Abstract
Background and aims
The concentration and the duration of exposure to low-density lipoprotein cholesterol (LDL-C) (LDL-C burden) is an important determinant of risk for cardiovascular disease and thresholds has recently been estimated. Individuals with familial hypercholesterolemia (FH) have increased risk of premature cardiovascular disease. The overall aim of the present study was to describe differences in LDL-C level and LDL-C burden in females and males with FH visiting an outpatient lipid clinic from a young age, using multiple LDL-C measurements during a follow-up time of 12 years. First, we aimed to study if the LDL-C concentration and the LDL-C burden is different between females and males at ages 0–10, 10–20, 20–30 and >30 years. Second, we aimed to estimate the subject-specific LDL-C burden at age 19 and 30 years, and the proportion of female and male patients that reach suggested LDL-C thresholds indicating high risk of ASCVD.
Methods
Data was retrospectively collected from medical records of 438 subjects (207 girls and 231 boys) with FH, referred to the Lipid Clinic, Oslo University Hospital below the age of 19 years. The LDL-C burden was estimated based on repeated LDL-C measurements over time.
Results
Subjects were followed over a period of mean 12.0 (SD 7.0) years, with median 10 years (7–17; 25–75 percentiles, minimum 2), with median 6 (4–9; 25–75 percentiles, minimum 2) available LDL-C measurements, starting at mean age 11 (SD 3.9) years. There was a difference in both LDL-C and LDL-C burden between sexes at different ages. On average, males had lower LDL-C over time, although this difference was less pronounced with age and males also had lower estimated LDL-C burden over time, and this difference was further exacerbated with age.
Conclusion
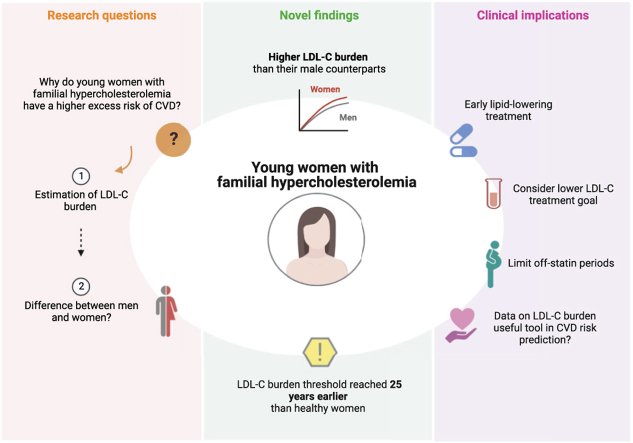
Our study shows that young women with FH have a higher LDL-C burden than their male counterparts, potentially explaining the increased excess CVD risk seen among these. It underscores the importance of careful-follow up and early treatment initiation both prior to and after pregnancies in order to limit statin-free periods.
Keywords: Low-density-lipoprotein exposure, Familial hypercholesterolemia, Cholesterol lowering, Sex-differences
Graphical abstract
Highlights
-
•
Concentration and duration of LDL-C exposure is an important determinant of CVD.
-
•
Women with FH had significantly higher LDL-C burden compared to men.
-
•
This difference increased with age.
-
•
At 33 years, all FH women had reached a cumulative LDL-C above 125 mM-year.
-
•
This is 25 years earlier than women from the general population.
Introduction
The atherosclerotic process begins early in life and is mainly driven by circulating low-density lipoproteins (LDL) and other apolipoprotein B-containing lipoproteins [1,2]. Both the magnitude and the duration of exposure to these lipoproteins (the LDL-cholesterol [LDL-C] burden), impacts the lifetime risk of atherosclerotic cardiovascular diseases (ASCVD) [[3], [4], [5], [6], [7]].
One condition that is characterized by higher LDL-C burdens is heterozygous familial hypercholesterolemia (FH), an autosomal dominant disease caused primarily by mutations in the LDL-receptor gene (LDLR), the apolipoprotein B gene (APOB) or the proprotein convertase subtilisin/kexin type 9 gene (PCSK9) [8,9]. As individuals with FH are exposed to higher LDL-C levels from birth and at increased risk of early ASCVD [8], treatment with statins is recommended from the age of 8–10 years, along with early adoption of a cholesterol-lowering diet and lifestyle guidance [10]. Recent evidence has shown that women treated for FH have a markedly higher excess risk of MI, coronary heart disease (CHD) and CVD morbidity than their male counterparts, especially at younger ages [7,11]. And interestingly, there is no sex-difference in age at first CVD event or age at time of death in an FH population [4], although women in the general population develops CVD 7–10 years later than men, and thus seem to be more ‘protected’ against CVD [4]. One might hypothesize that women with FH are exposed to a higher LDL-C burden through early life-course [12], leading to a higher excess risk of ASCVD. However, data on LDL-C burden in young men and women with FH has not yet been established, although it is highly relevant when recognizing the risk of ASCVD [13].
Previous studies have suggested theoretical thresholds of LDL-C burden for increased risk of myocardial infarction (MI) (125 mM-years or 5000 mg/dL-years) and an average threshold (220 mmol-years or 8500 mg/dL-years) at which an MI occurs [3]. To improve the understanding of the disease and the difference in ASCVD risk between men and women with FH, we need estimates of their LDL-C burden, based on repeated LDL-C data from an early age in genetically verified patients.
The overall aim of the present study was to describe differences in LDL-C level and LDL-C burden in females and males with FH visiting an outpatient lipid clinic from a young age, using multiple LDL-C measurements during a follow-up time of 12 years. First, we aimed to study if the LDL-C concentration and the LDL-C burden is different between females and males at ages 0–10, 10–20, 20–30 and >30 years. Second, we aimed to estimate the subject-specific LDL-C burden at age 19 and 30 years, and the proportion of female and male patients that reach suggested LDL-C thresholds indicating high risk of ASCVD.
Patients and methods
We retrospectively reviewed the medical records of 438 children with heterozygous FH that were below the age of 19 years between year 1990 and 2010, and who were followed-up at the Lipid Clinic, Oslo University Hospital, with at least two visits with available LDL-C data [14]. We collected LDL-C data from all visits up to latest July year 2019. Familial hypercholesterolemia was diagnosed by genetic testing (in 99% of the patients) and the remaining were diagnosed clinically based on the Dutch Lipid Clinic Network classification (World Health Organization publication no WHO/HGN/FH/CONS/99.2) where definite (certain) FH is defined with a score of 8 or more. The study complies with the Declaration of Helsinki and was approved by the Regional committee for medical and health research ethics, South-east Norway, as previously described [15].
Estimation of LDL-C burden in subjects with FH
For each subject, we estimated their LDL-C burden (determined by the plasma LDL-C concentration [mM] and duration [years] of the LDL-C concentration), based on repeated LDL-C measurements over time. The formula used for this calculation has previously been described [16]. First, a pre-treatment LDL-C burden was estimated for each subject by multiplying the LDL-C concentration (mmol/L) by the age of the subject at first visit before treatment was started. Second, a post-treatment LDL-C burden was estimated by annually imputing the LDL-C concentration measured at the next visit and adding these values together, and the same principal was followed for all the remaining LDL-C measurements. The pre-treatment LDL-C burden score and the post-treatment LDL-C score (mmol-years) were added together for the total estimated LDL-C burden.
Statistical analysis
Female and male subjects were compared in terms of demographics, follow-up at the Lipid Clinic, premature CVD, pre-treatment cholesterol levels, and latest lipid levels. Sex-differences were tested using Independent samples t-test for normally distributed data and Mann-Whitney U test for non-normally distributed data. A Chi-Square test was used for categorical data. For normally distributed data, means and standard deviations (SD) are provided, and for non-normally distributed data, medians and quartiles (1st and 3rd) are given.
To examine sex differences more in depth, we fitted linear mixed effects models by restricted maximum likelihood (REML) with the lmer function in the lme4/lmerTest packages, with random slope for age, and random intercept for subject. The outcome variables were LDL-C and estimated LDL-C burden, and the exposure variables were sex (male vs. female) and age (categorical variable binned at <10 years, 10–19 years, 20–29 years, and ≥30 years), as well as their interaction. We also performed a number of sensitivity analyses to examine sex differences, such as variation in adherence including pregnancy/lactation, use of high-potent statins, age at statin start, LDLR mutation type, and inheritance type (data not shown).
Statistical analyses were conducted in STATA version 16, (StataCorp, College Station, Texas, 77845 USA), SPSS version 26 (SPSS Inc., Chicago, IL, USA), and R version 4.1.2 (R Core Team, Vienna, Austria), all tests were two-sided and a significance level of 5% were set.
Comparison of LDL-C burden in a normal population and high-risk subjects
We provided a visual representation of the LDL-C burden distribution in a reference normal population to compare with the LDL-C burden estimated for the high-risk subjects included in our study, stratified by sex (Figure S1). The LDL-C burden distribution in the reference population was based on the age and sex-specific median plasma LDL-C (with given percentiles) from two normal populations, 1) the Netherlands (The Dutch Lifelines cohort study, n = 8071, 8-18y, percentiles: 5th, 10th, 25th, 50th, 75th, 90th, 95th) [17] and 2) Denmark (Copenhagen General population Study, n = ∼54 000 women and ∼42 000 men, 20-100y, percentiles: 2.5th, 25th, 50th, 75th, 97.5th) [18].
Results
Characteristics of patients
We included 438 children (207 girls and 231 boys) followed over a period of median (25–75 percentiles) 10 (7–17) years, with 6 (4–9, minimum 2) available LDL-C measurements, starting at mean age 11 (3.9) years (Table 1). Baseline and follow-up data for the total FH cohort, stratified by sex, are shown in Table 1, Table 2, respectively. Of those with genetically verified FH (433 out of 438), 98% (424 out of 433) carried an LDLR mutation (Table 1) and 2% (9 out of 433) carried a mutation in the APOB gene (data not shown). There was no difference between the sexes in the type of FH mutation that was carried (p = 0.67) or in the prevalence of LDLR null mutations (p = 0.45) (data not shown). At latest follow-up visit, 49% were prescribed a low or moderate-dose statin (atorvastatin 5, 10 and 20 mg, rosuvastatin 5 and 10 mg, simvastatin 10, 20 and 40 mg) and 36% were prescribed a high-dose statin (atorvastatin 40 and 80 mg, rosuvastatin 20 and 40 mg, simvastatin 80 mg). Also, one subject was prescribed a PCSK9-inhibitor. The percentage of female and male subjects that was prescribed a high-potent statin at latest visit was 36.2% and 36.8%, respectively (p = 0.90). Moreover, there were no differences between the sexes with regard to demographics, premature CVD, follow-up time, and age at statin start (Table 1). Women had higher pre-treatment total cholesterol, LDL-C and triglycerides, and higher HDL-C at latest visit compared to men (all p < 0.01, Table 1).
Table 1.
Baseline data of FH cohort stratified by sex.
| Total (n = 438) |
Females (n = 207) |
Males (n = 231) |
p value |
||||
|---|---|---|---|---|---|---|---|
| n | n | n | |||||
| Demographics | |||||||
| Age at first LDL-C measurement, y | 11.0 (3.9) | 438 | 11.2 (4.1) | 207 | 10.9 (3.8) | 231 | 0.50 |
| FH mutation, No. (%) | 433 (98.9) | 438 | 206 (99.5) | 207 | 227 (98.3) | 231 | 0.38 |
| LDL-R, No. (%) | 424 (97.9) | 433 | 201 (97.1) | 206 | 223 (96.5) | 227 | 0.74 |
| Premature CVDa | |||||||
| Premature CVD, No. (%) | 2 (0.5) | 438 | NA | 207 | 2 (0.9) | 231 | NA |
| Premature CVD in FH parent, No. (%) | 79 (18.0) | 438 | 37 (17.9) | 207 | 42 (18.2) | 231 | 0.52 |
| Premature CVD in FDR, No. (%) | 97 (22.1) | 438 | 46 (22.2) | 207 | 51 (22.1) | 231 | 0.76 |
| Pre-treatment lipids | |||||||
| TC, mM | 7.9 (1.8) | 438 | 8.2 (1.8) | 207 | 7.6 (1.7) | 231 | <0.001 |
| LDL-C, mM | 6.1 (1.7) | 438 | 6.4 (1.8) | 207 | 5.8 (1.6) | 231 | <0.01 |
| HDL-C, mM | 1.4 (0.9) | 432 | 1.3 (0.3) | 205 | 1.4 (1.2) | 227 | 0.35 |
| TGb, mM | 0.75 (0.60–1.05) | 429 | 0.80 (0.60–1.10) | 205 | 0.70 (0.50–1.00) | 224 | <0.01 |
Data are presented as mean (SD), unless otherwise stated. Abbreviations: CVD, cardiovascular disease; FDR, first-degree relative; FH, familial hypercholesterolemia; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LDL-R, the gene encoding low-density lipoprotein receptor; NA, not available; TC, total cholesterol; TG, triglycerides; y, years. Difference between sexes tested by Independent samples t-test for normally distributed data and Mann-Whitney U test for non-normally distributed data, and Chi-Square test for categorical data, statistically significant when p < 0.05.
Premature defined as age <55 y in men and <65 y in women.
Median (25–75 percentiles).
Table 2.
Follow-up data of FH cohort stratified by sex.
| Total (n = 438) | Females (n = 207) | Males (n = 231) | p value | ||||
|---|---|---|---|---|---|---|---|
| Follow-up at Lipid Clinic | |||||||
| Follow-up, y | 12.0 (7.0) | 438 | 12.6 (7.0) | 207 | 11.5 (6.9) | 231 | 0.12 |
| Number of LDL-C measurements, na | 6 (4–9) | 438 | 6 (5–9) | 207 | 5 (4–9) | 231 | <0.05 |
| Prescribed on statins, No. (%) | 381 (87.0) | 438 | 183 (88.4) | 207 | 198 (85.7) | 231 | 0.49 |
| Age at statin start, y | 15.5 (3.6) | 380 | 15.9 (3.4) | 182 | 15.2 (3.7) | 198 | 0.051 |
| Latest lipids | |||||||
| TC, mM | 5.7 (1.7) | 436 | 5.7 (1.6) | 206 | 5.7 (1.7) | 230 | 0.86 |
| LDL-C, mM | 3.9 (1.6) | 438 | 3.9 (1.5) | 207 | 4.0 (1.6) | 231 | 0.44 |
| HDL-C, mM | 1.3 (0.3) | 437 | 1.4 (0.3) | 206 | 1.2 (0.3) | 231 | <0.001 |
| TGb, mM | 0.90 (0.70–1.30) | 432 | 0.90 (0.70–1.10) | 203 | 0.90 (0.70–1.40) | 229 | 0.36 |
| LDL-C burden | |||||||
| 19 years, mM-years | 106.3 (26.3) | 313 | 111.7 (27) | 157 | 100.7 (24.6) | 156 | <0.001 |
| 30 years, mM-years | 166.5 (36.1) | 313 | 175.1 (36.4) | 42 | 156.7 (33.8) | 37 | <0.05 |
Data are presented as mean (SD), unless otherwise stated. Abbreviations: FH, familial hypercholesterolemia; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LDL-R, the gene encoding low-density lipoprotein receptor; NA, not available; TC, total cholesterol; TG, triglycerides; y, years. Difference between sexes tested by Independent samples t-test for normally distributed data and Mann-Whitney U test for non-normally distributed data, and Chi-Square test for categorical data, statistically significant when p < 0.05.
Premature defined as age <55 y in men and <65 y in women.
Median (25–75 percentiles).
Higher LDL-C burden in women compared to men
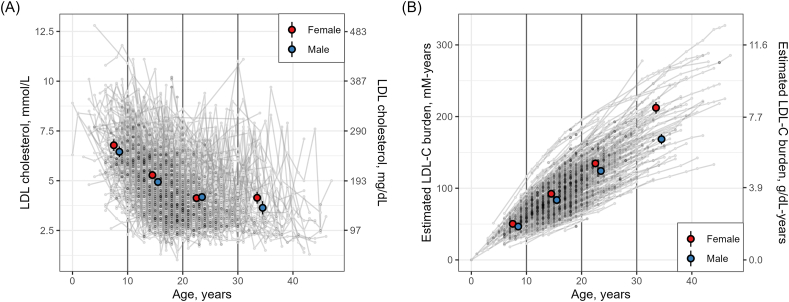
We examined the age-related changes in LDL-C and LDL-C burden and differences between sexes using exploratory figures (Fig. 1) and linear mixed effects models (Table 3). There was a difference in both LDL-C and LDL-C burden between sexes at different ages (Fig. 1, Table 3). On average, male subjects had lower LDL-C over time; the average difference at < 10 years was −0.609 mmol/l (95% CI = −1.088 to −0.130, p = 0.013). This difference was less pronounced with age, as the interaction terms for sex∗age were 0.255 mmol/L (95% CI = −0.155 - 0.664, p = 0.223) for 10–19 years, 0.696 mmol/L (95% CI = 0.187–1.205, p = 0.007) for 20–29 years, and 0.149 mmol/L (95% CI = −0.629 - 0.927, p = 0.707) for ≥30 years. Male subjects also had lower estimated LDL-C burden over time, with an average difference at < 10 years of −5.680 mM-years (95% CI = −9.115 to −2.245, p = 0.001). This difference was further exacerbated with age, as the interaction terms for sex∗age were −2.510 mM-years (95% CI = −4.099 to −0.921, p = 0.002) for 10–19 years, −3.095 mM-years (95% CI = −5.292 to −0.898, p = 0.006) for 20–29 years, and −4.008 mM-years (95% CI = −7.265 to −0.752, p = 0.016) ≥ 30 years.
Fig. 1.
Age-related changes in LDL-Cand estimated LDL-C burden. The figure shows measurements of LDL-C (Panel A) and estimated LDL-C burden (Panel B). Individual data points in grey are connected with lines to highlight the subject-specific trends. The figure also shows the sex-specific mean and 95% CI within strata of age (<10 years [n = 275], 10–19 years [n = 1528], 20–29 years [n = 843], and ≥30 years [n = 258]).
Table 3.
Linear mixed effects models for LDL-C and estimated LDL-C burden.
| LDL-C, mmol/L |
Estimated LDL-C burden, mM-years |
|||||
|---|---|---|---|---|---|---|
| Beta | 95% CI | p | Beta | 95% CI | p | |
| Sex | ||||||
| Female | – | – | – | – | ||
| Male | −0.609 | −1.088, −0.130 | 0.013 | −5.680 | −9.115, −2.245 | 0.001 |
| Age | ||||||
| <10 y | – | – | – | – | ||
| 10–19 y | −1.210 | −1.505, −0.915 | <0.001 | 9.590 | 8.450, 10.731 | <0.001 |
| 20–29 y | −2.111 | −2.475, −1.747 | <0.001 | 10.018 | 8.462, 11.573 | <0.001 |
| ≥30 y | −1.667 | −2.208, −1.126 | <0.001 | 8.408 | 6.153, 10.664 | <0.001 |
| Sex∗Age | ||||||
| Male ∗ 10–19 y | 0.255 | −0.155, 0.664 | 0.223 | −2.510 | −4.099, −0.921 | 0.002 |
| Male ∗ 20–29 y | 0.696 | 0.187, 1.205 | 0.007 | −3.095 | −5.292, −0.898 | 0.006 |
| Male ∗ ≥30 y | 0.149 | −0.629, 0.927 | 0.707 | −4.008 | −7.265, −0.752 | 0.016 |
We fitted linear mixed effects models by restricted maximum likelihood (REML) with the lmer function in the lme4/lmerTest packages in R, with random slope for age, and random intercept for subject. The outcome variables were LDL-C (in mmol/L) and estimated LDL-C burden (in mM-years), and the exposure variables were sex (male vs. female) and age (categorical variable), as well as their interaction. Abbreviations: CI, confidence interval; LDL-C, low-density lipoprotein cholesterol; y, years.
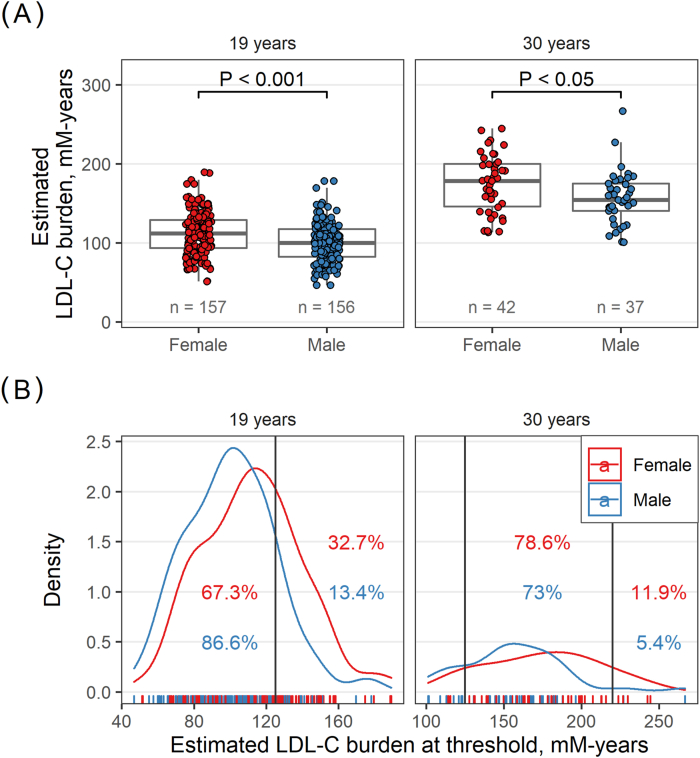
A threshold for LDL-C burden indicating higher risk of MI has been suggested [3]. We therefore subsequently compared the LDL-C burden between men and women at 19 years and 30 years to investigate and compare the proportions above the suggested LDL-C burden thresholds at these ages. At 19 years, women had a significantly higher LDL-C burden compared to men (111.7 [27] vs. 100.7 [24.6] mM-years, p < 0.001, Table 2 and Fig. 2) and consequently, a higher proportion of the women had reached the LDL-C threshold of 125 mM-years (32.7% vs. 13.4%, women and men respectively, p < 0.001 (Fig. 2 and Fig. S2). A larger mean difference was observed between women and men at 30 years, where the estimated LDL-C burden among women was 175.1 (36.4) mM-years vs. 156.7 [33.8] mM-years in men (p < 0.05, Table 2 and Fig. 2 and Fig. S2). At 30 years of age, totally 67 out of 79 patients (84.8%) were above the 125 mM-years LDL-C burden threshold, with 38 out of 42 women (90.5%) and 29 out of 37 men (78.4%) (no difference between sexes, p > 0.05, Fig. 2). Interestingly, of these 79 patients at 30 years, 7 (8.9%) had an LDL-C burden above 220 mM-years, 5 out of 42 women (11.9%) and 2 out of 37 men (5.4%) (no difference between sexes, p > 0.05, Fig. 2).
Fig. 2.
Proportion of women and men that reached LDL-C burden threshold. Distribution of estimated LDL-C burden in men and women at ages 19 and 30 years (Panel A) and the proportion above 125 mM-years and 200 mM-years (Panel B). At 19 years, in total n = 73/316 (23.1%), of which n = 52/159 (32.7%) women and n = 21/157 (13.4%) men had an LDL-C burden above 125 mM-years. At 30 years, in total n = 67/79 (84.8%), of which n = 33/42 (78.6%) women and n = 27/37 (73%) men had an LDL-C burden between 125 mM-years and 220 mM-years, and n = 5/42 (11.9%) women and n = 2/37 (5.4%) men had an LDL-C burden above 220 mM-years.
The proportion of men and women, at different ages, reaching the 125 and 220 mM-years thresholds are shown in Fig. S2. At age 36 years, a significantly higher proportion of women compared to men were above 220 mM-years, 43.8% vs. 6.2%, respectively (p < 0.05, Fig. S2). Moreover, all the women in our cohort had reached the 125 mM-year threshold at 33 years of age, and all the men had reached the same threshold at age 40 years (Fig. S1 and Fig. S2).
For comparison, the mean plasma LDL-C and LDL-C burden for the different age groups in the FH cohort and normal population are shown in Table S1. On average, women with FH reached the 125 mM-years threshold at age 22 years, compared to 47 years in the normal female population. Men with FH reached 125 mM-years threshold at 24 years on average, compared to 45 years in normal male population.
The LDL-C burden in high-risk subjects compared to a normal population
The estimated LDL-C burden distribution in a reference normal male and female population (data from the Dutch Lifelines cohort study and Copenhagen General population Study) are shown in Fig. S1, along the estimated LDL-C burden from all male and female subjects included in our study (from age one to maximum 47 years). The LDL-C burden in the FH population, both sexes, were considerably higher than for the reference population. The LDL-C burden trajectory of all subjects with FH were located above the age and sex-specific median LDL-C burden of the normal population (Fig. S1).
Discussion
In the present study, we provide data on the sex-specific distributions of LDL-C burden in a young FH cohort followed during childhood and early adulthood. Overall, women had higher LDL-C burden compared to men, most likely due to higher LDL-C in women before treatment start and until end of puberty, with the difference increasing over time. On average, women with FH reached the LDL-C burden threshold of 125 mM-years approximately 15 years earlier than the general population, and by the age of 30 years, the threshold was reached by as many as 90% of the women with FH. Importantly, 11% of these women had also reached the 220 mM-year threshold at 30 years of age, partly explaining the observed excess risk of CHD in this group of young women.
Sex differences in LDL-C burden
In the present study, we found that there was a difference in both LDL-C and LDL-C burden between sexes at different ages. On average, males had lower LDL-C over time, although this difference was less pronounced with age and males also had lower estimated LDL-C burden over time, and this difference was further exacerbated with age.
The LDL-C burden is one of the main determining factors of the lifetime risk of ASCVD [1,8]. The LDL-C burden threshold of 125 mM-years at which the risk of MI starts to increase in the population, and the average LDL-C burden at the time of an MI of 220 mM-years may serve as approximate estimates when considering LDL-C burden and differences in CVD risk. These threshold-estimates are based on the average LDL-C in the US population, and therefore, data on individual LDL-C at repeated time points and CVD outcomes are warranted. In the present study, 33% of female subjects with FH had an LDL-C burden above 125 mM-years at 19 years of age, compared to only 13% of the male subjects. Additionally, the sex-difference in LDL-C burden was present already from childhood and seemed to increase with age up to 42 years. Mundal et al. reported that the relative risk of acute MI and CHD in patients with FH was considerably higher than in the general population, with the highest excess risk in young women with FH between the ages of 25 and 39 years [7]. Also, Lyen et al. recently showed markedly elevated standardised morbidity ratios for composite CVD in young women with FH compared to men with FH, which were most pronounced among the younger age groups [11]. Our findings support the notion that young women with FH have a higher LDL-C burden and are thus at a higher risk of developing early CVD. We have previously shown that untreated LDL-C levels are higher in FH girls compared to boys through childhood and adolescence [12]. Although there was no difference in the age of treatment start between the sexes in our study, previous results on FH patients have shown that girls generally were started on statin treatment later than boys [19]. Moreover, women with FH are generally treated less aggressively than FH men [20], which may also contribute to the higher LDL-C burden seen in female subjects. During adulthood, periods of pregnancy and lactation also lead to longer off-statin periods of elevated LDL-C in women with FH. We have recently estimated that the length of pregnancy-related off-statin periods in women with FH was median 2.3 years and 20% of these had off-statin periods longer than 4 years. Interestingly, 8% of these women had experienced cardiovascular events already at median age 40 years [21]. Although there were no reported cardiovascular events among the women in our cohort, probably due to the low number of cases after 40 years (n = 9 women followed until age >40 years), we found that 11.9% had reached an LDL-C burden above 220 mM-years already at age 30 years. If these disparities between young men and women with FH are ignored, the consequence could be suboptimal treatment for women. Whether women with FH should have a lower LDL-C treatment goal or start statin treatment at an even younger age in order to compensate for the higher untreated LDL-C throughout childhood and future pregnancy-related off-statin periods, needs to be considered.
Inter-individual variations in LDL-C burden
The LDL-C burden was considerably higher in the FH cohort compared to a normal population, as expected. Our results also showed large inter-individual differences in LDL-C burden among the subjects. For example, at age 19 years, the LDL-C burden ranged from 47 to 189 mM-years. Variations in LDL-C burden is most likely due to the intensity and duration of lipid-lowering treatment, adherence to medication and diet. We have previously shown that approximately 30% of children and young adults with FH on statin treatment do not take their statins for a variety of reasons [14]. Longer off-statin periods may contribute to larger LDL-C burdens and a higher risk of experiencing early cardiovascular events. In fact, two male subjects in the present study had experienced premature ASCVD at age 31 and 39 years with an estimated LDL-C burden at age 30 years of 144 mM-years and 227 mM-years, respectively; both reported longer periods of non-adherence to medication. Individual data on LDL-C burden could therefore be an important tool in the CVD risk prediction and treatment goal decisions as it considers not only the absolute LDL-C level at a specific time, but the total exposure of LDL-C throughout life. As an example, we see that even if the absolute concentration of LDL-C between sexes approaches similar levels at the end of puberty, there is a significant difference in the LDL-C burden throughout the whole follow-up period. Data on LDL-C burden are highly relevant for high-risk subjects such as those with familial hypercholesterolemia (FH), as a useful tool in clinical practice to guide treatment goals and gain understanding of the disease [13]. Data on LDL-C burden may add to the patients’ understanding of the disease, potentially preventing longer periods of non-adherence and give a more precise picture of both short- and long-term ASCVD risk. Considering the patients LDL-C burden together with additional risk factors (for example high blood pressure, diabetes, elevated lipoprotein(a), family history of CVD, diet and smoking) may contribute to improve medical care and individualize the treatment of FH [13].
Strengths and limitations
Main strengths of the study were the large cohort of mainly genetically proven FH in children and the long follow-up time. Importantly, the LDL-C burden was calculated in both sexes and were based on several LDL-C measurements over time. However, there are some uncertainties around these estimates, especially for those with few LDL-C measurements. Also, we assumed the same LDL-C level from birth to first visit at the Lipid Clinic. We know that LDL-C levels usually fluctuate minimally until puberty, where a small decrease in LDL-C levels occurs. As some subjects had their first measurement of LDL-C in puberty or after puberty, the LDL-C burden may be slightly underestimated among those. Furthermore, it must be pointed out that we did not have information on how many females that were treated with birth control drugs and we do not have data on off-treatment periods due to low adherence. A limitation is also that our data do not take into account the LDL-C elevation during pregnancy, as the LDL-C is not routinely measured at the Lipid Clinic during this period, and therefore, the LDL-C burden among those having been pregnant is also an underestimation. Our data was collected retrospectively and included children below the age of 18 years in the period from 1990 to 2010. The guidelines regarding age of statin-start were different then and are reflected in the age at statin start in this population. Due to the young patient cohort, there were few subjects above 30 years of age, which limit the power to detect differences at higher age groups. Also, a limitation of the study is that in our cohort the patients were relatively young at baseline (mean age 11 years) and were followed over a period of mean 12 years. As a result, there were very few subjects above 35 years at latest follow-up visit (16 men and 16 women), and only two subjects in the current cohort had experienced a CHD event, making us unable to explore a correlation between LDL-C burden and CHD. However, the subjects that had experienced a CHD event were both male subjects (age 43 and 36 years at last visit) and had a cholesterol burden of 277 and 163 mmol-years, respectively. Finally, as the cohort consisted of mostly native Norwegians and our results cannot be generalized to multi-ethnic FH populations. We compared the lipid levels from our Norwegian cohort with lipid levels from a general population from the Netherlands [17] and Denmark [18]. We cannot exclude the possibility of differences in baseline lipid levels between these countries, but studies have shown similar lipid levels among children with FH and young women with FH in Norway and the Netherlands [21,22].
In conclusion, young women with FH are characterized by a higher LDL-C burden compared to men, potentially partly explaining the observed excess risk of CHD among young women with FH. Careful follow-up by lipid specialists and lower LDL-C treatment goals should be considered for young women with FH in order to compensate for the higher LDL-C burden obtained at young age.
Author contributions
Anja K. Johansen: Data collection, data analysis, drafting and revision of manuscript. Martin P. Bogsrud: Design and planning of the study, interpretation of data, revision of manuscript. Jacob J. Christensen: Data analysis and figures, revision of manuscript. Amanda Rundblad: Figures, revision of manuscript. Ingunn Narverud: Data collection, revision of manuscript. Stine Ulven: Discussion of data, revision of manuscript. Gisle Langslet: Interpretation of data, revision of manuscript. Kjetil Retterstøl: Interpretation of data, revision of manuscript. Kirsten B. Holven: Design and planning of the study, interpretation of data, revision of manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The study was supported by the South-Eastern Regional Health Authority, Oslo, Norway, the Norwegian National Advisory Unit on FH, Oslo University Hospital, Oslo, Norway, and the Throne-Holst Foundation for Nutrition Research, University of Oslo, Oslo, Norway and the University of Oslo, Oslo, Norway.
We thank Magne Thoresen at the Department of Biostatistics, Institute of Basic Medical Sciences, University of Oslo, Oslo, Norway for contributing on discussions regarding statistical analyses and figures.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.athplu.2023.01.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
The LDL-C burden in FH female and male subjects compared to normal population. The LDL-C burden (mM-years and g/dL-years) in 207 female and 231 male subjects with FH, along with the median (solid blue line) age and sex-specific LDL-C burden estimated from the LDL-C distributions in two normal populations [17,18]. Data for the normal populations are shown as the age- and sex-specific median LDL-C burden (solid blue line) with 10–90 percentiles at age 8–18 y and 25–75 percentiles at age 20–50 y (blue shading). Black solid lines represent the LDL-C burden trajectory assuming an annual average plasma concentration of LDL-C of 2 mM, 4 mM, 6 mM and 8 mM.
Proportion of male and female subjects (%) that crossed the 125 (Panel A) and 220 mM-years (Panel B) thresholds of LDL-C burden.
∗, p<0.05; ∗∗, p<0.01; ∗∗∗, p<0.001.
References
- 1.Ference B.A., Ginsberg H.N., Graham I., et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. Aug 21 2017;38(32):2459–2472. doi: 10.1093/eurheartj/ehx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stary H.C. Evolution and progression of atherosclerotic lesions in coronary arteries of children and young adults. Arteriosclerosis. Jan-Feb. 1989;9(1 Suppl):I19–I32. [PubMed] [Google Scholar]
- 3.Ference B.A., Graham I., Tokgozoglu L., Catapano A.L. Impact of lipids on cardiovascular health: JACC health promotion series. J Am Coll Cardiol. Sep 4 2018;72(10):1141–1156. doi: 10.1016/j.jacc.2018.06.046. [DOI] [PubMed] [Google Scholar]
- 4.Krogh H.W., Mundal L., Holven K.B., Retterstøl K. Patients with familial hypercholesterolaemia are characterized by presence of cardiovascular disease at the time of death. Eur Heart J. 2016;37(17):1398–1405. doi: 10.1093/eurheartj/ehv602. [DOI] [PubMed] [Google Scholar]
- 5.Mundal L., Veierød M.B., Halvorsen T., et al. Cardiovascular disease in patients with genotyped familial hypercholesterolemia in Norway during 1994–2009, a registry study. Eur j prevent cardiol. 2016;23(18):1962–1969. doi: 10.1177/2047487316666371. [DOI] [PubMed] [Google Scholar]
- 6.Mundal L., Igland J., Ose L., et al. Cardiovascular disease mortality in patients with genetically verified familial hypercholesterolemia in Norway during 1992-2013. Eur j prevent cardiol. Jan 2017;24(2):137–144. doi: 10.1177/2047487316676135. [DOI] [PubMed] [Google Scholar]
- 7.Mundal L.J., Igland J., Veierød M.B., et al. Impact of age on excess risk of coronary heart disease in patients with familial hypercholesterolaemia. Heart. 2018;104(19):1600–1607. doi: 10.1136/heartjnl-2017-312706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nordestgaard B.G., Chapman M.J., Humphries S.E., et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease. Eur Heart J. 2013;34(45):3478–3490. doi: 10.1093/eurheartj/eht273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leren T.P., Bogsrud M.P. Molecular genetic testing for autosomal dominant hypercholesterolemia in 29,449 Norwegian index patients and 14,230 relatives during the years 1993-2020. Atherosclerosis. 2021;322:61–66. doi: 10.1016/j.atherosclerosis.2021.02.022. Apr. [DOI] [PubMed] [Google Scholar]
- 10.Mach F., Baigent C., Catapano A.L., et al. ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2019;41(1):111–188. doi: 10.1093/eurheartj/ehz455. Jan 1 2020. [DOI] [PubMed] [Google Scholar]
- 11.Iyen B., Qureshi N., Weng S., et al. Sex differences in cardiovascular morbidity associated with familial hypercholesterolaemia: a retrospective cohort study of the UK Simon Broome register linked to national hospital records. Atherosclerosis. Dec. 2020;315:131–137. doi: 10.1016/j.atherosclerosis.2020.10.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holven K.B., Narverud I., van Lennep J.R., et al. Sex differences in cholesterol levels from birth to 19 years of age may lead to increased cholesterol burden in females with FH. J clin lipidol. May - Jun 2018;12(3):748–755.e2. doi: 10.1016/j.jacl.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 13.Langslet G., Holven K.B., Bogsrud M.P. Treatment goals in familial hypercholesterolaemia—time to consider low-density lipoprotein-cholesterol burden. Eur j prevent cardiol. 2021 doi: 10.1093/eurjpc/zwab228. [DOI] [PubMed] [Google Scholar]
- 14.Langslet G., Johansen A.K., Bogsrud M.P., et al. Thirty percent of children and young adults with familial hypercholesterolemia treated with statins have adherence issues. Am J Prev Cardiol. Jun. 2021;6 doi: 10.1016/j.ajpc.2021.100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narverud I., van Lennep J.R., Christensen J.J., et al. Maternal inheritance does not predict cholesterol levels in children with familial hypercholesterolemia. Atherosclerosis. 2015;243 1:155–160. doi: 10.1016/j.atherosclerosis.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt H.H., Hill S., Makariou E.V., Feuerstein I.M., Dugi K.A., Hoeg J.M. Relation of cholesterol-year score to severity of calcific atherosclerosis and tissue deposition in homozygous familial hypercholesterolemia. Am J Cardiol. Mar 15. 1996;77(8):575–580. doi: 10.1016/s0002-9149(97)89309-5. [DOI] [PubMed] [Google Scholar]
- 17.Balder J.W., Lansberg P.J., Hof M.H., Wiegman A., Hutten B.A., Kuivenhoven J.A. Pediatric lipid reference values in the general population: the Dutch lifelines cohort study. J clin lipidol. Sep - Oct 2018;12(5):1208–1216. doi: 10.1016/j.jacl.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Nordestgaard B.G., Langlois M.R., Langsted A., et al. Quantifying atherogenic lipoproteins for lipid-lowering strategies: consensus-based recommendations from EAS and EFLM. Atherosclerosis. 2020/02/01/2020;294:46–61. doi: 10.1016/j.atherosclerosis.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Langslet G., Bogsrud M.P., Halvorsen I., et al. Long-term follow-up of young adults with familial hypercholesterolemia after participation in clinical trials during childhood. J clin lipidol. 2015/11/01/2015;9(6):778–785. doi: 10.1016/j.jacl.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Balla S., Ekpo E.P., Wilemon K.A., Knowles J.W., Rodriguez F. Women living with familial hypercholesterolemia: challenges and considerations surrounding their care. Curr Atherosclerosis Rep. Aug 20 2020;22(10):60. doi: 10.1007/s11883-020-00881-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klevmoen M., Bogsrud M.P., Retterstøl K., et al. Loss of statin treatment years during pregnancy and breastfeeding periods in women with familial hypercholesterolemia. Atherosclerosis. 2021;335:8–15. doi: 10.1016/j.atherosclerosis.2021.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Narverud I., Retterstøl K., Iversen P.O., et al. Markers of atherosclerotic development in children with familial hypercholesterolemia: a literature review. Atherosclerosis. 2014;235(2):299–309. doi: 10.1016/j.atherosclerosis.2014.05.917. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The LDL-C burden in FH female and male subjects compared to normal population. The LDL-C burden (mM-years and g/dL-years) in 207 female and 231 male subjects with FH, along with the median (solid blue line) age and sex-specific LDL-C burden estimated from the LDL-C distributions in two normal populations [17,18]. Data for the normal populations are shown as the age- and sex-specific median LDL-C burden (solid blue line) with 10–90 percentiles at age 8–18 y and 25–75 percentiles at age 20–50 y (blue shading). Black solid lines represent the LDL-C burden trajectory assuming an annual average plasma concentration of LDL-C of 2 mM, 4 mM, 6 mM and 8 mM.
Proportion of male and female subjects (%) that crossed the 125 (Panel A) and 220 mM-years (Panel B) thresholds of LDL-C burden.
∗, p<0.05; ∗∗, p<0.01; ∗∗∗, p<0.001.