Abstract
Background and purpose
Watershed infarcts denote ischemic lesions involving the distal territories of two major arteries. For years, hypotheses on its pathophysiological mechanisms have been proposed. Yet, the cause is still widely debated. This study aimed to determine the mechanism of watershed strokes and compare their clinical outcomes to acute ischemic stroke from other causes and predict the factors affecting clinical outcomes in patients with watershed infarcts.
Methods
This single-center, comparative, six-years retrospective cohort study included patients with a diagnosis of Acute Ischemic Stroke. Patients were classified under watershed group or acute ischemic stroke based on their neuroimaging findings. Stroke mechanisms were determined between groups as well as the factors associated with clinical outcomes in watershed strokes.
Results
Among the 424 patients included in the study, large artery atherosclerosis was seen in greater frequency in patients with watershed infarcts regardless of the type (EWIs: n = 68, 73% vs IWIs: n = 89, 75%). No differences observed in the clinical outcomes between groups. Multiple variable analysis showed that age, female sex, high NIHSS score and presence of underlying malignancy were associated with clinical outcomes.
Conclusion
Clinical outcomes between watershed infarcts and acute ischemic strokes were similar. Hemodynamic compromise in the setting of severe stenosis is the underlying mechanism for both types of watershed strokes thus, the goal of treatment is to maintain adequate perfusion. High baseline NIHSS score, increased age, female gender and underlying malignancy were all poor predictors of clinical outcomes in patients with watershed strokes.
Keywords: Watershed infarcts, Clinical outcomes, Stroke mechanism, Predictive factors
Highlights
-
•
Large artery atherosclerosis is the most common underlying stroke etiology for both types of watershed infarcts.
-
•
No differences in the clinical outcomes between watershed strokes and ischemic stroke from other causes.
-
•
High baseline NIHSS score, increased age, female gender and underlying malignancy correlate to poor clinical outcomes in patients with watershed strokes.
1. Introduction
Watershed infarcts (WIs) denote ischemic lesions involving the distal territories of two major arteries. They represent approximately 10% of all ischemic strokes and most of the time related to carotid steno-occlusive disease [1] or hemodynamic compromise in the presence of severe intracranial stenosis [2]. For years, many classifications and hypotheses on its pathophysiological mechanisms have been proposed. Yet, the cause is still widely debated. To date, hemodynamic impairment from severe luminal stenosis is implicated as a determinant of internal watershed infarcts [3] with recent studies establishing stronger correlation between hemodynamic impairment and carotid steno-occlusion in its pathogenesis [4]. External watershed infarcts, conversely, are regarded as induced by micro embolism due to the lower level of cerebral vasoreactivity impairment and oxygen metabolism [5].
Current reports on the etiology and mechanism of this infarct are limited on small number of included patients and relatively short duration of data collection which could hinder precise estimate on the causal mechanism [[6], [7], [8]]. Although a registry of ischemic stroke patients provided a glimpse on the association of watershed infarcts on good outcome and low mortality [9], there are no comparative cohort study that compares the clinical outcome of watershed infarcts and ischemic stroke from other causes. Furthermore, most of these studies were conducted in European and American patients whose profile may be remarkably different from Asian.
Therefore, we aimed to determine the mechanism of watershed strokes to guide clinicians in the appropriate clinical management of these patients. In addition, we also aimed to compare clinical outcomes of watershed infarcts to acute ischemic stroke from other causes in terms of Modified Rankin Scale (MRS) score at 90 days, need for Intensive Care Unit (ICU) admission, readmission due to cerebrovascular cause and mortality. Lastly, to predict the different factors affecting clinical outcomes in patients with watershed infarcts.
2. Methodology
2.1. Study design, patient selection and cohort description
We performed a single-center, comparative, six-years retrospective cohort study involving all patients with a diagnosis of Acute Ischemic Stroke admitted on our institution. Inclusion criteria were: adults ≥19 years old, with retrieved neuroimaging findings in the Picture Archiving and Communication System (PACS) suggestive of watershed infarcts and patients with disposition (i.e. discharged, died) at the end of the study period. Individuals who had hemorrhagic conversion, venous infarction and incomplete or missing data were excluded from the study.
Identified patients had their neuroimaging findings retrieved through the institution's picture archiving system and were grouped based on the neuroimaging findings of the authors. Those who presented with watershed findings on imaging were classified under the watershed group while patients with acute ischemic stroke, age (+/− 5 years) and sex-matched to the watershed group, were classified under acute ischemic stroke.
2.2. Imaging criterion for watershed infarcts
Watershed infarcts were classified as external or internal based on their location. External watershed infarcts (EWIs) were defined radiographically by an ovoid or wedge-shaped infarct between the cortical territories of the anterior cerebral artery (ACA), middle cerebral artery (MCA) and posterior cerebral artery (PCA) while, internal watershed infarcts (IWIs), were defined as a series of three or more lesions arranged in a linear fashion parallel to the lateral ventricle or centrum semi-ovale [3].
2.3. Outcome variables
We obtained the following relevant patient outcomes: NIHSS score (patients with score of ≥15 were classified as severe using the classification of Brott et al [10]), mortality, functional outcome (patients with MRS score between 0 and 2 at 90 days were considered with good outcome whereas, those with score of 3–5 had poor outcome), ICU admission (stroke patients requiring care of higher intensity that cannot be provided in the Acute Stroke Unit, patients with neurological deterioration or cardio-respiratory compromise and those with communicable diseases) and readmission at 1 year due to a cerebrovascular cause (patients readmitted at 1 year because of another ischemic stroke).
2.4. Sample size
The calculation of the sample size, computed at 160 patients, was based on the proportion of watershed stroke patients using stroke recurrence as an outcome measure [11].
2.5. Statistical analysis
Inverse probability of treatment weighting (IPTW) method [12] using the propensity score was applied to balance the baseline demographics and clinical characteristics. Propensity scores were estimated using logistic regression that included age, sex and baseline comorbidities.
Demographic characteristics were summarized using frequencies and percentages for categorical variables and mean (SD) or median (IQR) for continuous variables. Independent t-test, Mann Whitney U test and Kruskal Wallis test were used to compare continuous data. Categorical data were compared using Chi square test or Fisher's exact test.
Multiple logistic regression analysis was done to determine the factors associated with the following outcomes: MRS score at 90 days, mortality, need for ICU admission, and readmission due to cerebrovascular cause. The use of simple logistic and linear regression analyses was done using the p < 0.20 criterion to screen for potential predictors [13] while, model building by a backward elimination technique was performed to account for all outcome predictors. All statistical analyses were conducted using the Stata MP version 16 software.
2.6. Ethical considerations
The study was evaluated and approved by the Institutional Ethics and Research Review Committee of St. Luke's Medical Center – Quezon City (SL-22120).
3. Results
3.1. Patients' characteristics
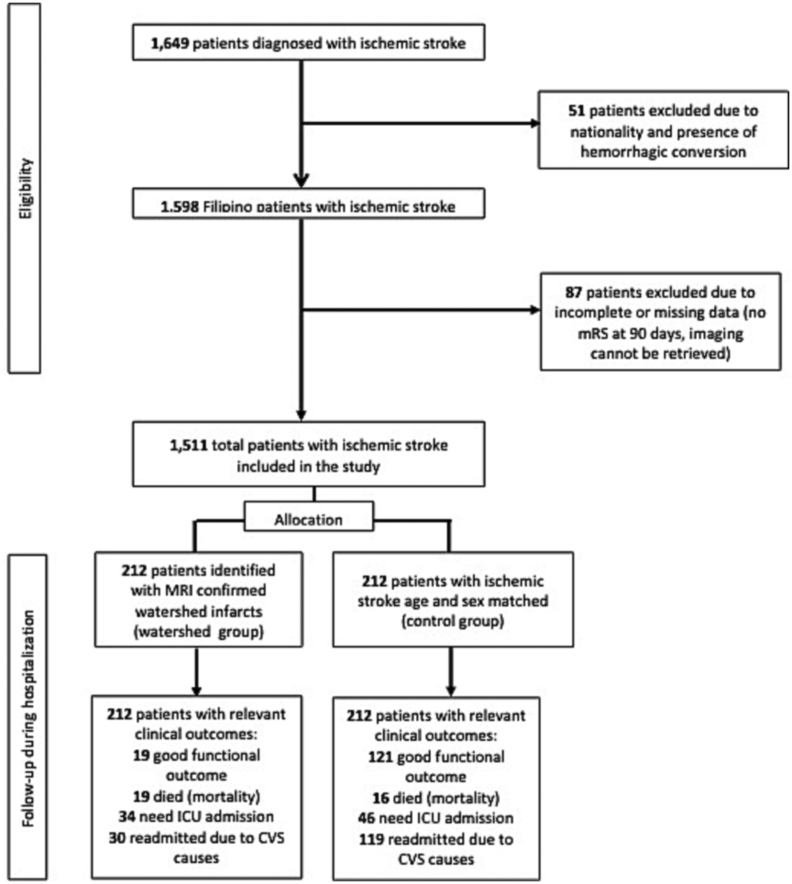
We identified a total of 1649 patients diagnosed with acute ischemic stroke from our stroke data bank. Before the weighting application, patients who had watershed strokes were older (65 [IQR: 56–76] vs 54 [IQR: 49–82]) than those with acute ischemic stroke from other causes. After applying the IPTW method, differences in baseline characteristics were balanced (all p < 0.05; absolute standardized difference < 0.1). A total of 424 were included in the qualitative and quantitative analyses. Two hundred twelve patients with MRI-confirmed watershed infarcts were classified under the watershed group, and another 212 ischemic stroke patients, age (±5 years) and sex-matched, served as the control (see Fig. 1).
Fig. 1.
Study flow of patients.
3.2. Demographic and clinical characteristics of included patients
The median age of the full cohort was 65 years old (24–103) mostly, males. Majority had hypertension (81%) while half had diabetes (50%). Regarding stroke severity, a lower proportion of internal watershed infarct patients had severe NIHSS on admission compared to the external watershed group and control (n = 7, 6% vs n = 15 vs 16% and n = 35, 17%). For etiologic investigations, large artery atherosclerosis was seen in greater frequency in patients with watershed infarcts regardless of the type (EWIs: n = 68, 73% vs IWIs: n = 89, 75%).
In the analysis of clinical outcomes between the three groups (EWIs, IWIs and control), no differences were observed except for the higher proportion of readmitted patients seen in the control (n = 44, 21% vs n = 12,13% vs n = 18,15%) (see Table 1, Table 2).
Table 1.
Baseline characteristics and clinical outcomes between the watershed and control group.
| Characteristics | Watershed infarct external (n = 93) n(%) |
Watershed infarct internal (n = 119) n(%) |
Control (n = 212) n(%) |
P Value |
|---|---|---|---|---|
| Age (in years), median | 66 [IQR: 56–75] | 64 [IQR: 56–76] | 64 [IQR: 55–76] | 0.9918a |
| Sex | ||||
| Female | 37 (40) | 54 (45) | 91 (43) | 0.717b |
| Male | 56 (60) | 65 (55) | 121 (57) | |
| Medical history | ||||
| Hypertension | 79 (85) | 97 (82) | 169 (80) | 0.558b |
| Diabetes | 37 (40) | 64 (54) | 109 (51) | 0.096b |
| Atrial fibrillation | 23 (25) | 22 (18) | 36 (17) | 0.279b |
| Hyperlipidemia | 29 (31) | 40 (34) | 68 (32) | 0.927b |
| CAD | 9 (10) | 6 (5) | 7 (3) | 0.069b |
| Malignancy | 9 (10) | 8 (7) | 14 (7) | 0.611b |
| Previous stroke | 13 (14) | 12 (10) | 34 (16) | 0.324b |
| Infection | 15 (16) | 17 (14) | 36 (17) | 0.814b |
| Smoking | 21 (23) | 34 (29) | 48 (23) | 0.439b |
| Alcohol intake | 9 (10) | 15 (13) | 37 (17) | 0.165b |
| TOAST classification | ||||
| Large artery atherosclerosis | 68 (73) | 89 (75) | 127 (59) | <0.0001*c |
| Cardio embolism | 21 (23) | 22 (18) | 378 (18) | |
| Small vessel disease | 3 (3) | 7 (6) | 45 (21) | |
| ESUS | 1 (1) | 1 (1) | 2 (1) | |
| NIHSS on admission, median | 5 [IQR: 2–11] | 4 [IQR: 2–7] | 4 [IQR: 2–8] | 0.2571a |
| Mild | 59 (63) | 94 (79) | 156 (74) | 0.004*b |
| Moderate | 19 (20) | 18 (15) | 21 (10) | |
| Severe | 15 (16) | 7 (6) | 35 (17) | |
Table 2.
Comparison of the clinical outcomes between watershed infarcts (external, internal) and control.
| Outcome | Watershed infarct external (n = 93) n(%) |
Watershed infarct internal (n = 119) n(%) |
Control (n = 212) n(%) |
P value |
|---|---|---|---|---|
| Functional Outcome (mRS at 90 days) | ||||
| Good outcome | 50 (83) | 64 (74) | 121 (79) | 0.435b |
| Poor outcome | 10 (17) | 22 (26) | 33 (21) | |
| Mortality | ||||
| Yes | 12 (13) | 7 (6) | 16 (8) | 0.159b |
| Need for ICU admission | ||||
| Yes | 18 (19) | 16 (13) | 46 (22) | 0.182b |
| Readmission for CVS cause | ||||
| Yes | 12 (13) | 18 (15) | 44 (21) | <0.0001*b |
eKruskal Wallis test was used; bchi square test was used, cfisher's exact test was used.
3.3. Factors related to clinical outcomes
Univariable analysis showed that age, female sex, high NIHSS score and presence of underlying malignancy were associated with clinical outcomes (see Table 3). Multiple variable analysis showed that age [OR 1.04 (1.00,1.08)], female sex [OR 3.40 (1.23, 9.38)] and high baseline NIHSS score (≥ 15) [OR 38.27 (10.98, 133.37)] were associated with poor functional outcome in 90 days. Likewise, a high baseline NIHSS score was associated with higher probability of ICU admission [OR 165.37 (38.08, 719.11)] and readmission due to cerebrovascular cause [OR 5.37 (1.88, 15.37)]. Malignancy was associated with higher odds of readmission in one year [OR 5.03 (1.53, 16.50)].
Table 3.
Factors affecting clinical outcomes in watershed infarcts.
| Adjusted or (95% CI) | P value | |
|---|---|---|
| A. Functional Outcome (mRS at 90 days) | ||
| Age (in years) | 1.04 (1.00–1.08) | 0.029* |
| Sex | ||
| Female | 3.40 (1.23–9.38) | 0.018* |
| NIHSS on admission | ||
| Severe | 249.03 (9.05–6855.10) | 0.001* |
| B. Need for ICU admission | ||
| NIHSS on admission | ||
| Severe | 165.37 (38.083–719.11) | <0.0001* |
| C. Mortality | ||
| NIHSS on admission | ||
| Severe | 38.27 (10.98–133.37) | <0.0001* |
| D. Readmission due to CVS cause | ||
| Medical history | ||
| Malignancy | 5.03 (1.53–16.50) | 0.008* |
| NIHSS on admission | ||
| Severe | 5.37 (1.88–15.37) | 0.002* |
4. Discussion
In this study, we compared the two types of watershed strokes – external and internal to ischemic stroke of other known etiology; and determined the factors affecting their clinical outcomes. We found that stroke mechanisms and clinical outcomes did not differ significantly between the two types of watershed strokes. In these group of patients, a high baseline NIHSS score, increased age, female gender and underlying malignancy correlate to poor clinical outcomes.
Interestingly, no significant difference in the clinical outcomes exist between the three groups. This could be attributed to the fact that unlike age and gender differences, wherein increased age and female sex were associated with multiple comorbidities and risk factors [14], stroke mechanism was not an independent predictor of clinical outcomes.
Among patients with watershed strokes, we separated the external from the internal border zone infarcts. Their stroke mechanisms and clinical outcomes were described, which contradict the results of earlier studies that showed differences between the two [3,9]. In our study, large artery atherosclerosis was prevalent in both types of infarcts suggesting that both severity of hemodynamic impairment and degree of stenosis played an important role on its pathophysiology [6]. Previous studies showed differences in the clinical outcomes between two types of watershed strokes. Internal border zone infarcts were associated with poorer prognosis and increased mortality [15] compared to the external border zone type. This dissimilarity in our study could be due to the differences of the included population, as hemodynamic involvement of the anterior and posterior circulation was excluded in the previous studies. Our study showed that baseline NIHSS score was an independent predictor of mortality, functional outcome and readmission in patients with watershed strokes. It supported earlier studies on the link between high baseline NIHSS score and increased probability of death and severe disability among ischemic stroke patients [16,17]. Stroke risk was highest at or soon after cancer diagnosis [18]. These cancer-mediated hypercoagulability peaks may partly explain why the likelihood of readmission is higher among watershed stroke patients with underlying malignancy.
There were several limitations in this study. First, since this was a single-center, retrospective study, there may have been selection bias. Second, the number of watershed patients was relatively small, although it was comparable and even higher to previously published studies [[19], [20], [21]] and lastly, patients with incomplete data were excluded from the study, which may introduce a selection bias.
5. Conclusion
In this study, we found that clinical outcomes between watershed infarcts and acute ischemic strokes were similar. Hemodynamic compromise in the setting of severe stenosis is the underlying mechanism for both types of watershed strokes thus, the goal of treatment is to maintain adequate perfusion. High baseline NIHSS score, increased age, female gender and underlying malignancy were all poor predictors of clinical outcomes in patients with watershed strokes.
Sources of funding
The authors received no financial support for the research, authorship, and/or publication of this article.
CRediT authorship contribution statement
Marian Irene C. Escasura: Conceptualization, Validation, Investigation, Formal analysis, Writing – original draft, Writing – review & editing, Visualization. Jose C. Navarro: Conceptualization, Methodology, Formal analysis, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that there is no conflict of interest.
Acknowledgement
The authors thank Dr. Jose Leonard R. Pascual V MD, FPNA and members of the Stroke Data Bank.
Data availability
Data not published in this article will be made available by request from the corresponding author.
References
- 1.Gülşah C.I., Corbacioglu S., Cevik Y. Watershed infarct due to massive pulmonary embolism: a rare diagnosis at the emergency department. J. Acad. Emerg. Med. 2016;7:34–36. [Google Scholar]
- 2.Howard R., Trend P., Russell R.W. Clinical features of ischemia in cerebral arterial border zones after periods of reduced cerebral blood flow. Arch. Neurol. 1987;44(9):934–940. doi: 10.1001/archneur.1987.00520210036016. [DOI] [PubMed] [Google Scholar]
- 3.Mangla R., Kolar B., Almast J., et al. Border zone infarcts: pathophysiologic and imaging characteristics. Radio Graph. 2011;31:1201–1214. doi: 10.1148/rg.315105014. [DOI] [PubMed] [Google Scholar]
- 4.Li Y., Li M., Zhang X., et al. Clinical features and the degree of cerebrovascular stenosis in different types and subtypes of cerebral watershed infarction. BMC Neurol. 2017;17:166. doi: 10.1186/s12883-017-0947-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weill C., Suissa L., Darcourt J., Mahagne M.H. The pathophysiology of watershed infarction: a three-dimensional time-of-flight magnetic resonance angiography study. J. Stroke Cerebrovasc. Dis. 2017 Sep;26(9):1966–1973. doi: 10.1016/j.jstrokecerebrovasdis.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 6.Moustafa R.R., Izquierdo-Garcia D., Jones P.S., et al. Watershed infarcts in transient ischemic attack/minor stroke with > or = 50% carotid stenosis: hemodynamic or embolic? Stroke. 2010;41(7):1410–1416. doi: 10.1161/STROKEAHA.110.580415. [DOI] [PubMed] [Google Scholar]
- 7.Momjian M., Isabelle B., Jean C. The pathophysiology of watershed infarction in internal carotid artery disease: review of cerebral perfusion studies. Stroke. 2005;36(3):567–577. doi: 10.1161/01.STR.0000155727.82242.e1. [DOI] [PubMed] [Google Scholar]
- 8.Yong S.W., Bang O.Y., Lee P.H., et al. Internal and cortical border-zone infarction: clinical and diffusion-weighted imaging features. Stroke. 2006;37:841–846. doi: 10.1161/01.STR.0000202590.75972.39. [DOI] [PubMed] [Google Scholar]
- 9.Moulin T., Tatu L., Vuillier F., et al. Role of a stroke data Bank in Evaluating Cerebral Infarction Subtypes: patterns and outcome of 1,776 consecutive patients from the Besan stroke registry. Cerebrovasc. Dis. 2000;10(4):261–271. doi: 10.1159/000016068. [DOI] [PubMed] [Google Scholar]
- 10.Brott T., Adams H.P., Jr., Olinger C.P., et al. Measurements of acute cerebral infraction: a clinical examination scale. Stroke. 1989 July;20(7):864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 11.Pu Y., Liu X., Wang Y., Meng X., et al. Higher early recurrence risk and potential benefit of dual antiplatelet therapy for minor stroke with watershed infarction: subgroup analysis of CHANCE. Eur. J. Neurol. 2020 May;27(5):800–808. doi: 10.1111/ene.14156. [DOI] [PubMed] [Google Scholar]
- 12.Stürmer T., Wyss R., Glynn R.J., et al. Propensity scores for confounder adjustment when assessing the effects of medical interventions using nonexperimental study designs. J. Intern. Med. 2014;275:570–580. doi: 10.1111/joim.12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenland S., Pearce N. Statistical foundations for model-based adjustments. Annu. Rev. Public Health. 2015 Mar;18(36):89–108. doi: 10.1146/annurev-publhealth-031914-122559. [DOI] [PubMed] [Google Scholar]
- 14.Fonarow G.C., Reeves M.J., Zhao X., et al. Age-related differences in characteristics, performance measures, treatment trends, and outcomes in patients with ischemic stroke. Circulation. 2010 Feb 23;121(7):879–891. doi: 10.1161/CIRCULATIONAHA.109.892497. [DOI] [PubMed] [Google Scholar]
- 15.Grange S., Grange R., Garnier P., et al. Boundary and vulnerability estimation of the internal border zone using ischemic stroke lesion mapping. Nat. Sci. Rep. 2020;10:1662. doi: 10.1038/s41598-020-58480-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams H.P., Jr., Davis P.H., Leira E.C., et al. Baseline NIH stroke scale score strongly predicts outcome after stroke: a report of the trial of org 10172 in acute stroke treatment (TOAST) Neurology. 1999 Jul doi: 10.1212/wnl.53.1.126. 13;53(1):126-31. [DOI] [PubMed] [Google Scholar]
- 17.Shrestha S., Poudel R., Khatiwada D., et al. Stroke subtype, age, and baseline NIHSS score predict ischemic stroke outcomes at 3 months: a preliminary study from Central Nepal. J. Multidiscip. Healthc. 2015;8:443–448. doi: 10.2147/JMDH.S90554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navi B.B., Iadecola C. Ischemic stroke in cancer patients: a review of an underappreciated pathology. Ann. Neurol. 2018 May;83(5):873–883. doi: 10.1002/ana.25227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nahas N.E., Shokri H., Abdulghani O., et al. Clinical characteristics of Borderzone infarction in Egyptian population. J. Stroke Cerebrovasc. Dis. 2019 May;28(5):1178–1184. doi: 10.1016/j.jstrokecerebrovasdis.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Isabel C., Lecler A., Turc G., et al. Relationship between watershed infarcts and recent intra plaque hemorrhage in carotid atherosclerotic plaque. PLoS One. 2014;9(10) doi: 10.1371/journal.pone.0108712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The E.Z., Ng M.Y., Ng G., et al. Predictors and outcomes of patients with watershed infarction: a longitudinal cohort analysis. J. Neurol. Sci. 2019;405 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data not published in this article will be made available by request from the corresponding author.