Highlights
-
•
The Khorana score VTE risk assessment model has not been validated in patients with uterine cancer.
-
•
Patients with uterine cancer undergoing chemotherapy had a VTE rate of 6.5%.
-
•
Uterine cancer patients that had a VTE during treatment had a non-statistically significant higher average Khorana score.
-
•
A high Khorana score is a poor predictor of VTE in patients with uterine cancer undergoing chemotherapy.
Keywords: Chemotherapy, Endometrial cancer, Khorana score, Thromboprophylaxis, Uterine cancer, Venous thromboembolism
Abstract
Objective
Gynecologic cancers are associated with a high risk of venous thromboembolism (VTE). The Khorana score is a validated tool to assess risk of VTE in cancer patients. The purpose of this study is to determine if the Khorana score can be used as a risk stratification tool for VTE in patients with uterine cancer undergoing chemotherapy.
Methods
A retrospective cohort study of patients with newly diagnosed uterine cancer receiving chemotherapy over a 4-year period was conducted. The patients were stratified based on their Khorana score as well as their chemotherapy sequence, neoadjuvant or definitive versus adjuvant.
Results
A total of 276 patients were included: 40 received neoadjuvant or definitive, 236 adjuvant chemotherapy. Most patients had advanced stage disease (64.5%). 18 (6.5%) patients developed VTE within 180 days of initiating chemotherapy. High Khorana score was associated with a non-significant increase in VTE (K ≥ 2 OR 1.17, CI 0.40–3.39, K ≥ 3 OR 1.69, CI 0.61–4.69) but had poor predictive accuracy based on area under the curve (K ≥ 2 0.51, K ≥ 3 0.55). The VTE rate was higher in the neoadjuvant/definitive chemotherapy group to adjuvant (12.5% vs 5.5%, p = 0.11). While the former group had a higher average Khorana score (2.35 vs 1.93, p = 0.0048), this was not predictive of VTE.
Conclusions
While validated in other cancer types, the Khorana score was found to be a poor predictor of VTE in patients with uterine cancer. The use of the Khorana score to guide routine thromboprophylaxis in these patients should be used with caution and further investigation is warranted.
1. Introduction
Gynecologic cancers are associated with a high risk of venous thromboembolism (VTE) with rates ranging between 3 and 25% (Barber and Clarke-Pearson, 2016, Cohen et al., 2017). Patients who develop VTE, diagnosed as either pulmonary embolism (PE) or deep vein thrombosis (DVT), have significant risk of morbidity and mortality, particularly when associated with malignancy (Khorana, 2010, Zhan and Miller, 2003, Rodriguez et al., 2011). Patients with uterine cancer often have comorbid conditions, such as obesity, further increasing the risk of VTE (Anderson and Spencer, 2003, Ageno et al., 2006). Therefore, risk stratification of patients is a key component when determining the appropriateness of VTE prophylaxis.
The Khorana score is a clinically validated tool to assess the risk of VTE in ambulatory cancer patients by utilizing the disease site, body mass index (BMI) and pre-chemotherapy blood counts (platelets, hemoglobin and WBC) (Khorana et al., 2008). The Khorana risk assessment model has been used by practice guidelines and clinical trials for over 10 years to determine which patients should receive primary VTE prophylaxis (Carrier et al., 2019, Khorana et al., 2019, Di Nisio et al., 2016). The 2019 American Society of Clinical Oncology (ASCO) Clinical Practice Guidelines updated their recommendations to include that patients with a Khorana score of ≥2 be offered pharmacologic thromboprophylaxis during systemic chemotherapy (Key et al., 2020). It is noted, however, that the Khorana score threshold of 2 is considered intermediate risk based on the original derivation and validation studies by Khorana et al. In those studies, the authors concluded patients in this intermediate group would be unlikely to benefit from thromboprophylaxis (Khorana et al., 2008). While patients with uterine cancer were included in the study, they were aggregated with other gynecologic malignancies and represented a relatively small portion of the study cohort. Furthermore, most available data regarding VTE in women with uterine cancer are focused on the perioperative setting due to the high rate of surgical treatment and minority receiving chemotherapy (Barber and Clarke-Pearson, 2016). Yet, it is well established that patients with advanced stage uterine cancer requiring chemotherapy are at the highest risk for VTE (Rodriguez et al., 2011, Ohashi et al., 2020), and the utility of primary thromboprophylaxis in this population is under-investigated. Our hypothesis is that the Khorana score will be able to predict the patients at highest risk for VTE. The purpose of this study is to determine if the Khorana score can be used as a risk stratification tool for VTE in ambulatory patients with newly diagnosed uterine cancer undergoing chemotherapy.
2. Methods
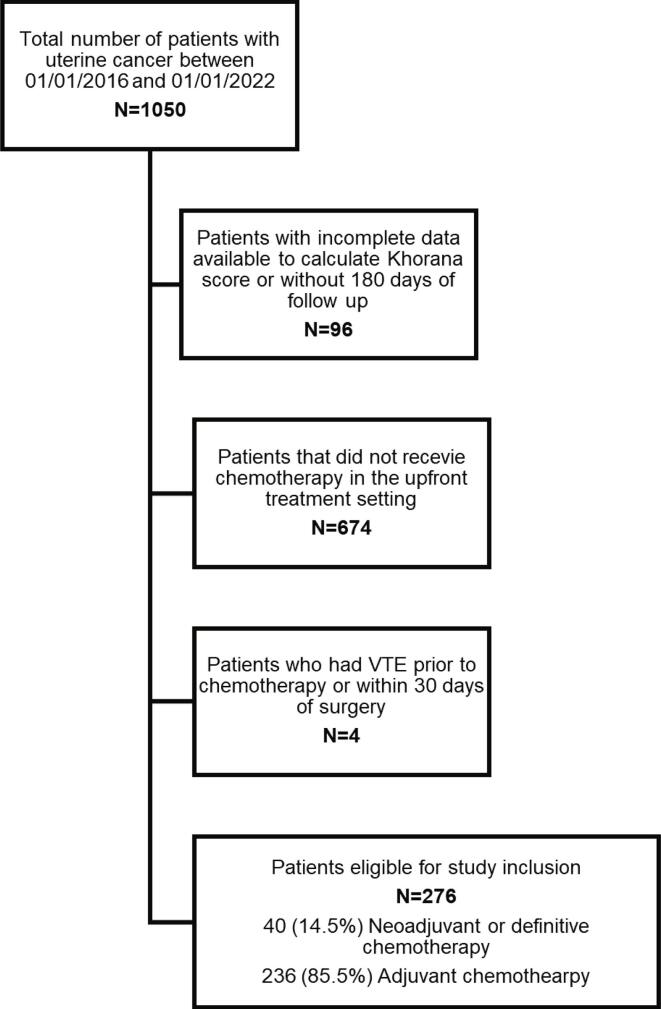
Following Institutional Review Board (IRB) approval, a single institution retrospective cohort study was performed. All patients with newly diagnosed uterine cancer treated at The Ohio State Wexner Medical Center from January 2016 to January 2020 were screened. Patients with complete clinical data and at least 180 days of follow up, receiving either adjuvant (ACT) chemotherapy or chemotherapy as a neoadjuvant or definitive treatment (NA/DCT) were included in the study. Patients receiving chemotherapy for recurrent disease were excluded. See Fig. 1 for STROBE diagram. A histologic diagnosis was confirmed by endometrial biopsy or surgical staging; all histologic subtypes, including carcinosarcomas and sarcomas, were included. Baseline demographic and clinical data were extracted from the medical chart including age, body mass index (BMI), race/ethnicity, smoking status, pathologic data including histology, International Federation of Gynecology and Obstetrics (FIGO) grade, FIGO stage, treatment details, as well as blood counts (platelet count, hemoglobin level and white blood cell count) within 2 weeks prior to initiation of chemotherapy. VTE events were recorded if documented by radiologic test or clinician report. The primary outcome of this study was the incidence of VTE within 180 days of initiation of chemotherapy. The duration of 180 days is based on it’s use in prospective VTE clinical trials in ambulatory cancer patients (Khorana et al., 2019) and similar retrospective studies. Due to concern for confounding postoperative VTE risk, patient’s with VTE within 30 days of surgery were excluded. During this time at the institution, routine chemoprophylaxis was not given for ambulatory patients undergoing chemotherapy.
Fig. 1.
STROBE diagram to illustrate patient eligibility for study inclusion.
The Khorana score was derived from the ASCO Clinical Practice Guideline (Key et al., 2020). The model includes five risk factors, each worth one point, consisting of: primary tumor site, platelet count ≥350 × 109/L, hemoglobin levels ≤100 g/L, white blood cell count >11 × 109/L and BMI ≥ 35 kg/m2. The Khorana score was calculated for each patient and the model performance for high-risk patients was compared between a score of ≥2 and ≥3. The patients were further stratified based on the sequence of chemotherapy (NA/DCT or ACT).
Descriptive statistics were reported for patient demographic and clinical data, stratified by NA/DCT vs ACT. In order to determine the prognostic utility of the Khorana score as well as other clinico-pathologic criteria on development of VTE, univariate analysis was performed using independent t test, chi-square test or Fisher’s exact test when appropriate. Measures of validity were also measured and compared including sensitive, specificity, positive predictive value (PPV), negative predictive value (NPV), area under the curve of the receiver-operator characteristic curve (AUC) and the positive likelihood ratio. All of the variables from the univariate analysis were then further assessed in a forward stepwise multivariate logistic regression model to evaluate which variables remained statistically significant when adjusting for other factors. A p-value of 0.05 was considered statistically significant in both the univariate analysis and as an inclusion threshold for the stepwise model. Data analysis was performed using SAS software. With 276 patients we calculated 80% power to detect a difference in risk groups with an estimated VTE rate of 10% in the high risk group and 2% in the low risk group with a 1:4 sampling ratio (high to low) and alpha set to 0.05 (estimates based on prior studies (Khorana et al., 2008, Austin et al., 2019).
3. Results
A total of 276 patients were included, 40 (14.5%) patients received NA/DCT, 236 (85.5%) ACT. Patient characteristics can be found in Table 1. The majority of patients were obese (BMI ≥ 30 kg/m2) (171, 61.9%) and 60 years or older (195, 70.6%). The most common histology was endometrioid (116, 42.0%) followed by serous (72, 26.1%). Most women had advanced-stage (stage 3–4) disease (178, 64.5%) and most underwent hysterectomy during treatment (253, 91.7%). Half of the patients in the NA/DCT subgroup underwent hysterectomy.
Table 1.
Baseline characteristics of the study population stratified by chemotherapy type.
| Variable | NA/DCT | ACT | All |
|---|---|---|---|
| Patients, n (%) | 40 (14.5) | 236 (85.5) | 276 (100) |
| VTE, n (%) | 5 (12.5) | 13 (5.5) | 18 (6.5) |
| Age (years) | |||
| Median | 62 | 65 | 64 |
| Range | 47–86 | 34–89 | 34–89 |
| BMI (kg/m2) | |||
| >30, n (%) | 23 (57.5) | 148 (53.6) | 171 (61.9) |
| Median | 31.3 | 32.3 | 32.1 |
| Range | 16.6–55.3 | 16.8–70.5 | 16.6–70.5 |
| Race/Ethnicity, n (%) | |||
| White | 32 (80) | 221 (93.6) | 256 (92.7) |
| Black | 6 (15) | 12 (5.1) | 18 (6.5) |
| Hispanic/Latino | 2 (5) | 1 (0.4) | 3 (1.1) |
| Other | 0 (0) | 2 (0.8) | 3 (1.1) |
| Current Smoker, n (%) | 1 (2.5) | 8 (3.4) | 9 (3.2) |
| Histology, n (%) | |||
| Endometrioid | 15 (37.5) | 101 (42.8) | 116 (42.0) |
| Serous | 13 (32.5) | 59 (25.0) | 72 (26.1) |
| Clear cell | 3 (7.5) | 3 (1.3) | 6 (2.2) |
| Mixed | 3 (7.5) | 35 (14.8) | 38 (13.8) |
| Carcinosarcoma | 2 (5) | 31 (13.1) | 33 (12.0) |
| Sarcoma | 1 (2.5) | 4 (1.7) | 5 (1.8) |
| Other* | 3 (7.5) | 3 (1.3) | 6 (2.2) |
| FIGO Grade, n (%) | |||
| 1 | 7 (17.5) | 52 (22.0) | 59 (21.3) |
| 2 | 3 (7.5) | 33 (14.0) | 36 (13.0) |
| 3 | 28 (70) | 148 (62.7) | 176 (63.8) |
| X | 2 (5) | 3 (1.3) | 5 (1.8) |
| FIGO Stage, n (%) | |||
| 1 | 2 (5) | 88 (37.3) | 90 (32.6) |
| 2 | 0 (0) | 8 (3.4) | 8 (2.9) |
| 3 | 4 (10) | 110 (46.6) | 114 (41.3) |
| 4 | 34 (85) | 30 (12.7) | 64 (23.2) |
| Hysterectomy, n (%) | 20 (50) | 233 (98.7) | 253 (91.7) |
| Residual Disease, n (%) | 25 (62.5) | 20 (8.5) | 45 (16.3) |
| Radiation, n (%) | 13 (32.5) | 146 (61.9) | 159 (57.6) |
| Blood Counts, n (%) | |||
| Platelet ≥ 350 x109/L | 18 (45) | 60 (25.4) | 78 (28.3) |
| Hgb ≤ 10 g/L | 14 (35) | 45 (19.1) | 59 (21.4) |
| WBC > 11 109/L | 11 (27.5) | 23 (9.7) | 34 (12.3) |
| Khorana Score ≥ 2, n (%) | 30 (75) | 161 (68.2) | 191 (69.2) |
| Khorana Score ≥ 3, n (%) | 18 (45) | 47 (19.9) | 65 (23.5) |
NA/DCT = neoadjuvant/definitive chemotherapy, ACT = adjuvant chemotherapy, FIGO = International Federation of Gynecology and Obstetrics.
*Other histologies includes dedifferentiated, undifferentiated and mucinous.
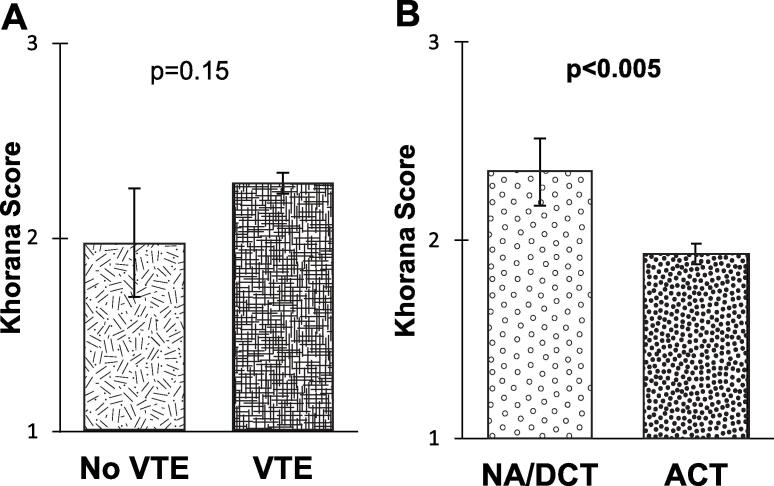
A total of 18 (6.5%) patients developed a VTE within 180 days of initiating chemotherapy. Of the patients with VTE, 8 were diagnosed with DVT, 9 with PE, and 1 patient with both DVT and PE. No patients died from VTE during the study period nor had a significant change to their treatment plan. Following VTE diagnosis, patients spent a mean time of 3.3 days in the hospital (range 0–21). The average Khorana score in the patients who had VTE was 2.28 compared to 1.97 for those without VTE, this difference was not statistically significant (p = 0.15, 95%CI −0.11–0.72) (Fig. 2). VTE rate was higher in the NA/DCT group (5, 12.5%) compared to ACT (13, 5.5%), but the difference was, again, not statistically significant (OR = 2.45, 95%CI 0.82–7.30, p = 0.11). The majority of patients (69.2%) had a Khorana score of ≥2 whereas only 23.5% had a score ≥ 3. Significantly more patients in the NA/DCT cohort had high risk Khorana scores (K ≥ 3, OR = 3.27, 95%CI 1.63–6.58, p = 0.001; K ≥ 2 OR = 1.40, 95%CI 0.65–3.01, p = 0.39) with an average score of 2.35 compared to an average score of 1.93 in the ACT group (p = 0.0048, 95%CI 0.13–0.71) (Fig. 2).
Fig. 2.
(A) Independent t test of the Khorana score between patients without venous thromboembolism (No VTE) and patients that were diagnosed with venous thromboembolism (VTE). (B) Independent t test of the Khorana score between the neoadjuvant/definitive chemotherapy (NA/DCT) subgroup and adjuvant chemotherapy (ACT) subgroup.
A high Khorana score (defined with threshold at either ≥2 or ≥3), was not able to significantly predict VTE (p > 0.05) in either the full chemotherapy cohort, or when analyzing the NA/DCT and ACT subgroups (the study was not powered to evaluate the subgroups). A high Khorana score was associated with a non-statistically significant elevated odds ratio of VTE (this association held in all subgroups except in the neoadjuvant group when the cutoff was ≥3). As expected, using a higher Khorana score cutoff for the high-risk group increased the specificity for predicting VTE at the expense of the sensitivity of the risk assessment. The negative predictive value of the Khorana score was >80% in all models however the positive predictive value was quite poor (<15%). This led to an overall poor predictive accuracy for VTE as measured by area under the curve of the receiver operating characteristic curve (AUC range between 0.51 and 0.60). A complete overview of screening validity tests for each group/subgroup is outlined in Table 2.
Table 2.
Odds ratio and measures of validity for each group/subgroup at both Khorana score cutoff of ≥2 and ≥3.
| Group/Subgroup | Khorana Cutoff | Odds Ratio | Confidence Interval | p Value | Sensitivity | Specificity | PPV | NPV | AUC | Likelihood Ratio (+) |
|---|---|---|---|---|---|---|---|---|---|---|
| All | K ≥ 2 | 1.17 | 0.40–3.39 | 0.77 | 0.72 | 0.31 | 0.068 | 0.94 | 0.51 | 1.05 |
| All | K ≥ 3 | 1.69 | 0.61–4.69 | 0.35 | 0.33 | 0.77 | 0.092 | 0.94 | 0.55 | 1.46 |
| ACT | K ≥ 2 | 1.05 | 0.31–3.53 | 0.94 | 0.69 | 0.32 | 0.056 | 0.95 | 0.51 | 1.02 |
| ACT | K ≥ 3 | 2.69 | 0.84–8.65 | 0.09 | 0.38 | 0.81 | 0.11 | 0.96 | 0.60 | 2.04 |
| NA/DCT | K ≥ 2 | 1.38 | 0.14–14.07 | 0.78 | 0.80 | 0.26 | 0.13 | 0.90 | 0.53 | 1.08 |
| NA/DCT | K ≥ 3 | 0.27 | 0.027–2.61 | 0.25 | 0.20 | 0.51 | 0.056 | 0.82 | 0.64 | 0.41 |
ACT = adjuvant chemotherapy, K = Khorana score, NA/DCT = neoadjuvant/definitive chemotherapy, NPV = negative predictive value, PPV = positive predictive value,
When analyzing risk factors for development of VTE within 180 days of chemotherapy, univariate analysis was performed on patient demographics, treatment factors, pathologic factors and patient blood counts. Of these factors, only residual or measurable disease and hemoglobin were found to be significantly correlated with VTE (p < 0.05) as outlined in Table 3. Only residual or measurable disease was found to be an independent risk factor in a forward stepwise multivariate logistic regression model.
Table 3.
Univariate logistic regression analysis of variables potentially associated with venous thromboembolism.
| Parameter | Odds Ratio | p Value | Confidence Interval |
|---|---|---|---|
| Age (Continuous) | 1.01 | 0.60 | 0.96–1.07 |
| BMI (Continuous) | 1.01 | 0.73 | 0.96–1.06 |
| Race (Nonwhite vs White) | 1.34 | 0.71 | 0.29–6.22 |
| Current Smoker (Yes vs No) | 1.84 | 0.58 | 0.22–15.56 |
| Non-endometrioid Histology (vs Endometrioid) | 1.15 | 0.78 | 0.43–3.06 |
| FIGO Grade (High vs Low) | 0.89 | 0.81 | 0.33–2.36 |
| FIGO Stage (III-IV vs I-II) | 2.01 | 0.23 | 0.64–6.27 |
| Radiation (Yes vs No) | 0.44 | 0.10 | 0.17–1.18 |
| NA/DCT (vs ACT) | 2.45 | 0.11 | 0.82–7.30 |
| Surgery with Laparotomy (Yes vs No) | 2.48 | 0.07 | 0.94–6.55 |
| Residual or Measurable Disease (Yes vs No) | 4.78 | 0.002* | 1.77–12.90 |
| Platelet ≥ 350 × 109/L (Yes vs No) | 1.29 | 0.62 | 0.47–3.57 |
| Hemoglobin ≤ 10 g/L (Yes vs No) | 3.25 | 0.02* | 1.22–8.64 |
| WBC > 11 × 109/L (Yes vs No) | 1.47 | 0.56 | 0.40–5.35 |
FIGO = International Federation of Gynecology and Obstetrics, NA/DCT = neoadjuvant/definitive chemotherapy, ACT = adjuvant chemotherapy.
*Denotes statistically significant (p < 0.05).
4. Discussion
VTE is a significant problem for women undergoing chemotherapy for uterine cancer with a rate of almost 7% in this population during the study period. At the highest risk, patients receiving either neoadjuvant or definitive chemotherapy had a >12% rate of VTE. Although no patients died from VTE in the study population, there was significant cost to the health care system and morbidity to the patients with an average hospital admission of 3 days attributable to VTE. The financial total “all-cause” inpatient and outpatient cost is cited between $36,000 to $82,000 per VTE per year in 2015 (Fernandez et al., 2015), which does not include additional complications for the patient. Furthermore, there are worse overall oncologic outcomes for patients that suffer VTE during treatment (Rodriguez et al., 2011, Wang et al., 2019, Matsuo et al., 2013).
Although validated in other cancer patient cohorts to predict chemotherapy-associated VTE (Khorana et al., 2008), the Khorana score was found to be non-statistically significant in the prediction of chemotherapy-associated VTE in this uterine cancer-specific cohort. Neither a high-risk Khorana score threshold of ≥2 (as recommended in ASCO guidelines) or ≥3 (as defined in the original Khorana model) was able to significantly predict VTE and had poor accuracy based on AUC. This held true when the cohort was subdivided by neoadjuvant/definitive and adjuvant chemotherapy (although the study was not powered for subgroups). A large majority of patients (69%) had a Khorana score ≥2, and, as expected, this translated to relatively low positive predictive value (7%) but high negative predictive value (94%). When we raised the threshold to ≥3, the negative predictive value remained stable (94%) and the positive predictive value was marginally improved, although still quite low (9%). The weak discrimination between those with and without VTE translates to poor clinical utility of the Khorana score in patients with uterine cancer.
Although In a similar retrospective study by Austin et al, the Khorana score was found to be predictive of VTE in an endometrial cancer population, there are some key differences between that study population and the current including that it was not a chemotherapy specific group and therefore a lower risk population (68.8% early stage and only 15.6% with Khorana ≥3) and the main outcome was VTE within a year of diagnosis (Austin et al., 2019). Additionally, the main conclusion of the study is the Khorana score was not predictive of VTE in most cancers. Lastly, these findings are mirrored in the recently presented data that the Khorana score does not accurately predict VTE in ovarian cancer patients undergoing chemotherapy (Fleming et al., 2020), strengthening the argument that alternative prediction tools are needed for gynecologic cancers.
Independent of Khorana score, patients having residual or measurable disease and hemoglobin level (≤10 g/L) were the only variables found to correlate with VTE in this population. Residual or measurable disease is likely a surrogate for the most advanced stage patients and the NA/DCT subgroup enriched for these patients. Advanced disease has previously been shown to have a significant impact on the prevalence of VTE in gynecologic malignancies (Ohashi et al., 2020), and anemia was established as a risk factor for VTE in the prospective study that led to development of the Khorana score (Khorana et al., 2008). The remaining patient demographics, treatment factors, additional blood counts and pathologic data were poor predictors of VTE in this study. This is in contrast to several studies which have found a variety of risk factors significantly associated with VTE including histology, platelets, WBC, coagulation factors and indwelling lines (Wang et al., 2019, Matsuo et al., 2013, Austin et al., 2019). Although some studies evaluating VTE in cancer patients have included uterine cancer patients as part of a larger cohort, this is one of few to look specifically at ambulatory patients with uterine cancer undergoing chemotherapy.
The strength of this study is the size of the disease-specific cohort, specifically considering only a minority of patients with uterine cancer receive chemotherapy and even fewer yet in the neoadjuvant or definitive setting. The obvious limitation is its retrospective nature. In addition, while oncology care was delivered at a large cancer hospital, patients often present to their local hospitals for acute issues, and therefore, VTE incidence may be underestimated. As this study was performed at a single, midwest academic institution with a small percentage of non-white patients, the ability to broadly generalize the results of this study are limited without ancillary studies.
5. Conclusions
At this time, use of the Khorana risk assessment model to guide routine thromboprophylaxis in patients undergoing chemotherapy for uterine cancer should be used with caution. Further investigation should be done to improve risk stratification for VTE as this has a high probability to decrease the morbidity and cost associated with VTE for these patients.
CRediT authorship contribution statement
Rachael N. Piver: Methodology, Data curation. Vincent M. Wagner: Conceptualization, Methodology, Data curation, Formal analysis. Monica D. Levine: Conceptualization, Methodology. Floor J. Backes: Conceptualization. Laura J. Chambers: Conceptualization. David E. Cohn: Conceptualization. Larry J. Copeland: Conceptualization. Casey M. Cosgrove: Conceptualization. Christa I. Nagel: Conceptualization. David M. O'Malley: Conceptualization. Kristin L. Bixel: Conceptualization, Methodology, Supervision.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Backes reports grants and personal fees from Clovis, Eisai, Merck, grants from Immunogen, personal fees from Agenus, AstraZeneca, Genentech, GlaxoSmithKline, all outside the submitted work. Dr. Copeland reports personal fees from Celsion Corporation, Corcept Therapeutics, Elevar Therapeutics, grants and personal fees from GSK, personal fees from Myriad Genetics, Inc, Rubius Therapeutics, Sorrento Therapeutics, Tarveda Therapeutics, Toray Industries, Inc, grants from Abbvie, Advaxis, Agenus, Ajinomoto, Array BioPharm, AstraZeneca, Bristol Myers Squbb, Clovis Oncology, Deciphera Parma, Eisai, EMD Serono Inc, ERGOMED Clinical Research, Exelixis, Genentech/Roche, Genmab, Hoffman-LaRoche, grants and personal fees from Immunogen, grants from Incyte Corporation, Iovance Biotherapeutics, InVentive Health Clinical, Jansen R&D, Leap Therapeutics, Ludwig Institute for Pharmaceuticals, Merck, Mersana Therapeutics, Novocure, Novartis Pharmaceuticals, OncoQuest, PRA International, Regeneron Pharmaceuticals, Seattle Genetics, Serono, Sutro Biopharm, Tesaro (GSK), Arcus Biosciences, Sumitomo Dainippon Pharma Oncology, Cerulean Pharma, Karyopharm, BeiGene USA, Ovagene, Pfizer, Pharma Mar USA, Precision Therapeutics, Sanofi, Stemcentrx, TRACON Pharm, Verastem, personal fees from VBL Therapeutics, OncoNova, Inx Med, Luzsana Biotechnology, all outside the submitted work. Dr. Cosgrove reports honoraria from UpToDateConsulting and personal fees from Agenus, all outside the submitted work. Dr. O'Malley reports personal fees for consulting and/or advisory boards from AstraZeneca, Tesaro/GSK, BBI, Immunogen, Ambry, Janssen/J&J, AbbVie, Regeneron, Amgen, Novocure, Genentech/Roche, GOG Foundation, Iovance Biotherapeutics, Myriad Genetics, Eisai, Agenus, Tarveda, Merck, SeaGen, Novartis, Mersana, Clovis, Rubius, Elevar; Research funding (all funding to institution): AstraZeneca, Tesaro/GSK, Immunogen, Janssen/J&J, AbbVie, Regeneron, Amgen, Novocure, Genentech/Roche, VentiRx, Array Biopharma, EMD Serono, Ergomed, Ajinomoto, Ludwig Cancer Research, Stemcentrx, Cerulean Pharma, GOG Foundation, NCI, BMS, Serono Inc., Yale University, New Mexico Cancer Care Alliance, INC Research, inVentiv Health Clinical, Iovance Biotherapeutics, PRA International, Eisai, Agenus, Merck, GenMab, SeaGen, Mersana, and Clovis; leadership or fiduciary role for BOD – GOG Foundation, and Editorial Board for Gynecologic Oncology. None of the authors have a significant conflict of interest related to the current study.
References
- Ageno W., Squizzato A., Garcia D., Imberti D. Epidemiology and risk factors of venous thromboembolism. Semin. Thromb. Hemost. 2006;32:651–658. doi: 10.1055/s-2006-951293. [DOI] [PubMed] [Google Scholar]
- Anderson F.A., Jr, Spencer F.A. Risk factors for venous thromboembolism. Circulation. 2003;107:I9–I. doi: 10.1161/01.CIR.0000078469.07362.E6. [DOI] [PubMed] [Google Scholar]
- Austin K., George J., Robinson E.J., Scully M., Thomas M.R. Retrospective cohort study of venous thromboembolism rates in ambulatory cancer patients: association with Khorana score and other risk factors. J. Hematol. 2019;8:17–25. doi: 10.14740/jh471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber E.L., Clarke-Pearson D.L. The limited utility of currently available venous thromboembolism risk assessment tools in gynecological oncology patients. Am. J. Obstet. Gynecol. 2016;215(445):e1–e9. doi: 10.1016/j.ajog.2016.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier M., Abou-Nassar K., Mallick R., Tagalakis V., Shivakumar S., Schattner A., Kuruvilla P., Hill D., Spadafora S., Marquis K., Trinkaus M., Tomiak A., Lee A.Y.Y., Gross P.L., Lazo-Langner A., El-Maraghi R., Goss G., Le Gal G., Stewart D., Ramsay T., Rodger M., Witham D., Wells P.S. AVERT investigators, Apixaban to prevent venous thromboembolism in patients with cancer. N. Engl. J. Med. 2019;380:711–719. doi: 10.1056/NEJMoa1814468. [DOI] [PubMed] [Google Scholar]
- Cohen A., Lim C.S., Davies A.H. Venous thromboembolism in gynecological malignancy. Int. J. Gynecol. Cancer. 2017;27:1970–1978. doi: 10.1097/IGC.0000000000001111. [DOI] [PubMed] [Google Scholar]
- Di Nisio M., Porreca E., Candeloro M., De Tursi M., Russi I., Rutjes A.W. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst. Rev. 2016;12:CD008500. doi: 10.1002/14651858.CD008500.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez M.M., Hogue S., Preblick R., Kwong W.J. Review of the cost of venous thromboembolism. Clinicoecon. Outcomes Res. 2015;7:451–462. doi: 10.2147/CEOR.S85635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming, N.D., Dal Molin, G.Z., Fellman, B., Cain, K.E., Taylor, J.S., Schmeler, K., Coleman, R.L., Afshar-Kharghan, V., Westin, S.N., Sood, A.K., 2020. Lack of utility of the Khorana score for predicting VTE in advanced ovarian cancer, in: SGO 2020 Annual Meeting on Women’s Cancer, SGO. <https://sgo.confex.com/sgo/2020/meetingapp.cgi/Paper/15519> (Accessed October 18, 2021).
- Key N.S., Khorana A.A., Kuderer N.M., Bohlke K., Lee A.Y.Y., Arcelus J.I., Wong S.L., Balaban E.P., Flowers C.R., Francis C.W., Gates L.E., Kakkar A.K., Levine M.N., Liebman H.A., Tempero M.A., Lyman G.H., Falanga A. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J. Clin. Oncol. 2020;38:496–520. doi: 10.1200/JCO.19.01461. [DOI] [PubMed] [Google Scholar]
- Khorana A.A. Venous thromboembolism and prognosis in cancer. Thromb. Res. 2010;125:490–493. doi: 10.1016/j.thromres.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorana A.A., Kuderer N.M., Culakova E., Lyman G.H., Francis C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902–4907. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorana A.A., Soff G.A., Kakkar A.K., Vadhan-Raj S., Riess H., Wun T., Streiff M.B., Garcia D.A., Liebman H.A., Belani C.P., O’Reilly E.M., Patel J.N., Yimer H.A., Wildgoose P., Burton P., Vijapurkar U., Kaul S., Eikelboom J., McBane R., Bauer K.A., Kuderer N.M., Lyman G.H. CASSINI investigators, rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer. N. Engl. J. Med. 2019;380:720–728. doi: 10.1056/NEJMoa1814630. [DOI] [PubMed] [Google Scholar]
- Matsuo K., Yessaian A.A., Lin Y.G., Pham H.Q., Muderspach L.I., Liebman H.A., Morrow C.P., Roman L.D. Predictive model of venous thromboembolism in endometrial cancer. Gynecol. Oncol. 2013;128:544–551. doi: 10.1016/j.ygyno.2012.12.014. [DOI] [PubMed] [Google Scholar]
- Ohashi Y., Ikeda M., Kunitoh H., Sasako M., Okusaka T., Mukai H., Fujiwara K., Nakamura M., Oba M.S., Kimura T., Ibusuki K., Sakon M. Venous thromboembolism in cancer patients: report of baseline data from the multicentre, prospective Cancer-VTE Registry. Jpn. J. Clin. Oncol. 2020;50:1246–1253. doi: 10.1093/jjco/hyaa112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A.O., Gonik A.M., Zhou H., Leiserowitz G.S., White R.H. Venous thromboembolism in uterine cancer. Int. J. Gynecol. Cancer. 2011;21:870–876. doi: 10.1097/IGC.0b013e31821a367e. [DOI] [PubMed] [Google Scholar]
- X. Wang, J. Huang, Z. Bingbing, S. Li, L. Li, Risk factors, risk assessment, and prognosis in patients with gynecological cancer and thromboembolism, J. Int. Med. Res. (2019) 300060519893173. [DOI] [PMC free article] [PubMed]
- Zhan C., Miller M.R. Excess length of stay, charges, and mortality attributable to medical injuries during hospitalization. JAMA. 2003;290:1868–1874. doi: 10.1001/jama.290.14.1868. [DOI] [PubMed] [Google Scholar]