Abstract
Immune diversification helps protect the host against a myriad of pathogens. CD8+ T cells are essential adaptive immune cells that inhibit the spread of pathogens by inducing apoptosis in infected host cells, ultimately ensuring complete elimination of infectious pathogens and suppressing disease development. Accordingly, numerous studies have been conducted to elucidate the mechanisms underlying CD8+ T cell activation, proliferation, and differentiation into effector and memory cells, and to identify various intrinsic and extrinsic factors regulating these processes. The current knowledge accumulated through these studies has led to a huge breakthrough in understanding the existence of heterogeneity in CD8+ T cell populations during immune response and the principles underlying this heterogeneity. As the heterogeneity in effector/memory phases has been extensively reviewed elsewhere, in the current review, we focus on CD8+ T cells in a “naïve” state, introducing recent studies dealing with the heterogeneity of naive CD8+ T cells and discussing the factors that contribute to such heterogeneity. We also discuss how this heterogeneity contributes to establishing the immense complexity of antigen-specific CD8+ T cell response.
Keywords: Naive T-cell, Heterogeneity, Homeostasis, Self-antigen, Cytokine
INTRODUCTION
The potent cytotoxic effector function of CD8+ T cells is crucial for controlling pathogenic infection. This function requires Ag-specific activation, proliferation, and effector differentiation of naive CD8+ T cells, which are regulated by the coordinated expression of various transcription factors (TFs) and epigenetic regulators, ultimately leading to phenotypic and functional heterogeneity within Ag-specific CD8+ T cell populations in response to pathogenic infection (1,2,3,4,5).
Heterogeneity observed in effector populations is an important defensive feature that protects the host by effectively coping with potential threatening situations caused by numerous pathogenic infections (1,6). In the early stages after pathogenic infections, most conventional effector cells and a minority of memory precursor effector cells (MPECs) coexist in the effector populations (7). While the majority of effector cells function as short-lived effector cells (SLECs) that disappear by apoptosis after pathogen clearance or, to a lesser extent, can differentiate into long-lived effector cells, MPEC detected in the effector phase differentiate into either central memory T (TCM) cells, which are distributed in secondary lymphoid organs (SLOs), or effector memory T (TEM) cells, which are circulating in peripheral tissues (8,9,10,11). In addition, resident memory T (TRM) cells, which reside persistently in peripheral tissues, and stem cell-like memory T cells, which are in a differentiated state much closer to naive cells, have also been added, complicating the heterogeneity of Ag-specific CD8+ T cell immune response (12,13).
Many studies have been conducted to elucidate the mechanisms that induce heterogeneity within the effector and memory cell populations (14,15,16). Our knowledge and understanding continue to expand as several important factors are identified, and their interactions and regulatory pathways are elucidated. In the early stages of an Ag-specific immune response, many different stimuli, such as TCR, costimulatory and coinhibitory molecules, and cytokines, as well as various contact signals derived from different anatomic locations within SLOs and/or different cell–cell interactions, are all potentially involved in forming heterogeneity in responding CD8+ T cell populations. As these factors inevitably lead to temporally and spatially random changes in a number of different combinations, many studies have focused on the priming and expansion phases of Ag-specific CD8+ T cell response to understand the underlying mechanisms of such heterogeneity, which has been intensively reviewed elsewhere (16,17,18,19).
Hence, in this review, we introduce recent studies dealing with interesting issues defining phenotypic and functional heterogeneity observed in “naive” CD8+ T cell populations under steady-state conditions. Based on the results from these studies, we will then discuss which changes can occur in naive CD8+ T cells, which factors cause these changes, and how these factors play a role and interact with each other. We also discuss how such heterogeneity observed in naive CD8+ T cells contributes to shaping diverse effector and memory T cell subpopulations after antigenic stimulation. Finally, we discuss the potential physiological basis of T cell-mediated immune diseases.
PHENOTYPIC HETEROGENEITY
Naive CD8+ T cells are generated in the thymus and continuously circulate SLOs through the blood and lymphatic systems. During peripheral maintenance, naive CD8+ T cells generally maintain a functional resting state for a long period without overt activation or cell division (20,21). Therefore, naive CD8+ T cells have long been recognized as a homogeneous population that is phenotypically and functionally relatively uniform in the G0 interphase, unless they are activated by exposure to a specific Ag (22,23). In fact, in most cases, the phenotype of naive CD8+ T cells appears homogeneous in terms of the expression of L-selectin (CD62L) and chemokine receptor CCR7, which are required for lymph node homing through high endothelial venules, and the cell-surface glycoprotein CD44, which is involved in cell–cell interactions, adhesion, and migration, and is closely related to the state of cellular activation (8,24). Based on these markers, naive CD8+ T cells in mice exhibit low expression (lo) level of CD44 (CD44lo) and high expression (hi) levels of CD62L and CCR7 (CD62Lhi CCR7hi), which are distinct from the effector and memory populations of the CD44hi CD62Lhi or CD44hi CD62Llo phenotypes (22,23). Similarly, human naive CD8+ T cells can also be distinguished using these markers, with the exception of CD45RA (and/or CD45RO), which exhibit the CD45RA+ CCR7+ (and also CD45RO− CD62L+) phenotype, from the effector and memory cells showing the CD45RA− CCR7+/− CD45RO+ CD62L+/− phenotype (8,25).
The above-mentioned phenotypic homogeneity of the naive CD8+ T cell population can be taken for granted considering the characteristics of these cells, which are kept in a constant resting state without exposure to specific stimulatory factors in a steady-state condition. However, several recent studies have reported a significant degree of heterogeneity in various surface proteins, and their expression levels exist in normal steady-state resting naive CD8+ T-cell populations in mice and humans (Fig. 1) (26,27,28,29,30,31,32,33,34). Notably, a subpopulation of naive CD8+ T cells exhibiting relatively high Ly6C and CXCR3 (CD183) expression levels was observed within the clearly defined CD44lo CD62Lhi naive pools of CD8+ T cells in the periphery (29,34,35). As these surface proteins are induced and upregulated in activated CD8+ T cells in a manner dependent on antigenic stimulations and thus are generally highly expressed in effector and memory cell populations, questions arise about this phenomenon: What are the stimuli that can drive the expression of such proteins in naive CD8+ T cells, and why are only certain subsets, but not all, of naive CD8+ T cells affected by the stimuli? What is the physiological significance of the existence of heterogeneous naive CD8+ T cells?
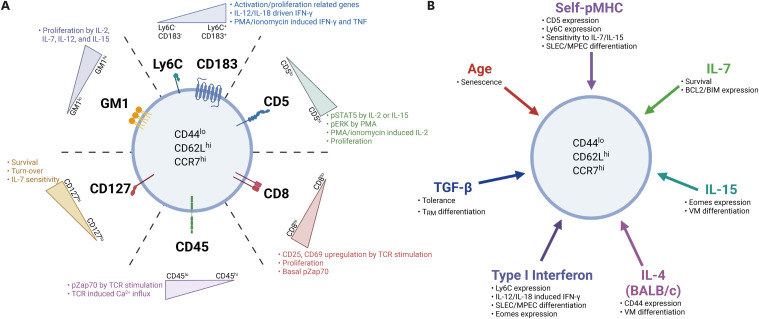
Figure 1. Heterogeneity of naive CD8+ T cells and factors shaping the heterogeneity. (A) Phenotypic and functional heterogeneity can be observed in naive CD8+ T cell subsets exhibiting different levels of various surface molecules. These proteins are all functionally relevant for variety of T cell responses, including T cell survival and basal turn-over in a steady state (CD127 and GM1), as well as T cell activation/proliferation (CD5, CD8, CD45, and GM1) and migration into non-lymphoid tissues (Ly6C and CD183) during antigenic stimulation. (B) Several factors contribute to shaping the heterogeneity of naive CD8+ T cells in a steady state, which includes tonic TCR signaling via self-ligand interactions (self-pMHC), homeostatic cytokines (IL-4, IL-7, IL-15, type I IFN, and TGF-β), as well as a certain age-associated cue.
In addition to the surface proteins mentioned above, there were significant differences in the transcriptional profiles within the naive CD8+ T cell population (29,30,32,34,35). Of these, genes encoding TFs, such as T-BET and EOMES, are of particular interest because these TFs are relatively highly expressed in activated T cells or differentiated effector and memory T cells that have experienced antigenic stimulation. However, the fact that such differences were observed not only in mice housed under specific pathogen-free conditions (29,30,32,34,35) but also in mice housed under germ-free and even Ag-free conditions (34), where antigenic stimulations derived from pathogenic infections are completely absent, completely rules out strong Ag-dependent TCR engagements, but rather emphasizes the role of homeostatic factors available under normal steady-state conditions. These potential homeostatic factors are discussed in detail below.
Although phenotypic heterogeneity within the naive CD8+ T cell population was confirmed at both the protein (e.g., Ly6C and CXCR3) and transcript (e.g., Tbx21 and Eomes) levels, whether these cells are truly naive may be questionable. In this regard, a recent study showed that CD44hi memory-phenotype (MP) CD8+ T cells, which generally express much higher levels of Ly6C, CXCR3, T-BET, and EOMES, undergo several rounds of active proliferation when treated with IL-2, IL-7, or IL-15 in culture. However, this response was not observed in CD44lo naive CD8+ T cells, although some naive cells included a subset that exhibited relatively high levels of Ly6C and CXCR3 (as well as T-BET and EOMES) (34). Moreover, while CD44hi MP CD8+ T cells were able to produce very high levels of IFN-γ upon short-term stimulation with PMA and ionomycin in vitro, such strong IFN-γ production was not observed in CD44lo naive CD8+ T cells (34). Clearly, naive CD8+ T cells consist of phenotypically heterogeneous subpopulations that maintain “naivety” at least in their functionality (based on their weaker responsiveness relative to CD44hi MP cells in response to the above stimuli) and therefore are defined as a truly naive. Notably, the phenotypic heterogeneity observed in murine naive CD8+ T cells was confirmed in both polyclonal (from C57BL/6 mice) and monoclonal TCR populations (from 2C, P14, or OT-I TCR transgenic mice) (26,29,31,32,34). All these findings emphasize that the phenotypic heterogeneity of naive CD8+ T cells is a common phenomenon that may occur independently of the diversity and specificity of their TCR repertoire.
Unlike the binary expression patterns of surface proteins such as Ly6C and CD183 (29,34,35), most proteins on the surface of naive CD8+ T cells displayed a continuum unimodal expression patterns, with relatively small differences in expression levels across the entire naive population. Despite such subtle differences, however, these surface proteins may include ligand or receptor molecules that are either stimulatory or inhibitory for different cellular functions in response to various stimuli and/or homeostatic cues, and thus may play a role in shaping different functionalities for individual naive CD8+ T cell subpopulations that are phenotypically heterogeneous. These issues, addressed in several recent studies, are discussed in the next section.
FUNCTIONAL HETEROGENEITY
Under steady-state conditions, naive CD8+ T cells maintain a stable quiescent state for a long time without showing any signs of overt activation or division (20,21). The maintenance of such homeostasis is not obtained as a default or in a predetermined manner for every individual cell within the naive CD8+ T cell population, but it is instead tightly controlled by several sophisticated regulatory mechanisms (22,23,36). Subtle differences in the expression levels of various surface proteins affect homeostasis and function of the naive CD8+ T cell population (Fig. 1A). Of these proteins, interesting results have been observed for naive CD8+ T cell subsets that exhibit differential levels of CD5 expression (26,28,29,30,31,34,35). CD5 is highly expressed in naive CD8+ T cells and its function is known as a negative regulator of TCR signaling (37). In addition, the amount of CD5 expression on naive CD8+ T cells is developmentally determined during thymic positive selection and is proportional to the overall strength of the TCR affinity for self-peptides bound to major histocompatibility complex molecules (self-peptide MHC [pMHC]) (38,39). Moreover, due to the “promiscuous” nature of the positive selection of self-peptides, expression levels of CD5 on naive CD8+ T cells may also exhibit a certain degree of variability in both polyclonal and monoclonal TCR populations (40).
Interestingly, the functional differences according to the level of CD5 expression were significant, even with relatively small differences in their expression levels. For example, a study showed that upon in vitro exposure to IL-2 or IL-15, naive CD8+ T cells with relatively CD5hi induced much higher signaling response (based on phosphorylation of STAT5) than cells with CD5lo (26). Consistent with this phenomenon, CD5hi cells showed significantly higher proliferative response than CD5lo cells when cultured with IL-2, IL-7, or IL-15 (26,31,34,41). Similar functional differences were also explained using different stimuli, such as PMA alone or PMA and ionomycin. The results clearly showed that CD5hi cells had significantly higher PMA-induced ERK activation (based on ERK phosphorylation) and PMA/ionomycin-induced IL-2 production than CD5lo cells (28). Similarly, another study showed that when comparing several monoclonal TCR transgenic naive T cells with different levels of CD5, constitutive basal CD3ζ chain phosphorylation, which indicates TCR signaling sensitivity, gradually increased in proportion to the level of CD5 (27,28,31). Together, these results emphasize that there are significant differences in intracellular signaling and response outcomes to various stimuli depending on the level of CD5 expression, providing a conceptual basis for increasing the functional heterogeneity of the naive CD8+ T cell population.
In addition to CD5, functional differences depending on the different levels of CD8 coreceptors in naive CD8+ T cells have also been reported (32). In this study, when treated with TCR stimulation (with anti-CD3 and -CD28) or PMA and ionomycin, CD8hi cells showed significantly enhanced activation profiles (based on upregulation of CD25 and CD69) as well as a proliferative response compared with CD8lo cells (32). Consistent with the response, higher levels of basal phosphorylation of zeta chain-associated protein kinase 70 (ZAP70) and ERK were also observed in CD8hi cells than CD8lo cells (32). Likewise, CD45, a surface protein with tyrosine phosphatase activity, is another example of a relationship between subtle differences in the expression levels of surface proteins and their divergent functionality (31,42). CD45 is highly expressed in naive CD8+ T cells and induces either the activation or inactivation of lymphocyte-specific protein tyrosine kinase by dephosphorylating 2 tyrosine residues, Y505 and Y394, respectively (43). Although CD45 has 2 opposing functions, the expression level of CD45 observed in normal steady-state T cell populations appears to act as a negative regulator of TCR signaling (42,44). In fact, a difference in the responsiveness to TCR (anti-CD3 and -CD28) stimulation was clearly observed in subsets of naive CD8+ T cells (derived from normal C57BL/6 mice) that have subtle differences in their CD45 levels; thus, significantly lower TCR-induced activation signaling (i.e., phosphorylation of ZAP70, phospholipase C gamma, ERK, and protein kinase B) and lower TCR-induced Ca2+ influx response were observed in CD45hi cells as compared with CD45lo cells (31).
Notably, in this study, CD45 expression level in naive CD8+ T cells was proportional to CD5 expression level (31). Therefore, similar to CD45hi cells, CD5hi cells showed significantly lower TCR-induced response than CD5lo cells exhibiting the CD45lo phenotype (31). However, these differences were no longer observed when treated with a pharmacochemical inhibitor that interfered with CD45 phosphatase activity, emphasizing that CD45, but not CD5, expression was the direct cause of the observed functional differences (31). Nevertheless, it seems debatable to completely rule out the potential direct role of different CD5 levels as a negative regulator of TCR signaling, based on observations showing relatively higher responsiveness to TCR stimulation in naive T cells from Cd5−/− mice than in those from wild-type control mice (28,37,45,46). However, unlike the results from these mutant mice, when the CD5 expression level was experimentally reduced in naive T cells by depriving of self-pMHC ligands, TCR-induced response of these cells with reduced CD5 levels was muted rather than enhanced (47,48,49,50). Therefore, the negative regulatory function of CD5 is perhaps limited to tuning the strength of the self-TCR signal for developing thymocytes rather than for steady-state resting naive CD8+ T cells in the periphery. Further studies using a mutant mouse strain that is genetically modified for conditional CD5 deletion in peripheral naive CD8+ T cells are necessary to clarify this issue.
In addition to the surface proteins that affect TCR signaling, interesting results have been obtained for other surface proteins or components that may regulate cellular functions related to naive CD8+ T cell survival and homeostasis. One study showed that subtle differences in IL-7Rα (CD127) expression levels within naive CD8+ T cell populations could result in differences in their survival and turnover, associated with differential sensitivity in response to IL-7 (41). In another study, differences in the level of GM1, a sphingolipid found mostly in a specialized plasma membrane microdomain called lipid rafts, were observed in the naive CD8+ T cell population (26). When treated with different combinations of cytokines, such as IL-2, IL-7, IL-12, and IL-15 in vitro, GM1hi cells showed a relatively high proliferative response compared with GM1lo cells (26). As such, receptor proteins for homeostatic cytokines, such as IL-2Rβ (CD122 and also shared with IL-15), as well as proteins that regulate functions related to lymph node homing (e.g., CD62L and CCR7), cell–cell adhesion (e.g., LFA-1, CD44, and Ly6C), metabolite sensing (e.g., CD71 and CD73), and migration into non-lymphoid tissues (e.g., CD103 and CXCR3) are all expected to differ in their expression levels among naive CD8+ T cells. The fact that even subtle differences in the amount of these proteins can lead to a wide range of functional differences warrants further research on many other surface molecules that have not yet been studied and will lead to a broader and much better understanding of how naive CD8+ T cells shape functional heterogeneity.
FACTORS SHAPING NAIVE CD8+ T CELL HETEROGENEITY
Under normal steady-state conditions, naive CD8+ T cells must meet the requirements for long-term survival in a stable quiescent state without any overt reactivity in response to a homeostatic factor or self-Ag that is continuously exposed for a long period of time (Fig. 1B). In addition, while maintaining a quiescent state, naive CD8+ T cells need to maintain a state of functional fitness so that an immediate response can be induced when an Ag-specific immune response is initiated. Therefore, to meet these key requirements, naive CD8+ T cells must rigorously and precisely regulate signals derived from exposure to various homeostatic factors. Thus, we now discuss thymic or post-thymic hemostatic cues as important players, which have been addressed in many studies, and discuss how they contribute to shaping the heterogeneity of naive CD8+ T cell populations.
Self-Ags
Continuous TCR contact with self-Ags is essential for the survival of peripheral naive CD8+ T cells. Therefore, under steady-state conditions, naive CD8+ T cells circulate SLOs through the blood and lymph and constantly interact with self-pMHC present on the surface of stromal cells or Ag-presenting cells within SLOs. The specificity of self-Ags that induce a tonic survival signal for peripheral naive CD8+ T cells is the same as the specificity of positively selecting self-pMHC for developing thymocytes (40,51). Thus, the strength of these self-signals appears to vary among individual naive CD8+ T cells in the pool, depending on the intrinsic TCR affinity for self-pMHC.
Variable tonic self-reactivity for individual naive CD8+ cells is not only necessary as a homeostatic survival cue for these cells, but it is also highly likely to have a significant impact on generating heterogeneity in these cells under steady-state conditions (52). In fact, many studies discussed in the previous section support this hypothesis. In particular, phenomena associated with CD5 expression are of great importance, as the level of this protein is proportional to the intrinsic TCR self-reactivity for naive CD8+ T cells. One of these studies showed that the expression levels of Ly6C and CD183 in naive CD8+ T cells varied greatly depending on the level of CD5 expression (29,34); while all CD5lo cells were Ly6C− cells, CD5hi cells contained both Ly6C+ and Ly6C− cells. Moreover, CD5hi Ly6C+ cells, unlike CD5hi Ly6C− cells, can be further divided into CD183+ cells and CD183− cells depending on CD183 expression (34). Interestingly, these naive subsets substantially differed in their transcriptomic profiles, including genes related to activation and proliferation, as well as effector and memory differentiation (34), which is consistent with the results from several other studies comparing gene expression profiles between CD5lo cells and CD5hi cells (29,30). In line with these differential gene expression profiles, these subsets of naive CD8+ T cells showed significant functional differences, with the highest response of CD5hi Ly6C+ cells, intermediate response of CD5hi Ly6C− cells, and the lowest response of CD5lo Ly6C− cells observed in the ability to induce IFN-γ production in response to IL-12 and IL-18 exposure as well as IFN-γ and TNF production upon PMA and ionomycine stimulation (34). Similar functional distinction has also been observed in CD5lo and CD5hi subsets of naive CD4+ T cell population in mice and humans (53,54,55,56).
These results strongly support the notion that functional differences in naive CD8+ T cells are dependent on signals derived from TCR contact with self-pMHC. In fact, in a particular condition where self-pMHC is limiting or depleted, the higher responsiveness characteristically observed in CD5hi cells was either markedly decreased or completely abrogated (29,34). For example, Ly6C expression level in naive OT-I cells that were adoptively transferred into TAP-deficient mice was significantly reduced compared with that in control wild-type mice (34). In addition, when the purified Ly6C− subsets of naive P14 (CD5lo relative to that of OT-I) and OT-I (CD5hi relative to that of P14) cells were adoptively co-transferred into C57BL/6 mice (no self-competition for OT-I TCR), the upregulation of Ly6C expression was much higher in OT-I cells than in P14 cells (34). Notably, when the same P14 and OT-I cells were adoptively co-transferred into congenically different OT-I recipient mice, where donor OT-I cells compete with host-derived OT-I cells to limit self-pMHC ligands, upregulation of Ly6C expression in OT-I donor cells was markedly reduced, and the expression level of Ly6C in P14 donor cells was significantly increased (34).
Although these results highlight the importance of a post-thymic tonic self-TCR signal, the possible role of thymic self-signals may not be overlooked. Indeed, the above functional differences between CD5hi and CD5lo subsets from peripheral naive CD8+ T cells were also observed mostly, if not all, between the 2 subsets from CD24lo mature CD4−CD8+ single-positive thymocytes (34), suggesting a role of a positively selecting thymic self-TCR signal. Further studies are needed to define the relative importance of both thymic and post-thymic self-TCR signals, which contribute to shaping the functional heterogeneity of peripheral naive CD8+ T cells.
Cytokines
Naive CD8+ T cells are exposed to various homeostatic cytokines under steady-state conditions. Of these cytokines, the gamma chain (γc) family cytokines, such as IL-2, IL-4, IL-7, and IL-15, are well known to broadly affect peripheral naive CD8+ T cell homeostasis (21,22,23,36). In particular, IL-7, together with self-ligands, is a crucial factor for naive CD8+ T cell survival; therefore, such a continuous IL-7 signal may variably affect the phenotype and function of naive CD8+ T cells. These effects may be attributed to the differential expression of cytokine receptors in naive CD8+ T cells (41). However, a more general view seems to be that this effect is due to differences in intrinsic self-reactivity rather than cytokine receptor expression. Despite comparing 2 subsets showing equivalent levels of IL-2 and IL-7 receptors (i.e., IL-2Rβ and IL-7Rα, respectively), CD5hi cells showed stronger functional response to these cytokines than CD5lo cells (26). Therefore, it is important to consider that the effects of homeostatic cytokines on naive CD8+ T cells largely differ from one subset to another depending on the intrinsic self-reactivity of individual T cells.
Among the various homeostatic effects, IL-15 plays a role in generating and maintaining CD44hi CD122hi MP (also called virtual memory [VM] cells) CD8+ T cells (57,58). In one study, conversion from subsets of naive CD8+ T cells to VM cells was investigated using adoptive transfer experiments, in which normal C57BL/6 mice were intravenously injected with either the CD5lo or CD5hi subset of naive CD8+ T cells (30). After several weeks under these conditions, CD5hi donor cells generated a much higher proportion of VM cells than CD5lo donor cells (30). Therefore, these results support the prevailing notion that naive cells with relatively higher intrinsic self-reactivity, such as CD5hi cells, have, in general, higher sensitivity to cytokine IL-15 (26,30,59). Similarly, IL-7 is crucial for keeping naive CD8+ T cells alive and inducing their homeostatic turnover at a slower rate in steady-state lymphoreplete conditions. The later slow turnover, however, becomes much more intense in lymphopenic conditions, leading to lymphopenia-induced homeostatic proliferation (HP) with much faster kinetics (21,23,60). Notably, under these lymphopenic conditions, CD5hi cells exhibited a much faster and greater HP with enhanced conversion into the CD44hi CD122hi VM phenotype than CD5lo cells (26). Such lymphopenic environments may physiologically occur during the very early stages of the neonatal period (61), and it is assumed that IL-7 plays a role in generating certain degrees of phenotypic and functional heterogeneity in naive CD8+ T cell subpopulations, that is, CD5hi cells and CD5lo cells. In this respect, the increased expression level of CD122 (IL-2/IL-15Rβ) in CD5hi cells (relative to CD5lo cells), which is attributed to higher IL-7-driven HP, is expected to respond better to a tonic homeostatic signal derived from IL-2 and/or IL-15, thereby rendering CD5hi cells more heterogeneous in their phenotype and function. In fact, for peripheral naive CD8+ T cells, anti- and pro-apoptotic molecules, BCL2 and BIM, both of which are known to be induced by IL-7 signaling (62,63,64), are expressed at much higher levels in CD5hi cells than in CD5lo cells (41). Likewise, the level of the TF EOMES, whose expression is dependent on IL-15 signaling, is also significantly higher in CD5hi cells than in CD5lo cells (29,30,34).
In addition to IL-7 and IL-15, IL-4 can also affect naive CD8+ T cell homeostasis under normal steady-state conditions (65), although the effect is largely limited to a specific mouse strain such as BALB/c. The relatively high physiological levels of IL-4 in BALB/c mice are NKT cell-dependent, and this phenomenon greatly increases the proportion of CD44hi CD122hi VM cells over that of CD44lo naive CD8+ T cells (66,67,68). Notably, unlike BALB/c mice, despite little or no evidence of NKT cell-derived IL-4 production in C57BL/6 mice, the CD5hi subset of naive CD8+ T cells from normal C57BL/6 mice showed significantly higher mean fluorescence intensity of CD44 expression than that of the CD5lo subset (29). Whether this phenomenon is due to the higher responsiveness of CD5hi cells to a trace amount of IL-4 in vivo remains unclear and warrants future studies on the homeostatic role of IL-4 in shaping naive CD8+ T cell heterogeneity.
The role of non-γc cytokines has recently been reported to act as homeostatic factors in naive CD8+ T cells. In particular, type I IFN, which is generally thought to be produced as a result of innate immune response after pathogenic infection, is of interest (69,70). Although the exact mechanism of type I IFN production under normal steady-state conditions remains elusive, this cytokine is thought to be produced in response to damage-associated molecular pattern (DAMP) signals derived from apoptotic cells during normal physiological processes (69,71,72). In fact, studies using a genetically modified mouse, which induces a luciferase reporter gene expression under the control of the type I IFN gene promoter, revealed that, among various organs analyzed, the thymus exhibited the highest levels of luciferase expression (73), suggesting a role for DAMP signals released from apoptotic thymocytes during the thymic selection process (74). The most direct and clear effect of tonic type I IFN signaling is its ability to induce Ly6C expression in naive CD8+ T cells (34,35). This expression was most prominent in CD5hi cells, but not in CD5lo cells, and was correlated with a higher responsiveness of CD5hi cells to type I IFN in vitro (based on enhanced type I IFN-induced STAT1 and STAT2 phosphorylation) (34). This phenomenon was observed not only in peripheral naive CD8+ T cells, but also in CD8+ SP thymocytes (34). Therefore, tonic type I IFN signaling likely has a constant influence on naive CD8+ T cells throughout their lifespan. In fact, results from bulk RNA-sequencing analysis comparing the CD5hi and CD5lo subsets of naive CD8+ T cells showed that approximately 30% of differentially expressed genes (DEGs) overlapped with DEGs regulated by type I IFN in vitro, with significant enrichment of genes associated with T cell activation and proliferation (34). Therefore, differences in transcriptional profiles driven by tonic type I IFN signaling likely contribute to shaping the phenotypic and functional heterogeneity of naive CD8+ T cells. In particular, EOMES, which is highly expressed in CD5hi cells compared to CD5lo cells, was more prominent in the CD5hi Ly6C+ subset than in the CD5hi Ly6C− subset, which was in close agreement with the highest sensitivity of CD5hi Ly6C+ cells in response to type I IFN (34,75). With respect to the functional ability to induce IFN-γ upon IL-12 and IL-18 treatment in vitro, CD5hi cells (and to a greater extent CD5hi Ly6C+ cells) that were deprived (either permanently or transiently) of tonic type I IFN signaling showed markedly reduced IL-12/IL-18-driven IFN-γ production (34). In line with these functional aspects, tonic type I IFN signaling is also likely to be involved in conditioning naive CD8+ T cells and allowing them to induce heterogeneous immune response upon pathogenic infection. This issue is discussed in the next section along with its physiological implications.
As another example of a non-γc homeostatic cytokine other than type I IFN, the role of TGF-β has been well demonstrated in CD8+ T cells (76,77). A mutant mouse strain with either T cell-specific deletion of TGF-βRII or dominant negative TGF-βRII results in autoimmune inflammatory diseases with altered T cell homeostasis and aberrant T cell activation (78,79,80). This finding highlights the importance of tonic TGF-β signaling as a key homeostatic regulator responsible for peripheral T cell tolerance. In addition to its regulatory role in T cell homeostasis, tonic TGF-β signaling plays a role in preconditioning naive CD8+ T cells to a steady state (81). Interestingly, TGF-β significantly affected the differentiation fate of naive CD8+ T cells into TRM cells after antigenic stimulation (81). In this study, whether tonic TGF-β signals differ in their sensitivity among different naive CD8+ T cell subpopulations was not addressed. Future studies will focus on defining the relationship between differential responsiveness to TGF-β depending on the intrinsic self-reactivity of individual naive CD8+ T cell subsets (particularly between CD5lo and CD5hi cells) and TRM cell differentiation after pathogenic infection.
Other extrinsic factors
In addition to the aforementioned key homeostatic signals derived from various cytokines and TCR contacts with self-pMHC ligands, environmental factors can also contribute to the heterogeneity of naive CD8+ T cell populations. Of these, the differential developmental origin of naive CD8+ T cells was recently shown to affect their phenotype and function (82). In this study, differences in the developmental history of naive CD8+ T cells were tracked using a mouse model capable of “timestamping” fate mapping from one day of the neonatal period after birth until 2 months of the young adult period (82). Notably, the fetal layer of CD8+ T cells, unlike the adult layer of CD8+ T cells, appears to have an increased ability to differentiate into VM cells (82). These findings strongly suggest that naive CD8+ T cells are composed of phenotypically and functionally multiple layers of subpopulations, depending largely on their developmental origin.
Changes in the homeostasis of naive CD8+ T cells with age have also been demonstrated (83,84). In particular, aging causes a decrease in the naive T cell compartment due to a decrease in thymic output, which in turn increases homeostatic turnover of the existing naive pools, resulting in their conversion and thus a gradual increase in the proportion of effector and MP cells (85,86). Thus, naive CD8+ T cells from aged mice appear to be in a functionally senescent state, probably due to prolonged exposure to various homeostatic factors, which induce a persistent low-grade stimulatory signal in an age-associated altered environment and repeated turnover (87). Further investigation of the phenotypic and functional differences between young and aged naive CD8+ T cells will help elucidate the mechanisms underlying age-related phenomena. In addition, differences in tissue compartmentalization (e.g., SLOs, blood, vs. mucosal-associated lymphoid tissues), anatomic location within SLOs (e.g., T cell zone vs. interfollicular spaces), interacting cell types, and dwell times (e.g., with dendritic cells [DCs] vs. fibroblastic reticular cells) are all potential environmental factors that affect naive CD8+ T cell response to homeostatic cues. In the future, all these variable spatiotemporal changes in the environment, where naive CD8+ T cells circulate and are exposed to various homeostatic factors, such as self-Ags, cytokines, chemokines, and metabolites, will need to be carefully evaluated to better understand naive CD8+ T cell heterogeneity.
PHYSIOLOGICAL IMPLICATIONS FOR IMMUNITY AND DISEASES
The fact that naive CD8+ T cells are phenotypically and functionally heterogeneous is especially important in terms of the efficiency and complexity of adaptive immunity. A single Ag-specific naive CD8+ T cell can differentiate into divergent subsets of effector and memory cells following antigenic stimulation (19,52). These results highlight that such heterogeneity in Ag-specific CD8+ T cell response is randomly formed during an immune response in a stochastic manner, which involves different types and/or strengths of stimuli, cytokines, or asymmetric cell division. However, as mentioned above, many homeostatic factors can affect naive CD8+ T cells at the transcriptional and epigenetic levels, perhaps pre-conditioning them to undergo a unique pattern of activation and differentiation upon Ag encounter. For example, under homeostatic conditions, tonic type I IFN signals can condition naive CD8+ T cells (especially the CD5hi Ly6C+ subset) with a transcriptional signature related to activation and proliferation (34,35). Upon LCMV infection, Ag-specific expansion of CD5hi naive cells was increased compared with CD5lo naive cells, and differentiation into SLEC (CD127− KLRG1+) and effector memory (TEM: CD44hi CD62Llo) cells was also greatly increased (34). Conversely, CD5lo naive cells showed enhanced differentiation into MPEC (CD127+ KLRG1−) and central memory (TCM: CD44hi CD62Lhi) cells (34). Notably, CD5hi cells that were deprived of type I IFN signaling for up to 7–10 days resulted a significant reduction in effector cell expansion as well as marked enhancement in MPEC/TCM differentiation after LCMV infection (34). These data indicate that type I IFN has a stable persistent effect on naive CD8+ T cells, allowing them to undergo better expansion and SLEC/TEM differentiation after Ag encounter.
In addition to type I IFN, naive CD8+ T cell conditioning by TGF-β has also been reported, showing that naive CD8+ T cells are epigenetically conditioned by continuous exposure to active TGF-β in lymph nodes (81). Under normal steady-state conditions, TGF-β is expressed abundantly in most tissues in its latent form by binding non-covalently to the latency-associated peptide (LAP) to confer latency. The cytokine becomes biologically active only when LAP binds to members of the αV class of integrins expressed on hematopoietic and non-hematopoietic cells, which promotes the release of active TGF-β. As such, it may precondition naive CD8+ T cells for epithelial TRM cell differentiation upon skin vaccination (81). In this study, TGF-β activation by αV integrin-expressing migratory DCs was crucial for naive CD8+ T cell preconditioning (81). Although a possible difference in the relative strength of such DC-T cell interactions was not evaluated in this study, TGF-β conditioning by DCs was shown to be MHC-dependent (81). Therefore, the effect of TGF-β may vary depending on the intrinsic TCR affinity for self-pMHC ligands (i.e., the difference between CD5lo and CD5hi cells); further studies are needed to confirm this hypothesis. Similarly, whether the aforementioned type I IFN conditioning also depends on the interaction with DCs and acts together with TGF-β will also be an interesting point to be further addressed.
The differentiation fate of naive CD8+ T cells could be imprinted differently depending on their developmental origin (82). Mechanistically, the fetal layer of CD8+ T cells showed a unique transcriptional profile, chromatin landscape, rapid proliferation, and SLEC differentiation response after pathogenic infection compared with the adult layer of CD8+ T cells (82). A similar phenomenon was observed in human neonatal T cells, which may be due to the relatively high frequency of recent thymic emigrants. In particular, IL-8, which is highly produced in neonatal naive T cells, has a pro-inflammatory function, and promotes naive T cell proliferation and differentiation into IFN-γ-producing Th1 cells (88). In addition, compared with human adult naive CD8+ T cells, neonatal naive CD8+ T cells exhibit a unique property to undergo continuous cell division, produce antimicrobial peptides and reactive oxygen species, and have a unique gene expression profile with high TLR3 and TLR5 expression levels (89). Moreover, human neonatal naive T cells appear to be more sensitive to innate stimuli than are adult naive T cells. It is not clear whether these phenomena are due to seemingly immature immune environments in the early stages of the neonatal period, including the type and/or level of homeostatic factors that may change during immune system development. Collectively, together with type I IFN and TGF-β, such environmental changes confer significant phenotypic and functional heterogeneity to naive CD8+ T cell pools at the transcriptional and even epigenetic levels, providing a crucial mechanistic insight into the generation of huge diversity and complexity of Ag-specific T cell immune response.
The fact that the homeostatic factors mentioned above are the main driving forces leading to the formation of naive CD8+ T cell heterogeneity requires the premise that these cells must be kept in check without causing uncontrolled abnormal activation by these homeostatic factors. Therefore, naive CD8+ T cells are likely to tolerate a certain threshold of physiological cues necessary for creating heterogeneity while maintaining a quiescent state in a normal homeostatic environment (90). In fact, a number of cell-intrinsic regulators, including TFs, such as FOXP1, FOXO1, and RUNX1, as well as non-TF proteins, such as PELI, PTPN2, and PTPN22, which are necessary for maintaining naive T cell quiescence, have been identified (91,92,93). In the absence of these regulators, naive T cells appear to lose tolerance to certain physiological amounts of homeostatic factors, spontaneously activate and proliferate, and ultimately lose their “naivety” and convert into cells with effector/memory-like properties. For example, a study showed that naive CD8+ T cells lacking FOXP1 lose the ability to regulate IL-7Rα (CD127) gene expression, resulting in persistently high levels of CD127 on naive CD8+ T cells, thereby causing hyper-responsiveness even to tonic physiological levels of IL-7 and massive proliferation accompanied by enhanced differentiation into effector/memory-like cells (94). Likewise, another study showed that naive CD8+ T cells lacking STAT1 failed to regulate the STAT4-driven lysosomal mTOR activation pathway induced by tonic type I IFN signaling, thereby losing naivety with aberrant cell proliferation and acquisition of effector/memory-like cell phenotypes (95). Another recent study also showed that naive CD4+ T cells that lack the V-domain Ig suppressor of T cell activation exhibit uncontrolled responsiveness to tonic self-ligands, thereby losing their quiescence with an elevated transition into effector/memory-like cells (96). Unrestrained T cell homeostasis resulting from either the lack of various regulators or improper homeostatic signals is associated with T cell-mediated autoimmune or inflammatory diseases, such as systemic lupus erythematosus (SLE), experimental autoimmune encephalomyelitis, inflammatory bowel disease, and graft-versus-host disease (91,96,97).
The immune-mediated pathologic outcome associated with the failure in naive T cell homeostasis has also been observed in humans. Naive T cells from patients with SLE and Sjogren’s syndrome exhibit DNA methylation patterns associated with activation and differentiation (98). Likewise, β-cell Ag-specific naive T cells from children with a propensity to develop β-cell autoimmunity exhibit partially active and differentiated (especially Th1, Th17, and Tfh) properties compared with those from healthy controls (99). Notably, these properties could be found even in infancy, long before the onset of autoimmune disease (100). Whether the phenomena observed in human naive T cells can be attributed to either failure of quiescence control in naive T cells or an atypical feature of a particular immune environment remains unclear. Moreover, whether this applies to other types of diseases, such as inflammation, chronic pathogenic infection, and cancer, is not fully understood. Nevertheless, these studies strongly suggest that individual naive T cell subsets have a unique epigenetic landscape that can change upon exposure to various homeostatic environmental cues, eventually affecting their differentiation fates and disease development.
Thus, it is important for naive T cells in a steady state to maintain an appropriate balance between quiescence and cellular changes (at the transcriptional and epigenetic levels) in response to continuous exposure to various homeostatic factors. However, when this balance is disturbed, naive T cells may exhibit unrestrained and excessive reactivity to a normally weak homeostatic signal, thereby resulting in inflammatory and autoimmune diseases. In the future, it will be interesting to study whether and how this balance is regulated differently among individual subpopulations of heterogeneous naive T cell pools. Elucidating the precise mechanisms underlying these phenomena will provide a better understanding of the development of various immune diseases associated with abnormal T cell homeostasis.
CONCLUDING REMARKS
Until recently, naive T cells were generally recognized as a phenotypically and functionally homogeneous population. The importance of naive T cells has been primarily limited to the enormous TCR repertoire diversity; thus, studies have focused on how such diverse naive T cells are maintained, and which factors are involved in their homeostasis. Therefore, the potential heterogeneity of the naive T cell population has not been a major consideration in the effort to understand diverse and complex Ag-specific T cell immune response. Indeed, studies showing that a single Ag-specific naive T cell can differentiate into various effector and memory T cell subsets after antigenic stimulation have cast doubt on the assumption that individual naive T cells possess unique differentiation properties. However, accumulating evidence indicates that naive T cells are not a homogeneous population but a phenotypically and functionally heterogeneous population, and that this heterogeneity can be formed through interactions with various homeostatic cues. Importantly, these interactions in a steady state contribute not only to creating heterogeneity, but also to preconditioning naive T cell pools either transcriptionally or epigenetically, rendering each cell predisposed to be distinct in terms of activation, proliferation, and differentiation properties, ultimately shaping the diversity and complexity of Ag-specific T cell immune response (Fig. 2). Considering the heterogeneous nature of naive T cells, comparative studies in different immune contexts (e.g., acute or chronic infections, autoimmunity, and cancer) will greatly expand our understanding of the physiological significance of naive T cell heterogeneity in immunity development and pathogenesis.
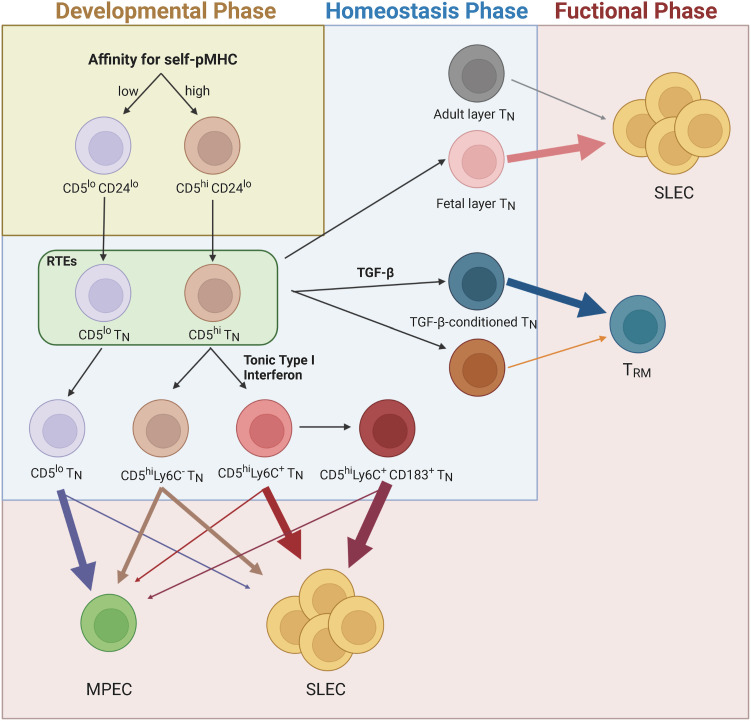
Figure 2. Schematic of how heterogeneity of naive CD8+ T cells is formed and its physiological implications. Naive CD8+ T cell heterogeneity can be formed by various factors derived from thymic developmental and peripheral homeostatic phases, which creates immense diversity and complexity of T cell immune responses. For example, the relatively low (CD5lo) or high (CD5hi) intrinsic self-reactivity of naive CD8+ T cell subsets shows enhanced MPEC and SLEC skewing, respectively. For SLEC skewing of CD5hi subsets (especially for Ly6C+ subset), preconditioning of tonic type I IFN is crucial. Likewise, tonic TGF-β preconditioning plays a role in TRM differentiation. Developmental origin (adult layer vs. fetal layer TN) of naive CD8+ T cells is also associated with shaping the fate of effector differentiation.
TN, naive T.
ACKNOWLEDGEMENTS
This work was supported by a grant from the National Research Foundation (NRF) funded by the Korean Ministry of Science and ICT (2020R1A5A2031185, 2020M3A9G3080281 and 2022R1A2C2009385) and a grant (HCRI 19001-1*HCRI20012) of Chonnam National University Hwasun Hospital and a new faculty research grant (2020-2029) of Chonnam National University. Figures were drawn using Biorender.com.
Abbreviations
- DAMP
damage-associated molecular pattern
- DC
dendritic cell
- DEG
differentially expressed gene
- hi
high expression
- HP
homeostatic proliferation
- LAP
latency-associated peptide
- lo
low expression
- MP
memory-phenotype
- MPEC
memory precursor effector cell
- pMHC
peptide major histocompatibility complex
- SLEC
short-lived effector cell
- SLE
systemic lupus erythematosus
- SLO
secondary lymphoid organ
- TCM
central memory T
- TEM
effector memory T
- TF
transcription factor
- TRM
resident memory T
- VM
virtual memory
- ZAP70
zeta chain-associated protein kinase 70
- γc
gamma chain
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Cho JH.
- Supervision: Cho JH.
- Writing - original draft: Lee SW, Cho JH.
- Writing - review & editing: Lee SW, Lee GW, Kim HO, Cho JH.
References
- 1.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang JT, Wherry EJ, Goldrath AW. Molecular regulation of effector and memory T cell differentiation. Nat Immunol. 2014;15:1104–1115. doi: 10.1038/ni.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y, Zander R, Khatun A, Schauder DM, Cui W. Transcriptional and epigenetic regulation of effector and memory CD8 T cell differentiation. Front Immunol. 2018;9:2826. doi: 10.3389/fimmu.2018.02826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henning AN, Roychoudhuri R, Restifo NP. Epigenetic control of CD8+ T cell differentiation. Nat Rev Immunol. 2018;18:340–356. doi: 10.1038/nri.2017.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi JO, Ham JH, Hwang SS. RNA metabolism in T lymphocytes. Immune Netw. 2022;22:e39. doi: 10.4110/in.2022.22.e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 7.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 8.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 9.Wherry EJ, Teichgräber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 10.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olson JA, McDonald-Hyman C, Jameson SC, Hamilton SE. Effector-like CD8+ T cells in the memory population mediate potent protective immunity. Immunity. 2013;38:1250–1260. doi: 10.1016/j.immuni.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masopust D, Vezys V, Marzo AL, Lefrançois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 13.Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31:859–871. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol. 2012;12:749–761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jameson SC, Masopust D. Understanding subset diversity in T cell memory. Immunity. 2018;48:214–226. doi: 10.1016/j.immuni.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Omilusik KD, Goldrath AW. Remembering to remember: T cell memory maintenance and plasticity. Curr Opin Immunol. 2019;58:89–97. doi: 10.1016/j.coi.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arsenio J. Single-cell analysis of CD8 T lymphocyte diversity during adaptive immunity. Wiley Interdiscip Rev Syst Biol Med. 2020;12:e1475. doi: 10.1002/wsbm.1475. [DOI] [PubMed] [Google Scholar]
- 19.Richard AC. Divide and conquer: phenotypic and temporal heterogeneity within CD8+ T cell responses. Front Immunol. 2022;13:949423. doi: 10.3389/fimmu.2022.949423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol. 2002;2:547–556. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 21.Takada K, Jameson SC. Naive T cell homeostasis: from awareness of space to a sense of place. Nat Rev Immunol. 2009;9:823–832. doi: 10.1038/nri2657. [DOI] [PubMed] [Google Scholar]
- 22.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Sprent J, Surh CD. Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nat Immunol. 2011;12:478–484. doi: 10.1038/ni.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weninger W, Crowley MA, Manjunath N, von Andrian UH. Migratory properties of naive, effector, and memory CD8+ T cells. J Exp Med. 2001;194:953–966. doi: 10.1084/jem.194.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 26.Cho JH, Kim HO, Surh CD, Sprent J. T cell receptor-dependent regulation of lipid rafts controls naive CD8+ T cell homeostasis. Immunity. 2010;32:214–226. doi: 10.1016/j.immuni.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandl JN, Monteiro JP, Vrisekoop N, Germain RN. T cell-positive selection uses self-ligand binding strength to optimize repertoire recognition of foreign antigens. Immunity. 2013;38:263–274. doi: 10.1016/j.immuni.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Persaud SP, Parker CR, Lo WL, Weber KS, Allen PM. Intrinsic CD4+ T cell sensitivity and response to a pathogen are set and sustained by avidity for thymic and peripheral complexes of self peptide and MHC. Nat Immunol. 2014;15:266–274. doi: 10.1038/ni.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fulton RB, Hamilton SE, Xing Y, Best JA, Goldrath AW, Hogquist KA, Jameson SC. The TCR’s sensitivity to self peptide-MHC dictates the ability of naive CD8+ T cells to respond to foreign antigens. Nat Immunol. 2015;16:107–117. doi: 10.1038/ni.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White JT, Cross EW, Burchill MA, Danhorn T, McCarter MD, Rosen HR, O’Connor B, Kedl RM. Virtual memory T cells develop and mediate bystander protective immunity in an IL-15-dependent manner. Nat Commun. 2016;7:11291. doi: 10.1038/ncomms11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho JH, Kim HO, Ju YJ, Kye YC, Lee GW, Lee SW, Yun CH, Bottini N, Webster K, Goodnow CC, et al. CD45-mediated control of TCR tuning in naïve and memory CD8+ T cells. Nat Commun. 2016;7:13373. doi: 10.1038/ncomms13373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balyan R, Gund R, Chawla AS, Khare SP, Pradhan SJ, Rane S, Galande S, Durdik JM, George A, Bal V, et al. Correlation of cell-surface CD8 levels with function, phenotype and transcriptome of naive CD8 T cells. Immunology. 2019;156:384–401. doi: 10.1111/imm.13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Simone G, Mazza EMC, Cassotta A, Davydov AN, Kuka M, Zanon V, De Paoli F, Scamardella E, Metsger M, Roberto A, et al. CXCR3 identifies human naive CD8+ T cells with enhanced effector differentiation potential. J Immunol. 2019;203:3179–3189. doi: 10.4049/jimmunol.1901072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ju YJ, Lee SW, Kye YC, Lee GW, Kim HO, Yun CH, Cho JH. Self-reactivity controls functional diversity of naive CD8+ T cells by co-opting tonic type I interferon. Nat Commun. 2021;12:6059. doi: 10.1038/s41467-021-26351-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jergović M, Coplen CP, Uhrlaub JL, Besselsen DG, Cheng S, Smithey MJ, Nikolich-Žugich J. Infection-induced type I interferons critically modulate the homeostasis and function of CD8+ naïve T cells. Nat Commun. 2021;12:5303. doi: 10.1038/s41467-021-25645-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawabe T, Yi J, Sprent J. Homeostasis of naive and memory T lymphocytes. Cold Spring Harb Perspect Biol. 2021;13:a037879. doi: 10.1101/cshperspect.a037879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tarakhovsky A, Kanner SB, Hombach J, Ledbetter JA, Müller W, Killeen N, Rajewsky K. A role for CD5 in TCR-mediated signal transduction and thymocyte selection. Science. 1995;269:535–537. doi: 10.1126/science.7542801. [DOI] [PubMed] [Google Scholar]
- 38.Azzam HS, Grinberg A, Lui K, Shen H, Shores EW, Love PE. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J Exp Med. 1998;188:2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Azzam HS, DeJarnette JB, Huang K, Emmons R, Park CS, Sommers CL, El-Khoury D, Shores EW, Love PE. Fine tuning of TCR signaling by CD5. J Immunol. 2001;166:5464–5472. doi: 10.4049/jimmunol.166.9.5464. [DOI] [PubMed] [Google Scholar]
- 40.Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see) Nat Rev Immunol. 2014;14:377–391. doi: 10.1038/nri3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palmer MJ, Mahajan VS, Chen J, Irvine DJ, Lauffenburger DA. Signaling thresholds govern heterogeneity in IL-7-receptor-mediated responses of naïve CD8+ T cells. Immunol Cell Biol. 2011;89:581–594. doi: 10.1038/icb.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McNeill L, Salmond RJ, Cooper JC, Carret CK, Cassady-Cain RL, Roche-Molina M, Tandon P, Holmes N, Alexander DR. The differential regulation of Lck kinase phosphorylation sites by CD45 is critical for T cell receptor signaling responses. Immunity. 2007;27:425–437. doi: 10.1016/j.immuni.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 43.Hermiston ML, Xu Z, Weiss A. CD45: a critical regulator of signaling thresholds in immune cells. Annu Rev Immunol. 2003;21:107–137. doi: 10.1146/annurev.immunol.21.120601.140946. [DOI] [PubMed] [Google Scholar]
- 44.Majeti R, Xu Z, Parslow TG, Olson JL, Daikh DI, Killeen N, Weiss A. An inactivating point mutation in the inhibitory wedge of CD45 causes lymphoproliferation and autoimmunity. Cell. 2000;103:1059–1070. doi: 10.1016/s0092-8674(00)00209-9. [DOI] [PubMed] [Google Scholar]
- 45.Pena-Rossi C, Zuckerman LA, Strong J, Kwan J, Ferris W, Chan S, Tarakhovsky A, Beyers AD, Killeen Negative regulation of CD4 lineage development and responses by CD5. J Immunol. 1999;163:6494–6501. [PubMed] [Google Scholar]
- 46.Voisinne G, Gonzalez de Peredo A, Roncagalli R. CD5, an Undercover Regulator of TCR Signaling. Front Immunol. 2018;9:2900. doi: 10.3389/fimmu.2018.02900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stefanová I, Dorfman JR, Germain RN. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002;420:429–434. doi: 10.1038/nature01146. [DOI] [PubMed] [Google Scholar]
- 48.Fischer UB, Jacovetty EL, Medeiros RB, Goudy BD, Zell T, Swanson JB, Lorenz E, Shimizu Y, Miller MJ, Khoruts A, et al. MHC class II deprivation impairs CD4 T cell motility and responsiveness to antigen-bearing dendritic cells in vivo . Proc Natl Acad Sci U S A. 2007;104:7181–7186. doi: 10.1073/pnas.0608299104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hochweller K, Wabnitz GH, Samstag Y, Suffner J, Hämmerling GJ, Garbi N. Dendritic cells control T cell tonic signaling required for responsiveness to foreign antigen. Proc Natl Acad Sci U S A. 2010;107:5931–5936. doi: 10.1073/pnas.0911877107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim HO, Cho JH. T cell’s sense of self: a role of self-recognition in shaping functional competence of naïve T cells. Immune Netw. 2017;17:201–213. doi: 10.4110/in.2017.17.4.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Surh CD, Sprent J. Homeostatic T cell proliferation: how far can T cells be activated to self-ligands? J Exp Med. 2000;192:F9–FF14. doi: 10.1084/jem.192.4.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eggert J, Au-Yeung BB. Functional heterogeneity and adaptation of naive T cells in response to tonic TCR signals. Curr Opin Immunol. 2021;73:43–49. doi: 10.1016/j.coi.2021.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zinzow-Kramer WM, Weiss A, Au-Yeung BB. Adaptation by naïve CD4+ T cells to self-antigen–dependent TCR signaling induces functional heterogeneity and tolerance. Proc Natl Acad Sci U S A. 2019;116:15160–15169. doi: 10.1073/pnas.1904096116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bartleson JM, Viehmann Milam AA, Donermeyer DL, Horvath S, Xia Y, Egawa T, Allen PM. Strength of tonic T cell receptor signaling instructs T follicular helper cell-fate decisions. Nat Immunol. 2020;21:1384–1396. doi: 10.1038/s41590-020-0781-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rogers D, Sood A, Wang H, van Beek JJ, Rademaker TJ, Artusa P, Schneider C, Shen C, Wong DC, Bhagrath A, et al. Pre-existing chromatin accessibility and gene expression differences among naive CD4+ T cells influence effector potential. Cell Reports. 2021;37:110064. doi: 10.1016/j.celrep.2021.110064. [DOI] [PubMed] [Google Scholar]
- 56.Sood A, Lebel MÈ, Dong M, Fournier M, Vobecky SJ, Haddad É, Delisle JS, Mandl JN, Vrisekoop N, Melichar HJ. CD5 levels define functionally heterogeneous populations of naïve human CD4+ T cells. Eur J Immunol. 2021;51:1365–1376. doi: 10.1002/eji.202048788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee JY, Hamilton SE, Akue AD, Hogquist KA, Jameson SC. Virtual memory CD8 T cells display unique functional properties. Proc Natl Acad Sci U S A. 2013;110:13498–13503. doi: 10.1073/pnas.1307572110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Truckenbrod EN, Jameson SC. The virtuous self-tolerance of virtual memory T cells. EMBO J. 2018;37:e99883. doi: 10.15252/embj.201899883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kieper WC, Burghardt JT, Surh CD. A role for TCR affinity in regulating naive T cell homeostasis. J Immunol. 2004;172:40–44. doi: 10.4049/jimmunol.172.1.40. [DOI] [PubMed] [Google Scholar]
- 60.Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Min B, McHugh R, Sempowski GD, Mackall C, Foucras G, Paul WE. Neonates support lymphopenia-induced proliferation. Immunity. 2003;18:131–140. doi: 10.1016/s1074-7613(02)00508-3. [DOI] [PubMed] [Google Scholar]
- 62.Wojciechowski S, Tripathi P, Bourdeau T, Acero L, Grimes HL, Katz JD, Finkelman FD, Hildeman DA. Bim/Bcl-2 balance is critical for maintaining naive and memory T cell homeostasis. J Exp Med. 2007;204:1665–1675. doi: 10.1084/jem.20070618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li WQ, Guszczynski T, Hixon JA, Durum SK. Interleukin-7 regulates Bim proapoptotic activity in peripheral T-cell survival. Mol Cell Biol. 2010;30:590–600. doi: 10.1128/MCB.01006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pearson C, Silva A, Saini M, Seddon B. IL-7 determines the homeostatic fitness of T cells by distinct mechanisms at different signalling thresholds in vivo . Eur J Immunol. 2011;41:3656–3666. doi: 10.1002/eji.201141514. [DOI] [PubMed] [Google Scholar]
- 65.Renkema KR, Lee JY, Lee YJ, Hamilton SE, Hogquist KA, Jameson SC. IL-4 sensitivity shapes the peripheral CD8+ T cell pool and response to infection. J Exp Med. 2016;213:1319–1329. doi: 10.1084/jem.20151359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Akue AD, Lee JY, Jameson SC. Derivation and maintenance of virtual memory CD8 T cells. J Immunol. 2012;188:2516–2523. doi: 10.4049/jimmunol.1102213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee YJ, Holzapfel KL, Zhu J, Jameson SC, Hogquist KA. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat Immunol. 2013;14:1146–1154. doi: 10.1038/ni.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kurzweil V, LaRoche A, Oliver PM. Increased peripheral IL-4 leads to an expanded virtual memory CD8+ population. J Immunol. 2014;192:5643–5651. doi: 10.4049/jimmunol.1301755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gough DJ, Messina NL, Clarke CJ, Johnstone RW, Levy DE. Constitutive type I interferon modulates homeostatic balance through tonic signaling. Immunity. 2012;36:166–174. doi: 10.1016/j.immuni.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Uccellini MB, García-Sastre A. ISRE-reporter mouse reveals high basal and induced type I IFN responses in inflammatory monocytes. Cell Reports. 2018;25:2784–2796.e3. doi: 10.1016/j.celrep.2018.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu Q, Katlinskaya YV, Carbone CJ, Zhao B, Katlinski KV, Zheng H, Guha M, Li N, Chen Q, Yang T, et al. DNA-damage-induced type I interferon promotes senescence and inhibits stem cell function. Cell Reports. 2015;11:785–797. doi: 10.1016/j.celrep.2015.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Härtlova A, Erttmann SF, Raffi FA, Schmalz AM, Resch U, Anugula S, Lienenklaus S, Nilsson LM, Kröger A, Nilsson JA, et al. DNA damage primes the type I interferon system via the cytosolic DNA sensor STING to promote anti-microbial innate immunity. Immunity. 2015;42:332–343. doi: 10.1016/j.immuni.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 73.Lienenklaus S, Cornitescu M, Zietara N, Łyszkiewicz M, Gekara N, Jabłónska J, Edenhofer F, Rajewsky K, Bruder D, Hafner M, et al. Novel reporter mouse reveals constitutive and inflammatory expression of IFN-β in vivo . J Immunol. 2009;183:3229–3236. doi: 10.4049/jimmunol.0804277. [DOI] [PubMed] [Google Scholar]
- 74.Xing Y, Wang X, Jameson SC, Hogquist KA. Late stages of T cell maturation in the thymus involve NF-κB and tonic type I interferon signaling. Nat Immunol. 2016;17:565–573. doi: 10.1038/ni.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martinet V, Tonon S, Torres D, Azouz A, Nguyen M, Kohler A, Flamand V, Mao CA, Klein WH, Leo O, et al. Type I interferons regulate eomesodermin expression and the development of unconventional memory CD8+ T cells. Nat Commun. 2015;6:7089. doi: 10.1038/ncomms8089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rubtsov YP, Rudensky AY. TGFβ signalling in control of T-cell-mediated self-reactivity. Nat Rev Immunol. 2007;7:443–453. doi: 10.1038/nri2095. [DOI] [PubMed] [Google Scholar]
- 77.Johnson LD, Jameson SC. TGF-β sensitivity restrains CD8+ T cell homeostatic proliferation by enforcing sensitivity to IL-7 and IL-15. PLoS One. 2012;7:e42268. doi: 10.1371/journal.pone.0042268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gorelik L, Flavell RA. Abrogation of TGFβ signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 79.Lucas PJ, Kim SJ, Melby SJ, Gress RE. Disruption of T cell homeostasis in mice expressing a T cell-specific dominant negative transforming growth factor β II receptor. J Exp Med. 2000;191:1187–1196. doi: 10.1084/jem.191.7.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455–471. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 81.Mani V, Bromley SK, Äijö T, Mora-Buch R, Carrizosa E, Warner RD, Hamze M, Sen DR, Chasse AY, Lorant A, et al. Migratory DCs activate TGF-β to precondition naïve CD8+ T cells for tissue-resident memory fate. Science. 2019;366:eaav5728. doi: 10.1126/science.aav5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smith NL, Patel RK, Reynaldi A, Grenier JK, Wang J, Watson NB, Nzingha K, Yee Mon KJ, Peng SA, Grimson A, et al. Developmental Origin Governs CD8+ T Cell Fate Decisions during Infection. Cell. 2018;174:117–130.e14. doi: 10.1016/j.cell.2018.05.029. [DOI] [PubMed] [Google Scholar]
- 83.Nikolich-Žugich J, Li G, Uhrlaub JL, Renkema KR, Smithey MJ. Age-related changes in CD8 T cell homeostasis and immunity to infection. Semin Immunol. 2012;24:356–364. doi: 10.1016/j.smim.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qi Q, Zhang DW, Weyand CM, Goronzy JJ. Mechanisms shaping the naïve T cell repertoire in the elderly - thymic involution or peripheral homeostatic proliferation? Exp Gerontol. 2014;54:71–74. doi: 10.1016/j.exger.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.den Braber I, Mugwagwa T, Vrisekoop N, Westera L, Mögling R, de Boer AB, Willems N, Schrijver EH, Spierenburg G, Gaiser K, et al. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity. 2012;36:288–297. doi: 10.1016/j.immuni.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 86.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 87.Goronzy JJ, Weyand CM. Mechanisms underlying T cell ageing. Nat Rev Immunol. 2019;19:573–583. doi: 10.1038/s41577-019-0180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Das A, Rouault-Pierre K, Kamdar S, Gomez-Tourino I, Wood K, Donaldson I, Mein CA, Bonnet D, Hayday AC, Gibbons DL. Adaptive from innate: human IFN-γ+CD4+ T cells can arise directly from CXCL8-producing recent thymic emigrants in babies and adults. J Immunol. 2017;199:1696–1705. doi: 10.4049/jimmunol.1700551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Galindo-Albarrán AO, López-Portales OH, Gutiérrez-Reyna DY, Rodríguez-Jorge O, Sánchez-Villanueva JA, Ramírez-Pliego O, Bergon A, Loriod B, Holota H, Imbert J, et al. CD8+ T Cells from human neonates are biased toward an innate immune response. Cell Reports. 2016;17:2151–2160. doi: 10.1016/j.celrep.2016.10.056. [DOI] [PubMed] [Google Scholar]
- 90.Hamilton SE, Jameson SC. CD8 T cell quiescence revisited. Trends Immunol. 2012;33:224–230. doi: 10.1016/j.it.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Neama AF, Looi CY, Wong WF. In: Lymphocyte Updates. Isvoranu G, editor. London: IntechOpen; 2017. 5. Multiple players in the mechanical control of T cell quiescence. [Google Scholar]
- 92.ElTanbouly MA, Noelle RJ. Rethinking peripheral T cell tolerance: checkpoints across a T cell’s journey. Nat Rev Immunol. 2021;21:257–267. doi: 10.1038/s41577-020-00454-2. [DOI] [PubMed] [Google Scholar]
- 93.Yusuf I, Fruman DA. Regulation of quiescence in lymphocytes. Trends Immunol. 2003;24:380–386. doi: 10.1016/s1471-4906(03)00141-8. [DOI] [PubMed] [Google Scholar]
- 94.Feng X, Wang H, Takata H, Day TJ, Willen J, Hu H. Transcription factor Foxp1 exerts essential cell-intrinsic regulation of the quiescence of naive T cells. Nat Immunol. 2011;12:544–550. doi: 10.1038/ni.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kye YC, Lee GW, Lee SW, Ju YJ, Kim HO, Yun CH, Cho JH. STAT1 maintains naïve CD8+ T cell quiescence by suppressing the type I IFN-STAT4-mTORC1 signaling axis. Sci Adv. 2021;7:eabg8764. doi: 10.1126/sciadv.abg8764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.ElTanbouly MA, Zhao Y, Nowak E, Li J, Schaafsma E, Le Mercier I, Ceeraz S, Lines JL, Peng C, Carriere C, et al. VISTA is a checkpoint regulator for naïve T cell quiescence and peripheral tolerance. Science. 2020;367:eaay0524. doi: 10.1126/science.aay0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Buang N, Tapeng L, Gray V, Sardini A, Whilding C, Lightstone L, Cairns TD, Pickering MC, Behmoaras J, Ling GS, et al. Type I interferons affect the metabolic fitness of CD8+ T cells from patients with systemic lupus erythematosus. Nat Commun. 2021;12:1980. doi: 10.1038/s41467-021-22312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Altorok N, Coit P, Hughes T, Koelsch KA, Stone DU, Rasmussen A, Radfar L, Scofield RH, Sivils KL, Farris AD, et al. Genome-wide DNA methylation patterns in naive CD4+ T cells from patients with primary Sjögren’s syndrome. Arthritis Rheumatol. 2014;66:731–739. doi: 10.1002/art.38264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Coit P, Dozmorov MG, Merrill JT, McCune WJ, Maksimowicz-McKinnon K, Wren JD, Sawalha AH. Epigenetic reprogramming in naive CD4+ T cells favoring T cell activation and non-Th1 effector T cell immune response as an early event in lupus flares. Arthritis Rheumatol. 2016;68:2200–2209. doi: 10.1002/art.39720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Heninger AK, Eugster A, Kuehn D, Buettner F, Kuhn M, Lindner A, Dietz S, Jergens S, Wilhelm C, Beyerlein A, et al. A divergent population of autoantigen-responsive CD4+ T cells in infants prior to β cell autoimmunity. Sci Transl Med. 2017;9:eaaf8848. doi: 10.1126/scitranslmed.aaf8848. [DOI] [PubMed] [Google Scholar]