based regimens.
Methods
The Meta-analyses Of Observational Studies in Epidemiology statement for meta-analyses was followed. Searches were conducted on MEDLINE, Embase, PubMed, Preprints, and the Cochrane Central Register of Controlled Trials from January 1990 to August 4, 2022. The Joanna Briggs Institute Critical Appraisal Checklist was used for quality assessment. The primary endpoint was the complete response (CR) rate and the secondary endpoints were relapse, pregnancy, and live birth rate.
Results
A total of 25 studies (821 women) were included. The CR rate of LIUS-based regimens was 63.4% (95% confidence interval [CI]=52.3%–73.2%), with 29.6% (95% CI=23.3%–36.8%) of cases experiencing recurrence during follow-up. In sensitivity analyses, patients younger than 45 years of age with a body mass index <30 kg/m2 who were treated with LIUS-based regimens achieved a high CR rate of 84.6% (95% CI=80.3%–88.1%) over a median follow-up of more than 24 months. Overall pregnancy and live birth rates were 37.9% (95% CI=24.1%–53.9%) and 39.3% (95% CI=24.0%–57.0%), respectively. No statistical differences were apparent in CR or relapse rates among the LIUS+GnRH agonist, LIUS+oral progesterone, or hysteroscopic resection followed by LIUS subgroups.
Conclusion
LIUS-based therapies are viable for the conservative management of early-stage endometrioid EC on CR and fertility outcome.
Trial Registration
PROSPERO Identifier: CRD42022352890
Keywords: Endometrial Cancer, Intrauterine Devices, Mirena, Progesterone, Conservative Management, Fertility Preservation
Synopsis
Women younger than 45 with body mass index <30 kg/m2 achieved a high complete response rate of 84.6% (95% confidence interval=80.3%–88.1%) with follow-ups of at least 24 months after levonorgestrel-releasing intrauterine system-based therapies of endometrioid endometrial cancer.
INTRODUCTION
Endometrial cancer (EC) is the most prevalent gynecological cancer in developed countries, with annual new diagnosis rates increasing globally. Among all ECs, endometrioid histology is the most common (80%), and obesity is a major underlying cause [1]. Surgical resection is the standard therapy for EC. However, this procedure may not be ideal for women with co-morbidities, when operating is difficult, or for preserving fertility. Thus, the search for viable non-surgical treatments for this population is ongoing. In the mid-20th century, progestational agents were successfully given as adjuvant or primary therapies in patients with metastatic endometrial carcinoma [2]. Later, these agents were used as an alternative to hysterectomy for young women with EC who had not completed childbearing. Systemic progestogens, such as medroxyprogesterone acetate (MPA) and megestrol acetates (MA), are efficacious in the treatment of hormone-sensitive, early-stage, well-differentiated endometrioid endometrial cancer (EEC), with a response rate of around 75.3%–88.7% and approximately 15.8%–35.2% of those cases experienced recurrence and requiring a hysterectomy [3]. However, progesterone receptors are often downregulated, leading to a relatively short therapeutic duration. In addition, systemic therapy is associated with low compliance rates due to adverse effects, including weight gain, nausea, abnormal uterine bleeding, and an increased risk of breast cancer [4]. Local progesterone delivery to uterus lesions could achieve the desired therapeutic outcomes for these individuals, concurrently avoiding any associated systemic adverse effects.
The levonorgestrel-releasing intrauterine system (LIUS, Mirena®; Bayer Global, Leverkusen, Germany) was initially introduced for contraceptive use in the mid-1970s. LIUS is a T-shaped device that releases high local concentrations of levonorgestrel (20 µg/day) into the uterine cavity over 5 years, with relatively low levels appearing in the serum. This leads to decidualization of the stroma, mucosal thinning, and, eventually, suppression of endometrial growth. In 2002, Montz et al. [5] first reported using a progesterone-containing intrauterine device in treating early-stage EEC with a favorable response rate and limited adverse effects. Since then, local progesterone devices have been increasingly utilized as an alternative to oral progesterone (OP) for fertility preservation in EEC patients. Despite the most common fertility-sparing option being systemic hormone therapy (HT), LIUS-based therapies combining LIUS with GnRH agonist (GnRHa), OP, an aromatase inhibitor, or hysteroscopic resection (HR) are continuously adopted for select EEC patients and represent promising conservative alternatives to the management of ECC. In fact, high response and pregnancy rates (75% and 47%) after LIUS-based treatment were observed in patients with G1 EEC [6]. However, some authors recommended interpreting the data with caution due to the highly variable response rate in the LIUS-based management of EEC [7,8,9,10]. Previously, no systematic reviews have focused on the outcomes of LIUS in treating early-stage EEC. Given the significantly different oncological outcomes of HT between atypical endometrial hyperplasia (AEH) and EC, this study aims to determine the effectiveness of LIUS-based therapies for conservative treatment on oncologic and reproductive outcomes in patients with EEC.
MATERIALS AND METHODS
1. Identification of literature
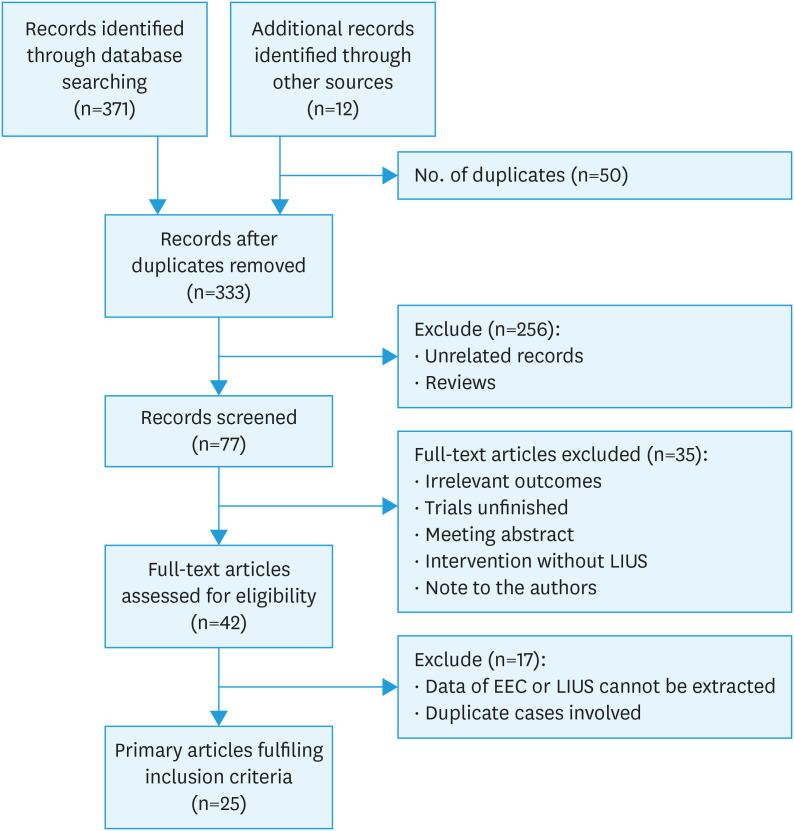
Meta-analyses Of Observational Studies in Epidemiology (MOOSE) guidance for systematic reviews of observational studies was followed (Fig. S1). The inclusion criteria for this systematic review were women with EC who received LIUS-based treatments with disease regression or relapse outcomes. The Embase, MEDLINE, PubMed, Preprints, and Cochrane Central Register of Controlled Trials databases were searched for articles published between January 1990 and August 4, 2022. The detailed study scheme and search strategies are described in Fig. 1 and Table S1. References in relevant primary publications were hand-searched to identify other eligible studies. Language or geographical restrictions were not applied during the search and selection process. Searches were independently conducted by investigators H.W. and N.P.
Fig. 1. Study flow diagram.
EEC, endometrioid endometrial cancer; LIUS, levonorgestrel-releasing intrauterine system.
2. Study selection
Studies were selected if the participants included women histologically diagnosed with EEC that was presumed stage IA, grade 1 or grade 2; the treatments included LIUS or LIUS-based regimens; and the outcome included histologic disease regression rate, as assessed via endometrial biopsy or hysterectomy specimen. Controlled and uncontrolled study designs were included. Non-English language manuscripts, disease with stage IB–IV, review articles, comments or those with the same patient information, posters, case series <5 patients, and articles where it was impossible to separate patients with AEH from those with EC were excluded. Studies were selected through a 2-stage process. First, the titles and abstracts from the electronic searches were scrutinized by 2 independent reviewers (H.W. and N.P.), and full manuscripts of all citations that met the predefined selection criteria were obtained. Secondly, final inclusion or exclusion decisions were made upon examination of the complete manuscripts (H.W. and W.G.). In cases of duplication, the most recent or complete publication was used. A third reviewer was consulted in cases without consensus (C.M.). Excluded studies and exclusion justification are presented in Table S2.
3. Data extraction
Data were extracted by 2 independent reviewers (H.W. and Z.D.). Study population (country, number of participants, age, body mass index [BMI], histologic grade, indications, treatment protocol, type of diagnostic method, and follow-up time), study period, study design, and the significant outcomes (complete response [CR] to treatment, recurrence, pregnancies, and live births) were collected.
4. Quality assessment
The quality of the included studies was assessed using the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for cohort/case series studies. Two independent reviewers (W.Z. and N.P.) performed quality assessments, and a third reviewer (G.X.) was consulted if no consensus could be reached.
5. Statistical analysis
The primary endpoint for this meta-analysis was the CR rate for different LIUS-based treatments for patients with EEC. The secondary endpoints were relapse rate, pregnancy, and live birth rate. The outcome incidence was explored using a random or fixed effects model to combine data. Event rates with a 95% confidence interval (CI) were extracted from each study. The I2 statistic was used to study heterogeneity. A random-effects model was used for I2 statistics >50%; otherwise, the fixed-effect model was employed. Sensitivity analyses were performed using the leave-one-out strategy, as well as by repeating the meta-analyses in subsets of studies defined by criteria related to age (≤45 years old), type of LIUS-based treatment, and follow-up time (6–12, 12–24, 24–36, and >36 months). In addition, publication bias on the primary endpoint was assessed by constructing funnel plots by Egger’s test (Fig. S2). All analyses were performed using Comprehensive Meta-Analysis statistical software, version 3.0 (Biostat, Englewood, NJ, USA).
6. Ethics approval
Ethical approval was not required for meta-analysis and systematic review.
7. Study registration
A protocol was prospectively registered in PROSPERO as CRD42022352890.
RESULTS
1. Basic characteristics of included studies
The search queries returned 371 records (50 duplicates), which were screened based on title and abstract. Twelve additional studies were identified through hand-searching. There were no unpublished studies included. Among these, 256 were excluded after the title and abstract review because they did not fulfill the selection criteria. Full manuscripts of the remaining 42 articles were assessed concerning their eligibility for inclusion, and 25 studies (821 women with clinical stage 1A, G1–G2, EEC managed with LIUS-based conservative treatment) were included in the systematic review (Table 1, Fig. 1). The studies had either a cohort (40%) or case series (60%) design. Seven studies were prospectively designed, and 18 were retrospectively designed. Trials originated in Italy (n=8), USA (n=6), Korea (n=5), Australia (n=3), Russia (n=2), and Spain (n=1). The median age across the studies ranged from 30 to 69, and 9 studies (36%) included women aged 45 or older with an average BMI >30 kg/m2, and mean BMI varied between 14.8–79.0 kg/m2. The mean follow-up length across the studies ranged from 6 to 92 months. LIUS insertion indications included medical co-morbidities, morbid obesity, desire for future childbearing potential, or patient choice. One study presented 20 patients with myometrium infiltration less than 2 mm on MRI [11]. The 18 studies enrolled the patients without myometrium invasion. The cases of myometrium involvement in 6 studies were unavailable [7,12,13,14,15,16]. Four progesterone treatment regimens were involved in these studies, including LIUS monotherapy and HR followed by LIUS, LIUS+GnRHa, and LIUS+OP. The grade of carcinoma (grade 1 or 2) being treated also varied between the studies. Quality assessment of the included studies was performed using the JBI Critical Appraisal Checklist for cohort studies and case series studies (Tables S3 and S4).
Table 1. The basic characteristics of included studies.
| Study (yr) | Country | Study design | No. of evaluable patients/Grade (n) | Age (yr) | BMI (kg/m2) | Study period | Indications | Treatment protocol | Follow-up (mo) | Type of diagnostic method during follow-up | Reported outcomes | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fertility | Co-morbidity and others | |||||||||||
| Pino et al. (2022) [6] | Italy | R | 75/G1 (75) | 34.7±5.5 | 25.2±6.7 | 2005–2020 | 75 | - | LIUS+GnRHa/LIUS+OP/LIUS+OP+Met | 45 (6–180) | Hysteroscopy | CR, PR, PD, SD relapse, FO |
| Lago et al. (2022) [12] | Spain | R | 73/G1 (66), G2 (7) | 34±5 | 28±9.5 | 2010–2020 | 74 | - | LIUS/OP/GnRHa | 57.8 (6–159) | Hysteroscopy, D&C, pipelle | CR, PR, PD, SD relapse, OS, DFS, FO |
| Westin et al. (2021) [7] | USA | P | 15/G1 (15) | 48 (19.1–84.1)* | 46 (21–79)* | 2008–2020 | 8 | 13 | LIUS | 12 | D&C, pipelle | CR, PR, PD, relapse, AD, QoL, biomarker |
| Sengal et al. (2021) [8] | Australia | R | 33/G1 (29), G2 (4) | <50: 5 | <30: 3 | 2006–2018 | 3 | 30 | LIUS/LIUS+OP | 524 days (386–1,286) | D&C, pipelle | CR, relapse, biomarkers |
| 50–60: 16 | 30–40: 6 | |||||||||||
| >60: 12 | >40: 23 | |||||||||||
| Unknown: 2 | ||||||||||||
| Roh et al. (2021) [13] | Korea | R | 43/G1 (36), G2 (7) | 32 (19–40) | 27 (14.8–39.0) | 2014–2017 | 43 | - | LIUS+OP/OP | 60 | D&C, hysteroscopy | CR, AD, OS, PFS, FO, relapse |
| Novikova et al. (2021) [11] | Russia | P | 190/G1 (170), G2(20) | 32 (19–46) | 24 (15.9–46.7) | 2009–2019 | 190 | - | LIUS+GnRHa+D&C/LIUS+OP+ GnRHa+ D&C/OP+D&C | 33 (3–136) | D&C, pipelle | CR, FO, relapse |
| Janda et al. (2021) [14] | Australia | P | 88/G1 (88) | 53±13.9* | >30 | 2012–2019 | + | + | LIUS± Met±WL | 6 | D&C, pipelle | CR, AD, biomarkers |
| Raffone et al. (2021) [9] | Italy | R | 7/G1 (7) | 33 (24–43) | NA | 2007–2019 | 7 | - | LIUS+HR | 69.9±36.3 | Hysteroscopy | CR, relapse, biomarkers |
| Falconeet al. (2020) [34] | Italy | P | 23/G2 (23) | 35 (28–44) | 28 (19.8–42) | 2004–2019 | + | - | OP/LIUS/LIUS+OP/OP+HR | 35 (9–148) | Hysteroscopy | CR, relapse, FO |
| Leone Roberti Maggiore et al. (2019) [32] | Italy | R | 20/G1 (16), G2 (4) | G1: 33±5.0 | G1: 23±4.6 | 2004–2017 | 20 | - | LIUS | G1:85.3±48.3 | Hysteroscopy | CR, PR, PD, relapse, FO |
| G2: 35±3.3 | G2: 31±14.5 | G2:115.5±2.6 | ||||||||||
| Kim et al. (2019) [29] | Korea | P | 35/G1 (35) | 33 (27–40) | 24.5 (15.1–37.5) | 2012–2017 | 35 | - | LIUS+OP | 6 | Biopsy, D&C | CR, PR, PD, SD |
| Giampaolino et al. (2019) [35] | Italy | R | 14/G1 (14) | 35 (20–44)* | 26 (20.2–44.8)* | 2007–2017 | 14 | - | LIUS+HR | 24 | Hysteroscopic biopsy | CR, PR, PD, relapse, FO |
| Casadio et al. (2019) [20] | Italy | R | 9/G1 (9) | 69 (57–85) | 33 (29–44) | 2009–2015 | - | 9 | LIUS+HR | 60 | Hysteroscopy | CR, relapse, AD |
| Chae et al. (2019) [30] | Korea | R | 118/G1–2 (NA) | 37 (28–45) | NA | 2005–2017 | 118 | - | LIUS+OP | 11.9 (4–49) | D&C | CR, relapse, FO |
| Pal et al. (2018) [21] | USA | R | 17/G1 (9), G2 (8) | 46 (18.5–85.2)* | 45 (20–74)* | 2003–2013 | + | + | LIUS | 4.2 yr (3.7 mo–12.0 yr) | Biopsy | CR, CR, PR, PD, FO, biomarker |
| Hwang et al. (2017) [36] | Korea | R | 5/G2 (5) | 30 (25–39) | 24 (18.5–30.5) | 2011–2015 | 5 | - | LIUS+OP | 44.4 (12–71) | D&C | CR, PR, PD, relapse, FO |
| Marnach et al. (2017) [22] | USA | R | 20/G1 (17), G2 (3) | OP: 51 (44–57) | OP: 31 (24.9–37.8) | 2007–2014 | + | + | LIUS/OP | 7.2 (IQR, 4.8–13.8) | Biopsy, D&C | CR |
| LIUS: 47 (41–59)* | LIUS: 37 (28.2–48.3)* | |||||||||||
| Baker et al. (2017) [23] | USA | R | 16/G1 (16) | 63 (49–92)* | 51 (22–84) | 2002–2013 | - | + | LIUS | 42 (3–118) | Biopsy | CR, PR, PD, relapse |
| Falcone et al. (2017) [18] | Italy | R | 28/G1 (27), G2 (1) | 36 (25–40) | 28 (20.9–53.5) | 2001–2016 | + | + | LIUS+HR/OP | 92 (6–172) | D&C, hysteroscopy | CR, SD, PD, relapse, FO |
| Kim et al. (2013) [19] | Korea | P | 16/G1 (16) | 35 (29–40) | 24 (17–36.3) | 2008–2012 | 16 | - | LIUS+OP | 31.1 (16–50) | D&C | CR, PR, PD, relapse, FO |
| Hubbs et al. (2013) [15] | USA | R | 39/G1–2 (NA) | OP: 70 (22–89) | OP: 41 (23.2–77.6) | 1999–2011 | 12 | 27 | LIUS/OP | OP: 24.5 (3.2–165.8) | Office biopsy or D&C | CR, PR, PD, relapse, FO |
| LIUS: 37 (22–83) | LIUS: 51 (28.8–67.8) | IUD: 29.3 (1.5–108.6) | ||||||||||
| Pashov et al. (2012) [16] | Russia | R | 11/G1 (11) | 30 (26–36) | NA | 2006–2012 | 11 | - | LIUS+GnRHa | 44.39 (24–72) | Pipelle, hysteroscopy | CR, PR, PD, relapse, FO |
| Minig et al. (2011) [28] | Italy | P | 14/G1 (14) | 34 (22–40)* | 21 (17–41)* | 1996–2009 | + | + | LIUS+GnRHa | 29 (4–102) | Pipelle, D&C | CR, AD, OS, FO, relapse |
| Cade et al. (2010) [25] | Australia | R | 16/G1 (16) | 35 (23–53) | NA | NA | 15 | 1 | LIUS/LIUS+OP/OP | 27 (3–134) | D&C | CR, PR, PD, relapse, FO |
| Wheeler et al. (2007) [24] | USA | R | 26/G1 (26) | Premenopausal: 34 (24–47) | NA | NA | + | + | LIUS/OP | 12 | Endometrial biopsy, D&C | CR, PR, PD, SD |
| Postmenopausal: 61 (50–77) | ||||||||||||
Values are presented as median (range).
BMI, body mass index; CR, complete response; D&C, dilation and curettage; FO, fertility outcomes; G1, grade 1; G2, grade 2; GnRHa, gonadotropin-releasing hormone agonist; HR, hysteroscopic resection; LIUS, levonorgestrel-releasing intrauterine system; Met, metformin; NA, not available; OP, oral progesterone; P, prospective; PD, progressive disease; PR, partial response; R, retrospective; WL, weight loss.
2. Primary outcomes
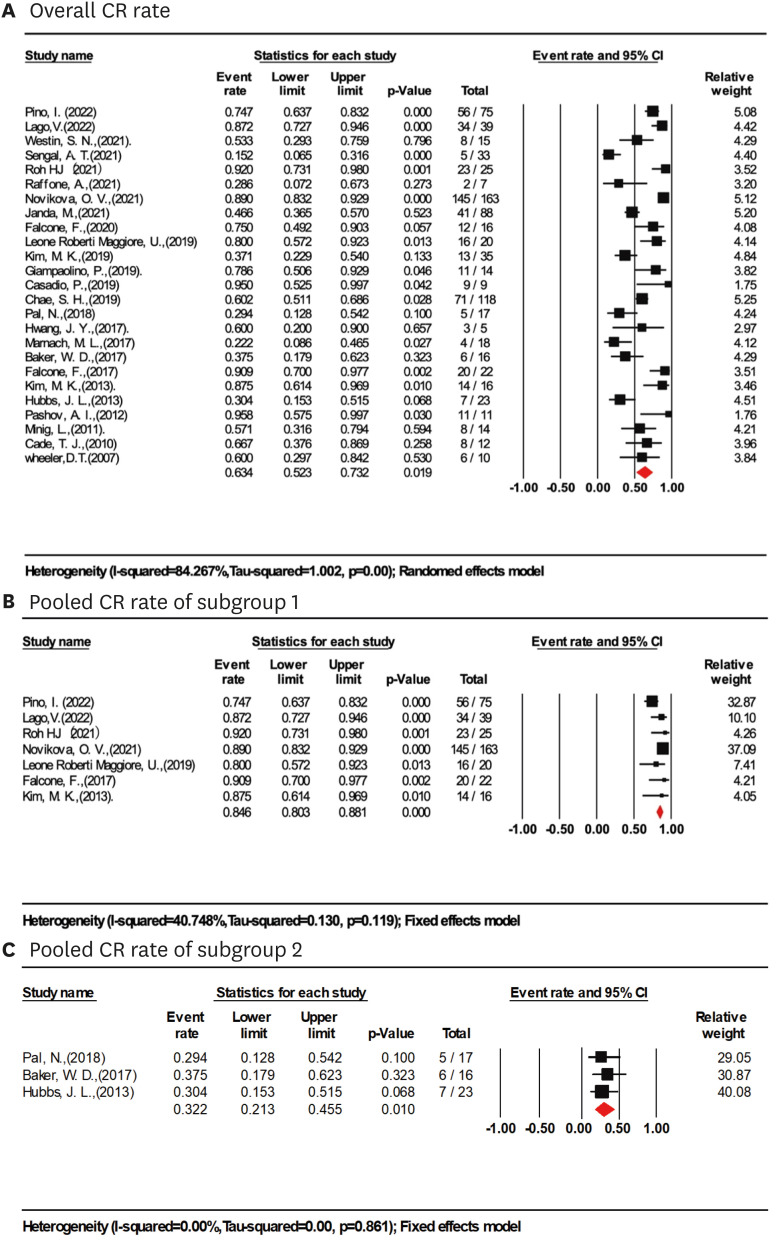
Based on a meta-analysis of 25 studies (821 women), the total CR rate of early EC (grade 1 and 2) treated with LIUS-based regimens was achieved in 63.4% of cases (95% CI=52.3%–73.2%; I2=84.3%; Fig. 2A).
Fig. 2. CR rate in women treated with LIUS-based therapies. (A) Overall CR rate in women treated with LIUS-based therapies. (B) Pooled CR rate of LIUS-based regimens in women younger than 45, BMI <30 kg/m2 with follow up time >24 months (study sample size >15). (C) Pooled CR rate of LIUS-based regimens in women older than 45, BMI ≥30 kg/m2 with follow up time >24 months (study sample size >15).
BMI, body mass index; CI, confidence interval; CR, complete response; LIUS, levonorgestrel-releasing intrauterine system.
Sensitivity analyses with the leave-one-out strategy did not significantly affect the results. The greatest difference was 2.2% in CR rate (65.6%; 95% CI=55.0%–74.8%; I2=82.4%) when excluding Sengal et al. [8], although this difference was not significant (Fig. S3). Further sensitivity analyses revealed that the most prominent differences from the primary meta-analyses were observed when pooling subsets of studies that included women of ages 45 or younger, BMI <30 kg/m2, LIUS-based treatment with HR, OP or GnRHa, or with at least 24 months of follow-up (Table 2). According to the results of sensitivity analyses, further subset meta-analysis of 7 studies (360 women with mean age <45, BMI <30 kg/m2, follow-up time >24 months, samples >15) [6,11,12,13,17,18,19] revealed the highest CR rate of 84.6% (95% CI=80.3%–88.1%; I2=40.7%; Fig. 2B). LIUS-based regimens varied in the 7 included studies. Two studies used LIUS alone, most commonly for at least 6 months; 2 administered LIUS with oral progestins; 2 combined LIUS with GnRHa and/or oral progestins, and the final survey, LIUS insertion was preceded by HR.
Table 2. Sensitivity analyses in subsets of studies on LIUS-based conservative management of early-stage endometrial cancer.
| Subset | CR | Relapse | Pregnancy | Live birth | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of women/Studies | Estimate (95% CI; %) | I2 (%) | No. of women/Studies | Estimate (95% CI; %) | I2 (%) | No. of women/Studies | Estimate (95% CI; %) | I2 (%) | No. of women/Studies | Estimate (95% CI; %) | I2 (%) | ||
| Mean age (yr) | |||||||||||||
| ≤45 (sample size >10) | 580/14 | 77.5 (67.2–85.3) | 79.9 | 155/7 | 25.8 (14.3–42.0) | 61.2 | 126/5 | 43.6 (34.7–53.0) | 65.0 | 110/4 | 45.4 (27.8–64.2) | 64.2 | |
| >45 | 229/9 | 38.0 (26.6–50.9) | 61.6 | 28/4 | 36.1 (18.9–57.8) | 22.0 | - | - | - | - | - | - | |
| Mean BMI (kg/m2) | |||||||||||||
| BMI <30 | 569/14 | 74.6 (63.3–83.4) | 80.9 | 155/8 | 28.7 (21.7–36.9) | 47.0 | 141/8 | 42.2 (24.9–61.6) | 63.8 | 125/7 | 45.1 (26.8–64.8) | 61.8 | |
| BMI ≥30 | 219/8 | 35.8 (24.3–49.4) | 63.4 | 35/5 | 41.4 (24.8–60.2) | 16.4 | - | - | - | - | - | - | |
| Type of treatment | |||||||||||||
| LIUS | 249/10 | 52.0 (32.6–55.8) | 75.1 | 48/6 | 45.1 (31.0–60.0) | 0.0 | 13/2 | 65.0 (2.9–99.1) | 81.1 | 13/2 | 57.3 (4.9–97.2) | 78.5 | |
| LIUS+HR | 68/5 | 76.9 (52.8–90.8) | 60.4 | 50/5 | 17.9 (9.0–32.4) | 0.0 | 17/2 | 62.9 (4.0–98.6) | 88.3 | 17/2 | 55.5 (6.3–95.9) | 79.4 | |
| LIUS+OP | 243/6 | 71.4 (52.5–84.9) | 80.7 | 21/4 | 23.4 (15.8–33.2) | 0.0 | 94/4 | 39.4 (29.8–49.9) | 25.9 | 78/3 | 39.9 (29.5–51.3) | 2.34 | |
| LIUS+GnRHa | 195/4 | 80.4 (52.4–93.8) | 85.9 | 19/2 | 16.9 (4.8–45.1) | 33.6 | 33/3 | 39.2 (23.0–58.2) | 54.9 | 33/3 | 33.3 (18.4–52.7) | 51.6 | |
| Mean follow-up (mo) | |||||||||||||
| 6–12 | 284/6 | 47.4 (36.6–58.5) | 69.8 | 79/2 | 26.7 (18.1–37.5) | 0.0 | - | - | - | - | - | - | |
| 12–24 | 47/2 | 44.0 (3.9–93.8) | 92.7 | 16/2 | 35.3 (7.8–77.9) | 60.4 | - | - | - | - | - | - | |
| 24–36 | 244/6 | 70.5 (45.3–87.4) | 86.5 | 47/5 | 32.9 (14.8–58.1) | 52.8 | 29/3 | 17.5 (7.5–35.7) | 0.0 | 13/2 | 15.7 (3.9–45.9) | 0.0 | |
| >36 | 246/11 | 73.5 (52.3–85.0) | 76.0 | 58/6 | 29.6 (17.8–45.0) | 44.1 | 79/6 | 50.7 (25.0–76.0) | 64.6 | 79/6 | 44.8 (20.3–72.0) | 68.2 | |
BMI, body mass index; CI, confidence interval; CR, complete response; GnRHa, gonadotropin-releasing hormone agonist; HR, hysteroscopic resection; LIUS, levonorgestrel-releasing intrauterine system; OP, oral progesterone.
The subgroup of 9 studies (229 women) with a mean age >45 [7,8,14,15,20,21,22,23,24] had a significantly lower CR rate of 38% (95% CI=26.6%–50.9%; I2=61.6%) and women in these subgroups also had a higher mean BMI greater than 30 kg/m2. In a subgroup of 9 studies with a BMI ≥30 kg/m2 (8 studies overlapped the subgroup of age >45), a lower CR rate of 35.8% was also observed (95% CI=24.3%–49.4%, I2=63.4%). LIUS monotherapy was used in 7 studies [7,8,15,21,22,23,24], LIUS+HR in one study[18], and LIUS±Metformin±Weight loss in one study [14]. Meta-analysis of 3 studies (56 women with mean age >45, BMI >30 kg/m2, follow-up time >24 months, samples >15) [15,21,23] revealed the lowest CR rate of 32.2% (95% CI=21.3%–45.5%; I2=0.0%, Fig. 2C). LIUS monotherapy was adopted in these 3 studies.
Conservative treatments of LIUS combined with HR, GnRHa, or OP were most commonly used in women younger than 45. CR rates of each combination therapy group did not show significant differences. The CR rate of the LIUS+HR, LIUS+OP, and LIUS+GnRHa subgroups were 76.9% (95% CI=52.8%–90.8%; I2=60.4%), 71.4% (95% CI=52.5%–84.9%; I2=80.7%), and 80.4% (95% CI=52.4%–93.8%, I2=85.9%), respectively. In those who received LIUS monotherapy (10 studies, 249 women), a relatively lower CR rate was observed, 52.0% (95% CI=32.6%–55.8%; I2=0.0%) [7,12,14,15,21,22,23,24,25]. Among these ten studies, the mean age in 7 studies was greater than 45. CR rate gradually increased from 47.4% (95% CI=36.6%–58.5; I2=69.8%) with a median follow-up of 6–12 months to 73.5% (95% CI=52.3%–85.0%; I2=76.0%) with a median follow-up over 36 months.
3. Secondary outcomes
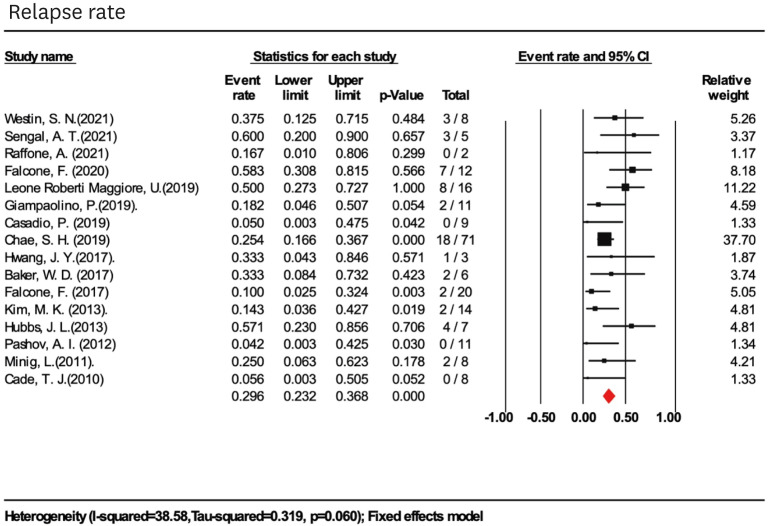
The overall pooled disease recurrence rate was 29.6% (95% CI=23.2%–36.8%; I2=38.58%) in 211 women (16 studies) with previous CRs (Fig. 3). In subgroup analysis, higher relapse rates were found in women with mean age >45 (36.1%; 95% CI=18.9%–57.8%; I2=22.0%), BMI ≥30 kg/m2 (41.4%; 95% CI=24.8%–60.2%; I2=16.4%), and the use of LIUS as progesterone treatment alone (45.1%; 95% CI=31.0%–60.0%; I2=0.0%). With longer follow-ups, the relapse rate did not increase. Recurrence rates did not differ among patients with conservative management of LIUS+HR, LIUS+OP, and LIUS+GnRHa, 17.9% (95% CI=9.0%–32.4%; I2=0.0%), 23.4% (95% CI=15.8%–33.2%; I2=0.0%), and 16.9% (95% CI=4.8%–45.1%; I2=33.6%), respectively (Table 2).
Fig. 3. Pooled relapse rate in women treated with levonorgestrel-releasing intrauterine system-based therapies.
CI, confidence interval.
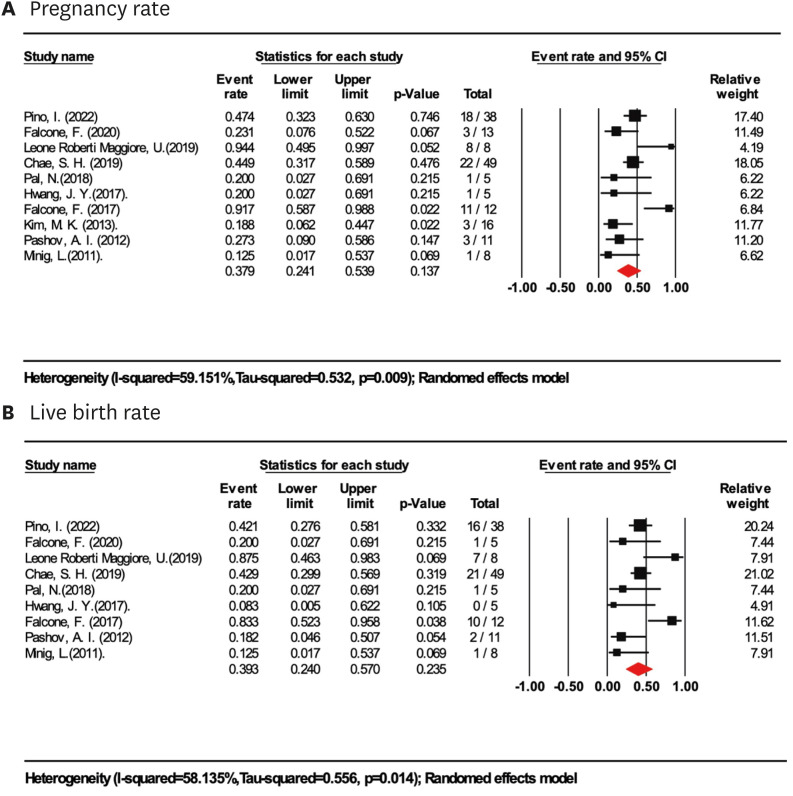
Based on a meta-analysis of 165 women in ten studies who tried to conceive, the pregnancy rate for women treated with LIUS-based regimens for fertility preservation was 37.9% (95% CI=24.1%–53.9%; I2=59.2%; Fig. 4A). The live birth rate after LIUS-based treatment was 39.3% (95% CI=24.0%–57.0%; I2=58.1%; Fig. 4B), based on a meta-analysis of 9 studies (141 women). Further sensitivity analyses revealed a mild increase in pregnancy and live birth rate in subgroups with sample size >10 and BMI <30 kg/m2, 43.6% (95% CI=34.7%–53.0%; I2=65.0%) and 42.2%(95% CI=24.9%–61.6%; I2=63.8%), respectively, however, the difference was not statistically significant. Subgroup analyses of different types of treatment showed high heterogeneity in pregnancy and live birth rates. In the subgroups of LIUS (2 studies, 13 women) and LIUS+HR (2 studies, 17 women), 65.0% (95% CI=2.9%–99.1%; I2=81.1%) and 62.9% (95% CI=4.0%–98.6%; I2=88.3%) of women got pregnant, 57.3% (95% CI=4.9%–97.2%; I2=78.5%) and 55.5% (95% CI=6.3%–95.9%; I2=79.4%) of them achieved at least one live birth. In the subgroup of LIUS+OP (4 studies, 94 women) and LIUS+GnRHa (3 studies, 33 women), the pregnancy and live birth rates were consistent with the main analyses. With extended follow-up, the highest pregnancy and live birth rate was achieved in at least 3 years of follow-up (50.7% pregnancy and 44.8% live birth).
Fig. 4. Fertility outcomes in women treated with LIUS-based therapies. (A) Pooled pregnancy rate in women treated with LIUS-based therapies. (B) Pooled live birth rate in women treated with LIUS-based therapies.
CI, confidence interval; LIUS, levonorgestrel-releasing intrauterine system.
DISCUSSION
This is the first meta-analysis to investigate the therapeutic effects of LIUS-based regimens, mainly focusing on early staged EC based on the current evidence. This review, which included 821 women with early-stage, grade 1 or 2, EEC treated with LIUS-based therapies, showed a CR rate of 63.4% overall. Further sensitivity analyses revealed that women younger than 45 with a BMI <30 kg/m2 treated with LIUS-based regimens achieved the highest CR rate of 84.6%. Women with a mean age >45 and BMI >30 kg/m2 had the lowest CR rate of 32.2%. There were 4 main types of LIUS-based treatment, including LIUS monotherapy, HR followed by LIUS, LIUS+GnRHa, and LIUS+OP. LIUS monotherapy was primarily used in women with a mean BMI >30 kg/m2 who were >45 years old with a relatively low CR rate of 52.0%. CR rates of other LIUS-based combination therapy groups did not show significant differences, although the LIUS+GnRHa group showed a higher CR rate (76.9% LIUS+HR; 71.4% LIUS+OP; 80.4% LIUS+GnRHa). Women treated with LIUS-based therapies have a 37.9% chance of achieving pregnancy and a 39.3% chance of having a live birth.
Although previous studies evaluating the effects of LIUS on endometrial hyperplasia and early-stage EEC have been reported, the focus on EEC only occupies a small portion, and the efficacy of LIUS therapy seems highly variable for these patients. Despite many studies supporting its effect on endometrial hyperplasia [26], more evidence is needed to prove LIUS as an effective and safe HT, especially for EEC patients. This meta-analysis thoroughly investigates LIUS and related agents in women with grade 1 and 2 EEC. In addition to study outcomes, several study-level variables are considered. Sensitivity analyses investigate possible effect modifiers and the stability of the findings in subgroups.
Limitations are mainly related to the available data sources. Fifteen studies are categorized as case series, and 3 studies included fewer than ten eligible women. Other sources of clinical heterogeneity include a wide age range, different indications of conservative management, and variable strategies of LIUS-based combination therapies. The random-effects model is applied for combined outcomes in cases of high heterogeneity, and subgroup meta-analysis is performed to reduce the heterogeneity. A further limitation is the lack of detailed obstetric data regarding the pregnancies after receiving fertility preservation treatments. Furthermore, the interpretation of these findings should also account for publication bias, which is likely to result in preferential reporting of cases with good outcomes, leading to a possible overestimation of the effect.
Previous studies showed that LIUS was superior to different forms of systemic progestins in managing patients with varying histological types of endometrial hyperplasia [26,27]. Our research indicates that LIUS-based therapies are also viable for the conservative management of early-stage EEC. The therapeutic benefit appeared to be the most pronounced in patients younger than 45 years old and with a BMI <30 kg/m2 (84.6%) but was less definitive for older, obese women (32.2%). The cause for these discrepancies might be the limitation of conservative treatment modalities for elderly patients with severe comorbidities.
Regarding treatment, LIUS-based combination therapy may be recommended as evidence suggests that LIUS combination therapy is a promising candidate for the treatment of EEC. LIUS combined with GnRHa has been proposed as a superior therapy to other progestin-based treatments, but there is insufficient consensus between the different studies [6,11,16,28]. Similarly, several authors have proposed the combination of oral and intrauterine progestins with limited evidence regarding its superiority to other progesterone-based treatments [1,6,29,30]. There’s only one study until now comparing the oncological outcomes of LIUS combined with either GnRHa or oral progestin: LIUS+GnRHa 65% regression, LIUS+MA 80% regression, and LIUS+MA+Metformin 83% regression (p>0.05) [6]. Although not statistically significant in CR rate, a statistically significant lower risk of recurrence in women treated with LIUS+MA+Metformin was detected when compared to LIUS+GnRHa regimen. The CR rate of HR followed by LIUS insertion (76.9%) was consistent with previous studies [31], with a 17.9% relapse rate. LIUS monotherapy was associated with a poor therapeutic response and a high recurrence rate in the treatment of EEC. Most patients treated with LIUS alone were postmenopausal and obese, with associated co-morbidities such as cardiovascular disease and metabolic syndrome. LIUS alone, used in younger patients, was associated with a higher CR rate of 81.3%–87.2% [12,32]. Differences in demographic characteristics likely cause these widely different clinical outcomes. For instance, older age and higher BMI may be associated with worse oncological outcomes [33]. Considering individual differences in the conservative management of EEC patients, future clinical investigations should focus on population-based combination therapies.
With extended follow-up, the CR, pregnancy, and live birth rates increased to some extent. This could be because endpoints, including response to treatment, pregnancies, and live births, naturally require time to occur. In contrast, conservative management of EEC is generally offered as a temporary therapy, and there is uncertainty about the safest time to attempt pregnancy or the ideal timing of hysterectomy. Moreover, female fertility decreases over time, and many patients have missed the optimal childbearing age of their lives. More information is needed to indicate the balance between oncological risk and fertility benefit in the conservative management of EEC. The live birth rate estimated in this meta-analysis is 39.3%, whereas live birth rates based on the utilization of MA or MPA treatment in patients with EEC were 19.6% (95% CI=12.8%–27.4%) according to a meta-analysis including 445 women [3]. Various medical or social factors may influence fertility outcomes in real-life settings, and effective fertility-sparing management of women with EEC requires multiple treatment stages over relatively long periods. Strict surveillance is recommended from disease treatment, pregnancy, and childbirth to post-childbearing management.
A small proportion of patients with myometrial invasion and G2 were included in the meta-analysis, for whom fertility-sparing treatment is not recommended in most guidelines. However, it applied in selected cases after counselling based on the evidence [20,34] for a shared decision with the patient. More studies are needed about optimized treatment protocol focusing on these patients.
Conservative management based on LIUS therapies is a viable option for patients with early-stage EEC who want to preserve fertility. Women younger than 45 with BMI <30 kg/m2 appeared to have the highest CR rate of about 85% with follow-ups of at least 24 months. Oncological and fertility outcomes among different types of combinations with LIUS were similar. A range of unmeasured confounders in observational studies may bias these results; therefore, our findings should be interpreted with caution, especially in patients with G2 and myometrial invasion.
Footnotes
Funding: This study was supported by the Key Projects of Philosophy and Social Sciences Research (21JZD032).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: W.H., P.N., X.G., D.Z., M.C.
- Data curation: W.H., P.N., Z.W., X.G., G.W., M.C.
- Formal analysis: W.H., P.N., Z.W., G.W., D.Z., M.C.
- Funding acquisition: M.C.
- Investigation: W.H., P.N., Z.W., M.C.
- Methodology: W.H., P.N., Z.W., X.G., G.W., D.Z., M.C.
- Project administration: W.H., P.N., G.W., M.C.
- Resources: P.N., M.C.
- Software: W.H., P.N., G.W., M.C.
- Supervision: W.H., X.G., G.W., M.C.
- Validation: W.H., G.W., D.Z., M.C. Visualization.
SUPPLEMENTARY MATERIALS
Search strategies (date of the search: August 4, 2022)
Characteristics of excluded studies
Quality assessment: Joanna Briggs Institute Critical Appraisal Checklist for cohort studies
Quality assessment: Joanna Briggs Institute Critical Appraisal Checklist for case series
Meta-analyses Of Observational Studies in Epidemiology checklist.
Publication bias.
Sensitivity analysis: leave one out.
References
- 1.Crosbie EJ, Kitson SJ, McAlpine JN, Mukhopadhyay A, Powell ME, Singh N. Endometrial cancer. Lancet. 2022;399:1412–1428. doi: 10.1016/S0140-6736(22)00323-3. [DOI] [PubMed] [Google Scholar]
- 2.Kelley RM, Baker WH. Progestational agents in the treatment of carcinoma of the endometrium. N Engl J Med. 1961;264:216–222. doi: 10.1056/NEJM196102022640503. [DOI] [PubMed] [Google Scholar]
- 3.Qin Y, Yu Z, Yang J, Cao D, Yu M, Wang Y, et al. Oral progestin treatment for early-stage endometrial cancer: a systematic review and meta-analysis. Int J Gynecol Cancer. 2016;26:1081–1091. doi: 10.1097/IGC.0000000000000723. [DOI] [PubMed] [Google Scholar]
- 4.Li CI, Beaber EF, Tang MT, Porter PL, Daling JR, Malone KE. Effect of depo-medroxyprogesterone acetate on breast cancer risk among women 20 to 44 years of age. Cancer Res. 2012;72:2028–2035. doi: 10.1158/0008-5472.CAN-11-4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montz FJ, Bristow RE, Bovicelli A, Tomacruz R, Kurman RJ. Intrauterine progesterone treatment of early endometrial cancer. Am J Obstet Gynecol. 2002;186:651–657. doi: 10.1067/mob.2002.122130. [DOI] [PubMed] [Google Scholar]
- 6.Pino I, Iacobone AD, Vidal Urbinati AM, Di Giminiani M, Radice D, Guerrieri ME, et al. Fertility-sparing treatment for endometrial cancer: oncological and obstetric outcomes in combined therapies with levonorgestrel intrauterine device. Cancers (Basel) 2022;14:2170. doi: 10.3390/cancers14092170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Westin SN, Fellman B, Sun CC, Broaddus RR, Woodall ML, Pal N, et al. Prospective phase II trial of levonorgestrel intrauterine device: nonsurgical approach for complex atypical hyperplasia and early-stage endometrial cancer. Am J Obstet Gynecol. 2021;224:191.e1–191.15. doi: 10.1016/j.ajog.2020.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sengal AT, Smith D, Rogers R, Snell CE, Williams ED, Pollock PM. Fibroblast growth factor receptor 2 isoforms detected via novel RNA ISH as predictive biomarkers for progestin therapy in atypical hyperplasia and low-grade endometrial cancer. Cancers (Basel) 2021;13:1703. doi: 10.3390/cancers13071703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raffone A, Travaglino A, Zullo FM, Gencarelli A, Micheli M, Miranda S, et al. Predictive accuracy of progesterone receptor B in young women with atypical endometrial hyperplasia and early endometrial cancer treated with hysteroscopic resection plus LNG-IUD insertion. J Minim Invasive Gynecol. 2021;28:1244–1253. doi: 10.1016/j.jmig.2020.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Chen X. The current situation of the levonorgestrel intrauterine system (LNG-IUS) in conservative treatment for patients with early-stage endometrial cancer and atypical hyperplasia. J Gynecol Oncol. 2019;30:e79. doi: 10.3802/jgo.2019.30.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novikova OV, Nosov VB, Panov VA, Novikova EG, Krasnopolskaya KV, Andreeva YY, et al. Live births and maintenance with levonorgestrel IUD improve disease-free survival after fertility-sparing treatment of atypical hyperplasia and early endometrial cancer. Gynecol Oncol. 2021;161:152–159. doi: 10.1016/j.ygyno.2021.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Lago V, Marina T, Laseca Modrego M, Gil-Ibañez B, Rodriguez JR, Domingo J, et al. Fertility sparing treatment in patients with endometrial cancer (FERT-ENC): a multicentric retrospective study from the Spanish Investigational Network Gynecologic Oncology Group (SPAIN-GOG) Arch Gynecol Obstet. 2022;306:821–828. doi: 10.1007/s00404-021-06375-2. [DOI] [PubMed] [Google Scholar]
- 13.Roh HJ, Yoon HJ, Jeong DH, Lee TH, Kwon BS, Suh DS, et al. Prognostic factors of oncologic outcomes after fertility-preservative management with progestin in early-stage of endometrial cancer. J Res Med Sci. 2021;26:48. doi: 10.4103/jrms.JRMS_103_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janda M, Robledo KP, Gebski V, Armes JE, Alizart M, Cummings M, et al. Complete pathological response following levonorgestrel intrauterine device in clinically stage 1 endometrial adenocarcinoma: results of a randomized clinical trial. Gynecol Oncol. 2021;161:143–151. doi: 10.1016/j.ygyno.2021.01.029. [DOI] [PubMed] [Google Scholar]
- 15.Hubbs JL, Saig RM, Abaid LN, Bae-Jump VL, Gehrig PA. Systemic and local hormone therapy for endometrial hyperplasia and early adenocarcinoma. Obstet Gynecol. 2013;121:1172–1180. doi: 10.1097/AOG.0b013e31828d6186. [DOI] [PubMed] [Google Scholar]
- 16.Pashov AI, Tskhay VB, Ionouchene SV. The combined GnRH-agonist and intrauterine levonorgestrel-releasing system treatment of complicated atypical hyperplasia and endometrial cancer: a pilot study. Gynecol Endocrinol. 2012;28:559–561. doi: 10.3109/09513590.2011.649813. [DOI] [PubMed] [Google Scholar]
- 17.Leone Roberti Maggiore U, Khamisy-Farah R, Bragazzi NL, Bogani G, Martinelli F, Lopez S, et al. Fertility-sparing treatment of patients with endometrial cancer: a review of the literature. J Clin Med. 2021;10:4784. doi: 10.3390/jcm10204784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falcone F, Laurelli G, Losito S, Di Napoli M, Granata V, Greggi S. Fertility preserving treatment with hysteroscopic resection followed by progestin therapy in young women with early endometrial cancer. J Gynecol Oncol. 2017;28:e2. doi: 10.3802/jgo.2017.28.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim MK, Seong SJ, Kim YS, Song T, Kim ML, Yoon BS, et al. Combined medroxyprogesterone acetate/levonorgestrel-intrauterine system treatment in young women with early-stage endometrial cancer. Am J Obstet Gynecol. 2013;209:358.e1–358.e4. doi: 10.1016/j.ajog.2013.06.031. [DOI] [PubMed] [Google Scholar]
- 20.Casadio P, Guasina F, Talamo MR, Paradisi R, Morra C, Magnarelli G, et al. Conservative hysteroscopic treatment of stage I well differentiated endometrial cancer in patients with high surgical risk: a pilot study. J Gynecol Oncol. 2019;30:e62. doi: 10.3802/jgo.2019.30.e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pal N, Broaddus RR, Urbauer DL, Balakrishnan N, Milbourne A, Schmeler KM, et al. Treatment of low-risk endometrial cancer and complex atypical hyperplasia with the levonorgestrel-releasing intrauterine device. Obstet Gynecol. 2018;131:109–116. doi: 10.1097/AOG.0000000000002390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marnach ML, Butler KA, Henry MR, Hutz CE, Langstraat CL, Lohse CM, et al. Oral progestogens versus levonorgestrel-releasing intrauterine system for treatment of endometrial intraepithelial neoplasia. J Womens Health (Larchmt) 2017;26:368–373. doi: 10.1089/jwh.2016.5774. [DOI] [PubMed] [Google Scholar]
- 23.Baker WD, Pierce SR, Mills AM, Gehrig PA, Duska LR. Nonoperative management of atypical endometrial hyperplasia and grade 1 endometrial cancer with the levonorgestrel intrauterine device in medically ill post-menopausal women. Gynecol Oncol. 2017;146:34–38. doi: 10.1016/j.ygyno.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Wheeler DT, Bristow RE, Kurman RJ. Histologic alterations in endometrial hyperplasia and well-differentiated carcinoma treated with progestins. Am J Surg Pathol. 2007;31:988–998. doi: 10.1097/PAS.0b013e31802d68ce. [DOI] [PubMed] [Google Scholar]
- 25.Cade TJ, Quinn MA, Rome RM, Neesham D. Progestogen treatment options for early endometrial cancer. BJOG. 2010;117:879–884. doi: 10.1111/j.1471-0528.2010.02552.x. [DOI] [PubMed] [Google Scholar]
- 26.Mittermeier T, Farrant C, Wise MR. Levonorgestrel-releasing intrauterine system for endometrial hyperplasia. Cochrane Database Syst Rev. 2020;9:CD012658. doi: 10.1002/14651858.CD012658.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Behnamfar F, Ghahiri A, Tavakoli M. Levonorgestrel-releasing intrauterine system (Mirena) in compare to medroxyprogesterone acetate as a therapy for endometrial hyperplasia. J Res Med Sci. 2014;19:686–690. [PMC free article] [PubMed] [Google Scholar]
- 28.Minig L, Franchi D, Boveri S, Casadio C, Bocciolone L, Sideri M. Progestin intrauterine device and GnRH analogue for uterus-sparing treatment of endometrial precancers and well-differentiated early endometrial carcinoma in young women. Ann Oncol. 2011;22:643–649. doi: 10.1093/annonc/mdq463. [DOI] [PubMed] [Google Scholar]
- 29.Kim MK, Seong SJ, Kang SB, Bae DS, Kim JW, Nam JH, et al. Six months response rate of combined oral medroxyprogesterone/levonorgestrel-intrauterine system for early-stage endometrial cancer in young women: a Korean Gynecologic-Oncology Group Study. J Gynecol Oncol. 2019;30:e47. doi: 10.3802/jgo.2019.30.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chae SH, Shim SH, Lee SJ, Lee JY, Kim SN, Kang SB. Pregnancy and oncologic outcomes after fertility-sparing management for early stage endometrioid endometrial cancer. Int J Gynecol Cancer. 2019;29:77–85. doi: 10.1136/ijgc-2018-000036. [DOI] [PubMed] [Google Scholar]
- 31.Alonso S, Castellanos T, Lapuente F, Chiva L. Hysteroscopic surgery for conservative management in endometrial cancer: a review of the literature. Ecancermedicalscience. 2015;9:505. doi: 10.3332/ecancer.2015.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leone Roberti Maggiore U, Martinelli F, Dondi G, Bogani G, Chiappa V, Evangelista MT, et al. Efficacy and fertility outcomes of levonorgestrel-releasing intra-uterine system treatment for patients with atypical complex hyperplasia or endometrial cancer: a retrospective study. J Gynecol Oncol. 2019;30:e57. doi: 10.3802/jgo.2019.30.e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen M, Jin Y, Li Y, Bi Y, Shan Y, Pan L. Oncologic and reproductive outcomes after fertility-sparing management with oral progestin for women with complex endometrial hyperplasia and endometrial cancer. Int J Gynaecol Obstet. 2016;132:34–38. doi: 10.1016/j.ijgo.2015.06.046. [DOI] [PubMed] [Google Scholar]
- 34.Falcone F, Leone Roberti Maggiore U, Di Donato V, Perrone AM, Frigerio L, Bifulco G, et al. Fertility-sparing treatment for intramucous, moderately differentiated, endometrioid endometrial cancer: a Gynecologic Cancer Inter-Group (GCIG) study. J Gynecol Oncol. 2020;31:e74. doi: 10.3802/jgo.2020.31.e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giampaolino P, Di Spiezio Sardo A, Mollo A, Raffone A, Travaglino A, Boccellino A, et al. Hysteroscopic endometrial focal resection followed by levonorgestrel intrauterine device insertion as a fertility-sparing treatment of atypical endometrial hyperplasia and early endometrial cancer: a retrospective study. J Minim Invasive Gynecol. 2019;26:648–656. doi: 10.1016/j.jmig.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Hwang JY, Kim DH, Bae HS, Kim ML, Jung YW, Yun BS, et al. Combined oral medroxyprogesterone/levonorgestrel-intrauterine system treatment for women with grade 2 stage IA endometrial cancer. Int J Gynecol Cancer. 2017;27:738–742. doi: 10.1097/IGC.0000000000000927. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategies (date of the search: August 4, 2022)
Characteristics of excluded studies
Quality assessment: Joanna Briggs Institute Critical Appraisal Checklist for cohort studies
Quality assessment: Joanna Briggs Institute Critical Appraisal Checklist for case series
Meta-analyses Of Observational Studies in Epidemiology checklist.
Publication bias.
Sensitivity analysis: leave one out.