Abstract
A liver abscess is an entity that is rarely observed in the emergency department; therefore, it requires timely diagnosis by the clinicians who support this service. The early diagnosis of a liver abscess is challenging as variable and non-specific symptoms are present; furthermore, symptoms may differ in patients with human immunodeficiency virus (HIV) infection. To date, reports on the presentation of diagnostic ultrasound with point-of-care ultrasonography (PoCUS) are limited. The present case report study describes a patient diagnosed with HIV and the presence of a liver abscess confirmed by PoCUS performed in an emergency department. The patient presented with abdominal pain upon palpation in the right hypochondrium and in the thoracoabdominal area, which became more severe with inspiration. PoCUS revealed a hypodense intrahepatic image observed between segments VII and VI, with internal echoes suggestive of a liver abscess. Moreover, it was decided to perform tomography-guided percutaneous drainage of the liver abscess. Antibiotic treatment with ampicillin/sulbactam and IV metronidazole was also commenced. The patient presented clinical improvement and was discharged on the third day.
Keywords: emergency department, emergency ultrasound, hepatic abscess, point-of-care ultrasound, pyogenic liver abscess, ultrasonography
Introduction
A liver abscess is an infectious condition that can have varying etiologies, including bacterial or amoebic. It is a rare entity with an incidence between 3 and 3.6 cases per 100,000 inhabitants in the United States; however, its incidence may be higher in Latin America, presenting with mortality rates of 25 to 30% (1,2).
The clinical presentation of liver abscesses in the emergency department requires prompt recognition in order to prevent morbidity and mortality (3). Point-of-care ultrasonography (PoCUS) is a useful tool in emergency services for the rapid detection of pathologies that require urgent intervention, including infections and abdominal complications. Furthermore, PoCUS presents a sensitivity between 85-92% (4,5). To date, reported literature on the use of PoCUS for the early detection of liver abscesses is limited; the lack of training in its management by clinical staff in emergency services has also been described, an issue that is still challenging for the physician due to the scarcity of symptoms and signs (6,7). Moreover, in human immunodeficiency virus (HIV)-positive patients, the usefulness of PoCUS is of utmost importance as a tool for the exclusion of other differential diagnoses, or common opportunistic infections in this population, concerns that can delay diagnosis and subsequent treatment.
The present case report study describes the importance of medical training in PoCUS in an emergency service, as it is a rapid strategy for the early diagnosis of liver abscess.
Case report
A 44-year-old male patient consulted the Hospital San Vicente Fundación (Rionegro, Colombia) in September, 2022. He reported a clinical condition of ~1 month of evolution consisting of an objective fever between 38-39˚C, associated with subjective weight loss. At 15 days prior to consultation, he presented with a dry cough and odynophagia, symptoms that were self-limited. At 3 days prior to the consultation, he presented with diarrhea (loose stool), not dysenteric. At 1 day prior to consulting the hospital, he had intense pain in the right thoracoabdominal region, with progressive onset; this reached maximum intensity and thus limited his ability to walk; for this reason, the patient decided to consult the hospital.
The patient reported a diagnosis of HIV infection in 2020, being adherent to treatment, without presenting opportunistic infections until then. The patient has been receiving treatment with tenofovir + emtricitabine (200/300 mg) every 24 h for 5 years. In the emergency department, the patient presented with the following vital signs: Blood pressure, 112/74 mmHg; mean blood pressure, 87 mmHg; heart rate, 89 beats per minute; respiratory rate, 16; oxygen saturation, 95%; temperature, 36˚C. Upon a physical examination, no dehydration and no lymphadenopathy were observed. He presented abdominal pain upon palpation in the right hypochondrium and in the thoracoabdominal area, which became more severe with inspiration.
It was decided to perform PoCUS (LOGIQ e, GE Healthcare) following previously established protocols (6). A hypodense intrahepatic image was observed between segments VII and VI, with internal echoes suggestive of a liver abscess (Fig. 1). An ultrasound of the liver and bile ducts performed by a radiologist was requested. A well-defined hypodense lesion between segments VII and VI was confirmed, measuring 81x78x76 mm, with an approximate volume of 253 cc. He presented internal echoes, faint posterior acoustic enhancement and minimal perilesional vascular flow, findings that were associated with the clinical suspicion of liver abscess (Fig. 2). On the same day, radiology and general surgery were consulted. It was decided to perform tomography-guided percutaneous drainage of the liver abscess (IQon Spectral CT, Philips) following formerly recognized protocols (6). A 230 cc drainage was obtained, with no complications (Fig. 3, Fig. 4 and Fig. 5).
Figure 1.
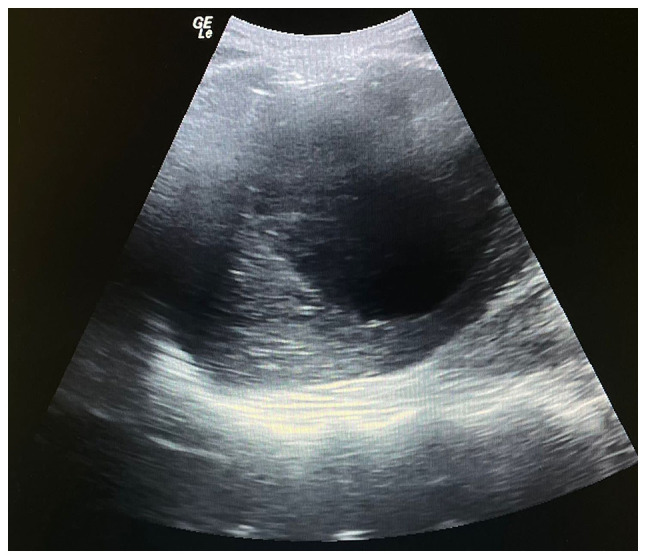
Point-of-care ultrasonography illustrating a hypodense intrahepatic image with internal echoes suggestive of an abscess.
Figure 2.
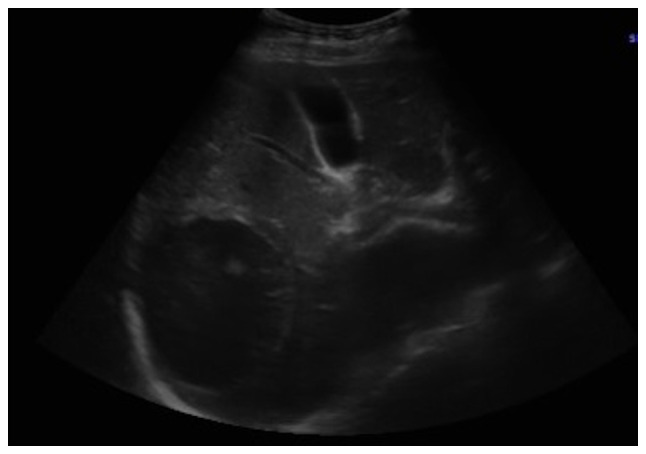
Ultrasound of the liver and bile ducts. A well-defined, hypodense lesion is observed. Gallbladder without lithiasis or dilatation of the common bile duct.
Figure 3.
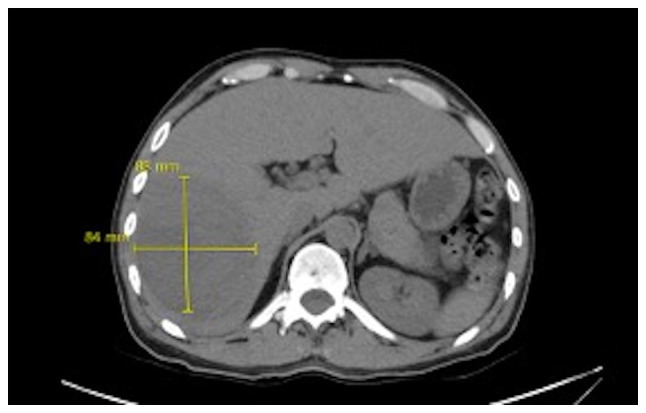
Simple abdominal tomography. Evidence of liver injury in segments VI and VII is compatible with an 84x88 mm abscess.
Figure 4.
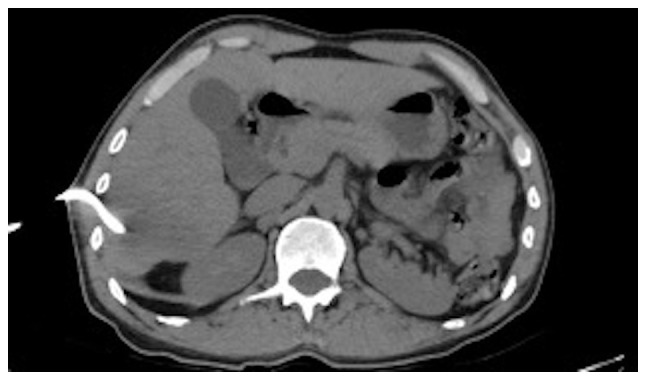
Simple axial projection abdominal tomography. Insertion and drainage site at the level of the right hypochondrium.
Figure 5.
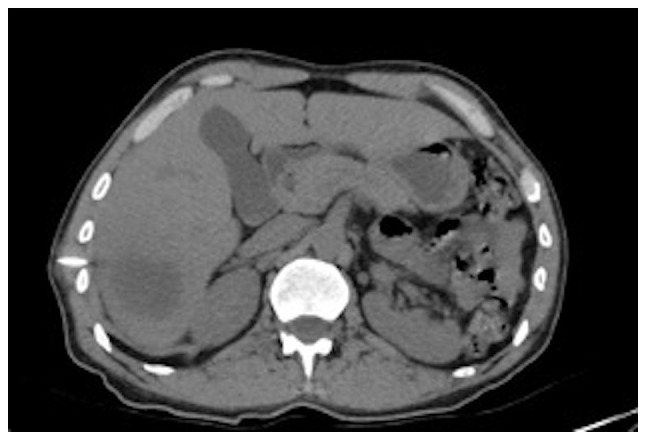
Simple axial slice abdominal tomography. Control image after liver abscess drainage.
Complementary laboratory analyses were performed, yielding the following results: Leukocytes, 8,700/ml (normal range, 4,500-11,500/ml); neutrophils, 69% (normal range, 40-60%); hemoglobin, 10.6 g/dl (normal range, 13.8-17.2 g/dl); hematocrit, 32% (normal range, 40.7-50.3%); platelets, 353,000/ml (normal range, 150,000-400,00/ml); international normalized ratio (INR), 1.4 (normal value, 1); partial prothrombin time (PTT), 30.6 sec (normal range, 24.2-36.3 sec); prothrombin time (PT), 16.3 sec (normal range, 12.7-16.3 sec); creatinine, 0.86 mg/dl (normal range, 0.7-1.3 mg/dl); C-reactive protein, 20.8 mg/dl (normal, <10 mg/dl); CD4, 345 cells per mm3 (normal range, 200-500 cells per mm3); and an undetectable HIV viral load. A chest X-ray was performed, which revealed the elevation of the right hemidiaphragm, without pneumoperitoneum, without the presence of consolidations that suggested pneumonia or the presence of pleural effusion (Fig. 6).
Figure 6.
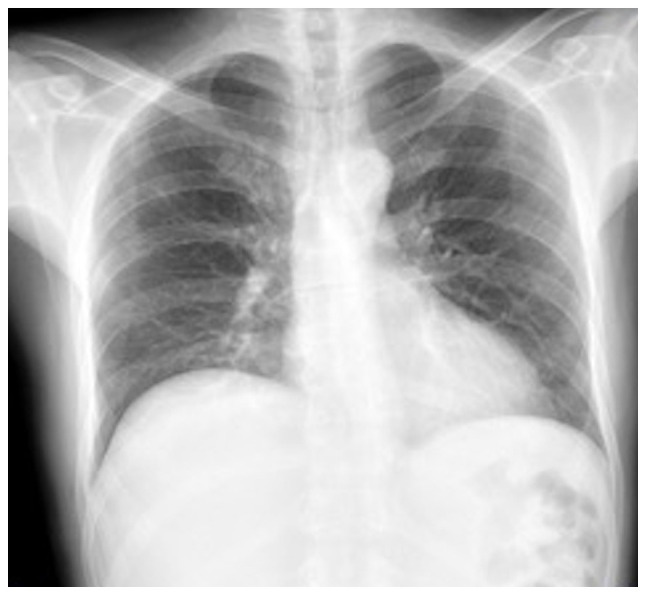
Chest X-ray. A rise of the right hemidiaphragm is observed. Visible lung parenchyma without alterations. There is no pleural effusion.
Considering all the aforementioned information, antibiotic treatment with ampicillin/sulbactam and IV metronidazole was commenced, and hospital management was considered. The patient presented clinical improvement (with drainage and antibiotic coverage performed) and was discharged on the third day. It was recommended antibiotic management at home (oral metronidazole) until completing 15 effective days of treatment.
Discussion
A liver abscess can be defined as a collection of purulent material encapsulated within the liver parenchyma caused by bacteria, fungi and/or parasites. Pyogenic liver abscess may be initiated by a diversity of microorganisms, involving Escherichia coli, Klebsiella pneumoniae and Burkholderia pseudomallei. The microbiology varies rendering to the assumed course of hepatic invasion. Infections can occur from the biliary tree, circulation, an adjacent point of infectivity and acute damage (1,8). The incidence of liver abscess varies according to the region, with ranges that occur from 2.3 cases per 100,000 inhabitants admitted to the hospitals in the United States, and up to 17.6 per 100,000 inhabitants admitted to hospitals in Taiwan (1,2). Mortality has been shown to be reduced from 50 to 10% following the use of percutaneous intervention guided by ultrasonography or tomography (8,9).
As observed in the patient presented herein, the majority of cases are observed in males, between the third and sixth decade of life. The etiology of liver abscess is multifactorial and is related to predisposing factors (1,6). The associated risk factors increase the presence of liver abscesses in individuals with oncological diseases, diabetes mellitus, immunosuppression, alcoholism and local factors, such as biliary causes (history of hepatobiliary surgery, chronic obstruction of the bile ducts), portal causes (chronic infectious focus drained by the portal) and the superimposition of pre-existing lesions (cyst and necrotic hepatic metastasis) (9,10).
The incidence of fungal abscesses is on the rise due to the increase in the number of immunocompromised patients. Candida species is the most common fungal infection, and bacterial co-infection is common (10). Cryptococcal liver abscess is frequently observed in the context of HIV, solid organ transplant, primary immune deficiency, or lymphoid hemopathies. The mode of presentation may consist of hepatitis, possibly fulminant, or diffuse micro abscesses (10-12).
Although episodes of liver abscess have been reported in HIV-positive patients, the specific symptoms remain unclear. In these patients, it is generated by the colonization of the colon by trophozoites that produce ulcerative lesions in the mucosa, due to an immune deficiency of CD4+ T-lymphocytes and macrophages. This leaves the capillaries exposed, generating their migration to the enteric-hepatic venous system; once it is in the parenchyma, it can remain and produce lysis, causing liver abscess, or it can be distributed in other organs with portal circulation (13-15).
As regards culture results, it has been indicated that these may be variable. The study conducted by Zhang et al (16) presented positive results in 16% of cases and the presence of positive blood cultures in 5.4% of patients. The microorganisms identified were Staphylococcus aureus and Staphylococcus haemolyticus (16).
The presence of a liver abscess in patients with HIV may be associated with co-infections, such as pneumonia, Candida infection and bacterial peritonitis; infections that did not occur in the present case (17). Hence, the importance of recognizing the probable microbiological etiology to guide therapy (18). On the other hand, HIV-positive patients may also present with other complications, such as the appearance of tumors including sarcomas located in various parts of the body (19) and hepatocellular carcinomas (20). Some of these tumors are associated with opportunistic infections that appear with a low CD4 count (19,20).
Imaging systems, including ultrasonography and computed tomography scanning are valuable implements to validate an area occupying lesion and corroborate the occurrence or non-existence of a liver abscess. Computed tomography has a superior sensitivity compared to ultrasound for the detection of liver abscess; however, this possibility can not permanently be available in low-middle income countries (21). Ultrasound is conventionally the initial image used in clinical practice for the evaluation of the liver, with a good performance to evaluate its lesions. Moreover, it is not expensive, it can be repeated multiple times, it is accessible and it does not generate radiation. It is widely used not only in the diagnosis of a liver abscess, but also in patient management in all phases of the diagnostic-therapeutic process (22); it is also appropriate to use ultrasound in various diseases of the liver, such as cirrhosis and tumors (23). Therefore, when the signs and symptoms indicate a liver abscess, ultrasound can be a guiding instrument to reach this diagnosis. This can identify lesions >2 cm in diameter, which shows a sensitivity of 85 to 95%, which can even increase when the study is improved with the addition of contrast. For its part, computed tomography has a sensitivity of 95% and can detect abscesses up to 0.5 cm (24,25).
Amebiasis is a common parasitic disease worldwide and is prevalent in all tropical countries (26,27). There are ultrasound protocols for parasitic infections. Upon an ultrasound, an amoebic liver abscess presents as a focal liver lesion that is unique in 60% of cases. It is located more frequently in the posterior part of the right lobe. The main clinical and ultrasound differential diagnosis of amoebic liver abscess is a pyogenic liver abscess (28,29). However, it is sometimes difficult to identify in the early stages due to the similar echogenicity of the surrounding liver tissue; pyogenic lesions become hypoechoic compared to the liver and may be more variable in shape and often have irregular walls (30).
As handled in the case described in the present study, antimicrobial guidelines usually advise empiric treatment, indicating both amoebic and pyogenic causes of liver abscess. As therapy is frequently managed prior to the compilation of suitable samples, the causal microorganism and occurrence of either sickness remain imprecise (21).
The mortality rate from liver abscesses in the 20th century was close to 75-80%. With the commencement of antibiotic coverage, the mortality rate was reduced, targeting microorganisms such as Gram-negative bacilli, Gram-positive cocci and anaerobic bacteria. Therefore, treatment with third-generation cephalosporins plus metronidazole or piperacillin/tazobactam has been shown to be effective. As regards the treatment of patients with HIV, if this is ineffective, the possibility of opportunistic infections should be suspected. Percutaneous drainage in these patients can reduce the mortality rate to 10-30% (31).
In conclusion, the clinical features and laboratory findings of HIV-infected patients with a liver abscess may be non-specific. Moreover, it is recommended to obtain a blood sample to perform a culture to determine the variety of microorganism involved in the infection and thus establish its origin. Likewise, the assessment of the etiology of the infection is very relevant. Liver abscess is not a rare condition in tropical countries; it is an emerging condition that carries high mortality if it is diagnosed incorrectly, or late and is not treated. The early identification and the use of PoCUS by the emergency physician may help to lead to timely management and interventions in the emergency department.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
MZG, DGA, RAOC, DNH and CMA contributed to the conception and design of the study. CMA wrote the manuscript. MZG, DGA, RAOC and DNH searched the literature. MZG, DGA, RAOC and DNH provided clinical assistance to the patient and were responsible for the treatments. MZG and CMA revised the manuscript. MZG and CMA confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The Bioethics Committee of San Vicente Fundación Hospital (Rionegro, Colombia) approved the publication of this case. Written informed consent for the publication of clinical details and images was obtained from the patient.
Patient consent for publication
Written informed consent for the publication of clinical details and images was obtained from the patient.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Johannsen EC, Sifri CD, Madoff LC. Pyogenic liver abscesses. Infect Dis Clin North Am. 2000;14:547–563. doi: 10.1016/s0891-5520(05)70120-3. vii. [DOI] [PubMed] [Google Scholar]
- 2.Meddings L, Myers RP, Hubbard J, Shaheen AA, Laupland KB, Dixon E, Coffin C, Kaplan GG. A population-based study of pyogenic liver abscesses in the United States: Incidence, mortality, and temporal trends. Am J Gastroenterol. 2010;105:117–124. doi: 10.1038/ajg.2009.614. [DOI] [PubMed] [Google Scholar]
- 3.Chia DWJ, Kuan WS, Ho WH, Sim TB, Chua MT. Early predictors for the diagnosis of liver abscess in the emergency department. Intern Emerg Med. 2019;14:783–791. doi: 10.1007/s11739-019-02061-z. [DOI] [PubMed] [Google Scholar]
- 4.Lin AC, Yeh DY, Hsu YH, Wu CC, Chang H, Jang TN, Huang CH. Diagnosis of pyogenic liver abscess by abdominal ultrasonography in the emergency department. Emerg Med J. 2009;26:273–275. doi: 10.1136/emj.2007.049254. [DOI] [PubMed] [Google Scholar]
- 5.Pearl R, Pancu D, Legome E. Hepatic abscess. J Emerg Med. 2005;28:337–339. doi: 10.1016/j.jemermed.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 6.McClure MB, Patel K, Cabrera G, Kalivoda EJ. Point-of-care ultrasound diagnosis of a pyogenic liver abscess in the emergency department. J Am Coll Emerg Physicians Open. 2021;2(e12412) doi: 10.1002/emp2.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mavilia MG, Molina M, Wu GY. The evolving nature of hepatic abscess: A review. J Clin Transl Hepatol. 2016;4:158–168. doi: 10.14218/JCTH.2016.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivero-León A, Núñez-Calatayud M. Modified amoebic liver abscess: Case report. Rev Colomb Gastroenterol. 2022;37:242–247. [Google Scholar]
- 9.Gallego A, Ramírez A, Gallego A. Pyogenic liver abscess, polycystic bag and chronic renal failure. Rev Elec Dr. Zoilo E: Marinello Vidaurreta 43: 2, 2018. https://revzoilomarinello.sld.cu/index.php/zmv/article/view/1233. [Google Scholar]
- 10.Rossi G, Lafont E, Rossi B, Dokmak S, Ronot M, Zarrouk V, Fantin B, Lefort A. Liver Abscess. EMC-Tratado de Medicina. 2018;22:1–10. In Spanish. https://www.sciencedirect.com/science/article/abs/pii/S1636541017878685. [Google Scholar]
- 11.Mourad MM, Liossis C, Algarni A, Kumar S, Bramhall SR. Primary hepatic tuberculosis in immunocompetent adults: A UK case series. Oxf Med Case Reports. 2014;2014:148–150. doi: 10.1093/omcr/omu056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stanley SL Jr. Amoebiasis. Lancet. 2003;361:1025–1034. doi: 10.1016/S0140-6736(03)12830-9. [DOI] [PubMed] [Google Scholar]
- 13.Rahimian J, Wilson T, Oram V, Holzman RS. Pyogenic liver abscess: Recent trends in etiology and mortality. Clin Infect Dis. 2004;39:1654–1659. doi: 10.1086/425616. [DOI] [PubMed] [Google Scholar]
- 14.Price JC, Thio CL. Liver disease in the HIV-infected individual. Clin Gastroenterol Hepatol. 2010;8:1002–1012. doi: 10.1016/j.cgh.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sáchez-Pobre P, Sáenz-López S, Salto E, Sanjuán R, Ibero C, Masedo A, Solís Herruzo JA. Amebic liver abscess with bacterial superinfection in a patient with no epidemiologic risk factors. Rev Esp Enferm Dig. 2004;96:796–800. doi: 10.4321/s1130-01082004001100007. (In English, Spanish) [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, Yu H, Luo N, Hu Z. Clinical characteristics and treatment outcomes in human immunodeficiency virus (HIV)-infected patients with liver abscess: A retrospective study of 53 patients. Med Sci Monit. 2020;26(e923761) doi: 10.12659/MSM.923761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rendón Unceta P, Macías Rodríguez MA, Correro Aguilar F, Prieto García JL, Díaz García F, Martín Herrera L. Hepatic abscesses: Is simple aspiration puncture with echography control an alternative to catheter drainage? Gastroenterol Hepatol. 2000;23:470–473. (In Spanish) [PubMed] [Google Scholar]
- 18.Boccatonda A, D'Ardes D, Cocco G, Cipollone F, Schiavone C. Ultrasound and hepatic abscess: A successful alliance for the internist. Eur J Intern Med. 2019;68:e19–e21. doi: 10.1016/j.ejim.2019.07.034. [DOI] [PubMed] [Google Scholar]
- 19.Colović N, Jurišić V, Terzić T, Jevtovic D, Colović M. Alveolar granulocytic sarcoma of the mandible in a patient with HIV. Onkologie. 2011;34:55–58. doi: 10.1159/000317351. [DOI] [PubMed] [Google Scholar]
- 20.Yuan Z, Ma R, Zhang Q, Zhao CS. doi: 10.2174/1570162X21666221128153942. Statistical inferences of HIVRNA and hepatocellular carcinoma based on the PAK1 expression via neural network model. Curr HIV Res: Nov 28, 2022 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 21.Khim G, Em S, Mo S, Townell N. Liver abscess: Diagnostic and management issues found in the low resource setting. Br Med Bull. 2019;132:45–52. doi: 10.1093/bmb/ldz032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cardona-Castro W, Zuluaga-Gómez M, González-Arroyave D, Ardila CM. Accuracy of point-of-care ultrasonography in the diagnosis of necrotizing fasciitis: A case report. Biomed Rep. 2022;17(98) doi: 10.3892/br.2022.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glišić TM, Perišić MD, Dimitrijevic S, Jurišić V. Doppler assessment of splanchnic arterial flow in patients with liver cirrhosis: Correlation with ammonia plasma levels and MELD score. J Clin Ultrasound. 2014;42:264–269. doi: 10.1002/jcu.22135. [DOI] [PubMed] [Google Scholar]
- 24.Elia F, Campagnaro T, Salacone P, Casalis S. Goal-directed ultrasound in a resource-constrained healthcare setting and a country Developing. Crit Ultrasonido J. 2011;3:51–53. In Spanish. https://www.medigraphic.com/pdfs/revcliescmed/ucr-2015/ucr155j.pdf. [Google Scholar]
- 25.Weinke T, Grobusch MP, Güthoff W. Amebic liver abscess-rare need for percutaneous treatment modalities. Eur J Med Res. 2002;7:25–29. [PubMed] [Google Scholar]
- 26.Vidal JE, da Silva PR, Schiavon Nogueira R, Bonasser Filho F, Hernandez AV. Liver abscess due to Salmonella enteritidis in a returned traveler with HIV infection: Case report and review of the literature. Rev Inst Med Trop Sao Paulo. 2003;45:115–117. doi: 10.1590/s0036-46652003000200014. [DOI] [PubMed] [Google Scholar]
- 27.Mortelé KJ, Segatto E, Ros PR. The infected liver: Radiologic-pathologic correlation. Radiographics. 2004;24:937–955. doi: 10.1148/rg.244035719. [DOI] [PubMed] [Google Scholar]
- 28.Malik AA, Bari SU, Rouf KA, Wani KA. Pyogenic liver abscess: Changing patterns in approach. World J Gastrointest Surg. 2010;2:395–401. doi: 10.4240/wjgs.v2.i12.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNeil T, Daniel S, Gordon DL. Management of pyogenic liver abscess: A South Australian experience. ANZ J Surg. 2020;90:2274–2278. doi: 10.1111/ans.15963. [DOI] [PubMed] [Google Scholar]
- 30.Chen MJ, Huang MJ, Chang WH, Wang TE, Wang HY, Chu CH, Lin SC, Shih SC. Ultrasonography of splenic abnormalities. World J Gastroenterol. 2005;11:4061–4066. doi: 10.3748/wjg.v11.i26.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuo SH, Lee YT, Li CR, Tseng CJ, Chao WN, Wang PH, Wong RH, Chen CC, Chen SC, Lee MC. Mortality in emergency department sepsis score as a prognostic indicator in patients with pyogenic liver abscess. Am J Emerg Med. 2013;31:916–921. doi: 10.1016/j.ajem.2013.02.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.


