Abstract
Objective
The purpose of this study was to assess the efficacy and tolerability of a paclitaxel, carboplatin and metformin regimen in the first-line treatment of advanced-stage ovarian, fallopian tube, and primary peritoneal carcinoma.
Methods
Eligible subjects underwent surgery and 6 cycles of neoadjuvant or adjuvant dose-dense intravenous paclitaxel (80 mg/m2), carboplatin (area under the curve 5 or 6 on Day 1), and oral metformin (850 mg daily). Study participants who completed their primary therapy and attained a clinically defined complete or partial response (PR) were treated with a planned 12 cycles of paclitaxel (135 mg/m2 every 21 days) and metformin (850 mg twice daily) maintenance therapy.
Results
Thirty subjects received a median of 6 cycles (range, 5–6) of primary induction chemotherapy and were eligible for response evaluation; twenty-three patients exhibited a complete response, while 3 study patients obtained a PR (an overall response rate of 86.7%). Grade 3–4 hematological toxicity included neutropenia (43.3%), thrombocytopenia (10%) and anemia (36.7%). There was no incidence of grade 3–4 neuropathy although 15 patients (50%) developed grade ≤2 neurotoxicity. Additionally, we observed grade ≤2 diarrhea in 20 (66.7%) subjects. The median progression-free survival was 21 months (range, 3–52) and overall median survival was 35 months (range, 15–61). The subjects also received an aggregate 103 cycles (median, 12; range, 6–12) of maintenance chemotherapy.
Conclusion
The study results suggest that the combination of paclitaxel, carboplatin and metformin is associated with moderate efficacy and a reasonable toxicity profile.
Keywords: Ovarian Cancer, Metformin, Chemotherapy, Survival
Synopsis
Standard of care for advanced-stage ovarian cancer comprises surgical cytoreduction, followed by primary chemotherapy. Nevertheless, 5-year patient overall survival rates are inauspicious. Our study findings indicate that metformin is reasonably well-tolerated, and potentially bolsters the efficacy of first-line platinum and taxane chemotherapy.
INTRODUCTION
Ovarian cancer is associated with the highest mortality rate amongst gynecologic malignancies in the United States [1]. The standard of care for patients diagnosed with advanced-stage ovarian cancer is optimal debulking surgery with concomitant taxane and platinum-based chemotherapy [2]. Despite favorable objective response rates, the risk for disease progression is high and 5-year survival rates approach 45% [3].
Numerous ovarian cancer studies have evaluated the inclusion of bevacizumab and PARP inhibitors to induction chemotherapy with the intent of further bolstering first-line treatment efficacy [4,5]. However, since many of these targeted therapies are indicated for very specified patient conditions (e.g., a homologous recombination repair deficiency) and accord cumulative toxicity, not to mention burgeoning costs that may preclude younger and financially disadvantageous patients [6,7], alternative medications should be considered.
Preclinical evaluations have indicated that metformin, a treatment for diabetes mellitus, inhibits the growth of cancer cells via insulin and non-insulin dependent mechanisms [8,9]. Metformin also impedes cell proliferation via activation of the AMP-activated protein kinase [10,11]. Accordingly, select epidemiologic studies have reported that metformin confers a reduced rate of cancer mortality in patients being treated with this medication [12].
Diabetes putatively increases the risk of several malignancies, namely colorectal, breast, and ovarian cancer [13]. Studies have also evaluated metformin because the treatment ostensibly impedes tumor growth, enhances chemosensitivity and is reasonably well-tolerated [14,15,16,17,18]. Since metformin is reasonably priced (i.e., the medication is generic), studying the drug in combination with chemotherapy for the treatment of ovarian cancer is a compelling endeavor. The purpose of this study was to assess the efficacy and toxicity of an incorporated paclitaxel, carboplatin, and metformin regimen in the first-line management of advanced-stage ovarian carcinoma.
MATERIALS AND METHODS
1. Study design
This was a phase II, single arm, 2-stage, open-label, non-randomized pilot study (NCT02437812). An Institutional Review Board (IRB) (Western IRB, GOA-TCOM1) approved this clinical investigation, and every participant signed a consent form prior to enrollment.
2. Inclusionary criteria
Patients were required to have a histologic diagnosis of advanced-stage (III–IV) epithelial ovarian, fallopian tube, or primary peritoneal carcinoma and undergone neoadjuvant chemotherapy and interval debulking surgery followed by adjuvant chemotherapy or surgical debulking and adjuvant chemotherapy. Optimal cytoreductive surgery was defined as tumor debulking with residual disease ≤1 cm [19]. Eligible patients had adequate bone marrow, renal, hepatic function, absolute neutrophil count of >1,5003, platelets of ≥100 k/uL, and a hemoglobin value >9 g/dL.
Patients had to obtain an European Cooperative Oncology Group performance status of ≤1 and commence with their first dose of paclitaxel, carboplatin and metformin within 4 weeks of their initial debulking surgery. Subjects may have a history of diabetes and be receiving treatment for their condition, metformin excepted.
3. Exclusionary criteria
Subjects with a history of treatment comprising radiotherapy or chemotherapy were excluded from study participation. Additionally, any subject with a concomitant or previous malignancy within the past 3 years (non-melanoma skin cancer notwithstanding) was excluded from study consideration. Participants could not be treated with metformin or have been on the medication within the previous 6 months. Study participants were also required to have an aspartate aminotransferase or alanine aminotransferase >2.5 × upper limit of normal and a serum creatinine 1.5 mg/dL. Also, subjects with sepsis, severe infection, borderline tumor diagnosis, acute hepatitis, severe gastrointestinal bleeding, history of congestive heart failure, angina, or myocardial infarction within the past 6 months were excluded from the study.
4. Treatment plan
Pre-medications (e.g., anti-emetics, hydration, antihistamines, corticosteroids, etc.) were administered according to institutional guidelines. Eligible subjects received 6 cycles of either neoadjuvant or primary induction chemotherapy encompassing intravenous dose-dense paclitaxel (80 mg/m2), which was infused over 60–90 minutes and carboplatin (area under the receiver operating characteristic curve 5 or 6) that was administered over approximately 30 minutes (Day 1 only). Paclitaxel (80 mg/m2) was repeated on Day 8 and Day 15. Metformin (850 mg oral) [20] was given once daily × 4 weeks, and then 850 mg oral twice daily (BID) every 21 or 28 days, thereafter.
5. Maintenance therapy
Patients who achieved a clinically defined complete response (CR) or partial response (PR) following primary induction chemotherapy received paclitaxel (135 mg/m2) [21] every 3 to 4 weeks for a planned 12 cycles. They also continued to receive metformin (850 mg oral BID) for up to 5 years. Subjects with persistent or progressive disease (PD) following cycle 6 were removed from the study and treated according to physician discretion.
6. Response evaluation
The overall response rate was determined following cycle 6. Clinical response was assessed by clinical, serologic, and radiographic means according to the RECIST criteria [22]. Chest X-ray and abdominal/pelvic computed tomography (CT) scans were performed prior to initial treatment and before cycles one and 2. CR was defined as no gross evidence of disease, resolution of measurable disease on CT scan or normalization of cancer antigen 125 (CA-125) levels from an elevated level [23]. PR was defined as a 30% reduction in lesions on CT scan or 50% reduction in CA-125. PD was defined as a 20% or greater increase in the lesions based on CT scan or doubling of CA-125 within 8 weeks of starting therapy. Stable disease (SD) was characterized by disease that does not fulfill the criteria for CR, PR, or PD. After cycle 6, a chest X-ray and CT scans of the abdomen and pelvis were repeated to determine the objective response rate.
7. Toxicity analysis
All patients who completed ≥3 cycles of chemotherapy were included in the toxicity analysis, which was graded using the Common Terminology Criteria for Adverse Events v5.0 [24]. Granulocyte colony-stimulating factor was initiated at the discretion of the treating physician. Patients were removed from the study if they incurred significant dose-limiting toxicity and were subsequently treated with every 3 weeks paclitaxel and carboplatin chemotherapy.
8. Survival assessment
Progression-free survival (PFS) was defined as the length of time from the date of initial induction chemotherapy until progression via clinical assessment, radiologic imaging, or CA-125 evaluation. Overall survival (OS) was defined as time from the date of entry until patient expiration, with all causes of death treated equally.
9. Study endpoints and assessments
The primary end point was investigator-assessed PFS for the patients treated with the experimental regimen. The key secondary endpoints were OS and toxicity for the patients treated with the experimental regimen. Response evaluation occurred at baseline and at protocol-defined intervals until the occurrence of imaging-based progression as assessed by the investigator.
10. Statistical analyses
All statistical analyses were conducted using MedCalc statistical software for biomedical research (version 18.10 for Windows; MedCalc Software, Ostend, Belgium). The initial data analysis was conducted by employing a descriptive statistical approach. Survival analyses were conducted via Kaplan Meier.
RESULTS
1. Patient demographics
From August 2013 until December 2016, we identified 37 advanced-stage ovarian patients for our study, 7 of whom withdrew from the study, primarily for elective reasons. Ultimately, we accrued 30 subjects, for whom the median age was 62 years (range, 26–88) and body mass index was 28.35 kg/mg2 (range, 19.2–51.35). Sixteen (53.3%) patients were treated with neoadjuvant chemotherapy and interval debulking surgery, and 14 (46.7%) subjects underwent primary surgery and adjuvant chemotherapy. Optimal cytoreductive surgery was achieved in 27 (90%) patients (Table 1). The median number of induction chemotherapy and maintenance therapy cycles administered was 6 (range, 5–6) and 12 (range, 6–12), respectively. In the study group, 7 patients completed the scheduled 18 cycles of treatment. The overall median duration of metformin treatment was 12 months (range, 5–61).
Table 1. Patient characteristics (n=30).
| Variables | Values | |
|---|---|---|
| Age (yr) | 62 (26–88) | |
| Body mass index (kg/m2) | 28.35 (19.2–51.35) | |
| Tumor type | ||
| Ovarian | 24 (80.0) | |
| Primary peritoneal tumor | 4 (13.3) | |
| Fallopian | 2 (6.7) | |
| Histology type | ||
| Papillary serous | 25 (83.3) | |
| Endometrioid | 2 (6.7) | |
| Mucinous | 1 (3.3) | |
| Mixed | 2 (6.7) | |
| Stage | ||
| 3A | 2 (6.7) | |
| 3B | 2 (6.7) | |
| 3C | 19 (63.5) | |
| IV | 7 (23.3) | |
| Grade | ||
| Poor | 28 (93.3) | |
| Moderate | 2 (6.7) | |
| Well | 0 (0) | |
| ECOG performance status | ||
| 0 | 22 (73.3) | |
| 1 | 8 (26.7) | |
| Cytoreductive surgery | ||
| Optimal | 27 (90.0) | |
| Sub-optimal | 3 (10.0) | |
| Chemotherapy | ||
| Neoadjuvant | 16 (53.3) | |
| Adjuvant | 14 (46.7) | |
Values are presented as number (%) or median (range).
ECOG, European Cooperative Oncology Group.
2. Response evaluation
In the group of evaluable patients, 23 (76.7%) achieved a CR and 3 (10%) subjects obtained a PR, which resulted in a CR rate was 86.7%. Additionally, 3 subjects (10%) had SD and one study participant (3.3%) had PD.
3. Toxicity
Grade 3–4 neutropenia was observed in 43.3% of patients, with no admissions for febrile neutropenia. Moreover, grade 3–4 thrombocytopenia and anemia were identified in 10% and 36.7% of subjects, correspondingly. Fifteen (50%) patients developed ≤2 neuropathy and 20 (66.7%) study participants exhibited ≤2 diarrhea. While there were no metformin-related dose reductions or delays, 2 patients were taken off metformin in accordance with physician discretion. Table 2 illustrates the hematological and non-hematological toxicity patient occurrences.
Table 2. Hematologic toxicity following primary induction chemotherapy (n=30).
| Grades | NP | TP | Anemia | PN | Diarrhea |
|---|---|---|---|---|---|
| 1 | 0 (0) | 7 (23.3) | 2 (6.7) | 11 (36.7) | 13 (43.3) |
| 2 | 4 (13.3) | 4 (13.3) | 9 (30.0) | 4 (13.3) | 7 (23.4) |
| 3 | 11 (36.7) | 3 (10.0) | 11 (36.7) | 0 (0) | 0 (0) |
| 4 | 2 (6.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Values are presented as number of patients (%).
NP, neutropenia; PN, peripheral neuropathy; TP, thrombocytopenia.
4. Patient survival
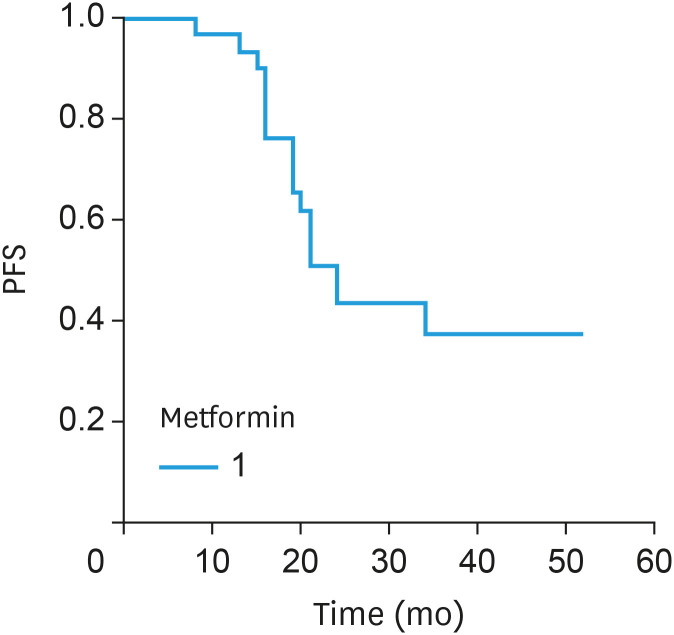
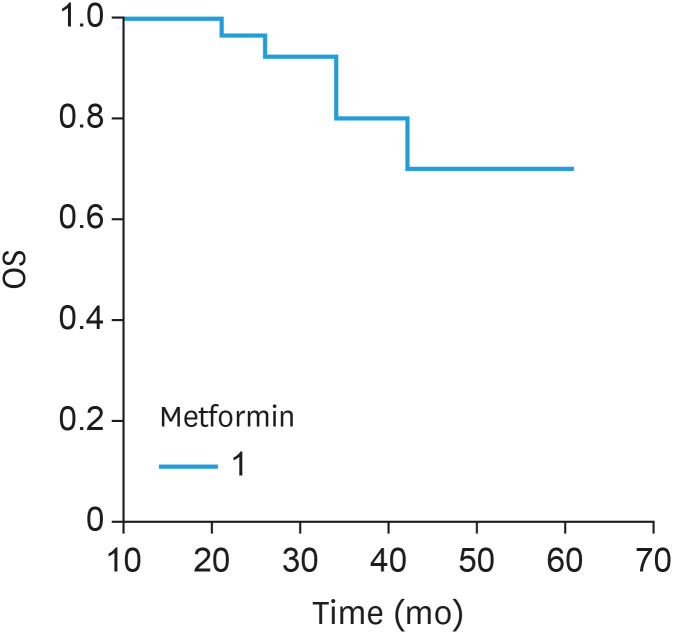
Currently, 20 patients have experienced PD, and the overall median PFS (Fig. 1) was 21 months (range, 3–52). The median disease-free interval for the patients throughout metformin maintenance therapy (i.e., the period following response evaluation) was 12 months (range, 4–40). In terms of OS (Fig. 2), 6 subjects died, and the median OS was 35 months (range, 15–61). Currently, patient follow-up has exceeded 41 months.
Fig. 1. PFS for the advanced-stage ovarian cancer patients treated with paclitaxel, carboplatin and metformin.
PFS, progression-free survival.
Fig. 2. OS for the advanced-stage ovarian cancer patients treated with paclitaxel, carboplatin and metformin.
OS, overall survival.
DISCUSSION
Metformin potentially confers intriguing anti-neoplastic activity via attenuated blood glucose levels, enhanced insulin resistance and decreased pro-inflammatory cytokines [25]. Retrospective and case-control ovarian cancer studies have indicated that metformin accords PFS benefits [26,27] although there have been limited prospective advanced-stage ovarian cancer studies evaluating the inclusion of metformin with paclitaxel and carboplatin chemotherapy [28].
In the current investigation, we observed a CR rate of 86.7%, which is comparable to the 86.2% response rate documented with paclitaxel, carboplatin and gemcitabine in advanced-stage ovarian cancer [29] but higher than the 67% described in the ICON7 trial that incorporated paclitaxel, carboplatin and bevacizumab [30]. We ascribe the reasonably high overall response rate in our study to metformin’s impact on chemotherapy potentiation, an enhanced effect that has been similarly reported with breast, prostate, and lung cancer cell line studies [31,32]. Nevertheless, we recognize that response was only assessed at the conclusion of induction therapy, and not during maintenance therapy.
We also encountered grade 3–4 neutropenia and thrombocytopenia in 43.3%, which were ultimately well-managed. While these rates are higher than what was described in the Perren et al. [30] phase 3 trial incorporating bevacizumab, carboplatin and paclitaxel (17% for grade 3–4 neutropenia and 3% for thrombocytopenia), bevacizumab occasions hypertension and wound healing complication. However, we also observed grade ≤2 diarrhea in in 66.7% of patients, with no incidence of grade 3 diarrhea, consistent with pancreatic and breast cancer treatment studies that included metformin [33,34]. While chemotherapy was the presumptive cause of the hematologic toxicity identified in this study, we acknowledge that without statistically analyzing the chemotherapy and metformin independently, we are precluded from remarking on the specific toxicities associated with metformin.
We ascertained a PFS of 21 months in the current study, which resembled the 23 months PFS described by Zheng et al. [28] in their prospective open-label pilot trial comparing ovarian cancer patients who were treated with either first-line paclitaxel, carboplatin and metformin or paclitaxel and carboplatin alone. However, they did not find any significant group PFS differences between the chemotherapy and metformin group and chemo alone group (23 months vs. 21 months; p=0.68).
Previous ovarian cancer studies have suggested that metformin is associated with improved OS [27,35,36,37]. Moreover, in a previous population-based cohort study and a recent phase II trial involving ovarian cancer patients, long-term use of metformin was associated with improved overall survival [36,37]. Alternatively, we recognize that a systematic review of metformin in ovarian cancer gainsaid any such improvements in OS [38]. The patients in the current study exhibited an OS of 35 months. While the survival findings were only moderately compelling, we do not minimize the potential impact of metformin; perhaps, higher doses of metformin should be further considered in conjunction with the collection of tumor and blood specimens to suss out any relationship between biomarker status and clinical response. Accordingly, we initially considered analyzing select patients for survival according to their duration of metformin use, but the limited sample size precluded this statistical undertaking.
When contemplating our modest findings, one could speculate that since most preclinical studies have evaluated metformin concentrations that are much higher than the pharmacological doses used in patients, this potentially confounds the therapeutic use of metformin as an anti-cancer agent [17]. Hence, at conventional doses, metformin may be insufficient at effectuating a significant clinical benefit in the management of advanced-stage ovarian cancer, a disease that is associated with a significant tumor burden [3].
We acknowledge the shortcomings of a single-arm investigation with a limited patient population. Moreover, the study would have benefitted from greater detail on the patients’ serious adverse events profile and an expanded survival analysis in accordance with the duration and dose of metformin. Perhaps, the study would have been further bolstered had the results included an analysis that incorporated patient responses after chemotherapy and metformin with respect to their histologic subtype and degree of cytoreductive surgery. However, we also refrained from this endeavor because of the limited number of study patients.
Footnotes
Funding: This study was supported by the Women’s Cancer Research Foundation, Joan and Len Rullo, in memory of Elizabeth Johnson, and Susan Berg.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: M.J.P.
- Data curation: B.R.D., G.B.H.
- Formal analysis: M.J.P., R.M.A., B.R.D.
- Investigation: M.J.P., R.M.A., G.B.H.
- Methodology: M.J.P.
- Supervision: M.J.P., R.M.A.
- Validation: M.J.P., B.R.D.
- Writing - original draft: M.J.P., R.M.A., B.R.D., G.B.H.
- Writing - review & editing: M.J.P., R.M.A., B.R.D., G.B.H.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Ledermann JA. First-line treatment of ovarian cancer: questions and controversies to address. Ther Adv Med Oncol. 2018;10:1758835918768232. doi: 10.1177/1758835918768232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falzone L, Scandurra G, Lombardo V, Gattuso G, Lavoro A, Distefano AB, et al. A multidisciplinary approach remains the best strategy to improve and strengthen the management of ovarian cancer (review) Int J Oncol. 2021;59:53. doi: 10.3892/ijo.2021.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daniele G, Raspagliesi F, Scambia G, Pisano C, Colombo N, Frezzini S, et al. Bevacizumab, carboplatin, and paclitaxel in the first line treatment of advanced ovarian cancer patients: the phase IV MITO-16A/MaNGO-OV2A study. Int J Gynecol Cancer. 2021;31:875–882. doi: 10.1136/ijgc-2021-002434. [DOI] [PubMed] [Google Scholar]
- 5.Lheureux S, Mirza M, Coleman R. The DNA repair pathway as a target for novel drugs in gynecologic cancers. J Clin Oncol. 2019;37:2449–2459. doi: 10.1200/JCO.19.00347. [DOI] [PubMed] [Google Scholar]
- 6.Vokinger KN, Hwang TJ, Grischott T, Reichert S, Tibau A, Rosemann T, et al. Prices and clinical benefit of cancer drugs in the USA and Europe: a cost-benefit analysis. Lancet Oncol. 2020;21:664–670. doi: 10.1016/S1470-2045(20)30139-X. [DOI] [PubMed] [Google Scholar]
- 7.Leighl NB, Nirmalakumar S, Ezeife DA, Gyawali B. An arm and a leg: the rising cost of cancer drugs and impact on access. Am Soc Clin Oncol Educ Book. 2021;41:1–12. doi: 10.1200/EDBK_100028. [DOI] [PubMed] [Google Scholar]
- 8.Cai D, Sun H, Qi Y, Zhao X, Feng M, Wu X. Insulin-like growth factor 1/mammalian target of rapamycin and AMP-activated protein kinase signaling involved in the effects of metformin in the human endometrial cancer. Int J Gynecol Cancer. 2016;26:1667–1672. doi: 10.1097/IGC.0000000000000818. [DOI] [PubMed] [Google Scholar]
- 9.Sivalingam VN, Myers J, Nicholas S, Balen AH, Crosbie EJ. Metformin in reproductive health, pregnancy and gynaecological cancer: established and emerging indications. Hum Reprod Update. 2014;20:853–868. doi: 10.1093/humupd/dmu037. [DOI] [PubMed] [Google Scholar]
- 10.Febbraro T, Lengyel E, Romero IL. Old drug, new trick: repurposing metformin for gynecologic cancers? Gynecol Oncol. 2014;135:614–621. doi: 10.1016/j.ygyno.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imai A, Ichigo S, Matsunami K, Takagi H, Yasuda K. Clinical benefits of metformin in gynecologic oncology. Oncol Lett. 2015;10:577–582. doi: 10.3892/ol.2015.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chae YK, Arya A, Malecek MK, Shin DS, Carneiro B, Chandra S, et al. Repurposing metformin for cancer treatment: current clinical studies. Oncotarget. 2016;7:40767–40780. doi: 10.18632/oncotarget.8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 2015;350:g7607. doi: 10.1136/bmj.g7607. [DOI] [PubMed] [Google Scholar]
- 14.Kang S, Kim BR, Kang MH, Kim DY, Lee DH, Oh SC, et al. Anti-metastatic effect of metformin via repression of interleukin 6-induced epithelial-mesenchymal transition in human colon cancer cells. PLoS One. 2018;13:e0205449. doi: 10.1371/journal.pone.0205449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De A, Kuppusamy G. Metformin in breast cancer: preclinical and clinical evidence. Curr Probl Cancer. 2020;44:100488. doi: 10.1016/j.currproblcancer.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Brown JR, Chan DK, Shank JJ, Griffith KA, Fan H, Szulawski R, et al. Phase II clinical trial of metformin as a cancer stem cell-targeting agent in ovarian cancer. JCI Insight. 2020;5:e133247. doi: 10.1172/jci.insight.133247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urpilainen E, Puistola U, Boussios S, Karihtala P. Metformin and ovarian cancer: the evidence. Ann Transl Med. 2020;8:1711. doi: 10.21037/atm-20-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan LY, Lu Y. New developments in molecular targeted therapy of ovarian cancer. Discov Med. 2018;26:219–229. [PubMed] [Google Scholar]
- 19.Eisenhauer EL, Abu-Rustum NR, Sonoda Y, Aghajanian C, Barakat RR, Chi DS. The effect of maximal surgical cytoreduction on sensitivity to platinum-taxane chemotherapy and subsequent survival in patients with advanced ovarian cancer. Gynecol Oncol. 2008;108:276–281. doi: 10.1016/j.ygyno.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 20.Heckman-Stoddard BM, DeCensi A, Sahasrabuddhe VV, Ford LG. Repurposing metformin for the prevention of cancer and cancer recurrence. Diabetologia. 2017;60:1639–1647. doi: 10.1007/s00125-017-4372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Micha JP, Goldstein B, Markman M. Optimism and the continued promise of maintenance chemotherapy. Cancer Chemother Pharmacol. 2017;80:879–880. doi: 10.1007/s00280-017-3428-0. [DOI] [PubMed] [Google Scholar]
- 22.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 23.Rustin GJ, Vergote I, Eisenhauer E, Pujade-Lauraine E, Quinn M, Thigpen T, et al. Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the Gynecological Cancer Intergroup (GCIG) Int J Gynecol Cancer. 2011;21:419–423. doi: 10.1097/IGC.0b013e3182070f17. [DOI] [PubMed] [Google Scholar]
- 24.National Cancer Institute Division of Cancer Treatment and Diagnosis, Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events (CTCAE 5.00) Washington, D.C.: U.S. Department of Health and Human Services; 2017. [Google Scholar]
- 25.Romero IL, McCormick A, McEwen KA, Park S, Karrison T, Yamada SD, et al. Relationship of type II diabetes and metformin use to ovarian cancer progression, survival, and chemosensitivity. Obstet Gynecol. 2012;119:61–67. doi: 10.1097/AOG.0b013e3182393ab3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar S, Meuter A, Thapa P, Langstraat C, Giri S, Chien J, et al. Metformin intake is associated with better survival in ovarian cancer: a case-control study. Cancer. 2013;119:555–562. doi: 10.1002/cncr.27706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang SB, Lei KJ, Liu JP, Jia YM. Continuous use of metformin can improve survival in type 2 diabetic patients with ovarian cancer: a retrospective study. Medicine (Baltimore) 2017;96:e7605. doi: 10.1097/MD.0000000000007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng Y, Zhu J, Zhang H, Liu Y, Sun H. Metformin plus first-line chemotherapy versus chemotherapy alone in the treatment of epithelial ovarian cancer: a prospective open-label pilot trial. Cancer Chemother Pharmacol. 2019;84:1349–1357. doi: 10.1007/s00280-019-03963-7. [DOI] [PubMed] [Google Scholar]
- 29.du Bois A, Herrstedt J, Hardy-Bessard AC, Müller HH, Harter P, Kristensen G, et al. Phase III trial of carboplatin plus paclitaxel with or without gemcitabine in first-line treatment of epithelial ovarian cancer. J Clin Oncol. 2010;28:4162–4169. doi: 10.1200/JCO.2009.27.4696. [DOI] [PubMed] [Google Scholar]
- 30.Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–2496. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 31.Whitburn J, Edwards CM, Sooriakumaran P. Metformin and prostate cancer: a new role for an old drug. Curr Urol Rep. 2017;18:46. doi: 10.1007/s11934-017-0693-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iliopoulos D, Hirsch HA, Struhl K. Metformin decreases the dose of chemotherapy for prolonging tumor remission in mouse xenografts involving multiple cancer cell types. Cancer Res. 2011;71:3196–3201. doi: 10.1158/0008-5472.CAN-10-3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nanni O, Amadori D, De Censi A, Rocca A, Freschi A, Bologna A, et al. Metformin plus chemotherapy versus chemotherapy alone in the first-line treatment of HER2-negative metastatic breast cancer. The MYME randomized, phase 2 clinical trial. Breast Cancer Res Treat. 2019;174:433–442. doi: 10.1007/s10549-018-05070-2. [DOI] [PubMed] [Google Scholar]
- 34.Kordes S, Pollak MN, Zwinderman AH, Mathôt RA, Weterman MJ, Beeker A, et al. Metformin in patients with advanced pancreatic cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2015;16:839–847. doi: 10.1016/S1470-2045(15)00027-3. [DOI] [PubMed] [Google Scholar]
- 35.Wen KC, Sung PL, Wu AT, Chou PC, Lin JH, Huang CF, et al. Neoadjuvant metformin added to conventional chemotherapy synergizes anti-proliferative effects in ovarian cancer. J Ovarian Res. 2020;13:95. doi: 10.1186/s13048-020-00703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park JY, Lim MC, Baek MH, Park YH, Kim S. Impact of metformin on survival outcome in ovarian cancer: a nationwide population-based cohort study. J Gynecol Oncol. 2021;32:e65. doi: 10.3802/jgo.2021.32.e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmed MF, Kanaan G, Mostafa JA. The role of metformin in ovarian cancer: does metformin increase survival in ovarian neoplasm? Cureus. 2021;13:e13100. doi: 10.7759/cureus.13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Liu X, Yan P, Bi Y, Liu Y, Zhang ZJ. No effect of metformin on ovarian cancer survival: a systematic review and meta-analysis of cohort studies. Curr Pharm Des. 2019;25:2595–2601. doi: 10.2174/1381612825666190716113126. [DOI] [PubMed] [Google Scholar]